Abstract
Infectious complications following interventional radiology (IR) procedures can cause significant patient morbidity and, potentially, mortality. As the number and breadth of IR procedures grow, it becomes increasingly evident that interventional radiologists must possess a thorough understanding of these potential infectious complications. Furthermore, given the increasing incidence of antibiotic-resistant bacteria, emphasis on cost containment, and attention to quality of care, it is critical to have infection control strategies to maximize patient safety. This article reviews infectious complications associated with percutaneous ablation of liver tumors, transarterial embolization of liver tumors, uterine fibroid embolization, percutaneous nephrostomy, percutaneous biliary interventions, central venous catheters, and intravascular stents. Emphasis is placed on incidence, risk factors, prevention, and management. With the use of these strategies, IR procedures can be performed with reduced risk of infectious complications.
Keywords: complication, infection, catheter-related, interventional radiology
Objectives: Upon completion of this article, the reader will be able to identify the incidence, risk factors, prevention, and management of infectious complications associated with commonly performed procedures in interventional radiology.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Infectious complications can occur following virtually any interventional radiology (IR) procedure. Despite new antimicrobial agents and an improved understanding of the immune system, these complications continue to have a profound impact on patient morbidity and mortality. This review focuses on the occurrence, prevention, and interventional management of infectious complications particular to the following percutaneous interventions: thermal ablation of liver tumors, transarterial embolization of liver tumors, uterine fibroid embolization (UFE), percutaneous nephrostomy, biliary interventions, central venous catheter (CVC) placement, and intravascular stents.
Infectious Complications Following Thermal Ablation
Thermal ablation of solid tumors utilizes extreme temperatures to induce irreversible cellular injury, apoptosis, and coagulative necrosis. Thermal ablation can be divided into high-temperature (radiofrequency ablation, RFA; microwave ablation, MWA) and low-temperature modalities (cryoablation), and is most commonly performed on tumors in liver, kidney, lung, and bone. Overall, development of postablation abscess is very rare. Development of hepatic abscess and/or cholangitis following RFA has been reported in ∼0.1 to 2% of cases,1 2 3 4 5 and abscess formation after thermal ablation of renal tumors is generally reported at <1%.6 7 Pulmonary abscess formation after thermal ablation of lung lesions is also extremely rare, occurring in ∼0.5 to 1.6%.8 9 While one series on cryoablation of bone lesions in 61 patients reported a single episode of osteomyelitis at the site of ablation, no reports of infection after RFA or MWA of bone lesions were identified.10
Postablation Syndrome
Patients undergoing thermal ablation can experience constitutional symptoms following the procedure including fever, malaise, nausea/vomiting, and pain. This postablation syndrome likely reflects a systemic inflammatory response to cytokines released from ablated tissues. This syndrome has been reported to occur with both hepatic and renal ablation in approximately one-third of patients.11 12 A prospective survey conducted by Wah et al in patients undergoing liver or kidney RFA reported low-grade fever in 42% of patients and flulike symptoms (malaise, myalgia, and nausea/vomiting) in 81% of patients.12 In that study, symptoms peaked on postprocedure day 3 and generally subsided by postprocedure day 10. Symptoms are expectantly managed with nonsteroidal anti-inflammatory drugs, analgesics, and antiemetics. High-grade or persistent fever following ablation could indicate an infectious complication such as abscess.
Hepatic Abscess
The most important risk factor for hepatic abscess development following thermal ablation of a hepatic tumor is a colonized biliary tract (e.g., biliary enteric anastomosis, endoscopic or percutaneous biliary drainage [PBD], pneumobilia, sphincterotomy).4 In the largest series to date, De Baere et al described RFA in nine patients who had a colonized biliary tract, with four (44%) patients developing a hepatic abscess.13 Notably, in this study various prophylactic antibiotic regimens were administered before and after the procedure. The efficacy of antibiotic prophylaxis to prevent abscess, however, was difficult to assess given the variety of agents used and small number of patients in the study. While no definitive antibiotic regimen can be recommended, the high incidence of postprocedure abscess in this population further corroborates prior biliary tract procedures as an important risk factor for abscess development following liver ablation.
While the exact mechanism of abscess formation following RFA is unknown, it has been suggested that bacterial colonization of bile can lead to colonization of the post-RFA ablation zone and abscess. The occurrence of abscess following RFA in patients with a history of biliary tract manipulation ranges from 13 to 62 days after ablation.13 The delay in abscess formation may indicate that bacterial superinfection of the ablated zone may play a role in abscess development.14 Regardless of the mechanism, the delay before abscess formation in patients with prior biliary manipulation is important to consider when following patients after liver RFA. Computed tomography (CT) findings of liver abscess are nonspecific, but include well-defined fluid-density collections, higher density foci indistinguishable from tumors, rim enhancement, and intralesional gas.15 However, benign gas bubbles can be seen in the RFA zone and surrounding vasculature (Fig. 1). An abscess should be suspected in the setting of continued enlargement of the ablation defect with newly formed or increasing gas collections (Fig. 2).16 In the postablation period, differentiation of infection from postablation syndrome can be difficult, since postablation syndrome includes fever. The reported prevalence of postablation fever is 19 to 34%, with a duration of 1 to 9 days,17 while the time interval between ablation and liver abscess ranges from 8 days to 5 months3 18 19; thus, some investigators suggest that if fever persists beyond 2 weeks, the possibility of an abscess should be entertained.1
Fig. 1.
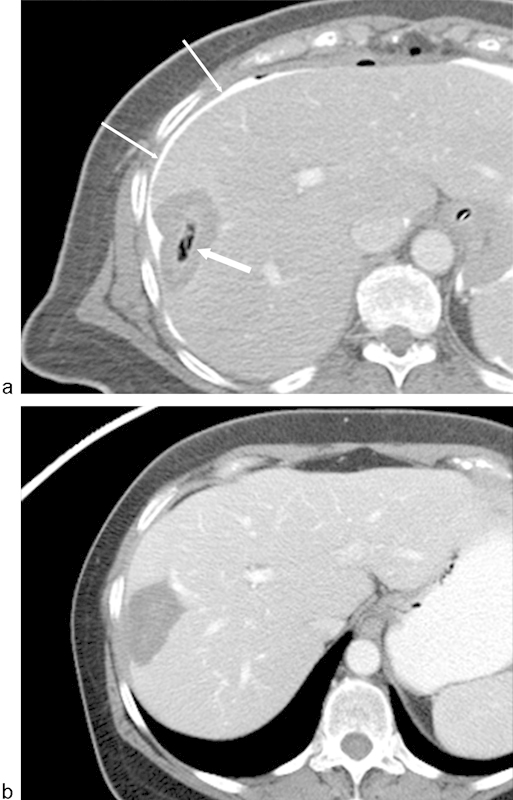
Gas in the region of ablation following radiofrequency ablation (RFA) of a liver metastasis from breast carcinoma. (a) Axial image from a CT scan with contrast immediately following RFA demonstrates gas within the zone of ablation (arrow). Because of the location of the metastasis adjacent to the liver capsule and chest wall, a solution of 5% dextrose water and iodinated contrast was instilled in the perihepatic space (thin arrows). Note that the gas bubbles do not correspond to the ablation margins. (b) Axial CT image with contrast 3 months following ablation demonstrates resolution of gas. There is also no evidence of enhancing tissue to suggest residual disease.
Fig. 2.
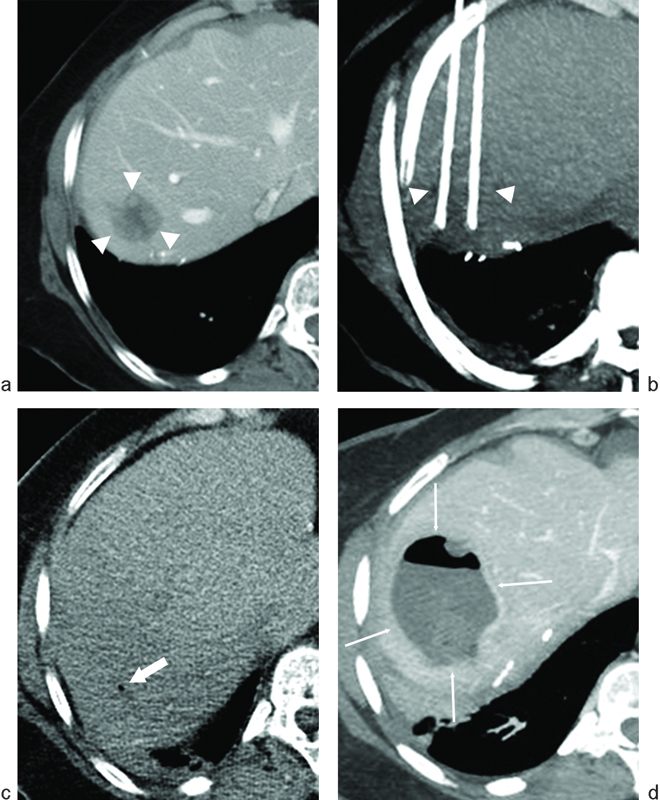
Hepatic abscess following radiofrequency ablation (RFA) of liver metastasis from pancreatic neuroendocrine carcinoma. The patient had previously undergone a Whipple procedure 2 years prior to RFA. (a) Axial image from a CT scan with contrast demonstrates a hypodense lesion near the hepatic dome (arrowheads) consistent with a hepatic metastasis. (b) Thick-slab reformatted axial CT image during ablation demonstrates the RFA probes along the medial and lateral aspects of the lesion (arrowheads). (c) Axial CT image without contrast immediately following ablation demonstrates a small focus of gas (arrow), which is normal following liver RFA. (d) Axial CT image with contrast performed 3 weeks following RFA demonstrates findings of a hepatic abscess following RFA (thin arrows): rim enhancement, low-density center, and increasing intralesional gas.
Management of Hepatic Abscess
The mainstay of management for hepatic abscess is catheter drainage.20 Smaller collections (<1 cm) are generally too small for catheter drainage and are thus aspirated for microbiology to allow optimization of antibiotic therapy. Larger collections (>1 cm) are treated with catheter drainage and antibiotics. Independent predictors of failure include the presence of yeast, biliary communication, and multiloculation.21 22 For abscesses as a result of prior RFA, in the study published by Elias et al, catheter drainage was successful in all four cases.13
Cholangitis
An additional infectious consideration following ablation of liver tumors is cholangitis.4 The Tokyo guidelines are generally used to make the definitive diagnosis of acute cholangitis.23 However, during the postablation period, the clinical picture is difficult to interpret as pain and elevated liver function enzymes are common.24 25 Shibata et al proposed the diagnosis of cholangitis when all the following criteria were met: fever > 38°C lasting more than 3 days, doubling of the baseline serum bilirubin level or doubling of the baseline serum alkaline phosphatase level, and new intrahepatic biliary ductal dilatation.4 It should be noted that this definition has not been validated. Nevertheless, in their study of 683 ablation procedures, there were 2 cases of cholangitis (0.3%). Generally, patients with acute cholangitis are treated with antibiotics though there is no consensus regarding the initial regimen. Typically, patients are given broad-spectrum antibiotics covering colonic bacteria, with therapy modified to reflect any organisms recovered in blood cultures. Seventy to 80% of patients with acute cholangitis will respond to conservative measures so that biliary decompression can be delayed 24 to 48 hours.26 27 28
Nonhepatic Abscesses
Given the extremely low incidence of postablation abscess formation of nonhepatic tumors, there is a paucity of literature and data on this topic. However, many of the principles of hepatic abscess are translatable to nonhepatic abscesses. In general, renal abscesses present with fever, leukocytosis, flank pain, and pyuria, and are generally managed with intravenous (IV) antibiotics and catheter drainage.29 30 Patients with a urinary tract infection at the time of or after ablation are theoretically at risk of seeding the necrotic tumor. The mainstay of treatment of pulmonary abscesses and osteomyelitis involves antibiotic therapy, although surgical management may be needed for refractory cases.
Antibiotic Prophylaxis
There is no consensus on the effectiveness of prophylactic antibiotics for patients undergoing thermal ablation to reduce the risk of postprocedural infection.31 32 If antibiotics are administered, they should be targeted at the most likely pathogens. For the liver, organisms potentially encountered include Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus species, Escherichia Coli Proteus species, Klebsiella species, and enterococcus species. Ampicillin/sulbactam IV can be given within 1 hour of starting the procedure, and for penicillin-allergic patients, vancomycin or clindamycin can be given for gram-positive coverage and an aminoglycoside (e.g., gentamicin) for gram-negative coverage.32 For kidney ablations, 1 g ceftriaxone IV can be considered.32 For bone ablations, 1 g cefazolin IV can be given.32
Infectious Complications Associated with Hepatic Arterial Embolotherapies
Bland transarterial embolization (TAE), transarterial chemoembolization (TACE), and radioembolization are palliative treatments for patients with unresectable liver malignancies. Embolization causes ischemia; TACE combines ischemia from embolization with local delivery of chemotherapy; and radioembolization with yttrium-90 (Y-90) causes radiation injury to liver tumors.
Postembolization Syndrome
In the postprocedural period, it is important to distinguish an infectious complication from postembolization syndrome (PES). PES is a spectrum of symptoms that can occur following transarterial embolization of various end organs, and can consist of abdominal pain, nausea/vomiting, malaise, and low-grade fever. These symptoms are commonly encountered after various hepatic arterial embolotherapies.32 33 34 PES may not be considered a complication, but rather an expected systemic reaction to the embolization. The exact pathophysiology of PES remains unknown, but is likely related to a systemic inflammatory reaction to tissue necrosis. Medical management of PES typically consists of supportive treatment using nonsteroidal anti-inflammatory agents, analgesics, and antiemetics. Specific therapeutic regimens vary among institutions and are dependent on drug availability and patient factors, although narcotic pain medications are commonly used. Prophylactic use of dexamethasone and scopolamine has not been shown to reduce the incidence of PES.35
Benign Intratumoral Gas
Imaging follow-up is generally obtained between 1 and 3 months after TAE, TACE, or radioembolization. CT or magnetic resonance imaging (MRI) scans are assessed for changes in tumor morphology, changes in tumor size, and additional liver lesions. As previously described, intralesional gas can be a sign of a liver abscess. However, if CT imaging is performed in the acute period within 2 weeks of embolization, this finding can also be seen following bland embolization and chemoembolization, as a result of tumor necrosis and injection of microscopic quantities of gas associated with embolic particles (Fig. 3).36 37 Such benign intratumoral gas tends to manifest as multiple tiny foci of gas throughout the tumor, rather than a larger focal collection of gas. In a study involving intrahepatic tumor embolization of three patients and an experiment using the V2 carcinoma implanted in the liver of a rabbit, Carroll and Walter found that benign intrahepatic gas persisted for 5 to 10 days following embolization, as monitored by serial ultrasound examinations.38 Thus, unless there are systemic signs and symptoms of infection, postprocedural intratumoral gas should not necessarily be regarded as a sign of abscess formation from gas-producing bacterial species, particularly in the first 1 to 2 weeks following embolization.
Fig. 3.
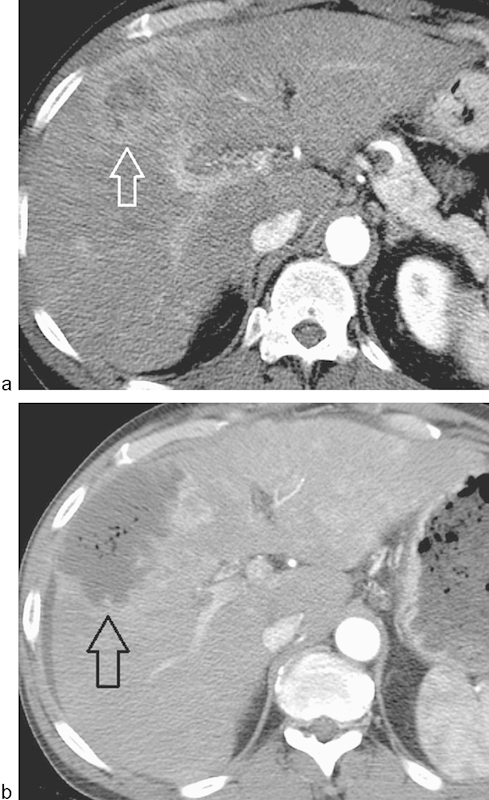
Benign intralesional gas postembolization. (a) A heterogeneous enhancing mass is present in the right lobe (arrow), which was treated with bland embolization using 150–250 µm polyvinyl alcohol particles to stasis. (b) 10 days later, the patient underwent contrast-enhanced CT due to persistent abdominal pain. A nonenhancing region measuring 20 Hounsfield units containing multiple bubbles was demonstrated (arrow). The patient was otherwise afebrile without leukocytosis and had an otherwise uneventful course without antibiotic therapy.
Hepatic Abscess Formation
Abscess formation is a rare complication following embolotherapies of the liver (Fig. 4). The reported rate is <1% in patients with a competent sphincter of Oddi.39 However, similar to liver ablation, the most important risk factor for developing a postembolization abscess is an incompetent sphincter of Oddi; in this particular patient cohort, the incidence of liver abscess following TACE is as high as 85 to 100%.40 41 Abscess formation appears to be a much rarer event following radioembolization even despite an incompetent sphincter of Oddi, with only case reports in the literature.42 43 44 Cholapranee et al compared a cohort of such patients following radioembolization with Y-90 resin microspheres with a cohort of patients following oily TACE.43 Both cohorts were administered the same antibiotic regimen before and after embolization. There were no abscesses among the 16 radioembolization patients (0%), while 3 of 13 (23%) patients developed an abscess following TACE (p = 0.078). While the study is limited by small sample size and heterogeneous tumor type, these results are compatible with the general evidence supporting a very low incidence of hepatic abscesses with radioembolization.
Fig. 4.
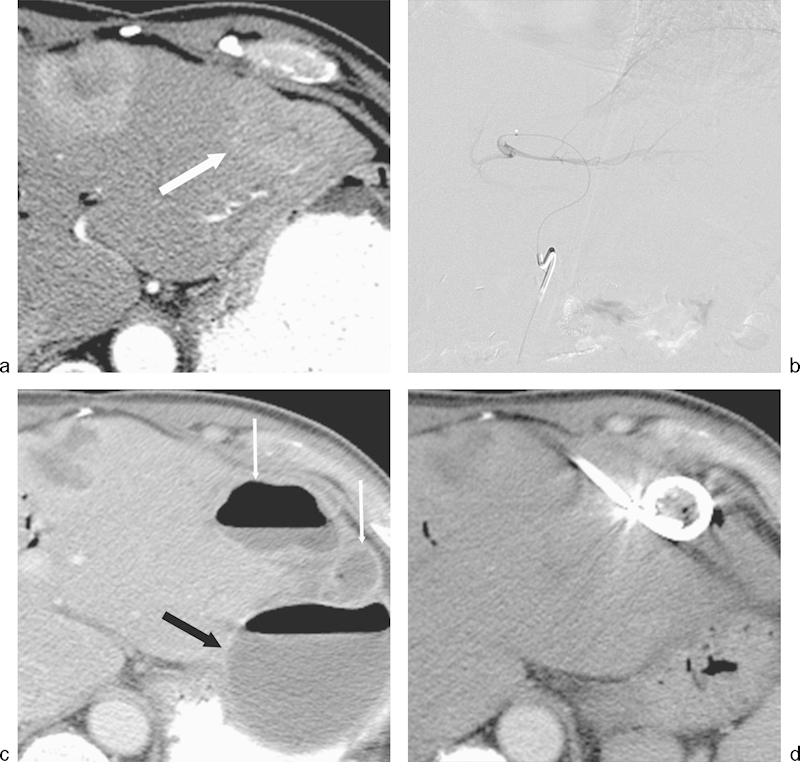
Hepatic abscess following transarterial bland embolization for metastatic neuroendocrine cancer. The patient had previously undergone a Whipple procedure 3 years prior to embolization. (a) Axial image from a CT scan during the arterial phase on enhancement demonstrates a hyperenhancing lesion in the left hepatic lobe (arrow) consistent with a hepatic metastasis. (b) Bland particle embolization of the segment 2 and 3 left hepatic artery was performed with 355–500 µm polyvinyl alcohol particles. Image from a digital subtraction angiogram demonstrates no significant residual enhancement of the left hepatic lobe lesion. (c) Axial CT image with contrast performed 3 weeks following RFA demonstrates findings of a complex hepatic abscess (thin arrows). A large extrahepatic abscess was also present (black arrow). The complex intrahepatic and extrahepatic abscesses were drained percutaneously. (d) Axial CT image performed 2 weeks following percutaneous drainage demonstrates interval resolution of both abscesses.
The pathophysiology behind abscess formation following chemoembolization is unclear. One potential mechanism is biliary ischemia by occlusion of the peribiliary plexus, which is supplied solely by the hepatic artery. Patients without an intact sphincter of Oddi have a biliary tree colonized by intestinal flora.43 The combination biliary ischemia/necrosis and bacterial colonization may provide the nidus for abscess development. On the other hand, bile duct injury was not seen on histologic examination of the liver during a swine study using resin Y-90 microspheres.45 While selection biases may certainly exist for the literature on the various therapies, the much more prolonged cellular death with radioembolization compared with TACE or bland embolization may be one contributory etiology.
Antibiotic Prophylaxis
The effectiveness of antibiotic prophylaxis for TAE, TACE, and radioembolization is unproven. Nonetheless, the Society of Interventional Radiology (SIR) Clinical Practice Guidelines recommends administration of antibiotic prophylaxis to cover skin flora and gram-negative enteric organisms. Common antibiotic regimens utilize either 1 g ceftriaxone or 1.5 to 3 g ampicillin/sulbactam, or for patient who are penicillin-allergic, vancomycin or clindamycin plus an aminoglycoside. In patients without an intact sphincter of Oddi, three commonly referenced antibiotic regimens are as follows:
Piperacillin/tazobactam sodium every 6 hours beginning 24 to 36 hours before treatment and bowel preparation (45 mL of oral fleet phospho soda, 1 g oral neomycin, 1 g oral erythromycin, 4 tablets oral bisacodyl, and 1 rectal suppository of bisacodyl).41
Oral levofloxacin daily and metronidazole twice daily, beginning 48 hours before the procedure; 1 g neomycin and 1 g erythromycin administered at 1 pm, 2 pm, and 11 pm on the day before the procedure; levofloxacin and metronidazole IV continued as an inpatient, and then transitioned to oral form for two weeks after discharge.46
Oral moxifloxacin 400 mg daily beginning 3 days before the procedure and continuing for 17 days after the procedure.47
While data for all three antibiotic regimens are sparse, the regimen proposed by Khan et al consisting of single agent moxifloxacin offers the distinct advantage of no bowel preparation. Bowel preparation not only is unpleasant for patients, but, in the case of phospho soda, also can cause electrolyte aberrations and dehydration.48
Management of Hepatic Abscess
The management of hepatic abscess following TAE, TACE, and radioembolization is catheter drainage and systemic antibiotics based on abscess culture results (see section on hepatic abscess management after thermal ablation). Following TACE, causative organisms include Enterococcus, Enterobacter, Citrobacter, Pseudomonas, Streptococcus, and E. Coli.40
Infectious Complications of Uterine Fibroid Embolization
UFE has become established as an effective method in the treatment of symptomatic uterine fibroids. In a recent systematic review analyzing 8,159 patients, the rate of infectious complications following UFE was 2.5%.49 Potential infectious sequelae include endometritis and pyomyoma. Endometritis, which presents with fever, pain, and bacteremia, is typically treated with antibiotics.50 In severe refractory cases, hysterectomy can be performed. Infection of a necrotic fibroid is extremely rare but can be life-threatening.51 The source of infection may be contiguous spread from the endometrial cavity or hematogenous colonization. Copious amounts of air within the fibroid and myometrium may be present.52 Hysterectomy is the definitive treatment. Routine IV antibiotic prophylaxis is recommended by the SIR Guidelines, with common choices including either 1 g cefazolin, 900 mg clindamycin with 1.5 mg/kg gentamycin, 3 g ampicillin, 1.5 to 3 g ampicillin/sulbactam, or vancomycin in a patient who is penicillin-allergic.32
As with hepatic embolotherapies, infectious sequelae after UFE must also be differentiated from PES, which is characterized by fever, pain, nausea, and vomiting.53 An increase in serum white blood cell count is commonly noted following UFE and likely reflects a systemic response to tissue ischemia.54 However, leukocytosis can also indicate infectious complications and cannot exclusively be attributed to PES. As with PES after hepatic embolotherapies, treatment is conservative. Patient-controlled analgesia pumps, nonsteroidal anti-inflammatory drugs such as ketorolac, and meperidine are commonly used for PES related to UFE.55 56
Infectious Complications of Percutaneous Nephrostomy
Infectious complications associated with percutaneous catheter nephrostomy (PCN) can occur during or after catheter placement. In the immediate postprocedural period, the most common and serious systemic complication is sepsis.57 Every obstructed system is potentially infected and the operator should be keenly aware that seemingly minor maneuvers, such as contrast media injection, can allow pressurization of bacteria retrograde across the nephron into the systemic circulation. The incidence of septic shock during PCN placement is 1.3 to 1.8%, although the incidence of bacteremia is likely much higher. In the setting of pyonephrosis, the incidence increases to 7%.57 58 59 Prophylactic antibiotics are typically given prior to PCN placement or exchange to minimize infectious episodes, although this has not been well studied in the medical literature. Current SIR Guidelines recommend prophylactic antibiotics in all cases except for routine exchange of noninfected, nonobstructed collecting systems in immunocompetent patients.32 Usually, the causative organisms are gram-negative bacteria, including E. coli, Proteus, and Klebsiella, though a common gram-positive pathogen is Enterococcus.60
Infectious complications after PCN insertion can also occur weeks to months following placement, with an incidence of 1.1 to 19%, although it should be noted that most studies are small, uncontrolled, and lack a unifying definition of nephrostomy-related pyelonephritis.61 62 63 64 Factors predisposing to infection include advanced age, diabetes, stones, bacteriuria, ureterointestinal conduits, and indwelling catheters.65 While essentially all initially sterile indwelling PCNs and collecting systems eventually become colonized with resulting bacteriuria or candiduria at a mean of 6 weeks postinsertion, patients are rarely symptomatic with infection unless catheter obstruction occurs.66 Catheter exchange is generally recommended, since clinically significant infection typically only occurs in the setting of catheter obstruction.66 Based on Foley urinary catheter studies, biofilm formation on the inner surface of these catheters provide a microenvironment suitable for high counts of microorganisms to embed and survive, which is also poorly penetrated by antimicrobials.67 68 69 However, the extent to which biofilm formation occurs with nephrostomy catheters, and thus applicability of this urinary catheter literature to nephrostomy catheters, is unknown.
Infectious Complications of Biliary Interventions
Percutaneous transhepatic cholangiogram (PTC) and PBD catheter insertion are performed in the setting of benign or malignant obstruction. In a retrospective review of 206 patients with biliary obstruction undergoing PTC and biliary drainage, bile cultures were positive in 43% of patients with malignancy and 68% of patients with benign disease.70 Clinical factors associated with an increased risk for a positive bile culture and/or sepsis include prior bilioenteric anastomosis, previous biliary instrumentation, advanced age (older than 70 years), obstructive jaundice, acute cholecystitis, and diabetes mellitus.71 72 73 74 75 From a procedural perspective, using ultrasound guidance to decrease the number of parenchymal passes and draining the biliary system before contrast injection are techniques to theoretically mitigate the risk of infectious complications.76 The risk of sepsis (defined as the presence of infection with systemic manifestations) following biliary interventional procedures ranges from 0.8 to 2.3%, although the incidence of bacteremia is likely much higher.74 77 78 Pressurization of bile that occurs in the setting of obstruction and during instrumentation can result in retrograde passage of bacteria (and bile) across the sinusoids, manifesting as bacteremia and sepsis.
In patients with indwelling PBD catheters, the biliary system will be colonized with bacteria, since the distal portion of the catheter is in the small bowel, thus allowing free migration of enteric organisms into the biliary tree. Generally, colonization is asymptomatic as long as the drainage catheter is not occluded. If patients develop signs or symptoms or infection (e.g., fever, hypotension, and leukocytosis) with a PBD catheter in place, the biliary drainage catheter should be placed to external gravity drainage and interrogated/exchanged as soon as possible. Catheter occlusion from debris, catheter kinking, or catheter malposition may be present. Catheter upsizing may be helpful in the setting of recurrent catheter obstruction.
Antibiotic Prophylaxis
Organisms often encountered in an infected biliary tree include Enterococcus, Candida, gram-negative aerobic bacilli, Streptococcus viridians, E. Coli, Clostridium, Klebsiella, Pseudomonas, Enterobacter cloacae, Bacteroides, and various yeasts.32 Given the high rate of infectious complications, which can be life-threatening, prophylactic antibiotic administration is recommended by the SIR Clinical Practice Guidelines.32 In the absence of prior positive culture results from bile to guide antimicrobial therapy, common empiric IV antibiotic choices include 1 g ceftriaxone, 1.5 to 3 g ampicillin/sulbactam, 1 g cefotetan with 4 g mezlocillin, 2 g ampicillin with 1.5 mg/kg gentamycin, or if penicillin-allergic, vancomycin or clindamycin with an aminoglycoside can be administered.32 It should be noted that blood stream infections have also been reported to occur following PTC and manipulations involving preexisting catheters,74 thus prophylactic antibiotics should be used prior to any biliary intervention.
Percutaneous Cholecystostomy
Percutaneous cholecystostomy is commonly performed for acute cholecystitis when surgery is considered high risk.79 As with biliary interventions, sepsis can occur immediately or within days following the procedure.80 Colonization of the gallbladder can also occur within days following the procedure and is most often asymptomatic. Positive bile culture results have been reported to vary between 16 and 49%.81 82 83 If the patient is not already receiving appropriate IV antibiotics, prophylactic antibiotics are recommended using the same regimen as for biliary drainage. Similar to drainage of the biliary ductal tree, mechanical agitation during placement of the percutaneous cholecystostomy catheter should be minimized to prevent bacteremia. Drainage of bile prior to injecting contrast is also prudent to prevent overpressurizing the gallbladder.
Central Venous Catheter–Related Infections
Catheter-related blood stream infections (CRBSIs) are an important source of morbidity, mortality, and cost.84 85 Implantable ports have the lowest incidence (0.1 per 1,000 catheter days) followed by peripherally inserted central catheters (1.1 per 1,000 catheter days), tunneled CVCs (1.6 per 1,000 catheter days), and nontunneled CVCs (2.7 per 1,000 catheter days).86 The pathogenesis of catheter infection may involve (1) colonization of the catheter at the skin exit site with migration along the outer catheter surface into the venous system; (2) contamination of the catheter hub that results in contamination of the lumen of the catheter; (3) hematogenous seeding from a remote source of infection; or (4) infusate contamination. The most common etiologic organisms are coagulase-negative Staphylococcus, S. aureus, Enterococci, and Candida.87
Prevention of Central Venous Catheter–Related Infection
Prevention of CRBSIs requires a multidisciplinary effort, involving the implanting physician, personnel who access the catheter, and the patient. Interventional radiologists should adhere to standard hand hygiene procedures, aseptic technique for catheter insertion and maintenance, maximum sterile barrier (i.e., cap, mask, sterile gown, sterile gloves, sterile body drape, and appropriate skin cleansing with >0.5% chlorhexidine preparation with alcohol or alternative agent).88 89 90 Femoral vein access for nontunneled catheters in patients with high body mass index is associated with higher rates of infection.91 Finally, greater numbers of lumens have been associated with a higher infection risk.92 93 Daily cleansing of the catheter skin exit site in ICU patients with a 2% chlorhexidine wash may be a simple way to reduce the rate of blood stream infections.94 Second, the choice of an antibiotic and/or antiseptic impregnated catheter and cuff has also been shown to reduce CRBSIs.95 However, because of costs associated with device acquisition and development of microbial resistance, multidisciplinary consensus guidelines limit the use of these catheters to patients needing central venous access for > 5 days and in facilities that have failed to reduce their blood stream infection rates despite implementation of comprehensive infection reduction strategies.96 Third, some physicians routinely administer prophylactic antibiotics prior to tunneled catheters and implantable ports. However, there is no level 1 data to support this practice.32 97 Unlike administering systemic antimicrobials, flushing and locking CVCs with a combined antibiotic and heparin solution appears to significantly reduce the risk of septicemia relative to a group of patients given heparin only (risk ratio 0.47, 95% confidence interval 0.28–0.80; p = 0.005).97 Thus, while no conclusive recommendation for systemic antimicrobials can be made, the judicious use of antibiotics in specific clinical scenarios (e.g., immunocompromised patients or those with a history of infection) may be reasonable.32
Management of Central Venous Catheter–Related Infection
Generally, the management of catheter-related infection requires a decision regarding whether the catheter should be removed, salvaged with antibiotic therapy, or exchanged. In general, IV antibiotics are promptly initiated upon diagnosis or suspicion of catheter-related infection. Immediate catheter removal is warranted in the setting of severe sepsis, hemodynamic instability, endocarditis/metastatic infection, persistent bacteremia (despite 72 hours of appropriate antimicrobial therapy), or exudate due to suppurative thrombophlebitis.98 It is generally accepted that catheter removal is the optimal and most definitive treatment for catheter-related infection. For implanted ports, whenever overlying cellulitis or purulence in the port pocket is encountered, the incision and pocket should be left open to heal by secondary intention, using packed gauze to prevent skin closure over the colonized granulation tissue followed by routine dressing changes. Otherwise, in the setting of a completely normal appearing skin and port pocket, the incision can be closed primarily, with close follow-up inspection for potential infection of the port pocket with abscess development. Conservative management for catheter salvage may be attempted in uncomplicated blood stream infections without evidence of tract infection (for tunneled catheters) or port-site infection (for port catheters) for pathogens other than S. aureus, Pseudomonas aeruginosa, fungi, or mycobacteria.98 Blood cultures should be obtained 72 hours after appropriate antimicrobial therapy with persistently positive culture results prompting catheter removal. In cases where continued catheter use is necessary but the risk of failed insertion at a new site is high (e.g., limited vascular access in patients with multiple central venous occlusions or severe coagulopathy), access salvage utilizing guidewire exchange may be acceptable. Several small and nonrandomized studies reported generally successful management of catheter-related infections with guidewire exchange of tunneled catheters.99 100 101 The National Kidney Foundation recommends initiation of antibiotic therapy for treatment of infected hemodialysis catheters, with catheter exchange as soon as possible and within 72 hours of initiating antibiotic therapy, and follow-up blood cultures 1 week after cessation of antibiotic therapy.102
Stent and Stent Graft Infection in Arteries, Veins, and Arteriovenous Grafts
Vascular endoprostheses (i.e., stents and stent grafts) are an indispensable component of any endovascular practice. Reports of infectious complications following bare metal stent placement for treatment of coronary, renal, and iliac atherosclerosis, as well as central venous stenosis, are relatively sparse within the literature and they remain a very rare complication.103 104 105 106 107 Infectious complications following placement of stent grafts are also rare. Vascular stents and stent graft infections may occur due to perioperative contamination or latent seeding from subsequent interventions, transient bacteremia, or erosion into adjacent structures. While presentation will be variable depending on the location of the stent, fever, chills, malaise, pain, petechiae, and persistent bacteremia will typically be present. Local manifestation of stent infection can include arteritis, abscess, mycotic aneurysm, vascular rupture, and septicemia. In the case of aortic endografts, delayed graft infection can be associated with aortoenteric fistulae.108 While there are no radiologic modalities shown to be both sensitive and specific for stent or stent graft infection, the use of stent grafts to treat prosthetic arteriovenous graft pseudoaneurysms has been shown to be associated with a particularly high risk of infection (42%) of the adjacent graft material, requiring graft resection.109 Unfortunately, stent infection is often a diagnosis of exclusion, as no radiologic methods have been proven to be effective for this diagnosis, although white blood cell scintigraphy has been suggested to be helpful.105
The definitive treatment of an infected endoprosthesis is surgical removal and IV antibiotics. However, this can be associated with significant perioperative risk, particularly with aortic endografts. Perigraft abscesses can be drained percutaneously or surgically; in cases of significant perioperative risk, conservative medical management may be most appropriate.110 Prophylactic antibiotics are typically administered prior to stent graft placement (e.g., endovascular aortic aneurysm repair and transjugular intrahepatic portosystemic shunt creation) given the theoretically increased risk of bacterial adherence to PTFE material used in stent grafts.32 Staphylococcal coverage with IV cefazolin or vancomycin is frequently used.
Conclusion
Infection can be an important complication following IR procedures. Successful prevention of infectious complications must begin at the initial preprocedural evaluation, with identification of relevant risk factors. High-risk patients (e.g., patients with prior biliary surgery undergoing hepatic RFA or embolization) and high-risk procedures (e.g., PTC, biliary drain in the setting of cholangitis) should be treated with prophylactic antibiotics. In some cases, however, patients may develop infectious complications despite appropriate intraprocedural and periprocedural care. It is important to follow these patients closely so that appropriate management, often from a multidisciplinary team, can be administered.
References
- 1.Rhim H Yoon K H Lee J M et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings Radiographics 2003231123–134., discussion 134–136 [DOI] [PubMed] [Google Scholar]
- 2.Livraghi T, Solbiati L, Meloni M F, Gazelle G S, Halpern E F, Goldberg S N. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 3.de Baère T, Risse O, Kuoch V. et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181(3):695–700. doi: 10.2214/ajr.181.3.1810695. [DOI] [PubMed] [Google Scholar]
- 4.Shibata T, Yamamoto Y, Yamamoto N. et al. Cholangitis and liver abscess after percutaneous ablation therapy for liver tumors: incidence and risk factors. J Vasc Interv Radiol. 2003;14(12):1535–1542. doi: 10.1097/01.rvi.0000099532.29957.4f. [DOI] [PubMed] [Google Scholar]
- 5.Mulier S, Mulier P, Ni Y. et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89(10):1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 6.Atwell T D, Schmit G D, Boorjian S A. et al. Percutaneous ablation of renal masses measuring 3.0 cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. AJR Am J Roentgenol. 2013;200(2):461–466. doi: 10.2214/AJR.12.8618. [DOI] [PubMed] [Google Scholar]
- 7.Schmit G D, Schenck L A, Thompson R H. et al. Predicting renal cryoablation complications: new risk score based on tumor size and location and patient history. Radiology. 2014;272(3):903–910. doi: 10.1148/radiol.14132548. [DOI] [PubMed] [Google Scholar]
- 8.Zheng A, Wang X, Yang X. et al. Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg. 2014;98(1):243–248. doi: 10.1016/j.athoracsur.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Kashima M, Yamakado K, Takaki H. et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol. 2011;197(4):W576-80. doi: 10.2214/AJR.11.6408. [DOI] [PubMed] [Google Scholar]
- 10.Callstrom M R, Dupuy D E, Solomon S B. et al. Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer. 2013;119(5):1033–1041. doi: 10.1002/cncr.27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodd G D III, Napier D, Schoolfield J D, Hubbard L. Percutaneous radiofrequency ablation of hepatic tumors: postablation syndrome. AJR Am J Roentgenol. 2005;185(1):51–57. doi: 10.2214/ajr.185.1.01850051. [DOI] [PubMed] [Google Scholar]
- 12.Wah T M, Arellano R S, Gervais D A. et al. Image-guided percutaneous radiofrequency ablation and incidence of post-radiofrequency ablation syndrome: prospective survey. Radiology. 2005;237(3):1097–1102. doi: 10.1148/radiol.2373042008. [DOI] [PubMed] [Google Scholar]
- 13.Elias D, Di Pietroantonio D, Gachot B, Menegon P, Hakime A, De Baere T. Liver abscess after radiofrequency ablation of tumors in patients with a biliary tract procedure. Gastroenterol Clin Biol. 2006;30(6-7):823–827. doi: 10.1016/s0399-8320(06)73327-9. [DOI] [PubMed] [Google Scholar]
- 14.Wood B J, Abraham J, Hvizda J L, Alexander H R, Fojo T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer. 2003;97(3):554–560. doi: 10.1002/cncr.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halvorsen R A, Korobkin M, Foster W L, Silverman P M, Thompson W M. The variable CT appearance of hepatic abscesses. AJR Am J Roentgenol. 1984;142(5):941–946. doi: 10.2214/ajr.142.5.941. [DOI] [PubMed] [Google Scholar]
- 16.Akahane M, Koga H, Kato N. et al. Complications of percutaneous radiofrequency ablation for hepato-cellular carcinoma: imaging spectrum and management. Radiographics. 2005;25 01:S57–S68. doi: 10.1148/rg.25si055505. [DOI] [PubMed] [Google Scholar]
- 17.Choi H, Loyer E M, DuBrow R A. et al. Radio-frequency ablation of liver tumors: assessment of therapeutic response and complications. Radiographics. 2001;21(Spec No):S41–S54. doi: 10.1148/radiographics.21.suppl_1.g01oc08s41. [DOI] [PubMed] [Google Scholar]
- 18.Zagoria R J, Chen M Y, Shen P, Levine E A. Complications from radiofrequency ablation of liver metastases. Am Surg. 2002;68(2):204–209. [PubMed] [Google Scholar]
- 19.Wood T F, Rose D M, Chung M, Allegra D P, Foshag L J, Bilchik A J. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7(8):593–600. doi: 10.1007/BF02725339. [DOI] [PubMed] [Google Scholar]
- 20.Huang C J Pitt H A Lipsett P A et al. Pyogenic hepatic abscess. Changing trends over 42 years Ann Surg 19962235600–607., discussion 607–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mezhir J J, Fong Y, Jacks L M. et al. Current management of pyogenic liver abscess: surgery is now second-line treatment. J Am Coll Surg. 2010;210(6):975–983. doi: 10.1016/j.jamcollsurg.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Lai K C, Cheng K S, Jeng L B. et al. Factors associated with treatment failure of percutaneous catheter drainage for pyogenic liver abscess in patients with hepatobiliary-pancreatic cancer. Am J Surg. 2013;205(1):52–57. doi: 10.1016/j.amjsurg.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Mayumi T, Takada T, Kawarada Y. et al. Results of the Tokyo Consensus Meeting Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14(1):114–121. doi: 10.1007/s00534-006-1163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGhana J P, Dodd G D III. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176(1):3–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- 25.Shibata T, Iimuro Y, Yamamoto Y. et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223(2):331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- 26.Kimura Y, Takada T, Kawarada Y. et al. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14(1):15–26. doi: 10.1007/s00534-006-1152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salek J, Livote E, Sideridis K, Bank S. Analysis of risk factors predictive of early mortality and urgent ERCP in acute cholangitis. J Clin Gastroenterol. 2009;43(2):171–175. doi: 10.1097/MCG.0b013e318157c62c. [DOI] [PubMed] [Google Scholar]
- 28.Hui C K, Lai K C, Yuen M F, Ng M, Lai C L, Lam S K. Acute cholangitis—predictive factors for emergency ERCP. Aliment Pharmacol Ther. 2001;15(10):1633–1637. doi: 10.1046/j.1365-2036.2001.01071.x. [DOI] [PubMed] [Google Scholar]
- 29.Siegel J F, Smith A, Moldwin R. Minimally invasive treatment of renal abscess. J Urol. 1996;155(1):52–55. [PubMed] [Google Scholar]
- 30.Best C D, Terris M K, Tacker J R, Reese J H. Clinical and radiological findings in patients with gas forming renal abscess treated conservatively. J Urol. 1999;162(4):1273–1276. [PubMed] [Google Scholar]
- 31.Beddy P, Ryan J M. Antibiotic prophylaxis in interventional radiology—anything new? Tech Vasc Interv Radiol. 2006;9(2):69–76. doi: 10.1053/j.tvir.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Venkatesan A M Kundu S Sacks D et al. Practice guidelines for adult antibiotic prophylaxis during vascular and interventional radiology procedures J Vasc Interv Radiol 201021111611–1630., quiz 1631 [DOI] [PubMed] [Google Scholar]
- 33.Słomka M, Radwan P. The evaluation of clinical results of hepatic artery embolization. Mater Med Pol. 1992;24(3):193–195. [PubMed] [Google Scholar]
- 34.Leung D A, Goin J E, Sickles C, Raskay B J, Soulen M C. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12(3):321–326. doi: 10.1016/s1051-0443(07)61911-3. [DOI] [PubMed] [Google Scholar]
- 35.Kogut M J, Chewning R H, Harris W P, Hippe D S, Padia S A. Postembolization syndrome after hepatic transarterial chemoembolization: effect of prophylactic steroids on postprocedure medication requirements. J Vasc Interv Radiol. 2013;24(3):326–331. doi: 10.1016/j.jvir.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Gates J, Hartnell G G, Stuart K E, Clouse M E. Chemoembolization of hepatic neoplasms: safety, complications, and when to worry. Radiographics. 1999;19(2):399–414. doi: 10.1148/radiographics.19.2.g99mr08399. [DOI] [PubMed] [Google Scholar]
- 37.Shah P A, Cunningham S C, Morgan T A, Daly B D. Hepatic gas: widening spectrum of causes detected at CT and US in the interventional era. Radiographics. 2011;31(5):1403–1413. doi: 10.1148/rg.315095108. [DOI] [PubMed] [Google Scholar]
- 38.Carroll B A, Walter J F. Gas in embolized tumors: an alternate hypothesis for its origin. Radiology. 1983;147(2):441–444. doi: 10.1148/radiology.147.2.6300960. [DOI] [PubMed] [Google Scholar]
- 39.Sakamoto I, Aso N, Nagaoki K. et al. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18(3):605–619. doi: 10.1148/radiographics.18.3.9599386. [DOI] [PubMed] [Google Scholar]
- 40.Kim W, Clark T W, Baum R A, Soulen M C. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12(8):965–968. doi: 10.1016/s1051-0443(07)61577-2. [DOI] [PubMed] [Google Scholar]
- 41.Geschwind J F, Kaushik S, Ramsey D E, Choti M A, Fishman E K, Kobeiter H. Influence of a new prophylactic antibiotic therapy on the incidence of liver abscesses after chemoembolization treatment of liver tumors. J Vasc Interv Radiol. 2002;13(11):1163–1166. doi: 10.1016/s1051-0443(07)61959-9. [DOI] [PubMed] [Google Scholar]
- 42.Mascarenhas N B, Mulcahy M F, Lewandowski R J, Salem R, Ryu R K. Hepatic abscess after yttrium-90 radioembolization for islet-cell tumor hepatic metastasis. Cardiovasc Intervent Radiol. 2010;33(3):650–653. doi: 10.1007/s00270-009-9617-4. [DOI] [PubMed] [Google Scholar]
- 43.Cholapranee A, van Houten D, Deitrick G. et al. Risk of liver abscess formation in patients with prior biliary intervention following yttrium-90 radioembolization. Cardiovasc Intervent Radiol. 2015;38(2):397–400. doi: 10.1007/s00270-014-0947-5. [DOI] [PubMed] [Google Scholar]
- 44.Mascarenhas N, Ryu R K, Salem R. Hepatic radioembolization complicated by abscess. Semin Intervent Radiol. 2011;28(2):222–225. doi: 10.1055/s-0031-1280669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bilbao J I, de Martino A, de Luis E. et al. Biocompatibility, inflammatory response, and recannalization characteristics of nonradioactive resin microspheres: histological findings. Cardiovasc Intervent Radiol. 2009;32(4):727–736. doi: 10.1007/s00270-009-9592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel S, Tuite C M, Mondschein J I, Soulen M C. Effectiveness of an aggressive antibiotic regimen for chemoembolization in patients with previous biliary intervention. J Vasc Interv Radiol. 2006;17(12):1931–1934. doi: 10.1097/01.RVI.0000244854.79604.C1. [DOI] [PubMed] [Google Scholar]
- 47.Khan W, Sullivan K L, McCann J W. et al. Moxifloxacin prophylaxis for chemoembolization or embolization in patients with previous biliary interventions: a pilot study. AJR Am J Roentgenol. 2011;197(2):W343-5. doi: 10.2214/AJR.10.6019. [DOI] [PubMed] [Google Scholar]
- 48.Ehrenpreis E D. Increased serum phosphate levels and calcium fluxes are seen in smaller individuals after a single dose of sodium phosphate colon cleansing solution: a pharmacokinetic analysis. Aliment Pharmacol Ther. 2009;29(11):1202–1211. doi: 10.1111/j.1365-2036.2009.03987.x. [DOI] [PubMed] [Google Scholar]
- 49.Toor S S, Jaberi A, Macdonald D B, McInnes M D, Schweitzer M E, Rasuli P. Complication rates and effectiveness of uterine artery embolization in the treatment of symptomatic leiomyomas: a systematic review and meta-analysis. AJR Am J Roentgenol. 2012;199(5):1153–1163. doi: 10.2214/AJR.11.8362. [DOI] [PubMed] [Google Scholar]
- 50.Kitamura Y, Ascher S M, Cooper C. et al. Imaging manifestations of complications associated with uterine artery embolization. Radiographics. 2005;25 01:S119–S132. doi: 10.1148/rg.25si055518. [DOI] [PubMed] [Google Scholar]
- 51.Payne J F, Haney A F. Serious complications of uterine artery embolization for conservative treatment of fibroids. Fertil Steril. 2003;79(1):128–131. doi: 10.1016/s0015-0282(02)04398-4. [DOI] [PubMed] [Google Scholar]
- 52.Rosen M L, Anderson M L, Hawkins S M. Pyomyoma after uterine artery embolization. Obstet Gynecol. 2013;121(2 Pt 2) 01:431–433. doi: 10.1097/aog.0b013e31827e8e8f. [DOI] [PubMed] [Google Scholar]
- 53.Hovsepian D M, Siskin G P, Bonn J. et al. Quality improvement guidelines for uterine artery embolization for symptomatic leiomyomata. Cardiovasc Intervent Radiol. 2004;27(4):307–313. doi: 10.1007/s00270-004-0087-4. [DOI] [PubMed] [Google Scholar]
- 54.Ganguli S, Faintuch S, Salazar G M, Rabkin D J. Postembolization syndrome: changes in white blood cell counts immediately after uterine artery embolization. J Vasc Interv Radiol. 2008;19(3):443–445. doi: 10.1016/j.jvir.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 55.Siskin G P, Stainken B F, Dowling K, Meo P, Ahn J, Dolen E G. Outpatient uterine artery embolization for symptomatic uterine fibroids: experience in 49 patients. J Vasc Interv Radiol. 2000;11(3):305–311. doi: 10.1016/s1051-0443(07)61422-5. [DOI] [PubMed] [Google Scholar]
- 56.Siskin G P, Bonn J, Worthington-Kirsch R L. et al. III. Uterine fibroid embolization: pain management. Tech Vasc Interv Radiol. 2002;5(1):35–43. doi: 10.1053/tvir.2002.124727. [DOI] [PubMed] [Google Scholar]
- 57.Wah T M, Weston M J, Irving H C. Percutaneous nephrostomy insertion: outcome data from a prospective multi-operator study at a UK training centre. Clin Radiol. 2004;59(3):255–261. doi: 10.1016/j.crad.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 58.Farrell T A, Hicks M E. A review of radiologically guided percutaneous nephrostomies in 303 patients. J Vasc Interv Radiol. 1997;8(5):769–774. doi: 10.1016/s1051-0443(97)70658-4. [DOI] [PubMed] [Google Scholar]
- 59.Larsen E H, Gasser T C, Madsen P O. Antimicrobial prophylaxis in urologic surgery. Urol Clin North Am. 1986;13(4):591–604. [PubMed] [Google Scholar]
- 60.McDermott V G, Schuster M G, Smith T P. Antibiotic prophylaxis in vascular and interventional radiology. AJR Am J Roentgenol. 1997;169(1):31–38. doi: 10.2214/ajr.169.1.9207497. [DOI] [PubMed] [Google Scholar]
- 61.Skolarikos A, Alivizatos G, Papatsoris A, Constantinides K, Zerbas A, Deliveliotis C. Ultrasound-guided percutaneous nephrostomy performed by urologists: 10-year experience. Urology. 2006;68(3):495–499. doi: 10.1016/j.urology.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 62.Rana A M, Zaidi Z, El-Khalid S. Single-center review of fluoroscopy-guided percutaneous nephrostomy performed by urologic surgeons. J Endourol. 2007;21(7):688–691. doi: 10.1089/end.2006.0281. [DOI] [PubMed] [Google Scholar]
- 63.Radecka E, Magnusson A. Complications associated with percutaneous nephrostomies. A retrospective study. Acta Radiol. 2004;45(2):184–188. doi: 10.1080/02841850410003671. [DOI] [PubMed] [Google Scholar]
- 64.Bahu R, Chaftari A M, Hachem R Y. et al. Nephrostomy tube related pyelonephritis in patients with cancer: epidemiology, infection rate and risk factors. J Urol. 2013;189(1):130–135. doi: 10.1016/j.juro.2012.08.094. [DOI] [PubMed] [Google Scholar]
- 65.Ryan J M, Ryan B M, Smith T P. Antibiotic prophylaxis in interventional radiology. J Vasc Interv Radiol. 2004;15(6):547–556. doi: 10.1097/01.rvi.000024942.58200.5e. [DOI] [PubMed] [Google Scholar]
- 66.Cronan J J, Marcello A, Horn D L, Robinson A, Dorfman G S, Opal S. Antibiotics and nephrostomy tube care: preliminary observations. Part I. Bacteriuria. Radiology. 1989;172(3, Pt 2):1041–1042. doi: 10.1148/172.3.1041. [DOI] [PubMed] [Google Scholar]
- 67.Raz R, Schiller D, Nicolle L E. Chronic indwelling catheter replacement before antimicrobial therapy for symptomatic urinary tract infection. J Urol. 2000;164(4):1254–1258. [PubMed] [Google Scholar]
- 68.Kunin C M, Steele C. Culture of the surfaces of urinary catheters to sample urethral flora and study the effect of antimicrobial therapy. J Clin Microbiol. 1985;21(6):902–908. doi: 10.1128/jcm.21.6.902-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nickel J C, Ruseska I, Wright J B, Costerton J W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27(4):619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yee A C, Ho C S. Complications of percutaneous biliary drainage: benign vs malignant diseases. AJR Am J Roentgenol. 1987;148(6):1207–1209. doi: 10.2214/ajr.148.6.1207. [DOI] [PubMed] [Google Scholar]
- 71.Chetlin S H, Elliott D W. Biliary bacteremia. Arch Surg. 1971;102(4):303–307. doi: 10.1001/archsurg.1971.01350040065012. [DOI] [PubMed] [Google Scholar]
- 72.Keighley M R. Micro-organisms in the bile. A preventable cause of sepsis after biliary surgery. Ann R Coll Surg Engl. 1977;59(4):328–334. [PMC free article] [PubMed] [Google Scholar]
- 73.Mueller P R, van Sonnenberg E, Ferrucci J T Jr. Percutaneous biliary drainage: technical and catheter-related problems in 200 procedures. AJR Am J Roentgenol. 1982;138(1):17–23. doi: 10.2214/ajr.138.1.17. [DOI] [PubMed] [Google Scholar]
- 74.Clark C D, Picus D, Dunagan W C. Bloodstream infections after interventional procedures in the biliary tract. Radiology. 1994;191(2):495–499. doi: 10.1148/radiology.191.2.8153328. [DOI] [PubMed] [Google Scholar]
- 75.Brody L A, Brown K T, Getrajdman G I. et al. Clinical factors associated with positive bile cultures during primary percutaneous biliary drainage. J Vasc Interv Radiol. 1998;9(4):572–578. doi: 10.1016/s1051-0443(98)70324-0. [DOI] [PubMed] [Google Scholar]
- 76.Lorenz J M, Leef J A, Chou C H, Funaki B, Straus C M, Rosenblum J D. Sonographic needle guidance in cholangiography in children. J Vasc Interv Radiol. 2001;12(3):342–346. doi: 10.1016/s1051-0443(07)61914-9. [DOI] [PubMed] [Google Scholar]
- 77.Hamlin J A, Friedman M, Stein M G, Bray J F. Percutaneous biliary drainage: complications of 118 consecutive catheterizations. Radiology. 1986;158(1):199–202. doi: 10.1148/radiology.158.1.3940380. [DOI] [PubMed] [Google Scholar]
- 78.Funaki B, Zaleski G X, Straus C A. et al. Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts. AJR Am J Roentgenol. 1999;173(6):1541–1544. doi: 10.2214/ajr.173.6.10584798. [DOI] [PubMed] [Google Scholar]
- 79.McGahan J P, Lindfors K K. Percutaneous cholecystostomy: an alternative to surgical cholecystostomy for acute cholecystitis? Radiology. 1989;173(2):481–485. doi: 10.1148/radiology.173.2.2678261. [DOI] [PubMed] [Google Scholar]
- 80.Akhan O, Akinci D, Ozmen M N. Percutaneous cholecystostomy. Eur J Radiol. 2002;43(3):229–236. doi: 10.1016/s0720-048x(02)00158-4. [DOI] [PubMed] [Google Scholar]
- 81.Boland G W, Lee M J, Leung J, Mueller P R. Percutaneous cholecystostomy in critically ill patients: early response and final outcome in 82 patients. AJR Am J Roentgenol. 1994;163(2):339–342. doi: 10.2214/ajr.163.2.8037026. [DOI] [PubMed] [Google Scholar]
- 82.Chopra S, Dodd G D III, Mumbower A L. et al. Treatment of acute cholecystitis in non-critically ill patients at high surgical risk: comparison of clinical outcomes after gallbladder aspiration and after percutaneous cholecystostomy. AJR Am J Roentgenol. 2001;176(4):1025–1031. doi: 10.2214/ajr.176.4.1761025. [DOI] [PubMed] [Google Scholar]
- 83.England R E, McDermott V G, Smith T P, Suhocki P V, Payne C S, Newman G E. Percutaneous cholecystostomy: who responds? AJR Am J Roentgenol. 1997;168(5):1247–1251. doi: 10.2214/ajr.168.5.9129421. [DOI] [PubMed] [Google Scholar]
- 84.Dimick J B, Pelz R K, Consunji R, Swoboda S M, Hendrix C W, Lipsett P A. Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch Surg. 2001;136(2):229–234. doi: 10.1001/archsurg.136.2.229. [DOI] [PubMed] [Google Scholar]
- 85.Blot S I, Depuydt P, Annemans L. et al. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis. 2005;41(11):1591–1598. doi: 10.1086/497833. [DOI] [PubMed] [Google Scholar]
- 86.Maki D G, Kluger D M, Crnich C J. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 87.Wisplinghoff H, Bischoff T, Tallent S M, Seifert H, Wenzel R P, Edmond M B. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 88.Abi-Said D, Raad I, Umphrey J. et al. Infusion therapy team and dressing changes of central venous catheters. Infect Control Hosp Epidemiol. 1999;20(2):101–105. doi: 10.1086/501597. [DOI] [PubMed] [Google Scholar]
- 89.Raad I I, Hohn D C, Gilbreath B J. et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15(4, Pt 1):231–238. [PubMed] [Google Scholar]
- 90.Maki D G, Ringer M, Alvarado C J. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet. 1991;338(8763):339–343. doi: 10.1016/0140-6736(91)90479-9. [DOI] [PubMed] [Google Scholar]
- 91.Parienti J J, Thirion M, Mégarbane B. et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA. 2008;299(20):2413–2422. doi: 10.1001/jama.299.20.2413. [DOI] [PubMed] [Google Scholar]
- 92.Yeung C, May J, Hughes R. Infection rate for single lumen v triple lumen subclavian catheters. Infect Control Hosp Epidemiol. 1988;9(4):154–158. doi: 10.1086/645820. [DOI] [PubMed] [Google Scholar]
- 93.Early T F, Gregory R T, Wheeler J R, Snyder S O Jr, Gayle R G. Increased infection rate in double-lumen versus single-lumen Hickman catheters in cancer patients. South Med J. 1990;83(1):34–36. doi: 10.1097/00007611-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 94.Bleasdale S C, Trick W E, Gonzalez I M, Lyles R D, Hayden M K, Weinstein R A. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med. 2007;167(19):2073–2079. doi: 10.1001/archinte.167.19.2073. [DOI] [PubMed] [Google Scholar]
- 95.Veenstra D L, Saint S, Saha S, Lumley T, Sullivan S D. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA. 1999;281(3):261–267. doi: 10.1001/jama.281.3.261. [DOI] [PubMed] [Google Scholar]
- 96.O'Grady N P, Alexander M, Burns L A. et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4) 01:S1–S34. doi: 10.1016/j.ajic.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 97.van de Wetering M D, van Woensel J B, Lawrie T A. Prophylactic antibiotics for preventing Gram positive infections associated with long-term central venous catheters in oncology patients. Cochrane Database Syst Rev. 2013;11:CD003295. doi: 10.1002/14651858.CD003295.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mermel L A, Allon M, Bouza E. et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Robinson D, Suhocki P, Schwab S J. Treatment of infected tunneled venous access hemodialysis catheters with guidewire exchange. Kidney Int. 1998;53(6):1792–1794. doi: 10.1046/j.1523-1755.1998.00954.x. [DOI] [PubMed] [Google Scholar]
- 100.Martínez E, Mensa J, Rovira M. et al. Central venous catheter exchange by guidewire for treatment of catheter-related bacteraemia in patients undergoing BMT or intensive chemotherapy. Bone Marrow Transplant. 1999;23(1):41–44. doi: 10.1038/sj.bmt.1701538. [DOI] [PubMed] [Google Scholar]
- 101.Shaffer D. Catheter-related sepsis complicating long-term, tunnelled central venous dialysis catheters: management by guidewire exchange. Am J Kidney Dis. 1995;25(4):593–596. doi: 10.1016/0272-6386(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 102.National Kidney Foundation KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: Hemodialysis adequacy, peritoneal dialysis adequacy and vascular access Am J Kidney Dis 20064801S1–S322.17045862 [Google Scholar]
- 103.Günther H U, Strupp G, Volmar J, von Korn H, Bonzel T, Stegmann T. Coronary stent implantation: infection and abscess with fatal outcome [in German] Z Kardiol. 1993;82(8):521–525. [PubMed] [Google Scholar]
- 104.DeMaioribus C A, Anderson C A, Popham S S, Yeager T D, Cordts P R. Mycotic renal artery degeneration and systemic sepsis caused by infected renal artery stent. J Vasc Surg. 1998;28(3):547–550. doi: 10.1016/s0741-5214(98)70143-0. [DOI] [PubMed] [Google Scholar]
- 105.Chalmers N, Eadington D W, Gandanhamo D, Gillespie I N, Ruckley C V. Case report: infected false aneurysm at the site of an iliac stent. Br J Radiol. 1993;66(790):946–948. doi: 10.1259/0007-1285-66-790-946. [DOI] [PubMed] [Google Scholar]
- 106.Guest S S, Kirsch C M, Baxter R, Sorooshian M, Young J. Infection of a subclavian venous stent in a hemodialysis patient. Am J Kidney Dis. 1995;26(2):377–380. doi: 10.1016/0272-6386(95)90661-4. [DOI] [PubMed] [Google Scholar]
- 107.Hogg M E, Peterson B G, Pearce W H, Morasch M D, Kibbe M R. Bare metal stent infections: case report and review of the literature. J Vasc Surg. 2007;46(4):813–820. doi: 10.1016/j.jvs.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 108.Heyer K S, Modi P, Morasch M D. et al. Secondary infections of thoracic and abdominal aortic endografts. J Vasc Interv Radiol. 2009;20(2):173–179. doi: 10.1016/j.jvir.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 109.Kim C Y, Guevara C J, Engstrom B I. et al. Analysis of infection risk following covered stent exclusion of pseudoaneurysms in prosthetic arteriovenous hemodialysis access grafts. J Vasc Interv Radiol. 2012;23(1):69–74. doi: 10.1016/j.jvir.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Blanch M, Berjón J, Vila R. et al. The management of aortic stent-graft infection: endograft removal versus conservative treatment. Ann Vasc Surg. 2010;24(4):5540–5.54E7. doi: 10.1016/j.avsg.2009.11.003. [DOI] [PubMed] [Google Scholar]


