Abstract
Iatrogenic injury to the urinary tract, including the kidneys, ureters, bladder, and urethra, is a potential complication of surgical procedures performed in or around the retroperitoneal abdominal space or pelvis. While both diagnostic and interventional radiologists often play a central and decisive role in the identification and initial management of a variety of iatrogenic injuries, discussions of these injuries are often directed toward specialists such as urologists, obstetricians, gynecologists, and general surgeons whose procedures are most often implicated in iatrogenic urinary tract injuries. Interventional radiologic procedures can also be a source of an iatrogenic urinary tract injury. This review describes the clinical presentation, risk factors, imaging findings, and management of iatrogenic renal vascular and urinary tract injuries, as well as the radiologist's role in the diagnosis, treatment, and cause of these injuries.
Keywords: iatrogenic injury, renal vascular, ureter, bladder, interventional radiology
Objectives: Upon completion of this article, the reader should be able to demonstrate knowledge of the clinical presentation, symptoms, risk factors, imaging findings, and initial management strategies of iatrogenic renal vascular and urinary tract injuries, as well as diagnostic and interventional radiologists' role in the diagnosis, management, and/or cause of such trauma.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Iatrogenic injury to the urinary tract, including the kidneys, ureters, bladder, and urethra, is a possible complication of surgical procedures performed in the retroperitoneal abdominal space or in or around the pelvis. Procedures performed by urologic surgeons, obstetricians, gynecologists, and general surgeons have traditionally accounted for the majority of iatrogenic injuries to this region.1 2 3 4 5 Thus, awareness of such injuries is of utmost importance for these specialists. Indeed, lack of recognition at time of injury precludes immediate repair, and additional procedures are often required at later intervals to address the complications of missed iatrogenic injuries. These procedures are associated with their own morbidities and run the risk of loss of renal function, or worse, of the renal unit.2 Thus, prompt investigation and treatment of suspected iatrogenic injury is crucial to lessen the occurrence of subsequent complications.
Advances in surgical techniques and approaches, particularly the shift toward minimally invasive applications such as robotic and laparoscopic surgery, have expanded the potential for iatrogenic injury. Application of energy-based tissue devices in close proximity to the urinary tract can result in delayed presentation of an iatrogenic injury.3 Of note, the management of iatrogenic injuries has similarly transformed in recent years to focus on conservative, noninvasive, or minimally invasive techniques. This shift, in addition to early recognition and awareness, has laid the groundwork for minimizing morbidity and loss of renal function associated with such injuries. Evidence suggests that delayed diagnosis of iatrogenic injury is a major factor negatively influencing a patient's outcome.6 The importance of early diagnosis is further emphasized, as a delay in identification of a procedural complication could be of medicolegal significance.7
Emphasis of awareness is often directed toward those specialists whose procedures are most implicated as the cause of iatrogenic renal and genitourinary (GU) injuries. Importantly, both diagnostic and interventional radiologists often play a central and decisive role in the identification and initial management of a variety of iatrogenic injuries. Moreover, there has been a recent expansion of interventional angiographic and nonangiographic procedures performed frequently in the kidneys and GU tract by radiologists. Examples include stent placement, percutaneous drain placements, parenchymal biopsies, and oncologic interventions.8 Notably, these radiologic procedures themselves carry a risk of iatrogenic harm. Thus, it is of critical importance that radiologists maintain awareness not only of their role in diagnosing and treating iatrogenic injuries, but also of the mechanisms and risks of iatrogenic injury due to radiologic procedures themselves.
In this review, the authors discuss the clinical presentations, symptoms, risk factors, imaging findings, and management of iatrogenic urinary tract injuries that are of particular interest to diagnostic and interventional radiologists, focusing on the radiologists role in the diagnosis, treatment, and/or cause of such injuries.2 3 4 5 9 10 11 Specifically, iatrogenic renal vascular, ureteral and bladder injuries, including the mechanisms of injury, clinical features, and management options are discussed.
Iatrogenic Renal Vascular Injury
Etiology
Iatrogenic injury to the kidneys is rare but carries the potential for significant morbidity.4 Iatrogenic renal injuries include but are not limited to hemorrhage (Fig. 1), hematoma, pseudoaneurysm (Figs. 2 3 4 5), arteriovenous fistula (AVF) (Fig. 2), injury and disruption of the renal pelvicaliceal system, arteriocaliceal fistula, and renal foreign bodies such as retained wires, sponges, or other surgical or endourological instruments. The most common iatrogenic renal injuries are vascular. These arise during a variety of surgical and endourological procedures, including percutaneous renal biopsy, percutaneous nephrostomy (PCN), percutaneous nephrolithotomy (PCNL), endopyelotomy, and partial nephrectomy. Iatrogenic injuries to renal allografts after transplantation, though not specifically discussed in this review, are more common and may even include arterial dissection. More rare iatrogenic causes of renal bleeding include angiography, percutaneous transluminal renal artery angioplasty, and Double-J catheter insertion via the ureter for urinary drainage.12 Both PCNL and PCN are associated with specific complications related to gaining access to the collecting system and the nephrostomy tract itself. Michel et al describe potential injuries, such as lacerations, bleeding, parenchymal, and vascular lesions, which occur during puncture and dilatation of the nephrostomy tract.13
Fig. 1.

Axial (a) and coronal (b) noncontrast CT images of a 59-year-old man who presented to the emergency room in hemodynamic shock on postoperative day 9, following open partial nephrectomies for renal cell carcinoma. Noncontrast CT scan of the abdomen shows high attenuation fluid in the perinephric area and layering of high attenuation material, consistent with acute hemorrhage (arrow). Note mass effect on the kidney displacing the kidney anteriorly.
Fig. 2.

(a) An 82-year-old man presented with persistent hematuria following left partial nephrectomy for renal cell carcinoma. Contrast-enhanced CT scan shows a large pseudoaneurysm (arrow) and simultaneous opacification of the renal artery and renal vein representing arteriovenous fistula in the surgical bed. (b) Digital subtraction angiogram of the left renal artery confirms the pseudoaneurysm (arrow) and high flow arteriovenous fistula. (c) Digital subtraction angiogram of the left renal artery showing successful occlusion of the left renal artery pseudoaneurysm and arteriovenous fistula using combination of microcoils and thrombin. During embolization of the pseudoaneurysm and arteriovenous fistula, venous outflow was occluded using a balloon catheter placed in the left renal vein via right common femoral approach to prevent the possibility of nontarget embolization.
Fig. 3.
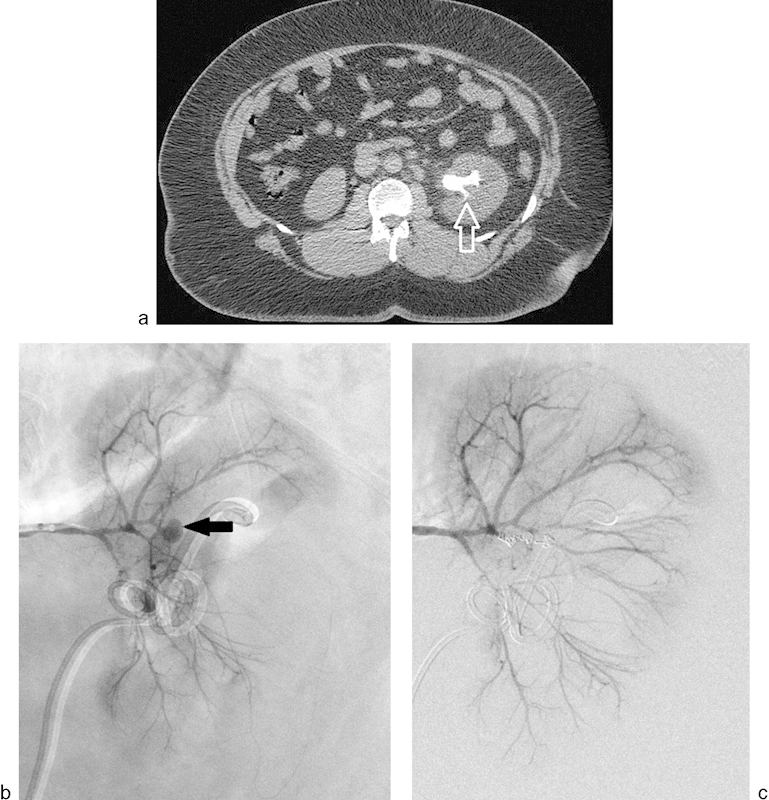
(a) Noncontrast CT scan of a 33-year-old man showing a staghorn calculus (arrow) within the left renal collecting system. The patient underwent nephrolithotomy for the stone and developed gross hematuria. (b) Left renal angiogram demonstrating an intrarenal pseudoaneurysm arising from a third-order branch of the left renal artery in the interpolar region (arrow). (c) Renal angiogram following embolization of the arterial supply using three 3-mm microcoils demonstrates complete occlusion of the pseudoaneurysm. Clinically, there was complete cessation of hematuria.
Fig. 4.
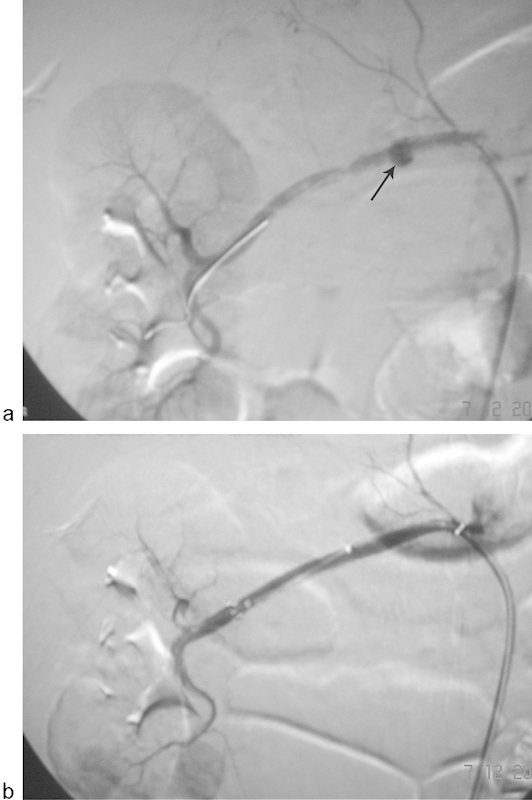
Digital subtraction angiography images of a 62-year-old woman following right renal artery angioplasty using 5 mm × 40 mm balloon catheter for renal artery stenosis. (a) Renal angiogram demonstrates development of a focal collection of contrast (arrow), consistent with a pseudoaneurysm. (b) Renal angiogram following deployment of 6 mm × 60 mm covered Fluency (Bard Peripheral Vascular, Inc. Tempe, AZ) stent. The pseudoaneurysm has been successfully excluded and good flow is noted in the artery.
Fig. 5.

A 76-year-old woman with history of rectal carcinoma underwent limited anterior resection and neoadjuvant chemoradiation therapy. Following chemoradiation and anterior resection, the patient developed bilateral hydronephrosis that was managed with bilateral ureteral stents. Six weeks after the stent placement, the patient presented with hematuria. (a) Contrast-enhanced CT scan demonstrates a pseudoaneurysm arising from the left external iliac artery (arrow), presumed to be due to erosion of the left distal ureter and external iliac artery by the ureteral stent. (b) Digital subtraction angiography of the left common iliac artery performed via a right common femoral artery approach confirms the above-described pseudoaneurysm (arrow) arising from the left external iliac artery. (c) Digital subtraction angiogram of the left common iliac artery following placement of an 8 mm × 50 mm Viabahn covered stent (Gore Inc., Flagstaff, AZ) across the above-described pseudoaneurysm demonstrating complete occlusion of the pseudoaneurysm. Clinically, following left common iliac artery endograft placement, there was complete cessation of hematuria.
Briefly, PCN and PCNL are procedures performed largely by interventional radiologists and urologists for a variety of indications. PCN is defined as image-guided insertion and placement of a catheter into the renal collecting system.14 This is successful if the catheter is of satisfactory size to permit drainage of the urinary collecting system or if a nephrostomy tract is adequately dilated to allow a planned intervention through the tract itself (such as PCNL). PCNL entails removal of a renal or proximal ureteral calculus through a rigid nephroscope placed in an adequately dilated nephrostomy tract. Sometimes, large stones may be fragmented (percutaneous nephrolithotripsy) before being extracted through the percutaneous access tract.
PCN is indicated for urinary obstruction caused by intrinsic or extrinsic sources (stones, malignancy, iatrogenic), infected hydronephrosis/pyonephrosis, urinary leakage or fistulas, access for other interventional procedures such as PCNL or antegrade ureteral stent placement, foreign body retrieval, chemotherapy delivery, urinary diversion for hemorrhagic cystitis, and emergent drainage in settings of severe shock due to urosepsis.12 14 Of note, PCNL has essentially replaced open surgery for nearly all types of renal calculi extraction in patients of all ages15 due to high technical success rates in the mid-high 90% range.12
However, despite the expanding role of percutaneous renal procedures as the initial choice in the treatment of multiple renal and ureteral pathologies, PCN and PCNL still carry a risk of multiple complications. In fact, these procedures have become the most common cause of renal vascular injury. Major complications of PCN include sepsis and septic shock, hemorrhage, vascular injury requiring embolization or nephrectomy, bowel transgression, pneumothorax, empyema, and hemothorax.14 Injuries due to PCNL are similar and can be divided into those due to nephrostomy access and those related to stone removal, such as renal pelvic injury.13 Of these, the most common and well-recognized issue is postprocedure bleeding due to renovascular or parenchymal injury.
The blood supply to the kidney is divided into anterior and posterior portions; these two territories are divided by a relatively avascular watershed zone known as Brödel's line.12 As the number of interventional procedures has increased, so too has the frequency of iatrogenic renal vascular injuries. The incidence of iatrogenic renal artery injury from percutaneous renal procedures has been reported to be 0.9 to 3.0%,16 17 while the rate of renal hemorrhage requiring intervention has been reported to be 0.3 to 1.4%.13 16 18 To avoid vascular damage, interventional radiologists traditionally target Brödel's line under fluoroscopic or ultrasound guidance for needle entry and access to the center of a posterior-facing, dorsolateral calyx.19 The renal vein and renal artery lie in the central part of the kidney, with the renal pelvis located posteriorly to these vessels. If this central portion of the renal pelvis is punctured at any time during access or stone removal, a major vascular structure may be injured, resulting in bleeding and other potential vascular complications. In addition, urinary leakage could occur due to incomplete sealing of the catheter tract, as the renal pelvis may not be completely surrounded by renal parenchymal tissue.12 An increased number of punctures and improper puncture site, for example, too medial of an approach, can be blamed for the development of vascular complications following percutaneous procedures. Therefore, it is of paramount importance to adhere to Brödel's line during initial access.19
Even correct technique can result in iatrogenic renal vascular injury.20 The most common cause of bleeding from renal vessels after these procedures is the formation of a pseudoaneurysm or AVF.21 These vascular lesions form when the nephrostomy tract passes close to or directly posterior to the renal hilum, which can cause laceration of interlobar and lower-pole arteries. The resulting damage in a high-pressure system will cause leakage into a lower pressure venous system, forming an AVF, or into renal parenchyma or hilar tissue, forming a pseudoaneurysm. On the other hand, laceration of larger anterior or posterior segmental arteries usually causes severe acute bleeding.22
Several authors have identified factors associated with an increased risk of forming vascular lesions and hemorrhage after PCNL.16 18 21 The presence of partial staghorn calculi, multiple nephrostomy tracts, the Amplatz method of dilation, and total stone burden/surface area of > 1,000 mm2 are significant predictors of blood loss.16 In one study, multiple nephrostomy tracts and the presence of staghorn calculi significantly predicted severe bleeding, defined as hemodynamic instability requiring blood transfusion and superselective embolization for treatment.18 The presence of a solitary kidney, inexperienced operator, and upper caliceal puncture are also significant risk factors for severe bleeding.16 In their report, El-Nahas and colleagues described physiologic compensatory hypertrophy and increased size of solitary kidneys leading to an increased risk of bleeding with more damage to renal tissue and vascular supply. In addition, upper caliceal punctures requiring a more oblique and longer nephrostomy tract often result in changes to the direction of the tract and a higher rate of injury to adjacent parenchyma and vasculature. Arteriosclerosis, diabetes mellitus, hypertension, and advanced age also predispose a patient to renal bleeding.22 Finally, Srivastava et al concluded that mean stone size significantly predicted severe vascular lesions following PCNL for symptomatic stone disease.21
Clinical Presentation
Clinically, patients with iatrogenic renal vascular injuries present with nonresolving postoperative gross hematuria, unremitting unilateral flank and/or loin pain, continued hemorrhage from the nephrostomy site, acute increase in serum creatinine, or a drop in hemoglobin/hematocrit that could manifest as hemodynamic instability.15 17 Vascular injuries such as AVF and pseudoaneurysm may manifest even 3 weeks after the initial percutaneous procedure.13
Imaging
Radiologists play a key role in the diagnosis of these injuries. Ierardi et al suggested that a postrenal procedure patient presenting with acute flank pain or gross hematuria should undergo color Doppler sonography, contrast-enhanced computed tomography (CT), or magnetic resonance (MR) angiography.17 Some authors describe renal angiography as the primary imaging study to evaluate those patients with significant postoperative hemorrhage because of the high likelihood of a positive angiogram and the opportunity to intervene.15 Retroperitoneal hematomas and AVFs can be detected using duplex ultrasound. However, Vignali et al reported that 4 of 10 pseudoaneurysms in their patient sample were not identified with duplex ultrasound.22 The development of multislice CT scanners has increased both the sensitivity and specificity in identifying renal vascular lesions. In fact, CT may be the first choice to detect iatrogenic kidney injury.23 CT confers the added advantage of imaging the entire GU tract in addition to the kidneys. Moreover, CT angiography is very highly sensitive in revealing renal vascular injuries in even the most peripheral sites. On CT angiography, hemorrhage is seen as active extravasation of contrast media,17 and AVFs can be detected during the arterial phase, during which there will be early or simultaneous opacification of one or several intrarenal arteries and veins. A pseudoaneurysm presents as an ovoid or round contrast-containing abnormality that communicates with a ruptured vessel wall. With these developments, renal angiography now shares a more limited diagnostic role and should be used primarily when planning a percutaneous intervention.22
Management
Most renal vascular injuries are minor and resolve spontaneously with conservative treatment.17 During PCNL itself, excessive bleeding is usually venous and easily controlled by noninvasive maneuvers, such as clamping the nephrostomy tube, Kaye balloon tamponade, adequate hydration, mannitol administration, or placement of a larger nephrostomy tube.21 Conversely, arterial injuries result in significant bleeding, which may not only result in formation of AVF or pseudoaneurysm but may also require invasive procedures for bleeding cessation and prevention of loss of renal function.
In the past, treatment of acute iatrogenic injury of the renal artery consisted of an open procedure with renal artery ligation followed by bypass graft placement or nephrectomy.17 However, technological advances in minimally invasive endourological procedures and other conservative treatments have shifted the paradigm of management away from initial surgery. One of the most widely accepted treatment strategies is now superselective renal artery embolization (SRAE). In fact, SRAE is now accepted as the most appropriate treatment of iatrogenic renal vascular pseudoaneurysms that arise peripherally in the kidney and does not respond first to conservative maneuvers22 24 (Figs. 2 and 3). On the other hand, pseudoaneurysms that arise from the main renal artery can be successfully excluded using covered stent graft (Fig. 4). In addition, this presents a unique instance in which diagnostic and interventional radiologists are enmeshed in the cause of iatrogenic injury (e.g., by PCN, PCNL, or percutaneous renal biopsy), subsequent diagnosis, and initial management.
Multiple series have demonstrated that SRAE for managing renal vascular injury has high technical and clinical success rates, with low complication rates.15 17 18 20 21 22 24 25 26 While surgery is generally fraught with more risk of tissue loss and other complications, SRAE often results in less parenchymal tissue sacrifice, when the alternative might be partial or complete nephrectomy.24 26 SRAE involves the precise identification of the site of the bleeding renal vascular lesion, to provide the highest selectivity possible. Superselective access of the target renal artery branch is achieved using hydrophilic catheters and low-profile microcatheters. An embolic agent is administered via the catheter to perform a distal and irreversible occlusion of the target bleeding vessel, with resulting complete hemostasis.22 By eliminating the pressure head to a vascular lesion such as an AVF or pseudoaneurysm, selective embolization works similarly to surgical ligation of a bleeding vessel, with a lower risk of further parenchymal injury.15
The choice of embolic agent varies widely in the published literature and is oftentimes reported as dependent on the operator's preference. Sole use or a combination of gelatin sponges, microcoils, polyvinyl alcohol particles, n-butyl cyanoacrylate iodized oil mixture, or microspheres has been reported.17 20 27 Each embolic material has its own advantages. Gelatin sponges are biodegradable and can be used repeatedly until the target lesion is occluded, while microcoils are known for greater efficacy and ability to be administered more distally and selectively to minimize parenchymal loss. Nevertheless, the embolic material should be chosen with consideration of the site, size, and flow pattern of the target vasculature, as well as institutional availability and experience of the operator.17
Often, renal angiography is employed to identify the target vessel for SRAE. In their retrospective study, Kluner et al demonstrated the utility of multislice CT and CT angiography to localize ongoing bleeding and precisely identify target vessels before attempted minimally invasive embolization.23 In one instance, multislice CT actually helped the radiologist detect a source of bleeding that would have been missed by renal angiography. Ultrasound was able to detect a hematoma in five of seven patients (71.5%), though it could not confirm ongoing bleeding or identify a target site.
Technical success in the majority of SRAE series is defined as complete occlusion of the targeted bleeding site or vascular lesion on angiography. Meanwhile, clinical success is described as the lack of clinical or imaging evidence of further bleeding after SRAE, such as the absence of hypotension, gross hematuria, or retroperitoneal hematoma. Two recent series illustrate the high technical and clinical success rates of SRAE.17 24 In a study sample of 50 patients with iatrogenic renal arterial injuries, Sam et al achieved technical success of SRAE in 98% of all patients.24 Clinical success rates increased steadily at 83, 94, and 98% at 24, 48, and 96 hours, respectively, after SRAE. There were no major complications directly linked to SRAE, though three patients suffered minor asymptomatic issues related to nontarget embolization. In addition, the authors analyzed data on 11 prior series of SRAE in iatrogenic renal injury and found technical success rates to range between 87 and 100%.18 20 21 22 24 In an article published in 2014, Ierardi et al demonstrated a technical success rate of 100% and clinical success rate of 95% in 21 SRAE procedures.17 No major complications were encountered during or after SRAE, and after a minimum 6-month follow-up, no recurrence of bleeding or complications related directly to SRAE were reported.
The most widely recognized and dreaded adverse effect of SRAE is nontarget embolization with resultant renal ischemia and loss of renal function. There is also a potential risk of hypertension from renin release due to ischemic renal parenchyma.24 There can be further iatrogenic renal vascular or parenchymal injury caused by procedural steps during SRAE, along with a risk for groin hematoma formation. In one series, Sam et al reported not only technical and clinical success rates of SRAE, but also the effect of the procedure on estimated glomerular filtration rate, National Kidney Foundation renal stage, systolic blood pressure (BP), and European Society of Hypertension BP stage. They showed no significant difference in any of these variables before and after SRAE. In a smaller study of four patients treated by SRAE for iatrogenic renal vascular injury, Chatziioannou et al demonstrated normalization of serum creatinine within 1 week of SRAE and at latest mean follow-up of 12 months.26
Limitations of prior studies often were due to the variety of patients and time frames of presentation of iatrogenic renal injury, as well as variation in embolization technique and choice of embolic materials. Zeng and colleagues reviewed over 19,000 PCNL procedures of which 117 underwent SRAE for iatrogenic PCNL injury.27 They identified that multiple nephrostomy tracts, greater than two bleeding sites on angiography, and use of gelatin sponges as the sole embolization material were significantly predictive factors for failure of initial SRAE, requiring a repeat attempt or nephrectomy. Like many of the other studies discussed previously, one limitation of this study was its retrospective nature. However, given the rarity of iatrogenic injury requiring intervention, designing a prospective study to obtain a large sample population would be arduous, if not impossible.
Regardless, SRAE of iatrogenic renal vascular injury has been proven to be of great utility in the control of iatrogenic bleeding. Both diagnostic and interventional radiologists should be cognizant of the potential iatrogenic harms of interventional renal procedures, such as PCN and PCNL. Moreover, bleeding injuries and AVF and pseudoaneurysm formation from these procedures present a unique instance in which the interventional radiologist's expertise will be critical in initial management. Indeed, the minimally invasive nature and capacity to rapidly stop hemorrhage, while sparing renal function, makes SRAE a valuable and accepted tool for treating such renal vascular injuries.
Iatrogenic Ureteral Injury
Due to their close proximity to vital abdominal and pelvic organs, the ureters are highly susceptible to iatrogenic injury. In fact, an estimated 75% of all ureteric injuries are iatrogenic.9 This is largely attributed to the close position of the ureters to vascular structures, combined with their course along virtually every level of the retroperitoneum and upper pelvis.2 Additionally, the ureters are difficult to identify during surgical procedures due to their close adherence to the posterior peritoneum and other confounding variables, such as unexpected congenital anomalies (e.g., ureteral duplication).
Etiology
There is a wide range of mechanisms of iatrogenic injury to the ureters. The most common injury is inadvertent ligation of the ureter, followed by ureteral kinking and obstruction by a suture.5 The ureter can also be completely or partially transected, perforated, crushed, or unintentionally devascularized by electrocoagulation, which leads to ureteral ischemia and stricture. The distal third of the ureter is the most susceptible to iatrogenic damage, accounting for 91% of injuries9; the proximal upper portion is more inaccessible, and with fewer procedures in this area, it is less likely to be injured.
With the rapid expansion of minimally invasive laparoscopic procedures, the leading cause of iatrogenic ureteric injury has shifted from urologic to gynecologic surgeries. A reported 64% of iatrogenic ureteral injuries are due to laparoscopic gynecologic surgeries, while the remaining 26 and 11% are due to general surgical procedures and urology procedures, respectively9 (Fig. 6). Though most reports concur that gynecologic surgeries are most fraught with inadvertent ureteric injury, specific incidences for particular surgeries vary widely.1 4 9 28 29 30 For example, Selzman et al reported a rate of ureteral injury in 2.2% of all hysterectomies and routine gynecologic pelvic procedures, but in up to 30% in all radical hysterectomies.1 The rate of ureteral injury during laparoscopic hysterectomy is as high as 6%.4 28 29 In a larger study of over 3,300 articles describing laparoscopic gynecologic complications, 70 cases of ureteral injury were observed.30 Of these, 20% were due to laparoscopic vaginal hysterectomy, with oophorectomy and pelvic lymphadenectomy being the next most implicated procedures. Ureteral injury occurs primarily during ligation of uterine and ovarian vessels, mobilization of the ureter, and during attempts to control profuse pelvic bleeding.
Fig. 6.

A single fluoroscopic image of a 62-year-old woman demonstrating complete right ureteral obstruction (arrow) after a laparoscopic partial nephrectomy for renal cell carcinoma. This was successfully drained using an 8 French nephrostomy tube, and 2 months later it was successfully treated with pyeloplasty.
From the urologist's perspective, ureteroscopy is the most common cause of ureteral damage.9 Ureteric perforation due to ureteroscopy is estimated in 0.6 to 1% of patients, and mucosal abrasion is estimated in 0.3 to 4.1% of cases.4 This can largely be attributed to the growing use of small-bore, semi-rigid, and flexible fiber optic technology. Similarly to PCN and PCNL, the ureter can be damaged at several stages of the procedure. Examples include damage during guidewire placement, passage of the ureteroscope, and manipulation of tools used for grasping stones or intraureteric lithotripsy. For general and colon and rectal surgeons, sigmoid colectomy, abdominoperineal resection, and division of the lateral ligaments of the rectum each carry a risk of ureteral injury.4 5
Interestingly, many patients who suffer iatrogenic ureteral injuries have few or no identifiable risk factors.31 However, due to the already tenuous state of ureteral identification during difficult pelvic surgeries, any disruption of normal pelvic anatomy would intuitively increase the susceptibility for iatrogenic injury. Therefore, endometriosis, large ovarian cysts, increased uterine size greater than 12 weeks gestation, other ovarian masses, urinary tract anomalies, pelvic malignancies, and prior pelvic radiation and/or surgical intervention increase the risk of iatrogenic ureteral injury.
Clinical Presentation
Patients with ureteral injury can present with a variety of signs and symptoms. They might have fever, hematuria, dysuria, anuria, flank pain, lower back pain, peritonitis with or without leukocytosis, incontinence, or even a vaginal urinary leak.4 5 9 They may show a rise in serum creatinine and blood urea nitrogen levels. Of note, with unilateral ureteral lesions, urine output will be of little clinical value in diagnosing the injury, as the urinary output will be essentially unchanged.
Diagnosis
The pitfall of delayed diagnosis of inadvertent ureteral injury is highlighted by an increase in the rate of ensuing complications.3 4 5 Failure to repair the injury can place the patient at risk for pain, systemic infection, abscess, urinoma, ureteral stricture, ureteric fistula, and ipsilateral renal unit loss. Overwhelmingly, evidence suggests that the key to improved outcomes is early intraoperative recognition of an iatrogenic injury, which facilitates prompt diagnosis and repair. To do so, it is of paramount importance that the operator maintains a low threshold for suspecting presence of an injury.9 Lack of timely recognition during the primary procedure often mandates adjunctive surgeries with their associated morbidities. Recognition during the surgical procedure reduces morbidity, and often provides for increased ease of the repair itself. Moreover, intraoperative repair commonly leads to a more straightforward postoperative course without notable sequelae.2
Intraoperative diagnosis of ureteral injury can be accomplished in several ways, and depends also on the type of injury suspected.2 5 9 Retrograde pyelography or ureteroscopy are commonly used for intraoperative diagnosis. Cystoscopy with bilateral retrograde pyelography is the gold standard for identifying ureteral narrowing or contrast extravasation.9 A retrograde pyelogram that demonstrates normal ureters without contrast leak or stricture precludes the need for further ureteroscopy. Ureteroscopy offers direct visualization of the ureters if retrograde pyelography is equivocal. If ureteral transection is suspected, one can administer intravenous indigo carmine or methylene blue with furosemide and observe for extravasation of blue-tinged urine. Alternatively, an on-table intravenous pyelogram can be performed by injecting 2.0 cc/kg of IV contrast and obtaining an abdominal plain film 10 minutes later to search for contrast extravasation.3 If suspecting ureteral ligation, the operator should dissect the target area and directly visualize the ureter. In addition, a ureteral catheter can be placed in a retrograde fashion to check for free passage to the renal pelvis, which indicates no complete transection or occlusion. If there is difficulty passing the catheter, there may be ureteral obstruction and the offending clip or suture should be removed. The surgeon should also check for ureteral viability and signs of ureteral devascularization, such as discoloration, absence of capillary refill, or lack of a bleeding edge.5 In general, active peristalsis of the area in question suggests viability and conservative treatment with observation can be initiated.
Nevertheless, only about a third of iatrogenic injuries to the ureter are identified during the primary surgical procedure.9 Laparoscopic procedures are most at risk for missed intraoperative diagnosis, with only a 12.5% reported detection rate. Intraoperative detection rates for open and endourological procedures are significantly higher at 43.5 and 62.5%, respectively.
In the delayed setting of missed ureteric injury, radiology plays a critical role in diagnosis. Patients will often present with the clinical signs and symptoms described earlier, often 48 to 72 hours after the primary procedure. Importantly, hematuria is absent in 15 to 45% of those with ureteral injuries.9 The selected investigative technique depends on the physician's impression of the patient's clinical presentation and context, but almost always includes a component of radiographic imaging. For example, though there is little to no evidence to support its routine use in this setting, ultrasound may reveal a triad of hydronephrosis, ascites, and absence of a ureteric jet into the bladder due to ureteral injury.5 9 Classically, intravenous urography (IVU) has been an accurate method of diagnosing delayed ureteral injuries. IVU requires adequate distention of the collecting system with complete opacification.32 It offers immediate visualization of the entirety of the GU system and can allow diagnosis of the smallest lesions at an early stage, due to its detailed visualization of the calyces and ureter.33 In fact, IVU was introduced in the 1930s as the primary method for evaluating the GU system until the arrival of cross-sectional imaging.
CT urography has become the most common method for diagnosing missed ureteral injuries over the past 20 years, despite a lack of published evidence regarding its sensitivity and specificity.9 To optimize identification of ureteral lesions, a triple-phase contrast-enhanced CT with both nephrographic and excretory phases (5–20 minutes after contrast administration) should be performed. Signs of ureteral injury include contrast medium extravasation, hydronephrosis, ureteric obstruction, urinary ascites, and localized fluid collections such as urinoma.5 9 However, the radiologist must take caution in interpreting these scans, as ordinary ascites, abscesses, hematomas, or cystic masses can resemble urine leaks and urinomas. Regardless, the diagnostic radiologist plays a pivotal role in ensuring that the appropriate imaging is planned and should help the referring physician by tailoring the performed study to the clinical question.33
If the diagnosis is still equivocal after IVU and/or CT urography, then bilateral retrograde pyelography should be performed.5 It is the most accurate imaging exam to determine the location, type, and degree of iatrogenic ureteric injury; limitations are that it is time-consuming and not often the first choice in an acute setting.
Management
When an iatrogenic ureteral injury is identified, initial management depends on the type, location, and degree of injury, as well as the patient's overall clinical condition, the time of discovery, and the urological expertise available.9 The goals of management principally include renal unit preservation with adequate drainage by stenting or nephrostomy and minimization of surgical morbidity. The required intervention is often dependent upon the location of the ureteral lesion. For upper and middle third injuries, first-line repair is often ureteroureterostomy, with transureteroureterostomy as second-line therapy. Lower third ureteral injuries call for direct reimplantation, possibly requiring a psoas hitch procedure if initial management fails. With complete ureteral loss, one may try autotransplantation, or nephrectomy may be required.
A variety of advanced surgical procedures can be employed by highly skilled operators to ensure the greatest chance for successful repair of ureteral injuries. In addition to those mentioned above, these include but are not limited to ureteroneocystostomy and Boari flap creation. The technical aspects of these procedures are outside the scope of this review, but have been highlighted comprehensively in publications by Delacroix et al, LaFontaine, and De Cicco et al.3 34 35
Interventional radiologists play a key role in the minimally invasive options that exist for treatment of iatrogenic ureteral injury, including chronic strictures that develop at the site of surgically created ureteroileal anastomosis after neobladder reconstruction (Fig. 7a–d). These techniques have been popularized as laparoscopic, percutaneous, and endourological technology has expanded.9 In partial ureteral transections, for example, cystoscopy with retrograde ureteral stent placement is considered among first-line treatment options. If this fails, an interventional radiologist may attempt anterograde ureteral stent placement. Ustunsoz et al demonstrated the utility of interventional radiologic techniques in managing 22 patients with distal ureteral injuries diagnosed late after caesarean section.6 Injury types included partial (n = 4) or complete (n = 3) transection, ureteral obstruction (n = 15), and ureterovaginal fistula (n = 2). All patients underwent PCN with subsequent anterograde Double-J ureteral catheter placement, balloon dilatation, or both. Of these, the authors were able to restore ureteral patency without the need for surgery in 13 of 15 (87%) ureteral obstructions and 5 of 9 (56%) transections and fistulas at median 11-month follow-up. There were no major complications associated with percutaneous management.
Fig. 7.
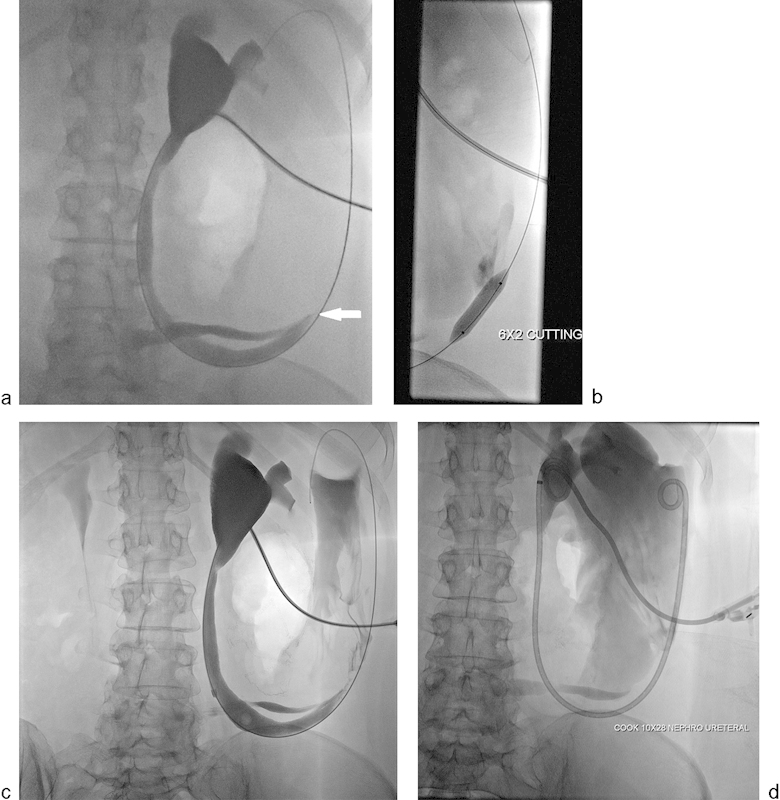
A 57-year-old woman with a history of interstitial cystitis underwent cystectomy and Indiana pouch urinary diversion. (a) Antegrade nephrostogram showing severe short segment, almost occlusive, stricture involving ureteroileal anastomosis (arrow). (b) Fluoroscopic image showing balloon dilation of the above-described stricture using 6 mm × 20 mm cutting balloon after failed regular balloon dilation. (c and d) Antegrade nephrostogram showing mild improvement in the appearance of the stricture to allow placement of a 10 mm × 28 cm nephroureteral stent placement.
Though there is a relative paucity of interventional radiologic literature comparing surgical versus percutaneous management of ureteral injuries, two studies have provided important insight.36 37 Ku et al retrospectively studied 30 patients with late ureteral injuries after obstetric and gynecological procedures.36 Of these, 13 were treated with primary operative repair and obtained primary healing. Of the remaining 17 treated with minimally invasive procedures, 9 (52.9%) received anterograde or retrograde stenting; 7 (41.2%) had PCN drainage, and 1 (5.9%) required both. While 11 patients (64.7%) were treated successfully with these procedures, 4 of those in the PCN group (47.1%) and 2 in the stented group (22.2%) required further surgical intervention due to worsening hydronephrosis and/or a decline in renal function. The authors concluded that minimally invasive strategies are not always successful in the delayed setting. However, urinary diversion alone without stenting did result in increased need for open operations. Thus, their study justified an initial attempt to bridge the affected area by anterograde or retrograde stent placement, and then proceed to open surgery if the clinical situation deteriorates. In a separate study, Lask et al reported on 44 patients with late diagnosed iatrogenic ureteral injuries.37 Of these, 24 patients underwent surgical ureteroneocystostomy or end-to-end uretero-ureteral anastomosis, while the remaining 20 patients underwent PCN. At an average of 32 days of follow-up, 16 patients (80%) demonstrated complete spontaneous recovery of the injured ureter with PCN alone. This strategy resulted in significantly reduced reoperation and morbidity rates.
Although there are discrepancies in the published literature, minimally invasive techniques are justified in the initial management of late diagnosed ureteral injuries, on the condition that the affected area is bridged by a stent.9 Because of the relative rarity of such injuries, it is difficult to develop randomized control trials or even prospective studies to fully elucidate the role of minimally invasive treatments over open surgical management. Nevertheless, advances in percutaneous, laparoscopic, and robotic techniques, highlighted by the role of interventional radiology, offer options to avoid major reconstructive procedures and accompanying surgical morbidity.
Iatrogenic Bladder Injury
Etiology
Bladder injuries are most often secondary to pelvic trauma; however, iatrogenic bladder injury is not uncommon.4 Iatrogenic bladder injuries can be divided into those attributable to internal bladder procedures and those that occur during external surgery near the bladder. Internal injuries often occur from endoscopic urological procedures.38 External injuries are often from pelvic gynecological, general, and urological surgery.39 40 41 Hysterectomy and transurethral resection of bladder tumor are two procedures most frequently implicated in bladder injury.10 At one institution, iatrogenic bladder injuries occurred from obstetric and gynecological, general, and urological surgery in 65, 22, and 13% of cases, respectively.42
Clinical Presentation
Ideally, intraoperative injuries are identified at the time they occur; in one study, ∼80% of injuries were recognized intraoperatively.43 In addition to direct visualization of tissue injury, external bladder injuries may be suspected with urine in the operative field, air in the Foley catheter collection bag, or direct visualization of the Foley catheter.44 Intraoperative internal injuries may result in new onset abdominal distension, difficulty maintaining bladder distension with instilled fluid, and visualization of urine external to the bladder.38 45
Perioperative bladder injury may present with a variety of clinical signs and symptoms such as suprapubic pain, hematuria, and oliguria.4 10 Gross hematuria and abdominal tenderness are two of the most common symptoms in patients with bladder injury.46 With extravasation of urine, patients may also note worsening abdominal pain, abdominal distension, peritonitis, and may even progress to sepsis if not treated.46 47
Diagnosis
With the appropriate suspicion of bladder injury and the inability to confirm diagnosis with immediate and direct visualization, there are a variety of tools to assist in the diagnosis. Intraoperative cystoscopy and intentional cystostomy have been deemed valuable methods to further visualize a suspected bladder injury.44 48 49 Additionally, during intraoperative injury that is either undefined or difficult to localize, the instillation of methylene blue dye into the bladder followed by careful observation for extravasation may prove useful for further management.44
A variety of radiographic methods can be used to diagnose bladder injury. Perioperatively, ultrasound examination is a quick and useful tool to identify bladder volumes and pelvic or abdominal fluid. However, ultrasound often lacks the ability to attribute abdominal or pelvic fluid to a specific source and thus is not considered sufficient to diagnose bladder injury.50 IVU had been historically utilized for evaluation of bladder injury but has been largely superseded by cross-sectional imaging modalities.33
In the perioperative setting, CT cystography has become the gold standard diagnostic study for clinically suspected iatrogenic bladder injury. In the setting of extraperitoneal injury, cystography may show extravasation of contrast into the pelvis (Fig. 8a, b), while intraperitoneal injury may show extravasated contrast outlining bowel loops and filling dependent intraabdominal spaces.11 Proper evaluation requires distension of the bladder with instillation of at least 350 mL of contrast and the inclusion of images after bladder drainage10 50 51; this technique has a reported accuracy of 85 to 100%.46 Conversely, improper imaging techniques, such as the use of 250 mL of contrast instillation to the bladder or exclusion of post–bladder drainage imaging, may result in excess false negative results.47 51 In the delayed diagnostic setting following pelvic or intraabdominal surgery, it may be difficult to suspect bladder injury without a broader differential diagnosis, and evaluation of the ureters may be necessary. In that scenario, CT urography may provide superior diagnostic information by allowing for contrast evaluation of the ureters and the bladder.4 10
Fig. 8.

A 70-year-old man underwent sigmoid colectomy for diverticulitis that was complicated by colorectal anastomotic stricture. During surgical repair of the colorectal stricture, the patient sustained a bladder laceration and right ureteral transection. (a) AP and (b) RAO cystography images showing extravasation of contrast adjacent to the bladder on right lateral and posterior aspects (arrows).
Management
Once diagnosed, bladder injuries are managed depending on their location (intraperitoneal or extraperitoneal). The majority of intraperitoneal injuries require immediate operative repair to prevent the development of sepsis. The occasional small intraperitoneal injury without resultant sepsis or ileus may be managed with conservative, nonoperative treatment.10 52 The standard repair is a two-layer closure including the mucosa with absorbable suture material. On the other hand, extraperitoneal injuries are often treated conservatively by decompression of the bladder with a Foley catheter and observation.4 10 However, if other associated injuries require operative repair, it is reasonable to repair an extraperitoneal bladder injury at the same time. Additionally, large extraperitoneal injuries or extraperitoneal injuries in patients that will require orthopedic hardware, such as in pelvic fractures, will benefit from operative repair rather than conservative bladder diversion.47
Urinoma
The term “urinoma” describes a collection of urine contained outside of the normal pathways that urine travels; urinomas therefore may arise anywhere from the upper abdomen (e.g., disruption of the renal calyx) to the lower pelvis (disruption of the urethra or bladder).53 The etiology of urinoma formation is varied and depends on the location of origin. For example, in the kidneys, renal urine leaks and urinomas most often result from blunt or penetrating trauma.54 Uncommonly, urinomas can be the result of a surgical procedure such as decortication of a renal cyst (Fig. 9). Iatrogenic renal injury is an uncommon cause of urinoma; however, in the ureter, urine leaks and urinomas most commonly occur from iatrogenic injury after pelvic, gynecologic, or urologic surgeries, including endourological procedures.55 56 Bladder leaks and urinomas may also be caused iatrogenically, but similarly to kidneys, injuries are most often secondary to blunt or penetrating trauma, particularly pelvic fractures.
Fig. 9.

A 52-year-old woman with urine leak following decortication of a renal cyst. (a, b) Select axial and coronal images from a CT of the abdomen and pelvis performed in the excretory phase show layering of extravasated contrast (arrow) in an otherwise low attenuation fluid collection in the decortication bed of the right kidney. The patient was initially managed conservatively but subsequently developed fever; percutaneous catheter drainage was placed into the urinoma.
Diagnostic radiologic imaging plays a major role not only in the identification of urine leaks and urinomas, but also in determining the source and extent of the leak. Ultrasound and contrast-enhanced CT imaging are widely accepted initial modalities to define a fluid collection, particularly if a urinoma is suspected.53 It is difficult to sonographically discern a fluid collection as a seroma, urinoma, or abscess, though a unidirectional jet of fluid into the collection on Doppler imaging may favor the diagnosis of urinoma.57 Contrast-enhanced CT imaging can sensitively detect urine flow into a fluid collection, as it demonstrates increased attenuation in the urinoma on delayed-phase imaging.54 Importantly, CT is useful in pinpointing the origin of the urine leak and portraying the extent of the leak itself. Furthermore, direct injection of contrast may better demonstrate the source of the leak. For instance, retrograde ureterograms, antegrade nephrostograms, and cystograms may be useful in particular situations, depending on the level of the leak.53 When imaging findings alone are not sufficient to identify urinoma over other fluid collections, direct image-guided aspiration of the fluid by the radiologist can aid in the final diagnosis. Urinomas typically contain creatinine levels significantly higher than serum creatinine levels; seromas, on the other hand, will have comparable creatinine measurements to serum levels.53 54
Clinically, urinomas may cause symptoms related to mass effect, such as pain and/or sensations of pressure. Urinomas may themselves cause ureteral obstruction, if large enough. In the majority of cases, small urinomas do not require drainage and will spontaneously reabsorb, particularly if urine leakage has stopped.58 Percutaneous image-guided drainage, however, is indicated for larger urinomas that place pressure on adjacent structures, urinomas that persist over several days, or if the patient develops fever or leukocytosis, suggesting that the urinoma is infected.53 54 Ultrasound-guided placement of drainage catheters confers the advantages of maintained access into the fluid collection while culture and laboratory analyses are being performed, and monitoring output until the urinoma is completely drained, which should occur in a few days if urine leakage is controlled.53
In treating urinomas, it is most crucial to address the underlying condition that caused urine leakage and urinoma formation in the first place. The principle basis of treatment is diversion of urine away from the initial defect, to allow the urothelium to heal and simultaneously prevent further flow into the urinoma.53 Depending on the location of the injury, the interventional radiologist may place a PCN catheter, usually in combination with ureteral stenting or nephroureterostomy catheterization, to divert urine away from the traumatic injury and leak, and to promote primary healing of the collecting system.54 If the urinoma is caused by complete or large ureteral transection, guidewires can be placed into the urinoma from above and below, with one wire exchanged for a snare that encircles the other wire and pulls it across the leak.59 An obstruction of the urinary tract causing a urinoma must be relieved, with the consideration for nephrostomy and ureteral stenting to provide drainage in difficult situations. Finally, if the urothelium is devitalized due to trauma, even adequate drainage will not allow primary healing. In these patients, arterial embolization of the renal parenchyma supplying the region with the urine leak could be considered.60
Conclusion
Diagnostic and interventional radiologists, along with urologists, gynecologists, and general or colon and rectal surgeons, must maintain awareness of the potential for iatrogenic urinary tract injuries. Each should be aware of the mechanisms of injury, presenting signs and symptoms, and diagnostic and therapeutic options. Studies prospectively comparing minimally invasive interventional radiologic approaches to open surgery in the initial management of these injuries, as well as to further elucidate the role of interventional radiology in iatrogenic bladder injury, are still needed.
Acknowledgment
The authors thank Megan Griffiths for editorial assistance in preparation of this manuscript.
Footnotes
Disclosures None.
References
- 1.Selzman A A, Spirnak J P. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol. 1996;155(3):878–881. doi: 10.1016/s0022-5347(01)66332-8. [DOI] [PubMed] [Google Scholar]
- 2.Hairston J C, Ghoniem G M. Urinary tract injuries: recognition and management. Clin Colon Rectal Surg. 2003;16:27–38. doi: 10.1055/s-0030-1263063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delacroix S E Jr, Winters J C. Urinary tract injuries: recognition and management. Clin Colon Rectal Surg. 2010;23(3):104–112. doi: 10.1055/s-0030-1263063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Summerton D J Kitrey N D Lumen N Serafetinidis E Djakovic N; European Association of Urology. EAU guidelines on iatrogenic trauma Eur Urol 2012624628–639. [DOI] [PubMed] [Google Scholar]
- 5.Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94(3):277–289. doi: 10.1111/j.1464-410X.2004.04978.x. [DOI] [PubMed] [Google Scholar]
- 6.Ustunsoz B, Ugurel S, Duru N K, Ozgok Y, Ustunsoz A. Percutaneous management of ureteral injuries that are diagnosed late after cesarean section. Korean J Radiol. 2008;9(4):348–353. doi: 10.3348/kjr.2008.9.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preston J M. Iatrogenic ureteric injury: common medicolegal pitfalls. BJU Int. 2000;86(3):313–317. doi: 10.1046/j.1464-410x.2000.00100.x. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor B S, Esparaz A, Levitin A, McLennan G, Moon E, Sands M. Nonvascular and portal vein applications of cone-beam computed tomography: current status. Tech Vasc Interv Radiol. 2013;16(3):150–160. doi: 10.1053/j.tvir.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Abboudi H, Ahmed K, Royle J, Khan M S, Dasgupta P, N'Dow J. Ureteric injury: a challenging condition to diagnose and manage. Nat Rev Urol. 2013;10(2):108–115. doi: 10.1038/nrurol.2012.254. [DOI] [PubMed] [Google Scholar]
- 10.Gomez R G, Ceballos L, Coburn M. et al. Consensus statement on bladder injuries. BJU Int. 2004;94(1):27–32. doi: 10.1111/j.1464-410X.2004.04896.x. [DOI] [PubMed] [Google Scholar]
- 11.Armenakas N A, Pareek G, Fracchia J A. Iatrogenic bladder perforations: longterm followup of 65 patients. J Am Coll Surg. 2004;198(1):78–82. doi: 10.1016/j.jamcollsurg.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Hausegger K A, Portugaller H R. Percutaneous nephrostomy and antegrade ureteral stenting: technique-indications-complications. Eur Radiol. 2006;16(9):2016–2030. doi: 10.1007/s00330-005-0136-7. [DOI] [PubMed] [Google Scholar]
- 13.Michel M S Trojan L Rassweiler J J Complications in percutaneous nephrolithotomy Eur Urol 2007514899–906., discussion 906 [DOI] [PubMed] [Google Scholar]
- 14.Ramchandani P, Cardella J F, Grassi C J. et al. Quality improvement guidelines for percutaneous nephrostomy. J Vasc Interv Radiol. 2003;14(9, Pt 2):S277–S281. [PubMed] [Google Scholar]
- 15.Tan T J, Teh H S, Pua U, Ho S H. Endovascular management of iatrogenic renal vascular injuries complicating percutaneous nephrolithotripsy: role of renal angiography and superselective coil embolisation. J HK Coll Radiol. 2008;11:103–107. [Google Scholar]
- 16.Turna B, Nazli O, Demiryoguran S, Mammadov R, Cal C. Percutaneous nephrolithotomy: variables that influence hemorrhage. Urology. 2007;69(4):603–607. doi: 10.1016/j.urology.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Ierardi A M, Floridi C, Fontana F. et al. Transcatheter embolisation of iatrogenic renal vascular injuries. Radiol Med (Torino) 2014;119(4):261–268. doi: 10.1007/s11547-013-0343-2. [DOI] [PubMed] [Google Scholar]
- 18.El-Nahas A R, Shokeir A A, El-Assmy A M. et al. Post-percutaneous nephrolithotomy extensive hemorrhage: a study of risk factors. J Urol. 2007;177(2):576–579. doi: 10.1016/j.juro.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 19.Lewis S, Patel U. Major complications after percutaneous nephrostomy-lessons from a department audit. Clin Radiol. 2004;59(2):171–179. doi: 10.1016/s0009-9260(03)00336-2. [DOI] [PubMed] [Google Scholar]
- 20.Phadke R V, Sawlani V, Rastogi H. et al. Iatrogenic renal vascular injuries and their radiological management. Clin Radiol. 1997;52(2):119–123. doi: 10.1016/s0009-9260(97)80104-3. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava A, Singh K J, Suri A. et al. Vascular complications after percutaneous nephrolithotomy: are there any predictive factors? Urology. 2005;66(1):38–40. doi: 10.1016/j.urology.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Vignali C, Lonzi S, Bargellini I. et al. Vascular injuries after percutaneous renal procedures: treatment by transcatheter embolization. Eur Radiol. 2004;14(4):723–729. doi: 10.1007/s00330-003-2009-2. [DOI] [PubMed] [Google Scholar]
- 23.Kluner C, Rogalla P, Gralla O, Elgeti T, Hamm B, Kroencke T. Value of dual-phase multislice CT prior to minimally invasive therapy of iatrogenic renal injuries. J Endovasc Ther. 2005;12(4):461–468. doi: 10.1583/04-1341MR.1. [DOI] [PubMed] [Google Scholar]
- 24.Sam K, Gahide G, Soulez G. et al. Percutaneous embolization of iatrogenic arterial kidney injuries: safety, efficacy, and impact on blood pressure and renal function. J Vasc Interv Radiol. 2011;22(11):1563–1568. doi: 10.1016/j.jvir.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Ueda J, Furukawa T, Takahashi S, Miyake O, Itatani H, Araki Y. Arterial embolization to control renal hemorrhage in patients with percutaneous nephrostomy. Abdom Imaging. 1996;21(4):361–363. doi: 10.1007/s002619900082. [DOI] [PubMed] [Google Scholar]
- 26.Chatziioannou A, Brountzos E, Primetis E. et al. Effects of superselective embolization for renal vascular injuries on renal parenchyma and function. Eur J Vasc Endovasc Surg. 2004;28(2):201–206. doi: 10.1016/j.ejvs.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Zeng G, Zhao Z, Wan S. et al. Failure of initial renal arterial embolization for severe post-percutaneous nephrolithotomy hemorrhage: a multicenter study of risk factors. J Urol. 2013;190(6):2133–2138. doi: 10.1016/j.juro.2013.06.085. [DOI] [PubMed] [Google Scholar]
- 28.Léonard F, Fotso A, Borghese B, Chopin N, Foulot H, Chapron C. Ureteral complications from laparoscopic hysterectomy indicated for benign uterine pathologies: a 13-year experience in a continuous series of 1300 patients. Hum Reprod. 2007;22(7):2006–2011. doi: 10.1093/humrep/dem111. [DOI] [PubMed] [Google Scholar]
- 29.Kalisvaart J F, Finley D S, Ornstein D K. Robotic-assisted repair of iatrogenic ureteral ligation following robotic-assisted hysterectomy. JSLS. 2008;12(4):414–416. [PMC free article] [PubMed] [Google Scholar]
- 30.Ostrzenski A, Radolinski B, Ostrzenska K M. A review of laparoscopic ureteral injury in pelvic surgery. Obstet Gynecol Surv. 2003;58(12):794–799. doi: 10.1097/01.OGX.0000097781.79401.0B. [DOI] [PubMed] [Google Scholar]
- 31.Akin Y, Basara I, Bozkurt A. Minimally invasive treatment of iatrogenic complete left ureter obstruction after hysterectomy. J Health Sci. 2012;2:144–147. [Google Scholar]
- 32.Nolte-Ernsting C, Cowan N. Understanding multislice CT urography techniques: Many roads lead to Rome. Eur Radiol. 2006;16(12):2670–2686. doi: 10.1007/s00330-006-0386-z. [DOI] [PubMed] [Google Scholar]
- 33.Van Der Molen A J Cowan N C Mueller-Lisse U G Nolte-Ernsting C C Takahashi S Cohan R H; CT Urography Working Group of the European Society of Urogenital Radiology (ESUR). CT urography: definition, indications and techniques. A guideline for clinical practice Eur Radiol 20081814–17. [DOI] [PubMed] [Google Scholar]
- 34.LaFontaine P. Management of ureteral injury. Oper Tech Gen Surg. 2007;9:167–174. [Google Scholar]
- 35.De Cicco C, Ret Dávalos M L, Van Cleynenbreugel B, Verguts J, Koninckx P R. Iatrogenic ureteral lesions and repair: a review for gynecologists. J Minim Invasive Gynecol. 2007;14(4):428–435. doi: 10.1016/j.jmig.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Ku J H, Kim M E, Jeon Y S, Lee N K, Park Y H. Minimally invasive management of ureteral injuries recognized late after obstetric and gynaecologic surgery. Injury. 2003;34(7):480–483. doi: 10.1016/s0020-1383(02)00412-6. [DOI] [PubMed] [Google Scholar]
- 37.Lask D, Abarbanel J, Luttwak Z, Manes A, Mukamel E. Changing trends in the management of iatrogenic ureteral injuries. J Urol. 1995;154(5):1693–1695. [PubMed] [Google Scholar]
- 38.Balbay M D Cimentepe E Unsal A Bayrak O Koç A Akbulut Z The actual incidence of bladder perforation following transurethral bladder surgery J Urol 200517462260–2262., discussion 2262–2263 [DOI] [PubMed] [Google Scholar]
- 39.Teerapong S, Rungaramsin P, Tanprasertkul C, Bhamarapravatana K, Suwannarurk K. Major complication of gynaecological laparoscopy in Police General Hospital: a 4-year experience. J Med Assoc Thai. 2012;95(11):1378–1383. [PubMed] [Google Scholar]
- 40.Lee J S, Choe J H, Lee H S, Seo J T. Urologic complications following obstetric and gynecologic surgery. Korean J Urol. 2012;53(11):795–799. doi: 10.4111/kju.2012.53.11.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGeady J B, Breyer B N. Current epidemiology of genitourinary trauma. Urol Clin North Am. 2013;40(3):323–334. doi: 10.1016/j.ucl.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cordon B H, Fracchia J A, Armenakas N A. Iatrogenic nonendoscopic bladder injuries over 24 years: 127 cases at a single institution. Urology. 2014;84(1):222–226. doi: 10.1016/j.urology.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 43.Adelman M R, Bardsley T R, Sharp H T. Urinary tract injuries in laparoscopic hysterectomy: a systematic review. J Minim Invasive Gynecol. 2014;21(4):558–566. doi: 10.1016/j.jmig.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Mendez L E. Iatrogenic injuries in gynecologic cancer surgery. Surg Clin North Am. 2001;81(4):897–923. doi: 10.1016/s0039-6109(05)70173-0. [DOI] [PubMed] [Google Scholar]
- 45.Traxer O, Pasqui F, Gattegno B, Pearle M S. Technique and complications of transurethral surgery for bladder tumours. BJU Int. 2004;94(4):492–496. doi: 10.1111/j.1464-410X.2004.04990.x. [DOI] [PubMed] [Google Scholar]
- 46.Carroll P R, McAninch J W. Major bladder trauma: the accuracy of cystography. J Urol. 1983;130(5):887–888. doi: 10.1016/s0022-5347(17)51551-7. [DOI] [PubMed] [Google Scholar]
- 47.Morey A F Hernandez J McAninch J W Reconstructive surgery for trauma of the lower urinary tract Urol Clin North Am 199926149–60., viii [DOI] [PubMed] [Google Scholar]
- 48.Tulikangas P K, Weber A M, Larive A B, Walters M D. Intraoperative cystoscopy in conjunction with anti-incontinence surgery. Obstet Gynecol. 2000;95(6, Pt 1):794–796. doi: 10.1016/s0029-7844(99)00655-9. [DOI] [PubMed] [Google Scholar]
- 49.Nezhat C H, Seidman D S, Nezhat F, Rottenberg H, Nezhat C. Laparoscopic management of intentional and unintentional cystotomy. J Urol. 1996;156(4):1400–1402. [PubMed] [Google Scholar]
- 50.Lynch T H, Martínez-Piñeiro L, Plas E. et al. EAU guidelines on urological trauma. Eur Urol. 2005;47(1):1–15. doi: 10.1016/j.eururo.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 51.Sandler C M, Goldman S M, Kawashima A. Lower urinary tract trauma. World J Urol. 1998;16(1):69–75. doi: 10.1007/s003450050028. [DOI] [PubMed] [Google Scholar]
- 52.Alperin M, Mantia-Smaldone G, Sagan E R. Conservative management of postoperatively diagnosed cystotomy. Urology. 2009;73(5):1.163E20–1.163E22. doi: 10.1016/j.urology.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 53.Lee J, Darcy M. Renal cysts and urinomas. Semin Intervent Radiol. 2011;28(4):380–391. doi: 10.1055/s-0031-1296080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Titton R L, Gervais D A, Hahn P F, Harisinghani M G, Arellano R S, Mueller P R. Urine leaks and urinomas: diagnosis and imaging-guided intervention. Radiographics. 2003;23(5):1133–1147. doi: 10.1148/rg.235035029. [DOI] [PubMed] [Google Scholar]
- 55.Gayer G, Zissin R, Apter S. et al. Urinomas caused by ureteral injuries: CT appearance. Abdom Imaging. 2002;27(1):88–92. doi: 10.1007/s00261-001-0052-5. [DOI] [PubMed] [Google Scholar]
- 56.Ghali A M, El Malik E M, Ibrahim A I, Ismail G, Rashid M. Ureteric injuries: diagnosis, management, and outcome. J Trauma. 1999;46(1):150–158. doi: 10.1097/00005373-199901000-00026. [DOI] [PubMed] [Google Scholar]
- 57.Testa A C, Gaurilcikas A, Licameli A. et al. Sonographic imaging of urinoma. Ultrasound Obstet Gynecol. 2009;33(4):490–491. doi: 10.1002/uog.6349. [DOI] [PubMed] [Google Scholar]
- 58.Lang E K, Glorioso L III. Management of urinomas by percutaneous drainage procedures. Radiol Clin North Am. 1986;24(4):551–559. [PubMed] [Google Scholar]
- 59.Anderson H, Alyas F, Edwin P J. Intra-urinoma rendezvous using a transconduit approach to re-establish ureteric integrity. Cardiovasc Intervent Radiol. 2005;28(1):95–97. doi: 10.1007/s00270-004-0051-3. [DOI] [PubMed] [Google Scholar]
- 60.Horikami K, Matsuoka Y, Nagaoki K. et al. Treatment of post-traumatic urinoma by means of selective arterial embolization. J Vasc Interv Radiol. 1997;8(2):221–224. doi: 10.1016/s1051-0443(97)70544-x. [DOI] [PubMed] [Google Scholar]


