Abstract
Percutaneous thoracic interventions are among the most common procedures in today's medical practice. From the simple placement of a pleural drain to the ablation of lung tumors, the advent of image guidance has revolutionized minimally invasive procedures and has allowed for the introduction of new techniques and widened the range of indications. It is therefore imperative to understand the complications associated with these interventions and their management. This article illustrates the common complications associated with these interventions and highlights the relative safety of these interventions.
Keywords: thoracic interventions, complications, percutaneous, nonvascular, interventional radiology
Objectives: Upon completion of this article, the reader will be able to describe the common complications encountered with nonvascular thoracic interventions.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Nonvascular, nonsurgical thoracic procedures were first introduced in the 19th century. The first percutaneous needle lung biopsy was performed in 1883 for the diagnosis of a pulmonary infection,1 but it was not until the second half of the 20th century that percutaneous interventions received wide acceptance.
The initial high rate of complications encountered with these procedures limited their utilization. Improved image guidance and the use of smaller gauge needles resulted in enhanced safety and significantly lowered the rate of complications, making minimally invasive procedures ubiquitous. Furthermore, the advancements in the management of diseases continue to expand the role of these interventions in the diagnosis and treatment of thoracic pathologies.
It is important to understand the risks associated with these procedures, to provide safe and effective patient management. The aim of this article is to discuss the most common complications, risk factors, and management of complications associated with minimally invasive percutaneous nonvascular thoracic interventions.
Nonvascular Complications
Pneumothorax
Pneumothorax is the most common complication after percutaneous thoracic interventions. In most large series, the reported rates of pneumothorax posttransthoracic biopsy, lung ablation, and thoracentesis are 8 to 54%, 30 to 60%, and 5 to 20%, respectively.2 3 4 5 6 7 8 9 10 11 12
Pneumothoraces typically occur during or immediately following the procedure. They are usually detectable on the postintervention chest radiograph or computed tomography (CT) scan.
Risk Factors
Several, albeit controversial, risk factors for pneumothorax after lung biopsy and ablation have been identified. These are related to the patient, lesion characteristics, and/or interventional techniques.
Patient-related risk factors include underlying lung conditions, in particular emphysema. In this population, the rate of pneumothorax increases with the severity of disease and is more likely to require chest tube placement for its management than in patients without underlying lung disease.10 13
Lesion characteristics also contribute to the likelihood of development of a pneumothorax. The most predictive influencing factors are lesion size, depth14 15 16 and contact with the pleura.17 Smaller lesions are more likely to be associated with the development of a pneumothorax, as they may require multiple attempts to target the lesions precisely. Furthermore, deeper lesions or longer needle paths impart a higher risk of pneumothorax. In contrast, if the needle does not traverse aerated lung, as is seen with lesions in the chest wall, mediastinum, pleura, or subpleural lung, there is a smaller risk of pneumothorax.
Technical factors influencing the incidence of pneumothorax include the increased number of pleural punctures, multiple repositionings of the needle, transgression of fissures, biopsies in the middle or lower lobe, and wider insertion angle of the needle at the level of the pleura.18 19 20
Management
Most pneumothoraces are small, nonprogressive, and asymptomatic and do not require any treatment. They are followed conservatively with serial chest radiographs. A stable small pneumothorax at 4 hours is unlikely to become larger.21
In 5 to 18% of cases, the pneumothorax is large (exceeding 30% of the lung volume), enlarges on serial chest radiographs, or becomes symptomatic (chest pain, dyspnea, oxygen desaturation, etc.); in these situations, chest tube insertion is required. Smaller catheters (7–10 French in size) are sufficient for evacuation of pneumothoraces with a success rate of 87 to 97%.14 15 16 20 22 Larger catheters are needed to prevent catheter occlusion if there is a coexisting complex fluid collection (Figs. 1 and 2).
Fig. 1.

Pneumothorax after percutaneous needle biopsy of a right lung lesion. (a) A small pneumothorax was identified after the biopsy (arrows), just before the withdrawal of the guiding needle. (b) Failed attempt of suction of the air through the guiding needle. Note the subcutaneous emphysema (open arrow). (c) In the recovery room, the patient complained of shortness of breath and demonstrated oxygen desaturation. A 6F pigtail catheter was therefore placed in the right pleural space. This chest radiograph demonstrates the pigtail catheter (open arrow), the small pneumothorax (white arrows), and the subcutaneous emphysema overlying the right chest wall (black arrow). (d) Chest radiograph obtained 48 hours after chest tube placement showing resolution of the pneumothorax and re-expansion of the right lung. The chest tube was subsequently removed.
Fig. 2.

A 63-year-old man with complex medical history including Hodgkin lymphoma, pulmonary fibrosis, and left lung transplant who developed a right pneumothorax postbronchoscopy. (a) Noncontrast CT of the chest demonstrating a large right pneumothorax (arrows). (b) Placement of pigtail catheter (arrow) for treatment of right-sided pneumothorax.
The major reason for treatment failure is catheter malposition or occlusion. Otherwise, no resolution of the pneumothorax suggests large air leaks, in which case surgical evaluation is warranted.
Prevention
Various techniques have been proposed to reduce the incidence of a significant pneumothorax after percutaneous transthoracic interventions, but their true efficacy remains unclear.10 23 24 25
Patient cooperation is integral for safe, uneventful thoracic interventions. Patients are instructed to refrain from moving, talking, coughing, or breathing deeply during and immediately after the procedure.
Preprocedural planning to choose the shortest path possible is essential to minimize the traversal of aerated lungs and avoid transgression of the fissures. Additionally, using a coaxial technique will usually limit the number of pleural punctures.
Recently, a new device composed of an expanding hydrogel has been used to plug the tract after CT-guided percutaneous transthoracic lung biopsy using 20 Gauge needles. In a prospective, multicenter, randomized, controlled clinical study, the device demonstrated significant reduction in the rates of pneumothorax, chest tube placement, and postprocedure hospital admission.26
Bronchopleural Fistula
Bronchopleural fistula is the formation of a sinus tract between the bronchial tree and the pleura. It is a rare complication that has a high associated mortality and morbidity. Pulmonary necrosis secondary to infections, chemotherapy, and radiation therapy are common causes of bronchopleural fistulas. However, the most common cause is postpulmonary surgical resection.27
In interventional radiology, this complication may be noted after lung biopsy and thermal ablative therapies. Patients may present immediately following the intervention or within weeks of ablation of a lung mass. The delayed presentation after ablation is related to the progressive necrosis of the ablated region, leading to fistula formation.
Risk Factors
Percutaneous intervention on peripheral lesions is more prone to the development of bronchopleural fistulas. In the case of thermal ablative therapies, the extension of an ablation zone to the pleural surface has been suggested to be a risk factor for the formation of a bronchopleural fistula.28
Management
Although most fistulas resolve spontaneously, their management can be challenging. If the fistula results in a symptomatic pneumothorax, immediate placement of a chest tube is required.28 If this fails as a long-term treatment option, the chest tube can be utilized to administer sclerosing agents to induce pleurodesis.29
Endobronchial techniques have been successfully used to occlude bronchopleural fistulas with different agents, including clot30 and cyanoacrylate glue.31 Video-assisted thoracic surgery and thoracotomy are more invasive options used in the management of this complication.
Diaphragmatic Injury
Diaphragmatic injury may complicate any percutaneous procedure involving the lower thorax or upper abdomen. For example, diaphragmatic injuries are a well-documented complication of thoracostomy tube placements and postthermal ablation of subphrenic hepatic tumors. Interestingly, it has rarely been reported following thoracic tumor ablations. It was first described in a large single-center case series, where it was observed in a single patient postablation of a pulmonary lesion in close proximity to the left diaphragm. The reported rate of diaphragmatic injury post–radiofrequency ablation of lung tumors is 0.1%.32 33
The clinical presentation of diaphragmatic injuries depends on the location and size of the defect. The injury may be identified incidentally on imaging examinations in asymptomatic patients, or may present with respiratory symptoms of dyspnea, shortness of breath, or pain, with other signs and symptoms relating to herniation of abdominal organs.
Diaphragmatic injury may be identified acutely or may have a delayed presentation months to years after the percutaneous procedure.
Risk Factors
Interventions performed close to the diaphragm are the major risk factors associated with this rare complication.
Management and Prevention
Careful preprocedural review of diagnostic imaging studies is paramount in identifying the anatomic location of the diaphragm in relation to the site of intervention. This will help in the planning of the intervention and reduce the risk of phrenic injury during the placement of thoracostomy tubes. However, this may not help in cases of thermal ablation of pulmonary lesions close to the diaphragmatic surface. In fact, given the rarity of this complication, there has not been any reported method of minimizing the risk of phrenic injury during ablation procedures. Two possible techniques that may prove useful in reducing the incidence of diaphragmatic injury include the creation of an iatrogenic intraprocedural pneumothorax, separating the lower thoracic lesion from the diaphragm prior to thermal ablation, and temperature monitoring of the diaphragm.
Diaphragmatic injuries are usually treated surgically upon diagnosis and preferably prior to the development of herniations or other complications.34
Chylous Leaks
Chylous leaks may occur at any anatomic level, from the intestinal lymphatic vessels to the thoracic duct. Iatrogenic chylous leaks are generally related to surgical procedures, radiation therapy,35 and rarely due to left subclavian venous access.36 37 However, given the role of interventional radiology in the management of chylous leaks, supradiaphragmatic leaks will be discussed briefly here.
Supradiaphragmatic leaks present as chylothorax, chylopericardium, and postsurgical chylous wound leaks. The fluid is usually turbid or white in appearance and has high triglyceride content. These are rare complications caused by direct injury to or occlusion of the thoracic duct; chylothorax has a reported incidence rate of 0.42% of patients undergoing major thoracic surgery, and 3.8% in postesophagectomy cases.38 39
Risk Factors
The major risk factor for the development of chyle leaks is iatrogenic injury to the thoracic duct during thoracic surgeries. Operative fields in close proximity to the thoracic duct are the major culprits in the development of significant chyle leaks. Esophageal surgery is one of the prime examples, given the proximity of the esophagus to the thoracic duct. Furthermore, in the context of esophageal cancer surgeries, a body mass index < 30 has been identified as a risk factor for the development of chyle leaks.39 Therefore knowledge of the anatomy of the surgical field is imperative to reduce the risk of thoracic duct injuries.39
Management
Initially, chylous leaks are treated conservatively. Nil by mouth, with parenteral nutrition together with somatostatin and octreotide, decreases the flow of chyle through the thoracic duct, allowing for the resolution of most leaks. Administration of low fat medium chain triglycerides by mouth, which are absorbed directly into the portal vein, is a described alternative albeit less effective than total parenteral nutritional therapy.40
Failure of conservative therapies is most often due to high output chylothoraces requiring more invasive procedures. High output leaks are associated with high morbidity and mortality; therefore, conservative management is only used judiciously.
Surgical ligation of the thoracic duct is the classical treatment of chylous leaks. However, this is associated with high morbidity and mortality rates. Therefore, other treatments have been proposed and have been used successfully. Video-assisted thoracoscopic surgery is a less invasive alternative that can also be used to ligate the thoracic duct.
Minimally invasive techniques are preferred to surgical techniques due to their lower morbidity and mortality rates. Lymphangiography followed by thoracic duct embolization is a technique that has been used to treat chylous leaks that have failed optimal conservative therapy.41 An alternative route for the embolization of the thoracic duct is via retrograde venous access.42
In addition to embolization, the placement of a thoracostomy tube is useful for the symptomatic improvement of the patient, and allows for measurement of the output. Furthermore, the chest tube may be used as a conduit to perform chemical pleurodesis.43
Vascular Complications
Hemorrhage
Iatrogenic hemorrhage can be the most serious complication following interventional thoracic procedures. It manifests as chest wall hematoma, mediastinal hemorrhage, hemoptysis, intraparenchymal hemorrhage, and/or hemothorax.
Hemorrhage after percutaneous transthoracic interventional procedures such as biopsy and tumor ablation is the second most commonly encountered complication, reported in up to 30% of cases.44 45 46 47 48
The most common manifestation of bleeding complications is intraparenchymal hemorrhage, which presents as perilesional ground glass opacity or needle track bleeding on CT scan.47 Opacification of the lung parenchyma may limit the visualization of the target lesion resulting in lower technical success of the procedure. Mild hemoptysis secondary to parenchymal hemorrhage or arterio-bronchial fistula reportedly occurs in 3 to 9% of cases.47 48 49 Fortunately, these are usually self-limiting and typically resolve with supportive care.
Hemothorax due to puncture of the heart or the great vessels, on the other hand, may result in catastrophic events, such as hemopericardium and cardiac tamponade.
Risk Factors
In addition to abnormal coagulation or pulmonary arterial hypertension, several lesion characteristics have been suggested as risk factors for hemorrhagic complications, including ablation of lesions larger than 2 cm in diameter; biopsy of lesions smaller than 1.5 cm; anatomic location of the target lesions centrally or in the mediastinum; long needle path through the lungs; traversal of pulmonary vasculature; multiple pleural punctures; the use of multi-tined ablative electrodes; and large needles.21 50 51
Management
Most parenchymal hemorrhages are self-limiting and resolve spontaneously with supportive care (Fig. 3). Rarely, they can be massive and fatal, especially after ablation.51 52 53 54 55 If significant hemorrhage occurs, the procedure should be terminated and the patient placed in the ipsilateral decubitus position to prevent aspiration of blood. However, if the patient is hemodynamically unstable, fluid resuscitation and blood transfusion may be required. In severe cases, transcatheter embolization may be warranted.
Fig. 3.
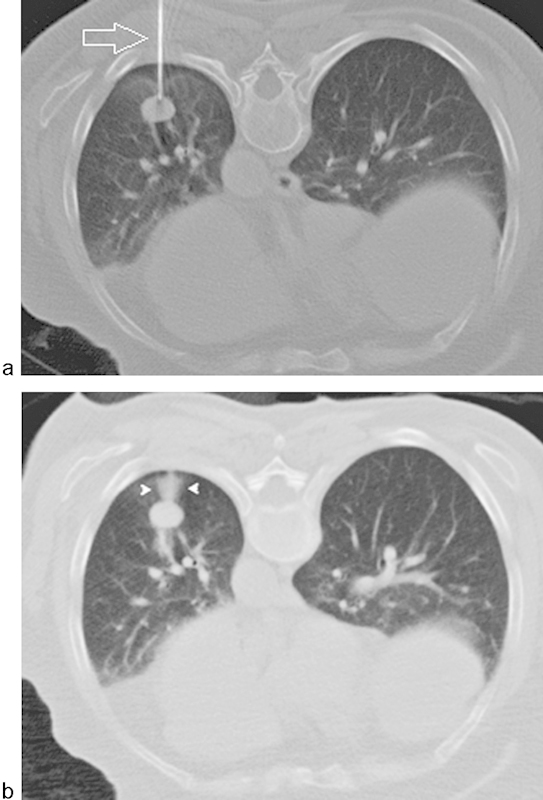
Perilesional and needle tract intraparenchymal hemorrhage after percutaneous needle biopsy of a left lower lobe nodule. (a) Axial CT-scan image showing a biopsy needle (arrow) at the outer margin of left lower lobe mass. (b) Postbiopsy axial image demonstrating perilesional and needle tract opacification compatible with intraparenchymal hemorrhage (arrowheads).
Hemothorax can be treated immediately with thoracostomy tube placement. Drainage may be facilitated by lytic therapy performed via the indwelling chest tube during the organizing phase of the hematoma. Surgical decortication and thoracoscopy are reserved for retained material and incomplete lung expansion.56 Similar to parenchymal hemorrhages, if there is hemodynamic compromise transcatheter embolization of the injured vessel may be necessary (Fig. 4).
Fig. 4.
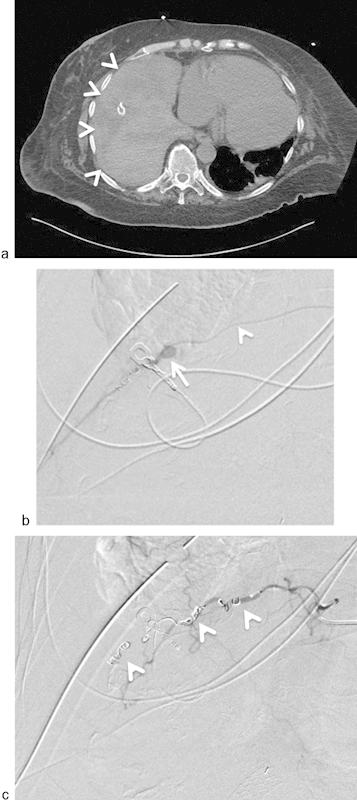
A 67-year-old woman developed severe hypotension and tachycardia following surgical chest tube placement for empyema at an outside hospital. Chest tube was noted to be draining bright red bloody fluid. (a) Axial image from an emergent CT scan revealed a large right hemothorax (arrowheads). (b) Selective catheterization (arrowhead = microcatheter) of the right ninth intercostal artery. Angiography revealed a pseudoaneurysm of the intercostal artery (arrow). (c) Successful coil (arrowheads) embolization of the intercostal artery distal and proximal to pseudoaneurysm.
Prevention
Patient selection and procedure planning are the most important factors in minimizing the incidence of hemorrhagic complications. Abnormal coagulation profiles should be corrected and anticoagulants should be withheld prior to the procedure. Careful review of patient history and baseline imaging are paramount in planning the procedure and in the anticipation of complications.
The availability and optimal use of imaging guidance systems are also helpful in preparing for the procedure and in avoiding vascular structures.
Air Embolism
Systemic air embolism is an extremely rare complication that can be fatal. Although systemic air embolism has been reported to occur in up to 0.07% of transthoracic needle biopsy procedures,57 58 it may also occur after lung tumor ablation.59 60 61 This low rate is most probably related to the underdiagnosis/underreporting of this complication, especially if patients do not demonstrate cardiac or neurologic dysfunction.
Presentation depends on the systemic vascular bed involved: air embolism to the coronary arteries may manifest with arrhythmias and/or cardiovascular collapse, while air embolism to the cerebral circulation may result in stroke. The diagnosis of air embolism is particularly challenging when deeper sedation or general anesthesia are utilized but should be suspected when there is an acute deterioration of the clinical status of the patient.
Several mechanisms have been proposed for air entry into the systemic circulation, with the common theme being access through a pulmonary venous lumen. Those mechanisms include direct puncture of a pulmonary vein with air injection through the needle, direct creation of a bronchovenous fistula by the needle, or the needle traversing an air-containing lesion and a pulmonary vein simultaneously. Furthermore, increased airway pressure such as coughing or positive ventilation and abnormal coagulation or impaired healing have also been described as possible pathways for air embolism.57
Risk Factors
A few risk factors have been considered but are difficult to confirm given the rarity of this complication. These include intraoperative coughing, changes in thoracic pressures, tissue friability, and the use of larger biopsy needles.
Management
When air embolism is suspected, supportive care with 100% oxygen should be initiated. Patient positioning is controversial; the authors recommend placing the patient in the left lateral decubitus and Trendelenburg position to trap air in the left atrium minimizes the risk of cerebral emboli.
Hyperbaric oxygen treatment may help in decreasing the volume of the gas bubble while increasing the solubility of air.55 62
Prevention
Implementation of intraprocedural precautions is essential to reducing the risk of systemic air embolism. To this end, patient cooperation with breath holding and minimizing cough are vital in reducing complications of percutaneous thoracic procedures. In addition, the operators should keep to a minimum the time where the needle is open to the atmosphere and choose the shortest avascular path to the target lesion.
Nerve Injury
Iatrogenic nerve injury is a rare complication reported in cases of ablation of tumors that are in close proximity to thoracic nerves. The most common nerves involved are the brachial and phrenic nerves,32 63 although other nerves described in the literature include intercostal and recurrent nerves. In a large series of radiofrequency ablation procedures, there was an iatrogenic neurological complication rate of 1.5%.63
Brachial nerves are usually affected in ablation of apical tumors. This usually involves the inferior component of the brachial plexus, including the C8 and the T1 nerve roots and the lower nerve trunk.64 Patients may complain of sensory discomfort/dysfunction intraoperatively or in the immediate postprocedural period. These deficits may be transient or have long-term sequela.
The phrenic nerves provide the motor innervation to the main muscle of respiration, the diaphragm. Therefore, damage of the phrenic nerves may result in significant physiologic impairment. This is augmented by the fact that a significant number of patients undergoing thoracic interventions have underlying compromised baseline pulmonary function. In interventional radiology, injury to the phrenic nerves has been described following thermal ablation of perimediastinal or mediastinal tumors that are in close proximity to the nerves.65 Other iatrogenic phrenic nerve injuries have been described in surgical patients and even in endovascular cardiac ablative procedures.66
Risk Factors
Nerve tissue is vulnerable to thermal injury; this is supported by the fact that the degree of nerve damage is related to the temperature and the duration of exposure.
The main risk factor is the anatomical location of the target lesion. Apical lesions are close to the brachial plexus while mediastinal lesions may be closely associated with the phrenic nerves. Therefore, thermal ablation of lesions close to thoracic nerves is the primary risk factor for injury.
Tumor size is the second most important risk factor. Larger tumors are associated with a higher risk of nerve injury.63 Secondary risk factors that correlate with tumor size are the use of larger electrodes and greater maximum power.67
Management
Nerve injuries may have devastating effects on patients. Management of nerve injuries is primarily supportive, and prevention remains the primary objective.
Prevention
Understanding the anatomy of the thoracic nerves is the best way to avoid injury. To this end, preprocedural review and analysis of diagnostic imaging studies and the ablation region is the major step in anticipating and avoiding nerve injury.
Nerve protection techniques have been described. These can be accomplished by the creation of a pneumothorax to separate the lesion from the nerve.65 Another technique is temperature monitoring of the area of the nerve of interest; thus, allowing for the protection of the nerves from temperature increases during the ablation.
Conclusion
Percutaneous thoracic interventions are among the most common interventions in today's interventional radiology practice. Although associated with the potential for serious complications, these are relatively rare. Careful evaluation of the patient, review of diagnostic imaging studies, and preprocedural planning are the most important steps in optimizing patients care. Furthermore, understanding the common complications, as well as their risk factors and management, will help operators improve patients' safety and ultimately improve outcomes.
References
- 1.Kocijančič I KK. CT-guided percutaneous transthoracic needle biopsy of lung lesions – 2-year experience at the Institute of Radiology in Ljubljana. Radiol Oncol. 2007;41:99–106. [Google Scholar]
- 2.Berger R. Iatrogenous pneumothorax. Chest. 1994;105(4):980–982. doi: 10.1378/chest.105.4.980. [DOI] [PubMed] [Google Scholar]
- 3.Klein J S Zarka M A Transthoracic needle biopsy Radiol Clin North Am 2000382235–266., vii [DOI] [PubMed] [Google Scholar]
- 4.Covey A M, Gandhi R, Brody L A, Getrajdman G, Thaler H T, Brown K T. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 2004;15(5):479–483. doi: 10.1097/01.rvi.0000124951.24134.50. [DOI] [PubMed] [Google Scholar]
- 5.vanSonnenberg E, Casola G, Ho M. et al. Difficult thoracic lesions: CT-guided biopsy experience in 150 cases. Radiology. 1988;167(2):457–461. doi: 10.1148/radiology.167.2.3357956. [DOI] [PubMed] [Google Scholar]
- 6.Arslan S, Yilmaz A, Bayramgürler B, Uzman O, Nver E, Akkaya E. CT-guided transthoracic fine needle aspiration of pulmonary lesions: accuracy and complications in 294 patients. Med Sci Monit. 2002;8(7):CR493–CR497. [PubMed] [Google Scholar]
- 7.Klein J S, Salomon G, Stewart E A. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology. 1996;198(3):715–720. doi: 10.1148/radiology.198.3.8628859. [DOI] [PubMed] [Google Scholar]
- 8.Brown K T, Brody L A, Getrajdman G I, Napp T E. Outpatient treatment of iatrogenic pneumothorax after needle biopsy. Radiology. 1997;205(1):249–252. doi: 10.1148/radiology.205.1.9314993. [DOI] [PubMed] [Google Scholar]
- 9.Cattelani L, Campodonico F, Rusca M. et al. CT-guided transthoracic needle biopsy in the diagnosis of chest tumours. J Cardiovasc Surg (Torino) 1997;38(5):539–542. [PubMed] [Google Scholar]
- 10.Collings C L, Westcott J L, Banson N L, Lange R C. Pneumothorax and dependent versus nondependent patient position after needle biopsy of the lung. Radiology. 1999;210(1):59–64. doi: 10.1148/radiology.210.1.r99ja1759. [DOI] [PubMed] [Google Scholar]
- 11.Simon C J, Dupuy D E, DiPetrillo T A. et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243(1):268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 12.Yan T D, King J, Sjarif A, Glenn D, Steinke K, Morris D L. Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol. 2006;13(11):1529–1537. doi: 10.1245/s10434-006-9101-1. [DOI] [PubMed] [Google Scholar]
- 13.Heck S L, Blom P, Berstad A. Accuracy and complications in computed tomography fluoroscopy-guided needle biopsies of lung masses. Eur Radiol. 2006;16(6):1387–1392. doi: 10.1007/s00330-006-0152-2. [DOI] [PubMed] [Google Scholar]
- 14.Kazerooni E A, Lim F T, Mikhail A, Martinez F J. Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology. 1996;198(2):371–375. doi: 10.1148/radiology.198.2.8596834. [DOI] [PubMed] [Google Scholar]
- 15.Cox J E, Chiles C, McManus C M, Aquino S L, Choplin R H. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology. 1999;212(1):165–168. doi: 10.1148/radiology.212.1.r99jl33165. [DOI] [PubMed] [Google Scholar]
- 16.Laurent F, Michel P, Latrabe V, Tunon de Lara M, Marthan R. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: incidence and risk factors. AJR Am J Roentgenol. 1999;172(4):1049–1053. doi: 10.2214/ajr.172.4.10587145. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Boiselle P M, Shepard J O, Trotman-Dickenson B, McLoud T C. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol. 1996;167(1):105–109. doi: 10.2214/ajr.167.1.8659351. [DOI] [PubMed] [Google Scholar]
- 18.Ko J P, Shepard J O, Drucker E A. et al. Factors influencing pneumothorax rate at lung biopsy: are dwell time and angle of pleural puncture contributing factors? Radiology. 2001;218(2):491–496. doi: 10.1148/radiology.218.2.r01fe33491. [DOI] [PubMed] [Google Scholar]
- 19.Haramati L B, Austin J H. Complications after CT-guided needle biopsy through aerated versus nonaerated lung. Radiology. 1991;181(3):778. doi: 10.1148/radiology.181.3.1947096. [DOI] [PubMed] [Google Scholar]
- 20.Hiraki T, Tajiri N, Mimura H. et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: incidence and risk factors. Radiology. 2006;241(1):275–283. doi: 10.1148/radiol.2411051087. [DOI] [PubMed] [Google Scholar]
- 21.Khankan A A, Al-Muaikeel M. Image-guided percutaneous transthoracic biopsy in lung cancer—emphasis on CT-guided technique. J Infect Public Health. 2012;5 01:S22–S30. doi: 10.1016/j.jiph.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Yamagami T, Nakamura T, Iida S, Kato T, Nishimura T. Management of pneumothorax after percutaneous CT-guided lung biopsy. Chest. 2002;121(4):1159–1164. doi: 10.1378/chest.121.4.1159. [DOI] [PubMed] [Google Scholar]
- 23.Billich C, Muche R, Brenner G. et al. CT-guided lung biopsy: incidence of pneumothorax after instillation of NaCl into the biopsy track. Eur Radiol. 2008;18(6):1146–1152. doi: 10.1007/s00330-008-0872-6. [DOI] [PubMed] [Google Scholar]
- 24.Moore E H. Technical aspects of needle aspiration lung biopsy: a personal perspective. Radiology. 1998;208(2):303–318. doi: 10.1148/radiology.208.2.9680552. [DOI] [PubMed] [Google Scholar]
- 25.Engeler C E, Hunter D W, Castaneda-Zuniga W, Tashjian J H, Yedlicka J W, Amplatz K. Pneumothorax after lung biopsy: prevention with transpleural placement of compressed collagen foam plugs. Radiology. 1992;184(3):787–789. doi: 10.1148/radiology.184.3.1509068. [DOI] [PubMed] [Google Scholar]
- 26.Zaetta J M Licht M O Fisher J S Avelar R L; Bio-Seal Study Group. A lung biopsy tract plug for reduction of postbiopsy pneumothorax and other complications: results of a prospective, multicenter, randomized, controlled clinical study J Vasc Interv Radiol 20102181235–430., 3 [DOI] [PubMed] [Google Scholar]
- 27.Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128(6):3955–3965. doi: 10.1378/chest.128.6.3955. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai J, Hiraki T, Mukai T. et al. Intractable pneumothorax due to bronchopleural fistula after radiofrequency ablation of lung tumors. J Vasc Interv Radiol. 2007;18(1, Pt 1):141–145. doi: 10.1016/j.jvir.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita T, Miyoshi S, Katoh M. et al. Intrapleural administration of a large amount of diluted fibrin glue for intractable pneumothorax. Chest. 2000;117(3):790–795. doi: 10.1378/chest.117.3.790. [DOI] [PubMed] [Google Scholar]
- 30.Lan R S, Lee C H, Tsai Y H, Wang W J, Chang C H. Fiberoptic bronchial blockade in a small bronchopleural fistula. Chest. 1987;92(5):944–946. doi: 10.1378/chest.92.5.944. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe S, Watanabe T, Urayama H. Endobronchial occlusion method of bronchopleural fistula with metallic coils and glue. Thorac Cardiovasc Surg. 2003;51(2):106–108. doi: 10.1055/s-2003-38981. [DOI] [PubMed] [Google Scholar]
- 32.Kashima M, Yamakado K, Takaki H. et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol. 2011;197(4):W576–80. doi: 10.2214/AJR.11.6408. [DOI] [PubMed] [Google Scholar]
- 33.Kesieme E B, Dongo A, Ezemba N, Irekpita E, Jebbin N, Kesieme C. Tube thoracostomy: complications and its management. Pulm Med. 2012;2012:256878. doi: 10.1155/2012/256878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soufi M, Meillat H, Le Treut Y P. Right diaphragmatic iatrogenic hernia after laparoscopic fenestration of a liver cyst: report of a case and review of the literature. World J Emerg Surg. 2013;8(1):2. doi: 10.1186/1749-7922-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McWilliams A, Gabbay E. Chylothorax occurring 23 years post-irradiation: literature review and management strategies. Respirology. 2000;5(3):301–303. doi: 10.1046/j.1440-1843.2000.00263.x. [DOI] [PubMed] [Google Scholar]
- 36.Ruggiero R P, Caruso G. Chylothorax—a complication of subclavian vein catheterization. JPEN J Parenter Enteral Nutr. 1985;9(6):750–753. doi: 10.1177/0148607185009006750. [DOI] [PubMed] [Google Scholar]
- 37.Anestis N, Christos F C, Ioannis P, Christos I, Lampros P, Stephanos P. Thoracic duct injury due to left subclavicular vein catheterization: a new conservative approach to a chyle fistula using biological glue. Int J Surg Case Rep. 2012;3(7):330–332. doi: 10.1016/j.ijscr.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyon S, Mott N, Koukounaras J, Shoobridge J, Hudson P V. Role of interventional radiology in the management of chylothorax: a review of the current management of high output chylothorax. Cardiovasc Intervent Radiol. 2013;36(3):599–607. doi: 10.1007/s00270-013-0605-3. [DOI] [PubMed] [Google Scholar]
- 39.Shah R D Luketich J D Schuchert M J et al. Postesophagectomy chylothorax: incidence, risk factors, and outcomes Ann Thorac Surg 2012933897–903., discussion 903–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos W, Faintuch J. Nutritional management of thoracic duct fistulas. A comparative study of parenteral versus enteral nutrition. JPEN J Parenter Enteral Nutr. 1986;10(5):519–521. doi: 10.1177/0148607186010005519. [DOI] [PubMed] [Google Scholar]
- 41.Chen E, Itkin M. Thoracic duct embolization for chylous leaks. Semin Intervent Radiol. 2011;28(1):63–74. doi: 10.1055/s-0031-1273941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mittleider D, Dykes T A, Cicuto K P, Amberson S M, Leusner C R. Retrograde cannulation of the thoracic duct and embolization of the cisterna chyli in the treatment of chylous ascites. J Vasc Interv Radiol. 2008;19(2, Pt 1):285–290. doi: 10.1016/j.jvir.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 43.McGrath E E, Blades Z, Anderson P B. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. 2010;104(1):1–8. doi: 10.1016/j.rmed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Khan M F, Straub R, Moghaddam S R. et al. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol. 2008;18(7):1356–1363. doi: 10.1007/s00330-008-0893-1. [DOI] [PubMed] [Google Scholar]
- 45.Hiraki T, Gobara H, Fujiwara H. et al. Lung cancer ablation: complications. Semin Intervent Radiol. 2013;30(2):169–175. doi: 10.1055/s-0033-1342958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winokur R S, Pua B B, Sullivan B W, Madoff D C. Percutaneous lung biopsy: technique, efficacy, and complications. Semin Intervent Radiol. 2013;30(2):121–127. doi: 10.1055/s-0033-1342952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeow K M, See L C, Lui K W. et al. Risk factors for pneumothorax and bleeding after CT-guided percutaneous coaxial cutting needle biopsy of lung lesions. J Vasc Interv Radiol. 2001;12(11):1305–1312. doi: 10.1016/s1051-0443(07)61556-5. [DOI] [PubMed] [Google Scholar]
- 48.Yeow K M, Su I H, Pan K T. et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126(3):748–754. doi: 10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]
- 49.Lucidarme O, Howarth N, Finet J F, Grenier P A. Intrapulmonary lesions: percutaneous automated biopsy with a detachable, 18-gauge, coaxial cutting needle. Radiology. 1998;207(3):759–765. doi: 10.1148/radiology.207.3.9609901. [DOI] [PubMed] [Google Scholar]
- 50.Shaham D. Semi-invasive and invasive procedures for the diagnosis and staging of lung cancer. I. Percutaneous transthoracic needle biopsy. Radiol Clin North Am. 2000;38(3):525–534. doi: 10.1016/s0033-8389(05)70182-2. [DOI] [PubMed] [Google Scholar]
- 51.Nour-Eldin N E, Naguib N N, Mack M, Abskharon J E, Vogl T J. Pulmonary hemorrhage complicating radiofrequency ablation, from mild hemoptysis to life-threatening pattern. Eur Radiol. 2011;21(1):197–204. doi: 10.1007/s00330-010-1889-1. [DOI] [PubMed] [Google Scholar]
- 52.Vaughn C Mychaskiw G II Sewell P Massive hemorrhage during radiofrequency ablation of a pulmonary neoplasm Anesth Analg 20029451149–1151., table of contents [DOI] [PubMed] [Google Scholar]
- 53.Dupuy D E, Mayo-Smith W W, Abbott G F, DiPetrillo T. Clinical applications of radio-frequency tumor ablation in the thorax. Radiographics. 2002;22(Spec No):S259–S269. doi: 10.1148/radiographics.22.suppl_1.g02oc03s259. [DOI] [PubMed] [Google Scholar]
- 54.Herrera L J, Fernando H C, Perry Y. et al. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg. 2003;125(4):929–937. doi: 10.1067/mtc.2003.18. [DOI] [PubMed] [Google Scholar]
- 55.Sano Y, Kanazawa S, Gobara H. et al. Feasibility of percutaneous radiofrequency ablation for intrathoracic malignancies: a large single-center experience. Cancer. 2007;109(7):1397–1405. doi: 10.1002/cncr.22541. [DOI] [PubMed] [Google Scholar]
- 56.Lorenz J, Blum M. Complications of percutaneous chest biopsy. Semin Intervent Radiol. 2006;23(2):188–193. doi: 10.1055/s-2006-941449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bou-Assaly W, Pernicano P, Hoeffner E. Systemic air embolism after transthoracic lung biopsy: a case report and review of literature. World J Radiol. 2010;2(5):193–196. doi: 10.4329/wjr.v2.i5.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomiyama N, Yasuhara Y, Nakajima Y. et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006;59(1):60–64. doi: 10.1016/j.ejrad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Okuma T, Matsuoka T, Tutumi S, Nakmura K, Inoue Y. Air embolism during needle placement for CT-guided radiofrequency ablation of an unresectable metastatic lung lesion. J Vasc Interv Radiol. 2007;18(12):1592–1594. doi: 10.1016/j.jvir.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 60.Ghaye B, Bruyère P J, Dondelinger R F. Nonfatal systemic air embolism during percutaneous radiofrequency ablation of a pulmonary metastasis. AJR Am J Roentgenol. 2006;187(3):W327–8. doi: 10.2214/AJR.06.0179. [DOI] [PubMed] [Google Scholar]
- 61.Jin G Y, Lee J M, Lee Y C, Han Y M. Acute cerebral infarction after radiofrequency ablation of an atypical carcinoid pulmonary tumor. AJR Am J Roentgenol. 2004;182(4):990–992. doi: 10.2214/ajr.182.4.1820990. [DOI] [PubMed] [Google Scholar]
- 62.Mirski M A, Lele A V, Fitzsimmons L, Toung T J. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007;106(1):164–177. doi: 10.1097/00000542-200701000-00026. [DOI] [PubMed] [Google Scholar]
- 63.Palussière J, Canella M, Cornelis F. et al. Retrospective review of thoracic neural damage during lung ablation - what the interventional radiologist needs to know about neural thoracic anatomy. Cardiovasc Intervent Radiol. 2013;36(6):1602–1613. doi: 10.1007/s00270-013-0597-z. [DOI] [PubMed] [Google Scholar]
- 64.Hiraki T, Gobara H, Mimura H. et al. Brachial nerve injury caused by percutaneous radiofrequency ablation of apical lung cancer: a report of four cases. J Vasc Interv Radiol. 2010;21(7):1129–1133. doi: 10.1016/j.jvir.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 65.Thornton R H, Solomon S B, Dupuy D E, Bains M S. Phrenic nerve injury resulting from percutaneous ablation of lung malignancy. AJR Am J Roentgenol. 2008;191(2):565–568. doi: 10.2214/AJR.07.3507. [DOI] [PubMed] [Google Scholar]
- 66.Sacher F, Monahan K H, Thomas S P. et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol. 2006;47(12):2498–2503. doi: 10.1016/j.jacc.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 67.Matsui Y, Hiraki T, Gobara H. et al. Phrenic nerve injury after radiofrequency ablation of lung tumors: retrospective evaluation of the incidence and risk factors. J Vasc Interv Radiol. 2012;23(6):780–785. doi: 10.1016/j.jvir.2012.02.014. [DOI] [PubMed] [Google Scholar]


