Abstract
As advances in surgical techniques and postoperative care continue to improve outcomes, the use of solid organ transplants as a treatment for end-stage organ disease is increasing. With the growing population of transplant patients, there is an increasing need for radiologic diagnosis and minimally invasive procedures for the management of posttransplant complications. Typical complications may be vascular or nonvascular. Vascular complications include arterial stenosis, graft thrombosis, and development of fistulae. Common nonvascular complications consist of leaks, abscess formation, and stricture development. The use of interventional radiology in the management of these problems has led to better graft survival and lower patient morbidity and mortality. An understanding of surgical techniques, postoperative anatomy, radiologic findings, and management options for complications is critical for proficient management of complex transplant cases. This article reviews these factors for kidney, liver, pancreas, islet cell, lung, and small bowel transplants.
Keywords: organ transplantation, complications, interventional radiology, angioplasty, percutaneous
Objectives: Upon completion of this article, the reader will be able to discuss the potential complications of solid organ transplant and the role of interventional radiology in the management of these complications.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Organ transplantation is often the only treatment available for end-stage organ failure. In 2013, a total of 28,935 solid organ transplants were performed in the United States.1 Given the large number of patients now living with transplanted organs, astute management of the inevitable complications of transplant surgery is an important aspect of patient care. These complications, often associated with the anastomotic sites, can often be treated using minimally invasive techniques. The purpose of this article is to review the most common complications associated with renal, liver, pancreas, islet cell, lung, and small bowel transplants and to discuss the minimally invasive techniques available to the interventional radiologist for the management of these complications.
Complications of Renal Transplants
For patients with end-stage renal disease, renal transplantation improves both quality of life and survival rates to a greater extent than hemodialysis or continuous ambulatory peritoneal dialysis.2 Recipients of cadaveric transplants have a 5-year survival rate of 82%, and living donor kidney recipients have a 5-year survival rate of 90%; patients with hemodialysis have an average survival of only 4 to 8 years.3 Refinement of surgical techniques, more effective immunosuppression, establishment of a nationwide coordinating network, and improved availability of HLA typing for donor–recipient matching have further improved survival rates for patients undergoing renal transplant. In 2013, there were 17,656 kidney transplants in the United States, more than any other organ.1 The number of transplants performed has remained relatively constant over the past 10 years, although the demand for kidneys far outstrips the supply.1
A variety of surgical techniques are employed for renal transplantation. The imaging appearances and postoperative complications encountered will vary depending on the surgical procedure performed; therefore, interventional radiologists must become familiar with the technique(s) used at their own institutions.4 Transplanted kidneys are typically placed in the extraperitoneal right iliac fossa (except in cases of pancreas-kidney transplants); vascular anastomoses are easier to perform on the right side because of the superficial and horizontal course of the right iliac vein. The type of arterial anastomosis created often depends on the available graft; cadaveric kidneys are harvested with an intact main renal artery and an attached portion of the aorta (Carrel patch) and are typically anastomosed end to side to the recipient external iliac artery (EIA).2 The kidney of a living donor cannot be harvested with a portion of the aorta, so either an end-to-side anastomosis of the donor renal artery to the recipient EIA or an end-to-end anastomosis to the recipient internal iliac artery (IIA) is performed.2 The donor renal vein is sutured end to side to the recipient external iliac vein. Techniques for urinary reconstruction vary among institutions, but the preferred method is generally ureteroneocystostomy, in which the ureter is anastomosed directly to the dome of the bladder.3 Ureteroureterostomy and ureteropyelostomy are additional surgical options.
The preservation of renal function after transplantation can be affected by several factors, including vascular and nonvascular complications. Postoperative complications occur in ∼12 to 20% of patients undergoing renal transplantation.5 One large study reported urologic complications in 4 to 8% of patients and vascular complications in % to 2% of patients.5 Surgical intervention for the management of such complications may be avoided thanks to advances in percutaneous diagnostic and interventional techniques.6
Vascular Complications
Vascular complications, including transplant renal artery stenosis (TRAS), renal graft thrombosis, arteriovenous fistulas (AVFs), flow-limiting dissection, and pseudoaneurysms, occur in 1 to 15% of renal transplant recipients and may be associated with significant morbidity.5
Transplant Renal Artery Stenosis
TRAS occurs most commonly at the anastomotic site but may also occur in a preanastomotic (iliac inflow arterial disease) or postanastomotic (extrarenal, segmental, or lobar) location.7 Wong et al reported that the incidence of documented TRAS at one institution increased from 2.4% in the era before Doppler ultrasound (DUS) to 12.4% in the DUS era; the authors suggested that this was due to the increased use of noninvasive imaging in renal transplant patients.8 TRAS accounts for 1 to 5% of posttransplant hypertension cases and is a curable cause of pharmacologic-refractory hypertension.9 Early TRAS (<2 months after transplant) is commonly related to operative complications (including suturing or clamp injury), whereas delayed complications (>2 months after transplant) are typically caused by vascular ischemia (vasa vasorum ischemia) and the progression of preexisting atherosclerotic disease.7
The clinical presentation of TRAS depends on the postoperative time frame. Within the first 2 weeks following transplant, TRAS may present with a continued need for dialysis and/or anuria; subsequently, accelerated renovascular hypertension and flash pulmonary edema secondary to fluid retention are most common.10 11 As the clinical presentation is often nonspecific, diagnostic imaging is typically necessary in these cases.
As with other vascular complications, DUS is often the first step in evaluation because of its widespread availability and cost-effectiveness.12 DUS has a reported sensitivity of 87 to 94% and specificity of 86 to 100% for TRAS.13 14 Diagnostic findings for TRAS include a peak systolic velocity (PSV) of >200 cm/s and a ratio of >2 for the PSVs of the stenotic to prestenotic segments (Fig. 1a). Suggestive indirect findings include color aliasing at the site of stenosis and a parvus tardus waveform with a prolonged systolic acceleration time.3
Fig. 1.
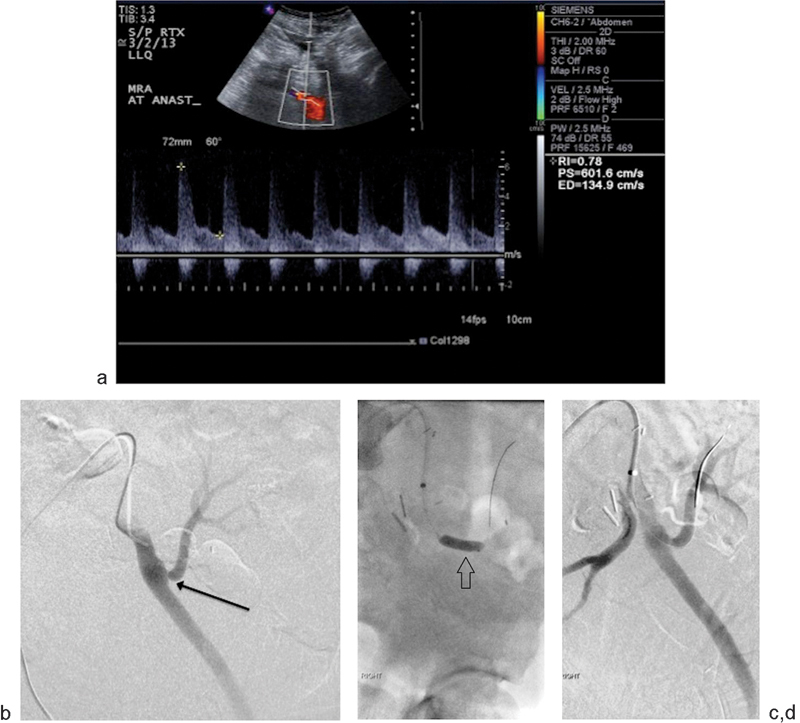
A 51-year-old man presented with deteriorating renal function 8 months after left lower quadrant renal transplant. (a) Doppler ultrasound demonstrates a markedly increased PSV of 601.6 cm/s at the arterial anastomotic site, consistent with severe stenosis. (b) Selective arteriography of the left external iliac artery demonstrates an end-to-side anastomosis and focal severe stenosis at the site of anastomosis (black arrow) and mild poststenotic dilatation. The pressure gradient across the anastomosis was 32 mm Hg. (c) Percutaneous transluminal angioplasty was performed with a 6 mm × 40 mm balloon (arrow) with significant improvement of the narrowing on follow-up arteriography (d). Postangioplasty angiogram revealed a pressure gradient of 5 mm Hg across the stenosis and < 30% residual narrowing.
Magnetic resonance angiography (MRA) provides superior anatomical detail, can assess other vascular and nonvascular complications, and may be used in patients with a body habitus unfavorable for US. Nonenhanced MR with steady-state free precession may be used when renal function or allergy does not permit gadolinium administration.
The gold standard for the diagnosis of TRAS is conventional transcatheter angiography.2 With this technique, carbon dioxide may be used to minimize the risk of contrast-induced nephropathy.12 Nonselective aortoiliac arteriography must be performed before selective transplant renal artery arteriography to exclude the possibility of an inflow preanastomotic stenosis.7
Treatment of TRAS may include angioplasty, stenting, or surgery depending on the clinical scenario. In some cases, conservative management is appropriate, especially when there is no decline in renal function and hypertension can be controlled medically.15 Hemodynamically significant TRAS is defined as narrowing of the luminal diameter >50% or pressure gradient >10 mm Hg across the stenosis. Initial management for these cases involves percutaneous transluminal angioplasty (PTA) with or without stent placement (Fig. 1b–d).12 13 16 A guidewire is typically placed through the stenosis, and dilation is performed with a balloon that has a diameter at least 1 mm greater than the normal segment of renal artery. End-to-side anastomoses are more amenable to PTA than end-to-end anastomoses, with technical success rates of 91 and 75%, respectively.17 Rates of early clinical success (within 1 month of intervention) with PTA, with or without stent placement, and long-term clinical success (>3 months) have ranged from 58 to 82% and 41 to 75%, respectively. Voiculescu et al18 found that restenosis occurred after PTA alone in 62% of patients, whereas the rate of restenosis after primary stent placement was only 30%. A study using drug-eluting stents adapted from coronary artery stenting reported technical success in all 17 cases and a mean decrease in systolic blood pressure of 24 mm Hg at 1 month; serum creatinine levels also decreased from a mean of 3.1 to 2.3 mg/dL.19
Anatomical vascular complications of endovascular treatment occur in 0 to 10.3% of patients and may include renal artery thrombosis (RAT), flow-limiting renal artery dissection, and renal artery pseudoaneurysm.17 20 21 22 23 24
Renal Artery Thrombosis
RAT is a rare but frequently catastrophic complication of kidney transplants, occurring in ∼0.3 to 2% of transplant cases. This condition has a variety of etiologies, including rejection, embolism, dissection, and kinking.3 25 26 RAT occurs in conjunction with renal vein thrombosis (RVT) in ∼11 to 15% of cases.27 28 RAT complication typically occurs within 2 weeks after transplant; 80% of cases occur within the first month.29 30
The kidney is very sensitive to ischemic injury. Human kidneys can safely tolerate 30 to 60 minutes of controlled clamp ischemia with mild structural changes.31 However, severe renal injury can occur within 1 to 2 hours of ischemia.32 33 Therefore, complete RAT is a clinical emergency, as there is no collateral supply to the affected kidney.
Patients with RAT often present with an abrupt decrease in urine output without pain or other symptoms. DUS may reveal a lack of or diminished arterial flow with possible echogenic thrombus filling defects within the renal artery. Angiography (transcatheter or MR based) should be performed for definitive diagnosis.
In these cases, urgent revascularization is critical to preserve the graft. Endovascular thrombectomy or thrombolysis can be attempted, but surgical thrombectomy is recommended in the immediate posttransplant period, as this method is quicker and avoids the risk of bleeding from thrombolytic therapy.34 Once flow is reestablished, the renal artery should be scrutinized for underlying anatomical defects such as kinks or stenoses.
Renal Vein Thrombosis
RVT is rare, occurring in only 0.1 to 0.3% of transplants.3 25 26 This complication typically occurs within 2 weeks after transplant; 80% of cases occur within 1 month.28 The clinical presentation is insidious, and graft dysfunction may be the initial manifestation of RVT. A patient with RVT may also have deep venous thrombosis (DVT) extending to the iliac veins.35
In the absence of RAT, RVT typically demonstrates graft edema with the absence of venous flow on DUX.2 Thrombus within the transplanted renal vein may be visualized, and the arterial resistive index (RI) is typically increased with reversal of diastolic flow (Fig. 2).
Fig. 2.
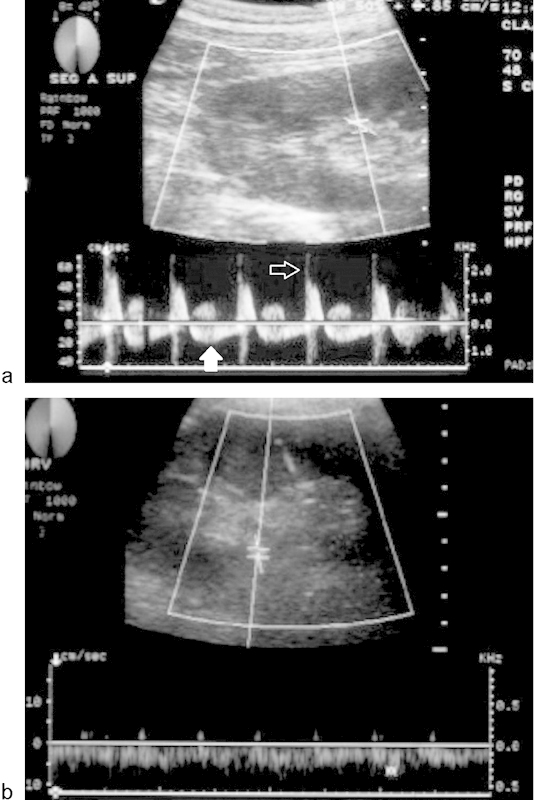
A 51-year-old man underwent a renal transplant and developed acute renal failure 1 week after the transplant. (a) Doppler ultrasound (DUS) demonstrates reversed, prolonged diastolic flow (solid arrow) as well as a highly resistant arterial flow with a spiked systolic component (open arrow) suggestive of renal vein thrombosis. No definite flow was seen in the renal vein. (b) After thrombectomy, DUS demonstrates normal venous flow.
In cases of isolated RVT, anticoagulation alone may be successful in treating this condition.36 In cases of complete RVT or RVT associated with femoro-iliac venous DVT, catheter-directed thrombolysis may be performed to decrease periprocedural morbidity.37
Renal Artery Dissection
This complication is almost always a result of iatrogenic injury from endovascular intervention, particularly after PTA with or without stent placement for TRAS.38 The presence of arterial kinks is a risk factor for dissection during endovascular procedures. The dissection rate after renal transplantation is <10%.17 20 21 22 23 24 Dissection may be difficult to detect on DUS; however, if arterial flow compromise or thrombosis is detected, angiography should be performed for further characterization.
Most dissections do not require intervention unless arterial blood flow is compromised. The interventionist may cross the dissection flap starting in and returning distally into the true lumen, using a balloon or stent to tack the dissection flap against the renal arterial wall.38
Arteriovenous Fistula
AVFs are almost always iatrogenic, with graft biopsy being the most common cause; the incidence of postbiopsy AVF ranges from 1 to 18%.5 39 40 41 Most AVFs are small and resolve spontaneously or persist with no clinical significance. Patients with large, persistent fistulas may present with gross hematuria, renal insufficiency, hypertension, and high cardiac output failure.
In cases of AVFs, DUS may demonstrate aliasing at the site of the AVF in the renal parenchyma, arterialization of a renal vein (increased velocity and arterial waveform), and decreased arterial resistive indices (Fig. 3a).42 In some cases, a lack of arterial Doppler flow distal to the AVF within the renal parenchyma may be seen.42 Contrast-enhanced MR will usually reveal a large AVF as a perfusion defect within the renal parenchyma or as an early draining vein during the arterial phase of enhancement. Transcatheter angiography remains the gold standard for the evaluation of AVFs, as this modality can be used to evaluate the hemodynamic significance of the lesion. Typically, a dilated, high-velocity, early draining vein is visualized.
Fig. 3.
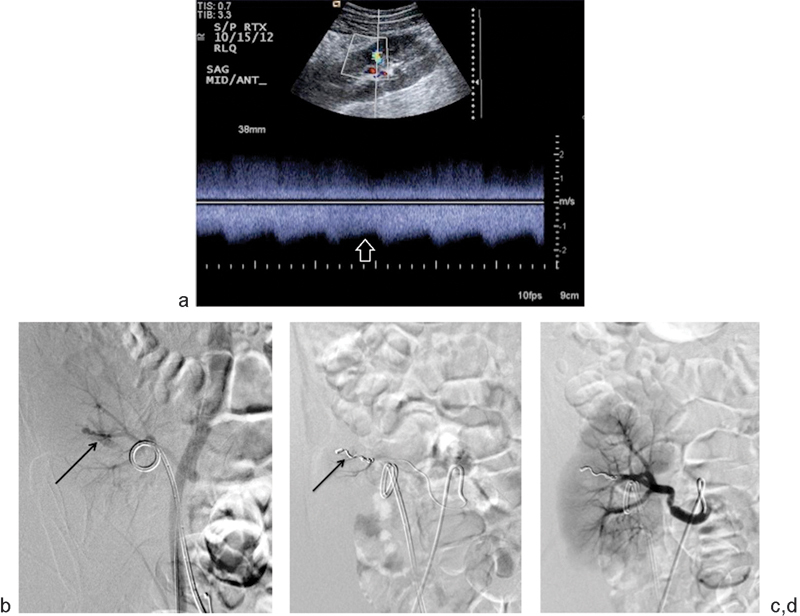
A 26-year-old man presented with hematuria after percutaneous transplant renal biopsy. (a) Doppler ultrasound in the interpolar region demonstrates spectral broadening (open arrow), suggesting an arteriovenous fistula (AVF). (b) Right common iliac arteriogram shows an early draining vein (black arrow) during the arterial phase, confirming the presence of an AVF. A double-J stent is incidentally noted within the ureter. (c) The arterial feeder supplying the AVF was successfully embolized using one 3-mm and one 4-mm detachable hydrocoils (arrow). Postembolization selective angiography of the arterial feeder confirmed occlusion of arteriovenous fistula and no opacification of the early draining vein. (d) After embolization, selective main renal arteriography again confirmed obliteration of arteriovenous fistula. There is incidental focal spasm of the main renal artery.
Treatment, which is only indicated if the AVF is symptomatic, typically involves transcatheter embolization using coils, a vascular plug, or glue (Fig. 3b–f).2 3 Selective embolization with a larger occlusive agent is preferred, as embolic particles may result in nontarget embolization and renal infarcts.2 Technical success rates of 71 to 100%, with alleviation of symptoms in 57 to 88% of cases, have been reported.43 44 45 46
Renal Artery Pseudoaneurysm
Intrarenal pseudoaneurysms, like AVFs, usually occur secondary to a percutaneous procedure such as biopsy or percutaneous nephrostomy tube placement.41 While AVFs result from injury to both an artery and an adjacent vein, pseudoaneurysms result from arterial wall injury only; however, pseudoaneurysms and AVFs may coexist in up to 30% of cases.35 37 Pseudoaneurysms occur in 0.1 to 0.3% of kidney transplant cases.26 47 Intrarenal pseudoaneurysms are often small and asymptomatic and may spontaneously resolve, but extrarenal pseudoaneurysms may rupture, leading to severe consequences.48 On grayscale US, these cystic-appearing fluid collections mimic a renal cyst; however, DUS may reveal the classic “yin-yang” flow due to jets of forward and reverse flow.2 3
Small pseudoaneurysms do not usually require intervention; follow-up imaging to ensure spontaneous resolution or lack of growth will generally suffice. Large and symptomatic pseudoaneurysms require endovascular management with embolization of intrarenal lesions or stent exclusion in extrarenal or segmental lesions.12 35 49
Ureteric Complications
The advent of ureteroneocystostomy has led to lower rates of urinary leaks and obstruction in patients undergoing renal transplant. Ureteric complications now occur in approximately 4 to 8% of cases, whereas earlier publications reported rates of 20 to 30%.50 51
Ureteral Obstruction
Ureteral obstruction occurs in 2 to 10% of kidney transplant cases and nearly always occurs within the first 6 months after transplant.52 Multiple etiologies, including ureteral kinking, perigraft fibrosis or fluid collections, calculi, and fungus balls, may lead to ureteral obstruction.
The incidence of ureteral strictures ranges from 2.9 to 4.6%.53 More than 90% of ureteral stenoses occur within the distal third of the ureter, most commonly at the site of ureteral implantation into the bladder, as this area is vulnerable to ischemia.41 Because the transplanted kidney is denervated, patients do not experience typical renal colic; rather, most present with increasing serum creatinine levels and/or oliguria.
Although US may confirm the presence of hydronephrosis, false negatives are not uncommon, particularly in cases of acute obstruction. On the other hand, false positives with mild to moderate dilation of the collecting system may be seen in patients with a denervated full bladder; therefore, emptying of the bladder and reimaging is important for accurate diagnosis.54 Echogenicities within the dilated collecting system are usually clinically significant and may represent pyonephrosis.55 Both computed tomography (CT) and MR imaging may help to exclude extrinsic compression from perigraft fluid collections; CT can also be used to detect ureteral calculi.
Antegrade nephrostography is effective in diagnosing ureteral obstruction and depicting the site and nature of the obstruction (Figs. 4a, b and 5a). The optimal approach with this technique is via the lateral calyx, as this method can eliminate the need for a transperitoneal approach, which is more painful, and also avoids vessels that may overlie the allograft.56 Although retrograde pyelography is optimal for ureteral evaluation in patients undergoing ureteropyelostomy or ureteroureterostomy, this technique is often difficult to perform in patients with a ureteroneocystostomy, as cannulation of the ureter is challenging in such instances. Once the ureteral obstruction is diagnosed, a nephrostomy catheter may be placed for decompression.
Fig. 4.
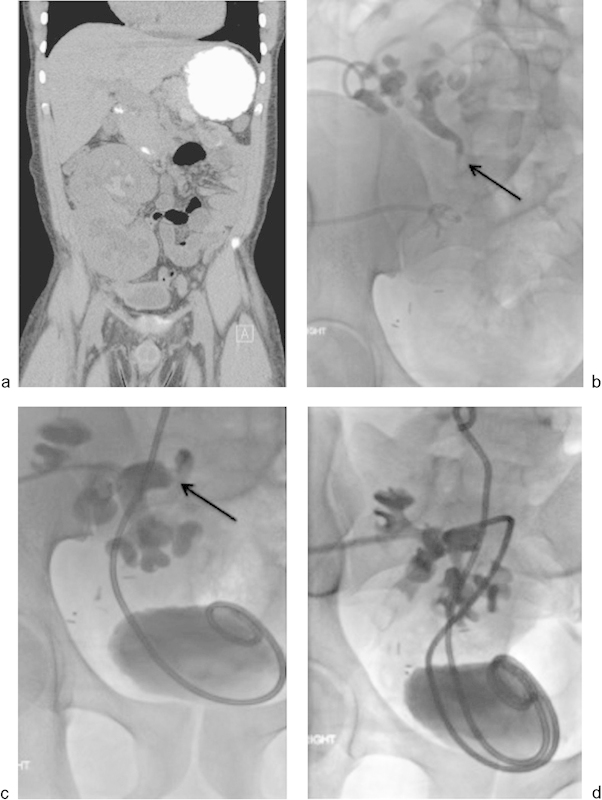
A 26-year-old man who had undergone en-bloc renal transplant presented with nausea, renal failure, and hyperkalemia. US revealed hydronephrosis. (a) Coronal unenhanced CT demonstrates marked dilatation of the collecting systems of both transplant kidneys. Percutaneous nephrostomy tubes were placed in both kidneys. Three days later, antegrade nephrostograms of both kidneys (b, c) demonstrate moderate hydronephrosis with moderate narrowing of the ureteropelvic junction (black arrows) involving both moieties. Eight-French 20-cm J-J ureteral stents were successfully placed into each collecting system (D).
Fig. 5.
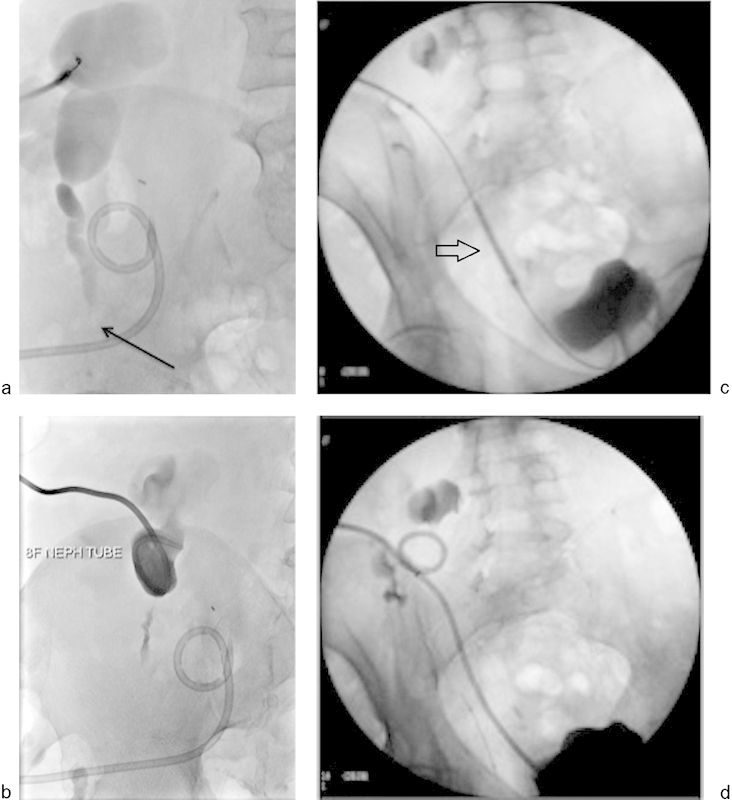
A 48-year-old man who had undergone renal transplant had developed a lymphocele adjacent to the transplant kidney. An 8-French pigtail catheter was placed for drainage of lymphocele. On follow-up, the patient presented with worsening hydronephrosis on ultrasound and elevated creatinine. (a) Antegrade nephrostogram demonstrates marked hydronephrosis and severe mid-ureteric stricturing (black arrow). The pigtail catheter is seen draining the lymphocele. (b) An 8-French percutaneous nephrostomy catheter was placed and (c) balloon dilatation was performed using 4 mm × 40 mm (arrow) and 5 mm × 40 mm balloons. (d) After balloon dilation, nephrostomy catheter was exchanged for a 10-French 20-cm nephroureteral catheter.
In cases of persistent obstructions after initial nephrostomy drainage and decompression are performed, an antegrade nephroureteral stent may be placed (Figs. 4c, d and 5b). A nephroureteral catheter may be left in place for subsequent dilations or nephrostography as needed. In such cases, a double-J stent may be placed to minimize the risk of infection and patient discomfort. The relatively new Memokath thermoexpandable stent (PNN Medical A/S, Kvistgaard, Denmark) expands after heating for placement; for removal, cold water causes the stent to contract and deform.57
Balloon ureteroplasty may be beneficial in cases of high-grade perianastomotic strictures, but is often not effective for strictures longer than 2 cm or for strictures caused by ischemia. Balloon dilation has a clinical success rate of only 58 to 62% for ureteral strictures but may be more successful for the treatment of fresh surgical strictures (Fig. 5c–f).58 59 Balloon dilation followed by endourotomy using the H:YAG laser can be useful for persistent strictures.60 The preferred surgical treatment is ureteropyelostomy, in which the native ureter is attached to the transplanted renal pelvis. This procedure is associated with good patency and a low rate of recurrent strictures.61
Urinary Leak and Urinoma
Urinary leak occurs in 1 to 5% of patients undergoing kidney transplant.6 The leak most commonly occurs at the distal ureter, related to necrosis caused by ischemia or rejection, or at the ureteroneocystostomy site, caused by problems at the time of surgery. Leaks occur less commonly in the proximal ureter or pelvicaliceal system as a result of rupture secondary to distal ureteral obstruction. Urinary leak most commonly presents during the first 3 months following transplant; a patient with a urinary leak may present with pain at the graft site, swelling, discharge from the wound, or urinoma. Urinary leaks and urinomas can be life threatening because of the risk of infection; therefore, prompt diagnosis and intervention are key.
US in cases of urinary leak may reveal a well-defined, anechoic fluid collection without septations that may increase in size rapidly. CT may better define the full extent of the perigraft fluid collection, as large urinomas can eventually rupture, leading to urinary ascites. US- or CT-guided drainage can provide a definitive diagnosis by demonstrating higher creatinine levels within the fluid than within serum, thereby differentiating a urinary leak from a seroma or lymphocele. Drainage may also prevent potential infection and reveal extrinsic compression. Antegrade nephrostography is necessary to provide detailed information regarding the site of origin of the urinoma and to allow clinicians to plan appropriate intervention.62
When the urine leak is significant, management options include placement of double-J stents to facilitate urine drainage, placement of a percutaneous urinoma drain, percutaneous nephrostomy to divert urinary flow, and surgical revision to repair the leak.2
To reduce the risk of ureteral obstruction or urinary leak, several institutions have investigated the use of routine stent placement in all transplant procedures. The most commonly used stent for these procedures is a double-J stent, which is a straight tube with anchoring J loops on either end. Whang et al studied the use of a shorter segment of ureter using the Lich-Gregoir technique (compared with the Politano-Leadbetter technique) and the routine use of indwelling stents, and reported an incidence of ureteral strictures and urinary leaks of 1.3 and 0.9%, respectively.63 Although a 1996 Cochrane review found a lower incidence of leak and stenosis in stented patients, there was a higher rate of urinary tract infections in the routine stenting group.64 65 Urinary tract infections associated with stents may be more difficult to treat than those in nonstented patients because of biofilm development.66 This risk of urinary tract infection can be decreased by shortening the duration of stent usage from 2 weeks to 1 week.67 In addition to urinary tract infections, there is an increase in the risk of BK viremia in stented patients.68
Compartment Syndrome
Retroperitoneal compartment syndrome is a unique complication of kidney transplant and occurs secondary to increased pressure within the retroperitoneal space. This syndrome occurs when tissue fluid within the retroperitoneal space accumulates in large volumes. It is similar to abdominal compartment syndrome and can cause organ ischemia and, eventually, graft loss. The incidence of this complication among patients undergoing kidney transplant is 1.2 to 2%. Compartment syndrome carries a high risk of morbidity because of its association with a rapid decline in graft function.69 70 Treatment of this condition involves surgical fasciotomy with replacement of the graft.69 70
Peritransplant Fluid Collections
Peritransplant fluid collections occur in up to 50% of renal transplant patients, and 15 to 20% of these cases become clinically significant.71 Patients with these collections most often present with local pain; however, transplant dysfunction secondary to extrinsic compression of the transplant vascular structures can also occur. Small hematomas and seromas may be seen in the immediate postoperative period. The size of these collections at baseline should be noted, as an increase in size may indicate vascular injury, abscess, or urinary leak.
The size, location, and potential growth of a peritransplant fluid collection determine its clinical significance.72 Aspiration is typically necessary for a specific diagnosis, as US findings are often nonspecific.
Lymphocele
Lymphoceles are the most common peritransplant fluid collections, usually occurring 4 to 8 weeks after transplant at an incidence of 0.6 to 18%.73 74 Patients undergoing kidney transplant are particularly at risk because of the typical use of corticosteroids after transplant and the potential for graft rejection. Lymphoceles form secondary to lymphatic leakage from the allograft itself or from lymphatic damage in the surgical bed. Chemical analysis of lymphoceles will reveal protein, urea nitrogen, electrolytes, and creatinine values similar to those in the serum, differentiating lymphoceles from urinomas, seromas, or abscesses.
In patients with lymphoceles, US typically demonstrates an anechoic collection with occasional septations; however, if a lymphocele is infected, a more complex appearance may be observed (Fig. 6a). CT usually reveals a sharply circumscribed collection with attenuation values similar to those of simple fluid but lower than values of recent hematoma or abscess. MR imaging typically demonstrates a T1 hypointense, T2 hyperintense collection with occasional septations (Fig. 6b).
Fig. 6.
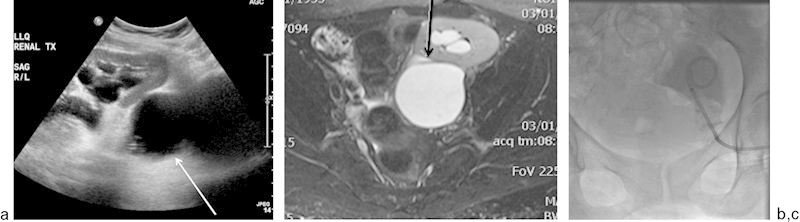
A 60-year-old woman who was 15 months post–renal transplant presented with recurrent lymphoceles. (a) US demonstrates an anechoic collection (white arrow) which does not appear to communicate with the ureter, surrounding the transplanted kidney, leading to moderate hydroureteronephrosis. (b) T2-weighted fat-saturated MR image demonstrates a T2 hyperintense collection adjacent to the transplanted kidney, which compresses the renal pelvis (black arrow) and results in hydronephrosis. (c) After a tractogram with injection of contrast, an 8.5-French × 25-cm drainage catheter was placed under fluoroscopic guidance. A total of 50 mL of Betadine was then instilled through the drainage catheter into the lymphocele.
Large lymphoceles require drainage; however, lymphoceles recur after percutaneous or surgical drainage in up to 80 to 90% of cases.75 Therefore, prolonged catheter drainage and transcatheter instillation of sclerosing agents, such as absolute alcohol, may be necessary for permanent resolution. These techniques have success rates of up to 97% (Fig. 6c).76 A sinogram should be performed prior to sclerotherapy to exclude a fistulous communication between the lymphocele and adjacent vital structures, such as the ureter or collecting system. Surgical marsupialization of lymphoceles into the peritoneal cavity may be performed, but this procedure is more invasive.
Hematoma
Hematomas occur frequently in the postoperative period, but the majority are small and asymptomatic. Enlarging hematomas in the immediate postoperative period may result from vessel injury in the graft bed, disruption of the vascular suture line, or spontaneous graft rupture.77
On US, a complex appearance is typical for hematomas. Acute hematomas are echogenic, with this echogenicity decreasing over time. Chronic hematomas may appear anechoic, and septations may develop.54 As with US, CT imaging characteristics of hematomas are time dependent, with acute hematomas demonstrating high-attenuation components and chronic hematomas containing liquefied and serous portions of intermediate attenuation.54 On MR imaging, acute hematomas demonstrate high signal intensity with both T1- and T2-weighted pulse sequences.
When a perigraft hematoma results in significant extrinsic compression on the renal allograft or when clinical and laboratory findings suggest that a hematoma is infected, percutaneous drainage with 12- to 14-French drains and periodic irrigation with saline solution to prevent drain clogging are generally successful treatment strategies.
Abscess
In a febrile transplant recipient, any peritransplant fluid collection must be presumed to be infected. Primary abscess development is likely uncommon; however, any of the aforementioned perigraft fluid collections may become secondarily infected. Local pain and fever are commonly present in such cases; however, some patients are relatively asymptomatic because of their immunosuppressed state. Most abscesses develop in the first several weeks after transplantation.
Diagnostic US findings in cases of abscess are often nonspecific, revealing a complex cystic appearance. In the case of emphysematous pyelonephritis, gas within the renal graft parenchyma may produce echogenic lines with distal reverberation artifacts. CT may also demonstrate gas, serving to differentiate abscesses from other perigraft fluid collections.
Prompt surgical or percutaneous drainage in combination with systemic antibiotics is mandated in cases of abscess because of the immunosuppressed state of the patient. Percutaneous drainage under US or CT guidance is associated with a high success rate and a low incidence of complications.78
Complications of Liver Transplants
The liver is the second most commonly transplanted organ, accounting for 21.2% (6,455 in total) of all organs transplanted in the United States in 2013; however, complications after liver transplant are more common than with other types of organ transplants.1 This is attributable to both the preoperative debilitation of these patients and the inherent complexity of the procedure. Multiple anastomoses are required, including the inferior vena cava (IVC), portal vein, hepatic artery, common bile duct, and the Roux-en-Y of the intestine, each of which can be associated with adverse events that can threaten both the graft and the patient.79
Inferior Vena Cava and Hepatic Vein Complications
Anastomosis of the IVC can be performed with a variety of techniques. The traditional bicaval technique involves anastomosis of the suprahepatic and infrahepatic portions of the recipient cava to the intrahepatic cava of the donor liver (Fig. 7). The piggyback technique preserves the recipient IVC, creating an end-to-side anastomosis between the donor and recipient suprahepatic cava while oversewing the infrahepatic donor cava. Recently, a side-to-side cavocavostomy has been used, in which the donor suprahepatic and infrahepatic cava are oversewn and a venotomy of the posterior donor cava is anastomosed to a venotomy along the anterior recipient cava.80 These techniques involve the creation of a common patch of the right, middle, and left hepatic veins. The incidence of IVC and hepatic vein outflow problems is closely related to these differences in technique.
Fig. 7.
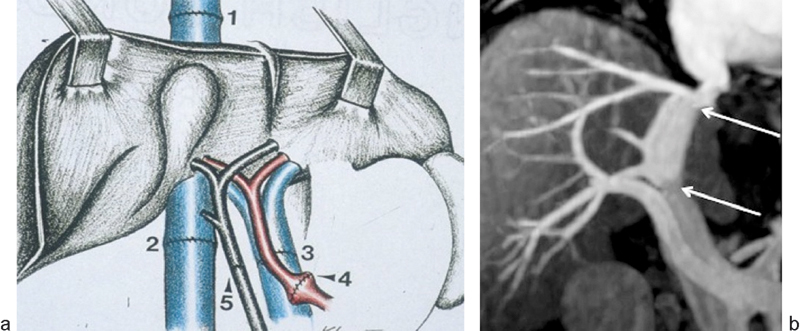
(a) Diagram of a transplanted liver (bicaval technique) shows the 4 end-to-end vascular anastomoses and the biliary anastomosis. 1 = suprahepatic inferior vena cava (IVC) anastomosis; 2 = infrahepatic IVC anastomosis; 3 = portal vein anastomosis; 4 = hepatic artery anastomosis; 5 = bile duct anastomosis. (b) MR image demonstrates the suprahepatic and infrahepatic IVC anastomoses (white arrows).
The incidence of caval obstruction with the bicaval and cavocavostomy techniques is 1 to 2%, whereas the piggyback technique is associated with a caval obstruction rate of 4%.81 Stenosis of the IVC is most commonly seen at the surgical anastomosis. IVC complications are generally related to graft rotation, a tight suture line, vessel size discrepancy, or kinking of the IVC.82 The resulting hepatic congestion manifests clinically as ascites, hepatomegaly, renal failure, pleural effusion, lower limb swelling, and abnormal liver function tests.
In most cases, IVC stenosis and thrombosis can be evaluated with DUS. A normal IVC and hepatic vein will demonstrate triphasic waveform because of variations in right atrial chamber pressures during systole and diastole. Grayscale US may demonstrate intraluminal echogenic thrombus within the IVC, whereas DUS may demonstrate a greater than threefold increase in the PSV of the stenotic to prestenotic segment, with turbulent flow in the stenotic segment.83 In cases of upper caval anastomotic stenosis, flow reversal in the hepatic veins and absence of phasicity in the hepatic venous Doppler waveform may be seen.
When US findings are concerning for stenosis or thrombosis, the hepatic veins and IVC can be mapped with a blood pool contrast agent and MR imaging.84 To select the most effective imaging approach, clinicians must know the type of IVC anastomosis used. A transjugular approach allows the best access to the hepatic veins if a piggyback technique was used, whereas both transjugular and femoral approaches can be used in cases of bicaval or cavocavostomy anastomosis. A transhepatic approach is reserved for cases in which these other methods are unsuccessful.85
Pressure measurements can be obtained across an area of stenosis. A gradient of >3 mm Hg within a hepatic vein is suggestive of the diagnosis. Hepatic vein stenosis can be treated with balloon angioplasty, but angioplasty of the IVC is less effective because of the elasticity of that vessel. Oversized stents, sized ∼2 mm greater than the lumen, can provide long-term patency, and prevents migration of the stent along the IVC (Fig. 8).86 87 However, overdilation of stents should be avoided, as the stents may fracture and migrate into the right atrium.88
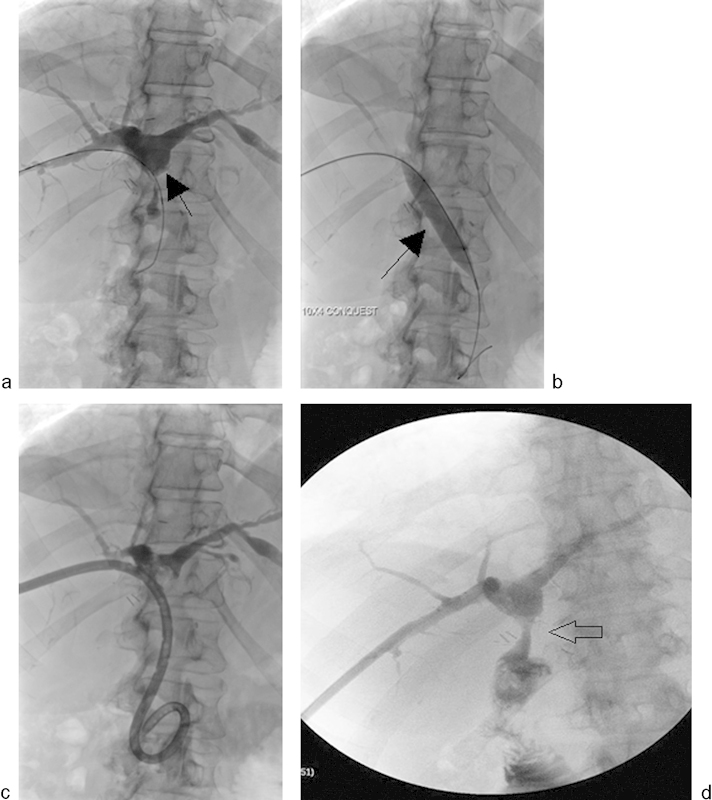
Fig. 8.
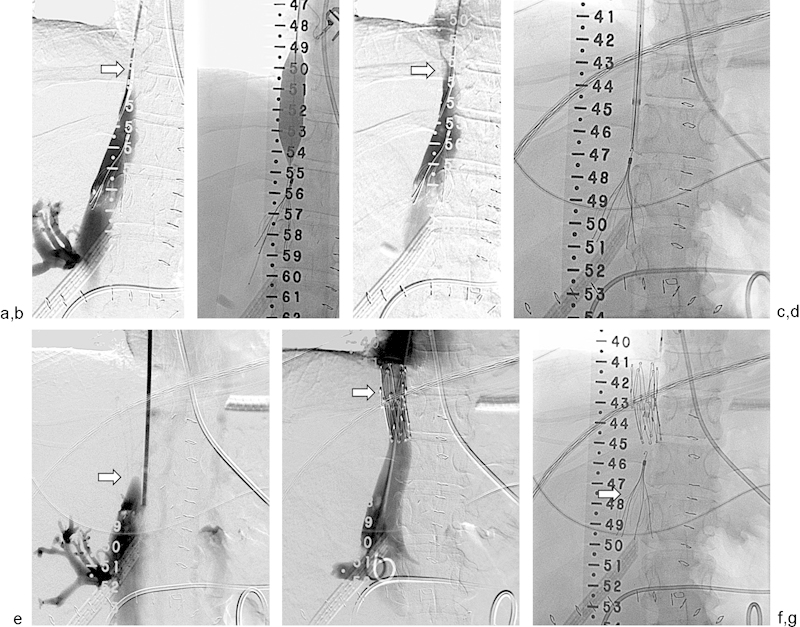
A 35-year-old patient underwent a whole liver transplant. (a) The infrahepatic inferior vena cava (IVC) is nearly occluded (arrow). A suprarenal IVC filter was previously placed for iliocaval thrombosis. (b) Percutaneous transluminal angioplasty was performed from a transjugular approach using an 18 mm × 40 mm balloon. (c) Repeat venogram shows modest improvement in flow but persistence of the caval stricture (arrow). (d) One week later, the filter was temporarily retrieved to allow for stent placement. (e) Venogram demonstrates recurrent IVC occlusion (arrow). (f) Cavagram performed after deployment of a 25 mm × 5 cm Gianturco Z stent (arrow) shows widely patent IVC. (g) Suprarenal filter (arrow) was replaced via right internal jugular vein approach.
IVC thrombosis is a rare but potentially serious complication related to IVC stenosis, occurring in 0.3% of transplantations. In the acute setting, this complication results in an urgent need for therapy and probable retransplantation.89 In the chronic setting, collaterals may develop and the patient may be relatively asymptomatic.
Portal Vein Complications
After caval anastomosis is completed, portal anastomosis can be performed before reperfusion of the transplanted liver. The portal anastomosis is usually end to end with a running suture.80 Portal vein complications, which are rare, may include stenosis and thrombosis. Portal vein stenosis occurs in 0.3 to 3.7% of liver transplant cases, although a higher incidence has been reported in patients with previous portal vein operations or portal vein thrombosis. Portal vein stenosis and thrombosis are usually the result of technical complications during the surgery, including vessel size mismatch, misalignment, or kinking.87 89 90 91 Clinically, patients with these complications may present with symptoms of portal hypertension, such as gastroesophageal varices and ascites.91
DUS is typically the initial diagnostic test in cases of suspected portal vein complications. The normal portal vein has a smooth wall with an anechoic lumen. Flow is monophasic and hepatopetal with respiratory variation, although turbulent flow can be normal in the early postoperative period.83 A peak anastomotic velocity of 125 cm/s or a 3:1 anastomotic to preanastomotic velocity ratio is suggestive of portal vein stenosis. If the portal vein is completely occluded, an echogenic intraluminal thrombus and lack of Doppler flow will be seen. Percutaneous transhepatic portography can be performed for direct measurement of pressure gradients across a stricture, with a 5-mm Hg gradient considered significant.92 A transjugular approach can also be used, but the transhepatic approach provides greater control.
PTA with or without stent placement is the primary treatment for portal vein stenosis (Fig. 9), with surgical intervention reserved for recalcitrant strictures. Endovascular therapy is more appropriate for early detection of thrombosis, resulting in a 40% success rate with overall 1-year graft survival of 33%.93 94
Fig. 9.
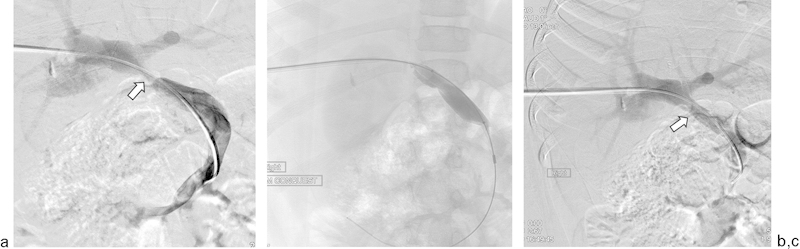
An 8-year-old male patient underwent a whole liver transplant. (a) Transhepatic portal venogram obtained with the catheter tip in the superior mesenteric vein shows a stricture of the portal anastomosis (arrow). (b) Through a 7-French sheath, venoplasty was performed with a 12 mm × 40 mm balloon. (c) Repeat venography reveals resolution of the stricture (arrow). The sheath was removed as Gelfoam pledgets were deployed into the parenchymal tract.
Hepatic Artery Complications
The donor hepatic artery is harvested as a Carrel patch of aorta containing the origin of the celiac axis or at the branch point of the common hepatic and splenic arteries, and is anastomosed in an end-to-end fashion to a branch patch created from the recipient hepatic artery bifurcation.80 Hepatic artery complications in liver transplant cases can include thrombosis, stenosis, and pseudoaneurysm.
Hepatic artery thrombosis (HAT), occurring in 4 to 12% of adult liver transplant recipients and in up to 40% of pediatric liver transplant recipients, is the most common vascular complication of liver transplantation.95 HAT is also the most serious vascular complication. Frequently cited risk factors for HAT include older donors, ABO incompatibility, cytomegalovirus status mismatch, recipient tobacco use, hypercoagulability, and prolonged graft ischemia time.96 The clinical presentation of HAT may range from mild transaminase elevation to delayed bile leak, bile duct stricture, sepsis, and fulminant hepatic necrosis.82
DUS is usually performed to confirm flow in the hepatic arteries after liver transplant. The normal hepatic artery demonstrates continuous diastolic flow with a sharp systolic upstroke. The RI, defined as (PSV − peak diastolic velocity)/PSV, ranges from 0.5 to 0.8. A low RI predicts vascular complications; a high RI can be normal in the first 72 hours after transplant and may be associated with prolonged ischemic times and advanced donor age, but is not predictive of vascular complications.97 DUS has a 92% accuracy rate in the diagnosis of HAT, which is seen on this imaging modality as an absence of flow in the proper and intrahepatic arteries.98 In some instances, intrahepatic flow may be detected in the presence of complete HAT secondary to arterial collateral vessels; consequently, the absence of arterial flow at the porta hepatis with tardus parvus waveform intrahepatically is also suggestive of HAT (see Fig. 13a).
Fig. 13.
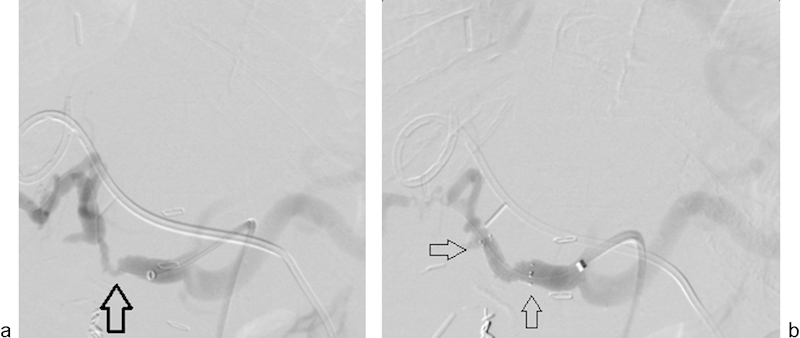
A 72-year-old man underwent orthotopic liver transplant for primary biliary cirrhosis. MRI performed 3 weeks later after transplant (not shown) revealed severe biliary dilatation and severe stenosis of the hepatic artery resulting in ischemic cholangiopathy. (a) A common hepatic arteriogram shows severe stenosis at the site of arterial anastomosis (arrow). (b) Arteriogram after successful stent placement (arrows) with re-establishment of patent lumen.
A strong predictor of HAT is a temporal progression from normal diastolic Doppler flow to absent diastolic flow with dampening of the systolic upstroke, with eventual complete loss of hepatic arterial flow.99 If HAT is suspected, selective catheter arteriography can be performed to confirm the diagnosis. On occasion, a false-positive diagnosis of HAT based on US results may occur in cases of severe hepatic edema with resulting markedly diminished hepatic arterial flow.
HAT is typically classified as early or late, occurring within 30 days of transplant or >30 days after transplant, respectively. Late HAT does not always lead to graft failure, and one-third of patients with this condition do well without any intervention.100 Those patients who go on to develop biliary necrosis or abscess formation can be treated with percutaneous biliary or abscess drainage. In some instances of early HAT, thrombolysis or thrombectomy may be performed for graft salvage; however, retransplantation is often required.
After thrombosis is confirmed by selective diagnostic arteriography, catheter-directed thrombolytic therapy and angioplasty or stent insertion of any underlying stenosis can be performed (Fig. 10b, c). Mechanical thrombolysis of the intra-arterial thrombus is performed with a guidewire or with an AngioJet Thrombectomy System.101 After debulking, an infusion catheter is placed into the thrombosed hepatic artery, ideally as deeply as possible without wedging in the distal hepatic artery.101 At this point, a thrombolytic agent can be infused. Repeat arteriographies should be performed to evaluate thrombolysis progress at 12, 24, or 36 hours depending on the individual case and the institutional protocol.
Fig. 10.
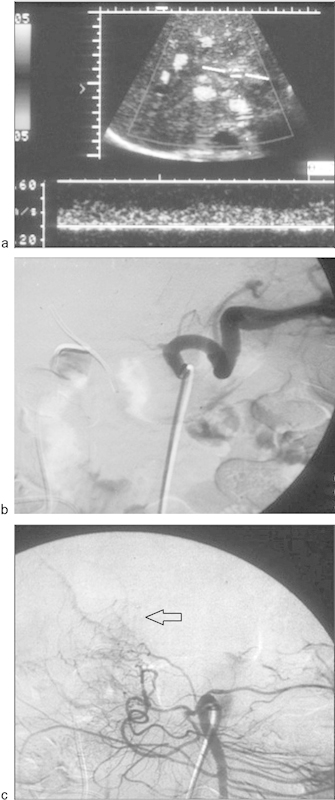
A 52-year-old woman underwent liver transplant and presented with increasing liver enzymes. A Doppler US was performed. (a) Doppler ultrasound of the right hepatic artery demonstrates a tardus parvus waveform (i.e., a low-resistance waveform with a resistive index <0.5 and a systolic acceleration time >0.1 second). A similar flow pattern was obtained in the left hepatic artery. No arterial flow could be identified in the proper hepatic artery. These findings are suggestive of hepatic artery thrombosis associated with formation of collateral vessels at the porta hepatis or high-grade stenosis with markedly diminished flow. (b) Arteriography obtained with injection of the celiac axis demonstrates complete occlusion of the common hepatic artery. (c) Arteriography obtained with a superior mesenteric artery injection shows reconstitution of intrahepatic arterial flow (arrow) from collateral vessels arising from the pancreaticoduodenal branches.
Hepatic artery stenosis (HAS) is the second most common vascular complication of liver transplantation, affecting between 1.2 and 9.5% of liver transplant cases.102 The stenosis most commonly occurs at the site of anastomosis, although narrowing both upstream and downstream to the anastomosis may also be seen. Rejection, faulty surgical technique, and clamp injury have all been cited as causes.
The median time of HAS presentation is 3 to 7 months after liver transplant, with clinical suspicion often raised by increases in bilirubin and alkaline phosphatase levels.103 The initial imaging modality for the diagnosis of HAS is usually DUS, which typically demonstrates a PSV >200 cm/s with associated turbulence at or immediately distal to the stenosis, tardus parvus intrahepatic arterial waveform, and a RI <0.5; however, DUS is not sensitive to low-grade narrowing (Fig. 11).97 CT angiography can also be used, although this modality sacrifices specificity (89%) for very high sensitivity (up to 100% in some studies); MRA demonstrates similar results.3
Fig. 11.
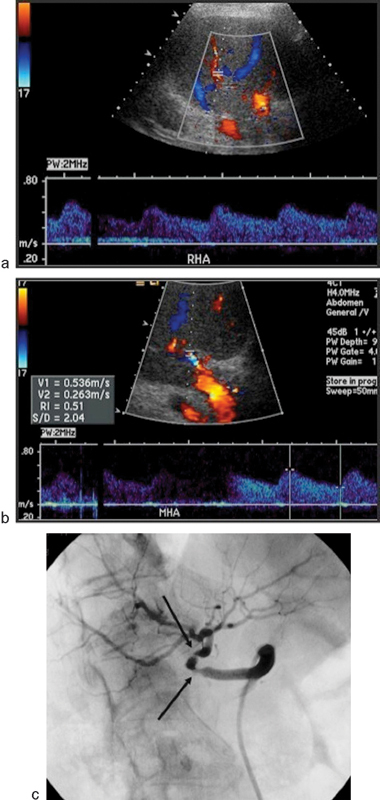
A 56-year-old man who underwent liver transplant presented with increasing liver enzymes. Doppler ultrasound of the right hepatic artery (a) and middle hepatic artery (b) distal to sites of stenoses demonstrates a low-resistance waveform and a systolic acceleration time >0.1 second (time from end diastole to first systolic peak). These findings are suggestive of significant hepatic artery stenosis. Selective angiogram demonstrates two distinct stenoses (black arrows) proximally within the proper hepatic artery.
The treatment for HAS is surgical revision or endovascular intervention. Although revision may be more definitive, the risk of recurrent stenosis still exists. Angioplasty and stenting are less invasive alternatives that are especially appropriate when the stenosis is short or intrahepatic, or when the abdomen is unreceptive because of multiple surgeries (Figs. 12 and 13).104 A recent meta-analysis of 263 procedures found similar short- and long-term patency and survival rates for stent placement and angioplasty.105 The most important considerations for the choice of treatment in this study were the shape of the stenosed vessel and the preference of the operator. The choice of stent type also depends on vessel character. Self-expanding stents are best suited for cases in which there is a size mismatch between the vessel proximal and distal to the lesion, and balloon expandable coronary stents are better suited for highly tortuous vessels.106 Technical success rates approach 100% for these procedures, although restenosis is not uncommon.103 Complications of endovascular intervention can include vasospasm, dissection, pseudoaneurysm, and ruptures, occurring in 5.7 to 9% of procedures.106 107
Fig. 12.
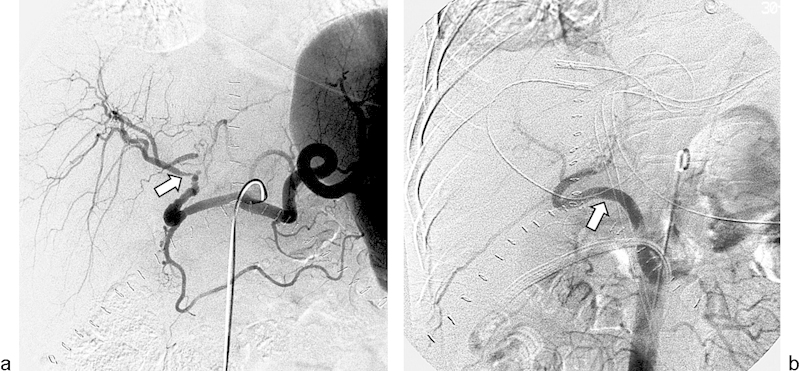
A 59-year-old patient underwent a whole liver transplant. (a) Celiac arteriogram shows nearly occluded transplant hepatic artery and an irregularly marginated stenosis (arrow). (b) Angiogram following surgical revascularization of the hepatic artery via an aortohepatic bypass (arrow) demonstrates complete resolution of the stenosis.
Pseudoaneurysm is another possible hepatic artery complication of liver transplant. This complication is infrequent but carries the risk of catastrophic rupture. Intrahepatic pseudoaneurysms commonly occur after intrahepatic and transhepatic procedures, such as biopsy or transhepatic cholangiography, whereas extrahepatic pseudoaneurysms are associated with technical issues at the anastomotic site.101 108 Overall, pseudoaneurysms most commonly occur at the anastomosis and the hilum.109 When extrahepatic, pseudoaneurysms are associated with HAT. Additionally, fistulization of the aneurysm with the portal vein or biliary tree may occur (i.e., mycotic aneurysms), which may result in hemobilia.
Although liver function tests can be abnormal with lesions in either location, extrahepatic pseudoaneurysms are more often associated with fever, sepsis, abnormal liver function tests, upper gastrointestinal or intra-abdominal bleeds, and mortality (78 vs. 50%).101
In cases of pseudoaneurysm, DUS may reveal a periportal or intrahepatic cystic structure with the classic “yin-yang” sign of bidirectional flow. During the arterial phase of a dynamic contrast-enhanced CT or MR, a pseudoaneurysm may appear as an enhanced focal enlargement of the hepatic artery. Angiography, however, is the definitive diagnostic test for pseudoaneurysm.108
Treatment of pseudoaneurysms includes surgical resection, coiling/embolization, placement of stent grafts, and retransplant. Coiling and embolization can be performed with a direct transhepatic percutaneous approach for intrahepatic lesions. Stent grafts are often complicated by endoleak; however, this problem can be partially overcome by the use of multilayer aneurysm repair stents.101 110 111 Nevertheless, mortality for patients with extrahepatic artery pseudoaneurysm is high, approaching 70% in one study.108
Splenic steal syndrome has recently been identified as another cause of hepatic functional ischemia in liver transplant recipients. This complication is characterized by arterial hypoperfusion of the graft with preferential flow to the spleen on conventional celiac arteriography.112 113 Patients with splenic steal syndrome may present with elevated liver function tests, and this condition is occasionally associated with thrombocytopenia, cholestasis, ascites, and graft failure.114 115 116 117 Left untreated, splenic steal syndrome can progress to graft failure.117 Proximal splenic arterial embolization can be an effective treatment for this condition, with immediate improvement of hepatic flow and minimal risk.
Biliary Complications
Ideally, the donor common bile duct is anastomosed to the recipient common hepatic duct; however, if the recipient common hepatic duct is too short, too narrow, or diseased, a choledochojejunostomy can be performed. A T-tube may be left in place to facilitate future cholangiography or other biliary procedures.
Biliary complications are referred to as the “Achilles heel” of liver transplantation, occurring in 21% of liver transplants. After liver transplant, the donor bile duct is entirely dependent on hepatic arterial blood supply; therefore, occlusion of the hepatic artery leads to bile duct ischemia and necrosis. Stricture (12.8% of transplants) and leakage (8.2% of transplants) are the most commonly observed biliary complications; these can result from ischemic damage (caused by an occluded or stenotic hepatic artery) or from technical issues during surgery (Figs. 14 and 15a).97 118 Additional complications can arise from dysfunction of the sphincter of Oddi.119 Bile duct complications are more common in split liver transplants, which complicate the surgical anastomosis, and after procedures in which the portal venous system is perfused before the hepatic artery, which increases the warm ischemic stress on the biliary system.120 121 Livers acquired from donors after cardiac death and from older donors have higher rates of biliary complications. Biliary complications can occur at any point from days to years after liver transplant.122
Fig. 14.
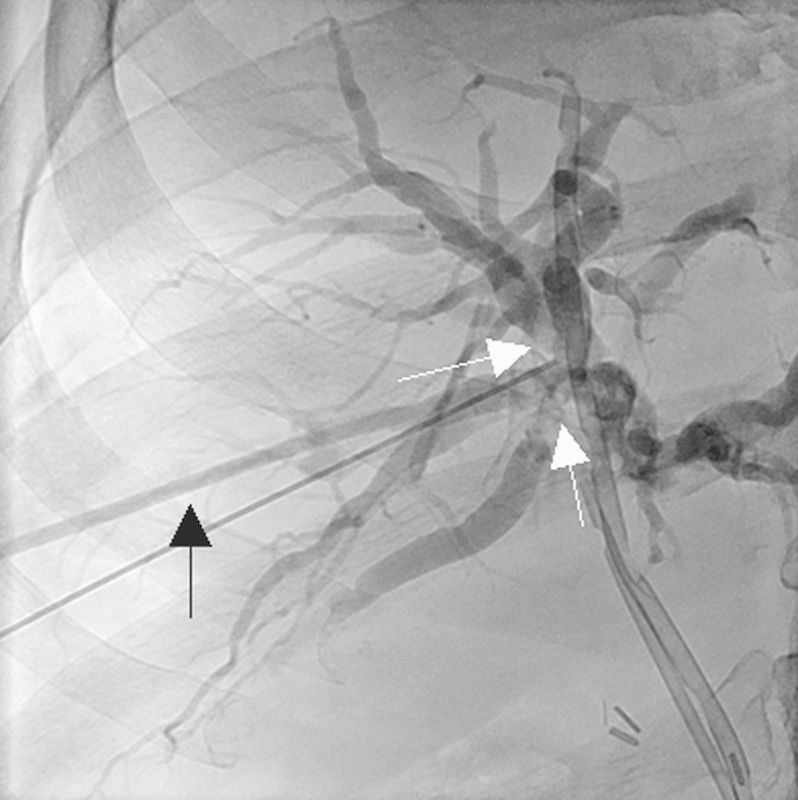
Image of a 64-year-old man with a liver transplant complicated by hepatic artery thrombosis. Cholangiogram shows biliary structure at the anastomosis between the donor and recipient bile biliary (white arrows) with dilatation of the intrahepatic bile ducts.
Fig. 15.
A 58-year-old man who underwent orthotopic liver transplant with common hepatic duct-to-jejunal anastomosis presented with increasing liver enzymes and serum bilirubin level. Dilated bile ducts were seen on ultrasound. (a) Cholangiogram shows a proximal common hepatic duct stricture (arrow). (b) Cholangioplasty of the stricture using 10 mm × 40 mm balloon (arrow). (c) Fluoroscopic image demonstrates internal/external biliary drainage catheter placement. (d) Six weeks after the biliary catheter placement, cholangiogram demonstrates that the anastomosis (arrow) is widely patent. The biliary catheter was removed.
Patients with biliary strictures may present with jaundice, fever, abdominal pain, and cholestasis, with dilated bile ducts visible on imaging.123 Biliary stricture is treated with a variety of techniques based on the preference of the institution and the clinical scenario; these therapies include endoscopic or percutaneous drainage, surgical revision, balloon dilatation, or stenting (Fig. 15b–d).118 Balloon dilation typically requires multiple treatments, with gradual expansion of the stricture over the course of a year.119 In cases of strictures not related to the anastomosis, there are often multiple stricture sites. The etiology of these strictures is often ischemic or autoimmune, and management is complicated by the presence of multiple lesions.119
A nonrandomized comparison of retrievable covered stents and indwelling drainage catheters found similar complication rates with stents and catheters, with stents requiring a shorter treatment duration but with a lower rate of clinical success.124 The most common complications for metal stents include restenosis and migration, although migration is often not clinically significant. Failure of stent treatment is usually related to scarring or overgrowth in the stented region or underlying pathophysiology, such as poor blood supply.125 Plastic stents can also be used, but these stents have a higher complication rate126; a 2014 randomized trial found that plastic stents required more procedures and had a lower rate of stricture resolution than metallic stents.127
Bile leak is the most common complication after liver transplant in patients with a choledochocholedochostomy. Patients with bile leak present with fever, abdominal pain, and signs of peritonitis. Most leaks occur at the T-tube site and occur after removal of the T-tube (Fig. 16).97 Fortunately, biliary drainage using an internal stent or nasobiliary drainage is usually sufficient for nonischemic leaks.119 CT- or US-guided aspiration is often necessary to confirm biloma.
Fig. 16.
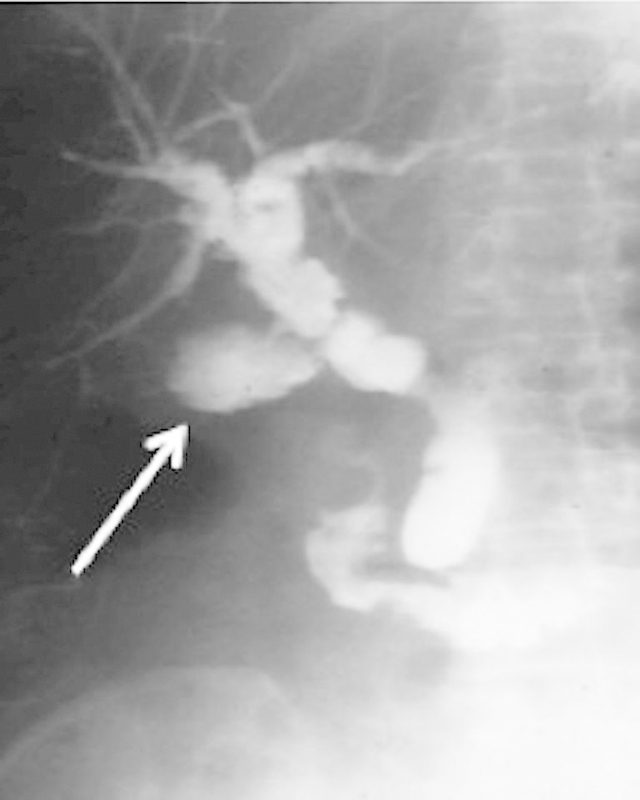
Bile leakage at T-tube site. Cholangiogram shows extravasated contrast material from the T-tube site resulting in a biloma (white arrow). Note the diffuse irregularity of the donor bile duct and dilatation resulting from bile duct ischemia.
Nonanastomotic leaks can also occur and may be related to ischemia. This type of leak is most often secondary to HAT and may be hilar, intrahepatic, or both. Again, percutaneous biliary decompression and drainage may be used for management; however, in cases of concomitant HAT, the outcome is often poor despite drainage.
Complications of Pancreas Transplants
A total of 1,018 pancreas transplants were performed in 2013.1 Pancreas transplants are used to treat the complications of type 1 diabetes related to hypoglycemia insensitivity. Combined pancreas–kidney transplants decrease mortality after 1 year when compared with mortality among patients on the transplant waiting list, whereas the transplant of a pancreas alone has no significant effect on mortality.3 128 Transplant recipients have improved HbA1c levels and lower insulin requirements, although insulin independence is not consistently achieved.129
The exocrine secretions from the transplanted pancreas are drained into either the bladder or the duodenum. In either case, the splenic and superior mesenteric arteries are joined to the iliac arteries using a cadaveric Y-graft. A bladder-drained pancreas is placed intraperitoneally, and venous drainage is into the systemic iliac veins; an enteric-drained pancreas empties its venous blood into the portal vein.3 Bladder drainage provides the advantage of easy identification of early transplant rejection, which will manifest as a decrease in exocrine secretions in the urine. However, bladder drainage has been associated with reflux pancreatitis from urinary retention, which is especially common in patients with diabetic autonomic neuropathy affecting the bladder.130
Pancreatic graft vascular thrombosis is the most common and the most devastating complication of pancreatic transplant. Literature on the subject is complicated by imprecise definition of the site of thrombosis, with arterial and venous thrombosis commonly reported as the same category of event.131 Incidence of this complication ranges from 5.8 to 13%, and this rate increases with longer organ preservation time.132 133 The cause is often unclear. Diagnosis of this complication usually occurs via US detection of graft enlargement (in the case of venous thrombosis) (Fig. 17) or necrosis (in arterial thrombosis).134 Thrombosis necessitates emergent removal of the graft.
Fig. 17.
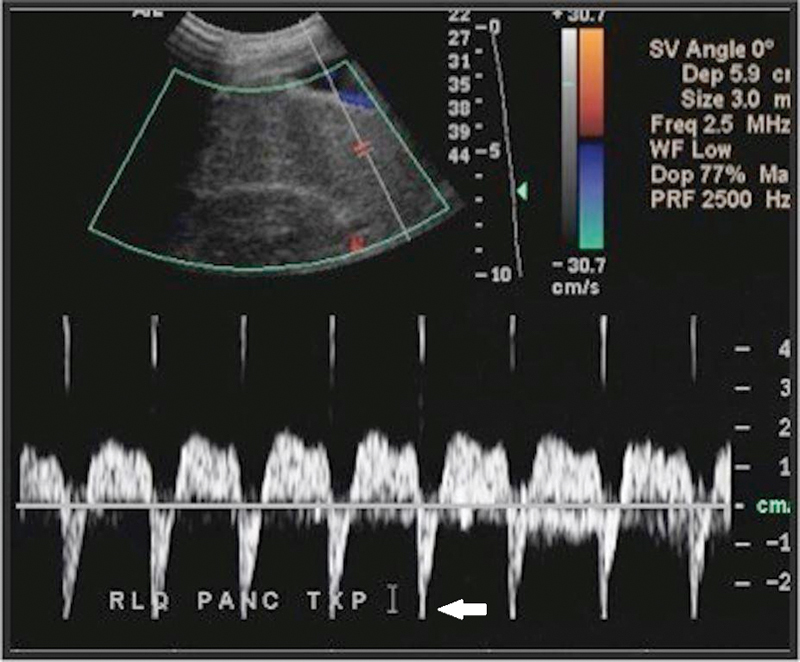
Doppler ultrasound of the right lower quadrant pancreatic transplant demonstrates a swollen, edematous pancreas and reversal of arterial diastolic flow (arrow), suggestive of splenic vein thrombosis.
AVFs and pseudoaneurysms are life-threatening complications in cases of pancreas transplant, occurring in approximately 1% of patients with pancreatic transplants.135 These complications, which typically arise at a vascular anastomotic or biopsy site,134 136 require rapid diagnosis and treatment.3 Endovascular embolization can be an effective treatment if no concomitant abscess has been detected.137
Arteroenteric fistulae are a complication of enterically drained pancreatic transplants that occur in 1.4% of transplant cases. These fistulae can be treated with either stent grafts or embolization.135
Complications of Islet Cell Transplants
Islet cell transplants are an alternative to whole-organ pancreas transplants for patients with uncontrolled type 1 diabetes. These transplants, which can be performed percutaneously, are indicated for patients with reduced awareness of hypoglycemia and for those with progressive secondary complications of diabetes.138 This procedure is performed less frequently than pancreas transplant, with only 571 islet cell transplants performed between 1999 and 2009, according to Collaborative Islet Transplant Registry Seventh Annual Report 2011. Islet cell transplants are less invasive and are associated with a lower morbidity risk than pancreas transplants.139 The development of a new glucocorticoid-free immunosuppressive regimen in 2000 and the insulin independence of more than 80% of islet cell transplant patients at 1 year have created considerable enthusiasm for this procedure; however, a gradual decline in islet cell dysfunction is usually observed in transplant recipients.140
For this transplant procedure, islet cells from donor pancreases must be purified. These purified cells are then inserted percutaneously into a second- or third-order portal vein branch. Patients undergoing islet cell transplant are monitored overnight for hemorrhage but are typically discharged the next day.141 142 143 Transjugular and transmesenteric insertion are less frequently employed.142 144 The islet cell transplant procedure is currently hampered by poor efficacy of islet cell harvest and poor postharvest cell viability. Most patients will not reach insulin independence and may require multiple transfusions. Additionally, this procedure requires several donor pancreases, which could alternatively be used for several pancreas transplant recipients.145 Islet cell transplantation is therefore not yet a first-line treatment, but this may change as techniques are further refined.146
Bleeding occurs in 10 to 13.6% of pancreatic islet cell transplants, with 25% of bleeding cases constituting serious hemorrhage. This bleeding usually occurs along the catheter tract and can largely be avoided by using tract sealants. Coiling along with tissue fibrin glue can be used to prevent bleeding, as can Gelfoam pledgets, powdered collagen, and thrombin-saturated gelatin sponges.140 141 147
Portal vein thrombosis is a serious complication of islet cell transplantation, occurring in 3 to 4% of procedures. This complication can be life threatening, and use of heparin has been reported to lower the risk of thrombosis in pancreatic transplant patients.140 141
Complications of Lung Transplants
As of 2011, more than 9,000 people were living with lung transplants according to the Scientific Registry of Transplant Recipients. Lung transplant is a treatment for end-stage vascular or parenchymal lung disease.148 The main indications for lung transplant are chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, and cystic fibrosis.149
Lung transplant is associated with a high rate of complications, including a 42.8% risk of infection, 10.0% risk of rejection, 5.4% risk of renal failure, and 1.9% risk of stroke. Approximately 19.9% of transplant recipients require reoperation.150 Recent advances in noninvasive imaging permit earlier detection of complications arising at either the bronchial or vascular anastomotic sites, as well as the use of minimally invasive procedures in the management of these complications.
Vascular Complications
Vascular complications after lung transplant have been reported in 1.8% of vascular anastomoses and are associated with high rates of morbidity and mortality.151 Surgical technique, donor–recipient size mismatch, twisting, stricture, and thrombosis have all been cited as potential causes of vascular complications.
The risk of pulmonary infarction is greatest during the immediate postoperative period, as the newly transplanted lung has no alternative pathway for bronchial circulation. Irreversible allograft damage may occur after just 4 to 6 hours of warm ischemia; therefore, early diagnosis and intervention for a perfusion abnormality is paramount. Lung perfusion scintigraphy with Tc-99 MAA may aid in making this diagnosis. In the early posttransplant period, unexplained hypoxia with pulmonary hypertension and hemodynamic compromise should raise suspicion for potential pulmonary vascular compromise.
Pulmonary artery stenosis has an incidence of 1.5 to 4.7% following lung transplant, and is more common than complications involving the pulmonary venous anastomosis, as arterial anastomoses are more prone to poor orientation, narrowing, or kinking.151 152 Because of the rarity of this complication, as well as the relative paucity of lung transplants as a whole, there are limited studies examining this phenomenon. However, case reports suggest that the stenosis most often occurs at the anastomotic site within a few months of surgery.
Pulmonary venous obstruction is rare, occurring in 0.4 to 2.7% of lung transplants.152 153 The use of an atrial cuff for the venous anastomosis makes it easier to orient in comparison to an arterial anastomosis, and therefore is generally less prone to complications. Clinical manifestations of pulmonary venous obstructions include hypoxia, pulmonary edema, hemoptysis, hemodynamic instability, and poor response to inotropic agents.
In the evaluation of pulmonary arterial anastomotic stenosis, multidetector CT (MDCT) pulmonary angiography is a noninvasive alternative to transcatheter angiography that has demonstrated diagnostic success.154 MDCT may be used to define the extent and degree of the stenosis and to identity the presence of collateral pathways. Additionally, this modality permits evaluation of the lung parenchyma and pleural spaces for other possible etiologies of hypoxemia. However, transcatheter arteriography remains the gold standard for diagnosis and simultaneously offers therapeutic options as well.
MDCT is not as useful in evaluating pulmonary venous anastomotic complications. Fortunately, venous anastomotic complications are less common than those on the arterial side. Distinguishing pulmonary venous obstruction from pneumonia, reperfusion injury, or rejection may be difficult. When pulmonary venous obstruction is suspected, the diagnosis can be confirmed by transesophageal echocardiography or angiography.
An algorithm for the management of vascular anastomotic complications suggests that mild obstructions to flow can be followed without intervention, particularly when only one lung is affected in the case of bilateral transplants.152 Anticoagulation should be the initial form of management in cases of anastomotic thrombosis unless significant thrombosis is present and there is concern for potential graft infarction, in which case surgical intervention is mandated.
When a significant anatomic lesion at the anastomotic site compromises the graft, the specific intervention will be dictated by the anatomy of the lesion, the time since transplantation, and the patient's ability to tolerate reoperation. Those cases identified after several weeks may be amenable to endovascular intervention. In the largest series of anastomotic stenoses managed with endovascular stents, Grubstein et al reported technical success in five out of five cases using balloon expandable stents with no residual stenosis.155 156 157 158 159 Catheter-based intervention is generally avoided when anastomotic complications are identified within the first 2 weeks after transplant because of concerns about the integrity of the anastomosis.155 160 Stent-related complications can include migration, thrombosis, restenosis, and embolization.
Bronchial Complications
Bronchial anastomotic complications occur in approximately 15% of lung transplant cases.161 The most common bronchial anastomotic complications after lung transplants consist of stenosis, dehiscence, tissue degeneration, and infection.
Because of improvements in surgical techniques, the incidence of bronchial stenoses has been significantly decreased from ∼60% to the current 5.8 to 14.5% after lung transplant.162 163 Bronchial anastomotic stenosis is usually noted within 4 months of transplantation. Ischemia in the bronchus intermedius, because of poor postoperative perfusion before the development of adequate collateral circulation, is commonly cited as a cause for the development of stenosis.164 In addition, necrosis, granulation tissue overgrowth, and malacia can progress to bronchial stenosis. CT of bronchial stenosis may demonstrate a fixed bronchial narrowing due to stricture, with a significant stenosis defined as a reduction of more than 50% in bronchial diameter.165
Bronchial strictures and bronchomalacia have been successfully managed with balloon dilatation, mechanical or laser debridement, and endobronchial stent placement, including self-expanding metallic stents. Self-expanding stents are mandated in cases of bronchomalacia because of their radial expansile force, which allows self-recovery after bronchial deformation with episodes of violent coughing. The same techniques used in crossing vascular stenoses are applicable to the management of endobronchial stenoses. There has been considerable controversy over the optimal type of stent for the bronchial system. Self-expanding uncovered metallic stents are currently favored by most interventional radiologists and pulmonologists.166 167 168 169 Metallic stents promote epithelialization of the stent, which aids in the prevention of stent migration. In a study by Burns et al,170 30 patients who underwent a total of 50 stentx insertions and 25 balloon dilatations demonstrated persistent improvements in forced expiratory volume in 1 second (FEV1) (compared with baseline) and a reduction in infection rates at 12-month follow-up.
Bronchial dehiscence is difficult to treat and is associated with a high mortality rate. This complication typically occurs within the first month after transplant due to necrosis at the anastomotic site, and occurs in 1.6% of transplants. Suggestive findings on CT include bronchial wall defects, fixed or dynamic bronchial narrowing, bronchial wall irregularity, extraluminal air, or a combination of these findings. Deployment of a self-expanding metallic stent is of great value in the management of bronchial dehiscence, as these stents are easily deployed without significant trauma to the anastomotic site and promote excessive granulation tissue formation that grows through the interstices of the stent.171 In a study by Mughal et al,171 symptomatic improvement was noted in six of seven (85.7%) patients with bronchial dehiscence managed with stent placement.
Complications of balloon dilatation and stent placement can include bronchial rupture, occlusion of side branches, malpositioned stents, stent migration, and stent crush/fracture. Isolated cases of bronchovascular fistulae have also been reported, including three cases reported by Knight et al.172 All three of these cases occurred after large mural dehiscence and positive fungal cultures. Metal stenting preceded two of the cases, with migration of the stents into the bronchial wall cited as a possible associated factor. Bronchovascular fistula formation has a reported 91% mortality rate.172
Complications of Small Bowel Transplants
Small bowel transplants are less commonly performed than kidney or liver transplants and are performed only at select institutions. A total of 109 small bowel transplants were performed in 2013, with 2,393 performed to date.1 Small bowel transplantation is indicated for patients with short-bowel syndrome with limited venous access for total parental nutrition (TPN), and for those with complete portomesenteric thrombosis. The goal of small bowel transplantation is to restore enteral absorption of ingested foods and fluids. In adults, the most common underlying etiologies include inflammatory bowel disease, bowel ischemia, and abdominal trauma, whereas in the pediatric population, the most common etiologies include midgut volvulus, intestinal atresia, necrotizing enterocolitis, and gastroschisis.
The surgical techniques for small bowel transplant are variable, vast, and complex. The isolated intestinal transplant is the most common procedure and typically includes the entire jejunum and ileum. The liver–intestinal transplant is performed in patients with intestinal failure and TPN-related cholestatic liver failure. In patients with irreversible failure of the small bowel and liver combined with portomesenteric thrombosis or Gardner syndrome with intra-abdominal desmoid tumor, multivisceral transplantation may be performed.173 The complexity of the procedure is paradoxically mitigated with multivisceral transplantation, as no hilar dissection of the graft is needed and there are therefore fewer risks for donor-related vascular or biliary complications.174
Small bowel transplants are associated with high complication rates in the immediate postoperative period and over long-term follow-up. Nearly 50% of intestinal transplant recipients develop at least one episode of rejection within the first year after transplantation, according to the Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) 2011 annual data report. Sepsis is one of the most frequent causes for readmission to the hospital among these patients and is the most frequent cause of death.175 Stenosis and thrombosis of the vascular anastomoses are uncommon in small bowel transplants, unlike in liver and kidney transplants.
Although interventional radiologists may not play a significant role in the treatment of graft rejection or sepsis among small bowel transplant recipients, these clinicians play a crucial role in supportive procedures and managing other complications. In a study of 43 small bowel transplants (25 small bowel transplants, 18 small bowel plus liver transplants), a total of 297 interventional radiology procedures were performed.176 Most (n = 120; 40%) of the procedures were gastrointestinal, including the placement of gastrojejunal, gastrostomy, jejunal, and Dobhoff tubes. Bowel dilatations and combined procedures with gastrointestinal endoscopy were also performed. Tailored feeding tubes and drainage catheters and transcutaneous bowel accesses were paramount in management. Central venous access and venography accounted for 102 procedures (34%) performed by interventional radiologists and included the placement of tunneled and nontunneled catheters and venograms. In many instances, nonroutine approaches, including translumbar approaches to the IVC, transhepatic access, and the use of collaterals, were used. Venous access was successful in all patients.
Conclusion
Organ transplantations are an increasingly utilized and effective treatment option for patients with end-stage organ failure. As with any elaborate surgical procedure, however, patients are vulnerable to a variety of associated complications. These expose transplant recipients to the risks of organ loss, serious morbidity, and mortality. Interventional radiology plays an important role in managing a variety of complications from transplantation and can further improve outcomes for patients after solid-organ transplantation.
Acknowledgment
The authors thank Megan Griffiths, scientific writer for the Imaging Institute, Cleveland Clinic, Cleveland, Ohio, for her help with the revision of the article.
References
- 1.OPTN: Organ Procurement and Transplantation Network. (n.d.) Retrieved from optn.transplant.hrsa.gov: http://optn.transplant.hrsa.gov/latestData/rptData.asp. Accessed November 1, 2014
- 2.Rajiah P, Lim Y Y, Taylor P. Renal transplant imaging and complications. Abdom Imaging. 2006;31(6):735–746. doi: 10.1007/s00261-006-9091-2. [DOI] [PubMed] [Google Scholar]
- 3.Norton P T, DeAngelis G A, Ogur T, Saad W E, Hagspiel K D. Noninvasive vascular imaging in abdominal solid organ transplantation. AJR Am J Roentgenol. 2013;201(4):W544–W553. doi: 10.2214/AJR.13.11306. [DOI] [PubMed] [Google Scholar]
- 4.Hanto D W, Simmons R L. Renal transplantation: clinical considerations. Radiol Clin North Am. 1987;25(2):239–248. [PubMed] [Google Scholar]
- 5.Orons P D, Zajko A B. Angiography and interventional aspects of renal transplantation. Radiol Clin North Am. 1995;33(3):461–471. [PubMed] [Google Scholar]
- 6.Bennett L N, Voegeli D R, Crummy A B, McDermott J C, Jensen S R, Sollinger H W. Urologic complications following renal transplantation: role of interventional radiologic procedures. Radiology. 1986;160(2):531–536. doi: 10.1148/radiology.160.2.3523596. [DOI] [PubMed] [Google Scholar]
- 7.Khaja M S, Matsumoto A H, Saad W E. Complications of transplantation. Part 1: renal transplants. Cardiovasc Intervent Radiol. 2014;37(5):1137–1148. doi: 10.1007/s00270-014-0851-z. [DOI] [PubMed] [Google Scholar]
- 8.Wong W, Fynn S P, Higgins R M. et al. Transplant renal artery stenosis in 77 patients—does it have an immunological cause? Transplantation. 1996;61(2):215–219. doi: 10.1097/00007890-199601270-00009. [DOI] [PubMed] [Google Scholar]
- 9.Luke R G, Curtus J. New York, NY: Raven; 1995. Biology and treatment of transplant hypertension; pp. 2471–2483. [Google Scholar]
- 10.Patel N H, Jindal R M, Wilkin T. et al. Renal arterial stenosis in renal allografts: retrospective study of predisposing factors and outcome after percutaneous transluminal angioplasty. Radiology. 2001;219(3):663–667. doi: 10.1148/radiology.219.3.r01jn30663. [DOI] [PubMed] [Google Scholar]
- 11.Osman Y, Shokeir A, Ali-el-Dein B. et al. Vascular complications after live donor renal transplantation: study of risk factors and effects on graft and patient survival. J Urol. 2003;169(3):859–862. doi: 10.1097/01.ju.0000050225.74647.5a. [DOI] [PubMed] [Google Scholar]
- 12.Hedegard W, Saad W E, Davies M G. Management of vascular and nonvascular complications after renal transplantation. Tech Vasc Interv Radiol. 2009;12(4):240–262. doi: 10.1053/j.tvir.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Pappas P, Zavos G, Kaza S. et al. Angioplasty and stenting of arterial stenosis affecting renal transplant function. Transplant Proc. 2008;40(5):1391–1396. doi: 10.1016/j.transproceed.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Loubeyre P, Abidi H, Cahen R, Tran Minh V A. Transplanted renal artery: detection of stenosis with color Doppler US. Radiology. 1997;203(3):661–665. doi: 10.1148/radiology.203.3.9169685. [DOI] [PubMed] [Google Scholar]
- 15.Geddes C C, McManus S K, Koteeswaran S, Baxter G M. Long-term outcome of transplant renal artery stenosis managed conservatively or by radiological intervention. Clin Transplant. 2008;22(5):572–578. doi: 10.1111/j.1399-0012.2008.00826.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghazanfar A, Tavakoli A, Augustine T, Pararajasingam R, Riad H, Chalmers N. Management of transplant renal artery stenosis and its impact on long-term allograft survival: a single-centre experience. Nephrol Dial Transplant. 2011;26(1):336–343. doi: 10.1093/ndt/gfq393. [DOI] [PubMed] [Google Scholar]
- 17.Raynaud A, Bedrossian J, Remy P, Brisset J M, Angel C Y, Gaux J C. Percutaneous transluminal angioplasty of renal transplant arterial stenoses. AJR Am J Roentgenol. 1986;146(4):853–857. doi: 10.2214/ajr.146.4.853. [DOI] [PubMed] [Google Scholar]
- 18.Voiculescu A, Schmitz M, Hollenbeck M. et al. Management of arterial stenosis affecting kidney graft perfusion: a single-centre study in 53 patients. Am J Transplant. 2005;5(7):1731–1738. doi: 10.1111/j.1600-6143.2005.00927.x. [DOI] [PubMed] [Google Scholar]
- 19.Abate M T, Kaur J, Suh H, Darras F, Mani A, Nord E P. The use of drug-eluting stents in the management of transplant renal artery stenosis. Am J Transplant. 2011;11(10):2235–2241. doi: 10.1111/j.1600-6143.2011.03652.x. [DOI] [PubMed] [Google Scholar]
- 20.Marini M, Fernandez-Rivera C, Cao I. et al. Treatment of transplant renal artery stenosis by percutaneous transluminal angioplasty and/or stenting: study in 63 patients in a single institution. Transplant Proc. 2011;43(6):2205–2207. doi: 10.1016/j.transproceed.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 21.Audard V, Matignon M, Hemery F. et al. Risk factors and long-term outcome of transplant renal artery stenosis in adult recipients after treatment by percutaneous transluminal angioplasty. Am J Transplant. 2006;6(1):95–99. doi: 10.1111/j.1600-6143.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 22.Benoit G, Moukarzel M, Hiesse C, Verdelli G, Charpentier B, Fries D. Transplant renal artery stenosis: experience and comparative results between surgery and angioplasty. Transpl Int. 1990;3(3):137–140. doi: 10.1007/BF00355459. [DOI] [PubMed] [Google Scholar]
- 23.Beecroft J R, Rajan D K, Clark T W, Robinette M, Stavropoulos S W. Transplant renal artery stenosis: outcome after percutaneous intervention. J Vasc Interv Radiol. 2004;15(12):1407–1413. doi: 10.1097/01.RVI.0000141338.62574.F4. [DOI] [PubMed] [Google Scholar]
- 24.Peregrin J H, Stríbrná J, Lácha J, Skibová J. Long-term follow-up of renal transplant patients with renal artery stenosis treated by percutaneous angioplasty. Eur J Radiol. 2008;66(3):512–518. doi: 10.1016/j.ejrad.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Aktas S, Boyvat F, Sevmis S, Moray G, Karakayali H, Haberal M. Analysis of vascular complications after renal transplantation. Transplant Proc. 2011;43(2):557–561. doi: 10.1016/j.transproceed.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Dimitroulis D, Bokos J, Zavos G. et al. Vascular complications in renal transplantation: a single-center experience in 1367 renal transplantations and review of the literature. Transplant Proc. 2009;41(5):1609–1614. doi: 10.1016/j.transproceed.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 27.Bakir N, Sluiter W J, Ploeg R J, van Son W J, Tegzess A M. Primary renal graft thrombosis. Nephrol Dial Transplant. 1996;11(1):140–147. [PubMed] [Google Scholar]
- 28.Robertson A J, Nargund V, Gray D W, Morris P J. Low dose aspirin as prophylaxis against renal-vein thrombosis in renal-transplant recipients. Nephrol Dial Transplant. 2000;15(11):1865–1868. doi: 10.1093/ndt/15.11.1865. [DOI] [PubMed] [Google Scholar]
- 29.Brown E D, Chen M Y, Wolfman N T, Ott D J, Watson N E Jr. Complications of renal transplantation: evaluation with US and radionuclide imaging. Radiographics. 2000;20(3):607–622. doi: 10.1148/radiographics.20.3.g00ma14607. [DOI] [PubMed] [Google Scholar]
- 30.Groggel G C. Acute thrombosis of the renal transplant artery: a case report and review of the literature. Clin Nephrol. 1991;36(1):42–45. [PubMed] [Google Scholar]
- 31.Parekh D J, Weinberg J M, Ercole B. et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol. 2013;24(3):506–517. doi: 10.1681/ASN.2012080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shabanah F, Connolly J, Martin D C. Acute renal artery occlusion. Surg Gynecol Obstet. 1970;131(3):489–494. [PubMed] [Google Scholar]
- 33.Collins G M, Taft P, Green R D, Ruprecht R, Halasz N A. Adenine nucleotide levels in preserved and ischemically injured canine kidneys. World J Surg. 1977;2(1):237–243. doi: 10.1007/BF01665093. [DOI] [PubMed] [Google Scholar]
- 34.Iwami D, Harada H, Miura M, Seki T, Togashi M, Hirano T. Successfully rescued renal graft artery thrombosis by ex vivo thrombectomy: a case report. Transplant Proc. 2009;41(5):1951–1953. doi: 10.1016/j.transproceed.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 35.Saad W E, Waldman D L. New York, NY: Taylor & Francis; 2006. Endovascular repair of vascular lesions in solid organ transplantation. [Google Scholar]
- 36.Ortega Herrera R, Medina Benítez A, Hernández Abad M J. [Renal vein partial thrombosis in 3 recipients of kidney transplantation] Arch Esp Urol. 2000;53(1):45–48. [PubMed] [Google Scholar]
- 37.Giustacchini P, Pisanti F, Citterio F, De Gaetano A M, Castagneto M, Nanni G. Renal vein thrombosis after renal transplantation: an important cause of graft loss. Transplant Proc. 2002;34(6):2126–2127. doi: 10.1016/s0041-1345(02)02876-2. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi M, Humke U, Girndt M, Kramann B, Uder M. Early posttransplantation renal allograft perfusion failure due to dissection: diagnosis and interventional treatment. AJR Am J Roentgenol. 2003;180(3):759–763. doi: 10.2214/ajr.180.3.1800759. [DOI] [PubMed] [Google Scholar]
- 39.Grenier N, Claudon M, Trillaud H, Douws C, Levantal O. Noninvasive radiology of vascular complications in renal transplantation. Eur Radiol. 1997;7(3):385–391. doi: 10.1007/s003300050171. [DOI] [PubMed] [Google Scholar]
- 40.Martinez T, Palomares M, Bravo J A. et al. Biopsy-induced arteriovenous fistula and venous aneurysm in a renal transplant. Nephrol Dial Transplant. 1998;13(11):2937–2939. doi: 10.1093/ndt/13.11.2937. [DOI] [PubMed] [Google Scholar]
- 41.Sandhu C, Patel U. Renal transplantation dysfunction: the role of interventional radiology. Clin Radiol. 2002;57(9):772–783. [PubMed] [Google Scholar]
- 42.Saad W E. Management of nonocclusive hepatic artery complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10(3):221–232. doi: 10.1053/j.tvir.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Maleux G, Messiaen T, Stockx L, Vanrenterghem Y, Wilms G. Transcatheter embolization of biopsy-related vascular injuries in renal allografts. Long-term technical, clinical and biochemical results. Acta Radiol. 2003;44(1):13–17. [PubMed] [Google Scholar]
- 44.Perini S, Gordon R L, LaBerge J M. et al. Transcatheter embolization of biopsy-related vascular injury in the transplant kidney: immediate and long-term outcome. J Vasc Interv Radiol. 1998;9(6):1011–1019. doi: 10.1016/s1051-0443(98)70442-7. [DOI] [PubMed] [Google Scholar]
- 45.deSouza N M, Reidy J F, Koffman C G. Arteriovenous fistulas complicating biopsy of renal allografts: treatment of bleeding with superselective embolization. AJR Am J Roentgenol. 1991;156(3):507–510. doi: 10.2214/ajr.156.3.1899745. [DOI] [PubMed] [Google Scholar]
- 46.Dorffner R, Thurnher S, Prokesch R. et al. Embolization of iatrogenic vascular injuries of renal transplants: immediate and follow-up results. Cardiovasc Intervent Radiol. 1998;21(2):129–134. doi: 10.1007/s002709900228. [DOI] [PubMed] [Google Scholar]
- 47.Srivastava A Kumar J Sharma S, Abhishek, Ansari M S Kapoor R Vascular complication in live related renal transplant: an experience of 1945 cases Indian J Urol 201329142–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saad N E, Saad W E, Davies M G, Waldman D L, Fultz P J, Rubens D J. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25 01:S173–S189. doi: 10.1148/rg.25si055503. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi K, Censullo M L, Rossman L L, Kyriakides P N, Kahan B D, Cohen A M. Interventional radiologic management of renal transplant dysfunction: indications, limitations, and technical considerations. Radiographics. 2007;27(4):1109–1130. doi: 10.1148/rg.274065135. [DOI] [PubMed] [Google Scholar]
- 50.Koçak T, Nane I, Ander H, Ziylan O, Oktar T, Ozsoy C. Urological and surgical complications in 362 consecutive living related donor kidney transplantations. Urol Int. 2004;72(3):252–256. doi: 10.1159/000077125. [DOI] [PubMed] [Google Scholar]
- 51.Göğüs C, Yaman O, Soygür T, Bedük Y, Göğüs O. Urological complications in renal transplantation: long-term follow-up of the Woodruff ureteroneocystostomy procedure in 433 patients. Urol Int. 2002;69(2):99–101. doi: 10.1159/000065555. [DOI] [PubMed] [Google Scholar]
- 52.Swierzewski S J III, Konnak J W, Ellis J H. Treatment of renal transplant ureteral complications by percutaneous techniques. J Urol. 1993;149(5):986–987. doi: 10.1016/s0022-5347(17)36274-2. [DOI] [PubMed] [Google Scholar]
- 53.Fayek S A, Keenan J, Haririan A. et al. Ureteral stents are associated with reduced risk of ureteral complications after kidney transplantation: a large single center experience. Transplantation. 2012;93(3):304–308. doi: 10.1097/TP.0b013e31823ec081. [DOI] [PubMed] [Google Scholar]
- 54.Akbar S A, Jafri S Z, Amendola M A, Madrazo B L, Salem R, Bis K G. Complications of renal transplantation. Radiographics. 2005;25(5):1335–1356. doi: 10.1148/rg.255045133. [DOI] [PubMed] [Google Scholar]
- 55.Gottlieb R H, Luhmann K IV, Oates R P. Duplex ultrasound evaluation of normal native kidneys and native kidneys with urinary tract obstruction. J Ultrasound Med. 1989;8(11):609–611. doi: 10.7863/jum.1989.8.11.609. [DOI] [PubMed] [Google Scholar]
- 56.Johnson S P, Berry R S. Interventional radiological management of the complications of renal transplantation. Semin Intervent Radiol. 2001;18(1):47–58. [Google Scholar]
- 57.Papatsoris A G, Buchholz N. A novel thermo-expandable ureteral metal stent for the minimally invasive management of ureteral strictures. J Endourol. 2010;24(3):487–491. doi: 10.1089/end.2009.0138. [DOI] [PubMed] [Google Scholar]
- 58.Bachar G N, Mor E, Bartal G, Atar E, Goldberg N, Belenky A. Percutaneous balloon dilatation for the treatment of early and late ureteral strictures after renal transplantation: long-term follow-up. Cardiovasc Intervent Radiol. 2004;27(4):335–338. doi: 10.1007/s00270-004-0163-9. [DOI] [PubMed] [Google Scholar]
- 59.Pauer W, Eckerstorfer G M. Use of self-expanding permanent endoluminal stents for benign ureteral strictures: mid-term results. J Urol. 1999;162(2):319–322. [PubMed] [Google Scholar]
- 60.Kristo B, Phelan M W, Gritsch H A, Schulam P G. Treatment of renal transplant ureterovesical anastomotic strictures using antegrade balloon dilation with or without holmium:YAG laser endoureterotomy. Urology. 2003;62(5):831–834. doi: 10.1016/s0090-4295(03)00655-1. [DOI] [PubMed] [Google Scholar]
- 61.Sandhu K, Masters J, Ehrlich Y. Ureteropyelostomy using the native ureter for the management of ureteric obstruction or symptomatic reflux following renal transplantation. Urology. 2012;79(4):929–932. doi: 10.1016/j.urology.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 62.Benedetti E, Hakim N S, Perez E M, Matas A J. Renal transplantation. Acad Radiol. 1995;2(2):159–166. doi: 10.1016/S1076-6332(05)80152-7. [DOI] [PubMed] [Google Scholar]
- 63.Whang M, Yballe M, Geffner S, Fletcher H S, Palekar S, Mulgaonkar S. Urologic complications in more than 2500 kidney transplantations performed at the Saint Barnabas healthcare system. Transplant Proc. 2011;43(5):1619–1622. doi: 10.1016/j.transproceed.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 64.Regan S M, Sethi A S, Powelson J A, Goggins W C, Milgrom M L, Sundaram C P. Symptoms related to ureteral stents in renal transplants compared with stents placed for other indications. J Endourol. 2009;23(12):2047–2050. doi: 10.1089/end.2009.0112. [DOI] [PubMed] [Google Scholar]
- 65.Wilson C H, Rix D A, Manas D M. Chichester, UK: John Wiley & Sons, Ltd; 2006. Routine intraoperative ureteric stenting for kidney transplant recipients. [Google Scholar]
- 66.Bonkat G, Rieken M, Siegel F P. et al. Microbial ureteral stent colonization in renal transplant recipients: frequency and influence on the short-time functional outcome. Transpl Infect Dis. 2012;14(1):57–63. doi: 10.1111/j.1399-3062.2011.00671.x. [DOI] [PubMed] [Google Scholar]
- 67.Parapiboon W, Ingsathit A, Disthabanchong S. et al. Impact of early ureteric stent removal and cost-benefit analysis in kidney transplant recipients: results of a randomized controlled study. Transplant Proc. 2012;44(3):737–739. doi: 10.1016/j.transproceed.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 68.Kayler L, Zendejas I, Schain D, Magliocca J. Ureteral stent placement and BK viremia in kidney transplant recipients. Transpl Infect Dis. 2013;15(2):202–207. doi: 10.1111/tid.12051. [DOI] [PubMed] [Google Scholar]
- 69.Ball C G, Kirkpatrick A W, Yilmaz S, Monroy M, Nicolaou S, Salazar A. Renal allograft compartment syndrome: an underappreciated postoperative complication. Am J Surg. 2006;191(5):619–624. doi: 10.1016/j.amjsurg.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Horrow M M, Parsikia A, Zaki R, Ortiz J. Immediate postoperative sonography of renal transplants: vascular findings and outcomes. AJR Am J Roentgenol. 2013;201(3):W479–W486. doi: 10.2214/AJR.12.10310. [DOI] [PubMed] [Google Scholar]
- 71.Irving H C, Kashi S H. Complications of renal transplantation and the role of interventional radiology. J Clin Ultrasound. 1992;20(8):545–552. doi: 10.1002/jcu.1870200808. [DOI] [PubMed] [Google Scholar]
- 72.Pozniak M A, Dodd G D III, Kelcz F. Ultrasonographic evaluation of renal transplantation. Radiol Clin North Am. 1992;30(5):1053–1066. [PubMed] [Google Scholar]
- 73.Greenberg B M, Perloff L J, Grossman R A, Naji A, Barker C F. Treatment of lymphocele in renal allograft recipients. Arch Surg. 1985;120(4):501–504. doi: 10.1001/archsurg.1985.01390280087019. [DOI] [PubMed] [Google Scholar]
- 74.Khauli R B, Stoff J S, Lovewell T, Ghavamian R, Baker S. Post-transplant lymphoceles: a critical look into the risk factors, pathophysiology and management. J Urol. 1993;150(1):22–26. doi: 10.1016/s0022-5347(17)35387-9. [DOI] [PubMed] [Google Scholar]
- 75.Brockis J G, Hulbert J C, Patel A S. et al. The diagnosis and treatment of lymphocoeles associated with renal transplantation. A report of 6 cases and a review of the literature. Br J Urol. 1978;50(5):307–312. doi: 10.1111/j.1464-410x.1978.tb03637.x. [DOI] [PubMed] [Google Scholar]
- 76.Amendola M A, Montalvo B M. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. Percutaneous management of lymphoceles; pp. 1487–1493. [Google Scholar]
- 77.Gillenwater J Y. Baltimore, MD: Lippincott Williams & Wilkins; 2002. Renal transplantation; pp. 941–954. [Google Scholar]
- 78.Bouali K, Magotteaux P, Jadot A. et al. Percutaneous catheter drainage of abdominal abscess after abdominal surgery. Results in 121 cases. J Belge Radiol. 1993;76(1):11–14. [PubMed] [Google Scholar]
- 79.Towbin A J, Towbin R B. Interventional radiology in the treatment of the complications of organ transplant in the pediatric population-part 2: the liver. Semin Intervent Radiol. 2004;21(4):321–333. doi: 10.1055/s-2004-861566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Welling T, Pelletier S. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. Hepatic transplantation; pp. 542–564. [Google Scholar]
- 81.Parrilla P, Sánchez-Bueno F, Figueras J. et al. Analysis of the complications of the piggy-back technique in 1,112 liver transplants. Transplantation. 1999;67(9):1214–1217. doi: 10.1097/00007890-199905150-00003. [DOI] [PubMed] [Google Scholar]
- 82.Pelletier S. Philadelphia, PA: Lippincott, Williams & Wilkins; 2006. Complications of liver transplantation; pp. 627–639. [Google Scholar]
- 83.Caiado A H, Blasbalg R, Marcelino A S. et al. Complications of liver transplantation: multimodality imaging approach. Radiographics. 2007;27(5):1401–1417. doi: 10.1148/rg.275065129. [DOI] [PubMed] [Google Scholar]
- 84.Strovski E, Liu D, Scudamore C, Ho S, Yoshida E, Klass D. Magnetic resonance venography and liver transplant complications. World J Gastroenterol. 2013;19(36):6110–6113. doi: 10.3748/wjg.v19.i36.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andrews J C. Vascular complications following liver transplantation. Semin Intervent Radiol. 2004;21(4):221–233. doi: 10.1055/s-2004-861556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guimarães M, Uflacker R, Schönholz C, Hannegan C, Selby J B. Stent migration complicating treatment of inferior vena cava stenosis after orthotopic liver transplantation. J Vasc Interv Radiol. 2005;16(9):1247–1252. doi: 10.1097/01.RVI.0000167586.44204.C8. [DOI] [PubMed] [Google Scholar]
- 87.Karakayali H, Boyvat F, Coskun M. et al. Venous complications after orthotopic liver transplantation. Transplant Proc. 2006;38(2):604–606. doi: 10.1016/j.transproceed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 88.Goelitz B W, Darcy M. Longitudinal stent fracture and migration of a stent fragment complicating treatment of hepatic vein stenosis after orthotopic liver transplantation. Semin Intervent Radiol. 2007;24(3):333–336. doi: 10.1055/s-2007-985746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akun E, Yaprak O, Killi R, Balci N C, Tokat Y, Yuzer Y. Vascular complications in hepatic transplantation: single-center experience in 14 years. Transplant Proc. 2012;44(5):1368–1372. doi: 10.1016/j.transproceed.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 90.Kim B S, Kim T K, Jung D J. et al. Vascular complications after living related liver transplantation: evaluation with gadolinium-enhanced three-dimensional MR angiography. AJR Am J Roentgenol. 2003;181(2):467–474. doi: 10.2214/ajr.181.2.1810467. [DOI] [PubMed] [Google Scholar]
- 91.Wozney P, Zajko A B, Bron K M, Point S, Starzl T E. Vascular complications after liver transplantation: a 5-year experience. AJR Am J Roentgenol. 1986;147(4):657–663. doi: 10.2214/ajr.147.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glockner J F, Forauer A R. Vascular or ischemic complications after liver transplantation. AJR Am J Roentgenol. 1999;173(4):1055–1059. doi: 10.2214/ajr.173.4.10511177. [DOI] [PubMed] [Google Scholar]
- 93.Ou H Y, Concejero A M, Huang T L. et al. Portal vein thrombosis in biliary atresia patients after living donor liver transplantation. Surgery. 2011;149(1):40–47. doi: 10.1016/j.surg.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 94.Duffy J P Hong J C Farmer D G et al. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients J Am Coll Surg 20092085896–903., discussion 903–905 [DOI] [PubMed] [Google Scholar]
- 95.Oh C K, Pelletier S J, Sawyer R G. et al. Uni- and multi-variate analysis of risk factors for early and late hepatic artery thrombosis after liver transplantation. Transplantation. 2001;71(6):767–772. doi: 10.1097/00007890-200103270-00014. [DOI] [PubMed] [Google Scholar]
- 96.Pareja E, Cortes M, Navarro R, Sanjuan F, López R, Mir J. Vascular complications after orthotopic liver transplantation: hepatic artery thrombosis. Transplant Proc. 2010;42(8):2970–2972. doi: 10.1016/j.transproceed.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 97.Bhargava P Vaidya S Dick A A Dighe M Imaging of orthotopic liver transplantation: review review AJR Am J Roentgenol 2011196(3, Suppl):WS15–WS25., Quiz S35–S38 [DOI] [PubMed] [Google Scholar]
- 98.Flint E W, Sumkin J H, Zajko A B, Bowen A. Duplex sonography of hepatic artery thrombosis after liver transplantation. AJR Am J Roentgenol. 1988;151(3):481–483. doi: 10.2214/ajr.151.3.481. [DOI] [PubMed] [Google Scholar]
- 99.Nolten A, Sproat I A. Hepatic artery thrombosis after liver transplantation: temporal accuracy of diagnosis with duplex US and the syndrome of impending thrombosis. Radiology. 1996;198(2):553–559. doi: 10.1148/radiology.198.2.8596865. [DOI] [PubMed] [Google Scholar]
- 100.Bhattacharjya S, Gunson B K, Mirza D F. et al. Delayed hepatic artery thrombosis in adult orthotopic liver transplantation-a 12-year experience. Transplantation. 2001;71(11):1592–1596. doi: 10.1097/00007890-200106150-00018. [DOI] [PubMed] [Google Scholar]
- 101.Saad W E, Davies M G, Saad N E. et al. Catheter thrombolysis of thrombosed hepatic arteries in liver transplant recipients: predictors of success and role of thrombolysis. Vasc Endovascular Surg. 2007;41(1):19–26. doi: 10.1177/1538574406296210. [DOI] [PubMed] [Google Scholar]
- 102.Huang M, Shan H, Jiang Z. et al. The use of coronary stent in hepatic artery stenosis after orthotopic liver transplantation. Eur J Radiol. 2006;60(3):425–430. doi: 10.1016/j.ejrad.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 103.Denys A L, Qanadli S D, Durand F. et al. Feasibility and effectiveness of using coronary stents in the treatment of hepatic artery stenoses after orthotopic liver transplantation: preliminary report. AJR Am J Roentgenol. 2002;178(5):1175–1179. doi: 10.2214/ajr.178.5.1781175. [DOI] [PubMed] [Google Scholar]
- 104.Ueno T, Jones G, Martin A. et al. Clinical outcomes from hepatic artery stenting in liver transplantation. Liver Transpl. 2006;12(3):422–427. doi: 10.1002/lt.20628. [DOI] [PubMed] [Google Scholar]
- 105.Rostambeigi N, Hunter D, Duval S, Chinnakotla S, Golzarian J. Stent placement versus angioplasty for hepatic artery stenosis after liver transplant: a meta-analysis of case series. Eur Radiol. 2013;23(5):1323–1334. doi: 10.1007/s00330-012-2730-9. [DOI] [PubMed] [Google Scholar]
- 106.Hamby B A, Ramirez D E, Loss G E. et al. Endovascular treatment of hepatic artery stenosis after liver transplantation. J Vasc Surg. 2013;57(4):1067–1072. doi: 10.1016/j.jvs.2012.10.086. [DOI] [PubMed] [Google Scholar]
- 107.Vignali C, Cioni R, Petruzzi P. et al. Role of interventional radiology in the management of vascular complications after liver transplantation. Transplant Proc. 2004;36(3):552–554. doi: 10.1016/j.transproceed.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 108.García-Criado A, Gilabert R, Berzigotti A, Brú C. Doppler ultrasound findings in the hepatic artery shortly after liver transplantation. AJR Am J Roentgenol. 2009;193(1):128–135. doi: 10.2214/AJR.07.3919. [DOI] [PubMed] [Google Scholar]
- 109.Marshall M M, Muiesan P, Srinivasan P. et al. Hepatic artery pseudoaneurysms following liver transplantation: incidence, presenting features and management. Clin Radiol. 2001;56(7):579–587. doi: 10.1053/crad.2001.0650. [DOI] [PubMed] [Google Scholar]
- 110.Elsharkawy A M, Sen G, Jackson R. et al. Use of a multilayered stent for the treatment of hepatic artery pseudoaneurysm after liver transplantation. Cardiovasc Intervent Radiol. 2012;35(1):207–210. doi: 10.1007/s00270-011-0335-3. [DOI] [PubMed] [Google Scholar]
- 111.Lu N N, Huang Q, Wang J F, Wei B J, Gao K, Zhai R Y. Treatment of post-liver transplant hepatic artery pseudoaneurysm with balloon angioplasty after failed stent graft placement. Clin Res Hepatol Gastroenterol. 2012;36(6):e109–e113. doi: 10.1016/j.clinre.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 112.Saad W E. Nonocclusive hepatic artery hypoperfusion syndrome (splenic steal syndrome) in liver transplant recipients. Semin Intervent Radiol. 2012;29(2):140–146. doi: 10.1055/s-0032-1312576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sanyal R, Shah S N. Role of imaging in the management of splenic artery steal syndrome. J Ultrasound Med. 2009;28(4):471–477. doi: 10.7863/jum.2009.28.4.471. [DOI] [PubMed] [Google Scholar]
- 114.Nüssler N C, Settmacher U, Haase R, Stange B, Heise M, Neuhaus P. Diagnosis and treatment of arterial steal syndromes in liver transplant recipients. Liver Transpl. 2003;9(6):596–602. doi: 10.1053/jlts.2003.50080. [DOI] [PubMed] [Google Scholar]
- 115.Mogl M T, Nüssler N C, Presser S J. et al. Evolving experience with prevention and treatment of splenic artery syndrome after orthotopic liver transplantation. Transpl Int. 2010;23(8):831–841. doi: 10.1111/j.1432-2277.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- 116.Pérez J, Grande L G, Achécar L. et al. Case report of splenic artery steal syndrome: demonstration of portal hyperflow mechanism by anatomic variant of the splenic artery and correlation with Doppler rates. Transplant Proc. 2011;43(6):2269–2271. doi: 10.1016/j.transproceed.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 117.De Carlis L, Sansalone C V, Rondinara G F. et al. Splenic artery steal syndrome after orthotopic liver transplantation: diagnosis and treatment. Transplant Proc. 1993;25(4):2594–2596. [PubMed] [Google Scholar]
- 118.Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24(4):379–392. doi: 10.1111/j.1432-2277.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 119.Kochhar G, Parungao J M, Hanouneh I A, Parsi M A. Biliary complications following liver transplantation. World J Gastroenterol. 2013;19(19):2841–2846. doi: 10.3748/wjg.v19.i19.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chok K S, Chan S C, Cheung T T. et al. A retrospective study on risk factors associated with failed endoscopic treatment of biliary anastomotic stricture after right-lobe living donor liver transplantation with duct-to-duct anastomosis. Ann Surg. 2014;259(4):767–772. doi: 10.1097/SLA.0b013e318294d0ce. [DOI] [PubMed] [Google Scholar]
- 121.Lu D, Xu X, Wang J. et al. The influence of a contemporaneous portal and hepatic artery revascularization protocol on biliary complications after liver transplantation. Surgery. 2014;155(1):190–195. doi: 10.1016/j.surg.2013.06.056. [DOI] [PubMed] [Google Scholar]
- 122.Axelrod D A, Lentine K L, Xiao H. et al. National assessment of early biliary complications following liver transplantation: incidence and outcomes. Liver Transpl. 2014;20(4):446–456. doi: 10.1002/lt.23829. [DOI] [PubMed] [Google Scholar]
- 123.Buis C I, Hoekstra H, Verdonk R C, Porte R J. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13(6):517–524. doi: 10.1007/s00534-005-1080-2. [DOI] [PubMed] [Google Scholar]
- 124.Kim J, Ko G Y, Sung K B. et al. Percutaneously placed covered retrievable stents for the treatment of biliary anastomotic strictures following living donor liver transplantation. Liver Transpl. 2010;16(12):1410–1420. doi: 10.1002/lt.22173. [DOI] [PubMed] [Google Scholar]
- 125.Cerecedo-Rodriguez J, Phillips M, Figueroa-Barojas P. et al. Self expandable metal stents for anastomotic stricture following liver transplant. Dig Dis Sci. 2013;58(9):2661–2666. doi: 10.1007/s10620-013-2703-0. [DOI] [PubMed] [Google Scholar]
- 126.Sauer P, Chahoud F, Gotthardt D. et al. Temporary placement of fully covered self-expandable metal stents in biliary complications after liver transplantation. Endoscopy. 2012;44(5):536–538. doi: 10.1055/s-0031-1291714. [DOI] [PubMed] [Google Scholar]
- 127.Kaffes A, Griffin S, Vaughan R. et al. A randomized trial of a fully covered self-expandable metallic stent versus plastic stents in anastomotic biliary strictures after liver transplantation. Therap Adv Gastroenterol. 2014;7(2):64–71. doi: 10.1177/1756283X13503614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gruessner R W, Sutherland D E, Gruessner A C. Mortality assessment for pancreas transplants. Am J Transplant. 2004;4(12):2018–2026. doi: 10.1111/j.1600-6143.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- 129.Fiorina P, Folli F, Zerbini G. et al. Islet transplantation is associated with improvement of renal function among uremic patients with type I diabetes mellitus and kidney transplants. J Am Soc Nephrol. 2003;14(8):2150–2158. doi: 10.1097/01.asn.0000077339.20759.a3. [DOI] [PubMed] [Google Scholar]
- 130.White S A, Shaw J A, Sutherland D E. Pancreas transplantation. Lancet. 2009;373(9677):1808–1817. doi: 10.1016/S0140-6736(09)60609-7. [DOI] [PubMed] [Google Scholar]
- 131.Khaja M S, Matsumoto A H, Saad W E. Vascular complications of transplantation: part 2: pancreatic transplants. Cardiovasc Intervent Radiol. 2014;37(6):1415–1419. doi: 10.1007/s00270-014-0867-4. [DOI] [PubMed] [Google Scholar]
- 132.Humar A, Kandaswamy R, Granger D, Gruessner R W, Gruessner A C, Sutherland D E. Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg. 2000;231(2):269–275. doi: 10.1097/00000658-200002000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reddy K S, Stratta R J, Shokouh-Amiri M H, Alloway R, Egidi M F, Gaber A O. Surgical complications after pancreas transplantation with portal-enteric drainage. J Am Coll Surg. 1999;189(3):305–313. doi: 10.1016/s1072-7515(99)00135-0. [DOI] [PubMed] [Google Scholar]
- 134.Dillman J R, Elsayes K M, Bude R O, Platt J F, Francis I R. Imaging of pancreas transplants: postoperative findings with clinical correlation. J Comput Assist Tomogr. 2009;33(4):609–617. doi: 10.1097/RCT.0b013e3181966988. [DOI] [PubMed] [Google Scholar]
- 135.Fridell J A, Johnson M S, Goggins W C. et al. Vascular catastrophes following pancreas transplantation: an evolution in strategy at a single center. Clin Transplant. 2012;26(1):164–172. doi: 10.1111/j.1399-0012.2011.01560.x. [DOI] [PubMed] [Google Scholar]
- 136.Verni M P, Leone J P, DeRoover A. Pseudoaneurysm of the Y-graft/iliac artery anastomosis following pancreas transplantation: a case report and review of the literature. Clin Transplant. 2001;15(1):72–76. doi: 10.1034/j.1399-0012.2001.150113.x. [DOI] [PubMed] [Google Scholar]
- 137.Saad W E, Darwish W E, Turba U C. et al. Endovascular management of vascular complications in pancreatic transplants. Vasc Endovascular Surg. 2012;46(3):262–268. doi: 10.1177/1538574412438949. [DOI] [PubMed] [Google Scholar]
- 138.Srinivasan P, Huang G C, Amiel S A, Heaton N D. Islet cell transplantation. Postgrad Med J. 2007;83(978):224–229. doi: 10.1136/pgmj.2006.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chhabra P, Brayman K L. Overcoming barriers in clinical islet transplantation: current limitations and future prospects. Curr Probl Surg. 2014;51(2):49–86. doi: 10.1067/j.cpsurg.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 140.Ryan E A, Lakey J R, Paty B W. et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51(7):2148–2157. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 141.Dixon S, Tapping C R, Walker J N. et al. The role of interventional radiology and imaging in pancreatic islet cell transplantation. Clin Radiol. 2012;67(9):923–931. doi: 10.1016/j.crad.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 142.Goss J A, Soltes G, Goodpastor S E. et al. Pancreatic islet transplantation: the radiographic approach. Transplantation. 2003;76(1):199–203. doi: 10.1097/01.TP.0000073976.26604.96. [DOI] [PubMed] [Google Scholar]
- 143.Hatziavramidis D T, Karatzas T M, Chrousos G P. Pancreatic islet cell transplantation: an update. Ann Biomed Eng. 2013;41(3):469–476. doi: 10.1007/s10439-012-0676-3. [DOI] [PubMed] [Google Scholar]
- 144.Osama Gaber A, Chamsuddin A, Fraga D, Fisher J, Lo A. Insulin independence achieved using the transmesenteric approach to the portal vein for islet transplantation. Transplantation. 2004;77(2):309–311. doi: 10.1097/01.TP.0000101509.35249.A0. [DOI] [PubMed] [Google Scholar]
- 145.Jamiolkowski R M, Guo L Y, Li Y R, Shaffer S M, Naji A. Islet transplantation in type I diabetes mellitus. Yale J Biol Med. 2012;85(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- 146.Medarova Z, Moore A. Non-invasive detection of transplanted pancreatic islets. Diabetes Obes Metab. 2008;10 04:88–97. doi: 10.1111/j.1463-1326.2008.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Villiger P, Ryan E A, Owen R. et al. Prevention of bleeding after islet transplantation: lessons learned from a multivariate analysis of 132 cases at a single institution. Am J Transplant. 2005;5(12):2992–2998. doi: 10.1111/j.1600-6143.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 148.de Perrot M, Chaparro C, McRae K. et al. Twenty-year experience of lung transplantation at a single center: Influence of recipient diagnosis on long-term survival. J Thorac Cardiovasc Surg. 2004;127(5):1493–1501. doi: 10.1016/j.jtcvs.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 149.Ng Y L Paul N Patsios D et al. Imaging of lung transplantation: review review AJR Am J Roentgenol 2009192(3, Suppl):S1–S13., quiz S14–S19 [DOI] [PubMed] [Google Scholar]
- 150.Kilic A George T J Beaty C A Merlo C A Conte J V Shah A S The effect of center volume on the incidence of postoperative complications and their impact on survival after lung transplantation J Thorac Cardiovasc Surg 201214461502–1508., discussion 1508–1509 [DOI] [PubMed] [Google Scholar]
- 151.Clark S C, Levine A J, Hasan A, Hilton C J, Forty J, Dark J H. Vascular complications of lung transplantation. Ann Thorac Surg. 1996;61(4):1079–1082. doi: 10.1016/0003-4975(96)00003-3. [DOI] [PubMed] [Google Scholar]
- 152.Siddique A, Bose A K, Özalp F. et al. Vascular anastomotic complications in lung transplantation: a single institution's experience. Interact Cardiovasc Thorac Surg. 2013;17(4):625–631. doi: 10.1093/icvts/ivt266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.González-Fernández C, González-Castro A, Rodríguez-Borregán J C. et al. Pulmonary venous obstruction after lung transplantation. Diagnostic advantages of transesophageal echocardiography. Clin Transplant. 2009;23(6):975–980. doi: 10.1111/j.1399-0012.2009.01078.x. [DOI] [PubMed] [Google Scholar]
- 154.Gaubert J Y, Moulin G, Thomas P, Reynaud-Gaubert M, Noirclerc M, Bartoli J M. Anastomotic stenosis of the left pulmonary artery after lung transplantation: treatment by percutaneous placement of an endoprosthesis. AJR Am J Roentgenol. 1993;161(5):947–949. doi: 10.2214/ajr.161.5.8273631. [DOI] [PubMed] [Google Scholar]
- 155.Grubstein A, Atar E, Litvin S. et al. Angioplasty using covered stents in five patients with symptomatic pulmonary artery stenosis after single-lung transplantation. Cardiovasc Intervent Radiol. 2014;37(3):686–690. doi: 10.1007/s00270-013-0758-0. [DOI] [PubMed] [Google Scholar]
- 156.Waurick P E, Kleber F X, Ewert R. et al. Pulmonary artery stenosis 5 years after single lung transplantation in primary pulmonary hypertension. J Heart Lung Transplant. 1999;18(12):1243–1245. doi: 10.1016/s1053-2498(99)00091-1. [DOI] [PubMed] [Google Scholar]
- 157.Gill R R, Poh A C, Camp P C. et al. MDCT evaluation of central airway and vascular complications of lung transplantation. AJR Am J Roentgenol. 2008;191(4):1046–1056. doi: 10.2214/AJR.07.2691. [DOI] [PubMed] [Google Scholar]
- 158.Chen F, Tazaki J, Shibata T. et al. Stent angioplasty for a kink in the pulmonary artery anastomosis soon after living-donor lobar lung transplantation. Ann Thorac Surg. 2011;92(5):e105–e106. doi: 10.1016/j.athoracsur.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 159.Lumsden A B, Anaya-Ayala J E, Birnbaum I. et al. Robot-assisted stenting of a high-grade anastomotic pulmonary artery stenosis following single lung transplantation. J Endovasc Ther. 2010;17(5):612–616. doi: 10.1583/10-3208R.1. [DOI] [PubMed] [Google Scholar]
- 160.Bousamra M II, Mewissen M W, Batter J, Presberg K W, Schlueter D P, Haasler G B. Pulmonary artery thrombolysis and stenting after a bilateral sequential lung transplantation. J Heart Lung Transplant. 1997;16(6):678–680. [PubMed] [Google Scholar]
- 161.Alvarez A, Algar J, Santos F. et al. Airway complications after lung transplantation: a review of 151 anastomoses. Eur J Cardiothorac Surg. 2001;19(4):381–387. doi: 10.1016/s1010-7940(01)00619-4. [DOI] [PubMed] [Google Scholar]
- 162.Wildevuur C R, Benfield J R. A review of 23 human lung transplantations by 20 surgeons. Ann Thorac Surg. 1970;9(6):489–515. doi: 10.1016/s0003-4975(10)65544-0. [DOI] [PubMed] [Google Scholar]
- 163.Dutau H, Cavailles A, Sakr L. et al. A retrospective study of silicone stent placement for management of anastomotic airway complications in lung transplant recipients: short- and long-term outcomes. J Heart Lung Transplant. 2010;29(6):658–664. doi: 10.1016/j.healun.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 164.Lari S M, Gonin F, Colchen A. The management of bronchus intermedius complications after lung transplantation: a retrospective study. J Cardiothorac Surg. 2012;7:8. doi: 10.1186/1749-8090-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Boiselle P M, Feller-Kopman D, Ashiku S, Weeks D, Ernst A. Tracheobronchomalacia: evolving role of dynamic multislice helical CT. Radiol Clin North Am. 2003;41(3):627–636. doi: 10.1016/s0033-8389(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 166.Gaer J A, Tsang V, Khaghani A. et al. Use of endotracheal silicone stents for relief of tracheobronchial obstruction. Ann Thorac Surg. 1992;54(3):512–516. doi: 10.1016/0003-4975(92)90445-a. [DOI] [PubMed] [Google Scholar]
- 167.Cooper J D, Pearson F G, Patterson G A. et al. Use of silicone stents in the management of airway problems. Ann Thorac Surg. 1989;47(3):371–378. doi: 10.1016/0003-4975(89)90375-5. [DOI] [PubMed] [Google Scholar]
- 168.Sonett J R, Keenan R J, Ferson P F, Griffith B P, Landreneau R J. Endobronchial management of benign, malignant, and lung transplantation airway stenoses. Ann Thorac Surg. 1995;59(6):1417–1422. doi: 10.1016/0003-4975(95)00216-8. [DOI] [PubMed] [Google Scholar]
- 169.Carré P, Rousseau H, Lombart L. et al. Balloon dilatation and self-expanding metal Wallstent insertion. For management of bronchostenosis following lung transplantation. Chest. 1994;105(2):343–348. doi: 10.1378/chest.105.2.343. [DOI] [PubMed] [Google Scholar]
- 170.Burns K E, Orons P D, Dauber J H. et al. Endobronchial metallic stent placement for airway complications after lung transplantation: longitudinal results. Ann Thorac Surg. 2002;74(6):1934–1941. doi: 10.1016/s0003-4975(02)04033-x. [DOI] [PubMed] [Google Scholar]
- 171.Mughal M M, Gildea T R, Murthy S, Pettersson G, DeCamp M, Mehta A C. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med. 2005;172(6):768–771. doi: 10.1164/rccm.200410-1388OC. [DOI] [PubMed] [Google Scholar]
- 172.Knight J, Elwing J M, Milstone A. Bronchovascular fistula formation: a rare airway complication after lung transplantation. J Heart Lung Transplant. 2008;27(10):1179–1185. doi: 10.1016/j.healun.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 173.Furukawa H, Todo S, Reyes J, Abu-Elmagd K, Starzl T E. Technical aspects of small bowel transplantation. Curr Opin Organ Transplant. 1998;3:279–285. [Google Scholar]
- 174.Langnas A N, Shaw B W Jr, Antonson D L. et al. Preliminary experience with intestinal transplantation in infants and children. Pediatrics. 1996;97(4):443–448. [PubMed] [Google Scholar]
- 175.Grant D, Abu-Elmagd K, Reyes J. et al. 2003 report of the intestine transplant registry: a new era has dawned. Ann Surg. 2005;241(4):607–613. doi: 10.1097/01.sla.0000157265.85388.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Culp W C, Vonk B N, McCowan T C, Sudan D L. Utilization and tailoring of interventional radiology procedures in small bowel transplants. Transplant Proc. 2002;34(3):929–930. doi: 10.1016/s0041-1345(02)02675-1. [DOI] [PubMed] [Google Scholar]


