Abstract
STUDY QUESTION
Can cultured follicles model the ovarian cycle, including follicular- and luteal-phase hormone synthesis patterns and ovulation?
SUMMARY ANSWER
Under gonadotrophin stimulation, murine follicles grown in an encapsulated three-dimensional system ovulate in vitro and murine and human follicle hormone synthesis mimics follicular and luteal phases expected in vivo.
WHAT IS KNOWN ALREADY
Studies of the human ovary and follicle function are limited by the availability of human tissue and lack of in vitro models. We developed an encapsulated in vitro follicle growth (eIVFG) culture system, which preserves 3D follicular structure. Thus far, the alginate system has supported the culture of follicles from mice, dog, rhesus macaque, baboon and human. These studies have shown that cultured follicles synthesize steroid hormones similar to those observed during the follicular phase in vivo.
STUDY DESIGN, SIZE, DURATION
Cultured murine follicles were treated with human chorionic gonadotrophin (hCG) and epidermal growth factor (EGF) and either assayed for luteinization or removed from alginate beads and assayed for ovulation. Human follicles were also cultured, treated with follicle-stimulating hormone (FSH), hCG and EGF to mimic gonadotrophin changes throughout the ovarian cycle, and culture medium was assayed for hormone production.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Murine and human follicles were cultured in alginate hydrogel and hormone production [17β-estradiol, progesterone, inhibin A, inhibin B, activin A and anti-Müllerian hormone (AMH)] was quantified in medium by enzyme-linked immuno assay (ELISA). Human ovarian tissue was acquired from females between 6 and 34 years of age with a cancer diagnosis. These participants were undergoing ovarian tissue cryopreservation at National Physicians Cooperative sites as part of the Oncofertility Consortium.
MAIN RESULTS AND THE ROLE OF CHANCE
When grown in this system, 96% of mouse follicles ovulated in response to hCG and released meiotically competent eggs. Ovulated follicles recapitulated transcriptional, morphologic and hormone synthesis patterns post-luteinizing hormone (LH/hCG). In addition to rodent follicles, individual human follicles secreted steroid and peptide hormones that mimicked the patterns of serum hormones observed during the menstrual cycle.
LIMITATIONS, REASONS FOR CAUTION
This was a descriptive study of an in vitro model of ovulation and the ovarian hormone cycle. The ovulation studies were limited to murine tissue and further studies are needed to optimize conditions using other species.
WIDER IMPLICATIONS OF THE FINDINGS
The eIVFG system reliably phenocopies the in vivo ovarian cycle and provides a new tool to study human follicle biology and the influence of cycling female hormones on other tissue systems in vitro.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by NIH U54 HD041857, NIH U54 HD076188, NIH UH2 E5022920, NIH UH3 TR001207 and F30 AG040916 (R.M.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Keywords: in vitro follicle growth, ovulation, luteinization, ovarian cycle, alginate
Introduction
Biomedical research of the female reproductive system is limited by ethical considerations related to the acquisition and use of healthy ovarian tissue (Kim et al., 2010; Tingen et al., 2010). There are few models to study the human ovary and no in vitro models exist that replicate a complete ovarian cycle. New research tools and techniques are needed, not only for fundamental research of ovarian follicle biology, but also for preclinical testing and toxicity screening. Moreover, there remains a gap in our knowledge about the environment in which the female gamete, the oocyte, develops. The broader goal of in vitro follicle growth is to develop methods that support maturation of a competent human egg for use by cancer survivors. Our hypothesis is that the development of systems that support the full repertoire of reproductive hormones provides new information about follicle development that can aid in this goal.
Follicle development underlies each ovarian cycle producing steroid and peptide hormones essential for female reproduction. As the basic unit of the ovary, the follicle is compartmentalized with interdependent somatic cells (granulosa and theca cells) and a central oocyte. Once a follicle is selected to resume growth, rising follicle-stimulating hormone (FSH) causes follicular-phase hormone synthesis, including estrogen and inhibin A and B. These hormones support oocyte and endometrial growth and provide feedback to the hypothalamus and pituitary. The mid-cycle luteinizing hormone (LH) surge sets the timing of female fertility by triggering ovulation. In addition to expulsion of a mature oocyte, ovulation leads to the terminal differentiation of the follicular somatic cells into the progesterone-producing corpus luteum (CL). The luteal-phase hormones facilitate eventual implantation or menstruation. Furthermore, the pattern of follicular and luteal hormones determines female fecundity and influences multiple organ systems.
Different approaches exist to study follicle development including ovarian hormone production and gamete maturation. The process of ovulation poses a unique investigative challenge and studies have been limited to in vivo or in vitro-perfused ovary models (Cajander et al., 1984; Brannstrom et al., 1987). Systems that support in vitro ovulation have been described with follicular rupture rates ranging from 7 to 80% (Boland et al., 1994; Hartshorne et al., 1994; Johnson et al., 1995; Rose et al., 1999). The development of a robust in vitro model of ovulation would provide a new tool to examine basic follicle biology and develop follicle-targeted contraceptives. In addition to ovulation, studies of hormone production require more than cell culture approaches. Patterns of follicular-phase hormone synthesis have been described in 3D follicle culture systems (Picton and Gosden 2000; Xu et al., 2006b, 2011b, 2013b; Kedem et al., 2011; Sanchez et al., 2012; Telfer and Zelinski, 2013), while in vitro investigations of luteinization have remained in 2D primary cell cultures (Oonk et al., 1989; Meidan et al., 1990; Engelhardt et al., 1991). Although much has been learned from these studies, granulosa or theca cells luteinized in vitro differ from those luteinized in vivo. Richards et al. (1986) showed that cells luteinized in vitro required LH/cAMP to maintain progesterone synthesis. In contrast, cells luteinized in vivo synthesized progesterone independent of gonadotrophin stimulation (Richards et al., 1986; Oonk et al., 1989). Moreover, a model supportive of complete luteinization in vitro would provide a more efficient approach to study the second half of the ovarian cycle.
Techniques in the emerging field of in vitro follicle growth include several culture environments supporting individual follicle development (Gutierrez et al., 2000; Telfer et al., 2000; Wu et al., 2001; Xu et al., 2006a; Dunning et al., 2011; Kedem et al., 2011; Vanacker et al., 2012). We developed an encapsulated in vitro follicle growth (eIVFG) culture system, which preserves 3D follicular structure with paracrine signaling maintained between oocyte, granulosa and theca cells (Kreeger et al., 2006; Tingen et al., 2011; Tagler et al., 2012; Hornick et al., 2013). In addition to murine follicles, success to date in the culture of larger mammalian species has been reported in the alginate system including follicles from dog, rhesus macaque, goat, baboon and human (Xu et al., 2009a,b, 2010, 2011b,c, 2013a; Songsasen et al., 2011; Camboni et al., 2013; Fisher et al., 2013; Araujo et al., 2014; Silva et al., 2014; Wang et al., 2014). Further, eIVFG has facilitated studies of inter- and intra-follicular mechanisms governing follicular-phase processes including antrum formation, oocyte maturation and granulosa cell proliferation (Kreeger et al., 2006; West-Farrell et al., 2009; Hornick et al., 2013).
The goal of this study was to develop and characterize an in vitro model that would recapitulate the hormone patterns and mid-cycle ovulation associated with the ovarian cycle. We hypothesized that eIVFG could support not only follicular-phase processes, but also the transformation associated with ovulation and luteinization. Such a model would provide a valuable tool to study follicle-targeted agents and human follicle dynamics. It would also provide a preclinical tool to assess the effects of drugs or toxins on reproductive function.
Materials and Methods
Animals
CD1 mice were housed in a temperature- and light-controlled environment (14 h light:10 h dark) and provided with food and water ad libitum. Animals were fed Teklad Global irradiated 2919 low-phytoestrogen chow (Harlan Laboratories, Indianapolis, IN, USA). To minimize differences in nutrient availability, eight females were housed per dam (if necessary pups were sacrificed at birth).
Murine follicle culture and in vitro ovulation and luteinization
Multi-layered secondary follicles (180–210 µm in diameter) were mechanically isolated from ovaries of 18-day-old mice and individually encapsulated in 0.5% (w/v) alginate (FMC BioPolymers, Philadelphia, PA, USA) as previously described (Kreeger et al., 2006). Alginate-encapsulated follicles were placed in individual wells of a 96-well plate containing 100 µl of growth medium [alpha minimum essential medium (alphaMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10 mIU/ml recombinant FSH (Organon, Roseland, NJ, USA), 3 mg/ml bovine serum albumin (BSA, MP Biomedicals, Irvine, CA, USA), 1 mg/ml bovine fetuin (Sigma-Aldrich, St. Louis, MO, USA), 5 µg/ml insulin (Sigma-Aldrich), 5 µg/ml transferrin (Sigma-Aldrich) and 5 ng/ml selenium (Sigma-Aldrich)] and cultured for 4 days in a 5% CO2:21% O2 atmosphere. Half of the culture medium was exchanged on day 2 and conditioned medium stored at −80°C. To remove them from alginate, follicles were transferred into 1 ml of Liebovitz L-15 medium (Invitrogen) containing 10 U/ml alginate lyase (Sigma-Aldrich) for 20 min at 37°C. Follicular rupture was induced by incubating follicles in 100 µl maturation medium [alphaMEM with 1.5 IU/mL human chorionic gonadotrophin (hCG; Sigma-Aldrich), 5 ng/mL epidermal growth factor (EGF; BD Biosciences, Franklin Lakes, NJ, USA), 3 mg/ml BSA, 5 µg/ml insulin, 5 µg/ml transferrin and 5 ng/ml selenium] for 14–16 h in an ultra-low attachment 96-well plate (Corning, Tewksbury, MA, USA). Rupture was assayed visually and via dissection (n = 70); unruptured follicles were those that required mechanical isolation of the cumulus oocyte complex (COC). Oocytes were isolated from a subset of follicles (n = 36) and scored visually [metaphase II (MII), germinal vesicle breakdown (GVBD) or degenerated]. For RNA or protein analysis, follicles were aspirated, transferred into microcentrifuge tubes, flash frozen in liquid nitrogen and stored at −80°C. For each protease inhibitor, dose–response curves were made and the concentration at which inhibition of ovulation occurred without cell death was used. The following compounds were used: 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), 500 µM (Sigma-Aldrich); SB-3CT, 250 µM (Santa Cruz, Dallas, TX, USA); urokinase plasminogen activator (uPA) inhibitor, 50 µM (Santa Cruz); matrix metalloproteinase-2 (MMP-2) IV, 250 µM (EMD Millipore, Billerica, MA, USA); mifepristone (RU-486), 100 µM (Sigma-Aldrich). Ulipristal acetate (UPA, 50 µM; also referred to as CDB-2914 or VA2914) was a gift from HRA Pharma (Paris, France). In the luteinization experiments, on day 10 half of the growth medium was replaced with maturation medium (as above) and follicles remained encapsulated in alginate. Follicles were matured for 16 h at 37°C and 5% CO2 at which point 50 µl of medium was replaced with growth medium (as above). Cultures were continued for four additional days, with medium exchanged and images taken every other day.
Acquisition of human ovarian tissue
Human ovarian tissue was obtained from three females with a cancer diagnosis (Supplementary data, Table SI) between 6 and 34 years of age. These participants were undergoing ovarian tissue cryopreservation at National Physicians Cooperative (NPC) sites as part of the Oncofertility Consortium. Following the surgical removal of ovarian tissue, 80% was cryopreserved for future clinical use and up to 20% was designated for research. The research tissue was transported to the laboratory in SAGE OFC holding Media (Cooper Surgical, Trumbull, CT, USA) at 4°C for 14–24 h. In all cases, the ovarian tissue was processed using a standard technique in ovarian tissue cryopreservation in which the ovarian cortex is separated from the medulla using a Thomas Stadie-Riggs Tissue Slicer (http://oncofertility.northwestern.edu/media/dissection-human-ovary-preparation-cryopreservation). For each of the described experiments, five follicles were used from three women (Supplementary data, Table SI).
Human follicle culture and in vitro luteinization
Human follicles were isolated and cultured as multiple follicles using a modified method as described previously (Abir et al., 1999; Hornick et al., 2013). Briefly, ovarian cortical strips were cut into 1 mm3 pieces in alpha-MEM Glutamax (Invitrogen) supplemented with 1% Pen-Strep, 1% Serum Protein Substitute (SPS, Cooper Surgical, Trumbull, CT, USA) and 1.5 IU/ml hCG. The tissue was then enzymatically digested by supplementing alpha-MEM Glutamax with 1% Liberase TM (Roche, Indianapolis, IN, USA) and 0.1% DNase (Worthington, Lakewood, NJ, USA) for 30 min at 37°C. After rinsing the cortex three times with fresh SAGE OFC holding medium (Cooper Surgical), follicles were then mechanically isolated from the cortex using insulin-gauge needles and encapsulated in 0.3% alginate (NovaMatrix, Philadelphia, PA, USA). Encapsulated human follicles were transferred to a 96-well plate containing 100 µl of growth media [alpha-MEM Glutamax (Invitrogen) supplemented with 0.5 mg/ml fetuin, 0.3% human serum albumin (Cooper Surgical), 5 µg/ml insulin, 5 µg/ml transferrin and 5 ng/ml selenium (Sigma-Aldrich) and 5 mIU recombinant FSH (A.F. Parlow, National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, USA)], and placed in an incubator at 37°C and 5% CO2. Half of the culture medium was exchanged every other day and stored at −20°C. Follicles were imaged at each medium change using a Leica DM IRB inverted microscope equipped with 4× and 20× objectives to assess growth and survival. Follicles that contained an oocyte surrounded completely by somatic cells and continued to increase in diameter were defined as live follicles. Dead follicles were defined by unhealthy oocytes, dark granulosa cells or lost structural integrity. Luteal conversion was triggered by hCG administration for 36 h and the follicles were cultured for an additional 15 days. After 36 h incubation in maturation media [alpha-MEM Glutamax (Invitrogen) supplemented with 20 IU/ml hCG, 5 ng/ml EGF, 0.3% human serum albumin (Cooper Surgical), 5 µg/ml insulin, 5 µg/ml transferrin and 5 ng/ml selenium (Sigma-Aldrich)] half of the medium was exchanged for growth medium without FSH.
Immunohistochemistry
In alginate beads follicles were fixed overnight at 48°C in solution containing 4% paraformaldehyde (Sigma-Aldrich), 0.1 M sodium cacodylate (Sigma-Aldrich), 0.1 M sucrose (Sigma-Aldrich) and 10 mM CaCl2 (Sigma-Aldrich) to prevent alginate degradation. Following fixation, follicles were dehydrated and embedded in paraffin. The follicles were sectioned at 5 µm and adhered to glass slides and stained with hematoxylin–eosin.
Hormone assays
17β-estradiol, progesterone, inhibin A, inhibin B, activin A and anti-Müllerian hormone (AMH) were measured in medium by commercially available enzyme-linked immuno assay (ELISA) kits [estradiol and progesterone (Calbiotech, Spring Valley, CA, USA); AMH and inhibin B, inhibin A (Beckman Coulter, Pasadena, CA, USA); activin A (Ansh Labs, Webster, TX, USA)]. The limits of sensitivity for estradiol, progesterone, inhibin A, inhibin B, activin A and AMH were 3.94 pg/ml, 0.22 ng/ml, <5.0 pg/ml, 7 pg/ml, 0.065 ng/ml, 0.006 ng/ml, respectively. Assays for mouse estradiol and progesterone were performed at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Ethical approval
Animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the established Institutional Animal Care and Use Committee (IACUC) protocol at Northwestern University. Human ovarian tissue was obtained following informed consent under Northwestern University Institutional Review Board-approved protocols.
Statistical analysis
All experiments were independently performed at least three times, unless otherwise noted. For comparisons between groups, a one-way ANOVA followed by the Bonferroni post hoc test was performed (Prism4; Graph Pad Software).
Results
An ovulatory stimulus elicits follicular rupture in vitro
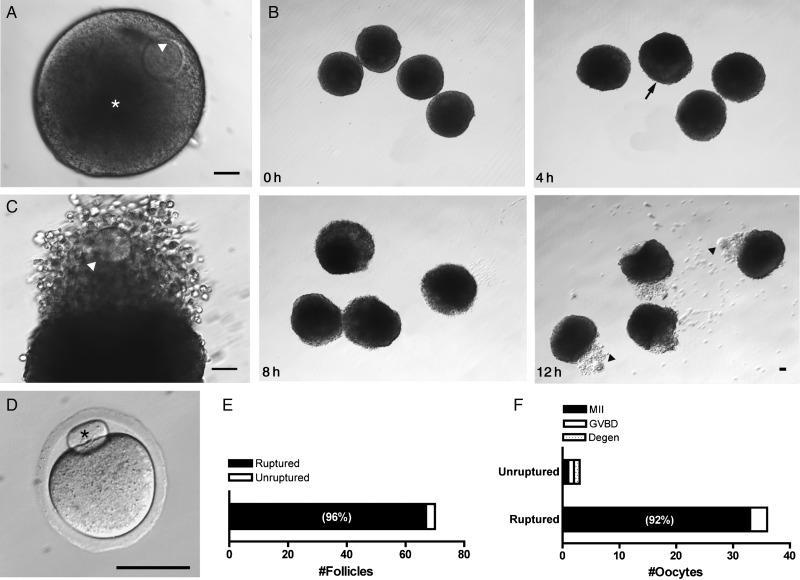
The encapsulated alginate system has been shown to support the development of secondary murine follicles, yielding fertilizable and developmentally competent oocytes (Xu et al., 2006a). To determine whether follicles grown in the alginate system ruptured and released a COC in response to an ovulatory stimulus, secondary murine follicles were mechanically isolated and encapsulated. After four days of culture, follicles grew from 203 ± 2 to 317 ± 3 µm (Fig. 1A) and were removed from alginate to permit COC extrusion. Follicles were treated with hCG and EGF for 14 h to mimic the LH surge and then assayed for rupture (Fig. 1B). The meiotic stage of the extruded gametes was also scored. After hCG administration, 96% (67/70) of follicles ruptured. Analysis of a subset of ruptured follicles showed that 92% (33/36) released mature MII eggs (Fig. 1D–F). The small fraction (4%) of follicles that did not rupture contained an equal distribution of MII, GVBD and degenerated oocytes (Fig. 1F). Cumulus expansion was visible by 4 h and extruded COCs visualized at 12 h for most follicles (Fig. 1B). Follicular rupture was highly localized to the area of COC expulsion and the remainder of the follicular structure remained intact (Fig. 1C). This morphology was conserved across ruptured follicles (Supplementary data, Fig. S1). Thus, in vitro grown follicles ruptured in response to hCG and recapitulated in vivo morphological changes.
Figure 1.
An ovulatory stimulus elicits follicular rupture in vitro. Murine follicles cultured in alginate rupture and release metaphase II (MII) eggs in response to hCG. (A) On the day of hCG administration, follicles contained an eccentric germinal vesicle (GV)-stage oocyte (arrowhead) and fluid-filled antrum (asterisk); scale bar = 50 µm. (B) Shown are representative images of follicles in the hours following hCG treatment (0, 4, 8 and 12 h). In some follicles, cumulus expansion was visible by 4 h (arrow). Extruded oocytes (arrowheads) and cumulus cells were visualized 12 h post-hCG; scale bar = 50 µm. (C) Image of a representative ruptured follicle 14 h post-hCG. The oocyte (arrowhead) is seen within the expanded cumulus matrix; scale bar = 50 µm. (D) Image of a representative MII egg collected from ruptured follicles with its extruded first polar body (asterisk); scale bar = 50 µm. (E) Overall, 96% of all follicles ruptured (n = 70). (F) Of the ruptured follicles (n = 36), 92% released MII stage eggs, while 8% were in the GVBD stage. Unruptured follicles were those that required mechanical isolation of the COC.
Effects of protease inhibitors and emergency contraceptives on ovulation in vitro
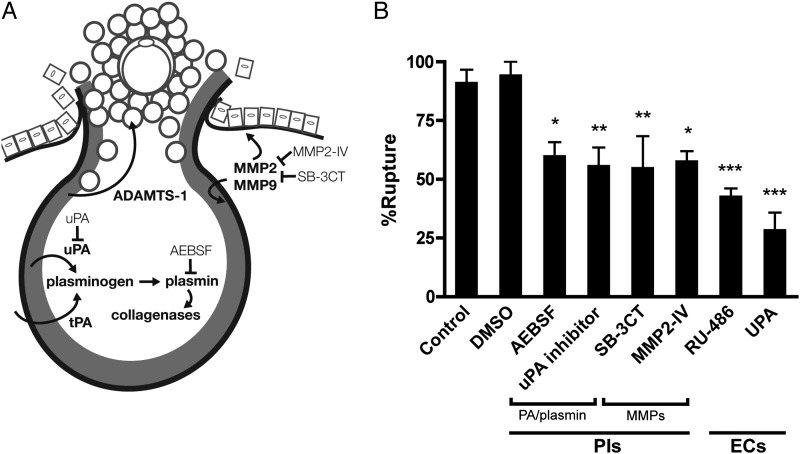
To further verify that our ovulation model mimicked the process of follicular rupture in vivo, we analyzed protease expression and action in vitro. First, we analyzed transcripts known to be regulated by LH/cAMP and important to ovulation: progesterone receptor (Pgr), tumor necrosis factor, alpha-induced protein 6 (Tnfaip6), prostaglandin-endoperoxidase protein synthase 2 (Ptgs2), plasminogen activator, urokinase (Plau) and a disintegrin and metalloproteinase with thrombospondin type 1 motif 1 (Adamts-1) (Supplementary data, Fig. S2). Importantly, temporal gene expression within hCG-treated follicles phenocopied expression observed in whole ovaries in vivo (Canipari et al., 1987; Camp et al., 1991; Park and Mayo, 1991; Espey et al., 2000; Yoshioka et al., 2000). We then treated follicles with protease inhibitors to examine their actions during in vitro ovulation. Follicles treated with either a serine protease inhibitor (AEBSF), uPA inhibitor, MMP-2/9 inhibitor (SB-3CT) or MMP-2 inhibitor ruptured at significantly lower rates when compared with those treated with vehicle alone (60%, P < 0.05; 56%, P < 0.01; 55%, P < 0.01; 58%, P < 0.05, respectively; vehicle, 94%) (Fig. 2A and B). These results are consistent with studies of knock-out models (uPA−/−/tPA−/−, MMP-2−/−, MMP-9−/−) showing that a reduction, but not complete suppression, of ovulation occurred with inhibition of the gelatinases or PA/plasmin (Leonardsson et al., 1995; Itoh et al., 1997; Vu et al., 1998).
Figure 2.
Effects of protease inhibitors and emergency contraceptives on ovulation in vitro. To assess the effects of the matrix metalloproteinases (MMPs) and PA/plasmin system on follicular rupture, inhibitors were added concomitant to hCG and rupture rates were determined. Similarly, the effects of emergency contraceptives (RU-486 and UPA) were assayed. (A) Schematic representation of putative proteases involved in ovulation and targeted action of protease inhibitors, see Materials and Methods. (B) Rupture rates of follicles treated with hCG and protease inhibitors (PIs) or emergency contraceptives (ECs). Control follicles are those treated with hCG only. Data are expressed as mean ± SEM, n = 3–4 cultures, 30–56 follicles; *P < 0.05, **P < 0.01, ***P < 0.001 according to one-way ANOVA followed by Bonferroni's Multiple Comparison Test.
We next assayed the effects of RU-486 and UPA on follicular rupture to validate the required role of progesterone receptor (PR) in the present ovulation model. Further, we aimed to investigate whether PR-targeted emergency contraceptives act directly on the follicle to inhibit ovulation in addition to their effects on the hypothalamic–pituitary–gonadotrophin axis. When follicles were treated with either progesterone agent, follicular rupture was significantly inhibited compared with vehicle-treated follicles (RU-486, 43%; UPA, 29%; P < 0.001) (Fig. 2B). Thus, these results validate the fidelity of our in vitro ovulation model as well as identify the direct effects PR emergency contraceptives may have on the follicle itself.
Alginate supports murine follicle luteinization
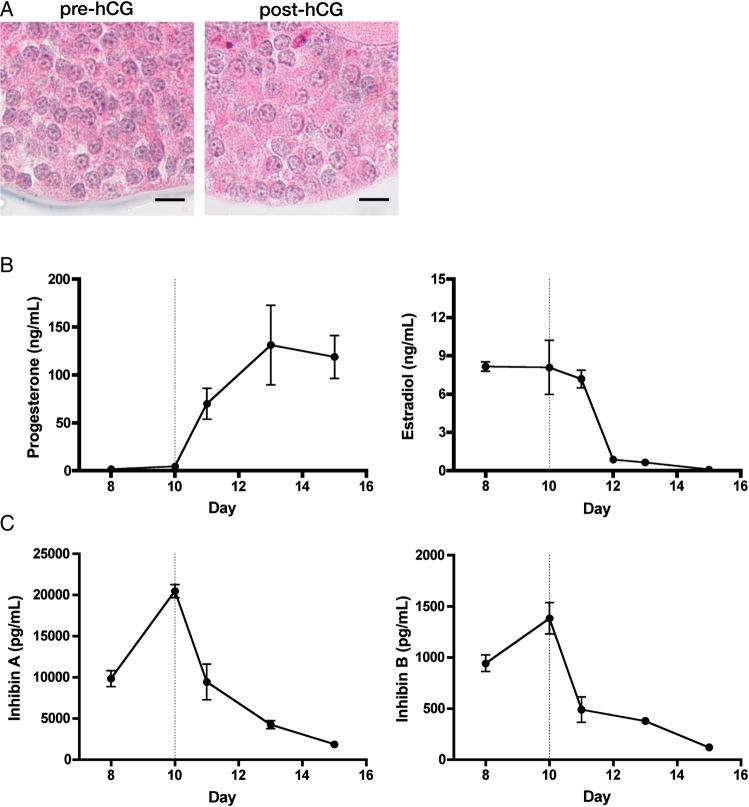
Once we established that cultured murine follicles could ovulate in vitro, we next assessed the capacity of these follicles to luteinize in vitro. Secondary follicles (153 ± 0.8 µm, n = 116) were isolated from mice and encapsulated in 0.5% alginate. Follicles were cultured for 10 days, treated with hCG and cultured for an additional 5 days. Medium was changed every other day, thus tapering the hCG concentration 16 h post-administration. Following hCG, follicles showed morphological signs of luteinization, marked by an increased granulosa cell cytoplasmic-to-nuclear ratio (Fig. 3A). Both steroid and peptide hormones were quantified to assess luteinization. As shown in Fig. 3B, progesterone rose steadily for 3 days post-hCG and progesterone synthesis remained elevated at the time of culture termination 5 days post-hCG. Estradiol synthesis sharply decreased upon hCG administration, with levels reaching nadir at the end of culture. For peptide synthesis, follicles produced increasing amounts of both inhibin A and inhibin B and as would be expected in vivo. Further, hCG decreased expression of both inhibin A and B by the end of culture.
Figure 3.
eIVFG supports murine follicle luteinization. Murine secondary follicles were cultured in alginate for 10 days, luteinized by hCG administration and cultured for an additional 5 days. (A) Follicles were fixed and stained with hematoxylin–eosin before and after hCG treatment (culture days 10 and 13, respectively). Follicles showed morphological signs of luteinization, marked by an increased granulosa cell cytoplasmic to nuclear ratio (scale bars = 10 µm). (B) Progesterone and estradiol and (C) inhibin A and inhibin B were quantified in the medium by enzyme-linked immuno assay (ELISA) (n = 3 cultures). Data are expressed as mean ± SEM.
To further validate the luteal phenotype of follicles in vitro, changes in key transcripts before and after hCG treatment were assessed including: steroidogenic acute regulatory protein (Star), cholesterol side-chain cleavage cytochrome P450 (Cyp11a1), 3β-hydroxysteroid dehydrogenase (Hsd3b1), 20α-hydroxysteroid dehydrogenase (20αHSD), 17α-hydroxylase (Cyp17a1), aromatase (Cyp19a1), follicle-stimulating hormone receptor (Fshr) and luteinizing hormone/choriogonadotrophin receptor (Lhcgr). Changes in these transcripts matched those observed in vivo with the up-regulation of steroidogenic enzymes Star, Cyp11a1 and 20αHSD and the down-regulation of Cyp17a1 and Cyp19a1 (Kidwell et al., 1966; Park and Mayo 1991; Kaynard et al., 1992) (Supplementary data, Fig. S3). While we observed a transient increase in Lhcgr expression 1 day post-hCG, it remained down-regulated 3 and 5 days post-treatment. Fshr was down-regulated at all time points post-hCG (Supplementary data, Fig. S3). Thus, cultured follicles luteinized in vitro as demonstrated by changes in hormone synthesis. Further, progesterone production was independent of constant gonadotrophin stimulation.
eIVFG supports the complete hormone cycle in human follicles
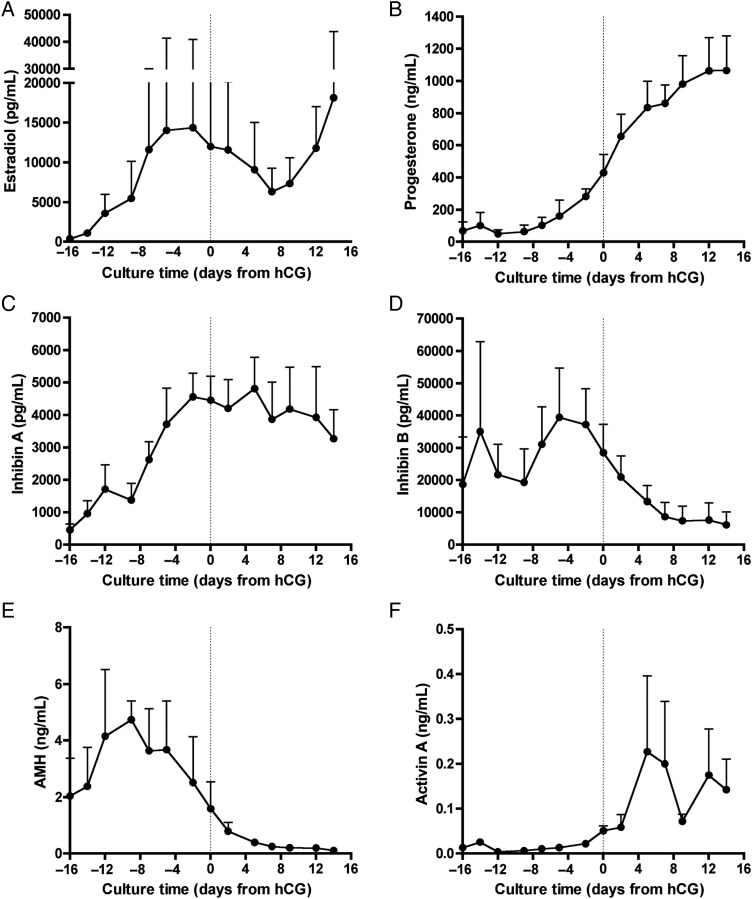
Combined with prior reports (Xu et al., 2006b; West-Farrell et al., 2009), we have shown that murine follicles cultured in an eIVFG system could provide a complete in vitro model of the ovarian cycle. We then sought to model the entire human ovarian cycle. To do so, primary to secondary stage human follicles (74–260 µm) were isolated from ovarian tissue (Supplemental data, Table SI and Fig. S4), encapsulated in alginate and monitored throughout growth via light microscopy. Follicle growth and morphology were used to assess viability (as described in Materials and Methods) and levels of estradiol, progesterone, inhibin A, inhibin B, AMH and activin A were measured in the medium throughout culture. As human follicle maturity cannot be precisely identified in vitro, follicle growth, morphology and estrogen synthesis were used as markers of terminal follicle development. Once estradiol levels plateaued, luteinization was triggered with the addition of hCG and EGF. The in vitro luteal phase was monitored for approximately 15 days post-hCG (similar in duration to the human in vivo luteal phase), resulting in total culture times ranging from 40 to 65 days.
The patterns of follicle hormone secretion mimicked those observed in serum levels in vivo. As the follicles matured, inhibin A and estradiol levels progressively increased as would be expected during the follicular phase in vivo (Fig. 4A and C). Inhibin B was the predominant form of inhibin produced by small antral follicles, known to rise in the early to mid-follicular phase and decline in the later follicular phase (Fig. 4D). Post-hCG, progesterone and activin A levels increased (Fig. 4B and F), mimicking patterns found in human serum (Muttukrishna et al., 1996). Inhibin A levels dropped transiently after addition of hCG, but then rose and remained elevated throughout the luteal phase (Fig. 4C). In contrast, inhibin B levels remained low during the luteal phase (Fig. 4D). Moreover, cultured follicles replicated the luteal phase discordance of inhibin A and inhibin B unique to primates (Klein et al., 2004; Welt et al., 2005).
Figure 4.
eIVFG supports the complete hormone cycle in human follicles. Secondary human follicles were isolated and cultured in alginate from 40 to 65 days. Steroid and peptide hormones were quantified in the medium throughout the culture period. The culture time on the x-axis indicates the time of hCG addition (day 0, dotted line); follicles were cultured for 14–15 days post-hCG. The concentration of (A) estradiol, (B) progesterone, (C) inhibin A, (D) inhibin B, (E) AMH and (F) activin A are reported throughout culture. Data are expressed as mean ± SD for n = 5 follicles. Follicles were collected from the ovaries of three women and cultured individually.
AMH, a hormone used clinically as a surrogate marker of small follicles, reached its peak in the early follicular phase and continued to decline through the luteal phase (Fig. 4E). Human serum AMH levels remain constant throughout the 28-day cycle, as a result of the steady recruitment of immature follicles into the growing pool (Durlinger et al., 2002; Weenen et al., 2004). For individual follicles, however, it has been shown that non-human primate follicles synthesize AMH through the time of antrum formation (Xu et al., 2010). Our in vitro results are consistent with this finding; individually cultured human follicles produced AMH approximately through the time of antrum formation. This finding underscores the value of in vitro systems to reveal aspects of human follicle biology that cannot be explored in vivo. Moreover, with gonadotrophin stimulation mimicking the timing of pituitary secretion, human follicles grown in vitro supplied a similar pattern of steroid and peptide hormone secretion observed in vivo.
Discussion
The ovarian cycle involves dynamic hormone changes that influence all of female physiology. Further, the mid-cycle release of a fertilizable oocyte involves transformative changes within the follicle determining the timing and rate of reproduction. The study of these events poses significant challenges due to the structural and paracrine requirements of growing follicles. Within an eIVFG system, we have shown that individual follicles can mimic the events associated with follicular growth, ovulation and luteinization—the complete ovarian cycle.
The field of in vitro follicle growth includes several cellular and biomaterial-based systems that support 3D follicular structure. These include approaches using V-shaped wells, droplets covered with oil, culture inserts and hydrogel encapsulation (Torrance et al., 1989; Nayudu and Osborn 1992; Boland et al., 1993; Hartshorne et al., 1994; Abir et al., 1997, 2001; Pangas et al., 2003; Shea et al., 2014). Ovulation was reported in some of these systems with rupture rates between 7 and 30% (Boland et al., 1994; Hartshorne et al., 1994; Johnson et al., 1995). With such low ovulatory rates in these early reports, in vitro systems could not serve as reliable ovulation models. Rose et al. reported that follicles grown on culture inserts had rupture rates between 15 and 80%, depending on the final diameter (Rose et al., 1999). Herein we report that 96% of follicles grown in alginate ovulate. This improvement in ovulation rate may reflect higher follicle quality or improved maturation protocols. Another possibility is that removal from alginate encapsulation weakens the follicle wall. Moreover, we have shown that the morphological and transcriptional changes associated with this model are characteristic of in vivo ovulatory patterns. Further, this model provides a high-fidelity system to study ovulatory mechanisms independent of the ovarian surface epithelium, vasculature and inflammatory changes.
Given significant species differences in periovulatory regulation, developing improved primate models will be imperative to clinical translation. To date, luteinization studies in primates have employed primary 2D cell culture (Chaffin et al., 2003; Duffy and Stouffer 2003) or in vivo models using stimulation protocols (Chandrasekher et al., 1994; Young et al., 2003; Xu et al., 2011a). Herein we report that human follicles undergo luteinization when treated with hCG in the alginate culture system, which provides a new model to study primate luteinization in real-time. With minimal animal use and subject harm, individual follicles can be tracked throughout the periovulatory period. Furthermore, immature oocytes and eggs may simultaneously be collected for clinical or research purposes. This model system may thus be used to address important questions regarding primate luteinization, a necessary process to support pregnancy.
Limitations to our study include a small number of human follicles, which restricted studies to hormone analysis. Ovulation was not assessed in human follicles due to limited human tissue and loss of oocyte visibility during human follicle culture. Future studies will investigate the effects of follicle hormone dynamics on other tissues within larger co-culture and microfluidic systems.
In summary, we have shown that eIVFG is able to recapitulate the capstone events of the ovarian cycle including hormone production, follicle growth, differentiation and ovulation. Our success in culturing human and mouse follicles in a system that permits both follicular- and luteal-phase hormone synthesis yields an important investigative tool not only in reproduction, but also toxicology, endocrinology and physiology.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org.
Authors' roles
R.M.S. and T.K.W. were responsible for study conception and design. R.M.S. and Y.X. carried out follicle cultures and acquired hormone data. R.M.S. prepared the manuscript with T.K.W. and L.D.S. providing critical review.
Funding
This work was supported by NIH U54 HD041857, NIH U54 HD076188, NIH UH2 E5022920, NIH UH3 TR001207 and F30 AG040916 (R.M.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
The authors have no competing interests.
Supplementary Material
Acknowledgements
We thank Kelly Whelan and Sarah Kiesewetter for their invaluable technical support and Megan Romero for her expertise in tissue processing and immunohistochemistry. HRA Pharma provided UPA, obtained by MTA.
References
- Abir R, Franks S, Mobberley MA, Moore PA, Margara RA, Winston RM. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril 1997;68:682–688. [DOI] [PubMed] [Google Scholar]
- Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, Ben Rafael Z. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod 1999;14:1299–1301. [DOI] [PubMed] [Google Scholar]
- Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril 2001;75:141–146. [DOI] [PubMed] [Google Scholar]
- Araujo VR, Gastal MO, Wischral A, Figueiredo JR, Gastal EL. In vitro development of bovine secondary follicles in two- and three-dimensional culture systems using vascular endothelial growth factor, insulin-like growth factor-1, and growth hormone. Theriogenology 2014;82:1246–1253. [DOI] [PubMed] [Google Scholar]
- Boland NI, Humpherson PG, Leese HJ, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod 1993;48:798–806. [DOI] [PubMed] [Google Scholar]
- Boland NI, Humpherson PG, Leese HJ, Gosden RG. The effect of glucose metabolism on murine follicle development and steroidogenesis in vitro. Hum Reprod 1994;9:617–623. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Johansson BM, Sogn J, Janson PO. Characterization of an in vitro perfused rat ovary model: ovulation rate, oocyte maturation, steroidogenesis and influence of PMSG priming. Acta Physiol Scand 1987;130:107–114. [DOI] [PubMed] [Google Scholar]
- Cajander S, Janson PO, LeMaire WJ, Kallfelt BJ, Holmes PV, Ahren K, Bjersing L. Studies on the morphology of the isolated perfused rabbit ovary. II. Ovulation in vitro after HCG-treatment in vivo. Cell Tissue Res 1984;235:565–573. [DOI] [PubMed] [Google Scholar]
- Camboni A, Van Langendonckt A, Donnez J, Vanacker J, Dolmans MM, Amorim CA. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology 2013;67:64–69. [DOI] [PubMed] [Google Scholar]
- Camp TA, Rahal JO, Mayo KE. Cellular localization and hormonal regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNAs in the rat ovary. Mol Endocrinol 1991;5:1405–1417. [DOI] [PubMed] [Google Scholar]
- Canipari R, O'Connell ML, Meyer G, Strickland S. Mouse ovarian granulosa cells produce urokinase-type plasminogen activator, whereas the corresponding rat cells produce tissue-type plasminogen activator. J Cell Biol 1987;105:977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin CL, Brogan RS, Stouffer RL, VandeVoort CA. Dynamics of Myc/Max/Mad expression during luteinization of primate granulosa cells in vitro: association with periovulatory proliferation. Endocrinology 2003;144:1249–1256. [DOI] [PubMed] [Google Scholar]
- Chandrasekher YA, Hutchison JS, Zelinski-Wooten MB, Hess DL, Wolf DP, Stouffer RL. Initiation of periovulatory events in primate follicles using recombinant and native human luteinizing hormone to mimic the midcycle gonadotropin surge. J Clin Endocrinol Metab 1994;79:298–306. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. Luteinizing hormone acts directly at granulosa cells to stimulate periovulatory processes: modulation of luteinizing hormone effects by prostaglandins. Endocrine 2003;22:249–256. [DOI] [PubMed] [Google Scholar]
- Dunning KR, Akison LK, Russell DL, Norman RJ, Robker RL. Increased beta-oxidation and improved oocyte developmental competence in response to l-carnitine during ovarian in vitro follicle development in mice. Biol Reprod 2011;85:548–555. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction 2002;124:601–609. [DOI] [PubMed] [Google Scholar]
- Engelhardt H, Gore-Langton RE, Armstrong DT. Luteinization of porcine thecal cells in vitro. Mol Cell Endocrinol 1991;75:237–245. [DOI] [PubMed] [Google Scholar]
- Espey LL, Yoshioka S, Russell DL, Robker RL, Fujii S, Richards JS. Ovarian expression of a disintegrin and metalloproteinase with thrombospondin motifs during ovulation in the gonadotropin-primed immature rat. Biol Reprod 2000;62:1090–1095. [DOI] [PubMed] [Google Scholar]
- Fisher TE, Molskness TA, Villeda A, Zelinski MB, Stouffer RL, Xu J. Vascular endothelial growth factor and angiopoietin production by primate follicles during culture is a function of growth rate, gonadotrophin exposure and oxygen milieu. Hum Reprod 2013;28:3263–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod 2000;62:1322–1328. [DOI] [PubMed] [Google Scholar]
- Hartshorne GM, Sargent IL, Barlow DH. Meiotic progression of mouse oocytes throughout follicle growth and ovulation in vitro. Hum Reprod 1994;9:352–359. [DOI] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD, Woodruff TK. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction 2013;145:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem 1997;272:22389–22392. [DOI] [PubMed] [Google Scholar]
- Johnson LD, Albertini DF, McGinnis LK, Biggers JD. Chromatin organization, meiotic status and meiotic competence acquisition in mouse oocytes from cultured ovarian follicles. J Reprod Fertil 1995;104:277–284. [DOI] [PubMed] [Google Scholar]
- Kaynard AH, Periman LM, Simard J, Melner MH. Ovarian 3 beta-hydroxysteroid dehydrogenase and sulfated glycoprotein-2 gene expression are differentially regulated by the induction of ovulation, pseudopregnancy, and luteolysis in the immature rat. Endocrinology 1992;130:2192–2200. [DOI] [PubMed] [Google Scholar]
- Kedem A, Hourvitz A, Fisch B, Shachar M, Cohen S, Ben-Haroush A, Dor J, Freud E, Felz C, Abir R. Alginate scaffold for organ culture of cryopreserved-thawed human ovarian cortical follicles. J Assist Reprod Genet 2011;28:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell WR, Balogh K, Jr, Wiest WG. Effects of luteinizing hormones on glucose-6-phosphate and 20-alpha-hydroxysteroid dehydrogenase activities in superovulated rat ovaries. Endocrinology 1966;79:352–361. [DOI] [PubMed] [Google Scholar]
- Kim AM, Tingen CM, Woodruff TK. Sex bias in trials and treatment must end. Nature 2010;465:688–689. [DOI] [PubMed] [Google Scholar]
- Klein NA, Houmard BS, Hansen KR, Woodruff TK, Sluss PM, Bremner WJ, Soules MR. Age-related analysis of inhibin A, inhibin B, and activin a relative to the intercycle monotropic follicle-stimulating hormone rise in normal ovulatory women. J Clin Endocrinol Metab 2004;89:2977–2981. [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 2006;27:714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardsson G, Peng XR, Liu K, Nordstrom L, Carmeliet P, Mulligan R, Collen D, Ny T. Ovulation efficiency is reduced in mice that lack plasminogen activator gene function: functional redundancy among physiological plasminogen activators. Proc Natl Acad Sci USA 1995;92:12446–12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meidan R, Girsh E, Blum O, Aberdam E. In vitro differentiation of bovine theca and granulosa cells into small and large luteal-like cells: morphological and functional characteristics. Biol Reprod 1990;43:913–921. [DOI] [PubMed] [Google Scholar]
- Muttukrishna S, Fowler PA, George L, Groome NP, Knight PG. Changes in peripheral serum levels of total activin A during the human menstrual cycle and pregnancy. J Clin Endocrinol Metab 1996;81:3328–3334. [DOI] [PubMed] [Google Scholar]
- Nayudu PL, Osborn SM. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil 1992;95:349–362. [DOI] [PubMed] [Google Scholar]
- Oonk RB, Krasnow JS, Beattie WG, Richards JS. Cyclic AMP-dependent and -independent regulation of cholesterol side chain cleavage cytochrome P-450 (P-450scc) in rat ovarian granulosa cells and corpora lutea. cDNA and deduced amino acid sequence of rat P-450scc. J Biol Chem 1989;264:21934–21942. [PubMed] [Google Scholar]
- Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng 2003;9:1013–1021. [DOI] [PubMed] [Google Scholar]
- Park OK, Mayo KE. Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol 1991;5:967–978. [DOI] [PubMed] [Google Scholar]
- Picton HM, Gosden RG. In vitro growth of human primordial follicles from frozen-banked ovarian tissue. Mol Cell Endocrinol 2000;166:27–35. [DOI] [PubMed] [Google Scholar]
- Richards JS, Hedin L, Caston L. Differentiation of rat ovarian thecal cells: evidence for functional luteinization. Endocrinology 1986;118:1660–1668. [DOI] [PubMed] [Google Scholar]
- Rose UM, Hanssen RG, Kloosterboer HJ. Development and characterization of an in vitro ovulation model using mouse ovarian follicles. Biol Reprod 1999;61:503–511. [DOI] [PubMed] [Google Scholar]
- Sanchez F, Romero S, Albuz FK, Smitz J. In vitro follicle growth under non-attachment conditions and decreased FSH levels reduces Lhcgr expression in cumulus cells and promotes oocyte developmental competence. J Assist Reprod Genet 2012;29:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea LD, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Annu Rev Biomed Eng 2014;16:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GM, Rossetto R, Chaves RN, Duarte AB, Araujo VR, Feltrin C, Bernuci MP, Anselmo-Franci JA, Xu M, Woodruff TK, et al. In vitro development of secondary follicles from pre-pubertal and adult goats cultured in two-dimensional or three-dimensional systems. Zygote 2014:1–10 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- Songsasen N, Woodruff TK, Wildt DE. In vitro growth and steroidogenesis of dog follicles are influenced by the physical and hormonal microenvironment. Reproduction 2011;142:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagler D, Tu T, Smith RM, Anderson NR, Tingen CM, Woodruff TK, Shea LD. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng Part A 2012;18:1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril 2013;99:1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer EE, Binnie JP, McCaffery FH, Campbell BK. In vitro development of oocytes from porcine and bovine primary follicles. Mol Cell Endocrinol 2000;163:117–123. [DOI] [PubMed] [Google Scholar]
- Tingen CM, Kim AM, Wu PH, Woodruff TK. Sex and sensitivity: the continued need for sex-based biomedical research and implementation. Womens Health (Lond Engl) 2010;6:511–516. [DOI] [PubMed] [Google Scholar]
- Tingen CM, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, Shea L, Woodruff TK. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction 2011;141:809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance C, Telfer E, Gosden RG. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J Reprod Fertil 1989;87:367–374. [DOI] [PubMed] [Google Scholar]
- Vanacker J, Luyckx V, Dolmans MM, Des Rieux A, Jaeger J, Van Langendonckt A, Donnez J, Amorim CA. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 2012;33:6079–6085. [DOI] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998;93:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TR, Yan LY, Yan J, Lu CL, Xia X, Yin TL, Zhu XH, Gao JM, Ding T, Hu WH, et al. Basic fibroblast growth factor promotes the development of human ovarian early follicles during growth in vitro. Hum Reprod 2014;29:568–576. [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 2004;10:77–83. [DOI] [PubMed] [Google Scholar]
- Welt CK, Hall JE, Adams JM, Taylor AE. Relationship of estradiol and inhibin to the follicle-stimulating hormone variability in hypergonadotropic hypogonadism or premature ovarian failure. J Clin Endocrinol Metab 2005;90:826–830. [DOI] [PubMed] [Google Scholar]
- West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod 2009;80:432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Emery BR, Carrell DT. In vitro growth, maturation, fertilization, and embryonic development of oocytes from porcine preantral follicles. Biol Reprod 2001;64:375–381. [DOI] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng 2006a;12:2739–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod 2006b;75:916–923. [DOI] [PubMed] [Google Scholar]
- Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod 2009a;24:2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod 2009b;81:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction 2010;140:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Stouffer RL, Muller J, Hennebold JD, Wright JW, Bahar A, Leder G, Peters M, Thorne M, Sims M, et al. Dynamics of the transcriptome in the primate ovulatory follicle. Mol Hum Reprod 2011a;17:152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod 2011b;26:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, Kiesewetter SE, Shea LD, Woodruff TK. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod 2011c;84:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, Zelinski MB. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod 2013a;28:2187–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu M, Bernuci MP, Fisher TE, Shea LD, Woodruff TK, Zelinski MB, Stouffer RL. Primate follicular development and oocyte maturation in vitro. Adv Exp Med Biol 2013b;761:43–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka S, Ochsner S, Russell DL, Ujioka T, Fujii S, Richards JS, Espey LL. Expression of tumor necrosis factor-stimulated gene-6 in the rat ovary in response to an ovulatory dose of gonadotropin. Endocrinology 2000;141:4114–4119. [DOI] [PubMed] [Google Scholar]
- Young KA, Chaffin CL, Molskness TA, Stouffer RL. Controlled ovulation of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod 2003;18:2257–2263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.