Abstract
STUDY QUESTION
Is consumption of fruits and vegetables with high levels of pesticide residues associated with lower semen quality?
SUMMARY ANSWER
Consumption of fruits and vegetables with high levels of pesticide residues was associated with a lower total sperm count and a lower percentage of morphologically normal sperm among men presenting to a fertility clinic.
WHAT IS KNOWN ALREADY
Occupational and environmental exposure to pesticides is associated with lower semen quality. Whether the same is true for exposure through diet is unknown.
STUDY DESIGN, SIZE, DURATION
Men enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort at an academic medical fertility center. Male partners (n = 155) in subfertile couples provided 338 semen samples during 2007–2012.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Semen samples were collected over an 18-month period following diet assessment. Sperm concentration and motility were evaluated by computer-aided semen analysis (CASA). Fruits and vegetables were categorized as containing high or low-to-moderate pesticide residues based on data from the annual United States Department of Agriculture Pesticide Data Program. Linear mixed models were used to analyze the association of fruit and vegetable intake with sperm parameters accounting for within-person correlations across repeat samples while adjusting for potential confounders.
MAIN RESULTS AND THE ROLE OF CHANCE
Total fruit and vegetable intake was unrelated to semen quality parameters. High pesticide residue fruit and vegetable intake, however, was associated with poorer semen quality. On average, men in highest quartile of high pesticide residue fruit and vegetable intake (≥1.5 servings/day) had 49% (95% confidence interval (CI): 31%, 63%) lower total sperm count and 32% (95% CI: 7%, 58%) lower percentage of morphologically normal sperm than men in the lowest quartile of intake (<0.5 servings/day) (P, trend = 0.003 and 0.02, respectively). Low-to-moderate pesticide residue fruit and vegetable intake was associated with a higher percentage of morphologically normal sperm (P, trend = 0.04).
LIMITATIONS, REASONS FOR CAUTION
Surveillance data, rather than individual pesticide assessment, was used to assess the pesticide residue status of fruits and vegetables. CASA is a useful method for clinical evaluation but may be considered less favorable for accurate semen analysis in the research setting. Owing to the observational nature of the study, confirmation is required by interventional studies as well.
WIDER IMPLICATIONS OF THE FINDINGS
To our knowledge, this is the first report on the consumption of fruits and vegetables with high levels of pesticide residue in relation to semen quality. Further confirmation of these findings is warranted.
STUDY FUNDING/COMPETING INTEREST(S)
Supported by National Institutes of Health grants ES009718, ES022955, ES000002, P30 DK046200 and Ruth L. Kirschstein National Research Service Award T32 DK007703-16. None of the authors has any conflicts of interest to declare.
Keywords: fruits and vegetables, pesticide, semen quality
Introduction
Infertility affects 12–16% of couples during the reproductive lifespan (Louis et al., 2013; Thoma et al., 2013), and male factor infertility is the sole etiology in up to 30% of couples seeking assistance with conception (Anderson et al., 2009). In addition, some data show a downward trend over time in semen quality (Swan et al., 2000), raising concerns that semen quality could be falling to levels which could affect fecundity at a population level. Among potential reproductive toxicants, pesticides may partially explain the decline in semen quality. In the 1970s, a number of cases of infertility were discovered in a pesticide factory and the observed effects, including azoospermia, oligospermia and higher serum levels of FSH and LH, appeared to be related to the longer occupational exposure to 1,2-dibromo-3-chloropropane (DBCP) (Whorton et al., 1977). More recent reports suggest that even low levels of pesticide exposure may have anti-androgenic effects (Kelce et al., 1994; Perry et al., 2011), potentially impairing human spermatogenesis. Two systematic literature reviews concluded that pesticide exposure, whether occupational or environmental, might be linked to decreased semen quality parameters, particularly sperm concentration (Perry, 2008; Martenies and Perry, 2013). Yet, studies directly addressing the impact of dietary pesticide exposure on male reproductive function are scarce.
Consumption of conventionally grown fruits and vegetables is a major source of non-occupational pesticide exposure. Previous studies have shown that vegetable intake is positively related to urinary metabolite levels of pyrethroid pesticides (Fortes et al., 2013) and that substituting conventionally grown produce with organic produce dramatically decreases the urinary metabolite levels of organophosphorus pesticides (Lu et al., 2006). In this study, we investigated the association of consumption of fruits and vegetables and their pesticide residues with semen quality. To accomplish this goal, we developed a novel approach to classify fruits and vegetables into high versus low-to-moderate pesticide residue groups based on data from the United States Department of Agriculture (USDA) Pesticide Data Program (PDP), a surveillance program that provides nationally representative data on pesticide residues in the US food supply (US Department of Agriculture, 2006–2012). We then evaluated the hypothesis that intake of fruits and vegetables with high pesticide residues is associated with poor semen quality (among men attending a fertility clinic).
Materials and Methods
Study population
The Environment and Reproductive Health (EARTH) Study is an ongoing prospective cohort study started in 2006 that recruits couples presenting to the Massachusetts General Hospital Fertility Center (Boston, MA, USA). Men are eligible if they are aged 18–55 years, without history of vasectomy, and are in a couple planning to use their own gametes for fertility treatment. In April 2007, diet assessment using a food frequency questionnaire (FFQ) was introduced into the study. For this analysis, we included men who completed the FFQ and subsequently provided semen samples up to 18 months after the completion of the FFQ. Of 246 men who met eligibility criteria, 58 men were excluded due to missing information on dietary questionnaire, 27 men were excluded because their semen analyses predated dietary assessment, 5 men were excluded because semen analysis data were incomplete (4 missing morphology; 1 missing concentration) and 1 man was excluded due to azoospermia. Of 385 semen samples from the 155 men retained in the analysis, we excluded 47 samples which were obtained more than 18 months after diet assessment to minimize misclassification due to changes in diet over time. Men excluded from the analysis were not significantly different from included men in terms of age, BMI, race, physical activity and reproductive history. After these exclusions, a total of 338 semen samples collected from 155 men between 2007 and 2012 were included in the analysis; 57 men contributed one sample to the analysis, 51 men provided two samples and the remaining 47 men provided three or more semen samples (maximum = 6 samples).
Men underwent an anthropometric assessment and completed a nurse-administered questionnaire at entry in which basic demographic and reproductive history data were collected. Participants also completed a detailed take-home questionnaire, which contained questions on various lifestyle factors and medical and reproductive history. The study was approved by the Human Subjects Committee of the Harvard School of Public Health and the Massachusetts General Hospital; informed consent was obtained from all participants.
Dietary assessment
Diet was assessed using a validated 131-item FFQ (Rimm et al., 1992). Men were asked to report how often, on average, they had consumed specified amounts of each food, beverage and supplement in the questionnaire over the past year. The serving sizes for fruits and vegetables were described specifically for each item in the FFQ using standard portion sizes (e.g. one apple, half avocado) or volumes (e.g. half cup of broccoli). In a validation study in a different cohort, the de-attenuated correlation (i.e. observed correlation corrected for random within-person variability) between two, 1-week diet records (Feskanich et al., 1993) and FFQ reports ranged from 0.27 for spinach to 0.95 for cantaloupe (mean r = 0.57). Two data-derived dietary pattern scores (Gaskins et al., 2012) were used to summarize overall food choices. Specifically, scores for a ‘prudent pattern’ (characterized by high intakes of fish, chicken, fruit, cruciferous vegetables, tomatoes, leafy green vegetables, legumes and whole grains) and a ‘western pattern’ (characterized by high intakes of red meat, processed meat, butter, high-fat dairy, refined grains, pizza, snacks, high-energy drinks, mayonnaise and sweets) were calculated for each subject using principal components analysis.
Pesticide residue assessment
We used the annual reports from the USDA PDP to classify fruits and vegetables according to their average pesticide residue status in the US food supply (US Department of Agriculture, 2006–2012). The PDP reports included data on pesticide residues for 35 of the 38 fruit and vegetable items included on the FFQ; data on pesticide residues for apricots, Brussels sprouts and mixed vegetables, which accounted for 4% of total fruit and vegetable intake, were unavailable, thereby excluding these foods from the pesticide classification. We considered three measures from the PDP to classify fruits and vegetables: (i) the percentage of samples tested with any detectable pesticides; (ii) the percentage of samples tested with pesticides exceeding the tolerance level and (iii) the percentage of samples with three or more types of detectable pesticides. The USDA does not sample every food every year, and therefore data were collected and averaged by annual reports from 2006 to 2012. When a FFQ item combined more than one food for which the PDP reported data separately (e.g. eggplant and summer squash) we used the weighted average of pesticide residue according to the ratio of consumption of each produce from the USDA reports (Gebhardt et al., 2008). Next, foods were categorized according to tertiles for each of the three measurements of contamination and assigned a score of 0 to each fruit and vegetable in the bottom tertile, 1 to fruits and vegetables in the middle tertile and 2 for fruits and vegetables in the top tertile. Scores for each fruit and vegetable were then summed across the three contamination measures. Fruits and vegetables with a total score ≥4 (i.e. at least one of three measurements was in the third tertile) were considered to be high pesticide residue foods while fruits and vegetables with a total score <4 were considered low-to-moderate pesticide residue foods. Based on these criteria 14 fruits and vegetables were categorized as high pesticide residue produce, and 21 as low-to-moderate pesticide produce (Supplementary data, Table S1). To evaluate the robustness of the pesticide residue classification we conducted sensitivity analyses where we modified the criteria for pesticide residue classification. First we created a score using the median level as cutoff for the three measures of contamination. The Spearman correlation between the pesticide contamination score based on tertile cutoffs and the score based on median cutoffs was 0.99. We also created a score where each food was ranked according to each measurement of contamination and the average rank across the three measures was calculated for each food. The Spearman correlation between the pesticide contamination score based on tertile cutoffs and the score based on average rankings was 0.92.
Semen analysis
Semen samples were obtained on site by masturbation into a sterile container. Men were instructed to abstain from ejaculation for at least 48 h, but no more than 5 days, before producing the semen sample. Samples were liquefied at 37°C for 20 min before analysis. Ejaculate volume was measured with a graduated serological pipet. Sperm concentration and motility were evaluated by computer-aided semen analysis (CASA; Hamilton-Thorne Biosciences Ceros, version 14), which correlates well with results by manual analysis (Akashi et al., 2010) and is considered adequate for routine diagnostic applications (World Health Organization, 2010). The percentage of sperm motility was classified as progressive and total (progressive + non-progressive) (World Health Organization, 2010). Sperm morphology was determined using Kruger's strict criteria (Kruger et al., 1988). Total sperm count (millions) was calculated as sperm concentration × ejaculate volume. Total normal count (millions) was defined as concentration × ejaculate volume × % morphologically normal, and total motile count (million) as concentration × ejaculate volume × % motile. In addition, we dichotomized semen quality into equal to or above versus below the World Health Organization (Whorton et al.) lower reference limits (<39 million per ejaculate for total sperm count, <15 million per ml for sperm concentration, <40% progressive and non-progressive motility for sperm motility, <4% morphologically normal sperm for morphology and <1.5 ml for ejaculate volume) (World Health Organization, 2010). We further defined excellent sperm quality samples as those with all semen parameters above the WHO lower reference limits.
Statistical analysis
Men were classified according to quartiles of total, high pesticide residue and low-to-moderate pesticide residue fruit and vegetable intake. The Kruskal–Wallis and Fisher's exact tests were used to evaluate differences in baseline characteristics according to fruit and vegetable intake for continuous measures and categorical variables, respectively. To evaluate the relation of fruit and vegetable intake with semen quality parameters, generalized linear mixed models with random intercepts, identity link for continuous outcomes or logit link for binary outcomes, were used to account for within-person correlations between repeated samples of the same individual. Robust estimators of the variance were used to compute 95% confidence intervals (CIs). Population marginal means were utilized to present population averages adjusted for the covariates in the model (Searle et al., 1980). Sperm concentration, total sperm count, total normal count and total motile count were log-transformed to meet normality assumptions of linear regression. Results for these parameters were back transformed to improve interpretability. Tests for linear trend were performed using median intake of fruits and vegetables in each quartile as a continuous variable. Non-linearity was also examined by fitting models with linear and quadratic terms.
Factors previously reported to be associated with semen quality as well as factors related to fruit and vegetable intake at P < 0.20, and factors that changed the exposure coefficient by more than 15% were considered as potential confounders. In addition, we also decided a priori that certain terms would be included regardless whether they met statistical properties of a confounder. Specifically, abstinence time was included regardless of statistical significance since this is a well-known predictor of most semen quality parameters thus helping to reduce the amount of unexplained random variability in the model (Schisterman et al., 2009). In addition, terms for smoking, BMI and dietary patterns were included regardless of statistical significance since these represent the best characterized modifiable risk factors for low semen quality (smoking and BMI) and in order to distinguish relations between fruit and vegetable intake from relations due to overall food choices. Based on these criteria, models were adjusted for age, BMI (kg/m2), smoking status (current/former or never), abstinence time (<2 days, 2–3 days, 3–4 days, >4 days, missing), moderate-to-vigorous physical activity (h/week), total energy intake (kcal/day), prudent and western dietary pattern scores (continuous) and history of varicocele (yes or no). The model for high pesticide residue fruit and vegetable intake was additionally adjusted for low-to-moderate pesticide fruit and vegetable intake, and vice versa. We further examined the possibility of residual confounding by dietary factors previously related to semen quality in this cohort (Chavarro et al., 2011; Attaman et al., 2012; Afeiche et al., 2014a,b).
To evaluate the robustness of our findings, we restricted the analyses to the first semen sample for each man and, separately, to samples collected within 1 year of FFQ completion. Effect modification by BMI (<25 and ≥25 kg/m2), smoking status (current/former and never smokers), study period (before and after 2010) and physical activity (moderate-to-vigorous activity <5.2 and ≥5.2 h/week) were tested using cross-product terms in the final model. Statistical analyses were performed with SAS v9.4 (SAS Institute, Cary, NC, USA). Two-sided P values <0.05 were considered significant.
Results
The median age was 36 years (range, 26–51). Most men were Caucasian (83%) and non-smokers (63%); 52% were overweight (25 ≤ BMI < 30 kg/m2) and 18% were obese (BMI ≥ 30 kg/m2). Forty-six percent of men had at least one semen analysis with a semen parameter below WHO lower reference limits. The median (25th, 75th percentile) values of semen parameters were 56.4 × 106/ml (26.1, 108.8) for concentration, 47% (25, 65) for total motility, 6.0% (4.0, 8.0) for normal sperm morphology and 2.6 ml (1.7, 3.8) for ejaculate volume. The median time (25th, 75th percentile) between diet assessment and semen sample collection was 158 (82 258) days for the first semen sample, and 266 (160 408) days for each man's last semen sample. The number of semen samples provided was not related to semen parameters, male infertility diagnosis or previous infertility examination (P > 0.10 in all cases). The median (25th, 75th percentile) intake of fruits and vegetables was 3.5 (2.2, 4.9) servings/day. Men had an average of 0.9 (0.6, 1.5) servings/day of high pesticide residue produce and 2.3 (1.4, 3.1) servings/day of low-to-moderate pesticide residue produce. Men who consumed more high pesticide residue fruits and vegetables tended to be older and have higher Prudent pattern scores, total caloric intake and physical activity (Table I).
Table I.
Baseline characteristics of the study population according to quartiles of fruit and vegetable intake.
| Characteristics | High pesticide residue fruit and vegetable intake |
|||||
|---|---|---|---|---|---|---|
| Total | Q1 | Q2 | Q3 | Q4 | P valuea | |
| Number of men | 155 | 38 | 39 | 39 | 39 | |
| Median, serving/d | 0.9 | 0.4 | 0.7 | 1.1 | 2.1 | |
| Range (min, max) | 0.2, 3.6 | 0.2, 0.6 | 0.6, 0.9 | 0.9, 1.5 | 1.5, 3.6 | |
| Median (IQR) or n (%) | ||||||
| Demographics | ||||||
| Age, years | 36.1 (33.0, 39.2) | 33.9 (31.8, 38.4) | 34.8 (32.9, 38.8) | 36.7 (33.8, 38.7) | 36.7 (35.1, 41.4) | 0.01 |
| BMI, kg/m2 | 27.0 (24.2,29.1) | 27.8 (26, 29.2) | 26.7 (23.7, 29.1) | 26.9 (24.2, 28.9) | 29.5 (23.7, 29.5) | 0.58 |
| Never smokers, n% | 98 (63) | 19 (50) | 26 (66.7) | 27 (69.2) | 26 (66.7) | 0.28 |
| Moderate-vigorous exercise, h/week | 3.2 (1.0, 7.2) | 2.4 (0.3, 4) | 2.5 (0.9, 5.7) | 5.0 (1.2, 7.5) | 4.0 (1.0, 8.0) | 0.11 |
| White, n% | 129 (83) | 34 (89.5) | 32 (82.1) | 32 (82.1) | 31 (79.5) | 0.68 |
| Abstinence time, hours | 58.5 (45.5, 81.0) | 51.0 (36.0, 81.0) | 61.0 (49.0, 77.0) | 56.5 (47.5, 81.5) | 56.5 (47.0, 84.0) | 0.78 |
| Time from FFQ completion to first semen collection, days | 158 (82, 258) | 185 (80, 248) | 112 (51, 296) | 118 (39, 253) | 199 (127, 256) | 0.31 |
| Season of sample collection (338 semen samples) | 0.72 | |||||
| Spring, n% | 79 (23) | 25 (30) | 18 (19) | 22 (25) | 14 (20) | |
| Summer, n% | 73 (22) | 15 (18) | 21 (22) | 20 (22) | 17 (24) | |
| Fall, n% | 92 (27) | 25 (30) | 27 (28) | 20 (22) | 20 (28) | |
| Winter, % | 94 (28) | 18 (22) | 29 (31) | 27 (30) | 20 (28) | |
| Diet | ||||||
| Alcohol, g/day | 9.9 (2.6, 19.3) | 9.7 (2.9, 15.8) | 10.6 (2.9, 23.5) | 9.9 (5.2, 17.3) | 8.9 (1.4, 17.6) | 0.67 |
| Caffeine, g/day | 143 (55, 245) | 114 (53, 224) | 151 (99, 281) | 243 (123, 299) | 120 (40, 244) | 0.007 |
| High-fat dairy intake, serv/d | 1.0 (0.6, 1.7) | 0.9 (0.6, 1.7) | 0.9 (0.6, 1.7) | 1.0 (0.6, 1.3) | 1.0 (0.5, 1.9) | 0.95 |
| Low-fat dairy intake, serv/d | 0.8 (0.3, 1.2) | 0.5 (0.1, 0.9) | 0.6 (0.2, 1.1) | 1.0 (0.6, 1.5) | 0.9 (0.3, 1.3) | 0.01 |
| Processed meat intake, serv/d | 0.4 (0.2, 0.6) | 0.4 (0.3, 0.6) | 0.4 (0.2, 0.6) | 0.4 (0.2, 0.5) | 0.4 (0.2, 0.7) | 0.72 |
| Total fish intake, serv/d | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.2) | 0.2 (0.2, 0.3) | 0.3 (0.1, 0.3) | 0.2 (0.1, 0.3) | 0.08 |
| Total carbohydrate, % energy | 48.3 (42.5, 54.6) | 46.9 (42.8, 55.8) | 46.4 (41.6, 53.5) | 50.9 (45, 54.4) | 49.5 (45, 56.4) | 0.22 |
| Total protein, % energy | 16.1 (14.5, 17.6) | 15.9 (13.6, 17.3) | 15.9 (14.5, 17.3) | 16.6 (15.2, 17.9) | 15.9 (14.7, 18.6) | 0.51 |
| Total fat, % energy | 32.0 (27.4, 35.6) | 32.3 (27.7, 35.4) | 32.8 (29.4, 36.8) | 30.5 (26.6, 33.2) | 33 (26.7, 35.7) | 0.41 |
| Total energy intake, kcal/day | 2033 (1634, 2486) | 1769 (1339, 2384) | 1913 (1429, 2258) | 2096 (1794, 2528) | 2258 (1879, 2808) | 0.005 |
| Prudent pattern scoreb | −0.1 (−0.7, 0.5) | −0.9 (−1.3, −0.6) | −0.5 (−0.7, −0.2) | 0.1 (−0.1, 0.5) | 1.0 (0.3, 1.7) | <0.0001 |
| Western pattern scoreb | −0.2 (−0.7, 0.5) | −0.2 (−0.7, 0.5) | −0.1 (−0.8, 0.7) | −0.2 (−0.9, 0.5) | −0.2 (−0.4, 0.6) | 0.80 |
| Self-reported reproductive history, n% | ||||||
| Male factor infertility diagnosis | 44 (28) | 9 (24) | 13 (33.3) | 9 (23.1) | 13 (33) | 0.86 |
| Previous infertility exam | 118 (76) | 26 (68.4) | 29 (74.4) | 32 (82.1) | 31 (79.5) | 0.52 |
| History of cryptorchidism | 6 (4) | 1 (2.6) | 2 (5.1) | 2 (5.1) | 1 (2.6) | 1.00 |
| History of varicocele | 15 (10) | 6 (15.8) | 5 (12.8) | 3 (7.7) | 1 (2.6) | 0.17 |
| Any reproductive surgeryc | 6 (5) | 2 (6.9) | 1 (3.2) | 3 (11.1) | 0 (0) | 0.36 |
IQR, interquartile range; FFQ, food frequency questionnaire; serv/d, serving/day.
aFor continuous variables, Kruskal–Wallis analyses were used to test for associations across quartiles of fruit and vegetable intake. For categorical variables, Fisher's exact tests were used to test the associations between quartiles of fruit and vegetable intake.
bDietary patterns were constructed by factor analysis as described in Gaskins et al. (2012). A higher score indicates higher adherence to the prudent or western dietary patterns.
cReport of any of the following: orchidopexy, varicocelectomy, hydrocelectomy, hernia repair, urethral repair, hypospadias repair, sympathectomy, bladder neck surgery or other reproductive surgery.
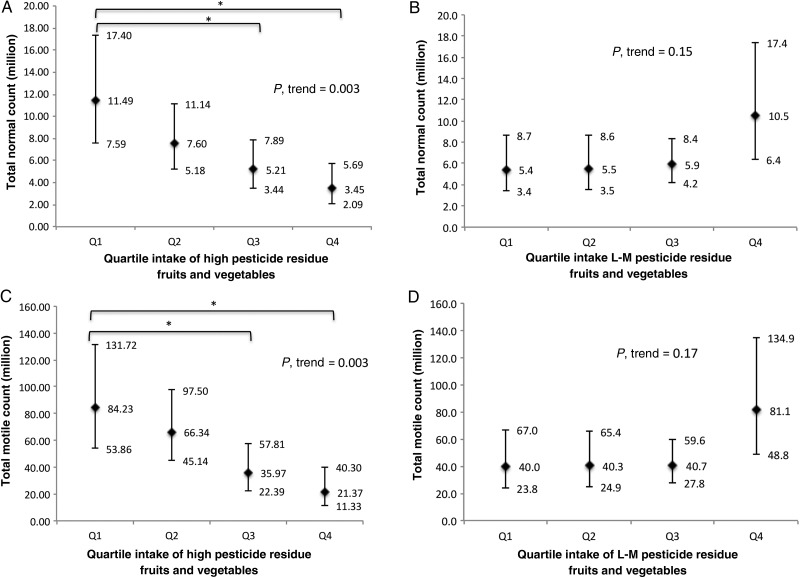
Total fruit and vegetable intake was not associated with semen quality (Table II). There were, however, inverse relations between intake of high pesticide residue fruits and vegetables and semen quality (Table III). On average, men in the highest quartile of high pesticide residue fruits and vegetables had 49% (95% CI: 31, 63) lower total sperm count, 32% (95% CI: 7, 58) fewer morphologically normal sperm and 29% (95% CI: 7, 52) lower ejaculate volume than men in the lowest quartile of intake. Furthermore, intake of high pesticide fruits and vegetables was associated with a significantly lower total motile count (P = 0.003) and lower total normal count (P = 0.003) (Fig. 1). On the other hand, there was a significant linear trend towards increasing percentage with morphologically normal sperm with higher intakes of low-to-moderate pesticide residue fruits and vegetables (P, trend = 0.04) (Table III). Intake of low-to-moderate pesticide residue fruits and vegetables was unrelated to other semen quality parameters.
Table II.
Adjusted semen quality parameters [mean (95% CI)] according to the intake of fruits and vegetables.
| Q1 | Q2 | Q3 | Q4 | P valuea | |
|---|---|---|---|---|---|
| Number of men | 38 | 39 | 39 | 39 | |
| Median intake of fruits and vegetables (min, max) | 1.5 (0.6, 2.2) | 2.8 (2.2, 3.5) | 4.1 (3.5, 4.8) | 6.0 (4.9, 12.9) | |
| Total sperm count (millions) | |||||
| Model 1b | 140 (107, 183) | 144 (107, 193) | 106 (81, 139) | 109 (83, 143) | 0.14 |
| Model 2c | 122 (89, 166) | 144 (109, 189) | 107 (86, 134) | 133 (93, 191) | 0.95 |
| Sperm concentration (millions/ml) | |||||
| Model 1b | 52.7 (38.9, 71.2) | 55.1 (40.8, 74.4) | 38.7 (30.1, 49.7) | 53.8 (40.7, 71.2) | 0.83 |
| Model 2c | 50.2 (35.2, 71.5) | 55.0 (41.1, 73.7) | 38.3 (30.6, 47.9) | 59.3 (41.1, 85.7) | 0.93 |
| Total motility (% PR + NP) | |||||
| Model 1b | 47.9 (40.5, 55.3) | 45.9 (38.4, 53.5) | 43.2 (36.6, 49.9) | 46.1 (39.5, 52.7) | 0.67 |
| Model 2c | 44.7 (36.5, 52.9) | 45.6 (38.6, 52.7) | 43.5 (37.3, 49.7) | 51.5 (41.1, 61.8) | 0.50 |
| Progressive motility (% PR) | |||||
| Model 1b | 27.3 (22.6, 32.0) | 25.5 (20.8, 30.3) | 27.3 (21.2, 33.4) | 26.2 (22.1, 30.3) | 0.84 |
| Model 2c | 26.2 (21.1, 31.4) | 25.8 (21.4, 30.3) | 27.3 (21.2, 33.4) | 28.0 (21.5, 34.4) | 0.66 |
| Sperm morphology (% normal) | |||||
| Model 1b | 5.9 (4.9, 6.9) | 5.9 (4.8, 7.0) | 6.5 (5.5, 7.5) | 6.9 (5.8, 7.9) | 0.14 |
| Model 2c | 6.2 (4.8, 7.6) | 6.0 (4.9, 7.2) | 6.4 (5.5, 7.2) | 6.6 (5.1, 8.1) | 0.71 |
| Ejaculate volume (ml) | |||||
| Model 1b | 3.0 (2.6, 3.4) | 2.8 (2.5, 3.2) | 3.1 (2.6, 3.5) | 2.4 (2.0, 2.8)* | 0.06 |
| Model 2c | 2.9 (2.3, 3.4) | 2.9 (2.5, 3.2) | 3.1 (2.7, 3.5) | 2.5 (2.0, 3.0) | 0.55 |
PR, progressive motility; NP, non-progressive motility; CI: confidence interval.
aEstimated using median intake in each quartile as a continuous variable.
bModel 1 was adjusted for total energy intake, abstinence time (missing, 0–2 days, 2–3 days, 3–4 days and ≥4 days).
cModel 2 was adjusted for total energy intake, abstinence time, age, BMI, moderate-vigorous physical activity, race, prudent and western dietary patterns, smoking status and history of varicocele.
*P-value for trend <0.05 compared with men in the lowest quartile of intake.
Table III.
Adjusteda semen quality parameters [mean (95% CI)] according to the intake of high or low-to-moderate pesticide residue fruits and vegetables.
| Quartile intake (range, servings/day) | Total sperm count (millions) | Sperm concentration (millions/ml) | Total motility (% PR + NP) | Progressive motility (% PR) | Sperm morphology (% normal) | Ejaculate volume (ml) |
|---|---|---|---|---|---|---|
| Quartile of high pesticide residue fruit and vegetable intakeb | ||||||
| Q1 [0.2, 0.6] | 171 (133, 222) | 66.1 (49.1, 90.2) | 51.7 (44.3, 59.0) | 29.4 (24.4, 34.4) | 7.5 (6.3, 8.7) | 3.0 (2.6, 3.5) |
| Q2 [0.6, 0.9] | 156 (123, 197) | 55.4 (43.5, 70.7) | 48.0 (42.2, 53.8) | 26.9 (23.2, 30.7) | 6.4 (5.2, 7.6) | 3.1 (2.7, 3.5) |
| Q3 [0.9, 1.5] | 103 (81, 132)* | 37.6 (28.5, 49.5)* | 45.4 (38.5, 52.3) | 25.9 (21.3, 30.5) | 6.0 (5.1, 7.0) | 3.0 (2.7, 3.3) |
| Q4 [1.5, 3.6] | 86 (63, 118)* | 43.4 (31.7, 59.6) | 38.9 (29.5, 48.4) | 24.8 (16.5, 33.0) | 5.1 (3.9, 6.2)* | 2.2 (1.7, 2.6)* |
| P, trendc | 0.003 | 0.14 | 0.07 | 0.47 | 0.02 | 0.003 |
| Quartile of low-to-moderate pesticide residue fruit and vegetable intaked | ||||||
| Q1 [0.2, 1.4] | 119 (89, 158) | 49.8 (35.6, 69.5) | 44.7 (37.0, 52.4) | 26.8 (21.4, 32.2) | 5.7 (4.5, 6.9) | 2.8 (2.3, 3.3) |
| Q2 [1.4, 2.3] | 135 (101, 180) | 47.0 (34.8, 63.3) | 39.7 (33.1, 46.4) | 24.1 (17.8, 30.4) | 5.4 (4.4, 6.4) | 3.1 (2.8, 3.5) |
| Q3 [2.3, 3.1] | 109 (87, 136) | 42.7 (33.4, 54.6) | 46.0 (39.4, 52.6) | 26.6 (22.1, 31.2) | 6.6 (5.6, 7.6) | 2.8 (2.4, 3.3) |
| Q4 [3.1, 8.8] | 147 (108, 200) | 64.3 (46.0, 90.0) | 56.4 (47.3, 65.5) | 30.5 (24.4, 36.6) | 7.8 (6.4, 9.2) | 2.6 (2.1, 3.1) |
| P, trendc | 0.67 | 0.56 | 0.10 | 0.44 | 0.04 | 0.61 |
aAdjusted for total energy intake, abstinence time, age, BMI, moderate-vigorous physical activity, race, prudent and western dietary patterns, smoking status and history of varicocele.
bHigh pesticide residue fruits and vegetables were defined as a final score = 4, 5 or 6. Models were additionally adjusted for low-to-moderate pesticide fruit and vegetable intake.
cEstimated using median intake in each quartile as a continuous variable.
dLow-to-moderate pesticide residue fruits and vegetables were defined as a final score = 0, 1, 2 or 3. Models were additionally adjusted for high pesticide fruit and vegetable intake.
*P-value for trend <0.05 compared with men in the lowest quartile of intake.
Figure 1.
Adjusted total normal sperm count and total motile sperm count according to quartile intake of high or low-to-moderate pesticide fruits and vegetables (n = 155 men, 338 semen samples). Values are adjusted normal count (95% CI) across (A) quartiles intake of high pesticide residue fruits and vegetables (P-trend = 0.003) and (B) intake of low-to-moderate pesticide residue fruits and vegetables (P-trend = 0.15); adjusted motile count (95% CI) across (C) quartiles intake of high pesticide residue fruits and vegetables (P-trend = 0.003) and (D) intake of low-to-moderate pesticide residue fruits and vegetables (P-trend = 0.17); the results are adjusted for total energy intake, abstinence time, age, BMI, physical activity, race, prudent and western dietary patterns, smoking status and history of varicocele. (A) and (C) were additionally adjusted for low-to-moderate pesticide fruit and vegetable intake. (B) and (D) were additionally adjusted for high pesticide fruit and vegetable intake. Tests for trend were conducted across quartiles using the median value of a variable in each quartile. Asterisks represented significant differences between groups, *P < 0.05. Error bar indicated 95% CI. L-M, low-to-moderate.
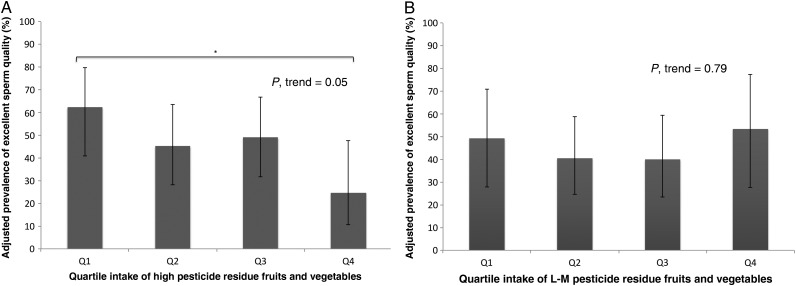
Results were similar when semen parameters were dichotomized according to WHO lower reference limits. The adjusted prevalence of low total sperm count and low normal morphology was positively related to high pesticide residue fruit and vegetable intake (Supplementary data, Table S2). Conversely, the prevalence of excellent sperm quality decreased with increasing intake of high pesticide residue fruits and vegetables (Fig. 2).
Figure 2.
Adjusted prevalence of excellent sperm quality according to quartile intake of high or low-to-moderate pesticide fruits and vegetables (n = 155 men, 338 semen samples). Excellent sperm quality is defined as semen sample that met the following criteria: total sperm count ≥39 million, sperm concentration ≥15 million/ml, total motility ≥40%, normal morphology ≥4% and ejaculate volume ≥1.5 ml. Values are adjusted prevalence of excellent sperm quality (95% CI) across (A) quartiles intake of high pesticide residue fruits and vegetables (P-trend = 0.05) and (B) intake of low-to-moderate pesticide residue fruits and vegetables (P-trend = 0.79); results are adjusted for total energy intake, abstinence time, age, BMI, physical activity, race, and western dietary patterns, smoking status and history of varicocele. (A) was additionally adjusted for low-to-moderate pesticide fruit and vegetable intake. (B) was additionally adjusted for high pesticide fruit and vegetable intake. Tests for trend were conducted across quartiles using the median value of a variable in each quartile. Asterisks represented significant differences between two groups, *P < 0.05. Error bar indicated 95% CI.
Results of the sensitivity analyses were consistent with those of the main analysis. Intake of high pesticide residue fruits and vegetables was inversely related to lower total sperm count, percentage of morphologically normal sperm and ejaculate volume in analyses based on median cutoffs (P, trend = 0.04, 0.03 and 0.02, respectively; Supplementary data, Table S3). Intake of higher average contamination rankings was also associated with lower sperm count and lower ejaculate volume, but not to sperm morphology (P, trend = 0.05, 0.03 and 0.78, respectively; Supplementary data, Table S3). Similarly, the associations between intake of high pesticide residue fruit and vegetable with semen quality were similar, albeit weaker, when each of the three contamination measures from the PDP was considered separately. The number of detectable pesticides was more strongly associated with total sperm count, while percentage exceeding the tolerance level was more strongly associated with sperm morphology and ejaculate volume (Supplementary data, Table S4). Intake of low-to-moderate pesticide residue fruits and vegetables was unrelated to semen parameters in all the sensitivity analyses. Results were also similar after additional adjustment for dietary factors previously related to semen quality in this cohort (Supplementary data Table S5), or when the analysis was restricted to the first semen sample per individual (Supplementary Table S6), or to samples collected within 1 year of diet assessment (Supplementary data Table S6).
Finally, there was no evidence of significant heterogeneity on the relation of high or low-to-moderate pesticide fruit and vegetable intake with semen quality according to study period (2007–2009 versus 2010–2012), BMI, smoking status or physical activity level (P, heterogeneity >0.10 in all cases).
Discussion
We evaluated the relation between intake of fruits and vegetables and their pesticide residue status among men presenting for evaluation and treatment at a fertility center. We found that intake of high pesticide residue fruits and vegetables was inversely associated with total sperm count, ejaculate volume and percentage of morphologically normal sperm, whereas intake of low-to-moderate pesticide residue fruits and vegetables was related to a higher percentage of morphologically normal sperm. Collectively, these findings suggest that dietary exposure to pesticides used in agriculture may impact semen quality in men.
Two consecutive systematic reviews (Perry, 2008; Martenies and Perry, 2013), which included a total of 37 studies published between 1991 and 2012, investigated the relationship between environmental and occupational pesticide exposure and sperm parameters, and 28 of the original studies reported significant findings. Specifically, pyrethroid pesticides and metabolites 3-phenooxybenzoic acid (3-PBA) and trans-3-(2,2-dichlorovinyl)-1-methylcyclopropane-1,2-dicarboxylic acid (TDCCA) were associated with a lower sperm concentration. The results from studies reporting on organochlorines were fairly consistent: higher p,p′-dichlorodiphenyldichloroethylene (DDE), primary metabolites of dichlorodiphenyltrichloroethane (DDT) and DDT concentrations increased the odds of low sperm concentration, low sperm motility and abnormal morphology. Organophosphates, the most frequently studied class of pesticides, were found to be associated with decreased sperm concentration, ejaculate volume and total sperm count. In brief, both environmental and occupational exposures to pesticides were most commonly associated with a decrease in sperm concentration. A decrease in motility was reported with exposure to certain pesticide classes, while an association with sperm morphology was less clear. However, limited information was available on the influence of exposure to multiple pesticide residues on semen quality or male reproduction.
While intake of pesticide-contaminated foods has not been examined in relation to semen quality before, some studies have evaluated the relation between organic food consumption and semen quality. Most relevant to our findings is a study among 256 farmers in Denmark (Juhler et al., 1999), which found that men who consumed conventional fruits and vegetables had a significantly lower proportion of morphologically normal spermatozoa than men who consumed organic diets (defined as ≥50% of fruits and vegetables being organic). Another study conducted among the members of a Danish association of organic farmers reported that sperm concentration was 43.1% higher among men eating organically produced food (Jensen et al., 1996). Although we did not directly assess consumption of organic produce in our FFQ, organically grown foods have been reported to have significantly lower pesticide residues than conventionally grown foods (Baker et al., 2002; Lu et al., 2006). This suggests that our findings and those among organic produce consumers are consistent with each other and that pesticide residues may impair spermatogenesis.
Several animal studies have suggested that pesticides have diverse mechanisms of action (Mehrpour et al., 2014). Pesticides may act as endocrine disrupting chemicals through hormonal or gonadotrophic pathways that affect male reproduction. For example, parathion and methylparathion, which structurally mimic estrogen, interact with hormone receptors and interfere with gene transcription (Mehrpour et al., 2014); 3,5,6-trichloro-2-pyridinol (TCPY) may interfere with the hypothalamic–pituitary–testes axis (Meeker et al., 2006). In addition, pesticides such as endosulfan, methamidophos, dimethoate and methylparathion have been shown to increase apoptosis during spermatogenesis (Recio-Vega et al., 2008). Quinalphos can impact sperm concentration by causing decreased lipid content in the testicular basement membrane, which in turn damages the germinal epithelium (Debnath and Mandal, 2000). Other indirect effects of organophosphates, bipyridyl herbicides and organochlorines included producing free radicals that induce oxidative stress and cause sperm dysfunction (Abdollahi et al., 2004). Although biological mechanisms for some pesticides, particularly organophosphates, have been investigated, toxicological research on synergistic effects of multiple chemicals is still lacking.
Despite these findings and potential biological mechanisms, our results must be interpreted with caution. First, diet was assessed only once and misclassification of true intake over time may have occurred. However, results were consistent when analyses were restricted to samples collected within 1 year of diet assessment or to the first sample collected for each man. Second, we did not have information on whether food items on the FFQ were organically or conventionally grown. Third, exposure to pesticides through diet may also be subject to misclassification since it was based on surveillance data rather than individual-level pesticide exposure measurement. It is important to highlight, however, that the most likely effect of the sources of error mentioned above is attenuation of the observed relation suggesting that the true effects may be stronger than those reported here. Our pesticide score also lacks specificity to individual chemicals, which could distort associations due to policy or agricultural practice changes. For example, carbofuran was banned for all crops in 2009 (Environmental Protection Agency, 2009). However, results were unchanged with additional adjustment for calendar year and there was no evidence of differences in the association between 2007–2009 and 2010–2012. As is the case for all observational studies, we cannot rule out the possibility of residual confounding and confirmation is required by interventional studies as well. However, adjustment for a large number of potential confounders had minimal impact of the effect estimates. We were also unable to assess polymorphisms in pesticide-metabolizing enzymes, such as paraoxonase 1 (PON1), which could be modifiers of the observed relations (Perez-Herrera et al., 2008; Costa et al., 2013). In addition, the participants were recruited through a fertility clinic, limiting the generalizability to men unaware of their fertility potential. However, results may still be useful to men seeking fertility care. Finally, CASA is a useful method clinically but may be considered less favorable for accurate semen analysis in the research setting.
A strength of this study is the novel method for classifying produce according to pesticide residues based on FFQ and PDP data, which directly addresses public health concerns while being an inexpensive and reasonable way to explore the effect of multiple pesticide-containing foods on health outcomes. In addition, the PDP collects data from all regions of the country and sampled produce reflect what is typically available to the consumer throughout the year, making the summary score potentially representative and generalizable to the US population. Additional strengths of the study include the use of multiple semen samples per individual, accounting for within-person variability in semen quality and detailed information on various lifestyle risk factors, reproductive history and results of a physical examination, which allowed adjustment for potential confounders.
In conclusion, consumption of high pesticide residue fruits and vegetables was associated with lower total sperm count, ejaculate volume and percentage of morphologically normal sperm among men attending a fertility clinic. On the other hand, intake of low-to-moderate pesticide residue produce was positively related to sperm morphology. These findings suggest that exposure to pesticides used in agricultural production through diet may be sufficient to affect spermatogenesis in humans. However, because our assessment of pesticide residues was based on data from the PDP, rather than on direct measurement of pesticides, further confirmation of these findings is warranted.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
J.E.C. and R.H. were involved in study concept and design, and critical revision for important intellectual content of the manuscript. The method of pesticide classification was developed by Y.H.C., R.H. and J.E.C. P.L.W contributed to method modification and provided statistical expertise. Y.H.C. analyzed data, drafted the manuscript and had a primary responsibility for final content; Y.H.C., M.C.A. and J.E.C. interpreted the data; M.C.A. contributed to statistical analyses. A.J.G. reviewed the statistical analysis; R.H, J.E.C., J.C.P. and C.T. were involved in acquisition of the data. All authors were involved in the critical revision of the manuscript and approved the final manuscript.
Funding
This study was supported by grants ES009718, ES022955 and ES000002 from the National Institute for Environmental Health Sciences (NIEHS), grants P30 DK046200 from the National Institutes of Health and Ruth L. Kirschstein, National Research Service Award T32 DK007703-16.
Conflict of interest
The authors have no conflicts of interest to declare.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the study participants, research nurses Jennifer B. Ford, B.S.N., R.N. and Myra G. Keller, R.N.C., B.S.N. and senior research assistant Ramace Dadd.
References
- Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit 2004;10:RA141–RA147. [PubMed] [Google Scholar]
- Afeiche MC, Bridges ND, Williams PL, Gaskins AJ, Tanrikut C, Petrozza JC, Hauser R, Chavarro JE. Dairy intake and semen quality among men attending a fertility clinic. Fertil Steril 2014a;101:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeiche MC, Gaskins AJ, Williams PL, Toth TL, Wright DL, Tanrikut C, Hauser R, Chavarro JE. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr 2014b;144:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi T, Watanabe A, Komiya A, Fuse H. Evaluation of the sperm motility analyzer system (SMAS) for the assessment of sperm quality in infertile men. Syst Biol Reprod Med 2010;56:473–477. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Farr SL, Jamieson DJ, Warner L, Macaluso M. Infertility services reported by men in the United States: national survey data. Fertil Steril 2009;91:2466–2470. [DOI] [PubMed] [Google Scholar]
- Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod 2012;27:1466–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BP, Benbrook CM, Groth E, 3rd, Lutz Benbrook K. Pesticide residues in conventional, integrated pest management (IPM)-grown and organic foods: insights from three US data sets. Food Addit Contam 2002;19:427–446. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Furtado J, Toth TL, Ford J, Keller M, Campos H, Hauser R. Trans-fatty acid levels in sperm are associated with sperm concentration among men from an infertility clinic. Fertil Steril 2011;95:1794–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Cole TB, Marsillach J, Furlong CE. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology 2013;307:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath D, Mandal TK. Study of quinalphos (an environmental oestrogenic insecticide) formulation (Ekalux 25 E.C.)-induced damage of the testicular tissues and antioxidant defence systems in Sprague-Dawley albino rats. J Appl Toxicol 2000;20:197–204. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency. Carbofuran Registration Review Status. Case 0101. December 2009. Washington, DC: Environmental Protection Agency; 2009. [Google Scholar]
- Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–796. [DOI] [PubMed] [Google Scholar]
- Fortes C, Mastroeni S, Pilla MA, Antonelli G, Lunghini L, Aprea C. The relation between dietary habits and urinary levels of 3-phenoxybenzoic acid, a pyrethroid metabolite. Food Chem Toxicol 2013;52:91–96. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod 2012;27:2899–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt S, Lemar L, Haytowitz D, Pehrsson P, Nickle M, Showell B, Thomas R, Exler J, Holden J. USDA National Nutrient Database for Standard Reference, release 21, 2008.
- Jensen TK, Giwercman A, Carlsen E, Scheike T, Skakkebaek NE. Semen quality among members of organic food associations in Zealand, Denmark. Lancet 1996;347:1844. [DOI] [PubMed] [Google Scholar]
- Juhler RK, Larsen SB, Meyer O, Jensen ND, Spano M, Giwercman A, Bonde JP. Human semen quality in relation to dietary pesticide exposure and organic diet. Arch Environ Contam Toxicol 1999;37:415–423. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE., Jr Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol 1994;126:276–285. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril 1988;49:112–117. [DOI] [PubMed] [Google Scholar]
- Louis JF, Thoma ME, Sorensen DN, McLain AC, King RB, Sundaram R, Keiding N, Buck Louis GM. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology 2013;1:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R. Organic diets significantly lower children's dietary exposure to organophosphorus pesticides. Environ Health Perspect 2006;114:260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martenies SE, Perry MJ. Environmental and occupational pesticide exposure and human sperm parameters: a systematic review. Toxicology 2013;307:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ryan L, Barr DB, Hauser R. Exposure to nonpersistent insecticides and male reproductive hormones. Epidemiology 2006;17:61–68. [DOI] [PubMed] [Google Scholar]
- Mehrpour O, Karrari P, Zamani N, Tsatsakis AM, Abdollahi M. Occupational exposure to pesticides and consequences on male semen and fertility: a review. Toxicol Lett 2014;230:146–156. [DOI] [PubMed] [Google Scholar]
- Perez-Herrera N, Polanco-Minaya H, Salazar-Arredondo E, Solis-Heredia MJ, Hernandez-Ochoa I, Rojas-Garcia E, Alvarado-Mejia J, Borja-Aburto VH, Quintanilla-Vega B. PON1Q192R genetic polymorphism modifies organophosphorous pesticide effects on semen quality and DNA integrity in agricultural workers from southern Mexico. Toxicol Appl Pharmacol 2008;230:261–268. [DOI] [PubMed] [Google Scholar]
- Perry MJ. Effects of environmental and occupational pesticide exposure on human sperm: a systematic review. Hum Reprod Update 2008;14:233–242. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Venners SA, Chen X, Liu X, Tang G, Xing H, Barr DB, Xu X. Organophosphorous pesticide exposures and sperm quality. Reprod Toxicol 2011;31:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio-Vega R, Ocampo-Gomez G, Borja-Aburto VH, Moran-Martinez J, Cebrian-Garcia ME. Organophosphorus pesticide exposure decreases sperm quality: association between sperm parameters and urinary pesticide levels. J Appl Toxicol 2008;28:674–680. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126; discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. The American Statistician 1980;34:216–221. [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect 2000;108:961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–1331. e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Agriculture. Pesticide data program, annual summary. URL: http://www.ams.usda.gov/AMSv1.0/PDP USDA, Agricultural Marketing Service, 2006–2012.
- Whorton D, Krauss RM, Marshall S, Milby TH. Infertility in male pesticide workers. Lancet 1977;2:1259–1261. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn Geneva: World Health Organization, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.