Abstract
STUDY QUESTION
Is use of depot medroxyprogesterone acetate (DMPA) a risk factor for or a protective factor against prevalent uterine leiomyoma?
SUMMARY ANSWER
Ever use of DMPA was associated with a decreased risk (adjusted risk ratio (RR): 0.8, 95% confidence interval (CI): 0.6, 0.9) of prevalent leiomyoma in young African American women.
WHAT IS KNOWN ALREADY
Although progesterone is associated with growth of leiomyoma, previous epidemiological studies have shown a protective association for DMPA use. These previous studies may have been biased by studying clinically diagnosed leiomyoma (DMPA may mask symptoms thus delaying diagnoses).
STUDY DESIGN, SIZE, DURATION
Cross sectional analysis of baseline data from a cohort study of 1696 African American women.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Community-based recruitment (e.g. letters, flyers, radio and TV announcements) were used to enroll African American women between 23 and 34 years old without a previous diagnosis of leiomyoma in the Metropolitan Detroit area. Extensive questionnaire data were used to determine DMPA use and screening ultrasound detected the presence of leiomyoma ≥0.5 cm in diameter. Relative risks with adjustment for covariates were calculated for the presence of leiomyoma based on ever use of DMPA as well as duration and recency of use.
MAIN RESULTS AND THE ROLE OF CHANCE
Among the 1696 volunteers who enrolled, 43% had used DMPA. Leiomyoma were detected in 17% of those who had ever used DMPA compared with 26% of those who had never used DMPA. The reduction in prevalence remained after adjustment for potential confounders and was highest among women who had used DMPA for more than 4 years (adjusted RR: 0.5, 95% CI: 0.3, 0.8). The reduction in risk was seen for women whose most recent use was up to 8 years prior to study enrollment.
LIMITATIONS, REASONS FOR CAUTION
The use of cross-sectional data means that the timing of initial fibroid development is not known, so the temporality of the association is uncertain. However in this sample of young women, most fibroids were small, suggesting that DMPA exposure may have occurred before leiomyoma development.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings are in agreement with previous epidemiological studies, but protected from the bias inherent in the use of clinically diagnosed leiomyoma. Although further studies will be needed to elucidate the mechanism, use of DMPA as a contraceptive appears to provide long lasting protection against uterine leiomyoma.
STUDY FUNDING/COMPETING INTEREST(S)
No competing interests. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, and in part by funds allocated for health research by the American Recovery and Reinvestment Act.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: leiomyoma, contraception, progesterone, African American women
Introduction
Uterine leiomyomata, or fibroids, are benign smooth muscle tumors of the uterus that develop during the reproductive years. Although frequently asymptomatic, fibroids can cause significant pain and bleeding which impair quality of life. The bleeding can be heavy enough to lead to anemia (Stewart et al., 2013). Fibroids can also impair fertility and complicate pregnancy (Klatsky et al., 2008). Although fibroids are common among all women, in the United States African American women have a higher prevalence. In African Americans, fibroids tend to appear at younger ages, and up to 80% of African American women have evidence of fibroids by the time of menopause (Baird et al., 2003). Initial treatment of fibroids is for symptom relief with pain control and the use of hormonal contraceptives (ACOG, 2008). Surgical treatment (myomectomy or hysterectomy), uterine artery embolization, focused ultrasound ablation or endometrial ablation can be used for women whose symptoms are not responsive to medication. While techniques to spare the uterus are effective, fibroids often recur and hysterectomy is the only definitive treatment. In the USA and Europe, fibroids are a leading cause of hysterectomy (Vessey et al., 1992; Debodinance, 2001; ACOG, 2008).
Identified risk factors for fibroids are largely non-modifiable including age, race/ethnicity and early age at menarche (reviewed in ref. Laughlin et al., 2010). Parity has a protective association, likely through post-partum uterine remodeling (Laughlin et al., 2011). Many other behavioral, dietary and environmental exposures have been examined but lack consistent evidence across studies.
Fibroids are hormonally dependent, developing during the reproductive ages and tending to shrink after menopause (reviewed in ref. Segars et al., 2014). In vivo and in vitro studies in laboratory animals and human tissue provide some insight into the role of hormones in fibroid growth. Although estrogen has traditionally been seen as the primary promoter of fibroid growth, laboratory animal and human data suggest that progesterone, in the context of normal estrogen levels, may also play an important role (reviewed in ref. Flake et al., 2003; Kim et al., 2013).
Although the biological evidence suggests a role for progesterone in fibroid growth, two epidemiological studies (Lumbiganon et al., 1996; Wise et al., 2004) have found an inverse association between the use of the progestin contraceptive, Depo-medroxyprogesterone acetate (DMPA, Depo-Provera™) and clinically diagnosed fibroids. DMPA is given as an injection every 3 months, and results in failure of follicular maturation, a reduction in the synthesis and secretion of ovarian estradiol (Speroff, 2014), thickening of the cervical mucus and thinning of the endometrium. After the first year of use, more than 50% of women experience amenorrhea (Speroff and Darney, 2005).
The two epidemiological studies (Lumbiganon et al., 1996; Wise et al., 2004) are consistent in finding DMPA use associated with a 40–60% decreased risk of fibroids. However, their use of clinically-diagnosed fibroids raises the possibility of bias: the long-term use of DMPA results in amenorrhea which may lessen the symptoms associated with fibroids and thereby reduce the likelihood of clinical diagnosis. The apparent protective association with clinically diagnosed fibroids therefore may be a result of factors related to diagnosis and not a true protective association with fibroids.
Despite the laboratory data indicating a proliferative effect of progesterone on fibroids, the epidemiological data suggest the possibility that an effective form of a progestin-only contraception may decrease the risk of fibroids. Thus, we sought to examine the association between DMPA use and ultrasound-detected fibroids in a cohort of young African American women.
Materials and Methods
Study population
The Study of Environment, Lifestyle and Fibroids (SELF) is a prospective cohort study of risk factors for the development and growth of fibroids in young African American women. The study recruited women from the Detroit, Michigan area using media, community advertisements and targeted letters through the Henry Ford Health System. African American women between 23 and 34 years of age who were premenopausal and had never been diagnosed with fibroids were invited to contact the SELF study coordinating center for further eligibility screening. Eligibility criteria included the following: at least part African American or black; no prior diagnosis of fibroids; no hysterectomy. Eligible women were excluded if they had a history of medically treated auto-immune disease (Systemic Lupus Erythematosus, Grave's Disease, Sjogren's Syndrome, Scleroderma, Multiple Sclerosis) or history of radiation or chemotherapy treated cancer. Women also needed to live in the USA, and be willing to visit Detroit for follow-up visits over 5 years and provide sufficient information for tracing. Eligible women were invited to attend an orientation session where, following a full briefing on the study, they could proceed with enrollment activities, which included an ultrasound to screen for fibroids. Recruitment and baseline data collection for SELF began in 2010 and was complete in early 2013. This study utilizes baseline data on the 1696 participants.
Ethical approval
The study was approved by the institutional review boards of the National Institute of Environmental Health Sciences and Henry Ford Health Systems. All participants provided written informed consent.
Assessment of DMPA use
Baseline data were collected using a telephone interview and a computer-based questionnaire. Both were computer assisted with prompts to minimize missing data. Women provided a lifetime history of all hormonal contraceptive use including age at initiation, duration of use and age at last use. At the time of clinic visit, which usually followed completion of interviews and questionnaires, women provided additional information on current medications including birth control and the use of any form of hormonal contraception in the 4 weeks preceding the clinic visit. Women were considered to have ever used DMPA if they reported ever having at least one DMPA shot. Duration of use and age at last use categories were based on the interview data.
Fibroid assessment
Participants underwent an ultrasound to detect fibroids. Experienced ultrasound technicians conducted transvaginal ultrasounds to detect, localize and measure fibroids of ≥0.5 cm in diameter. In cases where a transvaginal approach was not sufficient, a transvesical/abdominal approach was added. Images were archived, and an 8% sample for each sonographer (oversampled for sonograms with fibroids) was reviewed by the head sonographer.
Covariates
Identified confounders included age at menarche (≤10 years, >10 years), parity (nulliparous, 1 or 2 births, 3 or more births modeled as indicator variables) and age (years) at baseline ultrasound. Additional covariates explored included income, education, smoking, alcohol use, oral contraceptive use and body mass index (BMI). Height and weight for calculating BMI were measured at the clinic visit, all other covariates were self-reported.
Statistical analysis
The association between DMPA and prevalent fibroids was assessed with age-adjusted and multivariate-adjusted log linear models to estimate the prevalence risk ratio.
Initial models explored the association between the dichotomous exposure of ever/never use of DMPA and the presence of any fibroids with a diameter of at least 0.5 cm. In addition to a priori confounders of current age, age at menarche and parity, education was also included in the model. To examine effects of duration and recency of DMPA use, separate age-adjusted and multivariate-adjusted models were considered for categories of duration of DMPA use (≤9, 10–24, 25–48, >48 months), and time since most recent use of DMPA (current user, 1–4, 5–8, 9+ years). In each analysis, those categories were modeled using indicator variables, to allow a non-linear association. To examine the joint association for both duration of use and time since last use, each characteristic was dichotomized based on observed patterns. Individuals were assigned to the appropriate category considering both duration of use (short≤2 years, long >2 years) and time since last use (recent ≤8 years, past ≥9 years). The joint exposure categories were assigned as none (referent), short/past, short/recent, long/past or long/recent.
All analyses were conducted using SAS 9.3 (Cary, NC, USA).
Results
The SELF cohort included 1696 women. Twenty-two percent (n = 378) had fibroids detected at the clinic ultrasound. The use of DMPA was common in this cohort, with 43% of women reporting ever use of DMPA. Compared with women who had never used DMPA, women who had used DMPA were older and had lower income and education; a larger proportion were non-smokers, and reported an earlier age at menarche and more pregnancies (Table I).
Table I.
Baseline characteristics of 1696 participants in SELF 2010–2013 by use of depot medroxyprogesterone acetate.
| Characteristic | Overall |
DMPA use |
||||
|---|---|---|---|---|---|---|
| Never (N = 974) |
Ever (N = 722) |
|||||
| n | % | n | % | n | % | |
| Age (years) | ||||||
| 23–26 | 519 | 31 | 330 | 34 | 189 | 26 |
| 27–30 | 584 | 34 | 323 | 33 | 259 | 36 |
| 31–34 | 593 | 35 | 321 | 33 | 274 | 38 |
| Household incomea | ||||||
| <$20,000 | 768 | 46 | 382 | 39 | 386 | 54 |
| $20,000–$50,000 | 629 | 37 | 388 | 40 | 241 | 34 |
| ≥$50,000 | 287 | 17 | 198 | 20 | 89 | 12 |
| Educationb | ||||||
| HS/GED or less | 370 | 22 | 160 | 16 | 210 | 29 |
| Some college/associates/technical | 850 | 50 | 466 | 48 | 384 | 53 |
| Bachelors/masters/PhD | 475 | 28 | 348 | 36 | 127 | 18 |
| Body mass index (kg/m2) | ||||||
| <25 | 336 | 20 | 190 | 20 | 146 | 20 |
| 25–29 | 350 | 21 | 186 | 19 | 164 | 23 |
| 30–34 | 329 | 19 | 203 | 21 | 126 | 17 |
| ≥35 | 681 | 40 | 395 | 41 | 286 | 40 |
| Smoking history | ||||||
| Never smoked | 1247 | 74 | 756 | 78 | 491 | 68 |
| Former smoker | 125 | 7 | 59 | 6 | 66 | 9 |
| Current smoker | 324 | 19 | 159 | 16 | 165 | 23 |
| Alcohol usec | ||||||
| Non-drinker | 450 | 27 | 256 | 26 | 194 | 27 |
| Moderate | 554 | 33 | 341 | 35 | 213 | 30 |
| Heavyd | 692 | 41 | 377 | 39 | 315 | 44 |
| Age at menarche (years) | ||||||
| ≤10 | 310 | 18 | 165 | 17 | 145 | 20 |
| 11 | 334 | 20 | 197 | 20 | 137 | 19 |
| 12 | 459 | 27 | 282 | 29 | 177 | 25 |
| 13 | 287 | 17 | 173 | 18 | 114 | 16 |
| ≥14 | 306 | 18 | 157 | 16 | 149 | 21 |
| Reproductive history | ||||||
| Never pregnant | 452 | 27 | 363 | 37 | 89 | 12 |
| Nulliparous | 211 | 12 | 158 | 16 | 53 | 7 |
| 1 birth | 433 | 26 | 226 | 23 | 207 | 29 |
| 2 births | 313 | 18 | 140 | 14 | 173 | 24 |
| 3+ births | 287 | 17 | 87 | 9 | 200 | 28 |
DMPA, depot medroxyprogesterone acetate; HS, high school graduate; GED, general education development.
aMissing for income: no DMPA (n = 6), DMPA (n = 6).
bMissing for education: DMPA (n = 1).
cAt time in life when drank the most.
dHeavy drinking meets the criteria for binge drinking (4+ drinks at once 2–3 times per month).
Seventeen percent of women who had ever used DMPA had fibroids compared with 26% of women who had never used DMPA. The age-adjusted relative risk of fibroids for DMPA users was significantly reduced compared with never users and further adjustment for additional covariates showed a similar association (adjusted risk ratio (aRR): 0.76, 95% CI: 0.62, 0.93) (Table II).
Table II.
Association between DMPA use and prevalent fibroids in 1696 young African American women 2010–2013.
| DMPA use | Fibroids |
RR (95% CI) |
||
|---|---|---|---|---|
| N | % | Age adjusted | Fully adjusteda | |
| Never (N = 974) | 254 | 26 | Ref. | Ref. |
| Ever (N = 722) | 124 | 17 | 0.63 (0.52, 0.76) | 0.76 (0.62, 0.93) |
| Duration of use (months) | ||||
| ≤9 (N = 208) | 42 | 20 | 0.74 (0.55, 0.98) | 0.90 (0.67, 1.20) |
| 10–24 (N = 206) | 41 | 20 | 0.76 (0.57, 1.01) | 0.91 (0.68, 1.21) |
| 25–48 (N = 139) | 20 | 14 | 0.53 (0.35, 0.80) | 0.64 (0.42, 0.97) |
| >48 (N = 169) | 21 | 12 | 0.43 (0.29, 0.65) | 0.52 (0.35, 0.79) |
| Time since last use (years)b | ||||
| Current user (N = 146) | 22 | 15 | 0.64 (0.43, 0.94) | 0.77 (0.52, 1.15) |
| 1–4 (N = 188) | 26 | 14 | 0.56 (0.39, 0.81) | 0.65 (0.45, 0.94) |
| 5–8 (N = 174) | 25 | 14 | 0.57 (0.39, 0.82) | 0.68 (0.47, 0.99) |
| ≥9 (N = 213) | 50 | 23 | 0.69 (0.53, 0.90) | 0.87 (0.66, 1.14) |
DMPA, depot medroxyprogesterone acetate.
aAdjusted for age (continuous), parity (nulliparous, 1–2 births, 3+ births), menarche (≤10 years, 11 years or older), and education (Bachelor's degree or higher, all other levels of education).
bOne woman missing time since last use.
The strength of the association increased with increasing duration of use. Women who had used DMPA for 25–48 months (aRR: 0.64, 95% CI: 0.42, 0.97) or >48 months (aRR: 0.52, 95% CI: 0.35, 0.79) showed the strongest protective association. Women who had used DMPA within the last 8 years had a significantly reduced risk, but women who last used DMPA more than 8 years previously had a much weaker, non-significant association (aRR: 0.87, 95% CI: 0.66, 1.14) (Table II).
Considering the joint effects of duration and timing of use, women who used DMPA for at least 2 years (long duration) had a minimum estimated 34% decreased risk of fibroids regardless of time since last use (Fig. 1). In contrast, short duration DMPA use (<2 years) was not significantly associated with decreased risk of fibroids even for recent users.
Figure 1.
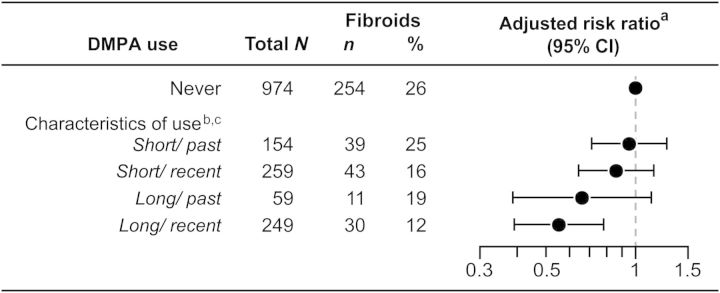
Association between characteristics of DMPA use and prevalent fibroids in 1696 young African American women 2010–2013. aAdjusted for age at enrollment (continuous), parity (nulliparous, 1 or 2 births, 3+ births) early age of menarche (≤10 years, 11 years or older) and education (Bachelor's degree or higher, all other levels of education). bDuration of use is characterized as short (≤2 years) or long (>2 years). Time since last use is characterized as recent (≤8 years) or past (≥9 years). cOne woman missing data for time since last use. DMPA, depot medroxyprogesterone acetate; CI, confidence interval.
Discussion
In this study of young African American women, we found that use of DMPA was associated with a decreased risk of fibroids detected on screening ultrasound. The protective association appeared to be strongest among women who had used DMPA for at least 24 months and had used it at some point within the 8 years before ultrasound. Considering the characteristics of the duration of use and time since last use jointly, it appeared that the duration of use was the most important factor.
Our findings are consistent with previous epidemiological findings (Lumbiganon et al., 1996; Wise et al., 2004). However, both previous studies relied on clinically diagnosed fibroids, which are often discovered following onset of symptoms such as heavy bleeding. The use of DMPA frequently results in amenorrhea and may mask symptoms and reduce the likelihood of fibroid diagnosis. Our study screened all participants for fibroids and was thereby protected from this bias. Therefore, the accumulating evidence suggests that the observed protective association is causal.
Although the factors involved in the initiation of fibroids are largely unknown, fibroid growth and development are highly dependent on both estrogen and progesterone (Kim and Sefton, 2012). Progesterone, in the presence of normal estrogen levels, may be particularly important for fibroid growth. In a xenograft mouse model, initial growth of human-derived leiomyoma tissue required both estrogen and progesterone, however subsequent growth could be arrested by withdrawal of progesterone or initiation of the anti-progesterone RU486 (Ishikawa et al., 2010). Two studies have identified increased mitotic activity, an indicator of cell division and growth, in response to progesterone. In one study, the mitotic activity was higher in fibroids removed from women during the luteal phase of the menstrual cycle when progesterone levels are high (Kawaguchi et al., 1989). In another study, fibroids removed following exposure to a progestin-only contraceptive showed increased mitotic activity compared with combined oral contraception use or no contraception use (Tiltman, 1985). Anti-progesterones such as RU486 and ulipristal acetate have also been used to reduce pain and bleeding associated with fibroids (Donnez et al., 2012; Kim and Sefton, 2012). Treatment with ulipristal acetate can result in reduced fibroid volumes that can be sustained for up to 6 months following treatment.
The apparent protective effect of a progestin-only contraceptive in face of the in vitro and in vivo studies may be a consequence of the concurrent changes in estrogen levels. While DMPA does result in periodic supra-physiologic serum levels of progestin (Speroff and Darney, 2005) it also results in decreased levels of endogenous estradiol (Clark et al., 2001). Low estrogen levels may both inhibit fibroid growth and down-regulate both estrogen and progesterone receptors (Kim and Sefton, 2012). Thus, the low estradiol experienced by women using DMPA may impact fibroid initiation directly and make the tissue less responsive to periodic boluses of progestin.
Our study has limitations. First, DMPA usage may be confounded by other factors that are associated with reduced fibroid risk. For example, the long use of an effective contraception like DMPA might be expected to decrease parity and thus increase risk because of the protective effect of parity (Laughlin et al., 2011). However, in this cohort, DMPA users were more likely to be parous, perhaps because DMPA is often started after a birth (Dozier et al., 2014). Though our adjusted analysis accounts for potential confounding with parity, in this data set the adjustment made little difference, indicating that the DMPA association we see is not heavily influenced by the contraceptive effects of DMPA per se. Our extensive data collection allowed us to evaluate several other potential confounders as well.
Secondly, reliance on retrospective self-reported contraception use over the life course may have led to misclassification of DMPA use characteristics. SELF tried to minimize misclassification through the use of life-events calendars, internal data consistency checks, and prompts to limit missing data. In addition, our young cohort was reporting contraceptive use in the relatively recent past.
Thirdly, our study excluded women with a known clinical diagnosis of fibroids, which has the potential to induce selection bias. In particular, a protective association could be falsely observed if women who were prescribed DMPA were routinely screened for fibroids. Anyone thus diagnosed would be ineligible for our study, leaving mostly DMPA users without fibroids. Under this hypothetical scenario, DMPA users who are in the study would not only be less likely to have fibroids, but would be more likely to have had a previous ultrasound. Among all study participants, 24% had had at least one previous ultrasound (outside of pregnancy). This rate did not differ by DMPA use. The median number of previous ultrasounds, time since last ultrasound and indication for ultrasound did not differ by DMPA use. Therefore, this form of selection bias seems unlikely. Other forms of selection bias that might result from differences in symptoms, clinical detection, prescribing habits or true direction of causation, may have attenuated our observed association, but are unlikely to have falsely identified the protective nature of the association.
This study has notable strengths. Compared with the Wise study (Wise et al., 2004), which collected baseline data on DMPA use within a few years of the licensing of DMPA (Speroff and Darney, 2005) in the USA, women in the SELF cohort have had access to DMPA as a contraceptive option for most of their reproductive life. This broad access to DMPA reduced the opportunity for confounding by indication.
Most importantly, this study used ultrasound screening to detect fibroids. The prevalence of fibroids of 0.5 cm or larger found in this population is similar to that reported in other studies (Laughlin et al., 2010). The use of screening ultrasound reduced bias by factors associated with symptoms that may bring women to clinical attention. As all women were free from clinically diagnosed fibroids at the time of study entry, and baseline questionnaire data were usually collected before the ultrasound results were revealed to participants, differential recall would also be reduced. However, the use of prevalent fibroids as the outcome limits our ability to firmly establish the temporality of the association because DMPA might be used as a treatment for heavy bleeding experienced by women with fibroids even before clinical diagnosis. Bias arising from reverse causality would, however, have tended to make DMPA positively and not negatively associated with fibroids and thus could not explain our findings.
The mechanism by which DMPA use might reduce the risk of fibroids remains unclear. Of note, women who had ever used DMPA were more likely than non-users to have single (69 versus 58%), and smaller (mean diameter of largest fibroid = 1.9 versus 2.4 cm) fibroids. This observation however does not point to a specific mechanism of action. DMPA may play a role in preventing initial tumor development or it may modify tumor growth. Examination of fibroid size before and after DMPA use in a large group of women with fibroids would be of interest. Continued surveillance of this cohort over the next several years will permit the investigation of these possible mechanisms as we will be documenting both future fibroid incidence and the growth of already existing tumors.
In summary, we found a strong protective association between ever use of DMPA and fibroids in young African American women. The estimated association was particularly strong among women who had used DMPA for at least 2 years, and the estimated reduction in risk persisted for several years after stopping DMPA.
Authors' roles
Q.H. participated in data analysis and interpretation, prepared the first draft of the manuscript and approved the final manuscript for publication. D.B. designed and implemented the SELF Study, participated in data analysis and interpretation of results, reviewed drafts of the manuscript and approved the manuscript for publication.
Funding
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, and in part by funds allocated for health research by the American Recovery and Reinvestment Act.
Conflict of interest
None declared.
References
- ACOG Practice Bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol 2008;112:387–400. [DOI] [PubMed] [Google Scholar]
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–107. [DOI] [PubMed] [Google Scholar]
- Clark MK, Sowers M, Levy BT, Tenhundfeld P. Magnitude and variability of sequential estradiol and progesterone concentrations in women using depot medroxyprogesterone acetate for contraception. Fertil Steril 2001;75:871–877. [DOI] [PubMed] [Google Scholar]
- Debodinance P. Hysterectomy for benign lesions in the north of France: epidemiology and postoperative events. J Gynécol Obstét Biol Reprod 2001;30:151–159. [PubMed] [Google Scholar]
- Donnez J, Tatarchuk T, Bouchard P, Puscasiu L, Zakharenko N, Ivanova T, Ugocsai G, Mara M, Jilla M, Bestel E, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012;366:409–420. [DOI] [PubMed] [Google Scholar]
- Dozier A, Nelson A, Brownell E, Howard C, Lawrence R. Patterns of postpartum depot medroxyprogesterone administration among low-income mothers. J Womens Health 2014;23:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flake G, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect 2003;111:1037–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ishi K, Serna V, Kakazu R, Bulun S, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010;151:2433–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol 1989;160:637–641. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Sefton EC. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol Cell Endocrinol 2012;358:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Kurita T, Bulun S. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev 2013;34:130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol 2008;198:357–366. [DOI] [PubMed] [Google Scholar]
- Laughlin S, Schroeder J, Baird D. New directions in the epidemiology of uterine fibroids. Semin Reprod Med 2010;28:204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SK, Hartmann KE, Baird DD. Postpartum factors and natural fibroid regression. Am J Obstet Gynecol 2011;204:496 e1–496.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbiganon P, Rugpao S, Phandhu-fung S, Laopaiboon M, Vudhikamraksa N, Werawatakul Y. Protective effect of depot-medroxyprogesterone acetate on surgically treated uterine leiomyomas: a multicentre case-control study. Br J Obstet Gynaecol 1996;103:909–914. [DOI] [PubMed] [Google Scholar]
- Segars J, Parrott E, Nagel J, Guo X, Gao X, Birnbaum L, Pinn V, Dixon D. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum Reprod Update 2014;20:309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speroff L. Medroxyprogesterone acetate. In: Gerald K, McEvoy PD. (ed). AHFS Drug Information® . Bethesda, MD: American Society of Health-System Pharmacists, Inc, 2014. [Google Scholar]
- Speroff L, Darney PD. A Clinical Guide for Contraception. Philadelphia, PA: Lippincott Williams & Wilkins, 2005. [Google Scholar]
- Stewart E, Nicholson W, Bradley L, Borah B. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health 2013;22:807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiltman AJ. The effect of progestins on the mitotic activity of uterine fibromyomas. Int J Gynecol Pathol 1985;4:89–96. [DOI] [PubMed] [Google Scholar]
- Vessey MP, Villard Mackintosh L, McPherson K, Coulter A, Yeates D. The epidemiology of hysterectomy: findings in a large cohort study. Br J Obstet Gynaecol 1992;99:402–407. [DOI] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, Rosenberg L. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol 2004;159:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]


