SUMMARY
SETTING
The cost of multidrug-resistant tuberculosis (MDR-TB) treatment is a major barrier to treatment scale-up in South Africa.
OBJECTIVE
To estimate and compare the cost of treatment for rifampicin-resistant tuberculosis (RR-TB) in South Africa in different models of care in different settings.
DESIGN
We estimated the costs of different models of care with varying levels of hospitalisation. These costs were used to calculate the total cost of treating all diagnosed cases of RR-TB in South Africa, and to estimate the budget impact of adopting a fully or partially decentralised model vs. a fully hospitalised model.
RESULTS
The fully hospitalised model was 42% more costly than the fully decentralised model (US$13 432 vs. US$7753 per patient). A much shorter hospital stay in the decentralised models of care (44–57 days), compared to 128 days of hospitalisation in the fully hospitalised model, was the key contributor to the reduced cost of treatment. The annual total cost of treating all diagnosed cases ranged from US$110 million in the fully decentralised model to US$190 million in the fully hospitalised model.
CONCLUSION
Following a more decentralised approach for treating RR-TB patients could potentially improve the affordability of RR-TB treatment in South Africa.
Keywords: MDR-TB, rifampicin-resistant tuberculosis, costing, budget impact, decentralization
LESS THAN 20% of the estimated number of multidrug-resistant tuberculosis (MDR-TB) emerging each year worldwide are diagnosed as such, and an even smaller percentage receive appropriate second-line treatment.1 The cost of MDR-TB treatment is a major barrier to treatment scale-up in many settings, with MDR-TB treatment estimated to cost around 26 times more than that for drug-susceptible TB.2 South Africa has a high burden of MDR-TB, with more than 14 000 cases notified in 2012.1 While scale-up of the Xpert® MTB/RIF test (Cepheid, Sunnyvale, CA, USA) has increased the case detection of rifampicin (RMP) resistant TB (RR-TB) substantially since 2010, access to appropriate second-line treatment has not kept pace. Routine data suggest that less than half of diagnosed cases initiate second-line anti-tuberculosis treatment.1 While data from the roll-out of Xpert indicate that 7% of TB cases in South Africa may have RR-TB,3 the cost of MDR-TB treatment is reported to encompass close to 55% of the total TB budget.1
The World Health Organization recommends ambulatory models of care for drug-resistant anti-tuberculosis treatment over hospital-based models.4 Before 2010, MDR-TB treatment was primarily centralised in specialist TB hospitals with mandatory in-patient admission. In 2011, faced with long waiting lists for admission and treatment initiation, the National Department of Health revised their policy to support the decentralisation of MDR-TB treatment.5 The revised policy removes the requirement to initiate treatment in hospital, but still suggests that sputum smear-positive patients be hospitalised. While the extent to which this policy has been implemented across South Africa’s provinces varies, the treatment gap for MDR-TB remains and may be increasing as case detection improves with Xpert.6
Previous studies in South Africa have estimated the cost of a centralised MDR-TB model of care.2,7 However, there is limited evidence on the impact of introducing a decentralised model of care on both the episode costs and the overall budget. Decentralisation of MDR-TB treatment is likely to be less costly than a fully hospitalised model, and can therefore potentially improve the capacity to scale up treatment for all diagnosed cases. We aimed to estimate the costs of treatment for RR-TB in South Africa across a range of models of care, based on the cost of treatment from a decentralised programme in Cape Town.8 We also estimated the likely budget impact of introducing decentralised MDR-TB treatment across South Africa.
METHODS
Estimating the costs of the decentralised model of care
In 2007, a decentralised model for the management of RR-TB was developed and piloted by Médecins Sans Frontières (MSF), the City of Cape Town and the Provincial Government of the Western Cape (PGWC) in Khayelitsha, the largest township in the Western Cape Province. This model of care permits initiation of treatment for RR-TB at primary health care clinics, provided the patient is sufficiently clinically stable to initiate MDR-TB treatment.9 The programme is associated with improved case detection and treatment initiation and results in treatment outcomes comparable to those seen in centralised specialist centres.8
Applying a cohort approach, we estimated the mean episode cost of managing a RR-TB patient from diagnosis to treatment outcome for each type of RR-TB patient by multiplying the unit cost of each treatment component by the number of times the cost was incurred by each patient in the cohort. The cohort included 467 RR-TB patients diagnosed and treated in Khayelitsha during the period from January 2009 to December 2011. This included all patients with a first episode of confirmed RR-TB for whom a treatment outcome was known. Cost data for clinic visits and hospital stay were collected from three primary health care clinics, one step-down facility and two TB hospitals. Sources of data included the PGWC, the local equipment and furniture suppliers, the Council for Scientific and Industrial Research for building and maintenance costs, and interviews with the facility manager. Where capital and overhead resources were shared between MDR-TB and other services, these joint clinic/hospital costs were allocated to MDR-TB on the basis of the proportion of total visits/in-patient days for which the MDR-TB patient accounted. Capital costs were annualised using a discount rate of 3%, and the assumption that the expected number of years of useful life was 20 years for buildings and 10 years for equipment and furniture.
The costs of diagnostic tests were estimated as part of a wider study into Xpert introduction, the XTEND study (Cunnama et al., paper in preparation). We used published literature for monitoring tests during treatment (Table 1).2 Drug costs were determined using the Western Cape Central Medical Depot tender price list, and computed based on the drug resistance profile and the duration of the intensive and continuation phases of treatment (Table 1). Where required, data were inflated to 2013 rates using the medical consumer price index of 6.4% for 2011 and 6.1% for 2012.10,11 Data were converted to US dollars using the 2013 average annual exchange rate of US$1 = South African rand (ZAR) 9.3 (OANDA Currency Converter 2014. Average exchange rate for January–December 2013. http://www.oanda.com).
Table 1.
Unit cost components for all scenarios, 2013 (in US$)
| Cost component | Unit cost US$ | Source |
|---|---|---|
| Clinic visit for initial diagnosis and monitoring | 10.88 | Study data |
| Clinic visit for direct drug collection/injections | 4.89 | Study data |
| In-patient day | 71.61* | Study data |
| Drugs† | 2015.27 | Study data |
| Xpert® MTB/RIF | 16.9 | Cunnama (paper in preparation) |
| Microscopy tests | 6.3 | Cunnama (paper in preparation) |
| Sputum liquid culture | 12.9 | Cunnama (paper in preparation) |
| First-line DST (LPA) | 20.3 | Cunnama (paper in preparation) |
| Second-line DST | 25.1 | Cunnama (paper in preparation) |
| X-ray | 24.01 | Cunnama (paper in preparation) |
| Kidney test | 12.45 | Pooran2 |
| Liver function test | 17.24 | Pooran2 |
| TSH | 23.94 | Pooran2 |
| Audiogram | 29.28 | Pooran2 |
| HIV rapid screening test | 6.03 | Pooran2 |
| CD4 count + viral load | 59.30 | Pooran2 |
In the base case, we used the average of US$71.61 but varied this estimate in a sensitivity analysis to factor in the economies of scale in larger urban hospitals. The cost per in-patient day in a large urban hospital in Cape Town and a smaller rural hospital in the Easter Cape was respectively US$44.44 and US$98.77.
Treatment regimens: RMP-monoresistant and MDR-TB patients are treated with KM in the intensive phase, and MFX, terizidone, ETH and pyrazinamide in both the intensive and continuation phases; pre-XDR fluoroquinolone patients are given KM in the intensive phase, and MFX, terizidone, ETH, PAS and CFZ in both the intensive and continuation phases; pre-XDR-TB INJ and XDR-TB patients use capreomycin in the intensive phase, and MFX, terizidone, ETH, PAS and CFZ in both intensive and continuation phases.
DST = drug susceptibility testing; LPA = line-probe assay; TSH = thyroid stimulating hormone; HIV = human immunodeficiency virus; KM=kanamycin; MFX=moxifloxacin; ETH=ethionamide; PAS=para-aminosalicylic acid; CFZ = clofazimine; INJ = injection drug resistant; XDR-TB = extensively drug-resistant tuberculosis.
Scenario analysis
Using the average treatment duration8 and the average cost per patient treated from the cohort analysis, but varying the duration of hospitalisation, we estimated the cost per patient treated in different models of care. The cost for each scenario assumed that the proportion of clinically unwell patients requiring hospitalisation was 19% (based on the proportion of RMP-monoresistant and MDR-TB cases who were hospitalised in the decentralised model) and that 44% of patients are sputum smear-positive (also based on the cohort data).
Four different potential scenarios for RR-TB treatment provision were considered: a fully decentralised model (as described for Khayelitsha above), a fully hospitalised model, a partially decentralised Model A and a partially decentralised Model B. In the fully hospitalised model, all patients are admitted to hospital until culture conversion (4 months), with long-term admission for extensively drug-resistant TB (XDR-TB) patients. Models A and B were based on recommendations from 2011 South African national policy5 and previously described models of care in South Africa.12,13 South African policy suggests that patients who are sputum smear-positive or have XDR-TB require admission for treatment initiation until two consecutive smear-negative results have been received. Alternative models of decentralised care describe short periods of hospitalisation of around 2 weeks at treatment initiation for the majority of patients, to ensure that the patients are stabilised on second-line medications.12
In the partially decentralised Model A, all patients are admitted for 2 weeks to initiate treatment, while the partially decentralised Model B required all sputum smear-positive patients to be hospitalised for 8 weeks or until smear conversion. In all models of care, once discharged from hospital, all patients were treated at clinic level.
Estimation of total costs of different models across nine provinces in South Africa
We estimated the total cost of treating all diagnosed cases of RR-TB in South Africa, and the contribution of hospitalisation to this cost using each scenario. As different scenarios may be more appropriate in different settings or different provinces in South Africa, we considered an additional scenario using the urban/rural population ratio per province and then applying this ratio to the number of diagnosed MDR-TB cases in each province. To assess variability, we used the 95% confidence intervals (CIs) for the average length of hospitalisation to vary the estimates of costs of treatment across the different scenarios.
Sensitivity analysis
Five univariate sensitivity analyses were performed. The first analyses address uncertainty in the cohort population. We also examined our assumptions about the models of care, in particular the ability to provide all out-patient visits at a fixed site and accessibility of hospital care. Lastly, we used a different cost of inpatient day to accommodate the economies of scale applicable in larger, urban specialised TB hospitals (we assumed that the economies of scale are not relevant at the primary care level).
Ethics statement
Ethics approval was not required for this study as it did not involve the participation of human subjects.
RESULTS
Cohort description
Among the cohort, 72% of patients were human immunodeficiency virus (HIV) infected. The average treatment duration was 482 days (95%CI 457–507), with 169 days (95%CI 143–195) of intensive phase treatment. The average length of hospitalisation at admission and during treatment was respectively 36 days (95%CI 29–42) and 8 days (95%CI 4–12). Overall treatment success was 49%, with 30% default.
Cost of treatment in different models of care
Based on the cohort costing, the average cost of treatment for RR-TB, combining all types of RR-TB patients in the decentralised model, was US$7753. The average costs of managing a RR-TB patient in different models are shown in Table 2. The fully hospitalised model was 42% more costly than the fully decentralised model. Partially decentralised Models A and B are also lower-cost models of care than the fully hospitalised model. A much shorter period of hospitalisation in the decentralised models of care, ranging from 44 to 57 days, compared to 128 days of hospitalisation in the fully hospitalised model, was the key contributor to the reduced cost of treatment.
Table 2.
Costs of managing a drug-resistant tuberculosis patient from diagnosis to completion of treatment, different scenarios, 2013 (in US$)
| Fully decentralised US$ (95%CI) | Fully hospitalised US$ (95%CI) | Partially decentralised Model A* US$ (95%CI) | Partially decentralised Model B† US$ (95%CI) | |
|---|---|---|---|---|
| Clinic visits for diagnosis and monitoring | 174 (152–207) | 131 (109–163) | 174 (152–207) | 152 (130–185) |
| Clinic visits for drug collection and injections | 1530 (1481–1582) | 1236 (1222–1411) | 1509 (1467–1558) | 1484 (1446–1526) |
| Hospital stay‡ | 3151 (2363–3867) | 9166 (6875–11 243) | 3580 (2650–4368) | 4082 (3079–5013) |
| Drugs | 2015 | 2015 | 2015 | 2015 |
| Diagnostic and monitoring tests | 883 | 883 | 883 | 883 |
| Total§ | 7753 (6917–8522) | 13 432 (11 165–15 494) | 8162 (7189–8999) | 8617 (7576–9590) |
Admission for all patients for 2 weeks to initiate treatment, extended hospitalisation for the proportion who were clinically unwell and ambulatory treatment for the rest.
Admission for all smear-positive patients for treatment initiation for 8 weeks, extended hospitalisation for a proportion who are clinically unwell and ambulatory treatment for the rest.
ALOS was calculated for each scenario based on the Khayelitsha patient cohort data: for a fully decentralised model, ALOS = 44 hospital days (36 days at admission + 8 days during treatment); for a fully hospitalised model, ALOS = 128 hospital days (120 days at admission + 8 days during treatment); for Model A, ALOS = 50 days (42 days at admission + 8 days during treatment); and for Model B, ALOS = 57 days (49 days at admission + 8 days during treatment).
Mean treatment duration of 482 (95%CI 457–507) was the same for all scenarios.
ALOS = average length of stay; CI = confidence interval.
Overall budget impact
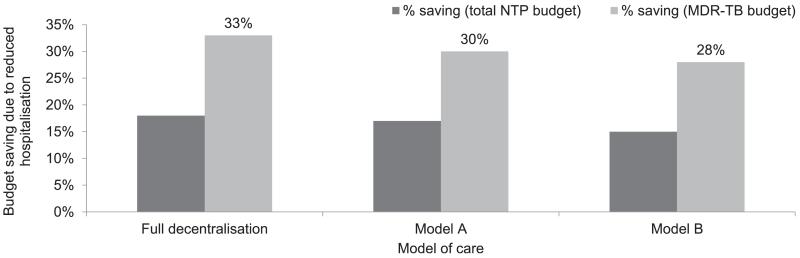
The total cost of treating all 14 161 diagnosed cases of RR-TB in 2012 in South Africa ranged from US$110 million in the fully decentralised model to US$190 million in the fully hospitalised model of care (Table 3). This translated to 23–40% of the 2013 total National Tuberculosis Programme (NTP) budget, and 42–73% of the total MDR-TB budget. Following a more decentralised approach for treating RR-TB patients could potentially reduce the costs by 15–32% (Figure). The urban-rural scenario could also potentially reduce the cost of treatment by 14% at the national level (Table 4). This scenario will have a larger impact in the more urban provinces such as Gauteng, the Western Cape and the Free State, where higher proportions of the population live in urbanised areas.
Table 3.
Total cost of treatment for all patients diagnosed and treated in South Africa in 2012 and the associated proportion of the budget spent in different scenarios, 2013 (in US$)
| Fully decentralised US$ |
Fully hospitalised US$ |
Partially decentralised Model A US$ |
Partially decentralised Model B US$ |
|
|---|---|---|---|---|
| Cost for all diagnosed cases* | 109 794 198 | 190 208 003 | 115 584 975 | 122 029 079 |
| Proportion of total NTP budget† | 23 | 40 | 24 | 26 |
| Proportion of MDR-TB budget‡ | 42 | 73 | 44 | 47 |
There were 14 161 cases diagnosed in 2012.14
2013 budget (WHO 2013).1
Using the 2010 and 2011 budget figures, 55% of the 2013 total NTP budget was allocated to MDR-TB (WHO 2013).1
NTP = National Tuberculosis Control Programme; MDR-TB = multidrug-resistant tuberculosis; WHO = World Health Organization
Figure.
Potential cost savings through reduced hospital stay, different scenarios. NTP = National Tuberculosis Control Programme; MDR-TB = multidrug-resistant tuberculosis.
Table 4.
Total cost of treatment for all diagnosed patients assuming different models of care for urban and rural populations, per province, 2013 (US$)
| Province | % urban/rural population | Diagnosed MDR-TB cases n | Total cost: fully decentralised model US$ | Total cost: fully hospitalised model US$ | Total cost: % urban fully decentralised and % rural fully hospitalised US$ |
|---|---|---|---|---|---|
| Eastern Cape | 36.6/63.4 | 2 205 | 17 095 982 | 29 617 163 | 25 034 414 |
| Free State | 68.6/31.4 | 390 | 3 023 779 | 5 238 410 | 3 918 238 |
| Gauteng | 97.0/3.0 | 1 198 | 9 228 429 | 16 091 320 | 8 795 179 |
| KwaZulu-Natal | 43.1/56.9 | 6 630 | 51 404 246 | 89 052 967 | 82 714 173 |
| Mpumalanga | 39.1/60.9 | 266 | 2 062 372 | 3 572 864 | 3 419 898 |
| Northern Cape | 70.1/29.9 | 760 | 5 892 493 | 10 208 183 | 7 521 590 |
| Limpopo | 11.0/89.0 | 373 | 2 891 973 | 5 010 069 | 5 793 957 |
| North West | 34.9/65.1 | 267 | 2 070 126 | 3 586 296 | 3 539 572 |
| Western Cape | 88.9/11.1 | 2 072 | 16 064 796 | 27 830 731 | 16 810 363 |
| South Africa | 53.7*/46.3 | 14 161 | 109 794 198 | 190 208 003 | 162 370 737 |
In 2011, 62% of South Africa’s population was urban. However, the estimates for provinces were not available.
MDR-TB = multidrug-resistant tuberculosis.
Sensitivity analysis
Varying the proportion of sputum smear-positive patients in Model B resulted in relatively small impacts of <5% of the total cost of treatment in that model of care (Table 5). If clinic visits for drug collection in Model A were replaced by home visits, the cost of treatment went up by 22%; however, total cost remained lower than with fully centralised care. The impact of the lower/upper cost of in-patient day on the total cost of treatment depended on the extent to which patients were hospitalised in each model. In the urban-rural scenario, replacing the fully hospitalised model with the partially decentralised Models A and B reduced the total cost of treatment by respectively 31% and 29%. The sensitivity analysis showed 17% higher/3% lower total costs for all scenarios when the relative proportion of XDR-TB and pre-XDR-TB patients was doubled/halved.
Table 5.
Sensitivity analysis using the total cost of treatment for all diagnosed cases, 2013 (in US$)
| Scenario | Fully decentralised US$ | Fully Hospitalised, US$ | Partially decentralised Model A US$ | Partially decentralised Model B US$ | Urban/rural divide US$ |
|---|---|---|---|---|---|
| Base-case estimate | 109 794 198 | 190 208 003 | 115 584 975 | 122 029 079 | 162 370 737 |
| Model B | |||||
| 20% smear-positive | – | – | – | 117 196 727 | – |
| 60% smear-positive | – | – | – | 125 763 169 | – |
| Model A | |||||
| Home visits at US$10.75* per patient per day | 141 187 047 | – | – | ||
| Using a range of costs for in-patient day | |||||
| Lower estimate at US$44.44† (applicable to larger urban hospitals due to economies of scale) | 92 868 141 | 140 959 464 | 96 347 256 | 100 098 080 | 115 133 858 |
| Upper estimate at US$98.77‡ (applicable to rural hospitals with fewer patients and higher transport costs) | 126 720 295 | 239 438 456 | 134 815 613 | 143 952 007 | 178 902 710 |
| Replacing centralised approach in rural patients with | |||||
| Model A | – | – | – | – | 112 471 859 |
| Model B | – | – | – | – | 115 455 086 |
| If relative % of XDR-TB and pre-XDR-TB | |||||
| Doubled | 128 129 829 | 221 972 740 | 134 887 666 | 142 407 935 | 189 486 650 |
| Halved | 106 390 578 | 184 311 555 | 112 001 841 | 118 246 178 | 157 337 244 |
Estimate.
Cost data from the urban TB hospital.
Cost data from the rural TB hospital.
TB = tuberculosis; XDR-TB = extensively drug-resistant TB.
DISCUSSION
We observed that the introduction of decentralised treatment in South Africa may reduce the overall cost to the NTP by between approximately 15% and 18% of the NTP budget, depending on the model adopted and on different assumptions about the characteristics of the MDR-TB patient cohort. Given that the overall cost of RR-TB treatment will likely increase with Xpert-driven increases in case detection,15 cost savings that could be achieved through the implementation of more decentralised treatment could absorb these increased overall costs. Moreover, a decentralised model of care has been shown to be as effective as a fully hospitalised model of care, and may improve patient access.9,12 Other benefits include earlier treatment initiation, resulting in improved early mortality and theoretically reduced community transmission.
Our episode cost findings are based on sound cohort data, and our unit and episode costs are consistent with previous cost analyses of MDR-TB treatment in South Africa ranging from US$6772 to US$17 164 between decentralised and fully hospitalised models.2,7 Nevertheless our estimates have some limitations. First, we focused on the efficiency in the delivery of RR-TB treatment in different models of care and did not consider the hospital capacity for each model. In high-burden settings, the bed capacity required even for Models A and B may not be feasible. In this analysis, we only included health service costs and did not consider costs borne by RR-TB patients, which may vary with different models of care. We are currently undertaking an RR-TB patient cost analysis to complement this study. We did not include the cost of home visits in our estimates, as home visits are not part of the Khayelitsha model.8 However, we added the cost of home visits in one of our sensitivity analyses (Table 5, Model A). Finally, there remains some uncertainty about the number of RR-TB cases diagnosed. In the absence of a system to record all diagnosed cases, notifications rely on laboratory data, which may lead to an overestimation of the number of cases diagnosed due to duplicate results for the same patient.
One of the study findings was that the cost between the decentralised models of care is very similar, and that, therefore, from the cost perspective, different approaches to decentralisation could be applied in different settings. A fully decentralised model is appropriate in high-burden, urban areas with an existing infrastructure (e.g., staff available to be trained on RR-TB treatment), where clinics have the capacity to increase the workload and where hospitalisation is required only for those patients who are clinically unwell. Hospitals could be used for patients who are not doing well on treatment and those in whom treatment is failing. This includes a considerable proportion of XDR-TB patients, although those who do respond to treatment can be treated on an ambulatory basis. In addition, there are some settings with low population density and low RR-TB burden that may be more suited to a model of care with more hospitalisation to enable patients to receive an appropriate standard of care. However, in all models of care, appropriate referral management systems between hospitals and primary care services are required to maintain continuity of care and ensure that patients are supported. Different models of care with different cost profiles may therefore be needed to enable all RR-TB patients to receive treatment. Model A seems appropriate in remote, rural settings, where it may be more advantageous to initiate treatment in hospital for the majority of patients for 2 weeks. Finally, Model B could be implemented in a TB unit as part of a district hospital, with only very complicated patients referred to a specialised TB hospital. Decentralised models of care in high-burden rural areas can achieve further cost savings by using both clinic and home visits, depending on the patient’s proximity to their nearest clinic. From the patient’s perspective, models of care that provide more flexibility may be more suitable.
Potential difficulties with more widespread implementation of decentralised care need to be acknowledged. The capacity to treat the increasing number of patients in a clinic setting may be lacking in some areas, particularly in rural areas struggling to retain staff and allocate resources effectively. Clinic staff will need to be trained in RR-TB treatment management, with ongoing supervision. Training might require additional resources in terms of staff time away from their usual activities and transport. Strong referral systems for complicated cases, along with good systems for data recording and reporting, are also required.
Acknowledgements
HC is supported by the Wellcome Trust (099818/Z/12/Z), London, UK.
The study was funded in part by a grant from the Bill & Melinda Gates Foundation (BMGF), Seattle, WA, USA, and in part by Médecins Sans Frontières (MSF), Paris, France. BMGF had no role in the study design, analysis, decision to publish or preparation of the manuscript. Authors from MSF were involved in the study design, data collection and analysis. However, final preparation of the manuscript and the decision to publish rests with the first and last authors.
Footnotes
Conflicts of interest: no disclosures beyond those mentioned above.
References
- 1.World Health Organization . Global tuberculosis report, 2013. WHO; Geneva, Switzerland: 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 2.Pooran A, Pieterson E, Davids M, Theorn G, Dheda K. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLOS ONE. 2013;8:e54587. doi: 10.1371/journal.pone.0054587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Health Laboratory Service . GeneXpert MTB/RIF Implementation Progress Report. NHLS; Pretoria, South Africa: [Accessed October 2014]. Oct, 2013. http://www.nhls.ac.za/?page=genexpert_implementation_progress_reports&id=75. [Google Scholar]
- 4.World Health Organization Guidelines for the programmatic management of drug resistant tuberculosis: 2011 update. WHO; Geneva, Switzerland: 2011. WHO/HTM/TB/2011.6. [PubMed] [Google Scholar]
- 5.South African National Department of Health . Management of drug-resistant tuberculosis: policy guidelines. DoH; Pretoria, South Africa: 2011. [Google Scholar]
- 6.Meyer-Rath G, Schnippel K, Long L, et al. The impact and cost of scaling up GeneXpert® MTB/RIF in South Africa. PLOS ONE. 2012;7:36966. doi: 10.1371/journal.pone.0036966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnippel K, Rosen S, Shearer K, et al. Costs of inpatient treatment for multi-drug resistant tuberculosis in South Africa. Trop Med Int Health. 2013;18:109–116. doi: 10.1111/tmi.12018. [DOI] [PubMed] [Google Scholar]
- 8.Cox H, Hughes J, Daniels J, et al. Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Int J Tuberc Lung Dis. 2014;18:441–448. doi: 10.5588/ijtld.13.0742. [DOI] [PubMed] [Google Scholar]
- 9.Médecins Sans Frontières . Scaling up diagnosis and treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Research Report. MSF; Cape Town: [Accessed October 2014]. 2011. http://www.msf.org.za/msf-publications/scaling-diagnosis-and-treatmentdrugresistanttuberculosis-khayelitsha-south. [Google Scholar]
- 10.Statistics South Africa . Statistical Release P0141 Consumer Price Index February 2012. Statistics South Africa; Pretoria, South Africa: [Accessed October 2014]. 2012. http://www.statssa.gov.za/publications/P0141/P0141February2012.pdf. [Google Scholar]
- 11.Statistics South Africa . Statistical Release P0141 Consumer Price Index February 2013. Statistics South Africa; Pretoria, South Africa: [Accessed October 2014]. 2013. http://www.statssa.gov.za/publications/P0141/P0141February2013.pdf. [Google Scholar]
- 12.Brust JC, Shah NS, Scott M, et al. Integrated, home-based treatment for MDR-TB and HIV in rural South Africa: an alternate model of care. Int J Tuberc Lung Dis. 2012;16:998–1004. doi: 10.5588/ijtld.11.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padayatchi N, Friedland G. Decentralised management of drugresistant tuberculosis (MDR- and XDR-TB) in South Africa: an alternative model of care. Int J Tuberc Lung Dis. 2008;12:978–980. [PubMed] [Google Scholar]
- 14.Ndjeka N. Pretoria. National Department of Health; South Africa: 2013. Drug-resistant tuberculosis in South Africa. [Google Scholar]
- 15.Menzies NA, Cohen T, Lin H-H, Murray M, Salomon JA. Population health impact and cost effectiveness of tuberculosis diagnosis with Xpert® MTB/RIF: a dynamic simulation and economic evaluation. PLOS MED. 2012;9:e1001347. doi: 10.1371/journal.pmed.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]