Abstract
Antimicrobial peptides (AMP), an important part of the innate immune system, are crucial for defense against invading micro-organisms. Whereas AMP have been extensively studied in adult skin little is known about the impact of AMP in the developing human skin. We therefore compared the expression and regulation of AMP in fetal, neonatal and adult keratinocytes (KC) in vitro. The constitutive expression of human beta defensin-2 (HBD-2), HBD-3, S100 protein family members and cathelicidin was significantly higher in KC from fetal skin than in KC from postnatal skin. The capacity to further increase AMP-production was comparable between pre- and postnatal KC. Analysis of skin equivalents (SE) revealed a strong constitutive expression of S100 proteins in fetal but not in neonatal and adult SE. The elevated AMP-levels correlated with reduced H3K27me3 (tri-methyl-lysine 27 on histone H3) levels and increased expression of the histone demethylase JMJD3. Knock-down of JMJD3 in fetal KC significantly down-regulated the expression of HBD-3, S100A7, S100A8, S100A9 and cathelicidin.
Our data indicate an important contribution of histone-modifications in the regulation of AMP-expression in the skin during ontogeny. The elevated AMP expression in prenatal skin might represent an important defense strategy of the unborn.
INTRODUCTION
Antimicrobial peptides (AMP) are a diverse group of mostly cationic polypeptides with antimicrobial cytotoxic activities against bacteria, fungi and viruses. The mode of action of AMP against bacteria include formation of transmembrane pores as well as metabolic interference (Brogden, 2005). The main groups of AMP identified so far in adult human skin include defensins, cathelicidins, and members of the RNase and the S100 families (Harder and Schroder, 2005;Schroder and Harder, 2006). Whereas some AMP, such as RNase 7 and S100 proteins, are constitutively expressed in the upper layers of the epidermis, others like human beta-defensin (HBD)-2 and HBD-3 are inducible in response to pathogen invasion of the skin or by inflammatory mediators (Harder and Schroder, 2002;Harder and Schroder, 2005;Schroder and Harder, 2006;Suter et al., 2009;Afshar and Gallo, 2013). In addition to their direct antimicrobial effect, AMP also have important immune-modulatory properties (Yang et al., 2004;Yang et al., 2001b;Yang et al., 2002). While beta-defensins are chemotactic for dendritic cells and memory T cells by binding to CCR6 (Yang et al., 2002), cathelicidins are chemotactic for neutrophils, monocytes and T cells but not for dendritic cells (Yang et al., 2001a). In addition, AMP not only attract immune cells but are also able to directly activate them. S100A7 for example has been shown to induce secretion of cytokines and chemokines from neutrophils, which in turn contribute to innate immunity through enhancing neutrophil host defence functions (Zheng et al., 2008).
Recent studies showed that histone modifications, especially lysine methylation, have an important function during cell differentiation and organ development. In particular, the methylation status of histone H3K27 has been associated with the activation and repression of a variety of developmental genes (Nottke et al., 2009). In addition, Sen and coworkers identified an important role of H3K27 trimethylation in epidermal cells during epidermal differentiation. KC lacking JMJD3 expression, the enzyme responsible for demethylation of H3K27, showed an impaired KC-differentiation process, resulting in reduced levels of KC-differentiation associated proteins such as certain keratins, involucrin and the AMP S100A8 (Sen et al., 2008). Furthermore, the importance of JMJD3 and H3K27me3 has also been demonstrated in tissue regeneration such as wound healing (Shaw and Martin, 2009).
In contrast to adult human skin, only few data have been published on AMP expression and regulation in developing prenatal human skin. Recently cathelicidin, human neutrophil defensins and S100A7 have been detected in vernix caseosa and full-term amniotic fluid (Marchini et al., 2002;Yoshio et al., 2003;Porre et al., 2005) and RNAse 7 and S100A7 have been found to be present in fetal epidermis (Schuster et al., 2013), indicating that AMP play a role in prenatal antimicrobial defense. Furthermore, systematic studies on the innate immune system during fetal development have recently demonstrated that prenatal skin expresses the same spectrum of pattern recognition receptors (PRRs) as adult skin and that Toll-like receptors (TLRs) 1-5, NODs 1/2, NALPs 1/3, DECTIN-1 are even more highly expressed in prenatal than in postnatal skin. Functional experiments revealed that fetal KC strongly react to the activation of TLRs, suggesting an enhanced innate immune response of prenatal skin (Iram et al., 2012). Together these data demonstrated that fetal KC are equipped with specific immune functions to combat invading pathogens.
Once born, the neonate is exposed to an abundance of microbial agents. Since the neonatal immune system is not yet fully responsive (Elbe-Burger and Schuster, 2010;Ginhoux and Merad, 2010;Chorro and Geissmann, 2010;Palin et al., 2013;PrabhuDas et al., 2011), innate immunity presumably plays a crucial role for protection of the newborn against invading pathogens. To further explore the role of AMP as a rapid first line of defense in the developing skin, we investigated the expression profile of several groups of AMP in pre- and postnatal KC.
RESULTS
Fetal KC express high levels of mRNA from antimicrobial peptides
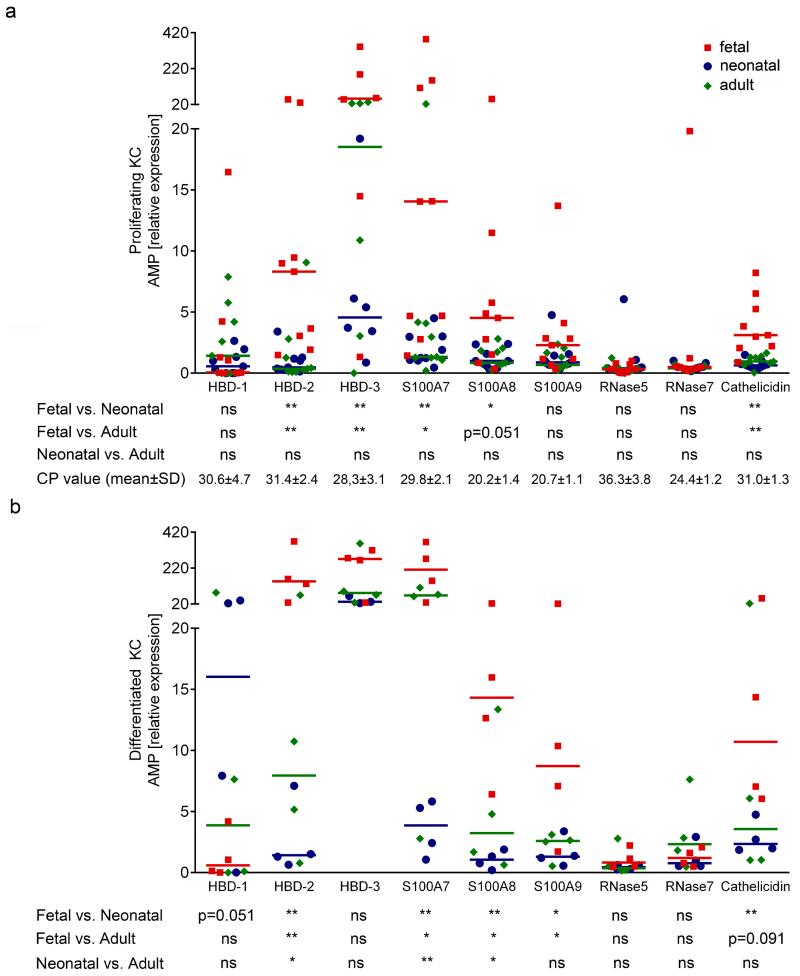
To comparatively assess the expression pattern of several AMP in pre- and postnatal, proliferating and differentiated KC, we performed real-time PCR for HBD-1, HBD-2, HBD-3, S100A7, S100A8, S100A9, RNase 5, RNase 7 and cathelicidin. As shown in figure 1, mRNA levels of HBD-2, HBD-3, S100A7, S100A8, S100A9 and cathelicidin were significantly higher in KC from several fetal donors as compared to KC derived from skin of neonatal and adult donors in proliferating (Figure 1a) as well as in differentiated KC (Figure 1b). In contrast to postnatal KC expression, the level of HBD-1 was only weakly inducible by differentiation of fetal KC. S100A8 and RNase 5 were not inducible at all in neonatal KC (Table 1). Differences between the AMP expression levels of proliferating neonatal and adult KC were not detected (Figure 1a), whereas in differentiated KC expression of HBD-2, S100A7 and S100A8 was constantly higher in adult KC (Figure 1b). Regulation of the KC-differentiation dependent proteins keratin 14, keratin 10, filaggrin and loricrin was comparable in all three KC groups before and after induction of KC-differentiation (Supplementary Figure 1), suggesting a comparable differentiation process in pre- and postnatal KC. These data demonstrate that despite comparable capacities of KC derived from different age groups to proliferate and differentiate, fetal KC constitutively express significantly higher levels of HBD-2, HBD-3, S100A7, S100A8, S100A9 and cathelicidin levels than adult and neonatal KC.
Figure 1. Fetal KC have a different mRNA expression pattern of AMP.
Fetal, neonatal and adult KC cultured under proliferating and differentiated conditions were investigated for AMP expression levels using quantitative real-time PCR. (a) Proliferating fetal KC expressed significantly higher levels of HBD-2, HBD-3, S100A7, S100A8 and cathelicidin as compared to neonatal and adult KC. (b) Differentiated fetal KC expressed significantly higher levels of HBD-2, S100A7, S100A8, S100A9 and cathelicidin as compared to neonatal and adult KC. The median and individual data points of nine different donors from each age group (a) and four donors from each age group (b) are shown. Each data point represents the mean of a measurement with three replicates; Mann Whitney test, *p<0.05, **p<0.005; CP value: mean PCR crossing point of all proliferating KC samples (for comparison: the CP of the housekeeping gene β2M was 19.3±1.1);
Table 1. Induction of AMP in differentiated KC.
Fold-induction of AMP in differentiated fetal (Fet.), neonatal (Neo.) and adult (Ad.) KC compared to proliferating KC is shown. Statistically significant values are highlighted in grey. The mean fold induction was calculated from four independent donors of each age group, each done in triplicate and statistical significance was calculated with Wilcoxon signed rank test:
| Gene | Fet. | Neo. | Ad. |
|---|---|---|---|
| HBD-1 | 2 | 14 * | 7 * |
| HBD-2 | 8 * | 5 * | 7 * |
| HBD-3 | 2 | 5 * | 8 * |
| S100A7 | 3 * | 2 * | 7 * |
| S100A8 | 6 * | 1 | 6 * |
| S100A9 | 5 * | 2 * | 4 * |
| RNase 5 | 2 | 1 | 3 * |
| RNase 7 | 2 * | 18 * | 7 * |
| Cathelicidin | 7 * | 5 * | 8 * |
| JMJD3 | 5 * | 1 | 1 |
p<0.05
Fetal KC constitutively produce high levels of HBD-2 and S100 proteins
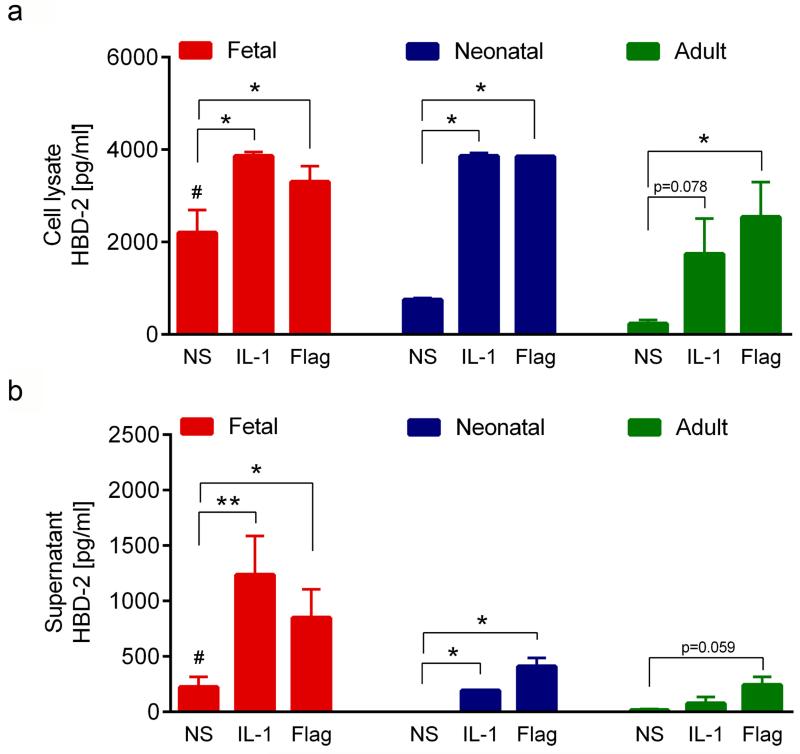
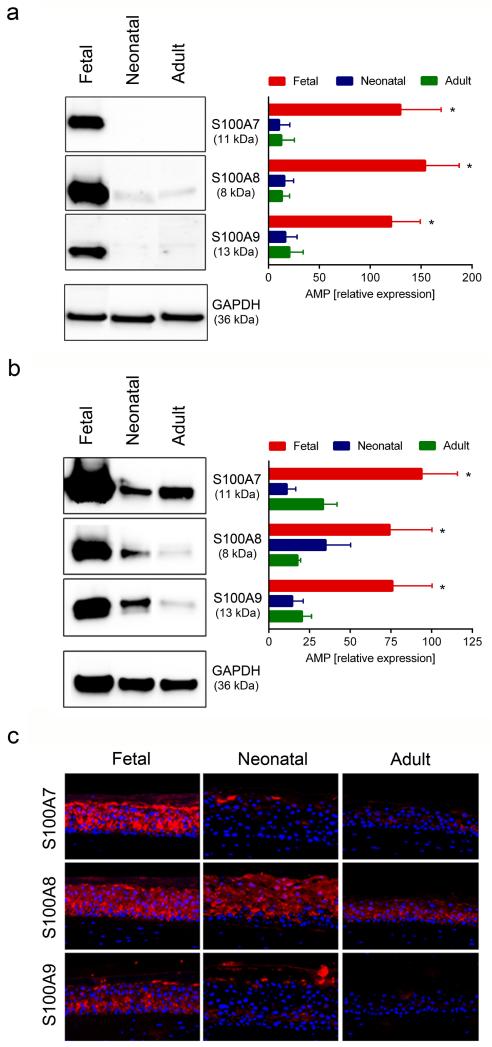
We next analyzed the production of AMP proteins by ELISA and Western blot analysis. Since HBD-2 belongs to a group of inducible AMP we also included IL-1α and the TLR5L flagellin, two known inducers of HBD-2 expression in postnatal KC (Abtin et al., 2008), as stimulants. In line with our real-time PCR data, significantly higher levels of HBD-2 protein were detected in cell lysates of non-stimulated fetal KC (2204±490 pg/ml) than in neonatal (754±35 pg/ml) and adult (232±8 pg/ml) KC (Figure 2a). The capacity of IL-1α and flagellin to upregulate HBD-2 was largely comparable in lysates of pre- and postnatal cells (Figure 2a). Surprisingly, considerable amounts (224±92 pg/ml) of HBD-2 were already released by unstimulated fetal KC but were not detectable in neonatal KC and only barely detectable in adult KC (Figure 2b). Both IL-1α and flagellin induced strong release of HBD-2 in fetal KC (1237±349 pg/ml and 850±256 pg/ml) (Figure 2b) but induction was much less pronounced in neonatal (193±21 pg/ml and 409±76 pg/ml) and adult (76±57 pg/ml and 243±73 pg/ml) KC (Figure 2b). Our mRNA results on S100A7-9 were corroborated by studying the production of S100 proteins. As shown in figure 3, Western blot analysis revealed strong baseline expression of S100A7, S100A8 and S100A9 in fetal proliferating KC, whereas their expression was marginal in neonatal and adult KC (Figure 3a). Since mRNA expression of the S100 proteins was induced in differentiated KC (Table 1), we also established SE cultures from KC of different age groups, and analyzed their protein production by Western blot analysis (Figure 3b) and immunofluorescence staining (Figure 3c). As in monolayer cultures expression of all three S100 proteins was significantly higher in SE established with fetal than with neonatal and adult KC (Figure 3b). This observation was confirmed by immuno-staining of SE. All three S100 proteins were strongly present throughout all epidermal layers in SE cultures of fetal KC. Whereas in SE cultures of neonatal and adult KC only the expression pattern of S100A8 was comparable with the one in fetal SE, S100A7 and S100A9 were only focally expressed (Figure 3c). Although cathelicidin mRNA was present in all KC tested, we could not detect protein expression of cathelicidin in monolayer cultures and SE using ELISA (data not shown).
Figure 2. HBD-2 expression and secretion is increased in fetal KC.
Fetal, neonatal and adult KC were cultured for 24 h in the presence or absence of IL-1α or the TLR5L flagellin and the concentration of HBD-2 in cell lysates (a) and supernatants (b) was determined by ELISA. Basal HBD-2 levels (NS) were significantly higher in supernatants and cell lysates from fetal KC as compared to neonatal and adult KC (#p<0.05). Stimulation with IL-1α or TLR5L induced HBD-2 expression in all KC groups (*p<0.05, **p<0.005). The mean and SEM of two to six independent experiments for each group are depicted; NS: non-stimulated control;
Figure 3. Fetal KC express high levels of S100A7, S100A8 and S100A9 protein in monolayer and SE cultures.
Fetal, neonatal and adult KC were cultured under proliferating conditions in monolayer cultures and analysed for the expression of S100 proteins (a). Western blot analysis of KC showed higher expression of S100 proteins in fetal KC, as compared to neonatal and adult KC. Western blots of representative samples and densitometric analysis of four to six independent experiments in each group are shown. *p<0.05 for fetal KC compared to neonatal and adult KC; SE from fetal, neonatal and adult KC were established and analyzed by Western blot analysis (b) and immunfluorescence staining (c). Western blot analysis showed higher expression of S100 proteins in SE from fetal KC, as compared to neonatal and adult KC. Western blots of representative samples and densitometric analysis of five to seven independent experiments in each group are shown. *p<0.05 for fetal KC compared to neonatal and adult KC. (c) Immunofluorescence staining of SE cultures showed higher expression of S100 proteins in SE cultured from fetal KC as compared to neonatal and adult KC; one representative experiment out of three is shown.
TLR-activation differently regulates expression of HBD-2, S100A7 and S100A8 mRNA in pre- and postnatal KC
To investigate the regulation of AMP after TLR-signaling in pre- and postnatal KC, proliferating cells were stimulated with the TLRL 1-9 and analyzed by real-time PCR. A regulation was only observed for HBD-2, HBD-3 and the S100 proteins but not for HBD-1, RNase 5 and 7 as well as cathelicidin independent of KC source and TLRL (Table 2). S100A8 showed the strongest response to cytokine and TLR-activation in fetal KC while HBD-2 and S100A7 were most potently induced in postnatal KC. Regulation of S100A9 was comparable between pre- and postnatal KC. HBD-3 was only weakly induced in fetal and neonatal KC after TLR-3 activation. IL-1α served as positive control and upregulated HBD-2 and the S100 proteins in fetal and adult KC, and slightly HBD-3 in fetal KC. The TLRL poly(I:C) (TLR3L), flagellin (TLR5L) and FSL1 (TLR6L) most potently upregulated AMP expression (Table 2, highlighted in grey). However, the total amount of HBD-2 mRNA was still slightly higher and the total amount of S100A7 and S100A8 mRNA was more than 100 fold higher in fetal KC compared to neonatal and adult KC (Supplementary Figure 2).
Table 2. Induction of AMP in response to IL-1α and TLRL.
Fold-induction of AMP in fetal (Fet.), neonatal (Neo.) and adult (Ad.) KC in response to stimulation with IL-1α or TLRLs 1-9 compared to non-stimulated controls is shown. Statistically significant values are highlighted in grey. The mean fold induction given in the table was calculated from five independent donors of each age group, each done in triplicate and statistical significance was calculated with Wilcoxon signed rank test:
| Gene | IL-1 | TLR1L (Pam3CSK4) | TLR2L (HKLM) | TLR3L (Poly I:C) | TLR4L (LPS) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| Fet. | Neo. | Ad. | Fet. | Neo. | Ad. | Fet. | Neo. | Ad. | Fet. | Neo. | Ad. | Fet. | Neo. | Ad. | |
| HBD-1 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 4 | 4 | 3 | 2 | 2 | 2 |
| HBD-2 | 78 * | 25 * | 160 * | 4 | 3 | 14 | 3 | 3 | 3 | 38 * | 154 * | 227 * | 2 | 2 | 2 |
| HBD-3 | 4 * | 2 | 1 | 1 | 2 | 1 | 4 | 1 | 1 | 4 * | 5 * | 1 | 1 | 1 | 1 |
| S100A7 | 8 * | 8 * | 54 * | 3 | 3 | 3 | 4 | 3 | 3 | 4 | 12 * | 14 * | 2 | 2 | 2 |
| S100A8 | 118 * | 4 | 8 * | 128 * | 3 | 3 | 40 * | 3 | 3 | 3 | 6 * | 7 * | 31 * | 2 | 2 |
| S100A9 | 7 * | 3 | 6 * | 3 | 2 | 2 | 3 | 2 | 2 | 5 * | 7 * | 8 * | 2 | 2 | 2 |
| RNase5 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| RNase7 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 3 | 2 | 1 | 2 | 2 |
| Cathelicidin | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| JMJD3 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 3 * | 4 * | 2 | 1 | 1 | 1 |
| Gene | TLR5L (Flaggelin) | TLR6L (FSL1) | TLR7L (Imiquimod) | TLR8L (ssRNA40) | TLR9L (ODN 2006) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| Fet. | Neo. | Ad. | Fet. | Neo. | Ad. | Fet. | Neo. | Ad. | Fet. | Neo. | Ad. | Fet. | Neo. | Ad. | |
| HBD-1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 5 | 3 |
| HBD-2 | 17 * | 24 * | 195 * | 19 * | 5 * | 42 * | 2 | 2 | 2 | 3 | 3 | 52 * | 2 | 2 | 2 |
| HBD-3 | 1 | 1 | 1 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | nd | nd | nd |
| S100A7 | 4 | 9 * | 14 * | 6 * | 3 | 6 * | 2 | 2 | 2 | 2 | 2 | 3 | 1 | 2 | 3 |
| S100A8 | 83 * | 4 | 6 * | 79 * | 3 | 3 | 52 * | 3 | 3 | 23 * | 3 | 3 | 7 | 5 | 5 |
| S100A9 | 6 * | 4 | 6 * | 6 * | 3 | 6 * | 4 | 3 | 3 | 3 | 2 | 3 | 3 | 2 | 4 |
| RNase5 | 2 | 2 | 2 | 6 | 1 | 2 | 6 * | 1 | 3 | 3 | 1 | 2 | 4 | 2 | 2 |
| RNase7 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 2 |
| Cathelicidin | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 3 |
| JMJD3 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 |
p<0.05; nd=not done
Expression of the histone demethylase JMJD3 influences the expression of HBD-3, S100A7, S100A8, S100A9 and cathelicidin
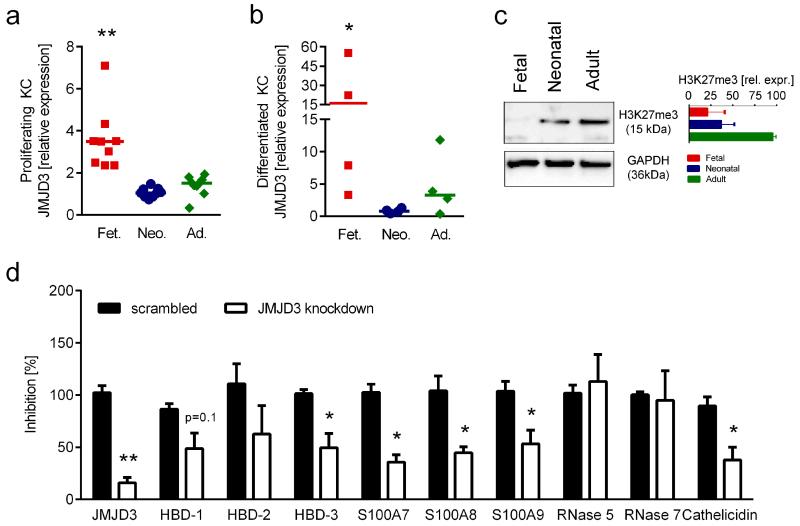
The histone demethylase JMJD3 is involved in gene-regulation during KC-differentiation by erasing H3K27me3 from diverse promoters including the S100A8 promoter (Sen et al., 2008). We therefore investigated the expression of JMJD3 mRNA in fetal, neonatal and adult KC and correlated it with the expression of several AMP. We found that JMJD3 expression was significantly higher in proliferating fetal KC as compared to both neonatal and adult KC (Figure 4a). Moreover, KC differentiation in monolayer cultures additionally increased JMJD3 expression in fetal cells, whereas no significant increase was observed with neonatal and adult KC (Figure 4b). Western Blot analysis revealed that in contrast to postnatal KC, H3K27me3 was barely detectable in fetal KC (Figure 4c). Spearman correlation between JMJD3 and the AMP tested in our study revealed a high correlation for HBD-3 (R=0.861), S100A7 (R=0.585), S100A8 (R=0.758) and cathelicidin (R=0.698) (Supplementary Figure 3). To further investigate the contribution of JMJD3 to AMP expression, we performed JMJD3 knock-down experiments using siRNA technology. Knock-down of JMJD3 in fetal KC led to significantly reduced expression levels of HBD-3, all S100 proteins tested and cathelicidin (Figure 4d), suggesting a direct impact of H3K27me3 demetylation on the regulation of certain KC-derived AMP.
Figure 4. JMJD3 knock-down influences the expression of HBD-3, S100A7, S100A8 S100A9 and cathelicidin.
Fetal, neonatal and adult KC cultured under proliferating (a) and differentiated (b) conditions were investigated for JMJD3 expression levels using quantitative real-time PCR. Fetal KC were found to express significantly higher levels of JMJD3 as compared to neonatal and adult KC (a,b Mann Whitney test, *p<0.05, **p<0.005). The median and individual data points of four to nine different donors per group are shown; each data point represents the mean of a measurement with three replicates. (c) Western blot analysis showed lower expression of H3K27me3 in fetal KC, as compared to neonatal and adult KC. Western blot of one representative experiment and densitometric analysis of two independent experiments are shown. (d) siRNA mediated knock-down of JMJD3 in fetal KC resulted in significantly lower expression of JMJD3, HBD-3, S100A7, S100A8, S100A9 and cathelicidin. The mean and SEM of two independent experiments with 3 different siRNAs each done in triplicates are depicted; paired t-test, *p<0.05, **p<0.005;
Discussion
The innate component of the skin immune system is the first line of defense, protecting the body from invasion of microbial pathogens. This first line consists of the physical barrier of the stratum corneum and the presence or induction of AMP produced by epithelial cells (Afshar and Gallo, 2013;Wiesner and Vilcinskas, 2010). Under physiological conditions, these mechanisms prevent microbial invasion on exposed body sites such as the skin but also colonization of sterile body sites such as the cerebrospinal fluid and the amniotic cavity by pathogenic microbes. Due to the fact that the adaptive immune system is not yet fully functional in utero (Elbe-Burger and Schuster, 2010;Ginhoux and Merad, 2010;Chorro and Geissmann, 2010;PrabhuDas et al., 2011;Palin et al., 2013), the innate immune system, especially AMP and PRRs, have been suggested to play central roles for the survival of the developing fetus and the newborns. In the present study we have investigated the expression and regulation of a variety of AMP in KC derived from fetal, neonatal and adult skin.
The sterility of the amniotic cavity is thought to be established and maintained by the action of multiple physical and chemical barriers such as the vaginal (Mildner et al., 2010b) and cervical epithelium (Svinarich et al., 1997), the cervical mucus plug (Hein et al., 2002) and the chorio-amniotic membranes (Kjaergaard et al., 2001). Although these epithelia and membranes represent a powerful defense against invading pathogens, some are still able to cross these barriers (Evaldson et al., 1983;Galask et al., 1984). While it is well established that amniotic fluids have strong antimicrobial activity and contain several AMP including human neutrophil peptides (HNP) 1-3, bactericidal/permeability increasing protein (BPI), S100A7 (Porre et al., 2005) and S100A8/A9 (Sampson et al., 1997;Espinoza et al., 2003) our knowledge of AMP expression in fetal skin is scarce. The facts that (i) KC are a rich source of AMP, (ii) S100A7 is primarily of epithelial origin, (iii) AMP of the cathelicidin and beta-defensin families are present in human newborn foreskin specimens (Dorschner et al., 2003) and (iv) RNase 7 is expressed in periderm and prenatal epidermis (Schuster et al., 2013) prompted us to investigate the contribution of fetal KC to the innate immune defense of the fetus. We found that already under normal culture conditions fetal KC produce significantly more HBD-2, HBD-3, S100A7-9 and cathelicidin than either neonatal or adult KC. Our finding was striking, since Schuster and coworkers did not find increased HBD-2 and S100A7 expression levels in fetal skin. Possible explanations for this discrepancy could be that in Schuste’s work the authors investigated supernatants from cell suspensions derived from full-thickness skin samples (dermis and epidermis), thus probably having a dilution effect. In addition, they cultured their cells in a medium (RPMI containing fetal bovine serum) which was not favorable for the survival and growth of KC. These two alterations in the experimental design might account for the observed differences between the in vitro results from Schuster and colleagues and ours. As to the differences seen in their in vivo stainings it is tempting to speculate that the aqueous environment in which the fetus is embedded together with the not yet fully established cornified layer of the epidermis favor a more rapid release of soluble factors, resulting in a weaker staining intensity. Also striking was the fact that fetal KC constitutively express much higher levels of AMP than neonatal KC, suggesting that the elevated levels seen in newborn skin specimens (Dorschner et al., 2003) are a result of exposure to the environment. The fact that the high constitutive production of AMP was maintained even after several passages under sterile conditions and in the presence of antibiotics, excludes the possibility that bacterial contamination accounts for activation of fetal KC in our cultures. It rather indicates that AMP production is due to cell-autonomous mechanisms which differ in the cells of different age. Indeed, when we transferred cell culture supernatants from fetal KC to adult KC we could not detect any changes in AMP expression (Supplementary figure 4). This finding is in line with our previously published secretome analysis of pre- and postnatal KC (Iram et al., 2012), were we could not detect any differences in the baseline expression levels of a variety of cytokines and chemokines, suggesting that AMP are not indirectly enhanced in fetal KC via the production and release of other soluble factors. Interestingly, the capacity to further increase AMP-production after stimulation with inflammatory triggers was largely comparable between pre- and postnatal KC. Our in vitro data of postnatal KC are in line with previously published in vivo data, showing that baseline-expression of most AMP is quite low or even absent in the epidermis, but can be strongly upregulated after pathogen contact or inflammation (Abtin et al., 2008;Glaser et al., 2009;Kim et al., 2006). The high baseline production and upregulation of AMP in response to inflammatory stimuli in fetal KC suggest an important defense strategy of the skin before birth.
We further present evidence for a methylation dependent regulation of at least some AMP during skin development. The histone demethylase JMJD3 has been shown to be involved in epidermal development and antimicrobial defense, since its expression is required for the transcription of the KC differentiation locus and the S100A8 gene (Sen et al., 2008). Transient histone methylation changes that are strongly tissue- and developmental-stage specific are constantly ongoing in the fetus, leading to the regulation of multiple genes (Yuen et al., 2011). Here we could demonstrate that proliferating as well as differentiated fetal KC express significantly higher JMJD3 mRNA levels than neonatal and adult KC. We further identified significant correlations between the expression of JMJD3 and the AMP HBD-3, S100A7, S100A8 and cathelicidin. Moreover, we found that the trimethylated form of H3K27 was indeed less abundant in fetal KC as compared to neonatal and adult KC. siRNA mediated knockdown of JMJD3 confirmed our assumption that histone methylation is involved in the regulation of AMP in fetal KC. These results show that in addition to S100A8 (Sen et al., 2008) also other AMP – HBD-3, S100A7, S100A9 and cathelicidin – are regulated by JMJD3 dependent de-methylation processes of their promoter regions. Furthermore, our observation of high JMJD3 and low H3K27me3 levels in fetal KC fit to an earlier study describing a similar phenomenon in healing epidermis (Shaw and Martin, 2009).
In summary, we have shown for the first time a quantitative comparison of several AMP in pre- and postnatal KC. The data presented expand our understanding of the complex mechanisms involved in maintaining sterility of the amniotic cavity, and show that fetal KC possess a high capacity to produce AMP, thereby contributing to the innate immune responses of the developing organism.
MATERIALS AND METHODS
Cell culture
KC prepared from fetal skin were purchased from Cellsystems (Troisdorf, Germany) and Tebu-bio (Offenbach, Germany). KC prepared from neonatal foreskin and adult skin were purchased from Lonza (Basel, Switzerland). All KC donors were cultured in serum-free KC growth medium (KGM; Lonza) as described previously (Mildner et al., 2006). Fetal KC were 20-23 weeks of estimated gestational age. For stimulation, third passage KC were cultured in 12-well tissue culture plates (Corning, NY, USA) and used at a confluence of 60–70%. Stimulation was performed in KC basal medium (KBM; Lonza). For the analysis of differentiated KC, cells were kept in culture for four days after reaching 100% confluence.
Reagents used for treatment of KC
For in vitro assays, 10 ng/ml recombinant interleukin-1α (IL-1α; R&D Systems, Minneapolis, MN, USA) and the following TLR ligands (TLRL) (InvivoGen, San Diego, CA, USA) were used: 1 μg/ml synthetic tripalmitoylated lipopeptide (Pam3CSK4; TLR1/2L), 108 cells/ml heat-killed Listeria monocytogenes (HKLM; TLR2L), 25 μg/ml poly(I:C), a synthetic analog of double-stranded RNA (TLR3L), 100 ng/ml ultrapure LPS from E. coli K12 (TLR4L), 500 ng/ml purified flagellin from Salmonella typhimurium (TLR5L), 500 ng/ml synthetic lipoprotein of Mycoplasma salivarium (FSL1; TLR6/2L), 10 μg/ml imiquimod (R837; TLR7L), 1 μg/ml single-stranded RNA40 (TLR8L), and 2.5 μM/ml ODN2006 (CpG oligonucleotide type B; (TLR9L). For some experiments supernatants from fetal and adult KC after 24 hours of culture were collected, mixed with the same volume of fresh medium and transferred on new cultures of adult KC. After 24 hours mRNA was prepared and RT-PCR was performed as described below.
siRNA mediated JMJD3 knock-down
Third-passage fetal KC of 50–60% confluence were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to a published protocol (Mildner et al., 2010a). 1 ml OPTI-MEM medium (Invitrogen) was mixed with 10 μl Lipofectamine 2000 and 20 μl of a 20 μM siRNA (Invitrogen) solution or the scrambled control RNA (Invitrogen) solution. Three different JMJD3 siRNAs were used (siRNA ID: HSS146206, HSS146208 and HSS177200). After incubation at room temperature for 30 min, the solution was added to 20 ml KGM (Lonza) and transferred to the KC. KC were then incubated for 48 h before mRNA was isolated.
RNA isolation, cDNA synthesis and quantitative real-time polymerase chain reaction
After stimulation for 16 h, KC were washed with PBS, and RNA was isolated using RNeasy 96 Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. For cDNA synthesis RNA was reverse-transcribed with iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) and real-time PCR was carried out with LightCycler480 SYBR Green I Master (Roche Applied Science, Penzberg, Germany) according to the manufacturer’s instructions. The primers used are listed in Supplementary Table 1. The relative expression of the target genes was calculated by comparing with the housekeeping gene β2-microglobulin (β2M) or Keratin 14 (for stimulation experiments in Table 2, since the expression of β2M was modulated by TLR3 stimulation) using a formula described previously (Pfaffl, 2001). All real-time PCR experiments were performed at least in triplicate and the specificity of the reactions was confirmed by sequencing of the PCR products.
Preparation of 3D skin equivalent (SE) cultures
In vitro 3D SE were generated as described previously (Mildner et al., 2010a). Briefly, a suspension of collagen type I (PureCol, Advanced Biomatrix, San Diego, CA, USA) containing 1×105 fibroblasts per ml was poured into cell-culture inserts (3 μm pore size; BD Bioscience, Bedford, MA, USA) and allowed to gel for 2 h at 37°C. After equilibration with KGM for 2 h, 1.5×106 KC, in a total volume of 2 ml KGM, were placed on the collagen gel. After overnight incubation the medium was removed from both the inserts and external wells, and replaced in the external wells only by serum-free KC-defined medium consisting of KGM but without bovine pituitary extract and supplemented with 1.3 mM calcium, 10 μg/ml transferrin, 50 μg/ml ascorbic acid, and 0.1% bovine serum albumin (all supplements from Sigma-Aldrich, Vienna, Austria). Serum-free KC-defined medium was changed every second day for 7 days.
Western Blot Analysis
For analysis of protein expression, monolayer cultured KC or epidermis taken from SE cultures were lysed in a buffer containing 50 mM Tris (pH 7.4) and 2% SDS. After sonication, insoluble cell debris was removed by centrifugation and the protein concentration was measured by BCA assay (Pierce, Rockford, IL, USA). After denaturing with 0.1 M DL-dithiothreitol (DTT; Sigma-Aldrich), SDS–PAGE was conducted on 8–18% gradient gels (GE Amersham Pharmacia Biotech, Uppsala, Switzerland). The proteins were then electro-transferred onto nitrocellulose membranes (Bio-Rad) and immunodetected with the following primary antibodies: mouse monoclonal anti-S100A7 (5 μg/ml; clone 47C1068, Abcam, Cambridge, UK), mouse monoclonal anti-S100A8 (2 μg/ml; clone S13.67, Acris, Hiddenhausen, Germany), mouse monoclonal anti-S100A9 (1μg/ml; clone 47-8D3, Abcam), anti-H3K27me3 (4 μg/ml; clone: mAbcam 6002m, Abcam) and mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, dilution 1:2000; Biogenesis, Poole, UK). Secondary antibody was sheep anti-mouse-HRP (1:10000, Amersham, Buckinghamshire, UK) and the reaction products were detected by chemiluminescence with the ImmunStar™ Western C™ Substrate kit (Bio-Rad) according to the manufacturer’s instructions. The band intensity of four to seven independent experiments was quantified by densitometric analysis using ImageJ software.
Immunofluorescence labeling
Five μm thin-sections of formalin-fixed, paraffin-embedded SE were staining by immunofluorescence labeling as described previously (Mildner et al., 2010a). Briefly, fixed sections were deparaffinized, rehydrated and washed in PBS (2×5 min), incubated with 10% goat serum at RT for 30 minutes and then with primary antibodies overnight at 4°C (the same antibodies as described for Western Blot were used). Sections were then washed in PBS (2×5 min) and incubated with goat anti–rabbit Alexa Fluor 488 (Alexa, Eugene, OR, USA). Slides were washed in PBS (2×5 min) and counterstained with Hoechst to visualize nuclei. Images were recorded using the AX70 microscope with the imaging software MetaMorph from Olympus (Hamburg, GER).
Enzyme-linked immunosorbent assay
After 24 h stimulation of KC, cell culture supernatants were harvested and KC were washed with PBS and lysed with 0.1% IGEPAL lysis buffer at 4°C for 15 min. Supernatants and cell lysates were stored at −20°C until further analyses. Concentrations of HBD-2 in the supernatant and in the cell lysate were determined by enzyme-linked immunosorbent assay (ELISA) Development Kit (Immundiagnostik AG, Bensheim, Germany) according to the manufacturer’s instructions. Optical density at 450 nm was measured using a microplate reader (Fluostar Optima, BMG Labtech, Offenburg, Germany).
Statistical analysis
Statistical analysis was performed using the program GraphPad Prism version 5 (GraphPad Software Inc., San Diego, CA, USA), and calculations are based on the means of independent experiments (each individual experiment with three to four replicates; Figures 1, 2, 4a, and Tables 1 and 2) or on the individual values of all experiments (Figures 3a-b, Figure 4d). Data are represented as individual values (mean of three to four replicates per experiment) and the median (Figure 1 and Figure 4 a,b) or as mean and SEM (Figures 2, 3 and 4d, Supplementary figures 1 and 2). Statistical significance was calculated using Mann-Whitney test (unpaired, non-parametric data, Figures 1 and 4 a,b), Wilcoxon signed rank test (paired, non-parametric data, Tables 1 and 2), unpaired t-test (Figure 2 comparison of non-stimulated controls, Figure 3 and Figure 4a) or paired t-test (Figure 2 comparison of stimulations and Figure 4d). The correlation coefficient R was calculated by Spearman correlation test (non-parametric data, Supplementary Figure 3). A p-value below 0.05 was regarded as significant and is depicted with *, and a p-value below 0.005 is depicted with **.
Supplementary Material
ACKNOWLEDGEMENTS
We are very grateful to Heidemarie Rossiter and Erwin Tschachler for critical reading of the manuscript and helpful discussions. This project was funded in part by Austrian Science Foundation grants (P19474-B13 to A. E-B. and T545-B19 to M.G.).
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Abtin A, Eckhart L, Mildner M, et al. Flagellin is the principal inducer of the antimicrobial peptide S100A7c (psoriasin) in human epidermal keratinocytes exposed to Escherichia coli. FASEB J. 2008;22:2168–76. doi: 10.1096/fj.07-104117. [DOI] [PubMed] [Google Scholar]
- Afshar M, Gallo RL. Innate immune defense system of the skin. Vet Dermatol. 2013;24:32–8. doi: 10.1111/j.1365-3164.2012.01082.x. [DOI] [PubMed] [Google Scholar]
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–50. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Chorro L, Geissmann F. Development and homeostasis of ‘resident’ myeloid cells: the case of the Langerhans cell. Trends Immunol. 2010;31:438–45. doi: 10.1016/j.it.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Dorschner RA, Lin KH, Murakami M, et al. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res. 2003;53:566–72. doi: 10.1203/01.PDR.0000057205.64451.B7. [DOI] [PubMed] [Google Scholar]
- Elbe-Bürger A, Schuster C. Development of the prenatal cutaneous antigen-presenting cell network. Immunol Cell Biol. 2010;88:393–9. doi: 10.1038/icb.2010.13. [DOI] [PubMed] [Google Scholar]
- Espinoza J, Chaiworapongsa T, Romero R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- Evaldson G, Malmborg AS, Nord CE, et al. Bacteroides fragilis, Streptococcus intermedius and group B streptococci in ascending infection of pregnancy. An animal experimental study. Gynecol Obstet Invest. 1983;15:230–41. doi: 10.1159/000299415. [DOI] [PubMed] [Google Scholar]
- Galask RP, Varner MW, Petzold CR, et al. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol. 1984;148:915–28. doi: 10.1016/0002-9378(84)90534-9. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Merad M. Ontogeny and homeostasis of Langerhans cells. Immunol Cell Biol. 2010;88:387–92. doi: 10.1038/icb.2010.38. [DOI] [PubMed] [Google Scholar]
- Gläser R, Meyer-Hoffert U, Harder J, et al. The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J Invest Dermatol. 2009;129:641–9. doi: 10.1038/jid.2008.268. [DOI] [PubMed] [Google Scholar]
- Harder J, Schröder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem. 2002;277:46779–84. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- Harder J, Schröder JM. Antimicrobial peptides in human skin. Chem Immunol Allergy. 2005;86:22–41. doi: 10.1159/000086650. [DOI] [PubMed] [Google Scholar]
- Hein M, Valore EV, Helmig RB, et al. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187:137–44. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- Iram N, Mildner M, Prior M, et al. Age-related changes in expression and function of Toll-like receptors in human skin. Development. 2012;139:4210–9. doi: 10.1242/dev.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Romero R, Chaiworapongsa T, et al. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49:506–14. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaergaard N, Hein M, Hyttel L, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94:224–9. doi: 10.1016/s0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- Marchini G, Lindow S, Brismar H, et al. The newborn infant is protected by an innate antimicrobial barrier: peptide antibiotics are present in the skin and vernix caseosa. Br J Dermatol. 2002;147:1127–34. doi: 10.1046/j.1365-2133.2002.05014.x. [DOI] [PubMed] [Google Scholar]
- Mildner M, Ballaun C, Stichenwirth M, et al. Gene silencing in a human organotypic skin model. Biochem Biophys Res Commun. 2006;348:76–82. doi: 10.1016/j.bbrc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Mildner M, Jin J, Eckhart L, et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol. 2010a;130:2286–94. doi: 10.1038/jid.2010.115. [DOI] [PubMed] [Google Scholar]
- Mildner M, Stichenwirth M, Abtin A, et al. Psoriasin (S100A7) is a major Escherichia coli-cidal factor of the female genital tract. Mucosal Immunol. 2010b;3:602–9. doi: 10.1038/mi.2010.37. [DOI] [PubMed] [Google Scholar]
- Nottke A, Colaiacovo MP, Shi Y. Developmental roles of the histone lysine demethylases. Development. 2009;136:879–89. doi: 10.1242/dev.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin AC, Ramachandran V, Acharya S, et al. Human neonatal naive CD4+ T cells have enhanced activation-dependent signaling regulated by the microRNA miR-181a. J Immunol. 2013;190:2682–91. doi: 10.4049/jimmunol.1202534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porre S, Heinonen S, Mantyjarvi R, et al. Psoriasin, a calcium-binding protein with chemotactic properties is present in the third trimester amniotic fluid. Mol Hum Reprod. 2005;11:87–92. doi: 10.1093/molehr/gah141. [DOI] [PubMed] [Google Scholar]
- PrabhuDas M, Adkins B, Gans H, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12:189–94. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- Sampson JE, Theve RP, Blatman RN, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol. 1997;176:77–81. doi: 10.1016/s0002-9378(97)80015-4. [DOI] [PubMed] [Google Scholar]
- Shaw T, Martin P. Epigenetic reprogramming during wound healing: loss of polycomb-mediated silencing may enable upregulation of repair genes. EMBO Rep. 2009;10:881–6. doi: 10.1038/embor.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder JM, Harder J. Antimicrobial skin peptides and proteins. Cell Mol Life Sci. 2006;63:469–86. doi: 10.1007/s00018-005-5364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C, Gläser R, Fiala C, et al. Prenatal human skin expresses the antimicrobial peptide RNase 7. Arch Dermatol Res. 2013;305:545–9. doi: 10.1007/s00403-013-1340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GL, Webster DE, Barragan DI, et al. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 2008;22:1865–70. doi: 10.1101/gad.1673508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MM, Schulze K, Bergman W, et al. The keratinocyte in epidermal renewal and defence. Vet Dermatol. 2009;20:515–32. doi: 10.1111/j.1365-3164.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- Svinarich DM, Wolf NA, Gomez R, et al. Detection of human defensin 5 in reproductive tissues. Am J Obstet Gynecol. 1997;176:470–5. doi: 10.1016/s0002-9378(97)70517-9. [DOI] [PubMed] [Google Scholar]
- Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. 2010;1:440–64. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- Yang D, Biragyn A, Kwak LW, et al. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–6. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Rosenberg HF, et al. Human ribonuclease A superfamily members, eosinophil-derived neurotoxin and pancreatic ribonuclease, induce dendritic cell maturation and activation. J Immunol. 2004;173:6134–42. doi: 10.4049/jimmunol.173.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chertov O, Oppenheim JJ. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37) J Leukoc Biol. 2001a;69:691–7. [PubMed] [Google Scholar]
- Yang D, Chertov O, Oppenheim JJ. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell Mol Life Sci. 2001b;58:978–89. doi: 10.1007/PL00000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshio H, Tollin M, Gudmundsson GH, et al. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res. 2003;53:211–6. doi: 10.1203/01.PDR.0000047471.47777.B0. [DOI] [PubMed] [Google Scholar]
- Yuen RK, Jiang R, Penaherrera MS, et al. Genome-wide mapping of imprinted differentially methylated regions by DNA methylation profiling of human placentas from triploidies. Epigenetics Chromatin. 2011;4:10. doi: 10.1186/1756-8935-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Niyonsaba F, Ushio H, et al. Microbicidal protein psoriasin is a multifunctional modulator of neutrophil activation. Immunology. 2008;124:357–67. doi: 10.1111/j.1365-2567.2007.02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.