Abstract
Sediments from a high-level nuclear waste plume were collected as part of investigations to evaluate the potential fate and migration of contaminants in the subsurface. The plume originated from a leak that occurred in 1962 from a waste tank consisting of high concentrations of alkali, nitrate, aluminate, Cr(VI), 137Cs, and 99Tc. Investigations were initiated to determine the distribution of viable microorganisms in the vadose sediment samples, probe the phylogeny of cultivated and uncultivated members, and evaluate the ability of the cultivated organisms to survive acute doses of ionizing radiation. The populations of viable aerobic heterotrophic bacteria were generally low, from below detection to ∼104 CFU g−1, but viable microorganisms were recovered from 11 of 16 samples, including several of the most radioactive ones (e.g., >10 μCi of 137Cs/g). The isolates from the contaminated sediments and clone libraries from sediment DNA extracts were dominated by members related to known gram-positive bacteria. Gram-positive bacteria most closely related to Arthrobacter species were the most common isolates among all samples, but other phyla high in G+C content were also represented, including Rhodococcus and Nocardia. Two isolates from the second-most radioactive sample (>20 μCi of 137Cs g−1) were closely related to Deinococcus radiodurans and were able to survive acute doses of ionizing radiation approaching 20 kGy. Many of the gram-positive isolates were resistant to lower levels of gamma radiation. These results demonstrate that gram-positive bacteria, predominantly from phyla high in G+C content, are indigenous to Hanford vadose sediments and that some are effective at surviving the extreme physical and chemical stress associated with radioactive waste.
As a result of World War II and the subsequent Cold War, a large nuclear complex was developed in the United States, including large land tracts in Nevada, Idaho, and Washington state. Over a 40-year period, approximately 104 metric tons of plutonium was extracted from irradiated uranium at various sites within this complex. The result of the fuel chemical reprocessing at the Hanford Site, near Richland, Washington, and the Savannah River Site, near Aiken, South Carolina, was an accumulation of approximately 90 million gallons of high-level radioactive waste (HLW). Most of the waste was stored in tanks of various sizes and designs at Hanford and Savannah River, with lesser amounts at other sites across the United States.
At Hanford alone, approximately 107,000 tons of nuclear fuel was irradiated in nine reactors. Pu was extracted from the irradiated fuel by three different reprocessing schemes: reduction-oxidation process, bismuth-phosphate, and plutonium-uranium extraction process (27). Much of the waste from irradiated fuel processing was stored in 177 single-shell and double-shell underground storage tanks that now contain approximately 55 million gallons of poorly characterized but highly radioactive waste. The tanks are below ground and are covered with approximately 3 m of soil and gravel. The earliest tanks, used since 1944, had a design life of 10 to 20 years; leaks were first suspected in 1956 and were confirmed in 1959. The amount and distribution of waste leakage from the Hanford tanks is unknown, but present estimates range from 0.6 to 1.5 million gallons. This waste contains approximately 1 million Ci of radiation, primarily from 137Cs, but the HLW soon after reprocessing contained high levels of short-lived radionuclides, including 106Ru, 144Ce, 147Pm, and others (28). The wastes leaked from these tanks have been in contact with surrounding soils and vadose sediments for decades and have undergone significant geochemical and radiological transformations. Wastes also contained an estimated 870 tons of chemicals.
Microorganisms in terrestrial subsurface environments play a major role in the cycling of elements as well as weathering of rocks and sediments and can affect the geochemical properties of groundwater (25) by modifying the fate and transport of organic and inorganic contaminants. While the vadose region of the subsurface generally does not support robust microbial populations, particularly in arid regions, there have been numerous reports of viable microorganisms associated with unsaturated zone soils and sediments (15, 21, 31, 33), including at the Hanford Site (9, 24, 30). Water potentials in the vadose zone generally do not directly restrict microbial activity, because many microorganisms are relatively tolerant to the matric water potentials typical of vadose sediments (30). Rather, it is relatively thin, discontinuous water films that retard the diffusion of solutes, including nutrients and metabolic waste products that restrict microbial metabolism (41).
During the summer of 2000, a slant borehole was drilled beneath tank SX-108 at Hanford's S-SX tank farm that intercepted a vadose zone contaminant plume of high-level nuclear waste. The purpose of this sampling effort was to assess the distribution of contaminants and to obtain scientific information regarding processes that may influence the fate and transport of the contaminants. The plume was characterized by high concentrations of radionuclide and chemical contaminants, elevated temperature, and low moisture content. Some samples exhibited the highest levels of radioactivity (>50 μCi g−1) of any soils or sediments yet collected at Hanford. As part of this effort, core samples were analyzed for viable microbial populations, and DNA from the isolates and sediments was subjected to phylogenetic analysis to identify the microorganisms. The main objectives of this research were to analyze the microbiological properties of SX-108 sediment samples in relation to sediment properties and contaminant distributions and to assess potential biogeochemical effects on contaminant fate and transport.
MATERIALS AND METHODS
Sampling location and procedures.
During late July and early August of 2000, core samples were collected from the vadose zone beneath the SX-108 tank located within waste management area S-SX on the U.S. Department of Energy's (DOE's) Hanford Site. Tank SX-108 first received waste from Hanford Site nuclear fuel reprocessing operations in 1955, and the first leaks were believed to have occurred around 1962. The leaked wastes contained high solute concentrations as a result of self boiling and evaporation in the tank induced by the decay of short-lived radioisotopes. The geology at this location has been described elsewhere (45).
Percussion (cable tool) drilling was used to advance the borehole, and core samples were collected by using split-spoon techniques (42). The borehole was drilled at a 30° angle to intercept the subsurface at locations directly below leaked tank SX-108 (Fig. 1). Subsurface vadose samples were collected by procedures that do not use circulating drilling fluids that can promote core contamination (26). Due to regulatory requirements to accurately define contaminant distributions without artifacts, considerable care was taken to prevent cross-contamination of core samples.
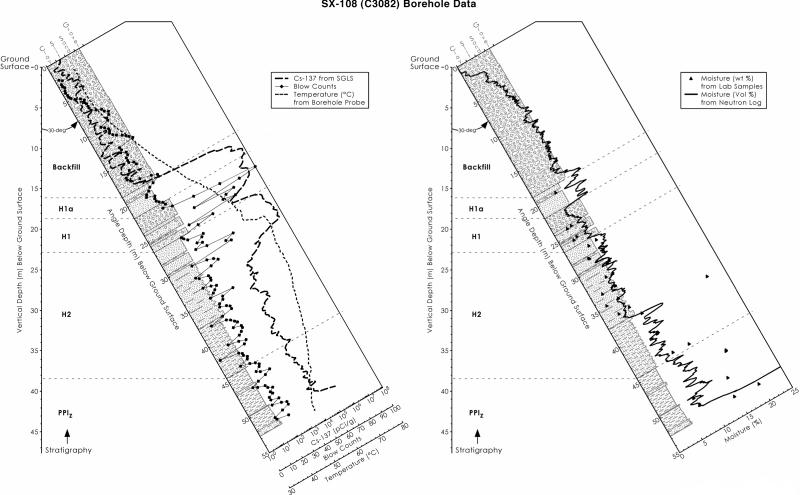
FIG. 1.
Field results from the SX-108 core, including stratigraphy, counts of 137Cs from down-hole spectral gamma logging, in situ temperature, and moisture content from down-hole neutron logging and laboratory measurements. High counts of 137Cs were observed immediately after samples were passed through the backfill surrounding the tank (see Fig. 2). Blow counts refers to the number of hammer strikes required to advance the borehole a given distance by cable tool drilling. SGLS, spectral gamma logging system. (Reprinted from reference 46 with permission of the publisher.)
In an effort to assess the effect of HLW contamination on the native vadose microbial population, two core samples from an adjacent uncontaminated borehole (299-W22-48) were obtained. These samples, designated RG1 and RG4, were collected from the same stratigraphic position as the SX-108 slant borehole cores. RG1 was from 25 m and RG4 from 27 m beneath the surface.
Sediment treatments.
Sediment was aseptically removed from the inner portion of core liners and was placed in sterile Whirlpak bags. Viable aerobic heterotrophic bacteria in untreated sediment were enumerated by dilution plate count methods (see below). Sediment was also used to directly inoculate liquid enrichment cultures. In addition, uncontaminated sediment (50 g) was irradiated at doses of 5 and 10 kGy with a 60Co source (MDS Nordion Inc., Kanata, Ontario, Canada) immediately prior to analysis by dilution plate count on peptone-tryptone-yeast extract-glucose (PTYG) agar medium (22). Sediment (50 g) was also placed inside an airtight vessel with desiccant (Drierite) to determine the effects of desiccation on the population of viable organisms. Moisture content (wt/wt) for both sediment samples decreased from 4.7% (RG1) and 9.0% (RG4) to 0.2% after 28 days, at which point the populations of viable aerobic heterotrophic bacteria were enumerated.
Culturing.
Untreated and treated vadose sediments were subjected to a variety of microbiological cultivation methods to determine the size and diversity of viable microbial populations. Based on the results of previous research involving vadose samples from the Hanford Site (5, 9, 24, 30), we focused our cultivation efforts on aerobic chemoheterotrophic bacteria but included enrichments for select physiological groups of anaerobic bacteria because of their potential for influencing contaminant chemical behavior. To this end, several types of agar and broth media were inoculated with each of 16 sediment samples obtained from the SX-108 borehole. Targeted microbial functional groups included aerobic heterotrophic bacteria, ammonia- and nitrite-oxidizing autotrophic bacteria, denitrifying bacteria, fermentative bacteria, Fe(III)-reducing bacteria, and sulfate-reducing bacteria. Details of these cultivation methods have been reported elsewhere (22, 38). Briefly, both dilution plate count and broth enrichment approaches were used. Broth media were inoculated directly with ∼1 g of sediment each. For dilution plates, sediment was suspended in the sterile pyrophosphate buffer, mixed vigorously, diluted, and spread on agar plates (22). Agar plates and enrichment broth were incubated at room temperature in the dark unless otherwise noted.
Agar plates were examined over a period of several months, but the number of bacterial colonies was determined at 14 days. Distinct colony types based on color, size, and morphology were noted, picked, and streaked onto fresh medium for isolation. For some core samples, bacterial colonies failed to develop on agar plates but growth was evident in broth enrichments. In these situations, a small volume of enrichment broth was transferred to fresh medium, including agar plates, in an attempt to isolate additional microorganisms. The cultures were preserved by freezing in 40% glycerol at −80°C. Culture stocks are maintained at Pacific Northwest National Laboratory and were also deposited with the DOE Subsurface Microbial Culture Collection at Florida State University (3).
Isolate 16S rRNA gene (rDNA) restriction fragment length polymorphism and phylogenetic analyses.
Bacterial cultures (isolates) were subjected to phylogenetic analysis by sequencing the 16S rRNA gene. The phylogenetic positions were analyzed by using distance matrix, maximum likelihood, and parsimony methods. Distance matrix analysis was performed with the PHYLIP group of computer programs (19). Distances were calculated with the method of Jukes and Cantor (29), and phylogenies were estimated with the FITCH option, which uses the Fitch-Margoliash criterion (20), and some related least-squares criteria. Maximum likelihood analysis was performed with the fastDNAml program (40). Parsimony analysis was carried out with the PAUP software package (PAUP* 4.0, beta version 4c) (47). A heuristic search was done first (using the standard program defaults), after which a bootstrap analysis (19) was used to assess the branch points of the resulting phylogenetic trees. A consensus tree was generated by bootstrapping at the greater-than-50% confidence limit, with 1,000 replications.
Community 16S rDNA analysis.
DNA was purified from sediment samples 3a, 5a, 6a, 8a, 12a, and 17a (Table 1). Ten 0.5-g aliquots of each sediment sample were processed using the FastDNA Spin kit for soil (Qbiogene), and the 10 50-μl eluants were pooled. PCR mixtures (50 μl) contained 1 μl of template, 1× PCR buffer, 1.5 mM MgCl2, 250 μM each deoxynucleoside triphosphate, 500 nM each primer, and 0.25 μl (1.25 U) of HotStar Taq (QIAGEN). Template (1 μl) was added to separate reaction mixtures at full strength and at 1:5, 1:15, 1:50, and 1:150 dilutions. rDNAs were amplified with universal primers 8f (5′-AGAGTTTGATCCTGGCTCAG-3′; 34) and 1390r (5′-ACGGGCGGTGTGTRCAA-3′; 50) and Archaea primers 21f (5′-TTCCGGTTGATCCYGCCGGA-3′) and 958r (5′-YCCGGCGTTGAMTCCAATT-3′) (18). Reaction mixtures were incubated in a Quadra thermal cycler (MJ Research) at 95°C for 10 min, followed by 35 cycles at 94°C for 1 min, 53°C for 45 s, and 72°C for 2 min and then a final extension of 10 min at 72°C. In some cases, 1 μl of amplified product was used as template in a seminested PCR with 518f (5′-CCAGCAGCCGCGGTAAT-3′) and 1390r primers. PCR products were verified by agarose gel electrophoresis, purified by using the QIAquick kit (QIAGEN), and ligated into pCR4-TOPO (Invitrogen). Ligations were shipped to the DOE Production Genomics Facility, where transformants were prepared and inserts were sequenced using standard protocols (http://www.jgi.doe.gov/Internal/prots_index.html). Sequence reads were analyzed against the Ribosomal Database Project database by blastN.
TABLE 1.
Chemical and physical characteristics of vadose samples from beneath Hanford waste tank SX-108c
| Sample | Vertical depth (m) | Water content (%) | pH | Conductivity (mS cm−1) | Detected amt of:
|
|||
|---|---|---|---|---|---|---|---|---|
| 137Cs (nCi g−1) | Cra (μg g−1) | NO3−a (mg liter−1) | NO2−a (mg liter−1) | |||||
| 1a | 16.6 | 4.3 | 9.2 | 0.40 | 3.06 × 103 | 0.02 | 7.0 | BDb |
| 3a | 20.5 | 2.8 | 9.6 | 0.70 | 1.95 × 104 | 0.98 | 29.1 | 0.4 |
| 4a | 21.8 | 2.8 | 9.5 | 0.58 | 1.38 × 103 | 0.86 | 23.5 | 0.3 |
| 5a | 23.1 | 4.7 | 9.8 | 0.88 | 6.52 × 103 | 3.64 | 92.8 | 0.3 |
| 6a | 24.4 | 3.7 | 8.0 | 16.71 | 5.31 × 104 | 483.83 | 11,740 | BD |
| 7a | 25.6 | 6.2 | 9.6 | 54.62 | 2.14 × 104 | 309.73 | 46,640 | BD |
| 8a | 26.9 | 6.0 | 7.9 | 49.01 | 5.55 × 102 | 829.76 | 39,710 | 87.5 |
| 9a | 28.2 | 2.4 | 7.9 | 31.76 | 0.17 | 512.62 | 22,850 | 57.1 |
| 10a | 29.5 | 1.9 | 8.2 | 25.56 | 0.45 | 398.13 | 18,990 | 59.0 |
| 11a | 30.8 | 3.2 | 8.4 | 13.93 | 0.91 | 0.90 | 9,520 | <10 |
| 12a | 32.0 | 21.4 | 8.0 | 2.36 | 0.34 | 0.29 | 1,530 | <1 |
| 13a | 34.5 | 7.6 | 8.0 | 29.78 | 0.52 | 430.95 | 22,200 | 72.5 |
| 14a | 37.0 | 12.0 | 7.8 | 30.24 | 0.84 | 297.83 | 21,500 | 46.3 |
| 15a | 39.5 | 17.4 | 7.5 | 40.01 | 0.59 | 336.50 | 34,600 | 34.4 |
| 16a | 41.9 | 7.5 | 7.2 | 5.80 | 0.01 | 0.11 | 4,190 | <10 |
| 17a | 43.9 | 19.7 | 7.2 | 3.74 | 0.18 | 0.09 | 2,390 | <1 |
Concentration in 1:1 water extract.
BD, below detection (0.1 mg liter−1).
Reprinted from reference 46 with permission of the publisher.
Ionizing radiation resistance.
Select isolates were analyzed for resistance to ionizing radiation from a 60Co source (MDS Nordion Inc.).
Cultures (50 ml) were grown in a medium of isolation, typically PTYG medium (22), to about mid-log to early stationary phase, and 10 ml was dispensed into triplicate 15-ml conical polypropylene tubes. Duplicate cultures were exposed to 2.5, 5, or 20 kGy while a single unexposed culture was used as a control. Cultures were kept on ice during irradiation to minimize growth. After exposure, 1-ml aliquots were removed from the tubes, diluted in sterile phosphate-buffered saline, and plated on agar medium. Agar plates were incubated at 30°C and examined for growth daily for up to 7 days. Percent survival was calculated as the population of cells surviving a given exposure relative to the unexposed control.
RESULTS
Vadose sediment physical and chemical properties.
The chemical and physical properties of the cored sediments (Table 1) reflect the complex effects of waste leakage from Hanford tank SX-108, subsequent migration of the tank liquor through the vadose zone, and geochemical reaction with vadose sediments. The slant borehole successfully traversed and allowed sampling of sediments beneath tank 108 that were contaminated with 137Cs and other chemical and radiological contaminants. Leaked wastes were very hot due to radioactive decay of short-lived isotopes during waste storage in the 1950s and 1960s and high concentrations of 137Cs associated with the HLW. Heating of the vadose sediments altered water seepage patterns in the subsurface and resulted in large-scale moisture redistributions. Thermal modeling of the SX tank farm and the SX-108 subsurface (43, 48) indicated that the temperature may have exceeded 100°C as deep as 24 m beneath the tanks at the time of the SX-108 leak (ca. 1962). At the time the samples were collected (2000), the temperatures had cooled from the estimated maximum (100°C) and ranged from near ambient (∼37°C) to 75°C (Fig. 1). The maximum subsurface temperature occurred near the lower depth of 137Cs penetration (e.g., ∼19 m). The effects of the thermal load were evident in the moisture contents of the various sediment samples as sediments were desiccated to depths of >20 m beneath the tanks (Table 1 and Fig. 1).
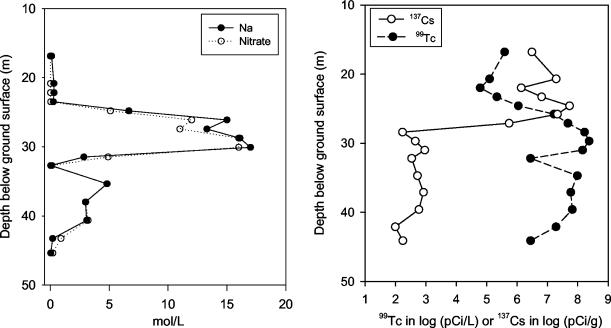
The pH of the sediments varied from 7.2 near the base of the borehole to >9 for several of the sediment samples collected from the upper region of the profile (Table 1). The moderately alkaline pH indicated that significant waste-sediment reaction had occurred that neutralized the high pH (>14) of the original waste from the reduction-oxidation process. The samples that were higher in the profile also contained the greatest concentrations of 137Cs, with sample 6a exceeding 50 μCi g−1 (Table 1 and Fig. 1 and 2). These high 137Cs concentrations resulted from the sorptive concentration of Cs+ by the abundant micaceous fraction of the sediment. These samples represent some of the most highly radioactive sediment samples yet collected at the Hanford Site. The highest concentrations of water-extractable Cr and nitrate are coincident and generally occur deeper in the profile than Cs, except in the cases of samples 6a to 8a. These differences result from the relative mobility of Cs+ and the negatively charged chromate and nitrate ions (for examples see references 36 and 49). The nitrate concentration in many of the samples was strikingly high, exceeding 10 g liter−1 in 1:1 water extracts in 50% of the samples. Computed pore water concentrations of NO3− based on the measured water contents of the sediments ranged between 5 and 15 mol liter−1 in the core of the plume (e.g., 24.4 to 29.5 m and 34.5 to 39.5 m; Fig. 2). Nitrite concentrations were substantially lower than those of nitrate but nonetheless exceeded 30 mg liter−1 in 1:1 water extracts in 6 out of 16 samples.
FIG. 2.
Porewater concentrations of Na, NO3, and 99Tc [Tc(VII)O4−] in the borehole samples determined by water extraction (46) and laboratory water content measurements. Also shown for reference is the sorbed concentration of 137Cs determined by high-resolution gamma energy analysis. (Reprinted from reference 46 with permission of the publisher.)
Technetium-99, the other major radiologic contaminant in the SX-108 vadose zone plume, existed deeper in the profile than 137Cs (Fig. 2). 99Tc is a long-lived mobile radionuclide (t1/2 = 2.13 × 105 years) that decays by beta emission in the form of the pertechnetate anion [Tc(VII)O4−]. The distribution of 99Tc was nearly identical to that of NO3− and defined the extent of the HLW vadose zone plume. The sorption status of 137Cs and 99Tc was distinct. 137Cs was strongly adsorbed as a high-affinity exchange complex on micaceous minerals that resist desorption except in saline electrolytes (35). In contrast, 99Tc was not adsorbed and existed as a solute in pore waters and as salt in air-filled pores.
Viable microbial populations.
In general, the populations of aerobic heterotrophic bacteria as determined by dilution plate counts were low, ranging from below detection to >104 CFU g−1 in the deepest sediment collected (17a) (Table 2). Of the three different agar media used in this study, PTYG yielded the highest populations of aerobic heterotrophic bacteria while R2A yielded fewer or no colonies for three samples; however, it provided for growth of a few colonies on two samples (10a and 15a) where PTYG agar did not.
TABLE 2.
Viable aerobic heterotrophic bacteria in vadose samples from beneath Hanford waste tank SX-108
| Sample | Vertical depth (m) | Viable plate counts (log CFU g−1)a
|
Growth in broth enrichments (PTYG/R2A) atc:
|
|||||
|---|---|---|---|---|---|---|---|---|
| PTYG | R2A | Actino | pH 7, 21°C | pH 10, 21°C | pH 7, 50°C | pH 10, 50°C | ||
| 1a | 16.6 | 4.0 | 4.0 | 4.0 | ++/++ | ++/++ | ±/++ | −/++ |
| 3a | 20.5 | BDb | BD | BD | ±/± | ±/± | −/− | −/− |
| 4a | 21.8 | 3.7 | BD | 2.9 | ++/++ | ++/++ | ±/± | ±/± |
| 5a | 23.1 | BD | BD | BD | ±/± | ±/± | ±/± | ++/± |
| 6a | 24.4 | BD | BD | BD | ±/± | −/± | −/− | −/− |
| 7a | 25.6 | 3.2 | 3.1 | 3.2 | ±/− | ±/± | −/− | ±/− |
| 8a | 26.9 | BD | BD | BD | ±/± | ±/± | ±/− | ±/− |
| 9a | 28.2 | 2.6 | BD | BD | ++/− | −/− | −/++ | ++/± |
| 10a | 29.5 | BD | 1.8 | BD | −/− | −/− | ±/− | ±/± |
| 11a | 30.8 | BD | BD | BD | −/− | −/− | ±/− | ±/− |
| 12a | 32.0 | 2.7 | 2.7 | 2.7 | ++/++ | ++/++ | ±/++ | ±/++ |
| 13a | 34.5 | BD | BD | BD | −/− | ++/± | ±/− | ++/− |
| 14a | 37.0 | BD | BD | BD | ±/− | ±/− | ±/− | ±/− |
| 15a | 39.5 | BD | 1.8 | BD | −/− | ++/− | ±/− | ±/− |
| 16a | 41.9 | 3.3 | 1.5 | BD | ++/− | −/− | −/− | ±/− |
| 17a | 43.9 | >4.3 | >4.3 | >4.3 | ++/++ | ++/++ | ±/− | ±/− |
Actino, growth on actinomycete isolation agar (DIFCO).
BD, below detection or <1.8 log CFU/g.
++, growth in original enrichment and transfer; ±, growth in original enrichment but not transfer; −, no growth. The backslashes separate results from PTYG and R2A enrichments.
Based on previous investigations, we anticipated relatively low population densities of aerobic heterotrophic bacteria in the contaminated vadose sediments. Therefore, liquid enrichments were included in the microbiological analyses. For a number of sediment samples, including highly radioactive sediments 3a, 5a, 6a, and 8a, positive broth enrichments were obtained where populations were below detection by dilution plate count techniques. Although most transfer attempts from the enrichments into fresh broth medium were unsuccessful, a number of isolates from the original enrichments were obtained by streak plate purification on agar medium, including several from the highly radioactive sediments. Many of the sediments that yielded successful enrichments at pH 7 also exhibited growth in the same medium where the pH was initially adjusted to 10. It is not possible from these analyses to establish whether the organisms that grew in the pH 10 enrichments were similar or distinct from those that grew at pH 7. Regardless, these results indicate the presence of organisms in the contaminated vadose sediments that were able to grow at alkaline pH values.
Because we anticipated elevated temperatures of the sediments beneath SX-108, replicate PTYG and R2A broth enrichments were also incubated at 50°C. Similar to the pH 10 enrichments, growth was common in many of the original enrichments but only a few of the cultures were successfully transferred (Table 2). Interestingly, the cultures that successfully transferred originated from some of the same samples for which the 21°C enrichment cultures also were successfully transferred; these included samples 1a, 9a, and 12a. A temperature of 50°C was selected for incubation of enrichment cultures, because it was estimated (e.g., Fig. 1) that this would approximate the in situ temperature for most of the sampled depths, although for some of the samples the temperatures were found to be higher (Fig. 1).
Because NO3− was a common tank waste constituent and the concentrations were remarkably high in a majority of the sediments examined, we initiated enrichments for denitrifying bacteria. Cores 12a and 17a were the only samples where the presence of viable denitrifying bacteria was confirmed. No sulfate-reducing or fermentative bacteria were cultured from any of the samples that were analyzed.
Uncontaminated vadose sediment microbial populations.
Two vadose samples were obtained from uncontaminated sediments from a borehole adjacent to the SX-108 slant borehole for comparison. These samples, designated RG1 and RG4, were from the same depths as the SX-108 samples that had the highest concentrations of 137Cs and, therefore, were stratigraphically similar. The population of viable aerobic heterotrophic bacteria in sample RG1 (Fig. 3) was low (2.4 log CFU g−1) but was comparable to the population size associated with sample 7a (Table 2) from the SX-108 borehole obtained from approximately the same depth. In contrast, the population from untreated RG4 sediment was relatively high at 5.5 log CFU g−1. This result is in considerable contrast to that for the sediment from SX-108 collected at approximately the same depth (8a), which exhibited no growth on PTYG agar even at the lowest dilution.
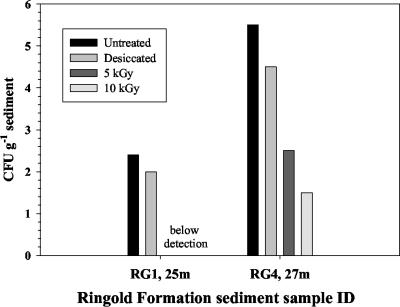
FIG. 3.
Influence of acute doses of ionizing radiation (60Co) on populations of viable aerobic heterotrophic bacteria in uncontaminated Hanford vadose zone sediments as determined by dilution plate counts on PTYG agar medium.
In order to assess the potential effects of drying and ionizing radiation on the population of viable vadose zone bacteria, uncontaminated sediments were subjected to desiccation or exposure to gamma radiation. The results from these experiments revealed that desiccation decreased the population sizes of aerobic heterotrophic bacteria in RG1 and RG4 by 2.5- and 10-fold, respectively (Fig. 3). Exposure to ionizing radiation had a much greater effect on the population size of viable aerobic bacteria, eliminating growth from the RG1 sample at both doses and decreasing the population size in RG4 by 3 and 4 orders of magnitude for acute exposures of 5 and 10 kGy, respectively.
Phylogeny and radiation resistance of isolates.
More than 110 cultures of aerobic heterotrophic bacteria were isolated and purified from the various enrichments and dilution plates (Table 3). To obtain insights into the genetic diversity and phylogeny of the isolates, the cultures were subjected to 16S rDNA gene sequencing.
TABLE 3.
Phylogenetic and gamma radiation resistance characteristics of isolates from contaminated (SX-108 slant borehole) and uncontaminated (299-W22-48) Hanford vadose sediments
| Core identity and sediment | Isolate identity | Nearest GenBank relative | SimRank | Accession no. | Dosea (kGy) | % Irradiation survival |
|---|---|---|---|---|---|---|
| SX-108 | ||||||
| 1a | 1b-1 | Arthrobacter globiformis | 0.982 | AY561524 | 2.5 | 0.24 |
| 1c-1 | Arthrobacter sp. strain CF-46 | 0.970 | AY561525 | 2.5 | 2.4 × 10−4 | |
| 1c-2 | A. globiformis | 0.967 | AY561526 | 2.5 | 9.4 × 10−3 | |
| 4a | 4a-1 | Rhodococcus fascians | 0.988 | AY561527 | ||
| 4a-2 | Clavibacter michiganense | 0.816 | AY561528 | |||
| 4a-3 | Microbacterium oxydans | 0.946 | AY561529 | 2.5 | 0 | |
| 4a-4 | Nocardia corynebacteroides | 0.991 | AY561530 | 2.5 | 0 | |
| 4b-1 | N. corynebacteroides | 0.983 | AY561531 | 2.5 | 0 | |
| 4b-2 | Staphylococcus warneri | 0.988 | AY561532 | |||
| 4b-3 | Nocardioides plantarum | 0.907 | AY561533 | |||
| 4c-1 | N. plantarum | 0.913 | AY561534 | |||
| 5a | 5L-1 | Arthrobacter agilis | 0.967 | AY561535 | 2.5 | 2.5 × 10−4 |
| 5L-2 | Agrococcus jenensis | 0.939 | AY561536 | 2.5 | 1.1 × 10−3 | |
| 5L-3 | Bacillus licheniformis | 0.977 | AY561537 | 2.5 | 0 | |
| 7a | 7b-1 | D. radiodurans | 0.980 | AY561538 | 20 | 0.21 |
| 7c-1 | D. radiodurans | 0.978 | AY561539 | 20 | 2.4 × 10−2 | |
| 7L-1 | M. luteus | 0.942 | AY561540 | 2.5 | 0.86 | |
| 8a | 8c-1 | Unnamed β-Proteobacterium | 0.871 | AY561542 | 2.5 | 0 |
| 8b-1 | Sphingomonas asaccharolytica | 0.968 | AY561541 | |||
| 9a | 9c-3 | Unnamed α-Proteobacterium | 0.966 | AY561544 | 2.5 | 0 |
| 9c-2 | Arthrobacter sp. strain CF-46 | 0.991 | AY561543 | |||
| 9c-4 | Dermabacter hominis | 0.966 | AY561545 | |||
| 9c-5 | Verrucosispora gifhornensis | 0.904 | AY561546 | |||
| 10a | 10c-1 | γ-Proteobacterial clone G21 | 0.929 | AY561547 | 2.5 | 1.7 × 10−3 |
| 10c-2 | Unnamed α-Proteobacterium | 0.877 | AY561548 | 2.5 | 0 | |
| 12a | 12a-1 | P. stutzeri | 0.997 | AY561549 | ||
| 12b-1 | P. stutzeri | 0.994 | AY561550 | 2.5 | 0 | |
| 15a | 15a-1 | Terrabacter tumescens | 0.948 | AY561551 | ||
| 15c-1 | Azospirillum lipoferum | 0.855 | AY561552 | 2.5 | 0 | |
| 16a | 16b-1 | Staphylococcus pasteuri | 0.986 | AY561553 | 2.5 | 4.1 × 10−2 |
| 16b-2 | S. warneri | 0.981 | AY561554 | |||
| 16b-4 | A. globiformis | 0.969 | AY561555 | |||
| 16b-5A | S. warneri | 0.999 | AY561556 | |||
| 16b-5B | S. warneri | 0.989 | AY561557 | |||
| 16c-1a | A. globiformis | 0.973 | AY561558 | |||
| 17a | 17a-1 | Arthrobacter nicotinovorans | 0.960 | AY561559 | 2.5 | 5.6 × 10−3 |
| 17a-2 | Arthrobacter sp. strain CF-46 | 0.959 | AY561560 | 2.5 | 3.5 × 10−2 | |
| 17a-3 | P. stutzeri | 0.994 | AY561561 | 2.5 | 0 | |
| 17a-4 | P. stutzeri | 0.990 | AY561562 | |||
| 17a-5 | Streptomyces sampsonii | 0.932 | AY561563 | |||
| 17b-1 | Arthrobacter sp. strain CF-46 | 0.909 | AY561564 | 2.5 | 6.7 × 10−4 | |
| 17b-2 | Pseudomonas sp. strain BRW1 | 0.998 | AY561565 | |||
| 17c-1 | Arthrobacter sp. strain CF-46 | 0.939 | AY561566 | 2.5 | 0 | |
| 17c-2 | Pseudomonas sp. strain BRW1 | 0.998 | AY561567 | |||
| 299-W22-48 | ||||||
| RG1 | RG-1 | Arthrobacter sp. strain CF-46 | 0.970 | AY561568 | ||
| RG-2 | Janibacter limosus | 0.858 | AY561569 | |||
| RG-3 | Variovorax sp. strain WFF52 | 0.927 | AY561570 | |||
| RG-4 | Variovorax sp. strain WFF52 | 0.931 | AY561571 | |||
| RG-5 | Arthrobacter sp. strain S2215 | 0.947 | AY561572 | |||
| RG-6 | Mycobacterium hodleri | 0.909 | AY561573 | |||
| RG-7 | M. hodleri | 0.908 | AY561574 | 5 | 8.8 × 10−4 | |
| RG-9 | Terrabacter sp. strain DPO 1361 | 0.840 | AY561575 | |||
| RG-59 | A. globiformis | 0.928 | AY561618 | |||
| RG-60 | Alcaligenes sp. strain 05-51 | 0.803 | AY561619 | |||
| RG-61 | A. globiformis | 0.936 | AY561620 | |||
| RG4 | RG-10 | Streptomyces sp. strain 254 | 0.936 | AY561576 | ||
| RG-11 | γ-Proteobacterial clone JAP412 | 0.973 | AY561577 | |||
| RG-12 | Arthrobacter sp. strain AC-48 | 0.905 | AY561578 | |||
| RG-13 | Arthrobacter sp. strain S22215 | 0.951 | AY561579 | |||
| RG-14 | R. fascians | 0.965 | AY561580 | |||
| RG-15 | A. agilis | 0.952 | AY561581 | |||
| RG-16 | Unnamed β-Proteobacterium | 0.865 | AY561582 | |||
| RG-17 | Bradyrhizobium sp. strain BDV 5840 | 0.874 | AY561583 | |||
| RG-18 | Unnamed β-Proteobacterium | 0.865 | AY561584 | |||
| RG-19 | Unnamed β-Proteobacterium | 0.867 | AY561585 | |||
| RG-20 | Detolaasinbacter tsukamotoae | 0.798 | AY561586 | 2.5 | 0 | |
| RG-21 | γ-Proteobacterial clone JAP412 | 0.964 | AY561587 | |||
| RG-22 | Arthrobacter sp. strain S22215 | 0.958 | AY561588 | |||
| RG-23 | Arthrobacter oxydans | 0.948 | AY561589 | |||
| RG-24 | Arthrobacter sp. strain S22215 | 0.958 | AY561590 | |||
| RG-67 | Stenotrophomonas maltophilia | 0.964 | AY561626 | 2.5 | 0 | |
| RG-68 | S. maltophilia | 0.965 | AY561627 | 2.5 | 0 | |
| RG1-10kGyb | RG-25 | Staphylococcus epidermidis | 0.985 | AY561591 | 2.5 | 2.6 × 10−2 |
| RG-26 | Brevibacillus agri | 0.965 | AY561592 | 2.5 | 0 | |
| RG-64 | M. luteus | 0.960 | AY561623 | 5 | 0.44 | |
| RG-65 | M. luteus | 0.896 | AY561624 | 5 | 0.51 | |
| RG-66 | Arthrobacter ramosus | 0.955 | AY561625 | |||
| RG1-des | RG-62 | A. ramosus | 0.918 | AY561621 | ||
| RG-63 | Microbacterium oxydans | 0.939 | AY561622 | |||
| RG-69 | Pseudomonas migulae | 0.958 | AY561628 | 2.5 | 0 | |
| RG-70 | P. migulae | 0.957 | AY561629 | 2.5 | 0 | |
| RG4-10kGyb | RG-29 | M. luteus | 0.955 | AY561593 | 5 | 0.46 |
| RG-30 | Brevibacillus agri | 0.955 | AY561594 | 2.5 | 0 | |
| RG-71 | M. oxydans | 0.940 | AY561630 | |||
| RG-72 | Arthrobacter sp. strain CF-46 | 0.919 | AY561631 | |||
| RG-72A | Arthrobacter sp. strain S21004 | 0.932 | AY561632 | |||
| RG-73 | Arthrobacter sp. strain CF-46 | 0.922 | AY561633 | |||
| RG4-des | RG-33 | A. oxydans | 0.958 | AY561595 | ||
| RG-34 | Streptomyces griseus | 0.936 | AY561596 | |||
| RG-35 | Arthrobacter sp. strain CF-46 | 0.933 | AY561597 | |||
| RG-36 | Streptomyces setonii | 0.888 | AY561598 | |||
| RG-37 | Pseudomonas migulae | 0.943 | AY561599 | 2.5 | 0 | |
| RG-38 | Arthrobacter sp. strain CF-46 | 0.941 | AY561600 | |||
| RG-39 | Arthrobacter globiformis | 0.987 | AY561601 | |||
| RG-40 | C. michiganense | 0.778 | AY561602 | |||
| RG-43 | Arthrobacter sp. strain 19B | 0.953 | AY561603 | 2.5 | 1.9 × 10−4 | |
| RG-45 | Arthrobacter sp. strain S21004 | 0.947 | AY561604 | 2.5 | 3.1 × 10−2 | |
| RG-46 | Unnamed β-Proteobacterium | 0.863 | AY561605 | |||
| RG-47 | Streptomyces sp. strain 254 | 0.944 | AY561606 | |||
| RG-48 | Rhodococcus sp. | 0.922 | AY561607 | |||
| RG-49 | A. agilis | 0.975 | AY561608 | |||
| RG-50 | Rhodococcus sp. | 0.939 | AY561609 | 2.5 | 0 | |
| RG-51 | Streptomyces sp. strain 254 | 0.920 | AY561610 | |||
| RG-52 | Unnamed β-Proteobacterium | 0.912 | AY561611 | |||
| RG-53 | Arthrobacter sp. strain 19B | 0.948 | AY561612 | |||
| RG-54 | A. agilis | 0.921 | AY561613 | |||
| RG-55 | Arthrobacter sp. strain CF-46 | 0.985 | AY561614 | |||
| RG-56 | Arthrobacter sp. strain 19B | 0.968 | AY561615 | |||
| RG-57 | Pseudomonas migulae | 0.973 | AY561616 | 2.5 | 0 | |
| RG-58 | Arthrobacter sp. strain 19B | 0.946 | AY561617 |
Dose to cultures provided via 60Co irradiator.
Before plating, sediment was subjected to 10 kGy of gamma radiation from a 60Co source.
The genera represented among the isolates from the SX-108 samples included gram-positive bacteria high in G+C content that are typical inhabitants of soil and vadose sediments. Isolates whose closest match was a member of the genus Arthrobacter were the most common for cultures from both SX-108 and 299-W22-48 boreholes (Table 3). Other gram-positive genera commonly represented among the isolates included Staphylococcus and Nocardia in addition to relatives to several unclassified bacteria high in G+C content. Gram-negative genera were less common, but representatives included Pseudomonas and Sphingomonas as well as close relatives to a number of unclassified α-, β-, and γ-Proteobacteria. Interestingly, several isolates from sample 7a, one of the most radioactive samples collected, were closely related to Deinococcus radiodurans, a bacterium that can withstand acute doses of ionizing radiation to 15 kGy without lethality (17).
There are several interesting observations regarding the phylogenetic distributions of the isolates in Table 3. Only gram-positive and/or organisms high in G+C content were cultured from the most highly radioactive sediments, 1a to 7a (Table 1). In contrast, below sample 7a organisms related to gram-negative bacteria were relatively common, representing ∼45% of the isolates. Many of the same genera in the SX-108 vadose sediments were also present in the uncontaminated vadose sediments from borehole 299-W22-48.
Nineteen of the 20 radiation-resistant isolates were gram-positive bacteria high in G+C content, 13 of which were phylogenetically related to members of Arthrobacter and its close relative Micrococcus. Only one (10c-1) of the 13 isolates related to gram-negative bacteria exhibited any resistance to 2.5 kGy of gamma radiation. Three of the four isolates with some resistance to 5 kGy were most closely related to an uncultured Micrococcus luteus-like bacterium identified in a clone library obtained from a sludge sample from a recirculating two-stage bioreactor (14). The two isolates (7b-1 and 7c-1) that exhibited the highest levels of radiation resistance, with >0.2% of the population of 7b-1 cells surviving exposure to 20 kGy, were most closely related to D. radiodurans, one of the most radiation-resistant organisms known. The source of these strains was sample 7a, which had the second highest concentration of 137Cs at 21.4 μCi g−1.
Community 16S rDNA analysis.
The direct extraction of nucleic acids from vadose sediments followed by PCR amplification, cloning, and sequencing allowed for a cultivation-independent analysis of microbial phylogeny to complement the characterization of sediment isolates. With the bacterial primers, the 1:5-diluted DNA template produced the strongest bands on agarose gels for samples 12a and 17a, with very weak bands present with template at full strength and no bands present at 1:50 and 1:150 dilutions. For samples 3a, 5a, 6a, and 8a, no PCR products were observed on gels regardless of template level. Use of a seminested PCR produced visible products in these samples, with the exception of 8a. The archaeal primers failed to produce a PCR product in any of the sample extracts, regardless of template concentration. Competitive PCR containing 1:5 dilutions of indigenous template spiked with various amounts of Escherichia coli genomic DNA showed (with the exception of sample 8) between 300 and 900 copies of indigenous 16S target in the reaction, equivalent to 150,000 to 450,000 copies on a per-gram-of-sediment basis (data not shown). The extent to which PCR was able to sample these low-biomass communities was poor because the detection level, determined to be 80,000 copies by spiking the 1:5 dilutions of indigenous template with known amounts of nonindigenous 16S target into PCRs, was only two- to sixfold lower than the indigenous template concentrations (data not shown). Nevertheless, blastN analysis of sequences revealed between 2 and 11 genera per sample and 22 genera across all samples.
There was relatively good agreement, at the genus level, between the bacterial phylogenies obtained by the cultivation-independent cloning and sequencing approach and the samples from which isolates were obtained and characterized. Gram-positive bacteria high in G+C content, including members of Arthrobacter, Bacillus, Streptomyces, and Nocardioides, were among the most common genera represented among the cloned sequences (Table 4) and were also represented among the isolates (Table 3), especially Arthrobacter. Among the gram-negative genera represented in the clone libraries, Sphingomonas and Pseudomonas were also present, including a sequence closely related to Pseudomonas stutzeri from sample 12a (Table 4), the same sample from which an isolate closely related to P. stutzeri was obtained (Table 3). A P. stutzeri-like sequence was also obtained from the 17a clone library that was phylogenetically similar to three of the nine isolates from this sample.
TABLE 4.
Phylogenetic association of clones from vadose sediments recovered from the SX-108 slant borehole
| Core identity | Clone | Nearest GenBank relative | Identitya (%) | Accession no. |
|---|---|---|---|---|
| 3a | RAY457.x1 | Bacillus sp. strain YY | 719/723 (99) | AY579781 |
| RAY473.x1 | Unidentified eubacterium clone BSV05 from anoxic soil (likely Bacillus) | 720/726 (99) | AY579782 | |
| RAY479.x1 | Uncultured soil bacterium clone 432-1 (likely Bacillus) | 735/753 (97) | AY579783 | |
| RAY516.x1 | Achromobacter xylosoxidans strain 2002-55549 | 729/729 (100) | AY579784 | |
| RAY554.x1 | Uncultured bacterium clone 623-1 (likely Arthrobacter) | 756/760 (99) | AY579785 | |
| RAY592.x1 | Arthrobacter sp. strain SMCC G968 | 593/600 (98) | AY579787 | |
| RAY651.x1 | Bacterium strain LMG 18435 (likely Bacillus) | 736/741 (99) | AY579788 | |
| RAY690.x1 | Bacterium K2-24 (likely Bacillus) | 543/553 (98) | AY579789 | |
| 5a | RAZ387.x1 | Methylobacterium extorquens ATCC14718 | 693/693 (100) | AY579790 |
| RAZ409.y1 | Nocardioides plantarum DSM 11054T | 612/621 (98) | AY579791 | |
| 6a | RBA441.y1 | Taxeobacter sp. strain SAFR-033 | 632/675 (93) | AY579792 |
| RBA464.x1 | Achromobacter xylosoxidans CIP 7132t | 652/654 (99) | AY579793 | |
| RBA468.y1 | β-Proteobacterium A0647 | 663/703 (94) | AY579794 | |
| RBA471.y1 | Sphingomonas phyllosphaerae FA2 | 740/741 (99) | AY579795 | |
| RBA480.y1 | Methylobacterium extorquens ATCC14718 | 563/563 (100) | AY579796 | |
| RBA484.y1 | Uncultured Alcaligenes sp. clone ON5 or Bordetella hinzii | 620/625 (99) | AY579797 | |
| RBA486.x1 | Bacterium strain 86356 (likely Sphingomonas) | 620/627 (98) | AY579798 | |
| RBA505.x1 | Uncultured β-Proteobacterium clone pA42B412 | 638/638 (100) | AY579799 | |
| RBA669.x1 | Uncultured bacterium clone cvf122070 (CFB group) | 578/582 (99) | AY579800 | |
| RBA761.y1 | Sphingomonas paucimobilis ATCC 29837 | 431/440 (97) | AY579801 | |
| 12a | RBB389.x1 | Achromobacter xylosoxidans strain 2002-55549 | 710/713 (99) | AY579802 |
| RBB392.y1 | Streptomyces sp. strain KN-1220 | 598/603 (99) | AY579803 | |
| RBB399.y1 | Streptomyces sp. strain VTT E-99-1326 (A4) | 711/711 (100) | AY579804 | |
| RBB431.x1 | Saccharothrix tangerinus strain MK27-91F2 | 623/625 (99) | AY579805 | |
| RBB518.x1 | P. stutzeri strain ASK-1 | 657/657 (100) | AY579806 | |
| RBB541.y1 | A. globiformis JCM 1332 | 361/362 (99) | AY579807 | |
| RBB579.x1 | S. paucimobilis strain ATCC 29837 | 652/753 (99) | AY579808 | |
| RBB612.x1 | A. agilis strain WED2.2 | 705/705 (100) | AY579809 | |
| RBB674.y1 | Arthrobacter sp. strain Fa21 | 538/549 (97) | AY579810 | |
| RBB697.x1 | Nocardiodes sp. strain NCFB3005 or Aeromicrobium sp. strain GWS-BW-H252 | 604/614 (98) | AY579811 | |
| RBB732.x1 | Stenotrophomonas maltophilia strain 6B2-1 | 621/624 (99) | AY579812 | |
| 17a | RBC394.x1 | Arthrobacter sp. strain pfB10 | 547/554 (98) | AY579813 |
| RBC400.x1 | Arthrobacter sp. strain 19503 | 717/717 (100) | AY579814 | |
| RBC407.x1 | Geodermatophilus sp. strain 4S | 594/607 (97) | AY579815 | |
| RBC412.y1 | Kocuria erythromyxa ATCC 187T | 565/568 (99) | AY579816 | |
| RBC413.y1 | Achromobacter xylosoxidans strain 2002-55549 | 676/676 (100) | AY579817 | |
| RBC423.y1 | Uncultured earthworm cast bacterium clone c276 (likely Amycolatopsis) | 661/681 (97) | AY579818 | |
| RBC435.x1 | Uncultured actinobacterium clone APe4_57 (likely Arthrobacter) | 663/666 (99) | AY579819 | |
| RBC437.x1 | Uncultured actinobacterium clone APe4_57 (likely Actinobispora) | 597/606 (98) | AY579820 | |
| RBC439.x1 | Nocardioides sp. strain NCFB3007 | 646/651 (99) | AY579821 | |
| RBC450.x1 | Arthrobacter crystallopoietes DSM 20117 | 555/564 (98) | AY579822 | |
| RBC489.x1 | P. stutzeri strain ASK-1 | 675/676 (99) | AY579823 | |
| RBC620.x1 | Blastococcus aggregatus strain DSM 4725T | 562/566 (99) | AY579824 | |
| RBC630.x1 | Arthrobacter sp. strain An5 | 632/632 (100) | AY579825 | |
| RBC645.x1 | A. agilis strain WED2.2 | 666/666 (100) | AY579826 | |
| RBC716.x1 | Streptococcus sanguis ATCC 10556 | 611/614 (99) | AY579827 | |
| RBC738.x1 | Phenanthrene-degrading bacterium 70-2 (likely Janthinobacterium) | 661/673 (98) | AY579828 | |
| RBC759.x1 | Arthrobacter aurescens | 685/688 (99) | AY579829 |
Nucleotides identical to nearest Genbank relative/total nucleotides of clone sequence.
DISCUSSION
In spite of harsh chemical and physical conditions imposed on vadose sediments by wastes leaked from tank SX-108 (Tables 1 and 2), viable aerobic heterotrophic bacteria were recovered from 11 of the 16 sediment samples. Due to low population densities it is difficult to discern trends in either population size or presence of aerobic heterotrophic bacteria in relation to sediment properties such as pH, water content, and contaminant concentration (Table 1). Several sediment samples, 1a, 4a, and 7a, with relatively low water contents and high radioactivity also contained moderate populations of heterotrophic bacteria. The highest viable populations were associated with samples that had not been subjected to heating and drying or severe contaminant exposure: 17a (>4.3 log CFU g−1) from SX-108 and RG4 (5.5 log CFU g−1) from the 299-W22-48 uncontaminated borehole. RG4 was from an uncontaminated region of the vadose zone, and 17a was among the least contaminated samples from SX-108. Because no attempts were made to measure total microbial biomass in these samples, it was not possible to draw any conclusions regarding relationships between total microbial biomass and sediment properties.
One of the caveats that must be recognized with a study of this type is the limitation associated with using cultivation-based methods exclusively for microbiological characterization. In some environments, the population size of the cultured prokaryotic community can be as much as 2 to 4 orders of magnitude below the population size determined by direct microscopic counting (2). In spite of their limitations, cultivation methods have previously been successfully applied to characterizing subsurface microbial populations in saturated (4, 23) and unsaturated (9, 24) nonradioactive subsurface sediments. The use of cultivation-based methods over sequence-based methods has the advantage that cultures can be used for physiologic and metabolic analyses (1). In this study, we applied both methods to investigate the phylogenetic composition of the microbial populations associated with contaminated subsurface sediments from the Hanford Site. We found the results (Tables 3 and 4) of both methods to be in reasonably good agreement, and they were consistent with previous findings (24, 30), supporting the idea that viable populations in Hanford vadose sediments are sparse but are typically higher in regions where the moisture contents are elevated.
Isolates related to members of the gram-positive bacteria high in G+C content dominated the cultures obtained from both the contaminated and uncontaminated vadose sediments, and they exclusively represented organisms isolated from either highly radioactive SX-108 samples or irradiated uncontaminated sediments (Table 3). The same group also dominated the phylogeny of cloned sequences obtained from sediment DNA extracts (Table 4). In contrast to the highly radioactive and gamma-irradiated samples, nearly half of the isolates from sediment samples 17a and RG4 that had little or no contamination and relatively high water contents were gram-negative Proteobacteria. Although the results are not quantitative, the phylogenetic diversity and the dominance of gram-positive bacteria high in G+C content was greater in the sequenced sediment DNA clones from samples 12a and 17a (Table 4) than was represented among the isolates from these same samples (Table 3). Desiccation alone did not eliminate the isolation of gram-negative bacteria from RG1 or RG4, as did gamma irradiation (Table 3), suggesting that ionizing radiation, perhaps in combination with other contaminants, may have had a significant effect on the phylogenetic composition of the vadose microbial population.
Previous studies have indicated that, in general, gram-positive bacteria such as Arthrobacter spp. are more drought tolerant than gram-negative organisms like Pseudomonas spp. (13, 32, 44). In fact, Arthrobacter members appear to be well adapted to life in arid soils (12), and some members are adept at surviving for extended periods of desiccation (8). Members of the genus Arthrobacter also appear to be well adapted to vadose sediments of the Hanford Site, as approximately one-third of the total isolates and a significant number of cloned sequences (11 out of 48) from this study were related to members of this genus. This is about the same proportion of total viable aerobic chemoheterotrophic bacteria as was isolated from pristine Ringold Formation sediments obtained from another location on the Hanford Site (6). Arthrobacter spp. were also common isolates in a third study of vadose zone sediments at the Hanford Site (10). The phylogeny of the Ringold Formation Arthrobacter strains has been investigated in detail, and many of the isolates appear to represent novel species within the genus (16). Additional genera represented among the vadose zone cultures and sediment DNA-cloned sequences from this study that were also found in previous analyses of uncontaminated subsurface sediments from the Hanford Site (6, 10) include Rhodococcus, Staphylococcus, Streptomyces, Nocardioides, Bacillus, and Sphingomonas.
One of the more intriguing results from this study was the isolation of two cultures from core 7a (25.6 m) that were resistant to extreme (20 kGy) laboratory doses of gamma radiation. This sample was obtained from the highest 137Cs concentration region of the plume. Both of these isolates were closely related to D. radiodurans, a bacterium that is well recognized for its remarkable ability to withstand high levels of ionizing radiation. To our knowledge, this is the first time that D. radiodurans-like strains have been isolated from a radionuclide-contaminated environment. It is possible that Deinococcus is indigenous to Hanford soils and vadose zone sediments and that the harsh environment of the SX-108 contaminant plume led to conditions that selected for this highly stress-resistant organism. The ecological habitat of deinococci is poorly defined, but they do appear to be widely distributed in soils (11, 39). Additional studies are presently under way to determine if Deinococcus is a cosmopolitan inhabitant of Hanford Site soils. Mattimore and Battista (37) have shown that in D. radiodurans some genes that are necessary to survive irradiation are also necessary for desiccation resistance. However, a recent report (7) has shown the existence of genes in D. radiodurans that affect desiccation resistance but not radiation resistance, indicating that resistance to these conditions may involve different mechanisms.
Although all the factors influencing the microbiological characteristics of the SX-108 vadose sediments are unclear at this time, finding viable aerobic heterotrophic bacteria in radioactive sediments beneath the SX-108 tank may have important implications for the fate and transport of waste-associated contaminants. Microorganisms, in general, have the capacity for a wide range of biogeochemical transformations, including various reactions with waste constituents. For example, microorganisms are capable of degrading a wide range of organic compounds, oxidizing and reducing multivalent metals and radionuclides, such as Cr, U, and Tc, oxidizing ammonium to nitrite and nitrate, reducing nitrate or nitrite to ammonium or N2, and for sorption and/or assimilation of a range of cations, including Cs and Sr. An important consideration for microbial-driven biogeochemical processes in the vadose sediments, including interactions with contaminants, is the availability of water. Assuming that the water contents measured on the core sediment samples accurately reflect in situ water distributions, it is clear that microbial processes in the upper 31 m are presently of little consequence to contaminant fate and transport because diffusion of solutes would be extremely limited and microbial cells are sparse and will likely be inactive or dormant. However, any future increases in moisture content due to either episodic natural or artificial (24) recharge or alteration in regional climate patterns could result in significant increases in the size and activity of microbial populations in vadose sediments. Indeed, moisture calculations for the S-SX tank farm indicate that subsurface water contents are increasing as the system slowly re-equilibrates from the extreme thermal loads imposed through HLW waste boiling. This high thermal load decreased in the early 1970s as the decay of short-lived radionuclides declined.
We have confirmed the presence of viable bacteria in vadose zone sediments contaminated with high-level radioactive waste beneath waste tank SX-108 on DOE's Hanford Site. The site has experienced extreme geochemical, thermal, and radiological conditions in the past and still represents a harsh chemical and radiological environment. The culturable microbiota was comprised predominantly of aerobic chemoheterotrophic bacteria, mainly gram-positive organisms, including several highly radiation-resistant isolates related to D. radiodurans. Although these organisms are likely inactive or dormant under present environmental conditions, the ability of these organisms to survive under extreme conditions for extended periods in vadose sediments indicates that they could influence contaminant fate and transport should moisture regimes be altered in the future.
Acknowledgments
Research was supported by the DOE through the Environmental Management Sciences Program (EMSP), the Natural and Accelerated Bioremediation Research Program (NABIR), the Microbial Genome Program, and the Hanford Science and Technology Program managed by the Groundwater/Vadose Zone Integration Project. Pacific Northwest National Laboratory is operated for the DOE by Battelle Memorial Institute under Contract DE-AC06-76RLO 1830.
Sequence data from sediments were produced by the DOE Joint Genome Institute (http://www.jgi.doe.gov/). We thank David Lanigan and Jeff Serne for providing Fig. 1.
REFERENCES
- 1.Achenbach, L. A., and J. D. Coates. 2000. Disparity between bacterial phylogeny and physiology—comparing 16S rRNA sequences to assess relationships can be a powerful tool, but its limitations need to be considered. ASM News 66:714-715. [Google Scholar]
- 2.Amann, R., W. Ludwig, and K.-H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill, D. 1993. DOE makes subsurface cultures available. ASM News 59:504-506. [Google Scholar]
- 4.Balkwill, D. L., J. K. Fredrickson, and J. M. Thomas. 1989. Vertical and horizontal variations in the physiological diversity of the aerobic chemoheterotrophic bacterial microflora in deep Southeast Coastal Plain subsurface sediments. Appl. Environ. Microbiol. 55:1058-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkwill, D. L., E. M. Murphy, D. M. Fair, D. B. Ringelberg, and D. C. White. 1998. Microbial communities in high and low recharge environments: implications for microbial transport in the vadose zone. Microb. Ecol. 35:156-171. [DOI] [PubMed] [Google Scholar]
- 6.Balkwill, D. L., R. H. Reeves, G. R. Drake, J. Y. Reeves, F. H. Crocker, M. Baldwin-King, and D. R. Boone. 1997. Phylogenetic characterization of bacteria in the subsurface microbial culture collection. FEMS Microbiol. Rev. 20:201-216. [DOI] [PubMed] [Google Scholar]
- 7.Battista, J. R., M. J. Park, and A. E. McLemore. 2001. Inactivation of two homologues of proteins presumed to be involved in the desiccation tolerance of plants sensitizes Deinococcus radiodurans R1 to desiccation. Cryobiology 43:133-139. [DOI] [PubMed] [Google Scholar]
- 8.Boylen, C. W. 1973. Survival of Arthrobacter crystallopoietes during prolonged periods of desiccation. J. Bacteriol. 113:33-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brockman, F. J., T. L. Kieft, J. K. Fredrickson, B. N. Bjornstad, S. W. Li, W. Spangenburg, and P. E. Long. 1992. Microbiology of vadose zone paleosols in south-central Washington state. Microb. Ecol. 23:279-301. [DOI] [PubMed] [Google Scholar]
- 10.Brockman, F. J., C. J. Murray, E. M. Murphy, B. N. Bjornstad, D. Balkwill, D. B. Ringelberg, S. Pfiffner, and R. Griffiths. 1997. Microbial life in the unsaturated subsurface under conditions of extremely low recharge: an extreme environment, p. 388-394. In R. B. Hoover (ed.), Instruments, methods, and missions for the investigation of extraterrestrial microorganisms. Proceedings of SPIE, vol. 3111. Society of Photo-optical Instrumentation Engineers, Bellingham, Wash. [Google Scholar]
- 11.Brooks, B. W., and R. G. E. Murray. 1981. Nomenclature for “Micrococcus radiodurans” and other radiation-resistant cocci: Deinococcaceae fam. nov. and Deinococcus gen. nov., including five species. Int. J. Syst. Bacteriol. 31:353-360. [Google Scholar]
- 12.Cacciarai, I., and D. Lippi. 1987. Arthrobacters: successful arid soil bacteria. Arid Soil Res. Rehab. 1:1-30. [Google Scholar]
- 13.Chen, M., and M. Alexander. 1973. Survival of soil bacteria during prolonged desiccation. Soil Biol. Biochem. 5:213-221. [Google Scholar]
- 14.Christensson, M., L. L. Blackall, and T. Welander. 1998. Metabolic transformations and characterisation of the sludge community in an enhanced biological phosphorous removal system. Appl. Microbiol. Biotechnol. 49:226-234. [Google Scholar]
- 15.Colwell, F. S. 1989. Microbiological comparison of surface soil and unsaturated subsurface soil from a semiarid high desert. Appl. Environ. Microbiol. 55:2420-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crocker, F. H., J. K. Fredrickson, D. C. White, D. B. Ringelberg, and D. L. Balkwill. 2000. Phylogenetic and physiological diversity of Arthrobacter strains isolated from unconsolidated subsurface sediments. Microbiology 146:1295-1310. [DOI] [PubMed] [Google Scholar]
- 17.Daly, M. J., O. Y. Ling, and K. W. Minton. 1994. Interplasmidic recombination following irradiation of the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 176:7506-7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLong, E. F. 1992. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 89:5685-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felsenstein, J. 1996. Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol. 266:418-427. [DOI] [PubMed] [Google Scholar]
- 20.Fitch, W. M., and E. Margoliash. 1967. Construction of phylogenetic trees. Science 155:279-284. [DOI] [PubMed] [Google Scholar]
- 21.Franzmann, P. D., L. R. Zappia, B. M. Patterson, J. L. Rayner, and G. B. Davis. 1998. Mineralisation of low concentrations of organic compounds and microbial biomass in surface and vadose zone soils from the Swan Coastal Plain, Western Australia. Aust. J. Soil Res. 36:921-939. [Google Scholar]
- 22.Fredrickson, J. K., and D. L. Balkwill. 1998. Sampling and enumeration techniques, p. 239-254. In R. S. Burlage, R. Atlas, D. Stahl, G. Geesey, and G. Sayler (ed.), Techniques in microbial ecology. Oxford University Press, New York, N.Y.
- 23.Fredrickson, J. K., D. L. Balkwill, J. M. Zachara, S. W. Li, F. J. Brockman, and M. A. Simmons. 1991. Physiological diversity and distributions of heterotrophic bacteria in deep Cretaceous sediments of the Atlantic Coastal Plain. Appl. Environ. Microbiol. 57:402-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredrickson, J. K., F. J. Brockman, B. N. Bjornstad, P. E. Long, S. W. Li, J. P. McKinley, J. V. Wright, J. L. Conca, T. L. Kieft, and D. L. Balkwill. 1993. Microbiological characteristics of pristine and contaminated deep vadose sediments from an arid region. Geomicrobiol. J. 11:95-107. [Google Scholar]
- 25.Fredrickson, J. K., and T. C. Onstott. 2001. Biogeochemical and geological significance of subsurface microbiology, p. 3-38. In J. K. Fredrickson and M. Fletcher (ed.), Subsurface microbiology and biogeochemistry. Wiley-LISS, Inc., New York, N.Y.
- 26.Fredrickson, J. K., and T. J. Phelps. 1996. Subsurface drilling and sampling, p. 526-540. In C. J. Hurst, G. R. Knudsen, M. J. McInerney, L. D. Stetzenbach, and M. V. Walter (ed.), Manual of environmental microbiology. American Society for Microbiology, Washington, D.C.
- 27.Gephart, R. E., and R. E. Lundgren. 1996. Hanford tank cleanup: a guide to understanding the technical issues. PNNL-10773 Pacific Northwest National Laboratory, Richland, Wash.
- 28.Jones, T. E., R. A. Watrous, and G. T. Maclean. 2000. Inventory estimates for single-shell tank leaks in S and SX tank farms RPP-6285. CH2M HILL Hanford Group, Inc., Richland, Wash.
- 29.Jukes, T. H., and C. R. Cantor. 1963. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, New York, N.Y.
- 30.Kieft, T. L., P. S. Amy, F. J. Brockman, J. K. Fredrickson, B. N. Bjornstad, and L. L. Rosacker. 1993. Microbial abundance and activities in relation to water potential in the vadose zones of arid and semiarid sites. Microb. Ecol. 26:59-78. [DOI] [PubMed] [Google Scholar]
- 31.Kieft, T. L., E. M. Murphy, D. L. Haldeman, P. S. Amy, B. N. Bjornstad, E. V. McDonald, D. B. Ringelberg, D. C. White, J. Stair, R. P. Griffiths, T. C. Gsell, W. E. Holben, and D. R. Boone. 1998. Microbial transport, survival, and succession in a sequence of buried sediments. Microb. Ecol. 36:336-348. [DOI] [PubMed] [Google Scholar]
- 32.Kieft, T. L., D. B. Ringelberg, and D. C. White. 1994. Changes in ester-linked phospholipid fatty acid profiles of subsurface bacteria during starvation and desiccation in a porous medium. Appl. Environ. Microbiol. 60:3292-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konopka, A., and R. Turco. 1991. Biodegradation of organic compounds in vadose-zone and aquifer sediments. Appl. Environ. Microbiol. 57:2260-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane, D. J. 1991. 16S/23S rRNA Sequencing, p. 115-147. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons Ltd., New York, N.Y.
- 35.Liu, C., J. M. Zachara, S. C. Smith, J. P. McKinley, and C. C. Ainsworth. 2003. Desorption kinetics of radiocesium from subsurface sediment at the Hanford Site, USA. Geochim. Cosmochim. Acta 67:2893-2912. [Google Scholar]
- 36.Liu, C. X., J. M. Zachara, O. Qafoku, and S. C. Smith. 2003. Effect of temperature on Cs+ sorption and desorption in subsurface sediments at the Hanford Site, USA. Environ. Sci. Technol. 37:2640-2645. [DOI] [PubMed] [Google Scholar]
- 37.Mattimore, V., and J. R. Battista. 1996. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 178:633-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinley, J. P., T. O. Stevens, J. K. Fredrickson, J. M. Zachara, F. S. Colwell, K. B. Wagnon, S. A. Rawson, and B. N. Bjornstad. 1997. The biogeochemistry of anaerobic lacustrine and paleosol sediments within an aerobic unconfined aquifer. Geomicrobiol. J. 14:23-39. [Google Scholar]
- 39.Murray, R. G. E. 1992. The family Deinococcaceae, p. 3732-3744. In A. Ballows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, vol. 4. Springer-Verlag, New York, N.Y. [Google Scholar]
- 40.Olsen, G. J., H. Matsuda, R. Hagstrom, and R. Overbeek. 1994. fastDNAmL: a tool for construction of phylogenetic trees of DNA sequences using maximum likelihood. Comput. Appl. Biosci. 10:41-48. [DOI] [PubMed] [Google Scholar]
- 41.Papendick, R. I., and G. S. Campbell. 1981. Theory and measurement of water potential, p. 1-22. In J. F. Parr, W. R. Gardner, and L. F. Elliott (ed.), Water potential relations in soil microbiology. Soil Science Society of America, Madison, Wis.
- 42.Phelps, T. J., and J. K. Fredrickson. 2002. Drilling, coring, and sampling subsurface environments, p. 679-695. In M. J. McInerney (ed.), Manual of environmental microbiology, 2nd ed. ASM Press, Washington, D.C.
- 43.Pruess, K., S. B. Yabusaki, C. I. Steefel, and P. C. Lichtner. 2002. Fluid flow, heat transfer, and solute transport at nuclear waste storage tanks in the Hanford vadose zone. Vadose Zone J. 1:68-88. [Google Scholar]
- 44.Robinson, J. B., P. O. Solonius, and F. E. Chase. 1965. A note on the differential response of Arthrobacter spp. and Pseudomonas sp. to drying in soil. Can. J. Microbiol. 11:746-748. [DOI] [PubMed] [Google Scholar]
- 45.Serne, R. J., G. V. Last, G. W. Gee, H. T. Schaef, D. C. Lanigan, C. W. Lindenmeir, R. E. Clayton, V. L. LeGorre, R. D. Orr, B. C. F., I. V. Kutnyakov, T. C. Wilson, and D. A. Myers. 2001. Geologic and geochemical data collected from vadose sediments from borehole SX 41-09-39 in the S/SX waste management area and preliminary interpretations. Pacific Northwest National Laboratory, Richland, Wash.
- 46.Serne, R. J., H. T. Schaef, G. V. Last, D. C. Lanigan, C. W. Lindenmeir, R. E. Clayton, V. L. LeGore, M. J. O'Hara, C. F. Brown, R. D. Orr, I. V. Kutnyakov, T. C. Wilson, D. B. Burke, B. A. Williams, and B. N. Bjornstad. 2001. Geologic and geochemical data collected from vadose zone sediments from the slant borehole under SX-108 in the S/SX waste management area and preliminary interpretations. PNNL-2001-4. Pacific Northwest National Laboratory, Richland, Wash.
- 47.Swofford, D. L. 2000. PAUP: phylogenetic analysis using parsimony, 4.0, beta version 4a ed. Sinauer Associates, Sunderland, Md.
- 48.White, M. D., S. B. Yabusaki, and K. Pruess. 2001. Nonisothermal multiphase fluid flow and transport: multitank modeling in the SX tank farm, p. D277-D305. In A. J. Knepp (ed.), Appendix D: digest of S&T program evaluations. CH2M HILL Hanford Group, Inc., Richland, Wash.
- 49.Zachara, J. M., C. C. Ainsworth, G. E. Brown, Jr., J. G. Catalano, J. P. McKinley, O. Qafoku, S. C. Smith, J. E. Szecsody, S. J. Traina, and J. A. Warner. 2004. Chromium speciation and mobility in a high level waste vadose zone plume. Geochim. Cosmochim. Acta 68:13-30. [Google Scholar]
- 50.Zheng, D., E. W. Alm, D. A. Stahl, and L. Raskin. 1996. Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Appl. Environ. Microbiol. 62:4504-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]