Abstract
Spiroplasma citri is transmitted from plant to plant by phloem-feeding leafhoppers. In an attempt to identify mechanisms involved in transmission, mutants of S. citri affected in their transmission must be available. For this purpose, transposon (Tn4001) mutagenesis was used to produce mutants which have been screened for their ability to be transmitted by the leafhopper vector Circulifer haematoceps to periwinkle plants. With one mutant (G76) which multiplied in leafhoppers as efficiently as S. citri wild-type (wt) strain GII-3, the plants showed symptoms 4 to 5 weeks later than those infected with wt GII-3. Thirty to fifty percent of plants exposed to leafhoppers injected with G76 remained symptomless, whereas for wt GII-3, all plants exposed to the transmission showed severe symptoms. This suggests that the mutant G76 was injected into plants by the leafhoppers less efficiently than wt GII-3. To check this possibility, the number of spiroplasma cells injected by a leafhopper through a Parafilm membrane into SP4 medium was determined. Thirty times less mutant G76 than wt GII-3 was transmitted through the membrane. These results suggest that mutant G76 was affected either in its capacity to penetrate the salivary glands and/or to multiply within them. In mutant G76, transposon Tn4001 was shown to be inserted into a gene encoding a putative lipoprotein (Sc76) In the ABCdb database Sc76 protein was noted as a solute binding protein of an ABC transporter of the family S1_b. Functional complementation of the G76 mutant with the Sc76 gene restored the wild phenotype, showing that Sc76 protein is involved in S. citri transmission by the leafhopper vector C. haematoceps.
Members of the class Mollicutes, phylogenetically related to gram-positive bacteria are the smallest known free-living organisms. They are characterized by small genome sizes (580 to 2,200 kbp), with low G+C contents (24 to 41 mol%). In the Mollicutes, the genus Spiroplasma is one of the largest genera and comprises 34 described species and 8 putative species (39). All of the spiroplasmas are characterized by helical morphology and motility. Although most of them are found only in arthropod hosts, especially in insects, three spiroplasmas, Spiroplasma citri (32), Spiroplasma kunkelii (38), and Spiroplasma phoeniceum (33), are also able to multiply in the sieve tubes of plants and are pathogenic.
S. citri was the first plant pathogenic mollicute to be cultured and characterized (31, 32). It is an important pathogen, causing citrus stubborn disease in the Mediterranean area and California (8) as well as horseradish brittle root disease in the United States (10).
S. citri is the most-studied plant pathogenic mollicute, and its mechanisms of pathogenicity have begun to be understood (for a review, see references 5, 6, and7). Recently, a nonphytopathogenic S. citri mutant (GMT 553) was obtained by random insertion of transposon Tn4001 into the genome of the wild-type (wt) GII-3. Periwinkle plants infected with the wt GII-3 show symptoms during the first week of the posttransmission period, and symptoms soon became severe. With mutant GMT 553, plants remained symptomless for 4 weeks of the posttransmission period and were symptomless at a time when the spiroplasma titer was high (12, 14). In mutant GMT 553, Tn4001 was inserted in the first of the three fructose operon genes, and the involvement of fructose metabolism in the phytopathogenicity of S. citri was demonstrated (16, 17).
Plant pathogenic spiroplasmas are transmitted from plant to plant by phloem-feeding leafhoppers in a persistent propagative manner (26). In leafhoppers, spiroplasmas ingested from the phloem sieve tubes traverse the insect gut wall and move into the hemolymph, where they multiply and circulate. They eventually invade the salivary glands, where they multiply further (22, 23). From there, they are introduced with saliva into the phloem of a new host plant. Some authors have proposed that, following ingestions, spiroplasmas adhere to receptors on the gut epithelium and then move into the hemolymph. To penetrate the salivary glands, the spiroplasmas have to cross the basal lamina and probably adhere to receptors on the plasmalemma outer surface of the salivary glands (11). Spiroplasmas are then released into the salivary duct by exocytosis. Observation of membrane-bound cytoplasmic vesicles of midgut epithelium and salivary gland cells suggests that the spiroplasmas may cross these physical barriers by endocytosis involving a receptor-ligand interaction (22). At the present time, the molecular and cellular interactions contributing to the crossing of these physical barriers in the insect vector are poorly understood.
With the aim of identifying proteins involved in the transmission mechanism of S. citri by the leafhopper Circulifer haematoceps, spiroplasma mutants unable to accomplish the complete life cycle in the insect must be available. In the present study, we used mutagenesis of S. citri with transposon Tn4001 to produce S. citri mutants. Mutants affected in their transmissibility by the leafhopper C. haematoceps were selected after transmission to periwinkle plants. One such mutant, named mutant G76, has been characterized and is described in this paper.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The S. citri wt strain GII-3 was cultured from its leafhopper vector, C. haematoceps, in Morocco (37). From an early passage of this isolate, a triply cloned strain was obtained and used in this study. Spiroplasmas were grown at 32°C in SP4 medium (36) from which fresh yeast extract was omitted.
Escherichia coli strains TG1 {supE hsdΔ5 thiΔ(lac-proAB) F′ [traD36 proAB+ lacIq lacZΔM15]}, an EcoK− derivative of JM110, and GM2163 [F− ara-14 leuB6 thi-1 fhuA31 lacY1 tsx-78 galK2 galT22 supE44 hisG4 rpsL136 (Strr) xyl-5 mtl-1 dam13::Tn9 (Camr) dcm-6 mcrB1 hsdR2 (rk−mk+) mcrA] were used as host strains for subcloning experiments and for propagation of plasmids. For transformation with plasmid DNA, competent E. coli cells were prepared according to the Hanahan procedure (34).
Plasmid pMUT harboring transposon Tn4001 previously used for insertional mutagenesis of S. citri (14) was propagated in E. coli GM2163. The replicative plasmid pBOT1 containing the S. citri origin of DNA replication (oriC) has been previously described (29).
Primers and PCR amplification.
Amplifications were carried out with Taq DNA polymerase (Invitrogen Life Technologies, Cergy Pontoise, France) The reaction mixture was prepared according to the instructions of the supplier. PCR products used as probes were labeled by the addition of 1 nmol of digoxigenin 11-dUTP (Roche Applied Science, Meylan, France) to 40 μl of reaction mixture. All primers used in this study and conditions of PCR are summarized in Table 1.
TABLE 1.
Synthetic oligonucleotides used in this study
| Function | Oligo- nucleotide | Accession no. | PCR cycle | No. of cycles | Sequence (5′-3′)a |
|---|---|---|---|---|---|
| Detection of G76 mutants | 94°C, 45 s | ||||
| AB1 | AY136815 | 55°C, 45 s | 30 | TATGGCGGGATGGATAATCC | |
| IS4 | X53952 | 72°C, 1 min | GTAAAAGTCCTCCTGGG | ||
| Detection of revertants | 94°C, 45 s | ||||
| AB1 | AY136815 | 60°C, 45 s | 30 | TATGGCGGGATGGATAATCC | |
| AB9 | AY136815 | 72°C, 1 min | GCCATACTTGGTGCTGATGC | ||
| Cloning of sc76 gene | 94°C, 30 s | ||||
| AB1 | AY136815 | 58°C, 30 s | 40 | TATGGCGGGATGGATAATCC | |
| AB2 | AY136815 | 72°C, 45 s | TAGTTACAACATAATCTTCGCC | ||
| Functional complementation | 94°C, 1 min | ||||
| AB20 | AY136815 | 58°C, 1 min | 30 | TATACTAGAATTCTGGTCTAGTGTTTCTAC | |
| AB21 | AY136815 | 72°C, 1 min 45 s | AATATTGAATTCCTCTATAAGTTCTTCATGG | ||
| Probe TetM | 94°C, 45 s | ||||
| Tet5 | X56353 | 50°C, 45 s | 35 | TTCGGATCCTTTGAATGGAGGAAAATCACATG | |
| Tet6 | X56353 | 72°C, 1 min | GAAAAGATCTTTATATAACAACTTAAATTACACTAAG | ||
| Probe IS256 | 94°C, 30 s | ||||
| IS1 | X53952 | 52°C, 30 s | 40 | ATATTCTGTAAAGGATGACG | |
| IS3 | X53952 | 72°C, 1 min | CTTTAACAGCTTCTCTG |
Underlined regions indicate the EcoRI restriction sites engineered into the oligonucleotides.
Transformation of S. citri.
Electrotransformation of S. citri with the nonreplicative plasmid pMUT (14) was done by using a previously described protocol (35). The S. citri transformants were selected by plating on solid SP4 medium supplemented with 100 μg of gentamicin per ml.
Transformations of S. citri with the replicative oriC plasmids were carried out with the same procedure. The transformants were selected as described above.
Functional complementation of mutant G76.
Gene sc76 with its promoter and terminator regions (nucleotides 696 to 2378) was recovered by PCR amplification with primers AB20 and AB21 containing EcoRI sites (Table 1). The amplified fragment obtained with the Deep VentR DNA polymerase (New England BioLabs, Inc., Beverly, Mass.) was inserted at the EcoRI site of the oriC replicative plasmid pBOT1 containing the gene tetM for tetracycline resistance. Plasmid pBOT1 was shown to replicate in S. citri as a free plasmid before it integrates into the spiroplasmal chromosome by recombination at the oriC region (29). The resulting complementing plasmid named pAB1 was used to transform G76 mutants. Transformants were selected by plating on SP4 medium containing 100 μg of gentamicin per ml and 2 μg of tetracycline per ml. Individual colonies were picked, and growth took place in broth medium containing 100 μg of gentamicin per ml and 2 μg of tetracycline per ml. During propagation, the tetracycline concentration was progressively increased up to 15 μg per ml. To determine whether the plasmid pAB1 was maintained extrachromosomally as a free plasmid or was integrated into the spiroplasmal genome, total DNA of transformants was hybridized with probes tetM and AB1-AB9 (Table 1).
The G76 mutant was also transformed with the pBOT1 plasmid. The spiroplasma in which pBOT1 was integrated into the chromosome was named G76-pBOT1 and was used as a control in the complemented mutant transmission experiments.
DNA manipulations and DNA sequence.
Total DNAs from spiroplasmas were isolated by using the Wizard genomic DNA purification kit (Promega, Madison, Wis.). Recombinant DNA manipulations were conducted according to standard techniques (34) and by following the manufacturer's recommendations. DNA was blotted onto positively charged membranes by the alkali transfer procedure (34). Hybridizations with appropriate probes were carried out by using the standard method described by the supplier (Roche Applied Science). Hybridization signals were detected by the alkaline phosphatase-conjugated antibody HNPP chemifluorescent system (Roche Applied Science) as described in the manufacturer's manual.
The DNA sequences were determined by the dideoxy chain termination method by using an automated DNA sequencer (model 377; Applied Biosystems, Perkin Elmer) according to the instructions of the supplier.
Sequence analyses.
A search for similarity in GenBank involved the BLAST program (1) and the PSI-BLAST program (2). Transmembrane helices and motifs were searched by using the TMpred and PROSITE ScanProsite programs. Analysis in the ABC transporter database was carried out by using the ABCdb database available at http://www.lcb.cnrs-mrs.fr/∼quentin/.
Construction and screening of S. citri genomic DNA libraries.
Genomic DNA of the S. citri mutant G76 was digested with HindIII, and the fragments were ligated to the HindIII-linearized pBS+ vector (Stratagene Cloning System, La Jolla, Calif.). The recombinant clones containing sequences adjacent to the IS256 ends of Tn4001 were selected by in situ hybridization of colonies with the digoxigenin dUTP-labeled probe IS256 according to standard procedures (34). The probe IS256 was generated by PCR amplification with primer pair IS1-IS3 (Table 1). Genomic DNA of the wt S. citri GII-3 was digested to completion with PstI, and the restriction fragments were ligated to the pBS+ vector digested with PstI. The bacterial clones containing S. citri gene sc76 were selected by in situ hybridization of colonies with digoxigenin dUTP-labeled AB1-AB2 probe. The AB1-AB2 probe (Table 1) was obtained by PCR amplification of the HindIII fragment of the mutant G76 DNA obtained previously.
Search for revertants.
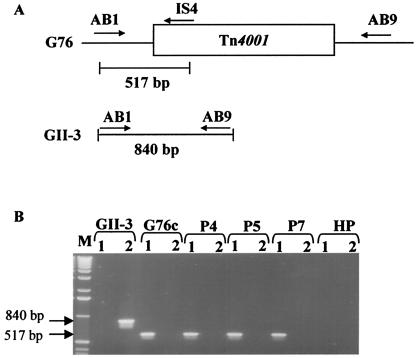
Total DNA from midribs of periwinkle plants infected with mutant G76 was extracted as previously described by using the cetyltrimethylammonium bromide extraction method (24). This DNA was used for PCR with primers AB1 and AB9 (Table 1) designed in regions surrounding the insertion site of Tn4001 in the genome (Fig. 1A). An amplicon of 840 bp should be obtained with spiroplasmas lacking Tn4001. In the presence of Tn4001, no amplification should occur because of the large size of the transposon (4.5 kbp). Using the primer pair AB1-IS4, mutants were detected by PCR amplification of a 517-bp DNA fragment (Table 1; Fig. 1A).
FIG. 1.
Search for revertants by PCR amplification. (A) Locations of primers on the chromosomal DNAs of wt GII-3 and G76; (B) ethidium bromide-stained agarose gel of DNAs amplified with primer pairs AB1-IS4 (lanes 1) and AB1-AB9 (lanes 2). GII-3, DNA from periwinkle plant infected by wt strain GII-3; G76c, DNA from a culture of mutant G76; P4, P5, P7, DNA from periwinkle plants P4, P5, and P7, respectively, infected by mutant G76 at week 11; HP, DNA from a healthy plant; M, 1-kb DNA ladder. Sizes are indicated in base pairs.
Experimental infection of periwinkle plants.
Intra-abdominal microinjections of S. citri into C. haematoceps leafhoppers and transmission to periwinkle plants (Catharanthus roseus) were previously described (12, 25). Briefly, CO2-anesthetized adult female leafhoppers were injected between the two last abdominal sternites, with hand-drawn glass needles. Approximately 5 × 104 cells of a spiroplasma culture was injected per insect. Injected leafhoppers were caged on young periwinkle plants (4 to 6 leaves) at a rate of 12 insects per plant. Leafhoppers were allowed to feed on periwinkle for a period of 12 to 15 days at 30 ± 2°C, representing the transmission period. After this period, the insects were killed with insecticide (dichlorvos) and the plants were kept in the greenhouse at 30 ± 2°C for symptom development and S. citri detection.
Culture of S. citri from periwinkle plants.
For 16 weeks after the end of the transmission period, old and young leaves were taken randomly on the periwinkle plants every 2 weeks. Midribs (0.2 to 0.5 g) were minced with a razor blade in 2 ml of liquid SP4 medium. After 30 min of incubation at room temperature, the extracts were filtered through 0.45-μm-pore-size filters. The number of CFU per 0.2 g of midribs was determined by plating 10-fold serial dilutions of the filtrate on solid SP4 medium (3 plates per dilution).
Culture of S. citri from insects.
Groups of 100 female C. haematoceps leafhoppers were microinjected with mutant G76 or wt GII-3 in SP4 medium and caged on two stock plants (Mathiola incana) (50 injected insects per plant). At different days after injection, 6 insects of each group were collected from the two stock plants and separated in 2 batches of 3 insects. Groups of 3 anesthetized leafhoppers were ground in 1 ml of SP4 medium in small conical Potter grinders. After 15 min of incubation at room temperature, the extracts were passed through filter membranes (pore diameter, 0.45 μm). The number of CFU was determined as described above.
Leafhopper heads, containing the salivary glands, were gently torn away from the thoraxes as described previously (22), rinsed in HEPES-sucrose buffer (8 mM HEPES [pH 7.4], 280 mM sucrose), and ground in groups of 5 in a Potter grinder with 1 ml of SP4 medium. After incubation (15 min at room temperature), the mixture was filtered through a 0.45-μm-pore-size membrane for CFU determination.
Transmission of S. citri through Parafilm membranes.
Female leafhoppers injected with S. citri were caged on healthy stock plants for a period of 12 days at 30 ± 2°C and transferred in groups of 4 to small cages on which a Parafilm membrane separated the leafhoppers from 5% sucrose SP4 medium as previously described (13, 28). When feeding through the Parafilm membrane, infected leafhoppers injected S. citri into the culture medium. After 24 h at room temperature, the SP4 medium was collected and plated on solid medium for CFU determination
Nucleotide sequence accession number.
The nucleotide sequence of the sc76 gene has been deposited in the EMBL and GenBank nucleotide sequence databases under accession no. AY136815.
RESULTS
Screening for S. citri mutants affected in leafhopper transmissibility and phytopathogenicity.
Tn4001 mutants of wt GII-3 were selected by hybridization with probe IS256 specific for the transposon. Each mutant was injected into the leafhopper vector C. haematoceps for transmission to two periwinkle plants as described in Materials and Methods. At the end of the 2-week transmission period, insects were killed and plants were observed for symptom development. The plants which had been in contact with S. citri-free leafhoppers remained symptomless. Plants infected by leafhoppers injected with wt GII-3 began to show marginal chlorosis during the second week following the end of the transmission period. With one mutant, named G76, the two inoculated plants remained symptomless and indistinguishable from the healthy control plants up to the end of the sixth week after the transmission period. Then in the seventh week, the symptoms developed, which suggest that mutant G76 is affected in either its multiplication, its pathogenicity, or its transmissibility by the leafhopper.
In vitro multiplication of mutant G76 and wt GII-3.
Growth of mutant G76 in gentamicin-SP4 medium was similar to that of wt GII-3 in gentamicin-free SP4 medium (data not shown). For both spiroplasmas, the titers of culture generally reached 5 × 108 CFU/ml. On solid SP4 medium (1% agar), colonies of mutant G76 and those of wt GII-3 had the same size (diameter, 0.15 mm) and same aspect, with satellite colonies indicating that motility was not affected (21).
Symptom development and multiplication of mutant G76 in periwinkle plants.
Periwinkle plants were infected with wt GII-3 and mutant G76 through leafhopper transmissions. Symptom development was recorded during a 16-week observation period following the 2-week transmission period (Table 2). As expected, plants (P1, P2, and P3) infected with wt GII-3 began to show marginal chlorosis within the second week of the observation period, developed general chlorosis during the fourth week, and died between weeks 8 (P2) and 13 (P1 and P3). With mutant G76, symptom development was delayed. Symptoms started to appear on plants P4 and P5 at the seventh week and a week later on plants P1, P6, and P7. At the 13th week, 6 of 9 plants presented typical symptoms of S. citri infection: asymmetric chlorotic young leaves and mild chlorosis on old leaves. At the 16th week, plant P4 was affected by lethal wilting, the last stage of the disease. Several plants (P2, P8, and P9) did not develop symptoms during the 16 weeks of observation.
TABLE 2.
Symptom development and spiroplasma titers in periwinkle plants at different weeks posttransmission
| S. citri strain | Plant no. | Symptomsa at wk no.
|
No. of CFUb at wk no.
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 5 | 7 | 8 | 9 | 13 | 16 | 5 | 9 | 13 | 16 | ||
| GII3 | P1 | − | + | +++ | +++ | +++ | +++ | ++++ | Dead | 1.3 × 106 | 1.8 × 106 | NDc | ND | |
| P2 | − | + | +++ | ++++ | ++++ | Dead | ND | ND | ND | ND | ||||
| P3 | − | + | +++ | +++ | +++ | +++ | ++++ | Dead | 1.1 × 106 | 106 | ND | ND | ||
| G76 | P1 | − | − | − | − | − | + | + | +++ | +++ | ND | ND | 3.8 × 104 | 104 |
| P2 | − | − | − | − | − | − | − | − | − | 0 | ND | ND | ND | |
| P3 | − | − | − | − | − | − | − | ++ | +++ | 0 | ND | ND | 104 | |
| P4 | − | − | − | − | + | ++ | ++ | +++ | ++++ | ND | 5.6 × 104 | 7.1 × 103 | 1.3 × 104 | |
| P5 | − | − | − | − | + | + | + | +++ | +++ | ND | 105 | 3.0 × 104 | 2.3 × 104 | |
| P6 | − | − | − | − | − | ++ | ++ | ++ | +++ | 0 | 6.9 × 104 | 7.0 × 104 | ||
| P7 | − | − | − | − | − | + | + | +++ | +++ | ND | 1.8 × 104 | 4.3 × 104 | 2.0 × 104 | |
| P8 | − | − | − | − | − | − | − | − | − | ND | ND | ND | ND | |
| P9 | − | − | − | − | − | − | − | − | − | ND | ND | ND | ND | |
| SP4 | P1 | − | − | − | − | − | − | − | − | − | 0 | ND | ND | ND |
| P2 | − | − | − | − | − | − | − | − | − | ND | ND | ND | ND | |
−, no symptoms; +, marginal chlorosis on young leaves; ++, asymmetrical and chlorotic young leaves, mild chlorosis on older leaves; +++, stunting and systemic leaf yellows; ++++, lethal wilting.
CFU per 0.2 g of midribs.
ND, not done.
The spiroplasma titers (in CFU) presented in Table 2 were determined on 0.2 g of midribs at weeks 5, 9, 13, and 16. For wt GII-3-infected periwinkle plants, the highest titer was reached 9 weeks after the transmission period and averaged 1.4 × 106 CFU per 0.2 g of midribs. During the same period, the average titer of mutant G76 was 20 times lower than that of wt GII-3 and ranged from 1.8 × 104 to 1.8 × 105 CFU/0.2 g of midribs. At weeks 13 and 16, the titers of spiroplasma G76 in periwinkle plants were similar to those found at week 9. Plants with no symptoms contained no spiroplasmas.
Search for reversion of S. citri mutant G76 in plants.
A search for putative revertants that lost the transposon during multiplication in plants, where no antibiotic selection pressure is present, was done by PCR amplification. Total DNA from G76-infected periwinkle plants P4, P5, and P7 11 weeks after the transmission period was used as described in Materials and Methods. Plants infected with wt GII-3 were used as controls. Results are shown in Fig. 1B. With primers AB1 and AB9, which encompass the insertion site of Tn4001 in the genome of mutant G76, a PCR product of 840 bp was obtained with DNA extracted from the wt GII-3-infected control plants. The size of this fragment agrees with the organization of the wt GII-3 genome (Fig. 1B, lane 2). As expected, no amplification occurred with DNAs extracted from plants P4, P5, and P7 infected with mutant G76 because of the large size of the inserted transposon (4.5 kbp) (Fig. 1B, lane 2). The same result was obtained when amplification with primers AB1-AB9 was carried out on a culture of mutant G76 (Fig. 1B, lane 2). With the primer pair AB1-IS4 (Fig. 1B, lane 1), amplification of a PCR product of 517 bp was obtained, revealing the presence of the Tn4001 in the spiroplasma genome at the insertion site. With DNA of control plants infected with wt GII-3, no amplification occurred (Fig. 1B, lane 1). These data showed that no revertants were present at week 11 in G76-infected plants and that the symptoms which developed on these plants were due to the mutant itself and not to a putative revertant.
Multiplication of mutant G76 and wt strain GII-3 in leafhoppers.
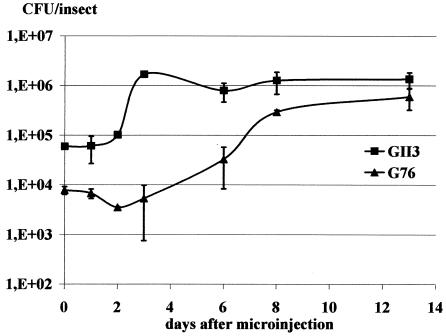
Multiplication of spiroplasmas in the leafhoppers was determined at 0, 1, 2, 3, 6, 8, and 13 days after injection on groups of 6 insects. Results are presented in Fig. 2.
FIG. 2.
Multiplication of wt strain GII-3 and mutant G76 in microinjected leafhoppers. Groups of 6 insects were used for CFU determinations. Standard deviations are indicated by error bars.
wt GII-3 grew from 6 × 104 CFU per insect at day 1 to 1.3 × 106 CFU per insect at day 13 after injection. The highest titer was reached at day 3 and remained more or less constant thereafter. For mutant G76, values increased from 8 × 103 CFU per insect at day 1 to 6 × 105 CFU per insect at day 13. An initial decrease in the titer was observed 2 days after injection and was followed by a gradual increase. The final titer reached a value that was half that of wt GII-3 at day 13 after injection.
Amount of G76 and GII-3 spiroplasmas in leafhopper heads.
To evaluate the amount of spiroplasma cells present in the salivary glands, cultures were done from leafhopper heads as a source of unbroken salivary glands.
Insects were microinjected with cultures of wt GII-3 or mutant G76 and were allowed to feed on stock plants for 2 weeks before head removal. Five culture assays were performed for mutant G76 and wt GII-3. As before, in the entire leafhopper the titer of mutant G76 was half that of wt GII-3 (1.3 × 106 and 2.5 × 106 CFU per insect, respectively). The mean CFU value per insect head was 1 × 104 ± 0.2 × 104 for mutant G76 and 15 × 104 ± 4 × 104 for wt GII-3, i.e., about 15 times lower for the mutant than for the wt GII-3.
Ability of leafhoppers to inject mutant G76 into SP4 medium.
Leafhoppers microinjected with a culture of mutant G76 or wt GII-3 were kept on 4 stock plants for 2 weeks. At the end of this period, the spiroplasma titers per insect were 1.4 × 105 for mutant G76 and 5 × 105 CFU for wt GII-3, as determined for 10 insects. Fifteen groups of 4 leafhoppers infected with mutant G76 and 18 groups of 4 leafhoppers infected with wt GII-3 were allowed to feed for 24 h on SP4 medium through Parafilm membranes as described in Materials and Methods. The media from 3 feeding chambers were mixed, and the number of spiroplasmas present in each batch was determined by plating on solid SP4 medium. In the case of leafhoppers injected with wt GII-3, spiroplasma cultures were obtained with all batches (Fig. 3) and the average CFU per culture was 1.2 × 102, i.e., 102 CFU for 10 insects. Cultures of mutant G76 were obtained only in 3 of the 5 batches, and the average CFU for 10 insects was 3.0, i.e., 30 times lower than that of wt GII-3.
FIG. 3.
Spiroplasmas present in culture medium after leafhopper inoculation through Parafilm membranes. CFU were inoculated by 12 insects in 24 h, 2 weeks after microinjection. Mean values are calculated from the results from six experiments for GII-3 (A, B, C, D, E, and F) and from five experiments for G76 (A, B, C, D, and E). Standard deviations are indicated by error bars.
Putative protein encoded by the disrupted gene.
Tn4001 is bordered at both ends by the insertion sequence IS256. Southern blot hybridization with the IS256-specific probe showed that Tn4001 was present as a single copy on the chromosomal DNA of mutant G76 (data not shown). DNA fragments encompassing the transposon insertion site were recovered from a genomic library of G76. The region corresponding to the insertion site of Tn4001 was cloned from the S. citri wt GII-3 as indicated in Materials and Methods. From a wt GII-3 PstI DNA library, a 3.2-kbp insert containing the entire gene of wt strain GII-3, mutated by insertion of Tn4001 in mutant G76, was isolated and named Pst3 (data not shown).
The complete nucleotide sequence (3,232 bp) of insert Pst3 was determined, and three regions were identified: two regions with no significant open reading frames, from nucleotide 1 to 844 and from nucleotide 2246 to 3262, and one region (1,400 bp) between nucleotides 845 and 2245 in which Tn4001 was inserted, coding for a putative protein of 466 amino acids (aa) with a molecular mass of 51.83 kDa, named SC76.
The putative initiation codon of this gene was an ATG located downstream of a possible ribosome binding site (AAAGGAG) starting at position 830. The termination codon was a TAA located at nucleotide 2245 upstream of an inverted repeat sequence between nucleotides 2260 and 2277.
BLASTp and PSI-BLAST analyses showed similarity between the putative protein SC76 and the lipoprotein MPN052 of Mycoplasma pneumoniae and lipoprotein MG040 of Mycoplasma genitalium. The highest similarity was in the first 319 aa, where 22% identity and 39% similar residues were found. These three lipoproteins have been noted in the ABCdb database as solute binding proteins of ABC transporters of the family S1_b.
As determined by Scan prosite analyses, the SC76 protein had a typical signal sequence and a signal peptidase II cleavage site followed by a cysteine residue. This putative signal peptide comprised 2 basic amino acids at positions 2 and 3, followed by 20 mainly hydrophobic amino acids and a cysteine in position 24 representing a potential acylation site. The composition of this signal peptide is in agreement with the consensus sequence described previously for bacterial lipoproteins (18).
The TMpred program indicated that protein SC76 had three transmembrane helices. The first one was predicted between aa 9 and 27 from the inner side to the outer side of the membrane, the second one was predicted from position 156 to 179 from the outer side to the inner side, and the last one was predicted from position 201 to 220 from the inner side to the outer side.
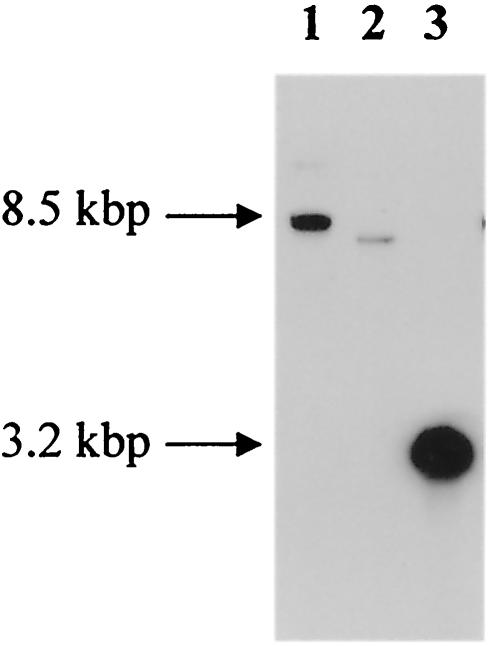
Southern blot hybridization with an sc76-specific probe AB1-AB9 was carried out with digested DNA of wt GII-3 (Fig. 4). Hybridizations revealed a KpnI fragment of approximately 8.5 kbp (lane 1), an NsiI fragment of 7.8 kbp (lane 2), and a PstI fragment of 3.2 kbp (lane 3), indicating that the sc76 gene is present in a single copy in the genome of wt GII-3.
FIG. 4.
Southern blot hybridization between probe AB1/AB9 and genomic DNA of wt GII-3 after digestion with KpnI (lane 1), NsiI (lane 2), and PstI (lane 3) restriction enzymes. Sizes are indicated in kilobase pairs.
Transmissibility of mutant G76 after complementation with the sc76 gene.
In insects or plants, spiroplasmas are not subjected to antibiotic selection pressure. For this reason, it is important to obtain stable complemented mutants in which the complementing plasmid pAB1 has integrated into the chromosome. From hybridizations between total DNAs of complemented mutants and probes specific to the tetM and sc76 genes (data not shown), integration of the plasmid was shown to be at the 5′ region of the sc76 gene. This event restores a complete copy of the sc76 gene. The selected complemented clone was named G76-comp. Mutant G76, mutant G76-comp, wt GII-3, and mutant G76-pBOT1, in which plasmid pBOT1 used for the construction of pAB1 was integrated into the chromosome, were transmitted by leafhoppers to periwinkle plants.
Plants infected with wt GII-3 or mutant G76-comp began to show asymmetric and chlorotic young leaves within the second week of the observation period and developed general chlorosis during the third week (Table 3). This result indicated that the recombination event which had occurred in G76 restored a functional copy of the sc76 gene in mutant G76-comp.
TABLE 3.
Transmission of the complemented mutant, G76-comp, by leafhopper to periwinkle plants
| S. citri strain | Plant no. | Symptomsa at wk no.
|
No. of CFUb at wk 6 | |||
|---|---|---|---|---|---|---|
| 2 | 3 | 6 | 9 | |||
| GII3 | P1 | + | +++ | +++ | ++++ | 4.3 × 106 |
| P2 | + | ++ | +++ | +++ | NDc | |
| P3 | + | ++ | +++ | ++++ | ND | |
| P4 | + | +++ | +++ | ++++ | ND | |
| P5 | + | ++ | +++ | +++ | ND | |
| G76-comp | P1 | + | +++ | +++ | +++ | 1.7 × 106 |
| P2 | − | + | +++ | +++ | ND | |
| P3 | + | +++ | +++ | +++ | ND | |
| P4 | − | ++ | +++ | +++ | ND | |
| P5 | + | +++ | +++ | +++ | ND | |
| G76 | P1 | − | − | − | − | ND |
| P2 | − | − | + | +++ | 8.0 × 104 | |
| P3 | − | − | + | +++ | ND | |
| P4 | − | − | ++ | +++ | ND | |
| P5 | − | − | − | − | ND | |
| G76-pBOT1d | P1 | − | − | + | +++ | ND |
| P2 | − | − | + | +++ | ND | |
| P3 | − | − | − | − | ND | |
| P4 | − | − | + | +++ | ND | |
| P5 | − | − | − | − | ND | |
| SP4 | P1 | − | − | − | − | ND |
| P2 | − | − | − | − | ND | |
−, no symptoms; +, marginal chlorosis on young leaves; ++, asymmetrical and chlorotic young leaves, mild chlorosis on older leaves; +++, stunting and systemic leaf yellows; ++++, lethal wilting.
CFU per 0.2 g of midribs.
ND, not done.
G76-pBOT1, mutant G76 transformed with pBOT1.
Plants infected with mutant G76 or with mutant G76-pBOT1 developed symptoms 6 weeks after the end of the transmission period, as shown in Table 3. Integration of plasmid pBOT1 in mutant G76 did not change the symptom evolution in comparison to that of G76.
Moreover, all of the plants infected with the complemented mutant, G76-comp, developed symptoms at 3 weeks after the transmission period, whereas only 3 of the 5 plants infected with either mutant G76 or mutant G76-pBOT1 developed symptoms.
The titer of spiroplasma G76-comp in periwinkle plants at week 6 of the observation period was not significantly different from that of wt GII-3 (1.6 × 106 CFU and 4.3 × 106 CFU per 0.2 g of midribs, respectively). The titer of mutant G76 in periwinkle plants at the same time was 8 × 104 CFU per 0.2 g of midribs, i.e., 50 times lower than that reached by wt GII-3 (Table 3).
To exclude the possibility that the G76-comp mutant could be a revertant, PCR amplifications with the primer pair AB1-IS4 (Table 1) were performed on DNA from a G76-comp-infected plant 6 weeks after the transmission period. No revertants could be detected (data not shown).
DISCUSSION
We have isolated a transpositional mutant G76 and determined its ability to be transmitted by the leafhopper vector C. haematoceps and to induce symptoms in periwinkle plants.
In all transmission experiments carried out with mutant G76, about 40% of the periwinkle plants remained symptomless and contained no spiroplasmas. The first symptoms on G76-infected periwinkle plants appeared with a delay of 7 or 8 weeks in comparison to those observed with wt GII-3. Spiroplasma titers of mutant G76 in symptomatic leaves never reached the titer of wt GII-3. The delay observed in symptom development could be due either to a lower multiplication of G76 in the plants compared to wt GII-3 or to a less-efficient insect transmission of the spiroplasmas. When mutant G76 and wt GII-3 were transmitted to periwinkle plants by graft inoculation, we observed the appearance of symptoms on both plants at the same time, even though the titer of G76 in the plants was lower than that of GII-3. This observation may indicate that the multiplication of mutant G76 in the plant is not responsible for the delay in symptom development and, thus, that G76 was affected in its transmissibility by the leafhopper.
In the insect, mutant G76 and wt GII-3 multiplied at the same titer, but the number of spiroplasmas was very different within the salivary glands, where the amount of mutant G76 was 15 times lower than that of wt GII-3. On the contrary, when the ratio of spiroplasmas present in salivary glands to spiroplasmas injected by a leafhopper into culture medium was determined, similar values were obtained for the mutant and wt GII-3. This indicated that poor transmission of mutant G76 by the leafhopper was due to the low G76 spiroplasma titer in the salivary glands and not to the capacity to transmit it through the stylets. As the titers of both spiroplasmas were similar in the whole insect, the weak number of spiroplasma G76 in the salivary glands suggested that G76 was affected in its ability to move from the hemolymph into the salivary glands and/or to multiply in the cells of salivary glands. Retardation of G76 entering the salivary glands and/or the low multiplication in the salivary glands is responsible for the symptom delay observed on periwinkle plants.
Functional complementation of mutant G76 with the sc76 gene restored completely the wild phenotype, showing that SC76 protein is involved in transmission of S. citri by the leafhopper vector C. haematoceps.
In silico analyses predicted that the putative lipoprotein SC76 was a solute binding protein of a sugar ABC transporter. The nature of the transported sugar is unknown and will be determined in future experiments. The superfamily of ABC transporters plays an important role in the export of proteins and polysaccharides and in the import of sugars, inorganic ions, and oligopeptides (27). We have demonstrated that multiplication of S. citri is highly dependent on carbohydrate utilization (17). In plants, the spiroplasma's preferred sugar is fructose, while the main sugars in the insect hemolymph and salivary glands are trehalose and glucose, respectively. If the ABC transporter mutated in G76 turns out to be a glucose transporter, the spiroplasma will multiply more slowly in the salivary gland cells. Another possibility, not exclusive of the previous one, is that SC76 functions as an adhesin and that the lack of this activity is responsible for poor invasion of the salivary glands.
Indeed, sequencing of mycoplasmal genomes indicated the presence in Mollicutes of a large number of ABC transporters (9, 15, 20), some of which have adhesion functions. In Mycoplasma hominis, the adherence-associated lipoprotein P100 was described as the substrate-binding protein of the oligopeptide permease (Opp), which is a member of the ABC transporter family (19). In the case of human and animal mycoplasmas, successful colonization of the host cells required adhesion as a first step. This event is mediated by mycoplasma surface proteins, and many of them are covalently modified by lipids. Among these proteins, adhesins play an important role in invasion and pathogenicity (3, 30). One S. citri adhesion-related protein, P89, was shown to be associated with spiroplasma adherence to insect cells (4, 40). Recently, it was shown that an S. citri mutant with no spiralin was transmitted, as G76, less efficiently by the leafhopper to the plants (9a). If adhesion mechanisms are involved in the poor transmissibility of both mutants (G76 and mutant with no spiralin), this would indicate that the passage through the salivary gland barriers required several spiroplasma proteins.
Acknowledgments
We thank J. M. Bové for critical reading of the manuscript and G. Fichant for help in the gene sequence analysis.
Support for A.B. was provided by the Ministère de l'Education Nationale de l'Enseignement Supérieur et de la Recherche.
REFERENCES
- 1.Altschul, R. W., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. H. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balish, M. F., and D. C. Krause. 2002. Cytadherence and the cytoskeleton, p. 491-518. In S. Razin and R. Herrman (ed.), Molecular biology and pathogenicity of mycoplasmas. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 4.Berg, M., U. Melcher, and J. Fletcher. 2001. Characterization of Spiroplasma citri adhesion related protein SARP1, which contains a domain of a novel family designated sarpin. Gene 275:57-64. [DOI] [PubMed] [Google Scholar]
- 5.Bové, J. M. 1993. Molecular features of Mollicutes. Clin. Infect. Dis. 17(Suppl. 1):S10-S31. [DOI] [PubMed] [Google Scholar]
- 6.Bové, J. M., P. Carle, M. Garnier, F. Laigret, J. Renaudin, and C. Saillard. 1989. Molecular and cellular biology of spiroplasmas, p. 243-364. In R. F. Whitcomb and J. G. Tully (ed.), The Mycoplasma, vol. 5. Academic Press, New York, N.Y. [Google Scholar]
- 7.Bové, J. M., J. Renaudin, C. Saillard, X. Foissac, and M. Garnier. 2003. Spiroplasma citri: discovery, properties and relationships with its two hosts, the plant and the leafhopper vector. Annu. Rev. Phytopathol. 41:483-500. [DOI] [PubMed] [Google Scholar]
- 8.Calavan, E. C., and J. M. Bové. 1989. Ecology of Spiroplasma citri, p. 425-485. In R. F. Whitcomb and J. G. Tully (ed.), The Mycoplasma, vol. 5. Academic Press, New York, N.Y. [Google Scholar]
- 9.Chambaud, I., R. Heilig, S. Ferris, V. Barbe, D. Samson, F. Galisson, I. Moszer, K. Dybvig, H. Wroblewski, A. Viari, E. P. C. Rocha, and A. Blanchard. 2001. The complete genome sequence of the murine respiratory pathogen Mycoplasma pulmonis. Nucleic Acids Res. 29:2145-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Duret, S., N. Berho, J. L. Danet, M. Garnier, and J. Renaudin. 2003. Spiralin is not essential for helicity, motility, or pathogenicity but is required for efficient transmission of Spiroplasma citri by its leafhopper vector Circulifer haematoceps. Appl. Environ. Microbiol. 69:6225-6234. [DOI] [PMC free article] [PubMed]
- 10.Fletcher, J., G. A. Schultz, R. E. Davis, C. E. Eastman, and R. M. Goodman. 1981. Spiroplasma citri is an etiological agent in brittleroot disease of horseradish. Phytopathology 71:874. [Google Scholar]
- 11.Fletcher, J., A. Wayadande, U. Melcher, and F. C. Ye. 1998. The phytopathogenic mollicute-insect vector interface: a closer look. Phytopathology 88:1351-1358. [DOI] [PubMed] [Google Scholar]
- 12.Foissac, X., J. L. Danet, C. Saillard, P. Gaurivaud, F. Laigret, C. Pare, and J. M. Bové. 1997. Mutagenesis by insertion of Tn4001 into the genome of Spiroplasma citri: characterization of mutants affected in plant pathogenicity and transmission to the plant by the leafhopper vector Circulifer haematoceps. Mol. Plant-Microbe Interact. 10:454-461. [Google Scholar]
- 13.Foissac, X., J. L. Danet, C. Saillard, R. F. Whitcomb, and J. M. Bové. 1996. Experimental infections of plant by spiroplasmas, p. 385-389. In S. Razin and J. G. Tully (ed.), Molecular and diagnostic procedures in mycoplasmology, vol. 2. Academic Press, New York, N.Y. [Google Scholar]
- 14.Foissac, X., C. Saillard, and J. M. Bové. 1997. Random insertion of transposon Tn4001 in the genome of Spiroplasma citri strain GII3. Plasmid 37:80-86. [DOI] [PubMed] [Google Scholar]
- 15.Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischmann, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, J. L. Fritchmann, J. F. Weidman, K. V. Small, M. Sandusky, J. Fuhrmann, D. Nguyen, T. R. Utterback, D. M. Saudek, C. A. Phillips, J. M. Merrick, J. F. Tomb, B. A. Dougherty, K. F. Bott, P. C. Hu, T. S. Lucier, S. N. Peterson, H. O. Smith, C. A. Hutchison III, and J. C. Venter. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. [DOI] [PubMed] [Google Scholar]
- 16.Gaurivaud, P., J. L. Danet, F. Laigret, M. Garnier, and J. M. Bové. 2000. Fructose utilization and phytopathogenicity of Spiroplasma citri. Mol. Plant-Microbe Interact. 13:1145-1155. [DOI] [PubMed] [Google Scholar]
- 17.Gaurivaud, P., F. Laigret, M. Garnier, and J. M. Bové. 2000. Fructose utilization and pathogenicity of Spiroplasma citri: characterization of the fructose operon. Gene 252:61-69. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi, S., and H. C. Wu. 1990. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22:451-471. [DOI] [PubMed] [Google Scholar]
- 19.Henrich, B., M. Hopfe, A. Kitzerow, and U. Hadding. 1999. The adherence-associated lipoprotein P100, encoded by an opp operon structure, functions as the oligopeptide-binding domain OppA of a putative oligopeptide transport system in Mycoplasma hominis. J. Bacteriol. 181:4873-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Himmelreich, R., H. Hilbert, H. Plagens, E. Pirkl, B. C. Li, and R. Herrmann. 1996. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 24:4420-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacob, C., F. Nouzieres, S. Duret, J. M. Bové, and J. Renaudin. 1997. Isolation, characterization, and complementation of a motility mutant of Spiroplasma citri. J. Bacteriol. 179:4802-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon, M. O., A. C. Wayadande, and J. Fletcher. 1999. Spiroplasma citri movement into the intestines and salivary glands of its leafhopper vector, Circulifer tenellus. Phytopathology 89:1144-1151. [DOI] [PubMed] [Google Scholar]
- 23.Liu, H. Y., D. J. Gumpf, G. N. Oldfield, and E. C. Calavan. 1983. The relationship of Spiroplasma citri and Circulifer tenellus. Phytopathology 73:585-590. [Google Scholar]
- 24.Maixner, M., U. Ahrens, and E. Seemüller. 1995. Detection of the German grapevine yellows (Vegilbungskrankheit) MLO in grapevine, alternative hosts, and a vector by a specific PCR procedure. Eur. J. Plant Pathol. 101:241-250. [Google Scholar]
- 25.Markham, P. G., and R. Townsend. 1979. Experimental vectors of spiroplasmas, p. 413-445. In K. Maramorosch and K. F. Harris (ed.), Leafhoppers vectors and plant disease agents. Academic Press, New York, N.Y.
- 26.Purcell, A. H. 1983. Insect vector relationships with procaryotic plant pathogens. Annu. Rev. Phytopathol. 20:397-417. [Google Scholar]
- 27.Quentin, Y., G. Fichant, and F. Denizot. 1999. Inventory, assembly and analysis of Bacillus subtilis ABC transport systems. J. Mol. Biol. 287:467-484. [DOI] [PubMed] [Google Scholar]
- 28.Rana, G. L., G. H. Kaloostian, G. N. Oldfield, A. L. Granett, E. C. Calavan, H. D. Pierce, I. M. Lee, and D. J. Grumpf. 1975. Acquisition of Spiroplasma citri through membranes by homopterous insects. Phytopathology 65:1143-1145. [Google Scholar]
- 29.Renaudin, J., A. Marais, E. Verdin, S. Duret, X. Foissac, F. Laigret, and J. M. Bové. 1995. Integrative and free Spiroplasma citri oriC plasmids: expression of the Spiroplasma phoeniceum spiralin in Spiroplasma citri. J. Bacteriol. 177:2870-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rottem, S. 2002. Invasion of mycoplasmas into and fusion with host cells, p. 391-401. In S. Razin and R. Hermann (ed.), Molecular biology and pathogenicity of mycoplasmas. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 31.Saglio, P., D. Laflèche, C. Bonisol, and J. M. Bové. 1971. Culture in vitro des mycoplasmes associés au stubborn des agrumes et leur observation au microscope électronique. C. R. Acad. Sci. (Paris) 272:1387-1390. [Google Scholar]
- 32.Saglio, P., M. Lhospital, D. Laflèche, G. Dupont, J. M. Bové, J. G. Tully, and E. A. Freundt. 1973. Spiroplasma citri gen. and sp. nov.: a mycoplasma-like organism associated with stubborn disease of citrus. Int. J. Syst. Bacteriol. 23:191-204. [Google Scholar]
- 33.Saillard, C., J. C. Vignault, J. M. Bové, A. Raie, J. G. Tully, D. L. Williamson, A. Fos, M. Garnier, A. Gadeau, P. Carle, and R. F. Whitcomb. 1987. Spiroplasma phoeniceum sp. nov., a new plant pathogenic species from Syria. Int. J. Syst. Bacteriol. 37:106-115. [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Stamburski, C., J. Renaudin, and J. M. Bové. 1991. First step toward a virus-derived vector for gene cloning and expression in spiroplasmas, organisms which read UGA as a tryptophan codon: synthesis of chloramphenicol acetyltransferase in Spiroplasma citri. J. Bacteriol. 173:2225-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tully, J. G., R. F. Whitcomb, H. F. Clark, and D. L. Williamson. 1977. Pathogenic mycoplasma: cultivation and vertebrate pathogenicity of a new spiroplasma. Science 195:892-894. [DOI] [PubMed] [Google Scholar]
- 37.Vignault, J. C., J. M. Bové, C. Saillard, R. Vogel, A. Farro, L. Venegas, W. Stemmer, S. Aoki, R. E. McCoy, A. S. Al-beldawi, M. Larue, O. Tuzco, M. Ozan, A. Nhami, M. Abassi, J. Bonfils, G. Moutous, A. Fos, F. Poutiers, and G. Viennot-Bourgin. 1980. Mise en culture de spiroplasmes à partir de matériel végétal et d'insectes provenant de pays circum méditerranéens et du Proche Orient. C. R. Acad. Sci. (Paris) 290:775-780. [Google Scholar]
- 38.Whitcomb, R. F., T. A. Chen, D. L. Williamson, C. Liao, J. G. Tully, J. M. Bové, C. Mouchès, D. L. Rose, M. E. Coan, and T. B. Clark. 1986. Spiroplasma kunkelii sp. nov.: characterization of the etiologic agent of corn stunt disease. Int. J. Syst. Bacteriol. 36:170-178. [Google Scholar]
- 39.Williamson, D. L., R. F. Whitcomb, J. G. Tully, G. E. Gasparich, D. L. Rose, P. Carle, J. M. Bove, K. J. Hackett, J. R. Adams, R. B. Henegar, M. Konai, C. Chastel, and F. E. French. 1998. Revised group classification of the genus Spiroplasma. Int. J. Syst. Bacteriol. 48:1-12. [DOI] [PubMed] [Google Scholar]
- 40.Yu, J., A. C. Wayadande, and J. Fletcher. 2000. Spiroplasma citri surface protein P89 implicated in adhesion to cells of the vector Circulifer tenellus. Phytopathology 90:716-722. [DOI] [PubMed] [Google Scholar]