Background: A significant non-neural, monoamine-independent mechanism underlies the antidepressant effect of amitriptyline.
Results: Amitriptyline-evoked GDNF production is mediated by pertussis toxin (PTX)-sensitive Gαi/o.
Conclusion: PTX-sensitive Gαi/o activation is critical for the cascade that underpins the biological effect of amitriptyline.
Significance: Further elaboration of the intracellular mechanism of amitriptyline could lead to greater understanding of depression and novel antidepressant treatments.
Keywords: astrocyte, biosensor, depression, G protein, glial cell, Cellkey assay, antidepressant
Abstract
Further elaborating the mechanism of antidepressants, beyond modulation of monoaminergic neurotransmission, this study sought to elucidate the mechanism of amitriptyline-induced production of glial cell line-derived neurotrophic factor (GDNF) in astroglial cells. Previous studies demonstrated that an amitriptyline-evoked matrix metalloproteinase (MMP)/FGF receptor (FGFR)/FGFR substrate 2α (FRS2α)/ERK cascade is crucial for GDNF production, but how amitriptyline triggers this cascade remains unknown. MMP is activated by intracellular mediators such as G proteins, and this study sought to clarify the involvement of G protein signaling in amitriptyline-evoked GDNF production in rat C6 astroglial cells (C6 cells), primary cultured rat astrocytes, and normal human astrocytes. Amitriptyline-evoked GDNF mRNA expression and release were inhibited by pertussis toxin (PTX), a Gαi/o inhibitor, but not by NF449, a Gαs inhibitor, or YM-254890, a Gαq inhibitor. The activation of the GDNF production cascade (FGFR/FRS2α/ERK) was also inhibited by PTX. Deletion of Gαο1 and Gαi3 by RNAi demonstrated that these G proteins play important roles in amitriptyline signaling. G protein activation was directly analyzed by electrical impedance-based biosensors (CellKeyTM assay), using a label-free (without use of fluorescent proteins/probes or radioisotopes) and real time approach. Amitriptyline increased impedance, indicating Gαi/o activation that was suppressed by PTX treatment. The impedance evoked by amitriptyline was not affected by inhibitors of the GDNF production cascade. Furthermore, FGF2 treatment did not elicit any effect on impedance, indicating that amitriptyline targets PTX-sensitive Gαi/o upstream of the MMP/FGFR/FRS2α/ERK cascade. These results suggest novel targeting for the development of antidepressants.
Introduction
Major depressive disorder (MDD)4 is a severe, chronic, and often life-threatening illness with a lifetime prevalence of more than 10% (1). Since the late 1950s, a wide range of antidepressants targeting the monoaminergic neurotransmitter system have been available to alleviate the symptoms of MDD. However, the efficacy of these antidepressants cannot be solely explained by their modulatory effects on brain monoamines. The limited success in understanding the pathophysiology of MDD and the equivocal therapeutic effects of antidepressants could be the result of excessive focus on dysfunctional neurons, with scant studies performed in other types of cells of the brain such as glia.
In the past decade, glial degeneration or dysfunction, especially of astrocytes, has been postulated to play a critical role in the pathogenesis of MDD (2). One of the major roles of astrocytes is the production of neurotrophic/growth factors, which support neurogenesis, gliogenesis, brain development, neural plasticity, and survival (3). Recently, both clinical and preclinical animal studies have demonstrated that multiple neurotrophic/growth factors, such as glial cell line-derived neurotrophic factor (GDNF), a member of the transforming growth factor β superfamily, have been proposed to play an important role in the therapeutic effect of antidepressants (4, 5). Glial cell line-derived neurotrophic factor has been shown to be decreased in the peripheral blood of patients with MDD (6), whereas blood levels of GDNF in MDD are increased after antidepressant treatment (7). These findings suggest that modulating GDNF production may be a key therapeutic effect of antidepressants. Therefore, understanding the mechanism of GDNF production in response to antidepressant treatment in astroglial cells could provide novel insights into the pathogenesis of MDD, the pharmacological effect of antidepressants and potentially novel treatments for MDD.
Previously, reports demonstrated that treatment with several different classes of antidepressants, such as tricyclic antidepressants, tetracyclic antidepressants, selective serotonin (5-HT) reuptake inhibitors, and 5-HT and noradrenaline reuptake inhibitors, increased GDNF production in normal human astrocytes (NHA), rat primary cultured astrocytes, and rat C6 astroglial cells (C6 cells, an astrocytic cell line) (8–10). The tricyclic antidepressant amitriptyline increased GDNF production by astroglial cells through a monoamine-independent mechanism (9). The FGF receptor (FGFR)/FGFR substrate 2α (FRS2α)/ERK signaling cascade plays a crucial role in amitriptyline-induced GDNF production, independent of monoamine activation (11). Fibroblast growth factor receptor activation evoked by amitriptyline is derived from a matrix metalloproteinase (MMP)-dependent shedding of FGFR ligands in astroglial cells (11). However, how amitriptyline triggers MMP and the subsequent signaling cascade remain unknown. Matrix metalloproteinase is known to be activated by intracellular signaling mediators, such as G proteins (12). Thus, this study attempts to clarify the involvement of G protein in the amitriptyline-evoked production of GDNF. The effect of amitriptyline on G protein activation in living C6 cells and rat astrocytes was analyzed using a novel biosensor technology, the CellKeyTM assay (MDS Sciex, Ontario, Canada). The CellKeyTM assay utilizes cellular dielectric spectroscopy, which is specifically tailored to G protein-coupled receptor (GPCR) detection and can distinguish signals between the Gαs (Gs), Gαi/o (Gi/o), and Gαq (Gq) subfamilies (13, 14).
Experimental Procedures
Reagents
Reagents were obtained from the following sources: amitriptyline (Wako Pure Chemical Industries, Ltd., Osaka, Japan); serotonin creatinine sulfate monohydrate, nor-binaltorphimine dihydrochloride (nor-BNI), and [d-Ala2,N-MePhe4,Gly-ol]enkephalin (DAMGO) (Sigma); FGF2 human recombinant (Roche Diagnostics); pertussis toxin, NF449, and U0126 (Calbiochem); GM6001, SU5402, and PD173074 (Merck KGaA, Darmstadt, Germany); acetylcholine and isoproterenol (Nacalai Tesque, Kyoto, Japan). YM-254890 was a gift from Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan).
Cell Culture
Culturing of C6 cells, and NHA has been described previously (8, 11). In brief, C6 cells were grown in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 2 mm l-glutamine and 5% fetal bovine serum (Sigma) in a 5% CO2 humidified atmosphere. Normal human astrocytes, male fetuses 18 weeks of age, were purchased from Cambrex (Walkersville, MD) and grown in ABMTM (Cambrex) in a 5% CO2 humidified atmosphere. More than 80% of NHA expressed glial fibrillary acidic protein. For drug treatment, the medium was replaced with serum-free Opti-MEM (Invitrogen) containing 0.5% bovine serum albumin (Sigma), and the cells were incubated for 24 h. The cells were then treated with various drugs of interest.
All experimental procedures and animal handling were performed according to both the Guiding Principles for the Care and Use of Laboratory Animals, as approved by the Japanese Pharmacological Society, and guidelines for the care and use of laboratory animals of Hiroshima University (Hiroshima, Japan). Primary cultured astrocytes were prepared from 1-day-old neonatal Wistar rats, as described previously (10, 15). Briefly, isolated cerebral cortices were minced and then incubated with trypsin and DNase I. Dissociated cells were suspended in DMEM supplemented with 10% fetal calf serum and penicillin/streptomycin (100 units/ml and 100 μg/ml, respectively). Thereafter, cell suspensions were plated in 75-cm2 tissue culture flasks (10 × 106 cells/flask) precoated with poly-l-lysine (10 μg/ml). The cells were maintained in a 10% CO2 incubator at 37 °C. After 8–12 days, the growth flasks containing mixed glial cells were shaken by a rotary shaker at 100 rpm for 15 h and washed with PBS to remove neuronal and microglial cells. Adherent cells were trypsinized (0.25%) and plated into 75-cm2 flasks. After the cells reached confluence (about 10 days), the confluent cells were shaken by hand for 10 min. Adherent cells were trypsinized (0.25%) and plated onto 35-mm diameter dishes (1 × 106 cells/dish). After 2 days, the medium was replaced with serum-free DMEM. The cells were used for experiments on the following day. For the CellKeyTM assay, rat astrocytes were prepared from the passaged cell cultures of 1-day-old rat cortex, as described previously (8, 16). Most of the cells (>95%) in culture are astrocytes, as they express the astrocytic marker glial fibrillary acidic protein.
Electrical Impedance-based Biosensors (CellKeyTM Assay)
The CellKeyTM assay has been described previously (17, 18). Cellular dielectric spectroscopy is a novel technology that employs a label-free, real time, cell-based assay approach for the comprehensive pharmacological evaluation of cells that exogenously or endogenously express GPCRs, and nuclear receptors on the same platform without any modification of the cells. Among the biosensors that use cellular dielectric spectroscopy technology, the CellKeyTM system not only detects the activation of GPCRs but also distinguishes between signals through different subtypes of the Gα protein (Gs, Gi/o, and Gq). Many reports have indicated that cell signaling induced by GPCR activation can induce changes in morphology, and there are well established links between Gs, Gi, and Gq signaling pathways (19–22). The activation of GPCR therefore changes the impedance (ΔZ), which can be interpreted as the differences of GPCR activation. In brief, the cells were cultured at a density of 2.0 × 104 cells/well for C6 cells, 5.0 × 104 cells/well for HEK293 cells, and 2.0 × 104 cells/well for rat astrocytes on a standard CellKeyTM 96-well microplate, which contained electrodes at the bottom of the wells, with 100 μl of growth medium. All experiments were performed in CellKeyTM assay buffer (Hanks' balanced salt solution (1.3 mm CaCl2·2H2O, 0.81 mm MgSO4, 5.4 mm KCl, 0.44 mm KH2PO4, 4.2 mm NaHCO3, 136.9 mm NaCl, 0.34 mm Na2HPO4, and 5.6 mm d-glucose) with 20 mm HEPES and 0.1% BSA) at 29 °C. Small voltages were applied to these electrodes every 10 s, and ΔZ values were measured. Just before assay, the cells were washed with assay buffer and allowed to equilibrate in the assay buffer for 30 min before starting the assay. The CellKeyTM instrument applied small voltages to these electrodes every 10 s and measured the ΔZ of the cell layer. In this study, a 5-min baseline was recorded; drugs were added, and then ΔZ was measured for 10 min. The extent of changes in ΔZ was expressed in terms of ΔZ maximum after drug injection.
Cells Stably Expressing Opioid Receptors
To visualize impedance changes from typical Gi/o-coupled receptors, as references, cells expressing the μ-opioid receptor were prepared (see “Electrical Impedance-based Biosensors (CellKeyTM Assay)” above). Human embryonic kidney 293 (HEK293) cells were plated in 35-mm dishes. After seeding for 24 h, the cells were transfected with μ-opioid receptor tagged at the N terminus with FLAG using X-tremeGENE HP DNA transfection reagent (Roche Diagnostics, Basel, Switzerland) according to the supplier's instructions. After transfection for 24 h, the cells were re-plated in a 10-cm dish and selected with 700 μg/ml G418 disulfate aqueous solution (Nacalai Tesque, Kyoto, Japan). These cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin mixed solution (Nacalai Tesque, Kyoto, Japan) in a 5% CO2 humidified atmosphere. Single clones expressing FL-MOR were then screened by both the conventional immunocytochemistry analysis using anti-FLAG (Wako Pure Chemical Industries, Ltd., Osaka, Japan) antibodies and a functional assay using CellKeyTM assay.
RNA Isolation
For the collection of total RNA, the cells were cultured at a density of 1.6 × 105/cm2 for C6 cells and 1.0 × 105/cm2 for primary cultured rat astrocytes on a 6-well plate with 3 ml of growth medium. After drug treatment, total RNA was isolated using an RNeasy mini kit (Qiagen, Valencia, CA) following the manufacturer's protocols. RNA quantity and purity were determined with a multi-spectrophotometer (Dainippon Sumitomo Pharma Co. Ltd., Osaka, Japan).
Real Time RT-PCR Assay
Real time RT-PCR assay has been described previously (10). In brief, the first strand cDNA was synthesized from 500 ng of total RNA by using an RNA PCR kit (AMV) Version 3.0 (Takara Bioscience, Shiga, Japan). Real time quantitative PCR was performed using the Thermal Cycler Dice® real time system II (Takara Bioscience), with TaqMan probes and primers for rat GDNF and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Applied Biosystems, Foster City, CA). The mRNA levels were normalized for GAPDH mRNA in the same samples by the 2(−ΔΔCT) method.
ELISA
For the GDNF release assay, C6 cells were cultured at a density of 1.3 × 105/cm2 on a 12-well plate with 0.5 ml of growth medium. After drug treatment, the concentrations of GDNF protein in cell-conditioned media were determined using a GDNF Emax® ImmunoAssay System (Promega, Madison, WI) according to the manufacturer's instructions.
ERK Activity Assay
The ERK activity assay has been described previously (11). In brief, the cells were cultured at a density of 1.6 × 105/cm2 for C6 cells, 0.8 × 105/cm2 for normal human astrocytes and 1.0 × 105/cm2 for primary cultured rat astrocytes on a 6-well plate with 2 or 3 ml of growth medium. After drug treatment, the cells were collected in a cell lysis buffer. The total amount of protein in each sample was measured by a bicinchoninic acid kit (Pierce), and it was adjusted to the same amount for all samples. ERK activity was determined using an assay kit according to the manufacturer's instructions (Cell Signaling Technology, Beverly, MA).
Western Blotting
Western blotting has been described previously (11). Western blotting was performed with the following antibodies: phospho-FGFR substrate 2α (FRS2α) (Tyr-196) antibody (Cell Signaling Technology); actin (C-2) antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA); and FRS2α (SNT-1) antibody (Sigma). Cells were collected in ice-cold phosphate-buffered saline (PBS) and solubilized in sample buffer (100 mm Tris-HCl (pH 6.8), 20% glycerol, 4% SDS). The total amount of protein between samples was adjusted to similar levels. After the addition of 1,4-dithiothreitol, the samples were boiled for 5 min. The proteins were separated by SDS-PAGE and transblotted to polyvinylidene difluoride membranes. The membranes were blocked with 5% (w/v) BSA or skim milk for 6 h at 4 °C and incubated with respective antibodies overnight at 4 °C. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. After incubation, the membranes were washed, and the desired proteins were detected with Immun-Star WesternCTM kit (Bio-Rad), using GE Healthcare Image Quant LAS 4000 (Waukesha, WI). Band intensity was quantified with the software ImageJ (National Institutes of Health, Bethesda).
RT-PCR Analysis
Total RNA from C6 cells and control tissues (rat cortex and spleen) was prepared by the methods of RNA isolation and used to synthesize cDNA with murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA) and a random hexamer primer. Polymerase chain reactions were performed with the specific primers and AmpliTaqGoldTM (Applied Biosystems) at 95 °C for 10 min followed by 35 (for Go1, Go2, and Gz) or 40 (for Gi1, Gi2, and Gi3) cycles of 95 °C for 30 s, 62 °C for 30 s, and 72 °C for 1 min with a final extension at 72 °C for 5 min. The sequences of the primers were as follows: Gi1, 5′-AAGGACAGCGGTGTGCAAGCCTGCTTCAAC-3′ (forward), and 5′-AATCTGTCATTCCGTACAAGGTACTTAACA-3′ (reverse); Gi2, 5′-AGTATGACGAGGCAGCCAGCTACATCCAGAGCAA-3′ (forward), and 5′-GTACTCCTCCAGACATAGGCCTTGGGAAACTCTGC-3′ (reverse); Gi3, 5′-TGCTAGGAGACGTCTAAGAGTATA-3′ (forward), and 5′-GCTTGCTTCCCAAAGCAGTTCTGA-3′ (reverse); Go1, 5′-CATCCTCCGAACCAGGGTC-3′ (forward), and 5′-CAAGCCACAGCCCCGGAG-3′ (reverse); Go2, 5′-CATCCTCCGAACCAGGGTC-3′ (forward), and 5′-GGCGATGATGACGTCCGT-3′ (reverse); and Gz, 5′-CACCTGGAGGACAACGCCGCT-3′ (forward), and 5′-TTTCGGTTTAGGTCCTCGAACTGA-3′ (reverse). The resulting PCR products were analyzed on a 1.5% agarose gel, and sizes were expected from known cDNA sequences.
RNAi Experiments
To suppress expression of Go1, Gi2, and Gi3, C6 cells were transfected with small interfering RNA (siRNA), which has been described previously (23). The cells were transfected with either siRNA, targeting rat Go1, Gi2, Gi3, or nontargeting siRNA (siGENOME Nontargeting siRNA Pool 5, Thermo Fisher Scientific) by using Lipofectamine 2000® (Life Technologies, Inc.) according to the manufacturer's directions. In these experiments, cells were used 48 h after transfection. The efficiency of the knockdown of the α-subunits of G proteins was determined by Western blotting.
Statistical Analysis
Data were expressed as the mean ± S.E. of at least three independent experiments. Statistically significant differences among the means were determined using one-way analysis of variance with pairwise comparison carried out by the Tukey's method. p values at less than 0.05 were taken as statistically significant.
Results
Effects of Pertussis Toxin, NF449, or YM-254890 on the GDNF Production Evoked by Amitriptyline in C6 Cells and Primary Cultured Rat Astrocytes
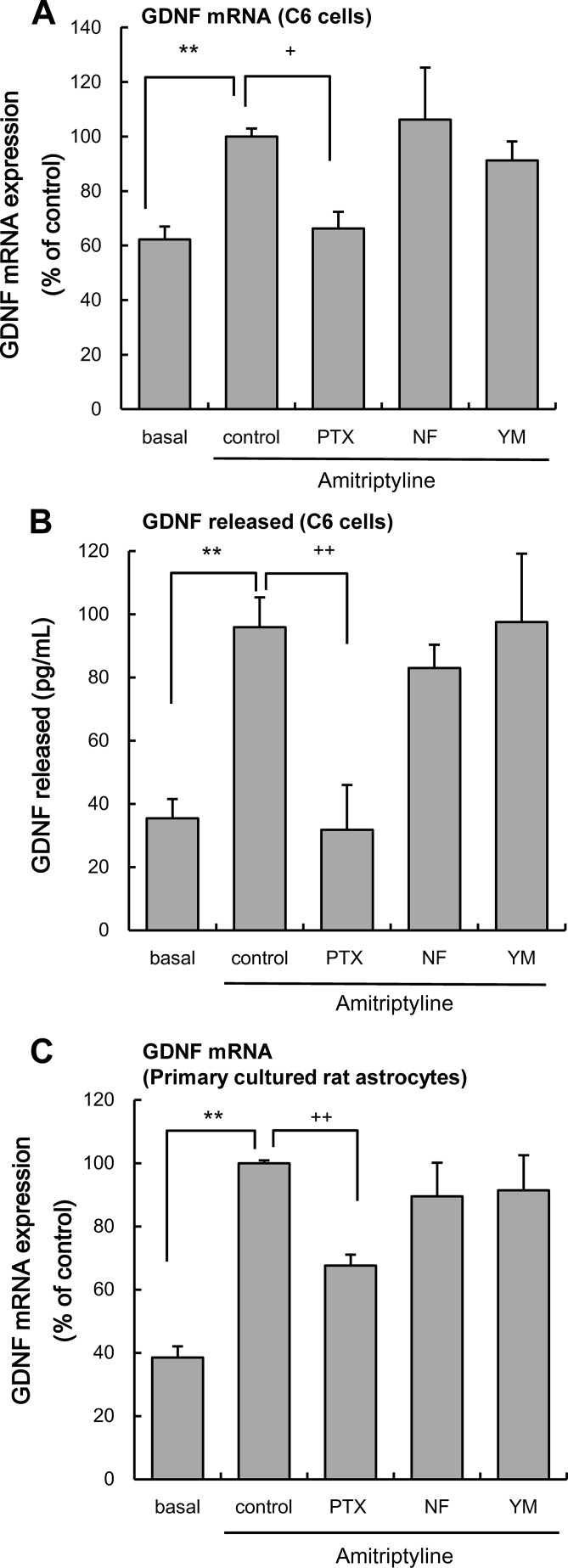
To clarify the involvement of G proteins in the amitriptyline-evoked production of GDNF, the effects of the following inhibitors of the α-subunits of G proteins were examined as described previously: pertussis toxin (PTX, 100 ng/ml; Gi/o inhibitor (24, 25)), NF449 (1 μm; Gs inhibitor (26)), and YM-254890 (100 nm; Gq inhibitor (23)). A 3-h treatment with amitriptyline (25 μm) significantly increased GDNF mRNA expression in C6 cells, and a 48-h amitriptyline treatment induced a significant release of GDNF from C6 cells. Both the amitriptyline-evoked expression of GDNF mRNA and release of GDNF were inhibited by PTX treatment. By contrast, neither NF449 nor YM-254890 had any effect on GDNF production (Fig. 1, A and B). Treatment with the inhibitors alone, without amitriptyline, had no significant effect on basal expression of GDNF mRNA (PTX only, 74.1 ± 8.3%; NF449 only, 73.8 ± 18.8%; YM-254890 only, 69.2 ± 7.6% of control) and GDNF release (PTX only, 19.1 ± 9.0 pg/ml; NF449 only, 32.0 ± 6.8 pg/ml; YM-254890 only, 47.9 ± 11.7 pg/ml).
FIGURE 1.
Effects of α-subunit of G protein inhibitors on amitriptyline-evoked GDNF production. A, effects of pertussis toxin (PTX), NF449 (NF), and YM-254890 (YM) on the amitriptyline-evoked GDNF mRNA expression. C6 cells were pretreated with 100 ng/ml PTX for 3 h and 1 μm NF449 or 100 nm YM-254890 for 0.5 h and subsequently treated with 25 μm amitriptyline for 3 h. Values are shown as the ratio of GDNF mRNA versus GAPDH mRNA (% of control). Data are expressed as the mean ± S.E. for three to seven independent experiments. **, p < 0.01 in comparison with the basal group; +, p < 0.05 in comparison with the control group (Tukey's test). B, effects of PTX, NF449, and YM-254890 on the amitriptyline-evoked GDNF release. C6 cells were pretreated with 100 ng/ml PTX for 3 h and 1 μm NF449 or 100 nm YM-254890 for 0.5 h and subsequently treated with 25 μm amitriptyline for 48 h. Values are expressed as the mean ± S.E. of released GDNF (pg/ml) for 4–12 independent experiments. **, p < 0.01 in comparison with the basal group; ++, p < 0.01 in comparison with the control group (Tukey's test). C, effects of pertussis toxin, NF449, and YM-254890 on the amitriptyline-evoked GDNF mRNA expression. Primary cultured rat astrocytes were pretreated with 100 ng/ml PTX for 3 h, 1 μm NF449, or 100 nm YM-254890 for 0.5 h and subsequently treated with 25 μm amitriptyline for 6 h. Values are shown as the ratio of GDNF mRNA versus GAPDH mRNA (% of control). Data are expressed as the mean ± S.E. for 8–11 independent experiments. **, p < 0.01 in comparison with the basal group; ++, p < 0.01 in comparison with the control group (Tukey's test).
In primary cultured rat astrocytes, a 6-h treatment with amitriptyline (25 μm) significantly increased GDNF mRNA expression (10). The amitriptyline-evoked expression of GDNF mRNA in primary cultured rat astrocyte was also inhibited by PTX, but not by NF449 or YM-254890 (Fig. 1C) Treatment with the inhibitors alone, without amitriptyline, had no significant effect on basal expression of GDNF mRNA (PTX only, 43.2 ± 5.0%; NF449 only, 48.0 ± 6.4%; YM-254890 only, 49.8 ± 9.0% of control).
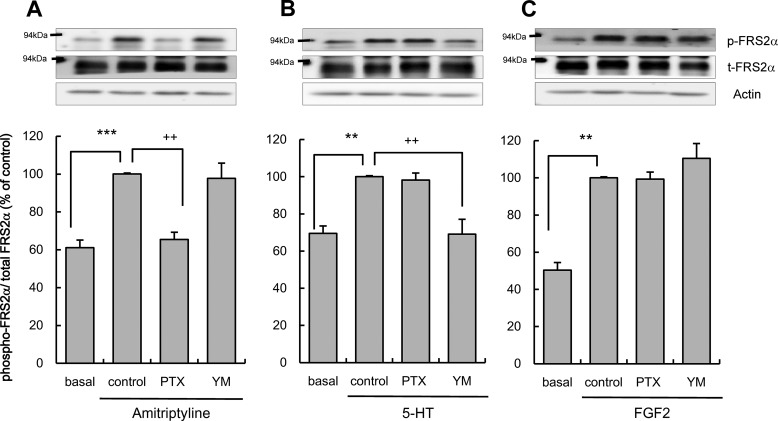
Effects of PTX or YM-254890 on the FRS2α Phosphorylation Evoked by Amitriptyline, 5-HT, or FGF2 in C6 Cells
The effect of Gi/o inhibition by PTX and Gq inhibition by YM-254890 on the GDNF production cascade was evaluated. FGFR and the related signaling cascade were previously shown to be essential for amitriptyline-evoked GDNF production (11). Fibroblast growth factor receptor substrate 2α is a member of the FRS family of lipid-anchored docking protein, and it is the primary substrate for the FGFR kinase and links the FGFR to Ras/MAPK signaling cascades (27, 28). Therefore, phosphorylation of FRS2α was utilized as a surrogate index of FGFR activation. The phosphorylation of FRS2α induced by amitriptyline treatment significantly increased and peaked 5 min after treatment in C6 cells (11). This increase was significantly reduced by PTX but not by YM-254890 treatment of C6 cells (Fig. 2A). A previous study showed that 5-HT increased GDNF production through FGFR transactivation by the 5-HT2 Gq-coupled receptor in C6 cells (23). 5-HT treatment acutely increased FGFR phosphorylation in C6 cells (23). To determine whether Gq is involved in the 5-HT-evoked FRS2α phosphorylation, unlike in the case of amitriptyline, the effects of PTX and YM-254890 on FRS2α phosphorylation evoked by 5-HT were examined. A 5-min treatment with 10 μm 5-HT significantly increased FRS2α phosphorylation in C6 cells. The 5-HT-induced phosphorylation of FRS2α was significantly reduced by YM-254890 but not by PTX (Fig. 2B). Next, to confirm the involvement of G proteins located upstream of FGFR, the effects of PTX and YM-254890 on exogenous FGF2 (a FGFR ligand)-evoked FRS2α phosphorylation was examined. FGF2 treatment acutely increased FGFR phosphorylation in C6 cells (29). In this study, treatment with FGF2 (10 ng/ml, 5 min treatment) also significantly increased FRS2α phosphorylation in C6 cells. The FGF2-evoked increase in FRS2α phosphorylation was not affected by either PTX or YM-254890 (Fig. 2C). The inhibitors alone had no significant effect on basal levels of FRS2α phosphorylation (PTX only, 99.0 ± 14.5%; YM-254890 only, 122.9 ± 26.3% of basal levels).
FIGURE 2.
Effects of pertussis toxin or YM-254890 on FRS2α phosphorylation evoked by amitriptyline, 5-HT, or FGF2. A, effects of pertussis toxin or YM-254890 (YM) on FRS2α phosphorylation evoked by amitriptyline in C6 cells. C6 cells were pretreated with 100 ng/ml PTX for 3 h; 100 nm YM-254890 for 0.5 h and subsequently treated with 25 μm amitriptyline for 5 min. Phosphorylated FRS2α, total FRS2α, and actin were quantified by Western blotting, and representative blots are shown. Values are shown as the ratio of phosphorylated FRS2α to total FRS2α (% of control). Data are expressed as the mean ± S.E. for four to seven independent experiments. ***, p < 0.001 in comparison with the basal group; ++, p < 0.01 in comparison with the control group (Tukey's test). B, effects of PTX or YM-254890 on FRS2α phosphorylation evoked by 5-HT in C6 cells. C6 cells were pretreated with 100 ng/ml PTX for 3 h, 100 nm YM-254890 for 0.5 h, and subsequently treated with 10 μm 5-HT for 5 min. Phosphorylated FRS2α, total FRS2α, and actin were quantified by Western blotting, and representative blots are shown. Values are shown as the ratio of phosphorylated FRS2α to total FRS2α (% of control). Data are expressed as the mean ± S.E. for six independent experiments. **, p < 0.01 in comparison with the basal group; ++, p < 0.01 in comparison with the control group (Tukey's test). C, effects of PTX or YM-254890 on FRS2α phosphorylation evoked by exogenous FGF2 in C6 cells. C6 cells were pretreated with 100 ng/ml PTX for 3 h, 100 nm YM-254890 for 0.5 h, and subsequently treated with 10 ng/ml FGF2 for 5 min. Phosphorylated FRS2α, total FRS2α, and actin were quantified by Western blotting, and representative blots are shown. Values are shown as the ratio of phosphorylated FRS2α to total FRS2α (% of control). Data are expressed as the mean ± S.E. for five to eight independent experiments. **, p < 0.01 in comparison with the basal group (Tukey's test).
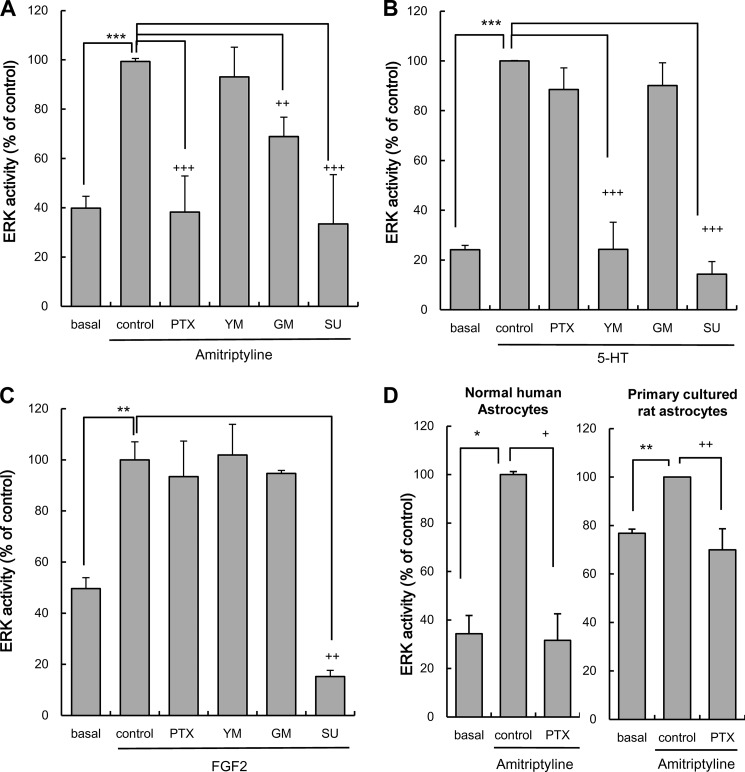
Amitriptyline and 5-HT Increase ERK Activation through Different Intracellular Signaling Pathways in C6 Cells
Amitriptyline-evoked ERK activation is elicited by FGFR activation through an MMP-dependent pathway that is coupled to GDNF production (11). To further determine whether PTX-sensitive Gi/o is specifically implicated in the cascade evoked by amitriptyline, the effect of PTX, YM-254890, MMP inhibitor (GM6001, 25 μm), and FGFR inhibitor (SU5402, 25 μm) on ERK activation evoked by either amitriptyline, 5-HT, or FGF2 was examined. The concentrations of inhibitors used were as described previously (23, 25, 30, 31). ERK activation by amitriptyline treatment significantly increased and peaked after 5 min of treatment in C6 cells (9). The increase of ERK activity evoked by amitriptyline (25 μm, 5 min treatment) was significantly inhibited by PTX, GM6001, and SU5402 but not by YM-254890 (Fig. 3A). The ERK activation by 5-HT treatment significantly increased and reached a maximum after 2 min of treatment in C6 cells (23). Serotonin-evoked ERK activation (10 μm, 2 min treatment) was significantly inhibited by YM-254890 and SU5402 but not by either PTX or GM6001 (Fig. 3B). The ERK activation by FGF2 treatment significantly increased and reached a maximum after 10 min of treatment in rat astrocytes (32). FGF2-evoked ERK activation (10 ng/ml, 10 min treatment) was significantly inhibited by SU5402 but not by either PTX, YM-254890, or GM6001 (Fig. 3C). A previous study confirmed that there was no effect on basal levels of ERK activation by the inhibitors alone (23).
FIGURE 3.
Effects of pertussis toxin, YM-254890, GM6001, and SU5402 on ERK activation evoked by amitriptyline, 5-HT, and FGF2. A, effects of pertussis toxin, YM-254890 (YM), GM6001 (GM), or SU5402 (SU) on ERK activation evoked by amitriptyline in C6 cells. C6 cells were pretreated with 100 ng/ml PTX for 3 h, 25 μm SU for 1 h, and 100 nm YM-254890 or 25 μm GM6001 for 0.5 h, and subsequently treated with 25 μm amitriptyline for 5 min. Values are shown as ERK activity (% of control). Data are expressed as the mean ± S.E. for four to five independent experiments. ***, p < 0.001 in comparison with the basal group; ++, p < 0.01; +++, p < 0.001 in comparison with the control group (Tukey's test). B, effects of PTX, YM-254890, GM6001, or SU5402 on ERK activation evoked by 5-HT in C6 cells. C6 cells were pretreated with 100 ng/ml PTX for 3 h, 25 μm SU5402 for 1 h, 100 nm YM-254890 or 25 μm GM6001 for 0.5 h, and subsequently treated with 10 μm 5-HT for 2 min. ERK activity (phosphorylation levels of ERK1/2) was quantified by Western blotting. Values are shown as ERK activity (% of control). Data are expressed as the mean ± S.E. for three independent experiments. ***, p < 0.001 in comparison with the basal group; +++, p < 0.001 in comparison with the control group (Tukey's test). C, effects of PTX, YM-254890, GM6001, and SU5402 on ERK activation evoked by FGF2 in C6 cells. C6 cells were pretreated with 100 ng/ml PTX for 3 h, 25 μm SU5402 for 1 h, 100 nm YM-254890, or 25 μm GM6001 for 0.5 h, and subsequently treated with 10 ng/ml FGF2 for 10 min. ERK activity (phosphorylation levels of ERK1/2) was quantified by Western blotting. Values are shown as ERK activity (% of control). Data are expressed as the mean ± S.E. for three to five independent experiments. **, p < 0.01 in comparison with the basal group; ++, p < 0.01 in comparison with the control group (Tukey's test). D, effects of PTX on ERK activation evoked by amitriptyline in NHA and primary cultured rat astrocytes. Normal human astrocytes were pretreated with 100 ng/ml PTX for 3 h and subsequently treated with 25 μm amitriptyline for 5 min. Primary cultured rat astrocytes were pretreated with 100 ng/ml PTX for 3 h and subsequently treated with 25 μm amitriptyline for 10 min. Values are shown as ERK activity (% of control). Data are expressed as the mean ± S.E. for three or five independent experiments. *, p < 0.05; **, p < 0.01 in comparison with the basal group; +, p < 0.05; ++, p < 0.01 in comparison with the control group (Tukey's test).
Effect of PTX on ERK Activation Evoked by Amitriptyline in NHA and Primary Cultured Rat Astrocytes
To confirm whether amitriptyline treatment evokes ERK activation through PTX sensitive G protein in both normal human and rat astrocytes, as it does in C6 cells, primary cultures of human and rat astrocytes were tested. In NHA, amitriptyline (25 μm, 5 min treatment) significantly increased ERK activity. The ERK activation evoked by amitriptyline in NHA was almost completely suppressed by PTX (Fig. 3C). In primary cultured rat astrocytes, amitriptyline (25 μm, 10 min treatment) significantly increased ERK activity. ERK activation evoked by amitriptyline in primary cultured rat astrocytes was also almost completely suppressed by PTX. The findings indicate that the intracellular mechanism observed in rat C6 cells is relevant to human astrocytes and primary cultured rat astrocytes.
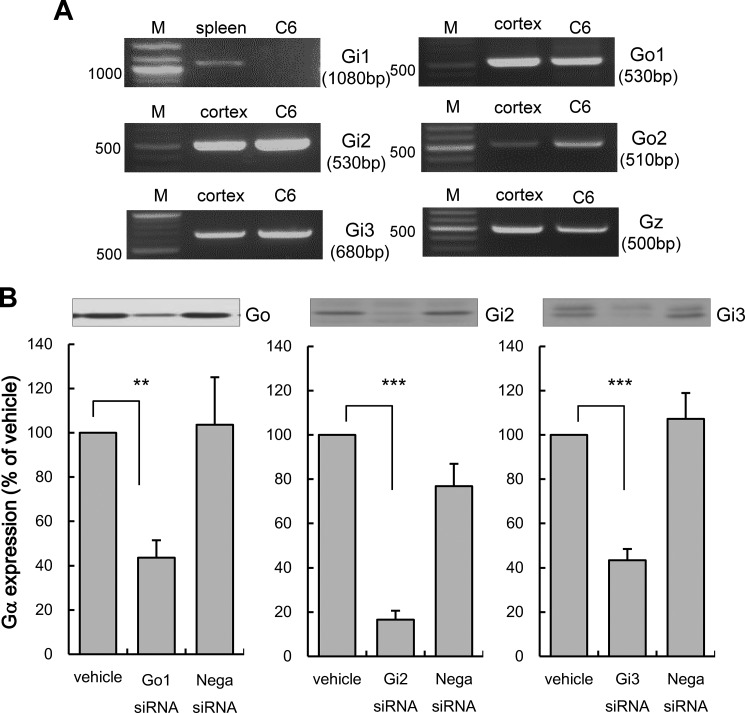
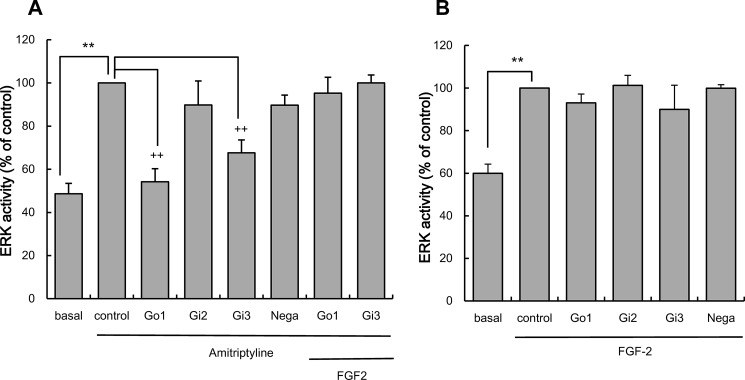
Effects of siRNA for Go1, Gi2, or Gi3 on the ERK Activation Evoked by Amitriptyline in C6 Cells
The effect of silencing PTX-sensitive α-subunit of the G protein was examined to determine the role of each subtype on amitriptyline's effect. It has been reported that C6 cells express PTX-sensitive α-subunits of the G protein, including Go1, Go2, Gi2, and Gi3 (33, 34). The presence of these α-subunits of G protein in C6 cells was confirmed by RT-PCR (Fig. 4A). Thus, the effect of siRNA specific for Go1, Gi2, and Gi3 on amitriptyline-induced ERK activation was examined. The effect of siRNA on Go2 was not examined because an siRNA for Go2 is not commercially available. Because ERK is the most sensitive intracellular signaling molecule that responds to amitriptyline, ERK activity was used as an index of intracellular signaling cascade activation. Forty eight h of transfection of 50 nm siRNAs against α-subunits of the G protein significantly reduced protein levels of individual subunits (Fig. 4B). An equivalent amount of negative control siRNA did not affect the expression levels of α-subunits of G protein (Fig. 4B). Amitriptyline (25 μm, 5 min treatment) significantly increased ERK activity in C6 cells. This activation was significantly blocked by Go1 siRNA and Gi3 siRNA transfection, whereas Gi2 siRNA transfection did not change ERK activity. Negative control siRNA had no effect on amitriptyline increased ERK activity (Fig. 5A). Thus, amitriptyline-evoked ERK activation appears to be more dependent on Go1 activity rather than Gi3 activity and not at all on Gi2 activity. Furthermore, addition of exogenous FGF2 increased ERK activity in cells that had Go1 and Gi3 knocked down by siRNA transfection (Fig. 5A). Knock-out of Go1 and Gi3 did not affect ERK activation by exogenous FGF2 (Fig. 5B), indicating that FGF2-induced ERK activation is independent of Go1 and Gi3.
FIGURE 4.
Expressions of α-subunits of G protein in C6 cells. A, RT-PCR analysis of α-subunits of G protein mRNA expression in C6 cells. Each lane represents the cDNA fragments of α-subunits of G protein amplified from the RNA of either C6 cells, rat cortex, or rat spleen. Lane M indicates the molecular size marker. B, effects of Go1, Gi2, Gi3, and negative control siRNA transfection on Go1, Gi2, and Gi3 expressions. C6 cells were transfected with either Go1, Gi2, Gi3 siRNA (50 nm) or negative control siRNA (Nega siRNA; 50 nm) for 48 h. The α-subunits of G protein were quantified by Western blotting. Representative blots are shown. Values shown are levels of α-subunits of G protein (% of vehicle). Data are expressed as the mean ± S.E. for 6–12 independent experiments. **, p < 0.01; ***, p < 0.001 in comparison with the vehicle group (Tukey's test).
FIGURE 5.
Effects of the α-subunits of G protein knockdown on amitriptyline and FGF2-evoked ERK activation. A, effect of either Go1, Gi2, or Gi3 knockdown on ERK activation evoked by amitriptyline. C6 cells were transfected with Go1 siRNA, Gi2 siRNA, or Gi3 siRNA (Go1, Gi2, or Gi3; 50 nm) or negative control siRNA (Nega; 50 nm) for 48 h, and subsequently treated with 25 μm amitriptyline alone or in conjunction with 10 ng/ml exogenous FGF2 for 5 min. Values are shown as ERK activity (% of control). Data are expressed as the mean ± S.E. for 3–12 independent experiments. **, p < 0.01 in comparison with the basal group; ++, p < 0.01 in comparison with the control group (Tukey's test). B, effect of either Go1, Gi2, or Gi3 knockdown on ERK activation evoked by FGF2. C6 cells were transfected with Go1 siRNA, Gi2 siRNA, or Gi3 siRNA (Go1, Gi2, or Gi3; 50 nm) or negative control siRNA (Nega; 50 nm) for 48 h, and subsequently treated with 10 ng/ml FGF2 for 10 min. Values are shown as ERK activity (% of control). Data are expressed as the mean ± S.E. for four to six independent experiments. **, p < 0.01 in comparison with the basal group (Tukey's test).
Effects of Amitriptyline on the Changes of Impedance (ΔZ) in C6 Cells and Rat Astrocytes Using Electrical Impedance-based Biosensors (CellKeyTM Assay)
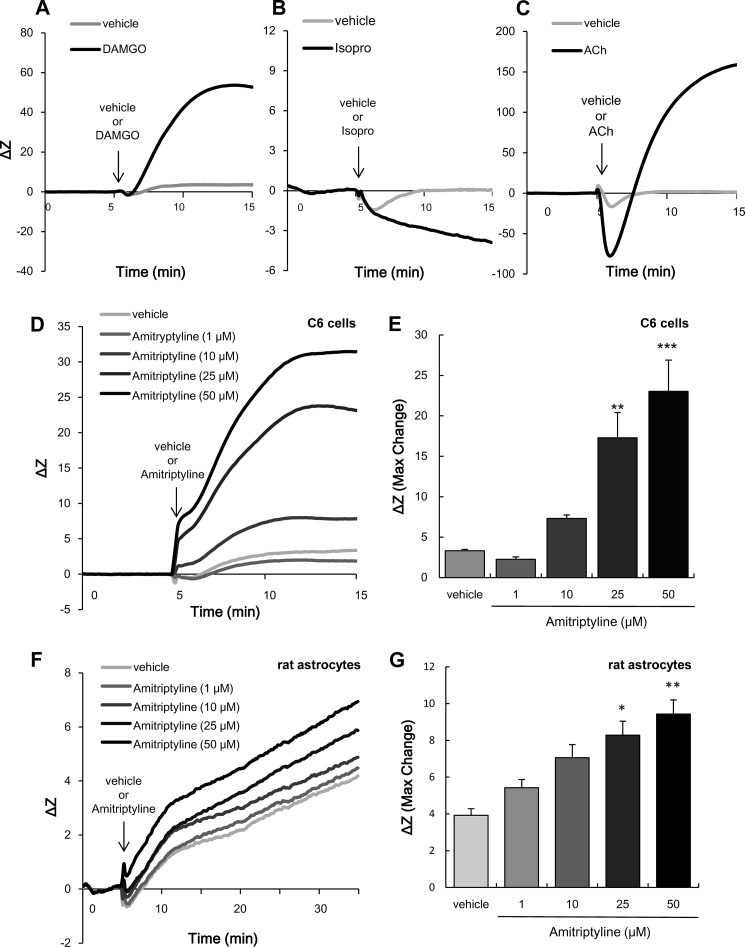
A number of reports have demonstrated the CellKeyTM assay's ability to distinguish the activation of Gα subfamily (Gi/o, Gs, and Gq) by patterns of ΔZ changes (19). To show typical different ΔZ values caused by each ligand to specific types of GPCRs, HEK293 cells expressing μ-opioid receptors, coupled to Gi/o, and C6 cells endogenously expressing β-adrenergic receptors, coupled to Gs, were used (14, 35). In addition, HEK293 cells, which endogenously express the muscarinic (M3) acetylcholine receptor, were used to show the activation of Gq-coupled receptors (36, 37). In Fig. 6A, DAMGO, an agonist of the μ-opioid receptor, induced an increase in ΔZ; the activation of Gi/o increases ΔZ. By contrast, isoproterenol, an agonist of β1- and β2-adrenergic receptors, induced a decrease in ΔZ; the activation of Gs decreases ΔZ (Fig. 6B). Acetylcholine, an M3 acetylcholine receptor ligand, induced a rapid decrease in ΔZ followed by a gradual increase in ΔZ; the activation of Gq leads to a unique ΔZ pattern-rapid decrease and subsequent increase (Fig. 6C). As shown in Fig. 6, D and E, amitriptyline in C6 cells increased ΔZ in a concentration-dependent manner. This increase peaked around 10 min after amitriptyline treatment and persisted for at least 30 min (data not shown). Amitriptyline treatment also increased ΔZ in a concentration-dependent manner in rat astrocytes (Fig. 6, F and G). Comparing the pattern of ΔZ induced by amitriptyline, the pattern suggests Gi/o activation (Fig. 6, A, D, and F).
FIGURE 6.
Effects of amitriptyline on impedance (ΔZ) in C6 cells and rat astrocytes using electrical impedance-based biosensors (CellKeyTM assay). A, effects of DAMGO on ΔZ in μ-opioid receptor-expressing HEK293 cells. The cells were treated with vehicle or 1 μm DAMGO for 10 min. The traces shown are representative of the mean increase in ΔZ of cells from each well. Similar results were obtained in at least three independent experiments. B, effects of a β-adrenergic receptor agonist isoproterenol (Isopro) on ΔZ in C6 cells endogenously expressing β-adrenergic receptors. The cells were treated with vehicle or 100 nm isoproterenol (Isopro) for 10 min. The traces shown are representative of the mean increase in ΔZ of cells from each well. Similar results were obtained in at least three independent experiments. C, effects of acetylcholine on ΔZ in HEK293 cells endogenously expressing muscarinic (M3) acetylcholine receptors. The cells were treated with vehicle or 1 μm acetylcholine (ACh) for 10 min. The traces shown are representative of the mean increase in ΔZ of cells from each well. Similar results were obtained in at least three independent experiments. D, effects of amitriptyline on ΔZ in C6 cells. C6 cells were treated with amitriptyline (1, 10, 25, and 50 μm) for 10 min. The traces shown are representative of the mean increase in ΔZ of cells from each well. Similar results were obtained in at least three independent experiments. E, extent of changes in ΔZ was presented as the maximum ΔZ after injection of vehicle or amitriptyline. Values are expressed as the mean ± S.E. of ΔZ (each group; n = 6). **, p < 0.01; ***, p < 0.001 in comparison with the vehicle group (Tukey's test). F, effects of amitriptyline on ΔZ in rat astrocytes. Rat astrocytes were treated with amitriptyline (1, 10, 25, and 50 μm) for 30 min. The traces shown are representative of the mean increase in ΔZ of cells from each well. Similar results were obtained in at least three independent experiments. G, extent of changes in ΔZ was presented as the maximum ΔZ after injection of vehicle or amitriptyline. Values are expressed as the mean ± S.E. of ΔZ (all each group; n = 12). *, p < 0.05; **, p < 0.01 in comparison with the vehicle group (Tukey's test).
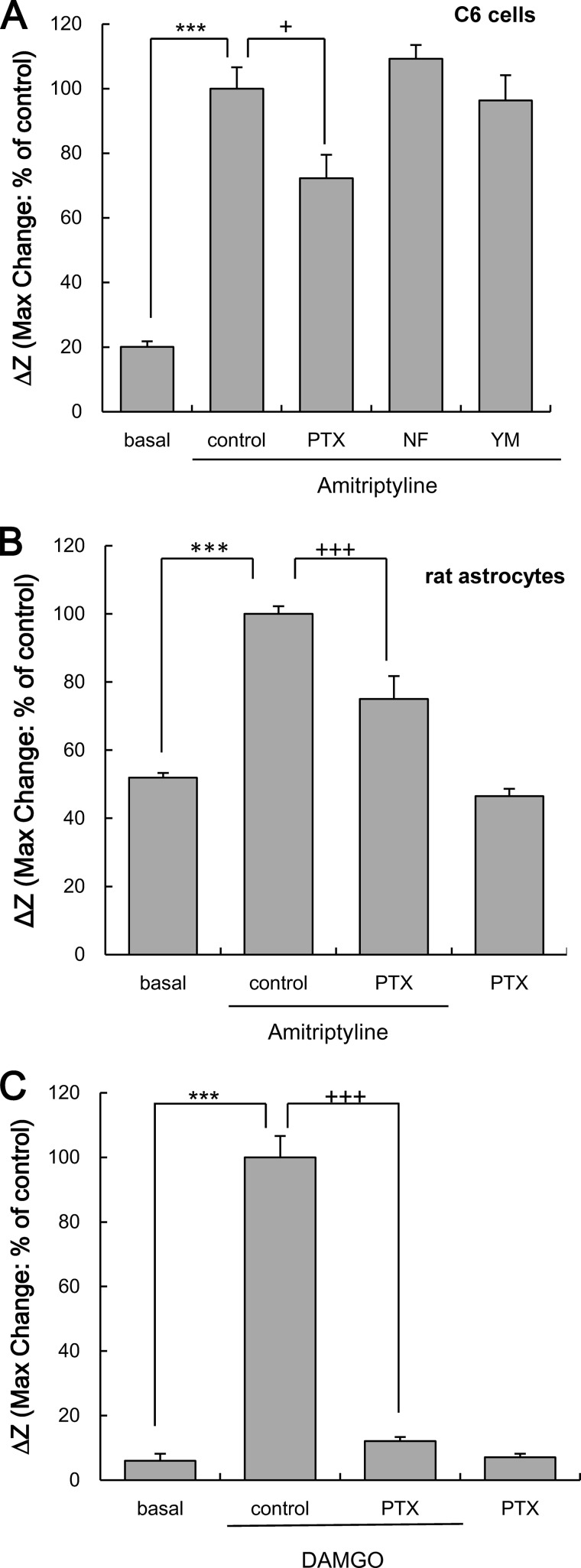
Effects of PTX, NF449, or YM-254890 on the Increase in ΔZ Evoked by Amitriptyline in C6 Cells and Rat Astrocytes
To pharmacologically characterize the increase of ΔZ evoked by amitriptyline, the effects of G protein α-subunit inhibitors were examined. The increase in ΔZ evoked by amitriptyline (25 μm) in C6 cells was significantly, but not completely, inhibited by PTX. However, neither NF449 nor YM-254890 inhibited the amitriptyline-induced increase in ΔZ (Fig. 7A). Application of each inhibitor alone did not affect in ΔZ (% of basal: PTX alone, 73.0 ± 5.2%; NF499 alone, 111.3 ± 24.7%; YM-254890 alone, 88.0 ± 8.0%). The increase in ΔZ evoked by amitriptyline (25 μm) in rat astrocytes was also significantly, but not completely, inhibited by PTX (Fig. 7B). In μ-opioid receptor-expressing HEK293 cells, the increase in ΔZ evoked by DAMGO (1 μm) was completely suppressed by a 3-h pretreatment of 100 ng/ml PTX (Fig. 7C). Application of PTX alone did not affect ΔZ (% of basal: PTX alone, 155.2 ± 47.8%).
FIGURE 7.
Effects of G protein inhibitors on the increase in ΔZ evoked by amitriptyline in C6 cells and rat astrocytes or μ-opioid receptor agonist DAMGO in HEK293 cells expressing μ-opioid receptors. A, effects of PTX, NF449 (NF), and YM-254890 (YM) on amitriptyline-evoked increase in ΔZ in C6 cells. C6 cells were pretreated with 100 ng/ml PTX for 3 h, 1 μm NF449 or 100 nm YM-254890 for 0.5 h, and subsequently treated with 25 μm of amitriptyline for 10 min. Values are expressed as the mean ± S.E. of ΔZ (basal, n = 21; control, n = 21; PTX, n = 9; NF449, n = 12; YM-254890, n = 9). ***, p < 0.001 in comparison with the basal group; +, p < 0.05 in comparison with the control group (Tukey's test). B, effects of PTX on amitriptyline-evoked increase in ΔZ in rat astrocytes. Rat astrocytes were pretreated with 100 ng/ml PTX for 3 h and subsequently treated with 25 μm amitriptyline for 30 min. Values are expressed as the mean ± S.E. of ΔZ (all each group; n = 6). ***, p < 0.001 in comparison with the basal group; +++, p < 0.001 in comparison with the control group (Tukey's test). C, effects of PTX on the increase in ΔZ evoked by the μ-opioid receptor agonist in HEK293 cells expressing μ-opioid receptors. HEK293 cells expressing μ-opioid receptors were pretreated with 100 ng/ml PTX for 3 h and subsequently treated with 1 μm DAMGO for 10 min. Values are expressed as the mean ± S.E. of ΔZ (all each group; n = 12). ***, p < 0.001 in comparison with the basal group; +++, p < 0.001 in comparison with the control group (Tukey's test).
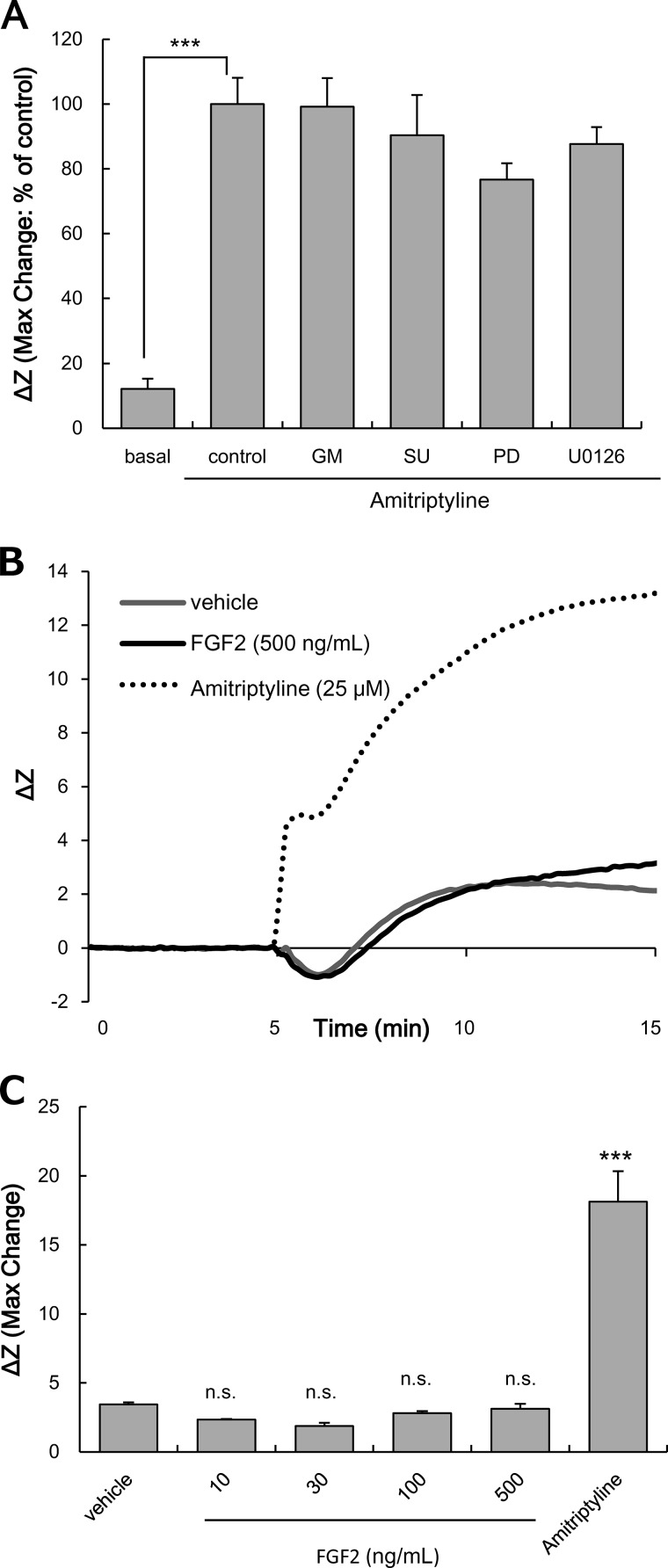
Effects of Inhibitors on the Cascade Related to GDNF Production on the Increase in ΔZ Evoked by Amitriptyline in C6 Cells
The effects of inhibitors on the cascade related to GDNF production (MMP/FGFR/FRS2α/ERK) on the increase in ΔZ evoked by amitriptyline were examined, to elucidate the point within the cascade that was responsible for the increase of ΔZ (Gi/o activation). The concentrations of inhibitors were used as described previously (30, 31, 38, 39). GM6001, SU5402, PD173074 (1 μm; FGFR inhibitor), and U0126 (10 μm; MEK inhibitor) had no effect on the amitriptyline-induced increase in ΔZ (Fig. 8A). Application of each inhibitor alone did not affect ΔZ (% of basal: GM6001 alone, 86.2 ± 9.7%; SU5402 alone, 83.3 ± 13.6%; PD173074 alone, 106.5 ± 9.3%; U0126 alone, 75.9 ± 6.9%). In addition, treatment with exogenous FGF2 (up to 500 ng/ml) did not change ΔZ in C6 cells (Fig. 8, B and C).
FIGURE 8.
Effects of inhibitors related to the GDNF production cascade on the amitriptyline-evoked increase of ΔZ in C6 cells. A, effects of GM6001 (GM), SU5420 (SU), PD173074 (PD), and U0126 on the increase of ΔZ evoked by amitriptyline in C6 cells. C6 cells were pretreated with 25 μm GM6001, 25 μm SU5402, 1 μm PD173074, or 10 μm U0126 for 0.5 h and subsequently treated with 25 μm amitriptyline for 10 min. Values are expressed as the mean ± S.E. of ΔZ (basal, n = 23; control, n = 23; GM6001, n = 9; SU5402, n = 5; PD173074, n = 10; U0126, n = 6). ***, p < 0.001 in comparison with the basal group (Tukey's test). B, changes in ΔZ evoked by incubation in vehicle, FGF2 (10, 30, 100, and 500 ng/ml), or amitriptyline (25 μm) for 10 min in C6 cells. The traces shown are representative of the mean increase in ΔZ of cells from each well. Similar results were obtained in at least three independent experiments. C, extent of changes in ΔZ was presented as the maximum ΔZ after injection of vehicle, FGF2, or amitriptyline. Values are expressed as the mean ± S.E. of ΔZ (vehicle, n = 9; FGF2 (each dose), n = 3; amitriptyline, n = 9). ***, p < 0.001 in comparison with the vehicle group (Tukey's test). n.s., no significant difference.
Increases of GDNF Production Evoked by Amitriptyline Are Independent of the κ-Opioid Receptor
Recently, it has been reported that amitriptyline exerts direct agonist activity at κ-opioid receptors, which is coupled to Gi/o (40). C6 cells are known to endogenously express κ-opioid receptors (41). To examine a potential involvement of κ-opioid receptors in GDNF production, we examined the effect of the κ-opioid receptor antagonist, nor-BNI (1 μm), as described previously (40). The increase of GDNF release evoked by amitriptyline (25 μm) was not affected by pretreatment with nor-BNI (basal, 15.9 ± 3.87 pg/ml; amitriptyline only, 43.5 ± 7.40 pg/ml (*, p < 0.05 in comparison with basal); amitriptyline + nor-BNI, 43.8 ± 8.50 pg/ml; nor-BNI only, 15.8 ± 4.83 pg/ml). Thus, κ-opioid receptors are not involved in GDNF production in C6 cells.
Discussion
The current data suggest that amitriptyline evokes GDNF production through a mechanism that is entirely independent of monoamines, via activation of a PTX-sensitive Gi/o/MMP/FGFR/FRS2α/ERK cascade in astroglial cells (Fig. 9). This study demonstrated that the PTX-sensitive Gi/o subunits, especially Go and Gi3, play important roles in the activation of ERK evoked by amitriptyline. The CellKeyTM assay confirmed the pharmacological and siRNA findings, in that the pattern of the change in impedance induced by amitriptyline was similar to that of activation of Gi/o-coupled receptors, and not of Gs- or Gq-coupled receptors (13, 14, 17, 18). Although the C6 cells is an astrocyte model cell line derived from rat glioma, activation of the MMP/FGFR/FRS2α/ERK cascade occurs in both normal human and rat astrocytes as well (9, 11), in a PTX-sensitive manner.
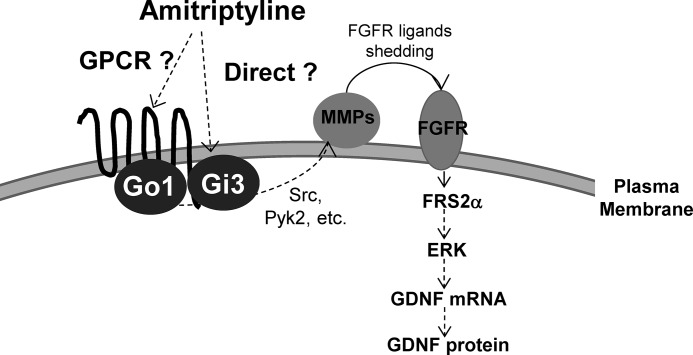
FIGURE 9.
Hypothesized monoamine-independent mode of action of amitriptyline-induced GDNF production in astroglial cells. Amitriptyline either directly or indirectly activates PTX-sensitive Gi/o (Gi3 or Go1) and MMP/FGFR/FRS2α/ERK cascade, leading to GDNF production in astroglial cells.
The Gα family of G proteins is composed of the Gs, Gi/o, and Gq subfamilies (14). Among the G protein inhibitors used in this study, only PTX inhibited amitriptyline-induced GDNF production and the amitriptyline-mediated response of the cascade (FRS2α and ERK), indicating that PTX-sensitive Gi/o plays a critical role in the intracellular response to amitriptyline. The concentrations of the selective inhibitors for the various intracellular signal cascade molecules were based on previous reports for cell culture systems (23–26, 30, 31, 38, 39). Based on the IC50 value of these inhibitors, these inhibitors are in fact selective for their respective targets (30, 42–47). Although 25 μm GM6001 was used, which is 1000-fold of the IC50, a previous study used the negative analog of GM6001 to confirm selectivity of GM6001. The negative analog, at 25 μm, showed no effect (11). To further confirm the involvement of PTX-sensitive Gi/o on the effects of amitriptyline, siRNAs specific for Go1, Gi2, and Gi3 were used. Amitriptyline-evoked ERK activation was significantly blocked in C6 cells in which either Go1 or Gi3 expression was reduced by siRNA. Although amitriptyline had no effect on ERK activation in these cells, treatment of the cells with exogenous FGF2 evoked ERK activation. PTX had no effect on the exogenous FGF2-mediated activation of FRS2α and ERK. Furthermore, FGFR activation by exogenous FGF2 did not change ΔZ (Gi/o activation). Conversely, FGFR inhibitor and ERK inhibitor had no effect on Gi/o activation by amitriptyline. These data indicate that activation of FGFR/FRS2α/ERK is well downstream of Go1 and Gi3 protein activation by amitriptyline, which is in line with our model system (Fig. 9). MMP is a key intermediary between Gi/o activation and FGFR/FRS2α/ERK activation. Activation of FGFR/FRS2α/ERK by amitriptyline was inhibited by a MMP inhibitor, whereas activation of FGFR/FRS2α/ERK by exogenous FGF2 was not affected by a MMP inhibitor (11). Furthermore, the increase of ΔZ (Gi/o activation) by amitriptyline was not affected by an MMP inhibitor. These results indicate that MMP lies between Gi/o and FGFR/FRS2α/ERK in the molecular signaling cascade evoked by amitriptyline. Thus, in our proposed pathway, although MMP and FGFR act as intermediaries, Go1 and Gi3 are crucial as the initiating signaling molecules that leads to GDNF production by amitriptyline in astroglial cells (Fig. 9).
The intracellular signaling pathway mediating GDNF production by amitriptyline has not been completely elucidated. The MMPs are a family of zinc-dependent endopeptidases with 24 identified members (48). It has been reported that MMP is activated by intracellular mediators such as Src family kinases, calcium (Ca2+), proline-rich tyrosine kinase 2 (Pyk2), and protein kinase C (PKC), which are regulated by G proteins (12). We previously showed that ERK activation evoked by amitriptyline was not inhibited by calphostin C (pan-PKC inhibitor), rottlerin (PKC δ inhibitor), EDTA (Ca2+ inhibitor), and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (intracellular Ca2+ inhibitor) (9). Thus, either Src family kinases or Pyk2 could be involved in the Gi/o/MMP/FGFR/FRS2α/ERK cascade activated by amitriptyline in this study. However, the molecular intermediaries between G protein and MMP in the cascade and the specific type of MMP involved have yet to be identified. Because of a potentially crucial role of MMP in mediating the physiological effect of amitriptyline, identification of the MMP in the cascade is currently under investigation.
In the CellKeyTM assay, the pattern of ΔZ evoked by amitriptyline indicated activation of Gi/o in C6 cells and rat astrocytes. Scott and Peters (19) reported that the CellKeyTM assay is a highly sensitive assay compared with traditional assays such as GTPγS binding assay, cAMP assay, and Ca2+ imaging assay. However, there is currently a lack of data demonstrating changes in terms of the amount, activity, or distribution of Gi/o in C6 cells following chronic antidepressant treatment (49–51). The discrepancy between the current findings and previous findings could stem from differences in experimental protocols, such as treatment period (acute versus chronic), sample preparation (e.g. living cells, membrane preparation, or fixed tissues or cells), and assay readout (adenylyl cyclase activity or CellKeyTM).
In this study, an acute treatment with amitriptyline activated Gi/o as indicated by increased ΔZ. However, pretreatment with 100 ng/ml PTX for 3 h only partially inhibited the increase in ΔZ. By contrast, the same treatment with PTX completely suppressed the DAMGO-induced increase of ΔZ. Other experiments in this study showed a robust suppression of the GDNF production cascade using the current PTX treatment procedure. Thus, the partial inhibition by PTX treatment suggests involvement of a PTX-insensitive Gi/o. In the Gi/o subfamily, Gi1, Gi2, Gi3, Go1, Go2, and Gz subunits have been identified and share similar properties in signal transduction (52). However, among these subunits, Gz lacks the C-terminal cysteine residue and is not inactivated by PTX treatment (53, 54). Wang et al. (55) reported that C6 cells express Gz. This study confirmed the expression of Gz in C6 cells by RT-PCR. Therefore, these data suggest that amitriptyline activates both PTX-sensitive Gi/o subunits, Go1 and Gi3, and PTX-insensitive Gi/o subunit, such as Gz. Although the ΔZ pattern following amitriptyline treatment was similar to that of DAMGO, indicating Gi/o activation, it was not the same. A rapid initial increase of ΔZ was observed following amitriptyline treatment, whereas a gradual initial increase of ΔZ was observed after DAMGO treatment. This differential response could be due to the type of Gi/o subunits activated by either ligand. Taken together, the predominant G protein subunit that mediates the effect of amitriptyline in this study is the PTX-sensitive Gi/o subunit, but it is possible, given the partial inhibition observed in this study, that amitriptyline also activates a PTX-insensitive Gi/o subunit such as Gz.
Although amitriptyline treatment acutely increased Gi/o activation in C6 cells and rat astrocytes, it is unknown whether amitriptyline directly or indirectly activates Gi/o. Amitriptyline may have two possible modes of action. One possibility is that amitriptyline directly acts on Gi/o associated with the plasma membrane, and another possibility is that amitriptyline activates Gi/o through a yet-to-be identified GPCR (Fig. 9). Yamamoto et al. (56) reported that tricyclic antidepressants increased GTPase activity of Go in bovine brain membranes reconstituted into a phospholipid mixture without receptors. The effect of tricyclic antidepressants on the GTPase activity of Go was abolished by pretreatment with PTX, indicating that tricyclic antidepressants, such as amitriptyline, could directly activate Go, bypassing the need for receptor activation (56). However, the mechanism of GDNF production evoked by amitriptyline is reminiscent of receptor tyrosine kinase transactivation by GPCR (12). In this study, the data indicate the involvement of PTX-sensitive Gi/o subunits, especially Go1 and Gi3. The histamine H1 and muscarinic M3 receptors, which are known to bind amitriptyline and are endogenously expressed in C6 cells, are functionally coupled to Gq (57–59). Like amitriptyline, 5-HT activated the FGFR/FRS2α/ERK cascade, but as demonstrated in this study, the effect of 5-HT was elicited through Gq, but not Gi/o. Furthermore, it was previously confirmed that amitriptyline-evoked GDNF production in C6 cells occurred independently of monoamine and muscarinic and histamine receptor activation (9). Thus, these GPCR for which amitriptyline has high affinity are not likely involved in GDNF production in C6 cells. Although it has been reported that amitriptyline exerts direct agonist activity at the κ-opioid receptor (40), this study demonstrated a lack of involvement of the κ-opioid receptor on GDNF production. Furthermore, it has been reported that in C6 cells, U69593, a κ-opioid receptor agonist, did not induce FGFR1 phosphorylation, which is a key step in the GDNF production cascade (Gi/o/MMP/FGFR/FRS2α/ERK) (29); amitriptyline increased both of FGFR1 and FGFR2 phosphorylation in C6 cells (11). Thus, the κ-opioid receptor is not involved in the activation of the cascade that leads to the production of GDNF. A GPCR with high affinity for amitriptyline could, however, be involved in GDNF production, as numerous GPCRs have been identified in astrocytes. The specific GPCR mediating amitriptyline's effect has yet to be identified and requires further investigation. Identifying the GPCR could facilitate understanding the source of the adverse effects as well as the beneficial effects of antidepressants.
Because of their high lipophilic properties, antidepressants accumulate in the brain at concentrations severalfold higher than that in blood. In fact, it has been previously shown that the concentration of amitriptyline in the brain is ∼10–35 times higher than the plasma concentration of amitriptyline (60, 61). The therapeutic plasma concentration of amitriptyline is commonly regarded to range from ∼0.36 to 0.9 μm (62). At therapeutic plasma concentrations, the brain concentration of amitriptyline can therefore be expected to be between 3.6 and 31.5 μm. Previous studies have shown that the minimum concentration of amitriptyline that induced GDNF release is 10 μm and a trend toward increased ERK activity with amitriptyline concentrations greater than 1 μm in astroglial cells was observed (9); these concentrations of amitriptyline are within the range of the estimated brain concentration that is achieved after treatment with clinically relevant doses. Furthermore, the concentrations of amitriptyline used in this study were not toxic to C6 cells and primary cultured rat astrocytes (9, 10). Amitriptyline inhibits the serotonin transporter and the norepinephrine transporter at a Ki value of 16 and 8.6 nm, respectively (63). Thus, the estimated brain concentration of amitriptyline is much higher than the Ki value for these transporters, indicating the likelihood that amitriptyline could interact with additional targets, such as Go1- and/or Gi3-coupled receptors or other membrane-binding sites.
G proteins are crucial mediators between extracellular signaling and the intracellular response, which, in turn, could lead to either neurological homeostasis or disorder. It remains unclear as to how amitriptyline-evoked Gi/o activation is related to the antidepressive effect of amitriptyline. An in vivo study showed that intracerebroventricular administration of PTX completely prevented the antidepressant-like effect of amitriptyline in the mouse forced swimming test (64). Furthermore, Galeotti et al. (64) showed that Gi1, Gi2, Gi3, and Go1, but not Go2, play important roles in the antidepressant-like effect of amitriptyline, administering antisense oligonucleotides against the α-subunits of the G protein. This study confirms the importance of PTX-sensitive Gi/o in the effect of antidepressants such as amitriptyline and further elaborates a novel mechanism that could explain the efficacy of antidepressants, increased GDNF production by astrocytes.
In conclusion, this study showed that the PTX-sensitive α-subunit of G proteins, Go1 and Gi3, plays an important role in a monoamine-independent production of GDNF evoked by amitriptyline. Previous studies have shown that several classes of antidepressants (tricyclic antidepressant, tetracyclic antidepressant, and selective 5-HT reuptake inhibitor) increase FRS2α phosphorylation, activate ERK, and stimulate GDNF production in both rat and human astrocytes (8, 9, 11). It is not yet clear whether these different antidepressants act similarly to amitriptyline. Further studies are needed to clarify whether the effect of amitriptyline on Gi/o is a feature shared among antidepressants. It is hoped that by elaborating the Gi/o-dependent mechanism that underlies amitriptyline's therapeutic effect, there will be greater understanding of the non-monoaminergic and non-neural mechanisms of antidepressant activity, thereby setting the stage for the development of antidepressants with truly novel mechanisms of action.
Acknowledgments
We thank the Analysis Center of Life Science, Hiroshima University, for the use of their facilities. We also thank Dr. Aldric T. Hama for careful editing of the manuscript.
This work was supported in part by Grants-in-aid for Young Scientists (B) 25870461 and 23591686 from the Japan Society for the Promotion of Science and the Takeda Science Foundation.
- MDD
- major depressive disorder
- GDNF
- glial cell line-derived neurotrophic factor
- MMP
- matrix metalloproteinase
- FGFR
- fibroblast growth factor receptor
- PTX
- pertussis toxin
- GPCR
- G protein-coupled receptor
- 5-HT
- serotonin
- NHA
- normal human astrocyte
- nor-BNI
- nor-binaltorphimine dihydrochloride
- DAMGO
- [d-Ala2,N-MePhe4,Gly-ol]enkephalin
- GTPγS
- 5′-3-O-(thio)triphosphate.
References
- 1. Belmaker R. H., Agam G. (2008) Major depressive disorder. N. Engl. J. Med. 358, 55–68 [DOI] [PubMed] [Google Scholar]
- 2. Rajkowska G., Stockmeier C. A. (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr. Drug Targets 14, 1225–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen N. J., Barres B. A. (2009) Neuroscience: Glia–more than just brain glue. Nature 457, 675–677 [DOI] [PubMed] [Google Scholar]
- 4. Schmidt H. D., Banasr M., Duman R. S. (2008) Future antidepressant targets: neurotrophic factors and related signaling cascades. Drug Discov. Today Ther. Strateg. 5, 151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bespalov M. M., Saarma M. (2007) GDNF family receptor complexes are emerging drug targets. Trends Pharmacol. Sci. 28, 68–74 [DOI] [PubMed] [Google Scholar]
- 6. Takebayashi M., Hisaoka K., Nishida A., Tsuchioka M., Miyoshi I., Kozuru T., Hikasa S., Okamoto Y., Shinno H., Morinobu S., Yamawaki S. (2006) Decreased levels of whole blood glial cell line-derived neurotrophic factor (GDNF) in remitted patients with mood disorders. Int. J. Neuropsychopharmacol. 9, 607–612 [DOI] [PubMed] [Google Scholar]
- 7. Zhang X., Zhang Z., Xie C., Xi G., Zhou H., Zhang Y., Sha W. (2008) Effect of treatment on serum glial cell line-derived neurotrophic factor in depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 886–890 [DOI] [PubMed] [Google Scholar]
- 8. Hisaoka K., Nishida A., Koda T., Miyata M., Zensho H., Morinobu S., Ohta M., Yamawaki S. (2001) Antidepressant drug treatments induce glial cell line-derived neurotrophic factor (GDNF) synthesis and release in rat C6 glioblastoma cells. J. Neurochem. 79, 25–34 [DOI] [PubMed] [Google Scholar]
- 9. Hisaoka K., Takebayashi M., Tsuchioka M., Maeda N., Nakata Y., Yamawaki S. (2007) Antidepressants increase glial cell line-derived neurotrophic factor production through monoamine-independent activation of protein tyrosine kinase and extracellular signal-regulated kinase in glial cells. J. Pharmacol. Exp. Ther. 321, 148–157 [DOI] [PubMed] [Google Scholar]
- 10. Kajitani N., Hisaoka-Nakashima K., Morioka N., Okada-Tsuchioka M., Kaneko M., Kasai M., Shibasaki C., Nakata Y., Takebayashi M. (2012) Antidepressant acts on astrocytes leading to an increase in the expression of neurotrophic/growth factors: differential regulation of FGF-2 by noradrenaline. PLoS One 7, e51197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hisaoka K., Tsuchioka M., Yano R., Maeda N., Kajitani N., Morioka N., Nakata Y., Takebayashi M. (2011) Tricyclic antidepressant amitriptyline activates fibroblast growth factor receptor signaling in glial cells: involvement in glial cell line-derived neurotrophic factor production. J. Biol. Chem. 286, 21118–21128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wetzker R., Böhmer F. D. (2003) Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat. Rev. Mol. Cell Biol. 4, 651–657 [DOI] [PubMed] [Google Scholar]
- 13. Peters M. F., Vaillancourt F., Heroux M., Valiquette M., Scott C. W. (2010) Comparing label-free biosensors for pharmacological screening with cell-based functional assays. Assay Drug Dev. Technol. 8, 219–227 [DOI] [PubMed] [Google Scholar]
- 14. Miyano K., Sudo Y., Yokoyama A., Hisaoka-Nakashima K., Morioka N., Takebayashi M., Nakata Y., Higami Y., Uezono Y. (2014) History of the G protein-coupled receptor (GPCR) assays from traditional to a state-of-the-art biosensor assay. J. Pharmacol. Sci. 126, 302–309 [DOI] [PubMed] [Google Scholar]
- 15. Morioka N., Suekama K., Zhang F. F., Kajitani N., Hisaoka-Nakashima K., Takebayashi M., Nakata Y. (2014) Amitriptyline up-regulates connexin43-gap junction in rat cultured cortical astrocytes via activation of the p38 and c-Fos/AP-1 signalling pathway. Br. J. Pharmacol. 171, 2854–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohta M., Mizuta I., Ohta K., Nishimura M., Mizuta E., Hayashi K., Kuno S. (2000) Apomorphine up-regulates NGF and GDNF synthesis in cultured mouse astrocytes. Biochem. Biophys. Res. Commun. 272, 18–22 [DOI] [PubMed] [Google Scholar]
- 17. Peters M. F., Knappenberger K. S., Wilkins D., Sygowski L. A., Lazor L. A., Liu J., Scott C. W. (2007) Evaluation of cellular dielectric spectroscopy, a whole-cell, label-free technology for drug discovery on Gi-coupled GPCRs. J. Biomol. Screen. 12, 312–319 [DOI] [PubMed] [Google Scholar]
- 18. Zysk J. R., Widzowski D., Sygowski L. A., Knappenberger K. S., Spear N., Elmore C. S., Dorff P., Liu H., Doherty J., Chhajlani V. (2011) Absence of direct effects on the dopamine D2 receptor by mGluR2/3-selective receptor agonists LY 354,740 and LY 379,268. Synapse 65, 64–68 [DOI] [PubMed] [Google Scholar]
- 19. Scott C. W., Peters M. F. (2010) Label-free whole-cell assays: expanding the scope of GPCR screening. Drug Discov. Today 15, 704–716 [DOI] [PubMed] [Google Scholar]
- 20. Lamb N. J., Fernandez A., Conti M. A., Adelstein R., Glass D. B., Welch W. J., Feramisco J. R. (1988) Regulation of actin microfilament integrity in living nonmuscle cells by the cAMP-dependent protein kinase and the myosin light chain kinase. J. Cell Biol. 106, 1955–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gräler M. H., Grosse R., Kusch A., Kremmer E., Gudermann T., Lipp M. (2003) The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J. Cell. Biochem. 89, 507–519 [DOI] [PubMed] [Google Scholar]
- 22. Davies S. L., Gibbons C. E., Vizard T., Ward D. T. (2006) Ca2+-sensing receptor induces Rho kinase-mediated actin stress fiber assembly and altered cell morphology, but not in response to aromatic amino acids. Am. J. Physiol. Cell Physiol. 290, C1543–C1551 [DOI] [PubMed] [Google Scholar]
- 23. Tsuchioka M., Takebayashi M., Hisaoka K., Maeda N., Nakata Y. (2008) Serotonin (5-HT) induces glial cell line-derived neurotrophic factor (GDNF) mRNA expression via the transactivation of fibroblast growth factor receptor 2 (FGFR2) in rat C6 glioma cells. J. Neurochem. 106, 244–257 [DOI] [PubMed] [Google Scholar]
- 24. Van Kolen K., Slegers H. (2004) P2Y12 receptor stimulation inhibits β-adrenergic receptor-induced differentiation by reversing the cyclic AMP-dependent inhibition of protein kinase B. J. Neurochem. 89, 442–453 [DOI] [PubMed] [Google Scholar]
- 25. van Corven E. J., Hordijk P. L., Medema R. H., Bos J. L., Moolenaar W. H. (1993) Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 90, 1257–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin Y. F., Tseng M. J., Hsu H. L., Wu Y. W., Lee Y. H., Tsai Y. H. (2006) A novel follicle-stimulating hormone-induced Gαh/phospholipase C-δ1 signaling pathway mediating rat Sertoli cell Ca2+-influx. Mol. Endocrinol. 20, 2514–2527 [DOI] [PubMed] [Google Scholar]
- 27. Kouhara H., Hadari Y. R., Spivak-Kroizman T., Schilling J., Bar-Sagi D., Lax I., Schlessinger J. (1997) A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89, 693–702 [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y., Zhang J., Lin Y., Lan Y., Lin C., Xuan J. W., Shen M. M., McKeehan W. L., Greenberg N. M., Wang F. (2008) Role of epithelial cell fibroblast growth factor receptor substrate 2α in prostate development, regeneration and tumorigenesis. Development 135, 775–784 [DOI] [PubMed] [Google Scholar]
- 29. Belcheva M. M., Haas P. D., Tan Y., Heaton V. M., Coscia C. J. (2002) The fibroblast growth factor receptor is at the site of convergence between μ-opioid receptor and growth factor signaling pathways in rat C6 glioma cells. J. Pharmacol. Exp. Ther. 303, 909–918 [DOI] [PubMed] [Google Scholar]
- 30. Mohammadi M., McMahon G., Sun L., Tang C., Hirth P., Yeh B. K., Hubbard S. R., Schlessinger J. (1997) Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276, 955–960 [DOI] [PubMed] [Google Scholar]
- 31. Tsai L. K., Leng Y., Wang Z., Leeds P., Chuang D. M. (2010) The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology 35, 2225–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurino M., Fukunaga K., Ushio Y., Miyamoto E. (1996) Cyclic AMP inhibits activation of mitogen-activated protein kinase and cell proliferation in response to growth factors in cultured rat cortical astrocytes. J. Neurochem. 67, 2246–2255 [DOI] [PubMed] [Google Scholar]
- 33. Castillo C. A., Albasanz J. L., Fernández M., Martín M. (2007) Endogenous expression of adenosine A1, A2 and A3 receptors in rat C6 glioma cells. Neurochem. Res. 32, 1056–1070 [DOI] [PubMed] [Google Scholar]
- 34. Lagriffoul A., Charpentier N., Carrette J., Tougard C., Bockaert J., Homburger V. (1996) Secretion of protease nexin-1 by C6 glioma cells is under the control of a heterotrimeric G protein, Go1. J. Biol. Chem. 271, 31508–31516 [DOI] [PubMed] [Google Scholar]
- 35. Morioka N., Sugimoto T., Tokuhara M., Dohi T., Nakata Y. (2010) Noradrenaline induces clock gene Per1 mRNA expression in C6 glioma cells through β(2)-adrenergic receptor coupled with protein kinase A-cAMP response element binding protein (PKA-CREB) and Src-tyrosine kinase-glycogen synthase kinase-3β (Src-GSK-3β). J. Pharmacol. Sci. 113, 234–245 [DOI] [PubMed] [Google Scholar]
- 36. Luo J., Busillo J. M., Benovic J. L. (2008) M3 muscarinic acetylcholine receptor-mediated signaling is regulated by distinct mechanisms. Mol. Pharmacol. 74, 338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen J. X., Cooper D. M. (2013) AKAP79, PKC, PKA and PDE4 participate in a Gq-linked muscarinic receptor and adenylate cyclase 2 cAMP signalling complex. Biochem. J. 455, 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Skaper S. D., Kee W. J., Facci L., Macdonald G., Doherty P., Walsh F. S. (2000) The FGFR1 inhibitor PD 173074 selectively and potently antagonizes FGF-2 neurotrophic and neurotropic effects. J. Neurochem. 75, 1520–1527 [DOI] [PubMed] [Google Scholar]
- 39. Tran M. D., Neary J. T. (2006) Purinergic signaling induces thrombospondin-1 expression in astrocytes. Proc. Natl. Acad. Sci. U.S.A. 103, 9321–9326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Onali P., Dedoni S., Olianas M. C. (2010) Direct agonist activity of tricyclic antidepressants at distinct opioid receptor subtypes. J. Pharmacol. Exp. Ther. 332, 255–265 [DOI] [PubMed] [Google Scholar]
- 41. Bohn L. M., Belcheva M. M., Coscia C. J. (2000) Mitogenic signaling via endogenous κ-opioid receptors in C6 glioma cells: evidence for the involvement of protein kinase C and the mitogen-activated protein kinase signaling cascade. J. Neurochem. 74, 564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hohenegger M., Waldhoer M., Beindl W., Böing B., Kreimeyer A., Nickel P., Nanoff C., Freissmuth M. (1998) Gsα-selective G protein antagonists. Proc. Natl. Acad. Sci. U.S.A. 95, 346–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takasaki J., Saito T., Taniguchi M., Kawasaki T., Moritani Y., Hayashi K., Kobori M. (2004) A novel Gαq/11-selective inhibitor. J. Biol. Chem. 279, 47438–47445 [DOI] [PubMed] [Google Scholar]
- 44. Grobelny D., Poncz L., Galardy R. E. (1992) Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry 31, 7152–7154 [DOI] [PubMed] [Google Scholar]
- 45. Mohammadi M., Froum S., Hamby J. M., Schroeder M. C., Panek R. L., Lu G. H., Eliseenkova A. V., Green D., Schlessinger J., Hubbard S. R. (1998) Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 17, 5896–5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Duncia J. V., Santella J. B., 3rd, Higley C. A., Pitts W. J., Wityak J., Frietze W. E., Rankin F. W., Sun J. H., Earl R. A., Tabaka A. C., Teleha C. A., Blom K. F., Favata M. F., Manos E. J., Daulerio A. J., et al. (1998) MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg. Med. Chem. Lett. 8, 2839–2844 [DOI] [PubMed] [Google Scholar]
- 47. Wilson K. M., Minneman K. P. (1990) Pertussis toxin inhibits norepinephrine-stimulated inositol phosphate formation in primary brain cell cultures. Mol. Pharmacol. 38, 274–281 [PubMed] [Google Scholar]
- 48. Nagase H., Woessner J. F. (1999) Matrix metalloproteinases. J. Biol. Chem. 274, 21491–21494 [DOI] [PubMed] [Google Scholar]
- 49. Chen J., Rasenick M. M. (1995) Chronic treatment of C6 glioma cells with antidepressant drugs increases functional coupling between a G protein (Gs) and adenylyl cyclase. J. Neurochem. 64, 724–732 [DOI] [PubMed] [Google Scholar]
- 50. Toki S., Donati R. J., Rasenick M. M. (1999) Treatment of C6 glioma cells and rats with antidepressant drugs increases the detergent extraction of G(sα) from plasma membrane. J. Neurochem. 73, 1114–1120 [DOI] [PubMed] [Google Scholar]
- 51. Donati R. J., Thukral C., Rasenick M. M. (2001) Chronic treatment of C6 glioma cells with antidepressant drugs results in a redistribution of Gsα. Mol. Pharmacol. 59, 1426–1432 [DOI] [PubMed] [Google Scholar]
- 52. Kowal D., Zhang J., Nawoschik S., Ochalski R., Vlattas A., Shan Q., Schechter L., Dunlop J. (2002) The C terminus of Gi family G-proteins as a determinant of 5-HT(1A) receptor coupling. Biochem. Biophys. Res. Commun. 294, 655–659 [DOI] [PubMed] [Google Scholar]
- 53. Fong H. K., Yoshimoto K. K., Eversole-Cire P., Simon M. I. (1988) Identification of a GTP-binding protein α subunit that lacks an apparent ADP-ribosylation site for pertussis toxin. Proc. Natl. Acad. Sci. U.S.A. 85, 3066–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Matsuoka M., Itoh H., Kozasa T., Kaziro Y. (1988) Sequence analysis of cDNA and genomic DNA for a putative pertussis toxin-insensitive guanine nucleotide-binding regulatory protein α subunit. Proc. Natl. Acad. Sci. U.S.A. 85, 5384–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang J., Tu Y., Woodson J., Song X., Ross E. M. (1997) A GTPase-activating protein for the G protein Gαz. Identification, purification, and mechanism of action. J. Biol. Chem. 272, 5732–5740 [DOI] [PubMed] [Google Scholar]
- 56. Yamamoto H., Tomita U., Mikuni M., Kobayashi I., Kagaya A., Katada T., Ui M., Takahashi K. (1992) Direct activation of purified Go-type GTP binding protein by tricyclic antidepressants. Neurosci. Lett. 139, 194–196 [DOI] [PubMed] [Google Scholar]
- 57. Peakman M. C., Hill S. J. (1994) Endogenous expression of histamine H1 receptors functionally coupled to phosphoinositide hydrolysis in C6 glioma cells: regulation by cyclic AMP. Br. J. Pharmacol. 113, 1554–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Singh S. P., Gao Y., Kunapuli S. P., Ravindra R. (1998) Glucose uptake by C6 glioma cells is mediated by G(q α). Neuroreport 9, 115–119 [DOI] [PubMed] [Google Scholar]
- 59. Pinkas-Kramarski R., Edelman R., Stein R. (1990) Indications for selective coupling to phosphoinositide hydrolysis or to adenylate cyclase inhibition by endogenous muscarinic receptor subtypes M3 and M4 but not by M2 in tumor cell lines. Neurosci. Lett. 108, 335–340 [DOI] [PubMed] [Google Scholar]
- 60. Glotzbach R. K., Preskorn S. H. (1982) Brain concentrations of tricyclic antidepressants: single-dose kinetics and relationship to plasma concentrations in chronically dosed rats. Psychopharmacology 78, 25–27 [DOI] [PubMed] [Google Scholar]
- 61. Miyake K., Fukuchi H., Kitaura T., Kimura M., Sarai K., Nakahara T. (1990) Pharmacokinetics of amitriptyline and its demethylated metabolite in serum and specific brain regions of rats after acute and chronic administration of amitriptyline. J. Pharm. Sci. 79, 288–291 [DOI] [PubMed] [Google Scholar]
- 62. O'Donnell J. M, Shelton R. C. (eds) (2011) Drug Therapy of Depression and Anxiety Disorders, Ed. 12, McGraw-Hill Book Co., New York [Google Scholar]
- 63. Owens M. J., Morgan W. N., Plott S. J., Nemeroff C. B. (1997) Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J. Pharmacol. Exp. Ther. 283, 1305–1322 [PubMed] [Google Scholar]
- 64. Galeotti N., Bartolini A., Ghelardini C. (2002) Role of Gi proteins in the antidepressant-like effect of amitriptyline and clomipramine. Neuropsychopharmacology 27, 554–564 [DOI] [PubMed] [Google Scholar]