Background: The liver produces both excessive glucose and lipids in type 2 diabetes.
Results: Glucose production is more vulnerable to insulin receptor (InsR) impairment ex vivo than is lipogenesis.
Conclusion: Selective insulin resistance arises due to inherent, cell-autonomous differences in the insulin responsiveness of glucose versus lipid metabolism.
Significance: InsR itself may represent a locus of treatment of diabetes.
Keywords: diabetes, FOXO, gluconeogenesis, glucose metabolism, lipogenesis, lipoprotein metabolism, atherosclerosis, insulin receptor, insulin resistance, lipid metabolism, liver
Abstract
The development of insulin resistance (IR) in the liver is a key pathophysiologic event in the development of type 2 diabetes. Although insulin loses its ability to suppress glucose production, it largely retains its capacity to drive lipogenesis. This selective IR results in the characteristic hyperglycemia and dyslipidemia of type 2 diabetes. The delineation of two branched pathways of insulin receptor (InsR) signaling to glucose versus triglyceride production, one through FoxO and the other through SREBP-1c, provides a mechanism to account for this pathophysiological abnormality. We tested the complementary hypothesis that selective IR arises due to different intrinsic sensitivities of glucose production versus de novo lipogenesis to insulin as a result of cell-autonomous down-regulation of InsR number in response to chronic hyperinsulinemia. We demonstrate in mouse primary hepatocytes that chronic hyperinsulinemia abrogates insulin's inhibition of glucose production, but not its stimulation of de novo lipogenesis. Using a competitive inhibitor of InsR, we show that there is a 4-fold difference between levels of InsR inhibition required to cause resistance of glucose production versus lipogenesis to the actions of insulin. Our data support a parsimonious model in which differential InsR activation underlies the selective IR of glucose production relative to lipogenesis, but both processes require signaling through Akt1/2.
Introduction
A conundrum facing clinicians is that hepatic lipogenesis is paradoxically increased in patients with insulin resistance (IR),2 engendering “selective” IR (1, 2). Thus, patients are exposed to the dual threats of poorly controlled glycemia and unchecked lipogenesis, with the latter contributing to non-alcoholic fatty liver disease and atherogenic dyslipidemia (3–5). Although the pathophysiology of selective IR remains unclear, it is generally acknowledged that the pathways to insulin regulation of glucose versus triglyceride production diverge downstream of Akt (6). However, it remains unclear why the divergence would result in selective resistance of one branch versus the other.
In this study, we tested the complementary hypothesis that the source of selective IR lies in an intrinsic difference of the sensitivities of these two pathways to insulin action. That is, the ED50 of insulin signaling required to blunt glucose production (GP) is lower than that required to stimulate de novo lipogenesis (DNL), such that IR overcomes the former more quickly than it does the latter. The primary cause of this IR may be compensatory CHI itself, which is known to dampen the insulin response in target cells (7–11). CHI that arises as an early manifestation of insulin resistance may trigger partial down-regulation of InsR in liver (12, 13), leading to a situation in which suppression of HGP is achieved at the price of stimulating DNL. In the case of complete IR, the hepatocyte is essentially indifferent to ambient insulin levels and therefore far below the ED50 for either arm (2).
To test this hypothesis in the absence of the effects of insulin in other tissues, we employed an InsR down-regulation model and a peptide blocker of InsR in primary murine hepatocytes. Although previous studies have also modeled CHI (8, 11, 14–16), they have not directly compared the simultaneous effect of partial InsR down-regulation on differential insulin signaling with lipid versus glucose metabolism.
Experimental Procedures
Chemicals
S961 insulin receptor antagonist was kindly supplied by Läuge Schaffer (Novo Nordisk), and its properties have been described previously (17). Akt inhibitor VIII (Akti-1/2, Sigma) has also been described previously (18).
Primary Hepatocyte Culture
Primary hepatocytes were isolated from wild-type C57Bl6/J mice aged over 10 weeks via collagenase perfusion as described (19). Following attachment to collagen-coated cultureware, cells were washed with PBS and incubated for 3 h in Medium 199 + 10% FBS (Life Technologies). Cells were then washed twice and incubated overnight in serum-free medium (see below) prior to the experimental assays described below. For CHI treatment, 100 nm insulin (Novolog) was added to the culture medium at each of the above steps.
Glucose Production Assay
Serum-free medium (Medium 199 + 1% BSA) was replaced with glucose production medium (glucose- and phenol red-free DMEM supplemented with 1% BSA, 3.3. g/liter sodium bicarbonate, 20 mm calcium lactate, and 2 mm sodium pyruvate). Cells were incubated with 100 μm 8-CPT-cAMP (Sigma), cAMP + insulin (1 or 10 nm), or vehicle for 5 h. Glucose released into the culture medium was measured via peroxidase-glucose oxidase assay (Sigma) and normalized to protein content. Cells to be incubated with cAMP + insulin were pretreated with insulin for 1 h in serum-free medium prior to the addition of glucose production cocktails. For S961 and Akti-1/2 treatment, inhibitors were added to serum-free medium for a 30-min pretreatment period prior to pretreatment with insulin (or vehicle), and then added again when medium was replaced with glucose production cocktail. Additional cells were treated in parallel to be used for measurement of gluconeogenic gene expression (see below).
De Novo Lipogenesis
Following overnight serum starvation (Medium 199 + 0.25% fatty acid-free BSA (Fisher)), medium was replaced with fresh serum-free medium containing insulin (1 or 10 nm) or vehicle. For S961 and Akti-1/2 treatment, inhibitors were added to serum-free medium for a 30-min pretreatment prior to medium replacement and then added again with subsequent medium changes. Following a 2-h insulin treatment period, cells were washed twice with PBS and radiolabeling was carried out in Medium 199 + 0.25% fatty acid-free BSA spiked with 0.6 μCi/ml [1,2-14C]acetic acid (PerkinElmer Life Sciences) with or without insulin and/or inhibitors over 3 h. Lipids were extracted using 3:2 hexane:isopropyl alcohol, dried in scintillation vials under N2 gas, and resuspended in 2:1 chloroform:methanol. Radiocarbon labeling of resuspended lipids was determined by liquid scintillation counting (PerkinElmer) and normalized to total cellular protein content. Additional cells were treated in parallel without radiolabeling to be used for measurement of lipogenic gene expression (see below).
Western Blotting
Following overnight serum starvation, cells were washed twice with PBS and replaced with serum-free medium (Medium 199 + 1% BSA) containing insulin at the concentrations indicated in the figures for 30 min. For S961 and Akti-1/2 treatment, inhibitors were added to serum-free medium 30 min prior to insulin treatment and then added again along with insulin. Following insulin treatment, cells were washed twice with ice-cold PBS and lysed in in ice-cold lysis buffer (20 mm Tris-HCl, 150 mm NaCl, 10% glycerol, 2% NP-40, 1 mm EDTA, 20 mm NaF, 30 mm Na4P2O7, 0.2% SDS, 0.5% sodium deoxycholate) supplemented with protease/phosphatase inhibitors (Cell Signaling). Protein concentration was assessed by bicinchoninic acid assay (Sigma). Antibodies used were all purchased from Cell Signaling with the exception of SREBP-1c (Novus Biologicals) and glucokinase, which was a gift of Mark Magnuson (Vanderbilt University). Densitometric analysis was performed using ImageJ software (National Institutes of Health).
mRNA Studies
Total RNA was isolated from hepatocytes treated as above using the RNeasy mini kit (Qiagen) following manufacturer's instructions. 1 μg of RNA was reverse-transcribed using the GoScript reverse transcription system (Promega). cDNAs were diluted 1:10, and RT-PCR was performed using a DNA Engine Opticon 2 System (Bio-Rad) with SYBR Green (Promega). Primer sequences are available upon request. Gene expression levels were normalized to TATA-binding protein (Tbp) using the 2−ΔΔCt method (20).
Results
Chronic Hyperinsulinemia Recapitulates Selective IR
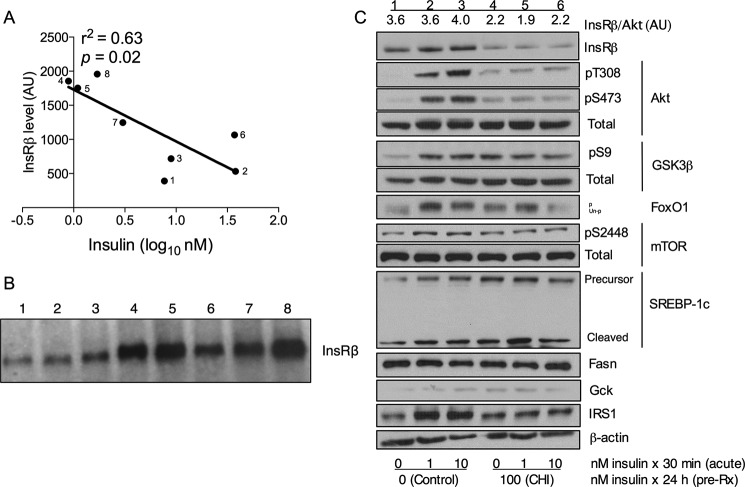
Although precedent exists for down-regulation of InsR in vivo in humans, there has been some controversy regarding its relevance in rodent models of obesity (21). Some of the variability in the data may stem from differences in the age of the mice, or the cause or degree of obesity. We have therefore assessed for liver expression of InsR in Insr+/− mice, a lean model of insulin resistance exhibiting compensatory hyperinsulinemia without hyperglycemia (22, 23). We found a significant correlation between circulating insulin levels and hepatic InsRβ protein content (Fig. 1, A and B). This finding suggests that compensatory hyperinsulinemia per se contributes to InsR down-regulation in the liver.
FIGURE 1.
Chronic hyperinsulinemia induces downregulation of the insulin receptor. A, linear regression analysis of liver InsRβ protein level as a function of the logarithm of the circulating insulin level in adult Insr+/− mice. AU, arbitrary units. B, InsRβ protein was quantified by densitometric analysis of the Western blot shown in B, in which the number of each lane corresponds to the same numbered point in A. C, Western blot analysis of InsR signaling in hepatocytes following chronic insulin treatment. Each lane contains equal amounts of protein pooled from three independent experiments. The densitometric quantification of InsR protein relative to Akt is labeled above each lane. pT308, phospho-Thr-308; pS473, phospho-Ser-473; pS9, phospho-Ser-9; pS2448, phospho-Ser-S2448; p, phosphorylated; un-p, unphosphorylated; Gck, glucokinase; pre-Rx, pretreatment.
The kinetics of CHI-induced InsR down-regulation in cultured hepatocytes is well described (8, 11, 14, 16, 24). Incubation of primary hepatocytes with 100 nm insulin (or lower concentrations) (24) recapitulates the effects of CHI, by decreasing InsR number by ∼50% in 24 h for up to 72 h (16). Removal of insulin from the culture medium results in a rapid rebound of InsR number to the level of untreated cells (16, 24). We treated mouse primary hepatocytes with 100 nm insulin for 24 h to mimic CHI, followed by acute (30-min) treatment with 10 nm insulin (10, 25). CHI treatment resulted in an ∼50% reduction in InsR protein levels, indicating InsR down-regulation (Fig. 1C). Expectedly, insulin-stimulated phosphorylation of Akt at Thr-308 and Ser-473 was markedly reduced, although basal levels of Akt phosphorylation were higher than in controls (Fig. 1C) (9, 10, 25). Phosphorylation of Akt substrates FoxO1 (as evidenced by electrophoretic mobility shift), GSK3β, and mTOR was similarly reduced, as was insulin-stimulated cleavage of SREBP-1c (Fig. 1C).
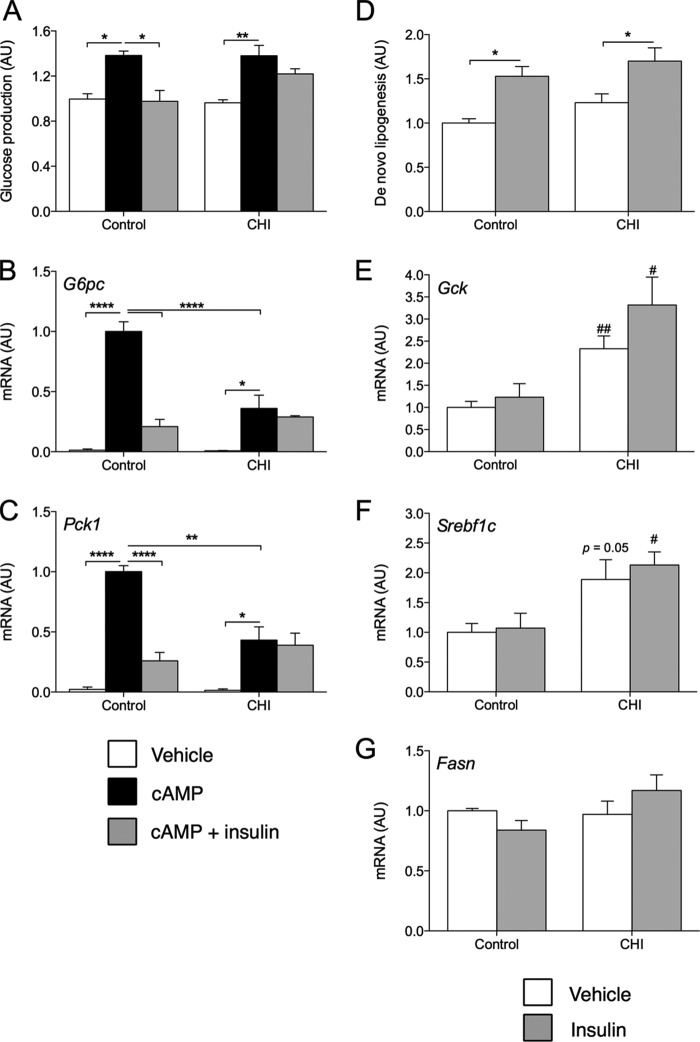
We next assessed insulin suppression of cAMP-induced GP. CHI treatment did not affect basal or maximal cAMP-induced GP but abrogated its suppression by insulin (Fig. 2A). Moreover, CHI treatment significantly dampened cAMP-induced expression of G6pc and Pck1 as well as their inhibition by insulin (Fig. 2, B and C). Conversely, insulin-stimulated de novo lipogenesis was unchanged relative to vehicle treatment (Fig. 2D), consistent with previous data in rat hepatocytes (11). Moreover, CHI-treated cells showed a non-significant trend toward increased rates of DNL in the basal and insulin-stimulated states relative to control cells. Although expression of the genes for glucokinase and SREBP-1c did not respond to insulin over the time course of the DNL assay in these cells, baseline expression of these genes increased following CHI (Fig. 2, E–G). These findings were borne out at the protein level (Fig. 1). Thus, we conclude from these experiments that CHI treatment renders primary hepatocytes selectively resistant to the effects of insulin on glucose production.
FIGURE 2.
Metabolic effects of chronic insulin treatment. A–C, relative glucose production (A) and gene expression (B and C) in control or CHI-treated primary hepatocytes incubated for 5 h with vehicle, cAMP, or cAMP and insulin. Data are normalized to vehicle-treated control. AU, arbitrary units. D–G, relative de novo lipogenesis (D) and gene expression (E–G) in control or CHI-treated primary hepatocytes incubated for 5 h with vehicle or 10 nm insulin. Data are normalized to vehicle-treated control and represent mean ± S.E. of at least three independent experiments performed in triplicate. *, p < 0.05, **, p < 0.01, ****, p < 0.0001 by Tukey's post hoc analysis following two-way analysis of variance. #, p < 0.05, ##, p < 0.01 relative to control by two-tailed, unpaired Student's t test.
Variable Acute InsR Antagonism Results in a Spectrum of IR
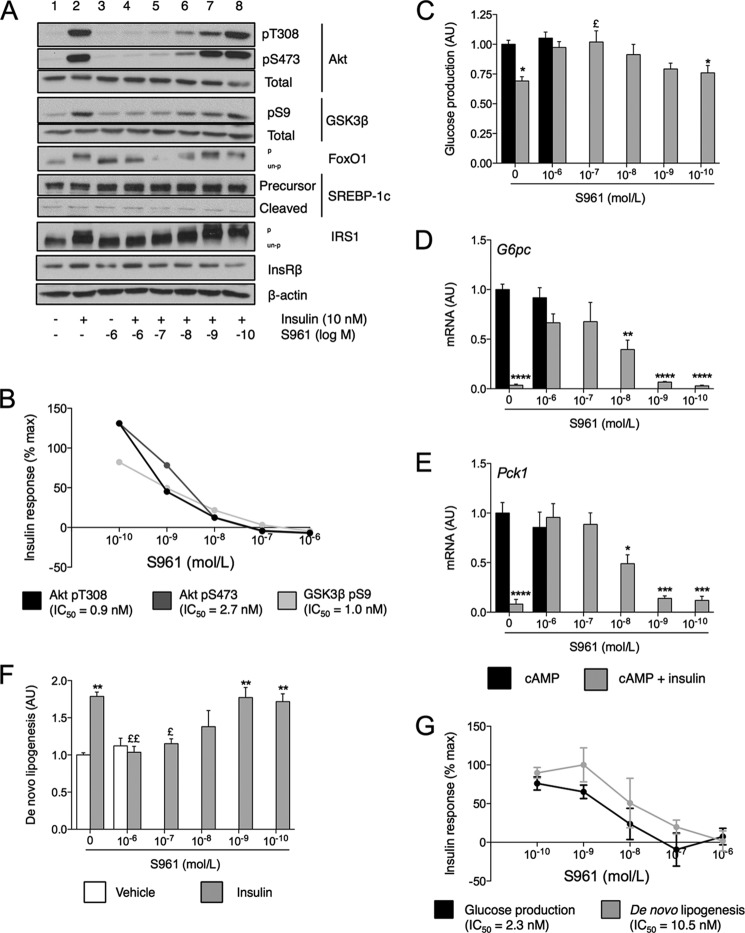
The recapitulation of selective IR in response to CHI treatment is consistent with findings in hyperinsulinemic humans (26) and mice (26, 27). To independently test the hypothesis, we used a competitive peptide inhibitor of InsR, S961 (17). We treated cells with a range of concentrations of S961 and assessed its inhibition of the actions of a fixed dose of insulin (10 nm) on glucose and lipid production. S961 treatment dose-dependently inhibited phosphorylation of IRS1 (as evidenced by electrophoretic mobility shift) and Akt at Thr-308 and Ser-473, as well as of Akt targets FoxO1 and GSK3β (Ser-9) (Fig. 3, A and B). Based on densitometric analysis of the Western blot findings in Fig. 3A, we determined that the IC50 of S961 for the phosphorylation of Akt was 0.9 nm at Thr-308 and 2.7 nm at Ser-473, and for the phosphorylation of GSK3β at Ser-9, the IC50 was 1.0 nm (Fig. 3B). Levels of total InsRβ were unaffected. SREBP-1c cleavage was also not notably affected by antagonist treatment.
FIGURE 3.
Insulin sensitivity of glucose and lipid metabolism. A, peptide-based InsR antagonism Western blots of InsR signaling components in response to treatment with 10 nm insulin and/or the indicated concentrations of S961. Each lane represents pooled samples of equivalent amounts of lysate from three independent experiments. pT308, phospho-Thr-308; pS473, phospho-Ser-473; pS9, phospho-Ser-9; pS2448, phospho-Ser-S2448; p, phosphorylated; un-p, unphosphorylated. B, graphical representation of densitometric analysis of data in A. IC50 is calculated as the log10 of the S961 concentration at which each dose-response curve intercepts 50% of maximal insulin response. C–E, relative glucose production (C) and gene expression (D and E) in primary hepatocytes incubated for cAMP with or without insulin and the indicated concentrations of S961. AU, arbitrary units. F, relative de novo lipogenesis in control or S961-treated primary hepatocytes incubated for 5 h with vehicle or 10 nm insulin. G, percent maximal insulin response of glucose production and de novo lipogenesis as a function of S961 concentration. Curves are calculated from data in panels C and F. IC50 is calculated as log10 of the S961 concentration at which each dose-response curve intercepts 50% maximal insulin response. Data in A–E are normalized to insulin-/S961-untreated control and represent mean ± S.E. of at least three independent experiments performed in triplicate. *, p < 0.05, **, p < 0.01, ***, p < 0.001, ****, p < 0.0001 relative to vehicle-treated control and £, p < 0.05, ££, p < 0.01 relative to insulin-treated control by Tukey's post hoc analysis following one-way analysis of variance.
These effects on the InsR phosphorylation cascade translated into reduced ability of insulin to suppress GP (Fig. 3C). Insulin decreased cAMP-induced GP by 31% in control cells, but this effect was lost in the presence of 10−6 and 10−7 m S961. Even at the lowest concentration of antagonist tested (10−10 m), insulin inhibition of GP was only 76% of control. Antagonist treatment did not affect maximal cAMP-induced GP. S961 treatment also dose-dependently blunted insulin's inhibition of G6pc and Pck1 expression (Fig. 3, D and E). However, unlike in the case of GP, insulin reduced expression of these genes to control levels even at 10−9 m S961. As in the case of GP, treatment with S961 did not alter basal de novo lipogenesis (Fig. 3F). In control cells, insulin stimulated lipogenesis by 78%. This effect was blocked by maximal doses of S961. However, unlike the lowering of GP, insulin-stimulated lipogenesis was unchanged from control even at 10−9 m S961.
The calculated IC50 values were 2.3 nm for GP and 10.5 nm for de novo lipogenesis (Fig. 3G). Thus, there exists a nearly 5-fold difference in the dose of antagonist required to affect insulin's action on GP as compared with lipogenesis. These findings reveal inherent differences in the insulin responsiveness of GP and de novo lipogenesis in isolated hepatocytes.
Inhibition of Akt1/2 Results in Pure IR
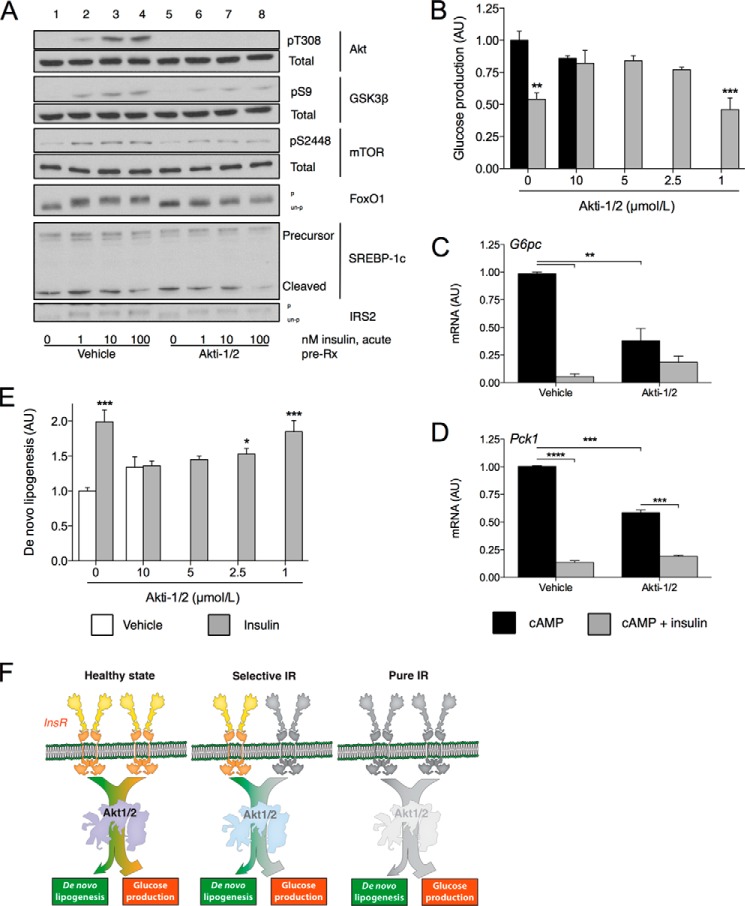
Previous studies indicate that Akt represents the most distal shared element of InsR signaling to glucose and lipid metabolism (28–30). We therefore tested the effect of inhibiting Akt on GP and DNL using the chemical inhibitor Akti-1/2 in primary hepatocytes (18). Akti-1/2 treatment blocked insulin-dependent Akt phosphorylation and decreased phosphorylation of FoxO1, GSK3β, and mTOR without affecting IRS2 (Fig. 4A). Of note, treatment with the inhibitor was without notable effect on SREBP-1c cleavage.
FIGURE 4.
Effect of Akt inhibition on glucose production and lipogenesis. A, Western blots of InsR cascade components in response to treatment with insulin and/or 5 μm Akti-1/2. Blots are representative of three independent experiments. pT308, phospho-Thr-308; pS9, phospho-Ser-9; pS2448, phospho-Ser-S2448; p, phosphorylated; un-p, unphosphorylated. B, relative glucose production in primary hepatocytes pretreated with the indicated concentrations of Akti-1/2 or vehicle and then incubated for 5 h with cAMP with or without 10 nm insulin. AU, arbitrary units. C and D, gene expression in primary hepatocytes pretreated with 5 μm Akti-1/2 or vehicle and incubated for 5 h with cAMP with or without 1 nm insulin. E, relative de novo lipogenesis in primary hepatocytes pretreated with the indicated concentrations of Akti-1/2 or vehicle and incubated for 5 h with or without 10 nm insulin. Data are mean ± S.E. of at least two independent experiments performed in triplicate. For B and E, *, p < 0.05, **, p < 0.01, ***, p < 0.001 relative to vehicle-treated control by Dunnett's post hoc analysis following one-way analysis of variance. For C and D, **, p < 0.01, ***, p < 0.001, ****, p < 0.0001 for designated comparison by Tukey's post hoc analysis following two-way analysis of variance. F, schematic illustrating the degree of intact InsR and Akt signaling to de novo lipogenesis and glucose production in the healthy state, selective IR, and complete IR.
Akti-1/2 treatment prevented insulin's ability to significantly inhibit cAMP-induced GP at all but the lowest concentration (1 μm) of inhibitor tested (Fig. 4B). Similar to the effect of CHI treatment, Akti-1/2 inhibition decreased the maximal cAMP-induced expression of G6pc and Pck1 (Fig. 4, C and D). Although Akti-1/2 prevented the lowering of G6pc expression to normal levels by insulin, it did allow for an ∼50% repression (Fig. 4C), whereas Akti-1/2 did not prevent the lowering of Pck1 expression to basal levels by insulin (Fig. 4D), similar to previous findings (31).
Finally, treatment with the highest dose of Akti-1/2 resulted in a non-significant trend to increased basal de novo lipogenesis. At this concentration of inhibitor, however, insulin treatment was unable to further induce DNL relative to vehicle-treated control. Unlike in the case of GP, insulin treatment significantly induced DNL at both 2.5 μm and 1 μm doses of Akti-1/2 relative to vehicle-treated control. This does not appear to be due merely to increased basal DNL in response to Akti-1/2 as treatment with 5 μm Akti-1/2 does not result in any discernible difference from vehicle-treated control (data not shown). These data support the idea that Akt signaling unites insulin's effects on GP and DNL in primary hepatocytes, and that a greater degree of intact Akt signaling may be required for DNL than for glucose production.
Discussion
This study supports the idea that InsR activation differentially modulates glucose and lipid metabolism because of inherent differences in the relative insulin responsiveness of each process. Specifically, as we show using a peptide inhibitor of InsR, a greater degree of intact InsR is required to halt HGP than to stimulate DNL. Thus, at intermediate levels of InsR activation, DNL is stimulated, whereas HGP fails to be suppressed. The loss of functional InsR in vivo may stem from down-regulation of receptor number and/or activity due to chronic hyperinsulinemia as seen in type 2 diabetes. Indeed, modeling of CHI in primary hepatocytes shows that these cells can be rendered selectively resistant to insulin's lowering of GP, while remaining able to engage in insulin-stimulated DNL. In other words, CHI and selective IR are not merely an epiphenomenon in garden variety diabetic patients; they are etiologically linked.
CHI induces IR through qualitative or quantitative defects in InsR itself. Selective IR, on the other hand, has generally been attributed to post-receptor defects (2, 27). Different potential explanations have been invoked, generally focusing on identifying a discrete branch point beyond which shared mediators of insulin signaling diverge to regulate specific metabolic processes (1, 2, 10, 32–35). These models posit that one branch (i.e. glucose metabolism) becomes resistant to the effects of insulin, whereas the other (i.e. lipid metabolism) remains sensitive, or even potentiated by hyperinsulinemia. The stumbling block in this explanation is that it has proved difficult to identify conditions in which signaling through one branch is impaired, whereas the other branch continues to function unhindered.
Our studies demonstrate that selective IR can be traced back directly to alterations in InsR number and/or function, without the need for additional inputs. This theory, obviously, does not mean that such inputs do not exist or do not play an important role in vivo (1). For example, our data support a branching model of insulin action whereby mediators downstream of Akt selectively alter lipid versus glucose metabolism (1, 34). However, in adipocytes, different Akt isoforms appear to serve distinct sub-functions, demonstrating that there is intrinsic heterogeneity in insulin signal transduction (9).
Our data favor a model in which CHI results from a decrement in InsR number and/or activity that leads to decreased signaling through IRS → PI3K → Akt en bloc rather than specifically affecting particular isoform combinations of these signaling intermediates (Fig. 4F). The partial loss of Akt activation is a mediator of the observed inherent differences in responsiveness of downstream pathways. Thus, when InsR signaling is partially blocked by CHI or low-dose S961 treatment, the degree of Akt activation is sufficient to activate lipogenesis but not to halt GP. In support of this possibility, treatment of hepatocytes with low-dose Akti-1/2 (i.e. at or below 2.5 μm) more efficiently prevents insulin's inhibition of GP than its induction of lipogenesis. This finding is mechanistically supported by previous studies in cultured cells in which low doses of Akti-1/2 are better able to block the phosphorylation of FoxO1 than of TSC2 (31), the latter representing the principal connection between Akt and the mTORC1 → SREBP-1c lipogenic pathway (36). On the other hand, when Akt signaling is completely abrogated, such as in high-dose Akti-1/2 (i.e. above 2.5 μm) and S961 experiments, or in published knock-out models, all pathways reliant on activation by Akt are shut off (31, 34, 37).
CHI-associated Insulin Resistance
Chronic hyperinsulinemia results from the attempt of pancreatic β cells to compensate for peripheral IR (38) but ends up exacerbating IR by causing receptor desensitization (7, 39), as seen in patients with insulinoma (39, 40) as well as mice overexpressing human insulin (41). That CHI may precede the development of hepatic IR is supported by the consensus that IR in skeletal muscle and white adipose tissue, the combination of which would induce CHI, antedates hepatic IR in the natural history of type 2 diabetes (12, 13, 42, 43). This is manifested clinically in the observation that post-prandial hyperglycemia, which partly reflects reduced muscle glucose disposal, precedes fasting hyperglycemia, a reflection of HGP (12, 43). One potential mechanism of CHI-induced IR is down-regulation of InsR in the hepatocyte. In a prior study, CHI treatment resulting in an ∼50% reduction in InsR number affected the maximal rates of insulin-stimulated lipogenesis and glycogen synthesis, but not their inherent sensitivities to insulin (i.e. ED50) (16). The studies described here are in agreement with this model. This paradigm suggests that most insulin-resistant patients should show a partial loss of functional InsR. Indeed, insulin binding to human liver plasma membranes was decreased by about 50%, similar to the extent of down-regulation in our model of CHI, in non-diabetic and diabetic obese patients as compared with non-obese controls (8, 44). Several studies provide evidence of InsR down-regulation in other human cell types such as adipocytes and myocytes (7, 45–47).
The decrease in total hepatocellular InsR levels does not necessarily reflect the relative contributions of intracellular versus cell surface receptors. Cell surface InsR may represent a minority of total InsR levels (8), but even this pool of InsR has been shown to be reduced in diabetic and non-diabetic obese livers (8). Moreover, insulin binding can acutely and reversibly alter subcellular localization of InsR (15, 48) even if not total InsR number (7), adding another layer of complexity (49). We did not measure insulin binding, and thus cannot rule out a post-binding defect as a contributor to this phenotype (8, 50–53). This is also reflected by our findings in CHI-treated cells of complete loss of Akt phosphorylation and regulation of G6pc and Pck1 in response to insulin, despite the reduction of InsR by only ∼45%. Also consistent with a qualitative effect of CHI on InsR function, treatment with increasing doses of S961, which does not alter total InsR levels acutely, resulted in the predicted dose-dependent changes in Akt phosphorylation.
Mechanism of Insulin-stimulated DNL in Primary Hepatocytes
A key finding of these studies is the preservation of insulin-stimulated DNL in the face of CHI-induced IR. Because of the fact that SREBP-1c regulation is not sensitive to insulin in isolated mouse hepatocytes (32, 54), we cannot examine whether compensatory hyperinsulinemia in insulin resistance drives DNL through the Akt → mTORC1 → SREBP-1c pathway (1, 32, 33, 55, 56). We did find an increase in SREBP-1c mRNA and precursor protein levels following CHI treatment, as reported previously in rat hepatocytes (10, 57). Thus, the steady-state level of cleaved SREBP-1c is likely higher than in control cells, especially as basal SREBP-1c cleavage appeared higher following CHI treatment. So, even if the acute insulin responsiveness of SREBP-1c cleavage and activity is decreased, it is still expected to remain elevated relative to insulin-stimulated FoxO1 phosphorylation and effects on GP. This may account for the trend toward higher basal DNL in CHI-treated cells.
On the other hand, acute inhibition of insulin signaling, either directly by S961 treatment or indirectly by Akt inhibition, may uncouple DNL from SREBP-1c expression as these treatments hindered DNL without systematically affecting SREBP-1c cleavage. We suspect that the time course of these experiments is too short to register changes in basal SREBP-1c expression. In these conditions, the increase in DNL may occur via posttranslational mechanisms (34, 58). Even so, these posttranslational mechanisms of insulin-stimulated DNL appear to be more sensitive to insulin than is the inhibition of GP. The latter process is also likely to be largely posttranslational given the fact that insulin remains able to efficiently inhibit the expression of G6pc and Pck1 at concentrations of S961 and Akti-1/2 that prevent its suppression of GP (59).
Our finding that CHI-induced IR spares DNL even in isolated hepatocytes represents an important conceptual step in our understanding of the connection between IR and hepatic steatosis. Our findings suggest that processes autonomous to the hepatocyte can contribute to steatosis independently of alterations in white adipose tissue-liberated FFA, namely both transcriptional and posttranslational changes in insulin-stimulated DNL (5). That the development of steatosis requires some participation of hepatic insulin signaling is consistent with data indicating that humans with pure IR due to INSR mutations do not develop fatty liver although their fasting FFA levels are elevated (2). Although previous studies investigating expression of lipogenic genes in primary hepatocytes suggested this possibility, these studies did not measure DNL directly (10, 32, 33, 57). On the other hand, older studies that also provide evidence that DNL continues unabated in the face of CHI did not correlate these data with lipogenic gene expression or compare them to GP (8, 11, 16).
Acknowledgments
We are grateful to members of the Accili laboratory for insightful data discussions. We thank Thomas Kolar and Ana Flete-Castro (Columbia University) for outstanding technical support. We thank Dr. Läuge Schaffer (Novo Nordisk) for providing us with S961 InsR antagonist and Dr. Mark Magnuson (Vanderbilt University) for providing us with glucokinase antibody.
This work was supported by National Institutes of Health Grants DK100038, DK57539 (to D. A.), and DK63608 (to Columbia University Diabetes Research Center).
- IR
- insulin resistance
- InsR
- insulin receptor
- IRS
- insulin receptor substrate
- mTOR
- mammalian target of rapamycin
- GSK3β
- glycogen synthase kinase-3β
- SREBP-1c
- sterol regulatory element binding protein-1c
- CHI
- chronic hyperinsulinemia
- GP
- glucose production
- HGP
- hepatic glucose production
- DNL
- de novo lipogenesis
- 8-CPT-cAMP
- 8-(4-chlorophenylthio)-adenosine-3′,5′-cyclic monophosphate, sodium salt.
References
- 1. Brown M. S., Goldstein J. L. (2008) Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 [DOI] [PubMed] [Google Scholar]
- 2. Semple R. K., Sleigh A., Murgatroyd P. R., Adams C. A., Bluck L., Jackson S., Vottero A., Kanabar D., Charlton-Menys V., Durrington P., Soos M. A, Carpenter T. A, Lomas D. J, Cochran E. K, Gorden P., O'Rahilly S., Savage D. B. (2009) Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J. Clin. Invest. 119, 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diraison F., Moulin P., Beylot M. (2003) Contribution of hepatic de novo lipogenesis and reesterification of plasma non-esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 29, 478–485 [DOI] [PubMed] [Google Scholar]
- 4. Donnelly K. L., Smith C. I., Schwarzenberg S. J., Jessurun J., Boldt M. D., Parks E. J. (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Postic C., Girard J. (2008) Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 118, 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haeusler R. A, Accili D. (2008) The double life of Irs. Cell Metab. 8, 7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gavin J. R., 3rd, Roth J., Neville D. M., Jr., de Meyts P., Buell D. N. (1974) Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc. Natl. Acad. Sci. U.S.A. 71, 84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caro J. F., Ittoop O., Pories W. J., Meelheim D., Flickinger E. G., Thomas F., Jenquin M., Silverman J. F., Khazanie P. G., Sinha M. K. (1986) Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes: insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J. Clin. Invest. 78, 249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzalez E., Flier E., Molle D., Accili D., McGraw T. E. (2011) Hyperinsulinemia leads to uncoupled insulin regulation of the GLUT4 glucose transporter and the FoxO1 transcription factor. Proc. Natl. Acad. Sci. U.S.A. 108, 10162–10167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimomura I., Matsuda M., Hammer R. E., Bashmakov Y., Brown M. S., Goldstein J. L. (2000) Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 6, 77–86 [PubMed] [Google Scholar]
- 11. Amatruda J. M, Newmeyer H. W, Chang C. L. (1982) Insulin-induced alterations in insulin binding and insulin action in primary cultures of rat hepatocytes. Diabetes 31, 145–148 [DOI] [PubMed] [Google Scholar]
- 12. Monnier L., Colette C., Dunseath G. J., Owens D. R. (2007) The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 30, 263–269 [DOI] [PubMed] [Google Scholar]
- 13. Weyer C., Bogardus C., Mott D. M., Pratley R. E. (1999) The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Invest. 104, 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caro J. F., Amatruda J. M. (1980) Functional relationships between insulin binding, action, and degradation. A reassessment. J. Biol. Chem. 255, 10052–10055 [PubMed] [Google Scholar]
- 15. Goodner C. J., Sweet I. R., Harrison H. C., Jr. (1988) Rapid reduction and return of surface insulin receptors after exposure to brief pulses of insulin in perifused rat hepatocytes. Diabetes. 37, 1316–1323 [DOI] [PubMed] [Google Scholar]
- 16. Kato S., Nakamura T., Ichihara A. (1982) Regulatory relation between insulin receptor and its functional responses in primary cultured hepatocytes of adult rats. J. Biochem. 92, 699–708 [DOI] [PubMed] [Google Scholar]
- 17. Schäffer L., Brand C. L., Hansen B. F., Ribel U., Shaw A. C., Slaaby R., Sturis J. (2008) A novel high-affinity peptide antagonist to the insulin receptor. Biochem. Biophys. Res. Commun. 376, 380–383 [DOI] [PubMed] [Google Scholar]
- 18. Lindsley C. W., Zhao Z., Leister W. H., Robinson R. G., Barnett S. F., Defeo-Jones D., Jones R. E., Hartman G. D., Huff J. R., Huber H. E., Duggan M. E. (2005) Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg. Med. Chem. Lett. 15, 761–764 [DOI] [PubMed] [Google Scholar]
- 19. Nakae J., Kitamura T., Silver D. L., Accili D. (2001) The Forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Invest. 108, 1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pajvani U. B., Qiang L., Kangsamaksin T., Kitajewski J., Ginsberg H. N., Accili D. (2013) Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med. 19, 1054–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hurrell D. G., Pedersen O., Kahn C. R. (1989) Alterations in the hepatic insulin receptor kinase in genetic and acquired obesity in rats. Endocrinology 125, 2454–2462 [DOI] [PubMed] [Google Scholar]
- 22. Accili D., Drago J., Lee E. J., Johnson M. D., Cool M. H., Salvatore P., Asico L. D., José P. A., Taylor S. I., Westphal H. (1996) Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat. Genet. 12, 106–109 [DOI] [PubMed] [Google Scholar]
- 23. Kido Y., Philippe N., Schäffer A. A., Accili D. (2000) Genetic modifiers of the insulin resistance phenotype in mice. Diabetes 49, 589–596 [DOI] [PubMed] [Google Scholar]
- 24. Blackard W. G., Guzelian P. S., Small M. E. (1978) Down regulation of insulin receptors in primary cultures of adult rat hepatocytes in monolayer. Endocrinology 103, 548–553 [DOI] [PubMed] [Google Scholar]
- 25. Matsumoto M., Han S., Kitamura T., Accili D. (2006) Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J. Clin. Invest. 116, 2464–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kahn C. R, Neville D. M, Jr., Gorden P., Freychet P., Roth J. (1972) Insulin receptor defect in insulin resistance: studies in the obese-hyperglycemic mouse. Biochem. Biophys. Res. Commun. 48, 135–142 [DOI] [PubMed] [Google Scholar]
- 27. Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. (1981) Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J. Clin. Invest. 68, 957–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsumoto M., Pocai A., Rossetti L., Depinho R. A., Accili D. (2007) Impaired regulation of hepatic glucose production in mice lacking the Forkhead transcription factor Foxo1 in liver. Cell Metab. 6, 208–216 [DOI] [PubMed] [Google Scholar]
- 29. Haeusler R. A., Pratt-Hyatt M., Welch C. L., Klaassen C. D., Accili D. (2012) Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 15, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haeusler R. A., Hartil K., Vaitheesvaran B., Arrieta-Cruz I., Knight C. M., Cook J. R., Kammoun H.L., Febbraio M. A, Gutierrez-Juarez R., Kurland I. J., Accili D. (2014) Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors. Nat. Commun. 5, 5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Logie L., Ruiz-Alcaraz A. J., Keane M., Woods Y. L., Bain J., Marquez R., Alessi D. R., Sutherland C. (2007) Characterization of a protein kinase B inhibitor in vitro and in insulin-treated liver cells. Diabetes 56, 2218–2227 [DOI] [PubMed] [Google Scholar]
- 32. Owen J. L., Zhang Y., Bae S. H., Farooqi M. S., Liang G., Hammer R. E., Goldstein J. L., Brown M. S. (2012) Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc. Natl. Acad. Sci. U.S.A. 109, 16184–16189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li S., Brown M. S., Goldstein J. L. (2010) Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 3441–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leavens K. F., Birnbaum M. J. (2011) Insulin signaling to hepatic lipid metabolism in health and disease. Crit. Rev. Biochem. Mol. Biol. 46, 200–215 [DOI] [PubMed] [Google Scholar]
- 35. Leavens K. F., Easton R. M., Shulman G. I., Previs S. F., Birnbaum M. J. (2009) Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 10, 405–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laplante M., Sabatini D. M. (2009) An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 19, R1046–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu M., Wan M., Leavens K. F., Chu Q., Monks B. R., Fernandez S., Ahima R. S., Ueki K., Kahn C. R., Birnbaum M. J. (2012) Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat. Med. 18, 388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Accili D. (2004) Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic. Diabetes 53, 1633–1642 [DOI] [PubMed] [Google Scholar]
- 39. Shanik M. H., Xu Y., Skrha J., Dankner R., Zick Y., Roth J. (2008) Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 31, Suppl. 2, S262–S628 [DOI] [PubMed] [Google Scholar]
- 40. Pontiroli A. E., Alberetto M., Pozza G. (1992) Patients with insulinoma show insulin resistance in the absence of arterial hypertension. Diabetologia. 35, 294–295 [DOI] [PubMed] [Google Scholar]
- 41. Marban S. L., Barr V., Roth J. (1990) Physiological effects of hyperinsulinemia: a transgenic mouse model of type-II diabetes. Clin Res. 38, A768–A [Google Scholar]
- 42. Brassard P., Frisch F., Lavoie F., Cyr D., Bourbonnais A., Cunnane S. C., Patterson B. W., Drouin R., Baillargeon J. P., Carpentier A. C. (2008) Impaired plasma nonesterified fatty acid tolerance is an early defect in the natural history of type 2 diabetes. J Clin Endocrinol Metab. 93, 837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petersen K. F., Hendler R., Price T., Perseghin G., Rothman D. L., Held N., Amatruda J. M., Shulman G. I. (1998) 13C/31P NMR studies on the mechanism of insulin resistance in obesity. Diabetes 47, 381–386 [DOI] [PubMed] [Google Scholar]
- 44. Arner P., Einarsson K., Backman L., Nilsell K., Lerea K. M., Livingston J. N. (1983) Studies of liver insulin receptors in non-obese and obese human subjects. J. Clin. Invest. 72, 1729–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bar R. S, Gorden P., Roth J., Kahn C. R., De Meyts P. (1976) Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. J. Clin. Invest. 58, 1123–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caro J. F., Sinha M. K., Raju S. M., Ittoop O., Pories W. J, Flickinger E. G, Meelheim D., Dohm G. L. (1987) Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J. Clin. Invest. 79, 1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olefsky J. M. (1976) Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J. Clin. Invest. 57, 1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fehlmann M., Carpentier J. L., Van Obberghen E., Freychet P., Thamm P., Saunders D., Brandenburg D., Orci L. (1982) Internalized insulin receptors are recycled to the cell surface in rat hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 79, 5921–5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caro J. F, Dohm L. G, Pories W. J, Sinha M. K. (1989) Cellular alterations in liver, skeletal muscle, and adipose tissue responsible for insulin resistance in obesity and type II diabetes. Diabetes Metab. Rev. 5, 665–689 [DOI] [PubMed] [Google Scholar]
- 50. Comi R. J., Grunberger G., Gorden P. (1987) Relationship of insulin binding and insulin-stimulated tyrosine kinase activity is altered in type II diabetes. J. Clin. Invest. 79, 453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Freidenberg G. R, Henry R. R, Klein H. H, Reichart D. R, Olefsky J. M. (1987) Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J. Clin. Invest. 79, 240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takayama S., Kahn C. R, Kubo K., Foley J. E. (1988) Alterations in insulin receptor autophosphorylation in insulin resistance: correlation with altered sensitivity to glucose transport and antilipolysis to insulin. J. Clin. Endocrinol. Metab. 66, 992–999 [DOI] [PubMed] [Google Scholar]
- 53. Arner P., Einarsson K., Ewerth S., Livingston J. (1986) Studies of the human liver insulin receptor in noninsulin-dependent diabetes mellitus. J. Clin. Invest. 77, 1716–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shimano H., Horton J. D, Shimomura I., Hammer R. E, Brown M. S, Goldstein J. L. (1997) Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J. Clin. Invest. 99, 846–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Porstmann T., Santos C. R, Griffiths B., Cully M., Wu M., Leevers S., Griffiths J. R, Chung Y. L, Schulze A. (2008) SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yecies J. L, Zhang H. H, Menon S., Liu S., Yecies D., Lipovsky A. I, Gorgun C., Kwiatkowski D. J, Hotamisligil G. S, Lee C. H, Manning B. D. (2011) Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 14, 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hegarty B. D., Bobard A., Hainault I., Ferré P., Bossard P., Foufelle F. (2005) Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory element-binding protein-1c. Proc. Natl. Acad. Sci. U.S.A. 102, 791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wan M., Leavens K. F., Saleh D., Easton R. M., Guertin D. A, Peterson T. R, Kaestner K. H, Sabatini D. M., Birnbaum M. J. (2011) Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab. 14, 516–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin H. V., Accili D. (2011) Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 14, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]