Abstract
Virus-like particles (VLPs) with the full-length VP2 and VP6 rotavirus capsid proteins, produced in the baculovirus expression system, have been evaluated as surrogates of human rotavirus in different environmental scenarios. Green fluorescent protein-labeled VLPs (GFP-VLPs) and particles enclosing a heterologous RNA (pseudoviruses), whose stability may be monitored by flow cytometry and antigen capture reverse transcription-PCR, respectively, were used. After 1 month in seawater at 20°C, no significant differences were observed between the behaviors of GFP-VLPs and of infectious rotavirus, whereas pseudovirus particles showed a higher decay rate. In the presence of 1 mg of free chlorine (FC)/liter both tracers persisted longer in freshwater at 20°C than infectious viruses, whereas in the presence of 0.2 mg of FC/liter no differences were observed between tracers and infectious rotavirus at short contact times. However, from 30 min of contact with FC onward, the decay of infectious rotavirus was higher than that of recombinant particles. The predicted Ct value for a 90% reduction of GFP-VLPs or pseudoviruses induces a 99.99% inactivation of infectious rotavirus. Both tracers were more resistant to UV light irradiation than infectious rotavirus in fresh and marine water. The effect of UV exposure was more pronounced on pseudovirus than in GFP-VLPs. In all types of water, the UV dose to induce a 90% reduction of pseudovirus ensures a 99.99% inactivation of infectious rotavirus. Recombinant virus surrogates open new possibilities for the systematic validation of virus removal practices in actual field situations where pathogenic agents cannot be introduced.
Rotaviruses are the single most important cause of severe viral gastroenteritis worldwide and are transmitted by the fecal-oral route (27, 28). The rotavirus virion consists of three concentric layers; the innermost layer is composed of protein VP2, the intermediate layer consists of protein VP6, and the outermost layer is composed of glycoprotein VP7 and the spike protein VP4 (12). Coexpression of capsid proteins in the baculovirus system results in the assembly of virus-like particles (VLPs) (10, 21). VLPs have been used to mimic rotavirus-host cell interactions (7, 24, 31) and analyzed for their immunogenicity (9, 40).
Environmental transmission of rotavirus occurs mainly through shellfish grown in polluted waters and contaminated drinking water (5, 16, 23, 25). Disinfection is an important treatment barrier between consumers and illness; however, current virus disinfection and/or removal practices often fail to adequately eliminate pathogenic viruses. The data on the stability of rotaviruses rely mostly on bench-scale studies performed with available cell-adapted strains (1, 3, 13, 15). A longstanding barrier to conducting actual field studies to evaluate the environmental behavior of human enteric viruses is the impossibility of introducing pathogens into the environment. As model systems, recombinant surrogates are perfectly adequate for field studies of microbial tracking, since they may be produced in extremely high numbers (several milligram amounts). In addition, their noninfectious nature makes them completely harmless and suitable to be used in scenarios in which the use of actual viruses is hampered by the impossibility of introducing potential pathogens into drinking water treatment plants, shellfish-growing waters, or selected foodstuffs. Recombinant norovirus particles have been used to investigate the influence of electrostatic interactions in the filtration of norovirus in quartz sand (30).
The aim of the present study was to verify whether recombinant rotavirus VLPs may be used as surrogates of actual human pathogenic viruses to model their behavior in the environment.
MATERIALS AND METHODS
Growth and assay of rotavirus.
The cytopathic strain Itor P13 of group A human rotavirus was propagated and assayed in MA104 cell monolayers as described elsewhere (1). Semipurified rotavirus stocks were obtained by two subsequent low-speed centrifugations of infected cell lysates. After ultracentrifugation of the supernatants at 100,000 × g for 2 h, the resulting pellets were resuspended in phosphate-buffered saline and stored at −80°C.
Infectious virus enumerations were performed by determining the 50% tissue culture infectious dose with eight wells per dilution and 20 μl of inoculum per well.
Production of rotavirus surrogate particles.
VLPs with the full-length VP2 and VP6 rotavirus capsid proteins (VLP2/6) were produced in the baculovirus expression system, as described previously (8, 10, 21).
Jellyfish green fluorescent protein (GFP) was inserted at the amino terminus of VP2 to produce fluorescent VLPs (GFP-VLPs), as described elsewhere (8, 11).
A heterologous RNA was introduced in rotavirus VLP2/6 to produce RNA-containing particles, which were termed “pseudoviruses.” These pseudovirus particles were produced after we coinfected insect cells with three recombinant baculoviruses. Two of them led to the expression at high level of rotavirus VP6 and rotavirus VP2 fused to an MS2 bacteriophage coat protein. This latter recombinant baculovirus contained, under the polyhedrin promoter control, a gene coding for a protein corresponding to the dimeric bacteriophage MS2 coat protein (29) fused to the amino terminus of rotavirus VP2 protein. The third recombinant baculovirus led to transcription, under the polyhedrin promoter, of an RNA corresponding to the binding domain of MS2 genome (5′-ACA UGA GGA UCA CCC AUG-3′) (18) fused to the 3′ end of the astrovirus genome (positions 6709 to 6797). The interaction between the MS2 coat protein and the RNA-binding domain of the MS2 genome enabled the production of pseudoviruses, i.e., rotavirus VLP2/6 with heterologous astrovirus RNA (J. Cohen, unpublished data).
All molecular tracer particles were purified by two isopycnic centrifugations on cesium chloride gradients. Pseudoviruses were additionally filtered through Centriplus YM-100 Amicon filters (Millipore, Bedford, Mass.).
Flow cytometry monitoring of GFP-VLPs.
A flow cytometry-based procedure was developed to trace the decay of GFP-VLPs, since the fluorescence signal is lost when the particle structure is damaged. Microspheres (0.74 μm in diameter; ProActive Microspheres, Bangs Laboratories, Inc., Fishers, Ind.), at a concentration of 104/ml, coated with goat anti-mouse immunoglobulin G were used to specifically adsorb the GFP-VLPs (at a 1:10 ratio of microspheres and test suspension, respectively) through reaction with the RV133 rotavirus mouse monoclonal antibody (MAb) directed against an immunodominant site in VP6 protein (19). The mixtures were then analyzed in a Coulter Epics XL flow cytometer (Beckman-Coulter, Miami, Fla.), equipped with a 488-nm Argon-ion laser at 15 mW, with a filter combination of 550 DL/525 BP in order to recover the green fluorescence. For each assay, a threshold of positivity was established by calculating the mean plus three standard deviations of the highest fluorometric readings of four negative controls, i.e., nonfluorescent VLP2/6. From this threshold value to the end of the fluorescence axis, a cursor was drawn, and the total fluorescence figured by multiplying the total counts within the established cursor by the mean fluorescence of the cells included in the aforementioned cursor.
Monitoring of pseudoviruses by antigen-capture PCR.
A total of 50 μl of RV133 MAb (diluted 1/100 in standard enzyme-linked immunosorbent assay coating buffer) was adsorbed onto 0.2-ml thin microtubes overnight at 4°C and used to immunocapture the pseudovirus particles in the test suspensions (50 μl) for 2 h at 37°C. After three conditioning washes with reverse transcription (RT) buffer, pseudoviruses were heat denatured for 5 min at 99°C, and 5-μl aliquots of the released RNA were used in an RT-PCR (26) targeted to the 3′ end of the astrovirus genome.
Test waters.
The freshwater used throughout these studies was obtained directly from the Sant Pere Martir well located in Esplugues de Llobregat, Barcelona, and natural seawater was collected in the Mediterranean coast at Premià de Mar. All water samples were allowed to settle in acid-washed 10-liter high-density polyethylene containers, filtered through 0.22-μm-pore-size filters, and kept at 4°C. Chemical analysis were performed according to procedures adapted from standard methods for the examination of water and wastewater (2). All containers used in the experiments were soaked in 12.5% nitric acid, rinsed with distilled water, and autoclaved prior to use.
Studies of thermal stability in seawater.
The comparative stabilities of infectious rotavirus, GFP-VLPs, and pseudoviruses in seawater were ascertained at 20 ± 1°C. Suspensions (1 ml) of infectious rotavirus (107 50% tissue culture infective doses), GFP-VLPs (1011 particles), and pseudoviruses (109 particles) were added to make up 10-ml aliquots of water, which were placed at the designated temperature with gentle agitation. At times 0, 1, 3, 7, 15, and 30 days, 1-ml sample aliquots were removed and kept refrigerated until assayed, always within 24 h after collection.
Survival of infectious rotavirus in the test water systems was determined by calculating the log10(Nt/N0), where N0 is the infectious titer of the virus at the time zero and Nt is the infectious titer at various time intervals. The stability of GFP-VLPs was figured by determining the log10(Nt/N0), where N0 is the total fluorescence detectable by flow cytometry at time zero, and Nt is the total fluorescence detectable by flow cytometry at the various time periods. Pseudovirus decay was also determined by the log10(Nt/N0), where N0 is in this case the reciprocal endpoint dilution detectable by antigen capture PCR at time zero, and Nt is the reciprocal endpoint dilution detectable by antigen capture PCR at various time intervals.
Studies of stability in the presence of FC.
Glassware used for systems involving free chlorine (FC) was made chlorine demand free by soaking it overnight in a solution of 0.8 mg of FC/liter. FC solutions of 0.2 and 1.0 mg/liter were prepared from a stock solution of sodium hypochlorite (5%). The test systems consisted of 10 ml of filtered freshwater with the appropriate FC concentration. Experiments were performed at 20 ± 1°C and pH 7.5 ± 0.2. As above, 1-ml suspensions with rotavirus infectious particles (107), GFP-VLPs (1011), or pseudoviruses (109) were added to the various disinfection systems and, at predetermined time intervals (0, 15, 30, 60, and 120 min), 1-ml samples were taken and neutralized with 14.6% sodium thiosulfate (1). FC concentrations were determined at each sampling time by the N,N-diethyl-p-phenylene diamine method (2) by using a test kit (Aquamerck 11735; Merck, Darmstadt, Germany).
The comparative resistance of rotavirus and the recombinant surrogates in the presence of FC was evaluated by determining the log10(Nt/N0) values for infectious viruses, GFP-VLPs, and pseudoviruses as described above. Raw data were subjected to linear regression analysis performed with Sigmaplot (version 8.0; SPSS, Inc., Chicago, Ill.) to calculate the predicted Ct values (i.e., the concentration of FC multiplied by time of contact for specific 1-, 2-, 3-, and 4-log removal [90, 99, 99.9, and 99.99% decay]).
Studies of stability after UV irradiation.
A collimated beam incorporating a 15-W low-pressure mercury UV lamp (Philips model G15T8) was used to irradiate the test suspensions at a 20-cm distance. The UV intensity at the irradiation site was measured at 254 nm with a radiometer (IL 1700; International Light, Inc., Newburyport, Mass.). Suspensions of 0.3 ml of the virus or the recombinant surrogates were added to 3 ml of filtered fresh and marine water in 60-by-15-mm petri dishes and kept at 20 ± 1°C under continuous gentle agitation. At designated times (0, 5, 15, 30, 60, and 120 s), 0.3-ml samples were taken, and the decay of viruses and surrogates was ascertained as the log10(Nt/N0), as described above. Raw data were subjected to linear regression analysis (Sigmaplot) to figure the UV doses, i.e., the product of the average intensity (in milliwatts per square centimeter) multiplied by the time (in seconds) of UV exposure to achieve 1-, 2-, 3-, and 4-log removal (90, 99, 99.9, and 99.99% decay) of viruses and surrogates.
All experimental procedures were performed at least in duplicate, and each sample was quantified in two independent assays. The analysis of variance test (33) was applied in log-transformed data to determine significant differences.
RESULTS
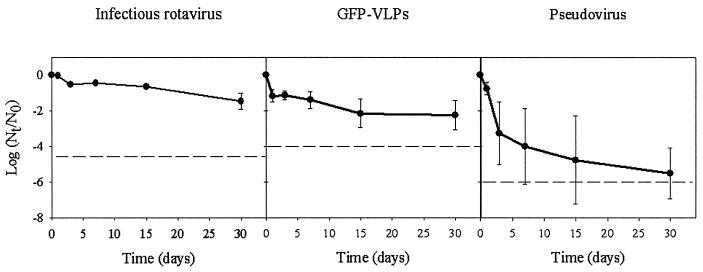
The behavior of surrogate VLPs in different environmental water scenarios was compared to that of infectious rotavirus. Table 1 depicts the physicochemical characteristics of the test fresh and marine waters used in these studies. The stability of infectious rotavirus and the recombinant particles suspended in marine water at 20°C was determined over 1 month (Fig. 1). No significant (P < 0.05) differences were observed between the behavior of GFP-VLPs and infectious rotavirus, whereas pseudovirus particles showed a significantly (P < 0.05) higher decay rate.
TABLE 1.
Water quality parameters of fresh and marine test waters
| Water type | pH | A254 | A650 | A720 | Conductivity (mS) | Salinity (%) |
|---|---|---|---|---|---|---|
| Freshwater | 7.80 | 0.0118 | 0.0078 | 0.0080 | 0.73 | 0 |
| Marine water | 7.92 | 0.0269 | 0.0094 | 0.0086 | 18.79 | 3.5 |
FIG. 1.
Comparative long-term stability of infectious rotavirus, GFP-labeled VLPs, and pseudoviruses in freshwater at 20°C. Dashed lines depict the detection limits.
Two chlorine treatments with 0.2 and 1 mg of FC/liter were carried out in freshwater spiked with human rotavirus, GFP-VLPs, or pseudovirus at 20°C (Fig. 2). FC concentrations, which were determined at each sampling time, markedly decreased throughout the duration of the experiments (data not shown), with the chlorine decay after 120 min ranging from 40 to 90%.
FIG. 2.
Comparative stability of infectious rotavirus, GFP-labeled VLPs, and pseudoviruses in freshwater in the presence of FC. Dashed lines depict the detection limits. Symbols: •, 1 mg/liter; ▾, 0.2 mg/liter.
In the presence of 1 mg of FC/liter, both recombinant surrogates persisted significantly (P < 0.05) longer than infectious viruses, whereas in the presence of 0.2 mg of FC/liter, no significant (P < 0.05) differences were observed between surrogates and infectious rotavirus at short contact times (up to 15 min). However, from 30 min of contact with FC onward, the decay of infectious rotavirus was significantly (P < 0.05) higher than that of recombinant particles. Pseudoviruses appear to be clearly more resistant (P < 0.05) than GFP-VLPs at contact times above 60 min in the presence of 1 mg of FC/liter. This unexpected differential decay rate observed may be a consequence of the detection methodologies used rather than of an actual different behavior of GFP-VLPs and pseudoviruses upon exposure to FC.
Ct values for specific 1-, 2-, 3-, and 4-log removal (90, 99, 99.9, and 99.99%) were used to assess the comparative sensitivities of viruses and surrogates (Table 2). By this approach it can be concluded that the predicted Ct value for a 90% (1-log) reduction of GFP-VLPs or pseudoviruses (4.5 and 7.2, respectively) induces a 99.99% (4-log) inactivation of infectious rotavirus.
TABLE 2.
Ct values for rotavirus chlorine inactivation
| Virus or VLP | Ct value ([mg/liter] • min) for reduction of:
|
|||
|---|---|---|---|---|
| 1 log10 | 2 log10 | 3 log10 | 4 log10 | |
| Infectious virus | 1.2 | 2.3 | 3.5 | 4.5 |
| GFP-VLP | 4.5 | 25 | 67 | 110 |
| Pseudovirus | 7.2 | 30 | 220 | NRa |
NR, not reached.
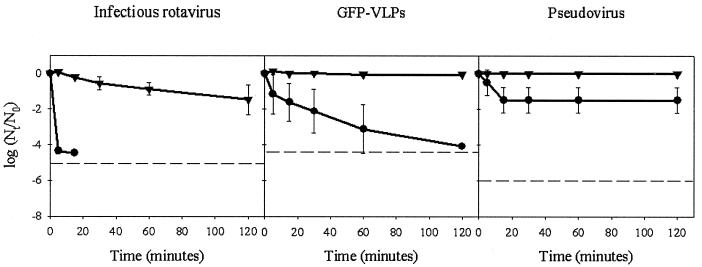
The curves of infectious rotavirus, GFP-VLP, and pseudovirus persistence after UV light irradiation of fresh and marine water at 20°C are shown in Fig. 3. In both types of water, both surrogates were more (P < 0.05) resistant to UV light exposure than rotavirus. Surprisingly, a slight decay of GFP-VLPs, devoid of nucleic acid, was observed in freshwater after UV irradiation. However, the effect of UV exposure was more pronounced on pseudovirus, although the differences were only significant (P < 0.05) in the case of seawater after 60 s of UV irradiation.
FIG. 3.
Comparative stability of infectious rotavirus, GFP-labeled VLPs, and pseudoviruses in freshwater and seawater after UV irradiation. Dashed lines depict the detection limits. Symbols: •, freshwater; ▾, seawater.
Predicted UV doses to achieve 1-, 2-, 3-, and 4-log removal (90, 99, 99.9, and 99.99%) of viruses and surrogates were calculated (Table 3). In all types of water, the UV dose to induce a 1-log reduction of pseudovirus ensures a 4-log inactivation of infectious rotavirus.
TABLE 3.
UV doses for rotavirus removal in freshwater and marine water
| Virus or VLP and water type | UV dose (mJ/cm2) for reduction of:
|
|||
|---|---|---|---|---|
| 1 log10 | 2 log10 | 3 log10 | 4 log10 | |
| Infectious viruses | ||||
| Freshwater | 20 | 80 | 140 | 200 |
| Seawater | 50 | 110 | 160 | 220 |
| GFP-VLPs | ||||
| Freshwater | NRa | NR | NR | NR |
| Seawater | NR | NR | NR | NR |
| Pseudoviruses | ||||
| Freshwater | 390 | 980 | NR | NR |
| Seawater | 410 | 900 | NR | NR |
NR, not reached.
DISCUSSION
VLPs with the full-length VP2 and VP6 rotavirus capsid proteins produced in the baculovirus expression system have been evaluated as surrogates of human rotavirus in different environmental scenarios. These recombinant particles are morphologically indistinct from actual rotavirus virions when visualized by electron microscopy (22). The lack of the double-stranded RNA genome, which is essential for rotavirus replication, makes these VLPs completely harmless since they are unable to infect any living cell. Further refinements on VLP2/6 constructs were the production of GFP-VLPs and particles enclosing a heterologous RNA (pseudoviruses), which may be monitored by flow cytometry and antigen capture RT-PCR, respectively.
The use of flow cytometry presents several advantages with respect to other fluorescence-based methods, the most important being the possibility to be automatable and standardizable. However, several critical issues must be sorted out in flow cytometry, such the minimum particle size required (0.3 to 0.4 mm), far beyond the size of a viral particle, and the threshold of fluorescence required to be detected, which is equivalent to 1,000 fluorescein molecules. To overcome these size and fluorescent detection limitations, microspheres and an indirect immunocapture procedure with rotavirus RV133 MAb were used. The detection limit of this method was established to be ∼104 GFP-VLPs/ml (data not shown). Since recognition by this RV133 MAb is lost when the rotavirus particle conformation is altered (36, 37) and since, in addition, the fluorescence signal also disappears when the GFP-VLPs structure is damaged, our flow cytometric assay specifically detects unaltered fluorescent surrogates. The same conformation-specific RV133 MAb was used for the monitoring of pseudovirus decay, with a threshold of positivity of 104 pseudovirus particles/ml. In any case, the capacity to produce very large (several milligrams) amounts of both types of recombinant surrogates allows experiments to ascertain the regulatory 99.99% reduction requirement (38) in large volumes of water.
The astrovirus RNA inside the pseudovirus particle is the target for amplification in the antigen capture RT-PCR used to monitor the surrogate decay. Coupling of the molecular procedure with capture with a conformationally dependent MAb is required, since the PCR technique alone fails to discern between intact and altered particles (1, 20). For instance, a partially degraded RNA may still be detected in nucleocapsids chemically altered by low levels of FC (1).
F. Loisy and coworkers (unpublished data) have found that enzyme-linked immunosorbent assays and Western blot assays on the short-term thermal alteration at 25°C of capsid proteins in recombinant VLP2/6 and the bovine strain RF reveal a similar pattern. In the present study, the stability of GFP-VLPs and infectious rotavirus suspended over 1 month in marine water at 20°C was similar and higher than that of pseudovirus under the same circumstances. FC and UV treatments, which are widely used for the routine inactivation of pathogenic microorganisms in water, were used to ascertain the validity of GFP-VLPs and pseudoviruses to monitor the efficiency of virus removal and/or inactivation practices.
Differences in absolute values of virus or recombinant surrogate levels may be largely a consequence of the methodologies used for their detection. However, any methodology, with its intrinsic limitations, is adequate provided that exactly the same procedure is used for each designated sample of either rotavirus, GFP-VLPs, or pseudoviruses.
Some of the curves of virus and surrogates decay after the disinfection treatments exhibited “tailing” or “flattening.” Tailing of inactivation curves has been described to result from particle aggregation, decrease of disinfectant concentrations, or adsorption to particulate materials, allowing protection from the disinfectant action (34, 35). Electron microscopy observation of the samples (data not shown) revealed the occasional occurrence of clumps or aggregates of viruses and surrogates, which prove the identical behavior of recombinant and actual virus particles.
Among critical factors that may be involved in virus resistance to UV exposure are the type and length of nucleic acid and the particle size. Although the mechanism by which UV inactivates microorganisms has not been completely elucidated, UV is known to cause pyrimidine dimer formation, i.e., thymine dimers and, in addition, intense irradiation can disrupt the virion structure (4). This capsid disruption by UV light may be the cause of the slight, although unexpected, decay observed after UV light exposure of nucleic acid free GFP-VLPs. Nevertheless, pseudoviruses appear to be better candidates for use as virus surrogates in UV disinfection processes.
The UV doses needed to achieve a 99.99% reduction of infectious Ito rotavirus in the present study are higher than those reported in a previous study with SA11 rotavirus (4). Differences in the methodology or in viral stocks may cause variability in virus inactivation kinetics. Nevertheless, the aim of the present study was to assess the validity of recombinant surrogates rather than to evaluate the behavior of a given virus strain in inactivation experiments. Disparities between viral disinfection studies make conclusions regarding the effectiveness of virucidal treatments hard to draw. Several factors, such as the state of the virus suspension or the suspending medium, are critical in determining virus resistance to disinfection. Hence, the manner in which experiments are designed greatly influences the outcome of the virus inactivation and/or removal treatment, and bench-scale studies may not necessarily reflect the reality of actual large-scale processes. The fact that GFP-VLPs or pseudoviruses are largely more stable than the actual virus provides a conservative, i.e., safer, evaluation of virological risk in a given setting. Although more studies are required to determine the most adequate molecular tracer for a given scenario, i.e., type of sample, applied treatment, etc., the use of recombinant virus surrogates may provide the tools for the systematic validation of virus removal practices in actual situations in which pathogenic agents cannot be introduced.
The environmental transmission of rotaviruses is well documented (6, 16, 23, 39). Microbial source tracking is imperative for the maintenance of the microbiological quality and safety of water systems used for drinking, for recreating, and in the harvesting of seafood since contamination of these systems can represent high risks to human health, as well as significant economic losses due to closures of beaches and shellfish-harvesting areas. Bacteriophages and other microorganisms of the fecal flora have been proposed as models of virus behavior (14, 17, 32). However, from the strictly structural point of view, there is no better surrogate of an actual virus pathogen to track their behavior in the environment than a noninfectious VLP of the same virus.
Acknowledgments
F.X.A. is the recipient of a PQS contract, partially supported by the Generalitat de Catalunya. This study was supported in part by grant QLRT-1999-0634 from the European Union, grant 1999SGR 00022 from the Generalitat de Catalunya, and the Centre de Referència de Biotecnologia de Catalunya, Generalitat de Catalunya.
We acknowledge the skillful assistance of Annie Charpilienne (CNRS-INRA) in the production of rotavirus VLP2/6 and pseudovirus. We thank Robert Atmar, Mary Estes, and Monique Pommepuy for advice and useful discussions. We acknowledge the technical expertise of Jaume Comas of the Serveis Científic-Tècnics of the University of Barcelona.
REFERENCES
- 1.Abad, F. X., R. M. Pintó, J. M. Díez, and A. Bosch. 1994. Disinfection of human enteric viruses in water by copper and silver in combination with low levels of chlorine. Appl. Environ. Microbiol. 60:2377-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association/American Water Works Association/Water Pollution Control Federation, Washington, D.C.
- 3.Ansari, S. A., V. S. Springhorpe, and S. A. Sattar. 1991. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev. Infect. Dis. 13:448-461. [DOI] [PubMed] [Google Scholar]
- 4.Battigelli, D. A., M. D. Sobsey, and D. C. Lobe. 1993. The inactivation of hepatitis A virus and other model viruses by UV irradiation. Water Sci. Technol. 27:339-342. [Google Scholar]
- 5.Bosch, A. 1998. Human enteric viruses in the water environment: a minireview. Int. Microbiol. 1:191-196. [PubMed] [Google Scholar]
- 6.Bosch, A., R. M. Pintó, A. R. Blanch, and J. Jofre. 1988. Detection of human rotavirus in sewage through two concentration procedures. Water Res. 22:343-348. [Google Scholar]
- 7.Charpilienne, A., J. Lepault, F. Rey, and J. Cohen. 2002. Identification of rotavirus VP6 residues located at the interface with VP2 that are essential for capsid assembly and transcriptase activity. J. Virol. 76:7822-7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charpilienne, A., M. Nejmeddine, M. Berois, N. Parez, E. Neumann, E. Hewat, G. Trugnan, and J. Cohen. 2001. Individual rotavirus-like particles containing 120 molecules of fluorescent protein are visible in living cells. J. Biol. Chem. 276:29361-29367. [DOI] [PubMed] [Google Scholar]
- 9.Ciarlet, M., S. E. Crawford, C. Barone, A. Bertolotti-Ciarlet, R. F. Ramig, M. K. Estes, and M. E. Conner. 1998. Subunit rotavirus vaccine administered parenterally to rabbits induces active protective immunity. J. Virol. 72:9233-9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford, S. E., M. Labbe, J. Cohen, M. H. Burroughs, Y. J. Zhou, and M. K. Estes. 1994. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J. Virol. 68:5922-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dundr, M., J. G. McNally, J. G. Mc Nally, J. Cohen, and T. Misteli. 2002. Quantitation of GFP-fusion proteins in single living cells. J. Struct. Biol. 140:92-99. [DOI] [PubMed] [Google Scholar]
- 12.Estes, M. K. 2001. Rotaviruses and their replication, p. 1747-1785. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 13.Estes, M. K., D. Y. Graham, E. M. Smith, and C. P. Gerba. 1979. Rotavirus stability and inactivation. J. Gen. Virol. 43:403-409. [DOI] [PubMed] [Google Scholar]
- 14.Gerba, C. P. 1987. Phage as indicators of fecal pollution, p. 197-209. In S. M. Goyal, C. P. Gerba, and G. Bitton (ed.), Phage ecology. Wiley-Interscience, New York, N.Y.
- 15.Girones, R., J. Jofre, and A. Bosch. 1989. Natural inactivation of enteric viruses in seawater. J. Environ. Qual. 18:34-39. [Google Scholar]
- 16.Gratacap-Cavallier, B., O. Genoulaz, K. Brengel-Pesce, H. Soule, P. Innicenti-Francillard, M. Bost, L. Gofti, D. Zmirou, and J. M. Seigneurin. 2000. Detection of human and animal rotavirus sequences in drinking water. Appl. Environ. Microbiol. 66:2690-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havelaar, A. H. 1993. Bacteriophages as models of human enteric viruses in the environment. ASM News 59:614-619. [Google Scholar]
- 18.Johansson, H. E., D. Dertinger, K. A. LeCuyer, L. S. Behlen, C. H. Greef, and O. C. Uhlenbeck. 1998. A thermodynamic analysis of the sequence-specific binding of RNA by bacteriophage MS2 coat protein. Proc. Natl. Acad. Sci. USA 95:9244-9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohli, E., L. Maurice, C. Bourgeois, J. B. Bour, and P. Pothier. 1993. Epitope mapping of the major inner capsid protein of group A rotavirus using peptide synthesis. Virology 194:110-116. [DOI] [PubMed] [Google Scholar]
- 20.Kopecka, H., S. Dubrou, J. Prévot, J. Maréchal, and J. M. López-Pila. 1993. Detection of naturally occurring enteroviruses in waters by reverse transcription, polymerase chain reaction and hybridization. Appl. Environ. Microbiol. 59:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labbe, M., A. Charpilienne, S. E. Crawford, M. K. Estes, and J. Cohen. 1991. Expression of rotavirus VP2 produces empty corelike particles. J. Virol. 65:2946-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawton, J. A., C. Q. Zeng, S. K. Mukherjee, J. Cohen, M. K. Estes, and B. V. Prasad. 1997. Three-dimensional structural analysis of recombinant rotavirus-like particles with intact and amino-terminal-deleted VP2: implications for the architecture of the VP2 capsid layer. J. Virol. 71:7353-7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Guyader, F., L. Haugarreau, L. Miossec, E. Dubois, and M. Pommepuy. 2000. Three-year study to assess human enteric viruses in shellfish. Appl. Environ. Microbiol. 66:3241-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liprandi, F., Z. Moros, M. Gerder, J. E. Ludert, F. H. Pujol, M. C. Ruiz, F. Michelangeli, A. Charpilienne, and J. Cohen. 1997. Productive penetration of rotavirus in cultured cells induces coentry of the translation inhibitor alpha-sarcin. Virology 237:430-438. [DOI] [PubMed] [Google Scholar]
- 25.Metcalf, T. G., J. L. Melnick, and M. K. Estes. 1995. Environmental virology: from detection of virus in sewage and water by isolation to identification by molecular biology: a trip of over 50 years. Annu. Rev. Microbiol. 49:461-487. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell, D. K., S. S. Monroe, X. Jiang, D. O. Matson, R. I. Glass, and L. K. Pickering. 1995. Virologic features of an astrovirus diarrhea outbreak in a day care center revealed by reverse transcriptase-polymerase chain reaction. J. Infect. Dis. 172:1437-1444. [DOI] [PubMed] [Google Scholar]
- 27.Oh, D.-Y., G. Gaedicke, and J. M. Schreir. 2003. Viral agents of acute gastroenteritis in German children: prevalence and molecular diversity. J. Med. Virol. 71:82-93. [DOI] [PubMed] [Google Scholar]
- 28.Parashar, U. D., R. C. Holman, M. J. Clarke, J. S. Bresee, and R. I. Glass. 1998. Hospitalizations associated with rotavirus diarrhea in the United States, 1993 through 1995: surveillance based on the new ICD-9-CM rotavirus-specific diagnostic code. J. Infect. Dis. 177:13-17. [DOI] [PubMed] [Google Scholar]
- 29.Peabody, D. S., and F. Lim. 1996. Complementation of RNA binding site mutations in MS2 coat protein heterodimers. Nucleic Acids Res. 24:2352-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redman, J. A., S. B. Grant, T. M. Olson, M. E. Hardy, and M. K. Estes. 1997. Filtration of recombinant Norwalk virus particles and bacteriophage MS2 in quartz sand: importance of electrostatic interactions. Environ. Sci. Technol. 31:3378-3383. [Google Scholar]
- 31.Rollo, E., K. Kumar, N. Reich, J. Cohen, J. Angel, H. Greenberg, R. Sheth, J. Anderson, B. Oh, S. Hempson, E. Mackow, and R. Shaw. 1999. The epithelial cell response to rotavirus infection. J. Immunol. 163:4442-4452. [PubMed] [Google Scholar]
- 32.Scott, T. M., J. B. Rose, T. M. Jenkins, S. R. Farrah, and J. Lukasik. 2002. Microbial source tracking: current methodology and future directions. Appl. Environ. Microbiol. 68:5796-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sokal, R. R., and E. J. Rohlf. 1973. Introduction to biostatistics. W. H. Freeman, San Francisco, Calif.
- 34.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, and C. P. Gerba. 2003. Chlorine inactivation of adenovirus type 40 and feline calicivirus. Appl. Environ. Microbiol. 69:3979-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, K. Riley, and C. P. Gerba. 2003. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 69:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thouvenin, E., G. Schoehn, F. Rey, I. Petitpas, M. Mathieu, M. C. Vaney, J. Cohen, E. Kohli, P. Pothier, and E. Hewat. 2001. Antibody inhibition of the transcriptase activity of the rotavirus DLP: a structural view. J. Mol. Biol. 307:161-172. [DOI] [PubMed] [Google Scholar]
- 37.Tosser, G., T. Delaunay, E. Kohli, J. Grosclaude, P. Pothier, and J. Cohen. 1994. Topology of bovine rotavirus (RF strain) VP6 epitopes by real-time biospecific interaction analysis. Virology 204:8-16. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Environmental Protection Agency. 1989. Guidance manual for compliance with the filtration and disinfection requirements for public water systems using surface water sources. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 39.Villena, C., W. M. El-Senousy, F. X. Abad, R. M. Pinto, and A. Bosch. 2003. Group A rotavirus in sewage samples from Barcelona and Cairo: emergence of unusual genotypes. Appl. Environ. Microbiol. 69:3919-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan, L., A. Geyer, D. C. Hodgins, Z. Fan, Y. Qian, K.-O. Chang, S. E. Crawford, V. Parreño, L. A. Ward, M. K. Estes, M. E. Conner, and L. J. Saif. 2000. Intranasal administration of 2/6-rotavirus-like particles with mutant Escherichia coli heat-labile toxin (LT-R192G) induces antibody-secreting cell responses but not protective immunity in gnotobiotic pigs. J. Virol. 74:8843-8853. [DOI] [PMC free article] [PubMed] [Google Scholar]