Abstract
Two series of self-assembled monolayers (SAMs) of ω-substituted alkanethiolates on gold were used to systematically examine the effects of varying substratum surface chemistry and energy on the attachment of two model organisms of interest to the study of marine biofouling, the bacterium Cobetia marina (formerly Halomonas marina) and zoospores of the alga Ulva linza (formerly Enteromorpha linza). SAMs were formed on gold-coated glass slides from solutions containing mixtures of methyl- and carboxylic acid-terminated alkanethiols and mixtures of methyl- and hydroxyl-terminated alkanethiols. C. marina attached in increasing numbers to SAMs with decreasing advancing water contact angles (θAW), in accordance with equation-of-state models of colloidal attachment. Previous studies of Ulva zoospore attachment to a series of mixed methyl- and hydroxyl-terminated SAMs showed a similar correlation between substratum θAW and zoospore attachment. When the hydrophilic component of the SAMs was changed to carboxylate, however, the profile of attachment of Ulva was significantly different, suggesting that a more complex model of interfacial energetics is required.
Upon submersion in a nonsterile aqueous liquid, most surfaces become rapidly colonized by collections of bacteria and other microorganisms. These attached cells, along with extracellular material they produce and other organic compounds adsorbed to the surface, comprise a structure referred to as a biofilm (17). Biofilms are ubiquitous in natural aqueous milieus and are increasingly considered to represent a separate developmental form of microorganisms (29). Similarly, a number of macroorganisms, such as algae, exploit a unicellular form for attachment and colonization of surfaces. Surface coverage by both macro- and microorganisms, therefore, depends initially on the ability of single cells to adsorb and adhere to the attachment substratum.
The tendency (driving force) for a microorganism to attach to a given surface is given by the free energy of adhesion (ΔGadh), which can be expressed by the following equation as a thermodynamic energy balance between the interfacial energies between the substratum, the organism, and the surrounding liquid (1): ΔGadh = γBS − γBL − γSL, where γBS is the interfacial tension between the organism (e.g., a bacterium) and substratum, γBL is the interfacial tension between the organism and the liquid, and γSL is the interfacial tension between the substratum and the liquid.
Experimental determination of the interfacial energy values for the above equation is controversial and has led to three different, yet complementary, models (8). All rely on estimation of interfacial energies by contact angles as indicated by Young's equation (2), which states that γSV (the vapor interfacial tensions [surface tensions] of the substratum) is related to the contact angle (θ) formed by a drop of liquid on the substratum such that γSV = γSL + γLVcosθ, where γSL is the interfacial tension between the surface and the liquid and γLV is the interfacial tension between the liquid and vapor phases.
The simplest and most elegant of these models invokes an equation of state, of the form γ12 = f(γ13,γ23) (28). Thus, γSL can be obtained as a function of γSV and γLV, γBS can be obtained as a function of γBV and γSV, and γBL can be obtained as a function of γBV and γLV, where γBV and γLV are the vapor interfacial tensions (surface tensions) of the bacterium and liquid, respectively. The empirically determined equation of state derived by Absolom et al. (1) leads to the following qualitative prediction: if γLV < γBV, bacterial attachment will increase with increasing γSV; conversely, if γLV > γBV, bacterial attachment will increase with decreasing γSV. Absolom et al. observed not only this relationship but also a linear relationship between γSV (as calculated from the water contact angle of the substratum) and bacterial attachment under both conditions.
A second model asserts that the contributions of Lifschitz-Van der Waals and polar interactions are the major components of interfacial energies and are most important to thermodynamic balance (8). Experimental systems based on this model require the measurement of contact angle with two liquids, one polar and one nonpolar, in order to estimate the relative interfacial energies. A variation on this model further divides the acid-base component into its electron donor and electron acceptor constituents. This model, called the extended DLVO (Derjaguin-Landau-Verwey-Overbeek) model, puts particular emphasis on electrostatic interactions as the major contribution to surface energy. These two models have also been empirically tested and, in certain cases, have been found to be valid under laboratory conditions (7).
The experimental results from these three different models, although predictive in certain cases, do not result in the same estimations of interfacial energy, and thus predict different values for ΔGadh. Systematic determination of the best model for γSV and the subsequent effect on microbial attachment has been difficult in the past, because the majority of studies have by necessity used either glass or polymeric substrata which can vary enormously in more than one energetic parameter from sample to sample (9). The use of self-assembled monolayers (SAMs) of ω-substituted alkanethiolates on gold has been an effective technique for the systematic investigation of the effect of substratum physicochemistry on protein adsorption (30, 31, 33) as well as on attachment of mammalian cells (15, 16, 27) and microbes (12, 23, 38, 39). The use of mixed monolayers has been particularly useful for the generation of surfaces constant in one parameter (e.g., contact angle or surface roughness) while varying the chemistry by changing the relative concentrations of different thiolates within the monolayer (3, 4). Furthermore, a series of samples can be produced in which one parameter (e.g., contact angle) is held constant while varying the chemical composition by using mixed monolayers with different thiolate components on different samples.
For this set of experiments, we sought to examine attachment by using the most simple of the models, derived from equations of state, which derives its estimations of interfacial energy on the contact angle of water. Using Absolom et al.'s interpretation of the equation of state, computer-generated tables give the value of γSV when θ and γLV are known (28), γSV being linear with regard to cosθ of water. Thus, the relationship between attachment and cosθ reflects the relationship between attachment and γSV.
In this study, we investigate the integrity of this model by examining the effect of varying the chemical composition of mixed monolayers on microbial attachment while keeping θAW and, thus, γSV approximately constant. Two series of mixed monolayers were produced, consisting of methyl- and hydroxyl-terminated and methyl- and carboxylic acid-terminated SAMs with identical, stepped contact angles. The attachment of bacterial cells and algal spores was then tested on each series. We used the gram-negative bacterium Cobetia marina (formerly Halomonas marina [3]) as a model marine biofouling bacterium (32). This organism was originally isolated from a marine biofilm and has many practical considerations that make it ideal for such studies: it is obligately aerobic, thus ensuring one mode of growth; it requires high salt concentrations, which inhibits contamination while growing in chemostat; and while in log phase, the cells are relatively large and easy to see during assays. Studies of this organism and its exopolysaccharide and motility mutants (32) have yielded a detailed picture of its biofilm developmental cycle, which allows for understanding of results, particularly for long-term experiments, in the context of this cycle. Previous studies have shown that, when grown in minimal medium with limiting carbon source, C. marina shows a greater affinity for attachment to hydrophobic surfaces (23, 24). The present study addresses this observation in detail.
We also examined attachment of zoospores of the marine alga Ulva linza (formerly Enteromorpha linza), an organism ubiquitous in marine biofilms. Dispersal and rapid colonization of substrata by this green fouling alga occurs mainly through the production of vast numbers of motile spores (11). Asexual zoospores are quadriflagellate, naked (i.e., lacking a cell wall), pyriform cells, the spore body being 7 to 10 μm in length. Critical events in the colonization of new substrata involve the swimming spore locating a suitable surface on which to settle (11, 13), followed by permanent adhesion through the rapid secretion and curing of a swollen, hydrophilic gel-like adhesive composed of an N-linked, polydisperse glycoprotein (molecular size of 110 kDa under denaturing reducing conditions) that anchors the spore to the substratum (10, 35).
Prior to adhesion, the swimming spore undergoes characteristic presettlement behavior that involves a searching pattern of exploration close to the substratum (13). A number of cues moderate the way in which a spore interacts with the substratum (11), and recent evidence suggests that the swimming spore is able to select suitable surfaces on the basis of surface characteristics, such as topography (14), or on the basis of physicochemical properties, such as contact angle (12). It was previously demonstrated that the number of Ulva zoospores settling and attaching increases with increasing contact angle on mixed monolayers containing hydroxyl- and methyl-terminated alkanethiolates (12, 20). In this work, we extend these studies by changing the hydrophilic component of the mixed SAMs to carboxylic acid in order to determine whether the equation of state model is predictive independent of chemistry.
MATERIALS AND METHODS
SAMs.
SAMs were prepared at the University of New Mexico (UNM) on gold films evaporated onto glass microscope slides. The glass slides (VWR Scientific) were cleaned by immersion in a solution (piranha etch) prepared by mixing 70% (vol/vol) concentrated H2SO4 with 30% H2O2 for 20 min to 1 h, thoroughly rinsing the slides in deionized water, and drying them under a stream of nitrogen. Piranha etch is a powerful oxidizer and can react violently when placed in contact with organics and should be stored in containers which prevent pressure build-up. The samples were then placed into the vacuum chamber of a metal evaporator. The system was evacuated to 10−7 torr, and 10 Å of chromium followed by 300 Å of gold was deposited on the substrata. The system was then restored to room pressure, and the samples were removed and submerged in 1 mM ethanolic solutions of dodecanethiol (referred to herein as CH3-thiol and obtained from Aldrich Chemical), 11-mercapto-1-undecanol (OH-thiol; Aldrich Chemical), 12-mercaptodecanoic acid (COOH-thiol; Aldrich Chemical), or mixtures of two of these thiols. The samples were immersed in thiol solution overnight at 4°C, after which they were rinsed in ethanol and dried under a stream of N2. The resulting surface (i.e., the SAMs of ω-terminated alkanethiolates) will be referred to as CH3-SAM, OH-SAM, COOH-SAM, or, in the case of mixed monolayers, COOH/CH3-SAM, OH/CH3-SAM, or COOH/OH-SAM.
Patterned SAMs were produced by serial electrochemical desorption and reformation of the SAMs as described previously (12, 36). Briefly, a CH3-SAM was formed on gold. A laser ablation system composed of a Nikon Diaphot inverted microscope, adapted with a computer-controlled, pulsed-nitrogen pumped-dye laser (λ = 390; 15 μJ pulse−1; 20 pulses s−1) was used to cut lines in the gold film to form electrically isolated regions in the film. The UV laser beam was focused through a 10× objective of the microscope and ablated the gold and supported SAMs generating lines of exposed glass approximately 15 μm wide. The slide was then placed in 0.5 M ethanolic KOH, and an anode was connected to one element. A cyclic current was then applied (−1.0 to 1.5 V versus Ag/AgCl; 500 mV s−1) to the element for 6 cycles. Desorption of CH3-SAM was monitored by cyclic voltammetry to ensure complete removal of the SAM. The exposed gold was then treated with a 10 mM ethanolic solution of the desired ω-substituted alkanethiol for 20 min. A series of elements could thus be addressed sequentially, resulting in a pattern of different SAMs upon a single surface.
Zoospore attachment studies were done at the University of Birmingham (UB), United Kingdom. For transportation, the SAMs were removed from the thiol solutions, rinsed, and placed into Coplin staining jars containing deionized water which had been deoxygenated in a stream of nitrogen for one hour. The lids of the jars were screwed on and sealed with Teflon tape. The jars were immediately packaged and sent via overnight delivery.
Surface characterization of SAMs.
X-ray photoelectron spectroscopy (XPS) was used to determine the surface composition of mixed monolayers. The analysis was conducted on an AXIS-HSi instrument from Kratos Analytical, Inc. (Ramsey, N.Y.) at UNM. An A1 Kα1,2 monochromatized X-ray source (hν = 1,486.7 eV) with an emission power of 225 W was used to stimulate photoelectron emission. The residual pressure in the analysis chamber was ∼4 × 10−10 torr during spectral acquisition. To minimize X-ray-induced sample damage, the exposure time for SAMs during analysis was limited to <60 min. The samples were loaded into the vacuum chamber less than 30 min after removal from the thiol solutions. Survey scans and high-resolution C1s and O1s spectra were recorded for each sample. The Au4f peak was referenced at 84 eV, which consistently located the main C1s hydrocarbon peak at 284.7 eV. Survey spectra were acquired by using a constant pass energy of 160 eV, whereas high-resolution spectra were acquired by using a pass energy of 80 eV. The spectral envelopes were resolved into Gaussian peaks to fit the spectra.
Contact angles of samples were measured both prior to and after shipment to ensure that the integrity of the samples was maintained in transit. Advancing water contact angles (θAW) were measured both immediately before packing and immediately before use in the adhesion assays after the following washing treatment: each slide was washed in ethanol, dried in a stream of nitrogen, and then washed in 0.1 M HCl followed by three rinses in deionized water.
Bacterial strain.
C. marina American Type Culture Collection (Manassas, Va.) strain 25374 (6, 19) was established as a chemostat culture in modified basal medium (200 mM NaCl, 50 mM MgSO4 · 7H2O, 10 mM KCl, 10 mM CaCl2 · 2H2O, 19 mM NH4Cl, 0.33 mM K2HPO4, 0.1 mM FeSO4 · 7H2O, 5 mM Tris-HCl [pH 7.5], and 2 mM glycerol [25]) as described previously (23). The chemostat was maintained at a flow rate of 1.2 ml min−1 with constant stirring. The cellular concentration of the subsequent culture was 5 × 107 cells ml−1.
Bacterial attachment studies.
SAMs prepared on gold films coated on 60- by 24-mm coverslips were placed into a flow-cell apparatus (23) which was then mounted onto the stage of an optical microscope (Labophot; Nikon) and connected to the outflow of the chemostat. The C. marina culture was allowed to flow through the cell at a rate of 1.2 ml min−1 for 2 h. Under these experimental conditions, the Reynolds number was ∼2 × 10−3, indicating laminar flow. The surface shear rate was 664 s−1. Bacterial attachment was monitored through a CCD camera attached to the microscope. The images were fed to a computer by using a Data Translations 3155 frame-grabber card and Image Tool imaging software (available from the University of Texas Health Science Center at San Antonio). At the end of the attachment time, images of 10 fields of view within 10 mm of the horizontal midline of the slide were captured, the number of attached bacteria were counted, and the average for each slide was determined.
Algal material.
Fertile plants of U. linza were collected from Wembury Beach, United Kingdom (50°18′N, 4°02′W). Zoospores were released and prepared for attachment experiments as described previously (12).
Zoospore adhesion assays.
Zoospore suspensions were standardized as described previously (13). The concentration of spores was adjusted to 1 × 106 to 2 × 106 ml−1 with natural seawater; the exact concentration is given for individual experiments. Each microscope slide was placed in a compartment of a polystyrene culture dish (Fisher Scientific Co.), and 10 ml of spore suspension was added. After incubation in the dark at 20°C for 60 min, the slides were washed by being passed back and forth 10 times in a beaker of seawater before being fixed in 2% glutaraldehyde in seawater for 10 min, followed by washing as described previously (12). Images of spores were recorded in 10 fields of view at 0.5-mm intervals through the mid-point of the long axis of each of three replicate slides by using a Zeiss epifluorescence microscope via a video camera. Spores were visualized by autofluorescence of chlorophyll. Spore counts were generated from the images by using an algorithm that had been calibrated against manual counts of spore numbers. Data are presented for the mean number of spores adhered at ±95% confidence limits (x = 30).
Zoospore adhesion to SAMs formed from mixtures of COOH- and OH-thiols.
Four slides of each SAM were shipped and treated immediately prior to use in the spore adhesion assay as described above. The SAMs that were used were formed with different solution mole fractions of COOH-thiol on a solvent-free basis (χCOOHsol) according to the formula χCOOHsol = (COOH-thiol)/(COOH-thiol + OH3-thiol). Three replicate slides were used for zoospore adhesion assays; the remaining slide was used to determine the contact angle. Samples were incubated for 1 h in a suspension of 1.0 × 106 ml−1 zoospores and were processed as described above for adhesion assays.
Measurement of spore adhesion to patterned SAMs.
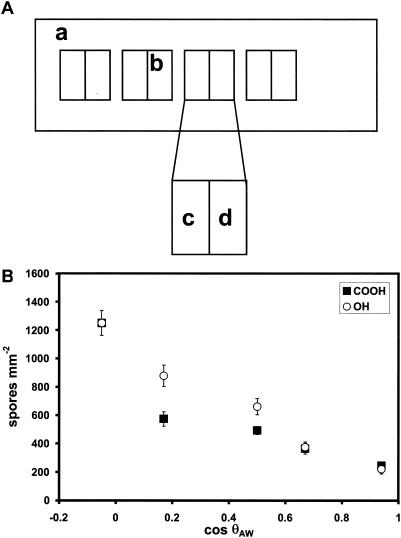
Three replicate patterned slides were prepared and incubated as described above. The pattern was arranged in pairs such that OH/CH3-SAM and COOH/CH3-SAM elements with comparable contact angles were in close proximity on the slide, with contact angle increments of ca. 20° between each pair of elements. A schematic of the patterns is shown in Fig. 5A. The spore concentration was 2 × 106 ml−1. Following incubation and rinsing, three replicates were fixed in 2% glutaraldehyde in seawater and spore counts were taken at 1-mm intervals along the middle of the long axis of each sector.
FIG. 5.
Attachment of Ulva zoospores to COOH/CH3- and OH/CH3-SAMs with similar cosθAW values. (A) Schematic representation of patterned COOH/CH3- and OH/CH3-SAMs on a single slide. A gold film supporting a CH3-SAM was laser etched to produce four divided squares (b), each 10 by 10 mm and containing two electrically isolated elements. Electrochemical desorption of the CH3-SAM from each element and subsequent exposure to solutions of either COOH/CH3-thiols (c) or OH/CH3-thiols (d) resulted in a series of adjacent paired SAMs with similar contact angles. (B) Attachment of Ulva zoospores as a function of cosθAW. Each point is the mean of 30 fields of view, 10 from each of three replicates; bars show 95% confidence limits.
RESULTS
Surface analysis.
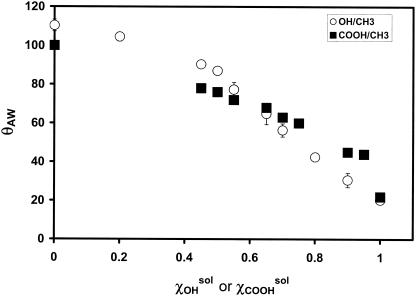
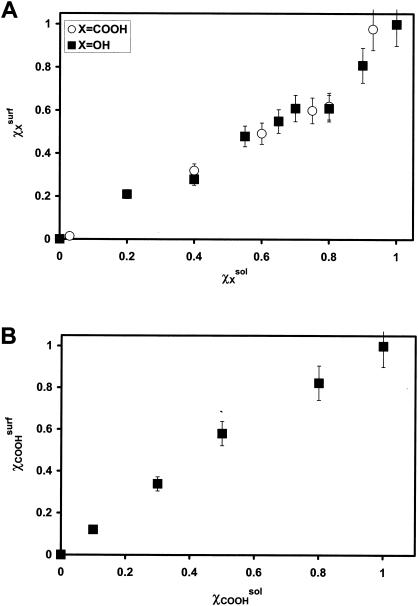
Figure 1 shows the contact angle data for OH/CH3- and COOH/CH3-SAMs in relation to the composition of the mixed thiol solutions from which they were formed. Surface composition data are shown in Fig. 2. For COOH/CH3- and OH/CH3-SAMs, the surface composition (χxsurf) was estimated by dividing the area of the O1s peak obtained by XPS by that obtained for the pure (i.e., χxsurf = 1) COOH- or OH-SAM, respectively. For the COOH/OH-SAMs, the mole fraction of COOH constituents was also estimated using the area of the O1s peaks; however, the area for a pure OH-SAM was first subtracted from the area of the mixed SAMs prior to division.
FIG. 1.
θAW of OH/CH3- and COOH/CH3-SAMs as a function of the mole fraction of the hydrophilic component (X) in the forming solution (χXsol). Data are the averages of at least three measurements on three individual samples ± standard errors.
FIG. 2.
Composition of mixed SAMs as a function of mole fractions of the forming thiol solutions as estimated by XPS. (A) Surface mole fraction of the hydrophilic component (χXsurf) as a function of the solution mole fraction of the forming thiol (χXsol) for COOH/CH3- and OH/CH3-SAMs. (B) Surface mole fraction of COOH-terminated alkanethiolate (χCOOHsurfl) as a function of the solution mole fraction of COOH-thiol in the forming solution (χCOOHsol) for COOH/OH-SAMs. Error bars reflect 10% error generally associated with XPS measurements.
The values of χCOOHsol used to form COOH/CH3-SAMs for initial zoospore adhesion assays were chosen to yield a series of SAMs for which θAW increased by increments of roughly 10°. For patterned SAMs used to compare adhesion to COOH/CH3-SAMs and for C. marina experiments, ∼20° increments were used. θAW was obtained prior to shipping from UNM and just before use at UB, and the values obtained in both locations were routinely in agreement (±1 to 5°), suggesting that degradation or contamination of the samples during transit was minimized. Subsequent discussion of θAW will refer to the measurements taken at UNM. For OH/COOH-SAM experiments, the series was produced such that the increments in the surface mole fraction of COOH (χCOOHsurf) were 0.2. The measured θAW for these samples was between 16 and 20°.
The surface roughness of these SAMs is predicted to be on the order of 4 to 5 nm based on atomic force microscopy studies done with SAMS on similar thicknesses of gold (22), and our own measurements have indicated that surface roughness of SAMs similar to those used in this experiment was <30 nm. As it is known that surface roughnesses of 50 nm or more can produce tangential electrostatic effects on microbial attachment (21), global electrostatic effects are unlikely in the present experiments. However, it is unknown how these could affect smaller, nanometer-sized structures, such as the apical tip of Ulva zoospores.
Bacterial attachment.
Attachment of C. marina to a series of COOH/CH3- and OH/CH3-SAMs with 20° increments is shown in Fig. 3. For both series of slides the number of cells increased with decreasing cosθAW and, thus, γSV (see below) in a linear manner (R2 > 0.95 for both series). Although small differences in the number of cells attached at each contact angle were observed, the profile was nearly identical for both COOH- and OH-containing surfaces.
FIG. 3.
Attachment of C. marina to COOH/CH3- and OH/CH3-SAMs as a function of cosθAW. Each data point represents the average of at least four experiments. Error bars represent standard errors.
C. marina grown under these experimental conditions is relatively hydrophobic. As part of ongoing strain monitoring in the laboratory, hydrophobic interaction chromatography on octyl-Sepharose (34) is performed regularly on the chemostat to monitor for possible changes in surface characteristics; only those cultures which show ∼85% of the cells retained by the hydrophobic column against artificial seawater were used in attachment experiments. Recent studies (data not shown) indicated that approximately 70% of the cells from a suspension in 0.1 M phosphate buffer (pH 7) segregate into the organic phase when mixed with decane.
Zoospore attachment.
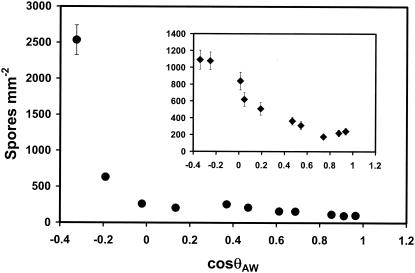
Attachment of Ulva zoospores to a series of COOH/CH3-SAMs with 10° θAW is shown in Fig. 4. Data from a previous experiment with OH/CH3-SAMs are shown in the inset (12). Although the OH/CH3 series shows approximately linear attachment between cosθAW and spore attachment (R2 = 0.89), that of the COOH/CH3-SAM series does not (R2 = 0.45). Because these data were obtained from spores collected at different times, we confirmed these results with patterned SAMs containing five pairs of elements (Fig. 5A). Each pair contained one element composed of a COOH/CH3-SAM and one of a OH/CH3-SAM which had the same (±1°) θAW. Attachment studies were done as described above, and the results are shown in Fig. 5B. Once again, there is a clear divergence between attachment to COOH/CH3- and OH/CH3-SAMs, with the OH/CH3 series once again exhibiting a nearly linear relationship between attachment and cosθAW (R2 = 0.97), while that of the COOH/CH3 series was significantly less linear (R2 = 0.79).
FIG. 4.
Attachment of Ulva zoospores to COOH/CH3-SAMs as a function of cosθAW. Attachment data for similar experiments performed on OH/CH3-SAMs (12) is shown in the inset. Attachment was for 1 h. Each point is the mean of 30 fields of view, 10 from each of three replicates. Bars show 95% confidence levels. Spore concentration was 2 × 106 ml−1.
To further study the effects of COOH-containing SAMs on zoospore attachment, we also examined COOH/OH-SAMs. The results of these investigations are shown in Fig. 6. Low numbers of zoospores attached to all the COOH/OH-SAMs, irrespective of χCOOHsurf. All surfaces were very hydrophilic, with cosθAW being within the range of 0.93 to 0.97.
FIG. 6.
Attachment of Ulva zoospores to COOH/CH3-SAMs. The number of zoospores is plotted versus χCOOHsurf. Spore concentration was 106 ml−1. Each point is the mean of 30 fields of view, 10 from each of three replicates; error bars show 95% confidence limits.
DISCUSSION
We have generated a matched series of COOH/CH3- and OH/CH3-SAMs which vary systematically in cosθAW and, thus, γSV, according to equation-of-state models for its estimation (1, 28). Analysis by XPS shows that θAW varies in accordance with the changes in the relative molar ratios of different alkanethiolates within the SAM (4, 5).
As has been qualitatively observed in previous studies, C. marina attached preferentially to hydrophobic surfaces (23, 24). In this study, we have shown a linear correlation between the attachment of this bacterium and decreasing cosθAW and, thus, γSV, as would be estimated by advancing water contact angles. As the cell surface of C. marina is also relatively hydrophobic, these results agree with the predictions of the equation-of-state models of bacterial attachment set forth by Absolom et al. (1). Attachment of Ulva zoospores to mixed COOH/CH3-SAMs was positively correlated with decreasing cosθAW. However, a dramatic response was observed only below a cosθAW of 0, and the relationship between attachment and cosθAW was nonlinear. The difference was especially apparent on patterned SAMs where direct comparison between attachment on OH- and COOH-containing SAMs of the same cosθAW was possible. This contrasts with the response to zoospore attachment on OH/CH3-SAMs, where the relationship between cosθAW and attachment was linear (Fig. 4, inset) (12). These results also differ from those obtained for C. marina in which the chemistry of the hydrophilic component of the SAMs did not alter the attachment profile. The global cell hydrophobicity of Ulva could not be reliably measured. Lacking a cell wall, the zoospore would be seriously disrupted by attempted partition into organic solvents; hydrophobic interaction chromatography uses what is essentially a redundant measurement of attachment to a solid and which is not determined by global cellular surface energy but rather by the sum of energies in a step-by-step process.
It is known that carboxylic acid-terminated SAMs change their surface energy in response to the pH of the surrounding medium (18, 26, 40). These SAMs can be titrated, with the range and position of the pH-dependent change in θAW varying with the surface concentration of COOH-containing thiolates. It is possible that, under our experimental conditions, the difference in attachment of Ulva zoospores on the OH/CH3- and COOH/CH3-SAMs might be attributable to a titration effect as the pH of the test medium (i.e., seawater) might be predicted to more strongly affect contact angles of SAMs with higher χCOOHsurf values (5). If this was indeed the case, attachment to the surfaces should also be titratable. We therefore tested the effect of pH on attachment of Ulva zoospores to COOH/CH3-SAMs with χCOOHsurf of 0, 0.25, 0.5, and 0.75 at pH 7.8 and 9.5. This set was chosen so that their titration curves spanned the range of pHs used (5); thus, we could expect different attachment profiles in response to pHs for different SAMs. The results of these experiments were inconclusive. While there were changes associated with attachment to the COOH-containing SAMs at different pHs, there were no obvious trends. Moreover, attachment to SAMs with χCOOHsurf of 0 (i.e., a pure CH3-SAM) was also influenced by pH. Because CH3-SAMs should not change their surface ionization or contact angle in response to pH, we concluded that different pHs affected not only the COOH-containing SAMs but also the surface charge and/or physiology of the zoospores. The effect could be on one of several presettlement processes of the zoospores, including motility, sensing, or secretion and/or polymerization of the adhesive.
As the equation-of-state model fell short of quantitatively modeling the attachment of Ulva to both kinds of surface, the suitability of the other models must be considered. Because the difference in attachment occurred on surfaces containing an ionizable group, one must consider the effects of electrostatic interactions on the attachment of Ulva zoospores to the surface. It should be noted that the molarity of artificial or natural seawater (ca. 250 mM) is sufficiently high to compress the double layer and to interrupt possible electrostatic repulsion (21). The potential for surface charge also requires that one consider the effect of Lewis acid-base interactions, which have been postulated to be the main driving force in microbial attachment (8, 37). Investigations are presently under way to examine this parameter of microbial attachment and will be presented in a subsequent report.
Our evaluation of the attachment of Ulva zoospores was obtained after washing and fixing of the slides, i.e., after the settled cells had undergone secondary, irreversible attachment. Successful attachment in this organism is the result of several steps, each of which involves cellular structures whose physicochemistry would be expected to exert a different influence during different times or processes in attachment (11, 13). It was previously shown that selection of the attachment site is affected by surface energy (12, 20), but the effect of surface energy is very likely influential at other specific points in the process of attachment as well. In this study, after settlement and secondary attachment, Ulva zoospores appeared to be less strongly adhered to COOH/CH3- than to OH/CH3-SAMs (data not shown), suggesting a direct effect of the physicochemical properties of the glycoprotein adhesive. We are presently investigating the effects of surface energy and chemistry on the spreading and firmness of attachment of the adhesive. Another possibility suggests itself when the spatial arrangement of the zoospores on various SAMs is considered. Ulva zoospores often settle gregariously (i.e., in groups) (13), and it has been observed that on OH/CH3-SAMs, an increase in the size of spore groups (group was defined as adjacent, touching zoospores) correlated with an increase in the number of spores settled as surface energy decreased (12, 20). On COOH/CH3-SAMs, however, a less marked effect was seen in regard to the size of attached groups versus cosθAW values. For this series of SAMs, 80% of the spores attached to all surfaces with cosθAW ≥ 0 were present in groups of no more than three spores. Larger spore groups (group sizes of up to 15 spores per group) were found only on the SAMs with cos θAW < 0°. Significantly, these were also the samples on which a marked increase in the total number of attaching spores was observed. It is possible, therefore, that the lack of attachment to COOH/CH3-SAMs with higher surface energy may be due to interference with mechanisms promoting gregarious settlement, such as cell-to-cell signaling.
Using SAMs, we were able to systematically investigate the accuracy of surface energy estimations based on equations of state for modeling the attachment of two quite different microorganisms, both of which use a unicellular means of substratum colonization. We have found that while estimations of surface energy by this approach would, indeed, accurately predict the pattern attachment of C. marina to the model solid surfaces under study, they were less accurate for attachment of Ulva zoospores. The exploration of other, more complex models must therefore be undertaken in order to generate a global model for microbial attachment, and such investigations are planned in future studies. Systematic studies of physicochemical properties such as these are important for understanding microbial attachment processes and may lead to the development of materials that resist or rapidly release microorganisms in order to control biofilm formation on submerged materials.
Acknowledgments
This work was supported through Office of Naval Research grants N00014-95-0901 and N0014-02-1-0377 to G.P.L. at UNM and grants N00014-95-0901 and N00014-02-1-0521 to M.E.C. and J.A.C. at UB.
We also thank Víctor Pérez-Luna and Lee Perry for help in obtaining and interpreting the XPS spectra and Dimiter Petsev for helpful discussions.
REFERENCES
- 1.Absolom, D. R., F. V. Lamberti, Z. Policova, W. Zingg, C. J. van Oss, and A. W. Neumann. 1983. Surface thermodynamics of bacterial adhesion. Appl. Environ. Microbiol. 46:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson, A. W. 1990. The solid-liquid interface-contact angle, p. 379-420. Physical chemistry of surfaces, 5th ed. Wiley and Sons, New York, N.Y.
- 3.Bain, C. D., J. Evall, and G. M. Whitesides. 1989. Formation of monolayers by the coabsorption of thiols on gold: variations in the head group, tail group, and solvent. J. Am. Chem. Soc. 111:7155-7164. [Google Scholar]
- 4.Bain, C. D., and G. M. Whitesides. 1988. Formation of two-component surfaces by the spontaneous assembly of monolayers on gold from solutions containing mixtures of organic thiols. J. Am. Chem. Soc. 110:6560-6561. [Google Scholar]
- 5.Bain, C. D., and G. M. Whitesides. 1989. A study by contact angle of acid-base behavior of monolayers containing ω-mercaptocarboxylic acids adsorbed on gold: an example of reactive spreading. Langmuir 5:1370-1378. [Google Scholar]
- 6.Baumann, L., R. D. Bowditch, and P. Baumann. 1983. Description of Deleya gen. nov. created to accommodate the marine species of Alcaligenes aestus, A. pacificus, A. cupidis, A. venustus, and Pseudomonas marina. Int. J. Syst. Bacteriol. 33:793-802. [Google Scholar]
- 7.Bellon-Fontaine, M.-N., J. Rault, and C. J. van Oss. 1996. Microbial adhesion to solvents: a novel method to determine the electron-donor/electron-acceptor or Lewis acid-base properties of microbial cells. Colloids Surf. B 7:47-53. [Google Scholar]
- 8.Bos, R., H. C. van der Mei, and H. J. Busscher. 1999. Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods of study. FEMS Microbiol. Rev. 23:179-230. [DOI] [PubMed] [Google Scholar]
- 9.Busscher, H. J., J. Sjollema, and H. C. van der Mei. 1990. Relative importance of surface free energy as a measure of hydrophobicity in bacterial adhesion to solid surfaces, p. •••. In R. J. Doyle and M. Rosenberg (ed.), Microbial cell surface hydrophobicity. American Society for Microbiology, Washington, D.C.
- 10.Callow, J. A., M. S. Stanley, R. Wetherbee, and M. E. Callow. 2000. Cellular and molecular approaches to understanding primary adhesion in Enteromorpha. Biofouling 14:141-150. [Google Scholar]
- 11.Callow, M. E., and J. A. Callow. 2000. Substratum location and zoospore behaviour in the fouling alga, Enteromorpha. Biofouling 15:49-56. [DOI] [PubMed] [Google Scholar]
- 12.Callow, M. E., J. A. Callow, L. K. Ista, S. E. Coleman, A. C. Nolasco, and G. P. Lopez. 2000. The use of self-assembled monolayers of different wettabilities to study surface selection and primary adhesion processes of green algal (Enteromorpha) zoospores. Appl. Environ. Microbiol. 66:3249-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callow, M. E., J. A. Callow, J. D. Pickett-Heaps, and R. Wetherbee. 1997. Primary adhesion of Enteromorpha (Chlorophyta, Ulvales) propagules: quantitative settlement studies and video microscopy. J. Phycol. 33:938-947. [Google Scholar]
- 14.Callow, M. E., A. R. Jennings, A. B. Brennan, C. E. Seegert, S. Gibson, L. Wilson, A. Feinberg, R. Baney, and J. A. Callow. 2002. Microtopographic cues for settlement of zoospores of the green fouling alga Enteromorpha. Biofouling 18:237-245. [Google Scholar]
- 15.Cooper, E., L. Parker, C. A. Scotchford, S. Downes, G. J. Leggett, and T. L. Parker. 2000. The effect of alkyl chain length and terminal group chemistry on the attachment and growth of murine 3T3 fibroblasts and human osteoblasts on self-assembled monolayers of alkanethiols on gold. J. Mater. Chem. 10:133-139. [Google Scholar]
- 16.Cooper, E., R. Wiggs, D. A. Hutt, L. Parker, G. J. Leggett, and T. L. Parker. 1997. Rates of attachment of fibroblasts to self-assembled monolayers formed by the adsorption of alkylthiols onto gold. J. Mater. Chem. 7:435-441. [Google Scholar]
- 17.Costerton, J. W., K. J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 18.Creager, S. E., and J. E. Clarke. 1994. Contact-angle titrations of mixed ω-mercaptoalkanoic acid/alkanethiol monolayers on gold. Reactive vs. nonreactive spreading and chain length effects on surface pKa values. Langmuir 10:3675-3684. [Google Scholar]
- 19.Dobson, S. J., and P. D. Franzmann. 1996. Unification of the genera Deleya (Baumann, et al. 1983), Halomonas (Vreeland et al. 1980), and Halovibrio (Robinson and Gibbons, 1952) into a single genus, Halomonas, and the placement of the genus Zymobacter in the family Halomonadaceae. Int. J. Syst. Bacteriol. 46:550-558. [Google Scholar]
- 20.Finlay, J. A., M. E. Callow, L. K. Ista, G. P. Lopez, and J. A. Callow. 2002. The influence of surface wettability on the adhesion strength of spores of the green alga Enteromorpha and the diatom Amphora. Integr. Comp. Biol. 42:1116-1122. [DOI] [PubMed] [Google Scholar]
- 21.Hermansson, M. 1999. The DLVO theory in microbial adhesion. Colloids and Surfaces Part B: Biointerfaces 14:105-119. [Google Scholar]
- 22.Huang, Y.-W., and V. K. Gupta. 2001. Effects of physical heterogeneity on adsorption of poly(ethylene glycol) at a solid-liquid surface. Macromolecules 34:3757-3764. [Google Scholar]
- 23.Ista, L. K., H. Fan, O. Baca, and G. P. López. 1996. Attachment of bacteria to model solid surfaces: oligo(ethylene glycol) surfaces inhibit bacterial attachment. FEMS Microb. Lett. 142:59-63. [DOI] [PubMed] [Google Scholar]
- 24.Ista, L. K., V. H. Perez-Luna, and G. P. López. 1999. Surface-grafted, environmentally responsive polymers for biofilm release. Appl. Environ. Microbiol. 65:1603-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kersters, K. 1992. The genus Deleya, p. 3189-3197. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schliefer (ed.), The prokaryotes, 2nd ed., vol. 4. Springer-Verlag, New York, N.Y. [Google Scholar]
- 26.Lee, T. R., R. I. Carey, H. A. Biebuyeck, and G. M. Whitesides. 1994. The wetting of monolayer films exposing acids and bases. Langmuir 10:741-749. [Google Scholar]
- 27.López, G. P., M. W. Albers, S. L. Schreiber, R. Carroll, E. Peralta, and G. M. Whitesides. 1993. Convenient methods for patterning the adhesion of mammalian-cells to surfaces using self-assembled monolayers of alkanethiolates on gold. J. Am. Chem. Soc. 115:5877-5878. [Google Scholar]
- 28.Neumann, A. W., D. R. Absolom, D. W. Francis, and C. J. van Oss. 1980. Conversion tables of contact angles to surface tension. Sep. Purif. Methods 9:69-163. [Google Scholar]
- 29.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as bacterial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 30.Prime, K. L., and G. M. Whitesides. 1991. Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces. Science 252:1164-1167. [DOI] [PubMed] [Google Scholar]
- 31.Roberts, C., C. S. Chen, M. Mrksich, V. Martichonok, D. E. Ingber, and G. M. Whitesides. 1998. Using mixed self-assembled monolayers presenting RGD and (EG3)OH groups to characterize long-term attachment of bovine capillary endothelium cells to surfaces. J. Am. Chem. Soc. 120:6548-6555. [Google Scholar]
- 32.Shea, C., L. J. Lovelace, and H. E. Smith-Somerville. 1995. Deleya marina as a model organism for studies of bacterial colonization and biofilm formation. J. Indust. Microbiol. 15:290-296. [Google Scholar]
- 33.Sigal, G. B., M. Mrksich, and G. M. Whitesides. 1998. Effect of surface wettability on the adsorption of proteins and detergents. J. Am. Chem. Soc. 120:3464-3473. [Google Scholar]
- 34.Sorongon, M. L., R. A. Bloodgood, and R. P. Burchard. 1991. Hydrophobicity, adhesion, and surface-exposed proteins of gliding bacteria. Appl. Environ. Microbiol. 57:3193-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanley, M. S., M. E. Callow, and J. A. Callow. 1999. Monoclonal antibodies to adhesive cell coat glycoproteins secreted by zoospores of the green alga Enteromorpha. Planta 210:61-71. [DOI] [PubMed] [Google Scholar]
- 36.Tender, L. M., R. L. Worley, H. Y. Fan, and G. P. López. 1996. Electrochemical patterning of self-assembled monolayers onto microscopic arrays of gold electrodes fabricated by laser-ablation. Langmuir 12:5515-5518. [Google Scholar]
- 37.van Oss, C. J. 1997. Hydrophobicity and hydrophilicity of biosurfaces. Curr. Opin. Colloid Interface Sci. 2:503-512. [Google Scholar]
- 38.Weincek, K. M., and M. Fletcher. 1995. Bacterial adhesion to hydroxyl- and methyl-terminated alkanethiol self-assembled monolayers. J. Bacteriol. 177:1959-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weincek, K. M., and M. Fletcher. 1997. Effects of substratum wettability and molecular topography on the initial adhesion of bacteria to chemically defined substrata. Biofouling 11:293-311. [Google Scholar]
- 40.Whitesides, G. M., H. A. Biebuyeck, J. P. Folkers, and K. L. Prime. 1991. Acid-base interactions in wetting. J. Adhesion Sci. Technol. 5:57-69. [Google Scholar]