Abstract
Literature on mirror neurons has shown that seeing someone preparing to move generates in the motor areas of the observers a brain activity similar to that generated when the subject prepares his own actions. Thus, the ‘mirroring’ of action would not be limited to the execution phase but also involves the preparation process. Here we confirm and extend this notion showing that, just as different brain activities prepare different voluntary actions, also different brain activities prepare to observe different predictable actions. Videos of two different actions from egocentric point of view were presented in separate blocks: (i) grasping of a cup and (ii) impossible grasping of a cup. Subjects had to passively observe the videos showing object-directed hand movements. Through the use of the event-related potentials, we found a cortical activity before observing the actions, which was very similar to the one recorded prior to the actual execution of that same action, in terms of both topography and latency. This anticipatory activity does not represent a general preparation state but an action-specific state, because being dependent on the specific meaning of the forthcoming action. These results reinforce our knowledge about the correspondence between action, perception and cognition.
Keywords: motor preparation, grasping, action observation, ERP
INTRODUCTION
Humans are able to interact with each other by recognizing and understanding other’s actions and behaviors but also by inferring others’ underneath intentions. Frequently, we experience the feeling to know in advance what other people are going to do, to ‘read their mind’ and anticipate their actions. This important feature in social cognition is probably due to a combination of aspects, such as the knowledge about other people, the association between specific actions with particular objects, the cultural aspects, etc. (Frith and Frith, 2006). However, the way such a function is achieved in the brain is still a matter of debate. The general aim of the present study is to investigate some mechanisms underlying social interactions, in particular those related to action anticipation and understanding.
Experimental evidences proved that actions are organized in a hierarchical manner (Jeannerod, 1994) and, therefore, can be described at multiple stages, from cognitive and abstract levels (i.e. intentions and goals) to lower levels (i.e. kinematics and motor aspects) (Hamilton and Grafton, 2007; Kilner et al., 2007; van Schie and Bekkering, 2007; van Elk et al., 2010; Kilner, 2011). For a proper social interaction, it is important that all these levels are processed and understood. Many authors have approached the study of action understanding by separating the recognition of an immediate action goal (the type of grip and action kinematic) from the final action goal (the outcome of the action). Creating expectancy, making the action predictable in their kinematics and/or final goal, they recorded different responses related to grip and goal action features (van Elk et al., 2008; Jacquet and Avenanti, 2015; Ondobaka et al., 2014), and concluded that action understanding is based on a predictive process that allows to infer the action kinematics based on the inference of the action goal (van Schie and Bekkering, 2007; van Elk et al., 2008). In addition, different cortical structures were reported in these processes: the parietal–occipital areas involved in grip-related information processing in contrast to frontal areas and the posterior cingulate cortex involved in the conceptual level for both action execution and observation tasks (van Schie and Bekkering, 2007; Ondobaka et al., 2014). In the present study, we focused on the processes occurring before the presentation of the action, when the observer just knows that the other person is about to move, to address the more abstract and conceptual levels of this hierarchical distinction, ruling out the kinematic aspects.
Since the discovery of the mirror neurons (MNs) and their property to fire during both action execution and observation, different motor theories on action understanding have been developed. According to the MNs view, this ‘motor resonance’, i.e. the activation of the motor system during action observation, translates the visual experience into an internal personal representation (Rizzolatti and Craighero, 2004; Shmuelof and Zohary, 2007; Mukamel et al., 2010). However, this ‘direct-matching’ hypothesis, which assumes a simulation of the action observed, has been challenged by alternative mechanisms for action understanding (Csibra and Gergely, 2007; Csibra, 2007; Hickok, 2009; Tomasino and Rumiati, 2013). Csibra and Gergely (2007) proposed an integration of different fundamental mechanisms subtending the goal attribution and action understanding, such as the action-effect association, the simulation procedures and the teleological reasoning, which represents a tendency to conserve energy and to estimate the most efficient way to achieve goals. An alternative model that integrates the above mechanisms is the ‘forward model’, which assumes that the central nervous system can learn to estimate the sensory outcome of a specific motor commands using an internal simulation (Wolpert and Kawato, 1998; Desmurget and Grafton, 2000) and this prediction is constantly updated through sensory feedback used for online action adjustments (Wolpert et al., 1995; Desmurget and Grafton, 2000). This top-down process can also be used to predict the outcome of others’ actions and, thus, the motor system activation does not imply the real understanding of other’s intentions but rather a prediction of the sensory input that a particular action will cause (Hickok, 2009). However, this can better account for the lower-level processes of the action hierarchy (the immediate action goal), considering input and output mechanisms taking place after movement initiation and assuming that higher-level aspects are estimated based on these bottom-up action representation.
It is worth noticing that the majority of these studies, and the subsequent models, have investigated the period between the movement onset and the reaching of the target, precueing participants with the action to attend and describing the brain activity and behavioral response during the action transport phase (Cuijpers et al., 2006; Hamilton and Grafton, 2006; Van Elk et al., 2008; Sartori et al., 2011). Therefore, the kind of action understanding and prediction previously studied were about the subsequent movement sequence participants were attending according to its match with provoked expectancies.
On the contrary, an outstanding experiment by Kilner and colleagues (2004) has proved the observers’ ability to anticipate the action of the actor by producing a motor preparation-like activity very similar to the one recorded before a real action execution. In this experiment, participants observed a hand waiting to perform a predictable action, and that elicited a readiness potential in the anterior motor areas, from 500 ms before the movement onset (Deecke et al., 1969). Interestingly, a follow-up study by Fontana and colleagues (2012) on patients with frontal or parietal brain lesions pointed out the role the posterior parietal areas also play in this anticipatory activity. They replicated the study and results by Kilner et al. (2004), but in this case, the observational readiness potential was present only in patients with frontal lesions, indirectly supporting the importance of parietal structures in the anticipatory activity for actions to be observed. Overall, they concluded that ‘for our motor system being ready to observe a movement may be equivalent to being ready to act’ (Fontana et al., 2012).
The importance of these findings lays on the discovery that the human brain is able not only to understand someone else’s action, but also to anticipate it ahead of its execution (Kilner et al., 2004). Nonetheless, despite these paramount results, not much attention has been given yet to the motor preparation phase before seeing someone performing an action. Thus, several questions are still to be answered. The first one is whether the premovement observation activity is limited to the premotor cortex and to the last part of the readiness potential, as previously described (Kilner et al., 2004; Fontana et al., 2012) or it also involves earlier activity in the parietal cortex, as indirectly suggested by the patients’ study (Fontana et al., 2012). Here, we address this question by measuring the brain activity during a time period before the observation of predictable object-oriented actions much larger than Kilner et al. (2004) and Fontana et al. (2012). In fact, previous studies on the execution of grasping actions have shown brain activity starting 3 s before the action initiation with an early involvement of the anterior intraparietal region (Bozzacchi et al., 2012a, b).
A second open question is whether this anticipatory activity is specific for the action to be observed or it just represents a general arousal or readiness for the observation of upcoming actions. To address this question, we used two actions previously studied in our laboratory and able to elicit different motor preparation activities, a grasp and an impossible grasp of a cup (Bozzacchi et al., 2012a). This approach is novel with respect to previous studies, given that neither Kilner et al. (2004) nor Fontana et al. (2012) compared brain activity preceding the observation of different actions. We assumed that if the activity preceding the observation of an action was actually index of anticipation for that specific action, then it would be different for the specific action observed. Moreover, such a difference should reflect the one described in the motor preparation for the real execution, in which earlier and stronger negativity was recorded before the grasp in comparison to the impossible grasp that, instead, was preceded by a prefrontal positivity. Alternatively, a lack of difference between the two conditions would indicate that waiting for an upcoming action produces a general arousal of the motor system, not an action-specific activity.
MATERIALS AND METHODS
Participants
Data were recorded from 14 subjects, all volunteers and university students (mean age 24.7 years; s.d. 6.2 years; 10 females). None presented neurological or psychiatric disease. All subjects were right handed and their manual preference was assessed using the Edinburgh Handedness Inventory (Oldfield, 1971) (LI > 60; mean score 85). After a full explanation of the procedures, all subjects provided written informed consent. The study was approved by the local ethical committee.
Stimuli
Video clips of an actor’s hands performing two types of actions toward a tea cup (called grasp and impossible grasp) were used as stimuli and filmed by the experimenters. The video presented one of the two hands moving towards the cup, grasping it and lifting it up (in the grasp condition), or just reaching the cup (in the impossible grasp condition) (see video frames in Figure 2). In the impossible grasp condition both hands were tied as fist by a white band. For each type of action, both right and left hand movements were shown, according to right/left orientation of the cup’s handle. To better match the stimuli for left and right hand movements, video clips were also flipped left to right and right to left using a video editing software (Ulead VideoStudio 9.0), and their presentation was counterbalanced so that 50% of the videos of one hand’s movement were mirrored movements of the other hand. In the video, only the table, the two hands, the distal portion of the arms of the actor and the tea cup were visible on the screen. The table was covered with a black tablecloth. The actor’s limbs wore yellow rubber gloves and a white coat covering arms and wrists making her unidentifiable. We selected the first frame of the video clip to create the initial static images of the two hands laid on the table in a resting position with the tea cup located in the middle. The size of the hands and the cup were created to simulate on the monitor the size of the real object when presented at a distance of 35 cm from the subject. Video clips started with the static image (duration 3.5 s) and then showed the movement from an egocentric point of view (duration 1.5 s), which is known to produce larger readiness potentials than allocentric presentation (Kilner et al., 2004).
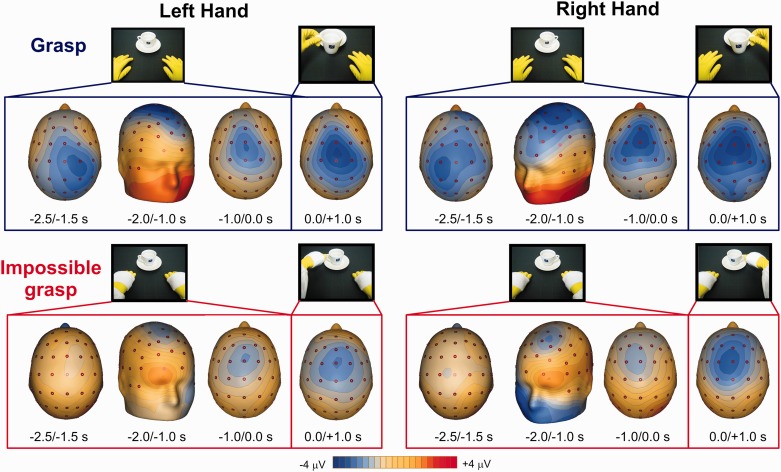
Fig. 2.
Tridimensional scalp topography of the activity during the tasks in the four studied time windows for left and right hand actions. Video frames above the maps show the scene observed by the subject in those time windows.
Each experimental condition had its own static image (with hands free or hands tied) that was followed by the video. This image made predictable whether the action to be observed was possible or impossible and which hand was about to move (based on cup’s handle position). Finally, the starting time of the video was constant and consequently predictable after few trials. The total duration of each trial was 5 s and the interval between trials was 1 s. Timing and presentation of the stimuli were controlled by the Presentation software (Neurobehavioral System, Davis, CA, USA).
Procedure
Subjects were comfortably seated on a chair in front of a monitor (at a distance of 35 cm) and were not required to perform any task except for observing with attention the videos presented. Since the model’s hands in the videos wore yellow gloves and a white coat, also the subjects were requested to wear the same gloves and coat to enhance an identification process. For the same reason, when subjects were presented with the impossible grasp videos block, they also wore the band over the gloves that blocked their hands as fist. The grasp and the impossible grasp videos were presented in separate blocks because subjects had to wear the band during the impossible grasp and it made impossible to set them up trial by trial. The tea cup was located in the middle, and participants were instructed to fixate on the cup logo (to avoid eye movements). Subjects did not see their own hands that were laid on their legs under the table.
The orientation of the cup handle (rightward or leftward) was displayed in a randomized order; accordingly, the corresponding hand moved. Each block included 10 runs and each run was composed by 24 trials (12 per hand). Every five runs the condition (grasp, impossible grasp) was switched. Half of the sample started the experiment with the grasp condition and the others with the impossible grasp condition.
Electrophysiological recording and data analysis
The electroencephalogram (EEG) was recorded during the experiment using a BrainVision™ 64-channel system (Brain Products GmbH, Munich, Germany) connected to an active sensor system (ActiCap™ by Brain Products GmbH), adopting the standard 10–10 international system montage. The left mastoid (M1) was used as initial reference electrode for all scalp channels. Two electrooculogram (EOG) channels located on the left outer canthi and below the right eye were used for horizontal eye movement and blinks detection.
Signal was digitized at 250 Hz, with an amplifier band-pass from 0.01 to 60 Hz with 50 Hz notch filter. Data were analyzed offline using BrainVision™ Analyzer 1.5 software (Brain Products). To obtain event-related potentials (ERPs), the onset of the static image was used as trigger and the EEG was segmented into nonoverlapping epochs of 5500 ms duration (500 ms: prestimulus baseline; 3500 ms: static image; 1500 ms: video of the moving hand). Before signal averaging, eye movement artifacts were reduced using the Gratton and Cole algorithm, and semiautomatic computerized artifacts rejection was performed to discard epochs with ocular or muscular contraction artifacts from further analysis. Blinks were found to be the most frequent cause for rejection (about 8% of trials). To further reduce high-frequency noise, the time-averaged ERPs were subsequently low-pass filtered at 8 Hz.
The epoch used for statistical analysis was 3.5 s, starting 1 s after the presentation of the still image (to avoid perceptual processing related to the onset of the static image) to 1 s after the action start. The time windows used for the statistical analysis were 1 s each and were chosen resembling our previous study on motor preparation for real grasping execution (Bozzacchi et al., 2012a). However, to increase the chance to record motor-related cortical potential (MRCP)-like activity, we extended them: from −2.5 to −1.5 s for the posterior BP peaking on parietal sites, from −1.0 to 0 s for the anterior BP and NS′ over anterior premotor areas, from 0 to 1.0 s for the activity related to the action observation (and, finally from −2.0 to −1.0 s for the prefrontal positivity) on lateralized prefrontal sites, as previously described for the impossible grasping condition only. Moreover, to allow a more clear graphical comparison between present data (observation) and the actual MRCP for real actions (execution) (data from Bozzacchi et al., 2012a), the action onset in the video was set as the time zero. Statistical differences in the ERP amplitudes between grasp and impossible grasp were initially assessed with sample-by-sample t-test on all electrodes to select the locations where the differences were consistently significant. This preliminary analysis allowed us to select 19 electrodes (F7, F8, FCz, FC1, FC2, FC3, FC4, Cz, C1, C2, C3, C4, Pz, P1, P2, P3, P4, P5 and P6). Lateralized readiness potential (LRP) was calculated considering pairs of symmetrical electrodes selected by the t-test analysis (see above), to verify the lateralization of the measured activity. Preliminary paired t-test on the LRP did not show any lateralization of the activity (P > 0.05) except for the F7–F8 electrodes (t(13) = 215, P < 0.05) in the impossible grasp condition. Because of the lack of lateralization on frontocentral and parietal sites, we run the statistical analysis on the more representative medial electrodes on the parietal (Pz), central (Cz) and frontocentral sites (FCz). These electrodes were used for a repeated measures 2×3×2 analysis of variance (ANOVA) with action (grasp and impossible grasp), electrodes (frontal, central and parietal) and hand (left and right) as main factors and run for each selected time window. Bonferroni correction was applied to post hoc comparisons. The assumption of sphericity was tested and, where necessary, the degree of freedom was corrected using the Geisser–Greenhouse correction. The minimum α-level was set at 0.05.
To visualize the voltage topography of the aforementioned ERP time windows, spline-interpolated 3D maps were generated using the Brain Electrical Source Analysis system (BESA 2000 version 5.18, MEGIS Software GmbH, Gräfelfing, Germany).
RESULTS
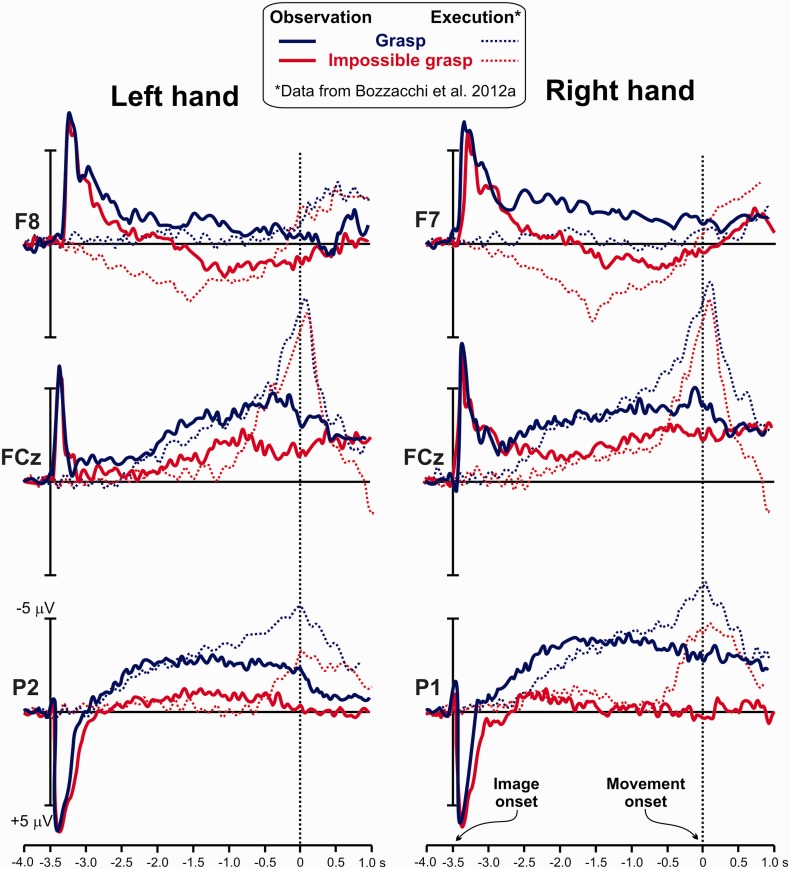
Figure 1 shows the ERP activity in the two conditions (grasping and impossible grasping; continuous thick lines) on representative prefrontal (F7 and F8), frontocentral (FCz) and parietal sites (P1 and P2). The time zero (dotted line) represents the action initiation in the video. Figure 2 shows the scalp topography in the selected time windows. The onset of the static image (at −3.5 s) produced large positive activities in occipital and parietal sites that terminate about 0.5 s after. Differently from previous results by Kilner and colleagues (2004), a slow-rising negative activity started on bilateral parietal sites around 3 s before the grasp onset, confirming the involvement of the posterior areas in this readiness potential (Fontana et al., 2012). From −2 s, a slow negative activity was also present on medial frontocentral sites in both conditions. For the impossible grasp only, peaking at −1.5 s, a positive activity on contralateral prefrontal sites was detected, indicating that this readiness potential activity was more action specific.
Fig. 1.
The ERPs on three relevant scalp sites obtained for observation of grasp and impossible grasp actions are represented by continuous thick lines. Time zero indicates the start of the observed movement. The sharp peak at the beginning of the waveform represents the visual response to the onset of the video. The thin dashed lines represent the MRCP obtained by the real execution of the same grasp and impossible grasp (from Bozzacchi et al., 2012a).
The ANOVA showed differences between the observations of the two actions. The earliest time windows (−2.0/−1.0 s before action onset) corresponding to the posterior BP, in the grasp condition produced larger negativity (F(1,13) = 6.946; P = 0.021) than the impossible grasp, but no effect of the hand (P = 0.19), electrode factors (P = 0.60) or interactions (P > 0.005) were found. This wide distributed activity was followed by a more anterior negativity, likely corresponding to the well-known anterior BP and NS′ components of the MRCP. Here the activity resulted to be more negative for the grasp condition (F(1,13) = 7.18; P = 0.019) peaking on the more anterior FCz electrode (F(1.218, 15.836) = 5.29; P = 0.030). No effect of hand was found (F(1,13) = 0.668; P = 0.42). For the following time window (from 0 s to 1 s), statistical analysis showed only effect of the electrode (F(1.086, 14.123) = 5.05; P = 0.039), with the activity stronger on the anterior areas than on posterior. No effect of condition (P = 0.11) and hand (P = 0.60) was found. A separate repeated measure 2×2×2 ANOVA for the lateral electrodes F7 and F8 was run for the time range from −2 to 1 s to assess the presence of the prefrontal positivity as already described in the previous study (Bozzacchi et al., 2012a). The ANOVA showed a significant interaction effect of the three factors (F(1,13) = 8.27; P = 0.013). The interaction showed a stronger positivity in the impossible grasping condition peaking on the electrode contralateral to the hand about to perform the action, F7 for the right hand and F8 for the left hand (respectively, P = 0.016 and P = 0.019 corrected for multiple comparisons).
To compare between observation and execution conditions, waveforms from a real execution condition are reported in Figure 1 from Bozzacchi et al. (2012a). This MRCP for actual grasp and impossible grasp actions (Execution, thin dashed lines) has been aligned to ERP from present experiment1 (Observation, continuous thick lines) so that the time zero represents the onset of both the observed and executed action. Comparing these waveforms, it is possible to note that the grasp action shows the presence of parietal activity and a larger frontocentral activity whereas only the impossible grasp shows the presence of prefrontal activity. These patterns of activity were similar for both action execution and observation.
DISCUSSION
The majority of literature on observation of object-oriented movement has focused on ongoing actions. In contrast, the present study investigated the activity preceding the observation of an action and extended the few previous studies on this topic (Kilner et al., 2004; Fontana et al., 2012) considering the brain activity in a broader time range (up to 3.5 s before movement observation) and comparing two different actions. For the first time, we could show that the brain anticipates a predictable action producing a complex premovement-like activity specific for that action.
The first question we posed concerned with whether the readiness potential activity before movement observation would only involve the anterior motor areas (Kilner et al., 2004) or, rather, whether it would be more representative of the real motor preparation for grasping actions that we know to involve different regions (Bozzacchi et al., 2012a; Fontana et al., 2012). Results showed an initial involvement of posterior parietal and prefrontal areas already from 3 s before the initiation of the observed action preceding the activity in premotor areas. This early activity in the posterior parietal cortex is a novel finding, consistent with recent studies on motor preparation for grasping execution (Wheaton et al., 2005; Bozzacchi et al., 2012a), and endorses what previously suggested by Fontana et al. (2012) from patients data.
Second, and more relevant, this premovement activity recorded was different for the two actions presented. In fact, although a readiness potential activity was observed in both conditions tested, (i) only the grasp condition (in the earlier phase) triggered an activity in the parietal structures whereas (ii) only for the impossible grasp condition we detected a prefrontal positive activity. Therefore, we conclude that this readiness potential activity does not represent a general arousal for the observation of upcoming actions but it is specific for the action attended, probably because of their different meaning (Bozzacchi et al., 2012a).
The main difference found between preparing to observe and preparing to move (Bozzacchi et al., 2012a) was a stronger signal for the latter task. Such a difference was particularly evident on motor and prefrontal areas (Figure 1). However, weaker brain activity in motor areas for observed actions is expected because no action is actually performed (Kilner et al., 2004; Fontana et al., 2012; Bozzacchi et al., 2012b). On the contrary, the parietal activity preceding observation or execution of the grasp action was similar in the two experiments. In a recent study (Bozzacchi et al., 2012b), we investigated the motor preparation related to two actions, which were different in terms of kinematics (a key press vs grasping) but aimed at the same goal (i.e. grasping a cup, virtually in former case and directly in the latter case). Although kinematically different, the two actions were preceded by the same parietal activity, likely localized in the anterior intraparietal area (AIP) specialized for grasping actions (e.g. Culham and Valyear, 2006). The parietal activity found in the present study likely corresponds to the same activity reported in the previous papers (Bozzacchi et al., 2012a, b), and was likely generated by the same source. Differently from what previously suggested (van Schie and Bekkering, 2007; Ondobaka et al., 2014), we proposed that the AIP involvement in the grasping early preparatory phase was related to the representation of the meaning of the action, regardless of the specific movement performed for its accomplishment. The present data support the view that at this stage of the actions, even when the subject has only to observe the action, the parietal activity is involved in coding and representing the outcome and the intention of an action, unrelated to mechanical features and constraints (Rice et al., 2006; Grafton and Hamilton, 2007; Tunik et al., 2007, 2008; Bozzacchi et al., 2012b), and forward this information to the motor areas where the action is then programmed.
This finding reinforces the -aforementioned interpretation of the activity as triggered by a top-down, hierarchically high, cognitive process, rather than a bottom-up activation, also because of its occurrence well ahead the action initiation.
As noted above, an overlap of premovement activities between observing and executing was evident also for the impossible grasp condition. Here we found a lack of parietal involvement but the presence of a prefrontal positivity corresponding to the one already described (Bozzacchi et al., 2012b). We linked this activity to the awareness of being unable to accomplish the action or processing strategies for resolving the ‘impossible’ task, or alternatively, to an inhibition to performing the action accurately (Bozzacchi et al., 2012a). The prefrontal cortex is associated with executive functions, predictions of outcomes and expectation based on actions. Its activity is also involved in abstract planning and inhibitory functions (Teffer and Semendeferi, 2012). Recently, studies have also linked the activity of this cortical structure to the perception of effort during the preparation of limb contraction. Berchicci and colleagues (2013) described a prefrontal positivity very similar to the one reported here, in terms of latency and localization, for those subjects who were reporting a high perception of effort in task performance. A similar effort perception was also reported by the subjects dealing with a complex motor behavior (impossible grasping) in our previous study, where we observed this prefrontal positivity for the first time (Bozzacchi et al., 2012a). The presence of this activity also in the present study, anticipating a pure action observation when no movement was requested and performed, supports the hypothesis that prefrontal positivity is involved in the awareness process about the action feasibility rather than an inhibitory one.
These results suggest that the activity preceding the observation of an action is actually index of anticipation for that specific action. That is, this activity actually specifies for the motor representations of what we have to either perform or observe. Mirroring activity detected during action observation was interpreted as a top-down mechanism in which the prediction of the goal is mapped onto the observer’s motor control system propagating downward to the motor code (Wolpert et al., 2003). In our study, participants were exposed to the video of the actions repeatedly and it made the following sensory experience predictable, as a sort of feedback. Moreover, because of the hands tied up in the impossible condition, such expectation was even enhanced. In our opinion, present findings support the top-down hypothesis, as well as a more integrative view of action understanding and perception. By showing a correspondence between observing and performing also before action observation (predictive mechanism), present results reinforce and extend the mirroring concept, accounting for the human ability to anticipate upcoming actions taking into consideration the particular circumstances (i.e. physical constraints) (Cibra, 2007). This premovement activity appears as modulated by the semantic knowledge of the action (van Elk et al., 2008) and a prior prediction of the goal (Kilner et al., 2004, 2007), rather than a direct mapping between the goal and the kinematics of the action (Rizzolatti and Craighero, 2004; Uithol et al., 2011; Cattaneo et al., 2013). This prediction ability seems to be essential in understanding others and interacting with them (Kilner et al., 2004); it minimizes surprise (Friston et al., 2006), thus shortening reaction time and enhancing accuracy of reaction to someone else’s actions. However, this predictability can be related and caused by different factors, such as the contest, environmental constraints, conceptual knowledge and previous associations between objects and actions and also personal expectancies (Frith and Frith, 2006; Ondobaka et al., 2014). It would be useful to disentangle between these multiple aspects, and future studies on this motor preparation phase preceding observation of upcoming action are needed to shed light on the specific role played by the motor system during this important social skill. In particular, brain activity before observation of less predictable actions than those studied here, and before actions presented from a third-person perspective should be studied. Others’ action prediction could be more supported by allocentric perspective, and experiments with allocentric point of view should be addressed in future.
In conclusion, we showed a tight correspondence between preparing to act and preparing to see in both timing and cortical localization. This activity likely supports a skill that has important social implications, facilitating interactions and reciprocal understanding. In particular, we showed that this activity is action specific, being different for two different upcoming actions. These results provide new insights and challenges in social and cognitive neurosciences for a theory of action prediction and anticipation.
Conflict of Interest
None declared.
Acknowledgments
Financial support: The work was funded by MIUR PRIN 2010-11 grant to FDR, research grant number 2010ENPRYE_002.
Footnotes
1 Participants of present experiment were age- and gender-matched to those of Bozzacchi et al. (2012a).
REFERENCES
- Berchicci M, Menotti F, Macaluso A, Di Russo F. The neurophysiology of contral and peripheral fatigue during sub-maximal lower limb isometric contractions. Frontiers in human neuroscience. 2013;7:135. doi: 10.3389/fnhum.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzacchi C, Giusti MA, Pitzalis S, Spinelli D, Di Russo F. Awareness affects motor planning for goal-oriented actions. Biological Psychology. 2012a;89(2):502–14. doi: 10.1016/j.biopsycho.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Bozzacchi C, Giusti MA, Pitzalis S, Spinelli D, Di Russo F. Similar cerebral motor plans for real and virtual actions. Plos ONE. 2012b;7(10):e47783. doi: 10.1371/journal.pone.0047783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L, Maule F, Barchiesi G, Rizzolatti G. The motor system resonates to the distal goal of observed actions: testing the inverse pliers paradigm in an ecological setting. Experimental Brain Research. 2013;231(1):37–49. doi: 10.1007/s00221-013-3664-4. [DOI] [PubMed] [Google Scholar]
- Csibra G. Sensorimotor Foundation of Higher Cognition: Attention and Performance. 2007. Action mirroring and action understanding: an alternative account. XXII, 435–80. [Google Scholar]
- Csibra G, Gergely G. ‘Obsessed with goals’: functions of mechanisms of teleological interpretation of actions in humans. Acta Psychologica. 2007;124:60–78. doi: 10.1016/j.actpsy.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Cuijpers RH, Schie HTV, Koppen M, Erlhagen W, Bekkering H. Goals and means in action observation: a computational approach. Neural Networks. 2006;19(3):311–22. doi: 10.1016/j.neunet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Current Opinion in Neurobiology. 2006;16:205–12. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Deecke L, Scheid P, Kornhuber HH. Distribution of readiness potential, pre-motion positivity, and motor potential of the human cerebral cortex preceding voluntary finger movements. Experimental Brain Research. 1969;7(2):158–68. doi: 10.1007/BF00235441. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends in Cognitive Science. 2000;4(11):423–31. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Fontana AP, Kilner JM, Rodrigues EC, et al. NeuroImage role of the parietal cortex in predicting incoming actions. Neuroimage. 2012;59:556–64. doi: 10.1016/j.neuroimage.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Frith C, Frith U. The physiological basis of theory of mind: functional neuroimaging studies. Understanding Other Minds: Perspectives from Developmental Cognitive Neuroscience. 2000:334–56. [Google Scholar]
- Frith CD, Frith U. How we predict what other people are going to do. Brain Research. 2006;1079:36–46. doi: 10.1016/j.brainres.2005.12.126. [DOI] [PubMed] [Google Scholar]
- Friston K, Kilner J, Harrison L. A free energy principle for the brain. Journal of Physiology (Paris) 2006;100(1–3):70–87. doi: 10.1016/j.jphysparis.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hamilton AFDC. Evidence for a distributed hierarchy of action representation in the brain. Human Movement Science. 2007;26(4):590–616. doi: 10.1016/j.humov.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AFDC, Grafton ST. Goal representation in human anterior intraparietal sulcus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(4):1133–7. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AFDC, Grafton ST. The motor hierarchy: form kinematics to goals and intentions. In: Haggard P, Rossetti Y, Kawato M, editors. Attention and Performance. 2007. XXII, 560–616. [Google Scholar]
- Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. Journal of Cognitive Neuroscience. 2009;21(7):1229–43. doi: 10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet PO, Avenanti A. Perturbing the action observation network during perception and categorization of actions' goals and grips: state-dependency and virtual lesion TMS effects. Cerebral Cortex. 2015;25(3):598–608. doi: 10.1093/cercor/bht242. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The representing brain: neural correlates of motor intention and imagery. Behavioral and Brain Sciences. 1994;17(02):187–202. [Google Scholar]
- Kilner JM, Vargas C, Duval S, Blakemore S, Sirigu A. Motor activation prior to observation of a predicted movement. Nature Neuroscience. 2004;7(12):1299–301. doi: 10.1038/nn1355. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD. Predictive coding: an account of the mirror neuron system. Cognitive Processing. 2007;8(3):159–66. doi: 10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM. More than one pathway to action understanding. Trends in Cognitive Neuroscience. 2011;15(8):352–7. doi: 10.1016/j.tics.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in human during execution and observation of actions. Current Biology. 2010;20(8):750–6. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness. The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ondobaka S, de Lange FP, Wottmann M, Frith CD, Bekkering H. Interplay between conceptual expectations and movement prediction underlies action understanding. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu056. doi:10.1093/cercor/bhu056. [DOI] [PubMed] [Google Scholar]
- Rice NJ, Tunik E, Grafton ST. The anterior intraparietal sulcus mediates grasp execution, independent of requirement to update: new insights from transcranial magnetic stimulation. The Journal of Neuroscience. 2006;26(31):8176–82. doi: 10.1523/JNEUROSCI.1641-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Sartori L, Becchio C, Castiello U. Cues to intention: the role of movement information. Cognition. 2011;119:242–52. doi: 10.1016/j.cognition.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Zohary E. Watching other’s actions: mirror representations in the parietal cortex. Nuroscientist. 2007;13(6):667–72. doi: 10.1177/1073858407302457. [DOI] [PubMed] [Google Scholar]
- Teffer K, Semendeferi K. Human prefrontal cortex: evolution, development, and pathology. Progress in Brain Research. 2012;195:191–218. doi: 10.1016/B978-0-444-53860-4.00009-X. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Rumiati RI. Frontiers in Human Neuroscience. 2013. Introducing the special topic: ‘When and why of sensorimotor processes in conceptual knowledge and abstract concepts’; pp. 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Rice NJ, Hamilton A, Grafton ST. Beyond grasping: representation of actions in human anterior intraparietal sulcus. Neuroimage. 2007;36:T77–86. doi: 10.1016/j.neuroimage.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Lo OY, Adamovich SV. Transcranial magnetic stimulation to the frontal operculum and supramarginal gyrus disrupts planning of outcome-based hand–object interactions. The Journal of Neuroscience. 2008;28(53):14422–7. doi: 10.1523/JNEUROSCI.4734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uithol S, van Rooij I, Bekkering H, Haselager P. Understanding motor resonance. Social Neuroscience. 2011;6(4):388–397. doi: 10.1080/17470919.2011.559129. [DOI] [PubMed] [Google Scholar]
- van Elk M, van Schie HT, Bekkering H. Conceptual knowledge for understanding other’s actions is organized primarily around action goals. Experimental Brain Research. 2008;189(1):99–107. doi: 10.1007/s00221-008-1408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M, van Schie HT, van den Heuvel R, Bekkering H. Semantics in the motor system: motor-cortical Beta oscillations reflect semantic knowledge of end-postures. Frontiers in Human Neuroscience. 2010;4:8. doi: 10.3389/neuro.09.008.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schie TH, Bekkering H. Neural mechanisms underlying immediate and final action goals in object use reflected by slow wave brain potentials. Brain Research. 2007;1148:183–97. doi: 10.1016/j.brainres.2007.02.085. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Shibasaki H, Hallett M. Temporal activation pattern of parietal and premotor areas during preparation and execution of praxic hand movements. Clinical Neurophysiology. 2005;116:1201–12. doi: 10.1016/j.clinph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science-AAAS-Weekly Paper Edition. 1995;269(5232):1880–2. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Kawato M. Multiple paired forward and inverse models for motor control. Neural Networks. 1998;11(7):1317–29. doi: 10.1016/s0893-6080(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Doya K, Kawato M. A unifying computational framework for motor control and social interaction. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2003;358(1431):593–602. doi: 10.1098/rstb.2002.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]