Abstract
A predominant expectation that social relationships with others are safe (a secure attachment style), has been linked with reduced threat-related amygdala activation. Experimental priming of mental representations of attachment security can modulate neural responding, but the effects of attachment-security priming on threat-related amygdala activation remains untested. Using functional magnetic resonance imaging, the present study examined the effects of trait and primed attachment security on amygdala reactivity to threatening stimuli in an emotional faces and a linguistic dot-probe task in 42 healthy participants. Trait attachment anxiety and attachment avoidance were positively correlated with amygdala activation to threatening faces in the control group, but not in the attachment primed group. Furthermore, participants who received attachment-security priming showed attenuated amygdala activation in both the emotional faces and dot-probe tasks. The current findings demonstrate that variation in state and trait attachment security modulates amygdala reactivity to threat. These findings support the potential use of attachment security-boosting methods as interventions and suggest a neural mechanism for the protective effect of social bonds in anxiety disorders.
Keywords: attachment, priming, fear, amygdala, fMRI, emotion
INTRODUCTION
The emotional bond that connects one person to another across time and space is called attachment (Bowlby, 1982) and an attachment figure is a person with whom we form such a bond. In infancy these are often our parents, whilst during adulthood these can be friends or partners. Attachment security is regarded as being vital for the development of stress resilience (Bowlby, 1982; Wyman et al., 1999).
Individuals who experience consistently sensitive and appropriate responses from their early attachment figures form a secure attachment style, which is built upon positive internal working models about themselves as lovable and effective and about others as available and responsive. Alternatively, individuals who experience insensitive or inconsistent responses from their attachment figure develop a negative internal working model in which they feel isolated and uncared for, and where help from attachment figures is unavailable or unreliable (Mikulincer and Shaver, 2004, 2007a). This forms the basis of an insecure attachment style, which can take the form of attachment anxiety or attachment avoidance (Fraley et al., 2006; Mikulincer and Shaver, 2007a). Attachment anxiety is predicted by the receipt of unreliable or unpredictable attachment caregiving, whereas experiences of rejection by attachment figures during times of need predict the development of an avoidant attachment style (Mikulincer and Shaver, 2007a; Simpson and Winterheld, 2012). Individuals high in attachment avoidance dismiss the importance of attachment bonds, whilst anxiously attached individuals are hypervigilant for signs of social rejection, and readily admit their longing for improved attachment relationships (Mikulincer and Shaver, 2004, 2007a). Both insecure styles are associated with reduced resilience (Wyman et al., 1999) and a higher propensity for mental health problems (Palitsky et al., 2013). However, the mechanisms by which secure attachment confers its protective effect on mental health are not yet fully understood. Although the overall stability of internal working models is such that attachment security can be considered a trait-level individual difference (Fraley, 2002; Mikulincer and Shaver, 2004, 2007a), perceptions of attachment resources can change on the basis of environmental signifiers of social support (Mikulincer and Shaver, 2007a,b).
Using numerous methods, it has been demonstrated that exposure to reminders of secure attachment (attachment-security priming) can temporarily increase accessibility to secure attachment representations, and has numerous resilience boosting effects including increased selfesteem, prosocial feelings and behaviours, positive affect and increased exploratory behaviour (Mikulincer and Shaver, 2001; Mikulincer et al., 2001a,b; Carnelley and Rowe, 2007; Mikulincer and Shaver, 2007a,b; Gillath et al., 2008; Canterberry and Gillath, 2013).
According to social baseline theory, a positive expectation of the availability of attachment figures leads to reduced activity in neural regions associated with threat appraisal, as potential threats are appraised in the context of a feeling of strength in numbers and a sense of the availability of an attachment figure from whom support can be expected (Coan, 2008, 2010). In support of this, functional magnetic resonance imaging (fMRI) studies of physical and social pain have found that providing participants with attachment-related stimuli reduces threat-related neural activation in the anterior cingulate and hypothalamus (Eisenberger et al., 2011; Karremans et al., 2011).
The amygdala consistently responds to threatening stimuli and, in the face of ambiguous stimuli, amygdala activation is associated with subjective appraisals of threat (Kim et al., 2003; Costafreda et al., 2008; Hariri and Whalen, 2011). Moreover, the degree of amygdala activation to threat predicts fear and stress-related physiological reactivity, and is associated with anxiety-related traits (Hariri, 2009; McEwen and Gianaros, 2010). Therefore, it could be argued that the amygdala is the key biomarker for threat-related neural activation (Hariri and Whalen, 2011), and that an investigation using validated amygdala provoking stimuli is an essential test of the notion that manipulating attachment security alters threat perception at the neural level. Previous neuroimaging studies of attachment priming have used tasks which do not typically evoke amygdala activation, and therefore these studies have not directly addressed this issue (Eisenberger et al., 2011; Karremans et al., 2011).
An attenuated amygdala response to social threat has been reported in individuals with secure attachment styles compared with relatively insecure individuals (Lemche et al., 2005; Buchheim et al., 2006; Vrtička et al., 2008, 2012). Given the putative role of the amygdala in the onset and maintenance of emotional disorders (Etkin and Wager, 2007; Shin and Liberzon, 2010; Hamilton et al., 2012), these findings suggest that regulation of amygdala reactivity may be one plausible neurobiological mechanism by which secure attachment confers resilience (Nolte et al., 2011). However, to date, no studies have investigated whether external attachment cues can attenuate amygdala responsivity to threat. Existing data on the association between attachment security and amygdala reactivity is correlational, and the nature of this relationship can only be assessed through the use of studies which aim to manipulate one or other of these variables.
In addition, normalisation of amygdala activation is a proposed mechanism by which psychopharmacological and psychotherapeutic methods produce symptom change (Furmark et al., 2002; Harmer et al., 2006; Murphy et al., 2009). Consequently, if the provision of external attachment-related cues (attachment-security priming) reduces threat-related amygdala reactivity, this would provide initial neuroimaging evidence in support of the potential for attachment-priming based interventions to be used in the treatment of disorders of mood and anxiety.
Therefore, the primary aim of this study was to investigate whether attachment-security priming would decrease threat-related neural activation in healthy participants, particularly in the amygdala. On the basis of prior research (Lemche et al., 2005; Buchheim et al., 2006; Vrtička et al., 2008, 2012), we also predicted that amygdala activation in two threat-reactivity tasks would correlate positively with trait attachment insecurity.
MATERIALS AND METHODS
Participants
Forty-two right-handed University of Exeter students (13 males) took part in this study in exchange for £10 reimbursement. Participants who had a history of neurological injury or psychiatric illness, or who were taking psychotropic medication, were excluded from the study. All participants met the Exeter MR Research Centre safety criteria. Ethical approval was granted by the University of Exeter School of Psychology Ethics Committee. Written informed consent was acquired prior to participation.
Materials and procedure
During the week preceding their scanning session, participants completed the State Trait Anxiety Inventory (STAI-Y) trait subscale (Spielberger et al., 1983) and the Relationships Structures questionnaire (ECR-RS) measure of attachment dimensions (Fraley et al., 2006).
During the scanning session, participants also completed Self-Assessment-Manikin (SAM) (Bradley and Lang, 1994) scales of state pleasure, arousal and dominance along. In addition, as a measure of state attachment security, anxiety and avoidance we choose the highest loading items from the state adult attachment measure (Gillath et al., 2009). This items used were ‘The idea of being emotionally close to someone makes me nervous’, ‘I need to feel loved’ and ‘I feel loved and safe’, and these were assessed via five-point Likert scales (1 = strongly disagree, 5 = strongly agree). Time one measurements were completed shortly before entering the scanner. Time two measurements were completed following the initial priming task. These measurements were included as manipulation checks.
Attachment-security priming task
We pseudorandomly allocated participants into two groups (attachment-security priming vs control group), whilst matching between groups for levels of trait anxiety. The attachment-security priming condition utilised 48 pictures depicting people engaging in caregiving behaviours and enjoying close attachment relationships (e.g. hugging loved ones). Seventeen of these photographs were selected from the International Affective Picture System (IAPS) (Lang et al., 2008), with the remainder purchased from private sources. The control condition used 48 pictures of household objects, 29 of which came from the IAPS library. In a small pilot study, our attachment-security priming images were rated as making people feel more loved, safe, calm and comforted than did the control images.1
Although participants lay in the scanner, six primes per block were presented to the left or right of the centre of the screen one at a time for 2.5 s with an interstimulus interval (ISI) of 0.5 s. Participants had to press a button to indicate the position of the image. This task therefore used an implicit attachment-security priming procedure. We use the term implicit priming as it is used elsewhere in the social neuroscience literature (Pichon et al., 2012; Powers and Heatherton, 2013) to refer to priming via incidental exposure within the context of a behavioural task, and to distinguish our attachment priming method from methods which involve the conscious recall of attachment experiences (Bartz and Lydon, 2004). For insecurely attached individuals, incidental exposure primes attachment security with greater success than does the explicit recall of attachment related experiences (Mikulincer et al., 2011). The six priming blocks were separated by 10 s rest periods during which participants were instructed to fixate on a cross presented in the centre of the screen.
In between the two threat-reactivity tasks (see below), participants completed an additional two blocks of their respective priming condition to refresh access to attachment representations, which may have weakened during the first threat-reactivity task.
First threat-reactivity task: dot probe
Following the priming and the completion of the ratings scales, participants completed the dot-probe paradigm (MacLeod et al., 1986) to evoke threat-related neural activation. Each trial began with the presentation of a word pair that remained onscreen for 500 ms (Lanteaume et al., 2009; El Khoury-Malhame et al., 2011a). One word from each pair was presented above the midpoint of the screen and the other below this midpoint. Sixteenthreat-neutral word pairings and 16 neutral-neutral word pairings were presented. All words were selected from the Affective Norms for English Words (Bradley and Lang, 1999) and each word pair was matched for word frequency and length (Kučera and Francis, 1967). Following the offset of each word pair, an asterisk probe replaced one of the two words for up to 2 s. The participants’ task was to indicate which of the words had been replaced by the probe as quickly and as accurately as possible. In total, 128 trials were completed. In 32 threat-congruent trials the probe replaced the threat word in a threat-neutral pair, whilst in 32 incongruent trials the neutral word was replaced by the probe. In addition, there were 64 neutral trials where the probe replaced one of the words in a neutral–neutral pairing. The intertrial interval (a black screen) randomly varied between 2 and 4 s, to optimise jittering in this rapid event-related design.
Second threat-reactivity task: emotional faces
Threat-related neural activation was also assessed using an emotional faces task, which is a validated probe of amygdala activation (Brown et al., 2005; Neumann et al., 2006; Fakra et al., 2009; Gianaros et al., 2009; Hariri et al., 2009; Cornelius et al., 2010; Manuck et al., 2010; El Khoury-Malhame et al., 2011b; Hyde et al., 2011; Carré et al., 2012). In the face-matching condition, participants were presented with sets of three faces. Their task was to match one of two faces (bottom of the screen) with the target face (top of the screen). Matching was performed according to a shared facial expression (fearful or angry). Sixty fearful and angry face images were used from the NimStim stimulus set (Tottenham et al., 2009). Each trio of faces was presented for 4 s, with an ISI of 0.5 s. Six blocks of the face-matching condition were completed, with each block containing 10 trials. Interleaved with these face-matching trials were six blocks of a sensorimotor control condition in which participants matched one of two shapes (ellipses and circles) with a target shape according to spatial orientation. Trial and block length were identical in both conditions. A 2-s interval between blocks presented the instructions ‘match faces!’ or ‘match shapes!’. Each block lasted 45.5 s, and the total task length was 9 min 24 s.
fMRI data acquisition
Scanning was conducted at the Exeter MR Research Centre using a 1.5-T Philips scanner fitted with an eight-channel SENSE head coil. For all tasks a T2*-weighted echoplanar imaging sequence was used. Acquisition parameters for the emotional faces task were: TR = 3000 ms, TE = 45 ms, 190 volumes, Voxel size = 3 × 3 × 3 mm3, Number of Slices = 39, FOV = 240 mm, flip angle = 90°. For the dot-probe task, acquisition parameters were: TR = 2400 ms, TE = 45 ms, 325 volumes, Voxel size = 3 × 3 × 3 mm3, Number of Slices = 39, FOV = 240 mm, flip angle = 90°. A shorter TR was used for the dot-probe task because of its rapid event-related design. For each participant, functional data were overlaid on a high-resolution T1-weighted anatomical image for registration into standard space and functional localisation (3D T1 FFE, TR = 25 ms, TE = 4.5 ms, Voxel size = 0.9 × 0.9 × 1.6 mm3, Number of Slices = 160, FOV = 230 mm, Flip angle = 30°).
Stimuli were projected on to a screen placed at the foot end of the scanner and viewed through a mirror attached to the head coil. Responses were made using index and middle fingers via a two-button fibre-optic response box placed in the right hand of participants. E-Prime 1.1 (Psychology Software Tools Inc.; www.pstnet.com/eprime) was used to control stimulus presentation and record responses.
Data analysis
Behavioural data preparation and analysis
In order to assess whether the priming tasks were associated with changes in emotion and attachment security, each state attachment item and item on the SAM was subjected to separate 2 × 2 mixed-design ANOVAs in which time (pre- vs post-priming) was the within-subjects variable and priming group (attachment versus control) was the between-subjects factor. For the dot-probe task, incorrect trials and trials where reaction times were >800 ms or <200 ms were removed (Monk et al., 2006; Telzer et al., 2008). A 2 × 2 mixed-design ANOVA was performed with trial type (threatcongruent vs threatincongruent) as the within-subjects variable and group (attachment-security priming vs control) as the between-subjects variable.
fMRI data preparation and analysis
fMRI data pre-processing and statistical analysis were carried out using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB’s Software Library). For each individual subject, standard pre-processing steps were performed. These were: motion correction (Jenkinson et al., 2002); removal of non-brain tissue (Smith, 2002); spatial smoothing (using a Gaussian kernel of FWHM 5 mm); normalisation based on grand-mean intensity; and highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, sigma = 100.0 s).
Registration of subjects’ functional data to high-resolution T1 structural images and subsequently to standard Montreal Neurological Institute space was achieved using FLIRT (Jenkinson and Smith, 2001; Jenkinson et al., 2002).
First level single-subject analyses were performed using a general linear model with local autocorrelation correction (Woolrich et al., 2001). For the face-matching task, the onset of the emotional faces condition was modelled as a box-car regressor convolved with a canonical haemodynamic response function, with the shape-matching condition modelled implicitly as a baseline.
In analysing the dot-probe task, we ran a contrast of neutral words>(blank screen) baseline, threat>baseline and threat>neutral at the single subject level. Threat trials included all trials where a threat word was presented. Excluded trials for this task were modelled as a subsequently ignored ‘nuisance’ variable. Participants showed equivalent amygdala activation to both threat and neutral trials, and therefore we focused our analyses on each trial type separately versus the baseline.
For the higher level analyses, we divided the participants into two groups according to the type of priming received. For both tasks, higher-level between-group analyses were carried out using the mixed-effects model FLAME 1 (Beckmann et al., 2003; Woolrich et al., 2004). FSL’s automatic outlier detection algorithm was used on higher level contrasts (Woolrich, 2008). Corrections for multiple comparisons were conducted at the cluster level using Gaussian Random field theory (z > 2.3, P < 0.05, corrected) (Worsley, 2001).
Region of interest analysis
Due to our a priori hypotheses regarding activation in the amygdala, we conducted planned analyses using anatomically defined regions-of-interests (ROIs). Hemisphere-specific ROIs of the ventral and dorsal amygdala, based upon those used in previous analyses of the emotional faces (Gianaros et al., 2009; Manuck et al., 2010; Hyde et al., 2011; Carré et al., 2012), were created using WFU-Pickatlas (http://www.fmri.wfubmc.edu/download.htm). Four distinct dorsal and ventral ROIs were used due to the functional heterogeneity of subnuclei within the amygdala, and to maintain continuity with previous studies which used the emotional faces task (Gianaros et al., 2009; Manuck et al., 2010; Hyde et al., 2011; Carré et al., 2012).
For the emotional faces task, mean % BOLD signal change from each of the ROIs was extracted from each participant’s lower level contrast map (faces>shapes) using the Featquery tool in FSL.
For the dot-probe task, mean % BOLD signal change for each of the ROIs was extracted from each participant’s lower-level maps for the contrasts threat>baseline and neutral>baseline. This allowed between group and correlational analyses with trait measures to be conducted in SPSS for Windows (version 17.0; SPSS Statistics Inc., Chicago, IL).
Moderation analyses
In order to test whether trait attachment anxiety and trait attachment avoidance moderated the effect of attachment-security priming on amygdala activation in both amygdala-reactivity tasks, we performed moderated regression analyses using modprobe (Hayes and Matthes, 2009). In these analyses, the independent variable was the type of priming received (dummy coded), and the dependent variables were BOLD signal changes in our amygdala ROIs in response to threat (emotional faces, and threat words). The moderator variables were scores on the state and trait anxiety, attachment anxiety and attachment avoidance measures.
RESULTS
Sample characteristics
Scores did not differ between groups for the STAI-S anxiety prior to priming [t(39) = 0.553, P = 0.597, 95% CI (−5.89, 3.43), d = −0.165], the STAI-T anxiety scale [t(40) = 0.325, P = 0.747, 95% CI (−8.60, 6.22), d = −0.100), attachment anxiety [t(38) = 0.312, P = 0.757, 95% CI (−0.436, −0.595), d = 0.009] or attachment avoidance [t(40) = 0.941, P = 0.352, 95% CI (−0.284, 0.778), d = −0.328) (see Table 1).
Table 1.
Demographic details for participants (s.d.s in parentheses)
| Attachment priming group | Neutral priming group | |
|---|---|---|
| Number of participants | 21 | 21 |
| Age | 20.42 (4.20) | 21.33 (1.81) |
| Sex | ||
| Male | 7 | 6 |
| Female | 14 | 15 |
| STAI state anxiety | 33.42 (7.26) | 32.20 (7.50) |
| STAI trait anxiety | 40.86 (13.34) | 39.67 (10.33) |
| ECR-RS Attachment anxiety | 1.97 (.85) | 2.05 (.76) |
| ECR-RS Attachment avoidance | 2.48 (.73) | 2.73 (.95) |
STAI, state trait anxiety inventory; ECR-RS, experience in close relationships—relationship structures.
Behavioural data were lost for one participant in the control group due to a technical fault, which prevented the analysis of the neural data for this participant in this task. Neural data for one participant in the attachment-security priming group during the dot-probe task were corrupted and unable to be analysed. One participant failed to fully complete the state segment of the STAI.
All questionnaire, behavioural and BOLD measures were checked for outliers defined as scores more than 3 s.d. from their group mean, and outlier values were removed accordingly. This resulted in the removal of two attachment anxiety scores. In the emotional faces task, one participant’s left and right ventral amygdala ROI values were removed. In the dot-probe task, two participants’ right dorsal amygdala responses to neutral word pairs and one participant’s left dorsal amygdala activation to neutral word pairs were removed. One participant showed more than 50% errors on the dot-probe so was excluded from analysis of this task.
The faster TR and reduced number of acquisition slices in the dot-probe task resulted in incomplete ventral amygdala coverage. Therefore, only the dorsal amygdala ROIs, which were completely covered, were analysed for the dot-probe task.
State attachment and self-reported valence, arousal and control
Significant main effects of time (pre- vs post-priming) were only observed for self-reported pleasure [F(1 40) = 32.76, P < 0.001, = 0.450] and arousal [F(1 40) = 40.48, P < 0.001, = 0.503). No other main effects or time by priming group interactions were significant (all P > 0.1). Contrary to our hypothesis, there were no significant interactions between time and priming group on self-reported attachment anxiety [F(1 40) = 0.462, P = 0.462, = 0.011], attachment avoidance [F(1 40) = 1.21, P = 0.277, = 0.029], or attachment security [F(1 40) = 0.473, P = 0.496, = 0.012] (see Table 2).
Table 2.
Mean changes in scores on the self-assessment manikin and the state attachment items taken before entering the scanner (pre-priming) and immediately post-priming
| Attachment priming group | Neutral priming group | Overall | |
|---|---|---|---|
| Self-Assessment Manikin | |||
| Pleasure | |||
| Time one | 1.81 (.87) | 2.10 (.77) | 1.95 (.82) |
| Time two | 3.00 (1.18) | 3.24 (.89) | 3.12 (1.04) |
| Arousal | |||
| Time one | 3.14 (1.11) | 3.52 (.93) | 3.33 (1.03) |
| Time two | 2.19 (.81) | 1.95 (.74) | 2.07 (.78) |
| Control | |||
| Time one | 3.29 (1.01) | 3.62 (.86) | 3.45 (.94) |
| Time two | 3.64 (.73) | 3.81 (.81) | 3.73 (.77) |
| Attachment items | |||
| Avoidant | |||
| Time one | 1.81 (.93) | 2.05 (1.12) | 1.93 (1.02) |
| Time two | 2.24 (1.34) | 2.24 (1.22) | 2.24 (1.27) |
| Anxious | |||
| Time one | 3.29 (1.01) | 3.14 (1.06) | 3.21 (1.02) |
| Time two | 3.29 (.90) | 3.00 (1.26) | 3.14 (1.09) |
| Secure | |||
| Time one | 4.14 (1.06) | 4.24 (.83) | 4.19 (.94) |
| Time two | 4.43 (.68) | 4.24 (.77) | 4.33 (.72) |
s.d.s in parentheses.
fMRI activation results: emotional faces
Group differences
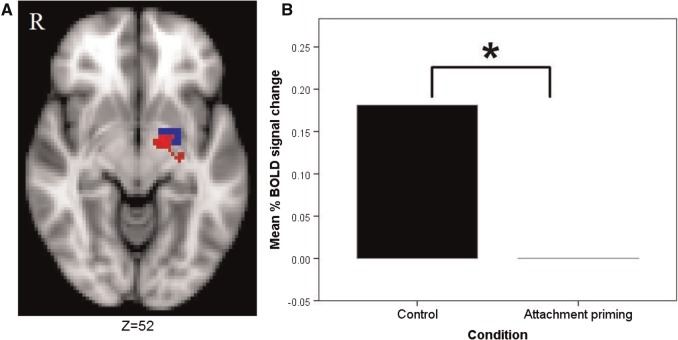
At the whole brain level, the control group showed greater activation to threatening faces (vs shapes) than the attachment primed group in a large cluster centred on the lentiform nucleus (Talairach-Tournoux Atlas coordinates x, y, z = −16, −6, −3, respectively), which contained portions of the left dorsal amygdala (see Figure 1A). The attachment-security priming group did not show any areas of increased activation compared with the control group. In addition, the control group showed significantly more activation within our left dorsal amygdala anatomical ROI [t(40) = 2.305, P = 0.026, 95% CI [0.022, 0.340], d = 0.711] (see Figure 1B). No significant effects were found for the left ventral amygdala [t(39) = 0.554, P = 0.583, 95% CI (−0.092, 0.163), d = 0.172], or the right dorsal amygdala [t(40) = 1.143, P = 0.260, 95% CI (−0.074, 0.268), d = 0.353] and right ventral amygdala [t(39) = 0.241, P = 0.811, 95% CI (−0.132, 0.168), d = 0.076].
Fig. 1.
Between-group differences in left dorsal amygdala activation in the emotional faces task. (A) Shows the cluster (red) in which the control group showed greater activation compared with the attachment primed group to emotional faces at the whole brain level, which overlaps with the left dorsal amygdala ROI (blue). Image thresholded at z-2.3, P < 0.05, corrected. (B) Shows the significant between-group difference in left dorsal amygdala activation to emotional faces.
Correlations with scales and moderation analyses
Left dorsal amygdala activation to faces correlated significantly with trait attachment anxiety [r(20) = 0.525, P = 0.017, 95% CI (0.107, 0.785)] and state anxiety [r(20) = 0.646, P = 0.002, 95% CI (0.285, 0.847)] in the control group, but not in the attachment-security priming group [attachment anxiety: r(20) = 0.318, P = 0.172, 95% CI (−0.145, 0.667); state anxiety: r(21) = −0.115, P = 0.620, 95% CI (−0.521, 0.333)]. Right dorsal amygdala activation was correlated with attachment anxiety in the controls [r(20) = 0.459, P = 0.042, 95% CI (0.021 0.749)] but not in the attachment-primed group [r(20) = −0.022, P = 0.924, 95% CI (−0.425 0.460)]. Similarly, attachment avoidance significantly correlated with right ventral amygdala activation in the control group [r(20) = 0.492, P = 0.028, 95% CI (0.063, 0.767)], but not in attachment-primed group [r(21) = −0.258, P = 0.259, 95% CI (−0.195, 0.621)]. Trait anxiety did not correlate with activation in any of our amygdala ROIs (all P > 0.1).
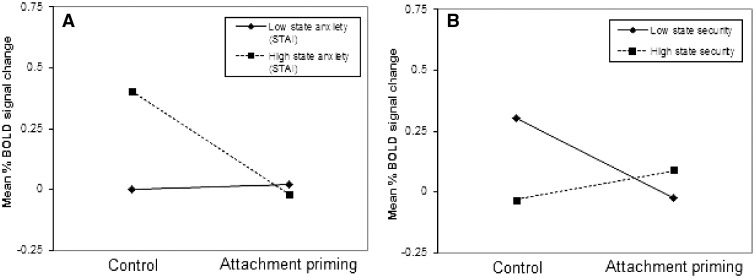
We examined whether trait anxiety and attachment dimensions moderated the association between priming effects and amygdala activation and found no significant effects. However, state anxiety prior to the priming moderated the effect of priming on left dorsal amygdala activity (t = −3.2, P = 0.028; ΔR2 = 0.166). High initial levels of state anxiety were associated with larger effects of attachment-security priming on reducing amygdala threat reactivity (β = −0.427; P < 0.001) than low levels of state anxiety (β = 0.020; P = 0.840) (Figure 2A). Moreover, state attachment security at time one (pre-scanning) significantly moderated the influence of attachment priming on amygdala reactivity to faces (t = −2.70, P = 0.010; ΔR2 = 0.15), with low initial levels of state attachment security associated with a larger effect of attachment priming on reducing right dorsal amygdala threat reactivity (β = −0.326; P = 0.008) relative to low levels of state attachment security (β = 0.121; P = 0.296) (Figure 2B).
Fig. 2.
State anxiety and state attachment security each interact with priming condition to predict amygdala threat reactivity. (A) Graph shows mean % BOLD signal change in the left dorsal amygdala in response to threatening faces (vs shapes) following neutral or attachment priming, in participants who have low or high levels of state anxiety (1 s.d. below or above the mean). (B) Graph shows mean % BOLD signal change in the right dorsal amygdala in response to threatening faces (vs shapes) following neutral or attachment priming (coded as a dummy variable), in participants who have low or high levels of state attachment security (1 s.d. below or above the mean).
Dot-probe behavioural data
As expected, participants showed an attentional bias towards threatening stimuli; i.e. there was a main effect for trial type [F(1 38) = 4.77, P = 0.035, = 0.112] with participants responding significantly more quickly to the threat-congruent trials (M = 425.32 ms, s.d. = 57.67) than to the incongruent trials (M = 432.14 ms, s.d. = 53.92). The group by trial type interaction failed to reach significance [F(1 38) = 3.58, P = 0.066, = 0.086) but interestingly participants in the attachment-security priming condition (M = −13.29, s.d. = 25.66) tended to show a larger attentional bias than control participants (M = −0.95, s.d. = 14.6).
fMRI activation results: dot probe
Group differences
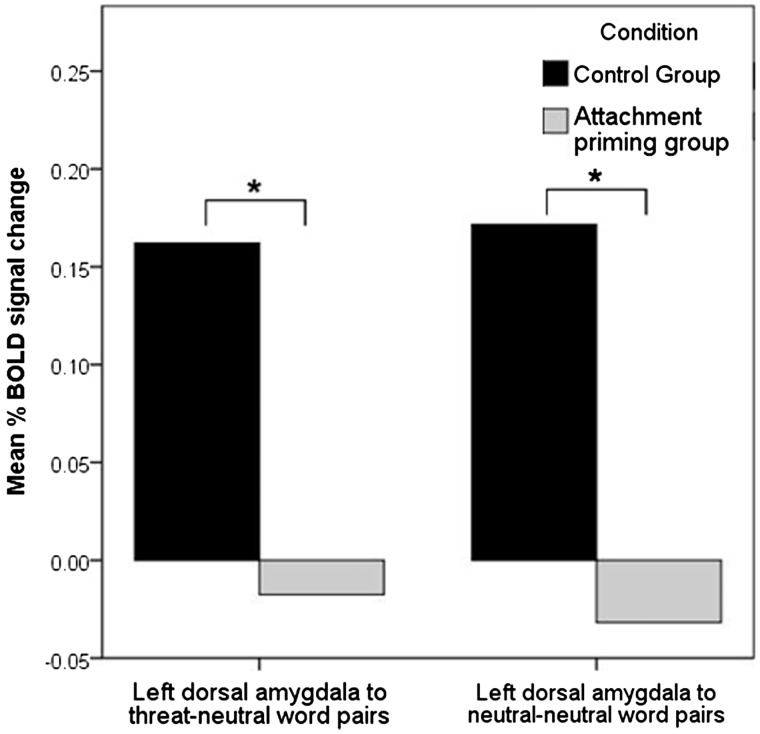
At the whole brain level, there were no between-group differences in activation to any contrast. Within our ROIs, an independent t-test revealed significant between-group differences (control > attachment primed group) in left dorsal amygdala ROI reactivity to both threat [t(37) = 2.47, P = 0.018, 95% CI (0.031, 0.313), d = 0.799] and neutral [t(36) = 2.60, P = 0.013, 95% CI (0.045, 0.362), d = 0.873] trials (see Figure 3). There were no significant differences found in the right dorsal amygdala for either the threat trials [t(37) = 1.28, P = 0.207, 95% CI (−0.050, 0.227), d = 0.419] or the neutral trials [t(35) = 0.644, P = 0.524, 95% CI (−0.076, 0.146), d = 0.214].
Fig. 3.
The attachment priming group show significantly less left dorsal amygdala activation in the dotprobe task. Graph shows the significant between-group differences in mean % BOLD signal change in the left dorsal amygdala in response to the threat and neutral trials in the dot-probe task.
Correlations with scales and moderation analysis
There were no positive correlations between amygdala activity during the dot-probe task and scores on any of the questionnaires (all P > 0.1), nor did we find any moderation effects of trait anxiety, attachment dimensions and state anxiety.
DISCUSSION
Our study extended previous research by investigating whether the provision of secure-attachment reminders can reduce threat-related neural activation. Supporting our hypothesis, we found that participants who viewed secure attachment-related stimuli prior to completing two threat-reactivity tasks showed attenuated amygdala responses to both threatening faces and threatening words. These findings add to previous attachment-security priming studies that have respectively reported attenuated limbic responses in the hypothalamus and anterior cingulate to social and physical pain following exposure to attachment reminders (Eisenberger et al., 2011; Karremans et al., 2011).
The current findings of reduced amygdala reactivity to threat following attachment-security priming are in line with recent theoretical accounts of attachment security, according to which reminders of secure attachment relationships act as safety cues which modulate threat appraisals and down-regulate neural responses to potential threats (Coan, 2008, 2010; Eisenberger et al., 2011). Decreased amygdala activation in the attachment-security priming group was observed in the absence of any areas of significantly greater activation group when compared with the control group. These findings therefore shed light on the mechanisms by which feelings of attachment security may regulate affective responding to signs of possible threat, and are consistent with the notion that attachment security regulates threat-reactivity through a bottom-up modulation of threat appraisal processes, rather than through top-down prefrontal mediated regulation (Coan, 2008, 2010).
Second, previous research exploring the therapeutic mechanisms of anxiolytic pharmacotherapies and psychotherapies has implicated amygdala desensitisation as an important therapeutic mechanism (Furmark et al., 2002; Harmer et al., 2006; Murphy et al., 2009). Therefore, our findings that attachment-security priming can modulate reactivity in this same structure raise the possibility that attachment-security priming methods may offer a novel therapeutic avenue for anxiety disorders.
In addition to an effect of attachment-security priming on amygdala reactivity, we replicated previous studies by finding a significant correlation between trait attachment insecurity and amygdala reactivity (Lemche et al., 2005; Buchheim et al., 2006; Vrtička et al., 2008, 2012). Given the hypothesised role of heightened amygdala responsivity in mediating anxious symptomatology and risk for the development of anxiety disorders (Etkin and Wager, 2007; Shin and Liberzon, 2010), these findings support the idea that increased risk for the development of anxiety disorders amongst insecurely attached individuals is partly mediated by increased threat reactivity in the amygdala. These findings are also broadly in line with previous findings of increased activation within neural threat systems in response to social threat in anxiously attached individuals (Gillath et al., 2005; DeWall et al., 2012), and are consistent with notion that anxiously attached individuals are more vigilant for signs of social threat (Mikulincer and Shaver, 2007a).
An unexpected finding was that, unlike in the emotional faces task, our measures of trait attachment security did not correlate with amygdala reactivity in the dot-probe task. Previously reported findings of threat-related amygdala hyperactivity in insecurely attached individuals have been to social threat stimuli (Lemche et al., 2005; Buchheim et al., 2006; Vrtička et al., 2008, 2012). This might indicate that attachment-security priming and trait attachment security have distinct modulatory effects, with trait security protecting against amygdala hyperactivity to socially relevant cues only, but attachment-security priming attenuating amygdala reactivity across multiple threat-relevant domains.
However, it should also be pointed out that the emotional faces used a block design with clearly delineated conditions (emotional faces vs shapes), whilst in the dot-probe task a rapid, intermixed, event-related design was used in which trials were temporally unpredictable, and the distinct trial types were not as automatically distinguishable. Our findings suggest that amygdala activation in the dot probe was not linked specifically to the detection of a threat-related stimulus, but may instead have occurred in response to the potential threat on each trial. Moreover, the two threat tasks differed not only in terms of the type of threat cues presented, but also in threat intensity, with threat-related photographs (emotional faces) considered to be more intense than threat-related words (Bradley et al., 1997). Therefore, one additional possibility is that attachment-security priming leads to a general gating of amygdala reactivity (both tasks), whereas trait-level attachment security specifically modulates amygdala responses to clearly delineated or highly threatening stimuli (emotional faces task only).
This study had some limitations. First, although it was important to test the mechanism first in healthy participants and although our findings are promising, they cannot yet be generalised. Attachment-security priming methods have not been tested in clinical samples, and it remains unclear whether they will be as effective in reducing amygdala reactivity in such populations. Importantly, clinical participants often report more severe attachment insecurities than do healthy controls (van IJzendoorn and Bakermans-Kranenburg, 1996; Mikulincer and Shaver, 2007a). A recent study (Rockliff et al., 2011) reported that activation of the attachment system by a combination of intranasal oxytocin and compassion-focused imagery was associated with heightened negative experience in individuals with high levels of attachment insecurity. Therefore, it remains a possibility that brief, single-session attempts at attachment-security priming may exacerbate attachment distress in patient groups. A replication of the study in a clinical sample is therefore warranted.
Second, we measured the effect of attachment-security priming on amygdala activation immediately following the end of the priming session. For attachment security boosting methods to have therapeutic potential, it must be established that they can modulate reactivity in threat circuitry over a longer time frame. Interestingly, previous studies have suggested that repeated attachment-security priming may lead to long term changes in attachment security (Carnelley and Rowe, 2007; Gillath et al., 2008). For instance, a study by Carnelly and Rowe (2007) found that repeating attachment-security priming over a period of three days led to an increase in attachment security which was detectable 2 days after the final priming session. Future research could use similar methods to determine whether repeated attachment-security priming might have a longer-term effect on amygdala activation to threat.
Despite these limitations, this study is the first to demonstrate that attachment-security priming can dampen amygdala reactivity to threat. Our findings inform our knowledge as to how reminders of our attachment figures help to alleviate distress in our day-to-day lives, and are supportive of existing theoretical accounts (Coan, 2008, 2010; Eisenberger et al., 2011). In order to determine whether attachment-security priming could be used as part of an intervention in clinical settings, future work should investigate whether repeated attachment-security priming can have longer term modulatory effects on limbic reactivity, as well as whether attachment-security priming can normalise amygdala reactivity in patient populations.
Conflict of Interest
None declared.
Acknowledgments
We thank all our participants for giving up their time for taking part in this study. The authors have no competing financial interests to declare regarding the current research project.
Footnotes
1 Thirteen University of Exeter undergraduate psychology students (3 male, 10 female) assessed the photographs on six-point Likert scales for the extent to which they made them feel loved, safe, happy, calm and comforted. Four participants rated the control images, and nine participants rated the attachment images. For the attachment stimuli, the mean ratings were loved = 4.39 (s.d. = 1.17), happy = 4.25 (s.d. = 1.01), safe = 4.63 (s.d. = 0.99), calm = 4.16 (s.d. = 0.95) and comforted = 4.29 (s.d. = 1.04). Lower ratings were provided for the control stimuli on the loved (M = 2.66, s.d. = 1.21), safe (M = 2.88, s.d. = 1.24), happy (M = 2.86, s.d. = 1.33), calm (M = 2.80, s.d. = 1.38) and comforted (M = 2.73, s.d. = 1.24) measures (all P < 0.001). Items were adapted from the felt security scale (Luke et al., 2012).
REFERENCES
- Bartz JA, Lydon JE. Close relationships and the working self-concept: implicit and explicit effects of priming attachment on agency and communion. Personality and Social Psychology Bulletin. 2004;30(11):1389–401. doi: 10.1177/0146167204264245. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and Loss. New York: Basic Books; 1982. [Google Scholar]
- Bradley BP, Mogg K, Millar N, et al. Attentional biases for emotional faces. Cognition and Emotion. 1997;11(1):25–42. [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Technical report C-1. 1999. Affective norms for English words (ANEW): instruction manual and affective ratings. The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Brown SM, Peet E, Manuck SB, et al. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol Psychiatry. 2005;10(9):805, 884–8. doi: 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Erk S, George C, et al. Measuring attachment representation in an fMRI environment: a pilot study. Psychopathology. 2006;39(3):144–52. doi: 10.1159/000091800. [DOI] [PubMed] [Google Scholar]
- Canterberry M, Gillath O. Neural evidence for a multifaceted model of attachment security. International Journal of Psychophysiology. 2013;88(3):232–40. doi: 10.1016/j.ijpsycho.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Carnelley KB, Rowe AC. Repeated priming of attachment security influences later views of self and relationships. Personal Relationships. 2007;14(2):307–20. [Google Scholar]
- Carré JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Social Cognitive and Affective Neuroscience. 2012;7(2):213–21. doi: 10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA. Toward a neuroscience of attachment. Handbook of Attachment: Theory, Research, and Clinical Applications. 2008;2:241–65. [Google Scholar]
- Coan JA. Adult attachment and the brain. Journal of Social and Personal Relationships. 2010;27(2):210–7. [Google Scholar]
- Cornelius JR, Aizenstein HJ, Hariri AR. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behaviour. 2010;35(6):644–6. doi: 10.1016/j.addbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Masten CL, Powell C, Combs D, Schurtz DR, Eisenberger NI. Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience. 2012;7(2):184–92. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Master SL, Inagaki TK, et al. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Sciences. 2011;108(28):11721–6. doi: 10.1073/pnas.1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury-Malhame M, Lanteaume L, Beetz EM, et al. Attentional bias in post-traumatic stress disorder diminishes after symptom amelioration. Behaviour Research and Therapy. 2011a;49(11):796–801. doi: 10.1016/j.brat.2011.08.006. [DOI] [PubMed] [Google Scholar]
- El Khoury-Malhame M, Reynaud E, Soriano A, et al. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011b;49(7):1969–73. doi: 10.1016/j.neuropsychologia.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164(10):1476. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakra E, Hyde LW, Gorka A, et al. Effects of HTR1A C (-1019) G on amygdala reactivity and trait anxiety. Archives of General Psychiatry. 2009;66(1):33. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley CR, Niedenthal PM, Marks M, Brumbaugh C, Vicary A. Adult attachment and the perception of emotional expressions: probing the hyperactivating strategies underlying anxious attachment. Journal of Personality. 2006;74(4):1163–90. doi: 10.1111/j.1467-6494.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Fraley RC. Attachment stability from infancy to adulthood: Meta-analysis and dynamic modeling of developmental mechanisms. Personality and Social Psychology Review. 2002;6(2):123–51. [Google Scholar]
- Furmark T, Tillfors M, Marteinsdottir I, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Archives of General Psychiatry. 2002;59(5):425. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biological Psychiatry. 2009;65(11):943–50. doi: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillath O, Bunge SA, Shaver PR, Wendelken C, Mikulincer M. Attachment-style differences in the ability to suppress negative thoughts: exploring the neural correlates. NeuroImage. 2005;28(4):835–47. doi: 10.1016/j.neuroimage.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Gillath O, Hart J, Noftle EE, Stockdale GD. Development and validation of a state adult attachment measure (SAAM) Journal of Research in Personality. 2009;43(3):362–73. [Google Scholar]
- Gillath O, Selcuk E, Shaver PR. Moving toward a secure attachment style: can repeated security priming help? Social and Personality Psychology Compass. 2008;2(4):1651–66. [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annual Review of Neuroscience. 2009;32:225. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, et al. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biological Psychiatry. 2009;66(1):9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Whalen PJ. The amygdala: inside and out. F1000 Biology Reports. 2011;3:2. doi: 10.3410/B3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biological Psychiatry. 2006;59(9):816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41(3):924–36. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hyde LW, Gorka A, Manuck SB, Hariri AR. Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia. 2011;49(4):651–6. doi: 10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Karremans JC, Heslenfeld DJ, van Dillen LF, Van Lange PAM. Secure attachment partners attenuate neural responses to social exclusion: an fMRI investigation. International Journal of Psychophysiology. 2011;81(1):44–50. doi: 10.1016/j.ijpsycho.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14(18):2317–22. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kučera H, Francis WN. Computational Analysis of Present-Day American English. Providence, RI: Brown Publishing; 1967. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical report A-8. 2008 [Google Scholar]
- Lanteaume L, Bartolomei F, Bastien-Toniazzo M. How do cognition, emotion, and epileptogenesis meet? A study of emotional cognitive bias in temporal lobe epilepsy. Epilepsy and Behavior. 2009;15(2):218–24. doi: 10.1016/j.yebeh.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Lemche E, Giampietro VP, Surguladze SA, et al. Human attachment security is mediated by the amygdala: Evidence from combined fMRI and psychophysiological measures. Human Brain Mapping. 2005;27(8):623–35. doi: 10.1002/hbm.20206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke MA, Sedikides C, Carnelley K. Your love lifts me higher! The energizing quality of secure relationships. Personality and Social Psychology Bulletin. 2012;38:721–33. doi: 10.1177/0146167211436117. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95(1):15. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Flory JD, Gorka A, Ferrell RE, Hariri AR. Salivary testosterone and a trinucleotide (CAG) length polymorphism in the androgen receptor gene predict amygdala reactivity in men. Psychoneuroendocrinology. 2010;35(1):94–104. doi: 10.1016/j.psyneuen.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186(1):190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulincer M, Gillath O, Halevy V, Avihou N, Avidan S, Eshkoli N. Attachment theory and rections to others’ needs: Evidence that activiation of the sense of attachment security promotes empathic responses. Journal of Personality and Social Psychology. 2001a;81(6):1205. [PubMed] [Google Scholar]
- Mikulincer M, Hirschberger G, Nachmias O, Gillath O. The affective component of the secure base schema: Affective priming with representations of attachment security. Journal of Personality and Social Psychology. 2001b;81(2):305. doi: 10.1037//0022-3514.81.2.305. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. Attachment theory and intergroup bias: evidence that priming the secure base schema attenuates negative reactions to out-groups. Journal of Personality and Social Psychology. 2001;81(1):97. [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. Security-based self-representations in adulthood. In: Rholes WS, Simpson JA, editors. Adult Attachment: Theory, Research, and Clinical Implications. New York: Guilford Press; 2004. [Google Scholar]
- Mikulincer M, Shaver PR. Attachment in Adulthood: Structure, Dynamics, and Change. New York: Guilford Press; 2007a. [Google Scholar]
- Mikulincer M, Shaver PR. Boosting attachment security to promote mental health, prosocial values, and inter-group tolerance. Psychological Inquiry. 2007b;18(3):139–56. [Google Scholar]
- Mikulincer M, Shaver PR, Rom E. The effects of implicit and explicit security priming on creative problem solving. Cognition and Emotion. 2011;25(3):519–31. doi: 10.1080/02699931.2010.540110. [DOI] [PubMed] [Google Scholar]
- Monk C, Nelson E, McClure E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163(6):1091–7. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, O’Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. The British Journal of Psychiatry. 2009;194(6):535–40. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann SA, Brown SM, Ferrell RE, Flory JD, Manuck SB, Hariri AR. Human choline transporter gene variation is associated with corticolimbic reactivity and autonomic-cholinergic function. Biol Psychiatry. 2006;60(10):1155–62. doi: 10.1016/j.biopsych.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Nolte T, Guiney J, Fonagy P, Mayes LC, Luyten P. Interpersonal stress regulation and the development of anxiety disorders: an attachment-based developmental framework. Frontiers in Behavioral Neuroscience. 2011;5:55. doi: 10.3389/fnbeh.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palitsky D, Mota N, Afifi TO, Downs AC, Sareen J. The Association Between Adult Attachment Style, Mental Disorders, and Suicidality: Findings From a Population-Based Study. The Journal of Nervous and Mental Disease. 2013;201(7):579–86. doi: 10.1097/NMD.0b013e31829829ab. [DOI] [PubMed] [Google Scholar]
- Pichon S, Rieger SW, Vuilleumier P. Persistent affective biases in human amygdala response following implicit priming with negative emotion concepts. NeuroImage. 2012;62(3):1610–21. doi: 10.1016/j.neuroimage.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Powers KE, Heatherton TF. Implicitly priming the social brain: failure to find neural effects. PLoS One. 2013;8(2):e56596. doi: 10.1371/journal.pone.0056596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockliff H, Karl A, McEwan K, Gilbert J, Matos M, Gilbert P. Effects of intranasal oxytocin on'compassion focused imagery. Emotion. 2011;11(6):1388. doi: 10.1037/a0023861. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JA, Winterheld HA. Person-by-situation perspectives on close relationships. In: Deaux K, Snyder M, editors. The Oxford Handbook of Personality and Social Psychology. New York: Oxford University Press; 2012. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory STAI (Form Y)(“Self-Evaluation Questionnaire”) Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- Telzer EH, Mogg K, Bradley BP, et al. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biological Psychology. 2008;79(2):216. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn MH, Bakermans-Kranenburg MJ. Attachment representations in mothers, fathers, adolescents, and clinical groups: a meta-analytic search for normative data. Journal of Consulting and Clinical Psychology. 1996;64(1):8. doi: 10.1037//0022-006x.64.1.8. [DOI] [PubMed] [Google Scholar]
- Vrtička P, Andersson F, Grandjean D, Sander D, Vuilleumier P. Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLoS One. 2008;3(8):e2868. doi: 10.1371/journal.pone.0002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtička P, Bondolfi G, Sander D, Vuilleumier P. The neural substrates of social emotion perception and regulation are modulated by adult attachment style. Social Neuroscience. 2012;7(5):473–93. doi: 10.1080/17470919.2011.647410. [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21(4):1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14(6):1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley K. Statistical analysis of activation images. Functional MRI: An Introduction to Methods. 2001;14:251–70. [Google Scholar]
- Wyman PA, Cowen EL, Work WC, Hoyt-Meyers L, Magnus KB, Fagen DB. Caregiving and developmental factors differentiating young at-risk urban children showing resilient versus stress-affected outcomes: a replication and extension. Child Development. 1999;70(3):645–59. doi: 10.1111/1467-8624.00047. [DOI] [PubMed] [Google Scholar]