Abstract
Socially anxious individuals tend to shift their attention away from external socially threatening cues and instead become highly self-focused. Such heightened self-focused attention has been suggested to be involved in the development and maintenance of social anxiety disorder. This study used functional magnetic resonance imaging to investigate the neural correlates of self-focused attention in 16 high socially anxious (HSA) and 16 low socially anxious (LSA) individuals. Participants were instructed to focus their attention either inwardly or outwardly during a simulated social situation. Results indicate hyperactivation of medial prefrontal cortex (mPFC), temporo-parietal junction (TPJ) and temporal pole during inward vs outward attention in HSA compared with LSA participants. Furthermore, activation of mPFC, right anterior insula, TPJ and posterior cingulate cortex was positively correlated with the trait of self-focused attention in HSA subjects. Results highlight the prominent role of the mPFC and other cortical structures in abnormal self-focused attention in social anxiety. Finally, findings for the insula suggest increased processing of bodily states that is related to the amount of habitual self-focused attention in social anxiety.
Keywords: social anxiety, self-focused attention, fMRI, mPFC, insula
INTRODUCTION
Individuals with social anxiety are characterized by pronounced fear of social interactions and performance situations. During social threat, it has been shown that individuals with social anxiety tend toward exceeded self-focused attention in a trait-like fashion (e.g. Clark and Wells, 1995; Rapee and Heimberg, 1997). Generally, self-focused attention is defined as becoming aware of self-referential and internally generated information (Ingram, 1990), which comprises information about bodily states, thoughts, memories, personal beliefs, attitudes, emotions and moods. The cognitive model of Clark and Wells (1995) suggests that abnormally increased self-focused attention during socially threatening situations is a central feature of social anxiety disorder (SAD). Accordingly, exceeded self-focused attention is suggested to negatively impact a person’s self-concept and enhance social anxiety (Bögels and Lamers, 2002; Bögels and Mansell, 2004). Furthermore, it might amplify the bias toward an uncomfortable perception of the observable self (Clark and Wells, 1995; Hackmann et al., 1998). Heightened self-focused attention seems to cause exaggerated negative self-evaluation, anxiety and arousal (e.g. Bögels et al., 1996; Woody, 1996; Wells and Papageorgiou, 1998; Woody and Rodriguez, 2000), as well as social withdrawal (Alden et al., 1992; Bögels and Mansell, 2004). Furthermore, increased self-focused attention occurs not only in patients with fully developed SAD (e.g. Alden et al., 1992; Mellings and Alden, 2000; Mansell et al., 2003) but also in individuals with subclinical social anxiety (Clark and McManus, 2002). Along these lines, it has been proposed that SAD can be located at the upper end of a continuum of social anxiety ranging from subclinical (e.g. shyness) to clinical manifestation (Stein et al., 2000).
Based on functional imaging studies, several parts of the medial prefrontal cortex (mPFC) have been associated with differential functions involved in the processing of self-referential information (Johnson et al., 2002; Mitchell et al., 2005; Amodio and Frith, 2006). It has been shown that especially the anterior part of the rostral mPFC is essential for self-knowledge, person perception and mentalizing the self (e.g. Johnson et al., 2002; Frith and Frith, 1999, 2006; Amodio and Frith, 2006; Van Overwalle, 2009). Furthermore, it seems to be involved in meta-cognitive processes of social cognition, which enable us to make assumptions about another individual’s motivation and their thoughts about us (see Amodio and Frith, 2006). In addition, other cortical midline structures such as the anterior cingulate cortex (ACC), posterior cingulate cortex (PCC) and the medial part of the orbitofrontal cortex (OFC) are assumed to be involved in self-referential processes as along with regions such as the insula, temporo-parietal junction (TPJ) and temporal pole (TP; Funnell, 2001; Johnson et al., 2002; Northoff and Bermpohl, 2004; Olson et al., 2007; van der Meer et al., 2010; Qin and Northoff, 2011). During self-referential processes, the OFC seems to be involved in emotional evaluations (Kelley et al., 2002; van der Meer et al., 2010), whereas the PCC is suggested to be important for the association of self-reflection with autobiographical information (van der Meer et al., 2010). Furthermore, ACC activation seems to be associated with cognitive-emotional interactions and action relevance (van der Meer et al., 2010). Additionally, TPJ and TP are generally involved in social cognition, including generating a coherent self-perception (Blanke and Arzy, 2005; van der Meer et al., 2010; Qin and Northoff, 2011). Finally, the insula is assumed to be involved in interoceptive perception in response to emotional stimuli and in emotional feelings by the perception of such changed bodily states (e.g. Damasio et al., 2000; Critchley, 2004; Craig, 2009).
In line with the hypothesis of increased self-focused attention in social anxiety, abnormal mPFC, OFC and ACC activation has been shown repeatedly in individuals with social anxiety during exposure to or anticipation of disorder-related stimuli or situations (e.g. Blair et al., 2008a, 2010, 2011; Quadflieg et al., 2008; Goldin et al., 2009; Goldin and Gross, 2010; Schmidt et al., 2010; Labuschagne et al., 2012; Boehme et al., 2013; Phan et al., 2013; Pujol et al., 2013), although it remains unclear to what extent these activations indeed represent increased self-focused attention in social anxiety. Previous imaging studies using self- vs other-related feedback stimuli showed heightened mPFC activation to self-related negative feedback in participants with SAD (Blair et al., 2008a, 2011). However, recent findings indicate that this may be specific for full-blown SAD, because mPFC hyperactivation could not be replicated in participants with subclinical social anxiety (Abraham et al., 2013).
According to the model of Clark and Wells (1995) and based on studies on the role of the insular cortex in SAD and other anxiety disorders (Craig, 2002, 2003, 2004; Critchley et al., 2004; Straube et al., 2004; Amir et al., 2005; Straube et al., 2005; Paulus and Stein, 2006; Gentili et al., 2008; Schmidt et al., 2010; Klumpp et al., 2012; Boehme et al., 2013; Ziv et al., 2013), enhanced activation of the insula should depend on the amount of self-focused attention and increased interoceptive awareness. However, studies on the direct modulation of the insula by self-focused attention are as yet lacking in social anxiety research.
There is a need for brain imaging studies, which explicitly manipulate the attentional focus in individuals with social anxiety during social interaction conditions to reveal the neural correlates of self-focused vs externally focused attention. Such experiments might be based on the procedures proposed in the therapy protocol of Clark and Wells (1995). There, patients are encouraged to take both perspectives on a given situation: on the external environment (external focus, which should be experienced as adaptive) and on the own responses (internal focus, which should be maladaptive). The internal focus is supposed to increase abnormal self-focused attention and feelings of anxiety in patients. Using an adaptation of this instructed attention conditions, the present functional magnetic resonance imaging (fMRI) study was set up to investigate the neural basis of internally vs externally focused attention and the association between the trait of self-focused attention and brain activation during social interaction in individuals with subclinical social anxiety.
MATERIALS AND METHODS
Participants
All participants were students recruited at the Friedrich Schiller University of Jena. To recruit subjects with high and low social anxiety, the Liebowitz Social Anxiety Scale (LSAS) questionnaire (German version, Stangier and Heidenreich, 2005) was applied via an internet portal to n = 639 individuals as a screening measure for social anxiety. Then, 33 female volunteers were chosen from this pool. Among these, participants with an LSAS score ≥ 60 were assigned to the high socially anxious (HSA) group (participants with high social anxiety) and those with a LSAS score ≤ 20 to the low socially anxious (LSA) group (participants with low social anxiety; Table 1). One person of the HSA group had to be discarded from further analysis due to exceeding head movements (>3 mm) during fMRI. Therefore, samples in the study consisted of 16 HSA and 16 LSA student participants. The two groups did not differ in age (Table 1). Furthermore, HSA and LSA participants filled in a questionnaire indicating the trait of self-focused attention during social situations (SFA; Bögels et al., 1996; a German version had been created via translation–retranslation). HSA showed significantly greater SFA-scores than LSA subjects (Table 1). All participants were right handed with normal or corrected-to-normal vision and had no history of neurological or psychiatric diseases. All participants provided written informed consent for the study according to the Helsinki declaration, and the study was approved by the Ethics Committee of the Friedrich Schiller University Jena.
Table 1.
Characteristics of participants
| HSA, M (SD) | LSA, M (SD) | t value | |
|---|---|---|---|
| Age | 23.50 | 22.94 | 0.67 |
| (2.76) | (1.91) | ||
| LSAS | 70.44 | 10.56 | 25.51* |
| (7.94) | (5.01) | ||
| SFA | 2.27 | 1.36 | 5.11* |
| (0.57) | (0.43) | ||
M, mean; SD, standard deviation; HSA, high socially anxious participants; LSA, low socially anxious participants; SFA, trait of self-focused attention.
*P < 0.05.
Procedure
During fMRI scanning, participants watched pre-prepared video clips. However, participants were told before the experiment proper that they would see and hear a conversation partner talking live to them by means of a live video session. Participants were further told that the speaker would be sitting in an adjoining room and would continue the conversation face to face with the participant after MR scanning. Each clip lasted 30 s and showed either a female or male speaker looking into a video camera while talking about a specific topic. The speaker’s face and upper part of the body were recorded in front of a white background and placed in the center of the camera screen. The content of the talk either focused on information about the last vacation, the favorite job, leisure activities or the favorite school subject. Preceding each video clip, the attentional focus was manipulated by instructing the participant either to focus attention inwardly, i.e. on bodily states, thoughts, emotions and moods that would come into his/her mind or to focus attention on the outer world (outwardly), i.e. to the actor, his/her appearance and performance and the content of the conversation. Prior to fMRI scanning, participants were familiarized with inward- and outward-focused attention. During scanning, participants watched two video clips with instructions on focusing attention inwardly and two video clips with instructions on focusing attention outwardly (order of instructions, topics and sex of actors was counterbalanced across the two groups). The onset of each video clip in the scanner was jittered between 20 and 30 s, and subjects were requested to fixate a cross with varying colors in the center of the screen while waiting for the next clip. The color of the fixation cross changed three times during each inter-stimulus interval (for 1 s to red, blue, green or yellow, counterbalanced; at least 2 s apart from each other). To avoid extended ruminating and inward focusing, participants were instructed to respond to each change in color of this fixation cross with a button press. After scanning, ratings of participant’s unpleasantness, arousal and anxiety sensation during the attention inward and outward conditions were measured using a nine-point Likert scale (unpleasantness: 1 = very pleasant to 9 = very unpleasant, whereas 5 = neutral; arousal: 1 = not arousing to 9 = very arousing; anxiety: 1 = not anxious to 9 = very anxious). Behavioral data were analyzed using repeated measures analyses of variance (ANOVA) and subsequent t-tests with SPSS software (Version 19.0.0.1, SPSS, Inc.). A probability level of P < 0.05 was considered statistically significant. For better interpretation of the results, effect sizes η2 were calculated using SPSS.
fMRI data acquisition and analysis
MRI scanning was performed in a 1.5 T magnetic resonance scanner (Magnetom Vision Plus; Siemens Medical Systems). After a high-resolution T1-weighted anatomical scan, a run of 167 volumes was conducted using a T2*-weighted echo-planar sequence (echo time = 50 ms, flip angle = 90°, matrix = 64 × 64, field of view = 192 mm, repetition time = 2.971 s). Each volume contained 30 axial slices (thickness = 3 mm; gap = 1 mm; in plane resolution = 3 × 3 mm). Before imaging, a shimming procedure was performed. To ensure that steady-state tissue magnetization was reached, the first three volumes were discarded from analysis.
fMRI-data preprocessing and analysis were performed using Brain Voyager QX software Version 2.1.2 (Brain Innovation, Maastricht, The Netherlands). The volumes of the functional data were realigned to the first volume to minimize effects of head movements. Further data preprocessing comprised spatial smoothing (performed with a Gaussian filter of 8 mm full-width half maximum) and temporal filtering (low-pass filter: 2.8 s; high-pass filter: 3 cycles/run; linear trend removal). The anatomical and functional images were coregistered with six degrees of freedom (three rotational and three translational) and transformed to the normalized Talairach space (Talairach and Tournoux, 1988).
Statistical analyses were performed by multiple linear regression of the signal time course at each voxel based on the general linear model. The expected blood oxygenation level-dependent (BOLD) signal change for each predictor was modeled by convolving the event- or epoch-related reference functions with a 2-gamma hemodynamic response function (HRF). Within-group statistical comparisons were conducted using a mixed-effect analysis. In the first step, predictor estimates (beta-weights) were generated for each individual. In the second step, predictor estimates were analyzed across subjects. Predictors of interest were the two video conditions (attention inward vs outward), while the color changes of the fixation cross/button presses and the two instruction cues served as predictors of no interest. Brain activation was analyzed time locked to onset of stimulus presentation for all predictors. Thus, for the predictors of interest (attention inward vs outward), a box-car function spanning the whole video presentation period was convolved with a 2-gamma HRF. Differences between groups (HSA vs LSA) for the contrast of predictor estimates of interest (inward vs outward condition) were analyzed in a priori defined regions of interest (ROIs). ROIs consisted of mPFC, insula, ACC, OFC and amygdala according to our previous studies in social anxiety (e.g. Straube et al., 2005; Schmidt et al., 2010; Boehme et al., 2013) as well as TPJ, TP and PCC, which were extracted from the Anatomical Automatic Labeling atlas included in WFU PickAtlas software (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003; Maldjian et al., 2004) and transformed into BrainVoyager-compatible Talairach coordinates via ICBM2tal (Lancaster et al., 2007) using MATLAB (Version 7.8; The MathWorks, Inc.).
Statistical parametric maps resulting from voxel-wise analyses were considered statistically significant for clusters that survived correction for multiple comparisons. For this purpose, we used the approach as implemented in Brain Voyager (see Goebel et al., 2006; based on a 3D extension of the randomization procedure described by Forman et al., 1995). First, the voxel-level threshold was set to P < 0.005 (uncorrected). Second, threshold maps were submitted to a ROI-based correction for multiple comparisons. The correction is based on the estimation of the cluster threshold that is the minimal number of voxels, which are necessary to control for multiple comparisons. The cluster threshold criterion was based on the estimate of the map’s spatial smoothness (Forman et al., 1995) and on an iterative procedure (Monte Carlo simulation). The Monte Carlo simulation used 1000 iterations to estimate the minimum cluster size threshold that yielded a cluster-level false-positive rate of 5%. The cluster size thresholds were applied to the statistical maps. For better interpreting the results, Cohen’s d was used to define the effect size of t-values from t-tests. These indices were calculated using the software package G*Power (http://www.psycho.uni-duesseldorf.de/aap/projects/gpower) with the following formula: d = (µ1−µ2)/σ, where µ1 and µ2 are the population means of HSA and LSA, respectively, and σ is the standard deviation of the population (see Faul et al., 2007). Cohen (1992) defines d = 0.2, d = 0.5 and d = 0.8 as ‘small’, ‘medium’ and ‘large’ effects, respectively. Finally, correlation analyses were conducted between brain activation within the ROIs and the trait of self-focused attention (via SFA questionnaire).
RESULTS
Rating data
Analyzing post-scanning rating data revealed a main effect of Condition only for ratings of unpleasantness (F[1,30] = 5.53, P < 0.050; η2 = 0.16). All participants rated the attention inward condition as less pleasant than the attention outward condition. Furthermore, we found effects of Group for ratings of arousal (F[1,30] = 8.33, P < 0.050; η2 = 0.22) and anxiety (F[1,30] = 11.25, P < 0.050; η2 = 0.27) and marginally for ratings of unpleasantness (F[1,30] = 4.16, P < 0.100; η2 = 0.12). HSA participants rated both attentional instructions as more arousing, more anxiety inducing and marginally more unpleasant than LSA participants. Table 2 lists the rating data.
Table 2.
Rating data
| Unpleasantness |
Arousal |
Anxiety |
||||
|---|---|---|---|---|---|---|
| Inward | Outward | Inward | Outward | Inward | Outward | |
| HSA | 4.81 | 3.69 | 3.63 | 3.50 | 2.56 | 2.50 |
| (0.34) | (0.33) | (0.42) | (0.44) | (0.49) | (0.35) | |
| LSA | 3.88 | 2.94 | 2.81 | 1.88 | 1.63 | 1.06 |
| (0.57) | (0.42) | (0.50) | (0.31) | (0.35) | (0.06) | |
HSA, high socially anxious participants; LSA, low socially anxious participants. Standard deviation values are given in parenthesis.
fMRI data
Group differences
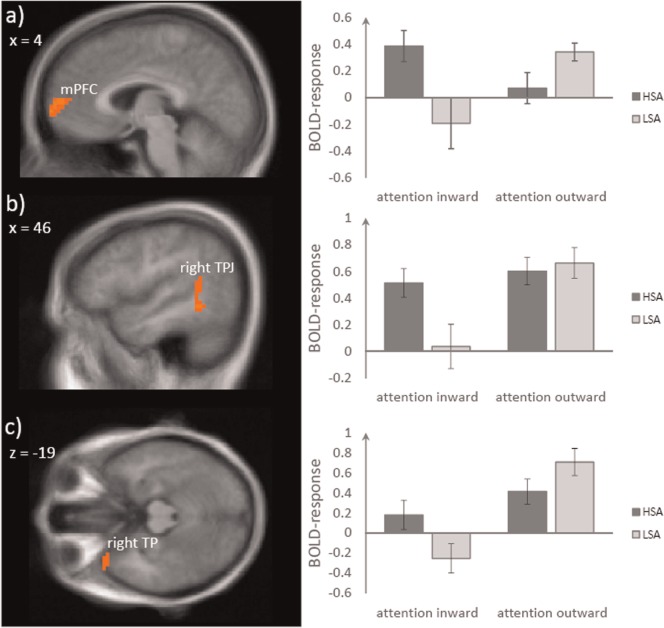
We investigated BOLD activation differences between attention inward and outward condition in HSA vs LSA participants in the specified ROIs. Significant differential brain activation was found in mPFC (peak voxel Talairach coordinates: x = 4, y = 59, z = 8; t[31] = 3.82, size = 786 mm3; P < 0.005; d = 1.31; see Figure 1a), right TPJ (peak voxel Talairach coordinates: x = 43, y = − 48, z = 8; t[31] = 3.84, size = 1343 mm3; P < 0.005; d = 1.32; see Figure 1b) and right TP (peak voxel Talairach coordinates: x = 40, y = 24, z − 22; t[31] = 3.40, size = 819 mm3; P < 0.005; d = 1.17; see Figure 1c). There were no further significant activation differences between the two groups in any of the other ROIs.
Fig. 1.
HSA when compared with LSA participants display an enhanced activation during the inward vs outward condition in mPFC—upper row, right TPJ—middle row and right TP—lower row. Statistical parametric maps (HSA > LSA for contrast attention inward–outward) are overlaid on a T1 scan. The plots on the right side display parameter estimates per condition (mean ± standard error for maximally activated voxel).
Correlation analyses
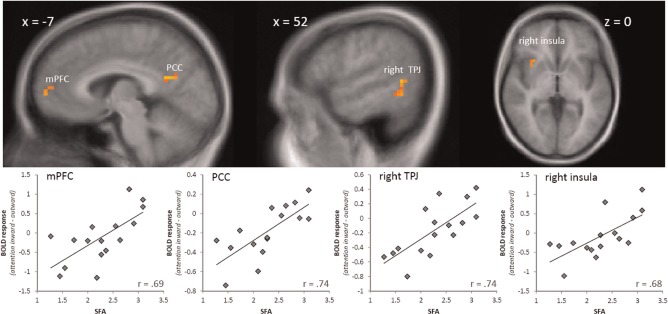
Then, BOLD activation for the contrast attention inward–outward was correlated with the trait of self-focused attention (as measured by SFA) in the HSA group. This analysis revealed a positive association between SFA-values and activation of a cluster in the mPFC that was similar in location to the cluster found in the analyses of significant group differences (peak voxel Talairach coordinates: x = −7, y = 58, z = 6; r = 0.69, size = 234 mm3, P < 0.005; see Figure 2). Furthermore, there was a positive correlation between SFA-values and BOLD activation in the right insula (peak voxel Talairach coordinates: x = 32, y = 13, z = 0; r = 0.68, size = 145 mm3, P < 0.005; see Figure 2), right TPJ (peak voxel Talairach coordinates: x = 54, y = −51, z = 2; r = 0.74, size =1171 mm3, P < 0.005; see Figure 2) and PCC (peak voxel Talairach coordinates: x = −6, y = − 56, z = 21; r = 0.74, size = 270 mm3, P < 0.005; see Figure 2) in HSA participants. There was no significant correlation between SFA-values and brain activation within the ROIs for the LSA group.
Fig. 2.
Brain activation in the mPFC, PCC, right TPJ and right insula correlated significantly with trait of self-focused attention (as measured by SFA) in HSA participants. Statistical parametric maps are overlaid on a T1 scan (radiological convention: left = right). The scatter plots on the lower row display the relationship between contrasts of parameter estimates (attention inward–outward) and SFA-scores in HSA participants.
DISCUSSION
This study investigated the neural correlates of experimentally induced self- vs external-focused attention in participants with social anxiety during a non-threatening simulated social interaction. Results showed that the mPFC, right TPJ and TP were more activated during the attention inward vs outward condition in HSA when compared with LSA participants. Moreover, the activation in mPFC, right insula, TPJ and PCC of HSA participants during self- vs external-focused attention was positively correlated with the trait of self-focused attention.
Findings for the mPFC are in line with the role of this region in introspective and self-referential processes (Mitchell et al., 2005; Northoff et al., 2006; van der Meer et al., 2010). When confronted with social stress, subjects with social anxiety tend toward exceeded focusing on introspective processes that is aimed to monitor and to adjust possible signs of anxiety. Negative self-referential information is used to build a cognitive representation of the observable self (Clark and Wells, 1995; Clark and McManus, 2002). In line with this, abnormal activation of the mPFC has previously been shown in participants suffering from SAD during the presentation of self-referential information (Blair et al., 2008a, 2011; Goldin and Gross, 2010). This suggests an abnormal functioning of mPFC in social anxiety in the context of processing of self-referential information, especially for other person’s viewpoints. Other studies have also reported abnormal mPFC activation during socio-emotional processing in patients with social anxiety (Stein et al., 2002; Straube et al., 2004; Amir et al., 2005; Blair et al., 2008a, 2008b, 2010, 2011, 2012; Goldin et al., 2009; Goldin and Gross, 2010; Labuschagne et al., 2012). However, those findings may not generalizable to subclinical anxiety. A very recent study (Abraham et al., 2013), which investigated neural correlates of self-referential processing in HSA individuals without SAD diagnosis, used a very similar design as Blair et al. (2008a) but could not replicate the finding of increased activation in the mPFC and other regions to negative vs neutral comments referring to the self. The authors suggest that subclinical social anxiety might not be associated with the abnormal brain function observed in full-blown SAD.
Our study provides direct evidence that not only the processing of negative self-referential beliefs or comments but also simply the instruction to focus attention on own thoughts, emotions, and responses induces abnormally increased mPFC activation during a social situation in individuals with high social anxiety. Increased self-focused attention is a central developmental and maintaining feature of SAD in the model of Clark and Wells (1995), and mPFC dysfunction seems to be the neural correlate of this uncomfortable process in social anxiety. Moreover, the present data show that the more pronounced the trait of self-focused attention is, the more the mPFC becomes activated. Heightened self-focused attention during social interactions obviously depends on individual differences in this trait variable. Notably, the location of mPFC cluster appears to fit the region that is suggested to encode self-knowledge during self-relevant information processing (Amodio and Frith, 2006) and has been also described in a meta-analysis of self-reflection (van der Meer et al., 2010).
We also found group effects and/or correlations with self-focused attention in other regions associated with self-referential processes, such as PCC, right TP and TPJ. These regions are hypothesized to have distinct functions during the processing of information referring to the self. The PCC seems to be involved in evaluating and deciding whether a perceived stimulus is self-related or not by consulting autobiographical memory (van der Meer et al., 2010; Qin and Northoff, 2011). The observed correlation between PCC activation and the trait of self-focused attention might indicate an exaggerated use of autobiographical information in HSA participants who tend toward heightened self-focused attention. Accordingly, socially anxious patients remember more negative self-related information (Mansell and Clark, 1999; Wong and Moulds, 2011) and more negative past events (Hinrichsen and Clark, 2003; but see Mellings and Alden, 2000) when social stress is anticipated. The TPJ and TP have mainly been discussed in Theory of Mind context (ToM; Frith and Frith, 1999; Moriguchi et al., 2007), but both regions are strongly involved in self-referential information processing (Qin and Northoff, 2011) and basically in the core representation of the bodily and emotional self (Blanke and Arzy, 2005; van der Meer et al., 2010). For these regions, generally increased activation should be also found for the outward attention condition, because these regions are strongly involved in monitoring the social environment. However, the differential activation between the inward vs outward condition in our study specified the increased involvement of these regions during the self-focus condition in HSA participants, an effect that was also supported by the correlation with the self-focused attention trait at least in the TPJ.
In addition, we also found a positive correlation between the trait of self-focused attention and activation of an anterior cluster in the right insula in HSA participants. This finding supports the role of this region in self-focused attention, which might be related to enhanced interoception (Critchley et al., 2000; Critchley et al., 2004). Insular involvement in the representation of visceral and autonomic responses to emotional stimuli (e.g. Damasio et al., 2000; Critchley, 2004) and in the integration of perceived feelings and other conditions of the current situation (Craig, 2009) has been suggested. The right anterior insula might be particularly involved in the re-representation of sympathetic activation (Critchley, 2004). Our findings, in line with previous studies, confirm that the insula represents a critical brain structure in social anxiety (e.g. Lorberbaum et al., 2004; Straube et al., 2004; Yoon et al., 2007; Schmidt et al., 2010; Boehme et al., 2013; Schulz et al., 2013). Increased processing of own bodily states has been suggested to amplify anxiety responses in social anxiety (see Clark and Wells, 1995). Socially anxious individuals are characterized by exaggerated perception of physical anxiety-related changes that may be visible by others (e.g. blushing; see Clark and Wells, 1995). This internal information in combination with external indicators of negative evaluation is used to form a mental representation of one’s external appearance and behavior (Clark and Wells, 1995; Rapee and Heimberg, 1997). However, in our study, social anxiety per se did not lead to differential insula activation in participants with high vs low social anxiety. Specifically, the trait of self-focused attention was associated with increased insula activation in the HSA group, suggesting that the trait variable moderates the association between insula activation and self-focused attention in social anxiety.
Furthermore, we did not observe differential BOLD activation between LSA and HSA participants in other areas such as the amygdala, ACC and OFC, although abnormal activation in these regions in participants with high social anxiety has been shown repeatedly (e.g. Stein et al., 2002; Straube et al., 2004; Phan et al., 2005; Straube et al., 2005; Etkin and Wager, 2007; Goldin et al., 2009; Schmidt et al., 2010; Labuschagne et al., 2012; Phan et al., 2013). This suggests that these regions are less involved in self-focused attention per se than in threat processing and emotional-cognitive interactions in social anxiety (LeDoux, 1998; Öhman and Mineka, 2001; Straube et al., 2004; Schmidt et al., 2010; Schulz et al., 2013).
We would like to mention several limitations of our study. Results were not controlled for effects of general IQ. Nevertheless, confounding effects of intelligence might be minimized due to the fact that only university students participated in the present study. A further concern is the missing behavioral indices of the dominant attentional focus during the different experimental conditions. Future studies should control for the attentional focus, e.g. as on-line measurement during the experiment. Furthermore, participants passively watched videos and did not participate in real conversation. Future studies might investigate effects during real social interactions. In addition, future studies should investigate the emotional and neural effects of experimentally manipulated self-focused attention also in patients with clinically manifested SAD. Although in subclinical social anxiety, increased self-focused attention seems not to be necessary to enhance negative emotions, abnormal self-focused attention might be a predisposition for the development of SAD (Bögels and Mansell, 2004). In patients with clinically relevant social anxiety, this might induce a vicious circle of exaggerated perception of bodily anxiety-related reactions, imagination of an uncomfortable mental image of the observable self during social threat, avoidant behavior and increased anxiety (e.g. Clark and Wells, 1995; Rapee and Heimberg, 1997; Stangier et al., 2006).
In conclusion, this study revealed emotional and neural correlates of self-focused attention in HSA and LSA participants. Results showed a hyperactivation in the mPFC, during attention inward vs outward in HSA when compared with LSA participants. Furthermore, activation in mPFC was positively correlated with the trait of self-focused attention in HSA participants. Results suggest that activation in the mPFC is a prominent neuronal correlate of excessive self-focused attention in social anxiety. In addition, TP, TPJ and PCC, brain regions commonly associated with self-referential processing, were involved in exceeded self-focused attention in socially anxious individuals. Furthermore, the observed association between right anterior insula hyperactivation and the trait of self-focused attention seems to reflect increased processing of interoceptive responses in relation to the amount of habitual self-focused attention in social anxiety. These outcomes are in accordance with cognitive models proposing abnormal self-focused attention as a key factor for the understanding of social anxiety.
Acknowledgments
We are grateful to the actors ant to all participants of the study. This work was partly supported by grants from the Deutsche Forschungsgemeinschaft (http://www.dfg.de/en/index.js; Project No. STR 987/3-2; SFB/ TRR 58: C06, C07).
REFERENCES
- Abraham A, Kaufmann C, Redlich R, et al. Self-referential and anxiety-relevant information processing in subclinical social anxiety: an fMRI study. Brain Imaging and Behavior. 2013;7:35–48. doi: 10.1007/s11682-012-9188-x. [DOI] [PubMed] [Google Scholar]
- Alden LE, Teschuk M, Tee K. Public self-awareness and withdrawal from social interactions. Cognitive Therapy and Research. 1992;16:249–67. [Google Scholar]
- Amir N, Klumpp H, Elias J, et al. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry. 2005;57:975–81. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Review Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, et al. Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry. 2008a;65:1176–84. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Shaywitz J, Smith BW, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. American Journal of Psychiatry. 2008b;165:1193–202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Hollon N, et al. Social norm processing in adult social phobia: atypically increased ventromedial frontal cortex responsiveness to unintentional (embarrassing) transgressions. American Journal of Psychiatry. 2010;167:1526–32. doi: 10.1176/appi.ajp.2010.09121797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Otero M, et al. Atypical modulation of medial prefrontal cortex to self-referential comments in generalized social phobia. Psychiatry Research: Neuroimaging. 2011;193:38–45. doi: 10.1016/j.pscychresns.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Smith BW, et al. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biological Psychiatry. 2012;72:476–82. doi: 10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Arzy S. The out-of-body experience: disturbed self-processing at the temporo-parietal junction. Neuroscientist. 2005;11:16–24. doi: 10.1177/1073858404270885. [DOI] [PubMed] [Google Scholar]
- Boehme S, Ritter V, Tefikow S, et al. Brain activation during anticipatory anxiety in social anxiety disorder. Social Cognitive and Affective Neuroscience. 2013;9:1413–18. doi: 10.1093/scan/nst129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögels SM, Lamers CTJ. The causal role of self-awareness in blushing-anxious, socially-anxious and social phobics individuals. Behaviour Research and Therapy. 2002;40:1367–84. doi: 10.1016/s0005-7967(01)00096-1. [DOI] [PubMed] [Google Scholar]
- Bögels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clinical Psychology Review. 2004;24:827–56. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Bögels SM, Alberts M, de Jong PJ. Self-consciousness, self-focused attention, blushing propensity and fear of blushing. Personality and Individual Differences. 1996;21:573–81. [Google Scholar]
- Clark DM, McManus F. Information processing in social phobia. Biological Psychiatry. 2002;51:92–100. doi: 10.1016/s0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social Phobia: Diagnosis, Assessment, and Treatment. New York: Guilford Press; 1995. pp. 69–93. [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–59. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Review Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends in Cognitive Sciences. 2004;8:239–41. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapp. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proceedings of the National Academy Science of the United States of America. 2004;101:6333–34. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds–a biological basis. Science. 1999;286:1692–95. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–34. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Funnell E. Evidence for scripts in semantic dementia: implications for theories of semantic memory. Cognitive Neuropsychology. 2001;18:323–41. doi: 10.1080/02643290042000134. [DOI] [PubMed] [Google Scholar]
- Gentili C, Gobbini MI, Ricciardi E, et al. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with Social Phobia and healthy subjects. Brain Res Bull. 2008;77:286–92. doi: 10.1016/j.brainresbull.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10:83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66:170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackmann A, Surawy C, Clark DM. Seeing yourself through other's eyes: a study of spontaneously occurring images in social phobia. Behavioural and Cognitive Psychotherapy. 1998;26:3–12. [Google Scholar]
- Hinrichsen H, Clark DM. Anticipatory processing in social anxiety: two pilot studies. Journal of Behavior Therapy and Experimental Psychiatry. 2003;34:205–18. doi: 10.1016/S0005-7916(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Ingram RE. Self-Focused Attention in Clinical Disorders: Review and a Conceptual Model. Washington, DC: ETATS-UNIS, American Psychological Association; 1990. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? an event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biological Psychology. 2012;89:273–276. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, et al. Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. The International Journal of Neuropsychopharmacology. 2012;15:883–96. doi: 10.1017/S1461145711001489. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: where have we been, and where are we going? Biological Psychiatry. 1998;44:1229–38. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, et al. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15:2701–5. [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–55. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–39. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mansell W, Clark DM. How do i appear to others? social anxiety and processing of the observable self. Behaviour Research and Therapy. 1999;37:419–34. doi: 10.1016/s0005-7967(98)00148-x. [DOI] [PubMed] [Google Scholar]
- Mansell W, Clark DM, Ehlers A. Internal versus external attention in social anxiety: an investigation using a novel paradigm. Behaviour Research and Therapy. 2003;41:555–72. doi: 10.1016/s0005-7967(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Mellings TM, Alden LE. Cognitive processes in social anxiety: the effects of self-focus, rumination and anticipatory processing. Behaviour Research and Therapy. 2000;38:243–57. doi: 10.1016/s0005-7967(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Mori T, Matsuda H, Komaki GEN. Changes of brain activity in the neural substrates for theory of mind during childhood and adolescence. Psychiatry and Clinical Neurosciences. 2007;61:355–63. doi: 10.1111/j.1440-1819.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–87. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Phan KL, Coccaro EF, Angstadt M, et al. Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biological Psychiatry. 2013;73:329–36. doi: 10.1016/j.biopsych.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Cortese BM, Seraji-Bozorgzad N, Tancer ME, Moore GJ. Anterior cingulate neurochemistry in social anxiety disorder: 1H-MRS at 4 Tesla. Neuroreport. 2005;16:183–86. doi: 10.1097/00001756-200502080-00024. [DOI] [PubMed] [Google Scholar]
- Pujol J, Giménez M, Ortiz H, et al. Neural response to the observable self in social anxiety disorder. Psychological Medicine. 2013;43:721–31. doi: 10.1017/S0033291712001857. [DOI] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–33. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Quadflieg S, Mohr A, Mentzel H, Miltner W, Straube T. Modulation of the neural network involved in the processing of anger prosody: the role of task-relevance and social phobia. Biological Psychology. 2008;78:129–37. doi: 10.1016/j.biopsycho.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy. 1997;35:741–56. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Mohr A, Miltner WHR, Straube T. Task-dependent neural correlates of the processing of verbal threat-related stimuli in social phobia. Biological Psychology. 2010;84:304–12. doi: 10.1016/j.biopsycho.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Schulz C, Mothes-Lasch M, Straube T. Automatic neural processing of disorder-related stimuli in social anxiety disorder (SAD): faces and more. Frontiers in Psychology. 2013;4:1–16. doi: 10.3389/fpsyg.2013.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangier U, Heidenreich T. Liebowitz social anxiety scale. In: Scalarum CIP, editor. Internationale Skalen für Psychiatrie (Internatioal Scales for Psychiatry) Weinheim: Beltz; 2005. pp. 299–306. [Google Scholar]
- Stangier U, Clark DM, Ehlers E. Soziale Phobie. Göttingen: Hogrefe; 2006. [Google Scholar]
- Stein M, Goldin P, Sareen J, Zorrilla L, Brown G. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–34. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stein MB, Torgrud LJ, Walker JR. Social phobia symptoms, subtypes, and severity: findings from a community survey. Archives of General Psychiatry. 2000;57:1046–52. doi: 10.1001/archpsyc.57.11.1046. [DOI] [PubMed] [Google Scholar]
- Straube T, Kolassa IT, Glauer M, Mentzel H, Miltner W. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biological Psychiatry. 2004;56:921–30. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel H, Miltner W. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–68. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. 1988. Stutgart: Thieme. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the mni mri single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience & Biobehavioral Reviews. 2010;34:935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30:829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A, Papageorgiou C. Social phobia: effects of external attention on anxiety, negative beliefs, and perspective taking. Behavior Therapy. 1998;29:357–70. [Google Scholar]
- Wong QJ, Moulds ML. Impact of anticipatory processing versus distraction on multiple indices of anxiety in socially anxious individuals. Behaviour Research and Therapy. 2011;49:700–6. doi: 10.1016/j.brat.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Woody SR. Effects of focus of attention on anxiety levels and social performance of individuals with social phobia. Journal of Abnormal Psychology. 1996;105:61–9. doi: 10.1037//0021-843x.105.1.61. [DOI] [PubMed] [Google Scholar]
- Woody SR, Rodriguez BF. Self-focused attention and social anxiety in social phobics and normal controls. Cognitive Therapy and Research. 2000;24:473–88. [Google Scholar]
- Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Research. 2007;154:93–98. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Ziv M, Goldin P, Jazaieri H, Hahn K, Gross J. Is there less to social anxiety than meets the eye? Behavioral and neural responses to three socio-emotional tasks. Biology of Mood & Anxiety Disorders. 2013;3:5. doi: 10.1186/2045-5380-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]