Abstract
Shiga-toxigenic Escherichia coli (STEC) strains were isolated from 7.4% of 1,440 fecal and farm environmental samples. Shiga toxin gene and STEC prevalences were significantly associated with animal production type and season. A range of serogroups were identified. Nine percent of isolates possessed all three principal virulence markers: stx2, eae, and ehx.
Shiga-toxigenic E. coli (STEC) strains are a significant public health threat in many industrialized countries (20, 38). STEC strains associated with human morbidity are generally referred to as enterohemorrhagic E. coli (EHEC) (25). The defining STEC virulence determinant is production of Shiga toxin (Stx), which is encoded by chromosomally located lambdoid prophage stx genes (2). Cattle are the primary reservoir for STEC, and human disease is most often associated with consumption of foods contaminated by cattle manure (5, 16, 38). E. coli O157:H7 is the predominant STEC serotype associated with human disease in the United States (3, 21). Non-O157 strains may also have the potential to be a public health threat in the United States, particularly when considering global trade practices and the relative importance of non-O157 STEC in many other countries (3). Non-O157 STEC strains have been associated with human disease and isolated from foods and beef carcasses in the United States (6, 15, 21, 30, 33). Such strains may be underreported, as diagnostic and surveillance procedures tend to target E. coli O157:H7 (1, 3, 15, 21, 33). Based on the prevalence and virulence characteristics of bovine STEC, it appears that only a subset of strains have the potential to be EHEC. Further exploration of the epidemiology and molecular biology of STEC is needed to determine what attributes confer enhanced public health risk. Comparative studies involving non-O157 STEC may answer critical questions on why E. coli O157:H7 is such a virulent EHEC serotype and the predominant strain in the United States.
Relatively few studies have addressed bovine non-O157 STEC epidemiology at the farm level in the United States. The first aim of this study was to determine crude prevalence rates for bovine non-O157 STEC in Washington State. Although a number of studies have determined associations between STEC excretion by cattle and various management or herd factors (12, 17, 32, 36, 37), few have directly compared the type of bovine production with STEC prevalence. Factors mediating STEC epidemiology at the preslaughter/harvest level may be exploited to mitigate food safety and public health risk (5, 16). Therefore, the second aim was to determine if the potential human health risk of bovine STEC was associated with production type.
Farms and animals.
Twelve farms were sampled: four of each of three production types (dairy, feedlot, and range cow-calf operations) (Table 1). Each production type was subcategorized on the basis of a major management/production difference in order to be representative of Washington cattle production. Farms within production category were ostensibly similar in terms of herd composition, management practices, etc., and these parameters reflected those typical for cattle production facilities in the Pacific Northwest. Dairy farms milked 400 to 800 cows, which were housed in dry lots and concentrate fed. During sampling, one farm switched to raising calves off-site and was substituted for a similar farm in the immediate location that reared calves on-site. Feedlots were >1,000-head facilities located within the central Columbia Basin. Range cow-calf operations were located in the eastern and western Columbia Basin. Farms were sampled twice: once in fall (September-October) and once in winter (January-February) between October 2001 and September 2002. Sixty samples were collected from each farm: 50 fecal (freshly passed manure) and 10 environmental (Table 1). Environmental samples comprised feeds, soils, mixed (i.e., water column and sediments) water from troughs and natural waterways, and dairy lagoon slurries.
TABLE 1.
Summary of farms sampled and sources of fecal samples collected
| Production type | Major management variable | Farms | No. and type of fecal samples collected |
|---|---|---|---|
| Dairy farms | Calves, raised replacement heifers on-site | D1, D2a | 20 milkers, 10 dry cows, 10 prebred heifers, 10 weaning-age calves |
| No calves, heifers raised/replaced off-site | D3, D4,a D5 | 30 milkers, 10 dry cows, 10 heifers | |
| Feedlots | Weaners, recently weaned calves (<300 kg)b | F1, F2 | 20 early on feed (within 2 wk of entry into feedlot), 20 late on feed (within 2 wk of slaughter), 10 hospital pen |
| Yearlings, yearling and stocker cattle (>300 kg)b | F3, F4 | ||
| Cow-calf ranches | Extensive, stocking density of ∼1 cow-calf pair/40 acres, summer grazed on native pasture | R1, R2 | 30 cows, 20 calves (of various ages depending on sampling point within production cycle) |
| Intensive, stocking densities of ∼1 pair/acre, irrigated and manure-spread pasture | R3, R4 |
D2 substituted for D4 between fall and winter samplings due to a change from on-site calf raising to off-site.
Body weight.
STEC isolation and characterization.
Microbiological methods have been described previously (11, 12). Briefly, samples were selectively enriched before PCR screening for stx. Culture media were supplied by Remel (Lenexa, Kans.), and antibiotics and other chemical reagents were supplied by Sigma (St. Louis, Mo.). PCR reagents were supplied by Invitrogen Life Technologies (Carlsbad, Calif.). STEC strains were isolated from stx-positive enrichments by hydrophobic grid membrane filtration and colony lysis DNA hybridization (Roche Diagnostics, Indianapolis, Ind.). Isolates were confirmed as E. coli based on triple-sugar iron agar morphology and oxidase and indole reactions (18). PCR (27) was used to detect principal EHEC virulence markers eae, representing the locus for enterocyte effacement (14, 22, 23), and ehx, which encodes an enterohemolysin (7). Serogrouping of isolates was performed with commercial kits and reference strains (Laboratorio de Referencia de E. coli, Lugo, Spain; and Pennsylvania State University E. coli Reference Center, University Park). STEC strains were designated putative EHEC on the basis of possession of both eae and ehx and/or being of a serogroup typically associated with human morbidity. Prevalences were compared by analysis of variance and t tests (least significant difference) with the SAS 8.02 GLM procedure (SAS Institute, Cary, N.C.). Differences with P ≥ 0.05 were considered significant. Confidence intervals (CIs) were estimated from the normal distribution or calculated from the binomial distribution (EpiInfo 6.04; Centers for Disease Control and Prevention, Atlanta, Ga.) where appropriate.
Prevalence of stx and STEC.
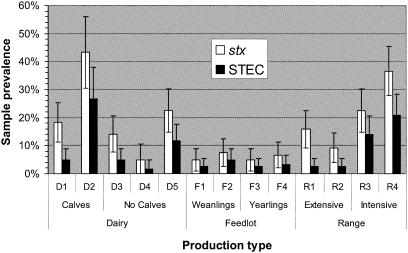
The percentages of samples positive for stx by PCR (stx+) and from which an STEC strain was isolated (STEC+) are summarized in Fig. 1. Prevalences represent those for both fecal and environmental samples collected on each study farm over fall and winter combined. STEC strains were isolated from 7% of fecal samples (dairy, 8%; feedlot, 3%; range cattle, 11%). While the data presented are preliminary in nature due to limited farm numbers and sampling frequency and comparisons are difficult to make between studies, cattle STEC prevalences from the current study were similar to those of previous U.S. investigations. In one such study on dairy farms (37), cow and heifer/calf prevalences were 8 and 19%, respectively, and a number of serotypes associated with human disease were isolated. Similarly, EHEC-associated serotypes were identified among E. coli strains isolated during the 1991-1992 U.S. Department of Agriculture National Animal Health Monitoring System study (13), which determined an stx+ rate of 5.9% and noted that the prevalence of non-O157 STEC with EHEC virulence markers exceeded that of E. coli O157:H7 prevalence. A group in Nevada studied STEC excretion in a herd of 23 beef heifers (35) and from 82 cows from eight ranches (19). Although methods of isolation were suboptimal (due to reliance on sorbitol-negative phenotype), up to 15% of samples were STEC+ and a variety of serogroups were recognized. Preliminary data from Nebraska indicated an STEC prevalence among feedlot and cow-calf operations of 25%, and wildlife were identified as potential sources of STEC (4).
FIG. 1.
Percentages of samples (fecal and environmental) positive for the stx gene and STEC from 13 farms representing bovine production systems in Washington State. Bars represent 95% CIs.
Cattle production type associations.
Both stx and STEC prevalences were significantly associated with production type (P = 0.01). The prevalences for stx (6%; CI, 4 to 8%) and STEC (3%; CI, 2 to 5%) in samples from feedlots was significantly lower than those from dairy (stx, 20%; CI, 16 to 23%; STEC, 9%; CI, 6 to 12%) and range (stx, 21%; CI, 17 to 25%; STEC, 10%; CI, 7 to 13%) facilities. Although many studies have examined the prevalence of STEC in dairy, feedlot, or grazed cattle, few have directly compared prevalences based on production system. Another survey conducted in Washington State (17) similarly determined that range cattle had higher prevalences for E. coli O157:H7 than feedlot cattle. Others have found the prevalences of E. coli O157:H7 in beef and dairy animals to be roughly equivalent (10, 36). In Canadian slaughter cattle (36), stx prevalence was not associated with cattle type (dairy or beef), although yearling cattle (mostly from feedlots) had a significantly lower stx prevalence than cull cows (dairy and beef). A smaller study in Brazil identified a higher stx prevalence in dairy cattle at slaughter compared to that in beef cattle (9). The finding that feedlot prevalence was significantly lower than range or dairy prevalences is somewhat counterintuitive, considering potential pathogen transmission risk factors associated with feedlots, such as high stocking density, cattle mixing, heavily soiled environmental sources, and stress (32). As feedlots primarily derive stock from cow-calf operations or dairies, it is interesting to consider why STEC excretion should decline when these cattle enter the feedlot. Conversely, increased STEC prevalence for range cattle is difficult to explain, considering the extensive nature of production. The use of irrigation on some range operations (particularly the more intensively managed ones) may contribute to enhanced STEC dissemination or maintenance via aquatic transmission (29, 31). Higher range and dairy cattle prevalence may directly impact food safety, as a significant proportion of beef, particularly ground beef, is derived from cull cows with dairy or range backgrounds (24).
EHEC virulence markers.
The prevalences of putative EHEC virulence markers eae and ehx in each production category varied greatly between farms (Table 2). This was also the case for the proportion of isolates possessing respective markers (data not shown). It appears that both shedding prevalence and isolate virulence can be highly variable between farms at various times, such that some herds intermittently provide a greater potential public health risk than others. Stx2 appears to be more cytopathic than Stx1 in animal and in vitro models and is more frequently associated with severe forms of human morbidity (8, 26, 34). Only 11 isolates (8.7%) possessed stx2 combined with eae and ehx, representing a sample prevalence of 0.8%. If this combination of virulence markers is exclusively associated with EHEC potential (8), it suggests that a minority of cattle strains constitute a significant public health risk. This does not account for strains that appear to be highly virulent in the absence of typical markers, e.g., eae− serogroups with stx2d mucus-inducible toxin (15, 28). Serogroups O26, O103, O113, O118, and O157 have been associated with human morbidity and were represented among isolates from a variety of farms.
TABLE 2.
Percentages of samples from cattle farms positive for EHEC virulence markers
| Production type and farm | % of samples with virulence marker
|
% EHECa | ||||
|---|---|---|---|---|---|---|
| stx1 | stx2 | stx1 and 2 | eae | ehx | ||
| Dairy | ||||||
| Calves | ||||||
| D1 | 0.8 | 1.7 | 3.3 | |||
| D2 | 9.7 | 6.5 | 12.9 | 9.7 | 19.4 | 9.7 |
| Mean | 3.8 | 3.3 | 6.6 | 3.3 | 6.6 | 3.3 |
| No calves | ||||||
| D3 | 5.0 | 0.8 | ||||
| D4 | 1.7 | |||||
| D5 | 2.4 | 5.7 | 5.7 | 5.7 | 0.8 | 0.8 |
| Mean | 3.0 | 2.3 | 3.0 | 2.3 | 0.3 | 0.3 |
| Mean | 3.3 | 2.7 | 4.3 | 2.7 | 2.7 | 1.4 |
| Feedlot | ||||||
| Weanling | ||||||
| F1 | 1.7 | 1.7 | ||||
| F2 | 2.5 | 3.3 | ||||
| Mean | 2.1 | 2.5 | ||||
| Yearling | ||||||
| F3 | 2.5 | 0.8 | ||||
| F4 | 0.8 | 2.5 | 1.7 | 1.7 | 1.7 | |
| Mean | 1.7 | 1.3 | 1.3 | 0.8 | 0.8 | |
| Mean | 1.9 | 1.9 | 0.6 | 0.4 | 0.4 | |
| Range | ||||||
| Extensive | ||||||
| R1 | 0.8 | 1.7 | 1.7 | |||
| R2 | 3.3 | 2.5 | ||||
| Mean | 0.4 | 2.5 | 2.1 | |||
| Intensive | ||||||
| R3 | 4.0 | 9.5 | 4.8 | 0.8 | 6.3 | 2.4 |
| R4 | 2.4 | 6.5 | 13.8 | 7.3 | 16.3 | 7.3 |
| Mean | 3.2 | 8.0 | 9.2 | 4.0 | 11.2 | 4.8 |
| Mean | 1.6 | 4.3 | 5.9 | 2.0 | 6.7 | 2.4 |
| Total | 1.6 | 2.9 | 4.0 | 1.8 | 3.3 | 1.4 |
Percentage of samples from which a putative EHEC strain was isolated.
Seasonal associations and environmental isolates.
Season was significantly associated with stx and STEC prevalence (P = 0.01). STEC+ sample prevalence in fall (9%; CI, 7 to 12%) was higher than that for winter (5%; CI, 4 to 7%). Similarly, stx prevalence was higher for fall (21%; CI, 18 to 24%) than for winter (10%; CI, 8 to 12%) samples. This matches a similar seasonal association determined for E. coli O157:H7 (10, 16, 36). It is not known whether this is a strictly climatic effect or relates to seasonal management practices and production cycles specific to the northern hemisphere. The proportion of isolates positive for various markers or that were putative EHEC was not affected by season. STEC and stx prevalences, respectively, were similar for both fecal (7.3 and 16%) and environmental (7.9 and 16%) samples. Although farm environmental contamination may simply reflect the degree of fecal excretion, environmental niches represent significant sources of STEC and are probably important in the epidemiology of STEC. The most frequently STEC+ environmental sample was bedding materials from dairy farms (4 of 12 positive). Water and soil samples were also STEC+ (9 of 100 and 6 of 77 positive, respectively). Despite cattle feeds being implicated as vehicles of dissemination of E. coli O157:H7 (16), no STEC strains were found in feed samples in this study.
Animal management or other practices specific to modern livestock production are suspected to be associated with the emergence of pathogens such as EHEC (5, 16). It seems likely that no single intervention or treatment will eliminate the public health threat of EHEC and that mitigation of risk through interventions at successive critical control points is necessary. By improving our understanding of the on-farm epidemiology of STEC, we hope to identify methods of control at the preslaughter/harvest level.
Acknowledgments
This work was supported by the Adler Endowment and USDA Animal Health Formula Funds.
We thank Jennifer Schaupp, Heather Wardell, Ernie Motteram, Jeffrey LeJeune, and the primary producers.
REFERENCES
- 1.Acheson, D. W. 2000. How does Escherichia coli O157:H7 testing in meat compare with what we are seeing clinically? J. Food Prot. 63:819-821. [DOI] [PubMed] [Google Scholar]
- 2.Acheson, D. W., A. V. Kane, and G. T. Keusch. 2000. Shiga toxins. Methods Mol. Biol. 145:41-63. [DOI] [PubMed] [Google Scholar]
- 3.Acheson, D. W., and G. T. Keusch. 1996. Which Shiga toxin-producing types of Escherichia coli are important? ASM News 62:302-306. [Google Scholar]
- 4.Acheson, D. W., T. Ngo, R. Chitrakar, D. G. Renter, J. R. Gillespie, and J. Ritchie. 2000. Prevalence and genetic characteristics of STEC in cattle and wild-life in Nebraska, p. 106. In Proceedings of the VTEC 2000: 4th International Symposium and Workshop on Shiga Toxin (Verocytotoxin)-Producing Escherichia coli Infections, Kyoto, Japan.
- 5.Armstrong, G. L., J. Hollingsworth, and G. Morris, Jr. 1996. Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiol. Rev. 18:29-51. [DOI] [PubMed] [Google Scholar]
- 6.Arthur, T. M., G. A. Barkocy-Gallagher, M. Rivera-Betancourt, and M. Koohmaraie. 2002. Prevalence and characterization of non-O157 Shiga toxin-producing Escherichia coli on carcasses in commercial beef cattle processing plants. Appl. Environ. Microbiol. 68:4847-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beutin, L., M. A. Montenegro, I. Ørskov, F. Ørskov, J. Prada, S. Zimmerman, and R. Stephan. 1989. Close association of verotoxin (Shiga-like toxin) production with enterohemolysin production in strains of Escherichia coli. J. Clin. Microbiol. 27:2559-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerqueira, A. M., B. E. Guth, R. M. Joaquim, and J. R. Andrade. 1999. High occurrence of Shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro State, Brazil. Vet. Microbiol. 70:111-121. [DOI] [PubMed] [Google Scholar]
- 10.Chapman, P. A., C. A. Siddons, A. T. Cerdan Malo, and M. A. Harkin. 1997. A 1-year study of Escherichia coli O157 in cattle, sheep, pigs and poultry. Epidemiol. Infect. 119:245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobbold, R., and P. Desmarchelier. 2001. Characterisation and clonal relationships of Shiga-toxigenic Escherichia coli (STEC) isolated from Australian dairy cattle. Vet. Microbiol. 79:323-335. [DOI] [PubMed] [Google Scholar]
- 12.Cobbold, R., and P. Desmarchelier. 2000. A longitudinal study of Shiga-toxigenic Escherichia coli (STEC) prevalence in three Australian dairy herds. Vet. Microbiol. 71:125-137. [DOI] [PubMed] [Google Scholar]
- 13.Cray, W. C., L. A. Thomas, R. A. Schneider, and H. W. Moon. 1996. Virulence attributes of Escherichia coli isolated from dairy heifer feces. Vet. Microbiol. 53:369-374. [DOI] [PubMed] [Google Scholar]
- 14.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fey, P. D., R. S. Wickert, M. E. Rupp, T. J. Safranek, and S. H. Hinrichs. 2000. Prevalence of non-O157:H7 shiga toxin-producing Escherichia coli in diarrheal stool samples from Nebraska. Emerg. Infect. Dis. 6:530-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock, D., T. Besser, J. Lejeune, M. Davis, and D. Rice. 2001. The control of VTEC in the animal reservoir. Int. J. Food Microbiol. 66:71-78. [DOI] [PubMed] [Google Scholar]
- 17.Hancock, D. D., T. E. Besser, M. L. Kinsel, P. I. Tarr, D. H. Rice, and M. G. Paros. 1994. The prevalence of Escherichia coli O157:H7 in dairy and beef cattle in Washington State. Epidemiol. Infect. 113:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holt, J. G. 1994. Bergey's manual of determinative bacteriology, 9th ed. Lippincott, Williams & Wilkins, Baltimore, Md.
- 19.Hussein, H. S., B. H. Thran, M. R. Hall, W. G. Kvasnicka, and R. C. Torell. 2003. Verotoxin-producing Escherichia coli in culled beef cows grazing rangeland forages. Exp. Biol. Med. 228:352-357. [DOI] [PubMed] [Google Scholar]
- 20.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein, E. J., J. R. Stapp, C. R. Clausen, D. R. Boster, J. G. Wells, X. Qin, D. L. Swerdlow, and P. I. Tarr. 2002. Shiga toxin-producing Escherichia coli in children with diarrhea: a prospective point-of-care study. J. Pediatr. 141:172-177. [DOI] [PubMed] [Google Scholar]
- 22.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKee, M. L., A. R. Melton-Celsa, R. A. Moxley, D. H. Francis, and A. D. O'Brien. 1995. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect. Immun. 63:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cattlemen's Beef Association. 2001. Cattle and beef industry statistics. National Cattlemen's Beef Association, Centennial, Colo.
- 25.Neill, M. A. 1997. Overview of verotoxigenic Escherichia coli. J. Food Prot. 60:1444-1446. [DOI] [PubMed] [Google Scholar]
- 26.Ostroff, S. M., P. I. Tarr, M. A. Neil, J. H. Lewis, N. Hargrett-Bean, and J. M. Kobayashi. 1989. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J. Infect. Dis. 160:994-998. [DOI] [PubMed] [Google Scholar]
- 27.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paton, A. W., M. C. Woodrow, R. M. Doyle, J. A. Lanser, and J. C. Paton. 1999. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renter, D. G., J. M. Sargeant, R. D. Oberst, and M. Samadpour. 2003. Diversity, frequency, and persistence of Escherichia coli O157 strains from range cattle environments. Appl. Environ. Microbiol. 69:542-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samadpour, M., M. Kubler, F. C. Buck, G. A. Depavia, E. Mazengia, J. Stewart, P. Yang, and D. Alfi. 2002. Prevalence of Shiga toxin-producing Escherichia coli in ground beef and cattle feces from King County, Washington. J. Food Prot. 65:1322-1325. [DOI] [PubMed] [Google Scholar]
- 31.Sargeant, J. M., J. R. Gillespie, R. D. Oberst, R. K. Phebus, D. R. Hyatt, L. K. Bohra, and J. C. Galland. 2000. Results of a longitudinal study of the prevalence of Escherichia coli O157:H7 on cow-calf farms. Am. J. Vet. Res. 61:1375-1379. [DOI] [PubMed] [Google Scholar]
- 32.Smith, D., M. Blackford, S. Younts, R. A. Moxley, J. Gray, L. Hungerford, T. Milton, and T. J. Klopfenstein. 2001. Ecological relationships between the prevalence of cattle shedding Escherichia coli O157:H7 and characteristics of the cattle or conditions of the feedlot pen. J. Food Prot. 64:1899-1903. [DOI] [PubMed] [Google Scholar]
- 33.Tarr, P. I., and M. A. Neill. 1996. Perspective: the problem of non-O157:H7 shiga toxin (verocytotoxin)-producing Escherichia coli. J. Infect. Dis. 174:1136-1139. [DOI] [PubMed] [Google Scholar]
- 34.Tesh, V. L., J. A. Burris, J. W. Owens, V. M. Gordon, E. A. Wadolkowski, A. D. O'Brien, and J. E. Samuel. 1993. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect. Immun. 61:3392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thran, B. H., H. S. Hussein, M. R. Hall, and S. F. Khaiboullina. 2001. Shiga toxin-producing Escherichia coli in beef heifers grazing an irrigated pasture. J. Food Prot. 64:1613-1616. [DOI] [PubMed] [Google Scholar]
- 36.van Donkersgoed, J., T. Graham, and V. Gannon. 1999. The prevalence of verotoxins, Escherichia coli O157:H7, and Salmonella in the feces and rumen of cattle at processing. Can. Vet. J. 40:332-338. [PMC free article] [PubMed] [Google Scholar]
- 37.Wells, J. G., L. D. Shipman, K. D. Greene, E. G. Sowers, J. H. Green, D. N. Cameron, F. P. Downes, M. L. Martin, P. M. Griffin, S. M. Ostroff, M. E. Potter, R. V. Tauxe, and I. K. Wachsmuth. 1991. Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing E. coli from dairy cattle. J. Clin. Microbiol. 29:985-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. 1998. Zoonotic non-O157 Shiga toxin-producing Escherichia coli (STEC): report of a WHO Scientific Working Group Meeting. World Health Organization—Berlin, Berlin, Germany.