Abstract
Humans have a fundamental need for strong interpersonal bonds, yet individuals differ appreciably in their degree of social integration. That these differences are also substantially heritable has spurred interest in biological mechanisms underlying the quality and quantity of individuals’ social relationships. We propose that polymorphic variation in the oxytocin receptor gene (OXTR) associates with complex social behaviors and social network composition through intermediate effects on negative affectivity and the psychological processing of socially relevant information. We tested a hypothesized social cascade from the molecular level (OXTR variation) to the social environment, through negative affectivity and inhibited sociality, in a sample of 1295 men and women of European American (N = 1081) and African American (N = 214) ancestry. Compared to European Americans having any T allele of rs1042778, individuals homozygous for the alternate G allele reported significantly lower levels of negative affectivity and inhibited sociality, which in turn predicted significantly higher levels of social support and a larger/more diverse social network. Moreover, the effect of rs1042778 variation on social support was fully accounted for by associated differences in negative affectivity and inhibited sociality. Results replicated in the African American sample. Findings suggest that OXTR variation modulates levels of social support via proximal impacts on individual temperament.
Keywords: oxytocin, OXTR, social support, personality, genetics
Humans possess a ‘fundamental’ need to form and maintain strong interpersonal bonds (Baumeister and Leary, 1995). These relationships are commonly characterized along two dimensions, social integration and social support (Brissette et al., 2000). Social integration reflects how extensively individuals are embedded in a social context, both by quantity of social contacts and diversity of social roles, whereas social support refers to the various types of assistance (e.g. emotional and tangible) that people may receive from others or perceive to be available from others (Cohen et al., 1985; Brissette et al., 2000). Despite the universal need to establish social ties, individuals vary markedly in their degree of social integration and in the extent and quality of social resources available to them (Sarason et al., 1986). Hence, many people maintain rich social connections, boasting high-contact relations across numerous family members, co-workers and friends, while others possess only a few close relationships, and in some instances, are largely socially isolated (Cacioppo et al., 2003). Understanding these individual differences in social integration is important, as both the quantity and quality of social support have significant implications for health and well-being (House et al., 1988; Holt-Lunstad et al., 2010).
Individual differences in social integration and quality of social support have been shown to be substantially heritable. Genetic differences are important in explaining variation not only in prosocial behaviors, but also in the etiology of social support and social network characteristics (Boomsma et al., 2005; Jackson, 2009). For instance, twin studies demonstrate substantial heritabilities (56%–72%) for prosocial behaviors such as empathy, altruism and nurturance (Rushton et al., 1984; Rushton, 2004), as well as for the major factors that comprise social support, with heritability estimates ranging from 28 to 75% for factors such as availability of confidants and friend/relative social support and the degree of social integration (Bergeman et al., 1990; Kessler et al., 1992; Kendler, 1997; Agrawal et al., 2002; Kendler and Baker, 2007). There are also considerable genetic influences on social network characteristics. For example, Fowler et al. (2009) showed that nearly half of the variation in in-degree nodes (the number of times a person is named as a friend) and transitivity nodes (the likelihood that two person’s contacts are connected to each other) of social networks is genetically attributable. Taken together, these data indicate that differences across individuals in quality of social support and social network characteristics cannot be construed merely as environmental provisions (Sarason et al., 1986). Rather, individuals play a crucial role in creating their own social environments (Plomin et al., 1977; Scarr and McCartney, 1983). We are endowed with certain traits that affect our ability to form and maintain close relationships with others.
Thus, the fact that some people have fewer social contacts than others may reflect attributes of temperament or personality that can hamper social exchange, such as a general propensity to experience negative emotions (e.g. anxiety and anger), or that impede sociality in particular, such as shyness or social anxiety. These two trait clusters—negative affectivity (or neuroticism) and inhibited sociality—are known to influence both social networks and quality of social support (Monroe and Steiner, 1986; Sarason et al., 1986; Windle, 1992). High negative affectivity, for instance, is associated with reduced levels of sociability and social competence (Digman, 1990), problematic relationships with friends and relatives, fewer confidants, poorer social integration (Monroe and Steiner, 1986; Sarason et al., 1986; Kendler, 1997), and smaller social networks (Kalish and Robins, 2006). Furthermore, inhibited sociality associates inversely with levels of social support and social integration (Jones and Carpenter, 1986; Sarason et al., 1986; Eisenberg et al., 1995; Fordham and Stevenson-Hinde, 1999; Mounts et al., 2006). Individuals high on neuroticism/negative affectivity and inhibited sociality thus find it particularly challenging to form and maintain close relationships with others.
Much recent biological research on social relationships has focused on actions of the neuropeptide oxytocin (OXT). Although the relationship between OXT in the brain and peripherally circulating OXT levels remains poorly understood (Feldman et al., 2012), plasma OXT concentrations have been reported to covary with a number of socially relevant behaviors and cognitions. For instance, lower levels of plasma OXT have been linked to reduced trust and trustworthiness (Zak et al., 2004, 2005), heightened hormonal responses to a psychosocial stressor (Taylor, 2006), and increased incidence of Autism spectrum disorders (Green et al., 2001). Furthermore, experimental studies have shown that intranasal OXT administration increases trust behavior (Kosfeld et al., 2005), cooperation (Declerck et al., 2010), generosity (Zak et al., 2007) and in-group altruism (De Dreu et al., 2010), and decreases anxiety and neuroendocrine responses to stress in social interactions (Heinrichs et al., 2003).1 Thus, variation in OXT may ultimately affect the quality and quantity/diversity of social relationships by influencing more proximal mechanisms, such as interpersonal reactivity style and associated traits of temperament. This is consistent with the notion of an evocative gene–environment correlation (Plomin et al., 1977; Scarr and McCartney, 1983) or the idea that ‘individuals' genes predispose them not only to particular behaviors, but also to the social consequences of their behaviors’ (Burt, 2008, p. 113).
Like intranasal and plasma OXT, molecular variation in the gene encoding the oxytocin receptor (OXTR), located on chromosome 3p25.3, has been found to predict complex human social behavior (Bartz et al., 2011). Two OXTR single nucleotide polymorphisms (SNPs) have emerged as particularly promising candidates: rs1042778 (located in the 3′ untranslated region, an area often containing regulatory elements that govern mRNA expression) and rs53576 (located within intron 3). Variation in these SNPs has been linked to both sociability and indices of negative affectivity, including sensitive parenting (rs1042778; Feldman et al., 2012), prosocial fund allocations in the dictator game (rs1042778; Israel et al., 2009 cf. Apicella et al., 2010), sociality (rs53576; Tost et al., 2010), empathy and stress reactivity as assessed by the startle response (rs53576; Rodrigues et al., 2009), negative affectivity and loneliness (rs53576; Lucht et al., 2009), and, in interaction with perceived threat, prosocial behavior outside of the laboratory (rs53576; Poulin et al., 2012). Although, to our knowledge, there is as yet no evidence that these OXTR polymorphisms also predict size and diversity of social networks or availability of social supports, OXTR variation has been shown to modulate the effectiveness of social support as a buffer against stressful experience (rs53576; Chen et al., 2011) and to influence social support seeking during distress (rs53576; Kim et al., 2010). Moreover, twin research has shown the co-variation of low social support with aspects of negative affect (depressive symptomatology) and problematic interpersonal style (hostility) to be largely attributable to shared genetic influences (heritability estimates of 0.61–0.72; Raynor et al., 2002).
Two central hypotheses put forth to explain OXT’s effects on prosocial behavior are that it stimulates affiliative behavior (Uvnäs-Moberg et al., 2005) and, in conjunction with positive affiliative contacts, produces anxiolytic-like effects in response to social stress (Taylor et al., 2006). The latter hypothesis has received considerable research support. OXT attenuates the stress response through its interactions with the hypothalamo-pituitary-adrenal axis and the amygdala–cingulate circuit, and this inhibitory effect of OXT is especially prominent for socially relevant anxiety (Bartz and Hollander, 2006; Campbell, 2010). OXT shows significant binding in the amygdala (Huber et al., 2005), thereby decreasing reactivity to social fear (Labuschagne et al., 2010) and decreasing anxiety and neuroendocrine responses to stress in social interactions (Heinrichs et al., 2003; Holt-Lunstad et al., 2008). Furthermore, neuroimaging studies show that genetic variation in OXTR is associated with the functioning, as well as the size of, the amygdala (Inoue et al., 2010; Tost et al., 2010; Furman et al., 2011). Results from these imaging genetics studies support a model in which OXTR variants might ultimately affect the quality and quantity of social relationships by modulating the more proximal mechanisms of interpersonal reactivity style (i.e. reduced social anxiety and negative affect and increased social affiliative behavior).
We propose that OXTR variation associates with complex social behaviors and social network topology through intermediate effects on negative affectivity and the psychological processing of socially relevant information. Therefore, we sought to test a hypothesized social cascade from the molecular level (i.e. OXTR variation) to the social environment, through negative affectivity and inhibited social temperament in a sample of 1295 midlife men and women of European American (N = 1081) and African American (N = 214) ancestry. Consistent with prior research on OXTR and social behaviors, we predicted that individuals homozygous for the G allele (GG) of rs53576 would report higher levels of social support and a larger and more diverse social network compared to individuals with one or two copies of the A allele (AA/AG) of rs53576, and that these relationships would be mediated by lower levels of negative affectivity and inhibited sociality. Because of inconsistencies in the literature regarding whether the G or T allele of rs1042778 is associated with inhibited sociality (Lerer et al., 2007; Israel et al., 2009; Campbell et al., 2011), we tested our hypotheses by each of the three possible combinations of rs1042778 genotypes (i.e. GG vs GT vs TT; GG vs GT/TT and GG/GT vs TT). Given reported sex differences in the behavioral effects of these two SNPs (e.g. Israel et al., 2009; Lucht et al., 2009), we also tested for sex-dependent moderation of the paths between OXTR and socially relevant phenotypes.
METHOD
Participants
Study data were derived form a sample of 1081 non-Hispanic Caucasian men and women (50.9% female) and 214 African American men and women (62.2% female) who had participated in the University of Pittsburgh Adult Health and Behavior (AHAB) project. The AHAB project provides a registry of behavioral and biological measurements, including DNA, on mid-life community volunteers (30–54 years of age) recruited via mass-mail solicitation from communities of southwestern Pennsylvania (principally Allegheny County; see Halder et al., 2010). Participants had no history of atherosclerotic cardiovascular disease, chronic kidney or liver disease, cancer treatment within the preceding year, major neurologic disorders, schizophrenia or other psychotic illness. Data collection occurred over multiple sessions, and informed consent was obtained in accordance with the University of Pittsburgh IRB.
Genotyping
Genomic DNA was isolated from peripheral white blood cells to ensure optimal quality and purity of DNA using the PureGene kit (Gentra Systems, Minneapolis, MN). The OXTR SNPs rs1042778 (G/T) and rs53576 (A/G) were genotyped by polymerase chain reaction amplification using the primers F-5′-GTAGAAGTACGTTAGGAG-3 and R-5′-GCTGTGCTGGCATAAGTG-3, and F-5′-CACCATGCTCTCCACATC-3 and R-5′-CATCTGCAGGAGCGTTG-3, respectively, followed by genotyping using fluorescent polarization according to Chen et al. (1999). Genotypes were assigned using the program AlleleCaller by comparison to controls of known genotype. A 5% resample was tested to confirm the reproducibility of the genotypes.
Overall, 96.0% and 97.1% of the individuals were genotyped successfully for rs1042778 and rs53576, respectively. This yielded a final study sample of 1040 Caucasians (51% female) and 203 (62% female) African Americans for rs1042778, and 1048 (50% female) Caucasians and 209 (63% female) African Americans for rs53576. The distribution of OXTR genotypes for both SNPs conformed to Hardy–Weinberg Equilibrium in the Caucasian (Ps > 0.31) and African American samples (Ps > 0.17). Consistent with prior reports (e.g. Lerer et al., 2007), alleles were not found to be in linkage disequilibrium (LD) (D′ = 0.38, r2 = 0.05). For purposes of analysis, rs1042778 genotypes were tested additively, as well as grouped by presence of any T allele vs GG homozygote and by presence of any G allele vs TT homozygote, and rs53576 genotypes were grouped by presence of any A allele vs GG. Analyses were run separately for Caucasians and African Americans, as allele frequencies for the SNPs included in this study vary across different racial populations (e.g. Kim et al., 2010).
Measures
Negative affectivity
Negative affectivity was defined by the six neuroticism facets of the Revised NEO Personality Inventory [NEO-PI-R; (Costa, and McCrae, 1992)], which conformed adequately to a single factor model in this sample [ = 71.09, P < 0.001; root-mean-square error of approximation (RMSEA) = 0.07, 90% confidence interval (CI) = 0.058–0.090; comparative fit index (CFI) = 0.98; standardized root mean residual (SRMR) = 0.03].
Inhibited sociality
Inhibited sociality was assessed by three variables. The first was ‘shyness with others’, indexed as a component of Harm Avoidance on the Temperament and Character Inventory (Cloninger et al., 1993), which has good psychometric properties. The second was social anxiety, as assessed by the Self-Consciousness Scale-Revised (SCS-R; Scheier and Carver, 1985), which measures levels of social anxiety and dispositional private/public self-consciousness. The psychometric properties of the SCS-R compare favorably to the original (Scheier and Carver, 1985). The third was the Social Inhibition scale of the short version of the Inventory of Interpersonal Problems (IIP-32; Barkham et al., 1996), which measures distress arising from interpersonal sources. The brief version of this scale has good psychometric properties.
Social networks
Social network diversity was measured by the social network index (Cohen et al., 1997), which assesses aspects of social integration, including participation in 12 types of social relationships and frequency of contact with family, friends and participation in social/religious groups. One point is assigned for each type of relationship (possible score of 12) for which respondents indicate that they speak (in person or on the phone) to someone in that social role at least once every 2 weeks (high contact social roles). The total number of persons with whom they speak at least once every 2 weeks (number of network members) is assessed.
Social support
Availability of social support was assessed by the Interpersonal Support Evaluation List (ISEL; Cohen et al., 1985), which measures perceived availability of supportive functions across four specific domains: appraisal support (availability of having someone to talk to about one’s problems), belonging support (availability of people with whom one can do things), tangible support (availability of material aid) and self-esteem (availability of a positive comparison when comparing one’s self to others). The ISEL has excellent internal reliability and test–retest reliability (Cohen et al., 1985). Importantly, the construct validity of the ISEL has also been demonstrated. It is significantly correlated with several criterion measures, including number of close friends and relatives and quality of marital or living partner relationships (Cohen et al., 1985; Heitzmann and Kaplan, 1988). Based on study hypotheses, three subscales were used in the present study (i.e. appraisal, belonging and tangible support).
RESULTS
Table 1 shows participant characteristics and descriptive statistics for the observed variables by race/ethnicity. As shown, there were no differences in age, NEO-neuroticism subscale scores, appraisal support or tangible support across the two samples. However, Caucasians had a higher proportion of females, more reported years of schooling, higher family incomes, more shyness, social anxiety and social inhibition, more high contact social roles and people in their social network, and less belonging support than African Americans.
Table 1.
Participant characteristics and descriptive statistics of observed variables across racial groups
| Caucasians | African Americans | F/χ2 | P value | |
|---|---|---|---|---|
| Age | 44.7 | 44.3 | 0.7 | 0.40 |
| Female gender | 50.9% | 62.1% | 9.1 | 0.002 |
| Education | 16.0 | 14.1 | 85.2 | <0.001 |
| Income | 5.7 | 4.0 | 122.3 | <0.001 |
| Negative Affectivity | ||||
| NEO-Anxiety | 13.0 | 12.8 | 0.5 | 0.49 |
| NEO-Depression | 11.6 | 11.9 | 1.2 | 0.26 |
| NEO-Anger/Hostility | 11.6 | 11.2 | 0.9 | 0.32 |
| NEO-Impulsiveness | 14.0 | 13.5 | 2.4 | 0.12 |
| NEO-Stress Vulnerability | 15.2 | 15.0 | 0.5 | 0.46 |
| NEO-Self-Consciousness | 9.0 | 8.6 | 2.4 | 0.13 |
| Inhibited Sociality | ||||
| Shyness | 3.0 | 2.4 | 11.8 | 0.001 |
| Social Anxiety | 6.9 | 6.2 | 4.1 | 0.04 |
| Social Inhibition | 3.4 | 2.6 | 11.9 | 0.001 |
| Social Network | ||||
| High Contact Roles | 6.2 | 5.8 | 7.8 | 0.005 |
| People Network | 21.0 | 18.6 | 9.9 | 0.002 |
| Social Support | ||||
| Appraisal | 9.6 | 9.7 | 0.4 | 0.53 |
| Belonging | 8.8 | 9.3 | 6.2 | 0.01 |
| Tangible | 9.7 | 9.5 | 1.9 | 0.16 |
Education = years of schooling; Income was graded over six bracketed ranges from <$25,000 to >$80,000.
Table 2 shows frequencies of OXTR alleles and genotypes, and final categorization of genotypes for each SNP across racial groups. The distribution of genotypes varied significantly across racial groups for both SNPs [for rs1042778, χ2(2, n = 1243) = 46.8, P < 0.001; for rs53576, χ2(2, n = 1257)=8.8, P < 0.02]. Primary analyses were conducted on the larger Caucasian sample, with supplementary analyses conducted on the African American sample to test for homogeneity of effects.
Table 2.
Frequencies of OXTR alleles/genotypes and dichotomized genotypes for analysis
| Caucasians |
African Americans |
||||||
|---|---|---|---|---|---|---|---|
|
OXTR rs1042778 |
OXTR rs53576 |
OXTR rs1042778 |
OXTR rs53576 |
||||
| Allele | n (%) | Allele | n (%) | Allele | n (%) | Allele | n (%) |
| T | 865 (41.6) | A | 639 (30.5) | T | 239 (58.9) | A | 102 (24.4) |
| G | 1215 (58.4) | G | 1457 (69.5) | G | 167 (41.1) | G | 316 (75.6) |
| Genotype | Genotype | Genotype | Genotype | ||||
| TT | 172 (16.5) | AA | 91 (8.7) | TT | 74 (36.5) | AA | 16 (7.7) |
| TG | 521 (50.0) | AG | 457 (43.6) | TG | 91 (44.8) | AG | 123 (58.9) |
| GG | 347 (33.5) | GG | 500 (47.7) | GG | 38 (18.7) | GG | 70 (33.5) |
| Dichotomized Genotypes |
Dichotomized Genotypes |
||||||
| TT/TG | 693 (66.6) | AA/AG | 548 (52.3) | TT/TG | 165 (81.3) | AA/AG | 139 (66.5) |
| GG | 347 (33.4) | GG | 500 (47.7) | GG | 38 (18.7) | GG | 70 (33.5) |
Structural equation modeling (SEM) and power
Analyses were conducted in an SEM framework, which allowed us to estimate latent variables to account for the unreliability of measurement and to test mediational questions by comparison of fit indices across competing models (Tomarken and Waller, 2005). Latent factors were estimated for ‘Negative Affectivity’, ‘Inhibited Sociality’, ‘Social Network’ and ‘Social Support’ constructs. All models were estimated in Mplus 7. Due to the extreme sensitivity of the χ2 test to negligible sources of ill fit in large samples using real world data, we follow convention and rely on multiple alternative fit indices as a reasonable but conservative approach to evaluate model fit (Brown, 2006). These include the RMSEA and its 90% CI, with values <0.08 indicating acceptable fit, and <0.05 indicating good fit, the CFI and Tucker–Lewis index (TLI) for which values of 0.95 or greater indicate excellent fit, and the SRMR for which values of <0.05 indicate good model fit. An a priori power analysis showed our study to be adequately powered (power = 0.81) to detect small effects (that is, a Cohen’s d of 0.20 or 1% of the variance explained) in the structural paths of interest. Primary analyses were conducted in the Caucasian sample, given insufficient power to detect small effect sizes in the African American sample. However, the final model was also estimated in the African American sample to allow for comparison of parameter values across races.
Measurement model and regressions on genotype
A measurement model with four latent factors corresponding to study constructs evidenced good fit to the data ( = 350.63, P < 0.001; RMSEA = 0.061, 90% CI = 0.055–0.067; CFI/TLI = 0.97/0.96; SRMR = 0.03). Due to a very large modification index and conceptual overlap, we allowed the NEO-PI-R Self-Consciousness scale to cross load on to ‘Inhibited Sociality’. To establish the relationship between OXTR genotype and the latent constructs, we estimated two models such that latent ‘Negative Affectivity’, ‘Inhibited Sociality’, ‘Social Network’ and ‘Social Support’ were simultaneously regressed on variation in rs1042778 in one model and on rs53576 in the other. Only the dichotomization of GG vs GT/TT proved significant for rs1042778, and thus other classifications were dropped in subsequent models. OXTR rs1042778 variation (GG individuals coded as 1 and TT/TG individuals coded as 0) significantly predicted ‘Negative Affectivity’ (β = −0.16, SE = 0.07, P = 0.02), ‘Inhibited Sociality’ (β = −0.21, SE = 0.07, P = 0.002), and ‘Social Support’ (β = 0.16, SE = 0.07, P = 0.02), but not ‘Social Network’ (β = 0.10, SE = 0.07, P = 0.14), although this latter effect was in the hypothesized direction. This model achieved good fit to the data ( = 355.33, P < 0.001; RMSEA = 0.058, 90% CI = 0.051–0.064; CFI/TLI = 0.97/0.95; SRMR = 0.03). None of the relationships was significant for rs53576 (all Ps > 0.50). Therefore, only rs1042778 genotype (referred to as OXTR throughout) was included in subsequent SEM analyses.
Gender moderation
To test for possible gender moderation in the relationship between OXTR rs1042778 and social phenotypes, we compared multi-group SEMs with the regression pathways of study constructs on OXTR freely estimated (as opposed to fixed across gender groups). Chi-square difference tests indicated that constraining parameters across groups did not result in a significant degradation in fit, suggesting that gender was not a significant moderator of these associations (‘Negative Affectivity’ = 3.62, P = 0.06; ‘Inhibited Sociality’ = 1.04, P = 0.31; ‘Social Network’ = 0.06, P = 0.80; ‘Social Support’ = 0.10; P = 0.76). Thus, the remaining analyses were conducted on the combined gender sample.
Mediational analyses
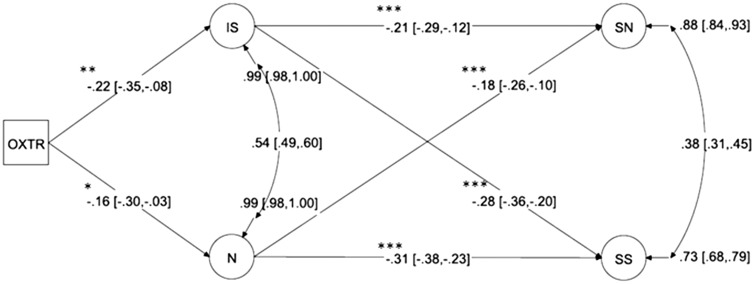
We then determined whether the effect of OXTR on ‘Social Support’ was mediated by ‘Negative Affectivity’ and ‘Inhibited Sociality’. To do so, we examined a full nested structural model in which ‘Social Network’ and ‘Social Support’ were each regressed on ‘Negative Affectivity’ and ‘Inhibited Sociality’, and all latent variables but ‘Social Network’ were regressed on variation in OXTR genotype. This resulted in a non-significant decrement in model fit ( = 0.19, P = 0.66) and revealed that ‘Negative Affectivity’ and ‘Inhibited Sociality’ fully mediated the relationship between OXTR and ‘Social Support’ (β = 0.05, SE = 0.06, P = 0.46). Therefore, for the final model, we removed the non-significant direct pathway between OXTR and ‘Social Support’ leaving only the significant meditational effect through the temperament traits (Figure 1). Model parameter estimates are presented in Figure 1. The rs1042778 GG genotype, compared with TT/TG genotypes, was a significant negative predictor of both ‘Negative Affectivity’ and ‘Inhibited Sociality’, which in turn were each significant incremental predictors of both ‘Social Network’ and ‘Social Support’ factors.2
Fig. 1.
Final structural model with OXTR rs1042778 genotype predicting temperament indices, which in turn predict social functioning. Note: OXTR = rs1042778; N = NEO-PI-R neuroticism; IS = inhibited sociality; SN = social network; SS = social support. All parameters presented in standardized values. We include significance notation for the primary paths of interest, *P < 0.05, **P < 0.01, ***P < 0.001
Relative proximity of negative affectivity vs. inhibited sociality to OXTR effects
To determine the relative importance of ‘Negative Affectivity’ vs. ‘Inhibited Sociality’ on OXTR effects’ on ‘Social Support’, we estimated two statistically equivalent models to the hypothesized model in Figure 1 (i.e. model fit was identical). Instead of allowing ‘Negative Affectivity’ and ‘Inhibited Sociality’ factors to freely covary, we treated them as covariates of each other in separate models (i.e. we partialled out the effect of one variable on the other). In the model in which ‘Negative Affectivity’ was treated as a covariate of ‘Inhibited Sociality’, the pathway between OXTR genotype and ‘Inhibited Sociality’ remained significant (β = −0.13, SE = 0.06, P = 0.04), indicating that ‘Negative Affectivity’ did not fully account for the relationship between OXTR genotype and ‘Inhibited Sociality’. In contrast, when ‘Inhibited Sociality’ was included as a covariate of ‘Negative Affectivity’, the remaining pathway was non-significant (β = −0.04, SE = 0.06, P = 0.46), indicating that ‘Inhibited Sociality’ fully accounted for the relationship between OXTR genotype and ‘Negative Affectivity’.
Directionality of effects and ordering of scales
To address whether ‘Social Support’ is best placed downstream from ‘Negative Affectivity’ and not the other way around, we examined the fit indices of a model that reversed the ordering of scales, such that ‘Negative Affectivity’ and ‘Inhibited Sociality’ were treated as outcomes to perceived ‘Social Support’ and ‘Social Network’. We structured the model this way because merely replacing the position of ‘Social Support’ and ‘Negative Affectivity’ would result in an identical model. Because these are non-nested models, we relied on the Akaike (AIC) and Bayesian (BIC) Information Criteria to adjudicate among models (lower is better in both cases; Brown, 2006). Both the AIC (73 107.67 vs 73 110.75) and BIC (73 359.97 vs. 73 363.04) favored the current model, with perceived ‘Social Support’ downstream to ‘Negative Affectivity’ and not the other way around. We then pared down the model to remove ‘Inhibited Sociality’ and ‘Social Network’ and compared a model in which ‘Negative Affectivity’ mediated the effect of OXTR on ‘Social Support’ without a direct effect (AIC = 48 026.41; BI = 48 169.88) as opposed to the other way around (i.e. a model in which ‘Social Support’ mediated the effect of OXTR on ‘Negative Affectivity’; AIC = 48 026.74; BIC = 48 170.20). Again, the directionality of both fit indices favored the model as it is currently structured.
Supplementary analyses
As a supplementary analysis, we estimated this model in the African American sample, which provided good fit to the data ( = 117.76, P < 0.01; RMSEA = 0.046, 90% CI = 0.026–0.064; CFI/TLI = 0.98/0.97; SRMR = 0.04). The main coefficients of interest and their 95% CIs are catalogued in Table 3. The pathways linking ‘Negative Affectivity’ and ‘Inhibited Sociality’ to ‘Social Support’ and ‘Network’ factors were highly consistent across races, and all were also significant. The pathway linking ‘Inhibited Sociality’ to OXTR in African Americans was larger than in Caucasians, but the 95% CIs were largely overlapping, indicating that the strength of the relationship was similar across racial groups. Furthermore, although the pathway of OXTR predicting ‘Negative Affectivity’ was not significant in African Americans, the path coefficient was virtually identical (and stronger) to that found in the Caucasian sample, indicating that the lack of significance was likely a function of sample size. Finally, the path coefficients linking OXTR to ‘Social Support’ and ‘Social Network’ were similar in magnitude to those found in the Caucasian sample. We did not test for gender moderation in the African American sample due to insufficient power.
Table 3.
Regression parameters from structural model across racial groups
| Regression Pathways | Race |
|||||
|---|---|---|---|---|---|---|
| Caucasians |
African Americans |
|||||
| Coeff | 95% CI | P | Coeff | 95% CI | P | |
| Inhibited Sociality on OXTR | −0.22 | −0.35, −0.08 | <0.001 | −0.45 | −0.82, −0.08 | 0.02 |
| Negative Affectivity on OXTR | −0.16 | −0.30, −0.03 | 0.02 | −0.22 | −0.59, −0.15 | 0.25 |
| Social Support on Inhibited Sociality | −0.28 | −0.36, −0.20 | <0.001 | −0.22 | −0.39, −0.06 | 0.01 |
| Social Support on Negative Affectivity | −0.31 | −0.38, −0.23 | <0.001 | −0.47 | −0.62, −0.31 | <0.001 |
| Social Network on Inhibited Sociality | −0.21 | −0.29, −0.13 | <0.001 | −0.2 | −0.38, −0.02 | 0.03 |
| Social Network on Negative Affectivity | −0.18 | −0.26, −0.10 | <0.001 | −0.28 | −0.46, −0.11 | 0.01 |
Coeff = standardized coefficient; CI = confidence interval.
DISCUSSION
We provide evidence of the OXTR rs1042778 SNP’s cascading effect on complex social phenotypes, with the molecular variation accounting for some portion of individual differences in socially relevant temperaments and these in turn accounting for differences in participants’ social environments. Specifically, we found that individuals homozygous for the G allele of rs1042778, compared with those with one or two copies of the T allele, reported significantly lower levels of negative affectivity and inhibited sociality, which in turn predicted significantly higher levels of social support and a larger/more diverse social network. Furthermore, negative affectivity and inhibited sociality fully mediated the relationship between rs1042778 and social support. These findings build on prior research linking rs1042778 to sensitive parenting (Feldman et al., 2012) and prosocial fund allocations in the dictator game (Israel et al., 2009) and suggest that rs1042778 variation plays a significant role not only in the promotion of social affiliative behavior, but also in the creation of social environments (Plomin et al., 1977; Scarr and McCartney, 1983; Burt, 2008). Importantly, this study follows the best practices guidelines for conducting candidate gene studies by providing an independent replication of our exact results in the African American sample (Hewitt, 2012), disclosing all analyses conducted (i.e. both genetic variants; Little et al., 2009), and using the ‘new statistics’ to avoid problems with null-hypothesis significance testing (Cumming, 2014; Eich, 2014).
Twin studies underscore the importance of genetic influences on prosocial behavior (Rushton, 2004), social support (Kendler, 1997), and social network characteristics (Fowler et al., 2009), but it has been difficult to identify specific genes and polymorphisms related to such distal and complex social phenotypes (Ebstein et al., 2010). Although prior studies have shown that OXTR is important in modulating the effectiveness of social support as a buffer against stressful experience (Chen et al., 2011), to our knowledge, this is the first study to directly link OXTR variation to differences in quality of available social support. Importantly, we also found that the effect of rs1042778 variation on social support was completely mediated by differences in negative affectivity and inhibited sociality. This is consistent with the notion that OXTR variation exerts its effect on complex social environmental factors by modulating neural circuitry involved in the processing of social information and the experience and expression of negative emotions (Meyer-Lindenberg and Tost, 2012). Although in the expected direction, we did not find evidence of a direct effect of rs1042778 on social network size/diversity of social roles. This indicates that OXTR variation may either be only a distal contributor to factors that influence network size/diversity of social roles or that it may be more important in explaining differences in the quality rather than the quantity/diversity of social relationships. While one prior study reported results suggestive of sex specific effects (Israel et al., 2009), we did not find gender moderation in the relationship between rs1042778 variation and social phenotypes (although there was a trend for the effects to be stronger in females). Despite limited power, our cascade model produced very similar effects in the African American sample, indicating that observed relationships between OXTR and social phenotypes is consistent across these racial groups and providing a direct replication of study results.
Prior research has led to two central hypotheses regarding the function of OXT (i.e. to promote social affiliation and to reduce socially relevant stress reactivity). We found evidence of both, as rs1042778 variability contributed to decreased social inhibition and lower negative affectivity. Furthermore, in a series of mediation analyses, we demonstrated that decreased social inhibition seems to be more proximal to OXTR effects than is negative affectivity. Of course, the cross-sectional study design precludes drawing definitive conclusions about the temporal relationship among the OXTR rs1042778 polymorphism, temperament factors and social environmental variables. However, we do provide statistical evidence (by comparison of model fit indices across competing models) in support of the directionality of the effects we report in our model. Furthermore, our social cascade hypothesis is consistent with the idea that individuals’ genotypes can evoke certain social outcomes (e.g. increased social support; Burt, 2008), as well as longitudinal research supporting a causal relationship between differences in interpersonal style/personality functioning and subsequent social functioning impairments (Morey et al., 2012). Nonetheless, future studies using prospective designs are indicated.
The present research had other limitations. We selectively measured the constructs of inhibited sociality and negative affectivity, and thus it remains unclear whether rs1042778 exerts its effect on perceived social support via effects on more generalized temperament variables (e.g. stress reactivity) that overlap with the constructs examined in this study. Further, our data were found to fit a model in which we grouped participants by the presence or absence of the T-allele (T carriers vs GG), rather than the presence or absence of the G allele found in two smaller, prior studies (Israel et al., 2009; Feldman et al., 2012). Importantly, though, the current study used a sample size 4.5–6 times larger than these prior studies and, despite differences in sample characteristics, our results are consistent with family-based association tests showing a significant link between the T allele and increased incidence of Autism spectrum disorders (ASD) (Lerer et al., 2007; given that our results are consistent with the interpersonal difficulties characteristic of ASD). In addition, like most SNPs reported in the literature, the functional effect of the rs1042778 polymorphism is currently unknown. However, there is some evidence that it may play a regulatory role in OXTR transcription and translation based on its location in the 3′ untranslated region, an area that usually contains several regulatory elements governing transcriptional efficiency and stability of mRNA (Kuersten and Goodwin, 2003; Israel et al., 2009). Furthermore, one prior study found evidence that the T allele of rs1042778 was associated with lower plasma OXT levels, indicating that this genetic marker may play a functional role in regulating OXT signaling pathways and/or peripheral OXT activity (Feldman et al., 2012). Consistent findings reported here across independent samples will hopefully motivate future molecular studies to elucidate the possible functional effect of the rs1042778 polymorphism.
We measured multiple dimensions of social support, but the ISEL is a self-report instrument, and thus item endorsement reflects participants’ perceptions of support availability. Although perceived availability of social support, rather than mere membership in a social group or number of social contacts, is a better predictor of a number of positive health outcomes (Cohen and Wills, 1985), future studies should also examine actual (i.e. received) social support. Finally, none of our socially relevant phenotypes were predicted by rs53576, and it is unclear why associations were only obtained for rs1042778 (it is important to note, however, that these two polymorphisms are in low LD). Our null findings are consistent with prior reports of null association between rs53576 variation and attachment style (e.g. attachment anxiety/avoidance; Rodrigues et al., 2009) and perceived quality of a supportive interaction with a partner or friend (Chen et al., 2011). However, null findings observed in this study in the link between the G allele and higher levels of social support quality seem inconsistent with prior reports linking the G allele to socio-emotional sensitivity, such as less loneliness and greater empathy (Lucht et al., 2009; Rodrigues et al., 2009). Future studies should clarify the exact nature of the relationship between rs53576 variation and social phenotypes.
Human beings possess a basic need to form and maintain close relationships with others (Baumeister and Leary, 1995), and the absence of such close relationships increases mortality risk at a magnitude rivaling other well-established risk factors (e.g. smoking and physical activity level; Holt-Lunstad et al., 2010). Yet, some individuals find it particularly challenging to navigate the social environment and report deficits in available social support. While previous studies demonstrate that there is a substantial heritable component to the ability to form and maintain relationships, specific sources of genetic variation underlying these heritable influences have not yet been identified. This study demonstrates that OXTR variation, previously shown to be important in the regulation of adaptive, prosocial behavior, affects interpersonal reactivity and associated temperament traits in a manner accounting for OXTR-dependent variation in quality of social support. These findings have important implications for understanding how variation in the OXTR system can ultimately affect complex social network systems and may provide insight into the development of innovative therapies to improve the quality of one’s social environment.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Acknowledgments
This study was primarily supported by National Heart Lung Blood Institute Grants PO1 HL040962 and RO1 HL065137, as well as National Institute of Mental Health Grants T32MH018269 and F32MH097325 and National Heart Lung Blood Institute Grant HL093220.
Footnotes
1 It is important to note that the effects of OXT are often moderated by contextual factors (i.e. situational features in which OXT is administered) or by individual difference factors (e.g. trait aggressiveness) of the individuals to whom OXT is administered (Bartz et al., 2011; DeWall et al., forthcoming).
2 We also ran an SEM model augmenting ‘Inhibited Sociality’ with the Extraversion facet scales of the NEO-PI-R, and results remained the same (i.e. path coefficients and significance levels were virtually identical). See the Supplementary Material for additional details on these analyses and an explanation for why this model was not retained.
REFERENCES
- Agrawal A, Jacobson KC, Prescott CA, Kendler KS. A twin study of sex differences in social support. Psychological Medicine. 2002;32:1155–64. doi: 10.1017/s0033291702006281. [DOI] [PubMed] [Google Scholar]
- Apicella CL, Cesarini D, Johannesson M, et al. No association between oxytocin receptor (OXTR) gene polymorphisms and experimentally elicited social preferences. PLoS One. 2010;5(6):e11153. doi: 10.1371/journal.pone.0011153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkham M, Hardy GE, Startup M. The IIP-32: a short version of the inventory of interpersonal problems. British Journal of Clinical Psychology. 1996;35(1):21–35. doi: 10.1111/j.2044-8260.1996.tb01159.x. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Hormones and Behavior. 2006;50:518–28. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15(7):301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497–529. [PubMed] [Google Scholar]
- Bergeman CS, Plomin R, Pedersen NL, McClearn GE, Nesselroade JR. Genetic and environmental influences on social support: the Swedish Adoption/Twin Study of Aging. Journal of Gerontology. 1990;45:101–6. doi: 10.1093/geronj/45.3.p101. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Willemsen G, Dolan CV, Hawkley LC, Cacioppo JT. Genetic and environmental contributions to loneliness in adults: the Netherlands Twin Register Study. Behavior Genetics. 2005;35(6):745–52. doi: 10.1007/s10519-005-6040-8. [DOI] [PubMed] [Google Scholar]
- Brissette I, Cohen S, Seeman TE. Measuring social integration and social networks. In: Cohen S, Underwood L, Gottlieb B, editors. Measuring and Intervening in Social Support. New York: Oxford University Press; 2000. [Google Scholar]
- Brown TA. Confirmatory factor analysis for applied research. New York: Guilford Press; 2006. [Google Scholar]
- Burt SA. Genes and popularity: evidence of an evocative gene-environment correlation. Psychological Science. 2008;19:112–3. doi: 10.1111/j.1467-9280.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG. The anatomy of loneliness. Current Directions in Psychological Science. 2003;12(3):71–4. [Google Scholar]
- Campbell A. Oxytocin and human social behavior. Pers Soc Psychol Rev. 2010;14:281–95. doi: 10.1177/1088868310363594. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Datta D, Jones ST, Batey Lee E, Sutcliffe JS, Hammock EAD, Levitt P. Association of oxytocin receptor (OXTR) gene variants with multiple phenotype domains of autism spectrum disorder. Journal of Neurodevelopmental Disorders. 2011;3(2):101–12. doi: 10.1007/s11689-010-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proceedings of the National Academy of Sciences. 2011;108(50):19937–42. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Levine L, Kwok PY. Fluorescence polarization in homogeneous nucleic acid analysis. Genome Research. 1999;9(5):492–8. [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50(12):975–90. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. Journal of American Medical Association. 1997;277(24):1940–4. [PubMed] [Google Scholar]
- Cohen S, Mermelstein R, Kamarck T, Hoberman HM. Measuring the functional components of social support. In: Hoberman HM, Sarason IG, Sarason BR, editors. Social Support: Theory, Research and Applications. Dordrecht, The Netherlands: Martinus Nijhoff; 1985. pp. 73–94. [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98(2):310–57. [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Normal personality assessment in clinical practice: the NEO personality inventory. Psychological Assessment. 1992;4(1):5–13. [Google Scholar]
- Cumming G. The new statistics why and how. Psychological Science. 2014;25(1):7–29. doi: 10.1177/0956797613504966. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Handgraaf MJJ, Shalvi S, Van Kleef GA, Baas M, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Hormones and Behavior. 2010;57(3):368–74. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Gillath O, Pressman SD, et al. When the love hormone leads to violence: oxytocin increases intimate partner violence inclinations among high trait aggressive people. Social and Personality Psychological Science. 2014;5(6):691–7. [Google Scholar]
- Digman JM. Personality structure: emergence of the five-factor model. Annual Review of Psychology. 1990;41(1):417–40. [Google Scholar]
- Ebstein RP, Israel S, Chew SH, Zhong S, Knafo A. Genetics of human social behavior. Neuron. 2010;65(6):831–44. doi: 10.1016/j.neuron.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Eich E. Business not as usual. Psychological Science. 2014;25(1):3–6. doi: 10.1177/0956797613512465. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy BC. Relations of shyness and low sociability to regulation and emotionality. Journal of Personality and Social Psychology. 1995;68(3):505–17. doi: 10.1037//0022-3514.68.3.505. [DOI] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, et al. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biological Psychiatry. 2012;72(3):175–81. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Fordham K, Stevenson-Hinde J. Shyness, friendship quality, and adjustment during middle childhood. Journal of Child Psychology and Psychiatry. 1999;40(5):757–68. [PubMed] [Google Scholar]
- Fowler JH, Dawes CT, Christakis NA. Model of genetic variation in human social networks. Proceedings of the National Academy of Sciences. 2009;106(6):1720–24. doi: 10.1073/pnas.0806746106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Chen MC, Gotlib IH. Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology. 2011;36:891–7. doi: 10.1016/j.psyneuen.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biological Psychiatry. 2001;50:609–13. doi: 10.1016/s0006-3223(01)01139-8. [DOI] [PubMed] [Google Scholar]
- Halder I, Marsland AL, Cheong J, Muldoon MF, Ferrell RE, Manuck SB. Polymorphisms in the CRP gene moderate an association between depressive symptoms and circulating levels of C Reactive Protein. Brain, Behavior, and Immunity. 2010;24(1):160–7. doi: 10.1016/j.bbi.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–98. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heitzmann CA, Kaplan RM. Assessment of methods for measuring social support. Health Psychology. 1988;7(1):75–109. doi: 10.1037//0278-6133.7.1.75. [DOI] [PubMed] [Google Scholar]
- Hewitt JK. Editorial policy on candidate gene association and candidate gene-by-environment interaction studies of complex traits. Behavioral Genetics. 2012;42:1–2. doi: 10.1007/s10519-011-9504-z. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosomatic Medicine. 2008;70:976–85. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–8. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Inoue H, Yamasue H, Tochigi M, et al. Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biological Psychiatry. 2010;68:1066–72. doi: 10.1016/j.biopsych.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, et al. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PLoS One. 2009;4(5):e5535. doi: 10.1371/journal.pone.0005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MO. Genetic influences on social network characteristics. Proceedings of the National Academy of Sciences. 2009;106(6):1687–8. doi: 10.1073/pnas.0813169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WH, Carpenter BN. Shyness, social behavior, and relationships. In: Jones WH, Cheek JM, Briggs SR, editors. Shyness. New York: Springer US; 1986. pp. 227–38. Available: http://link.springer.com/chapter/10.1007/978-1-4899-0525-3_17. [Google Scholar]
- Kalish Y, Robins G. Psychological predispositions and network structure: The relationship between individual predispositions, structural holes and network closure. Social Networks. 2006;28(1):56–84. [Google Scholar]
- Kendler KS. Social support: a genetic-epidemiologic analysis. American Journal of Psychiatry. 1997;154(10):1398–404. doi: 10.1176/ajp.154.10.1398. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. Psychological Medicine. 2007;37:615–26. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Kendler KS, Heath A, Neale MC, Eaves LJ. Social support, depressed mood, and adjustment to stress: a genetic epidemiologic investigation. Journal of Personality and Social Psychology. 1992;62:257–72. doi: 10.1037//0022-3514.62.2.257. [DOI] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Sasaki JY, et al. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proceedings of the National Academy of Sciences. 2010;107(36):15717–21. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kuersten S, Goodwin EB. The power of the 3′ UTR: translational control and development. Nature Reviews Genetics. 2003;4(8):626–37. doi: 10.1038/nrg1125. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–13. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Molecular Psychiatry. 2007;13(10):980–8. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Little J, Higgins JPT, Ioannidis JPA, et al. Strengthening the reporting of genetic association studies (STREGA): an extension of the STROBE Statement. Human Genetics. 2009;125:131–51. doi: 10.1007/s00439-008-0592-7. [DOI] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, et al. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(5):860–6. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nature Neuroscience. 2012;15(5):663–8. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Steiner SC. Social support and psychopathology: Interrelations with preexisting disorder, stress, and personality. Journal of Abnormal Psychology. 1986;95(1):29–39. doi: 10.1037//0021-843x.95.1.29. [DOI] [PubMed] [Google Scholar]
- Morey LC, Hopwood CJ, Markowitz JC, et al. Comparison of alternative models for personality disorders, II: 6-, 8- and 10-year follow-up. Psychological Medicine. 2012;42(8):1705–13. doi: 10.1017/S0033291711002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounts NS, Valentiner DP, Anderson KL, Boswell MK. Shyness, sociability, and parental support for the college transition: relation to adolescents’ adjustment. Journal of Youth & Adolescence. 2006;35:68–77. [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–22. [PubMed] [Google Scholar]
- Poulin MJ, Holman EA, Buffone A. The neurogenetics of nice: Receptor genes for oxytocin and vasopressin interact with threat to predict prosocial behavior. Psychological Science. 2012;23(5):446–52. doi: 10.1177/0956797611428471. [DOI] [PubMed] [Google Scholar]
- Raynor DA, Pogue-Geile MF, Kamarck TW, McCaffery JM, Manuck SB. Covariation of psychosocial characteristics associated with cardiovascular disease: Genetic and environmental influences. Psychosomatic Medicine. 2002;64(2):191–203. doi: 10.1097/00006842-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences. 2009;106(50):21437–41. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton JP. Genetic and environmental contributions to pro-social attitudes: a twin study of social responsibility. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2004;271(1557):2583–5. doi: 10.1098/rspb.2004.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton JP, Fulker DW, Neale MC, Blizard RA, Eysenck HJ. Altruism and genetics. Acta Genet Med Gemellol. 1984;33:265–71. doi: 10.1017/s0001566000007315. [DOI] [PubMed] [Google Scholar]
- Sarason IG, Sarason BR, Shearin EN. Social support as an individual difference variable: its stability, origins, and relational aspects. Journal of Personality and Social Psychology. 1986;50(4):845–55. [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype-environment effects. Child Development. 1983;54:424–35. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS. The Self-Consciousness Scale: A revised version for use with general populations. Journal of Applied Social Psychology. 1985;15(8):687–99. [Google Scholar]
- Taylor SE. Tend and befriend: Biobehavioral bases of affiliation under stress. Current Directions in Psychological Science. 2006;15(6):273–7. [Google Scholar]
- Tomarken AJ, Waller NG. Structural equation modeling: strengths, limitations, and misconceptions. Annu Rev Clin Psych. 2005;1:31–65. doi: 10.1146/annurev.clinpsy.1.102803.144239. [DOI] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences. 2010;107(31):13936–41. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: the role of the oxytocinergic system. International Journal of Behavioral Medicine. 2005;12(2):59–65. doi: 10.1207/s15327558ijbm1202_3. [DOI] [PubMed] [Google Scholar]
- Windle M. Temperament and social support in adolescence: interrelations with depressive symptoms and delinquent behaviors. Journal of Youth and Adolescence. 1992;21:1–21. doi: 10.1007/BF01536980. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. The neurobiology of trust. Annals of the New York Academy of Sciences. 2004;1032(1):224–7. doi: 10.1196/annals.1314.025. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Hormones and Behavior. 2005;48:522–7. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.