Abstract
In developing countries, rheumatic fever and carditis still constitutes a major public health problem. Patients have special characteristics that differ from those with rheumatic mitral valve disease we still see in developed countries. They are usually young, poor, uneducated, and have low compliance to prophylaxis / therapy. In addition, they usually have great difficulty in accessing medical care. In these situations, the rate of complications associated to valve replacement is significantly increased. Alternatively, mitral valve repair is now known to achieve better long-term results in this pathology, but this was not widely recognized three or four decades ago, when first reports showed worse results after repair of rheumatic regurgitation than with degenerative valves. This has been reported by several groups in developing countries in different continents, with high incidence of repairs and excellent long term results. It is, therefore, becoming increasingly clear that, although, the results may not compare to those obtained with degenerative pathology, repair of rheumatic valves, when feasible, is the procedure of choice, especially in these underprivileged populations.
Keywords: rheumatic carditis, mitral valvuloplasty, developing countries
Introduction
In developing countries, rheumatic fever and carditis still constitutes a major public health problem. This is a disease of poor people with difficult access to health care.
“… The combination of lack of resources, lack of infrastructure, political, social and economic instability, poverty, overcrowding, malnutrition and lack of political will contributes to the persistence of a high burden of rheumatic fever, rheumatic valvular heart diseases and infective endocarditis.”1
These patients have special characteristics that differ from those we generally see in the economically developed countries. They are usually young, poor, uneducated, and have low compliance to prophylaxis/therapy. In addition, they usually experience great difficulty in accessing medical care.
In these situations, the rate of complications arising from valve replacement is significantly increased. Mechanical prostheses are prone to thromboembolic complications, the incidence of which is magnified several fold by the poor adherence to anticoagulation protocols.2 On the other hand, bioprostheses, which do not require anticoagulation, especially in young patients mostly in sinus rhythm, hence initially considered ideal for these populations, degenerate fast, sometimes within only a few years after implantation.3
By contrast, rheumatic fever has mostly disappeared in developed countries, except in those with large immigrant contingents, because medical care is usually easily accessible. In these regions, we now rarely see new cases of acute rheumatic carditis, but older patients, who had the acute form of the disease some decades ago, still present with sequelae of rheumatic disease, in their fifties and sixties, requiring valve surgery. As discussed below, these chronic forms have different macroscopic characteristics.
As an alternative to replacement, mitral valve repair is now known to achieve better long-term results in this pathology, but this was not widely recognized three or four decades ago, when first reports showed worse results after repair of rheumatic regurgitation than after repair of degenerative valves. My experience in Johannesburg, South Africa, in the 1980's, predominantly in a rheumatic black population, with a mean age of 21.5+11.8 years, demonstrated a significantly better survival in patients with mitral valve repair by comparison with replacement, either with mechanical valves or with bioprostheses (Figure 1).4 This experience has been replicated and confirmed, more recently, in Mozambique in annual surgical missions undertaken in the in Maputo Heart institute.
Figure 1.
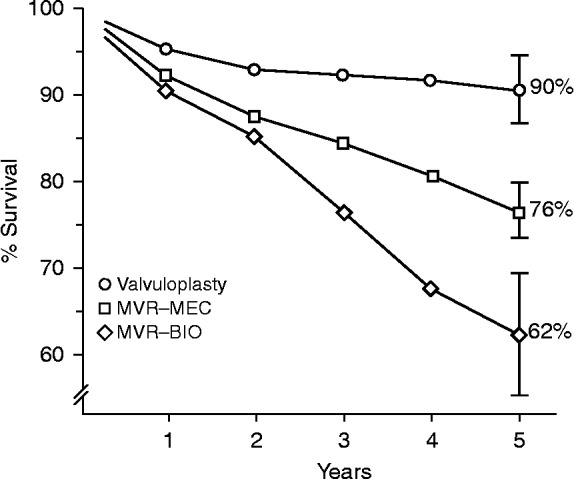
Five-year survival of patients submitted to mitral valvuloplasty, and to mitral valve replacement with mechanical valves (MVR-MEC) or bioprostheses (MVR-BIO).
In this work, I aim at analysing the specificities of rheumatic mitral valve disease, with special emphasis on valve repair, its techniques and results, by comparison with those used and obtained in non-rheumatic cases.
The pathology of rheumatic mitral valve disease
Rheumatic mitral valves exhibit a different set of lesions by comparison with degenerative valves, because of the characteristic inflammatory process, which results in thickening of the leaflets and other components of the mitral valve apparatus, of variable degrees, and distorts and impairs the movements of the valve. Hence, the disease appears here in two forms - stenosis and regurgitation, or a combination of both.
In the case of regurgitant valves, the most frequent lesion, by far, is prolapse of the anterior leaflet, which is present in more than 90% of young patients and is most often caused by elongated chordae to its central and medial areas (A2 and A3). By contrast with degenerative disease, posterior leaflet prolapse is practically non-existing, except in cases with ruptured chordae due to infective endocarditis. On the contrary, this leaflet is often shortened in its width, sometimes resumed to a very narrow and thick strip of tissue. In more complex cases, the chordae may be thick and retracted, as may also be the papillary muscles, and the commissures may be fused in varying degrees. Often, the leaflets and subvalvular apparatus are a continuous mass of fibrous tissue (Figure 2). Finally, the annulus is dilated in 95% of the patients. It is widely accepted that dilatation occurs essentially in the posterior segment of the annulus, although there is some evidence that it may also occur in the anterior segment, especially in dilated cardiomyopathy.5 It is still subject to some degree of speculation whether dilatation is only a functional phenomenon or the result of direct involvement by the rheumatic process, as the annulus does not appear to retain the normal capacity of changing its shape during the cardiac cycle.
Figure 2.
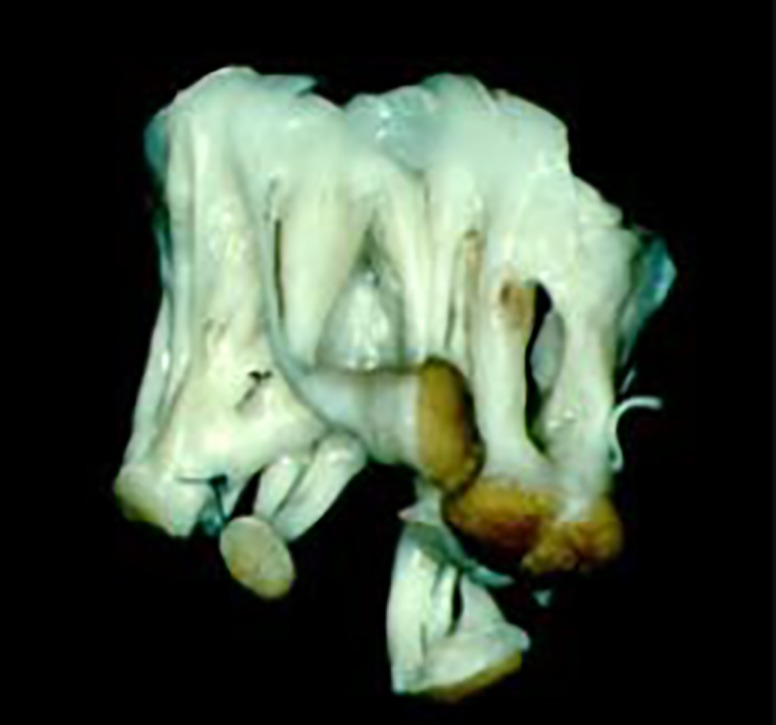
Surgically removed mitral valve with intense fibrosis and fusion of the leaflets and subvalvular apparatus.
In any case, the hallmark of the rheumatic pathology is simultaneous involvement of all components of the mitral apparatus (Table 1).
Table 1.
Spectrum of pathology in the rheumatic mitral valve disease in a South African population (1982-86). In the majority of cases, all components of the mitral apparatus are involved (reproduced from Antunes MJ, Doctoral Thesis 1987). MR = mitral regurgitation, MS = mitral stenosis.
| Pathology | MR (N = 128) | MR/MS (N = 47) | MS (N = 66) | |||
| N | % | N | % | N | % | |
| Annulus | ||||||
| - Dilatation | 121 | 94.5 | 27 | 57.4 | 8 | 12.1 |
| Leaflets | ||||||
| - Moderate thickening | 5 | 3.9 | 35 | 74.5 | 27 | 40.9 |
| - Marked thickening | - | - | 6 | 12.8 | 15 | 22.7 |
| - Redundant | 15 | 11.7 | - | - | - | - |
| - Fused commissures | 5 | 3.9 | 47 | 100.0 | 66 | 100.0 |
| - Calcium | - | - | 6 | 12.8 | 8 | 12.1 |
| Chordae tendineae | ||||||
| - Elongated | 107 | 83.6 | 21 | 44.7 | - | - |
| - Ruptured | 13 | 10.2 | 2 | 4.3 | - | - |
| - Thickened/Fused/Shortened | 23 | 18.0 | 36 | 76.6 | 43 | 65.2 |
In very young patients, some appearing at as early as 3 or 4 years of age with acute rheumatic fever, the disease is marked by a very acute and active inflammatory process with oedema of the tissues, and the characteristic rheumatic vegetations/nodules in the free edge of the leaflets or in the chordae tendineae, and in the other valves, especially the tricuspid (Figure 3).
Figure 3.
Characteristic rheumatic vegetations in the free edge of the leaflets of the tricuspid valve.
In these patients, the predominant valve dysfunction is regurgitation. The very inflamed and oedematous tissues make repair challenging and subjected to a long and painful learning curve, and the medium and long-term results are usually not satisfactory, reoperation being frequently required, especially when the acute carditis subsists or recurs. However, the very young age makes repair even more essential in these cases, if for no other reason to delay prosthetic valve implantation by a few years until the patient reaches a more “mature” age.
By contrast, in the cases of stenosis, usually appearing later after the rheumatic fever, the leaflets may be pliable and the chordae normal, the valve involvement being limited to fusion of the free edge of the leaflets, commencing at commissural level and extending centrally, which makes treatment by commissurotomy a simple procedure. This was exemplified by the closed commissurotomy used in the years 50 to 80 with excellent results, thereafter replaced by percutaneous balloon commissurotomy, currently the most used method, because of its much less invasive nature, although open (surgical) commissurotomy still produces the best results in our experience, especially in more complex anatomies, because the direct exposure of the valve permits not only de commissural split but also the division of fused chordae and papillary muscles (Figure 4).6
Figure 4.
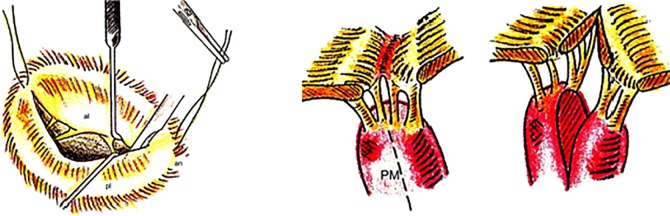
Open mitral commissurotomy, with splitting of the papillary muscles.
A completely different group are the patients who are still presenting in their fifties with valves that are late sequels of rheumatic fever. Most of these patients have severely fibrotic and/or calcified valves, with variable degrees of stenosis and/or regurgitation, mostly impossible to repair, but some still have pliable leaflets, often with no prolapse and only a dilated annulus in approximately half of the cases, where implantation of a remodelling ring is all that is necessary to correct the regurgitation. In these cases, the repair is much more durable. Di Bardino and co-workers, from Boston, were even able to demonstrate similar long-term survival after repair for rheumatic and for valves with degenerative disease.7
How far can we go in repairing rheumatic mitral valves?
The variety and complexity of the rheumatic lesions make valve repair much more demanding than that of degenerative disease, thus far less accepted by the majority of cardiac surgeons.8 In fact, initial experiences showed less good results after repair of rheumatic than with degenerative valves. However, as discussed above, reports coming from different parts of the world confirmed better results with repair than with replacement of the mitral valve in these populations. Furthermore, recent reports show better rates of repair and improved results, derived from increased experience and newer and refined techniques, as described later in this work.
The results of mitral valve repair for rheumatic disease vary with the age of the patients, which is different from series to series as a consequence of the different stages of the disease described above. Wang et al, from Taiwan, compared the midterm results of rheumatic mitral repair versus replacement (mean age at operation 49.7 ± 13.2 years) in a more economically developed society, demonstrating better outcomes after repair.9 In these conditions, the patients are older and the rheumatic process is stabilized, hence the longer term durability of the repair when compared to those obtained in the younger patient groups of the less developed countries. However, Talwar et al, from New Delhi, India, have similarly produced excellent results in children with a mean age of 11 years, having observed a 90% survival and up to 15 years of follow-up.10 Other groups, from different parts of the world, have also recently reported excellent results in these young populations (see Results below). However, a greater incidence of recurring regurgitation requiring reoperation is usually observed in these patients.
Techniques of repair
The foundations for the modern surgical treatment of mitral regurgitation were laid in the late 1970's by Carlos Duran and Alain Carpentier. These were based on a comprehensive methodology aimed at treating all components of the mitral valve apparatus involved in the disease: leaflets, chordae/papillary muscles and annulus (Figure 5).
Figure 5.
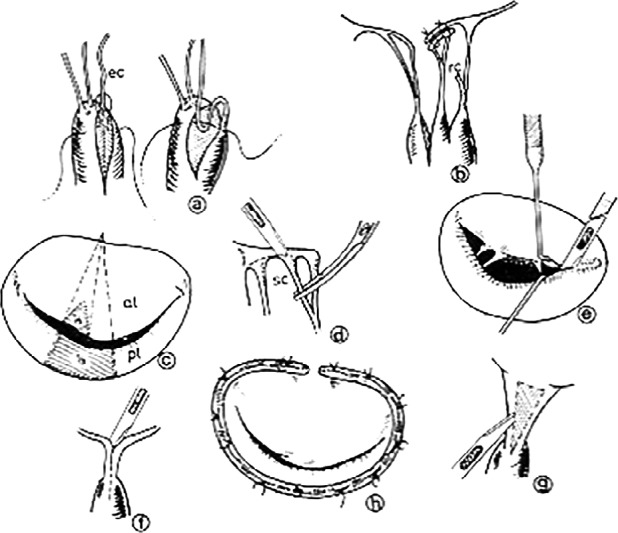
– Carpentier's comprehensive approach to mitral valve repair: a) shortening of chordae; b) transfer; of chordae; c) resesction of leaflet tissue; d) excision of secondary chordae; e) commissurotomy; f) splitting of fused chordae/ papillary muscles; g) fenestration of fused chordae; h) implantation of annuloplasty ring.
Working in Spain and France, respectively, these two surgeons were dealing with diverse pathology, but including a significant proportion, if not a majority, of rheumatic cases, still appearing in large numbers in Europe in those times. Their pioneer experiences were not easily accepted initially and it took a couple of decades before they were more universally adopted. Still now, the rate of mitral repair worldwide is probably inferior to 50% for all types of pathology, and even much lower for rheumatic valves, as can be inferred from the database of the Society of Thoracic Surgeons.11
The so-called “French repair”12 has since undergone several modifications and additions by Carpentier himself and by contributions from several surgical groups and individual surgeons, which have made the techniques more effective and reproducible, hence leading to better results.
With regards to repair of rheumatic mitral valve regurgitation, the following technical aspects deserve special consideration.
Leaflet prolapse
As indicated above, the main lesion in rheumatic regurgitation of young patients is anterior leaflet prolapse. The prolapse is usually more prominent or localized in the medial half of the leaflet (A2, A3) and results from elongation of the chordae originating from the respective head of the posteromedial papillary muscle. Elongation of the paramedial chordae originating from the anterolateral papillary muscle may also occur. The initial techniques for correction of this lesion included triangular resection of the central portion of the anterior leaflet, and/or chordal shortening. However, the amount of leaflet tissue is quite restricted in rheumatic cases, hence resection is rarely used here and repair is currently based solely on correction of the elongation of the chordae.
In this regard, Carpentier's initial techniques were oriented towards creating a trench in the corresponding papillary muscle head where the elongated portion of the chorda was buried. The chordae of the posterior leaflet, usually not elongated, were used for comparison. The techniques were relatively easy to perform and to replicate, with good initial results. But the progression of the disease frequently resulted in continued elongation, or the chordae had become too thin to sustain the tension and tended to rupture subsequently (Figure 6). Hence, this technique had an important rate of failure leading to recurrent mitral regurgitation.
Figure 6.
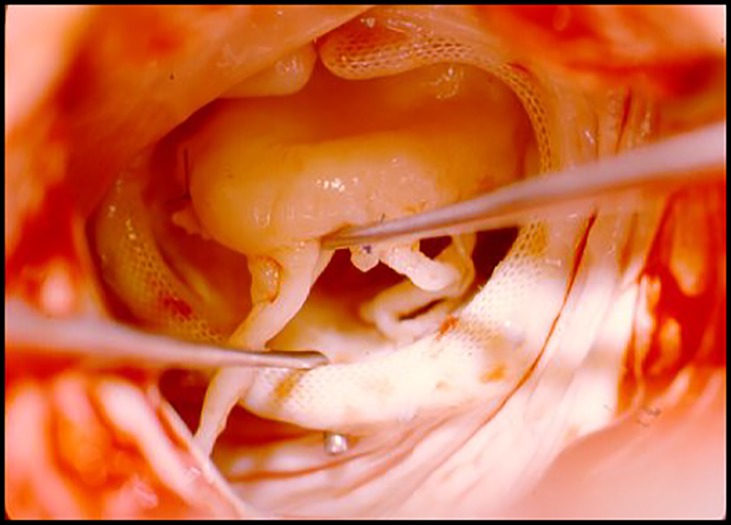
Rupture of chordae tendineae approximately 3 years after repair.
Today, the majority of surgeons treating rheumatic valves have opted for the implantation of artificial chordae for substitution or reinforcement of the ruptured or elongated chordae. These neo-chordae, originally suggested by Frater and colleagues, from New York, and made of autologous or heterologous pericardium, are now fashioned from poly(tetrafluorethylene) (PTFE) sutures, size 4/0 or 5/0.13,14 This material has been proven to be very pliable, inextensible and durable for more than twenty years in the treatment of degenerative mitral regurgitation,15 although isolated cases of rupture have been reported.16 Importantly, we have observed this durability even in very young patients operated on during growing age. It is as if the chordae had the capacity to grow with them.
The principal technical problem with the PTFE is the difficulty in adjusting the appropriate length and in knot-tying, because of the very smooth and slippery characteristics of the material. Several manoeuvers have been described by different authors to overcome these technical difficulties and the procedure has become standardized and reproducible, and can now be relatively easily performed after some experience (Figure 7).
Figure 7.

Neo-chordae made from poly(tetrafluorethylene) (PTFE) suture material (reproduced, with permission, from Zussa et al.17)
Leaflet restriction
Due to the relative shortage of leaflet tissue to bridge the mitral orifice, characteristic of rheumatic valves, several groups have advocated leaflet extension to enlarge the coaptation area of the leaflets and permit the use of larger annuloplasty rings (see below). Posterior leaflet extension was initially used and is still practiced by some, but anterior leaflet extension appears to be preferred by others. Good results have been reported with both techniques.18,19 In either case, the technique is relatively easy to master, the main principle is that it must be extended from commissure to commissure, using a continuous polypropylene suture.
The yet unresolved problem is the choice of material for the extension, which still lies between autologous (glutaraldehyde-fixed) and heterologous (bovine) pericardium. Both are subjected to calcification which, however, appears to be a much later occurrence by comparison with the untreated autologous pericardium that was used in the early days. Currently, the treated autologous pericardium appears to be the preferred material for most surgeons.
Annular dilatation
The vast majority of significantly regurgitant rheumatic mitral valves have annular dilatation and deformation of different degrees. The importance of annular dilatation in the genesis of the regurgitation is demonstrated by the fact that, by contrast to degenerative valves, rheumatic mitral valves are very rarely competent after completion of the leaflet component of the repair.
Hence, some kind of annular restriction and re-shaping is required to complete the correction. In contrast with degenerative disease, where all sorts of devices, from bands to soft and rigid or semi-rigid rings, complete or incomplete, or even no-rings have been successfully used, it is my belief that complete, pre-shaped rings are necessary in rheumatic valves, because of the need to reduce the antero-posterior diameter of the annulus. In our practice, we still use the original Carpentier-Edwards rings, while reserving the physio model for other types of pathology. Other manufacturers make models that are similar to these and, probably, giving identical results. The classic ring type has a shorter antero-posterior diameter, which helps to increase leaflet coaptation (Figure 8).
Figure 8.

Carpentier-Edwards classic mitral annuloplasty ring.
Some authors have used other types of rings with apparent good short and medium-term results, but these may not be sustainable in the long-term.
The use of ring sizes 30-34 is advised. When in doubt between two sizes, the larger size should be used. The use of too small rings is ill-advisable because the natural and progressive thickness of the leaflets makes them very restrictive to blood flow. Hence, I do not recommend the use of sizes smaller than 28. It is in these conditions where leaflet extension techniques may be especially indicated, permitting the use of larger rings.
Also by contrast with degenerative regurgitation, systolic anterior motion (SAM), which results from a large posterior leaflet pushing the anterior leaflet towards the left ventricular outflow tract, making it obstructive, occurs very rarely in rheumatic valves, because of the characteristic small width of the posterior leaflet.
Current results
Our recent experience in Coimbra, mostly with expatriates and/or patients referred from African countries for treatment, and in Maputo, Mozambique, as well as that acquired in occasional demonstrations in several other developing countries, have demonstrated that repair of mitral regurgitation is possible in most cases. From 1992 to 2012, 1,514 of the 1,874 rheumatic valves operated on first time in Coimbra were repaired, for a repair rate of nearly 81%, with excellent immediate and medium-term results. Long-term results are less easy to ascertain because of the traditional difficulty to follow these elusive populations. However, in a relatively well-controlled situation we were able to demonstrate a survival of 89% and survival free from reoperation of 85%, at 15 years (Figure 9).
Figure 9.
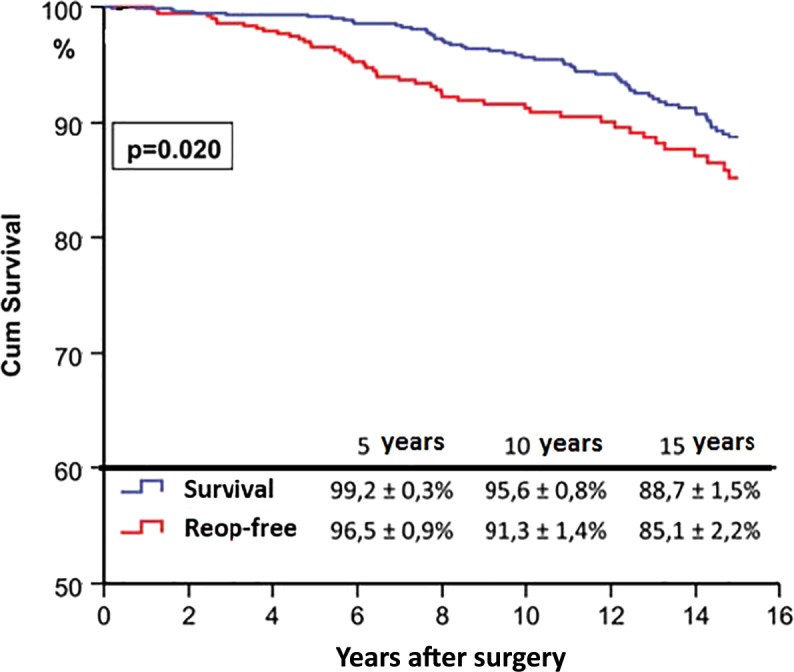
Overall survival and survival free from reoperation after mitral valve repair in Coimbra (1988–2013).
Other surgeons have confirmed this experience, even in younger patients of developing countries. The group of Khumar, from New Delhi, India, has repeatedly reported excellent results in young rheumatic patients, with a mean age of 11 years, with a 90% survival up to 15 years of follow-up (Figure 10).10,20
Figure 10.

Freedom from reoperation (upper curve) and from all events (lower curve (adapted from Talwar et al. 10).
Several groups from Brazil, South Africa, and other southern hemisphere countries have similarly reported excellent long-term survivals and good survival free from reoperation.21 Remenyi et al, from Auckland, New Zealand, recently reported their experience with patients with rheumatic heart disease under 20 years of age, with a follow-up to 19 years. They concluded that “repair offers a survival advantage, greater freedom from valve-related morbidity, and long-term durability that equals that of mitral valve replacement”.22
When reoperation is required for recurrent regurgitation, the valve is most of the times very fibrosed and distorted, thus with some degree of stenosis which, in some cases, may even be the predominant lesion (Figure 11).
Figure 11.
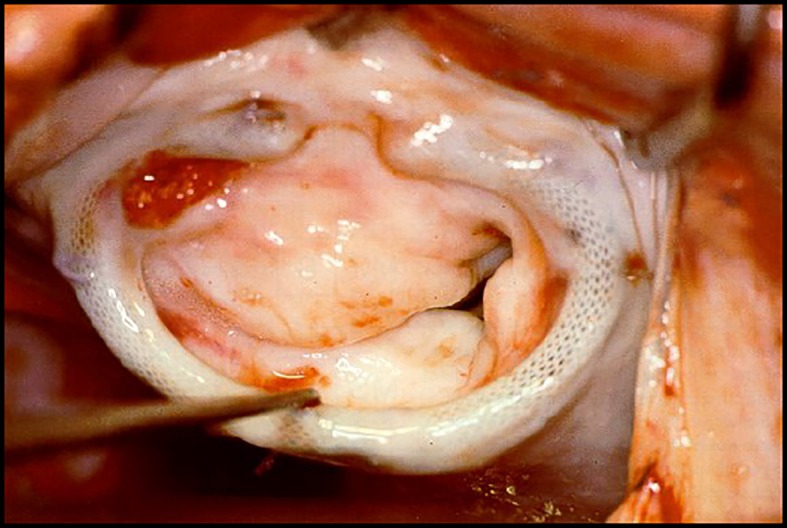
Mitral valve four years after valvuloplasty in a 23-year old patient, showing severe fibrosis and retraction of the leaflets.
Thus, re-repair is not feasible in the majority of cases, unless the regurgitation results from an obvious technical error during the initial surgery or there is another easily repairable abnormality. But replacement of a previously repaired valve is, generally speaking, technically much easier than that of a previously replaced valve and is associated to lower morbidity and mortality rates, another advantage of an initial repair policy.
Conclusions
The evolution of the techniques, including avoidance of anterior leaflet resection, use of PTFE chordae vs. shortening procedures, use of pre-shaped (rigid) rings, together with surgeons' greater experience, have greatly contributed to continuous improvement in the rate of repair and better results of surgery for rheumatic mitral valve regurgitation. In parallel, better anti-failure therapy and improved rheumatic fever prophylaxis contributed to altering the course of the disease, also contributing to better long-term outcomes.
As recently written by Dr Khumar, “…in children with rheumatic heart disease, conservative surgery is the best option whenever feasible. Surgeons must develop an attitude and interest in valve repair techniques that can be easily learned. Patients who undergo valve repair at an early age are at risk of requiring additional surgery over time. Mechanical valve replacement, nonetheless, should be reserved for situations where more conservative approaches are not feasible”.23
It is certainly worthwhile repairing the rheumatic mitral valve.24 The numbers of valves requiring replacement decreases as the experience of surgeons with mitral valve repair increases. Willingness to perform these challenging repairs is a fundamental challenge.
References
- 1.Nkomo VT. Epidemiology and prevention of valvular heart diseases and infective endocarditis in Africa. Heart. 2007;93:1510–1519. doi: 10.1136/hrt.2007.118810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antunes MJ, Wessels A, Sadowski RJ, Schutz JG, Vanderdonck KM, Oliveira JM, Fernandes LE. Medtronic Hall valve replacement in a third world population group. A review of the performance of 1,000 valves. J Thorac Cardiovasc Surg. 1988;95:980–93. [PubMed] [Google Scholar]
- 3.Antunes MJ, Santos LP. Performance of glutaraldehyde-preserved porcine bioprosthesis as a mitral valve substitute in a young population group. Ann Thorac Surg. 1984;37:387–392. doi: 10.1016/s0003-4975(10)60761-8. [DOI] [PubMed] [Google Scholar]
- 4.Antunes MJ, Magalhães MP. Colsen PR, Kinsley RH. Valvuloplasty for rheumatic mitral valve disease. A surgical challenge. J Thorac Cardiovasc Surg. 1987;94:44–56. [PubMed] [Google Scholar]
- 5.Hueb AC, Jatene FB, Moreira LF, Pomerantzeff PM, Kallas E, de Oliveira SA. Ventricular remodeling and mitral valve modifications in dilated cardiomyopathy: new insights from anatomic study. J Thorac Cardiovasc Surg. 2002;124:1216. doi: 10.1067/mtc.2002.125342. [DOI] [PubMed] [Google Scholar]
- 6.Antunes MJ, Vieira H, de Oliveira JF. Open mitral commissurotomy: the “golden standard”. J Heart Valve Dis. 2000;9:472–477. [PubMed] [Google Scholar]
- 7.Di Bardino DJ, El Bardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg. 2010;139:76–84. doi: 10.1016/j.jtcvs.2009.08.058. [DOI] [PubMed] [Google Scholar]
- 8.Antunes MJ. Repair of rheumatic mitral valve regurgitation: how far can we go? Eur J Cardio-thorac Surg. 2013;44:689–691. doi: 10.1093/ejcts/ezt012. [DOI] [PubMed] [Google Scholar]
- 9.Wang YC, Tsai FC, Chu JJ, Lin PJ. Midterm Outcomes of Rheumatic Mitral Repair Versus Replacement. Int Heart J. 2008;49:565–576. doi: 10.1536/ihj.49.565. [DOI] [PubMed] [Google Scholar]
- 10.Talwar S, Rajesh MR, Subramanian A, Saxena A, Kumar AS. Mitral valve repair in children with rheumatic heart disease. J Thorac Cardiovasc Surg. 2005;129:875–879. doi: 10.1016/j.jtcvs.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Gammie JS, Sheng S, Griffith BP, Peterson ED, Rankin JS, O'Brien SM, Brown JM. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2009;87:1431–1437. doi: 10.1016/j.athoracsur.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 12.Carpentier A. Cardiac valve surgery–the “French correction”. J Thorac Cardiovasc Surg. 1983;86:323–337. [PubMed] [Google Scholar]
- 13.Frater RWM, Gabbay S, Shore D, Factor S, Strom J. Reproducible replacement of elongated or ruptured mitral valve chordae. Ann Thorac Surg. 1983;35:14. doi: 10.1016/s0003-4975(10)61426-9. [DOI] [PubMed] [Google Scholar]
- 14.Bortolotti U, Milano AD, Frater RW. Mitral valve repair with artificial chordae: a review of its history, technical details, long-term results, and pathology. Ann Thorac Surg. 2012;93:684–691. doi: 10.1016/j.athoracsur.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Salvador L, Mirone S, Bianchini R, Regesta T, Patelli F, Minniti G, Masat M, Cavarretta E, Valfrè C. A 20-year experience with mitral valve repair with artificial chordae in 608 patients. J Thorac Cardiovasc Surg. 2008;135:1280–1287. doi: 10.1016/j.jtcvs.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Coutinho GF, Carvalho L, Antunes MJ. Acute mitral regurgitation due to ruptured ePTFE neo-chordae. J Heart Valve Dis. 2007;16:278–281. [PubMed] [Google Scholar]
- 17.Zussa C, Frater RW, Polesel E, Galloni M, Valfré C. Artificial mitral valve chordae: experimental and clinical experience. Ann Thorac Surg. 1990;50:367–373. doi: 10.1016/0003-4975(90)90476-m. [DOI] [PubMed] [Google Scholar]
- 18.Zegdi R, Khabbaz Z, Chauvaud S, Latremouille C, Fabiani JN, Deloche A. Posterior leaflet extension with an autologous pericardial patch in rheumatic mitral insufficiency. Ann Thorac Surg. 2007;84:1043–1044. doi: 10.1016/j.athoracsur.2006.12.058. [DOI] [PubMed] [Google Scholar]
- 19.Dillon J, Yakub MA, Nordin MN, Kong PK, Moorthy PSK. Leaflet extension in rheumatic mitral valve reconstruction. Eur J Cardiothorac Surg. 2013;44:682–689. doi: 10.1093/ejcts/ezt035. [DOI] [PubMed] [Google Scholar]
- 20.Kumar AS, Rao PN, Saxena A. Results of mitral valve reconstruction in children with rheumatic heart disease. Ann Thorac Surg. 1995;60:1044–1047. doi: 10.1016/0003-4975(95)00486-5. [DOI] [PubMed] [Google Scholar]
- 21.Severino ES, Petrucci O, Vilarinho KA, Lavagnoli CF, Silveira Filho Lda M, Oliveira PP, Vieira RW, Braile DM. Late outcomes of mitral repair in rheumatic patients. Rev Bras Cir Cardiovasc. 2011;26:559–564. doi: 10.5935/1678-9741.20110045. [DOI] [PubMed] [Google Scholar]
- 22.Remenyi B, Webb R, Gentles T, Russell P, Finucane K, Lee M, Wilson N. Improved long-term survival for rheumatic mitral valve repair compared to replacement in the young. World J Pediatr Congenit Heart Surg. 2013;4:155–164. doi: 10.1177/2150135112474024. [DOI] [PubMed] [Google Scholar]
- 23.Kumar AS. Surgical options in rheumatic mitral valve disease in children. A surgeon's perspective. World J Ped Congenit Heart Surg. 2014;5:180–184. doi: 10.1177/2150135113512333. [DOI] [PubMed] [Google Scholar]
- 24.Antunes MJ. Valve repair for rheumatic mitral regurgitation: still worthwhile? J Heart Valve Dis. 2011;20:254–256. [PubMed] [Google Scholar]



