Abstract
Three patients with the clinical and investigation features of facial onset sensory and motor neuronopathy (FOSMN) syndrome are presented, one of whom came to a post-mortem examination. This showed TDP-43-positive inclusions in the bulbar and spinal motor neurones as well as in the trigeminal nerve nuclei, consistent with a neurodegenerative pathogenesis. These data support the idea that at least some FOSMN cases fall within the spectrum of the TDP-43 proteinopathies, and represent a focal form of this pathology.
Key Words: Facial onset sensory and motor neuronopathy, Motor neurone disease, TDP-43 proteinopathy
Introduction
Facial onset sensory and motor neuronopathy (FOSMN) is a slowly progressive disorder characterised by facial sensory deficits which may spread to affect the neck, the upper trunk and the limbs, with the development later in the course of the disease of bulbar symptoms such as dysarthria and dysphagia, muscle weakness, cramps and fasciculation [1]. Because of these latter features, parallels between FOSMN and motor neurone disease (MND) have been drawn, although sensory features are not a feature of MND and the onset and progression of FOSMN appear to be slower than those of MND [2].
The pathogenesis of FOSMN is uncertain. Based on the limited currently available clinical and investigative evidence [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12] and the lack of therapeutic response to immunosuppressive agents in most (but not all) [4] cases, an underlying neurodegenerative process has been suggested in FOSMN, although cases with neuropathological examinations are very few [2, 6, 9]. We report 3 further cases of FOSMN, one with a post-mortem examination, suggesting that at least some of these cases fall within the spectrum of the transactive response DNA binding protein 43 (TDP-43) proteinopathies.
Case 1
A 62-year-old man presented with a 3-year history of perioral numbness and paraesthesia progressing to involve all divisions of the trigeminal nerve bilaterally. Over a similar interval, he also developed bilateral global upper limb weakness, muscle twitching and cramps. Twelve months prior to presentation, he described progressive slurring of speech, dysphagia and weight loss of 7 kg as well as complete loss of taste and smell. There was no past medical history of note.
A neurological examination showed a decreased sensation to pinprick and light touch in all divisions of the trigeminal nerve bilaterally. Corneal reflexes were absent. Eye movements were normal. He had a spastic dysarthria with tongue atrophy and fasciculation. There was wasting of the periscapular muscles bilaterally, with widespread fasciculation in the upper limbs and occasional fasciculation in the quadriceps bilaterally. Power was preserved throughout. Reflexes were globally brisk with bilateral flexor plantar responses.
Normal or negative investigations included an autoantibody screen, anti-ganglioside antibodies, serum immunoglobulins, cerebrospinal fluid (CSF) analysis (opening pressure, cell count, protein, glucose), and MRI of the brain and the cervical spine. Needle electromyography and nerve conduction studies (EMG/NCS) showed widespread neurogenic changes without evidence of a neuropathy. Motor evoked potentials were normal.
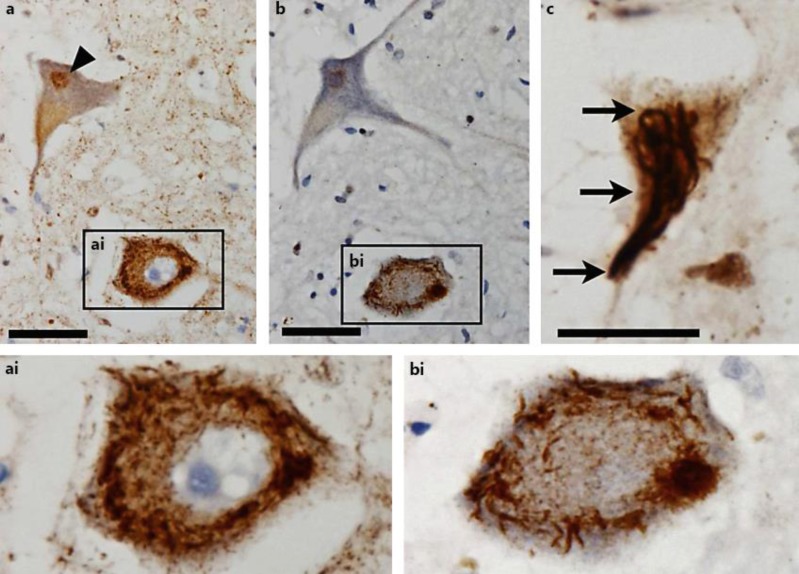
The patient died at the age of 64, approximately 6 years after symptom onset. A neuropathological examination exhibited that the number of cervical, thoracic, and lumbar spinal motor neurones was reduced by approximately 40%. Some of the remaining spinal cord motor neurones displayed characteristic intracytoplasmic extranuclear inclusions that stained positive for TDP-43 (fig. 1a) as well as for p62 antibodies (fig. 1b); TDP-43 inclusions were also seen in hypoglossal nucleus motor neurones (fig. 1c). In addition, p62 and TDP-43-positive inclusions could be detected in neurones of the trigeminal nuclei (not shown).
Fig. 1.
Motor neurones in the spinal cord (a, ai, b, bi) and hypoglossal nucleus (c) of case 1. Immunohistochemistry for TDP-43 (a, ai, c) and p62 (b, bi). Physiological intranuclear TDP-43 staining (arrowhead in a) and pathological aggregations of phosphorylated TDP-43 (arrows in c) are seen, with strong intracytoplasmic extranuclear positivity for TDP-43 (ai) and p62 (bi). Original magnification: ×400 (a, b), ×600 (c); scale bars: 50 μm (a, b), 20 μm (c).
Case 2
A 38-year-old woman presented with a 3-month history of right lower facial and oral cavity numbness. Over the next 3 years, she developed sensory loss involving the upper and lower limbs as well as a wasting of the small muscles of the hand. She described decreased upper limb strength, and it progressed to a point when she was unable to wash her hair or dress without help. There was a progressive spread of sensory loss to her arms, neck and trunk. Over the next 8 years, she developed lower limb weakness, more prominent distally than proximally; she mobilised with a frame.
A neurological examination showed mild dysarthria, but no tongue weakness. Pinprick sensation was reduced in all divisions of the trigeminal nerve bilaterally. Horizontal saccades were slow, with normal vertical saccades and smooth pursuit eye movements. There was fasciculation and weakness of the facial muscles bilaterally. Her neck flexion was weak. In the upper limbs, there was fasciculation, global muscle wasting and flaccid weakness with finger drop of the ring finger and little finger on the right. In the lower limbs, the weakness was more marked distally than proximally and with areflexia. There was a reduced pinprick and temperature sensation to the knees and elbows and over the trunk, with preserved joint position and vibration sensation.
Normal or negative investigations included autoantibody screen, anti-neuronal and anti-GM1 antibodies, serum protein electrophoresis, lysosomal storage enzymes, HIV and syphilis serology, CSF examination, MRI of the brain, and neurogenetic tests (PMP-22, P0, CMT-X, mitofusin, SCA 1, 2, 3, 6, 7, 17, and frataxin). An MRI of the spinal cord showed an atrophy of the cervical and thoracic cord with no evidence of a syrinx. A muscle biopsy (left quadriceps) showed denervation changes only, with no evidence of COX negative or abnormal NADH staining and no polyglucosan bodies. EMG/NCS showed neurogenic changes and a loss of sensory action potentials.
There was no clinical response to 3 monthly intravenous immunoglobulin (IVIg) infusions given over 12 months.
Case 3
A 69-year-old man presented with a 4-month history of numbness affecting his left cheek, gradually progressing to involve the right cheek, tongue, jaw, and forehead, associated with difficulty swallowing.
A neurological examination showed a reduced sensation to pinprick and light touch throughout the distribution of the trigeminal nerve bilaterally, and also over the tongue. Speech was dysarthric. An initial examination was otherwise normal, but over the following year, he developed wasting and fasciculation of the tongue and widespread wasting of the upper limbs. Reflexes remained symmetrical with flexor plantar responses. His dysphagia deteriorated over the same interval, causing a weight loss of 19 kg, requiring a percutaneous endoscopic gastrostomy insertion for nutritional support.
Normal or negative investigations included autoantibody screen, thyroid autoantibodies, onconeural and anti-glutamic acid decarboxylase antibodies, serum electrophoresis, serum angiotensin-converting enzyme, complement, HIV and lyme serology, brain MRI, CT of the chest/abdomen/pelvis, bronchoscopy, and CSF analysis. EMG/NCS showed no neurogenic changes 1 year after symptom onset; further electrophysiological examination after onset of the tongue and upper limb fasciculation was declined.
There was no response to treatment with steroids and IVIg; treatment with riluzole was subsequently commenced.
Discussion
We have presented 3 cases with clinical and investigation features consistent with the syndrome of FOSMN first described by Vucic et al. in 2006 [1], namely trigeminal sensory symptoms followed by a lower motor neurone disorder with bulbar onset (table 1). None of our patients had any clinical features of autonomic dysfunction.
Table 1.
Summary of clinical characteristics and treatment response
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Gender/age at onset, years | Male/62 | Female/38 | Male/69 |
| Disease duration, years | Deceased at 6 years | Alive at 16 years | Alive at 6 years |
| Trigeminal sensory symptoms | Yes: first symptom | Yes: first symptom | Yes: first symptom |
| Bulbar symptoms | Yes | Yes | Yes |
| Neck flexion weakness at last follow-up | No | Yes | No |
| Fasciculation and wasting at last follow-up | Yes | Yes | Yes |
| Upper limb weakness at last follow-up | No | Yes | No |
| Lower limb weakness at last follow-up | No, but fasciculation present | Yes | No |
| Upper motor neurone signs at last follow-up | No, except brisk reflexes | No | No |
| Immunotherapy | No treatment | No improvement with IVIg | No improvement with steroids/IVIg |
The characteristic features in the limited number of cases reported to date [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12] are those of a sensory and motor neurone disorder, with sensory loss in the trigeminal nerve distribution, with subsequent peripheral facial weakness which may spread to affect other bulbar muscles, neck, upper and lower limbs. The disease is, with exceptions, usually slowly progressive (but with exceptions) [8]. Onset is usually in later life, although childhood onset has been described [11].
Neurophysiological findings in FOSMN syndrome include diminished sensory nerve action potential amplitudes in the upper limbs. There are also reports of widespread axonal loss on needle EMG testing. Blink reflexes have also been noted to be abnormal in the majority of patients identified with FOSMN (not examined in our cases). A selective loss of myelinated trigeminal nerve fibres with sparing of unmyelinated fibres has been reported [12].
The nosology of FOSMN is uncertain. Despite the facial sensory onset, some authors conceptualise FOSMN as a lower motor neurone form of MND. Sensory impairment is detected in some patients with familial MND associated with SOD1 gene mutations [13, 14]. A case with the clinical and neurophysiological features of FOSMN with a heterozygous D90A-SOD1 mutation has been reported [10]. Some cases of FOSMN have gone on to develop upper motor neuron signs [5]. Other authors argue that mechanisms unrelated to MND underpin the pathophysiology of FOSMN.
Neuropathological data on FOSMN are sparse. One case reported in the abstract by Bosch et al. [6] had neuronal loss and gliosis with TDP-43-positive cytoplasmic neuronal and glial inclusions in the hypoglossal nucleus and cervical motor neurones, as well as incidental changes of Lewy body disease. The case presented by Sonoda et al. [9] showed a severe degeneration of the nuclei of the right trigeminal nerve and the right facial nerve as well as widespread TDP-43-positive glial inclusions in the brainstem tegmentum. Neurones in the hypoglossal nerve nuclei were also shrunken and lost, with TDP-43-positive neuronal inclusions. Neuronal loss and gliosis were prominent in the anterior horns, predominantly in the cervical cord, with TDP-43-positive skein-like inclusions. These cases support a neurodegenerative pathogenesis, akin to that in MND. However, in contrast, Vucic et al. [2] found a widespread degeneration of sensory and motor neurones in 5 cases, but with no evidence of intraneuronal inclusions such as TDP-43, Bunina bodies or ubiquitin inclusions, nor inflammation or amyloid deposition, and felt that novel mechanisms, unrelated to MND, were involved.
The neuropathological findings in our case 1 are similar to those reported by Sonoda et al. [9], with a widespread loss of spinal motor neurones and characteristic TDP-43 inclusions in some motor neurones, which also stained positive for p62 antibodies. In addition, TDP-43-and p62-positive inclusions could be detected in some neurones of the trigeminal nuclei. These data suggest that at least some FOSMN cases fall within the spectrum of the TDP-43 proteinopathies, which encompasses MND and some forms of frontotemporal dementia with or without MND [15]. FOSMN may therefore represent a focal form of MND (progressive course, affecting contiguous regions, fasciculation and denervation), but with slow onset and progression and a relatively good prognosis with the appropriate supportive care. To pursue this potential nosology, it might be of interest to examine cognitive function in FOSMN cases, specifically looking for subtle subclinical signs of frontal dysfunction as found in some MND cases [16].
However, since FOSMN has rightly been described as a syndrome [1], this does not exclude the possibility that other examples of FOSMN may have a different pathogenesis, including possibly inflammatory disorders which may on occasion respond to immunotherapy [4]. Anti-ganglioside antibodies were checked in 2 of our patients (cases 1 and 2) and were negative. Nevertheless, an assay of anti-ganglioside antibodies, especially those associated with facial and bulbar weakness (GD1a and GT1a respectively), might be reasonable in FOSMN cases, although unlike FOSMN, the associated clinical syndromes are not chronic progressive disorders.
Further neuropathological studies are critical to clarify disease mechanisms. We suggest that this might be facilitated by identifying possible cases of FOSMN, for example cases currently being followed up with a diagnostic label of ‘atypical’ or ‘benign’ bulbar MND. The possibility of identifying a subpopulation of patients with a better prognosis mandates searching MND clinic databases for such patients as well as a careful review of the history for sensory symptoms in each new ‘bulbar MND’ case.
Disclosure Statement
The authors declare no competing interests.
Acknowledgments
We thank Dr. Radhika Manohar for the electrophysiological assessments. This work was supported by the Wellcome Trust (grant No. 089893/Z/09/A).
References
- 1.Vucic S, Tian D, Chong PS, et al. Facial onset sensory and motor neuronopathy (FOSMN syndrome): a novel syndrome in neurology. Brain. 2006;129:3384–3390. doi: 10.1093/brain/awl258. [DOI] [PubMed] [Google Scholar]
- 2.Vucic S, Stein TD, Hedley-White ET, et al. FOSMN syndrome: novel insight into disease pathophysiology. Neurology. 2012;79:73–79. doi: 10.1212/WNL.0b013e31825dce13. [DOI] [PubMed] [Google Scholar]
- 3.Isoardo G, Troni W. Sporadic bulbospinal muscle atrophy with facial-onset sensory neuropathy. Muscle Nerve. 2008;37:659–662. doi: 10.1002/mus.20991. [DOI] [PubMed] [Google Scholar]
- 4.Hokonohara T, Shigeto H, Kawano Y, Ohyagi Y, Uehara M, Kira J. Facial onset sensory and motor neuronopathy (FOSMN) syndrome responding to immunotherapies. J Neurol Sci. 2008;275:157–158. doi: 10.1016/j.jns.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Fluchere F, Verschueren A, Cintas P, et al. Clinical features and follow-up of four new cases of facial-onset sensory and motor neuronopathy. Muscle Nerve. 2011;43:136–140. doi: 10.1002/mus.21884. [DOI] [PubMed] [Google Scholar]
- 6.Bosch EP, Tracy J, Goodman B, Dyck JB, Tora C, Giannini C. Facial onset sensory and motor neuronopathy: a neurodegenerative TDP-43 proteinopathy? Neurology. 2012;78(Meeting Abstracts 1):P03–183. [Google Scholar]
- 7.Dobrey D, Barohn RJ, Anderson NE, et al. Facial onset sensorimotor neuronopathy syndrome: a case series. J Clin Neuromuscul Dis. 2012;14:7–10. doi: 10.1097/CND.0b013e31825f82b3. [DOI] [PubMed] [Google Scholar]
- 8.Barca E, Russo M, Mazzeo A, Terranova C, Toscano A, Girlanda P. Facial onset sensory motor neuronopathy: not always a slowly progressive disorder. J Neurol. 2013;260:1415–1416. doi: 10.1007/s00415-013-6894-2. [DOI] [PubMed] [Google Scholar]
- 9.Sonoda K, Sasaki K, Tateishi T, et al. TAR DNA-binding protein 43 pathology in a case clinically diagnosed with facial-onset sensory and motor neuronopathy syndrome: an autopsied case report and a review of the literature. J Neurol Sci. 2013;332:148–153. doi: 10.1016/j.jns.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Dalla Bella E, Rigamonti A, Mantero V, et al. Heterozygous D90A-SOD1 mutation in a patient with facial onset sensory motor neuronopathy (FOSMN) syndrome: a bridge to amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2014;85:1009–1011. doi: 10.1136/jnnp-2013-307416. [DOI] [PubMed] [Google Scholar]
- 11.Karakis I, Vucic S, Srinivasan J. Facial onset sensory and motor neuronopathy (FOSMN) of childhood onset. Muscle Nerve. 2014;50:614–615. doi: 10.1002/mus.24299. [DOI] [PubMed] [Google Scholar]
- 12.Truini A, Provitera V, Biasiotta A, et al. Differential trigeminal myelinated and unmyelinated nerve fiber involvement in FOSMN syndrome. Neurology. 2015;84:540–542. doi: 10.1212/WNL.0000000000001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakabayashi K, Horikawa Y, Oyake M, Suzuki S, Morita T, Takahashi H. Sporadic motor neuron disease with severe sensory neuronopathy. Acta Neuropathol. 1998;95:426–430. doi: 10.1007/s004010050820. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg-Vos RM, Visser J, Franssen H, et al. Sporadic lower motor neuron disease with adult onset: classification of subtypes. Brain. 2003;126:1036–1047. doi: 10.1093/brain/awg117. [DOI] [PubMed] [Google Scholar]
- 15.Geser F, Lee VM, Trojanowski JQ. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: a spectrum of TDP-43 proteinopathies. Neuropathology. 2010;30:103–112. doi: 10.1111/j.1440-1789.2009.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology. 2003;60:1094–1097. doi: 10.1212/01.wnl.0000055861.95202.8d. [DOI] [PubMed] [Google Scholar]