Abstract
The heart is subject to multiple sources of stress. To maintain its normal function, and successfully overcome these stresses, heart muscle is equipped with fine-tuned regulatory mechanisms. Some of these mechanisms are inherent within the myocardium itself and are known as intrinsic mechanisms. Over a century ago, Otto Frank and Ernest Starling described an intrinsic mechanism by which the heart, even ex vivo, regulates its function on a beat-to-beat basis. According to this phenomenon, the higher the ventricular filling is, the bigger the stroke volume. Thus, the Frank-Starling law establishes a direct relationship between the diastolic and systolic function of the heart. To observe this biophysical phenomenon and to investigate it, technologic development has been a pre-requisite to scientific knowledge. It allowed for example to observe, at the cellular level, a Frank-Starling like mechanism and has been termed: Length Dependent Activation (LDA).
In this review, we summarize some experimental systems that have been developed and are currently still in use to investigate cardiac biophysical properties from the whole heart down to the single myofibril. As a scientific support, investigation of the Frank-Starling mechanism will be used as a case study.
Introduction
For centuries, the heart has fascinated philosophers, medical doctors and scientists. This is a complex organ highly regulated by several “external” systems (central nervous, neurohormonal) and by an important “internal” system. Failures of these regulation systems are a major cause of death in our societies. Investigating its contractile properties has always been an important, but challenging goal. In 1866, Carl Ludwig and Elias Cyon, decided to take up the challenge to develop the first ex-vivo beating whole heart system1. They used this system to keep an excised and perfused frog heart beating for a relatively long period of time (i.e. several minutes) whilst isolated from the body. This major discovery has motivated other investigators to develop a variety of excised whole heart experimental apparatus. To date, “the Langendorff Heart” is by far the most famous heart perfusion setup that is still being used in laboratories. By using his system, Oscar Langendorff, in the 19th century, was able to maintain a perfused mammalian heart in a complete isolated state for several hours. By taking advantage of a similar experimental system, Otto Frank in Germany (1895) and Ernest Starling (1918) in England, discovered a crucial mechanism by which the left ventricle senses variations in its filling volume (i.e. pre-load) and responds by increasing its stroke volume2,3. This was the first experiment that established a direct relationship between the end-diastolic volume and the end-systolic pressure and has been known, as the Frank-Starling Law of the heart. Since then, numerous studies have shown alterations of this relationship to be associated with heart failure4. Based on these observations, the Frank-Starling law of the heart constitutes a crucial intrinsic mechanism by which the heart maintains its normal function on a beat-to-beat basis. The technological development has been a pre-requisite in the understanding of cardiac regulation, as demonstrated by Langendorff heart system.
Since the success of the whole heart perfusion apparatus, researchers have developed other experimental systems to explore different myocardium components from trabeculae to single myofibrils. It is of note however, that myocardium mechanical properties significance depends on the sample being used in a given experiment. For instance, a single myofibril preparation is preferred for actin-myosin interaction kinetics measurement. This is because calcium binding to the thin filament significantly reduces the apparent diffusion rate of this ion. Therefore, due to the small dimension of the single myofibril, contraction kinetics are not biased by calcium diffusion. Trabeculae and papillary muscles, on the other hand, are more appropriate for force-frequency relationship, and slow force response studies. Moreover, each preparation type requires a particular hardware setup in respect to its physiological working range. In the past decades, investigators have developed different experimental systems adapted to the various types of cardiac specimens (trabeculae muscles, papillary muscles, single cardiac cells, and single myofibrils)5. Many of them were unsatisfactory, too damaging for the tissue and too difficult to handle and were finally given up. Only a few techniques are now used around the world to investigate cardiac contractile properties and these will be discussed in this review. Towards this aim, we will be focusing on Length Dependent Activation as an example of a biophysical parameter that can be studied on these types of preparations.
According to Frank-Starling's law, under healthy conditions, increasing venous return, and therefore the filling pressure of the LV, results in increased stroke volume. Figure 1 shows a typical pressure-volume relationship (Fig. 1; solid curve). Following increased end-diastolic volume, the volume of blood ejected is increased beat-by-beat, this curve is shifted to the right and enlarged (Fig. 1; dashed curve). Interestingly, single cardiac cells, single myofibril, trabeculae and papillary muscles all show a positive correlation between sarcomere length and generated tension named Length Dependent Activation (LDA). Since LDA supports the Frank-Starling law of the heart6, unraveling the cellular and sub-cellular mechanisms involved in its regulation may help better understand the Frank-Starling Law alterations observed during physiological and pathophysiological stresses.
Figure 1.

Frank-Starling Law as observed on a typical pressure-volume loop. Solid curve; control pressure-volume loop shape at rest. Dashed curve; pressure-volume loop shape following increased diastolic volume. Dashed double arrow; increased stroke volume in response to increased ventricular volume (Frank-Starling Law). The ESPVR (End Systolic Pressure-Volume Relationship) is often used to estimate systolic function of the heart. The EDPVR (End Diastolic Pressure-Volume Relationship) is an indication diastolic function of the heart.
In this review article, we propose to provide an overview of some experimental approaches employed to investigate biophysical properties of myocardium samples. We will particularly emphasize on how researchers have progressed from studying the Frank-Starling law at the tissue level (i.e. trabeculae and papillary muscles), to single cell contractile biology, and finally to LDA and activation/relaxation dynamics at the single myofibril level.
Multicellular preparation (trabeculae and papillary muscles)
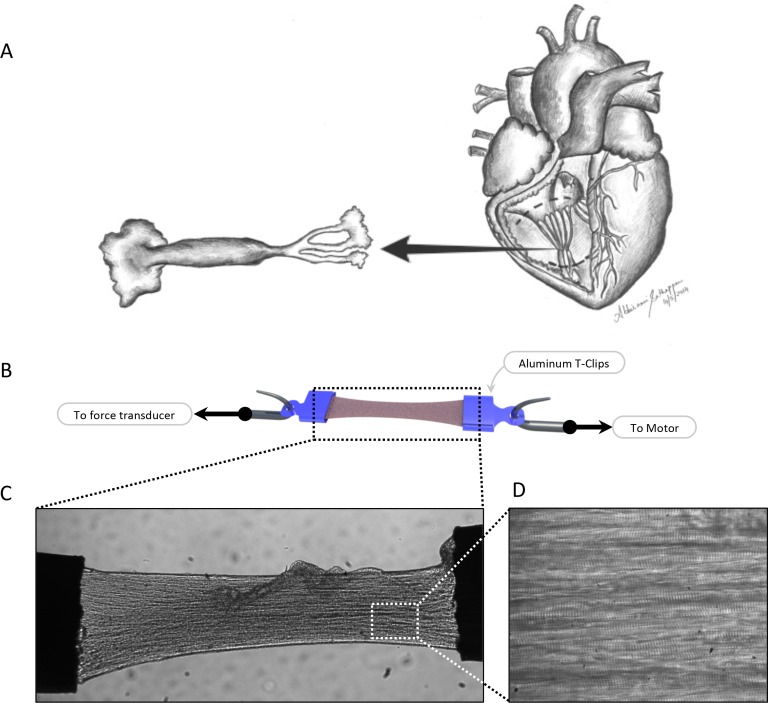
In the intact heart, the parameters measured in vivo such as pressure and volume relate to the parameters of force, length or velocity measured in less complex preparations such as cardiac multicellular preparations. Papillary muscles consist of major muscular trunks from which an average of six cordae tendinae project (Fig. 2). Located in both the right and the left ventricle, their function is to support valves and to prevent regurgitation7. Trabeculae muscles are divided into two types: the trabeculae carneae and the trabeculae septomarginalis. In the ventricle they help to reduce blood turbulence during systole. The trabeculae carneae cover the inside of the ventricle and are the structures used for research purposes of mechanical investigation8. Accordingly, in this review, the word trabeculae will be referring to the trabeculae carneae.
Figure 2.
Myocardium multicellular preparation. Single trabeculae and papillary muscles are isolated from the right ventricle (A). Using aluminum T-clips, the samples are mounted between a force transducer and a piezo actuator (B and C). The muscle quality can be estimated based on the overall muscle shape (10 × magnification; C) and presence of clear and uniform striations pattern (40 × magnification; D).
Trabeculae and papillary muscles offer a complete cardiac multicellular structure where one can measure the developed active and passive properties under different conditions. In these preparations, LDA can be estimated by acquiring developed tension while varying sarcomere length. Due to muscle thickness and non-uniformity, researchers had difficulties in acquiring accurate and dynamic sarcomere length in these preparations. In the mid-1970's, Krueger and Pollack employed the laser diffraction technique to achieve proper dynamic sarcomere length measurement on isolated rat papillary muscles9. In the early 80's, based on Krueger and Pollack's technique, Ter Keurs' group used small trabeculae muscles isolated from the right ventricle of rat heart to estimate LDA. In these experiments, trabeculae were mounted on spring-loaded stainless steel clips and force measured with a capacitive force transducer. A servo motor was used to vary the muscle length. The entire experimental setup was built on top of a modified inverted microscope to allow simultaneous microscopic and laser diffraction measurement. Since these muscles were thick and do not transmit light, a laser diffraction technique was used to estimate sarcomere length. When the muscle is exposed to the laser, it produces a particular diffraction pattern8,9. With a known calibration factor, the intensity distribution of the first diffraction order is used to estimate sarcomere length. The developed isometric twitching force was then correlated to both sarcomere length and extracellular calcium concentration.
With this technical approach many new mechanisms were revealed such as the first evidence that calcium release from sarcoplasmic reticulum and calcium binding to the myofilaments could both be responsible for LDA regulation8. Later, Allen and Kurihara showed that LDA is composed of an immediate response (few milliseconds) followed by a slower response (several minutes) if the stretch is maintained10. The early response is due to increase is myofilament calcium sensitivity and the slow force response is due to increased sarcoplasmic reticulum (SR) calcium release11,12.
Since then, the laser diffraction technique has been combined with other experimental techniques to explore additional mechanisms. For instance, along with the X-Ray diffraction technique, this experimental procedure has been used to explore the myofilament structural arrangement at different SL's13. With this approach researchers obtained information about the lattice spacing between actin and myosin filaments and even the position of the myosin head in the sarcomere13. Some old hypotheses were challenged and new ones were raised. For instance, based on the fact that a cardiac cell has a constant volume, it has long been thought that stretch would induce cell compression, reduction in interfilament lattice spacing that could increase acto-myosin formation probability and, consequently, contraction14. Using the X-Ray diffraction technique, Irving et al. observed that although lattice spacing decreases with length it does not correlate with the extent of developed force15.
During contraction, at a given SL, the extent of force generated by the myocardium is correlated with the number of active thin filament units (i.e. troponins); the number of strongly bound cross-bridges; and to a feedback combination between thin and thick filaments16–18. Knowing that each myosin head consumes one ATP molecule per cycle, by measuring ATP consumption, one can estimate the energy cost for each active contraction. Accordingly, cross-bridge performance can be estimated. To verify this hypothesis, researchers have improved Krueger's system described above to include a U.V. light source and PMT (PhotoMultiplier Tube) as part of the enzyme-coupled optical Unit. The updated system was capable of performing simultaneous measurements of force, sarcomere length, and energy consumption on skinned (i.e. permeabilized) trabeculae and papillary muscles19–21. This experimental setup is used to measure how many ATP molecules are converted to ADP molecules per contraction. The most direct way to estimate this reaction kinetics is to measure the NADH oxidation into NAD+. This oxidation is coupled with conversion of one molecule of ATP to one molecule of ADP and one inorganic phosphate. Knowing that NADH absorbs light at 340 nm, and NAD+ does not, one can practically measure the amount of NADH molecules being converted into NAD+ by measuring light absorbance at 340 nm. Accordingly, this approach revealed that stretch improves weak to strong forces generating cross-bridge formations improving thus, the developed tension20.
Multicellular preparations provided important information for cardiac biophysical contraction, particularly in relation to the role of the collagen extracellular matrix22 and also for the sub-molecular structure during contraction. However as summarized by Brady5 and Garnier23, interpretation of data from these preparations is limited by their complicated structure, non-uniformity and attachment-induced alteration. These complexities led to the development of cellular and molecular approaches providing a more simplified system.
Single cardiomyocyte preparations
Attaching a single cardiomyocyte (∼ 20 μm thick and ∼200 μm long) at both ends to tiny needle tips connected to mechanical transducers has been a significant challenge for researchers. The membrane of the cardiomyocytes is very fragile and attaching it to stainless steel needles or glass pipettes can easily cause irreversible damage to the membrane's structure and/or to the contractile apparatus. Moreover, cardiomyocytes are stretch/stress sensitive due to stretch-activated channels (SAC)24. In intact cardiomyocytes, damages or activation of SACs result in perturbation of intracellular calcium homeostasis leading to spontaneous contractions and potentially to cell death. Next, we discuss experimental systems developed to measure biophysical parameters on both intact and skinned cardiomyocytes.
Intact cardiomyocyte
Single intact cardiomyocytes offer a unique ‘stand-alone’ system that would tolerate variations in physiological calcium concentration. Due to their cellular integrity, single intact cardiac cells are useful in cases where simultaneous measurement of electrical, metabolic, and mechanical properties is needed (see for review5). In the late 80s and early 90s, different groups tried to attach a single intact cardiomyocyte while preserving its sarcolemmal integrity. These techniques used different strategies: suction micropipettes, silicon glue, impalement pipettes23. At that time, the most advanced technique was developed by Garnier and Le Guennec. Using carbon fibers, they established an easy to reproduce, yet efficient, method of attaching and stretching a single intact mammalian cardiac cell25. Later, Le Guennec's colleagues employed this technique to simultaneously measure electrical, calcium and mechanical properties of a single intact cell at different cell lengths26. Below is a brief description of the experimental system used. Attachment was performed by approaching a cell membrane with two carbon fibers with different pre-determined compliances. The more compliant fiber (∼80 μm/μN) was used to report the developed active and passive forces. Whereas, the less compliant (∼4 μm/μN) fiber was used for cell positioning and mechanical stretching. This experimental procedure has been commonly known as the ‘carbon fibers technique’ and has been successfully used by others to investigate biophysical properties of single intact cardiac cells27–31. In this system, the force developed by the cardiomyocyte was determined by following the position of the compliant carbon fiber by optical contrast; knowing the compliance (distance/force), the force can be estimated. Sugiura et al. have published a detailed protocol for single cardiomyocyte attachment procedures using the carbon fiber technique29.
This technique has been used to study LDA in intact myocytes25,27. To this aim, special attention to the adhesion efficiency must be considered. Although carbon fiber adhesion to the cell membrane allows twitch force measurement (Fig. 3), it is not strong enough to stretch the cell up to the required length for LDA evaluation (∼20 % from slack length). Rather, the cells start detaching at 10 to 15 % stretch from slack length. This may be due to different weaknesses including: the attachment cell-fiber capacity itself that is too weak to support such forces, the stretch-induced damages on the membrane at the level of the attachment, or to stretch activated channels and calcium entry. Consequently, researchers have put more effort into investigation of other attachment alternatives to counter balance these weaknesses. Recently, a biological adhesive known as MyoTak has been used to attach intact cardiac and skeletal myocytes32. This technique uses glass micro-rods coated with the MyoTak to attach single cardiac cells. When compared to the carbon fibers technique25, the results obtained with this technique show higher force recordings32. This may be due to the low compliance of glass micro-rods along with better signal processing units used with the MyoTak and the stronger adhesion between the cell membrane and the micro-rods. It is worth noting however, that although efficient, this technique is relatively new and suffers from a lower success rate. Despite the advantages and inconveniences of both methods, various studies have employed them to investigate the effect of stretch on cellular contractility. Confirming results in multicellular preparations, stretch of isolated myocytes has been shown to induce an immediate increase of passive and active tensions, the latter being attributed to improvement of myofilament responsiveness to calcium32. Given that, the early force response appears to be independent of calcium transient amplitude. However, it would be interesting to investigate whether calcium homeostasis is involved in the slow force response observed on multicellular specimens discussed earlier11. Unfortunately, due to the limitations previously discussed, researchers have not been able to record forces over a longer period of time (i.e. several minutes) on a single intact cardiomyocyte at higher SL. Indeed, in the case of weak attachment at long sarcomere length (∼2.1 μm), each active twitch causes the cell to slightly detach from the supporting tips. This phenomenon can be clearly visible on the force recording that shows sudden force drops. Future improvement of single cell attachment procedures will certainly help investigating contraction properties over longer periods of time and at longer sarcomere length.
Figure 3.
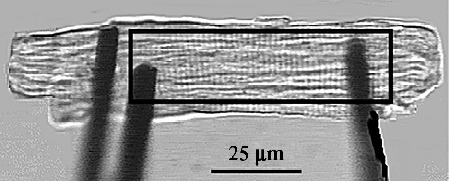
A typical attached intact cardiomyocyte with carbon fibers. The black box defines a ROI (Region of Interest) that is used by the FFT (Fast Fourier Transform) algorithm to compute sarcomere length. The edge detection algorithm is used to follow the compliant carbon fiber's displacement (right tip on the picture). The optical displacement is then converted to force27.
Mechanically and chemically permeabilized (i.e. skinned) cells
In an intact preparation the amplitude of contraction is influenced by several factors such as action potential properties, calcium homeostasis, myofilament properties, intracellular pH, and reactive oxygen species production33. To limit the influence of these parameters on contraction, the myocytes has been transformed and permeabilized to control the intracellular environment of the myofilaments. To this aim, researchers have developed the skinned cell experimental procedure (Fig. 4). This technique consists of altering the cellular membranes to expose the contractile machinery. The obtained cardiomyocytes are known as permeabilized or skinned cells.
Figure 4.

Single skinned cardiac cell attachment apparatus. Typical experimental system employed to investigate single skinned cardiac mechanical properties. The system consists of an inverted microscope surrounded by two attachment stainless iron needles. A, one needle is connected to a tiny force transducer (AE801 from Kronex), where the other is connected to a piezo actuator. In some cases, a rapid change (2 milliseconds) is needed to evaluate contraction kinetics, hence, the use of a piezo actuator. B, a perfusion system is used to expose the attached permeabilized cell to different calcium containing solutions. The inset image shows a typical attached cardiac cell at rest (∼ 1.9 microns).
First approaches used to remove the cell membrane were based on a challenging mechanical procedure34. This technique consists of approaching the sarcolemma with pulled glass pipettes, and to gently tear it off the cell; hence, the challenging aspect. Chemical skinning of cardiomyocytes is another method to remove the cellular membrane. It consists of incubating myocardium specimens in a solution containing a detergent (i.e. Triton X-100)35. Given its reproducibility and ease, the later approach has gained popularity in the field36.
Once skinned, the cardiomyocyte is exposed to custom-made intracellular solutions, containing various known calcium concentration, to activate the myofilaments and steady state developed tensions were measured. The resulted Tension-[Ca2+] relationship can be fitted to a Hill equation and the myofilament active properties estimated36,37.
To attach a skinned cardiomyocyte to a force transducer and motor, different types of glue are commonly used. The first is expanding polyurethane foam called GREAT STUFF® manufactured by The Dow Chemical Company that may not be so great for the cell due to the release of toxic solvents during the polymerization phase. The other is silicone-based glue manufactured by Dow Corning Corp classically in acetic acid solvent used as aquarium sealant. Finally, the group of Vassort has developed a non-injurious approach based on, UV-curing, optical adhesive37.
Experiments based on skinned cardiomyocytes attachment are used to estimate myofilament passive and active properties such as calcium sensitivity, maximal active force, and Hill coefficient. All these parameters can be extracted from the Force-[Ca2+] described above (Fig. 5). Myofilament sensitivity to calcium is derived from the pCa50 (-Log [Ca2+]) which is the calcium concentrations producing half maximal active tension. By evaluating the pCa50 at short (∼1.9 μm) and long (2.3 μm) sarcomere length, one can estimate ΔpCa50 = pCa50 at long SL – pCa50 at short SL. In skinned cardiac cells, ΔpCa50 is the main parameter upon which LDA is estimated. Increased LDA is manifested by a leftward shift of the force-pCa curve following stretch to lower calcium concentrations and upward to higher generated force (Fig. 5).
Figure 5.
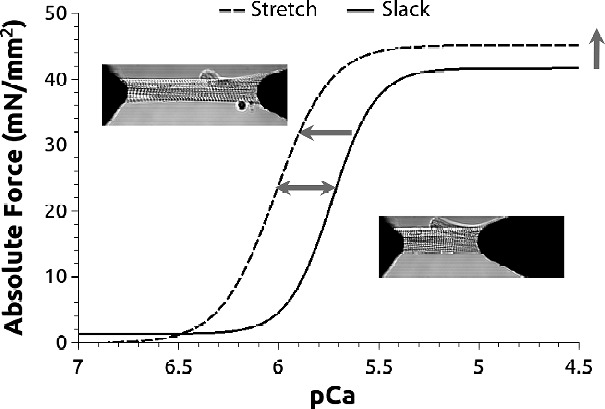
Force-Calcium relationship. Force is measured at slack (1.9 mm) and after stretch (2.3 mm) at different calcium concentration. At a given calcium concentration, the attached cardiac cell (insets) generates a corresponding active force. When this active force is potted against calcium concentration, a typical Tension-pCa curve is obtained. This curve can be fitted with a Hill equation and both pCa50 (calcium concentration generating 50 % maximal force) and nH (hill coefficient) are estimated. Stretch induces a leftward shift of this relationship (dashed curve; horizontal arrow). This shift reflects an increased myofilament sensitivity to calcium. DpCa50 is used to estimate LDA, the myofilament basis of Frank-Starling law of the heart. It is obtained by computing the difference between pCa50 at long and short sarcomere length (double horizontal arrow).
Experimentations performed on a single cardiac cell (intact and skinned) were helpful to specifically study a region within the LV thus unraveling specific mechanical properties across the left ventricular wall38,39. In addition to the spatial heterogeneity (i.e. fiber orientation)40, a mechanical heterogeneity spreads across the left ventricle from the epicardium (i.e. the LV outer layer) to the endocardium (i.e. the LV inner layer) (Fig. 6; solid line). Using a similar experimental system, previous studies have demonstrated that alterations of this transmural mechanical heterogeneity are associated with heart failure38,41–44. Interestingly, heart failure eliminates this heterogeneity mainly by altering the endocardium contractility (Fig. 6; red arrow). This property can be used by researchers to elaborate on treatment that specifically targets the altered endocardium (Fig. 6; green arrow)41,42,45.
Figure 6.

Transmural contractile heterogeneity across the mammalian left ventricular free wall. A, DpCa50: index of myofilament sensitization to calcium with stretch. Solid line shows the correlation between DpCa50 and passive tension in healthy conditions. Ischemic heart failure (HF) eliminates this gradient (dashed red line) by mainly affecting the Endocardium contractility (downward red arrow). Physiological (exercise) and pharmacological (SR33805) treatment both reverse HF effect by restoring the altered Endocardium cellular contractility (upward green arrow and dashed green line).
Although highly specific in terms of biophysical mechanics investigation, single skinned cardiomyocytes cannot answer all the questions related to the myofilament contractility. For instance, questions such as how fast the myofilaments activate/deactivate cannot be accurately addressed with this type of preparation. The main barrier to this is the calcium diffusion rate that induces a bias on the kinetics data. Accordingly, an isolated single myofibril is preferred to perform myofilament activation/deactivation kinetics. Next, we discuss experimental apparatus used on single myofibrils.
Single myofibril
The myofibril is the thinnest smallest contractile element found in the myocardium46. A cardiomyocyte is composed of a multiple myofibrils that contracts together to produce global cell contraction. Due to their size, myofibrils are ideal to measure rapid myofilaments activation/deactivation kinetics (i.e. in a millisecond range). Recently, Mateja and deTombe showed, in a mammalian single myofibril, that the rapid phase of myofilament activation develops within 5 milliseconds of calcium infusion47. This experiment wouldn't be feasible on a single cardiomyocyte due to longer calcium diffusion phenomena.
A single myofibril is composed of many sarcomeres. When observed under an electron microscope, the myofibril appears as a uniform alternation of dark (A-band) and light (I-band) bands that result in a striated appearance. The sarcomere defines one elementary contractile unit (Fig. 7). The alternation between the A and I bands is used to estimate sarcomere length. Due to their size and force ranges, researchers have developed specific experimental systems to isolate and collect mechanical information on mammalian myofibrils46,48–52. Figure 8 shows a typical single myofibril experimental system. The myofibril is mounted on two small tips. Similar to single cardiomyocytes, one tip is employed to apply mechanical strains (low compliance) the other tip is used to report developed force (high compliance). The myofibril is then approached with a double barrel perfusion pipette. Each barrel contains either activation (high calcium) or relaxation (low calcium) solutions (see blue and red flow jets on Fig. 8). The myofibril is then exposed to either solution at a given time. The perfusion pipette is mounted on a rapid actuator allowing rapid solution switching. The rapid solution switching, together with the double barrel pipette, help reduce any unwanted limitation on myofilament contraction kinetics that might be due to calcium ion diffusion. By applying this experimental protocol, the acquired force will be mostly the result of mechanical properties inherent within the contractile apparatus itself.
Figure 7.

Sarcomere structure. A) Electron microscope image revealing the contractile machinery organization in a cardiomyocyte. B) Schematic of the contractile apparatus (i.e. sarcomere). The contractile machinery is composed of thick (myosin) and thin (actin) filament. During contraction, thin filament slide over thick filament to produce cellular shortening. The shown dimensions are in micrometers (according to65).
Figure 8.
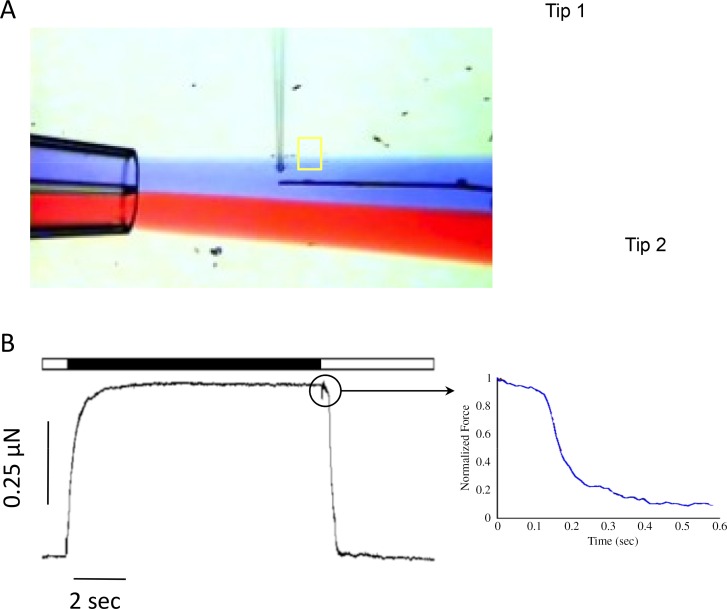
Single cardiac myofibril force measurement system. To measure force generated by a single myofibril, the sample is attached to two tips with distinguish compliance. A steep tips (A; Tip 1) is connected to a piezo motor where the more compliant tip (A; Tip 2) is used to report developed active and passive forces. A double barrel pipette is used to expose the myofibril to relax (low [Ca2+]; blue flow on panel A) and active (higher [Ca2+]; red flow on panel A) solutions. Perfusion solutions are quickly changed by rapidly moving the pipette's position back and forth between the two solutions. A typical resulted force record is shown on panel B. the horizontal bare shows current perfusion solution; white: relaxing solution and black: activating solution. Due to the sample's size and solution rapid switching, parameter such as activation and relaxation kinetics can be measured (B; zoom trace).
Clinical implications
Heart failure is a complex phenomenon involving multiple factors. It has long been known that excitation-contraction coupling is altered during HF. Hence, most early HF treatments are based on β-adrenergic receptor blockers and Angiotensin Converting Enzyme (ACE) inhibitors. These agents, although efficient to some extent, suffer from their non-specific actions. Indeed, heart failure might originate from specific sub-cellular components such as the contractile machinery (see for review53). Development of experimental systems has helped investigators conduct studies on the specific impacts of heart failure relating to myofilament biophysical properties54,55. Accordingly, researchers found that contractile protein mutations50,56,57, post-transcriptional contractile protein modifications (i.e. phosphorylation, nitrosylation, glutathionylation, and oxidation), and isoform expression may all induce organ-level heart failure through myofilament apparatus alteration (see for review58). Consequently, researchers have oriented their efforts toward the discovery of drugs that specifically improve altered myofilament contractility during heart failure. Thus, a whole new class of drugs known as calcium sensitizers has been discovered53. Examples of these drugs include Levosimendan59; Pimobendan60; EMD-5703361; MCI-15462; and SR3380542,63. Most of these drugs have been tested using similar systems to the one described in this review and have been shown to improve myocardium function by specifically altering myofilament sensitivity to calcium, hence, the term calcium sensitizers.
Concluding remarks
Experimental system developments are important steps in fundamental disease mechanisms understanding as well as drug discovery. In this review, we provided an overview of some experimental systems employed to investigate biophysical properties of myocardium samples. We particularly emphasized on how researchers have progressed from studying the Frank-Starling law at the whole organ level, to single cell contractile biology, and finally to length dependent activation and activation/relaxation dynamics at the single myofibril level. By employing these systems, one can study contractile properties in various regions of the LV muscle, and this has led to the discovery of a gradient of contractility across the left ventricle wall36,38,41,43,64. As discussed earlier, cardiomyocytes isolated from the inner layer of the LV (i.e. Endocardium(ENDO)) have different biophysical properties as the ones isolated from the outer layer (i.e. Epicardium (EPI)). That established a contractile heterogeneity that can only be evaluated at the single cardiac cell level. Interestingly, during heart failure, these contractile heterogeneities disappear mainly because of reduced LDA in the ENDO layer36,38,42. These findings may be of great help in specific drug synthesis to specifically improve the ENDO altered LDA. Consequently, the contraction heterogeneity across the left ventricle free wall can be restored and heart failure improved. Similarly, we have previously shown that physiological treatment (i.e. exercise training), improves global heart pump function by specifically restoring altered LDA in ENDO layer41. This constitutes a proof of concept showing that the heart pump dysfunction can, indeed, be reversed by specifically acting upon the altered ENDO layer. To conduct cutting edge scientific research, medical investigators and engineers must always push the limits of what can be measured and how these measurements can be accomplished. This review shows how researchers have progressed from studying the Frank-Starling law on the whole organ, to length dependent activation on a single myofibril.
Despite their importance in disease diagnostic, cardiac biophysical exploration systems still suffer from their luck of popularity. Due to their complexity and time requirement, very few clinicians utilize these systems in their disease diagnostic routines. It would be of great interest to orient these systems development toward a more clinical approach.
Acknowledgements
The authors thank Abbirami Sathappan for kindly providing the drawing in Figure 2A. The authors also thank Brian P. Mitchelson for the grammar proofing.
References
- 1.Zimmer H-G. The Isolated Perfused Heart and Its Pioneers. News Physiol Sci. 1998;13:203–210. doi: 10.1152/physiologyonline.1998.13.4.203. [DOI] [PubMed] [Google Scholar]
- 2.Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. J Physiol. 1914;48(5):357–379. doi: 10.1113/jphysiol.1914.sp001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagawa K, Lie RK, Schaefer J. Translation of Otto Frank's paper “Die Grundform des Arteriellen Pulses”. Zeitschrift fur Biologie. 1990;22(3):253–254. doi: 10.1016/0022-2828(90)91459-k. [DOI] [PubMed] [Google Scholar]
- 4.Huke S, Knollmann BC. Familial hypertrophic cardiomyopathy: is the Frank-Starling law kaput? Circ Res. 2013;112(11):1409–1411. doi: 10.1161/CIRCRESAHA.113.301406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady AJ. Mechanical properties of isolated cardiac myocytes. Physiol Rev. 1991;71(2):413–428. doi: 10.1152/physrev.1991.71.2.413. [DOI] [PubMed] [Google Scholar]
- 6.Shiels HA, White E. The Frank-Starling mechanism in vertebrate cardiac myocytes. The Journal of experimental biology. 2008;211(Pt 13):2005–2013. doi: 10.1242/jeb.003145. [DOI] [PubMed] [Google Scholar]
- 7.Roberts WC, Cohen LS. Left ventricular papillary muscles. Description of the normal and a survey of conditions causing them to be abnorma. Circulation. 1972;46(1):138–154. doi: 10.1161/01.cir.46.1.138. [DOI] [PubMed] [Google Scholar]
- 8.ter Keurs HE, Rijnsburger WH, van Heuningen R, Nagelsmit MJ. Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ Res. 1980;46(5):703–714. doi: 10.1161/01.res.46.5.703. [DOI] [PubMed] [Google Scholar]
- 9.Krueger JW, Pollack GH. Myocardial sarcomere dynamics during isometric contraction. J Physiol. 1975;251(3):627–643. doi: 10.1113/jphysiol.1975.sp011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- 12.Kurihara S, Allen DG. Intracellular Ca++ transients and relaxation in mammalian cardiac muscle. Japanese circulation journal. 1982;46(1):39–43. doi: 10.1253/jcj.46.39. [DOI] [PubMed] [Google Scholar]
- 13.Konhilas JP, Irving TC, de Tombe PP. Frank-Starling law of the heart and the cellular mechanisms of length-dependent activation. Pflugers Arch. 2002;445(3):305–310. doi: 10.1007/s00424-002-0902-1. [DOI] [PubMed] [Google Scholar]
- 14.McDonald KS, Moss RL. Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length. Circ Res. 1995;77(1):199–205. doi: 10.1161/01.res.77.1.199. [DOI] [PubMed] [Google Scholar]
- 15.Irving TC, Konhilas J, Perry D, Fischetti R, de Tombe PP. Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. Am J Physiol Heart Circ Physiol. 2000;279(5):H2568–H2573. doi: 10.1152/ajpheart.2000.279.5.H2568. [DOI] [PubMed] [Google Scholar]
- 16.de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC. Myofilament length dependent activation. J Mol Cell Cardiol. 2010;48(5):851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith L, Tainter C, Regnier M, Martyn DA. Cooperative cross-bridge activation of thin filaments contributes to the Frank-Starling mechanism in cardiac muscle. Biophys J. 2009;96(9):3692–3702. doi: 10.1016/j.bpj.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzsimons DP, Moss RL. Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circ Res. 1998;83(6):602–607. doi: 10.1161/01.res.83.6.602. [DOI] [PubMed] [Google Scholar]
- 19.Witayavanitkul N, Ait Mou Y, Kuster DWD, Khairallah RJ, Sarkey J, Govindan S, Chen X, Ge Y, Rajan S, Wieczorek DF, Irving T, Westfall MV, de Tombe PP, Sadayappan S. Myocardial infarction-induced N-terminal fragment of cMyBP-C impairs myofilament function in Human myocardium. J Biol Chem. 2014;289(13):8818–8827. doi: 10.1074/jbc.M113.541128. PMID: 24509847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Tombe PP, Stienen GJ. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ Res. 1995;76(5):734–741. doi: 10.1161/01.res.76.5.734. [DOI] [PubMed] [Google Scholar]
- 21.Guth K, Wojciechowski R. Perfusion cuvette for the simultaneous measurement of mechanical, optical and energetic parameters of skinned muscle fibres. Pflugers Arch. 1986;407(5):552–557. doi: 10.1007/BF00657515. [DOI] [PubMed] [Google Scholar]
- 22.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68(3):1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garnier D. Attachment procedures for mechanical manipulation of isolated cardiac myocytes: a challenge. Cardiovasc Res. 1994;28(12):1758–1764. doi: 10.1093/cvr/28.12.1758. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, Naruse K. Stretch-activated BK channel and heart function. Progress in biophysics and molecular biology. 2012;110(2–3):239–244. doi: 10.1016/j.pbiomolbio.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Le Guennec JY, Peineau N, Argibay JA, Mongo KG, Garnier D. A new method of attachment of isolated mammalian ventricular myocytes for tension recording: length dependence of passive and active tension. J Mol Cell Cardiol. 1990;22(10):1083–1093. doi: 10.1016/0022-2828(90)90072-a. [DOI] [PubMed] [Google Scholar]
- 26.White E, Le Guennec JY, Nigretto JM, Gannier F, Argibay JA, Garnier D. The effects of increasing cell length on auxotonic contractions; membrane potential and intracellular calcium transients in single guinea-pig ventricular myocytes. Exp Physiol. 1993;78(1):65–78. doi: 10.1113/expphysiol.1993.sp003671. [DOI] [PubMed] [Google Scholar]
- 27.Cazorla O, Pascarel C, Garnier D, Le Guennec JY. Resting tension participates in the modulation of active tension in isolated guinea pig ventricular myocytes. J Mol Cell Cardiol. 1997;29(6):1629–1637. doi: 10.1006/jmcc.1997.0402. [DOI] [PubMed] [Google Scholar]
- 28.King NMP, Methawasin M, Nedrud J, Harrell N, Chung CS, Helmes M, Granzier H. Mouse intact cardiac myocyte mechanics: cross-bridge and titin-based stress in unactivated cells. J Gen Physiol. 2011;137(1):81–91. doi: 10.1085/jgp.201010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiura S, Nishimura S, Yasuda S, Hosoya Y, Katoh K. Carbon fiber technique for the investigation of single-cell mechanics in intact cardiac myocytes. Nat Protoc. 2006;1(3):1453–1457. doi: 10.1038/nprot.2006.241. [DOI] [PubMed] [Google Scholar]
- 30.Iribe G, Kohl P. Axial stretch enhances sarcoplasmic reticulum Ca2+ leak and cellular Ca2+ reuptake in guinea pig ventricular myocytes: experiments and models. Progress in biophysics and molecular biology. 2008;97(2–3):298–311. doi: 10.1016/j.pbiomolbio.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RA, Garny A, Morphew MK, Hoenger A, Lederer WJ, Kohl P. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res. 2009;104(6):787–795. doi: 10.1161/CIRCRESAHA.108.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333(6048):1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 33.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 34.Fabiato A, Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen DG, Kentish JC. Calcium concentration in the myoplasm of skinned ferret ventricular muscle following changes in muscle length. J Physiol. 1988;407:489–503. doi: 10.1113/jphysiol.1988.sp017427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ait Mou Y, le Guennec J-Y, Mosca E, de Tombe PP, Cazorla O. Differential contribution of cardiac sarcomeric proteins in the myofibrillar force response to stretch. Pflugers Arch. 2008;457(1):25–36. doi: 10.1007/s00424-008-0501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puceat M, Clement O, Lechene P, Pelosin JM, Ventura-Clapier R, Vassort G. Neurohormonal control of calcium sensitivity of myofilaments in rat single heart cells. Circ Res. 1990;67(2):517–524. doi: 10.1161/01.res.67.2.517. [DOI] [PubMed] [Google Scholar]
- 38.Cazorla O, Szilagyi S, Le Guennec J-Y, Vassort G, Lacampagne A. Transmural stretch-dependent regulation of contractile properties in rat heart and its alteration after myocardial infarction. FASEB J. 2005;19(1):88–90. doi: 10.1096/fj.04-2066fje. [DOI] [PubMed] [Google Scholar]
- 39.Tan JH, Liu W, Saint DA. Differential expression of the mechanosensitive potassium channel TREK-1 in epicardial and endocardial myocytes in rat ventricle. Exp Physiol. 2004;89(3):237–242. doi: 10.1113/expphysiol.2003.027052. [DOI] [PubMed] [Google Scholar]
- 40.LeGrice IJ, Smaill BH, Chai LZ, Edgar SG, Gavin JB, Hunter PJ. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. The American journal of physiology. 1995;269(2 Pt 2):H571–H582. doi: 10.1152/ajpheart.1995.269.2.H571. [DOI] [PubMed] [Google Scholar]
- 41.Ait Mou Y, Reboul C, Andre L, Lacampagne A, Cazorla O. Late exercise training improves non-uniformity of transmural myocardial function in rats with ischaemic heart failure. Cardiovasc Res. 2009;81(3):555–564. doi: 10.1093/cvr/cvn229. [DOI] [PubMed] [Google Scholar]
- 42.Ait Mou Y, Toth A, Cassan Cec, Czuriga D, de Tombe PP, Papp Z, Lacampagne A, Cazorla O. Beneficial effects of SR33805 in failing myocardium. Cardiovasc Res. 2011;91(3):412–419. doi: 10.1093/cvr/cvr096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su JB, Cazorla O, Blot Sep, Blanchard-Gutton N, Ait Mou Y, Barth\'e lemIes, Sambin L, Sampedrano CC, Gouni V, Unterfinger Y, Aguilar P, Thibaud J-L, Biz\'e A, Pouchelon J-L, Dabir\'e H, Ghaleh B, Berdeaux A, Chetboul Ver, Lacampagne A, Hittinger L. Bradykinin restores left ventricular function, sarcomeric protein phosphorylation, and e/nNOS levels in dogs with Duchenne muscular dystrophy cardiomyopathy. Cardiovasc Res. 2012;95(1):86–96. doi: 10.1093/cvr/cvs161. [DOI] [PubMed] [Google Scholar]
- 44.LeGrice IJ, Takayama Y, Holmes JW, Covell JW. Impaired subendocardial function in tachycardia-induced cardiac failure. The American journal of physiology. 1995;268(5 Pt 2):H1788–H1794. doi: 10.1152/ajpheart.1995.268.5.H1788. [DOI] [PubMed] [Google Scholar]
- 45.Betts TR, Gamble JHP, Khiani R, Bashir Y, Rajappan K. Development of a technique for left ventricular endocardial pacing via puncture of the interventricular septum. Circ Arrhythm Electrophysiol. 2014;7(1):17–22. doi: 10.1161/CIRCEP.113.001110. [DOI] [PubMed] [Google Scholar]
- 46.Colomo F, Piroddi N, Poggesi C, te Kronnie G, Tesi C. Active and passive forces of isolated myofibrils from cardiac and fast skeletal muscle of the frog. J Physiol. 1997;500(Pt 2):535–548. doi: 10.1113/jphysiol.1997.sp022039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mateja RD, de Tombe PP. Myofilament length-dependent activation develops within 5 ms in guinea-pig myocardium. Biophys J. 2012;103(1):L13–L15. doi: 10.1016/j.bpj.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cecchi G, Colomo F, Poggesi C, Tesi C. A force transducer and a length-ramp generator for mechanical investigations of frog-heart myocytes. Pflugers Arch. 1993;423(1–2):113–120. doi: 10.1007/BF00374968. [DOI] [PubMed] [Google Scholar]
- 49.Colomo F, Nencini S, Piroddi N, Poggesi C, Tesi C. Calcium dependence of the apparent rate of force generation in single striated muscle myofibrils activated by rapid solution changes. Adv Exp Med Biol. 1998;453:373–381. doi: 10.1007/978-1-4684-6039-1_42. discussion 381–372. [DOI] [PubMed] [Google Scholar]
- 50.Belus A, Piroddi N, Ferrantini C, Tesi C, Cazorla O, Toniolo L, Drost M, Mearini G, Carrier L, Rossi A, Mugelli A, Cerbai E, van der Velden J, Poggesi C. Effects of chronic atrial fibrillation on active and passive force generation in human atrial myofibrils. Circ Res. 2010;107(1):144–152. doi: 10.1161/CIRCRESAHA.110.220699. [DOI] [PubMed] [Google Scholar]
- 51.Belus A, Piroddi N, Scellini B, Tesi C, D'Amati G, Girolami F, Yacoub M, Cecchi F, Olivotto I, Poggesi C. The familial hypertrophic cardiomyopathy-associated myosin mutation R403Q accelerates tension generation and relaxation of human cardiac myofibrils. J Physiol. 2008;586(Pt 15):3639–3644. doi: 10.1113/jphysiol.2008.155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piroddi N, Belus A, Scellini B, Tesi C, Giunti G, Cerbai E, Mugelli A, Poggesi C. Tension generation and relaxation in single myofibrils from human atrial and ventricular myocardium. Pflugers Arch. 2007;454(1):63–73. doi: 10.1007/s00424-006-0181-3. [DOI] [PubMed] [Google Scholar]
- 53.Endoh M. Cardiac Ca2+ signaling and Ca2+ sensitizers. Circ J. 2008;72(12):1915–1925. doi: 10.1253/circj.cj-08-0838. [DOI] [PubMed] [Google Scholar]
- 54.de Tombe PP, Solaro RJ. Integration of cardiac myofilament activity and regulation with pathways signaling hypertrophy and failure. Ann Biomed Eng. 2000;28(8):991–1001. doi: 10.1114/1.1312189. [DOI] [PubMed] [Google Scholar]
- 55.Hamdani N, Kooij V, van Dijk S, Merkus D, Paulus WJ, Remedios CD, Duncker DJ, Stienen GJM, van der Velden J. Sarcomeric dysfunction in heart failure. Cardiovasc Res. 2008;77(4):649–658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 56.Morimoto S. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res. 2008;77(4):659–666. doi: 10.1093/cvr/cvm084. [DOI] [PubMed] [Google Scholar]
- 57.Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev. 2005;10(3):237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- 58.Hernandez OM, Housmans PR, Potter JD. Invited Review: pathophysiology of cardiac muscle contraction and relaxation as a result of alterations in thin filament regulation. Journal of applied physiology. 2001;90(3):1125–1136. doi: 10.1152/jappl.2001.90.3.1125. [DOI] [PubMed] [Google Scholar]
- 59.Cleland JGF, Nikitin N, McGowan J. Levosimendan: first in a new class of inodilator for acute and chronic severe heart failure. Expert Rev Cardiovasc Ther. 2004;2(1):9–19. doi: 10.1586/14779072.2.1.9. [DOI] [PubMed] [Google Scholar]
- 60.Sata M, Sugiura S, Yamashita H, Aoyagi T, Momomura S, Serizawa T. Pimobendan directly sensitizes reconstituted thin filament to slide on cardiac myosin. Eur J Pharmacol. 1995;290(1):55–59. doi: 10.1016/0922-4106(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 61.Soergel DG, Georgakopoulos D, Stull LB, Kass DA, Murphy AM. Augmented systolic response to the calcium sensitizer EMD-57033 in a transgenic model with troponin I truncation. Am J Physiol Heart Circ Physiol. 2004;286(5):H1785–H1792. doi: 10.1152/ajpheart.00170.2003. [DOI] [PubMed] [Google Scholar]
- 62.Sata M, Sugiura S, Yamashita H, Fujita H, Momomura S, Serizawa T. MCI-154 increases Ca2+ sensitivity of reconstituted thin filament. A study using a novel in vitro motility assay technique. Circ Res. 1995;76(4):626–633. doi: 10.1161/01.res.76.4.626. [DOI] [PubMed] [Google Scholar]
- 63.Cazorla O, Lacampagne A, Fauconnier J, Vassort G. SR33805, a Ca2+ antagonist with length-dependent Ca2+-sensitizing properties in cardiac myocytes. Br J Pharmacol. 2003;139(1):99–108. doi: 10.1038/sj.bjp.0705221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cazorla O, Le Guennec JY, White E. Length-tension relationships of sub-epicardial and sub-endocardial single ventricular myocytes from rat and ferret hearts. J Mol Cell Cardiol. 2000;32(5):735–744. doi: 10.1006/jmcc.2000.1115. [DOI] [PubMed] [Google Scholar]
- 65.Jewell BR. A reexamination of the influence of muscle length on myocardial performance. Circ Res. 1977;40(3):221–230. doi: 10.1161/01.res.40.3.221. [DOI] [PubMed] [Google Scholar]