Summary
Maturation of a vascular plexus is a critical and yet incompletely understood process in organ development, and known maturation factors act universally in all vascular beds. In this study, we show that CXCL12 is an organ-specific maturation factor of particular relevance in coronary arterial vasculature. In vitro, CXCL12 does not influence nascent vessel formation but promotes higher order complexity of preinitiated vessels. In the heart, CXCL12 is expressed principally by the epicardium and its receptor CXCR4 is expressed by coronary endothelial cells. CXCL12 is not a chemotactic signal for endothelial cell migration, but rather acts in a paracrine manner to influence the maturation of the coronary vascular plexus. Mutants in CXCL12 signaling show an excess of immature capillary chains and a selective failure in arterial maturation, and become leaky with the onset of coronary perfusion. Failed maturation of the coronary system explains the late-gestation lethality of these mutants.
Keywords: heart, epicardium, coronary vessels, chemokines, stromal derived factor-1, CXCL12/CXCR4, mouse
Introduction
Formation of the vascular system is tightly regulated by the spatial and temporal expression of various factors (Jain, 2003). Vascular endothelial growth factor (VEGF) promotes the assembly of endothelial progenitors into a nascent immature plexus (Ferrara et al., 1996; Carmeliet et al., 1996) and also promotes specification to an arterial fate (Hong et al., 2006; Lawson et al., 2002; Mukouyama et al., 2005). The newly formed plexus then undergoes a complex and incompletely understood process of vascular maturation that involves pruning and reorganization into a hierarchical network of larger and smaller arteries and veins; for arteries, plexus maturation also involves assembly of a surrounding layer of smooth muscle. Connection to systemic circulation initiates perfusion and additional remodeling processes that continue through life. Several signaling pathways have been implicated in plexus maturation, including angiopoietin (Suri et al., 1998), ephrin (Wang et al., 1998), and Notch (Duarte et al., 2004), although these appear to be universally required in all vascular compartments. In contrast, there is also evidence for organ-specific maturation processes that are thought to be controlled by unique locally expressed molecules (Jain, 2003; Wang et al., 2012), although few if any such factors have been identified.
In mouse heart development, cells from the proepicardium migrate onto and adhere to the outer myocardial surface between embryonic days E9.5–10.5, forming the epicardium (Sengbusch et al., 2002). Coronary endothelial cells first assemble into a plexus in the space between the epicardium and ventricular myocardium, then associate with epicardium-derived smooth muscle cells as the nascent tubes coalesce and become incorporated into the ventricular wall (Red-Horse et al., 2010). Coronary veins remain close to the outer surface whereas coronary arteries become more deeply positioned. Ingrowth of the anterior ends of the arteries into the ascending aorta at E14 initiates perfusion and coronary circulation (Chen et al., 2014; Tian et al., 2013a). The molecular signals that promote differentiation of the primitive plexus to assemble the mature coronary vasculature of the embryonic ventricular wall are largely unknown.
The chemokine CXCL12, also known as SDF1, has chemotactic and mitogenic activity on many cell types (Nagasawa, 2014). Various nonexclusive roles for CXCL12 signaling in vasculogenesis have been proposed, including endothelial cell migration (Siekmann et al., 2009), arterial-nerve alignment (Li et al., 2013), and mediation of plexus connections to systemic arteries (Ara et al., 2005). CXCL12 primarily acts through the G-protein coupled receptor CXCR4; global mouse knockouts of Cxcl12 or of Cxcr4 die shortly before birth with vascular deficiencies in the gut, kidney, and skin, and with a number of additional hematopoietic and neural defects (Ma et al., 1998; Nagasawa et al., 1996; Tachibana et al., 1998; Zou et al., 1998). Endothelium-specific Tie2Cre/Cxcr4 mutants replicate the vascular phenotype of global mutants (Ara et al., 2005; Takabatake et al., 2009). Except for an unexplained ventricular septal defect, cardiac development was not closely examined. Importantly, the known defects cannot explain the late gestation lethality seen in the global or conditional mutants.
In the present study, we found that CXCL12 has potent activity in vascular plexus maturation, although not in the initial assembly of the vascular plexus. We demonstrate that this function is particularly relevant in the maturation of the coronary arterial vasculature. Absence of CXCL12/CXCR4 signaling results in a poorly formed coronary system and insufficient myocardial perfusion, and accounts for late gestation lethality seen in these mutants.
Results
CXCL12 promotes higher order vascular plexus organization in vitro
Many aspects of early vascular plexus formation and maturation are modeled by the behavior of HUVEC endothelial cells in fibrin gels (Nakatsu et al., 2003), including the formation of vascular sprouts, lumenized tubes, branches, and anastomoses of branches with other tubes (Fig. S1A–E). Using this assay, we measured initial endothelial cell sprouting at 3 days and the complexity of lumenized tubes at 5 days to test the role of CXCL12 and its possible interaction with VEGF. In the absence of added factors (Fig. 1A,E,I) or with CXCL12 alone (Fig. 1B,F,J), no outgrowth occurred even as late as 7 days after plating. VEGF induced sprouting at 3 days (Fig. 1C) and formation of tubes at 5 days (Fig. 1G), as previously reported (Nakatsu et al., 2003). Addition of the CXCR4 antagonist AMD3100 to VEGF-treated cultures had no effect (Fig. S1F), indicating that CXCL12 is not made by endogenous components of this assay and that CXCR4 is not required for the VEGF response. The number of primary sprouts that originated from each bead was unchanged when CXCL12 was added with VEGF (Fig. 1D,M). CXCL12 also did not influence VEGF-induced changes in HUVEC arterial or venous gene expression (Fig. S1I). However, the combination of CXCL12 with VEGF dramatically increased the complexity of the endothelial tube network by day 5, as manifest both by the number of tubes (i.e., primary tubes plus branched tubes) and by the number of anastomoses between tubes (Fig. 1H,M). Thus, in vitro, CXCL12 does not initiate vasculogenesis or alter arterial-venous specification but rather promotes further remodeling of a plexus that is initially induced by VEGF (Fig. S1H).
Figure 1. CXCL12 promotes plexus maturation in vitro.
(A–L) Representative images of HUVEC-covered beads in fibrin gels after 3, 5 and 7 days of culture. Negative control was endothelial basal media (EBM-2) plus 2% FBS with no added growth factors; VEGF and CXCL12 were added at 20 ng/ml and 100 ng/ml respectively. (M) Quantitation of total number of sprouts, tubes, branching points, and anastomosis in the fibrin bead assay. The data are normalized to the number of beads present in each microscopic field. Data are represented as mean ± SEM. * p<0.001 versus VEGF alone. See also Fig. S1.
CXCL12 expression during the period of mouse coronary vessel formation
The majority of coronary endothelial cells of the embryonic ventricular wall are thought to originate from the sinus venosus and migrate between the epicardium and myocardium ((Red-Horse et al., 2010); see also below). In mice, this process initiates at embryonic day E11.5 on the dorsal side of the heart at the atrioventricular canal, with expansion and maturation of the plexus occurring over the next several days. Cxcl12 was expressed throughout the E10.5–16.5 interval in the epicardium (Fig. 2A–F); this included the epicardium of the atrioventricular canal and over the ventricle. Cxcl12 may also be expressed at a uniform low level in the myocardium; we measured 4-fold lower expression in isolated primary embryonic ventricular cardiomyocytes compared to primary ventricular epicardial cells (Fig. S2A). Cxcl12 expression was also detected in the wall of the aorta during this period (Fig. S2B), by cells of presumptive neural crest origin (Choudhary et al., 2009). Consistent with epicardial expression in vivo, CXCL12 is secreted at an abundant level by immortalized MEC1 mouse embryonic ventricular epicardial cells (Fig. S2C). In MEC1 and in primary mouse embryonic epicardial cells, the Cxcl12 gene was induced by hypoxia (Fig. S2D), as observed in other cell types (Santiago et al., 2011; Sun et al., 2010). Cxcl12 was expressed later by epicardium-derived smooth muscle cells surrounding mature coronary blood vessels (Fig. 2F). The receptor Cxcr4 was expressed at E11.5 in sinus venosus endothelium, in endothelial cells sprouting from the sinus venosus towards the ventricle, and in heart endocardium (Fig. 2G–I, Fig. S2E–G), a pattern identical to the venous marker endomucin (Emcn) (Fig. S2H–N). Thus, the spatial and temporal expression of Cxcl12 and Cxcr4 are consistent with a role in coronary vascular development.
Figure 2. CXCL12 and CXCR4 expression during coronary vessel development.
(A–F) In situ hybridization with antisense probe for Cxcl12 shows expression in the epicardium in wild-type embryos. (A) Cxcl12 is expressed in the epicardium surrounding the atrioventricular canal at E10.5. (B) Hybridization with a sense control. A′ and B′ are magnified views of the boxed region in A and B. (C–F) Cxcl12 is expressed in the epicardium through E11.5–16.5, and in epicardium-derived smooth muscle surrounding coronary blood vessels (arrowheads) at E16.5. (G–I) CXCR4 is expressed in endothelial cells sprouting from the sinus venosus at E11.5 (arrowheads). The dotted lines outline the pericardial cavity. CD31 (Pecam1) is a pan-endothelial marker. Abbreviations: oft: outflow tract, ra: right atria, rv: right ventricle, avc: atrioventricular canal, sv, sinus venosus, li: liver, epi: epicardium. See also Fig. S2.
CXCL12/CXCR4 signaling is required for coronary vascular maturation
We evaluated global Cxcl12−/− embryos and Tie2Cre/Cxcr4 conditional mutant embryos to determine the role of CXCL12/CXCR4 in coronary vasculogenesis. At E12.0, the number of ventricular subepicardial endothelial cells, as visualized by CD31 immunostaining (a pan-endothelial marker), was normal in mutant embryos (Fig. S3A–B), implying that CXCL12 signaling is not required for chemotactic migration of endothelial cells to the ventricle. Using whole mount CD31 immunohistochemistry to visualize the forming vascular plexus, we noted an expansion in mutant hearts compared to littermate controls, on the dorsal ventricular surface at E11.5 and on the ventral (anterior) surface at E12.5 (Fig. 3A,E, Fig. S3C,D,G,K, quantitated in Fig. S3E). The expanded plexus in mutants included the peritruncal endothelium (Fig. S3K) from which the proximal coronary stems are thought to originate (Tian et al., 2013a). Thus, just as addition of CXCL12 does not promote initial vascular assembly in vitro (Fig. 1), absence of CXCL12 signaling does not impede initial coronary plexus assembly in vivo. The expanded plexus in mutant embryos is characteristic of a deficiency in maturation, since plexus maturation includes pruning (elimination) of a subset of capillary sprouts pursuant to remodeling into higher order vessels. As the coronary plexus matures, arteries migrate deeper into the myocardium whereas veins remain close to the ventricular surface. Using endomucin (Emcn) as a venous marker, in mutant hearts we observed many examples of CD31+Emcn− vessels at the subepicardial ventricular surface, and CD31+Emcn+ vessels in deeper intramyocardial arterial positions, whereas such mispositioned vessels were never seen in control embryos (Fig. 3J,L). Mispositioning is consistent with persistence of vessels that should have been eliminated from the earlier expanding plexus. Moreover, intramyocardial vessels in mutants showed only weak expression of the arterial marker NRP1 (Fig. S3N–Q), and there were fewer intramyocardial arteries that displayed an SM22+ smooth muscle lining in mutants relative to controls (Fig. 3M–N), both implying compromised arterial differentiation. Thus, maturation of the coronary plexus is compromised in the absence of CXCL12-CXCR4 signaling, which is manifest by a transiently expanded but ultimately unreorganized and poorly differentiated vascular network.
Figure 3. Defective coronary vessel organization in embryos with disrupted CXCL12 signaling.
(A–H) Whole mount CD31 immunostaining of hearts (dorsal surfaces) from control and conditional Tie2Cre/Cxcr4 mutants at the indicated stages. Dotted lines indicate the extent of endothelial cell plexus organization within the ventricle. The diffuse staining at E11.5 on the ventricular surface corresponds to CD31-positive endocardium (open arrowheads). Views of the ventral surfaces of these same hearts are in Fig. S3. (I–L) Histological sections of control and mutant hearts at E13.5 immunostained with CD31 and endomucin (EMCN, venous marker). In the control, superficial veins are EMCN-positive (arrows). In the mutant, open arrowhead in center points to an intramyocardial vessel ectopically expressing endomucin, and the open arrowhead at right to a superficial dilated EMCN-negative vessel. (M–N) Reduced number of major coronary artery branches (SM22+) in mutant hearts at E15.5. (O–P) Whole mount EMCN staining at E15.5, indicating normal assembly of higher order venous structures (arrowheads). (Q–T) Visualization of the coronary arterial vasculature by aortic ink perfusion at E15.5 and E16.5. Arrowheads point to perfused vessels of abnormal branching organization in the mutant hearts. (U–V) Reduced number of major coronary artery branches in mutant hearts. U and V are low magnification views of control and mutant hearts at E17.5 showing only SM22 (smooth muscle marker) staining. Close-up views of the boxed regions are shown in U′-U″ and V′-V″ with CD31 and SM22 immunostaining. Endothelial cell density is similar between control and mutant (compare U′ to V′). Note smaller and fewer intramyocardial major branches (solid arrowheads) and dilated superficial vessels (open arrowheads) in the mutant. (W–X) Close up views of hearts from control (same as in Q) and Cxcl12 KO (shown in Fig. S4C). In the mutant, note perfusion of the proximal coronary artery from the aorta into the adjacent persisting capillary plexus. Abbreviations: Ao, aorta, endo, endocardium, lca, left coronary artery, rca, right coronary artery, sb, septal branch, co, coronary ostia. See also Fig. S3.
Embryos with deficient CXCL12 signaling have severely compromised coronary arterial vasculature
Whole mount endomucin staining of mutant E15.5 hearts indicated normal higher order organization of the venous component of the coronary vasculature (Fig. 3O–P). Thus, CXCL12 signaling is not required for venous coronary maturation. We performed aortic ink perfusion to visualize the organization of the coronary arterial vasculature in control and mutant embryos in late gestation (Fig. 3Q–T, Fig. S4A–C). In normal hearts, the main proximal coronary branches give rise to arteries of progressively smaller diameter that perfuse the entire ventricle to the apex. In mutant hearts, in the vicinity of the aorta we observed either a poorly differentiated plexus that quickly terminated, or when main proximal branches were present these also quickly terminated. The deficiency of the peripheral arterial network in late gestation mutant embryos was not the result of absent vascular elements, because in sections an abundance of small vessels was apparent in the ventricular wall (Fig. 3U–V). Many of these intramyocardial vessels lacked a SM22+ smooth muscle lining and were therefore either mispositioned veins or immature vessels persisting from the vascular plexus of earlier stages, although some SM22+ arteries were also detected, consistent with the presence of some arteries as visualized by ink perfusion. Importantly, at least some proximal components of the coronary system were perfused with ink in all mutant embryos (Fig. 3R,T, Fig. S4B,C), implying connection to the aorta, and coronary ostia and proximal branches were readily observed in mutant embryos (Fig. 3W–X, Fig. S4C,D–F). CXCL12/CXCR4 signaling does not therefore appear to be required for coronary ostia formation.
Perfusion of the coronary arteries initiates around E14 (Tian et al., 2013a). The ventricular myocardium in mutants appeared normal through E15 (Fig. S3R–U), but in late-gestation mutant embryos, we commonly observed ventricular hemorrhaging (Fig. S4G–H), which we interpret as the result of an immature, leaky vascular network following the onset of coronary perfusion. As previously reported, Cxcl12 global and Tie2Cre/Cxcr4 mutants appeared macroscopically normal through E14.5; growth deficiency started at E15.5 and embryos varied in time of lethality between E16.5–18.5. The compromised coronary arterial network in mutant embryos is expected to result in impaired heart growth and function after the E14 onset of coronary perfusion, which would result in diminished embryo growth and ultimately in late-gestation embryo lethality.
Coronary arterial endothelial cells are primarily derived from an extracardiac origin
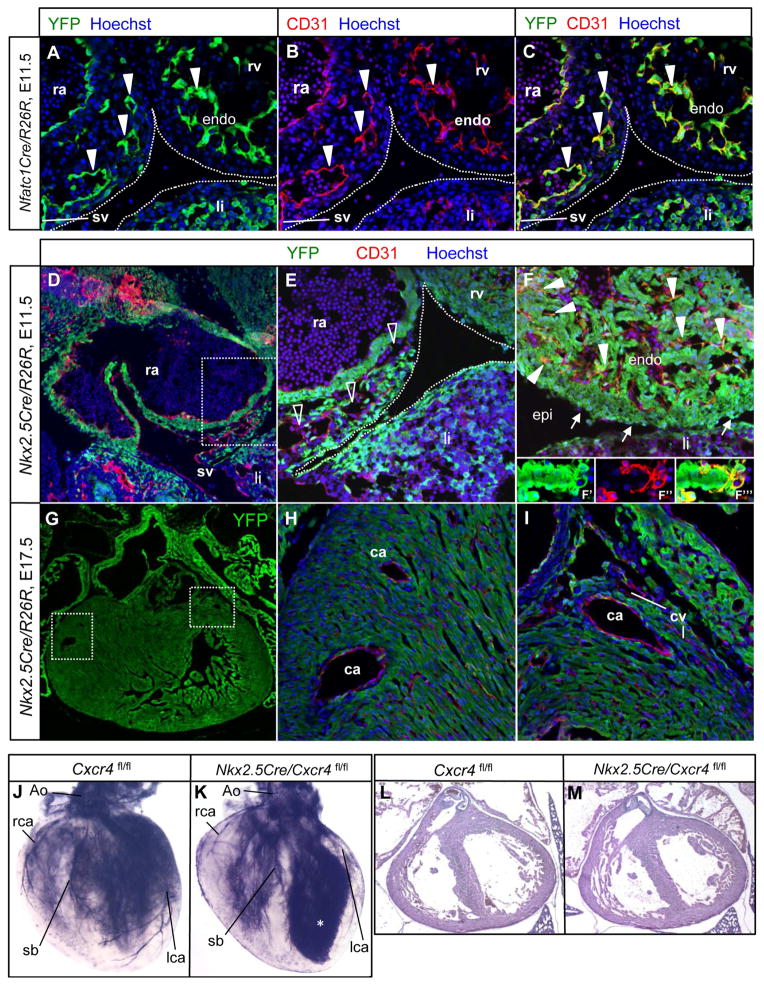
The endocardium is the source of coronary endothelium that forms after birth in the ventricular septum and inner ventricular wall (Tian et al., 2014). However, there is uncertainty regarding the cell lineage origin of embryonic coronary endothelial cells, with the two principal sources argued to be either the sinus venosus (Red-Horse et al., 2010; Tian et al., 2013b) or the endocardium (Wu et al., 2012). Tie2Cre is active in both and so does not address this issue; Nfatc1Cre, which has been used in past studies to conclude an endocardial origin of coronary arterial endothelium (Wu et al., 2012), is also expressed in both (Fig. 4A–C). Nkx2.5Cre is active in the first and second heart fields and therefore efficiently labels the atrial and ventricular endocardium, myocardium, and epicardium, but is not active in most vascular endothelium, including that of the sinus venosus (Fig. 4D–F, Fig. S4I–L). In normal hearts, the great majority (>90%) of coronary endothelial cells in the ventricular wall were unlabeled with the Nkx2.5Cre lineage marker, whereas the great majority (>90%) of endocardial cells and virtually all myocardial and epicardial cells were labeled. The absence of labeling was clearly evident in large and small caliber coronary arteries, and in coronary veins (Fig. 4G–I, Fig. S4K,L). The approx. 10% Nkx2.5Cre lineage-labeled coronary endothelial cells implies a degree of heterogeneity in the origin of this lineage, which was also noted in previous studies (Red-Horse et al., 2010). Furthermore, unlike the invariable late gestation lethality of Tie2Cre/Cxcr4 mutants, Nkx2.5Cre/Cxcr4 mutants survived normally through adulthood with no apparent deficiencies. Ink injection in late gestation Nkx2.5Cre/Cxcr4 hearts demonstrated a normal coronary arterial pattern (Fig. 4J–K) and the myocardium was also normal (Fig. 4L–M). These results support the model that most coronary arterial and venous endothelial cells in the ventricular wall, and the endothelial cell lineage that responds to CXCL12 signaling through CXCR4 during maturation of the coronary vasculature, originate from outside the Nkx2.5Cre domain of the heart (i.e., originate from the sinus venosus) rather than from the endocardium.
Figure 4. Coronary endothelial cells are derived from the sinus venosus.
(A–C) Nfatc1Cre recombines in sinus venosus endothelial cell sprouts (three arrowheads at left) and in all endocardial cells at E11.5. (D–E) Nkx2.5Cre recombination at E11.5. Panel E corresponds to the boxed area in D. Nkx2.5Cre does not recombine in endothelial cells (CD31+) of the sinus venosus and in endothelial cell sprouts of the sinus venosus moving towards the ventricle at the atrioventricular groove (open arrowheads). (F) Nkx2.5Cre recombines efficiently in the myocardium, epicardium (arrows) and the majority of endocardial cells (solid arrowheads). A close-up confocal view of a trabecular element is shown in separate (F′ and F″) and merged (F‴) channels in the insets to emphasize that endocardial cells are labeled. (G–I) Nkx2.5Cre recombination at E17.5. Panel G shows a low magnification view of the heart (green channel only). H and I correspond to the boxed areas in G. Nkx2.5Cre does not recombine in coronary artery or vein endothelium throughout the mature ventricle. (J–K) Normal coronary vasculature in E17.5 Nkx2.5Cre/Cxcr4 mutants; the asterisk in K indicates leaking of excess ink into the ventricular cavity. (L–M) H&E staining of frontal sections showing normal heart morphology in both control and mutant hearts at E17.5. Abbreviations: ra, right atria; sv, sinus venosus, rv, right ventricle, endo, endocardium, li, liver; ca, coronary artery, cv, coronary vein, lca, left coronary artery, rca, right coronary artery, sb, septal branch. See also Fig. S4.
Discussion
Maturation of the vascular plexus is critically required for adequate end-organ perfusion. Our studies show that CXCL12 is a necessary factor for coronary plexus maturation. Cxcl12 and Cxcr4 mutant embryos displayed a poorly formed coronary arterial network that resulted in late gestation underperfusion of the heart, compromised heart growth and function, and embryo lethality, all manifestations of a failure in plexus maturation. For several reasons, we exclude a role for CXCL12 in earlier steps of coronary development. A previous study showed that signals from the ventricle are responsible for inducing migration of coronary endothelial cells from the sinus venosus (Red-Horse et al., 2010), but as we observed a normal density of endothelial cells at all stages in normal vs. mutant embryos, this signal is not CXCL12. In Cxcl12 or Cxcr4 mutant embryos, initial plexus formation was normal (and was even expanded because of the lack of subsequent pruning), and in vitro, addition of CXCL12 did not influence VEGF-induced vascular sprouting. At the terminal phase of coronary vasculogenesis, ingrowth of anterior arterial stems into the aorta results in the initiation of perfusion, and this too occurred normally in mutant embryos with arterial elements that had properly matured in the vicinity of the aorta. We thus conclude that CXCL12 acts in the ventricle as a vascular plexus maturation factor needed for arterial differentiation (with VEGF) and for hierarchical arterial assembly during coronary development.
Interestingly, hierarchical organization of the coronary venous system was normal in Cxcl12 and Cxcr4 mutants, implicating a specific role for this signaling axis in arterial maturation. The basis for this selectivity is unclear, but suggests that coronary arterial and venous maturation in the initial plexus involve distinct molecular programs. This may be because the coronary endothelium originates from a venous source (the sinus venosus), such that coronary arterial endothelium must first be respecified to an arterial fate. This may necessitate not only VEGF signaling (to initiate arterialization) but also CXCL12 to promote further maturation.
Several features distinguish the role of CXCL12 in coronary vasculogenesis from previously described maturation factors such as Notch, angiopoietin, and ephrin. First, unlike these general factors, CXCL12 is clearly not required in all or even in most vascular compartments. We examined coronary maturation in mutant embryos, and previous studies have noted vascular defects in the gut (Ara et al., 2005), kidney (Takabatake et al., 2009), and skin (Li et al., 2013), but yolk sac and most embryonic vessel beds were normal, and embryo growth, morphology, and viability were normal until late gestation when coronary perfusion becomes required. Second, CXCL12 is not absolutely required for vessel maturation, since coronary artery formation was evident although greatly limited in mutant embryos, and those arteries that did form were able to assemble a smooth muscle layer and become perfused with systemic blood. Importantly, though, coronary vascular defects in Cxcl12/Cxcr4 mutants were manifest prior to the initiation of perfusion, implying that these defects are not the secondary consequence of impaired flow. CXCL12 therefore appears to facilitate the process of vascular maturation in select organ systems including the heart. This probably occurs in conjunction with more general factors; in this light, Notch signaling has been reported to control Cxcr4 and Cxcl12 expression in myeloma and mesenchymal cells (Mirandola et al., 2013; Xie et al., 2013). This role of facilitating vascular maturation may also explain the phenotype observed in the developing gut (Ara et al., 2005) and possibly also in kidney (Takabatake et al., 2009). Finally, unlike Notch, angiopoietin, and ephrin signaling in which both ligand and receptor are expressed by endothelial cells and control vessel maturation largely by autocrine interactions, CXCL12 functions as a paracrine factor that influences adjacent CXCR4-expressing endothelial cells. In coronary development, CXCL12 is expressed by the epicardium and epicardium-derived mesenchymal cells and to a lesser extent by the myocardium (Fig. 2), and in the kidney CXCL12 is expressed by podocytes (Takabatake et al., 2009). The requirement for a nearby source of CXCL12 might explain why only some organ systems utilize it for vascular maturation.
Our prior results demonstrated that the epicardium expresses IGF2, which is required for cardiomyocyte proliferation and expansion of the ventricular wall (Li et al., 2011; Shen et al., 2015). IGF2 has no apparent role in coronary formation or maturation, and in the present study, CXCL12 signaling had no apparent role in the myocardium. These two epicardial factors therefore independently regulate different aspects of heart development. The expansion of the ventricular wall in response to IGF2 initiates from E10 (the time of formation of the epicardium) when growth substrates such as oxygen and glucose can diffuse into the myocardium from the ventricular lumen. As this process progresses, diffusion across the thickening ventricular wall becomes increasingly more limiting. CXCL12 during the E11.5–14.5 period promotes the assembly of the coronary vasculature, so that the onset of coronary perfusion around E14 provides a new means of distributing materials to the myocardium and thereby supporting heart and embryonic growth to the end of gestation.
Experimental Procedures
Fibrin bead assay
Dextran-coated Cytodex 3 microcarriers were coated with HUVECs, resuspended in fibrinogen, and added to wells of a 24-well plate containing thrombin to allow clotting. 1ml of endothelial basal media containing 2% FBS with or without VEGF, CXCL12, or AMD3100 was added to each well, and dermal fibroblasts were plated on top of the clot. Beads were photographed at 5× magnification after 3–7 days in culture; sprout number, tube number, branching points, and anastomoses were manually quantified from multiple microscopic fields per well.
Mice
All mouse lines used in this study have been described previously: Cxcl12 mutant (Ara et al., 2003), conditional Cxcr4 (Nie et al., 2004), Tie2Cre transgenic (Kisanuki et al., 2001), Nfatc1Cre (Zhou et al., 2002), Nkx2.5Cre knock-in (Moses et al., 2001), and conditional R26lacZ and R26YFP (Soriano, 1999).
India ink coronary artery perfusion
Hearts were carefully dissected from the thoracic regions of E15.5–E17.5 embryos and transferred to DMEM/10%FBS/heparin on ice. India ink diluted in PSB/heparin was then injected via the ascending aorta by mouth pipetting with a borosilicate glass tube. Hearts were fixed, dehydrated, cleared in benzyl alcohol:benzyl benzoate (1:1) and imaged with a stereomicroscope.
Additional and detailed experimental procedures are described in the on-line supplement.
Supplementary Material
Highlights.
CXCL12 is a vascular maturation factor important in coronary artery development.
CXCL12 acts in an organ-specific and paracrine manner.
Defective coronary formation explains late-gestation lethality of CXCL12 mutants.
The sinus venosus is the primary origin of embryonic coronary endothelium.
Acknowledgments
This work was supported by NIH grant HL070123 to H.M.S., and grant 14BGIA20500059 to S.R.K. from the American Heart Assn. S.C. was supported by a postdoctoral fellowship from the American Heart Assn. H.S. was supported by a postdoctoral fellowship from the California Institute for Regenerative Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, Kishimoto T, Matsui Y, Nagasawa T. Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1) Proc Natl Acad Sci U S A. 2003;100:5319–5323. doi: 10.1073/pnas.0730719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T. The role of CXCL12 in the organ-specific process of artery formation. Blood. 2005;105:3155–3161. doi: 10.1182/blood-2004-07-2563. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Chen HI, Poduri A, Numi H, Kivela R, Saharinen P, McKay AS, Raftrey B, Churko J, Tian X, Zhou B, et al. VEGF-C and aortic cardiomyocytes guide coronary artery stem development. J Clin Invest. 2014;124:4899–4914. doi: 10.1172/JCI77483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary B, Zhou J, Li P, Thomas S, Kaartinen V, Sucov HM. Absence of TGFbeta signaling in embryonic vascular smooth muscle leads to reduced lysyl oxidase expression, impaired elastogenesis, and aneurysm. Genesis. 2009;47:115–121. doi: 10.1002/dvg.20466. [DOI] [PubMed] [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18:2474–2478. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Hong CC, Peterson QP, Hong JY, Peterson RT. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol. 2006;16:1366–1372. doi: 10.1016/j.cub.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Li P, Cavallero S, Gu Y, Chen THP, Hughes J, Hassan AB, Bruning J, Pashmforoush M, Sucov HM. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development. 2011;138:1795–1805. doi: 10.1242/dev.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kohara H, Uchida Y, James JM, Soneji K, Cronshaw DG, Zou YR, Nagasawa T, Mukouyama YS. Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin. Dev Cell. 2013;24:359–371. doi: 10.1016/j.devcel.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirandola L, Apicella L, Colombo M, Yu Y, Berta DG, Platonova N, Lazzari E, Lancellotti M, Bulfamante G, Cobos E, et al. Anti-Notch treatment prevents multiple myeloma cells localization to the bone marrow via the chemokine system CXCR4/SDF-1. Leukemia. 2013;27:1558–1566. doi: 10.1038/leu.2013.27. [DOI] [PubMed] [Google Scholar]
- Moses KA, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2-5/Cre gene using ROSA26 reporter mice. Genesis. 2001;31:176–180. doi: 10.1002/gene.10022. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- Nagasawa T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J Mol Med (Berl) 2014;92:433–439. doi: 10.1007/s00109-014-1123-8. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez-del-Pulgar S, Carpenter PM, Hughes CC. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res. 2003;66:102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200:1145–1156. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–553. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago B, Calonge E, Del Rey MJ, Gutierrez-Canas I, Izquierdo E, Usategui A, Galindo M, Alcami J, Pablos JL. CXCL12 gene expression is upregulated by hypoxia and growth arrest but not by inflammatory cytokines in rheumatoid synovial fibroblasts. Cytokine. 2011;53:184–190. doi: 10.1016/j.cyto.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Sengbusch JK, He W, Pinco KA, Yang JT. Dual functions of [alpha]4[beta]1 integrin in epicardial development: initial migration and long-term attachment. J Cell Biol. 2002;157:873–882. doi: 10.1083/jcb.200203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Cavallero S, Estrada KD, Sandovici I, Kumar SR, Makita T, Lien CL, Constancia M, Sucov HM. Extracardiac control of embryonic cardiomyocyte proliferation and ventricular wall expansion. Cardiovasc Res. 2015;105:271–278. doi: 10.1093/cvr/cvu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann AF, Standley C, Fogarty KE, Wolfe SA, Lawson ND. Chemokine signaling guides regional patterning of the first embryonic artery. Genes Dev. 2009;23:2272–2277. doi: 10.1101/gad.1813509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sun X, Wei L, Chen Q, Terek RM. CXCR4/SDF1 mediate hypoxia induced chondrosarcoma cell invasion through ERK signaling and increased MMP1 expression. Mol Cancer. 2010;9:17. doi: 10.1186/1476-4598-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, Nagasawa Y, Hamano T, Matsui I, Kawada N, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20:1714–1723. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Hu T, He L, Zhang H, Huang X, Poelmann RE, Liu W, Yang Z, Yan Y, Pu WT, et al. Peritruncal coronary endothelial cells contribute to proximal coronary artery stems and their aortic orifices in the mouse heart. PLoS One. 2013a;8:e80857. doi: 10.1371/journal.pone.0080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, Yang Z, Yan Y, Yang X, et al. De novo formation of a distinct coronary vascular population in neonatal heart. Science. 2014;345:90–94. doi: 10.1126/science.1251487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, Yang Z, Zhang Z, Zhong TP, et al. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res. 2013b;23:1075–1090. doi: 10.1038/cr.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O’Rourke BP, Sharp DJ, et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151:1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Wang W, Si JW, Miao XY, Li JC, Wang YC, Wang ZR, Ma J, Zhao XC, Li Z, et al. Notch signaling regulates CXCR4 expression and the migration of mesenchymal stem cells. Cell Immunol. 2013;281:68–75. doi: 10.1016/j.cellimm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Zhou B, Cron RQ, Wu B, Genin A, Wang Z, Liu S, Robson P, Baldwin HS. Regulation of the murine Nfatc1 gene by NFATc2. J Biol Chem. 2002;277:10704–10711. doi: 10.1074/jbc.M107068200. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.