SUMMARY
Interruption of coronary blood supply severely impairs heart function with often-fatal consequences for heart disease patients. However the formation and maturation of these coronary vessels is not fully understood. Here we provide a detailed analysis of coronary vessel development in zebrafish. We observe that coronary vessels form in zebrafish by angiogenic sprouting of arterial cells derived from the endocardium at the atrioventricular canal. Endothelial cells express the CXC-motif chemokine receptor Cxcr4a and migrate to vascularize the ventricle under the guidance of the myocardium-expressed ligand Cxcl12b. cxcr4a mutant zebrafish fail to form a vascular network, whereas ectopic expression of Cxcl12b ligand induces coronary vessel formation. Importantly, cxcr4a mutant zebrafish fail to undergo heart regeneration following injury. Our results suggest that chemokine-signaling has an essential role in coronary vessel formation by directing migration of endocardium-derived endothelial cells. Poorly developed vasculature in cxcr4a mutants likely underlies decreased regenerative potential in adults.
INTRODUCTION
The maintenance of systemic blood flow around the body places incredible demands on the heart such that it requires a continuous supply of oxygen and nutrients by an intricate network of coronary vasculature. Coronary disease is the leading cause of death worldwide (Alwan, 2011), and malformation of coronary vasculature can often cause sudden death in young adults (Taylor et al., 1992). Despite this critical importance, the processes and factors required for coronary vessel development remain to be elucidated.
The developmental origin of coronary vascular cells in birds and mammals has been an area of intense research and debate. Based on anatomical observations, coronary arteries were once thought to bud from the aorta in mammals (Hutchins et al., 1988). This was first questioned with experiments using chick-quail chimeras, which suggested that proepicardial cells spread over the heart and undergo EMT to differentiate and assemble into endothelial tubes (Perez-Pomares et al., 2002). More recent work using mouse histological and clonal analyses suggest that proepicardial cells only make a small contribution to the coronary vasculature (Katz et al., 2012). Instead, vessels are thought to derive from existing endothelial cells that either sprout directly from the sinus venosus (SV) to cover and vascularize the heart (Chen et al., 2014; Red-Horse et al., 2010), form an intermediate subepicardial endothelial cell population that arises from the endocardium of SV and atrium (Tian et al., 2013) or derive from budding ventricular endocardial cells (Chen et al., 2014; Wu et al., 2012). The relative contribution of different sources appears to vary between different regions and vessels of the heart (Chen et al., 2014) and is complicated further by partial contributions of both endocardial and proepicardial cells to the sinus venosus (SV) prior to coronary vessel development (Katz et al., 2012; Wu et al., 2012).
Zebrafish has become a major model for the study of heart development and disease. Development of the zebrafish heart begins during gastrulation with the specification of endocardial and myocardial progenitor cells (Stainier et al., 1993). These progenitor cells undergo cardiogenic differentiation and heart morphogenesis after which a third cell layer, the epicardium, covers the outer surface (Figure S1B and F) (Serluca, 2008). The result is a fully functioning two contractile-chambered heart at hatching (at 5 days post-fertilization (dpf)) that supplies blood to the body via a single circulatory loop (Hu et al., 2000). Here, we report that coronary endothelial cells in zebrafish form by angiogenic sprouting from endocardial-derived cells during post-embryonic development and identify Cxcr4a-Cxcl12b signaling as a key regulator of this process.
CXC-chemokines have a well-established role in regulating cell adhesion and migration during zebrafish development (Raz and Mahabaleshwar, 2009). Zebrafish have two CXCL12 (also known as SDF1) encoding genes (cxcl12a and cxcl12b) and two receptors (Cxcr4a and Cxcr4b), which act as discrete pairs in the embryo to simultaneously direct cell migration during development (Boldajipour et al., 2011). Cxcl12a-Cxcr4b signaling is required for directed migration of individual germ cells or the collective migration of the lateral line primordium. In both cases Cxcr4b-positive cells migrate along a Cxcl12a gradient (David et al., 2002; Doitsidou et al., 2002). Both axes of the signaling pathway are utilized in the step wise formation of the truck lymphatic network, where Cxcl12a first guides the dorsal migration of lymphatic progenitors then Cxcl12b guides the growth of the lymphatic sprouts along the intersegmental vessels (Cha et al., 2012). Independent Cxcl12b-Cxcr4a signaling has been implicated in the tethering of Cxcr4a–positive endodermal to the underlying Cxcl12b-experssing mesoderm during zebrafish gastrulation (Nair and Schilling, 2008) and later in the formation of the lateral dorsal aorta (Siekmann et al., 2009) and the capillary network of the zebrafish brain (Bussmann et al., 2011). Similarly, mouse knockouts of CXCR4 and CXCL12 show defects during embryonic vessel development particularly in the gastrointestinal tract (Ara et al., 2005; Tachibana et al., 1998) and kidney (Takabatake et al., 2009). We have found that Cxcl12/Cxcr4 chemokine signaling is necessary for coronary vessel formation in zebrafish and also sufficient to provide the positional cues for this process. Furthermore, cxcr4a mutant hearts lose the potential to regenerate in adulthood, suggesting poorly developed vasculature in cxcr4a mutants likely underlies decreased regenerative potential in adult zebrafish.
RESULTS
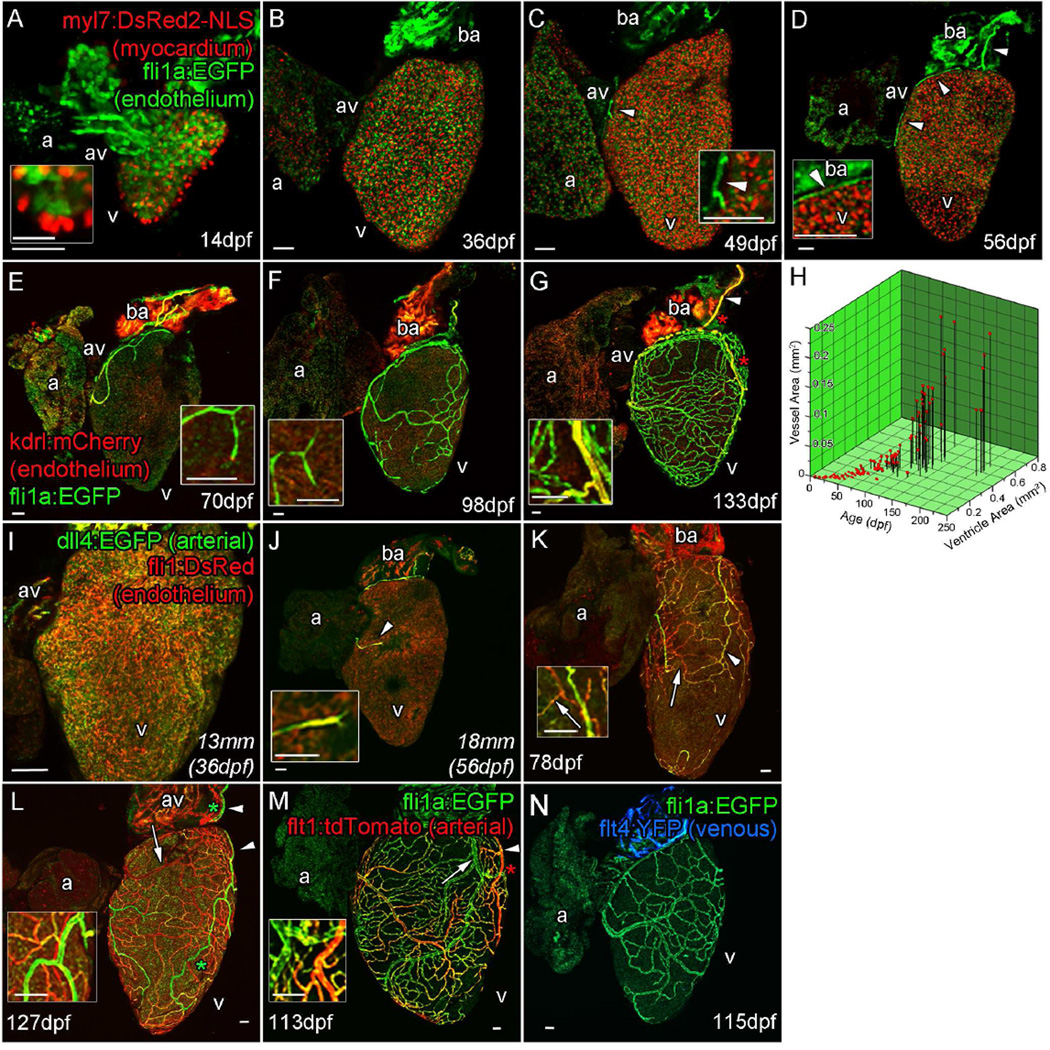
In the two months following hatching, the zebrafish juvenile ventricular myocardium undergoes significant expansion (Figure 1A–C, Figure S1A–L) (Gupta and Poss, 2012; Hu et al., 2000). Using a transgenic line that specifically labels endothelial cells (fli1a:EGFP), we have observed that zebrafish did not develop coronary vasculature until after this expansion had started; they hatched without endothelial cells or vessels on the surface of the heart (Figure 1A–C and Figure S1A–L). This is in striking contrast to sharks, birds and mammals for which coronary vessel development occurs during embryogenesis (De Andres et al., 1993; Hutchins et al., 1988; Perez-Pomares et al., 2002; Red-Horse et al., 2010; Wu et al., 2012). The formation of coronary vasculature began 1–2 months post hatching (~14mm in body length) with the emergence of endothelial cells between the atrium and ventricle (Figure 1C and Figure S1M–O). The newly emerged endothelial cells then migrated rostrally on to the bulbous arteriosus (BA) to form a vascular connection to the gills (Figure 1D, Figure S1P–R). This provides a supply of freshly oxygenated blood to the expanded myocardial layer via the main coronary artery over the BA which strongly expresses kdrl:mCherry (Figure 1D–G), dll4:EGFP (Figure 1L and S1II, asterisk denotes artery) and flt1:tdTomato (Figure 1M and S1GG, asterisk denotes artery) indicative of its arterial fate (Bussmann et al., 2010; Torres-Vazquez et al., 2003; Wythe et al., 2013). As the heart continued to grow, angiogenic sprouts appeared to progressively spread over the ventricle in later juvenile stages (Figure 1E and F, Figure S1S–Z). The result was the formation of a dense network of vessels that covers the ventricle in adult zebrafish (Figure 1G, Figure S1AA–FF). The larval endocardium expresses arterial marker dll4:EGFP prior to the emergence to vessel sprouts (Figure 1I). These emerging sprouts also appear to be comprised of arterial endothelial cells (Figure 1J). As the coronary vasculature network forms, some of these endocardial-derived cells appear to progressively down-regulate dll4:EGFP while others maintain a high level of expression. The result is the formation of a network of arteries (arrowheads) and non-arterial vessels (arrows) (Figure 1K–M and S1GG and II). Unlike other vasculature systems such as in the fin these non-arterial vessels do not appear up-regulate venous markers (Figure 1N and S1GG-LL), nor do they express lymphatic marker prox1a:RFP by this stage (113–127 dpf) (Figure S1KK).
Figure 1. Zebrafish coronary vessels develop during juvenile stages and respond to increased heart size.
Confocal images of whole larval, juvenile and adult double transgenic hearts, Tg(fli1a:EGFP; myl7:DsRed2-NLS (A–D) and Tg(fli1a:EGFP; kdrl:mCherry) (EG). flila:EGFP-positive endothelial (endocardial) cells are visible only on the inside of the myocardium (labeled with myl7:DsRed2-NLS) and bulbous arteriosus after hatching (A and inset 14 dpf and B, 36 dpf). Five to seven weeks later, endothelial cells are found on the surface of the heart (C, 49 dpf, arrowhead and inset), forming a vessel connection between the gills and AV-boundary after one month (D, 56 dpf, arrowheads and inset). The major vessel over the bulbous arteriosus expresses high levels of kdrl:mCherry (marked by arrowhead in G). Branches from these early vessels appear to progressively cover and interconnect over the juvenile heart into adulthood; however, the majority express low kdrl:mCherry levels (E, 70 dpf to G, 133 dpf, insets). The emergence and coverage of these vessels is stochastic, but correlates strongly with age and the area of the ventricle (H). Endocardial cells prior to the emergence of vessels express arterial marker dll4:EGFP (I). Emerged endothelial cells on the ventricle surface also have an arterial identity (J, arrowhead and inset), which is selectively down-regulated in some vessels as they form (K, arrow). A subset of vessels maintain dll4:EGFP (L, arrowhead) and express flt1:tdTomato confirming they are arteries (M, arrowhead). Vessels which down-regulate arterial makers appear to not progress to full venous identity as flt4:YFP expression is not observed at significant levels (N). a, atrium; av, AV canal; v, ventricle. Zebrafish age listed as days post fertilization (dpf) or italicized total body lengths with equivalent age in brackets when zebrafish were raised under non-standard conditions. Asterisk denotes artery. Scale bars, 50 µm except for that in inset A, which is 10 µm.
The time of vessel emergence and rate of formation was variable, but correlated strongly with age, fish length, and ventricle size when zebrafish were maintained under controlled conditions (Figure 1H, Figure S2G – I). Consistent with the stochastic nature of vessel emergence and the correlation between heart size and vessel area during development, the adult zebrafish coronary vasculature appears to respond to increasing heart size. Adult zebrafish that continued to grow had larger hearts and increased coronary vessel area and density (Figure S2A – F). Zebrafish raised at high density (20 fish per 3L) until 8mpf and then switched to a lower density (4 fish per 3L) for 4 months became significantly larger and had a corresponding increase in heart size (Figure S2J and K). Coronary vessels of these fish appeared to adapt to these changes by increasing vessel area and vessel density despite their relatively old age (8–12mpf, Figure S2L – O), demonstrating the dynamic nature of vessel development and its capability to adapt to environmental changes.
Endocardial cells migrate onto the surface of the ventricle
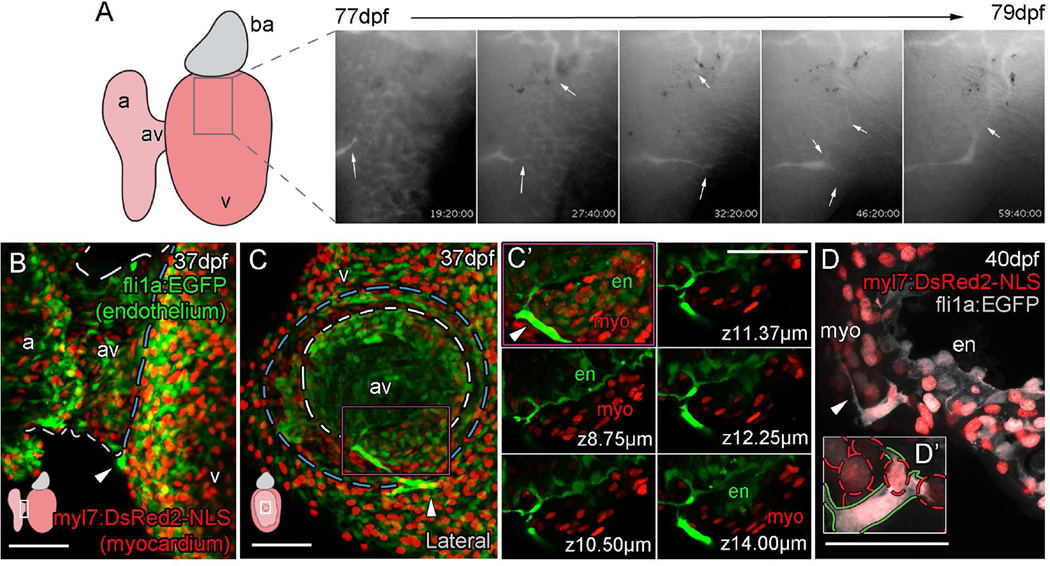
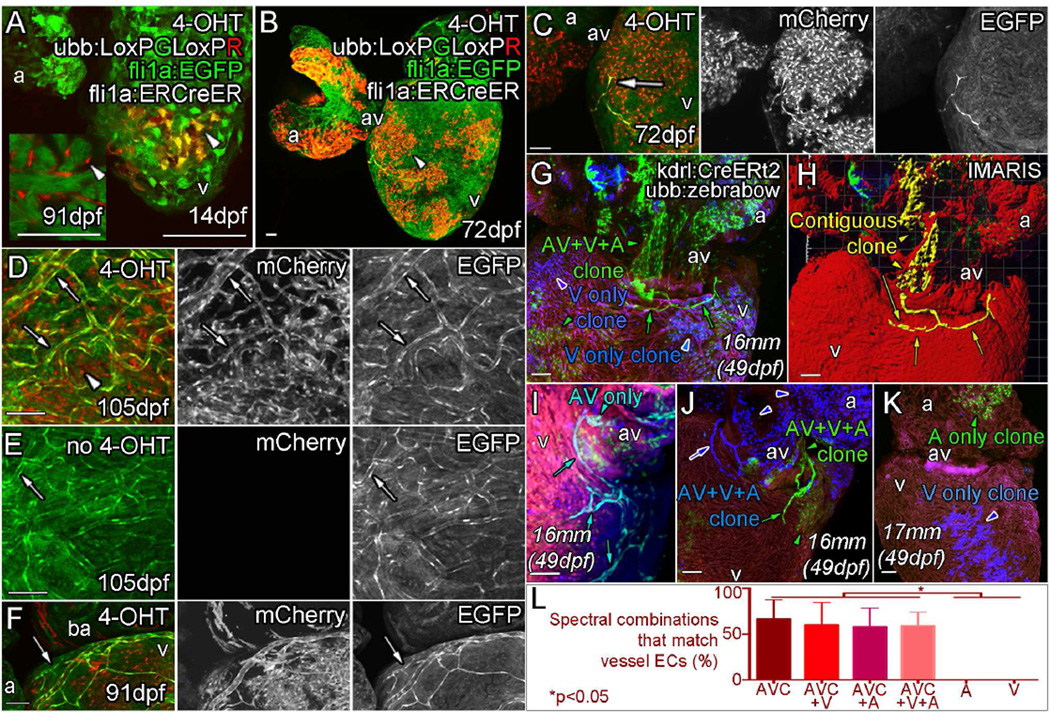
The progressive nature of vessel formation was indicative of an angiogenic process in which interlinked endothelial cells migrate over the heart to form interconnecting vessels. Time lapse imaging of live juvenile hearts confirmed that endothelial sprouts actively migrated over the ventricle and made connections between endothelial cells (Figure 2A, arrows, Movie S1). To investigate the source of these angiogenic sprouts, we analyzed hearts at the earliest stage of vessel development at around 5 weeks post fertilization (Figure 2B–D). We consistently observed one or two fli1a:EGFP positive endothelial cells on the ventricle proximal to the atrioventricular (AV) canal at the groove between the two (Figure 2B and C). Cells in this position had direct connections to the endocardial layer suggesting that they emerged from this point (Figure 2C’). In some cases, single cells spanned the thin myocardium while projecting processes across the surface of the heart (Figure 2D and D’). To test whether the endocardial endothelium was the source of vessel endothelial cells, embryonic endocardial cells were genetically labeled from 0–5 days post fertilization. We generated clonal patches of mCherry-positive endocardial cells in the juvenile heart (prior to the emergence of coronary vessels) by inducing recombination of a transgenic reporter [Tg(ubb:LoxPEGFPLoxPmCherry)] with a fli1a endothelial cell specific enhancer driven ERt2CreERt2 (Das and Crump, 2012) that was temporarily activated by 4-OHT in the embryo (Figure 3A and B, Figure S3A–I). Endothelial cells emerging from the AV canal in juvenile hearts were also mCherry-positive (39% of hearts with endocardium labeling, n=23; Figure 3B, C and S3G–I), suggesting that these cells were derived from embryonic endothelial cells, most likely from the labeled endocardium adjoining the AV canal (Figure 3C, S3G and H). Live and fixed imaging suggested that vessels form through an angiogenic process, where endothelial cells divide and migrate over the heart (Risau, 1997) (Figure 1 and Figure 2A, Figure S1, Movie S1). Consistent with these observations, the majority of developed vessels (fli1a:EGFP positive) on the adult heart were also continuously mCherry-positive along labeled vessels, indicating that they were derived from the early angiogenic sprouts observed in juvenile fish (Figure 3D, arrow, 3E, no 4OHT control). Some unlabeled vessels were observed (Figure 3F, arrow) suggesting either that multiple endocardial cells transmigrate onto the surface of the ventricle, or that a secondary minor source of cells contributes to formation of these vessels. We quantified the contribution of the clonal patches to coronary endothelial cells and found that the majority of coronary endothelial cells were labeled in hearts with only partial endocardial labeling (9/23 labeled hearts, Figure S3I). We also found some hearts that had a large portion of the endocardium labeled, but lack vascular labeling (Figure S3E, F and I). These data suggest that coronary endothelial cells were likely derived from a restricted population rather than many different regions of the endocardium. In addition, no vessel labeling was observed in hearts that, although treated with 4OHT, lack endocardial labeling due to the stochastic nature of recombination (n=6, not included in quantification).
Figure 2. Vessels are formed from angiogenic sprouts of cells emerging from the AV canal.
Live imaging stills shows the angiogenic migration of the endothelial cells that migrate over the ventricle surface and form interconnections between forming vessels (A; angiogenic sprouts marked by arrows). The first emerging endothelial cells are visible on the surface of the ventricle at the juncture of the atrium and ventricle (arrowheads, B, ventral view, C, lateral view of the same heart, diagram denotes heart orientation). Cells at this point have a direct connection to the endocardium (C, dashed white line demarks AV canal, blue line marks invagination of the ventricular myocardium; C’, higher magnification of region demarcated by purple box with select individual planes in z, labeled with distance from the first plane of projection). Individual endothelial cells span the myocardium (D; D’, higher magnification with red dashed lines demarcating myocardial nuclei, green line demarcates endothelial cell) and then project filopodia over the ventricle surface (D, arrowhead). Scale bars, 50 µm
Figure 3. Angiogenic coronary endothelial cells are derived from a subpopulation of the endocardium.
Triple transgenic fish, Tg (ubb:LoxPEGFPLoxPmCherry (ubb:LoxPGLoxPR); fli1aep:ERt2CreERt2 (fli1a:ERCreER); fli1a:EGFP) were treated with 4-OHT during embryogenesis (0-5dpf) to label the endocardium as mCherry positive clonal patches (arrowheads) and imaged at 14dpf (A), 72dpf (B and C), 91dpf (F) and 105dpf (D). E, no 4-OHT control. A (inset), 91dpf section with no fli1a:EGFP showing mCherry expression in endocardial cells lining the trabecular myocardium. Emerging endothelial cells (fli1a:EGFP-positive, bright green) are also mCherry-positive (C and D, arrow, labeled endothelial cells; arrowhead, labeled endocardial cells). The majority of subsequently formed vessels are found to be similarly labeled in adult zebrafish treated with 4-OHT as embryos (D, arrows), but not in those that were not exposed to 4-OHT during embryogenesis (E, arrow). Unlabeled vessels (arrowhead) have been observed on hearts with labeled vasculature consistent with the hypothesis that multiple cells give rise to vessels by an angiogenic process (F, arrow). Clonal analysis with multispetral fluorescent proteins (ubb:Zebrabow; ubb:Lox2272-LoxP-RFP-Lox2272-CFP-LoxP-YFP) is used to follow the fate of restrictedly labeled endocardial cells (G–L). Image of the ventral side of the AV canal, with ventricle below and atrium above showing labeling of the endocardial cells as a single green clone (G, green arrowhead) which gives rise to the forming vessel on the ventricle surface (G, green arrows). Part of this green clone is contiguous with the surface endothelial cells, extending through the myocardium to the AV canal and atrium (H, Yellow). Analysis of multiple clones, including AV endocardium specific clones (I), shows a clonal relationship between AV endocardial cells and endothelial cells on the surface (G–J). Forming vessels can be derived from more than one clone, but always share the same labeling with the AV canal (J). Non-AV clones are found not to give rise to surface endothelium (K). Quantification of the endocardial spectral combinations found in different heart regions that match those of the vessel endothelium (L, *p<0.05 ANOVA/Tukey, ±SEM). Zebrafish age listed as days post fertilization (dpf) or italicized total body lengths with equivalent age in brackets when zebrafish were raised under non-standard conditions. AVC, Atrioventricular Canal; A, Atrium; V, Ventricle. Scale bars, 50 µm.
To further characterize the origin of coronary endothelial cells in zebrafish, we employed a clonal analysis using the multispectral ubb:zebrabow line (ubb:Lox2272-LoxP-RFP-Lox2272-CFP-LoxP-YFP) and kdrl:CreERt2 to label and then follow specific endocardial clones (Figure 3G–L, Figure S3J–O, Movie S2) (Pan et al., 2013; Zhao et al., 2014). Embryonic induction (32–56 hpf) of CreERt2 resulted in spectrally distinct patches of labeled endocardium in juvenile fish (Figure 3G–L, arrowheads, Figure S3J–O). Endothelial sprouts observed on the surface of the heart often displayed the same spectral combination as endocardial clones within the AV canal (Figure 3G–J, arrows, Figure S3M–O). Such endocardial spectral labeling often extends into the atrium, ventricle or both, but those restricted to the atrium or ventricle endocardium did not appear to give rise to emerging endothelial cells on the surface of the heart (Figure 3K and L, Figure S3M–O). Furthermore, vessel endothelial cells on the ventricle surface were often found to be contiguous with endocardial clones at the AVC (Figure 3G–J, Figure S3M). Multiple angiogenic sprout clones were observed (an average 1.2 vessel forming clones in hearts with labeled sprouts) suggesting that multiple AV endocardial cells migrate onto the surface of the heart to form the coronary vasculature (Figure 3J; arrowhead, endocardium; arrow, angiogenic sprout). Together, these data strongly suggest that the AV canal endocardium is the major source of the angiogenic sprouts when coronary endothelial cells first emerge on the surface of the ventricle.
Coronary vessel formation is regulated by CXC-chemokines
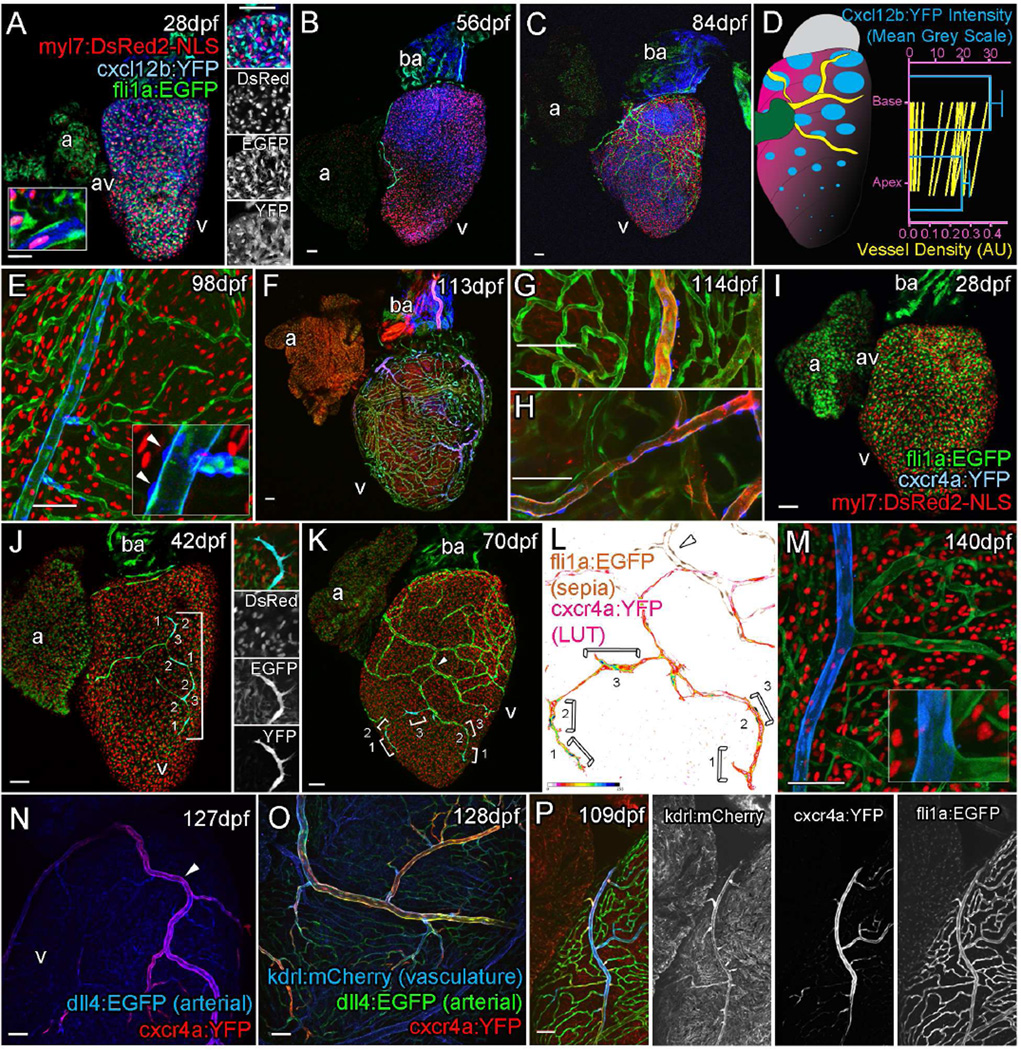
Homeostatic chemokines have a well-established role in directing the migration and positioning of receptor expressing cells (Cha et al., 2012; David et al., 2002; Doitsidou et al., 2002; Nair and Schilling, 2008; Raz and Mahabaleshwar, 2009). CXC motif-chemokines are one such family of molecules that act on endothelial cells during angiogenesis (Ara et al., 2005; Bussmann et al., 2011; Kiefer and Siekmann, 2011; Siekmann et al., 2009; Tachibana et al., 1998; Takabatake et al., 2009). During formation of both the lateral aortae and brain capillaries, endothelial cells expressing chemokine (CXC-motif) receptor 4a (Cxcr4a) migrate towards or over chemokine (CXC-motif) ligand 12b (Cxcl12b) expressed in the underlying endoderm and neural keel midline respectively (Bussmann et al., 2011; Siekmann et al., 2009). During juvenile heart development in zebrafish, we found that a cxcl12b:YFP reporter was expressed in ventricular, but not atrial, cardiomyocytes prior to the emergence of endothelial cells onto the ventricular surface (Figure 4A and Figure S4A–C). This expression was maintained as the endothelial cells migrated over the ventricle (Figure 4B and C, Figure S4D). cxcl12b:YFP expression was generally observed to be strongest in the base of the ventricle in the region that tends to be vascularized first (Figure 1C and D, Figure 4D and Figure S4E and F). However, levels and regions of myocardial expression varied markedly between hearts (Figure 4A–C, Figure S4A–D).
Figure 4. Cxcr4a–Cxcl12b expression during coronary vessel formation.
Confocal images of transgenic fish Tg(myl7:DsRed2-NLS; fli1:EGFP; cxcl12b:YFP) (A–C, E), Tg(kdrl:mCherry; fli1:EGFP; cxcl12b:YFP) (F–H), Tg(myl7:DsRed2-NLS; fli1:EGFP; cxcr4a:YFP) (I–M), Tg(dll4:EGFP; cxcr4a:YFP) (N), Tg(dll4:EGFP; cxcr4a:YFP; kdrl:mCherry) (O), and Tg(kdrl:mCherry; fli1:EGFP; cxcr4a:YFP) (P). Ventricular, but not atrial cardiomyocytes express cxcl12b:YFP prior to and during vessel formation (A, 28 dpf, B, 56 dpf, C, 77 dpf). cxcl12b:YFP labeling is found within the cytoplasm of cells containing a myl7-positive nuclei (red) and flanked by fli1a-positive (green) endothelial cells (A, inset, from confocal section, side panel). Levels of cxcl12b:YFP expression vary over the ventricle and between hearts, but are generally higher towards the base (A–D). Quantification of cxcl12b:YFP intensity (blue, normalized mean 14dpf to 84dpf, ±SEM) and vessel density (green, mean values of individual time points 7dpf to 420dpf) between base and apex of ventricular myocardium (D), both p<0.0001 (Wilcoxon signed rank test on paired values). In addition, expression is observed on the surface of the bulbous arteriosus (ba, A–C, F). cxcl12b:YFP expression is down regulated following establishment of the vessel network in cardiomyocytes, but is later expressed in the mural cells of mature vessels (E, inset arrowhead). cxcl12b:YFP-expressing mural cells surround large arterial vessels (F–H, blue), which express high levels of kdrl:mCherry (F–H, red). cxcr4a:YFP is not expressed prior to the emergence of endothelial cells on the surface of the ventricle (I, 28 dpf). Endothelial cells of angiogenic sprouts express cxcr4a:YFP as they migrate over the surface of the myocardium (J, 42dpf, panel showing tip cell; K, 70 dpf; brackets label cells displaying active migration morphology; L, intensity of cxcr4a:YFP in endothelial cells). The majority of vessels do not express cxcr4a:YFP after their formation (K–P). Larger arterial vessels do maintain cxcr4a:YFP expression, which overlaps with high levels of kdrl:mCherry and dll4:EGFP expression in these vessels (N–P). Scale bars 50µm.
Strong expression of cxcl12b:YFP was also observed in an unknown cell population on the surface of the BA prior to and after the formation of the major arterial vessel over the BA (Figure 4A–C, F Figure S4A–C). Following the formation of the coronary vasculature, cxcl12b:YFP was downregulated in cardiomyocytes, but was upregulated in epicardial derived perivascular cells which line the kdrl:mCherry expressing arteries (Figure 4E–H, inset arrowheads, Figure S4G and H). Tcf21:CreERt2 was used to label the epicardium in embryos (Kikuchi et al., 2011) and the descendants of these cells were found to give rise to the cxcl12b:YFP expressing mural cell population of arterial vessels (Figure S4I).
cxcr4a:YFP was only expressed after the emergence of endothelial cells and was more strongly expressed by migratory endothelial cells at the angiogenic sprouts (Figure 4I–L, Figure S4J–L). Expression was maintained in migrating cells, but became largely downregulated as vessels formed (Figure 4K and L, brackets demarcate migrating cells; arrowhead, formed vessel and Figure S4M) suggesting a role for CXC-signaling during the migration of these cells. Consistent with cxcl12b:YFP expression after vessel formation, cxcr4a:YFP receptor expression was maintained in endothelial cells of the mural cell lined arteries, which express high levels of kdrl:mCherry and dll4:EGFP (Figure 4M–P and Figure S4N–Q). These data suggest that Cxcr4a–Cxcl12b signaling not only guides the newly emerged endocardial derived endothelial cells, but also may later stabilize and maintain the arterial vascular network over the ventricle.
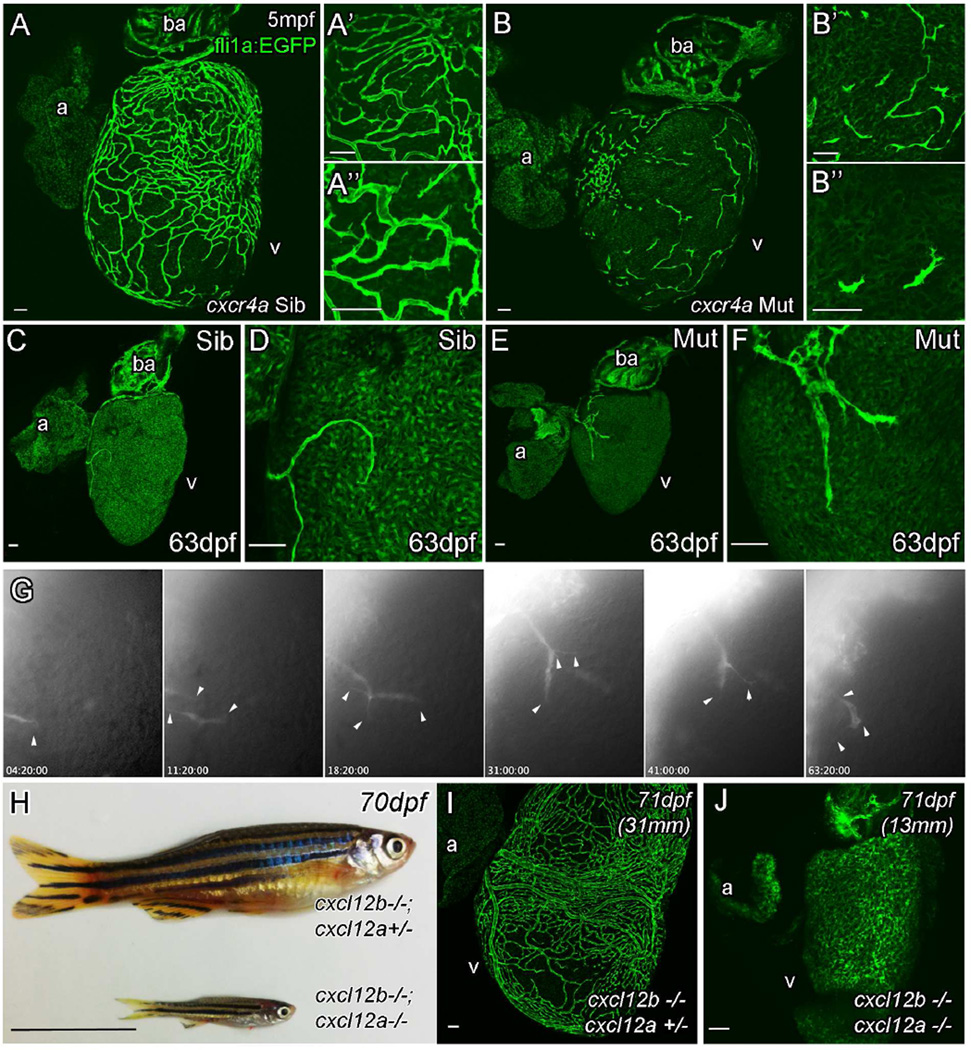
Mutant zebrafish lacking a functional Cxcr4a receptor failed to develop coronary vasculature (5mpf, Figure 5A and B, Figure S5A–H). cxcr4a–mutant endothelial cells migrated onto the surface of the ventricle suggesting that initiation of the sprout occurs normally, but the mutant sprouts appeared abnormal (Figure 5C–F) and failed to migrate in a coordinated way, resulting in isolated cells or groups of cells that did not resolve to form a functional vasculature network (Figure 5A and B, Figure S5A–H). Angiographs suggested that there was no major supply of blood to the surface of the heart in the mutant other than systemic flow (Figure S5I–K). Mutant endothelial cells appeared to be less consolidated in the direction of their migration, simultaneously extending multiple processes in different directions (Movies S3, Figure 5G), suggesting that these endothelial cells were unable to correctly interpret the cues of their external environment. In contrast, the ligand Cxcl12b was not required for vessel formation, indicating that there may be a compensatory mechanism for coronary vessel development in cxcl12b–mutants (Figure S6A and B). Evidence from other development paradigms suggests that the Cxcr4a and Cxcr4b receptors show strong affinity for Cxcl12b and Cxcl12a respectively, but in the absence of one they can respond to the other (Boldajipour et al., 2011). In addition, during fin regeneration in adult zebrafish, Cxcl12a rather than Cxcl12b appears to be the relevant ligand for Cxcr4a–dependent vascular plexus remodeling (Xu et al., 2014). Consistent with this cxcl12a:DsRed was indeed expressed by ventricular cardiomyocytes in cxcl12b–mutant zebrafish (Figure S6E–H). To test for such compensation, cxcl12a/b double mutants were raised. Unlike their siblings that had at least one functional copy of cxcl12a or cxcl12b, double mutant zebrafish were found to not survive past larval stages using standard husbandry approaches (Figure S6I), suggesting broad functional redundancy between cxcl12a and cxcl12b. Using optimized husbandry (see methods), a small number of cxcl12a/b double mutants were able to survive into juvenile stages (7 out of 136 selected double mutants survived beyond 1 month, the oldest of which died at 93dpf). Surviving double mutant zebrafish were much smaller than siblings raised under the same conditions (Figure 5H), but reached a size (>13mm) and age at which coronary vessel development should have started. No endothelial cells, angiogenic sprouts or formed vessels were observed on the surface of the cxcl12a/b double mutant heart (Figure 5I and J, S5J and K). Although their stunted growth could be a contributing factor to the lack vessel development in cxcl12a/b double mutants, some cxcl12b−/−; cxcl12a+/− adult fish show abnormal patterning and positioning of coronary vessels suggesting that Cxcl12 signaling is required for proper development of the coronary vasculature (Figure S6L–O). Independently, Cxcl12a and Cxcr4b appeared not to be required for coronary vessel development as these mutant zebrafish developed vasculature normally (Figure S6A, C and D).
Figure 5. Cxcr4a and Cxcl12 are required for coronary vessel formation.
Endothelial cells migrate over the surface of the heart, but fail to form a vascular network in adult cxcr4a mutant fish (A, Sibling control and B, Mutant). As cxcr4a mutant endothelial cells emerge onto the surface of the heart in juvenile zebrafish they have an uncharacteristic shape appearing truncated and highly spiculated (C–F). Movie stills show abnormal migration of endothelial cells in the mutant (G). Unlike wildtype cells, cxcr4a mutant cells project and retract multiple processes in different directions while migrating over the ventricle (arrowheads). They often migrate individually in different changing vectors and fail to make and maintain connections between each other. cxcl12a; cxcl12b double mutants show general retardation in growth in comparison with siblings raised under the same (optimized) husbandry conditions (H). Unlike their siblings, no endothelial cells are observed on the surface of heart of cxcl12a/b double mutant fish (I, sibling; J, cxcl12a; cxcl12b double mutant). Italicized age indicates zebrafish are raised using optimized husbandry, body length in brackets. Scale bars 50, µm except in H, which is 10mm.
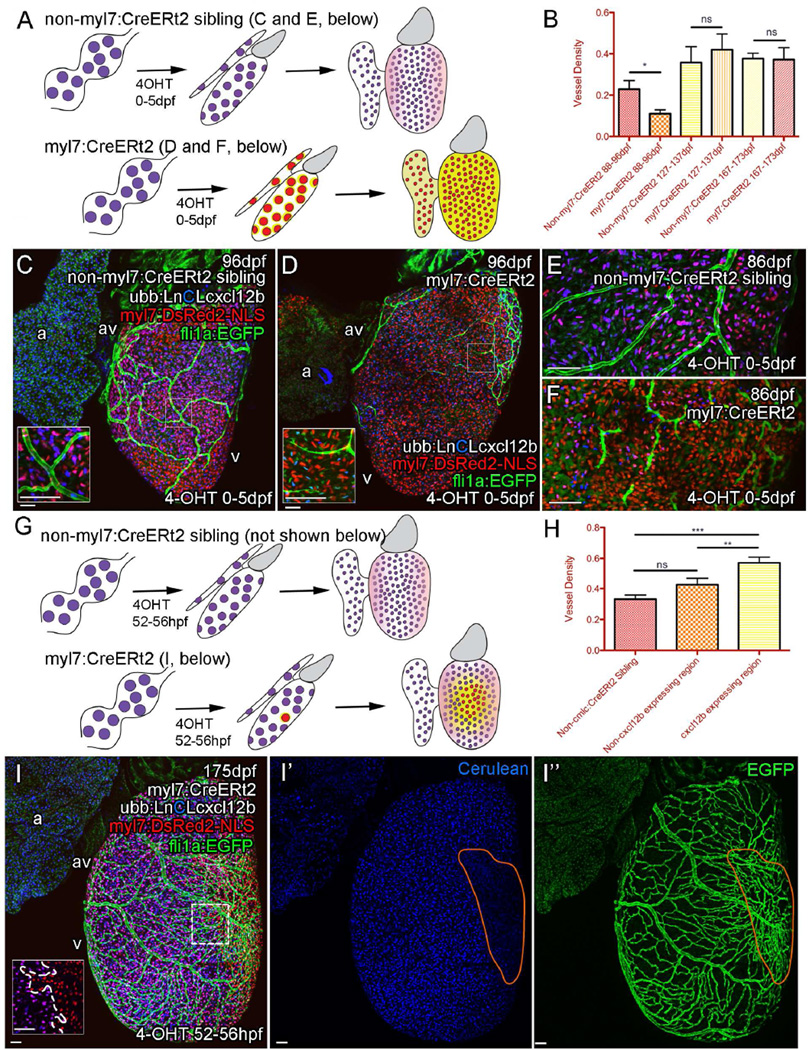
To investigate if the underlying myocardium was using Cxcl12b-Cxcr4a signaling to provide positional information to the endothelial cells, cxcl12b was overexpressed in all cardiomyocytes to dilute or disrupt the local gradients and positional cues given by the endogenous ligand (Figure 6A–F). Uniform overexpression of cxcl12b in the myocardium resulted in a disruption of vessel formation in juvenile zebrafish, suggesting that the location and/or dynamics of cxcl12b expression may be important for correct vessel formation (Figure 6A–F). Zebrafish overexpressing cxcl12b had less vessel coverage between 88 and 96dpf with endothelial cells appearing elongated or spiculated and irregularly positioned over the heart (Figure 6C–F). Later at around 127dpf, these overexpressing zebrafish did appear to successfully construct a vasculature network and vessel coverage becomes comparable with non-overexpressing siblings (Figure 6B, Figure S7A–D). It is possible that the endogenous signal reaches a level at which it can be interpreted over the exogenous signal. Ectopic expression in the atrium was insufficient to attract endothelial cells and induce vessel formation. This ectopic expression may not be strong enough to compete with the ventricular expression or additional factors may be required to vascularize the atrium (Figure S7C’–D’). When the developing myocardium is partially exposed to 4OHT, it resulted in localized and stable cxcl12b overexpression in clonal patches of the juvenile myocardium (Figure 6G). In contrast to the disruption caused by uniform overexpression, the expression of cxcl12b from patches of ventricular myocardial cells resulted in greater vessel density within and around the cxcl12b expressing region (Figure 6G–I and Figure S7E–I). Taken together these results indicate that Cxcl12b directs the localized formation of blood vessels on the surface of the heart by directing the migration and/or retention of cxcr4a-positive endothelial cells.
Figure 6. Cxcl12b likely provides positional information for coronary endothelial cells.
Uniform overexpression of Cxcl12b in quadruple transgenic fish Tg(Ubb:LoxPH2b-Cerulean-LoxP-cxcl12b(ubb:LnCLcxcl12b); myl7:DsRed2-NLS; fli1a:EGFP; myl7:CreERt2) (A–E). Schematic illustration of uniform overexpression of Cxcl12b. Prolonged treatment with 4OHT between 0 and 5 dpf resulted in efficient removal of the floxed H2b-Cerulean (blue) and expression of cxcl12b (yellow) in myl7-positive cardiomyocytes (red) such that any endogenous Cxcl12b signaling pattern may be obscured (pink) (A). Such overexpression of cxcl12b in cardiomyocytes also resulted in an initial disruption of vessel formation (B, quantification of vessel density, *p<0.05 t-test, ±SEM); C, non-myl7:CreERt2 sibling control; D, cxcl12b overexpression) with endothelial cells becoming elongated and appearing spiculated (C, and D, inset, E (control) and F (overexpression)). Clonal overexpression of Cxcl12b in quadruple transgenic fish Tg(Ubb:LoxPH3B–Cerulean-LoxP-cxcl12b (ubb:LnCLcxcl12b); myl7:DsRed2-NLS; fli1a:EGFP; myl7:CreERt2) (G–I). A 4 hour pulse of 4OHT results in partial activation of the myl7:CreERt2 transgene prior to cardiomyocyte clonal expansion. cxcl12b is then expressed from a localized source in adult zebrafish [G, I; inset from box; boundary between exogenous-cxcl12b expressing (DsRed-NLS positive) and non-expressing (H2b-Cerulean and DsRed-NLS double positive) cardiomyocytes is marked with a dashed line]. There is an increase in density of vessels over and proximal to the region of cxcl12b expressing cardiomyocytes (H, quantification of vessel density, **/***p<0.01/0.0001 t-test, ±SEM). Representative image of clonal overexpression (I, I′ and I″) with region of cxcl12b-expressing (Cerulean-negative) cardiomyocytes demarcated by orange. Scale bars, 50 µm.
cxcr4a mutant hearts fail to regenerate following resection
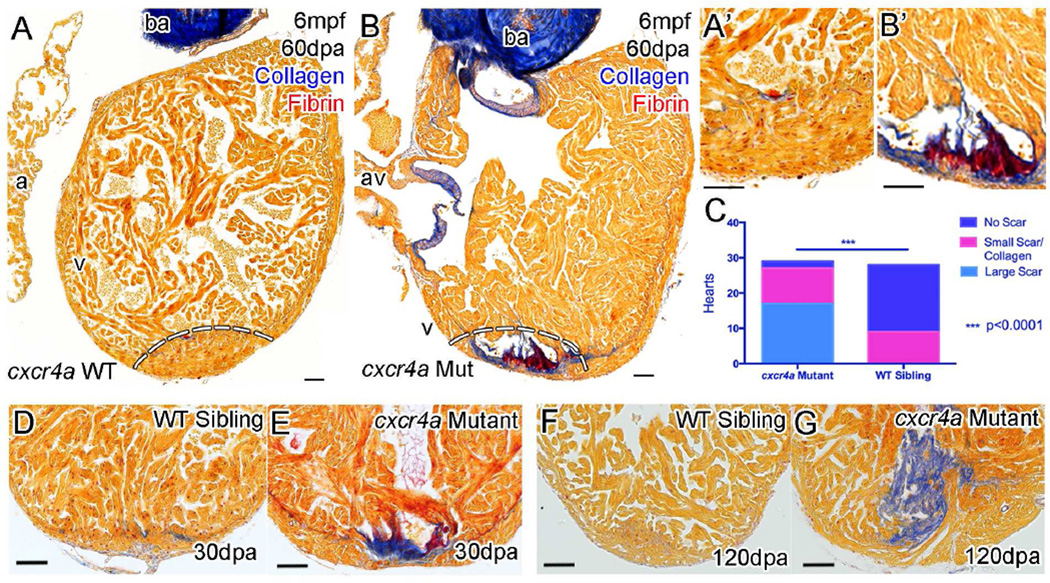
Surprisingly, some cxcr4a mutant zebrafish lacking coronary vasculature are viable as adults. Although grossly normal, mutants appear to be at a competitive disadvantage, having a range of ability to adapt to the loss of coronary vessels. Following incross of cxcr4a heterozygous parents, only 16 out of 294 adult offspring were found to be homozygous cxcr4a mutants, or 58 fewer than expected with Mendelian inheritance. Although some mutants survive, the majority of these cxcr4a mutant zebrafish failed to undergo heart regeneration as adults perhaps because of compromised development during juvenile stages. cxcr4a mutant zebrafish failed to regenerate following amputation of the apex forming a scar instead at 30dpa (Figure 7D and E), 60dpa (Figure 7A and B) and 120dpa (Figure 7F and G). Previous studies into Cxcr4-Cxcl12 signaling during heart regeneration suggest that Cxcr4b, but not Cxcr4a, is required for correct migration and contribution of regenerating cardiomyocytes to the regenerating region (Itou et al., 2012). We demonstrated that cxcr4a is required during development and our results suggest that developmental disruption of coronary vascularization results in a zebrafish heart that is unable to respond to injury in the typical manner.
Figure 7. cxcr4a mutant hearts fail to regenerate following resection of the ventricle apex.
Zebrafish with functional Cxcr4a-Cxcl12b signaling and normal coronary vessel development completely regenerate resected ventricle tissue after 60 days (A, dashed line represents amputation plane, and A’). Adult cxcr4a mutants with disrupted coronary vessel development fail to regenerate the resected tissue resulting in the deposition and persistence of collagen (blue) and fibrin (red) (B, B’, E and G). Quantification of scar formation at 60 dpa (C). cxcr4 mutant zebrafish have significantly more collagen deposition and scar formation compared to the siblings (***P<0.0001, χ2). Scar formation is observed by 30dpa (D and E) and persists beyond 120dpa (F and G) with no apparent effect on survival. Scale bars 50, µm.
DISCUSSION
Here we have described for the first time the vascularization of the ventricle in zebrafish by angiogenesis of endothelial cells from the endocardium. The presence of coronary vessels in all studied elasmobranchs suggests they have origins early in vertebrate heart evolution coinciding with the emergence of compact myocardium (Sedmera and Wang, 2012). Consistent with this, in zebrafish only the ventricle becomes vascularized after the compact myocardium has formed post-embryonically and the more spongy and thinner-walled atrium never develops coronary vessels (Figure 1 and Figure S3P and Q). Unlike mammalian cardiac muscle, the fish myocardium is not strictly dependent on coronary circulation for survival (Figure 5). During evolution, coronary vessels have been lost in many teleost species, and some amphibians have only a vestigial non-penetrating coronary vessel on the surface of the outflow tract (Sedmera and Wang, 2012). It appears that coronary vessels evolved initially as a supplement of cardiac oxygen supply, but later became strictly required for the function and survival of the myocardium. Our study suggests that angiogenesis could be the ancestral method of coronary vessel development and this mechanism is at least partially conserved in mammals (Chen et al., 2014; Red-Horse et al., 2010; Tian et al., 2013).
Divergent cell populations have been identified as the source of coronary vascular endothelial cells (Chen et al., 2014; Perez-Pomares et al., 2002; Red-Horse et al., 2010; Tian et al., 2014; Tian et al., 2013; Wu et al., 2012). Our results suggest that zebrafish coronary endothelial cells derive from the endocardium at the atrioventricular canal. Although the source of coronary vascular endothelial cells remains unclear in elasmobranchs such as the dogfish (MunozChapuli et al., 1996), the process of coronary vascular development is thought to be initialed by the formation of a diverticulum from the sinuous venous (SV) (De Andres et al., 1993). This is consistent with observations in mice demonstrating the SV endocardium as a major source of vascular endothelial cells (Chen et al., 2014; Red-Horse et al., 2010; Tian et al., 2013). Interestingly, the site of the future coronary sinus in both dogfish and mice may match this point of origin (the SV becoming atrialized in mammals). By contrast, zebrafish appear to lack such a coronary sinus on the SV and this chamber also seems to largely lack cardiomyocytes (Figure S3 P and Q). Furthermore, both the SV and the atrium remain unvascularized in adult zebrafish, and at no point did we observe endothelial cells on their surface during development (Figure 1 and Figure S3 P and Q). In addition, observed early angiogenic sprouts on the surface of the heart appear to be arterial (as are the endocardial endothelial cells) rather than venous (Figure 1). Taken together, we think it unlikely that the sinuous venous (SV) has a major direct contribution to the developing vasculature. However, earlier developmental contributions to the endocardium of the AV canal from the SV, proepicardium or liver primordium in zebrafish is still possible (Katz et al., 2012; Poelmann et al., 1993; Tian et al., 2013).
Given the evolutionary distance between dogfish and zebrafish it is conceivable that such anatomical changes could occur and this reinforces the notion that there is a degree of developmental plasticity for the coronary vasculature. Nonetheless, in these species coronary vessel formation remains fundamentally consistent with what is known to occur in mammals, in which it develops from existing endothelial cell populations residing within the heart (Chen et al., 2014; Red-Horse et al., 2010; Tian et al., 2014; Tian et al., 2013; Wu et al., 2012). The ventricular endocardium has also been shown to give rise to coronary endothelial cells during postnatal trabecular compaction in the mouse inner myocardial wall (Tian et al., 2014). The zebrafish myocardium remains trabecular and the coronary vasculature remains superficial in subepicardial space, further suggesting that coronary vasculature formation is a versatile process that evolves responsively to fulfill the need of myocardium.
Observations in birds led to the initial hypothesis that the major mode of coronary vessel formation was by vasculogenesis of epicardial-derived cells (Perez-Pomares et al., 2002). This is inconsistent with a number of our observations in zebrafish including the nature of endothelial cell migration (Movie S1); the progressive formation of vessels over the heart (Figure 1); the continuous lineage-based labeling along vessels derived from fli1a-expressing endothelial cells (Figure 3); and the clonal nature of the early angiogenic sprouts on the ventricle surface (Figure 3). The epicardium does, however, provide a source of vascular support cells to the coronary arteries that express cxcl12b and may have an important role in maintaining the integrity of the coronary vasculature (Figure S1 and Figure S4) (Kikuchi et al., 2011).
Although the coronary vasculature is a supplemental cardiac oxygen supply, it has an important function in zebrafish. Most cxcr4a mutants lacking coronary vessels do not survive to adulthood, but those that do survive appear to lack the normal regenerative response to injury (Figure 7). What physiological and developmental adaptations take place in the zebrafish myocardium in response to the loss of this oxygen supply remains to be elucidated. How these factors impinge on the regenerative process are fascinating areas of future study and is of relevance in patients whose coronary vessel function in is compromised by occlusion or abnormal development. Understanding the underlying molecular and cellular basis of these adaptations will prove beneficial to patients whose endogenous regenerative responses to myocardial damage could be harder to stimulate by clinical intervention. Furthermore, enhancing collateral blood vessel formation by Cxcl12/Cxcr4 guided angiogenesis might be beneficial after ischemic heart injuries.
Given the medical burden associated with compromised coronary vasculature function, finding new avenues of therapy for coronary heart disease is imperative (Alwan, 2011; Taylor et al., 1992). Stimulating endogenous endothelial cells with exogenous factors or introducing analogous cells is a promising strategy for alleviating pathologies associated with coronary artery disease and malformation. Here we have identified CXC-chemokines as one such potential exogenous factor. As discussed, cxcr4a is required for vessel formation in juvenile zebrafish (Figure 5) and modulation of cxcl12b–expression can influence the formation and density of coronary vessels (Figure 6). Interestingly an SNP in the region of human CXCL12 has consistently been associated with early onset myocardial infarction and coronary artery disease in GWA studies of patients, suggesting that the mechanisms uncovered here in zebrafish may be conserved in humans and gives promise to their effective modulation in a clinical setting (Myocardial Infarction Genetics Consortium et al., 2009; Samani et al., 2007; Schunkert et al., 2011).
EXPERIMENTAL PROCEDURES
Zebrafish strains
Tg(fli1a:EGFP)y1; Tg(fli1a.ep:DsRedEx)um13 (referred to as fli1a:DsRed); Tg(−5.1myl7:DsRed2-NLS)f2;, Tg(kdrl:GRCFP)zn1; Tg(−6.5kdrl:mCherry)ci5; Tg(−3.5ubb:LOXP-EGFP-LOXP-mCherry)cz1701; Tg(cryaa:DsRed,-5.1myl7:Cre-ERT2)pd10 (referred to as myl7:CreERt2);, TgBAC(tcf21:DsRed2)pd37;, TgBAC(cryaa:EGFP,tcf21:Cre-ERT2)pd42 (referred to as tcf21:CreERt2);, Tg(cxcl12a:DsRed2)umn1;, Tg(wt1b:EGFP)li1;, Tg(Mmu.Dll4F2-ADV.E1B:EGFP)sgf1 (referred to as dll4:EGFP);, Tg(−0.8flt1:RFP)hu5333 (referred to as flt1:tdtomato);, TgBAC(flt4:Citrine)hu7135 (referred to as flt4:YFP);, TgBAC(prox1aBAC:KalTA4-4xUAS-ADV.E1b:TagRFP)nim5 (referred to as prox1a:RFP);, Tg(ubb:LOX2272-LOXP-RFP-LOX2272-CFP-LOXP-YFP)a132 (referred to as ubb:zebrabow);, Tg(kdrl:Cre-ERT2)fb13 transgenic lines have been described previously (Bussmann et al., 2010; Cross et al., 2003; Glass et al., 2011; Kikuchi et al., 2011; Kikuchi et al., 2010; Lawson and Weinstein, 2002; Mably et al., 2003; Mosimann et al., 2011; Pan et al., 2013; Perner et al., 2007; Proulx et al., 2010; van Impel et al., 2014; Wythe et al., 2013; Zhao et al., 2014), as have mutant lines cxcr4aum20, cxcl12bmu100, cxcr4bt26035, cxcl12at30516 (Bussmann et al., 2011; Knaut et al., 2003; Siekmann et al., 2009; Valentin et al., 2007). Details of zebrafish lines not previously described, along with husbandry and Cre induction protocols are described in Supplemental Experimental Procedures.
Confocal imaging
Hearts were isolated from terminally anesthetized zebrafish, rinsed in PBS and fixed briefly (30 seconds) in 4%PFA/PBS. Whole isolated hearts were then mounted in 1% low-melting point agarose/PBS in a grass bottom dish for confocal microscopy. A Z-stack of both restricted band-pass channel and lambda images was then acquired using LSM700 and LSM710 microscopes (Ziess). Coronary vessel, endocardial patch and ventricle area was calculated from max projected confocal images of the hearts using ImageJ/Fiji, surface volume and contiguous pixel analysis was carried out using IMARIS software. The ventricle or individual patches were traced and pixels counted using differential threshold limits to measure surface vessel endothelial cell, endocardial patch and total area. Quantification of cxcl12b:YFP intensity was carried out using the mean grey value measurement function in ImageJ/Fiji outlining the base for the ventricle (defined as the area above the AVC connection) and apex (below the AVC connection) and normalizing both to the (cxcl12b–negative) atrium. Quantification of cxcr4a:YFP intensity was carried using a 16-color look up table calibrated across the intensity spectrum of cxcr4a:YFP in ImageJ/Fiji.
Heart explant culture and live imaging
Hearts were isolated from terminally anesthetized fli1a:EGFP transgenic zebrafish and placed in Ringer’s solution containing 100µg/ml Primocin and 150U/ml heparin and transferred to glass bottomed dishes containing L-15 media supplemented with 10%FCS, 100µg/ml Primocin, 1.25mM CaCl2 and 800 mg/l glucose. Hearts were then imaged using a Leica DM IRE2 equipped with a 20x/0.40 objective, Leica GFP filter cube (ex470/40, em525/50) and a Hamamatsu ORCA-ER camera. µManager acquisition software (Edelstein et al., 2010) is used to capture a predefined z-stack of images every 20 minutes over 4 days. As the heart was still beating, images were selected that contained the area of interest within the focal plane for each given time point and arranged to form a time-lapse movie.
Amputation, Angiography and AFOG
Ventricular amputations and adult zebrafish angiography were carried out as previously described (Poss et al., 2002; Pugach et al., 2009) and both in accordance with CHLA IACUC animal care protocols. Tissue collection, preparation and histological AFOG (Acid Fuchsin Orange-G) were carried out using standard protocols (Poss et al., 2002).
Supplementary Material
Highlights.
Zebrafish develop coronary vessels during late juvenile development.
Coronary vessel endothelial cells are derived from the endocardium.
Endothelial cells form coronary vessels under the guidance of CXC-signaling.
Cxcr4a–mutant zebrafish hearts lack coronary vessels and are unable to regenerate.
ACKNOWLEDGMENTS
We are grateful to G. Crump for providing the fli1a endothelial enhancer, S. Megason for providing the H2b-Cerulean fusion construct and for zebrafish lines from various groups. We also thank M. Chao, J. Chen, G. Crump, V. Kaartinen, C. Pearson for critical reading of the manuscript. We also wish to acknowledge the assistance of E. Fernandez and the facilities of the CHLA microscopy core S. Sumida and D. Warburton for helpful comments and discussion. This work was supported by National Heart, Lung and Blood Institute Grant (R01HL096121 to C.-L. L.), a postdoctoral fellowship from the California Institute for Regenerative Medicine (TG2-01168 to D.W; CIRM Scholar: M.H.) and a Research Career Development Fellowship from the Saban Research Institute (to M.H.). This work was furthermore funded by the Max Planck Society (A.F.S.), the Deutsche Forschungsgemeinschaft (DFG SI-1374/3-1; A.F.S.), the Sonderforschungsbereich (SFB) 629 and an ERC starting grant (260794-ZebrafishAngio; A.F.S.). This work was supported by the Deutsche Forschungsgemeinschaft (DFG) Cells-in-Motion Cluster of Excellence (EXC 1003-CIM), University of Münster, Germany. Jeroen Bussmann was supported by a European Molecular Biology Organization (EMBO) long-term fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Additional supplemental figures are found in Harrison Supplemental Data.pdf
REFERENCES
- Alwan A. World Health Organization Global status report on noncommunicable disaeses 2010. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T. The role of CXCL12 in the organ-specific process of artery formation. Blood. 2005;105:3155–3161. doi: 10.1182/blood-2004-07-2563. [DOI] [PubMed] [Google Scholar]
- Boldajipour B, Doitsidou M, Tarbashevich K, Laguri C, Yu SR, Ries J, Dumstrei K, Thelen S, Dorries J, Messerschmidt EM, et al. Cxcl12 evolution--subfunctionalization of a ligand through altered interaction with the chemokine receptor. Development. 2011;138:2909–2914. doi: 10.1242/dev.068379. [DOI] [PubMed] [Google Scholar]
- Bussmann J, Bos FL, Urasaki A, Kawakami K, Duckers HJ, Schulte-Merker S. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development. 2010;137:2653–2657. doi: 10.1242/dev.048207. [DOI] [PubMed] [Google Scholar]
- Bussmann J, Wolfe SA, Siekmann AF. Arterial-venous network formation during brain vascularization involves hemodynamic regulation of chemokine signaling. Development. 2011;138:1717–1726. doi: 10.1242/dev.059881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YR, Fujita M, Butler M, Isogai S, Kochhan E, Siekmann AF, Weinstein BM. Chemokine signaling directs trunk lymphatic network formation along the preexisting blood vasculature. Developmental cell. 2012;22:824–836. doi: 10.1016/j.devcel.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HI, Sharma B, Akerberg BN, Numi HJ, Kivela R, Saharinen P, Aghajanian H, McKay AS, Bogard PE, Chang AH, et al. The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development. 2014;141:4500–4512. doi: 10.1242/dev.113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross LM, Cook MA, Lin S, Chen JN, Rubinstein AL. Rapid analysis of angiogenesis drugs in a live fluorescent zebrafish assay. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:911–912. doi: 10.1161/01.ATV.0000068685.72914.7E. [DOI] [PubMed] [Google Scholar]
- Das A, Crump JG. Bmps and id2a act upstream of Twist1 to restrict ectomesenchyme potential of the cranial neural crest. PLoS genetics. 2012;8:e1002710. doi: 10.1371/journal.pgen.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David NB, Sapede D, Saint-Etienne L, Thisse C, Thisse B, Dambly-Chaudiere C, Rosa FM, Ghysen A. Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16297–16302. doi: 10.1073/pnas.252339399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Andres AV, Munoz-Chapuli R, Sans-Coma V. Development of the coronary arteries and cardiac veins in the dogfish (Scyliorhinus canicula) Anat Rec. 1993;235:436–442. doi: 10.1002/ar.1092350312. [DOI] [PubMed] [Google Scholar]
- Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. Computer control of microscopes using microManager. Curr Protoc Mol Biol Chapter. 2010;14 doi: 10.1002/0471142727.mb1420s92. Unit14 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass TJ, Lund TC, Patrinostro X, Tolar J, Bowman TV, Zon LI, Blazar BR. Stromal cell-derived factor-1 and hematopoietic cell homing in an adult zebrafish model of hematopoietic cell transplantation. Blood. 2011;118:766–774. doi: 10.1182/blood-2011-01-328476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Poss KD. Clonally dominant cardiomyocytes direct heart morphogenesis. Nature. 2012;484:479–484. doi: 10.1038/nature11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Sedmera D, Yost HJ, Clark EB. Structure and function of the developing zebrafish heart. Anat Rec. 2000;260:148–157. doi: 10.1002/1097-0185(20001001)260:2<148::AID-AR50>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hutchins GM, Kessler-Hanna A, Moore GW. Development of the coronary arteries in the embryonic human heart. Circulation. 1988;77:1250–1257. doi: 10.1161/01.cir.77.6.1250. [DOI] [PubMed] [Google Scholar]
- Itou J, Oishi I, Kawakami H, Glass TJ, Richter J, Johnson A, Lund TC, Kawakami Y. Migration of cardiomyocytes is essential for heart regeneration in zebrafish. Development. 2012;139:4133–4142. doi: 10.1242/dev.079756. [DOI] [PubMed] [Google Scholar]
- Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, Tabin CJ. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Developmental cell. 2012;22:639–650. doi: 10.1016/j.devcel.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Siekmann AF. The role of chemokines and their receptors in angiogenesis. Cellular and molecular life sciences : CMLS. 2011;68:2811–2830. doi: 10.1007/s00018-011-0677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Gupta V, Wang J, Holdway JE, Wills AA, Fang Y, Poss KD. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development. 2011;138:2895–2902. doi: 10.1242/dev.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaut H, Werz C, Geisler R, Nusslein-Volhard C, Tubingen Screen C. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 2003;421:279–282. doi: 10.1038/nature01338. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Developmental biology. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Mably JD, Mohideen MA, Burns CG, Chen JN, Fishman MC. heart of glass regulates the concentric growth of the heart in zebrafish. Current biology : CB. 2003;13:2138–2147. doi: 10.1016/j.cub.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Mosimann C, Kaufman CK, Li P, Pugach EK, Tamplin OJ, Zon LI. Ubiquitous transgene expression and Cre-based recombination driven by the ubiquitin promoter in zebrafish. Development. 2011;138:169–177. doi: 10.1242/dev.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MunozChapuli R, Macias D, Ramos C, Gallego A, DeAndres V. Development of the subepicardial mesenchyme and the early cardiac vessels in the dogfish (Scyliorhinus canicula) Journal of Experimental Zoology. 1996;275:95–111. [Google Scholar]
- Myocardial Infarction Genetics Consortium. Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nature genetics. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Schilling TF. Chemokine signaling controls endodermal migration during zebrafish gastrulation. Science. 2008;322:89–92. doi: 10.1126/science.1160038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YA, Freundlich T, Weissman TA, Schoppik D, Wang XC, Zimmerman S, Ciruna B, Sanes JR, Lichtman JW, Schier AF. Zebrabow: multispectral cell labeling for cell tracing and lineage analysis in zebrafish. Development. 2013;140:2835–2846. doi: 10.1242/dev.094631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, Atencia G, Wessels A, Munoz-Chapuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. The International journal of developmental biology. 2002;46:1005–1013. [PubMed] [Google Scholar]
- Perner B, Englert C, Bollig F. The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Developmental biology. 2007;309:87–96. doi: 10.1016/j.ydbio.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Gittenberger-de Groot AC, Mentink MM, Bokenkamp R, Hogers B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circulation research. 1993;73:559–568. doi: 10.1161/01.res.73.3.559. [DOI] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- Proulx K, Lu A, Sumanas S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Developmental biology. 2010;348:34–46. doi: 10.1016/j.ydbio.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Pugach EK, Li P, White R, Zon L. Retro-orbital injection in adult zebrafish. Journal of visualized experiments : JoVE. 2009 doi: 10.3791/1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz E, Mahabaleshwar H. Chemokine signaling in embryonic cell migration: a fisheye view. Development. 2009;136:1223–1229. doi: 10.1242/dev.022418. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–553. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, et al. Genomewide association analysis of coronary artery disease. The New England journal of medicine. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nature genetics. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmera D, Wang T. Ontogeny and phylogeny of the vertebrate heart. New York: Springer; 2012. [Google Scholar]
- Serluca FC. Development of the proepicardial organ in the zebrafish. Developmental biology. 2008;315:18–27. doi: 10.1016/j.ydbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Standley C, Fogarty KE, Wolfe SA, Lawson ND. Chemokine signaling guides regional patterning of the first embryonic artery. Genes & development. 2009;23:2272–2277. doi: 10.1101/gad.1813509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DY, Lee RK, Fishman MC. Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development. 1993;119:31–40. doi: 10.1242/dev.119.1.31. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, Nagasawa Y, Hamano T, Matsui I, Kawada N, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. Journal of the American Society of Nephrology : JASN. 2009;20:1714–1723. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. Journal of the American College of Cardiology. 1992;20:640–647. doi: 10.1016/0735-1097(92)90019-j. [DOI] [PubMed] [Google Scholar]
- Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Yan Y, et al. Vessel formation. De novo formation of a distinct coronary vascular population in neonatal heart. Science. 2014;345:90–94. doi: 10.1126/science.1251487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Zhang Z, et al. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell research. 2013;23:1075–1090. doi: 10.1038/cr.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Vazquez J, Kamei M, Weinstein BM. Molecular distinction between arteries and veins. Cell and tissue research. 2003;314:43–59. doi: 10.1007/s00441-003-0771-8. [DOI] [PubMed] [Google Scholar]
- Valentin G, Haas P, Gilmour D. The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Current biology : CB. 2007;17:1026–1031. doi: 10.1016/j.cub.2007.05.020. [DOI] [PubMed] [Google Scholar]
- van Impel A, Zhao Z, Hermkens DM, Roukens MG, Fischer JC, Peterson-Maduro J, Duckers H, Ober EA, Ingham PW, Schulte-Merker S. Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development. 2014;141:1228–1238. doi: 10.1242/dev.105031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O’Rourke BP, Sharp DJ, et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151:1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wythe JD, Dang LT, Devine WP, Boudreau E, Artap ST, He D, Schachterle W, Stainier DY, Oettgen P, Black BL, et al. ETS factors regulate Vegf-dependent arterial specification. Developmental cell. 2013;26:45–58. doi: 10.1016/j.devcel.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Hasan SS, Schmidt I, Rocha SF, Pitulescu ME, Bussmann J, Meyen D, Raz E, Adams RH, Siekmann AF. Arteries are formed by vein-derived endothelial tip cells. Nature communications. 2014;5:5758. doi: 10.1038/ncomms6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Borikova AL, Ben-Yair R, Guner-Ataman B, MacRae CA, Lee RT, Burns CG, Burns CE. Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1403–1408. doi: 10.1073/pnas.1311705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.