Abstract
Sepsis can lead to multiple organ dysfunction, including the Acute Respiratory Distress Syndrome (ARDS), due to intertwined, dynamic changes in inflammation and organ physiology. We have demonstrated the efficacy of Chemically-Modified Tetracycline 3 (CMT-3) at reducing inflammation and ameliorating pathophysiology in the setting of a clinically realistic porcine model of ARDS. Here, we sought to gain insights into the derangements that characterize sepsis/ARDS and the possible impact of CMT-3 thereon, by combined experimental and computational studies. Two groups of anesthetized, ventilated pigs were subjected to experimental sepsis via placement of a peritoneal fecal clot and intestinal ischemia/reperfusion by clamping the superior mesenteric artery for 30 min. The treatment group (n = 3) received CMT-3 at 1 hour after injury (T1), while the control group (n = 3) received a placebo. Multiple inflammatory mediators, along with clinically relevant physiologic and blood chemistry variables, were measured serially until death of the animal or T48. Principal Component Analysis (PCA) and Dynamic Bayesian Network (DBN) inference were used to relate these variables. PCA revealed a separation of cardiac and pulmonary physiologic variables by principal component, and a decreased rank of oxygen index and arterial PO2/FiO2 ratio in the treatment group compared to control. DBN suggested a conserved network structure in both control and CMT-3 animals: a response driven by positive feedback between interleukin-6 and lung dysfunction. Resulting networks further suggested that in control animals, acute kidney injury, acidosis, and respiratory failure play an increased role in the response to insult compared to CMT-3 animals. These combined in vivo and in silico studies in a high fidelity, clinically applicable animal model suggest a dynamic interplay between inflammatory, physiologic, and blood chemistry variables in the setting of sepsis and ARDS that may be dramatically altered by pleiotropic interruption of inflammation by CMT-3.
Keywords: Sepsis, shock, MODS, ARDS, CMT-3, data-driven, model, networks, porcine
Introduction
Acute respiratory distress syndrome (ARDS), with approximately 200,000 annual cases in the United States, is one of the leading causes of death in young adults and a major concern in victims of combat trauma in the military [1]. The accompanying cost for each case is roughly $150,000 [2]. The pathophysiology of ARDS causes death in 30-60% of affected patients, despite treatment with the standard of care low tidal volume (LTV) ventilation strategy [3]. Diagnostically, the principal feature of ARDS, according to the 2011 ESICM “Berlin” definition, is a PaO2/FiO2 ratio of less than 300 mmHg. Additionally, respiratory symptoms must be new (< 1 week), lung imaging must indicate bilateral opacities, and alternative causes of acute hypoxemic respiratory failure must be ruled out.
ARDS, resulting from heterogeneous etiologies, is a common endpoint of sepsis, hemorrhagic shock, and trauma. In the hospital setting, ARDS often occurs concurrently with Multiple Organ Dysfunction Syndrome (MODS). Biologically, ARDS results from the systemic manifestation of the acute inflammatory response in conjunction with local mechanical trauma delivered to lung tissue by inappropriate mechanical ventilation, which our group has shown to be critical in ARDS pathogenesis [1,2]. Unchecked inflammation causes barrier failure and subsequent accumulation of fluid in the alveoli of the lungs, leading to reduced gas exchange [4].
ARDS poses a significant challenge for the clinician and to our knowledge, there are no medications approved by the U. S. Food and Drug Administration to treat this condition. By the time ARDS appears, the current standard of care treatment is only supportive in the form of mechanical ventilation, which is only minimally effective [3]. Furthermore, mechanical ventilation, while necessary in the short term, contributes to ARDS pathology via a secondary, ventilator-induced lung injury (VILI), thus significantly increasing mortality [5].
The lack of insight into the complex workings of the human acute inflammatory response has been a primary obstacle for developing effective treatments for ARDS. The inflammatory response comprises dynamic networks that can include hundreds of mediators derived from multiple cell types, all of which vary over time. Most variables are interrelated due to the feedback structure of the system, and this complexity is compounded by frequent pleiotropy and redundancy, as well as the multiscale aspect inherent in a system that affects multiple tissues and organs. The traditional reductionist experimental approach, in which a single variable is isolated and its role inferred from the results, has been insufficient in elucidating the workings of this system. We and others have gained insights into trauma and sepsis-based on data from cells, animals, and humans-using quasi-mechanistic data-driven computational modeling based on tools such as principal component analysis and various forms of dynamic network analysis [6-10].
Separately from challenges posed by the complexity of critical illness, the lack of applicability of commonly-used rodent models to the phenotype and mechanisms of human ARDS has significantly hindered the development of effective treatment [11]. We speculate that this discrepancy is due, at least in part, to the fundamental differences between the presentation of ARDS in rodents and in man and the fact that most rat models do not use mechanical ventilation, which is a large component of ARDS pathogenesis [1,2]. Rodent ARDS can be characterized as an “all-or-none” response, with the animal quickly transitioning from health to severe respiratory failure and death [12,13]. Human ARDS, however, exhibits a wider spectrum of disease and a longer time-course [14]. A porcine ARDS model using standard of care mechanical ventilation presents many advantages over rodent models. The anatomy and physiology of swine mirror man much more closely than rodents can. The increased size of swine compared to rodents allows for repeated measurements and the ability to perform experiments in a human clinical setting using standard human equipment and procedures. Finally, unlike rodents, swine do not exhibit an “all-or-none” response, but rather a human-like progression of pathology in the lung to ARDS [14]. The porcine model used in this work is one we have developed to reliably produce ARDS [15] and have used in numerous previous studies [1,2,16-18].
We have used this porcine model previously to test experimental therapies for ARDS. Chemically-Modified Tetracycline 3 (CMT-3) is a non-antimicrobial tetracycline that inhibits multiple inflammatory mediators, including TNF-α and IL-6 [19,20]. This drug has been reported to prevent ARDS in our porcine model both before and after insult [16]. Herein, we sought to leverage these studies in order to gain further insights into human ARDS pathophysiology, using a combination of 1) our clinically-relevant porcine model, including perturbation with CMT-3; 2) extensive time course measurements of biochemistry, physiology, and inflammation; and 3) computational analysis aimed at discerning principal characteristics and interconnected networks.
Because CMT-3 has demonstrated efficacy in a clinically relevant timeframe (1 h after insult) in our porcine model, we chose to investigate the effect of this drug on the development of ARDS using in silico methods, with the hypothesis that this combined experimental/computational approach will define dynamic networks of disease as well as how a beneficial treatment affects those networks. Indeed, we demonstrate that networks combining inflammation, physiology, and blood chemistry data, with the novel use of PCA as a filter for variable inclusion in Dynamic Bayesian network (DBN) inference, help us define novel and possibly therapeutically-relevant interactions.
Materials and methods
The animal experiments generating the data for the mathematical analysis were previously published [17]. Thus, the comprehensive and specific description of animals used and procedural methods can be found in this publication [17]. An overview of these methods has been restated below as reference for the mathematical analysis. At the time of the aforementioned work, a subset of three randomly selected animals in both the control and treatment groups were selected to have inflammatory mediators measured. Thus, only those six animals are analyzed in the work presented herein. The experiments were performed in compliance with the National Institutes of Health’s Guidelines on the Use of Laboratory, and the Committee for the Humane Use of Animals at Upstate University Hospital approved the study protocol.
Animals
The animal model protocol has been described in full detail in earlier studies [15] and specifically in the study in which we tested CMT-3 [17]. Briefly, female Yorkshire pigs (Keystone Farms, Ithaca, NY) weighing between 25 and 35 kg were pretreated with glycopyrrolate, tiletamine hydrochloride and zolazepam hydrochloride, and xylazine prior to intubation. Anesthesia after intubation was maintained using a continuous infusion of ketamine/xylazine. Animals were monitored for 48 h or until death.
Surgical procedures
An open tracheostomy was performed, and the animal was connected to a Galileo ventilator (Hamilton Medical, Reno, NV) with initial settings during the surgical preparation as follows: tidal volume of 12 mL/kg, respiratory rate of 15 breaths/min, FiO2 of 21%, and positive end-expiratory pressure of 3 cm H2O. Respiratory rate was titrated to maintain PaCO2 within the reference range (35-45 cm H2O); FiO2 was titrated to maintain O2 saturation of greater than 88%.
Under sterile conditions, a carotid arterial catheter and two external jugular central venous catheters were placed. A Foley catheter was inserted directly into the bladder.
A midline laparotomy was performed for placement of a gastrostomy tube and induction of a two-hit injury, peritoneal sepsis and ischemia/reperfusion, described in detail in prior publications from this model [21]. The superior mesenteric artery (SMA) was clamped for 30 min to induce intestinal ischemia. During the 30 min ischemic time, 0.5 mL/kg of feces was harvested from a cecotomy and mixed with 2 mL/kg of blood to create a fecal clot. After releasing the clamp on the SMA, reperfusion was confirmed by the appearance of the mesenteric pulse and return of normal color to the bowl. The clot was then implanted into the lower portion of the abdominal cavity. The abdomen was closed with a running monofilament fascial suture and skin staples.
Ringer’s lactate was used for fluid resuscitation and maintenance. Maintenance fluid requirements were calculated on a per-kilo basis according to clinical guidelines. Fluid boluses were given as indicated by deteriorations in hemodynamic parameters or decreases in urine output less than 0.5 mL/kg per hour. Broad-spectrum antibiotics were delivered intravenously following closure of the abdomen (ampicillin 2 g i.v. and metronidazole 500 mg i.v.). This antibiotic regimen was repeated at 12, 24, and 36 h after injury.
Measurements
Baseline (BL) measurements were taken following vascular access before injury. Time 0 (T0) measurements were taken immediately after the induction of injury (i.e., removal of SMA clamp and placement of fecal clot) upon closure of the abdomen.
Hemodynamic parameters were measured (CMS-2001 System M1176A, with Monitor M1094B; Agilent, Bobingen, Germany) using Edwards transducers (Pressure Monitoring Kit [PXMK1183]; Edwards Lifesciences, Irvine, CA). Measurement of blood gases and chemistries was made with a Roche blood gas analyzer (Cobas b221; Basel, Switzerland). Pulmonary parameters were measured or calculated by the Galileo ventilator (Hamilton Medical). After centrifugation at 15°C for 10 min, plasma fluid inflammatory mediators IL-1β, IL-6, IL-4 (Affymetrix, Santa Clara, CA), IL-10 (R & D Systems, Minneapolis, MN) were measured using enzyme-linked immunosorbent assay according to the manufacturer’s recommendation and NO3 -/NO2 was measured by the nitrate reductase method using a commercially available kit (Cayman Chemical, Ann Arbor, MI).
Variables and time points
The following physiologic variables were measured at baseline (BL) and each hour from 0 (T0) to 48 h (T48): heart rate (HR); mean arterial pressure (MAP); pulmonary artery pressure (PAP); central venous pressure (CVP); cardiac output (CO); peak airway pressure (Ppeak); plateau pressure (Pplat); mean airway pressure (Pmean); static compliance (Cstat); tidal volume (Vt); respiratory rate (RR); positive-end expiratory pressure (PEEP); body temperature (Temp).
The following blood chemistry variables were measured at BL and each hour from T0 to T6, then every 3 h from T9 to T48: arterial pH (pH_art); arterial PCO2 (PCO2_art); arterial PO2 (PO2_art); arterial oxygen saturation (SaO2); arterial hematocrit (Hct_art); arterial hemoglobin (Hgb_art); arterial base excess (BE_art); arterial Na+ (Na+_art); arterial K+ (K+_art); arterial Cl- (Cl-_art); arterial Ca++ (Ca++_art); arterial glucose (Glu_art); arterial BUN (BUN_art); arterial lactate (Lact_art); venous pH (pH_ven); venous PCO2 (PCO2_ven); venous oxygen saturation (SvO2). Additionally, two other calculated variables: P/F Ratio (PF, Arterial PO2/FiO2) and Oxygenation Index (OI, [Mean Airway Pressure x FiO2]/Arterial PO2) were included in the blood chemistry analysis, due to their blood chemistry component variables limiting the number of time points at which they could be calculated.
Inflammatory mediators were measured at BL and each hour from T0 to T6, then every 3 h from T9 to T 24, then every 6 h from T30 to T48: IL-6; IL-4; NO3 -/NO2; IL-1β; IL-10.
Treatment groups
Animals were randomized into two groups. The treatment group (n = 3) received an orally active dose (200 mg/kg) of the modified tetracycline CMT-3 (6-demythyl-6-deoxy-4dedimentylamino-tetracycline; CollaGenex Pharmaceuticals Inc, Newton, PA) delivered per gastrostomy. The placebo group received the same dose of a vehicle (carboxymethylcellulose) for CMT-3 delivered per gastrostomy. Doses were delivered one hour after injury for both groups.
Principal component analysis
PCA is a nonparametric statistical method of reducing the dimensionality of a dataset used to identify the subsets of variables (in the form of orthogonal normalized linear combinations of the original variables, called principal components) that are most strongly correlated with overall variability of the dataset, and thereby might be considered principal drivers of each response [10,22]. We have recently demonstrated the utility of PCA for elucidating differences in inflammatory responses between a treatment and control group in the same porcine ARDS model used in this work [18], and have extensively used this method to study inflammatory networks in mouse [23], rat [24], and porcine [24,25] models of sepsis.
PCA was performed on physiologic and blood chemistry datasets obtained from each treatment group. Additionally, for the physiologic dataset an initial PCA was performed on data from both groups combined. To perform PCA, the data were first normalized for each variable (i.e., a given value divided by the maximum value for that variable), so that all variable levels were converted into the same scale (from 0 to 1). In this way, any artifactual effects on variance due to the different ranges of concentration observed for different variables were eliminated. Only sufficient components to capture at least 95% of the variance in the data were considered. From these leading principal components, the coefficient (weight) associated with each variable was multiplied by the eigenvalue associated with that principal component. This product represented the contribution of a given variable to the variance accounted for in that principal component. The overall score given to each variable is the sum of its scores in each component. This gives a measure of a variable’s contribution to the overall variance of the system. The variables with the largest scores are the ones who contributed most to the variance of the process being studied. More specifically, the overall PCA score was calculated in the following way: Pj = Σi|ei · Wij|, where i is the index of component and j is the index of variable. Wi, j is the amount that the jth mediator contributes to the ith component. The complete MATLAB code for this analysis can be found as supplementary material to our previously published work [23].
PCA-based variable reduction
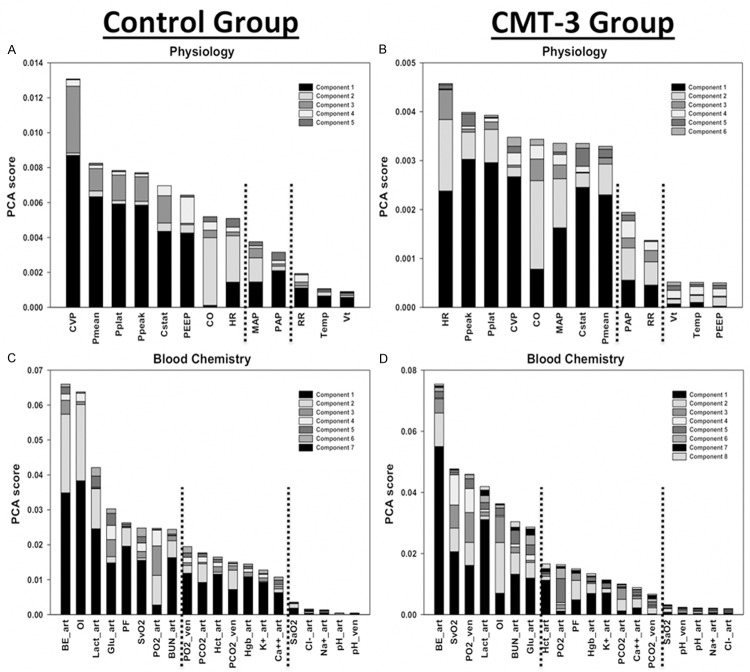
PCA results were compared between treatment and control groups, separately for physiologic and blood chemistry analyses. Variables that were revealed to provide little information content in the analyses for both groups were removed prior to proceeding with DBN inference. Two strategies were employed when choosing cutoffs: Strategy 1 only excluded variables shown by PCA to be of minimal importance, judged by being in the bottom tier of the PCA bar chart in both groups; Strategy 2 was more aggressive, also excluding variables that for both groups were in the bottom or second lowest tier. Tiers were chosen by the experimenters based on the relative magnitude of the contribution of a given mediator across all components. A complete summary of variables excluded for each strategy is shown in Table 1, and PCA bar chart results can be seen in Figure 2.
Table 1.
Summary of variable exclusion for Dynamic Bayesian Network analysis. PCA was used as an information content filter to determine which variables could be excluded from DBN analysis. Strategy 1 excluded variables that for both control and CMT-3 groups were in the bottom tier of PCA results. Strategy 2 excluded variables that were both in either the bottom or second lowest tier of the PCA results for both groups. The percent reduction in variable count from the complete set of physiologic (n = 13) or blood chemistry (n = 20) variables is shown
| Strategy 1 | Strategy 2 | |||
|---|---|---|---|---|
|
|
||||
| ANALYSIS | Variables excluded | % reduction in variable count | Additional variables excluded | Additional/Total % reduction in variable count |
| Physiology | Vt | 15.4 | RR | 15.4/30.8 |
| Temp | PAP | |||
| Chemistry | SaO2 | 25.0 | K+_art | 30.0/55.0 |
| pH_art | Ca++_art | |||
| pH_ven | PCO2_art | |||
| Na+_art | PCO2_ven | |||
| Cl-_art | Hct_art | |||
| Hgb_art | ||||
Figure 2.
Principal Component Analysis by group and variable type. The control and CMT-3 group were treated in identical fashion, except 1 h after injury control pigs (n = 3) received a placebo, while CMT-3 pigs (n = 3) received chemically modified tetracycline 3. Physiology = only physiologic variables were included in the analysis. Blood Chemistry = only blood chemistry variables, and derived variables OI and PF, were included in the analysis. Variables are ordered by the sum of the absolute values of their contributions to all components, with contributions to individual components represented by different colored sections of the bars. Dashed lines represent cutoffs chosen for variable tiers used in determining exclusion from DBN analysis. In physiology, the separation of cardiac variables into the second principal component remains apparent after the dataset has been divided by group. The physiology analyses also revealed PEEP to be the lowest ranked variable in the CMT-3 group, compared to being ranked 6th of 13 variables in control. In blood chemistry, the oxygen index (OI) and PF ratio decrease markedly in rank and tier from control to CMT-3.
For each strategy and for each treatment group, DBN inference (see below) was performed on a combined set of the included physiologic and blood chemistry variables along with the complete set of inflammatory variables. Since inflammatory variables were measured at the fewest time points, only physiologic and blood chemistry data taken at the time points used for inflammatory variables were included in this analysis.
Dynamic bayesian network inference
Given time-series data, DBN inference is a method for suggesting causal relationships among variables based on probabilistic measure. Unlike standard correlative approaches, DBNs consider the joint distribution of the entire data set when making inferences about the dependencies among variables or nodes in the network. In a DBN, variables are shown as nodes, and the interconnections are shown as edges. The values of each node are assumed to be distributed according to a chosen model (e.g., Gaussian), and the relationships among nodes are defined by the structure of the directed network and the corresponding conditional probability distributions of the interacting nodes. Network structure is inferred by a sampling technique that iteratively proposes candidate structures and evaluates them based on how well they explain the observed data using a specified scoring criterion, until reaching convergence on a network structure with the highest score.
Our analysis was carried out in MATLAB (The MathWorks, Inc, Natick, MA), using an algorithm adapted from Grzegorczyk and Husmeier [26] and revised recently by our group [18,27,28]. Briefly, the algorithm uses an inhomogeneous dynamic change-point model, with a Bayesian Gaussian with score equivalence scoring criterion. For each node, a new set of parent nodes was sampled directly from the posterior distribution, and the local scores computed using the Bayesian Gaussian with score equivalence scoring model. Each node was subject to a fan-in restriction of three parent (i.e., effector) nodes. The marginal posterior edge probability, the likelihood of observing each edge (interaction) in the network, was estimated by calculating the average occurrence of each edge in the highest scoring networks that were sampled. The inference procedure was run individually for each pig, and the marginal edge probabilities averaged across all runs. The thickness of edges was weighted by this number, and only edges with an averaged marginal edge probability greater than 0.5 were included in the final consensus network for each condition. We note that the program is probabilistic and thus applying this hard cutoff can lead to minor differences in results from different runs.
Results
We have demonstrated previously the parallel use of PCA and DBN on a single dataset to gain independent insights into the intertwined roles of inflammation and pathophysiology in the setting of porcine sepsis/ARDS, and the impact of a multimodal therapy (peritoneal suction to remove inflammatory ascites) thereon [18]. In the current study, we sought to discern the impact of a drug (CMT-3) on more comprehensive networks involving interconnected biochemical, physiological and inflammatory variables. Additionally, given this goal and our recent work demonstrating the applicability of PCA to datasets containing a large number of variables [29], we sought to determine the utility of PCA as an information content filter prior to DBN inference.
We first sought to determine if PCA could segregate among variables, and furthermore if this methodology could define a natural separation between experimental groups. An analysis of physiologic data combined for both the control and CMT-3 groups revealed an overall trend of separation of cardiac and pulmonary variables by principal component (Figure 1). Cardiac output, heart rate, and mean arterial pressure were all found to make distinctly greater contributions to the second principal component than the first, and this was reversed for the pulmonary variables of mean, peak, and plateau pressure as well as static compliance.
Figure 1.
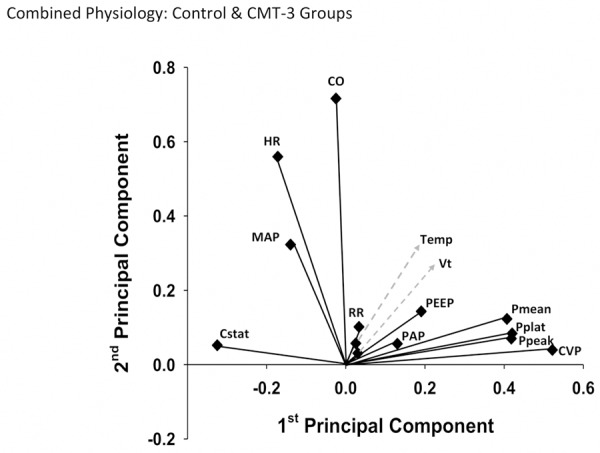
Two-dimensional physiology Principal Component Analysis results for entire dataset. For each physiologic variable, contributions to the 1st and 2nd principal components were plotted in an (x, y) format. In this 2-dimensional view, the separation of cardiac and pulmonary variables by component, and grouping of certain pulmonary variables, can be better visualized.
The results of PCA for physiologic and blood chemistry data separately for each group can be seen in Figure 2. Of note, after the physiology data were analyzed separately by group, the splitting of cardiac and pulmonary variables by component could still be observed in each individual analysis (Figure 2A and 2B). Interestingly, this result was more distinct in the control group, in which the second principal component is almost exclusively derived from cardiac variables. The physiology analyses also revealed PEEP to be the lowest ranked variable in the CMT-3 group, compared to being ranked 6th of 13 variables in control. The analyses of blood chemistry variables revealed the Oxygen Index (OI) and P/F ratio to both be markedly lower in rank in the CMT-3 group as compared to control (Figure 2C and 2D).
We next sought to infer dynamic networks of the interconnections among variables relevant to the overall response to insult in our animal model. We hypothesized that we could utilize PCA as an information content filter to reduce the number of variables included in DBN inference. Accordingly, we selected variables and tiers for this filtering process as described in the Materials and Methods. Resultant tiers for variable selection for DBN can be seen in Figure 2. DBN results for Strategy 2 can be seen in Figure 3. Importantly, DBN results for Strategy 1 (Figure 4) appeared similar to Strategy 2, indicating that the filtering process did not alter the underlying results obtainable by DBN. More specifically, the same overall network structure was maintained. Furthermore, all variables excluded in the transition from Strategy 1 to Strategy 2 were either not included in the Strategy 2 results, or were included only as outputs (did not show self-feedback or outward links to other nodes).
Figure 3.
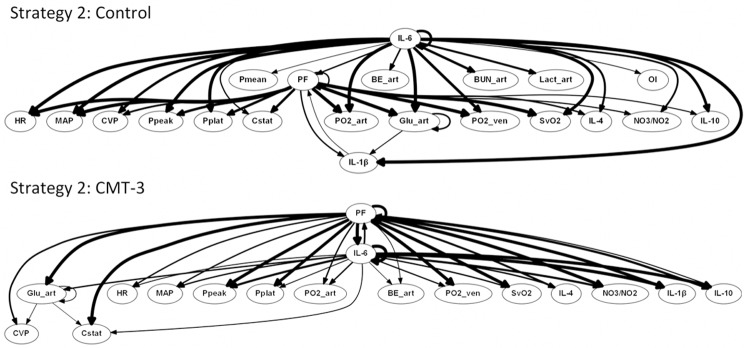
Dynamic Bayesian Network analysis suggests conserved network driven by inflammation and lung dysfunction in porcine ARDS model, but with blunting of systemic damage in CMT-3 group. The control and CMT-3 group were treated in identical fashion, except 1 h after injury control pigs (n = 3) received a placebo, while CMT-3 pigs (n = 3) received chemically modified tetracycline 3. Numerous inflammatory, physiologic, and blood chemistry variables were found to be interrelated using DBN analysis. The weight of the arrows denotes strength of interaction. Of note is the absence of BUN, lactate, and OI as outputs, decrease in weighting of BE as an output, and decreased involvement of IL-1β in the CMT-3 group compared to control.
Figure 4.
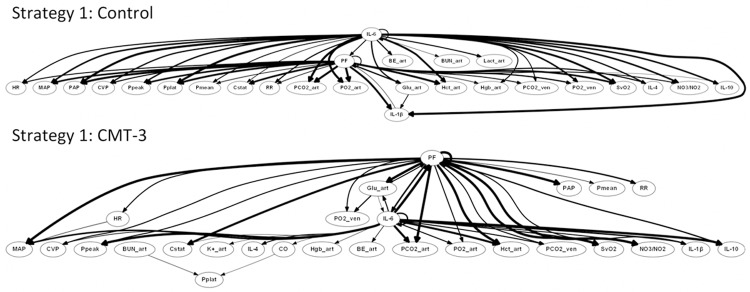
Dynamic Bayesian Network analysis of Strategy 1 suggests lack of network alteration by filtering process. The control and CMT-3 group were treated in identical fashion, except 1 h after injury control pigs (n = 3) received a placebo, while CMT-3 pigs (n = 3) received chemically modified tetracycline 3. Strategy 1 excluded fewer variables (see Table 1) than Strategy 2 (see Figure 3). Comparing the results for the two strategies, the same overall IL-6 and PF driven network structure is seen and all variables excluded in the transition from Strategy 1 to Strategy 2 were either not included in the Strategy 2 results, or were included only as outputs (did not show self-feedback or outward links to other nodes).
Numerous inflammatory, physiologic, and blood chemistry variables were interrelated based on DBN inference. This analysis suggested an overall response coordinated by the pro-inflammatory mediator IL-6 and lung dysfunction (PF), with lung dysfunction feeding back into a multifaceted inflammatory response (IL-6, IL-1β, IL-4, IL-10, and NO3 -/NO2), as we have suggest previously [18,25]. Within this general network structure, differences were observed between experimental groups. These differences included the suggestion that PF is affected by IL-6 and IL-1β in control animals, while PF is affected solely by IL-6 in CMT-3 animals. Another inferred difference was the lack of feedback from PF back to IL-6 in control animals. IL-1β, in addition to appearing to have a bidirectional relationship with PF, was inferred to be an output of arterial glucose in control animals. These findings suggest that in an untreated ARDS response IL-1β, in response to hyperglycemia, plays a key role affecting processes that ultimately result in pulmonary dysfunction.
The interplay between inflammation and blood chemistry in the DBN inferred from control animals is also noteworthy for outputs from IL-6 to BUN, arterial lactate, and OI that are not present in the CMT-3 DBN. In fact, these three markers of acute kidney injury (AKI), acidosis, and respiratory failure, respectively, are not present anywhere in the CMT-3 DBN, suggesting a reduced degree of multiple organ dysfunction in response to the initiating insult. Across all animals and time points, the average base excess in control data was -2.83, and in CMT-3 data was -2.10, supporting the DBN-based inference of increased relevance of acidosis in control animals.
Also of note is the role of arterial glucose in both DBN models. Aside from IL-6 and PF, arterial glucose is the only other variable to exhibit self-feedback. Moreover, arterial glucose was connected to more outputs than any other variable in our dataset. This finding may support recent emphasis on glucose control in sepsis [30,31], as well as the relation of glucose to stress and cortisol [32].
Discussion
We have focused much of our work on dynamic networks of systemic inflammation in diverse settings [18,23,27-29,33-35]. These studies have helped elucidate novel mechanisms and interactions, as well as suggesting novel disease biomarkers. In the present study, we sought to utilize these in silico methods to help link the numerous physiologic and blood chemistry information parameters available to the clinician, in order to gain further insights in ARDS, and to help define the multi-factorial mechanisms by which a drug such as CMT-3 exerts its beneficial effects. Using a clinically applicable model of ARDS, biochemical/physiological data of the type that could be readily available to clinicians, as well as data on circulating inflammation biomarkers, we compared a group of control and CMT-3-treated swine in order to gain insights into the mechanisms of sepsis/ARDS pathogenesis. This work is not meant to suggest the clinical use of CMT-3. Rather, this particular treatment was chosen because CMT-3 is, in essence, a multi-factorial perturbation of ARDS with previously defined, major effects on ARDS [14].
A key aspect of our work revolved around the integration of multiple data elements collected over time, with the goal of gaining translationally-useful knowledge. Data-driven computational modeling can help infer mechanisms operant in biological systems, leading to hypotheses that can be tested in the laboratory [9,10,22,36]. For researchers interested in testing a particular hypothesis or searching for new targets, this approach can narrow down an excessive number of potential reductionist experiments. The wide variety of currently available techniques allows for modeling, mapping of networks, and dimensionality reduction to suggest principal characteristics or drivers of a response [9]. We have previously employed PCA and DBN, among other data-driven modeling methods, to study networks of inflammatory mediators in rodents, swine, and humans [18,23,25,27-29,33]. In the present study, we combined PCA and DBN to gain insights into the multi-compartment dynamics of our porcine ARDS model, extending our prior work [18] to assess the impact of CMT-3.
We utilized PCA to include multiple physiologic and blood chemistry variables in addition to inflammatory mediators. Within the physiology analyses, the PCA-based separation of cardiac and pulmonary variables is a particularly interesting finding. One of the traditional uses of PCA in the social sciences has been separation of variables by category into different principal components, and we see this trend clearly in both the combined and group-specific results. The decrease in rank of OI and PF from control to CMT-3 treatment groups within the blood chemistry analyses mirrors our previous work, which found OI and PF to decrease in rank from control to peritoneal suction treatment groups within a set of inflammatory variables in septic swine [18]. The conservation of this significant decrease in the ranking of these two variables across different treatments and classes of comparison variables suggests their broad utility as metrics of pulmonary dysfunction, and further supports the theory of intertwined physiological, biochemical, and inflammatory networks in sepsis/ARDS. The OI is the only routine measure that takes into account both oxygenation and lung pressure [37]; OI can be predictive of acute hypoxic respiratory failure in children [38]. We have also shown in our previous work that OI correlates with predicted overall “damage”, a simulated index of whole-animal health status in a two-compartment ordinary differential equation, mechanistic model of porcine endotoxemia [25].
The present study supports the current hypothesis that mechanical ventilation is a primary ‘driver’ of acute lung injury pathogenesis in normal lungs before the development of ARDS [1,2]. It has recently been shown that patients are risk of developing ARDS (e.g. sepsis, trauma, hemorrhagic) but with normal lungs when placed on mechanical ventilation had a significantly higher incidence of ARDS if they were on non-protective high tidal volume ventilation [39]. PCA shows that both OI and PF ratio decrease markedly in rank and tier between the control and CMT-3 groups. OI and PF ratio are markers of lung pathology, and the airway pressure component in OI suggests a role for VILI in this lung pathology (i.e. the higher the airway pressure the more potential for VILI). Thus, we hypothesize, based on recent clinical studies, that severe pulmonary inflammation in the control group, combined with non-protective mechanical ventilation strategies, act synergistically to drive the disease process forward culminating ultimately in ARDS [39,40]. We further hypothesize that upon treatment with CMT-3 and concomitant alteration of inflammatory patterns, the injurious impact of non-protective ventilation is reduced and ARDS is prevented. Also of interest is that PEEP, thought to play a key role in protective ventilation, was in the lowest tier in the CMT-3 group. This finding also supports the hypothesis that, upon treatment with the CMT-3, alteration of the inflammatory patterns driven by VILI is no longer an important mechanism driving acute lung injury. Our DBN analysis further supports this hypothesis, since OI, and presumably VILI, is no longer an output node in the CMT-3 group.
In further support of this hypothesis, our computational analysis suggested that IL-1β, in addition to appearing to have a bidirectional relationship with PF, was inferred to be an output of arterial glucose in control animals. These findings suggest that in an untreated ARDS response IL-1β, in response to hyperglycemia, plays a key role affecting processes that ultimately result in pulmonary dysfunction. Our prior computational modeling work, based on studies of acute lung injury in endotoxemic swine, suggested that IL-1β drives the release of the damage-associated molecular pattern molecule HMGB1, setting in motion a feed-forward loop of inflammation à organ dysfunction à inflammation [25].
There are multiple limitations to our study. The number of animals was relatively small, due to the nature and expense of large animal studies. However, this limitation is justified by the increased applicability, compared to rodent models, of a porcine model to man, and is mitigated by the extensive number of time points and data elements obtained. The number of inflammatory variables was also small, as this study aimed to only include a reduced number of key variables of this type so that physiologic and blood chemistry variables could be focused on, and since the availability of pig-specific analytical reagents is lacking in comparison to those available for rodents or humans. A weakness of our DBN algorithm used here is its inability to determine directly the positive or negative nature of edges connecting the nodes, as well as whether links represent a linear or other type of relationship. Nonetheless, DBN inference is still capable of providing valuable information regarding the interconnectedness of variables that methodologies such as PCA cannot infer, and that can be supplemented and interpreted in context by the experimenter. Finally, data-driven modeling techniques are not mechanistic and must be further validated by in vivo or in vitro experiments in the laboratory, or by in silico mechanistic models.
Conclusion
In conclusion, we suggest that our studies reveal a dynamic interplay between inflammatory, physiologic and blood chemistry variables in the setting of acute systemic inflammation and ARDS. We have demonstrated the use of PCA on large data sets as an information content filter for DBN inference, without detracting from the nature of DBN results, and have expanded our use of both techniques to include two new classes of clinically applicable variables. Thus, the data analysis framework presented herein, combined with the clinically-realistic nature of the animal model from which these data were obtained, may serve as a translationally useful discovery platform.
Acknowledgements
This work was supported by NIH grants R33-HL-089082, R42HL065030 and NIH P50-GM53789.
Disclosure of conflict of interest
None.
Abbreviations
- AKI
Acute kidney injury
- ARDS
Acute Respiratory Distress Syndrome
- BE_art
Arterial base excess
- BL
Baseline
- BUN_art
Arterial BUN
- Ca++_art
Arterial Ca++
- Cl-_art
Arterial Cl-
- CO
Cardiac output
- COL-3
CMT-3, Chemically-Modified Tetracycline 3
- Cstat
Static compliance
- CVP
Central venous pressure
- DBN
Dynamic Bayesian Network
- ESCIM
European Society of Intensive Care Medicine
- FiO2
Fraction of inspired oxygen
- Glu_art
Arterial glucose
- Hct_art
Arterial hematocrit
- Hgb_art
Arterial hemoglobin
- HR
Heart rate
- IL
Interleukin
- K+_art
Arterial K+
- Lact_art
Arterial lactate
- MAP
Mean arterial pressure
- MODS
Multiple Organ Dysfunction Syndrome
- Na+_art
Arterial Na+
- OI
Oxygenation Index ([Mean Airway Pressure x FiO2]/Arterial PO2)
- P/F Ratio
Pulmonary Function Ratio (Arterial PO2/FiO2)
- PAP
Pulmonary artery pressure
- PCA
Principal Component Analysis
- PCO2_art
Arterial PCO2
- PCO2_ven
Venous PCO2
- PEEP
Positive-end expiratory pressure
- pH_art
Arterial pH
- pH_ven
Venous pH
- Pmean
Mean airway pressure
- PO2_art
Arterial PO2
- Ppeak
Peak airway pressure
- Pplat
Plateau pressure
- RR
Respiratory rate
- SaO2
Arterial oxygen saturation
- SMA
Superior mesenteric artery
- SvO2
Venous oxygen saturation
- Temp
Body temperature
- TNF
Tumor necrosis factor
- VILI
Ventilator-induced lung injury
- Vt
Tidal volume
References
- 1.Roy S, Habashi N, Sadowitz B, Andrews P, Ge L, Wang G, Roy P, Ghosh A, Kuhn M, Satalin J, Gatto LA, Lin X, Dean DA, Vodovotz Y, Nieman G. Early airway pressure release ventilation prevents ARDS-a novel preventive approach to lung injury. Shock. 2013;39:28–38. doi: 10.1097/SHK.0b013e31827b47bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy S, Sadowitz B, Andrews P, Gatto LA, Marx W, Ge L, Wang G, Lin X, Dean DA, Kuhn M, Ghosh A, Satalin J, Snyder K, Vodovotz Y, Nieman G, Habashi N. Early stabilizing alveolar ventilation prevents acute respiratory distress syndrome: a novel timing-based ventilatory intervention to avert lung injury. J Trauma Acute Care Surg. 2012;73:391–400. doi: 10.1097/TA.0b013e31825c7a82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, Ambros A, Gandia F, Carriedo D, Mosteiro F, Basaldua S, Fernandez RL, Kacmarek RM. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 4.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 5.Emr B, Gatto LA, Roy S, Satalin J, Ghosh A, Snyder K, Andrews P, Habashi N, Marx W, Ge L, Wang G, Dean DA, Vodovotz Y, Nieman G. Airway pressure release ventilation prevents ventilator-induced lung injury in normal lungs. JAMA Surg. 2013;148:1005–1012. doi: 10.1001/jamasurg.2013.3746. [DOI] [PubMed] [Google Scholar]
- 6.Buchman TG. Nonlinear dynamics, complex systems, and the pathobiology of critical illness. Curr Opin Crit Care. 2004;10:378–382. doi: 10.1097/01.ccx.0000139369.65817.b6. [DOI] [PubMed] [Google Scholar]
- 7.Vodovotz Y, Csete M, Bartels J, Chang S, An G. Translational systems biology of inflammation. PLoS Comput Biol. 2008;4:e1000014. doi: 10.1371/journal.pcbi.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An G. Closing the scientific loop: bridging correlation and causality in the petaflop age. Sci Transl Med. 2010;2:41ps34. doi: 10.1126/scitranslmed.3000390. [DOI] [PubMed] [Google Scholar]
- 9.An G, Nieman G, Vodovotz Y. Computational and systems biology in trauma and sepsis: current state and future perspectives. Int J Burns Trauma. 2012;2:1–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Vodovotz Y, Billiar TR. In silico modeling: methods and applications to trauma and sepsis. Crit Care Med. 2013;41:2008–2014. doi: 10.1097/CCM.0b013e31829a6eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med. 2009;37:S30–37. doi: 10.1097/CCM.0b013e3181922bd3. [DOI] [PubMed] [Google Scholar]
- 12.Bastarache JA, Blackwell TS. Development of animal models for the acute respiratory distress syndrome. Dis Model Mech. 2009;2:218–223. doi: 10.1242/dmm.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubiak BD, Albert SP, Gatto LA, Vieau CJ, Roy SK, Snyder KP, Maier KG, Nieman GF. A clinically applicable porcine model of septic and ischemia/reperfusion-induced shock and multiple organ injury. J Surg Res. 2011;166:e59–69. doi: 10.1016/j.jss.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg J, Halter J, Schiller H, Gatto L, Nieman G. The development of acute respiratory distress syndrome after gut ischemia/reperfusion injury followed by fecal peritonitis in pigs: a clinically relevant model. Shock. 2005;23:129–137. doi: 10.1097/01.shk.0000148053.66645.2e. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg J, Halter J, Schiller H, Gatto L, Carney D, Lee HM, Golub L, Nieman G. Chemically modified tetracycline prevents the development of septic shock and acute respiratory distress syndrome in a clinically applicable porcine model. Shock. 2005;24:348–356. doi: 10.1097/01.shk.0000180619.06317.2c. [DOI] [PubMed] [Google Scholar]
- 17.Roy SK, Kubiak BD, Albert SP, Vieau CJ, Gatto L, Golub L, Lee HM, Sookhu S, Vodovotz Y, Nieman GF. Chemically modified tetracycline 3 prevents acute respiratory distress syndrome in a porcine model of sepsis + ischemia/reperfusion-induced lung injury. Shock. 2012;37:424–432. doi: 10.1097/SHK.0b013e318245f2f9. [DOI] [PubMed] [Google Scholar]
- 18.Emr B, Sadowsky D, Azhar N, Gatto L, An G, Nieman G, Vodovotz Y. Removal of inflammatory ascites is associated with dynamic modification of local and systemic inflammation along with prevention of acute lung injury: In vivo and in silico studies. Shock. 2014;41:317–323. doi: 10.1097/SHK.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy SK, Kendrick D, Sadowitz BD, Gatto L, Snyder K, Satalin JM, Golub LM, Nieman G. Jack of all trades: pleiotropy and the application of chemically modified tetracycline-3 in sepsis and the acute respiratory distress syndrome (ARDS) Pharmacol Res. 2011;64:580–589. doi: 10.1016/j.phrs.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Y, Lee HM, Simon SR, Golub LM. Chemically modified tetracycline-3 (CMT-3): a novel inhibitor of the serine proteinase, elastase. Pharmacol Res. 2011;64:595–601. doi: 10.1016/j.phrs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Kubiak BD, Albert SP, Gatto LA, Snyder KP, Maier KG, Vieau CJ, Roy S, Nieman GF. Peritoneal negative pressure therapy prevents multiple organ injury in a chronic porcine sepsis and ischemia/reperfusion model. Shock. 2010;34:525–534. doi: 10.1097/SHK.0b013e3181e14cd2. [DOI] [PubMed] [Google Scholar]
- 22.Janes KA, Yaffe MB. Data-driven modelling of signal-transduction networks. Nat Rev Mol Cell Biol. 2006;7:820–828. doi: 10.1038/nrm2041. [DOI] [PubMed] [Google Scholar]
- 23.Mi Q, Constantine G, Ziraldo C, Solovyev A, Torres A, Namas R, Bentley T, Billiar TR, Zamora R, Puyana JC, Vodovotz Y. A dynamic view of trauma/hemorrhage-induced inflammation in mice: principal drivers and networks. PLoS One. 2011;6:e19424. doi: 10.1371/journal.pone.0019424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Namas RA, Namas R, Lagoa C, Barclay D, Mi Q, Zamora R, Peng Z, Wen X, Fedorchak MV, Valenti IE, Federspiel WJ, Kellum JA, Vodovotz Y. Hemoadsorption reprograms inflammation in experimental gram-negative septic peritonitis: insights from in vivo and in silico studies. Mol Med. 2012;18:1366–1374. doi: 10.2119/molmed.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieman G, Brown D, Sarkar J, Kubiak B, Ziraldo C, Dutta-Moscato J, Vieau C, Barclay D, Gatto L, Maier K, Constantine G, Billiar TR, Zamora R, Mi Q, Chang S, Vodovotz Y. A two-compartment mathematical model of endotoxin-induced inflammatory and physiologic alterations in swine. Crit Care Med. 2012;40:1052–1063. doi: 10.1097/CCM.0b013e31823e986a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grzegorczyk M, Husmeier D. Improvements in the reconstruction of time-varying gene regulatory networks: dynamic programming and regularization by information sharing among genes. Bioinformatics. 2011;27:693–699. doi: 10.1093/bioinformatics/btq711. [DOI] [PubMed] [Google Scholar]
- 27.Azhar N, Ziraldo C, Barclay D, Rudnick D, Squires R, Vodovotz Y. Analysis of serum inflammatory mediators identifies unique dynamic networks associated with death and spontaneous survival in pediatric acute liver failure. PLoS One. 2013;8:e78202. doi: 10.1371/journal.pone.0078202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaaqoq AM, Namas R, Almahmoud K, Azhar N, Mi Q, Zamora R, Brienza DM, Billiar TR, Vodovotz Y. Inducible protein-10, a potential driver of neurally controlled interleukin-10 and morbidity in human blunt trauma. Crit Care Med. 2014;42:1487–1497. doi: 10.1097/CCM.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fontes P, Marsh JW, Lopez R, Vodovotz Y, van der Plaats A, Minervini M, Scott V, Soltys K, Shiva S, Paranjpe S, Sadowsky D, Barclay D, Zamora R, Stolz D, Demetris A, Michalopoulos G. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant. 2015;15:381–94. doi: 10.1111/ajt.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali NA, O’Brien JM Jr, Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF Jr, Preiser JC. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 32.Gearhart MM, Parbhoo SK. Hyperglycemia in the critically ill patient. AACN Clin Issues. 2006;17:50–55. doi: 10.1097/00044067-200601000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Ziraldo C, Vodovotz Y, Namas RA, Almahmoud K, Tapias V, Mi Q, Barclay D, Jefferson BS, Chen G, Billiar TR, Zamora R. Central role for MCP-1/CCL2 in injury-induced inflammation revealed by in vitro, in silico, and clinical studies. PLoS One. 2013;8:e79804. doi: 10.1371/journal.pone.0079804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf M, Vodovotz Y, Tottey S, Brown B, Badylak S. Inferring in vivo responses to biomaterials via combined in vitro and in silico analysis. Tissue Engineering Part C Methods. 2015;21:148–59. doi: 10.1089/ten.tec.2014.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Namas RA, Vodovotz Y, Almahmoud K, Abdul-Malak O, Zaaqoq AM, Namas R, Mi Q, Barclay D, Zuckerbraun B, Peitzman AB, Sperry J, Billiar TR. Temporal patterns of circulating inflammation biomarker networks differentiate susceptibility to nosocomial infection following blunt trauma in humans. Ann Surg. 2014 doi: 10.1097/SLA.0000000000001001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azhar N, Mi Q, Ziraldo C, Buliga M, Constantine G, Vodovotz Y. Integrating Data Driven and Mechanistic Models of the Inflammatory Response in Sepsis and Trauma. In: Vodovotz Y, An G, editors. Complex Systems and Computational Biology Approaches to Acute Inflammation. New York: Springer; 2013. [Google Scholar]
- 37.Funk DJ, Lujan E, Moretti EW, Davies J, Young CC, Patel MB, Vaslef SN. A brief report: the use of high-frequency oscillatory ventilation for severe pulmonary contusion. J Trauma. 2008;65:390–395. doi: 10.1097/TA.0b013e31817f283f. [DOI] [PubMed] [Google Scholar]
- 38.Trachsel D, McCrindle BW, Nakagawa S, Bohn D. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172:206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 39.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, Damasceno MC, Schultz MJ. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 40.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant JY, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin JE, Pereira B, Jaber S, Group IS. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]