Abstract
The influence of irradiance on bacterial incorporation of [3H]leucine was evaluated at Station ALOHA in the oligotrophic North Pacific subtropical gyre. Six experiments were conducted on three cruises to Station ALOHA to examine how [3H]leucine incorporation varied as a function of irradiance. Two experiments were also conducted to assess the photoautotrophic response to irradiance (based on photosynthetic uptake of [14C]bicarbonate) in both the upper and lower photic zones. Rates of [3H]leucine incorporation responded to irradiance in a photosynthesis-like manner, increasing sharply at low light and then saturating and sometimes declining with increasing light intensity. The influence of irradiance on bacterial growth was evaluated in both the well-lit (5 to 25 m) and dimly lit regions of the upper ocean (75 to 100 m) to determine whether the bacterial response to irradiance differed along the depth-dependent light gradient of the photic zone. [3H]leucine incorporation rates were analyzed with a photosynthesis-irradiance model for a quantitative description of the relationships between [3H]leucine incorporation and irradiance. Maximum rates of [3H]leucine incorporation in the upper photic zone increased 48 to 92% relative to those of dark-incubated samples, with [3H]leucine incorporation saturating at light intensities between 58 and 363 μmol of quanta m−2 s−1. Rates of [3H]leucine incorporation in the deep photic zone were photostimulated 53 to 114% and were susceptible to photoinhibition, with rates declining at light intensities of >100 μmol of quanta m−2 s−1. The results of these experiments revealed that sunlight directly influences bacterial growth in this open-ocean ecosystem.
Approximately half of the total global primary production occurs in the marine environment, with the majority of marine production occurring in the open ocean (13, 30). Subtropical ocean gyres constitute roughly 40% of the Earth's surface area, and the sunlit regions of these oceanic ecosystems are often characterized by low concentrations of inorganic nutrients and plankton biomass. The North Pacific subtropical gyre is one of the largest subtropical ocean ecosystems on the planet and forms one of Earth's most expansive biomes. In the upper ocean of the North Pacific subtropical gyre, organic matter remineralization represents one of the dominant biologically mediated fluxes of carbon (10, 19, 55).
Planktonic bacteria constitute the largest inventory of biogenic carbon in the sea, and bacterial carbon production and respiration form globally significant carbon fluxes (10, 11, 54). Oxygen-evolving photoautotrophic cyanobacteria such as Prochlorococcus spp. are the most abundant photosynthetic organisms on Earth, and in the North Pacific subtropical gyre, Prochlorococcus growth supports the majority of photoautotrophic production (5, 6, 16, 18, 42). In addition to photoautotrophic cyanobacteria, both cultivation and cultivation-independent studies suggest that previously uncharacterized photoheterotrophic bacteria could also play important roles in upper ocean carbon cycling (3, 22). Several cultivated marine bacteria appear to derive some of their cellular energy from sunlight and fulfill some (or all) of their carbon, nutrient, and energy demands through metabolism of organic matter (22, 23, 47). To date, however, the physiological capabilities of these organisms in the marine environment remain unknown. In addition, several other groups of potentially photoheterotrophic bacteria include the aerobic anoxygenic photosynthetic bacteria (22, 48, 56), proteorhodopsin-containing α- and γ-proteobacteria (3, 4, 7), and facultative photoautotrophic cyanobacteria such as Prochlorococcus spp. (57).
A number of studies have examined how sunlight influences bacterial production in the oceans. Many of these studies have focused on how UV radiation affects bacterial growth and may alter the cycling of dissolved organic matter by bacteria (35, 36). Several studies have also examined whether photosynthetically available radiation influences marine bacteria (1, 15, 37, 40, 49). Results from these different studies suggest that the influences of photosynthetically available radiation on marine bacteria may vary depending on the habitat and plankton community structure. For example, in the oligotrophic Mediterranean Sea, photosynthetically available radiation appeared to stimulate bacterial growth (37), while in more productive marine waters, photosynthetically available radiation sometimes appears to be detrimental to or to have no influence on bacterial growth (1, 49).
While considerable research has focused on how irradiance influences plankton photoautotrophic production, to date little is known about the direct effects of light intensity on heterotrophic production in the sea. The present study was conducted to better understand how bacterial production in the North Pacific subtropical gyre is influenced by sunlight. In this study, we evaluated the direct influences of irradiance on bacterial protein synthesis with photosynthetron experiments; our results suggest that light intensity could play an important role in regulating bacterial growth and productivity in the North Pacific subtropical gyre.
MATERIALS AND METHODS
Sample collection and photosynthetron experiments.
Photosynthetron experiments were conducted on three cruises to the Hawaii Ocean Time Series Station ALOHA (158°00′W, 22°45′N) between February and May 2002. Water was collected in 12-liter polyvinylchloride bottles mounted to a 24-bottle conductivity-temperature-depth rosette from predawn hydrocasts. Upon completion of the hydrocast, water was sampled from the rosette into darkened 2-liter polycarbonate bottles. Bottles were transferred to a radioisotope lab van, where the [3H]leucine experiments were initiated.
A photosynthetron incubator, designed for studies of photosynthesis-irradiance characteristics (27), was utilized to examine the response of [3H]leucine incorporation to irradiance (Leu-E). Seawater samples were spiked with [3H]leucine and incubated at 24 different light intensities ranging from approximately 0 to 1,500 μmol of quanta m−2 s−1. All incubations were conducted in 40-ml polycarbonate centrifuge tubes. Water samples were placed in a metal cooling block plumbed to a large-capacity refrigerated waterbath to keep the samples at or near in situ temperatures. Samples were placed inside incubation wells that had been bored into the cooling block; the cooling block was placed atop the light source, illuminating the samples from below. The photosynthetron incubations lasted 1 to 2 h.
A single 1,500-W halogen bulb served as the light source for the photosynthetron. Light was transmitted through a blue Plexiglas shield prior to entering the incubation chamber. Neutral density screens were used to produce 24 different light intensities. The light intensity of each sampling well was measured by placing a Biospherical QSL-100 photosynthetically available radiation sensor inside a polycarbonate incubation tube, and the photosynthetically available radiation flux inside each well was measured.
[3H]leucine incorporation into bacterial biomass.
The response of [3H]leucine to irradiance was based on the incorporation of [3H]leucine into bacterial protein (21). Seawater was subsampled from collection bottles into acid-cleaned, 40-ml polycarbonate centrifuge tubes and inoculated with 20 nmol of [3H]leucine (specific activities of [3H]leucine stocks were 150 to 180 Ci mmol−1; New England Nuclear 460A) liter−1. Centrifuge tubes were capped, placed in the photosynthetron, and incubated at the different light intensities. Two time-zero treatments were prepared for each experiment and immediately filtered onto a mixed-cellulose ester (MCE) filter (25 mm by 0.2 μm), and the time-zero filters were frozen in 15-ml centrifuge tubes until processed in the laboratory. Replicate dark controls were wrapped in aluminum foil and placed in a waterbath plumbed to the photosynthetron. At the end of the experiments, the 40-ml samples were filtered onto MCE filters and frozen in 15-ml centrifuge tubes.
In the laboratory, bacterial incorporation of [3H]leucine into protein was determined via an alkaline hydrolysis procedure (17). Filters were initially solubilized in 5 ml of an ice-cold acetone slurry (0.5 g of diatomaceous earth plus 5 ml of 100% acetone), after which sample tubes were placed in a refrigerated (2°C) bench-top centrifuge and spun at 1,500 × g for 10 min. Supernatants were removed by vacuum aspiration, and 5 ml of ice-cold 5% trichloroacetic acid (TCA) was added to each sample. The tubes were vortex-mixed, placed back into the centrifuge, and spun for an additional 10 min. This process was repeated twice with 5% TCA, followed by two rinses with ice-cold 95% ethanol. Following the second ethanol rinse, the tubes were placed in a heating block (80°C), and the samples were dried. Two milliliters of 5% TCA was then added to each sample, and the tubes were placed back in the heating block at 100°C for 30 min to hydrolyze nucleic acids. The 5% TCA was then aspirated from each tube, followed by two additional 5% TCA rinses and one additional 95% ethanol rinse. Samples were dried again, and 2 ml of 1-mol liter−1 NaOH was added to each sample. Samples were placed in a waterbath at 37°C for 18 h, after which 1 ml of base hydrolyzed proteins was removed from each tube and placed in a 20-ml glass scintillation vial with 1 ml of 1-mol liter−1 HCl and 10 ml of Aquasol II. Samples were counted on a TRI-CARB 4640 liquid scintillation counter (Packard Instruments Co.) with external quench standards and luminescence correction.
Photosynthesis versus irradiance.
To evaluate the response of photoautotrophic production to irradiance, [14C]bicarbonate was added to seawater samples, and the rate of 14C uptake into plankton biomass was measured on the May 2002 cruise with the photosynthetron incubator described above. Seawater for these experiments was subsampled into 40-ml polycarbonate incubation tubes and spiked with approximately 400 μCi of 14C-labeled sodium bicarbonate. A 250-μl subsample was removed from two different samples and placed into a scintillation vial containing 1 ml of β-phenylethylamine to measure the 14C activity in each sample. Independent measurements of total dissolved inorganic carbon (by coulometry) were used to calculate the sample specific activities. One time-zero treatment was processed immediately after spiking, and one sample was incubated in the dark to evaluate dark 14C uptake. Values for dark controls were subtracted from the resulting photosynthesis rates to correct for dark inorganic carbon uptake.
At the end of the incubation, the entire 40-ml sample was filtered onto MCE filters. Filters were placed into glass scintillation vials and stored frozen until processed. In the laboratory, filters were acidified with 1 ml of 2-mol liter−1 HCl and vented for 24 h, followed by addition of 10 ml of Aquasol II scintillation cocktail. Samples were counted on a TRI-CARB 4640 liquid scintillation counter. Chlorophyll a concentrations were determined fluorometrically with standard protocols from the Hawaii Ocean Time Series program (http://hahana.soest.hawaii.edu).
In situ [3H]leucine incorporation and primary production.
To evaluate the influence of sunlight on bacterial incorporation of [3H]leucine, seawater was collected from eight discrete depths in the upper ocean from a predawn conductivity-temperature-depth cast and incubated in situ. [3H]leucine experiments were conducted in triplicate 40-ml polycarbonate centrifuge tubes identical to those used for the photosynthetron experiments. Seawater was inoculated with 20 nmol of [3H]leucine liter−1 and incubated in situ both under natural irradiance and in darkened opaque bags. Time-zero controls were collected from each depth, spiked with 20 nmol of [3H]leucine liter−1, and filtered immediately. Triplicate samples were attached to a surface-tethered array and incubated throughout the daylight period (averaging 12 h) in both light and dark. At the end of the incubation period, samples were filtered onto MCE filters and frozen in 15-ml centrifuge tubes. In the laboratory, samples were processed in the same manner as the samples collected from the photosynthetron experiments.
In situ rates of photosynthetic uptake of [14C]bicarbonate were also determined on the three cruises to Station ALOHA. Seawater for photosynthetic production experiments was collected from the same predawn hydrocasts sampled for [3H]leucine experiments. Triplicate seawater samples were collected in acid-cleaned 500-ml polycarbonate bottles and spiked with 52 μCi of [14C]bicarbonate liter−1. Triplicate samples were incubated on the same in situ array used for [3H]leucine experiments, and samples were incubated for the daylight period. At the end of the experiment, seawater was filtered onto 25-mm glass fiber filters, and filters were frozen in 20-ml glass scintillation vials. In the laboratory, filters were processed in the same way as samples from the photosynthetron experiments.
The penetration of photosynthetically available radiation into the upper ocean was measured at approximately noon during each experiment with a Biospherical Instruments profiling reflectance refractometer (PRR 600). Downwelling photosynthetically available radiation fluxes (400 to 700 nm) were measured throughout the upper water column (0 to 200 m).
Data analyses.
The measured [3H]leucine incorporation rates were fitted by least-squares regressions to a photosynthetic model (43) that included a term for dark [3H]leucine incorporation (leuD):
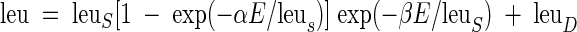 |
(1) |
where leu is the predicted rate of [3H]leucine incorporation (picomoles of leucine per liter per hour), leuS is the maximum [3H]leucine incorporation rate in the absence of photoinhibition (i.e., β = 0), α is the initial slope of the [3H]leucine incorporation rate at light intensities approaching zero (picomoles of leucine per liter per hour [micromoles of quanta per square meter] per second), E is the experimental irradiance (micromoles of quanta per square meter per second), and β describes the slope of the photoinhibited region of the leu-E response (picomoles of leucine per liter per hour [micromoles of quanta per square meter] per second). In addition to determining the parameters for equation 1, the maximum leucine incorporation rate, leumax (inclusive of photoinhibition), was calculated as:
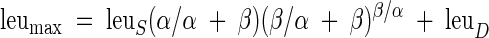 |
(2) |
Finally, parameters derived from equations 1 and 2 were used to calculate Ek, an index of photoadaptation, describing the irradiance required to saturate [3H]leucine incorporation:
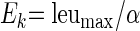 |
(3) |
The photoautotrophic responses to irradiance were fitted with the model of Platt et al. (43):
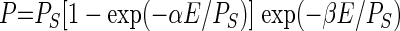 |
(4) |
where P is the photosynthetic carbon fixation rate, PS is the maximum rate of photosynthesis in the absence of photoinhibition, α is the initial slope of the photosynthetic response at low light intensities, E is the light flux, and β is the rate of photoinhibition.
RESULTS
Upper ocean physical characteristics and productivity.
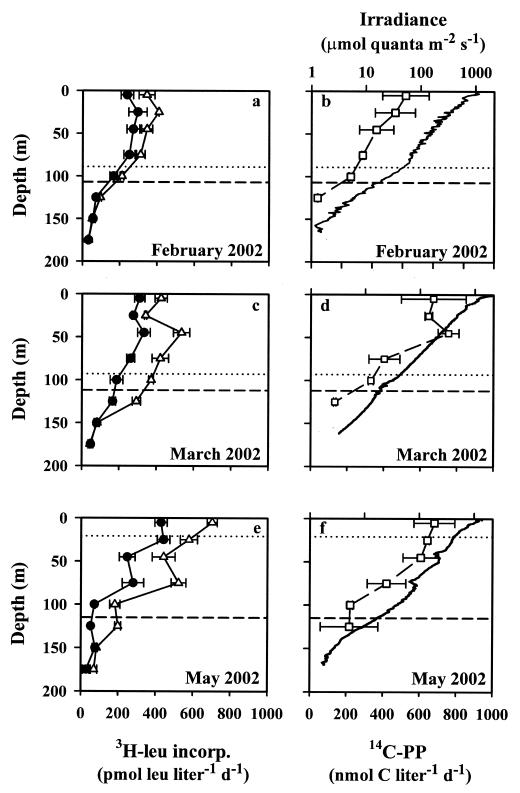
Maximum photic zone depths (1% surface isopleth) were 107, 112, and 115 m for the February, March, and May cruises, respectively (Fig. 1). Mixed layer depths for these three cruises, based on a 0.125 potential density criterion (31), were 89, 93, and 27 m, respectively (Fig. 1). Photosynthetron experiments were conducted on water collected from the upper (5 and 25 m) and lower (75 and 100 m) photic zones. Surface photosynthetically available radiation fluxes ranged between 1,000 and 1,990 μmol of quanta m−2 s−1 (Fig. 1), and photosynthetically available radiation fluxes at the depths where experiments were conducted in the upper photic zone ranged from 300 to 800 μmol of quanta m−2 s−1, while irradiance in deep photic zone ranged from 25 to 81 μmol of quanta m−2 s−1 (Table 1). Chlorophyll a concentrations at the depths where experiments were conducted ranged from 0.07 to 0.24 μg liter−1 (Table 1), with elevated concentrations in the lower photic zone due to the presence of the deep chlorophyll maximum.
FIG. 1.
Depth profiles of in situ [3H]leucine incorporation (panels a, c, and e). 14C-labeled photoautotrophic production (14C-PP, squares) and downwelling photosynthetically available radiation (solid line) (panels b, d, and f) were determined on three cruises to Station ALOHA. [3H]leucine incorporation was measured in the light (open triangles) and in the dark (solid circles); values are means of replicate treatments, and error bars represent standard deviations of the means. Downwelling photosynthetically available radiation was determined at approximately local noon. Dotted lines indicate the base of the mixed layer (0.125 potential density criterion); dashed lines are the bases of the photic zones (1% surface isopleths).
TABLE 1.
Upper-ocean properties at Station ALOHA during this studya
| Cruise and depth (m) | Ez (μmol quanta m−2 s−1) | Chlorophyll a (μg liter−1) | 3H-leuL (pmol liter−1 h−1) | 3H-leuD (pmol liter−1 h−1) | 14C-PP (nmol of C liter−1 h−1) |
|---|---|---|---|---|---|
| 20 February 2002 | |||||
| 25 | 302 | 0.08 | 39 (4.9) | 26 (4.2) | 48 (11) |
| 100 | 25 | 0.24 | 18 (0.87) | 15 (1.8) | 23 (1.2) |
| 12 March 2002 | |||||
| 25 | 480 | 0.06 | 28 (0.48) | 22 (0.63) | 53 (1.7) |
| 100 | 31 | 0.17 | 30 (1.0) | 14 (2.8) | 27 (1.1) |
| 20 May 2002 | |||||
| 5 | 887 | 0.05 | 55 (2.1) | 34 (2.6) | 52 (8.5) |
| 75 | 81 | 0.11 | 41 (7.1) | 22 (3.1) | 32 (8.0) |
3H-leuL and 3H-leuD are in situ [3H]leucine incorporation from daytime incubations conducted in the light and dark, respectively; 14C-PP, measured rates of photoautotrophic production. Ez, maximum photosynthetically available radiation flux based on measured attenuation coefficients from the noon cast. Numbers in parentheses are standard deviations of measured properties.
Photoautotrophic production, as estimated by [14C]bicarbonate uptake, ranged from 26.7 to 52.5 μmol of C liter−1 h−1 at the discrete depths where photosynthetron experiments were conducted (Table 1). The rates of photoautotrophic production in the upper 75 m of the photic zone ranged from 550 to 671 nmol of C liter−1 day−1 and declined toward the base of the photic zone (Fig. 1). In situ profiles of [3H]leucine incorporation demonstrated consistent photostimulation through the upper 125 m of the photic zone (Fig. 1).
[3H]leucine incorporation versus irradiance in the upper photic zone.
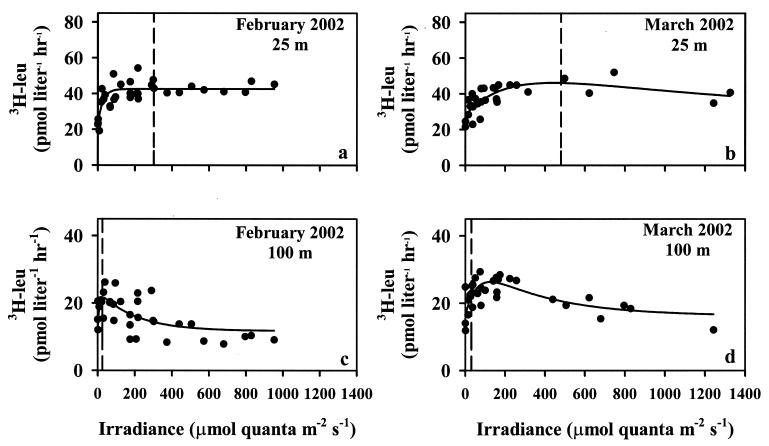
In total, six experiments were conducted to determine the response of [3H]leucine incorporation to irradiance. Three experiments were conducted on water collected in the upper 25 m of the water column (Fig. 2a and b), and three experiments were conducted on seawater collected from the lower photic zone (75 to 100 m) (Fig. 2c and d). In all experiments, [3H]leucine incorporation increased with irradiance in a nonlinear fashion, often resembling a photosynthesis-like response to irradiance. Application of a photosynthetic model provided estimates of the photophysiological parameters describing the leu-E relationships (Table 2).
FIG. 2.
Relationships between [3H]leucine incorporation and irradiance in the upper photic zone (panels a and b) and deep photic zone (panels c and d) at Station ALOHA. Fitted lines are least-squares nonlinear regressions (see the text); parameters defining the lines are given in Table 2. Dashed vertical lines represent maximal (local noon) photosynthetically available radiation flux measured at each depth for the cruises shown.
TABLE 2.
Summary of photophysiological parameters describing the response of [3H]-leucine incorporation to irradiance at Station ALOHAa
| Cruise and depth (m) | α | β | leuD | leuS | leumax | Ek | R2 (P) |
|---|---|---|---|---|---|---|---|
| February 2002 | |||||||
| 25 | 0.74 (0.33) | 0.00b (0.00) | 23 (2.8) | 43 (4.3) | 43 | 58 | 0.62 (<0.0001) |
| 100 | 1.2b (1.3) | 0.062 (0.014) | 12 (1.9) | 24 (3.8) | 22 | 18 | 0.41 (0.0015) |
| March 2002 | |||||||
| 25 | 0.13 (0.05) | 0.023b (0.041) | 28 (2.2) | 58 (6.0) | 46 | 363 | 0.57 (<0.0001) |
| 100 | 0.25 (0.11) | 0.049b (0.057) | 16 (1.7) | 34 (8.7) | 26 | 106 | 0.54 (<0.0001) |
| May 2002 | |||||||
| 5 | 0.36 (0.13) | 0.025b (0.024) | 32 (3.8) | 71 (10) | 62 | 175 | 0.68 (≤0.0001) |
| 75 | 0.64 (0.31) | 0.19 (0.032) | 28 (5.8) | 92 (13) | 60 | 94 | 0.54 (<0.0001) |
Values are regression-derived parameters; values in parentheses are standard errors. See the text for units.
Not significantly different from zero, P > 0.05.
[3H]leucine incorporation in the upper ocean generally increased asymptotically with irradiance; rates increased roughly linearly at low light intensities (0 to 150 μmol of quanta m−2 s−1), with incorporation saturating and remaining nearly constant above ∼200 μmol of quanta m−2 s−1 (Fig. 2). Fifty to sixty-eight percent of the variance in the leu-E relationships was described with the Platt et al. (43) model of the photosynthesis irradiance relationship (Table 2). [3H]leucine incorporation in the upper photic zone was stimulated 48 to 92% by irradiance.
In February, [3H]leucine incorporation was maximal at relatively low light flux (∼58 μmol of quanta m−2 s−1), while in March and May [3H]leucine incorporation was maximal at higher light fluxes (363 and 175 μmol of quanta m−2 s−1, respectively) (Fig. 2, Table 2). Consistent with these results, fluxes of photosynthetically available radiation to the upper ocean were lower in February than in either March or May (Table 1). In both February and May, the derived Ek values of [3H]leucine incorporation (58 and 175 μmol of quanta m−2 s−1, respectively) corresponded to isolume depths of ∼82 and 23 m, respectively, approximately coincident with the base of the mixed layer during these cruises (89 and 22 m, respectively). In contrast, in March, the 363 μmol of quanta m−2 s−1 isolume was positioned at ∼30 m, well above the base of the mixed layer (87 m). None of the experiments conducted in the upper photic zone demonstrated significant photoinhibition within the range of experimental photosynthetically available radiation fluxes tested (Table 2), indicating a high-light-adapted population. Incorporation of [3H]leucine in the dark was significantly greater in May than in either February or March (t test, P < 0.05), but there were no significant differences (at the 95% confidence level) in leumax or Ek in the upper photic zone among the three experiments conducted.
Leucine incorporation versus irradiance in the deep photic zone.
The responses of [3H]leucine incorporation to irradiance in the deep photic zone were different from those observed in the upper photic zone. In the deep photic zone, 41 to 54% of the variance in the leu-E relationships was described with the photosynthesis irradiance model (Table 2). In both February and May, [3H]leucine incorporation in the deep photic zone was reduced at light intensities of 18 and 94 μmol of quanta m−2 s−1, respectively (Fig. 3, Table 2). Deep photic zone estimates of α were 62 to 92% greater than estimates derived for the upper photic zone, and values of Ek were 1.9- to 3.4-fold lower than those derived from upper photic zone experiments (Table 2).
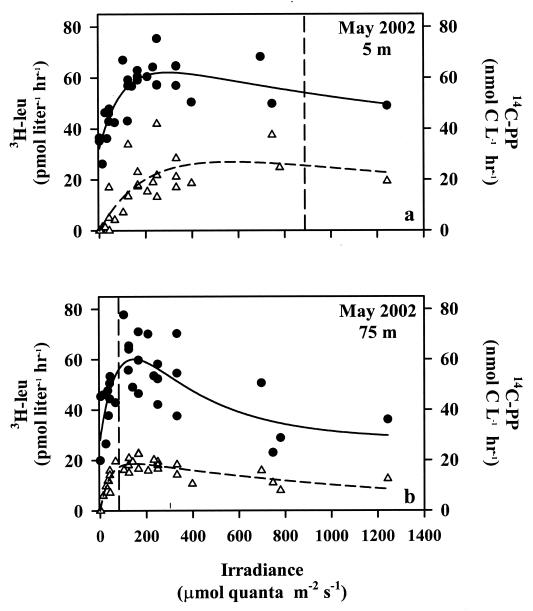
FIG. 3.
Responses of [3H]leucine incorporation (solid circles) and 14C-labeled photoautotrophy products (open triangles) to irradiance in the upper (a) and deep (b) photic zones. Experiments conducted in May 2002. Fitted lines are least-squares nonlinear regression (see the text), and parameters defining the lines are given in Tables 2 and 3. Dashed vertical lines represent the maximal (noon) photosynthetically available radiation flux measured at each depth.
During the February cruise, [3H]leucine rates in the lower photic zone saturated at 18 μmol of quanta m−2 s−1 (Fig. 3, Table 2), equivalent to an isolume depth of 110 m. In March and May, rates saturated at higher light fluxes than in February (106 and 94 μmol of quanta m−2 s−1, respectively); these light fluxes occurred at ∼67 and 73 m, respectively. The response of [3H]leucine incorporation in the lower photic zone during the March cruise was very similar to the response in the upper photic zone; [3H]leucine rates increased gradually at low light fluxes and never demonstrated significant photoinhibition (Table 2).
Photosynthesis as a function of irradiance.
Two experiments were conducted in the upper and deep photic zones during the cruise in May to evaluate how photosynthetic rates responded to irradiance (Fig. 3). The photosynthetic rates from both the upper and lower photic zones were similar to the responses of [3H]leucine incorporation. In the upper photic zone, 14C-labeled products of photosynthesis increased asymptotically at low light fluxes, saturating at 162 μmol of quanta m−2 s−1 (Table 3). The lower photic zone P-E response was similar in appearance to that of the upper photic zone, but photosynthesis in the lower photic zone was inhibited at light fluxes of >36 μmol of quanta m−2 s−1 (Fig. 3). Based on measured photosynthetically available radiation flux at noon, light intensities in both the upper and lower photic zones were sufficient to saturate photosynthetic production.
TABLE 3.
Photophysiological parameters describing the responses of 14C uptake to irradiance at Station ALOHA during the May 2002 cruisea
| Depth (m) | α | β | PS | Pm | Ek | R2 (P) |
|---|---|---|---|---|---|---|
| 5 | 0.17 (0.05) | 0.01b (0.05) | 35 (12) | 27 | 161 | 0.80 (<0.0001) |
| 75 | 0.52 (0.08) | 0.02 (0.00) | 21 (1.6) | 18 | 36 | 0.82 (<0.0001) |
See Table 2, footnotes a and b. Values are regression-derived parameters; values in parentheses are standard errors.
DISCUSSION
The results of this study suggest that both primary and secondary productivities in the North Pacific subtropical gyre are partly controlled by irradiance. Sunlight stimulated rates of [3H]leucine incorporation throughout the photic zone at Station ALOHA, with depth profiles of [3H]leucine incorporation demonstrating photostimulation across a range of irradiances spanning more than two orders of magnitude. The characteristics of the light-stimulated [3H]leucine incorporation were similar to the well-studied response of photosynthesis to irradiance; [3H]leucine incorporation increased linearly at low light intensities before saturating or declining with increasing irradiance. These phased responses are identical to light-limited, light-saturated, and light-inhibited processes that are characteristic in photosynthesis-irradiance experiments (43).
In all of the experiments, both [3H]leucine incorporation and 14C photoautotrophic production increased sharply at low light fluxes; however, the nature of the responses varied with depth and among cruises. In total, a model of [3H]leucine incorporation that included a photoinhibition parameter described 41 to 68% of the variance in [3H]leucine incorporation. This model has been applied to describe several irradiance-driven planktonic responses, including nutrient uptake (44) and fixation of nitrogen by cyanobacteria (28).
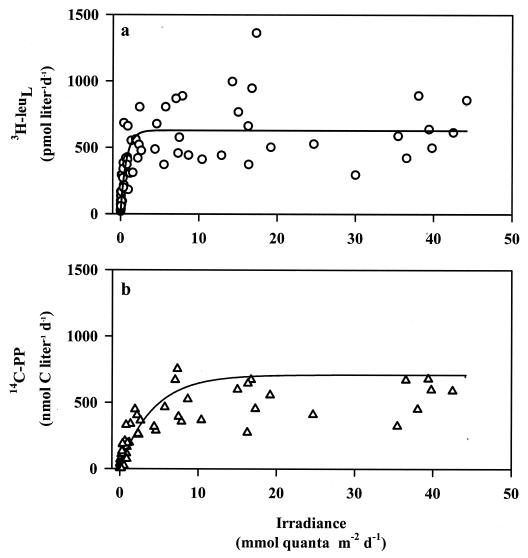
To evaluate how in situ [3H]leucine incorporation and 14C photoautotrophic production varied in their responses to light, in situ [3H]leucine incorporation rates and 14C-primary production were plotted against the measured daily light fluxes, as done by Letelier et al. (26) (Fig. 4). In situ rates of [3H]leucine incorporation were measured on nine cruises to Station Aloha between February 2000 and May 2002, and the results were fitted to the model of Platt et al. (43). Similar to the photosynthetron results, in situ [3H]leucine incorporation rates increased at low light, saturating at light intensities of >1 mol of quanta m−2 day−1, and maximum [3H]leucine incorporation at this light flux was ∼640 pmol liter−1 day−1. In situ primary production increased gradually at low light intensities and saturated at photosynthetically available radiation fluxes roughly 10-fold greater than in situ [3H]leucine incorporation rates. While [3H]leucine incorporation and 14C photoautotrophic production appeared to respond similarly to irradiance in the photosynthetron experiments, this relationship was not observed in situ, suggesting that across the continuum of temperature and nutrient gradients in the photic zone at Station ALOHA, [3H]leucine incorporation and 14C primary production have different responses to irradiance.
FIG. 4.
In situ relationships between daily photosynthetically available radiation flux and [3H]leucine incorporation (3H-leuL) (a) and 14C- photoautotrophic production (14C-PP) (b) on nine Hawaii Ocean Time-Series cruises between March 2000 and May 2002. Line fits are least-squares nonlinear regressions. Equations describing the relationships are: [3H]leucine = 561 (1 − e−1.1E) + 66, R2 = 0.64, P < 0.0001; and photoautotrophic production = 511 (1 − e−0.46E), R2 = 0.47, P < 0.0001, where E is the in situ irradiance.
The results of this study provide some insight into the potential mechanisms of light-stimulated bacterial growth in this ecosystem. Among the possibilities, heterotrophic bacterial growth may be tightly regulated by photosynthetic production of dissolved organic matter; alternatively, incorporation of amino acids by obligate or facultative photoheterotrophic bacteria could result in light-stimulated bacterial production; finally, photodynamic transformation of nutrients might indirectly stimulate heterotrophic activity in this ecosystem.
Indirect photostimulation of bacterial growth (via exudation of labile dissolved organic matter) could have contributed to the observed photostimulated bacterial growth (20); however, both direct observations and trophic-flow models suggest that bacterial growth in oligotrophic marine ecosystems may be largely supported by fluxes of dissolved organic matter arising from protozoan grazing (2, 14, 38). Assuming that grazing is a major source of dissolved organic matter in the North Pacific subtropical gyre, it would seem unlikely that grazing-mediated dissolved organic matter release would demonstrate the rapid response to irradiance observed in our experiments. Similarly, indirect stimulation of bacterial production by phototransformation of dissolved organic matter appears an unlikely mechanism in our experiments, as all of the experiments in this study excluded UV radiation and photochemical alteration of dissolved organic matter is largely determined by UV radiation (35). Finally, the photoinhibition observed in our experiments from the deep photic zone would appear to be inconsistent with an indirect stimulatory mechanism.
The observed relationships between [3H]leucine incorporation and irradiance in this study likely imply that the bacterial assemblage at Station ALOHA demonstrated a direct photophysiological response to sunlight. Several studies have demonstrated utilization of nanomolar concentrations of amino acids by photoautotrophic cyanobacteria (including Prochlorococcus spp.) (24, 39, 52, 57). Moreover, the recently reported genomes of both Prochlorococcus and Synechococcus spp. revealed amino acid transport systems, suggesting that these organisms have the capacity to utilize amino acids in the marine environment (12, 41, 46). Prochlorococcus populations are abundant and productive components of the upper ocean bacterial assemblage at Station ALOHA (16, 25, 29), and facultative photoheterotrophy by assemblages of Prochlorococcus spp. could explain the observed responses of both [3H]leucine incorporation and 14C photoautotrophic production to irradiance.
There were several notable differences in [3H]leucine responses between the upper and lower photic zones; in particular, protein production rates in the lower photic zone appeared more susceptible to photoinhibition than the upper photic zone. [3H]leucine incorporation in the lower photic zone was photoinhibited at relatively low light fluxes (18 to 94 μmol of quanta m−2 s−1), while [3H]leucine rates in the upper photic zone never demonstrated significant photoinhibition. Various groups of marine bacteria appear to utilize different light-harvesting strategies to maximize growth along the light gradient in the upper ocean. For example, divergent light-harvesting strategies have been observed among populations of Prochlorococcus and Synechococcus (33, 45, 50); similarly, proteorhodopsin-containing proteobacteria also appear to “tune” their light acquisition (3). The results from these [3H]leucine experiments suggest depth-dependent differences in the responses of bacterial protein synthesis to irradiance at Station ALOHA. Either the bacterioplankton assemblages at Station ALOHA have flexible photophysiologies and acclimate to the available light flux or vertically segregated bacterial populations have different physiological responses to sunlight, similar to the differences observed among vertically segregated Prochlorococcus ecotypes (32, 34, 51). Unfortunately, the results of the present study do not allow us to differentiate between these possible mechanisms.
Several previous studies on the response of heterotrophic bacteria to sunlight differ from our results, suggesting that the relationship between bacterial production and irradiance may vary depending on the structure of the plankton assemblage. For example, in the Mediterranean Sea, Morán et al. (37) found that [3H]leucine incorporation rates were greater in darkened incubations than when samples were incubated in situ in the light; however, Prochlorococcus abundance during this study in the Mediterranean Sea was approximately two orders of magnitude lower than typically observed in the upper ocean at Station ALOHA. Similarly, in studies conducted in the Gulf of Mexico and the Adriatic Sea, sunlight appeared to repress [3H]leucine incorporation by planktonic bacteria (1, 49). The basis of the differences between our study in the North Pacific subtropical gyre and these studies in other marine systems are not known; however, the similarities between the [3H]leucine and 14C photosynthetic responses to irradiance (Fig. 3) suggest that facultative heterotrophy may partly support Prochlorococcus growth in this oligotrophic ecosystem.
Until the photodynamic processes that influence bacterial growth are better understood, production estimates in oligotrophic marine environments should include coupled determinations of bacterial growth in both the light and the dark. One important implication of this study is that photodynamic processes may stimulate bacterial production in the North Pacific subtropical gyre by 48 to 114% more than would be suggested by dark incubations alone. Such results are important because bacterial growth directly regulates the metabolic state of the upper ocean in oligotrophic ecosystems (8, 9, 53). The paucity of inorganic nutrients in the upper ocean of the North Pacific subtropical gyre may select phototrophic bacteria capable of supplementing or entirely meeting their cellular nutrient requirements from the relatively large pool of dissolved organic matter. Such phototrophic bacteria could harvest sunlight for energy while obtaining carbon, nutrients, and energy from dissolved organic matter; this physiological capability would alter our understanding of biologically mediated carbon fluxes in this system. For example, ecosystem models that derive production based on the turnover of inorganic nutrient pools would not accommodate facultative heterotrophy by presumed photoautotrophs such as Prochlorococcus spp. Such an increase in bacterial growth efficiency could substantially reduce the total flux of dissolved organic matter required to sustain heterotrophic production in this ecosystem.
Acknowledgments
We thank the captain and crew of the R/V Kaimikai-O-Kanaloa. R. Letelier (Oregon State University) graciously provided the photosynthetically available radiation data. We are grateful to W. O. Smith (Virginia Institute of Marine Science) for use of the photosynthetron. Two anonymous reviewers and the comments of I. Anderson, J. Bauer, E. Canuel, and W. O. Smith (Virginia Institute of Marine Science) greatly improved this paper.
Funding for this project was supported by NSF grants to H. Ducklow (OCE98-19581) and to D. Karl (OCE96-17409) and R. Lukas (OCE93-03094).
Footnotes
This is School of Ocean and Earth Science (SOEST) contribution 6399 and Joint Global Ocean Flux Study (JGOFS) contribution 1045.
REFERENCES
- 1.Aas, P., M. M. Lyons, R. Pledger, D. L. Mitchell, and W. H. Jeffrey. 1996. Inhibition of bacterial activities by solar radiation in nearshore waters and the Gulf of Mexico. Aquat. Microb. Ecol. 11:229-238. [Google Scholar]
- 2.Anderson, T. R., and H. W. Ducklow. 2001. Microbial loop carbon cycling in ocean environments studied using a simple steady-state model. Aquat. Microb. Ecol. 26:37-49. [Google Scholar]
- 3.Beja, O., L. Aravind, E. V. Koonin, M. T. Suzuki, A. Hadd, L. P. Nguyen, S. Jovanovich, C. M. Gates, R. A. Feldman, J. L. Spudich, E. N. Spudich, and E. F. DeLong. 2000. Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science 289:1902-1906. [DOI] [PubMed] [Google Scholar]
- 4.Beja, O., E. N. Spudich, J. L. Spudich, M. Leclerc, and E. F. DeLong. 2001. Proteorhodopsin phototrophy in the ocean. Nature 411:786-789. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, L., H. A. Nolla, and D. Vaulot. 1994. The importance of Prochlorococcus to community structure in the central North Pacific Ocean. Limnol. Oceanogr. 39:954-961. [Google Scholar]
- 6.Chisholm, S. W., R. J. Olson, E. R. Zettler, R. Goericke, J. B. Waterbury, and N. A. Welschmeyer. 1988. A novel free-living Prochlorophyte abundant in the oceanic euphotic zone. Nature 334:340-343. [Google Scholar]
- 7.de la Torre, J. R. C., L. M. Christianson, O. Beja, M. T. Suzuki, D. M. Karl, J. Heidelberg, and E. F. DeLong. 2003. Proteorhodopsin genes are distributed among divergent marine bacterial taxa. Proc. Natl. Acad. Sci. USA 100:12830-12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.del Giorgio, P. A., J. J. Cole, and A. Cimbleris. 1997. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature 385:148-151. [Google Scholar]
- 9.Duarte, C. M., and S. Agusti. 1998. The CO2 balance of unproductive aquatic ecosystems. Science 281:234-236. [DOI] [PubMed] [Google Scholar]
- 10.Ducklow, H. W. 1999. The bacterial component of the oceanic euphotic zone. FEMS Microbiol. Ecol. 30:1-10. [Google Scholar]
- 11.Ducklow, H. W., D. A. Purdie, P. J. L. Williams, and J. M. Davies. 1986. Bacterioplankton—a sink for carbon in a coastal marine plankton community. Science 232:865-867. [DOI] [PubMed] [Google Scholar]
- 12.Dufresne, A., M. Salanoubat, F. Partensky, F. Artiguenave, I. M. Axmann, V. Barbe, S. Duprat, M. Y. Galperin, E. V. Koonin, F. Le Gall, K. S. Makarova, M. Ostrowski, S. Oztas, C. Robert, I. B. Rogozin, D. J. Scanlan, N. T. de Marsac, J. Weissenbach, P. Wincker, Y. I. Wolf, and W. R. Hess. 2003. Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc. Natl. Acad. Sci. USA 100:10020-10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field, C. B., M. J. Behrenfeld, J. T. Randerson, and P. Falkowski. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237-240. [DOI] [PubMed] [Google Scholar]
- 14.Hagstrom, A., F. Azam, A. Andersson, J. Wikner, and F. Rassoulzadegan. 1988. Microbial loop in an oligotrophic pelagic marine ecosystem: possible roles of cyanobacteria and nanoflagellates in the organic fluxes. Mar. Ecol. Prog. Ser. 49:171-178. [Google Scholar]
- 15.Helbling, E. W., E. R. Marguet, V. E. Villafane, and O. Holm-Hansen. 1995. Bacterioplankton viability in Antarctic waters as affected by solar ultraviolet-radiation. Mar. Ecol. Prog. Ser. 126:293-298. [Google Scholar]
- 16.Karl, D. M. 1999. A sea of change: biogeochemical variability in the North Pacific Subtropical Gyre. Ecosystems 2:181-214. [Google Scholar]
- 17.Karl, D. M. 1982. Selected nucleic acid precursors in studies of aquatic microbial ecology. Appl. Environ. Microbiol. 44:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karl, D. M., R. R. Bidigare, and R. M. Letelier. 2001. Long-term changes in plankton community structure and productivity in the North Pacific Subtropical Gyre: the domain shift hypothesis. Deep-Sea Res. II 48:1449-1470. [Google Scholar]
- 19.Karl, D. M., J. R. Christian, J. E. Dore, D. V. Hebel, R. M. Letelier, L. M. Tupas, and C. D. Winn. 1996. Seasonal and interannual variability in primary production and particle flux at Station ALOHA. Deep-Sea Res. II 43:539-568. [Google Scholar]
- 20.Karl, D. M., D. V. Hebel, K. Bjorkman, and R. M. Letelier. 1998. The role of dissolved organic matter release in the productivity of the oligotrophic North Pacific Ocean. Limnol. Oceanogr. 43:1270-1286. [Google Scholar]
- 21.Kirchman, D., E. Knees, and R. Hodson. 1985. Leucine incorporation and its potential as a measure of protein-synthesis by bacteria in natural aquatic systems. Appl. Environ. Microbiol. 49:599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolber, Z. S., F. G. Plumley, A. S. Lang, J. T. Beatty, R. E. Blankenship, C. L. VanDover, C. Vetriani, M. Koblizek, C. Rathgeber, and P. G. Falkowski. 2001. Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science 292:2492-2495. [DOI] [PubMed] [Google Scholar]
- 23.Kolber, Z. S., C. L. Van Dover, R. A. Niederman, and P. G. Falkowski. 2000. Bacterial photosynthesis in surface waters of the open ocean. Nature 407:177-179. [DOI] [PubMed] [Google Scholar]
- 24.Leekaden, J., and W. Simonis. 1982. Amino acid uptake and energy coupling dependent on photosynthesis in Anacystis nidulans. J. Bacteriol. 151:229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letelier, R. M., R. R. Bidigare, D. V. Hebel, M. Ondrusek, C. D. Winn, and D. M. Karl. 1993. Temporal variability of phytoplankton community structure based on pigment analysis. Limnol. Oceanogr. 38:1420-1437. [Google Scholar]
- 26.Letelier, R. M., J. E. Dore, C. D. Winn, and D. M. Karl. 1996. Seasonal and interannual variations in photosynthetic carbon assimilation at Station ALOHA. Deep-Sea Res. II 43:467-490. [Google Scholar]
- 27.Lewis, M. R., and J. C. Smith. 1983. A small volume, short-incubation-time method for measurement of photosynthesis as a function of incident irradiance. Mar. Ecol. Prog. Ser. 13:99-102. [Google Scholar]
- 28.Lewis, W. M., and S. N. Levine. 1984. The light response of nitrogen fixation in Lake Valencia, Venezuela. Limnol. Oceanogr. 29:894-900. [Google Scholar]
- 29.Liu, H. B., H. A. Nolla, and L. Campbell. 1997. Prochlorococcus growth rate and contribution to primary production in the equatorial and subtropical North Pacific Ocean. Aquat. Microb. Ecol. 12:39-47. [Google Scholar]
- 30.Longhurst, A., S. Sathyendranath, T. Platt, and C. Caverhill. 1995. An estimate of global primary production in the ocean from satellite radiometer data. J. Plankton Res. 17:1245-1271. [Google Scholar]
- 31.Monterey, G., and S. Levitus. 1997. Seasonal variability of mixed layer depth for the world ocean. NOAA Atlas NESDIS 14:5.
- 32.Moore, L. R., and S. W. Chisholm. 1999. Photophysiology of the marine cyanobacterium Prochlorococcus: ecotypic differences among cultured isolates. Limnol. Oceanogr. 44:628-638. [Google Scholar]
- 33.Moore, L. R., R. Goericke, and S. W. Chisholm. 1995. Comparative physiology of Synechococcus and Prochlorococcus—influence of light and temperature on growth, pigments, fluorescence and absorptive properties. Mar. Ecol. Prog. Ser. 116:259-275. [Google Scholar]
- 34.Moore, L. R., G. Rocap, and S. W. Chisholm. 1998. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature 393:464-467. [DOI] [PubMed] [Google Scholar]
- 35.Mopper, K., and D. J. Kieber. 2002. Photochemistry and the cycling of carbon, sulfur, nitrogen, and phosphorus, p. 456-508. In D. A. Hansell and C. A. Carlson (ed.), Biogeochemistry of marine dissolved organic matter. Academic Press, San Diego, Calif.
- 36.Moran, M. A., and R. G. Zepp. 2000. UV radiation effects on microbes and microbial processes, p. 201-228. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, New York, N.Y.
- 37.Morán, X. A. G., R. Massana, and J. M. Gasol. 2001. Light conditions affect the measurement of oceanic bacterial production via leucine uptake. Appl. Environ. Microbiol. 67:3795-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagata, T. 2000. Production mechanisms of dissolved organic matter, p. 121-152. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, New York, N.Y.
- 39.Paerl, H. W. 1991. Ecophysiological and trophic implications of light-stimulated amino acid utilization in marine picoplankton. Appl. Environ. Microbiol. 57:473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pakulski, J. D., P. Aas, W. Jeffrey, M. Lyons, L. Von Waasenbergen, D. Mitchell, and R. Coffin. 1998. Influence of light on bacterioplankton production and respiration in a subtropical coral reef. Aquat. Microb. Ecol. 14:137-148. [Google Scholar]
- 41.Palenik, B., B. Brahamsha, F. W. Larimer, M. Land, L. Hauser, P. Chain, J. Lamerdin, W. Regala, E. E. Allen, J. McCarren, I. Paulsen, A. Dufresne, F. Partensky, E. A. Webb, and J. Waterbury. 2003. The genome of a motile marine Synechococcus. Nature 424:1037-1042. [DOI] [PubMed] [Google Scholar]
- 42.Partensky, F., W. R. Hess, and D. Vaulot. 1999. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol. Mol. Biol. Rev. 63:106-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Platt, T., C. L. Gallegos, and W. G. Harrison. 1980. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 38:687-701. [Google Scholar]
- 44.Priscu, J. C. 1989. Photon dependence of inorganic nitrogen transport by phytoplankton in perennially ice-covered Antarctic lakes. Hydrobiologia 172:173-182. [Google Scholar]
- 45.Rocap, G., D. L. Distel, J. B. Waterbury, and S. W. Chisholm. 2002. Resolution of Prochlorococcus and Synechococcus ecotypes by using 16S-23S ribosomal DNA internal transcribed spacer sequences. Appl. Environ. Microbiol. 68:1180-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rocap, G., F. W. Larimer, J. Lamerdin, S. Malfatti, P. Chain, N. A. Ahlgren, A. Arellano, M. Coleman, L. Hauser, W. R. Hess, Z. I. Johnson, M. Land, D. Lindell, A. F. Post, W. Regala, M. Shah, S. L. Shaw, C. Steglich, M. B. Sullivan, C. S. Ting, A. Tolonen, E. A. Webb, E. R. Zinser, and S. W. Chisholm. 2003. Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature 424:1042-1047. [DOI] [PubMed] [Google Scholar]
- 47.Shiba, T., Y. Shioi, K. Takamiya, D. C. Sutton, and C. R. Wilkinson. 1991. Distribution and physiology of aerobic-bacteria containing bacteriochlorophyll alpha on the east and west coasts of Australia. Appl. Environ. Microbiol. 57:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiba, T., U. Simidu, and N. Taga. 1979. Distribution of aerobic bacteria which contain bacteriochlorophyll a. Appl. Environ. Microbiol. 38:43-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sommaruga, R., I. Obernosterer, G. J. Herndl, and R. Psenner. 1997. Inhibitory effect of solar radiation on thymidine and leucine incorporation by freshwater and marine bacterioplankton. Appl. Environ. Microbiol. 63:4178-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urbach, E., D. J. Scanlan, D. L. Distel, J. B. Waterbury, and S. W. Chisholm. 1998. Rapid diversification of marine picophytoplankton with dissimilar light-harvesting structures inferred from sequences of Prochlorococcus and Synechococcus (cyanobacteria). J. Mol. Evol. 46:188-201. [DOI] [PubMed] [Google Scholar]
- 51.West, N. J., W. A. Schonhuber, N. J. Fuller, R. I. Amann, R. Rippka, A. F. Post, and D. J. Scanlan. 2001. Closely related Prochlorococcus genotypes show remarkably different depth distributions in two oceanic regions as revealed by in situ hybridization using 16S rRNA-targeted oligonucleotides. Microbiology 147:1731-1744. [DOI] [PubMed] [Google Scholar]
- 52.Wheeler, P. A., and D. L. Kirchman. 1986. Utilization of inorganic and organic nitrogen by bacteria in marine systems. Limnol. Oceanogr. 31:998-1009. [Google Scholar]
- 53.Williams, P. J. L. B. 1998. The balance of plankton respiration and photosynthesis in the open oceans. Nature 394:55-57. [Google Scholar]
- 54.Williams, P. J. L. B. 1981. Microbial contribution to overall marine plankton metabolism—direct measurements of respiration. Oceanol. Acta 4:359-364. [Google Scholar]
- 55.Williams, P. J. L. B., and D. A. Purdie. 1991. In vitro and in situ derived rates of gross production, net community production and respiration of oxygen in the oligotrophic subtropical gyre of the North Pacific Ocean. Deep-Sea Res. I 38:891-910. [Google Scholar]
- 56.Yurkov, V. V., and J. T. Beatty. 1998. Aerobic anoxygenic phototrophic bacteria. Microbiol. Mol. Biol. Rev. 62:695-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zubkov, M. V., B. M. Fuchs, G. A. Tarran, P. H. Burkill, and R. Amann. 2003. High rate of uptake of organic nitrogen compounds by Prochlorococcus cyanobacteria as a key to their dominance in oligotrophic oceanic waters. Appl. Environ. Microbiol. 69:1299-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]