Abstract
Objective
To assess effects of chronic antidepressant drug treatment on serotonin type-1A receptor (5-HT1AR) binding potential (BP) in major depressive disorder.
Methods
Depressed subjects (n=27) were imaged using PET and [11C]WAY-100635 at baseline and following a median of 9.4 weeks of treatment with selective serotonin reuptake inhibitor or dual reuptake inhibitor antidepressant agents. Fifteen subjects had complete pre- and post-treatment scan data. The 5-HT1AR BP was derived from the tissue time-radioactivity concentrations from regions-of-interest defined a priori using a simplified reference tissue model (SRTM), and in a subset of subjects, compartmental modeling (CMOD).
Results
Chronic treatment had no effect on pre- or postsynaptic 5-HT1AR BP, as confirmed by both the SRTM and CMOD analyses. These results were unaffected by treatment response status and were consistent across brain regions. Among the 22 subjects for whom the clinical response-to-treatment was established, the treatment non-responders (n=7) had higher baseline BP values in the left (p=0.01) and right orbital cortex (p=0.02) than the responders (n=15).
Conclusions
Chronic antidepressant drug treatment did not significantly change cerebral 5-HT1AR binding, consistent with preclinical evidence that the alterations in serotonergic function associated with antidepressant drug administration are not accompanied by changes in 5-HT1AR density. Higher baseline 5-HT1AR binding was associated with poorer response to treatment.
Keywords: raphe nucleus, hippocampus, major depression, selective serotonin reuptake inhibitor
Introduction
Alterations in serotonin type-1A receptor (5-HT1AR) function have been associated with the pathophysiology (Arango et al. 2001; Bowen et al. 1989; Drevets et al. 2000; Lopez et al. 1998; Stockmeier et al. 1998) and treatment (Artigas et al. 1996; Chaput et al. 1991; Cowen 2000; Frazer et al. 1990; Haddjeri et al. 1998) of major depressive disorder (MDD). Several groups (Drevets et al. 1999; Meltzer et al. 2004a; Sargent et al. 2000), but not all (Parsey et al. 2006), reported abnormal reductions of in vivo pre- and postsynaptic 5-HT1AR binding potential (BP; proportional to Bmax x affinity, where Bmax = receptor density) in depressives versus controls using PET and [carbonyl-11C]WAY-100635 ([11C]WAY). There has been minimal study, however, of whether antidepressant treatment reverses the 5-HT1AR binding abnormalities observed in MDD. Although serotonin reuptake inhibitor (SRI) induced increases in intrasynaptic 5HT concentrations are not associated with increases in 5-HT1AR density and mRNA expression in experimental animals, SRI effects in depressed humans may be unique. For example, the down-regulation of 5-HT1AR mRNA expression and density in MDD has been hypothesized to result from glucocorticoid hormone hypersecretion, a process which may be interrupted by antidepressant drug treatment (Lopez et al. 1998).
In one study of selective SRI (SSRI) treatment effects on human 5-HT1AR BP using [11C]WAY-PET (Sargent et al. 2000), no treatment-associated changes in BP were evident in regions where the 5-HT1A receptor is expressed postsynaptically. In the raphe, where 5-HT1AR expression occurs presynaptically, the BP showed a nonsignificant trend toward being lower in the post-SRI-scans (effect size of 0.26). This latter observation was notable because the presynaptic 5-HT1AR population becomes desensitized during chronic SRI administration (see Discussion). The present study investigated the effects of SRI treatment in an independent MDD sample, using both a reference tissue model and an arterial input function-based kinetic model to quantify 5-HT1AR BP. Preliminary data also were acquired in a subgroup of subjects treated with venlafaxine, an agent expected to inhibit the reuptake of norepinephrine as well as serotonin (Beique et al. 2000; Debonnel et al. 2006; Harvey et al. 2000), based upon preclinical evidence that antidepressant agents with potent norepinephrine reuptake inhibiting effects proved more effective than SSRI at preventing stress-induced decreases in 5-HT1AR binding and mRNA expression (Lopez et al. 1998).
In addition, we characterized the relationship between the clinical response to antidepressant drug treatment and the regional 5-HT1AR binding in the pretreatment, baseline condition. Most studies of MDD found abnormal reductions in the postsynaptic 5-HT1AR receptor binding in unmedicated depressed patients (Bowen et al. 1989; Drevets et al. 1999; Sargent et al. 2000; Lopez et al. 1998). It is thus noteworthy that chronic administration of antidepressant agents from various drug classes increases post-synaptic 5-HT1AR function (without altering receptor density) in rodents (Chaput et al. 1991; Haddjeri et al. 1998). If this effect also extends to depressed humans, then it is conceivable that the depressed subjects with the greatest reduction in post-synaptic 5-HT1AR binding prior to treatment will benefit most from the potentially compensatory effect of enhancing postsynaptic 5-HT1AR transmission. We tested this hypothesis by comparing the pretreatment 5-HT1AR binding between the subjects who proved responsive to antidepressant treatment versus those who did not.
Materials and Methods
Subjects were enrolled between January 1998 and October 2002, after being recruited through media advertisements and psychiatric services (inpatient/outpatient/emergency) at the University of Pittsburgh. Subjects provided written informed consent as approved by the University of Pittsburgh Biomedical IRB. Subjects were included if they met DSM-IV criteria for recurrent MDD based upon an unstructured interview with a psychiatrist and the Structured Clinical Interview for DSM IV, and had a 17-item Hamilton Rating Scale for Depression (HRSD17) score ≥ 18. Subjects were excluded if they had medical or neurological illnesses likely to affect cerebral physiology or anatomy, gross abnormalities of brain structure evident in MRI scans, suicidal intent, substance abuse within 1 year, lifetime history of substance dependence (other than nicotine), or exposure to psychotropic or other medications likely to alter cerebral physiology or monoamine function within 3 weeks (8 weeks for fluoxetine).
Twenty-seven depressed subjects (55.6% female; mean age=34.3±8.7 yrs) were imaged at unmedicated baseline. Seven subjects withdrew from the study after the baseline scan (5 discontinued treatment, one continued treatment but failed to return for rescanning, and one elected to receive a non-SRI antidepressant drug). The remaining 20 subjects were imaged after a median of 9.4 weeks (range: 7 to 63 weeks) of treatment. Medication-naïve subjects and prior SSRI responders were treated with citalopram (n=14; mean dose=41±9.5 mg/d, range 20–60 mg). Prior SSRI non-responders were treated with venlafaxine (n=4; doses were 187.5, 225, 375, and 375 mg/d). An additional subject was treated with combined citalopram 20 mg/d plus venlafaxine 225 mg/d. Treatment response was defined as a 50% reduction in HRSD17 score.
The PET scans were acquired on an ECAT HR+ PET scanner (CTI-PET systems, Knoxville, TN) in 3D mode [63 transaxial planes 2.4-mm thick; in-plane resolution=4.1 mm full-width at half-maximum (FWHM) over a 15.2-cm field-of-view]. Radiosynthesis of [carbonyl-11C]WAY was performed as described by (McCarron et al. 1996). A transmission scan was obtained to correct the PET emission scan for attenuation effects. A dynamic emission scan (29 frames of increasing length over 60 min) then was initiated following i.v. bolus administration of 9 to 19 mCi of high specific activity [11C]WAY (1.58 +/− 0.71 mCi/nmol at time of injection). Thirty minutes of additional post-injection emission data (total scan duration=90 min) was collected in a subset of 8 subjects to ensure the comparability of the results obtained using either scan length (Parsey et al. 2000). Arterial blood was sampled and corrected for radiolabeled metabolites to compute the plasma input function of [11C]WAY in 17 of the 27 subjects pretreatment, and in 8 subjects post-treatment.
To provide an anatomical framework for analysis of the PET data, MRI scans were obtained using a 1.5 T GE Medical Systems (Milwaukee, WI) Signa Scanner and a 3D spoiled gradient recalled (SPGR) sequence. The MRI and PET images were aligned using automated image registration (AIR; (Woods et al. 1993). Regions-of-interest (ROI) were manually traced on the MRI scan using a modified version of the IDL-based (Interactive Data Language, Boulder, CO) computer program, ROITOOL (CTI PET Systems; Knoxville, TN). The ROIs were defined in the raphe nucleus (RN), the mesiotemporal cortex [MTC; hippocampus, amygdala, and parts of the parahippocampal and periamygdaloid cortices; Brodmann area (BDA) 34/27/28], the right (RLO; BDA 45/47) and left lateral orbital (LLO; BDA 45/47) cortex, the postcentral gyrus (PCG; BDA 1/2/3) and the occipital cortex-posterior cingulate gyrus (OCC-PC; BDA 17/18/31), as previously described (Drevets et al. 1999). These ROI were chosen on the basis of association with 5-HT1AR reductions in prior PET (Drevets et al. 1999; Sargent et al. 2000) and post mortem studies of depression (Arango et al. 1995; Bowen et al. 1989; Lopez et al. 1998). A reference region for assessing nonspecifically bound and free radioligand was defined in the cerebellar gray matter (CER) using anatomical guidelines that excluded the vermis (Parsey et al. 2005) and minimized the influence of spill-in effects from the temporal-occipital cortex (Bailer et al. 2005; Drevets et al. 1999; Meltzer et al. 2001; Meltzer et al. 2004b; Price et al. 2002b). Because the RN is inadequately visualized in MR images, the ROI for this structure was defined on summed late PET image frames which predominantly reflect 5-HT1AR -specific binding on 7 planes spanning the pontine and midbrain raphe nuclei (see Drevets et al. 1999).
Tissue time-activity concentrations were obtained from the dynamic PET image for each ROI. Regional 5-HT1AR-BP values were determined using a simplified reference tissue method (SRTM; (Gunn et al. 1998; Lammertsma et al. 1996). In subjects for whom arterial blood was sampled, a 2-tissue compartmental model (CMOD) was applied to the arterial input function and regional tissue time-activity concentrations to derive the 5-HT1AR distribution volumes (DV). Regional measures of 5-HT1AR BP were determined for the compartmental model as BP = (DVROI/DVCER)−1= (Bmax/Kd)f2 = k3/k4 (equivalent to SRTM BP measure), where Bmax is the available 5-HT1AR density, KD is the equilibrium dissociation constant, f2 is the free fraction of [11C]WAY in tissue, k3 is the association rate of [11C]WAY to 5-HT1AR, and k4 is the dissociation rate of [11C]WAY from 5-HT1AR (Lammertsma et al. 1996; Mintun et al. 1984).
Statistical inference for 5-HT1AR BP was conducted with the Wilcoxon signed ranks test or the Mann-Whitney U test, for related and independent samples respectively, with SPSS (SPSS Software Version 13, SPSS Inc, Chicago, IL). Possible relationships between the baseline HRSD17 scores and the 5-HT1AR-BP values and between the treatment-associated changes in these measures also were explored using Spearman’s rho correlation coefficients. Mixed-effects statistical analysis with repeated measures was performed post-hoc on the SRTM-derived BP data using SAS Proc Mixed (SAS/STAT Software Version 8 2000 SAS institute, Cary, NC), with subjects as the random term. This method offered the benefit of employing the maximal sample size (Brown et al. 1999) to test treatment-by-region, treatment-by-gender, and treatment-by-response interactions in the SRTM-derived BP data. The SRTM- and CMOD-derived BP values for the 60 and 90 minute dynamic scans were compared to assess the possibility of bias, and were correlated within subjects using Pearson correlation coefficients.
Results
The demographic and clinical data for the subjects are listed in table I. Comorbid disorders present in the depressed sample were: panic disorder (2), bulimia (1), alcohol/substance abuse in remission >1 year (11), specific phobia (4), post-traumatic stress disorder (2), and social phobia (2). Of the 27 subjects entered, 22 (81.5%) had sufficient clinical data obtained during the treatment phase to determine response status (these included the 20 cases scanned both pre- and post-treatment plus two cases who withdrew before re-scanning). Of these, 15 (68%) were treatment responders (>50% reduction in HRSD17 score). The mean interscan interval did not differ between responders (16±16 wks) and nonresponders (16±11 wks; p=0.99). The interscan intervals were not correlated with the changes in 5HT1AR BP measured using SRTM. The subgroups with and without post-treatment scan data were demographically and clinically similar.
TABLE I.
Demographic and clinical characteristics of subjects
| All Subjects | Usable Post-treatment scan data | Unusable or unavailable Post-treatment scan data | |
|---|---|---|---|
| N | 27 | 18 | 9 |
| Proportion of females (%) | 55.6 | 50 | 66.7 |
| Mean age (SD) | 34.3 (8.7) | 34.8 (7.8) | 33.2 (10.7) |
| Baseline HRSD17 [mean (SD), range] | 21.0 (4.3), 14–31 | 21.1 (3.8), 14–28 | 20.8 (5.4), 17–31 |
| Post-treatment HRSD17 [mean (SD), range] | 9.5 (8.5), 0–28 (n=22) | 8.6 (8.0), 0–28 | 11.0 (11.1), 0–28 (n=4)* |
post-treatment HRSD missing for four subjects who discontinued medications before completion of a therapeutic trial
Of the 20 subjects scanned both pre- and post-treatment, the pretreatment scans were excluded from analysis for 2 subjects due to excessive movement and for 1 subject for showing outlying values [defined as x > mean ± 3(SD)]. The post-treatment scans from 2 additional subjects were excluded due to excessive movement. A complete set of technically usable pre- and post-treatment images thus was obtained for 15 subjects. In the subjects for whom arterial blood was sampled both pre- and post-treatment (n=8), the arterial input function from one subject’s pretreatment scan and the image reconstruction from another subject’s post-treatment scan proved unusable due to technical problems. Thus, 6 subjects had complete pre- and post-treatment scan data with technically usable arterial input functions.
From the paired analyses, the mean [11C]WAY BP values derived using SRTM appear in table II, and the mean [11C]WAY DV and BP values derived via CMOD appear in table III. The pre- and post-treatment BP or DV values did not differ significantly in any ROI in either analysis (Tables I & II; Figure 1). Inclusion of the outlier (see above) in the SRTM analysis did not alter these results. When the subjects treated with venlafaxine were excluded from the paired analysis, the SSRI alone group (n=10) showed no significant differences in the pre- versus post-treatment BP values in any region (Table II; Figure 1). In subjects treated with venlafaxine (n=5), there also were no significant differences in the pre- versus post-treatment BP values in any region (Table II).
TABLE II.
Paired comparisons of regional 5-HT1AR binding potential (BP) values (mean±SD) derived using simplified reference tissue modeling in the pre- versus post-treatment conditions.
| Entire Sample (n=15) | SSRI alone (n=10) | Venlafaxine +/− SSRI (n=5) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ROI | Pre-treatment | Post-treatment | W; p-valueb | Pre-treatment | Post-treatment | W; p-valueb | Pre-treatment | Post-treatment | W; p-valueb |
| MTCa | 5.62 ± 1.37 | 5.14 ± 1.39 | −0.85;0.43 | 5.59 ± 1.63 | 5.11 ± 1.51 | −0.53;0.65 | 5.68 ± 0.90 | 5.19 ± 1.29 | −0.67;0.63 |
| RN | 2.02 ± 0.40 | 1.92 ± 0.44 | −0.97;0.36 | 2.05 ± 0.47 | 2.02 ± 0.45 | −0.36;0.77 | 1.94 ± 0.24 | 1.73 ± 0.40 | −1.21;0.31 |
| LLO | 3.44 ± 0.51 | 3.50 ± 0.71 | −0.28;0.80 | 3.35 ± 0.55 | 3.38 ± 0.73 | −0.15;0.92 | 3.61 ± 0.43 | 3.74 ± 0.67 | −0.67;0.63 |
| RLO | 3.51 ± 0.48 | 3.53 ± 0.65 | −0.06;0.98 | 3.42 ± 0.52 | 3.54 ± 0.71 | −0.46;0.70 | 3.69 ± 0.37 | 3.51 ± 0.59 | −0.94;0.44 |
| OCC-PC | 2.02 ± 0.40 | 2.11 ± 0.57 | −1.08;0.30 | 2.08 ± 0.35 | 2.13 ± 0.50 | −0.46;0.70 | 1.91 ± 0.52 | 2.10 ± 0.77 | −0.94;0.44 |
| PCG | 2.77 ± 0.59 | 2.90 ± 0.68 | −0.85;0.42 | 2.54 ± 0.41 | 2.74 ± 0.66 | −0.87;0.43 | 3.23 ± 0.67 | 3.23 ± 0.64 | −0.14;1.00 |
Abbreviations: ROI=region of interest; MTC=mesiotemporal cortex; RN=raphe nucleus; LLO=left lateral orbital cortex; RLO=right lateral orbital cortex; OCC-PC =occipital cortex-posterior cingulate gyrus; PCG=post central gyrus.
one subject’s MTC-BP data were excluded because the model failed to provide stable curve fits for this region’s time-activity concentrations.
W=Wilcoxon signed ranks test statistic.
TABLE III.
Mean regional [11C]WAY distribution volume (DV) and binding potential (BP) values in depressed subjects, pre and post-treatment derived using compartmental modeling (CMOD) and arterial input functions [mean ± SD; paired data, n=6].
| ROI | CMOD DV | CMOD BP | ||||
|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | W; p-value | Pre-treatment | Post-treatment | W; p-value | |
| CER | 0.44 ± 0.12 | 0.51 ± 0.11 | −0.73;0.56 | n.a. | n.a. | n.a. |
| MTCa | 7.50 ± 2.98 | 6.81 ± 1.95 | −0.73;0.63 | 15.67 ± 2.74 | 11.36 ± 4.21 | −1.46;0.25 |
| RN | 2.03 ± 0.68 | 2.11 ± 0.50 | −0.11;1.00 | 3.68 ± 1.62 | 3.21 ± 1.09 | −0.52;0.69 |
| LLO | 2.86 ± 0.85 | 3.14 ± 0.88 | −0.52;0.69 | 5.47 ± 1.40 | 5.17 ± 1.34 | −0.73;0.56 |
| RLO | 2.78 ± 0.74 | 3.11 ± 0.53 | −0.1.15;0.31 | 5.41 ± 1.59 | 5.21 ± 1.28 | −0.31;0.84 |
| OCC-PC | 1.79 ± 0.60 | 2.00 ± 0.50 | −0.94; 0.44 | 3.06 ± 1.20 | 2.95 ± 0.78 | −0.31;0.84 |
| PCG | 2.29 ± 0.69 | 2.60 ± 0.49 | −0.94;0.44 | 4.17 ± 0.96 | 4.17 ± 1.01 | −0.11;1.00 |
two subjects’s MTC-BP data were excluded because the model failed to provide stable curve fits for this region’s time-activity concentrations.
Abbreviations as in table II
Figure 1.
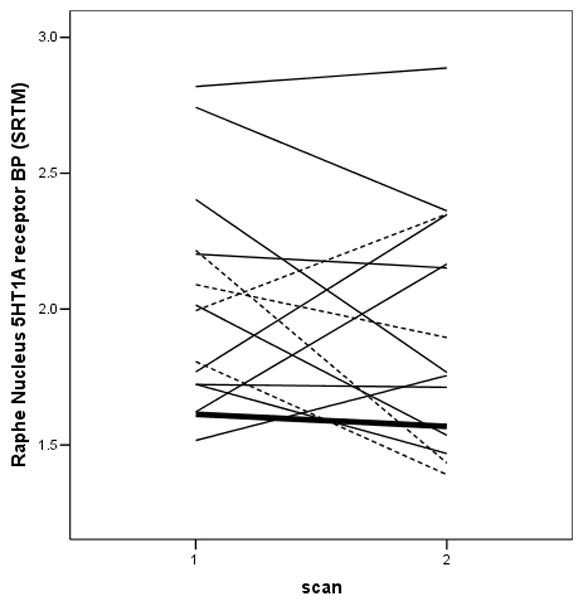
Pattern of 5-HT1A receptor BP changes in the raphe nucleus during treatment with selective serotonin reuptake inhibitors (SSRI; solid lines), venlafaxine alone (dashed lines), or venlafaxine plus an SSRI (heavy solid line).
Analysis of the data from the entire intention-to-treat sample (n=27) using the mixed-effects model also revealed no significant effects of treatment on the SRTM-derived BP values in any region either when including [F(1,14) = 2.07, p = 0.17; Figure 2] or excluding the subjects treated with venlafaxine ([F(1,9)=2.99, p=.12]. There also were no significant treatment-by-region interactions [F(5,69)=0.05, p=1.00] or treatment-by-gender interactions evident in the these analyses [F(1,13)=1.51, p=0.24].
Figure 2. Boxplots of observed 5HT1A receptor BP before and following SRI treatment for data obtained from the entire study sample.
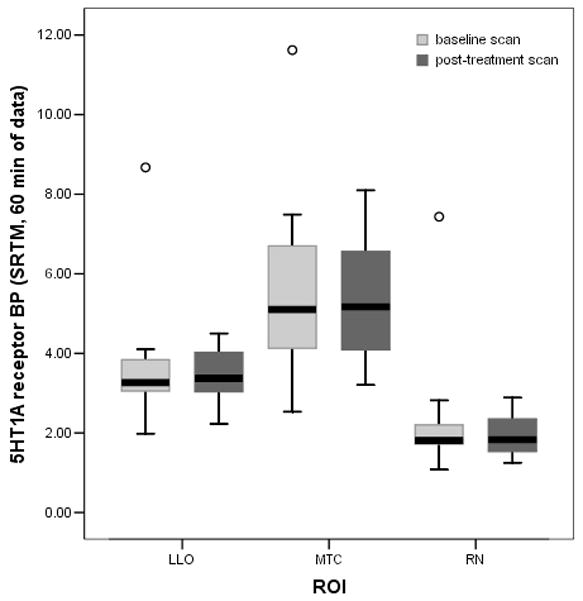
This graph shows pre- and post-treatment 5-HT1A receptor BP in three representative regions-of-interest using all available pre and post-treatment SRTM data (including the outlier). LLO = left lateral orbital cortex; baseline scan n=26, post-treatment scan n=18. MTC = mesiotemporal cortex; baseline scan n=24, post-treatment scan n=16. RN = raphe nucleus: baseline scan n=26, post-treatment scan n=18). ○ denotes the outlier.
The regional BP values did not differ significantly between pre- and post-treatment scans for the treatment responsive subgroup alone, and the mean treatment-associated changes in BP did not differ significantly between responders and nonresponders. The interactions between treatment response status and treatment-associated changes in regional BP were not significant [F(1,13)=3.10, p=0.10]. Changes in the HRSD17 scores did not correlate with changes in SRTM-derived BP values in any ROI (RN: r=0.004, p=0.99; LLO: r= −0.34, p=0.22; RLO: r = −0.18; p=0.53; OCC-PC: r =0.06, p=0.82; PCG: r= −.11, p=0.69; MTC: r= −0.37, p=0.20).
Among the 22 subjects for whom the clinical response-to-treatment was established, the treatment non-responders (n=7) had higher baseline BP values in the left (3.82 ± 0.39 versus 3.20 ± 0.55; exact MWU =88; p=0.01) and right orbital cortex (3.83 ± 0.33 versus 3.26 ± 0.55; exact MWU =71; p=0.02) than the treatment-responders (n=15). The baseline BP values did not differ significantly between responders and nonresponders in the PCG (p=0.08), OCC-PC (p=0.12), RN (p=0.97) or MTC (p=0.98). The baseline BP values were not correlated with baseline HRSD17 scores in any region (RN: r= 0.29, p=0.17; LLO: r= 0.06, p=0.78; RLO: r= 0.18, p=0.39; OCC-PC: r = 0.17, p=0.44; PCG: r= −0.09, p=0.67; MTC: r= 0.16, p=0.49).
For the scans for which technically usable arterial input functions were available (n=15), the CMOD-derived BP values were positively correlated with the SRTM-derived BP values (r; 95% confidence interval; p value) in RN (0.83; 0.54–0.94; <0.001), LLO (0.83; 0.54–0.94; <0.001) RLO (0.81; 0.50–0.93; <0.001), OCC-PC (0.85; 0.60–0.95; <0.001), PCG (0.81; 0.52–0.94; <0.001), and MTC (0.59; 0.02–0.87;0.045; n=12). The SRTM-derived BP-values were biased toward being lower than the CMOD-derived BP-values, as previously described (Parsey et al 2000). [SRTM BP values were lower than CMOD BP values in every ROI (p<.001).]
In the subjects with 90 min emission scans (n=6), the SRTM-derived BP values obtained using the 90 min time-radioactivity concentration curves correlated tightly with those obtained using only the 60 min curves in all regions, as follows (r; 95% confidence interval; p value): RN (0.99; 0.91–1.0;<0.001), LLO (0.99; 0.91–1.0; <0.001), RLO (0.96; 0.67–1.0; 0.003), OCC-PC (0.96; 0.67–1.0; 0.003), PCG (0.99; 0.91–1.0; <0.001), and MTC (0.98; 0.82–1.0;0.001). [SRTM 60 min BP values were lower than SRTM 90 min BP values as follows: LLO 4%, p=.063; MTC 11%, p=.031; OCC 0.2%, NS; PCG 3.8%, p=.031; RLO 4.4%, p=.063; RN 9.4%, p=.094.]
In subjects for whom both 90 min emission scans and arterial input functions were obtained (n=7), the CMOD derived BP values also were highly correlated between the 60 and 90 min data in RN (0.94; 0.62–0.99; <0.002), LLO (0.93; 0.61–0.99; p<0.002), RLO (r=0.87; 0.35–0.98; <0.01), OCC-PC (0.96; 0.75–0.99; p<0.001), PCG (r=0.75; 0.0–0.96; <0.05), and MTC (0.91;0.50–0.99; 0.004). [CMOD 60 min BP values were non-significantly lower (p>.10) than CMOD 90 min BP values as follows: LLO 3%, MTC 4.7%, OCC 3.7%, PCG 2.3%, RLO 5.5%, RN 7.3%.]
Discussion
The mean 5-HT1AR binding did not change significantly following chronic SRI treatment in the orbital, mesiotemporal, parietal, or occipital cortices or the midbrain/pontine raphe nuclei, as measured using [11C]WAY-PET. These negative results are consistent with Sargent et al. (2000), who found no significant changes in [11C]WAY binding in MDD subjects following chronic paroxetine (n=16) or sertraline (n=4) treatment. These data also appear compatible with preclinical evidence that postsynaptic 5-HT1AR density and mRNA expression remain unchanged during SRI-induced increases in intrasynaptic serotonin concentrations (Carli et al. 1996; Hensler et al. 1991; Spurlock et al. 1994; Welner et al. 1989), and that the SRI-induced 5-HT1A autoreceptor desensitization occurs through receptor internalization or G-protein changes (Hensler 2003; Zimmer et al. 2004) rather than through alterations in receptor density (Frazer & Hensler 1990). Notably, [11C]WAY binding is insensitive to competition from endogenous 5HT (Hume et al. 2001; Parsey et al. 1998), and as a lipophilic, high-affinity 5-HT1AR antagonist, [11C]WAY is likely to bind internalized receptors without showing significant changes in site affinity (Laruelle 2000).
A limitation of our study was that the sample for which usable pre- and post-treatment scan data were available consisted of only 15 subjects, so we could not rule out the possibility that the small and nonsignificant differences observed in tables II and III might become significant in a much larger sample. However, the small effect sizes across regions for the full sample (0.01 – 0.35; 0.5 – 9% changes) suggests that any treatment-associated effect may be within the range of measurement variability. Too few subjects (n=5) were re-imaged following venlafaxine treatment in order to establish whether NRI agents alter the 5-HT1AR BP, however. A future study involving a larger subject sample treated with NRI agents thus may be warranted.
The negative results obtained with respect to changes in 5-HT1AR binding were primarily based upon BP values derived using SRTM and 60 min emission scan data. Our study was initiated using this approach as validated for [11C]WAY by Gunn et al. (1998). When Parsey et al. (2000) reported evidence that the SRTM approach to analyzing [11C]WAY images introduced bias (consistently underestimating BP) and that longer dynamic scans were needed to reach equilibrium conditions in some regions, we revised our methods to add arterial blood sampling and increase the scan duration. Nevertheless, the bias introduced by the SRTM method (Parsey et al. 2000) is inconsequential to the comparisons of pre- versus post-treatment 5-HT1AR BP data, because both measures are subject to identical bias. Moreover, the greater test-retest reliability of the SRTM method compared to CMOD or other approaches (Parsey et al. 2000) enhanced the sensitivity of our pre-versus post-treatment comparisons. Moreover, the SRTM- and CMOD-derived 5-HT1AR BP values were correlated in the subset of subjects for whom arterial blood was sampled, supporting the emphasis of the SRTM-derived BP values as the largest available data set of pre- and post-SRI treatment 5-HT1AR binding data (table II).
Another limitation of the current study was that the analyses were restricted to a few, relatively large, predefined ROIs. We thus could not exclude the possibility that SRI-induced changes in 5-HT1AR BP may have occurred outside the regions sampled, or may have been limited to subsections of these ROI.
Although chronic SRI administration did not appear to correct the abnormal reduction in 5-HT1AR binding reported in MDD, such treatments may nevertheless compensate for deficient 5-HT1AR function in depression. By desensitizing the presynaptic 5-HT1A autoreceptor and thereby increasing the amount of 5-HT release (Artigas et al. 1996; Blier et al. 1983; Blier et al. 1986; Chaput et al. 1988; Chaput et al. 1991) chronic SRI administration appears to increase postsynaptic 5-HT1AR function (Chaput et al. 1991; Price et al. 1989). Likewise, chronic treatment with NRI or tricyclic antidepressant agents results in tonic activation of postsynaptic 5-HT1AR in the absence of alterations in receptor density (Chaput et al. 1991; Haddjeri et al. 1998).
These effects of antidepressant drugs of increasing postsynaptic 5-HT1AR function is noteworthy in view of our finding that the pre-treatment 5-HT1AR binding was lower in the orbital cortex in subjects who proved responsive to treatment versus subjects who proved nonresponsive (effect size of 1.3 in the LLO). This observation is compatible with a previous report that MDD cases with a lower raphe 5-HT1AR binding in the pretreatment, baseline condition showed more rapid rates of symptom remission during antidepressant drug treatment (Meltzer et al. 2004a). Conversely, MDD cases who expressed the G allele of the C(−1019)G 5-HT1A promoter region polymorphism showed decreased response rates to SSRI treatment (Lemonde et al. 2004) and increased 5-HT1AR BP in one study (Parsey et al. 2006), although a second study could not confirm the association between this polymorphism and regional 5-HT1AR BP (David et al. 2005). Nevertheless, concurrent assessment of genotype, treatment response and neuroimaging binding measures ultimately may provide fruitful research directions for elucidating pathophysiology and guiding treatment development.
Acknowledgments
We would like to thank members of the PET Facility Staff who carried out the acquisition of PET data and care of all subjects during PET procedures. We thank Andrea Confer for assistance with preparation of this manuscript. We are grateful to the NIMH for support of this work (MH59769 and MH64561) and to the research subjects who participated in this study.
Footnotes
Conflicts of Interest: David J. Kupfer serves on the advisory board of Eli Lilly & Company and is a consultant for Servier Amerique. Michael E. Thase is a consultant and on the speakers bureau for AstraZeneca, Eli Lilly & Company, GlaxoSmithKline, Bristol-Myers Squibb, and Wyeth Pharmaceuticals and also is a consultant for Organon, Inc., Cephalon, Inc., Forest Pharmaceuticals, Inc., Janssen Pharmaceutica, Novartis, and Pfizer Pharmaceutical. Chester A. Mathis receives royalty payments and is an advisor to GE Healthcare.
References
- Arango V, Underwood M, Gubbi A, et al. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontial cortex of suicide victims. Brain Research. 1995;688:121–33. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Artigas F, Romero L, de Montigny C, et al. Acceleration of the effects of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–83. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Frank GK, Henry SE, et al. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measures by positron emission tomography and [11C]WAY 100635. Arch Gen Psychiatry. 2005;62:1032–41. doi: 10.1001/archpsyc.62.9.1032. [DOI] [PubMed] [Google Scholar]
- Beique J, de Montigny C, Blier P, et al. Effects of sustained administration of the serotonin and norepinephrine reuptake inhibitor venlafaxine: I. in vivo electrophysiological studies in the rat. Neuropharmacology. 2000;39(10):1800–12. doi: 10.1016/s0028-3908(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Electrophysiological investigations on the effect of repeated zimelidine administration on serotonergic neuortransmission in the rat. J Neurosci. 1983;3:1270–8. doi: 10.1523/JNEUROSCI.03-06-01270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C, Azzaro AJ. Modification of serotonergic and noradrenergic neurotransmissions by repeated administration of monoamine oxidase inhibitors: electrophysiological studies in the rat central nervous system. J Pharmacol Exp Ther. 1986;237:987–94. [PubMed] [Google Scholar]
- Bowen DM, Najlerahim A, Procter AW, et al. Circumscribed changes of the cerebral cortex in neuropsychiatric disorders of late life. Proc Natl Acad Sci. 1989;86:9504–8. doi: 10.1073/pnas.86.23.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied mixed models. Chichester: John Wiley & Sons, LTD; 1999. Repeated measures data; pp. 199–261. [Google Scholar]
- Carli M, Afkhami-Dastjerdian S, Reader TA. [3H]8-OH-DPAT binding and serotonin content in rat cerebral cortex after acute fluoxetine, desipramine, or pargyline. J Psychiatry Neurosci. 1996;21:114–22. [PMC free article] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C. Effects of the 5-hydroxytryptamine receptor antagonist, BMY 7378, on 5-hydtroxytryptamine neurotransmission: electrophysiological studies in the rat central nervous system. J Pharmacol Exp Ther. 1988;246:359–70. [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Presynaptic and postsynaptic modifications of the serotonin system by long-term administration of antidepressant treatments. An in vivo electrophysiologic study in the rat. Neuropsychopharmacology. 1991;5:219–29. [PubMed] [Google Scholar]
- Cowen PJ. Psychopharmacology of 5HT1A Receptors. Nuclear Medicine and Biology. 2000;27:437–9. doi: 10.1016/s0969-8051(00)00108-6. [DOI] [PubMed] [Google Scholar]
- David SP, Murthy NV, Rabiner EA, et al. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. Journal of Neuroscience. 2005;25(10):2586–90. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debonnel G, Saint-Andre E, Hebert C, et al. Differential physiological effects of a low dose and high doses of venlafaxine in major depression. Int J Neuropsychopharmacol. 2006 doi: 10.1017/S1461145705006413. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, et al. Serotonin Type-1A receptor imaging in depression. Nuclear Med Biology. 2000;27(5):499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–87. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Frazer A, Hensler JG. 5-HT1A receptors and 5-HT1A-mediated responses: effect on treatments that modify serotonergic neurotransmission. In: Whitaker-Azmitia PM, Peroutka SJ, translators and editors. The Neuropharmacology of Serotonin. New York: The New York Academy of Sciences; 1990. pp. 460–75. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Sargent PA, Bench CJ, et al. Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY-100635 for PET. Neuroimage. 1998;8:426–40. doi: 10.1006/nimg.1998.0379. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, De Montigny C. Long-term antidepressant treatments result in tonic activation of forebrain 5HT1A receptors. J Neuroscience. 1998;18(23):10150–6. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AT, Rudolph RL, Preskorn SH. Evidence of the dual mechanisms of action of venlafaxine. Archives of General Psychiatry. 2000;57(5):503–9. doi: 10.1001/archpsyc.57.5.503. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Regulation of 5HT1A receptor function in brain following agonist or antidepressant administration. Life Science. 2003;72:1665–82. doi: 10.1016/s0024-3205(02)02482-7. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Kovachich GB, Frazer A. A quantitative autoradiographic study of serotonin 1A receptor regulation. Effect of 5, 7-dihydroxytryptamine and antidepressant treatments. Neuropsychopharmacology. 1991;4:131–44. [PubMed] [Google Scholar]
- Hume S, Hirani E, Opacka-Juffry J, et al. Effect of 5-HT on binding of [(11)C] WAY 100635 to 5-HT(IA) receptors in rat brain, assessed using in vivo microdialysis nd PET after fenfluramine. Synapse. 2001;41(2):150–9. doi: 10.1002/syn.1069. [DOI] [PubMed] [Google Scholar]
- Lammertsma A, Bench C, Hume S, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding copetition techniques: A critical review. Journal of Cerebral Blood Flow and Metabolism. 2000;20:423–51. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, et al. Association of the C(−1019)G 5-HT1A functional promoter polymorphism with antidepressant response. International Journal of Neuropsychopharmacology. 2004;7(4):501–6. doi: 10.1017/S1461145704004699. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, et al. Regulation of serotonin 1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: Implications for the neurobiology of depression. Biological Psychiatry. 1998;43:547–73. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- McCarron JA, Turton D, Pike VW, et al. Remotely controlled production of the 5HT1A receptor radioligand, [carbonyl-11C]WAY-100635, via 11C-carboxylation of an immobilized Grignard reagent. J Labelled Comp Radiopharm. 1996;38:941–53. [Google Scholar]
- Meltzer CC, Drevets WC, et al. Gender-Specific Aging Effects on the Serotonin 1A Receptor. Brain Research. 2001;895(1–2):9–17. doi: 10.1016/s0006-8993(00)03211-x. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, et al. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004a;29(12):2258–65. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, et al. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004b;29(12):2258–65. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Kilbourn MR, et al. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–27. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Arango V, Olvet DM, et al. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–93. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hwang D, Simpson N, et al. Kinetic derivation of serotonin 5-HT1A receptors binding potential with [C-11]Carbonyl-Way 100635 and competition studies with endogenous serotonin. J Nuclear Med. 1998;39(5 Suppl):167P. [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, et al. Altered serotonin 1A binding in major depression: a {carbonyl-C-11]WAY 100635 positron emission tomography study. Biol Psychiatry. 2006;59(2):106–13. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Slifstein M, Hwang DR, et al. Validation and Reproducibility of Measurement of 5HT1A Receptor Parameters With [carbonyl-11C]WAY-100635 in Human: Comparison of Arterial and Reference Tissue Imput Functions. Journal of Cerbral Blood Flow and Metabolism. 2000;20:1111–33. doi: 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- Price JC, Kelley DE, Ryan CM, et al. Evidence of Increased Serotonin-1A Receptor Binding in Type 2 Diabetes: A Positron Emission Tomography Study. Brain Res. 2002b;927(1):97–103. doi: 10.1016/s0006-8993(01)03297-8. [DOI] [PubMed] [Google Scholar]
- Price LH, Charney DS, Delgado PL, et al. Effects of desipramine and fluvoxamine treatment on the prolactin response to tryptophan. Arch Gen Psychiatry. 1989;46:625–31. doi: 10.1001/archpsyc.1989.01810070051009. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, et al. Brain serotonin 1A receptor binding measured by positron emission tomography with [11C]WAY-100635: Effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–80. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Spurlock G, Buckland P, O’Donovan M, et al. Lack of effect of antidepressant drugs on the levels of mRNAs encoding serotonergic receptors, synthetic enzymes and 5HT transporter. Neuropharmacology. 1994;33:433–40. doi: 10.1016/0028-3908(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, et al. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-Postmortem evidence for decreased serotonin activity. J Neuroscience. 1998;18(18):7394–401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welner SA, DeMontigny C, Desroches J, et al. Autoradiographic quantification of serotonin1A receptors in rat brain following antidepressant drug treatment. Synapse. 1989;4:347–52. doi: 10.1002/syn.890040410. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–46. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Zimmer L, Riad M, Rbah L, et al. Toward brain imaging of serotonin 5-HT1A autoreceptor internalization. NeuroImage. 2004;22:1421–6. doi: 10.1016/j.neuroimage.2004.03.020. [DOI] [PubMed] [Google Scholar]


