Abstract
Introduction
Endovascular aortic repair has revolutionized the management of traumatic blunt aortic injury (BAI). However, debate continues about the extent of injury requiring endovascular repair, particularly with regard to minimal aortic injury (MAI). Therefore, we conducted a retrospective observational analysis of our experience with these patients.
Methods
We retrospectively reviewed all BAI presenting to an academic Level I trauma center over a ten-year period (2000–2010). Images were reviewed by a radiologist and graded according to Society for Vascular Surgery (SVS) guidelines (Grade I–IV). Demographics, injury severity, and outcomes were recorded.
Results
We identified 204 patients with BAI of the thoracic or abdominal aorta. Of these, 155 were deemed operative injuries at presentation, had grade III-IV injuries, or aortic dissection and were excluded from this analysis. The remaining 49 patients had 50 grade I–II injuries. We managed 46 grade I injuries (intimal tear or flap, 95%), and 4 grade II injuries (intramural hematoma, 5%) nonopertively. Of these, 41 patients had follow-up imaging at a mean of 86 days post-injury and constitute our study cohort. Mean age was 41 years and mean length of stay was 14 days. The majority (48 of 50, 96%) were thoracic aortic injuries and the remaining 2 (4%) were abdominal. On follow-up imaging, 23 of 43 (55%) had complete resolution of injury, 17 (40%) had no change in aortic injury, and 2 (5%) had progression of injury. Of the 2 patients with progression, one progressed from grade I to grade II and the other progressed from grade I to grade III (pseudoaneurysm). Mean time to progression was 16 days. Neither of the patients with injury progression required operative intervention or died during follow-up.
Conclusions
Injury progression in grade I–II BAI is rare (∼5%) and did not cause death in our study cohort. Since progression to grade III injury is possible, follow-up with repeat aortic imaging is reasonable.
INTRODUCTION
Blunt traumatic aortic injury (BAI) is associated with significant mortality. It was historically estimated that over 75% of patients experienced pre-hospital mortality, and of those arriving to the hospital alive, up to 50% died within the first 24 hours following injury.1, 2 Contemporary data drawn from a recent analysis of the National Trauma Databank suggest that approximately 4% of patients die during transport to the hospital and that 20% of these patients die early in their hospital course.3 Patients present with a wide range of concomitant injuries that pose significant challenges for management of BAI: 29% of patients present with major abdominal injury and 31% present with major head injury.3
BAI presents as a wide range of pathology, from small intimal defects to full-thickness aortic transections with rupture. The currently accepted grading system for these injuries was proposed in 20094 and has been adopted by the Society for Vascular Surgery (SVS) in the clinical practice guidelines for management of thoracic BAI.5 In this grading system, injuries are assigned one of four categories: grade I (intimal tear), grade II (intramural hematoma), grade III (pseudoaneurysm), and grade IV (rupture). Current guidelines from the SVS recommend endovascular repair of grade II-IV injuries of the thoracic aorta.5 Current clinical practice guidelines do not include recommendations for the management of abdominal aortic injuries, which represent a minority of BAI.
A recent trend in the management of BAI has been evolution towards nonoperative management of “minimal aortic injury” (MAI).6–8 This category includes grade I injuries. However, the natural history of these injuries remains poorly defined and the risk of injury progression to dissection, pseudoaneurysm, aneurysm, and rupture remains poorly quantified in patients managed nonoperatively. Grade II injuries (intramural hematoma), while still “minimal” in nature, are often managed more aggressively, reflected by current practice guidelines which recommend endovascular repair of these injuries5 despite a lack of supportive data. The experience with nonoperative management of grade I–II injuries is limited to small retrospective case series with short follow-up.4, 6–15
Our institution practices nonoperative management of grade I–II BAI. It is our practice to manage these injuries medically with surveillance imaging to evaluate for progression. Therefore, we analyzed our experience with nonoperative management of grade I–II BAI.
METHODS
Following approval from the Institutional Review Board of Vanderbilt University Medical Center, we performed a retrospective review of BAI presenting to our institution from January 1, 2000 through September 1, 2010. We retrospectively searched institutional radiology and trauma databases (which are prospectively maintained) to identify all patients presenting with BAI using the ICD-9-CM codes 901.0 (injury to thoracic aorta) and 902.0 (injury to abdominal aorta). We identified 205 aortic injuries in 204 patients. Of these, 111 were managed operatively and were excluded: 5 patients with grade I injuries and one patient with a grade II injury were selected for open or endovascular repair based on surgeon preference between 2000 and 2005. Based on our institutional algorithm established during the middle of the study period in which we manage grade I–II BAI nonoperatively, no patients with grade I or II BAI were selected for operative repair from 2005 to 2010. An additional 105 patients underwent open or endovascular repair for grade III-IV injuries and were excluded. The remaining 93 patients were managed nonoperatively. Of these, 40 patients had grade III-IV aortic injuries and were excluded. In addition, 4 patients presented with traumatic aortic dissection and were excluded. The remaining 49 patients had 50 grade I (n=46) or II (n=4) BAI; one patient presented with two grade I injuries. These 49 patients constitute our study cohort (Figure 1).
Figure 1.
Diagram summarizing selection of patients for inclusion in this analysis.
Demographics, injury severity score (ISS), hemodynamic parameters, and clinical outcomes were captured. Demographics included age, gender, length of stay, and concomitant injuries. Injury severity score (ISS) was calculated according to a well-described grading system.16 We recorded institution of pharmacologic agents including beta-blockers, calcium channel blockers, and vasodilators. Daily values for systolic blood pressure (SBP) and heart rate (HR) were recorded during hospitalization and clinical follow-up. A daily hemodynamic value for SBP and HR during inpatient hospitalization was captured from daily reports generated by our computer system on inpatients with daily mean SBP and HR averaged over a 24-hour period. Follow-up SBP and HR measurements recorded during follow-up clinic visits were additionally captured for this analysis and all values were averaged. Complications of BAI were captured from the medical record; thromboembolic events and complications related to end-organ or limb ischemia were noted when present.
All contrasted computed tomography (CT) scans of the chest, abdomen, and pelvis were retrospectively reviewed by a radiologist and BAI grade was assigned according to SVS guidelines.4, 5 According to this system, BAI is graded as follows (Table I): grade I (intimal tear); grade II (intramural hematoma); grade III (pseudoaneurysm); and grade IV (rupture). In addition, the anatomic location of the injury was defined as follows: ascending aorta (proximal to innominate artery); aortic arch (innominate artery to left subclavian artery); isthmus (initiating <1 cm of the ligamentum arteriosum); descending thoracic aorta (initiating >1 cm from the ligamentum arteriosum to the diaphragmatic hiatus); and abdominal aorta (diaphragmatic hiatus to aortic bifurcation). If the ligamentum arteriosum was not definitively identified on CT imaging, its position was inferred from common normal anatomy and the location of the ductus bump on oblique sagittal (candy cane) or sagittal multiplanar reformatted images. The 1-cm distance was a rough estimate from the expected location of the ductus. Helically acquired axial images were obtained on either a 16-slice CT scanner with 3.75 to 5 mm axial reconstructions (2000–2004) or 40 to 64-slice CT scanner (2004–2010) with 2 to 3 mm axial reconstructions. Multiplanar reformations were performed on all examinations to aid in BAI characterization. We reviewed all subsequent CT images for injury evolution (See Table I for definitions): in particular, the size of the injury was recorded (from superior to inferior extent in grade I–II injuries; and from superior to inferior extent as well as from anterior to posterior extent in grade III injuries), as well as associated thrombus, contrast extravasation, or mediastinal hematoma. Factors possibly associated with injury progression and resolution were assessed and included age, injury severity score, length of hospital stay, gender, antiplatelet therapy, average HR and SBP over follow-up, associated thrombus, presence of periaortic hematoma, and anatomic region of injury in aorta. Univariate correlations were performed to assess factors predictive of injury resolution or progression.
Table I.
Definitions used in this study regarding aortic injury grade and aortic injury evolution.
| Aortic injury grades |
| Grade I aortic injury – aortic intimal tear or flap |
| Grade II aortic injury – aortic intramural hematoma without change in external contour of aorta |
|
Grade III aortic injury – contained aortic pseudoaneurysm with concurrent increase in external contour of the aorta but without extravasation of intravenous contrast |
|
Grade IV aortic injury – full-thickness aortic injury resulting in rupture with extravasation of intravenous contrast on imaging |
| Aortic injury evolution |
|
Injury resolution – interval injury resolution with aorta of normal diameter; absence of external contour abnormality or intraluminal filling defect; no identifiable aortic injury |
| Stable injury – no interval change in aortic injury |
|
Injury progression – interval enlargement of injury, either by increase in injury grade, or by increase in size of injury with no change of injury grade |
Mortality was determined by reviewing the institutional medical records of patients who died during their initial hospitalization or follow-up. For the remaining patients, a search of the social security death index was conducted to rule out death in the remaining patients. For patients who died in our institution, cause of death was determined by reviewing the medical record and death summary.
Primary outcomes included BAI progression and all-cause mortality. Numeric data are summarized as mean, median, range, and interquartile range where appropriate. Statistical univariate analysis was performed using a bivariate Pearson’s Correlation Coefficient (SPSS Statistics, IBM Corp., Armonk, NY) to assess factors correlated with injury resolution and progression. We compared mean HR and SBP of patients with injury resolution and those with stable injuries using nonparametric student’s t-test; patients with injury progression could not be compared in this analysis due to small sample size (n=2). P values <0.05 were considered statistically significant.
RESULTS
Patients
Clinical demographics of our study cohort were as follows (Table II): mean age was 41 years (range, 16–87), 66% were male, mean length of hospital stay was 14 days (range, 1– 46), and mean ISS was 33.3. The distribution of injuries by location was as follows (Table III): ascending aorta (n=1, 2%); aortic arch (n=3, 6%); aortic isthmus (n=19, 36%); descending thoracic aorta (n=27, 49%), and abdominal aorta (n=4, 8%). Medical management included pharmacologic control of HR (goal <70 beats/min) and SBP (goal <110–120 mmHg) using intravenous infusion of beta-blocking agents and/or vasodilators with eventual transition to oral agents to achieve target heart rate and blood pressure control in 30 patients; the remaining 19 patients were deemed unsuitable for hemodynamic control due to comorbid conditions or traumatic injuries precluding hemodynamic restriction such as traumatic brain injury or spinal cord injury, or did not complete pharmacologic therapy. Patients initiated on intravenous infusions of antihypertensive agents were transitioned to orally administered beta-blocking agents when deemed medically appropriate. In patients with injury resolution, antihypertensives were discontinued. In patients with stable injuries or injury progression, orally administered beta-blockers were continued as outpatients. Antiplatelet therapy was recommended for injuries associated with thrombus or at the discretion of the consultant; 10 of 49 patients were administered antiplatelet therapy. No patients suffered complications related to end-organ or limb ischemia or thromboembolic complications.
Table II.
Clinical demographics of patients in this study.
| Characteristic | Average value (range) |
|---|---|
| Age, years | 41 (16–87) |
| Male, percent | 66% |
| Length of hospital stay, days | 14 (1–46) |
| Injury severity score | 33.3 (16–50) |
Table III.
Distribution of aortic injuries by location in aorta.
| Location | Number (Percent) |
|---|---|
| Ascending aorta | 1 (2%) |
| Aortic arch | 3 (6%) |
| Aortic isthmus | 19 (38%) |
| Descending thoracic aorta | 25 (50%) |
| Abdominal aorta | 2 (4%) |
Mortality
All-cause mortality was 14%. Of 49 patients, there were 7 deaths during a mean follow-up of 4.9 years (median, 5.3 years; interquartile range, 3.9 years). Mortality occurred at a median of 6 days (range, 1–1043), and 6 of 7 deaths occurred in-hospital within 13 days. Patients who died were significantly older compared with patients who survived (51.6 vs. 39 years, p=0.05), and there was a trend toward higher ISS among patients who died (34.6 vs. 33, p=0.06). No deaths occurred secondary to BAI: deaths resulted from multiple system organ failure (MSOF, n=4); traumatic brain injury (TBI, n=2); and one patient had an unknown cause of death 2.9 years following injury. However, repeat CTA demonstrated complete BAI resolution in this patient (Table IV).
Table IV.
Characteristics of seven patients with Grade I–II aortic injuries managed nonoperatively who died during follow-up.
| Patient | Age (years) |
Injury severity score (ISS) |
Aortic Injury Grade at Presentation |
Aortic Injury Grade on Follow-up Imaging |
Last Follow-up Imaging, Days Post- Presentation |
Cause of death | Death, days post- injury |
|---|---|---|---|---|---|---|---|
| 1 | 19 | 29 | I | Resolution (No sign of injury) | 1 | TBI | 2 |
| 2 | 45 | 29 | I | Resolution (No sign of injury) | 131 | Unknown | 1043 |
| 3 | 46 | 32 | I | I (No change in injury) | 3 | MSOF | 4 |
| 4 | 31 | 36 | I | I (No change in injury) | 2 | MSOF | 13 |
| 5 | 87 | 50 | I | I (No change in injury) | 8 | MSOF | 9 |
| 6 | 50 | 45 | I | Not performed | -- | TBI | 1 |
| 7 | 81 | 21 | II | Not performed | -- | MSOF | 6 |
Injury Evolution on Follow-up Imaging
An average of 2.7 imaging studies were performed to evaluate BAI (range, 1–9). Following the admission CT, follow-up imaging was performed in 41 of 49 patients (84%) to evaluate BAI evolution. Patients who received follow-up imaging had an average of 2 additional imaging studies to evaluate BAI progression. The remaining 8 patients did not have follow-up imaging performed, either due to death from other traumatic injuries (n=2) or because of loss to follow-up (n=6). The mean interval from admission imaging to last imaging study was 86 days (range, 1–826), and the median was 29 days (interquartile range, 108 days).
Injury Resolution on Follow-up Imaging
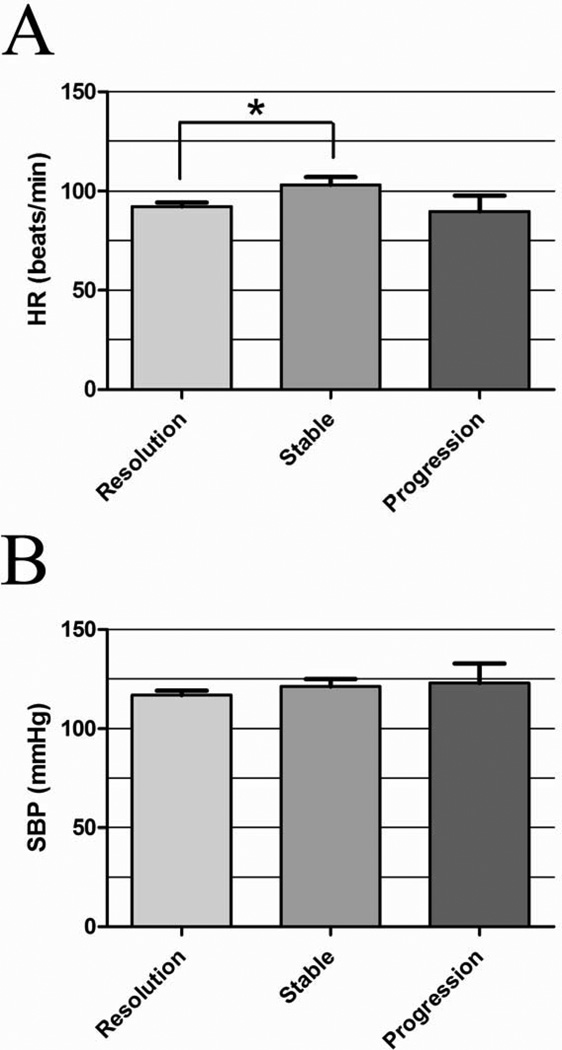
Twenty-three of 42 injuries (55%) demonstrated BAI resolution (Figure 2) with normal aortic contour and diameter and absence of intraluminal filling defect. The mean time to radiographic identification of injury resolution was 74 days (median, 24 days; interquartile range, 85.5 days). On univariate analysis, higher mean HR over follow-up was inversely correlated with injury resolution, with lower HR correlating with injury resolution by univariate analysis (p=0.018). The mean HR of patients with injury resolution was also significantly lower than the mean HR of patients with stable injuries (Figure 3A, p=0.014). The mean duration to last follow-up imaging among patients with injury resolution was 118.5 days (median, 54 days; interquartile range, 139.5 days).
Figure 2.
Evolution of injury following grade I–II BAI. Between the time of injury and last follow-up imaging, of 55% of patients demonstrated complete injury resolution, 40% had stable injuries, and 5% of patients developed injury progression to higher injury grade.
Figure 3.
Mean HR (A) and SBP (B) between the time of injury and last follow-up imaging in patients with injury resolution, stable injuries, and injury progression after grade I–II BAI. The mean HR is significantly lower in patients with injury resolution compared with stable injuries (*p=0.01).
Stable Injuries on Follow-up Imaging
Seventeen of 42 injuries (40%) were stable without radiographic change on follow-up imaging (Figure 2). The mean duration to last follow-up imaging was 43.3 days in this group (median, 5.5 days; interquartile range, 13.5 days).
Injury Progression on Follow-up Imaging
Two of 42 injuries (5%) progressed to higher grade (Figure 2). The mean time to radiographic identification of injury progression was 16 days. The first of these injuries was in a male aged 25 years with an ISS of 29 whose BAI progressed from grade I to II (intramural hematoma measuring 1.1×2.0 cm) at 3 days and was stable on last follow-up imaging at 81 days; this patient is currently alive 10.8 years later. The second of these injuries was in a female aged 21 years with an ISS of 24 whose BAI progressed from grade I to III (pseudoaneurysm measuring 0.5×1.3 cm) at 29 days; this patient was managed nonoperatively and was subsequently lost to follow-up but is currently alive 5.5 years later. Both of these injuries were located at the aortic isthmus. In both cases, intravenous infusion of beta blocking agents was initiated at admission and had been transitioned to oral beta blockers prior to recognition of injury progression. Both patients have been nonoperatively managed with pharmacologic HR and SBP reduction and serial imaging and have been documented to have stable injuries. The last follow-up imaging was performed 81 and 29 days following injury, respectively. Both patients are alive at 10.8 and 5.5 years of follow-up, respectively.
On univariate analysis, there were no statistically significant correlates of injury progression, but the sample size was extremely limited (2 patients), precluding a powerful analysis. Patients with injury progression had similar mean HR (Figure 3A) and SBP (Figure 3B) equal to or lower than patients with stable BAI or BAI resolution.
DISCUSSION
Herein we present our 10-year experience with nonoperative management of grade I–II BAI. While limited to only 49 patients, our study represents the largest experience with nonoperative management of grade I–II BAI to our knowledge in the literature. Several small, single-institution, retrospective reviews have analyzed outcomes with nonoperative management of Grade I BAI.4,6–15 However, the majority of these studies have been limited by small sample size and short follow-up (Table V). The use of diverse imaging modalities for BAI diagnosis and follow-up (CTA, transesophageal echocardiogram (TEE), aortography, intravascular ultrasound (IVUS)) and the use of diverse grading systems for BAI classification preclude any unified conclusions to be made.
Table V.
Summary of retrospective studies examining the natural history of BAI.
| Study | Patients | Injury Grade |
Injury Location |
Imaging Modality at Diagnosis and Followup |
Follow-up imaging |
Clinical Follow up |
Aortic Injury Evolution Among Survivors |
Aortic-Related Mortality |
All-Cause Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Fabian et al., 199812 |
n=6 | I (intimal flap with <10%lumen compromise) |
Not specified | CTA, aortography |
Not specified | Not specified | Resolution in 5/6 (83%) Stable in 1/6 (17%) |
0/6 (0%) | Not specified |
| Malhotra et al., 20017 |
n=8 | I (intimal tear <1 cm) |
Thoracic aorta | CTA, aortography, IVUS |
<8 weeks | Not specified | Resolution in 2/6 (33%) Stable in 1/6 (17%) Progression to pseudoaneurysm in 3/6 (50%) |
0/8 (0%) | 2/8 (25%) -MSOF (n=1) -PE (n=1) |
| Kepros et al., 200210 |
n=5 | I (intimal tears 5–20 mm) |
Thoracic aorta | TEE | 9.4 days (mean) | 16.8 months (mean) |
Resolution in 5/5 (100%) | 0/5 (0%) | 0/5 (0%) |
| Holmes et al., 200215 |
n=6 | I (intimal tear) | Thoracic aorta | CTA, aortography |
Not specified | 2.5 years (median) | Resolution in 1/4 (25%) Stable in 3/4 (75%) |
0/5 (0%) | 1/5 (20%) -TBI |
| n=2 | II (intramural hematoma) |
Thoracic aorta | CTA, aortography |
Not specified | 2.5 years (median) | Resolution in 2/2 (50%) | 0/2 (0%) | 0/2 (0%) | |
| Hirose et al., 200611 |
n=3 | I (intimal tear) | Thoracic aorta | CTA, aortography |
60 days (median) |
4.4 years (mean) | Resolution in 3/3 (50%) | 0/3 (0%) | 0/3 (0%) |
| Azizzadeh et al., 20094 |
n=10 | I (intimal tear) | Thoracic aorta | CTA, IVUS | None | Not specified | No follow-up imaging | 0/10 (0%) | 0/10 (0%) |
| Caffarelli et al, 201013 |
n=6 | I (intraluminal thrombus/intim al injury) |
Thoracic aorta | CTA | 81 days (mean) for entire cohort |
1.8 years (median) for entire cohort |
Resolution in 4/6 (66%) Stable in 2/6 (33%) |
Not specified |
Not specified |
| n=2 | II (intramural hematoma) |
Thoracic aorta | CTA | 81 days (mean) for entire cohort |
1.8 years (median) for entire cohort |
Resolution in 1/2 (50%) Stable in 1/2 (50%) |
Not specified |
Not specified | |
| Paul et al., 20116 |
n=11 | I (intimal tear <1 cm) |
Thoracic aorta | CTA | 4 days (median) | 16 days (mean) | Not specified | 0/11 (0%) | 0/11 (0%) |
| Mosquera et al., 20128 |
n=9 | I (intimal tear <1 cm) |
Thoracic and abdominal aorta |
CTA, TEE, aortography | Not specified | 27 months (median) | Resolution in 6/7 (86%) Progression to pseudoaneurysm in 1/7 (14%) |
0/9 (0%) | 2/9 (22%) -MSOF (n=1) -TBI (n=1) |
| Starnes et al., 20129 |
n=20 | I (intimal tear <1 cm) |
Thoracic and abdominal aorta |
CTA, TEE, aortography |
71 days (mean) | 71 days | Resolution in 14/16 (87.5%) Stable in 2/16 (12.5%) |
0/20 (0%) | 3/20 (15%) -MSOF (n=3) |
| n=2 | I (intimal flap >1 cm) |
Thoracic and abdominal aorta |
CTA, TEE, aortography |
7 days (mean) | 7 days | Stable in 2/2 (100%) | 0/2 (0%) | 0/2 (0%) | |
| Shalhub et al., 201214 |
n=6 | I (intimal tear <1 cm) |
Abdominal aorta | CTA | <72 hours | 6 days (median) | Resolution in 5/6 (83%) Stable in 1/6 (17%) |
0/6 (0%) | 0/6 (0%) |
| n=3 | I (intimal flap >1 cm) |
Abdominal aorta | CTA | <72 hours | Not specified | Resolution in 1/3 (33%) Stable in 2/3 (66%) |
0/3 (0%) | 0/3 (0%) |
Over the past decade, there has been a transition in the management of aortic trauma from open surgical repair to endovascular repair. The rapid evolution of endovascular technology has enabled operative repair of BAI in a broader patient population with significant reduction in morbidity and mortality compared with open surgical technique.17–20 However, endovascular repair is not without risks as this procedure is associated with small but measurable rates of stroke (as high as 2%), spinal cord ischemia (as high as 0.9%), procedure-related mortality (as high as 3.6%) and overall mortality (as high as 16%) reported in the trials and meta-analyses investigating thoracic endovascular aneurysm repair (TEVAR).18–21 Additionally, endovascular procedures are associated with higher rates of device-related complications, they are costly, and require extended follow-up imaging. Moreover, the longevity and durability of this technology over the lifespan of young patients remains to be elucidated. These factors pose challenges in an underinsured patient population with a high prevalence of noncompliance.19
The increased utilization of CT imaging at the time of admission of trauma patients has increased the frequency of diagnosis of grade I and grade II BAI,7, 9 and there remains substantial institution-to-institution variation in management of these injuries. Many institutions practice an approach of endovascular repair for all BAI and many of the trials and meta-analyses comparing outcomes in open repair versus TEVAR included patients with grade I BAI in both operative arms.18, 20, 21 Many surgeons decide to repair MAI because of concern over eventual injury progression with potential for aneurysm development or rupture in a noncompliant patient population with a high risk of loss to follow-up. Other surgeons have chosen to manage these patients nonoperatively despite a lack of supportive data.
Our results indicate that there is a very low risk of injury progression in patients with grade I–II BAI (Figure 2). Among the 84% of patients with follow-up imaging, we observed injury progression in 2 of 42 injuries (5%), although this was assessed over a relatively short mean follow-up of 86 days (median follow-up, 29 days). When injury progression was noted, it occurred relatively early at an average of 16 days. This is consistent with the few existing reports of BAI progression which have noted the majority of these events to occur early (Table V). Malhotra et al. reported three cases of injury progression from grade I to pseudoaneurysm, all of which occurred within 8 weeks following injury;7 Mosquera et al. similarly noted one case of grade I injury progressing to pseudoaneurysm diagnosed one year following injury.8 It is worth mentioning that 16% of our patients were lost to follow-up and did not return for surveillance imaging. While we can only speculate about the eventual fate of injury in these patients, they did not develop injury progression to the point of aortic rupture, as all are still alive at the time of this study (mean follow-up, 4.9 years). However, if all 6 of these injuries progressed, the progression rate in our cohort would have increased from 5% to 16%. It remains to be determined whether longer follow-up with repeat imaging would identify additional patients with injury resolution or progression.
The vast majority (95%) of Grade I–II BAI resolve or remain stable (Figure 2). Seventeen injuries (40%) remained stable, and 23 injuries resolved (55%). Overall, 52.5% of patients with grade I injuries resolved and 100% of patients (2/2 patients) with grade II injuries resolved. Univariate analysis identified a statistically significant inverse correlation between HR and injury resolution (Figure 3A); the same trend was not, however, observed with SBP (Figure 3B). Pharmacologic therapy for HR and SBP reduction remains a cornerstone in the medical management of BAI, although these modalities have never been proven to successfully delay BAI progression. However, there is good evidence that pharmacologic HR and SBP reduction delays aneurysmal aortic degeneration in other aortic pathologies, including type B aortic dissection22 and Marfan Syndrome.23 Based on our results, this type of pharmacologic therapy may promote injury resolution. However, it is not clear yet whether withholding pharmacologic BP and HR control promotes injury progression. We noted patients with injury progression to have similar hemodynamics (Figure 3A-3B) to patients with injury resolution, however the sample size in our progression group (n=2) precluded a powerful statistical analysis. Clearly, further work is needed to validate the role of pharmacologic therapy in patients with BAI. In our opinion, it is advisable to continue this practice unless contraindicated (i.e. traumatic brain injury, spinal cord injury, shock).
We did not observe any deaths secondary to grade I–II BAI over an average follow-up was 4.9 years (median, 5.3 years). The predominant causes of death were other traumatic injuries, as in prior reports of grade I–II BAI.4, 6–15 The majority of deaths occurred within two weeks (Table IV). The only death occurring from a non-traumatic etiology was an unknown cause of death occurring four years following injury. This patient presented with grade I BAI and had a follow-up CTA demonstrating BAI resolution; therefore, we do not suspect this death was related to BAI. It has been our practice to discontinue aortic surveillance and pharmacologic SBP and HR control after documenting normal imaging, and it will remain so.
The alternative to nonoperative management of grade I–II BAI is to perform immediate or delayed endovascular repair. Proponents of operative intervention do so out of concern for eventual injury progression in a patient population prone to limited follow-up. However, endovascular repair poses measurable risks of procedure-related morbidity and mortality, requires extended imaging, and incurs significant costs. The risks posed by operative management (paraplegia, CVA, endoleak, endograft migration, death) must be weighed against the risks posed by nonoperative management (i.e. unrecognized injury progression, aneurysmal degeneration, death). Either approach requires follow-up imaging, albeit to varying extents. Comparing the endpoint of mortality between these two approaches, the nonoperative approach poses a BAI-related mortality of 0% at 4.9 years of clinical follow-up, versus a TEVAR-related mortality as high as 3.6% in contemporary studies. The overall mortality in our cohort was 14%, which is slightly better than the overall mortality of 16% described in a large meta-analysis of TEVAR.18 Therefore, our experience with nonoperative management of grade I–II BAI does not convey greater risk for mortality, spares patients the costs and risks associated with the procedure, and may well shorten the duration of follow-up imaging required.
BAI of the ascending aorta, aortic arch, and abdominal aorta have received relatively little attention. Based on our results, it appears that these injuries behave similar to aortic injuries in other locations, albeit our sample size was very small. The largest series of nonoperatively managed grade I–II abdominal BAI reported by Shalhub et al. (Table V),14 reported similar findings. Combining these authors’ experience with abdominal BAI and ours, no patients were noted to have injury progression; all patients developed injury regression or have stable injuries on repeat imaging. Therefore, abdominal BAI appears to behave similarly to thoracic BAI.
This study has several limitations. The retrospective nature of data collection limits the quality of the data and the consistency of follow-up. The variable selection of patients with grade I–II injuries for operative versus nonoperative management prior to the year 2005 introduced selection bias, although it was uniform practice at our institution to manage these injuries nonoperatively for the latter half of the period we investigated (2005–2010). Also, a significant proportion of our cohort was lost to follow-up (16%) and did not have repeat imaging. Among those who did have repeat imaging, there was significant variability in duration of clinical and radiographic follow-up; this variability was introduced by practitioner-to-practitioner differences in clinical management, not to mention the challenges inherent in follow-up among the trauma population. Finally, assessment of injury progression by repeat imaging was limited by relatively short duration of follow-up (average, 86 days; and median, 29 days).
CONCLUSIONS
Our 10-year experience with management of grade I–II BAI is the largest experience reported in the literature. The majority of these injuries healed or remained unchanged on repeat imaging and nonoperative management did not result in aortic-related death or require intervention. Based on these results, it is apparent that nonoperative management of Grade I–II BAI is a management strategy that does not pose increased mortality, although this approach warrants prospective validation. Nonoperative management spares patients the costs and device-related complications incurred by TEVAR, not to mention the unproven durability of TEVAR over the lifespan of young patients. It is reasonable to obtain follow-up imaging within one month after injury, with interval surveillance thereafter. Follow-up imaging is likely unnecessary in patients whose injuries resolve.
ACKNOWLEDGEMENTS
Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Vanderbilt University Medical Center.24 REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
FUNDING SOURCES
This work was supported by NIH NRSA F32HL104965 (MJO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
T.N.: Consultant, WLGore, Clinical Events Committee; Consultant, CvRX, Data Monitoring Committee.
REFERENCES
- 1.Fabian TC, Richardson JD, Croce MA, Smith JS, Jr, Rodman G, Jr, Kearney PA, et al. Prospective study of blunt aortic injury: Multicenter Trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42(3):374–380. doi: 10.1097/00005373-199703000-00003. discussion 80-3. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson WR, Janusz MT, Gudas VM, Burr LH, Fradet GJ, Henderson C. Traumatic rupture of the thoracic aorta: third decade of experience. Am J Surg. 2002;183(5):571–575. doi: 10.1016/s0002-9610(02)00851-6. [DOI] [PubMed] [Google Scholar]
- 3.Arthurs ZM, Starnes BW, Sohn VY, Singh N, Martin MJ. Andersen CA. Functional and survival outcomes in traumatic blunt thoracic aortic injuries: An analysis of the National Trauma Databank. J Vasc Surg. 2009;49(4):988–994. doi: 10.1016/j.jvs.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 4.Azizzadeh A, Keyhani K, Miller CC, 3rd, Coogan SM, Safi HJ, Estrera AL. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg. 2009;49(6):1403–1408. doi: 10.1016/j.jvs.2009.02.234. [DOI] [PubMed] [Google Scholar]
- 5.Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53(1):187–192. doi: 10.1016/j.jvs.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Paul JS, Neideen T, Tutton S, Milia D, Tolat P, Foley D, et al. Minimal aortic injury after blunt trauma: selective nonoperative management is safe. J Trauma. 2011;71(6):1519–1523. doi: 10.1097/TA.0b013e31823b9811. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra AK, Fabian TC, Croce MA, Weiman DS, Gavant ML, Pate JW. Minimal aortic injury: a lesion associated with advancing diagnostic techniques. J Trauma. 2001;51(6):1042–1048. doi: 10.1097/00005373-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Mosquera VX, Marini M, Gulias D, Cao I, Muniz J, Herrera-Norena JM, et al. Minimal traumatic aortic injuries: meaning and natural history. Interact Cardiovasc Thorac Surg. 2012;14(6):773–778. doi: 10.1093/icvts/ivs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starnes BW, Lundgren RS, Gunn M, Quade S, Hatsukami TS, Tran NT, et al. A new classification scheme for treating blunt aortic injury. J Vasc Surg. 2012;55(1):47–54. doi: 10.1016/j.jvs.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 10.Kepros J, Angood P, Jaffe CC, Rabinovici R. Aortic intimal injuries from blunt trauma: resolution profile in nonoperative management. J Trauma. 2002;52(3):475–478. doi: 10.1097/00005373-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Hirose H, Gill IS, Malangoni MA. Nonoperative management of traumatic aortic injury. J Trauma. 2006;60(3):597–601. doi: 10.1097/01.ta.0000205044.99771.44. [DOI] [PubMed] [Google Scholar]
- 12.Fabian TC, Davis KA, Gavant ML, Croce MA, Melton SM, Patton JH, Jr, et al. Prospective study of blunt aortic injury: helical CT is diagnostic and antihypertensive therapy reduces rupture. Ann Surg. 1998;227(5):666–676. doi: 10.1097/00000658-199805000-00007. discussion 76-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caffarelli AD, Mallidi HR, Maggio PM, Spain DA, Miller DC, Mitchell RS. Early outcomes of deliberate nonoperative management for blunt thoracic aortic injury in trauma. J Thorac Cardiovasc Surg. 2010;140(3):598–605. doi: 10.1016/j.jtcvs.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 14.Shalhub S, Starnes BW, Tran NT, Hatsukami TS, Lundgren RS, Davis CW, et al. Blunt abdominal aortic injury. J Vasc Surg. 2012;55(5):1277–1285. doi: 10.1016/j.jvs.2011.10.132. [DOI] [PubMed] [Google Scholar]
- 15.Holmes JHt, Bloch RD, Hall RA, Carter YM, Karmy-Jones RC. Natural history of traumatic rupture of the thoracic aorta managed nonoperatively: a longitudinal analysis. Ann Thorac Surg. 2002;73(4):1149–1154. doi: 10.1016/s0003-4975(01)03585-8. [DOI] [PubMed] [Google Scholar]
- 16.Baker SP, O'Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 17.Hong MS, Feezor RJ, Lee WA, Nelson PR. The advent of thoracic endovascular aortic repair is associated with broadened treatment eligibility and decreased overall mortality in traumatic thoracic aortic injury. J Vasc Surg. 2011;53(1):36–42. doi: 10.1016/j.jvs.2010.08.009. discussion 3. [DOI] [PubMed] [Google Scholar]
- 18.Xenos ES, Abedi NN, Davenport DL, Minion DJ, Hamdallah O, Sorial EE, et al. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. 2008;48(5):1343–1351. doi: 10.1016/j.jvs.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 19.Azizzadeh A, Charlton-Ouw KM, Chen Z, Rahbar MH, Estrera AL, Amer H, et al. An outcome analysis of endovascular versus open repair of blunt traumatic aortic injuries. J Vasc Surg. 2013;57(1):108–114. doi: 10.1016/j.jvs.2012.05.110. discussion 15. [DOI] [PubMed] [Google Scholar]
- 20.Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, et al. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries: results of an American Association for the Surgery of Trauma Multicenter Study. J Trauma. 2008;64(3):561–570. doi: 10.1097/TA.0b013e3181641bb3. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- 21.Tang GL, Tehrani HY, Usman A, Katariya K, Otero C, Perez E, et al. Reduced mortality, paraplegia, and stroke with stent graft repair of blunt aortic transections: a modern meta-analysis. J Vasc Surg. 2008;47(3):671–675. doi: 10.1016/j.jvs.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Onitsuka S, Akashi H, Tayama K, Okazaki T, Ishihara K, Hiromatsu S, et al. Long-term outcome and prognostic predictors of medically treated acute type B aortic dissections. Ann Thorac Surg. 2004;78(4):1268–1273. doi: 10.1016/j.athoracsur.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 23.Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan's syndrome. N Engl J Med. 1994;330(19):1335–1341. doi: 10.1056/NEJM199405123301902. [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]