Abstract
Purpose
RB94, a truncated form of RB110, has enhanced tumor suppressor potency and activity against all tumor types tested to date, including bladder carcinoma. However, efficient, systemic delivery of the gene encoding RB94 specifically to tumors is an obstacle to clinical application as an anti-cancer therapeutic. We have developed a systemically administered, nanosized liposome DNA delivery system that specifically targets primary and metastatic disease. The ability of RB94, delivered via this nanocomplex, to sensitize bladder carcinoma to chemotherapy in vitro and in vivo was assessed.
Experimental Design
The nanocomplex is an RB94 plasmid encapsulated by a cationic liposome (Lip), the surface of which is decorated with a tumor-targeting moiety, either transferrin (Tf) (Tf/Lip/RB94), or an anti-transferrin receptor single-chain antibody fragment (TfRScFv) (TfRScFv/Lip/RB94). The ability of the complex to sensitize human bladder carcinoma HTB-9 cells to chemotherapeutics was assessed in vitro by XTT. In vivo tumor specificity, and efficacy were tested in mice carrying HTB-9 tumors by PCR, and tumor growth inhibition, respectively.
Results
Transfection with Tf/Lip/RB94 significantly sensitized HTB-9 cells to chemotherapeutic agents in vitro. Tumor specificity of the complex was demonstrated in an orthotopic bladder tumor model by immunohistochemistry and PCR. Moreover, in mice bearing subcutaneous HTB-9 tumors, the combination of systemically administered Tf/Lip/RB94 or TfRScFv/Lip/RB94 plus gemcitabine resulted in significant (p<0.0005) tumor growth inhibition/regression and induction of apoptosis.
Conclusions
Use of our tumor-targeting nanocomplex to specifically deliver the potent tumor suppressor RB94 efficiently to tumors has potential as a more effective treatment modality for genitourinary and other cancers.
Keywords: RB94, Nanocomplex, liposomal delivery, tumor-targeting, chemosensitization
INTRODUCTION
There is increasing emphasis on the development and use of non-viral delivery methods for cancer gene therapy, including cationic liposomes. Features of cationic liposomes that make them versatile and attractive for DNA delivery include: lack of immunogenicity or biohazardous activity (reviewed in 1-3). Moreover, cationic liposomes have been proven to be safe and efficient for in vivo gene delivery (reviewed in 4, 5). More than 102 clinical trials using cationic liposomes for gene delivery, 78 in the US alone, have been approved (6, 7). At least 6 liposome-based products are on the market (8).
The transfection efficiency of cationic liposomes can be dramatically increased when they bear a ligand recognized by a cell surface receptor, such as transferrin (Tf), which facilitates entry of DNA into cells through internalization of the complex via receptor-mediated endocytosis, a highly efficient internalization pathway (9, 10). TfR levels are elevated in various types of cancer, recycle during internalization in rapidly dividing cells (11-13), and correlate with the aggressive or proliferative ability of tumor cells, making TfR a potential target for anti-cancer drug deliver.
Studies employing Tf-cationic liposome complexes as tumor-targeting systemic delivery vehicles for wtp53 gene therapy of head and neck, prostate and breast cancer have been successfully undertaken in vitro and in vivo (14-16). Use of this complex resulted in a 70%-80% in vitro transfection efficiency in JSQ-3 cells (derived from a radiation resistant squamous cell carcinoma of the head and neck [SCCHN]), and was at least 2-3 fold more efficient than transfection with the same liposome lacking Tf (14, 15).
Using the β-galactosidase reporter gene, we demonstrated that expression of this systemically delivered ligand-liposome complex has a high degree of tumor selectivity. Strong β-gal staining was present in both the primary xenograft tumors, and metastases, with little staining evident in normal tissues or organs including liver, lung, bone marrow or gut (14-16). Whereas neither p53 gene therapy nor radiation alone was sufficient to eliminate tumors long term, replacement of the normal p53 gene via this systemically delivered complex rendered head and neck xenograft tumors significantly more sensitive to radiation and chemotherapy in vivo, resulting not only in growth inhibition, but in long term (18 month) tumor elimination (14). These data demonstrate a pronounced synergistic effect of the combination therapy, and provide proof-of-principle for the utility of this ligand-facilitated cationic liposome delivery system in cancer gene therapy. Combining gene therapy with more conventional cancer treatment may represent a significant improvement over traditional therapies alone.
In addition to the use of ligands such as Tf, specific antibodies can also be attached to the liposome surface enabling them to deliver therapeutic drugs to a specific target cell population (8, 17-19). Immunoliposomes are being employed for a variety of therapeutic uses including delivery of antisense molecules as anti-HIV agents (20), chemotherapeutics (21) and plasmid DNA (22). Thus, the combination of cationic liposome-gene transfer and immunoliposome techniques appears to be a promising system for targeted gene therapy.
While the majority of antibody targeted molecules in the clinic and in clinical trials contain intact Mab, including chimeric and humanized forms (8), progress in biotechnology has permitted the construction of specific recognition domains derived from MAb that have better pharmacokinetic profiles while simultaneously reducing the immunogenicity associated with whole antibodies. These include Fab’ and scFv fragments (23). The recombination of the variable regions of heavy and light chains and their integration into a single polypeptide provides the possibility of employing single-chain antibody derivatives (designated scFv) for targeting purposes. The binding site of an scFv can replicate both the affinity and specificity of its parent antibody (21).
A scFv based on the anti-TfR MAb 5E9 (24,25) contains the complete antibody binding site for the epitope of the TfR recognized by this MAb as a single polypeptide chain of ~ 28 kDa (TfRscFv). This TfRscFv is formed by connecting the component VH and VL variable domains from the heavy and light chains, respectively, with an appropriately designed linker peptide. The linker bridges the C-terminus of the first variable region and N-terminus of the second, ordered as either VH-linker-VL or VL-linker-VH. We have modified our Tf ligand targeting lipoplex, replacing Tf with this TfRscFv fragment. Previous studies using the TfRscFv targeting lipoplex showed that this nanosized immunoliposome was able to deliver the wtp53 gene specifically and efficiently to tumor cells in vitro and in vivo (26, 27) resulting in increased survival (26). We have also used this complex to deliver siRNA (28, 29) and imaging agents (30).
The tumor suppressor protein RB94 is produced by translation of the wild-type RB gene from the second in-frame AUG codon, and lacks the N-terminal 112 amino acids present in RB110 (31). RB94 has markedly increased tumor suppressor potency compared to RB110 and is active against all tumor types examined to date, despite their specific genetic defects, including both RB (+) and RB (−) tumors (31-33). Moreover, no resistance to RB94 has been found in any cancer cells or cancer cell types examined to date, based on the fact that a cancer cell has never been able to be isolated more than three weeks after transfection with, and expression of, RB94. In addition, no cytotoxicty to normal human cells has been associated with RB94 (31-33). Therefore its therapeutic index should be high.
In this paper we have focused on the delivery of the RB94 gene by our nanoliposome complexes (both Tf and TfRscFv targeted), and have examined the ability of these complexes to sensitize human bladder carcinoma cells to conventional chemotherapeutic agents in vitro and in vivo. We have undertaken our studies in bladder cancer since a Phase 1 clinical trial is planned using our targeted systemic delivery approach with the RB94 gene primarily in patients with metastatic RB negative bladder cancer.
MATERIALS AND METHODS
Cell Culture
RB negative human bladder carcinoma cell line, HTB-9 and normal human umbilical vein endothelial cell line CRL1730 (HUV-EC-L) were obtained from ATCC (Manassas, VA). HTB-9 was cultured in RPMI 1640 (Invitrogen Carlsbad, CA) supplemented with 2mmol/L L-glutamine, 50ug/ml each of penicillin, streptomycin and neomycin (PSN), plus 10% heat-inactivated Fetal Bovine Serum (FBS). CRL1730 was cultured in HAM's F12K medium (Invitrogen, Carlsbad, CA) with 0.1mg/ml Heparin, 0.03-0.05 mg/ml endothelial cell growth supplement, plus L-glutamine, PSN and FBS as above.
pSCMV-RB94 Clones
A 3.1 kb Bam HI-Hind III restriction digest fragment of the original RB94 clone (PEW 13) (31) was cloned into a high expression vector where the RB94 gene is under the control of a modified CMV promoter to yield pSCMV-RB94 (Fig. 1A), and propagated in bacterial host TOP 10 F1 (Invitrogen, Carlsbad, CA). Plasmid DNA was prepared using the Qiagen endofree Kit (Valencia, CA) with a resultant 260:280 ratio of > 1.9, >80% supercoiled molecules, and endotoxin levels of 3-4 Eu/mg DNA.
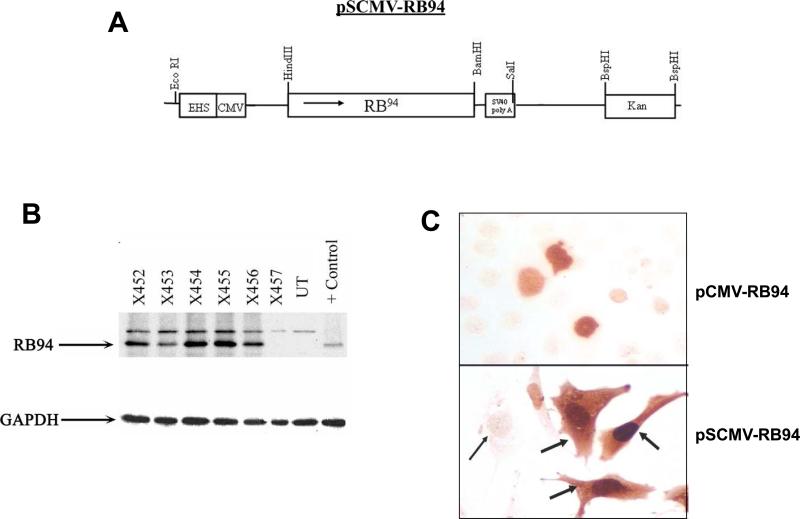
Figure 1. RB94 expression in HTB-9 cells.
Panel A: Diagram of pSCMV-RB94.
Panel B: Western blot analysis of RB94 negative HTB-9 cells following transfection with various Tf/Lip encapsulated pSCMV-RB94 clones (X452-X456) and the parental plasmid construct containing a different CMV promoter. (X457). UT = untreated HTB-9 cells; Control = purified protein to confirm position of RB94.
Panel C: RB94 staining in HTB-9 cells transfected with the Tf/Lip complex carrying either the original pCMV-RB94 (X457) or the pSCMV-RB94 (X455) construct 24 hr post- transfection. Top = transfection with pCMV-RB94; Bottom = Transfection with pSCMV. The magnification of both images is 400X. The broad arrows indicate strongly RB94 expressing cells; the thin arrow shows a non-transfected cell.
Complex Preparations and In Vitro Transfections
The Tf-liposome-DNA complexes were prepared and transfection performed as previously described (14). The complexes using TfRscFv as the targeting moiety were prepared and transfection performed as previously described (34). The sizes of the complexes were measured by dynamic light scattering using a Malvern Zetasizer 3000HS.
Western Blot and Immunochemical Analysis
For in vitro Western blot analysis, the cells were seeded in 6 well plates at 2 × 105 cells/well. After 24 hours, they were transfected as previously described (14, 34). The detached cells were collected by centrifugation of the culture medium at 200 x g and 4°C for 7 minutes. The adherent cells were mechanically detached by scraping, suspended in PBS and pelleted as above. The pellets were combined, washed once with PBS, and incubated at 4°C in 50-100ul of cold lysis (RIPA) buffer for 20 minutes. The resulting lysate was passed several times through a 21 gauge needle and protein concentration measured (Pierce Micro BCA protein assay reagent, Rockford, IL). The proteins were denatured, separated by discontinuous 10% polyacrylamide/SDS gel electrophoresis and transferred to nitrocellulose membrane. RB94 protein was identified using 1:10,000 dilution of RB monoclonal antibody (QED Bioscience Inc., San Diego CA), followed by a peroxidase-conjugated antimouse IgG antibody (Santa Cruz, Santa Cruz CA), ECL (Amersham, Pascataway NJ) detection and exposure to film.
For Western blot analysis of in vivo samples, the tumor, lungs and liver were excised from the mice, flash frozen in liquid N2, pulverized using a Bessman tissue pulverizer (Fisher Scientific, Waltham MA) and homogenized in RIPA buffer. The protein was isolated and RB94 expression analyzed as described above for cell culture. Paraffin-sections from the same tissues were examined for RB94 positive cells as previously described (32).
For the immunohistochemical studies, the HTB9 cells were cultured at 2 × 105 cells/per well in 6 well plates containing a coverslip. At 24 hours post- transfection cells on the coverslip were fixed and stained for RB94 as previously described (32).
XTT Assay
For the XTT in vitro drug sensitivity assay either HTB-9 bladder cancer or normal CRL1730 endothelial cells were seeded in 96 well plates at 5×103 cells/well. After 24 hours, the cells were transfected with the specific complex or control as previously described (14, 34). Twenty-four hours post-transfection, the medium was replaced with complete medium without drug or with varying concentrations of CDDP (0.1 to 10μM) or gemcitabine (0.01 to 100μM), followed by incubation at 37°C for an additional 72 hours, at which time untreated cells reached approximately 100% confluency. Cell viability was determined by via an XTT-based assay following the manufacturer's protocol (Roche Applied Sciences, Indianapolos, IN). The IC50 value, the drug concentration resulting in 50% cell kill, was interpolated from the graph of the log of drug concentration vs. the fraction of surviving cells.
PCR for Detection of Exogenous RB94 DNA in Mouse Tissues
DNA was isolated from mouse tissues using the high pure PCR template kit (Roche Applied Science, Indianapolis, IN ) per manufacturer's instructions. To specifically amplify exogenous RB94 DNA, the forward primer (5’-ATG GTGATG CGG TTT TG-3’) is a sequence in the CMV promoter in the vector backbone, while the reverse primer (5’-ACA TGG GAG GTG AGA GTT TA-3’) is a sequence in the RB94 gene insert, yielding an ~850 bp fragment. DNA PCR was performed using Biolase DNA polymerase (Bioline Co., Randolph, MA) and a 25 cycle amplification using the following conditions: 94°C 1min. for 1cycle; 94°C 30sec, 53°C 1min., 72°C 1min. for 25 cycle; 72°C for 10min. for extension. The PCR products were run on an Agarose gel, stained with Ethidium Bromide and photographed.
Detection of Cleaved Caspase 3 17kDa Subunit as an Indicator of Apoptosis
Equal volumes of blood were obtained from mice in the presence of Sodium Heparin (0.015 USP units/μl blood collected) and centrifuged twice at 0.1 x g, and 4°C for 10 minutes to separate the plasma from the blood cells. The 17kDa fragment of cleaved caspase-3 was isolated from the plasma via chromatography. Equal volumes were analyzed by Western blot analysis using the Cleaved Caspase-3 (Asp175) Antibody (Cell Signaling, Danvers MA) and the ECL Western blot kit (Amersham, Pascataway NJ). This polyclonal antibody is specific for the large fragment (17kDa) of activated caspase-3.
Induction of Bladder Tumors in Mice
Orthotopic HTB-9 bladder tumors were induced in female nude mice as previously described (35, 36).
In Vivo Chemosensitization of HTB-9 Xenograft Tumors
Female athymic nude (NCR nu/hu) mice (4-6 week old) were subcutaneously inoculated on the lower back above the tail with 5×106 HTB-9 cells suspended in Matrigel® collagen basement membrane (BD Biosciences, Franklin Lakes, NJ,). Approximately 10-13 days post-injection the tumors averaged 50-100mm3.
Mice bearing subcutaneous xenograft tumors of ~100 mm3 were divided into groups (4-10 mice/group). The targeted liposome complex (either Tf/Lip/DNA or TfRscFv/Lip/DNA) carrying the pSCMV-RB94 plasmid DNA was intravenously injected 3x/week via the tail vein. 10-20ug pSCMV-RB94 plasmid DNA was administered/injection/mouse. Gemcitabine administration was initiated 6-20 hours after the first i.v. injection and was given i.v. twice weekly at a dose of 60 mg/kg/mouse/injection. Tumor sizes were measured weekly by a third party in a blinded manner at the Georgetown University Medical Center animal facility. Tumor volume (L x W x H) in mm3 is given as the mean + Standard Error. Statistical differences in tumor volumes were determined using the Students t-test (SigmaStat, Systat Softwate, San Jose, CA). All animal experiments were approved and performed in accordance with Georgetown University and MD Anderson Cancer Center institutional guidelines for the care and use of laboratory animals.
RESULTS
Size Determination
The size of the ligand-targeted liposome RB94 complex was analyzed by dynamic light scattering on a Malvern Zetasizer 3000HS. The unliganded liposome/RB94 complex measured approximately 166nm with a zeta potential of 32. The Tf/Lip/RB94 complex was approximately 108nm with a zeta potential of 9.4 while TfRscFv/Lip/RB94 was approximately 207nm with a zeta potential of 6.6, demonstrating that these complexes are in the nanosize range. The polydispersity, or width of the complex size distribution, is within the recommended range for all of the complexes. The zeta potential of these complexes is positive, as desired for optimal interactions with cell membranes.
RB94 Expression in HTB-9 Cells
After subcloning a 3.1kb Bam HI-Hind III fragment of the original RB94 clone (PEW 13) into the pSCMV high expression vector (Fig.1A), we analyzed several of the resulting pSCMV-RB94 clones by restriction enzyme digestion and partial DNA sequence analysis to verify the orientation and sequence authenticity of the inserts, and chose several clones for further study.
Using the established Tf-targeted liposomal delivery system (14, 15) and the luciferase gene as a reporter, we tested a series of different liposomes to determine which compositions would yield the highest transfection efficiency in the human bladder carcinoma cell line HTB-9. We found that liposome compositions A (DOTAP:DOPE, ) and D (DOTAP: cholesterol) were the most efficient (data not shown). HTB-9 cells were subsequently transfected with the various RB94 plasmids complexed with Tf/LipD. Twenty-four hours post-transfection RB94 expression by assessed by Western analysis using an anti-RB monoclonal antibody (QED Bioscience Inc, San Diego CA). Purified RB94 protein is included on the gel as a positive control and for verification of RB94 positioning. In all cases, a significantly higher level of RB94 protein expression was observed with the pSCMV-RB94 clones (X452-X456) compared to that of the original construct (X457) (Fig.1B). An RB94 band is present in X457 upon longer exposure (data not shown). GAPDH levels demonstrate equal protein loading. Moreover, RB94 expression was not observed in normal endothelial cell line CRL1730 after transfection with Tf/LipD/RB94, indicating the tumor cell specificity of this complex (not shown).
We chose pSCMV-RB94 clone X455 for use in the remainder of the studies. High RB94 expression level achieved using this clone when compared to the original pCMVRB94 construct is shown at the single cell level by immunochemical analysis in Fig.1C. Clear nuclear staining is evident in the HTB-9 cells transfection with Tf/Lip complex carrying the original pCMV-RB94 construct (Fig. 1C, top panel). However, after transfection under identical conditions using Tf/Lip encapsulating the construct with the high expression promoter, strong staining is detected not only in the nucleus, but also in cytoplasm (Fig. 1C, bottom panel). The broad arrows indicate the RB94 expressing cells with strong nuclear and cytoplasmic staining.
RB94-mediated Sensitization of Bladder Tumor Cells to Chemotherapy
We assessed the ability of RB94 delivered via the Tf-targeted liposome complex to sensitize RB94 negative HTB-9 cells to conventional chemotherapeutic agents gemcitabine and CDDP (cisplatin) by means of the XTT cytotoxicity assay. IC50 values, the drug concentration yielding 50% growth inhibition, were calculated and represent the degree of chemosensitization. In these experiments LipD was employed. A dramatic increase in sensitization of HTB-9 cells to both drugs with the Tf/LipD/RB94 complex was observed (Fig. 2A, 2B).
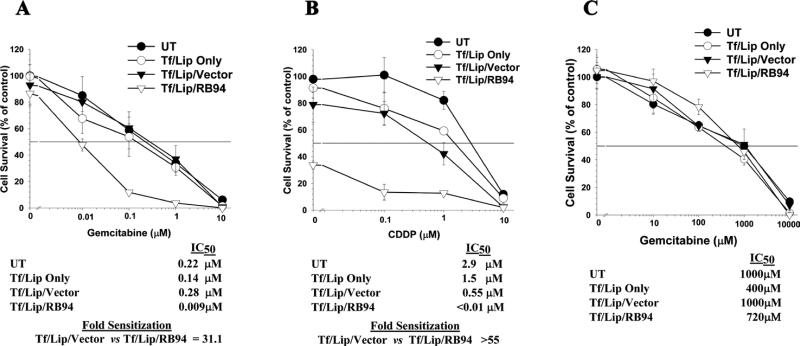
Figure 2. Chemosensitization of tumor cells by nanocomplex delivered RB94. XTT cell survival assays.
Panel A: Evaluation of the degree of sensitization of bladder cancer cell line HTB-9 to gemcitabine by the Tf/LipD delivered RB94 gene or empty vector (0.05μg plasmid DNA). Tf/Lip only was used to assess the influence of non-specific cytotoxicity. Fold sensitization is a comparison of the IC50 values of Tf/Lip/Rb94 vs. Tf/Lip/empty vector. Panel B: Similar experiment as in Panel A in HTB-9 using the chemotherapeutic agent cisplatin (CDDP). The DNA dose was 0.1μg.
Panel C: Assessment of the effect of nanocomplex Tf/LipD/RB94 (0.1μg) on the response of normal human endothelial cell line CRL1730 to gemcitabine. Note that for this normal cell line the gemcitabine concentration range is 1000 fold higher that that used with the tumor cell line.
The HTB-9 cells transfected with the Tf/Lip/RB94 complex at 0.05μg RB94 plasmid DNA have an IC50 for gemcitabine of 0.009μM, compared to 2.8μM for cells transfected with the complex carrying empty vector (Tf/Lip/Vector), a 31.1-fold increase in sensitivity of the bladder cancer cells to the chemotherapeutic agent (Fig. 2A). This increased sensitivity was not present in cells treated with the complex minus DNA (Tf/Lip only). These results demonstrate that the observed sensitization is RB94 related and is not due to non-specific cytotoxicity from the delivery system.
A similar high degree of chemosensitization of HTB-9 cells to CDDP occurred after treatment with Tf/LipD/RB94 at a DNA dose of 0.1ug. In this instance, even without CDDP, the effect of Tf/LipD/RB94 on the tumor cells is so potent that only 40% of the cells survive, thus an IC50 value could not be calculated (Fig 2B). However, if we assume that the IC50 for Tf/LipD/RB94 is <0.01 (the lowest dose of CDDP used), then transfection of HTB-9 cells with this complex results in a >55 fold increase in cell killing by CDDP compared to that observed with cells treated with the complex carrying empty vector.
To demonstrate the tumor cell specificity of Tf/LipD/RB94 induced chemosensitization, a similar assay using normal human endothelial cell line CRL1730 and gemcitabine was performed. In this experiment, the DNA dose was 0.1ug, twice that used above with HTB-9 cells. In addition, the range of gemcitabine concentration (0-10,000μM) was 1000 fold higher with these normal cells than that used with the tumor cell line (0-10μM). No significant sensitization over that seen with gemcitabine alone (UT) was observed after transfection with Tf/LipD/RB94 or the control complexes (Fig 2C). These findings in normal cells also indicate the safety and potential for reduced side effects with our tumor cell specific delivery system.
Tumor Specific Targeting and In Vivo RB94 Expression
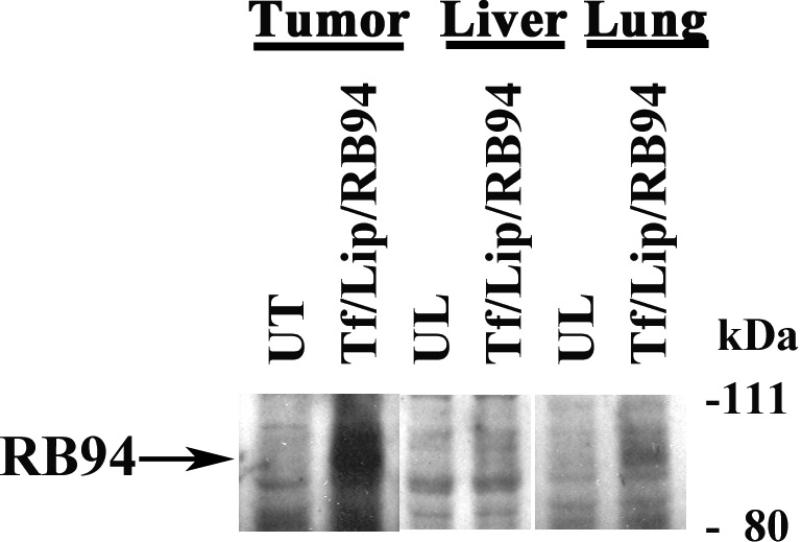
To begin to assess the potential of this ligand-liposome-RB94 complex as a clinical agent, we tested the ability of the systemically administered complex to deliver RB94 specifically and efficiently to tumor tissue in an animal model. Tf/Lip/RB94 (with liposome formulation D) was i.v. administered via the tail vein to nude mice bearing subcutaneous HTB-9 xenograft tumors. The animals received two i.v. injections within 24 hours at 60ug RB94 plasmid DNA/injection. As a control we also injected mice with LipD-RB94 minus the targeting Tf ligand (UL). Approximately 16 hours after the second injection we sacrificed the mice, harvested tumor, liver and lung and isolated protein for Western analysis. 80 ug protein was loaded per lane. There was strong expression of RB94 in the tumor from the animal that received Tf/LipD/RB94 complex, while there was no clear signal evident in the tumor from the mouse injected with the unliganded complex (Fig. 3A). More importantly, there was no RB94 expression in the liver and only minimal expression in the lungs of the animal treated with Tf/LipD/RB94. These findings demonstrate the tumor specificity of this complex. Actin expression, as an internal control for protein loading, was comparable in all samples. It has previously been shown using Ponceau Red staining that although equal amounts of protein are present on the membrane, actin levels are lower in the liver as compared to other organs (37).
Figure 3. In vivo expression of RB94 in nanocomplex transfected HTB-9 tumors.
Panel A: Mice bearing subcutaneous RB94 negative HTB-9 human bladder cancer tumors were i.v. tail vein injected with TL/RB94 or the complex minus the Tf ligand (UL) as described in Materials and Methods. Sixteen hours post-injection, the tumor liver and lungs were excised, protein isolated and RB94 expression determined by Western Blot analysis using an anti-RB monoclonal antibody (QED Bioscience Inc., San Diego CA).
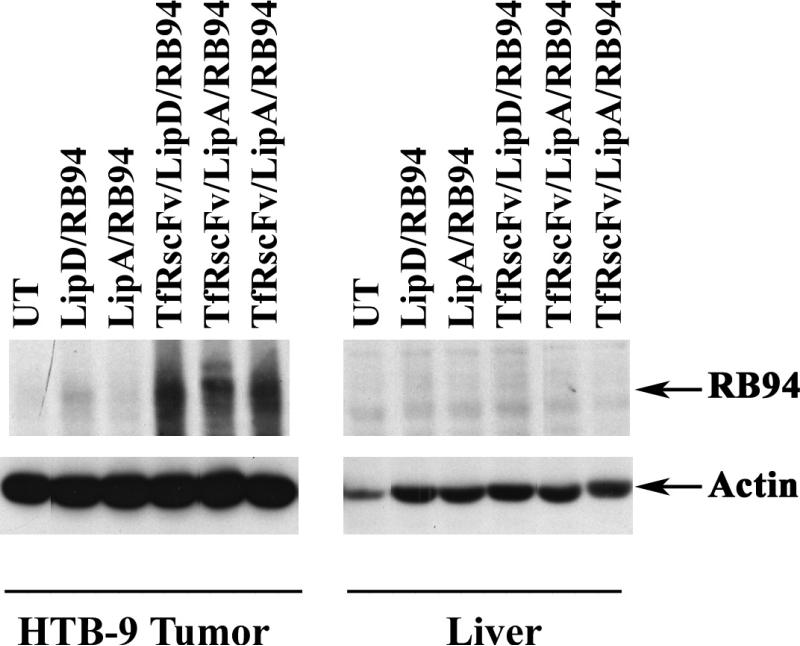
Panel B: Mice bearing subcutaneous RB94 negative HTB-9 human bladder cancer tumors were i.v. tail vein injected with scL/RB94 or the complex minus the TfRscFv targeting moiety. Lipsome formulations A and D were both used in this experiment. Forty-eight hours post-injection the tumor and liver were excised, protein isolated and RB expression assessed as in Panel A. UT= untreated; Arrow indicates the position of the RB94 protein.
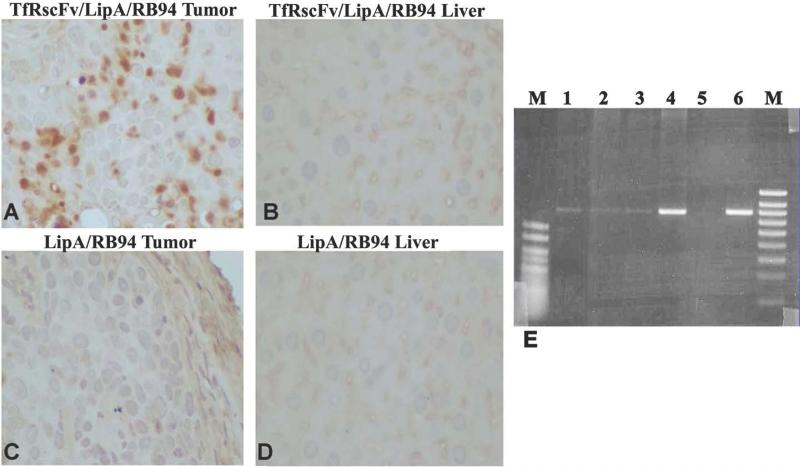
The properties of the anti-transferrin receptor single chain antibody fragment (TfRscFv) make it attractive as a potential targeting moiety for tumor specific delivery. We have demonstrated that the tumor specificity and transfection efficiency of the nanocomplex with TfRscFv are at least as good, if not better, than those observed when Tf is used (26, 27). Therefore, we assessed the ability of the i.v. administered liposome-RB94 complex, targeted by the TfRscFv molecule (TfRscFv/Lip/RB94), to deliver RB94 specifically to the tumor. In this study we compared the targeting ability and transfection efficiency of the complex using either liposome formulation A or formulation D. The complex without TfRscFv (Unliganded) was also i.v. injected as a control. The mice received three i.v. tail vein injections over 24 hours at 40ug RB94 plasmid DNA/injection. Forty-eight hours after the last injection, tumor and liver were excised and protein isolated for Western blot analysis. As seen with the Tf-targeted complex, there was a high level of RB94 expression evident in the tumor and not in the liver of the animals receiving the i.v. TfRscFv/Lip/RB94 complex (Fig.3B). A high incidence of tumor specific RB94 transfer and expression was also seen by immunohistochemical analysis of tissue from the same experiment. Numerous RB94 staining cells were observed in the tumor from the mouse that received TfRscFv/Lip/RB94 (Fig.4A), but none were evident in liver from the same animal (Fig.4B). This high expression was evident with both Liposome A as well as Liposome D. In addition no RB94 positive tumor or liver cells were evident if the TfRscFv was not present (Unliganded) (Fig.4C, and 4D), demonstrating the importance of the targeting molecule in obtaining high frequency and levels of targeted gene expression in the tumor.
Figure 4. In vivo expression of RB94 in tumor and liver at the single cell level by immunohistocemical analysis and PCR.
In Panels A-D, HTB-9 tumor and liver cells from the mice shown in Figure 3B were immunohistochemically stained for RB94 expression.
Panel A: Tumor from mouse injected with TfRscFv/LipA/RB94 in Figure 3B
Panel B: Liver from the same mouse whose tumor is shown in Panel A.
Panel C: Tumor from a mouse that received the untargeted LipA/RB94 complex in Figure 3B
Panel D: Liver from the same mouse whose tumor is shown in Panel C
Panel E: PCR analysis of tumor bearing bladder, and normal tissues from two individual mice bearing RB94 negative HTB-9 tumors which had been injected three times over 24 hours with the TfRscF/LipA/RB94 complex at 24ugDNA/mouse/injection. Lanes 1-4 are from mouse 1 while lane 6 is from a separate tumor bearing mouse. Lane 1 = liver; Lane 2 = large intestine; Lane 3 = kidney; Lanes 4 and 6 = bladder with tumor; Lane 5 = water blank. M = size markers (500bp and 1000bp hyperladder V and IV, respectively,) (Bioline Co., Randolph, MA).
In a separate experiment, tumor specificity was also demonstrated by DNA PCR using samples from tumor containing bladders, as well as from normal tissues, of mice i.v. injected three times over 24 hours with the TfRscF/LipA/RB94 complex at 24ug DNA/mouse/injection. DNA was isolated from the tissues and PRC performed as described in Materials and Methods. As shown in Fig. 4E, a strong RB94 specific signal was evident in the bladder tumors from two individual mice (lanes 4 and 6). In contrast, only a very weak RB94 DNA band is detectable in the normal liver (lane 1), large intestine (lane 2) or kidney (lane 3).
Thus complexes with both LipA and LipD, and with both Tf and TfRscFv can lead to tumor specific transfection and expression of RB94 after i.v. administration in tumors located both subcutaneously or within the bladder.
Cleaved Caspase 3 in Plasma as a Measure of RB94 Induced Apoptosis
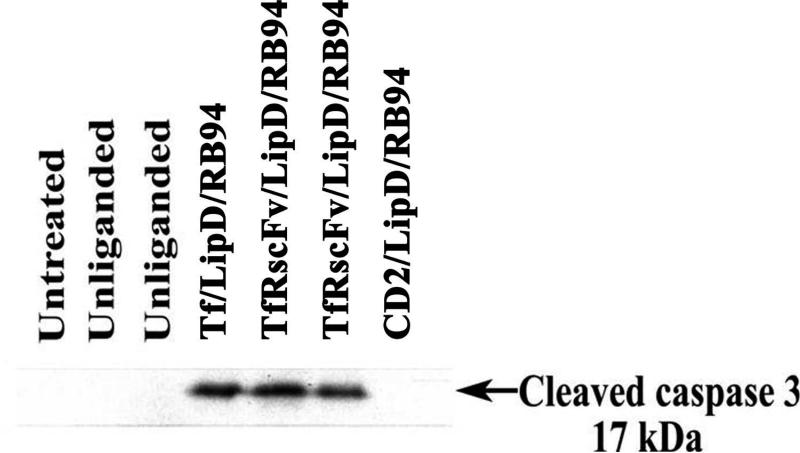
We have recently shown in vitro that TUNEL positivity is an early marker of RB94 induced cancer cell death whereas caspase 3 cleavage is a later event1 Thus, delivery and expression of RB94 to tumor cells in vivo should result in induction of apoptosis. Detection of the 17 kDa fragment of cleaved caspase 3 in the plasma of tumor-bearing mice treated with RB94 would be indicative of ongoing apoptosis. Athymic nude mice bearing subcutaneous HTB-9 xenograft tumors were i.v. injected three times within 24 hours with the liganded-liposome D complex carrying the RB94 gene (40 μg RB94 plasmid DNA/mouse/injection), with either Tf or TfRscFv as the targeting molecule. As controls, other mice were i.v. injected with the complex without targeting ligand (Unliganded), or with a non-tumor specific molecule (CD2) as the ligand. Sixteen hours after the last injection the animals were sacrificed, blood taken and plasma isolated as described in Materials and Methods. Western analysis of the expression of the 17 kDa fragment of cleaved caspase 3 is shown in Figure 5. The 17 kDa cleaved caspase 3 protein was evident in the plasma from all three of the mice that received the RB94 gene complexed with either of the tumor targeting moieties. However, no cleaved caspase 3 expression was present in any of the untreated mice or those injected with the unliganded complex or with the non-tumor cell specific CD2 ligand. Therefore, targeted delivery of the RB94 gene to tumors results in the induction of apoptosis as indicated by the presence of cleaved caspase 3 in the plasma.
Figure 5. Detection of cleaved caspase 3 in vivo after treatment with TfRscFv/LipD/RB94.
Western blot analysis of the level of the 17kDa cleaved caspase 3 protein, a marker for apoptosis, in plasma for HTB-9 tumor bearing mice 16 hours after treatment with complete tumor targeting nanocomplex (TF/Lip/RB94 and TfRscFv/Lip/RB94), complex minus the targeting moiety (Unliganded) or the complex bearing a non- tumor specific ligand (CD2). The mice received three i.v. tail vein injections over 24 hours at 40μg DNA/mouse/injection. Protein was obtained and Western blot analysis performed as described in Materials and Methods.
In Vivo Response of HTB-9 Xenograft Tumors to the Combination of Ligand-Liposome-RB94 and Gemcitabine
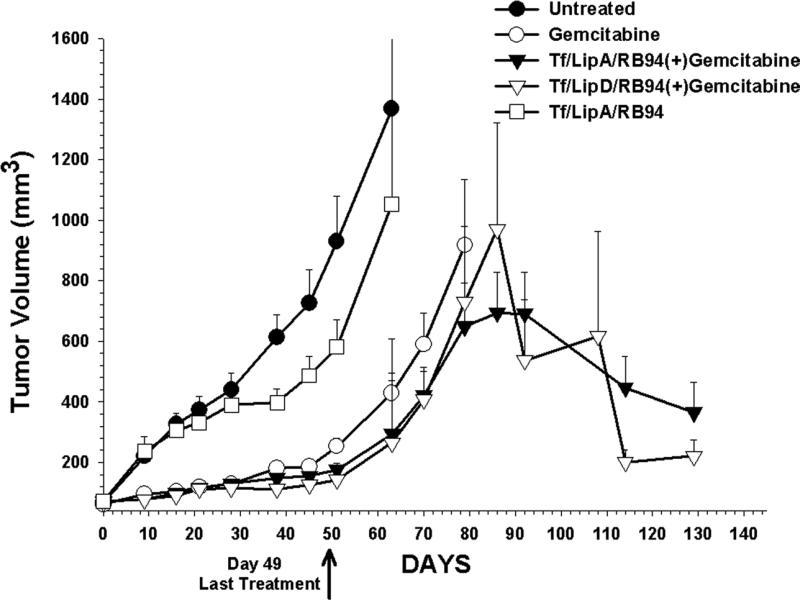
The response of subcutaneous HTB-9 xenograft tumors to the combination of targeted-liposome-RB94 and gemcitabine was assessed in a nude mouse tumor model. This study also compared the efficacy of the complex made with LipA and LipD, as well as the effect of the combination treatment with that of single agent therapy (gemcitabine or Tf/LipA/RB94 alone). Mice (4-10/group) bearing xenograft tumors of approximately 100 mm3 were treated with the combination of i.v. administered Tf/LipA/RB94 or Tf/LipD/RB94 plus gemcitabine. Additional groups of animals received no treatment, or treatment with gemcitabine or TF/LipA/RB94 only. Each group received a total of 20 i.v. injections of the complex (10 ug DNA/injection) and 14 i.p. injections of gemcitabine (60 mg/kg). A low DNA dose of 10ug was used here to be able to assess the presence of synergy between the immunoliposome and gemcitabine. Treatment ended on day 49. At this low DNA dose, less than one month after the end of treatment (Day 78) the animals in both single agent groups were sacrificed due to excessive tumor burden (thus, statistical analysis could not be performed) (Fig. 6A). In contrast, three months post-treatment (Day 130) the tumors in the groups treated with the combination of either Tf/LipA/RB94 or Tf/LipD/RB94 plus gemcitabine were showing regression. These results not only demonstrate an improved tumor response to combination therapy, but also show that both LipA and LipD work equally well in vivo.
Figure 6. Tumor response of the HTB-9 xenograft tumor model to ligand-targeted liposome delivery of RB94. HTB-9 tumors were induced in female athymic nude mice as described in Materials and Methods.
Panel A: Mice bearing tumors of approximately 100mm3 were i.v. tail vein injected with Tf/LipA or TF/LipD complexed RB94 plasmid DNA (10μg DNA/mouse/injection) alone or in combination with gemcitabine (Gem). The last injection was on day 49. Points are the mean of 4-10 tumors/group ± S.E.
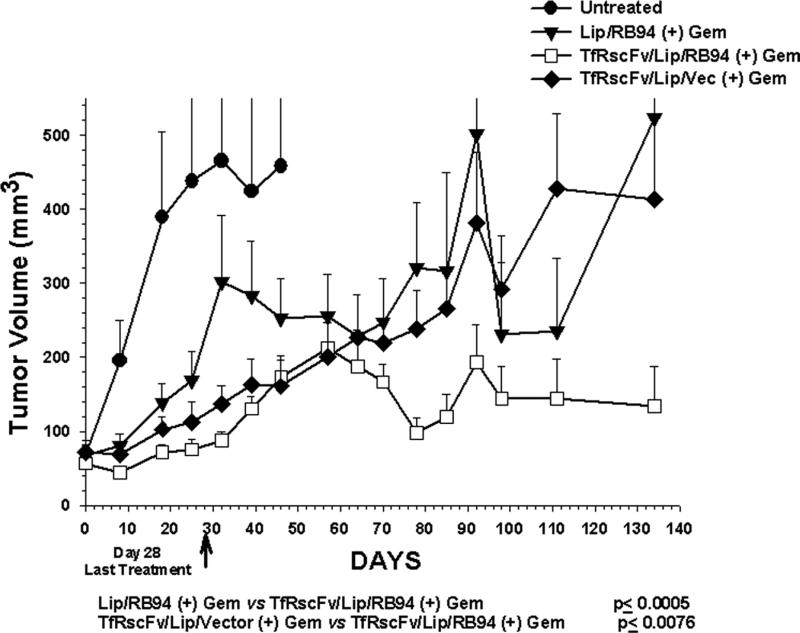
Panel B: Mice bearing tumors of 50-100mm3 were i.v. tail vein injected with scL complexed RB94 plasmid DNA or empty vector (20μg DNA/mouse/Injection) in combination with gemcitabine. Unliganded Liposome/RB94 complex was also administered in combination with the chemotherapeutic agent. The last injection was on day 28. Points are the mean of 4-10 tumors/group ± S.E.
A second in vivo study was performed in which TfRscFv was used as the targeting moiety (Fig 6B). Liposome formulation D was used in this experiment. In addition to the group of mice that received the combination of TfRscFv/LipD/RB94 plus gemcitabine, groups of mice remained untreated or were treated with unliganded LipD/RB94 plus gemcitabine or with the combination of the TfRscFv/LipD complex carrying empty vector plus gemcitabine. This latter group was included to verify that the tumor response observed was due to the presence of RB94 and not to a liposomal effect. Each group consists of 4-10 mice. The mice received 13 i.v. injections of the complex (20ug of RB94 plasmid DNA/mouse/injection) and 9 i.p. administrations of gemcitabine (60 mg/kg/injection). Treatment ended on day 28. As was observed with TF/Lip/RB94, the tumors in the group receiving the combination of TfRscFv/LipD/RB94 plus gemcitabine showed no increase in tumor size, and even evidenced tumor regression on day 150, approximately 4 months after the end of treatment. In contrast, the untreated group was humanely euthanized by day 50 due to tumor burden. The group that received the unliganded complex plus gemcitabine or the complex carrying empty vector plus gemcitabine experienced a significant increase in tumor size. The differences in tumor volumes between the TfRscFv/LipD/RB94 plus gemcitabine and both control groups, Lip/RB94 plus gemcitiabine, and TfRscFv/LipD/vector plus gemcitabine, were highly statistically significant by student's t-test, at p <0.0005 and p <0.0076, respectively. The decrease in tumor size shown at 90 days for the two control groups was the result of removal of fluid that had accumulated in the tumors. Similar massive fluid accumulation was not observed in the tumors that received TfRscFv/LipD/RB94 plus gemcitabine.
Discussion
Although many Mab-based agents are in use as anti-cancer therapeutics, they possess some drawbacks and limitations. Adverse toxic reactions can occur due to interactions between Fc receptors on normal tissues and the MAb. Moreover, with regards to use as therapeutic agents in the treatment of cancer, the large size of the intact MAb (approximately 155kDa) limits their ability to diffuse from the capillaries into the solid tumor (38) and thus their potential efficacy. Thus, new approaches employing antibody fragments such as scFv molecules are being developed.
For a number of reasons the anti-transferrin receptor single chain antibody fragment (TfRscFv) has advantages in human use over the full MAb or even the Tf molecule itself in targeting liposomes to cancer cells with elevated TfR levels: 1) the size of the scFv (~28kDa) is much smaller than that of the Tf molecule (~80kDa) or the parental MAb (~155kDa). The scFv-liposome-DNA complex may thus exhibit better penetration into small capillaries characteristic of solid tumors. The nanosize of this complex lends support to this theory. 2) the scFv also has practical advantages related its production as a recombinant protein since large scale production of the TfRscFv will be required for the Phase 1 RB94 trial. 3) the scFv is a recombinant molecule (not a blood product like Tf) and therefore presents no issues related to potential contamination with blood borne pathogens. 4) without the Fc region of the MAb, the problem of non-antigen-specific binding through Fc receptors is eliminated.
We have previously shown that this single chain antibody fragment can target an intravenously administered cationic liposome/payload complex preferentially to tumors (26-30) resulting, e.g. in increased survival in various mouse models of human cancer (26, 29, 34). Moreover, our TfRscFv/LipD/DNA nanocomplex carrying the wtp53 gene has been approved to enter Phase I clinical trials for gene therapy of cancer.
The efficacy of our approach can be attributed in part to the nanosize of the complex carrying payloads as diverse as plasmid DNA (26, 27, 34, 37), siRNA (28, 29) and even imaging agents (30). The encapsulation of the payload within the ligand-decorated liposome helps maintain this small size. As it has been shown that the payload is indeed encapsulated within the targeting molecule decorated liposome for other molecules such as imaging agents (30), siRNA (29) and other plasmid DNAs (37), it is likely that this is also the case with the RB94 constructs.
Here we further demonstrate the potential of this platform nanotechnology by systemically delivering the RB94 tumor suppressor gene preferentially and efficiently to tumors in a mouse model of human bladder cancer. The specificity was demonstrated not only by Western analysis, but also by immunohistochemistry and DNA PCR. The very faint bands seen in the normal tissues with PCR are likely a result of the complex still in circulation in the blood stream and/or the presence of macrophages in the tissues, particularly liver and kidney as we have previously shown (14, 16). Non-sterically stabilized liposomes are known to be taken up by the macrophages of the reticulo-endothelial system (39).
Furthermore, the results of the in vivo experiments presented here not only demonstrated the ability of the combination of nanoliposome complex delivered RB94 and a conventional chemotherapeutic agent to inhibit bladder tumor growth, but also the importance of the targeting molecule and that this anti-tumor effect is RB94 specific. They also indicate the potential of this approach as an anti-cancer treatment in the clinic.
We believe that RB94 may be an ideal gene to consider for this type of systemically administered gene therapy since it has been effective in all tumor types studied to date. No human cancer cells have yet been identified that have survived transfection with an RB94 construct (31-33). Therefore no resistance to this therapeutic has yet been observed. In addition, RB94 has not been found to be cytotoxic to various normal cell types, including fibroblasts, endothelial cells as shown here, or urothelial cells. Furthermore, as demonstrated with the CRL1730 cells, the use of the tumor-targeting delivery system did not affect this lack cytotoxicity in normal cells.
Our initial planned Phase 1 study will involve the systemic treatment of RB negative tumors such as bladder and prostate tumors with TfRscFv/Lip/RB94 as a single agent to demonstrate safety and proof of principle for tumor specific targeting. It is our plan, however, to add gemcitabine in future studies since we believe the combination will provide an enhanced therapeutic modality for the treatment of genitourinary and other cancers.
ACKNOWLEDGEMENTS
Grant Support: NCI grant R01 CA103579-01 (E. H. Chang and K. F. Pirollo), SynerGene Therapeutics Inc (K. F. Pirollo), NCI SPORE grant P50 CA091846 (W. F. Benedict and E.H. Chang), and NCI grant R01 CA097127 (W. F. Benedict). These studies were conducted in part using the Lombardi Comprehensive Cancer Center Animal Core Facilities supported by NCI Center Support grant 1S10 RR15768-01. This investigation was conducted in part in a Lombardi Comprehensive Cancer Center facility constructed with support from Research Facilities Improvement grant C06 RR14567 from the National Center for Research Resources, NIH.
We thank Sophia Kim and Jeff Young for assistance in preparation of the manuscript.
Footnotes
Zhou J, Zhang X-Q, Ashoori F, Dong L, McConkey DJ, and Benedict WF. The truncated retinoblastoma protein RB94 produces caspase-independent DNA fragmentation and cell death in human bladder cancer cells. Submitted to Cancer Research.
REFERENCES
- 1.Huang L, Viroonchatapan E. Non-viral Vectors for Gene Therapy. In: Huang L, Hung M, Wagner E, editors. Introduction. Academic Press; San Diego: 1999. pp. 3–22. [Google Scholar]
- 2.Lasic DD, Papahadjopoulos D, editors. Medical Applications of Liposomes. Elsevier; Amsterdam: 1998. [Google Scholar]
- 3.Torchilin VP, Weissig V, editors. Liposomes, Practical Approach. Oxford Univ. Press; Oxford: 2003. [Google Scholar]
- 4.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 5.Wasungu L, Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. Journal of Controlled Release. 2006;116:255–64. doi: 10.1016/j.jconrel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 6.The Journal of Gene Medicine Clinical Trials Database. 2007 Jan; hellottp:/www.wiley.co.uk/wileychi/genmed/clinical.
- 7.NIH Clinical Trials Database. http://clinicaltrails.gov.
- 8.Sapra P, Tyagi P, Allen TM. Ligand-targeted liposomes for cancer treatment. Current Drug Delivery. 2005;2:369–81. doi: 10.2174/156720105774370159. [DOI] [PubMed] [Google Scholar]
- 9.Cristiano RJ, Curiel DT. Strategies to accomplish gene delivery via the receptor-mediated endocytosis pathway. Cancer Gene Ther. 1996;3:49–57. [PubMed] [Google Scholar]
- 10.Cheng PW. Receptor ligand-facilitated gene transfer: enhancement of liposome-mediated gene transfer and expression by transferring. Human Gene Ther. 1996;7:275–82. doi: 10.1089/hum.1996.7.3-275. [DOI] [PubMed] [Google Scholar]
- 11.Thorstensen K, Romslo I. The transferrin receptor: its diagnostic value and its potential as therapeutic target. Scan J Clin Lab Inves. 1993;215:113–20. doi: 10.3109/00365519309090703. [DOI] [PubMed] [Google Scholar]
- 12.Ponka P, Lok CN. The transferrin receptor: role in health and disease. International J of Biochem Cell Biol. 1999;31:1111–37. doi: 10.1016/s1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 13.Singh M. Transferrin As A targeting ligand for liposomes and anticancer drugs. Current Pharmaceutical Design. 1999;5:443–51. [PubMed] [Google Scholar]
- 14.Xu L, Pirollo K, Tang W, Rait A, Chang EH. Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Human Gene Ther. 1999;10:2941–52. doi: 10.1089/10430349950016357. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Pirollo KF, Chang EH. Transferrin-Liposome-Mediated p53 Sensitization of Squamous Cell Carcinoma of the Head and Neck to Radiation in vitro. Human Gene Therapy. 1997;8:467–75. doi: 10.1089/hum.1997.8.4-467. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Pirollo K, Rait A, Murray A, Chang EH. Systemic p53 gene therapy in combination with radiation results in human tumor regression. Tumor Targeting. 1999;4:92–104. doi: 10.1089/10430349950016357. [DOI] [PubMed] [Google Scholar]
- 17.Allen TM, Hansen CB, Zalipsky S. Antibody-targeted stealth liposomes. In: Lasic DMF, editor. Stealth Liposomes. CRC Press; London: 1995. pp. 233–44. [Google Scholar]
- 18.Dass CR, Choong PF. Selective gene delivery for cancer therapy using cationic liposomes: in vivo proof of applicability. Journal of Controlled Release. 2006;113:155–63. doi: 10.1016/j.jconrel.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Park JW, Benz CC, Martin FJ. Future directions of liposome- and immunoliposome-based cancer therapeutics. Seminars in Oncology. 2004;31:196–205. doi: 10.1053/j.seminoncol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Selvam MP, Buck SM, Blay RA, Mayner RE, Mied PA, Epstein JS. Inhibition of HIV replication by immunoliposomal antisense oligonucleotide. Antiviral Research. 1996;33:11–20. doi: 10.1016/s0166-3542(96)00993-x. [DOI] [PubMed] [Google Scholar]
- 21.Pirollo KF, Xu L, Chang EH. Immunoliposomes: a targeted delivery tool for cancer treatment. In: Curiel D, editor. Vector Targeting for Therapeutic Gene Delivery. Wiley Press; 2002. pp. p33–61. [Google Scholar]
- 22.Gunther M, Wagner E, Ogris M. Specific targets in tumor tissue for the delivery of therapeutic genes. Current Medicinal Chemistry - Anti-Cancer Agents. 2005;5:157–71. doi: 10.2174/1568011053174855. [DOI] [PubMed] [Google Scholar]
- 23.Poon RY. In: Biotechnology International: International Developments in the Biotechnology Industry. Fox F, Connor TH, editors. Universal Medical Press; San Francisco, CA: 2000. pp. 113–28. [Google Scholar]
- 24.Haynes BF, Hemler M, Cotner T, et al. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J of Immunology. 1981;127:347–51. [PubMed] [Google Scholar]
- 25.Batra JK, Fitzgerald DJ, Chaudhary VK, Pastan I. Single-chain immunotoxins directed at the human transferrin receptor containing Pseudomonas exotoxin A or diphtheria toxin: anti-TFR(Fv)-PE40 and DT388-anti-TFR(Fv). Mol Cel Biol. 1991;11:2200–5. doi: 10.1128/mcb.11.4.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Tang WH, Huang CC, et al. Systemic p53 Gene Therapy of Cancer with Immunolipoplexes Targeted by Anti-Transferrin Receptor scFv. Mol Med. 2001;7:723–34. [PMC free article] [PubMed] [Google Scholar]
- 27.Xu L, Huang CC, Huang WQ, et al. Systemic Tumor-targeted Gene Delivery by Anti-Transferrin Receptor scFv-Immunoliposomes. Mol Cancer Ther. 2002;1:337–46. [PubMed] [Google Scholar]
- 28.Pirollo KF, Zon G, Rait A, Zhou Q, Yu W, Hogrefe R, Chang EH. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Human Gene Therapy. 2006;17:117–24. doi: 10.1089/hum.2006.17.117. [DOI] [PubMed] [Google Scholar]
- 29.Pirollo KF, Rait A, Zhou Q, et al. Materializing the potential of small interfering RNA via a tumor-targeting nanodelivery system. Cancer Research. 2007;67:2938–43. doi: 10.1158/0008-5472.CAN-06-4535. [DOI] [PubMed] [Google Scholar]
- 30.Pirollo KF, Dagata J, Wang P, et al. A tumor-targeted nanodelivery system to improve early MRI detection of cancer. Molecular Imaging: Official Journal of the Society for Molecular Imaging. 2006;5:41–52. [PubMed] [Google Scholar]
- 31.Xu HJ, Xu K, Zhou Y, Li J, Benedict WF, Hu SX. Enhanced tumor cell growth suppression by an internal AUG codon initiated retinoblastoma protein. Proc Natl Acad Sci. 1994;91:9837–41. doi: 10.1073/pnas.91.21.9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu HJ, Zhou Y, Seigne J, et al. Enhanced tumor suppressor gene therapy via replication-deficient adenovirus vectors expressing an N-terminal truncated retinoblastoma protein. Cancer Res. 1996;56:2245–9. [PubMed] [Google Scholar]
- 33.Zhang X, Multani AS, Zhou JH, et al. Adenoviral-mediated retinoblastoma 94 produces rapid telomere erosion, chromosomal crisis, and aspase-dependent apoptosis in bladder cancer and immortalized human urothelial ells but not in normal urothelial cells. Cancer Res. 2003;63:760–5. [PubMed] [Google Scholar]
- 34.Yu W, Pirollo KF, Yu B, et al. Enhanced transfection efficiency of a systemically delivered tumor-targeting immunolipoplex by inclusion of a pH-sensitive histidylated oligolysine peptide. Nucleic Acids Research. 2004;32:e48. doi: 10.1093/nar/gnh049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe T, Shinohara N, Sazawa A, Harabayashi T, Ogiso Y, Koyanagi T, et al. An improved intravesical model using human bladder cancer cell lines to optimize gene and other therapies. Cancer Gene Therapy. 2000;7:1575–80. doi: 10.1038/sj.cgt.7700261. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita M, Rosser CJ, Zhou JH, Zhang XQ, Connor RJ, Engler H, et al. Syn3 provides high levels of intravesical adenoviral-mediated gene transfer for gene therapy of genetically altered urothelium and superficial bladder cancer. Cancer Gene Therapy. 2002;9:687–91. doi: 10.1038/sj.cgt.7700488. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Frederik P, Pirollo KF, et al. Self-Assembly of a Virus-Mimicking Nanostructure System for Efficient Tumor-Targeted Gene Delivery. Human Gene Ther. 2002;13:469–81. doi: 10.1089/10430340252792594. [DOI] [PubMed] [Google Scholar]
- 38.Jain RK, Baxter LT. Mechanisms of heterogenous distribution of monoclonal antibodies and other macro-molecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988;48:7022–32. [PubMed] [Google Scholar]
- 39.Medina OP, Zhu Y, Kairemo K. Targeted liposomal drug delivery in cancer. Current Pharmaceutical Design. 2004;10:2981–9. doi: 10.2174/1381612043383467. [DOI] [PubMed] [Google Scholar]