Abstract
During official shellfish control for the presence of marine biotoxins in Greece in year 2012, a series of unexplained positive mouse bioassays (MBA) for lipophilic toxins with nervous symptomatology prior to mice death was observed in mussels from Vistonikos Bay–Lagos, Rodopi. This atypical toxicity coincided with (a) absence or low levels of regulated and some non-regulated toxins in mussels and (b) the simultaneous presence of the potentially toxic microalgal species Prorocentrum minimum at levels up to 1.89 × 103 cells/L in the area’s seawater. Further analyses by different MBA protocols indicated that the unknown toxin was hydrophilic, whereas UPLC-MS/MS analyses revealed the presence of tetrodotoxins (TTXs) at levels up to 222.9 μg/kg. Reviewing of official control data from previous years (2006–2012) identified a number of sample cases with atypical positive to asymptomatic negative MBAs for lipophilic toxins in different Greek production areas, coinciding with periods of P. minimum blooms. UPLC-MS/MS analysis of retained sub-samples from these cases revealed that TTXs were already present in Greek shellfish since 2006, in concentrations ranging between 61.0 and 194.7 μg/kg. To our knowledge, this is the earliest reported detection of TTXs in European bivalve shellfish, while it is also the first work to indicate a possible link between presence of the toxic dinoflagellate P. minimum in seawater and that of TTXs in bivalves. Confirmed presence of TTX, a very heat-stable toxin, in filter-feeding mollusks of the Mediterranean Sea, even at lower levels to those inducing symptomatology to humans, indicates that this emerging risk should be seriously taken into account by the EU to protect the health of shellfish consumers.
Keywords: Aegean Sea, emerging biotoxins, Mediterranean Sea, cultured mussels, Mytilus galloprovincialis, Prorocentrum minimum, tetrodotoxin, toxic episode, UPLC-MS/MS, venerupin shellfish toxin
1. Introduction
Tetrodotoxin (TTX) is an extremely potent neurotoxin that can block sodium channels and thus inhibit propagation of action potentials in muscle and nerve cells [1]. Named after the Tetraodontidae puffer fish family, TTX is perhaps most notorious as the toxin that causes puffer fish poisoning. TTX can cause death by muscular paralysis, respiratory depression and circulatory failure [2]. The minimum lethal and minimum acute doses of TTX to human (wt. 50 kg) are estimated to be around 2 mg and 0.2 mg, respectively. Symptoms usually appear within 10–45 min of exposure, depending upon the amount of the toxin ingested; however, some of the reported cases were asymptomatic until as much as 3 to 6 h after exposure. The initial symptom is usually oral paresthesia, which gradually spreads to the extremities and trunk. Other early symptoms comprise taste disturbance, dizziness, headache, diaphoresis, and pupillary constriction, potentially accompanied by gastrointestinal symptoms of salivation, hypersalivation, nausea, vomiting, hyperemesis, hematemesis, hypermotility, diarrhea and abdominal pain [3,4].
Tetrodotoxin is known to occur in a variety of fish species and other organisms including arthropods, echinoderms, an alga, mollusks, worms, newts, frogs and toads [5,6,7,8,9]. To date, however, reported occurrences of TTX in bivalve mollusks (clams, cockles, mussels, oysters, scallops and others) are very few. TTX has been determined in New Zealand Paphies australis clams [10] and in Japanese scallops [11]. Similarly, reported occurrences in European seafood are very limited. TTX was first detected in 2007, in the course of a non-fatal human intoxication following consumption of contaminated sea snails Charonia lampas lampas (a gastropod) harvested in Spain [12]. Very recently, TTX presence has been reported in bivalve mollusks (mussels and Pacific oysters) harvested from the south coast of England, along the Channel [13].
Due to the wide TTX distribution among genetically-unrelated animals, combined with individual, regional and seasonal variation in TTX levels, the exact origin of TTX in the food chain has been long debated, but still the source of the toxin remains practically unknown [5,14]. In contrast to the rest of biotoxins that accumulate in fishery products, TTX production has not been linked until now to any microalgal organism. Contribution of symbiotic bacteria has been suggested to play a role in TTX genesis for marine animals [15]. Such symbiotic bacteria, including Shewanella algae, S. putrefaciens, Vibrio sp., Pseudomonas sp., and Alteromonas tetraodonis, are suggested to accumulate in the subcutaneous mucus, or in the intestine, releasing the TTX [16,17]. This was later confirmed by the isolation of TTX-producing bacteria from different TTX-bearing animals [13,18,19].
Prorocentrum minimum (Pavillard) Schiller (previously reported also as Exuviaella mariae-lebouriae Parke and Ballantine and P. mariae-lebouriae), a phytoplanctonic dinoflagellate known to cause red tides, is widely distributed in the seas and oceans of the northern hemisphere. P. minimum is generally considered harmless; however, episodes of shellfish and human poisoning have been so far reported in Japan, Portugal and Norway. In Japan, a mass-poisoning incident occurred at Lake Hamana in March 1942 after consumption of short-necked clams (Venerupis semidecussata), causing death in 114 out of 324 persons affected [20]. A total of seventy-one deaths in the same region were later attributed to ingestion of toxic oysters (Crassostrea gigas) in March 1943 and of toxic clams in March 1949. Symptoms included heavy liver injury (necrosis and fatty degeneration), hemorrhagic diathesis with frenzy, unconsciousness and coma, and death occurring within 24–48 h of symptoms initiation [21]. P. minimum (E. mariae-lebouriae) was attributed as the toxin source, after establishing a relationship between shellfish toxicity and seawater abundance of these dinoflagellates [20,22,23,24]. A nitrogenous toxic substance, which produced symptoms in mice similar to those observed in humans, was isolated by Akiba and Hattori [25] from the mid-gut of the short-necked clam. This substance was named “venerupin” and the associated syndrome was assigned as Venerupin Shellfish Poisoning (VSP) [21]. Much later, P. minimum (identified initially as Exuviaella baltica and later as Prorocentrum balticum) was incriminated for a number of human poisoning episodes in Portugal (Obidos Lagoon) following shellfish consumption. The symptoms were characterized as resembling those of paralytic shellfish poisoning (PSP) [26,27,28]. Reported events with PSP-like symptomatology in the Zhengjiang and Fujiang provinces from 1978 to 1985 were also attributed to P. minimum, but details are scarce [29]. Similarly, P. minimum was identified as the cause in a case of poisoning after mussels consumption in the Oslofjord, Norway in 1979, as it had bloomed several weeks before in the mussel harvesting area. The symptoms (especially nausea and late gastrointestinal disorders) resembled in nature and development to those of VSP. Preliminary toxin bioassays on mussels sampled from the fjord showed signs of toxicity, with a symptomatology very similar to that of VSP, but less severe [30,31]. Toxin presence has been occasionally investigated at the same time with a P. minimum bloom. Kimor et al. [32], for instance, failed to find any toxicity in Baltic Sea dinoflagellates, whereas mouse tests revealed only very minor symptoms of toxicity in Black Sea mussels [33]. Red tides caused by P. minimum or E. baltica (actually P. minimum) have also been associated with various effects on marine organisms. Blooms were regarded as highly toxic, since fish and other marine animals died or were forced to flee [27,34,35]. In one case, however, these deaths occurred three days after reduction of dissolved oxygen [34]. It should be noted that in both of these cases, further studies were not undertaken to clarify the source of toxicity.
Effects of algal blooms on shellfish and aquaculture have been reviewed by Shumway [36], who suggested that toxic effects have been often associated with P. minimum blooms and that the toxin accumulated in shellfish was able to affect both shellfish harvested from bloom water and humans consuming the shellfish. So far, however, the only unambiguous link between blooms of P. minimum and shellfish toxicity has been documented by Grzebyk et al. [37] in a French Mediterranean site. In that study, a number of P. minimum axenic clones, producing a water-soluble neurotoxic component that rapidly killed mice, were isolated. Prior to that, mussel toxicity had been detected in the Sete region on the French Mediterranean coast during a large bloom of P. minimum and P. micans [37]. The symptomatology observed in mouse bioassays indicated a rapid neurological effect; however, it was not possible to provide clear evidence that the dinoflagellates were actually the source of this toxicity. Subsequently though, Denardou-Queneherve et al. [38] documented the same toxic fraction obtained from shellfish during a large P. minimum bloom (56 × 106 cells/L), which occurred in the Salses-Leucate lagoon on the French Mediterranean coast. Despite the fact that this research group provided a substantial contribution towards clarification of the P. minimum toxin properties, the nature and structure of the toxin was never identified.
During official shellfish control for the presence of marine biotoxins in Greece in year 2012, a series of unexplained positive mouse bioassays (MBA) for lipophilic toxins with nervous symptomatology prior to mice death was observed in mussels from Vistonikos Bay–Lagos, Rodopi. This atypical toxicity coincided with the absence or presence in trace levels of all known lipophilic, PSP and ASP group toxins in mussels, as well as with the presence of P. minimum at levels up to 1.89 × 103 cells/L in the area’s seawater, whereas further analyses by different MBA protocols indicated that the unknown toxin was of hydrophilic nature. In this context, the purpose of this work was: (a) to investigate this episode with regard to the nature of the toxin(s) causing this atypical toxicity and (b) to investigate toxin profiles of shellfish from previous years (2006–2012) from different Greek production areas, collected in time periods when P. minimum was present at high abundances.
2. Results
2.1. The 2012 P. minimum Episode in Vistonikos Bay–Lagos, Rodopi, Greece
2.1.1. Episode History and Preliminary Investigation
In late spring-early summer of 2012, a series of four consecutive mussel samples (1770/2012; 1774/2012; 1786/2012 and 1798/2012) derived from the sampling site Zone 6, Vistonikos Bay–Lagos (regional unit of Rodopi, Greece; Table 1, Figure 1A) tested positive in the Yasumoto 1978 (Yas’78) protocol for lipophilic toxins [39], presenting an atypical nervous symptomatology prior to mice death, whereas all four tested negative to the MBA for PSP toxins and below the limit of detection (0.1 mg/kg) for the presence of ASP toxins. During the same time period, phytoplankton counts for all known species associated with the production of regulated marine biotoxin groups (lipophilic, paralytic and amnesic toxins) were all either undetectable or present at low levels not adequate to account for any shellfish toxicity. The only microalgal species present at considerable concentrations was Prorocentrum minimum (syn. = P. cordatum, as the currently taxonomically accepted name; [40]) reaching levels up to 1.89 × 103 cells/L (see Table 2).
Table 1.
Locations of sampling stations included in the study for each coastal area expressed by geographical coordinates (longitude and latitude).
| Coastal Area | Regional Unit | Sampling Station | Latitude (N) | Longitude (E) |
|---|---|---|---|---|
| Vistonikos Bay–Lagos | Rodopi | Zone 6 | 40°58'23.73" | 25°07'06.56" |
| Keramoti Bay | Kavala | Keramoti | 40°52'00.70" | 24°40'34.60" |
| Strymonikos Bay | Serres | S1—Nea Kerdyllia | 40°46'46.49" | 23°49'50.00 |
| Chalkidiki | X2—Olympiada | 40°33'00.60" | 23°50'00.00" | |
| Thermaikos Gulf | Thessaloniki | Kymina | 40°30'00.22" | 22°41'00.06" |
| Saronikos Gulf | Attiki/Nisson | Zone I—Vasilika–Faneromeni | 37°59'42.29" | 23°27'50.06" |
| Zone IV—Agios Georgios | 37°58'09.89" | 23°25'53.92" | ||
| Dytiki Attiki/Megara | Neraki | 38°01'30.19" | 23°28'52.79" | |
| Drepano | 37°59'01.02" | 23°24'30.09" |
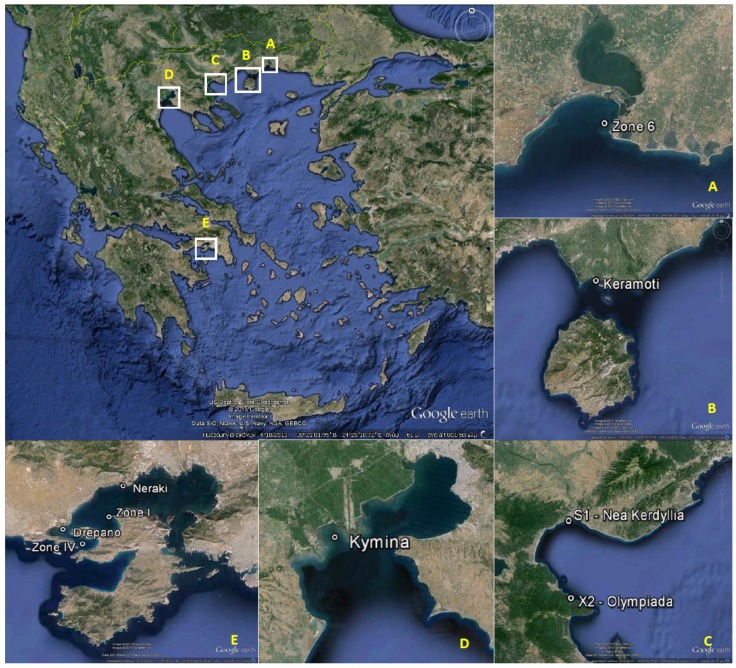
Figure 1.
Maps showing locations of sampling stations: (A) Vistonikos Bay–Lagos (Rodopi); (B) Keramoti Bay (Kavala); (C) Strymonikos Bay (Serres, Chalkidiki); (D) Thermaikos Gulf (Thessaloniki); and (E) Saronikos Gulf (Dytiki Attiki/Megara, Attiki/Nisson). Round marks (•) depict the location of sampling stations.
Table 2.
Sampling data and analysis results of the 2012 P. minimum episode in Vistonikos Bay–Lagos, Rodopi, Greece.
| Sampling data | Prorocentrum minimum (cells/L) | MBA (Yasumoto1978 [39]) | MBA (Other) | UPLC-MS/MS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample code | Date of collection | Regional Unit | Site | Species | Result | Symptomatology | Protocol | Result | Symptomatology | Tissue tested | TTX (μg/kg) | 4-epi-TTX (μg/kg) | 4,9-anhydro-TTX (μg/kg) | Sum of TTXs (μg/kg) | |
| 1727/2012 | 7 May 2012 | Rodopi | Zone 6 | Mussels | Presence | Negative (− − −) | No symptomatology | N/A | N/A | N/A | DG | 46.2 | ND | ND | 46.2 |
| 1770/2012 | 22 May 2012 | Rodopi | Zone 6 | Mussels | 8.26× 102 | Positive (+ + +) | 3/3 mice dead in 1:00–1:30 h | CRL-MBA-SOP v.5 (DEE fraction) | Negative (− − −) | None | DG | 202.9 | 7.6 | <LOQ (12.4) * | 222.9 |
| WF | 179.1 | <LOQ (4.2) * | <LOQ (10.3) * | 193.6 | |||||||||||
| 1774/2012 | 28 May 2012 | Rodopi | Zone 6 | Mussels | 1.78 × 103 | Positive (+ + +) | 3/3 mice dead in 1:30–2:00 h | CRL-MBA-SOP v.5 (DEE fraction) | Negative (− − −) | 2/3 mice with mild diarrhea | DG | 185.9 | <LOQ (4.5) * | <LOQ (15.9) * | 206.3 |
| 1786/2012 | 30 May 2012 | Rodopi | Zone 6 | Mussels | 1.70 × 103 | Positive (+ + +) | 3/3 mice dead (1/3 in 1:15–1:30 h, 1/3 in 1:48 h & 1/3 in 1:51 h) | CRL-MBA-SOP v.5 (DEE fraction) | Negative (− − −) | 1/3 mice with mild diarrhea | DG | 180.0 | <LOQ (6.8) * | <LOQ (14.5) * | 201.4 |
| CRL-MBA-SOP v.5 (aqueous fraction) | Positive (+ + +) | 3/3 mice dead in 7–10 min | |||||||||||||
| 1798/2012 | 6 Jun 2012 | Rodopi | Zone 6 | Mussels | 1.89 × 103 | Positive (+ + +) | 3/3 mice dead (1/3 in 1:31 h, 1/3 in 2:30 h & 1/3 in 2:31) | CRL-MBA-SOP v.5 (DEE fraction) | Negative (− − −) | None | DG | 186.2 | 15.6 | <LOQ (14.7) * | 216.6 |
| 1801/2012 | 11 Jun 2012 | Rodopi | Zone 6 | Mussels | 5.65 × 102 | Negative (− − −) | 2/3 mice with diarrhoea | CRL-MBA-SOP v.5 (ether fraction) | Negative (− − −) | 1/3 mice with diarrhea | DG | 56.5 | 8.8 | 22.9 | 88.2 |
MBA = Mouse bioassay, TTX = tetrodotoxin, DG = digestive glands, WF = whole flesh, DEE = diethylether, N/A = not applicable, ND = not detected. Values with an asterisk (*) indicate concentrations between the calculated method LOD and LOQ and are shown as contributions to the sum of TTXs.
According to the documented procedures of the Greek National Reference Laboratory on Marine Biotoxins (NRLMB) at that time and due to the fact that Regulation (EC) 15/2011 [41] was already in place, according to which regulatory monitoring decisions with regard to lipophilic toxins have to be based on the results of the LC-MS/MS method (EU-Harmonised SOP-LIPO-LC-MS/MS, Version 4, European Reference Laboratory for Marine Biotoxins, Vigo, Spain) in case of conflict, samples exhibiting atypical responses in the routine Yas’78 MBA protocol, were as a rule also tested the following ways.
(a) The harmonized MBA protocol of the EU Reference Laboratory on Marine Biotoxins (CRL-MBA-SOP, version 5; [42]), which includes a partitioning step between diethylether (DEE) and water. This partitioning step is necessary to confirm the lipophilic nature of a toxin, in case of a positive result. In case of a negative result, however, it could be indicative of interferences due to the presence of hydrophilic toxins (e.g., PSPs), which are quite common and documented for the Yasumoto1978 protocol; and (b) the UPLC-MS/MS in order to confirm the presence of regulated lipophilic biotoxin groups (okadaic acid (OA) group comprising OA, dinophysistoxins (DTXs) and pectenotoxins (PTXs) together, yessotoxin (YTX) group and azaspiracids (AZA) group), as well as non-regulated ones, which can be determined by this method, such as gymnodimines (GYM), spirolides (SPX) and pinnatoxins (PnTXs).
Results of the harmonized MBA protocol tests (injection of DEE fraction) on the four positive samples (1770/2012, 1774/2012, 1786/2012 and 1798/2012) are presented in Table 2. In all four samples, a negative result was obtained, with mice surviving the whole 24-h observation period and either being completely asymptomatic or presenting minor clinical symptoms, such as diarrhea. In one of the samples (1786/2012), the aqueous fraction from the CRL-MBA-SOP protocol was also tested, producing rapid deaths in all three injected mice (<10 min) with severe symptomatology from the nervous system before death. Analysis by UPLC-MS/MS for the presence of lipophilic toxins, on the other hand, showed only trace amounts of OA, GYM, SPX and PnTXs (OA: <20 μg/kg, GYM: <0.2 μg/kg, SPX: <0.2 μg/kg and PnTXs: <0.1 μg/kg), which cannot account for mouse toxicity. The samples were thus designated as negative to the presence of regulated lipophilic toxins and therefore safe for human consumption, in terms of regulatory monitoring. Based on these results, no sanitary measures could be taken by the respective regional competent authority. All the above findings, could not justify the toxicity observed in the Yasumoto 1978 MBA protocol, but also strongly indicated that the toxic substance(s) producing the deaths was most probably of a hydrophilic nature.
The fact that a series of four consecutive samples presented: (a) positive MBA responses in the Yas’78 protocol with similar atypical nervous symptomatology and death times; (b) similar results in the preliminary investigations by the CRL-MBA-SOP protocol and by UPLC-MS/MS or lipophilic toxins; and (c) all coinciding with a bloom of a species bibliographically documented as potentially toxic, at least in a few, even limited, cases, triggered our interest in further investigation of the episode. For this reason, methanolic extracts of all samples and whole tissue of sample 1786/2012 were forwarded to the University of Santiago de Compostela (USC) for cross-checking and further testing. Analysis for the presence of lipophilic toxins by UPLC-MS/MS confirmed the NRLMB findings, similarly detecting the presence of OA, GYM, SPX and PnTXs in trace amounts. On the other hand, analysis by HPLC-FLD for the presence of PSP toxins in sample 1786/2012 revealed the presence of GTX5 at a concentration equivalent to 1.32 μg STX eq/kg, thus not able to account for the presence of toxicity in the MBA. Finally, analysis by LC-MS/MS for the presence of tetrodotoxins by the method of Rodriguez et al. [8], resulted in the detection of TTX analogues in that sample and thus a relevant UPLC-MS/MS method for the presence of TTXs was developed in NRLMB to be employed for a full investigation of the episode.
2.1.2. Performance Parameters and Quality Control of the UPLC-MS/MS Method for TTXs
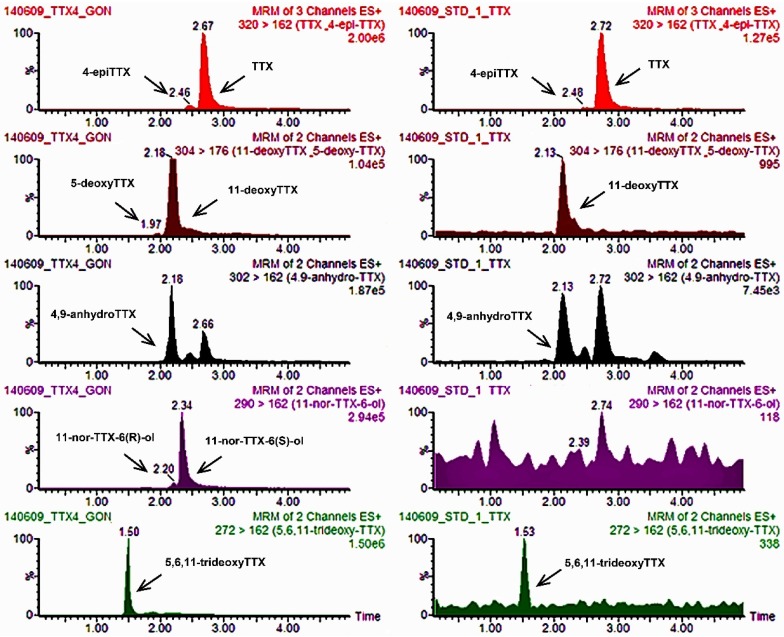
The certified TTXs (standard containing TTX), 4-epiTTX and 4,9-anhydroTTX, together with naturally contaminated gonad and muscle samples from Lagocephalus sceleratus pufferish used in former works [8], provided us with the retention times (RTs) of TTX and its analogues. Values presented for each compound are the mean RT and range from minimum to maximum (in brackets): TTX = 2.73 min (2.56–2.85 min); 4-epiTTX = 2.53 min (2.43–2.65 min); 4,9-anhydroTTX = 2.20 min (2.10–2.34 min); 5,6,11-trideoxyTTX = 1.53 min (1.42–1.61 min); 5-deoxyTTX = 2.02 min (1.92–2.10 min); 11-deoxyTTX = 2.24 min (2.13–2.34 min); 11-norTTX-6(R)-ol = 2.26 min (2.15–2.36 min); and 11-norTTX-6(S)-ol = 2.41 min (2.28–2.49 min) (Figure 2). RTs of TTXs in tested samples when compared to those of the reference standards and naturally contaminated materials were always within the ±2.5% tolerance limit allowed for LC-MS methods in compliance to the requirements of EU Decision 2002/657/EEC [43] for each day of analysis.
Figure 2.
UPLC-MS/MS chromatograms (TIC) of: (a) naturally contaminated gonads ample from L. sceleratus pufferfish; and (b) the certified tetrodotoxins (TTXs) reference standard (TTX = 2000 ng/mL; 4-epiTTX = 49.86 ng/mL; 4,9-anhydroTTX = 467.4 ng/mL). Peak scale has been adjusted in some of the chromatograms to allow presentation of lower concentration peaks.
Solutions of TTXs certified reference standard showed excellent linearity within the whole range of standard concentrations for all three analogues contained in the reference standard (TTX, 4,9-anhydroTTX and 4-epiTTX), with R2 values higher than 0.995 in all analysis days. The limits of detection (LOD; S/N > 3) and limits of quantification (LOQ; S/N > 10) of the method obtained from the standard solutions were: TTX = 0.6 and 2.2 ng/mL; 4-epiTTX = 0.7 and 2.3 ng/mL and 4,9-anhydroTTX = 1.9 and 6.5 ng/mL, respectively, corresponding to TTX = 2.2 and 7.2 μg/kg; 4-epiTTX = 2.3 and 7.6 μg/kg and 4,9-anhydroTTX = 6.2 and 21.1 μg/kg in shellfish tissue, respectively.
Trueness of the method, assessed through TTX recovery (%) was within the acceptable levels indicated by the EU Decision 2002/657/EEC [43] in all five levels tested and for both types of tissue (DG or WF) used in the study (Table 3).
Table 3.
Mean TTX recovery data for mussel digestive gland (DG) and whole flesh (WF) fortified at five concentrations (n = 6).
| TTX Concentration and Recovery | Tissue | TTX Fortification Level | ||||
|---|---|---|---|---|---|---|
| 100 μg/kg | 200 μg/kg | 400 μg/kg | 800 μg/kg | 1200 μg/kg | ||
| Mean TTX detected (μg/kg) | DG | 90.1 | 211.2 | 431.5 | 833.9 | 1171.5 |
| Mean Recovery (%) | 90.1 | 105.6 | 107.9 | 104.2 | 97.6 | |
| Mean TTX detected (μg/kg) | WF | 94.3 | 209.3 | 384.3 | 754.6 | 1211.0 |
| Mean Recovery (%) | 94.3 | 104.6 | 96.1 | 94.3 | 100.9 | |
2.1.3. Application of the UPLC-MS/MS Method to the Mussel Samples of the P. minimum Episode under Investigation
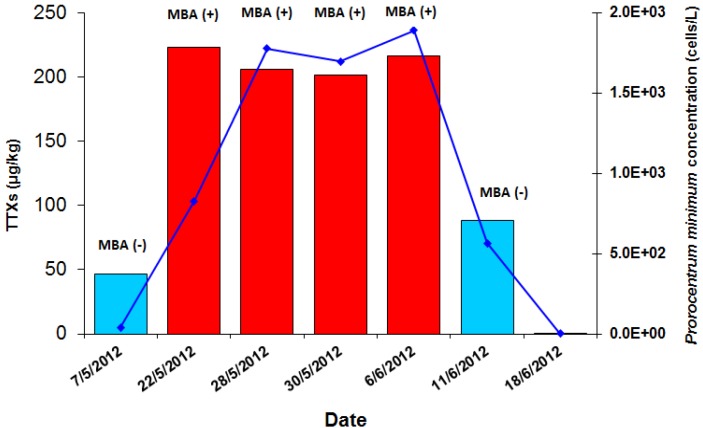
Results of the UPLC-MS/MS analyses for the presence of TTXs with regard to mussel samples obtained from the episode in question are presented in Table 2. The sum of TTXs concentrations (TTX, 4-epiTTX and 4,9-anhydroTTX) exceeded the level of 200 μg/kg in all four consecutive samples that tested positive in the Yas’78 protocol whereas the sum of TTXs in the two MBA negative ones (one sampled prior and one sampled after the positive ones) were much lower, with the highest concentration being 88.2 μg/kg, possibly coinciding with the decline of the episode. All remaining TTX analogues were either not detected or below the limit of quantification. A quite evident correlation can be observed when these results are graphically compared to the P. minimum concentrations recorded during that time period, indicating a possible link between the presence of this potentially toxic species and the presence of TTXs (Figure 3). Figure 4 shows a typical chromatogram of a sample containing TTX.
Figure 3.
TTXs concentrations (μg/kg) and mouse bioassay (MBA; Yasumoto 1978 protocol) results compared to Prorocentrum minimum concentrations (cells/L) of the 2012 shellfish toxicity episode in Vistonikos Bay–Lagos, Rodopi, Greece.
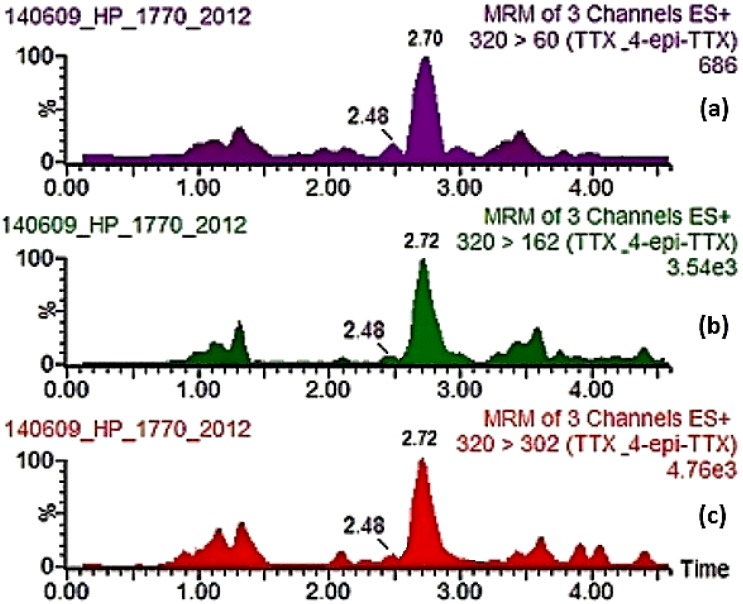
Figure 4.
UPLC-MS/MS chromatogram of mussel digestive gland sample 1770/2012 from Vistonikos Bay–Lagos, Rodopi, Greece, containing TTX and 4-epiTTX. (a) m/z 320 > 60; (b) m/z 320 > 162; and (c) m/z 320 > 302.
2.1.4. Experimentally TTX-Contaminated Digestive Gland Tissue Tested by MBA
Results of mouse testing with the Yasumoto1978 protocol on DG tissue experimentally contaminated with TTX at five levels are presented in Table 4. Mouse toxicity was observed down to the fortification level of 100 μg/kg, whereas the 50-μg/kg concentration was non-detectable. Mouse survival times of the experimentally contaminated tissue at the level of 200 μg/kg correlated rather well with those of the MBA positive samples of the 2012 Rodopi episode in which TTXs concentrations were at similar levels.
Table 4.
Mouse bioassay (MBA) data for blank mussel digestive gland fortified with TTX at five concentrations and tested by the Yasumoto1978 MBA protocol (n = 6).
| TTX Fortification Level (μg/kg) | Mice Dead | Mouse Survival Time | |
|---|---|---|---|
| Median | Range | ||
| 0 | 0/6 | >24 h | >24 h |
| 50 | 0/6 | >24 h | >24 h |
| 100 | 4/6 | 310 min | 260 min→24 h |
| 200 | 6/6 | 85 min | 72–98 min |
| 360 | 6/6 | 33 min | 22–37 min |
| 1000 | 6/6 | 9 min | 7.5–10 min |
2.2. Monitoring of Vistonikos Bay–Lagos and Neighboring Coastal Areas in 2014
Due to its previous history with the 2012 aforementioned episode, the coastal area of Vistonikos Bay–Lagos, Rodopi (sampling site Zone 6, Figure 1A) was selected for intensive monitoring of TTXs concentrations for a period of 3 months (8 April to 16 July, 2014), in combination with MBA testing by the Yasumoto1978 protocol and UPLC-MS/MS routine testing for lipophilic toxins, at a weekly basis according to the national monitoring program. The monitoring period was specifically chosen to include the respective time period (end of May–beginning of June) that the previous episode had occurred, in order to also comply with the requirements of Regulation (EC) 15/2011 [41] regarding the “periodic monitoring of production areas and relaying areas for detecting new or unknown marine toxins”. Two samples from the wider coastal area of North Aegean Sea (Strymonikos Gulf, Figure 1C) were also tested for the same purpose. It should be noted that during this period there were no significant occurrences of P. minimum indicating a bloom.
MBA results and TTX concentrations of the samples obtained during this 3-month monitoring period are presented in Table 5. During the whole monitoring period, there was only one MBA positive sample (2290/2014) and a negative one (2310/2014) with mice presenting a bad clinical condition. These results were not attributed to the presence of TTXs, as their concentrations were below 50 μg/kg, thus non-detectable by the MBA protocol employed, but could be due to either a synergistic action of the lipophilic toxin groups determined by UPLC-MS/MS analysis in these two samples ((a) sample 2290/2014: OA group = 41.8 μg/kg, YTXs = 0.79 mg/kg, GYMs = 4.8 μg/kg, SPXs = 1.1 μg/kg and PnTX-G = 5.1 μg/kg and (b) 2310/2014: OA group = 51.1 μg/kg, GYMs = 4.7 μg/kg, SPXs = 1.6 μg/kg and PnTX-G = 7.0 μg/kg) or to the presence of other toxins, non-detectable by this method. Nevertheless, it should be noted that low TTXs concentrations (<50 μg/kg) were recorded in all tested samples from this coastal area, indicating a steady presence of this toxin group in shellfish produced in this location.
Table 5.
Sampling data and analysis results of the 2014 three-month monitoring of Vistonikos Bay–Lagos, Rodopi, Greece and neighboring areas.
| Sampling Data | MBA (Yasumoto1978) | UPLC-MS/MS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Sample Code | Date of Collection | Regional Unit | Site | Species | Result (24 h) | Symptomatology | Tissue Tested | TTX (μg/kg) | 4-epi-TTX (μg/kg) | 4,9-anhydro-TTX (μg/kg) | Sum of TTXs (μg/kg) |
| 2014 | 2084/2014 | 08 April 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | None | WF | 43.4 | ND | ND | 43.4 |
| 2014 | 2126/2014 | 24 April 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | None | WF | 38.9 | ND | ND | 38.9 |
| 2014 | 2141/2014 | 29 April 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | None | WF | 34.9 | ND | ND | 34.9 |
| 2014 | 2167/2014 | 07 May 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | None | WF | 37.7 | ND | ND | 37.7 |
| 2014 | 2172/2014 | 08 May 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | None | WF | 34.8 | ND | ND | 34.8 |
| 2014 | 2203/2014 | 20 May 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | None | WF | 30.1 | ND | ND | 30.1 |
| 2014 | 2239/2014 | 28 May 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | 2/3 mice with mild diarrhea | WF | 25.8 | ND | ND | 25.8 |
| 2014 | 2232/2014 | 28 May 2014 | Serres | S1: Nea Kerdyllia | Mussels | Negative (− − −) | None | WF | 37.9 | 9.1 | ND | 47.0 |
| 2014 | 2234/2014 | 28 May 2014 | Chalkidiki | X2: South Strymonikos Gulf—Olympiada | Mussels | Negative (− − −) | None | WF | 30.6 | <LOQ (3.2) * | ND | 33.8 |
| 2014 | 2270/2014 | 11 June 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | 3/3 mice with diarrhea | WF | 28.4 | ND | ND | 28.4 |
| 2014 | 2290/2014 | 19 June 2014 | Rodopi | Zone 6 | Mussels | Positive (− + +) | 1/3 mice dead in 3:30–19:30 h, 1/3 dead in 21:30–22:00 h | WF | 36.8 | ND | ND | 36.8 |
| 2014 | 2310/2014 | 25 June 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | 3/3 mice with bad clinical condition | WF | 25.1 | ND | ND | 25.1 |
| 2014 | 2319/2014 | 02 July 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | 3/3mice with bad clinical condition and diarrhea | WF | 33.3 | ND | ND | 33.3 |
| 2014 | 2336/2014 | 08 July 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | 3/3 mice with diarrhea | WF | 25.0 | ND | ND | 25.0 |
| 2014 | 2362/2014 | 16 July 2014 | Rodopi | Zone 6 | Mussels | Negative (− − −) | None | WF | 37.6 | ND | ND | 37.6 |
MBA = Mouse bioassay, TTX = tetrodotoxin, WF = whole flesh, ND = not detected. Values with an asterisk indicate concentrations between the calculated method LOD and LOQ and are shown as contributions to the sum of TTXs.
2.3. Investigation of Routine Shellfish Samples from 2006–2012 Obtained in Periods of P. minimum Increased Presence
Reviewing of official control data from the years 2006–2012 identified 17 shellfish samples with atypical positive to asymptomatic negative Yas’78 MBAs for lipophilic toxins, obtained from sampling sites of different Greek production areas (Figure 1) near periods where P. minimum blooms or high concentrations (>1000 cells/L) were recorded. UPLC-MS/MS analysis of retained digestive gland or whole flesh sub-samples from these cases already showed the presence of TTXs (TTX, 4-epiTTX and 4,9-anhydroTTX) in Greek shellfish in 2006, in concentrations ranging between 61.0–194.7 μg/kg (Table 6). All remaining TTX analogues were either not detected or below the limit of quantification. A connection of the TTXs concentrations determined with the presence of P. minimum could not be shown in these cases, as presence of this potentially toxic species at that time was not steadily reported in terms of the weekly monitoring program. However, the fact that TTXs concentrations in all these cases were higher than 60 μg/kg, in contrast to the results presented in Section 2.2, in combination with the fact that the highest TTXs concentration (194.7 μg/kg) was recorded in an MBA positive sample with death times and symptomatology resembling those of the samples from the 2012 episode in Rodopi (see Section 2.1.3) could point to a possible relationship between P. minimum presence in seawater and TTX accumulation in shellfish.
Table 6.
Sampling data and analysis results of routine shellfish samples from 2006–2012 obtained in periods of P. minimum presence.
| Sampling Data | Prorocentrum minimum (cells/L) | MBA (Yasumoto1978) | UPLC-MS/MS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Sample Code | Date of Collection | Regional Unit | Site | Species | Result (24 h) | Symptomatology | Tissue Tested | TTX (μg/kg) | 4-epi-TTX (μg/kg) | 4,9-anhydro-TTX (μg/kg) | Sum of TTXs (μg/kg) | |
| 2006 | 1357/2006 | 20 March 2006 | Dytiki Attiki/ Megara | Drepano | Mussels | No data provided | Positive (+ + +) | 3/3 mice dead in <15 h | DG | 70.7 | <LOQ (6.7) * | ND | 77.4 |
| 2006 | 1366/2006 | 11 April 2006 | Dytiki Attiki/ Megara | Neraki | Mussels | 3.50 × 104 | Positive (+ + −) | 2/3 mice dead in <15 h | DG | 68.4 | <LOQ (3.4) * | ND | 71.8 |
| 2006 | 1367/2006 | 11 April 2006 | Dytiki Attiki/ Megara | Drepano | Mussels | 7.50 × 104 | Negative (+ − −) | 1/3 mice dead in <15 h, 1/3 mild diarrhea | DG | 61.2 | <LOQ (7.5) * | ND | 68.8 |
| 2008 | 1547/2008 | 9 September 2008 | Dytiki Attiki/ Megara | Drepano | Mussels | 7.50 × 104 | Positive (+ + −) | 1/3 mice dead in 2:00–12:30 h, 1/3 in 23:00–23:45 h, 1/3 with bad clinical condition and diarrhea (and death in 26:00–36:30 h) | DG | 55.4 | <LOQ (4.7) * | <LOQ (12.4) * | 72.5 |
| 2008 | 1583/2008 | 6 October 2008 | Dytiki Attiki/ Megara | Neraki | Mussels | No data provided. Bloom in previous month | Negative (− − −) | 3/3 mice with bad clinical condition and diarrhea (and death 2/3 in 26:00–35:30 h and 1/3 in 42:30–44:00 h) | DG | 52.9 | <LOQ (4.3) * | <LOQ (10.8) * | 68.0 |
| 2008 | 1590/2008 | 13 October 2008 | Dytiki Attiki/ Megara | Neraki | Mussels | No data provided. Bloom in previous month | Positive (+ + +) | 1/3 mice dead in 2:00–2:30 h, 1/3 in 2:20–2:40 h, 1/3 in 2:40–3:30 h | DG | 65.6 | <LOQ (4.7) * | <LOQ (15.1) * | 85.4 |
| 2008 | 1594/2008 | 14 October 2008 | Attiki/Nisson | Salamina—Zone Ι—Vasilika–Faneromeni | Venus clams | Bloom in the wider area during the previous month (Neraki, Drepano) | Positive (+ + +) | 3/3 mice dead in 2:00–2:30 h | DG | 176.5 | <LOQ (3.9) * | <LOQ (14.3) * | 194.7 |
| 2008 | 1607/2008 | 23 October 2008 | Dytiki Attiki/ Megara | Neraki | Mussels | 3.68 × 105 | Negative (− − −) | 2/3 bad clinical condition and diarrhea, 1/3 bad clinical condition and mild diarrhea | DG | 62.6 | <LOQ (4.9) * | <LOQ (18.6) * | 86.2 |
| 2008 | 1625/2008 | 4 November 2008 | Attiki/Nisson | Salamina—Zone ΙV—Agios Georgios | Mussels | Simultaneous bloom in the wider area (Neraki: 1.11 × 104) | Negative (− − −) | 1/3 mice with bad clinical condition and diarrhea, 2/3 bad clinical condition and mild diarrhea (and death 1/3 in 27:35–37:10 h and 1/3 in 45:00–46:20 h) | DG | 57.2 | <LOQ (3.8) * | ND | 61.0 |
| 2009 | 1544/2009 | 16 June 2009 | Rodopi | Zone 6 | Mussels | Bloom in the previous month (6 May 2009: 1.13 × 105 & 12 May 2009: 8.24 × 103) | Positive (+ + +) | 2/3 mice dead in 13:00–21:00 h, 1/3 in 21:00–23:00 h | DG | 46.7 | <LOQ (5.4) * | <LOQ (19.2) * | 71.3 |
| 2009 | 1552/2009 | 22 June 2009 | Rodopi | Zone 6 | Mussels | No data provided | Negative (− − −) | 3/3 mice with bad clinical condition and diarrhea (and death 3/3 in 29:20–46:20 h) | DG | 48.1 | <LOQ (5.0) * | <LOQ (15.9) * | 69.0 |
| 2009 | 1556/2009 | 24 June 2009 | Rodopi | Zone 6 | Mussels | No data provided | Positive (+ + −) | 2/3 mice dead in 1:30–2:00 h, 1/3 with bad clinical condition and diarrhea (and death in 30:00–48:00 h) | DG | 52.6 | <LOQ (3.5) * | <LOQ (8.6) * | 64.8 |
| 2012 | 2057/2012 | 6 November 2012 | Rodopi | Zone 6 | Mussels | No data provided. Bloom reported in the following 2 weeks, max. 5.36 × 105 | Negative (− − −) | 2/3 mice with bad clinical condition and mild diarrhea, 1/3 mice with bad clinical condition | DG | 68.5 | <LOQ (3.4) * | <LOQ (12.0) * | 83.9 |
| 2012 | 1216/2012 | 22 November 2012 | Kavala | Keramoti | Mussels | No data provided. Bloom reported in the following weeks | Positive (+ − +) | 2/3 mice dead in 4:00–20:00 h, 1/3 mice with bad clinical condition | DG | 71.0 | <LOQ (3.4) * | <LOQ (20.7) * | 95.0 |
| 2012 | 1224/2012 | 10 December 2012 | Kavala | Keramoti | Mussels | 3.13 × 104 | Negative (− − +) | 1/3 mice dead in 2:00–18:00 h, 2/3 mice with bad clinical condition and mild diarrhea | DG | 71.2 | <LOQ (5.2) * | <LOQ (11.7) * | 88.2 |
| 2012 | 1227/2012 | 17 December 2012 | Kavala | Keramoti | Mussels | 1.56 × 104 | Negative (− − −) | 2/3 mice with bad clinical condition and mild diarrhea, 1/3 mice with bad clinical condition | DG | 91.8 | <LOQ (4.3) * | <LOQ (13.2) * | 109.2 |
| 2012 | 1241/2012 | 3 December 2012 | Thessaloniki | Kymina | Mussels | 174 (in wider area 1.75 × 105) | Negative (− − −) | None | WF | 79.5 | <LOQ (5.6) * | <LOQ (18.0) * | 103.1 |
MBA = Mouse bioassay, TTX = tetrodotoxin, DG = digestive glands, WF = whole flesh, ND = not detected. Values with an asterisk (*) indicate concentrations between the calculated method LOD and LOQ and are shown as contributions to the sum of TTXs.
2.4. Investigation of Random Routine Shellfish Samples Obtained in June–July 2014
Digestive gland and whole flesh samples from a number of randomly selected samples obtained in June and July 2014 from the same areas (except Rodopi) where previous presence of TTXs was recorded (see Section 2.2 and Section 2.3) were also analyzed by UPLC-MS/MS for the presence of TTXs (Table 7). TTXs were not detectable or below the limit of quantification in these samples, some of which served as negative tissue material for the fortification experiments of the study.
Table 7.
Sampling data and analysis results of random routine shellfish samples obtained in June–July 2014.
| Sampling Data | MBA (Yasumoto1978) | LC-MS/MS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Sample Code | Date of Collection | Regional Unit | Site | Species | Result (24 h) | Symptomatology | Tissue Tested | TTX (μg/kg) | 4-epi-TTX (μg/kg) | 4,9-anhydro-TTX (μg/kg) |
| 2014 | 2303/2014 | 23 June 2014 | Chalkidiki | X2: South Strymonikos Gulf—Olympiada | Mussels | Negative (− − −) | 3/3 mice with diarrhea | HP/WF | <LOQ | ND | ND |
| 2014 | 272/2014 | 30 June 2014 | Thessaloniki | Kymina | Mussels | Negative (− − −) | None | HP/WF | ND | ND | ND |
| 2014 | 2313/2014 | 30 June 2014 | Dytiki Attiki/ Megara | Drepano | Mussels | Negative (− − −) | 3/3 mice with bad clinical condition and diarrhea | HP | ND | ND | ND |
| 2014 | 2315/2014 | 30 June 2014 | Dytiki Attiki/ Megara | Neraki | Mussels | Negative (− − −) | None | HP/WF | ND | ND | ND |
| 2014 | 1303/2014 | 01 July 2014 | Kavala | Keramoti | Mussels | Negative (− − −) | None | HP/WF | <LOQ | ND | ND |
| 2014 | 2323/2014 | 01 July 2014 | Serres | S1: Nea Kerdyllia | Mussels | Negative (− − −) | None | HP/WF | <LOQ | ND | ND |
| 2014 | 283/2014 | 07 July 2014 | Thessaloniki | Kymina | Mussels | Negative (− − −) | 1/3 mice with mild diarrhea | HP/WF | ND | ND | ND |
| 2014 | 2332/2014 | 07 July 2014 | Dytiki Attiki/ Megara | Drepano | Mussels | Negative (− − −) | 3/3 mice with bad clinical condition | HP | ND | ND | ND |
| 2014 | 2334/2014 | 07 July 2014 | Dytiki Attiki/ Megara | Neraki | Mussels | Negative (− − −) | None | HP/WF | ND | ND | ND |
| 2014 | 2337/2014 | 07 July 2014 | Chalkidiki | X2: South Strymonikos Gulf—Olympiada | Mussels | Negative (− − −) | 1/3 mice with bad clinical condition | HP | <LOQ | ND | ND |
| 2014 | 1307/2014 | 08 July 2014 | Kavala | Keramoti | Mussels | Negative (− − −) | None | HP | ND | ND | ND |
| 2014 | 2342/2014 | 08 July 2014 | Serres | S1: Nea Kerdyllia | Mussels | Negative (− − −) | 1/3 mice with diarrhea | HP | ND | ND | ND |
| 2014 | 297/2014 | 14 July 2014 | Thessaloniki | Kymina | Mussels | Negative (− − −) | 1/3 mice with diarrhea, 2/3 mild diarrhea | HP | ND | ND | ND |
| 2014 | 1311/2014 | 15 July 2014 | Kavala | Keramoti | Mussels | Negative (− − −) | 1/3 mice with bad clinical condition | HP | ND | ND | ND |
| 2014 | 2360/2014 | 15 July 2014 | Dytiki Attiki/ Megara | Drepano | Venus clams | Negative (− + −) | 1/3 mice dead in 1:05 h, 1/3 diarrhea | HP/WF | ND | ND | ND |
| 2014 | 2368/2014 | 15 July 2014 | Serres | S1: Nea Kerdyllia | Mussels | Negative (− − −) | None | HP/WF | ND | ND | ND |
3. Discussion
3.1. UPLC-MS/MS Method Performance
TTXs reference standard solutions exhibited excellent linearity in the developed UPLC-MS/MS method for the determination of TTXs over the whole calibration ranges of all three analogues, with R2 values higher than 0.995 for all analysis days. Mean TTX recovery at the five fortification levels (100, 200, 400, 800 and 1200 μg/kg) selected in the present study and for both tested matrices (mussel DG and WF) was good, ranging between 90.1% and 107.9%. These values are in compliance with the 90%–110% range indicated as acceptable recovery levels according to the requirements of EU Decision 2002/657/EEC [43], when recovery is assessed by fortification.
3.2. Presence of TTXs in Greek Shellfish
In the present study, concentrations of the sum of TTXs in a total of 38 shellfish samples selected for UPLC-MS/MS analysis ranged from 25.0 μg/kg up to a maximum of 222.9 μg/kg. TTX was the most abundant of all TTXs and was present in all 38 samples, at concentrations ranging from 25.0 to 202.9 μg/kg. The analogues 4-epiTTX and 4,9-anhydro TTX were quantified in lower concentrations, in 24 and 19 out of 38 samples, respectively. On the other hand, 5,6,11-trideoxyTTX and the remaining analogues were not found at quantifiable concentrations, a fact attributable to either their absence in our samples, as they probably result by metabolism in shellfish [13], or to possible differences in method sensitivity for these compounds. It should also be noted that a number of mussel samples negative to the presence of TTXs were found among the routine samples of regulatory monitoring (data not shown) and these were used for the fortification experiments conducted in terms of method recovery assessment.
The TTXs levels of our study are substantially higher than those recently reported by Turner et al. [13] in bivalve mollusks (blue mussels and pacific oysters) harvested in 2013–2014 from the south coast of England, along the Channel. This is expectable, taking into account that TTX is traditionally occurring in warmer tropical or subtropical waters and that Greece is located more southern compared to England, with a substantially warmer climate. Furthermore, the present work is mostly based on analyses of targeted and specifically selected samples, either connected to the presence of P. minimum blooms or derived from coastal areas with a previous history of TTXs present in increased levels.
Various TTXs levels have been reported in gastropods and other bivalve species in Europe and internationally. Rodriguez et al. [12] found very high TTXs concentrations (TTX: 315 mg/kg and 5,6,11-trideoxyTTX: 1004 mg/kg) in the digestive glands of the trumpet shell Charonia lampas lampas sample harvested from the south coast of Portugal and purchased from Malaga market. Such increased TTXs concentrations in this specific sample, however, are not common but were expectable due to the fact that this sample was implicated in a food poisoning incident. Indeed, Nzoughet et al. [44] reported much lower TTX levels of 22.4 and 66.6 μg/kg in viscera and muscle, respectively, of naturally contaminated trumpet shells from the same species, harvested from Angeiras Coast, Portugal. Similarly, the presence of TTXs at low levels was also reported in two more gastropod species harvested from Portuguese coasts: Gibbula umbilicalis (monodeoxyTTX: 63.81 μg/kg) and Monodonta lineata (TTX: 90 μg/kg and 4-epiTTX: 21 μg/kg) [45]. The only other reported occurrences of TTX in bivalve mollusks are those by McNabb et al. [10],who found TTX in the New Zealand clam Paphies australis at levels up to 800 μg/kg and by Kodama et al. [11] who reported the presence of TTX in the Japanese scallop Patinopecten yessoensis.
Our study constitutes, to our best knowledge, the earliest report (March 2006) for TTXs detection in European bivalve mollusks, mussels and venus clams, as well as the first report for shellfish harvested in the Mediterranean Sea. Although the TTXs levels found in our samples are relatively low when compared to other species implicated in human intoxication incidents, the possibility of increase in their concentrations in the near future cannot be excluded. TTXs presence in bivalves is not routinely monitored or regulated in Europe or anywhere else in the world, given the absence so far of published data demonstrating a risk of TTX intoxication from bivalves. In the present study, TTX presence was revealed due to positive results and atypical responses in the MBA for lipophilic toxins. Until mid-2011, this situation would result in a regulatory closure of the affected area, thus the consumer would be protected from exposure to this toxin. This is not anymore the case, as due to the replacement of MBA by LC-MS/MS as a reference method for the presence of lipophilic toxins, such “false positive” results are not detectable, thus exposing the consumers to TTX and analogues together with all possible toxins not detectable by the currently existing routine testing methods.
Taking into account the absence of any formal regulatory guidance for the presence of TTX in shellfish, the maximum concentration of 222.9 μg/kg TTX in the present study, equates to ca. 28% of the maximum permitted level for saxitoxin (STX) equivalents (800 μg STX equivalents/kg shellfish tissue), noting the similarity in biological activity between the two toxin groups. The TTXs level of 222.9 μg/kg would also equate to a low level dose of toxin in comparison to the proposed minimum lethal dose (MLD) for TTX of between 0.5 to 2 mg [19], but would be quite near to the minimum acute dose (0.2 mg) for induction of symptomatology to humans (wt. 50 kg) [3]. In the same context, consumption of 500 g of shellfish contaminated with 222 μg/kg of TTXs would equate to the intake of ca. 110 μg TTX, i.e., almost22% of the proposed MLD if taken as 0.5 mg TTX for a 60 kg human [46]. Such rough calculations, of course, do not incorporate any additional safety factors as applied by the European Food Standards Agency (EFSA) in their risk assessment methods, taking into account measurement or toxicity-related uncertainties [47], and/or the possibility of high toxin content variability in bulk samples of shellfish across harvesting areas.
Consequently, while the human health risk arising from the specific samples analyzed in our study is rather low, there is the potential for health impacts, especially if TTX levels were significantly higher at other times or in other areas associated with shellfish harvesting. It is important to note that while bacterial pathogens may be eliminated in shellfish products following effective cooking, TTXs are heat stable and will thus not be destroyed in the food preparation process [13].
3.3. Comparison of Tetrodotoxin to Venerupin Shellfish Poisoning Toxin
Unfortunately no unpreserved seawater samples containing alive P. minimum cells were available in order to obtain cultures of the specific strain causing the 2012 episode in Rodopi, which would allow an in-depth study of its toxin production potential. Certain results from the present study, however, combined with published findings from previous works, point to the suspicion that Venerupin Shellfish Poisoning (VSP) toxin associated with P. minimum blooms could be related to tetrodotoxin. Relevant facts supporting this hypothesis would be: (a) the increase and decline of TTXs concentrations in mussels of the 2012 episode in Rodopi almost in parallel with increase and decline of P. minimum concentrations (Table 2 and Figure 3); (b) the presence of TTXs in a number of samples selected according to instances of P. minimum blooms at levels higher than 60 μg/kg and up to a maximum of 194.7 μg/kg (Table 6); and (c) the presence of TTXs at much lower levels (<50 μg/kg) in a coastal area with a previous history of TTXs presence in increased levels, when no blooms of P. minimum were recorded (Table 5).
Where data available in the bibliography are concerned, important facts include: (a) previous incrimination of P. minimum for several episodes of human poisoning subsequent to the consumption of shellfish, with a symptomatology characteristic of paralytic shellfish poisoning (PSP) [26,27,28,29], which is rather difficult to distinguish with that of TTX poisoning [19]; (b) mussel toxicity associated to the presence of P. minimum by a water soluble neurotoxic component, different to PSP but also inhibiting calcium channels, that rapidly killed mice [37]; (c) the subsequent isolation of the same toxic fraction from shellfish during a large P. minimum [38]; and (d) the strain specific and stimulated by associated bacteria character of the toxin production by P. minimum [37], also taking into account the generally accepted bacterial origin of tetrodotoxin. All these findings are in agreement with our observations with regard to the hydrophilic and neurotoxic nature of the compound causing shellfish toxicity in the 2012 episode in Vistonikos Bay, where TTX was detected. However, it would be rather premature to conclude that TTX and VSP are indeed identical, as a number of experiments would be necessary to verify this hypothesis. Upon occurrence of a future P. minimum toxic episode, therefore, further research will have to be undertaken, including obtaining seawater field samples, establishing cultures of the specific strain and TTX analysis of the experimental materials in order to elucidate the actual mode of P. minimum toxin production and to establish the exact, if any, role of this species regarding the presence of TTXs in shellfish.
4. Experimental Section
4.1. Standards and Reagents
A certified reference standard for tetrodotoxins (CRM-03-TTXs; purity >96%), purified from puffer fish, was obtained from Laboratorio CIFGA S.A. (Lugo, Spain). The standard has a certified reference concentration of 25.7 ± 2.1 μg/mL for tetrodotoxin (TTX) and 3.0 ± 0.4 μg/mL for 4,9-anhydro tetrodotoxin (4,9-anhTTX) and a non-certified reference concentration of 0.32 ± 0.08 μg/mL for 4-epi tetrodotoxin (4-epiTTX). Certified reference standards for lipophilic toxins analysis (okadaic acid (NRC-CRM-OA-c), yessotoxin (NRC-CRM-YTX), pectenotoxin-2 (NRC-CRM-PTX2), azaspiracid-1 (NRC-CRM-AZA1), gymnodimine-A (NRC-CRM-GYM) and 13-desmethyl-spirolide C (NRC-CRM-SPX1)), were purchased from the National Research Council of Canada (Halifax, Canada). The pre-certified reference standard for pinnatoxin-G (RM-PnTX-G) was a generous gift from Pearce McCarron (NRC, Halifax, Canada). Acetic acid was of analytical grade, methanol of HPLC grade, acetonitrile and water of LC-MS grade, whereas formic acid and ammonium formate were of mass spectrometry grade. Chemicals were obtained from Fisher Scientific UK (Loughborough, UK), Panreac (Barcelona, Spain), Fluka (Sigma Aldrich group, Schnelldorf, Germany) and Alfa Aesar (Karlsrube, Germany).
4.2. Sampling and Test Materials
Samples and respective data on P. minimum counts and presence of lipophilic toxins were derived from the Greek “National Programme for Monitoring of Bivalve Molluscs” Production Areas for the presence of Marine Biotoxins” during the years 2006–2014; coordination was provided by the Ministry of Productive Reconstruction, Environment and Energy (former Ministry of Rural Development and Food [48,49]). All samplings were conducted by the relevant Prefectural Veterinary and/or Fisheries authorities. Cell counts of toxic and/or potentially toxic phytoplankton in seawater, and specifically of the species P. minimum, were conducted by the Laboratory Unit of Toxic Marine Microalgae (LUTMM), Department of Biology, Aristotle University of Thessaloniki (scientific coordinator: G. Nikolaidis (until February 2010) and M. Arsenakis (March 2010–to date), and data were forwarded to the NRLMB initially by the prefectural authorities and later on by the conducting laboratory, as indicated by the monitoring program requirements. Mussel (Mytilus galloprovincialis) samples and additional seawater samples, in the case of the main investigated episode of Rodopi were collected from the sampling site “Zone 6” in Vistonikos Gulf, North Aegean Sea (Figure 1). The shellfish samples tested from previous years (mussels: Mytilus galloprovincialis and clams: Venus verrucosa) were obtained from various sampling sites along the Greek coasts. Random samples from these latter sites, obtained in June and July 2014, were also tested in order to check TTXs background levels in these areas and to provide negative tissue material for fortification experiments (see Figure 1 and Table 1, Table 5, Table 6 and Table 7).
4.3. Seawater Analyses
Seawater samples were analyzed for the presence of toxic and/or potentially toxic phytoplankton by Utermöhl’s sedimentation method [50], using inverted microscopy for identification and enumeration of the phytoplankton cells. Briefly, after thorough homogenization, sub-samples were allowed to settle on 25 mL sedimentation chambers. Cells were counted in an inverted microscope, at 100× and 400× magnifications. It should be noted that until November 2012, individual concentrations of P. minimum cells were not regularly reported by the testing laboratory (LUTMM), as they were not perceived as potentially toxic in the context of the bivalve mollusks’ monitoring program, but only mentioned as responsible for ichthyotoxicity. For this reason, additional seawater samples obtained for investigation of the 2012 Rodopi episode had to be enumerated ad hoc during the episode development, in order to obtain a full dataset with regard to P. minimum presence for each sample obtained at that period.
4.4. Shellfish Preparation and Sub-Sampling
Shellfish samples were washed and shucked upon arrival to the laboratory; digestive glands (DG) and whole flesh (WF) were separated and homogenized. All samples were tested for the presence of lipophilic toxins by mouse bioassay (MBA), according to the protocol of Yasumoto et al. (1978) [39] (see Section 4.5), whereas a number of samples were also further investigated using the EU-harmonized MBA protocol of the European Reference Laboratory on Marine Biotoxins (version 5; [42]). According to the NRLMB laboratory practice, DG and/or WF sub-samples were retained for each tested sample, for at least one year after the analysis. However, in the case of samples with positive results or negative results with severe symptomatology, with an emphasis being paid on atypical responses, sub-samples are retained as a rule for indefinite time under deep freezing conditions (−70°C),for the purpose of further investigation in due time.
4.5. Mouse Bioassay Tests
Mussel DG samples (routine and fortified with TTX at various levels) were tested for the presence of lipophilic toxins by the mouse bioassay (MBA) protocol of Yasumoto et al. (1978). Briefly, a 20 g portion of DG was extracted thrice with 50 mL acetone each time and filtered through a cellulose filter. The combined toxin extract was evaporated to dryness and resuspended in 1% Tween-60 to a final volume of 4 mL (5 g DG/mL). Each one of three mice (Albino Swiss, 18–20 g body weight) was injected intraperitoneally with 1 mL of this solution.
Where further investigation was required, mussel whole flesh samples were also tested for the presence of lipophilic toxins using the harmonized EU-MBA protocol (version 5; [42]). An aliquot of 100 g of whole flesh tissue homogenate was weighed into 500 mL plastic centrifuge containers (Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany), to which acetone (300 mL) was added. The mixture was homogenized for 2 min using an Ultra turrax (15 mm shaft, 10,000 rpm; IKA, Staufen, Germany) and centrifuged at 1200× g for 10 min at 4 °C (Centrifuge Sigma 4K15C; Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany). The supernatant was transferred through a filter paper into a labeled 1 L round-bottom flask, while the tissue residue was re-extracted in the same way with 200 mL of acetone. The combined 500 mL filtrate was rotary evaporated under vacuum at 42 °C ± 2 °C (Rotavapor R-200, Buchi, Flawil, Switzerland), until complete removal of acetone. The aqueous residue of the round-bottom flask was transferred to a 100 mL glass cylinder and volume was adjusted to 100 mL by addition of de-ionized water, while 100 mL of diethyl ether were used to rinse off the residue of the round bottom flask. Both aqueous and diethyl ether extracts were transferred to a 500 mL separatory funnel. The mixture was agitated and following separation of the aqueous and diethyl ether phases, the aqueous layer was transferred back into the round-bottom flask. The water was re-extracted twice with 100 mL of diethyl ether and separated in the separatory funnel yielding ca. 300 mL of diethyl ether extract, which was backwashed twice with 20 mL de-ionized water. The ether phase was collected in another round-bottom flask and rotary evaporated to dryness under vacuum at 42 °C ± 2 °C. The dry residue was resuspended in 1% Tween-60 to a final volume of 4 mL (25 g whole flesh/mL). When necessary, the resuspended extract was further homogenized using an Ultra turrax (8 mm shaft) prior to injection. Each one of three mice (Albino Swiss, 18–20 g body weight) was injected intraperitoneally with 1 mL of this solution.
In both protocols, the criterion of toxicity established by the EU Regulation 2074/2005 (2005) was employed, which is the death of two out of three mice within 24 h of injection with an extract equivalent to 5 g of DG or 25 g of whole flesh tissue. This constitutes a positive result for the presence of the lipophilic toxins mentioned in the above Regulation. The mice were allowed laboratory feed and water ad libitum throughout the observation period. All animal manipulations were performed in accordance with the EU Directive 86/609/EEC (1986) and the EU Recommendation 2007/526/EC (2007), under official license from the Prefectural Veterinary Service of Thessaloniki, Greece (licensed facilities: for conduction of MBAs no. EL54BIO07 and for breeding mice no. EL54BIO08).
4.6. UPLC-MS/MS Analysis (Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry)
4.6.1. Sample Treatment
Aliquots of 2 g of sample tissue (DG or WF) were extracted in 6 mL of 1% acetic acid in methanol by vortex mixing for 5 min (Multi Reax, Heidolph Instruments, Schwabach, Germany), then further homogenized for 1 min using an Ultra turrax (18 mm shaft, 24,000 rpm; IKA, Staufen, Germany) and ultrasonic waterbath (10 min, 40 kHz) (T910DH, Elma Transsonic Digitals, Singen, Germany). The tubes containing the homogenized extracts were tightly closed with parafilm® (3M) to prevent solvent evaporation and were placed in a waterbath for 10 min at 100°C. After cooling to room temperature, volume was adjusted to 7.5 mL, where necessary, with the methanolic acetic acid solution and extracts were centrifuged at 3000× g for 10 min at 4°C (4K15C, Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany). The supernatants were filtered through 0.20 μm methanol compatible nylon syringe filters (Titan 3, Thermo Scientific, Nashville TN, USA) and were placed in vials for UPLC-MS/MS analysis.
4.6.2. UPLC-MS/MS Method
An Acquity UPLC system coupled to a TQD mass spectrometer (Waters, Manchester, UK) equipped with a Z-Spray ESI source was used for sample analysis. The UPLC system was equipped with an Acquity UPLC BEH Amide column (2.1 × 150 mm, 1.7 μm) and an Acquity UPLC BEH VanGuard Pre-Column (2.1 × 5 mm, 1.7 μm, 130 Å), with column oven maintained at 25 °C. The mobile phase consisted of 100% water in channel A and 95% acetonitrile (ACN) in channel B, both containing 2 mM ammonium formate and 50 mM formic acid. An isocratic elution was used with a flow rate of 0.4 mL/min at a ratio of 35% A and 65% B for a total of 5 min and the injection volume was 10 μL (partial loop with needle overfill mode). The mass spectrometer operated in electrospray positive mode with the capillary voltage set at 2.8 kV. The TQD mass spectrometer operated with the following optimized source-dependent parameters (ESI source): capillary voltage 2.8 kV, cone voltage 40 V, desolvation temperature 350 °C, desolvation gas flow 800 L/h N2, cone gas flow 50 L/h N2, source temperature 120 °C, collision gas flow 0.10 mL/min Argon. The cone voltage and collision energy were optimized for TTX by standard infusion. MassLynx 4.1 with QuanLynx software (Waters, Manchester, UK) was used for data processing.
The mass spectrometer operated in MRM, detecting in positive mode, analyzing at least two product ions per compound: one for quantification (the most abundant) and another for confirmation. The transitions employed were: TTX and 4-epiTTX (m/z 320 > 302/162/60), 4,9-anhTTX (m/z 302 > 256/162), 5,6,11-trideoxyTTX (272 > 254/162), 11-deoxyTTX and 5-deoxyTTX (304 > 286/176) and 11-norTTX-6(S)-ol and 11-norTTX-6(R)-ol (290>272/162) and were selected according to Yotsu-Yamashita et al. [51], Rodriguez et al. [8] and McNabb et al. [10] following optimization, where possible, by infusion of reference standards.
For the calibration curve, serial dilutions from the certified TTXs stock standard were performed using 1% acetic acid in methanol to provide eight calibration standards. The concentration ranges were: TTX: 15.63–2000 ng/mL, 4-epiTTX: 0.39–49.86 ng/mL and 4-9-anhTTX: 3.65–467.4 ng/mL. These compounds were quantified using their peak areas to calculate amounts and using the relevant curve obtained from the standard, while the remaining analogues were quantified using the TTX curve and assuming an equal molar response factor. TTXs concentrations in samples were expressed in μg/kg. The calibration range was chosen in order to include concentrations of TTX currently found in various aquatic species [7,8,12,13,44,45,52,53,54] and also to encompass the EU regulatory limit (800 μg/kg) for PSP toxins set by Regulation (EC) no 853/2004 [55]. To overcome the challenge of the lack of standards for some of the TTX analogues, gonad and muscle samples of a naturally-contaminated Lagocephalus sceleratus pufferish used in former works [8] were injected in the UPLC-MS/MS, this way determining the respective retention times (RT) (Figure 2).
4.6.3. UPLC-MS/MS Method Quality Control
Negative mussel DG and WF tissues (i.e., free from TTX) were fortified at five concentrations, i.e.,100, 200, 400, 800 and 1200 μg/kg, equivalent to, respectively, 0.125, 0.25, 0.5, 1 and 1.5 times the regulatory limit for PSP toxins for the purpose of evaluating the method performance. Fortified materials were extracted following the extraction procedure described in Section 4.6.1 with six independent replicates for each concentration. The mean method recovery for each concentration and tissue was calculated. Linearity of calibration curves, as well as limit of detection (LOD) and limit of quantification (LOQ) based on signal to noise ratios for all three analogues included in the certified TTXs standard were also assessed.
5. Conclusions
The present work, to our knowledge, constitutes the earliest report (March 2006) for the presence of TTXs in European bivalve mollusks, mussels and venus clams, as well as the first report for shellfish harvested in the Mediterranean Sea. Taking into account the evidence presented for TTXs occurrence in European bivalve mollusks, and the traditional occurrence of these toxins in warm tropical waters, an important question is whether this is linked to climate change. TTXs have until recently been assumed not to occur in bivalve mollusks, particularly in temperate waters. Since these bivalve species are largely consumed, the probability of human health hazards seems to be increasing. Therefore, despite the low concentrations detected, ranging from 25.0 to 222.9 μg/kg, it is evident that the presence of TTX and analogues should be monitored in all species that can potentially accumulate the toxins and can be used as human food, especially in combination with monitoring of P. minimum blooms.
In the present work, preliminary indications were found for the existence of a possible link between P. minimum blooms and presence of TTXs in shellfish. Although reported association between P. minimum blooms and human toxicity are rare, further research is required, when another episode of P. minimum combined with shellfish toxicity re-occurs in the future, in order to elucidate the mode of P. minimum toxin production and to establish the exact, if any, role of this species regarding the presence of TTXs in shellfish.
Acknowledgments
The research leading to USC results has received funding from the following FEDER cofunded-grants. From CDTI and Technological Funds, supported by Ministerio de Economía y Competitividad, AGL2012-40185-CO2-01 and Consellería de Cultura, Educación e Ordenación Universitaria, GRC2013-016, and through Axencia Galega de Innovación, Spain, ITC-20133020 SINTOX. In addition from the European Union’s Seventh Framework Programme managed by REA—Research Executive Agency (FP7/2007–2013) under grant agreement 315285 CIGUATOOLS and 312184 PHARMASEA. Inés Rodriguez is supported by a fellowship from Subprograma de Formación de Personal Investigador (AGL2012-40185-CO2-01), Spain.
In depth investigation of the toxic episodes leading to the results and publication of the present work was undertaken by the Greek National Reference Laboratory of Marine Biotoxins (NRLMB) to fulfill the requirements of EU Regulation 178/2002/EC (Articles 6 and 7) regarding risk analysis and communication and scientific information needed for risk assessment and EU Regulation 882/2004/EC (article 7) with regard to transparency and information to the public. Collaboration of all the staff of the NRLMB is greatly appreciated. Thanks are also expressed to all the Greek regional veterinary services for their contribution to the shellfish samplings and for provision of the seawater analyses results for the presence of potentially toxic microalgae. The use of cell counts’ data within the period 2006–2009 and 2012 regarding P. minimum presence in seawater, derived from the Greek “National Programme for Monitoring of Bivalve Molluscs’ Production Areas for the presence of Marine Biotoxins” and conducted by the Laboratory Unit of Toxic Marine Microalgae (LUTMM), Department of Biology, Aristotle University of Thessaloniki (scientific coordinator: G. Nikolaidis (until February 2010) and M. Arsenakis (March 2010–to date)), as well as the restrictions of LUTMM regarding the use of data produced within 2013–2015 due to contract terms and ISO 17025 requirements are acknowledged. Thanks are also expressed to the anonymous reviewers of the manuscript for all the improvements introduced according to their productive comments.
Author Contributions
Aristidis Vlamis conducted the seawater analyses for re-enumeration of the presence of P. minimum of the 2012 Rodopi episode in the additional seawater samples taken. He was responsible for all preparations of standards and shellfish samples and the relevant analyses by UPLC-MS/MS for the presence of TTXs. He handled UPLC-MS/MS and microalgae data processing and interpretation and also contributed to experimental design, UPLC-MS/MS method development, the manuscript preparation and completion. Panagiota Katikou was responsible for the experimental design, contributed to UPLC-MS/MS method development and manuscript preparation and handled the submission. Angelos Papazachariou participated in UPLC-MS/MS method development and was responsible together with Thetis Zacharaki and Panagiota Katikou for the conduction and data collection of all MBA analyses. Inés Rodriguez, Verónica Rey and Amparo Alfonso were in charge of USC analysis LC-MS/MS and HPLC-FLD). Luis M. Botana was co-responsible for the experimental design and overall supervision of the research work included in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Narahashi T. Pharmacology of tetrodotoxin. J. Toxicol. Toxin Rev. 2001;20:67–84. doi: 10.1081/TXR-100102537. [DOI] [Google Scholar]
- 2.Field J. Puffer fish poisoning. J. Accid. Emerg. Med. 1998;15:334–336. doi: 10.1136/emj.15.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noguchi T., Ebesu J.S.M. Puffer poisoning: Epidemiology and treatment. J. Toxicol. Toxin Rev. 2001;20:1–10. doi: 10.1081/TXR-100103080. [DOI] [Google Scholar]
- 4.Yin H.L., Lin H.S., Huang C.C., Hwang D.F., Liu J.S., Chen W.H. Tetrodotoxication with Nassauris glans: A possibility of tetrodotoxin spreading in marine products near Pratas Island. Am. J. Trop. Med. Hyg. 2005;73:985–990. [PubMed] [Google Scholar]
- 5.Noguchi T., Arakawa O. Tetrodotoxin—Distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs. 2008;6:220–242. doi: 10.3390/md20080011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mebs D., Yotsu-Yamashita M., Yasumoto T., Lotters S., Schluter A. Further report of the occurrence of tetrodotoxin in Atelopus species (family: Bufonidae) Toxicon. 1995;33:246–249. doi: 10.1016/0041-0101(94)00149-3. [DOI] [PubMed] [Google Scholar]
- 7.Katikou P., Georgantelis D., Sinouris N., Petsi A., Fotaras T. First report on toxicity assessment of the Lessepsian migrant pufferish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece) Toxicon. 2009;54:50–55. doi: 10.1016/j.toxicon.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez P., Alfonso A., Otero P., Katikou P., Georgantelis D., Botana L.M. Liquid chromatography–mass spectrometry method to detect Tetrodotoxin and its analogues in the puffer fish Lagocephalus sceleratus (Gmelin, 1789) from European waters. Food Chem. 2012;132:1103–1111. doi: 10.1016/j.foodchem.2011.11.081. [DOI] [Google Scholar]
- 9.Bane V., Lehane M., Dikshit M., O’Riordan A., Furey A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins. 2014;6:693–755. doi: 10.3390/toxins6020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNabb P.S., Taylor D.I., Ogilvie S.C., Wilkinson L., Anderson A., Hamon D., Wood S.A., Peake B.M. First detection of tetrodotoxin in the bivalve Paphies australis by liquid chromatography coupled to triple quadrupole mass spectrometry with and without precolumn reaction. J. AOAC Int. 2014;97:325–333. doi: 10.5740/jaoacint.SGEMcNabb. [DOI] [PubMed] [Google Scholar]
- 11.Kodama M., Sato S., Ogata T. Alexandrium tamarense as a source of Tetrodotoxin in the scallop Patinopecten yessoensis. In: Smayda T.J., Shimizu Y., editors. Toxic Phytoplankton Blooms in the Sea. Elsevier Science Publishers; Amsterdam, The Netherlands: 1993. pp. 401–406. [Google Scholar]
- 12.Rodriguez P., Alfonso A., Vale C., Alfonso C., Vale P., Tellez A., Botana L.M. First toxicity report of tetrodotoxin and 5,6,11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008;80:5622–5629. doi: 10.1021/ac800769e. [DOI] [PubMed] [Google Scholar]
- 13.Turner A.D., Powell A., Schofield A., Lees D.N., Baker-Austin C. Detection of the pufferfish toxin tetrodotoxin in European bivalves, England, 2013 to 2014. [(accessed on 25 February 2015)];Euro Surveill. 2015 20 doi: 10.2807/1560-7917.es2015.20.2.21009. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21009. [DOI] [PubMed] [Google Scholar]
- 14.McNabb P., Selwood A.I., Munday R., Wood S.A., Taylor D.I., MacKenzie L.A., van Ginkel R., Rhodes L.L., Cornelisen C., Heasman K., et al. Detection of tetrodotoxin from the grey side-gilled sea slug—Pleurobranchaea maculata, and associated dog neurotoxicosis on beaches adjacent to the Hauraki Gulf, Auckland, New Zealand. Toxicon. 2010;3:466–473. doi: 10.1016/j.toxicon.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Yotsu-Yamashita M., Mebs D., Flachsenberger W. Distribution of tetrodotoxin in the body of the blue-ringed octopus (Hapalochlaena maculosa) Toxicon. 2007;49:410–412. doi: 10.1016/j.toxicon.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi T., Arakawa O., Takatani T. TTX accumulation in pufferfish. Comp. Biochem. Physiol. 2006;1:145–152. doi: 10.1016/j.cbd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Croci L., Cozzi L., Suffredini E., Ciccaglioni G., Toti L., Milandri A. Characterization of microalgae and associated bacteria collected from shellfish harvesting areas. Harmful Algae. 2006;5:266–274. doi: 10.1016/j.hal.2005.08.001. [DOI] [Google Scholar]
- 18.Wu Z., Xie L., Xia G., Zhang J., Nie Y., Hu J., Wang S., Zhang R. A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon. 2005;45:851–859. doi: 10.1016/j.toxicon.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi T., Onuki K., Arakawa O. Tetrodotoxin poisoning due to pufferfish and gastropods, and their intoxication mechanism. [(accessed on 25 February 2015)];ISRN Toxicol. 2011 doi: 10.5402/2011/276939. Available online: http://www.hindawi.com/journals/isrn/2011/276939/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakazima M. Studies on the source of shellfish poison in Lake Hamana. I. Relation of the abundance of a species of dinoflagellate, Prorocentrum sp. to shellfish toxicity. Bull. Jpn. Soc. Sci. Fish. 1965;31:198–203. doi: 10.2331/suisan.31.198. [DOI] [Google Scholar]
- 21.Heil C.A., Glibert P.M., Fan C. Prorocentrum minimum (Pavillard) Schiller: A review of a harmful algal bloom species of growing worldwide importance. Harmful Algae. 2005;4:449–470. doi: 10.1016/j.hal.2004.08.003. [DOI] [Google Scholar]
- 22.Nakazima M. Studies on the source of shellfish poison in Lake Hamana. II. Shellfish toxicity during the “red-tide”. Bull. Jpn. Soc. Sci. Fish. 1965;31:204–207. doi: 10.2331/suisan.31.204. [DOI] [Google Scholar]
- 23.Nakazima M. Studies on the source of shellfish poison in Lake Hamana. III. Poisonous effects of shellfish feeding on Prorocentrum sp. Bull. Jpn. Soc. Sci. Fish. 1965;31:281–285. doi: 10.2331/suisan.31.281. [DOI] [Google Scholar]
- 24.Nakajima M. Studies on the source of shellfish poison in Lake Hamana. IV. Identification and collection of the noxious dinoflagellates. Bull. Jpn. Soc. Sci. Fish. 1968;34:130–131. doi: 10.2331/suisan.34.130. [DOI] [Google Scholar]
- 25.Akiba T., Hattori Y. Food poisoning caused by eating asari and oyster-toxic substance, venerupin. Jpn. J. Exp. Med. 1949;20:271–284. [Google Scholar]
- 26.Silva E.S. Les “red waters” à la lagune d’ Obidos. Ses causes probables et ses rapports avec la toxicité des bivalves. Notas Est. Inst. Biol. Mar. 1963;27:265–275. (In French) [Google Scholar]
- 27.Silva E.S. As grandes populacòes de dinoflagelados tóxicos na lagoa de óbidos. Arq. Inst. Nac. Saude. 1980;4:253–262. [Google Scholar]
- 28.Silva E.S., Sousa I. Experimental Work on the Dinoflagellate Toxin Production. Arq. Inst. Nac. Saude. 1981;6:381–387. [Google Scholar]
- 29.Chen Y.Q., Gu X.G. An ecological study of red tides in the East China Sea. In: Smayda T.J., Shimizu Y., editors. Toxic Phytoplankton Blooms in the Sea. Elsevier; Amsterdam, The Netherlands: 1993. pp. 217–221. [Google Scholar]
- 30.Tangen K. Brunt vann i Oslofjordi September 1979, forarsaket av den toksiske Prorocentrum minimum og andre dinoflagellater. Blyttia. 1980;38:145–158. (In Norwegian) [Google Scholar]
- 31.Tangen K. Shellfish poisoning and the occurrence of potentially toxic dinoflagellates in Norwegian waters. Sarsia. 1983;68:1–7. [Google Scholar]
- 32.Kimor B., Moigis A.G., Dohms V., Stienen C. A case of mass occurrence of Prorocentrum minimum in the Kiel Fjord. Mar. Ecol. Prog. Ser. 1985;27:209–215. doi: 10.3354/meps027209. [DOI] [Google Scholar]
- 33.Moncheva S.P. On the toxicity of Exuviaella cordata Ost. Blooming in Black Sea. Rev. Int. Océanogr. Méd. 1991;101/104:124–126. [Google Scholar]
- 34.Rabbani M.M., Rehman A.U., Harms C.E. Mass mortality of fishes caused by dinoflagellate bloom in Gwdar Bay, Southwestern Pakistan. In: Granéli E., Sundstrom B., Edler L., Anderson D.M., editors. Toxic Marine Phytoplankton. Elsevier; New York, NY, USA: 1990. pp. 209–214. [Google Scholar]
- 35.Tseng C.K., Zhou M.J., Zou J.Z. Toxic phytoplankton studies in China. In: Smayda T.J., Shimizu Y., editors. Toxic Phytoplankton Blooms in the Sea. Elsevier; New York, NY, USA: 1993. pp. 347–352. [Google Scholar]
- 36.Shumway S. A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquacult. Soc. 1990;21:65–104. doi: 10.1111/j.1749-7345.1990.tb00529.x. [DOI] [Google Scholar]
- 37.Grzebyk D., Denardou A., Berland B., Pouchus Y.F. Evidence of a new toxin in the red-tide dinoflagellate Prorocentrum minimum. J. Plankton Res. 1997;19:1111–1124. doi: 10.1093/plankt/19.8.1111. [DOI] [Google Scholar]
- 38.Denardou-Queneherve D., Grzebyk D., Pouchus Y.F., Sauviat M.P., Alliot E., Biard J.F., Berland B., Verbist J.F. Toxicity of French strains of the dinoflagellate Prorocentrum minimum experimental and natural contaminations of mussels. Toxicon. 1999;37:1711–1719. doi: 10.1016/S0041-0101(99)00113-0. [DOI] [PubMed] [Google Scholar]
- 39.Yasumoto T., Oshima Y., Yamaguchi M. Occurrence of a new type of shellfish poisoning in the Tohoku district. Bull. Jpn. Soc. Sci. Fish. 1978;44:1249–1255. doi: 10.2331/suisan.44.1249. [DOI] [Google Scholar]
- 40.Moestrup Ø., Akselman R., Cronberg G., Elbraechter M., Fraga S., Halim Y., Hansen G., Hoppenrath M., Larsen J., Lundholm N., et al. 2014 IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. [(accessed on 25 February 2015)]. Available online: http://www.marinespecies.org/hab/aphia.php?p=taxdetails&id=232376.
- 41.EU Regulation 15/2011 Commission Regulation (EU) No 15/2011 of 10 January 2011 amending Regulation (EC) No 2074/2005 as regards recognized testing methods for detecting marine biotoxins in live bivalve molluscs. Off. J. Eur. Union. 2011;L6:3–6. [Google Scholar]
- 42.Community Reference Laboratory for Marine Biotoxins . EU-HarmonisedStandard Operating Procedure for Determination of Lipophilic Toxins by Mouse Bioassay, Version 5. Community Reference Laboratory for Marine Biotoxins (CRLMB); Vigo, Spain: 2009. [Google Scholar]
- 43.EU Decision 2002/657/EC Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Commun. 2002;L221:8–36. [Google Scholar]
- 44.Nzoughet J.K., Campbell K., Barnes P., Cooper K.M., Chevallier O.P., Elliott C.T. Comparison of sample preparation methods, validation of an UPLC–MS/MS procedure for the quantification of tetrodotoxin present in marine gastropods and analysis of pufferfish. Food Chem. 2013;136:1584–1589. doi: 10.1016/j.foodchem.2012.01.109. [DOI] [PubMed] [Google Scholar]
- 45.Silva M., Azevedo J., Rodriguez P., Alfonso A., Botana L.M., Vasconcelos V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar. Drugs. 2012;10:712–726. doi: 10.3390/md10040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arakawa O., Hwang D.-F., Taniyama S., Takatani T. Toxins of pufferfish that cause human intoxications. In: Ishimatsu A., Lie H.-J., editors. Coastal Environmental and Ecosystem Issues of the East China Sea. Nagasaki University/Terrapub; Tokyo, Japan: 2010. pp. 227–244. [Google Scholar]
- 47.European Food Safety Authority Scientific Opinion: Marine Biotoxins in Shellfish—Saxitoxin group. EFSA J. 2009;1019:1–76. [Google Scholar]
- 48.Ministry of Rural Development and Food . MRDF Decision 313153/20-12-2002: National Program for Monitoring of Bivalve Molluscs’ Production Areas for the Presence of Marine Biotoxins. Ministry of Rural Development and Food; Athens, Greece: 2002. p. 16. (In Greek) [Google Scholar]
- 49.Ministry of Rural Development and Food . MRDF Circular 1558/45842/12-04-2012: Guidelines for Categorization and Monitoring of Production/Relay Areas of Live Bivalve Molluscs. Ministry of Rural Development and Food; Athens, Greece: 2012. p. 16. (In Greek) [Google Scholar]
- 50.Utermöhl H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt. Int. Verein. Limnol. 1958;9:1–38. (In German) [Google Scholar]
- 51.Yotsu-Yamashita M., Jang J.H., Cho Y., Konoki K. Optimization for simultaneous analysis of tetrodotoxin, 4-epitetrodotoxin, 4,9-anhydrotetrodotoxin and 5,6,11-trideoxytetrodotoxin by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Forensic Toxicol. 2011;29:61–64. doi: 10.1007/s11419-010-0106-x. [DOI] [Google Scholar]
- 52.Deeds J.R., White K.D., Etheridge S.M., Landsberg J.H. Concentrations of saxitoxin and tetrodotoxin in three species of puffers from the Indian river lagoon, Florida, the location for multiple cases of Saxitoxin Puffer Poisoning from 2002 to 2004. Trans. Am. Fish. Soc. 2008;137:1317–1326. doi: 10.1577/T07-204.1. [DOI] [Google Scholar]
- 53.Huang H.N., Lin J., Lin H.L. Identification and quantification of tetrodotoxin in the marine gastropod Nassarius by LC–MS. Toxicon. 2008;51:774–779. doi: 10.1016/j.toxicon.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Jang J.H., Lee J.S., Yotsu-Yamashita M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine Puffer Fish Fugu niphobles from the Southern Coast of Korea, and in the Brackish water Puffer Fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar. Drugs. 2010;8:1049–1058. doi: 10.3390/md8041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.EU Regulation 853/2004 Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union. 2004;226:22–82. [Google Scholar]