Abstract
The concept of Endothelial Progenitor Cells (EPCs) therapy for adult neovascularization has continuously received attention. They are believed to participate in endothelial repair and post natal angiogenesis due to their abilities in differentiating into endothelial cells and producing protective cytokines and growth factors. Abundant evidence supports the involvement of EPCs in capillary growth and in participating in the formation of collateral vessels, which lead to improved vascular perfusion and functional recovery in target tissue. Autologous EPC now is becoming a novel treatment option for therapeutic revascularization and vascular repair in ischemic diseases. However, various diseases such as diabetes, heart disease and ischemic diseases are related to EPC dysfunction and give rise to additional challenges of autologous EPC therapy. A novel strategy to enhance the number and function of EPCs is needed to be established to provide successful autologous EPCs therapy. Currently, clinical trials for the new generation of EPC therapy in treating peripheral ischemic diseases are underway. In this review we provide an overview and the limitations of current EPCs therapy with an introduction to the new strategies of next generation EPC therapy for more promising vascular and tissue regeneration therapy.
Keywords: Endothelial progenitor cell therapy, vasculogenesis, clinical application, ex vivo expansion
Introduction
After the discovery of bone marrow derived vascular stem cells called endothelial progenitor cells (EPCs), many investigators have extensively explored the potential of cell based therapeutic angiogenesis in wide range of cardiovascular-related ischemic diseases. EPCs were shown to derive from the bone marrow (BM) which has ability to promote vascular repair by migration, homing into target tissue and incorporate into neovascularization [1-5]. BM-derived EPCs (BM-EPCs) has been identified as population of selected hematopoietic stem/progenitor cells expressing CD34+ or CD133+ cells in human and c-Kit+/Sca-1+/Lineage negative (KSL) cells in mouse [1,6-9]. These population of cells demonstrated their regenerative capacity by their involvement in neovascularization for functional recovery in ischemic condition [1,2,8,10]. Autologous application of CD34+ and or CD133+ enriched EPCs in early clinical trials of ischemic limb disease have indicated the promising of EPCs therapy [6,11,12].
The prospect of EPC therapy is still facing difficulty in clinical application for the low quantity and quality of EPCs isolated from bone marrow and peripheral blood of certain patient populations. Since EPC population is decreased in numbers and functional activity related to age and cardiovascular risk factor [13-16], isolation and application of EPCs from patients with these backgrounds have high chance of receiving EPCs with low therapeutic effect. To further augment the efficacy of EPCs therapy, several methodological approaches’ to enhance EPC survival and longevity are currently being developed [17-21]. In this review we will focus on the overview and the limitations of current EPC therapy on peripheral arterial disease with our thoughts for novel and ideal style of EPC therapy in the future.
Identification and characterization of EPCs
Endothelial progenitor cells (EPCs) were first initially discovered by Asahara et al., who isolated human putative endothelial cells (CD34+ cells) using magnetic micro beads [1]. EPCs have been identified through several methods, such as colony formation assay [7,22,23], detection of acetylated low-density lipoprotein uptake and lectin binding [1,7,22], as well as flow cytometry technique based on their surface markers [20,23]. Current concept of circulating EPCs has been identified as two major categories, hematopoietic lineage EPCs and non-hematopoietic EPCs. Hematopoietic EPCs are population of cells derived from BM hematopoietic stem cells (HSCs) [24-26] which are able to migrate into peripheral blood due to stimulation of vascular injured. It is believed that HSCs and EPCs have the same origin from hemangioblast and therefore they have common surface markers (CD34 and CD133) [24,25,27]. Current characterization and identification of EPCs are related to surface markers such as membrane receptors like CD34, CD133, Flk-1/KDR, CXCR4 and CD105 for human and receptors such as c-Kit, Sca-1 and CD34 +Flk-1(VEGFR2)+ for mice [28]. The benefit of hematopoietic EPCs is to full fill the clinical application related to medical regulatory in terms of the feasibility of cell isolation from blood cells by targeting antibodies and the potential effectiveness of primary cells through vasculogenic and angiogenic mechanism.
Non-hematopoietic EPCs are not derived from HSCs cells; it is derived from possibly as organ or tissue-derived EPCs, including blood cells. This population of cells can be obtained after consecutive culture, which demonstrated the ability to differentiate into endothelial cell-like cells (ECs) [29]. This EPCs population is also named endothelial outgrowth cells (EOCs) and was first described by Ingram et al., and Yorder et al., [30,31]. In vitro EOCs represent more distinct ECs lineage phenotype (EC-like cells) which appears 7 days after culture as polygonal cells in a confluent cobblestone monolayer. This EPCs type express CD31, CD34, CD105, CD146, VE-cadherin and VEGFR-2 but not hematopoietic surface markers such as CD133 [28,32]. Functionally, this EPC also enables to develop capillary tube formation, produce nitric oxide and enhanced neovascularization in hind limb ischemia model [33,34]. Although these cells showed vasculogenic potential when applied into animal model, the caveat of this EPCs type is that their quality and quantity is restricted by less proliferative activity and the possibility of progressive senescence during culture in attached EPC phenotype [20]. Also considering the culturing method involved in isolating technique, these non-hematopoietic EPCs are considered less applicable for clinical.
Vasculogenic potential of EPCs
EPCs demonstrated to accumulate in ischemic injured tissues and repair injured tissue following cluster formation [1,35], therefore colony-forming potential of EPCs is important for angiogenic therapy as well as the assay system in which colony-forming potential of EPCs can be assessed. Recently, the culture methods of colony-forming unit-endothelial cells (CFU-ECs) [36] or endothelial colony-forming cells (ECFCs) were established on mononuclear cells from peripheral blood or cord blood [30,32,33]. However, it was reported that CFU-ECs were not EPCs but were myeloid cells that differentiate into phagocytic macrophages [32]. Moreover by this ECFCs system we could not distinguish the different differentiation levels as well as the differentiation capacities of immature stem cells.
A novel of EPCs colony forming assay (EPC-CFA) system has been developed to estimate the number of primitive progenitor cells and to assess the differential quality of colonies [22]. Using EPCs-CFA system for progenitor- enriched population such as CD34+ or CD133+ cells in human or c-Kit+/Sca-1+/Lineage negative (KSL cells) in mice; have enable us to identify two distinct forms of cell colony type namely primitive (small)-EPCs (pEPC-CFUs) colony and definitive (large)-EPCs (dEPC-CFUs) colony. These two types of cells also represent two different EPCs populations, primitive (small) EPCs which contained primitive cells with highly proliferative capacity; and definitive (large) EPCs contained definitive form of EPCs which tend to differentiate and promote vascular repair [7,22,37-39].
Further in vivo studies has demonstrated that EPC-CFA can be used for defining EPC status in response to pathological conditions such as ischemia and wound healing [7,21,23]. Mouse ischemia model showed to promote differentiation of PB-MNCs, BM-MNCs or BM-KSL cells into mature EPC-CFUs. Ischemia signal has potentially induced differentiation into dEPC-CFUs and suggested that dEPC-CFU as a more mature EPCs which possible play important role in repairing ischemic tissue [7,23]. Moreover transplantation of dEPC-CFU markedly increased limb perfusion ad capillary density compare to pEPC-CFUs [23]. EPC-CFA functions as a tool to identify the vasculogenic function of the cells such as MNC or CD34 cells used for EPC therapy. Our experiences in clinical trials have shown that the patients transplanted with CD34 cells with low EPC-CFUs have lower efficacy in cell therapy. Therefore we believe that higher efficacy of EPC therapy can be obtained by transplanting cells with higher EPC-CFUs.
Application of EPCs therapy
EPC transplantation in animal limb ischemia model
The potential of EPCs therapy in peripheral ischemia limb disease is currently being extensively studied. This is a new hope for ischemia limb disease patients who fail to respond to pharmacological therapy and are not suitable for surgical procedures which lead to no option other than amputation. Therapeutic approaches on EPCs-based therapy in ischemia animal model are varies ranging from utilizing fresh isolated cells, culture-expanded cells and combination with cytokine/growth factor mobilizing-agents. Asahara et al., has shown in vivo mobilization and incorporation of autologous cells application into site of tissue ischemia and neovascularization in a rabbit hindlimb ischemia model [3,4]. This was first step in defining the EPCs and confirmed that new blood vessel formation in adult was through mobilization of vascular progenitor cells from bone marrow, distinct from the pre-existing vasculature. Since then, accumulating studies in wide range of animal model including rat, rabbit and mice have proved that exogenously administration of EPCs could restore impaired neovascularization in hind limb ischemia model [17,40,41]. These experiments demonstrated that in response to ischemia the administered cells were incorporated into capillaries and support improvement in tissue perfusion.
Because HSCs and EPCs share common surface marker which is not possible to distinguish between them with current available method, recently several studies have investigated the potential of CD34+ cells as EPC-enriched fraction [8,42]. Local injection of CD34+ cells on ischemic limbs of diabetic mice has demonstrated to improve wound healing and promote vascular growth in part through increased sensitivity to VEGF [8,42]. Moreover Yang et al., showed higher recruitment capacity of transplanted fresh isolated BM-derived CD34+ into injured area [8]. Schatteman et al., transplanted freshly isolated human CD34+ cell into diabetic nude mice with hind limb ischemia and demonstrated significantly blood flow recovery in ischemic area [43].
EPC transplantation in clinical trials
The main purpose of therapeutic angiogenesis is to robust adaptive blood vessel formation and improves tissue perfusion in ischemic tissue. The promising results from animal studies have led to early clinical trials. Promotion of collateral vessel formation and angiogenesis in ischemia limb disease patients is an important therapeutic strategy to minimize tissue injury associated with severe ischemia.
Based on the cell source, recent EPCs therapy could be divided into non-selective EPCs therapy using bone marrow-derived mononuclear cells (BM-MNCs) in total which including EPCs fraction and non-EPCs fraction; and the selective EPCs therapy using isolated and purified EPCs from peripheral blood (PB) or BM-MNCs [6,8,44]. Purified EPCs such as CD34+ cells is performed by magnetic cell separator. To be able to participate in neovascularization and endothelial repair, BM-EPC must respond to signals which lead their mobilization and home into the site of ongoing vascular development and differentiate into mature endothelial cells (ECs). Because the number of EPC in circulation is very low, G-CSF is used to mobilize BM-EPCs to peripheral blood. Thus facilitating BM-EPCs mobilization and homing will enhance EPCs-mediated vasculogenesis [45,46].
Therapeutic Angiogenesis using Cell Transplantation (TACT) trial was first report on the use of bone marrow-derived mononuclear cells to treat peripheral arterial disease (PAD). This study was randomized controlled trial which demonstrated that intramuscular injection of autologous BM mononuclear cells significantly improved in leg pain scale, ulcer size, and pain-free walking distance, and was maintained during at least 2 year [47]. Furthermore, a three years follow-up assessment showed significantly lowered amputation rates [46]. After TACT first publication increased general interest in cell therapy for angiogenesis and lead to the use of this method for peripheral ischemia in worldwide with the advantages in results and without serious side effect reported. The search of the literature yielded a total of more than 50 early-phase clinical trials for a total of almost 2000 enrolled patients. A recent overview regarding the clinical results of cell therapy is ischemia limb disease was well summarized in Botti et al. [48].
In further, Kawamoto et al., was the first to report the efficacy of autologous and purified CD34+ cells transplant in patients with chronic ischemia in the lower extremities [45]. Recently Kawamoto et al., also reported a phase I/II clinical trial of intramuscular transplantation of autologous and G-CSF-mobilized CD34+ cells in patients with intractable critical limb ischemia (CLI). G-CSF was used to efficiently mobilize BM-EPCs into the PB, and the mobilized CD34+ cells were isolated as an EPC-enriched fraction. Moreover, all subjects with primary endpoint of efficacy score at week 12 were positive indicating improvement of lower limb ischemia after cell therapy. In addition, both subjective and objective parameters of lower limb ischemia improved significantly after CD34+ cells transplantation [45]. However because this study was not a controlled randomized study, it needs evaluation in a large-scale trial of the possibility of a placebo effect after transplantation of CD34+ cells.
The current clinical study by our group demonstrated the first clinical trial of transplantation of autologous GCSF-mobilized PB CD34+ cells in non-healing diabetic foot patients as prospective clinical trial phase I/IIa. Diabetic patients with non-healing foot ulcer were treated with 2 x 10^7 cells of G-CSF mobilized PB CD34+ cells as EPC-enriched population. The results showed that although minor amputation and recurrence were seen in three out of five patients, no death, other serious adverse events, or major amputation was seen following transplantation [47]. Importantly the patients who received cells with higher total number of EPC-CFU and CD34/KDR double positive cells were followed by higher clinical improvement as demonstrated by accelerating in wound healing and positive prognosis without recurrence or heterotopic ulcers [47]. These results indicate that vasculogenic potential of EPCs and the numbers of EPCs directly affects the efficacy of cell therapy. Therefore we believe that transplanting cells with higher EPC-CFU and CD34/KDR+ cells is the key to effective EPC therapy. Overall the clinical trials of reported studies indicate safety and feasibility of cell-based therapy in patients with CLI but with certain limitations.
The challenges in EPCs therapy application
The final success of clinical improvement of progenitor cell-mediated vascular repair and angiogenic therapy depends on a better understanding of EPC biology. The main challenges in EPCs therapy may represent its quantity and quality status. Due to its scarcity in circulating, EPCs therapy enclose a quantity problem due to less cell yielding during isolation and required more attempt to process BM cells or more PB to obtain sufficient number which is a burden for the patients. In fact study by Iwasaki et al. described that PB CD34+ cell transplantation demonstrated dose-dependent effect to concurrent vasculogenesis for functional regenerative recovery [48].
With regard to vasculogenic property of EPCs, certain circumstances including advanced age, diabetes, and classic risk factors of cardiovascular disease demonstrates impair in EPCs function [13,49,50]. Increasing age is associated with decreased number [13] and impaired function [51] of EPCs leads to increased risk of cardiovascular events [15,52]. Aging also related with endogenous alteration of vessel wall with notion that endothelial turnover and regeneration is involved in the dysfunction of endothelial. Scheubel et al. described that preoperative number of circulating EPCs in patients with stable coronary artery disease (CAD) is reduced with increasing age followed by decreased in plasma VEGF levels. In line with age, there is association and correlation between reduced EPCs and cardiovascular risk factors. Multivariate analysis revealed that reduced EPCs levels were significant and independent predictor of poor prognosis for cardiovascular events [15,52] and that correction for the risk factors are able to restore circulating EPCs towards normal level [49].
Diabetes is associated with EPCs impairment lead to wide range of vascular complication. Lack in endothelial regeneration and impaired in vasculogenesis were the underlying cause of micro and macro-vascular complication in diabetes. Moreover the endogenous microenvironment in diabetic is demonstrated having overproduction of reactive oxygen species (ROS) caused by increased activation of NADPH oxidase which involved in the initiation and progression of diabetic vascular complications by decreasing the bioavailability of NO. Blockade of NADPH oxidase restores the functional activity of human CD34+ including its migration and homing capacity into target tissue [53].
Therefore in the setting where there is impairment of endogenous EPCs regarding both quantity and functional, autologous EPCs therapy is thought to be challenged and less effective compare to healthy subjects. These circumstances were described by some studies including autologous transplantation of PB CD34+ cells for diabetic foot ulcer that demonstrated less effective compare to control [10], decreased EPCs mobilization in older patients due to less responsive of the EPCs [13] and that ischemic vascular damage can be repaired by healthy human CD34+ cells, but not diabetic one [54]. To overcome this issue the EPCs therapy should be equipped with cellular and molecular tools to increase their survival and enhance proliferation of the cells. Their migration to the site of repair and the ability of homing, differentiating, and engrafting into tissue target should be optimal.
Furthermore the long step procedure needed to collect and isolate the cells could become a physical burden for the patients [55]. The additional step to obtain adequate numbers before cell transplantation such as ex vivo progenitor cells propagation or pre-treatment to facilitate cell mobilization also means that there is a delay in treatment. Taken together, although clinical trials demonstrated the safety and efficacy in autologous EPCs therapy, there are still some limitations to overcome such as: 1) isolation technique and procedures, 2) cell dysfunction and 3) cell number.
Expansion methods to augment EPCs therapy
Specific approach that will recover potential EPCs dysfunction and improve its bioactivity for the optimal treatment of ischemic disorders should be considered, particularly in regard of the current findings indicating that there is impairment of the EPCs function in certain diseases. In order to further enhance the efficacy of EPC therapy, several groups have developed ex vivo expansion system which enriched with cytokines and growth factors pre-administered of EPCs. These accumulating studies demonstrated improvement results in neovascularization compare to pre-expansion EPCs (Table 1).
Table 1.
Expansion culture methods of EPC to enhance its bioactivity
| Authors | Surface markers | Methods | Result | Reference |
|---|---|---|---|---|
| Ott et al., | CD34+ Blood | Expansion in medium enriched with growth factors (SCF, VEGF, SCGFβ) | Improved in LV after myocardial infarct | [58] |
| Lipross et al., | CD133+ CD34+ | Medium enriched with bFGF and platelet released growth factor | PRGF constitutes a highly effective supplement that boosts the growth medium expansion of cultured EPCs | [59] |
| Bernard | UCB CD133+ CD34+ | Medium enriched with growth factors (SCF, TPO, Flt3, IL-6) | Increased ejection fraction after myocardial infarction | [60] |
| Janic et al., | Human UCB CD AC133+ | Stemline medium enriched with Flt3 and TPO | Long term invitro culture preserved EPCs characteristics | [19] |
| Senegaglia et al., | Human UCB CD133+ | Medium enriched with bFGF, ILF-1, VEGF | Improve LV ejection after myocardial infarct | [61] |
| Masuda et al., | UCB CD133+ | Serum free medium enriched with growth factors (TPO, VEGF, SCF, Flt3-ligand, IL-6) | Preserved LV function in nude rat after myocardial infarct | [20] |
Abreviations: SCGFβ: stem cell growth factor-β; PRGF; platelet released growth factor; UCB: umbilical cord blood; TPO: Thrombopoietin.
However the crucial limitation of these expansion methods was hampered by the lack of qualitative and quantitative measures of regenerative EPCs. Despite of those limitations, recently our group has reported newly established expansion method of EPCs to develop the culture system in order to enhance the number and function of EPCs for vascular repair by optimized growth factor and cytokine combinations [20]. Using our quality and quantity culture (QQc) system (Figure 1) enable to optimize EPCs therapy by augment both qualitative and quantitative vasculogenic properties of EPCs and also provide measureable regenerative capacity as represent by both types of primitive and definitive EPCs colony.
Figure 1.
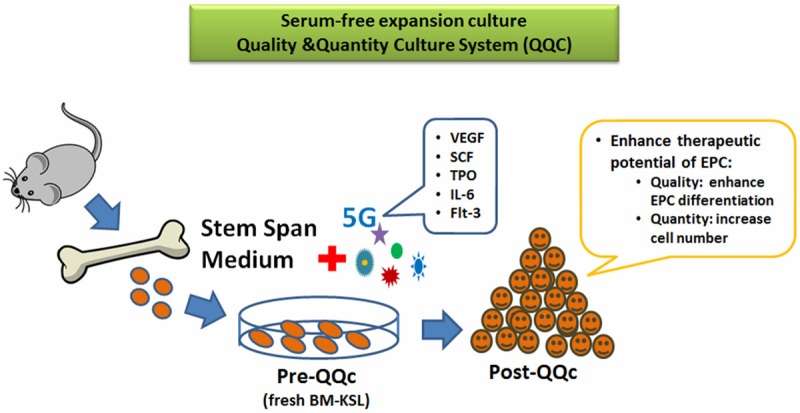
Serum-free quality and quantity control culture (QQc) system. Ex-vivo expansion method enriched with optimized growth factors and cytokines to enhance EPC bioactivity.
The advantages of QQc culture system to enhance EPCs therapy
Serum free ex vivo expansion culture has been intensively studied in the field of hematology for hematopoietic stem cells (such as CD133+ cells or CD34+ cells in cord blood) in order to reconstitute hematopoiesis following bone marrow ablation by chemotherapy for malignancies [19,56,57]. Based on this ex vivo hematopoietic expansion cultures, the development of EPC expansion culture (Figure 2) has also been at interest recently. The reason is that EPC share common surface markers with hematopoietic stem cells and the fact that the successful clinical application of cell based therapy is limited by low quantities of EPC that can be generated from a patient. Hence the in vitro method to amplify numbers of autologous EPC while preserving its angiogenic potential is crucial for developing EPC based therapies.
Figure 2.
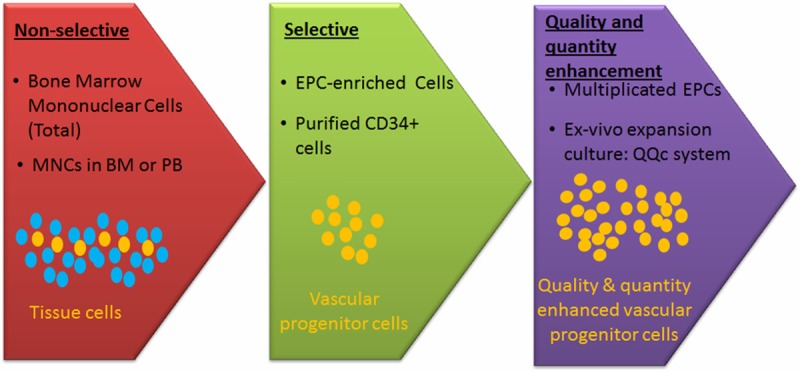
Development of EPC-based therapy. The methodology of EPC-based therapy has been developed to increase the number and bioactivity function for vascular regeneration.
Recently Masuda et al has developed newly serum free expansion method enriched with optimal cytokine and growth factors (stem cell factor (SCF), thrombopoietin (TPO), vascular endothelial growth factor (VEGF), interleukin-6 (IL-6) and flt-3 ligand) to enhance therapeutic potential of EPC in both qualitative and quantitative aspects, namely quality and quantity culture system (QQc) [20]. Seven days after cultured in QQc the post-QQc cells showed augment differentiation of EPC into endothelial cell lineage as represented by increase in definitive EPC-CFU and enhanced endothelial expressions of VEGFR-2, CD146 and vWF compare to pre-QQc EPC (fresh EPC). Post-QQc cells also enhanced the capacity of tube-like formation and production of pro-angiogenic growth factors such as VEGF and HGF compare to pre-QQc cells [20]. Moreover this system also enhanced the sprouting capability and predominant potential in cell proliferation [20]. One week of QQc culture also significantly increased number of murine diabetic EPCs and its vasculogenic potential with significant enhancement in wound closure [21]. Therefore this QQc method has positively augment in almost all aspects of vasculogenic potential of EPCs.
Tanaka et al demonstrated the efficacy of QQc in mice diabetic wound healing model. QQc system significantly increased the number of bone marrow-derived EPCs (c-Kit+/Sca-1+/Lin- (KSL) cells) in both diabetic and control groups followed by increased in vasculogenic potential of diabetic KSL above the fresh control KSL level. Moreover post-QQc diabetic EPC form tube-like formation greater than fresh control KSL cells. Importantly adoptive transplantation of post-QQc diabetic KSL cells significantly enhances wound closure compared with fresh diabetic KSL cells and comparable to post-QQc control KSL cells [21].
This system may facilitate cell-based therapies for diabetic patients since it rapidly expands the number of diabetic EPCs. Our QQc system has also demonstrated that post-QQc cells were exhibited the potential to augment and restore diabetic EPCs dysfunction through increased the capability to form and to incorporate in tube-like formation and also increased the proliferation capacity. Moreover it also up regulated the autocrine and paracrine actions of pro-angiogenic factors which enhance vasculogenic potential of EPCs in further [20,21]. Therefore, with regard of safety, suspended stem cells in serum-free conditions may be readily applicable for clinical application. Furthermore the post-QQc EPCs population which has rapid expanded property could be stored in cryopreserved until it be used.
Future perspective
Despite the wide range outcomes, research and development in stem cell applications worldwide is moving rapidly towards clinical application. Both experimental and clinical results indicate a therapeutic potential for EPCs in the treatment of the ischemic limb disease. Although new strategies are developing to enhance survival and longevity of EPCs prior to deliver, the technique that is minimal invasive but effective therapy is demanded by the patients. Since our QQc can generate large amount of functional cells from small number of cells after one week of serum free culture, the development of our QQc culture system may offer promising method with qualitative and quantitative advantages for all ischemic diseases. Our future goal in EPC therapy is to collect enough number of functional cells from small volume of peripheral blood for autologous application of EPCs for effective therapeutic vasculogenesis and tissue regeneration. We are now modifying our QQc method so that in the future outpatient EPC therapy may become possible with just a small amount of blood draw.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–7. [PubMed] [Google Scholar]
- 3.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner M, Isner JM. Bone Marrow Origin of Endothelial Progenitor Cells Responsible for Postnatal Vaculogenesis in Physiologycal and Pathological Neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 5.Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105:1068–77. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 6.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–9. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka R, Wada M, Kwon SM, Masuda H, Carr J, Ito R, Miyasaka M, Warren SM, Asahara T, Tepper OM. The effects of flap ischemia on normal and diabetic progenitor cell function. Plast Reconstr Surg. 2008;121:1929–42. doi: 10.1097/PRS.0b013e3181715218. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Ii M, Kamei N, Alev C, Kwon SM, Kawamoto A, Akimaru H, Masuda H, Sawa Y, Asahara T. CD34+ cells represent highly functional endothelial progenitor cells in murine bone marrow. PLoS One. 2011;6:e20219, 1–14. doi: 10.1371/journal.pone.0020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Lee SH, Yoo SY, Asahara T, Kwon SM. CD34 Hybrid Cells Promote Endothelial Colony-Forming Cell Bioactivity and Therapeutic Potential for Ischemic Diseases. Arterioscler Thromb Vasc Biol. 2013;33:1622–1634. doi: 10.1161/ATVBAHA.112.301052. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka R, Masuda H, Kato S, Imagawa K, Kanabuichi K, Nakashioya C, Yoshiba F, Fukui T, Ito R, Kobori M, Wada M, Asahara T, Miyasaka M. Autologous G-CSF mobilized peripheral blood CD34(+) cell therapy for diabetic patients with chronic non-healing ulcer. Cell Transplant. 2014;23:167–79. doi: 10.3727/096368912X658007. [DOI] [PubMed] [Google Scholar]
- 11.Kajiguchi M, Kondo T, Izawa H, Kobayashi M, Yamamoto K, Shintani S, Numaguchi Y, Naoe T, Takamatsu J, Komori K, Murohara T. Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J. 2007;71:196–201. doi: 10.1253/circj.71.196. [DOI] [PubMed] [Google Scholar]
- 12.Burt RK, Testori a, Oyama Y, Rodriguez HE, Yaung K, Villa M, Bucha JM, Milanetti F, Sheehan J, Rajamannan N, Pearce WH. Autologous peripheral blood CD133+ cell implantation for limb salvage in patients with critical limb ischemia. Bone Marrow Transplant. 2010;45:111–6. doi: 10.1038/bmt.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells inpatients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and Migratory Activity of Circulating Endothelial Progenitor Cells Inversely Correlate With Risk Factors for Coronary Artery Disease. Circ Res. 2001;89:e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 15.Hill JM, Zalos G, Halcox JPJ, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 16.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human Endothelial Progenitor Cells From Type II Diabetics Exhibit Impaired Proliferation, Adhesion, and Incorporation Into Vascular Structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 17.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–7. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O E, Lee BH, Ahn HY, Shin JC, Kim HK, Kim M, Park IY, Park YG, Joe YA. Efficient nonadhesive ex vivo expansion of early endothelial progenitor cells derived from CD34+ human cord blood fraction for effective therapeutic vascularization. FASEB J. 2011;25:159–69. doi: 10.1096/fj.10-162040. [DOI] [PubMed] [Google Scholar]
- 19.Janic B, Guo AM, Iskander ASM, Varma NRS, Scicli AG, Arbab AS. Human cord blood-derived AC133+ progenitor cells preserve endothelial progenitor characteristics after long term in vitro expansion. PLoS One. 2010;5:e9173, 1–11. doi: 10.1371/journal.pone.0009173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda H, Iwasaki H, Kawamoto A, Akimaru H, Ishikawa M, Ii M, Shizuno T, Sato A, Ito R, Horii M, Ishida H, Kato S, Asahara T. Development of serum-free quality and quantity control culture of colony-forming endothelial progenitor cell for vasculogenesis. Stem Cells Transl Med. 2012;1:160–71. doi: 10.5966/sctm.2011-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka R, Vaynrub M, Masuda H, Ito R, Kobori M, Miyasaka M, Mizuno H, Warren SM, Asahara T. Quality Control Culture System Restores Diabetic Endothelial Progenitor Cell Vasculogenesis and Accelerates Wound Closure. Diabetes. 2013;62:1–13. doi: 10.2337/db12-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masuda H, Alev C, Akimaru H, Ito R, Shizuno T, Kobori M, Horii M, Ishihara T, Isobe K, Isozaki M, Itoh J, Itoh Y, Okada Y, McIntyre BA, Kato S, Asahara T. Methodological development of a clonogenic assay to determine endothelial progenitor cell potential. Circ Res. 2011;109:20–37. doi: 10.1161/CIRCRESAHA.110.231837. [DOI] [PubMed] [Google Scholar]
- 23.Tsukada S, Kwon SM, Matsuda T, Jung SY, Lee JH, Lee SH, Masuda H, Asahara T. Identification of mouse colony-forming endothelial progenitor cells for postnatal neovascularization: a novel insight highlighted by new mouse colony-forming assay. Stem Cell Res Ther. 2013;4:20, 1–13. doi: 10.1186/scrt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelosi E, Valtieri M, Coppola S, Botta R, Gabbianelli M, Lulli V, Marziali G, Masella B, Müller R, Sgadari C, Testa U, Bonanno G, Peschle C. Identification of the hemangioblast in postnatal life. Blood. 2002;100:3203–8. doi: 10.1182/blood-2002-05-1511. [DOI] [PubMed] [Google Scholar]
- 25.Grant MB, May WS, Caballero S, Brown GAJ, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 26.Bailey AS, Jiang S, Afentoulis M, Baumann CI, Schroeder DA, Olson SB, Wong MH, Fleming WH. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood. 2004;103:13–9. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 27.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development. 1988;102:471–8. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 28.Timmermans F, Plum J, Yöder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13:87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fändrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–9. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- 30.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 31.Yoder MC. Defining human endothelial progenitor cells. J Thromb Haemost. 2009;7(Suppl 1):49–52. doi: 10.1111/j.1538-7836.2009.03407.x. [DOI] [PubMed] [Google Scholar]
- 32.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–93. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 34.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–27. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 35.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone Marrow Origin of Endothelial Progenitor Cells and Pathological Neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 36.Hill JM, Zalos G, Halcox JPJ, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 37.Kwon SM, Eguchi M, Wada M, Iwami Y, Hozumi K, Iwaguro H, Masuda H, Kawamoto A, Asahara T. Specific Jagged-1 signal from bone marrow microenvironment is required for endothelial progenitor cell development for neovascularization. Circulation. 2008;118:157–65. doi: 10.1161/CIRCULATIONAHA.107.754978. [DOI] [PubMed] [Google Scholar]
- 38.Kwon SM, Suzuki T, Kawamoto A, Ii M, Eguchi M, Akimaru H, Wada M, Matsumoto T, Masuda H, Nakagawa Y, Nishimura H, Kawai K, Takaki S, Asahara T. Pivotal role of lnk adaptor protein in endothelial progenitor cell biology for vascular regeneration. Circ Res. 2009;104:969–77. doi: 10.1161/CIRCRESAHA.108.192856. [DOI] [PubMed] [Google Scholar]
- 39.Kamei N, Kwon SM, Alev C, Ishikawa M, Yokoyama A, Nakanishi K, Yamada K, Horii M, Nishimura H, Takaki S, Kawamoto A, Ii M, Akimaru H, Tanaka N, Nishikawa SI, Ochi M, Asahara T. Lnk deletion reinforces the function of bone marrow progenitors in promoting neovascularization and astrogliosis following spinal cord injury. Stem Cells. 2010;28:365–75. doi: 10.1002/stem.243. [DOI] [PubMed] [Google Scholar]
- 40.Hirata K, Li TS, Nishida M, Ito H, Matsuzaki M, Kasaoka S, Hamano K. Autologous bone marrow cell implantation as therapeutic angiogenesis for ischemic hindlimb in diabetic rat model. Am J Physiol Heart Circ Physiol. 2003;284:H66–70. doi: 10.1152/ajpheart.00547.2002. [DOI] [PubMed] [Google Scholar]
- 41.He T, Smith L, Harrington S, Nath K. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke. 2004;35:2378–2384. doi: 10.1161/01.STR.0000141893.33677.5d. [DOI] [PubMed] [Google Scholar]
- 42.Awad O, Dedkov EI, Jiao C, Bloomer S, Tomanek RJ, Schatteman GC. Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler Thromb Vasc Biol. 2006;26:758–64. doi: 10.1161/01.ATV.0000203513.29227.6f. [DOI] [PubMed] [Google Scholar]
- 43.Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000;106:571–8. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tongers J, Roncalli JG, Losordo DW. Role of Endothelial Progenitor Cells During ischemia-induced Vasculogenesis and Collateral Formation. Microvasc Res. 2011;79:200–206. doi: 10.1016/j.mvr.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawamoto A, Katayama M, Handa N, Kinoshita M, Takano H, Horii M, Sadamoto K, Yokoyama A, Yamanaka T, Onodera R, Kuroda A, Baba R, Kaneko Y, Tsukie T, Kurimoto Y, Okada Y, Kihara Y, Morioka S, Fukushima M, Asahara T. Intramuscular Transplantation of G-CSF-Mobilized CD34 1 Cells in Patients With Critical Limb Ischemia: A Phase I/IIa, Multicenter, Single-Blinded, Dose-Escalation Clinical Trial. Stem Cells. 2009;27:2857–2864. doi: 10.1002/stem.207. [DOI] [PubMed] [Google Scholar]
- 46.Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S, Yamamoto Y, Onodera R, Teramukai S, Fukushima M, Matsubara H. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010–8. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka R, Masuda H, Kato S, Imagawa K, Kanabuchi K, Nakashioya C, Yoshiba F, Fukui T, Ito R, Kobori M, Wada M. Autologous G-CSF-Mobilized Peripheral Blood CD34+ Cell Therapy for Diabetic Patients With Chronic Nonhealing Ulcer. Cell Transplant. 2014;23:167–179. doi: 10.3727/096368912X658007. [DOI] [PubMed] [Google Scholar]
- 48.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–25. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 49.Fadini G, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–37. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tepper OM, Carr J, Allen RJ, Chang CC, Lin CD, Tanaka R, Gupta SM, Levine JP, Saadeh PB, Warren SM. Decreased Circulating Progenitor Cell Number and Failed Mechanisms of Stromal Cell-Derived Factor-1α Mediated Bone Marrow Mobilization Impair Diabetic Tissue Repair. Diabetes. 2010;59:1974–1983. doi: 10.2337/db09-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–8. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–7. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 53.Jarajapu YPR, Caballero S, Verma A, Nakagawa T, Lo MC, Li Q, Grant MB. Blockade of NADPH oxidase restores vasoreparative function in diabetic CD34+ cells. Invest Ophthalmol Vis Sci. 2011;52:5093–104. doi: 10.1167/iovs.10-70911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, Kern TS, Grant MB. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56:960–7. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation. 2010;122:517–26. doi: 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piacibello W, Sanavio F, Severino a, Danè A, Gammaitoni L, Fagioli F, Perissinotto E, Cavalloni G, Kollet O, Lapidot T, Aglietta M. Engraftment in nonobese diabetic severe combined immunodeficient mice of human CD34(+) cord blood cells after ex vivo expansion: evidence for the amplification and self-renewal of repopulating stem cells. Blood. 1999;93:3736–49. [PubMed] [Google Scholar]
- 57.Kobari L, Pflumio F, Giarratana M, Li X, Titeux M, Izac B, Leteurtre F, Coulombel L, Douay L. In vitro and in vivo evidence for the long-term multilineage (myeloid, B, NK, and T) reconstitution capacity of ex vivo expanded human CD34(+) cord blood cells. Exp Hematol. 2000;28:1470–80. doi: 10.1016/s0301-472x(00)00557-9. [DOI] [PubMed] [Google Scholar]
- 58.Ott I, Keller U, Knoedler M, Götze KS, Doss K, Fischer P, Urlbauer K, Debus G, von Bubnoff N, Rudelius M, Schömig A, Peschel C, Oostendorp RA. Endothelial-like cells expanded from CD34+ blood cells improve left ventricular function after experimental myocardial infarction. FASEB J. 2005;19:992–4. doi: 10.1096/fj.04-3219fje. [DOI] [PubMed] [Google Scholar]
- 59.Lippross S, Loibl M, Hoppe S, Meury T, Benneker L, Alini M, Verrier S. Platelet released growth factors boost expansion of bone marrow derived CD34(+) and CD133(+) endothelial progenitor cells for autologous grafting. Platelets. 2011;22:422–32. doi: 10.3109/09537104.2011.559559. [DOI] [PubMed] [Google Scholar]
- 60.Schlechta B, Wiedemann D, Kittinger C, Jandrositz A, Bonaros NE, Huber JC, Preisegger KH, Kocher AA. Ex-Vivo Expanded Umbilical Cord Blood Stem Cells Retain Capacity for Myocardial Regeneration. Circ J. 2010;74:188–194. doi: 10.1253/circj.cj-09-0409. [DOI] [PubMed] [Google Scholar]
- 61.Senegaglia AC, Barboza LA, Dallagiovanna B, Aita CAM, Hansen P, Rebelatto CLK, Aguiar AM, Miyague NI, Shigunov P, Barchiki F, Correa A, Olandoski M, Krieger MA, Brofman PRS. Are purified or expanded cord blood-derived CD133+ cells better at improving cardiac function? Exp Biol Med. 2010;235:119–29. doi: 10.1258/ebm.2009.009194. [DOI] [PubMed] [Google Scholar]


