Abstract
Blood stasis syndrome (BSS) is an important pathologic condition in traditional East Asian medicine, characterized by multiple signs and symptoms, including sublingual varicosis, angiotelectasis, slow and choppy pulse, local fixed pain, nyctalgia, menstrual cramps, dark-purple tongue and infra-orbital darkness. However, recent studies have been restricted to the circulatory disorder and could not suggest the pathologic core to explain all of the characteristics of BSS. Here, we review the current research on the senescence of red blood cells (RBCs), focusing on the correlation between the pathologic properties of senescent RBCs and BSS-specific manifestations. The accumulation of senescent RBCs and their products induce pathological conditions that affect blood flow resistance and cause thrombosis, vasoconstriction and methemoglobinemia. These pathological alterations are identical to the characteristics of BSS, therefore supporting the hypothesis that accelerated RBC aging could be considered as a novel pathologic mechanism of BSS.
Keywords: Blood stasis syndrome, red blood cell, senescence
Introduction
Blood stasis is a pathological concept in traditional East Asian medicine and refers to stagnant blood that has lost its physiological function within the body [1,2]. It develops into a blood stasis syndrome (BSS) that is characterized by multiple signs and symptoms, such as sublingual varicosis, angiotelectasis, slow and choppy pulse, local fixed pain, nyctalgia, menstrual cramps, dark-purple tongue or infra-orbital darkness [1,3]. In the clinic, these manifestations are frequently observed in patients with ischemic heart disease, cerebral vascular accident, diabetes mellitus, chronic renal failure, severe traumatic injury and dysmenorrhea [2,4]. Many herbal formulas have been shown to be effective for relieving the BSS-specific manifestations and the severity of these diseases [5-8]. In recent decades, many preclinical and clinical studies have been conducted to reveal the underlying pathogenic correlation between BSS and these diseases. However, almost all of these studies have been restricted to ischemic heart disease and could not suggest the pathologic core to explain all of the characteristics of BSS [9-13].
In this review, we present the current research progress on the senescence of red blood cells (RBCs), focusing on the correlation between the pathologic properties of senescent RBCs and the signs and symptoms of BSS, and we suggest that the accelerated RBC aging process could be considered to be a novel pathologic mechanism of BSS.
Pathologic properties of senescent RBCs
RBCs are naturally exposed to various stressful situations during their lifespan, such as oxidative stress in the lungs, osmotic shock in the kidneys and squeezing through capillary beds [14]. During RBC aging, the accumulation of damage by these stressors induces biochemical and structural alterations that derange the functions of the RBCs.
Decreased deformability
Deformability is the ability of an RBC to change its shape in response to a deforming force without hemolysis, and it is determined by the geometric and viscoelastic properties of the plasma membrane [15,16]. The lipid bilayer contents of the membrane and the sub-membrane cytoskeletal network of spectrin molecules are primarily responsible for the discocyte morphology of RBCs and provide the membrane with its viscoelastic properties [16,17]. Oxidative damage to the membrane lipids and the spectrin network occur during RBC aging, which causes morphological changes and decreased membrane viscoelasticity, resulting in impaired deformability [16,18,19]. Therefore, older RBCs demonstrate a decreased deformability compared to younger RBCs [20,21].
Release of microparticles
Microparticles (MPs) are membrane vesicles of less than 1 μm that are released into the blood flow by various types of cells, including platelets, RBCs, white blood cells and endothelial cells [22]. Their cell membrane consists of a phospholipid bilayer, and phosphatidylserine (PS) and phosphatidylethanolamine are specifically enriched in the inner membrane, while phosphatidylcholine and sphingomyelin are enriched in the outer membrane. This asymmetric distribution of phospholipids is actively maintained by three major enzyme systems: a flippase, a floppase and a scramblase [23]. However, various stimuli, such as cell activation, shear stress or apoptosis, induce negatively charged PS externalization on the membrane through the impairment of these enzyme functions and cytoskeletal degradation via Ca2+-dependent proteolysis. This process causes sufficient membrane fluctuation, allowing the formation and release of MPs containing hemoglobin (Hb) and membrane lipids [24,25]. MPs have also been implicated in RBC senescence [26,27]. In actuality, RBCs lose approximately 20% of their volume during their lifespan, and this lost volume could be caused by the shedding of MPs from their membrane during RBC aging [28,29].
Increased methemoglobin
Each human RBC contains approximately 270-million Hb molecules, which are oxygen-transporting metalloproteins that contain four iron atoms. Each ferrous iron (Fe2+) in Hb reversibly binds to one O2 molecule to provide the oxygen supply for the body. On the converse, methemoglobin (MetHb) is a form of oxidized hemoglobin in which the ferrous iron is oxidized to ferric iron (Fe3+) [30]. It is normally maintained as a very small proportion of the total hemoglobin (1%), primarily by the reduction of nicotinamide adenine dinucleotide (NADH)-dependent MetHb reductase [31]. Previous studies have reported that senescent RBCs are characterized by an increased proportion of MetHb as a result of reduced NADH-dependent MetHb reductase activity and accumulated oxidative membrane damage [32,33].
Pathogenetic mechanisms of senescent RBCs and their products
Increased blood flow resistance
The circulatory resistance of the blood has two major components, the rheological properties of the blood and the geometric features of the blood vessels [15,34]. The rheological properties of the blood are determined by the hematocrit, plasma viscosity, cell aggregation and cell deformability [15]. Especially in the microcirculation, the deformability of an RBC is the principal factor in maintaining normal flow, allowing their transit through capillaries as small as 2-3 μm in diameter because their size, which is approximately 7.5-8.7 μm in diameter and 1.7-2.2 μm in thickness, is larger than the capillary diameter [15,16]. Therefore, senescent RBCs with impaired deformability increase the local resistance to the blood flow [35,36].
Thrombosis
RBC-derived MPs in the plasma are involved in several pathological processes, including thrombosis and hemostasis [37,38]. MPs provide a membrane surface for the initiation of blood coagulation because negatively charged PSs on their outer membrane facilitate the assembly of components of the clotting cascade through an electrostatic interaction with the positively charged γ-carboxyglutamic acid domains in the clotting proteins [37]. In addition, the nitric oxide (NO) scavenging activity of RBC-derived MPs contributes to platelet activation and aggregation through the decreased NO signaling in the platelets, promoting clot formation [39-41].
Vasoconstriction
NO is a critical regulator of basal blood vessel tone. It is synthesized in the endothelial cells and then diffuses to the smooth muscles to activate soluble guanylyl cyclase, ultimately leading to vasodilation and increased blood flow in the tissues [42-45]. As oxygenated Hb reacts with NO to form nitrate and methemoglobin, the NO can be scavenged by Hb in the blood [46,47]. However, a physical barrier produced by the RBC membrane inhibits the endothelial diffusion of NO to the intracellular Hb [48,49]. Additionally, the laminar flow within the blood vessel pushes the RBCs inward and away from the endothelial cells, forming a cell-free zone and blocking the access of the RBCs to the endothelium [49-51]. Because of these diffusional barriers, sufficient NO can reach the smooth muscles to sustain vascular homeostasis before being scavenged by Hb. Interestingly, the Hb from RBC-derived MPs are able to more effectively scavenge NO than the Hb from RBCs [52] because the MPs can enter the cell-free zone and easily access the endothelial NO, even in the laminar blood flow [53]. Therefore, the reduction of the endothelial NO concentration by RBC-derived MPs induces vasoconstriction and decreases the blood flow in the tissues.
Methemoglobinemia
Generally, methemoglobinemia, which is due to abnormally increased MetHb, causes only a grayish pigmentation of the skin and brownish lips and mucous membranes when the MetHb levels are between 3 and 15% of the total hemoglobin. Above 15%, patients develop central cyanosis that is non-responsive to oxygen therapy [30]. The reason is that the ferric iron of MetHb cannot bind to oxygen, which enhances the binding affinity of the remaining ferrous irons to oxygen, shifting the oxygen dissociation curve to the left and decreasing the delivery of oxygen to the tissues [54].
Alterations to the human body during the accelerated RBC aging process
Under normal circumstances, the lifespan of a circulating human RBC is approximately 120 days [14], indicating that approximately 1% of all circulating RBCs are destroyed and newly generated from hematopoietic stem cells each day [55]. Therefore, the RBC’s composition and counts are maintained within the normal range for a particular age. However, under highly stressful situations, such as oxidative stress due to severe trauma [56-59] or exhaustive physical exercise [60-62], shear stress from a cardiovascular device [63,64], hyperlipidemia [65-67] or hyperglycemia [68,69], the RBC aging process is accelerated due to the increased damage to the cellular components [63,70]. The rapid accumulation of senescent RBCs and their products can lead to pathological alterations in the human body.
Circulatory disturbances
The impaired deformability of RBCs and thrombosis increase the blood flow resistance in the tissues, which leads to the development of circulatory disturbances. Impaired deformability is correlated with coronary artery disease, claudication and diabetes mellitus-associated vascular complications [71-75], and the procoagulant activity of RBC-derived MPs has also been observed in the patients with atherosclerosis, ischemic heart disease and nephrotic syndrome [76-78]. These pathologic properties of senescent RBCs can cause BSS-specific signs, such as a slow and choppy pulse, sublingual varicosis and angiotelectasis, depending on the circulatory disturbance.
Local pain
Thrombosis and vasoconstriction decrease the blood flow in the tissues, and MetHb inhibits the oxygen delivery from the RBCs to the tissues. In this case, hypoxia-induced pain [79,80] can lead to the development of BSS-specific symptoms, including local fixed pain, nyctalgia and menstrual cramps, depending on the site of the lesion.
Discoloration
Skin discoloration, dark-purple tongue and infra-orbital darkness are unique signs of BSS; however, previous studies have been unable to determine the mechanism for the observed discolorations. If the MetHb levels increase to more than 3% of the total Hb in BSS patients, the grayish skin color could be explained by the presence of methemoglobinemia.
Taken together, highly stressful situations should be correlated with the etiology of BSS, and BSS-associated diseases and manifestations are also correlated with accelerated RBC senescence. Furthermore, we explained the characteristics of BSS that arise from the pathologic mechanisms of accelerated RBC senescence (Figure 1).
Figure 1.
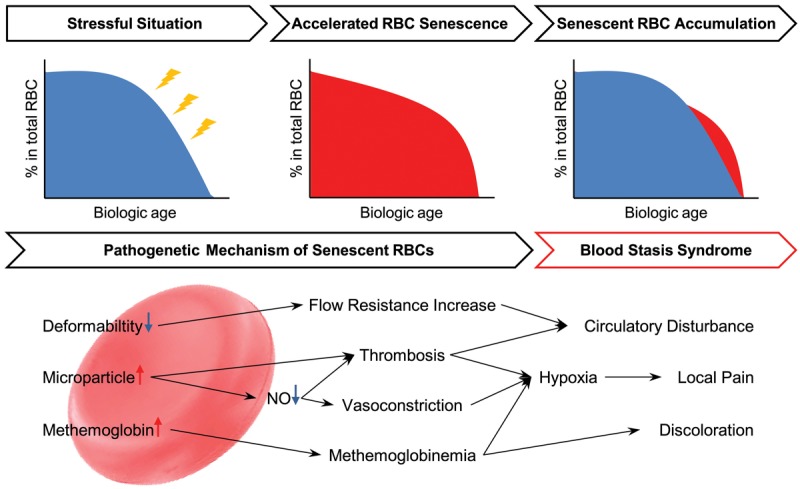
The hypothetical scheme illustrating the pathobiological effects of senescent RBCs on blood stasis syndrome. When the RBC aging process is accelerated during highly stressful situations, the senescent RBCs play pathogenetic roles. The impaired deformability of the RBCs increases the blood flow resistance in the tissues and induces circulatory disturbances. Additionally, increased RBC-derived microparticles activate the body’s procoagulant and NO-scavenging activities, therefore facilitating thrombosis and vasoconstriction. This process causes hypoxia in the tissues and ischemic pain, as well as circulatory disturbances. The elevation of methemoglobin leads to a grayish skin color and also contributes to the hypoxia. These pathological alterations caused by senescent RBCs are identical to the diagnostic characteristics of blood stasis syndrome.
Conclusion
BSS is an important pathologic condition in traditional East Asian medicine, and it is correlated with diseases such as ischemic heart disease, cerebral vascular accident, diabetes mellitus, chronic renal failure, severe traumatic injury and dysmenorrhea, which have been reported in several preclinical and clinical studies [4-8]. However, these studies have been unable to reveal the pathologic mechanism behind the characteristics of BSS. Here, we presented the pathogenetic mechanisms of senescent RBCs, which explain all of the BSS-specific manifestations, such as circulatory disorders and local pain, as well as the grayish skin color.
As RBCs lack a nucleus to synthesize new proteins for repairing stress-induced damage, they lose their deformability and enzyme activity during their aging process, leading to the formation of MPs and methemoglobin. Senescent RBCs in the circulation are selectively removed by macrophages in the liver and spleen, while a proportion of aging RBCs are normally maintained in a healthy individual. However, under highly stressful situations, the RBC aging process is accelerated, and the number of senescent RBCs in the circulatory system increases [63,70]. The rapid accumulation of senescent RBCs and their products induce pathological conditions that affect the blood flow resistance and lead to thrombosis, vasoconstriction and methemoglobinemia, which lead to pathological alterations, circulatory disturbances, local pain and discoloration of the skin. These findings support the hypothesis that the accelerated RBC aging process may be a novel pathologic mechanism of BSS.
Although intense clinical studies are still necessary to prove the relationship between accelerated RBC senescence and BSS, this new approach could provide an opportunity to develop diagnostic tools using biological markers and contribute to a more accurate diagnosis and effective treatment of BSS.
Acknowledgements
This research was supported by the Korea Institute of Oriental Medicine (K14281).
Disclosure of conflict of interest
None.
References
- 1.Li SM, Xu H, Chen KJ. The diagnostic criteria of blood-stasis syndrome: considerations for standardization of pattern identification. Chin J Integr Med. 2014;20:483–489. doi: 10.1007/s11655-014-1803-9. [DOI] [PubMed] [Google Scholar]
- 2.Park B, You S, Jung J, Lee JA, Yun KJ, Lee MS. Korean Studies on Blood Stasis: An Overview. Evid Based Complement Alternat Med. 2015;2015:316872. doi: 10.1155/2015/316872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park YJ, Yang DH, Lee JM, Park YB. Development of a valid and reliable blood stasis questionnaire and its relationship to heart rate variability. Complement Ther Med. 2013;21:633–640. doi: 10.1016/j.ctim.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Chen KJ. Blood stasis syndrome and its treatment with activating blood circulation to remove blood stasis therapy. Chin J Integr Med. 2012;18:891–896. doi: 10.1007/s11655-012-1291-5. [DOI] [PubMed] [Google Scholar]
- 5.Jin X, Shen G, Gao F, Zheng X, Xu X, Shen F, Li G, Gong J, Wen L, Yang X, Bie X. Traditional Chinese drug ShuXueTong facilitates angiogenesis during wound healing following traumatic brain injury. J Ethnopharmacol. 2008;117:473–477. doi: 10.1016/j.jep.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Du JP, Shi DZ, Li TC, Xu H, Chen H. [Correlation between blood stasis syndrome and pathological characteristics of coronary artery in patients with coronary heart disease] . Zhong Xi Yi Jie He Xue Bao. 2010;8:848–852. doi: 10.3736/jcim20100908. [DOI] [PubMed] [Google Scholar]
- 7.Tang H, Gu BL, Zhou XM. [Study on the scores of blood stasis syndrome of acute ischemic stroke and its correlation with TOAST subtypes] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:1500–1502. [PubMed] [Google Scholar]
- 8.Liu P, Duan JA, Bai G, Su SL. [Network pharmacology study on major active compounds of siwu decoction analogous formulae for treating primary dysmenorrhea of gynecology blood stasis syndrome] . Zhongguo Zhong Yao Za Zhi. 2014;39:113–120. [PubMed] [Google Scholar]
- 9.Yuan ZK, Wang LP, Huang XP. [The screening and the functional pathway analysis of differential genes correlated with coronary heart disease of blood stasis syndrome] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:1313–1318. [PubMed] [Google Scholar]
- 10.Guo S, Chen J, Chuo W, Liu L, Feng X, Lian H, Zheng L, Wang Y, Xie H, Luo L, Zheng C, Fu B, Wang W. A New Biomarkers Feature Pattern Consisting of TNF-alpha, IL-10, and IL-8 for Blood Stasis Syndrome with Myocardial Ischemia. Evid Based Complement Alternat Med. 2013;2013:130702. doi: 10.1155/2013/130702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Wang JS, Yin HJ, Chen KJ. The Expression of CD14(+)CD16(+) Monocyte Subpopulation in Coronary Heart Disease Patients with Blood Stasis Syndrome. Evid Based Complement Alternat Med. 2013;2013:416932. doi: 10.1155/2013/416932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Yu G. A Systems Biology Approach to Characterize Biomarkers for Blood Stasis Syndrome of Unstable Angina Patients by Integrating MicroRNA and Messenger RNA Expression Profiling. Evid Based Complement Alternat Med. 2013;2013:510208. doi: 10.1155/2013/510208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Yu G. Biomedical mechanisms of blood stasis syndrome of coronary heart disease by systems biology approaches. Chin J Integr Med. 2014;20:163–169. doi: 10.1007/s11655-013-1461-3. [DOI] [PubMed] [Google Scholar]
- 14.Allison AC. Turnovers of erythrocytes and plasma proteins in mammals. Nature. 1960;188:37–40. doi: 10.1038/188037a0. [DOI] [PubMed] [Google Scholar]
- 15.Chien S. Red cell deformability and its relevance to blood flow. Annu Rev Physiol. 1987;49:177–192. doi: 10.1146/annurev.ph.49.030187.001141. [DOI] [PubMed] [Google Scholar]
- 16.Diez-Silva M, Dao M, Han J, Lim CT, Suresh S. Shape and Biomechanical Characteristics of Human Red Blood Cells in Health and Disease. MRS Bull. 2010;35:382–388. doi: 10.1557/mrs2010.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Oliveira S, Saldanha C. An overview about erythrocyte membrane. Clin Hemorheol Microcirc. 2010;44:63–74. doi: 10.3233/CH-2010-1253. [DOI] [PubMed] [Google Scholar]
- 18.Ando K, Beppu M, Kikugawa K. Evidence for accumulation of lipid hydroperoxides during the aging of human red blood cells in the circulation. Biol Pharm Bull. 1995;18:659–663. doi: 10.1248/bpb.18.659. [DOI] [PubMed] [Google Scholar]
- 19.Mazzulla S, Schella A, Gabriele D, Baldino N, Sesti S, Perrotta E, Costabile A, de Cindio B. Oxidation of human red blood cells by a free radical initiator: Effects on rheological properties. Clin Hemorheol Microcirc. 2014 doi: 10.3233/CH-141841. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Tillmann W, Levin C, Prindull G, Schroter W. Rheological properties of young and aged human erythrocytes. Klin Wochenschr. 1980;58:569–574. doi: 10.1007/BF01477168. [DOI] [PubMed] [Google Scholar]
- 21.Shiga T, Maeda N, Suda T, Kon K, Sekiya M. The decreased membrane fluidity of in vivo aged, human erythrocytes. A spin label study. Biochim Biophys Acta. 1979;553:84–95. doi: 10.1016/0005-2736(79)90032-4. [DOI] [PubMed] [Google Scholar]
- 22.Diamant M, Tushuizen ME, Sturk A, Nieuwland R. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest. 2004;34:392–401. doi: 10.1111/j.1365-2362.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 23.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 24.Manno S, Takakuwa Y, Mohandas N. Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc Natl Acad Sci U S A. 2002;99:1943–1948. doi: 10.1073/pnas.042688399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An X, Guo X, Sum H, Morrow J, Gratzer W, Mohandas N. Phosphatidylserine binding sites in erythroid spectrin: location and implications for membrane stability. Biochemistry. 2004;43:310–315. doi: 10.1021/bi035653h. [DOI] [PubMed] [Google Scholar]
- 26.Bosman GJ, Werre JM, Willekens FL, Novotny VM. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med. 2008;18:335–347. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 27.Bosman GJ, Lasonder E, Groenen-Dopp YA, Willekens FL, Werre JM. The proteome of erythrocyte-derived microparticles from plasma: new clues for erythrocyte aging and vesiculation. J Proteomics. 2012;76 Spec No.:203–210. doi: 10.1016/j.jprot.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Willekens FL, Werre JM, Groenen-Dopp YA, Roerdinkholder-Stoelwinder B, de Pauw B, Bosman GJ. Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol. 2008;141:549–556. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 29.Stewart A, Urbaniak S, Turner M, Bessos H. The application of a new quantitative assay for the monitoring of integrin-associated protein CD47 on red blood cells during storage and comparison with the expression of CD47 and phosphatidylserine with flow cytometry. Transfusion. 2005;45:1496–1503. doi: 10.1111/j.1537-2995.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 30.do Nascimento TS, Pereira RO, de Mello HL, Costa J. Methemoglobinemia: from diagnosis to treatment. Rev Bras Anestesiol. 2008;58:651–664. doi: 10.1590/s0034-70942008000600011. [DOI] [PubMed] [Google Scholar]
- 31.Hare GM, Tsui AK, Crawford JH, Patel RP. Is methemoglobin an inert bystander, biomarker or a mediator of oxidative stress-The example of anemia? Redox Biol. 2013;1:65–69. doi: 10.1016/j.redox.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Signorini C, Ferrali M, Ciccoli L, Sugherini L, Magnani A, Comporti M. Iron release, membrane protein oxidation and erythrocyte ageing. FEBS Lett. 1995;362:165–170. doi: 10.1016/0014-5793(95)00235-2. [DOI] [PubMed] [Google Scholar]
- 33.Arashiki N, Kimata N, Manno S, Mohandas N, Takakuwa Y. Membrane peroxidation and methemoglobin formation are both necessary for band 3 clustering: mechanistic insights into human erythrocyte senescence. Biochemistry. 2013;52:5760–5769. doi: 10.1021/bi400405p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuart J, Nash GB. Red cell deformability and haematological disorders. Blood Rev. 1990;4:141–147. doi: 10.1016/0268-960x(90)90041-p. [DOI] [PubMed] [Google Scholar]
- 35.Pantely GA, Swenson LJ, Tamblyn CH, Seaman GV, Anselone CG, Johnson WB, Bristow JD. Increased vascular resistance due to a reduction in red cell deformability in the isolated hind limb of swine. Microvasc Res. 1988;35:86–100. doi: 10.1016/0026-2862(88)90052-0. [DOI] [PubMed] [Google Scholar]
- 36.Cabrales P. Effects of erythrocyte flexibility on microvascular perfusion and oxygenation during acute anemia. Am J Physiol Heart Circ Physiol. 2007;293:H1206–1215. doi: 10.1152/ajpheart.00109.2007. [DOI] [PubMed] [Google Scholar]
- 37.Owens AP 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin O, Delobel J, Prudent M, Lion N, Kohl K, Tucker EI, Tissot JD, Angelillo-Scherrer A. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2013;53:1744–1754. doi: 10.1111/trf.12008. [DOI] [PubMed] [Google Scholar]
- 39.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 40.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 41.Schafer A, Wiesmann F, Neubauer S, Eigenthaler M, Bauersachs J, Channon KM. Rapid regulation of platelet activation in vivo by nitric oxide. Circulation. 2004;109:1819–1822. doi: 10.1161/01.CIR.0000126837.88743.DD. [DOI] [PubMed] [Google Scholar]
- 42.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 44.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 45.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 46.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 47.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 48.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J Biol Chem. 2000;275:2342–2348. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 49.Deonikar P, Kavdia M. Contribution of membrane permeability and unstirred layer diffusion to nitric oxide-red blood cell interaction. J Theor Biol. 2013;317:321–330. doi: 10.1016/j.jtbi.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butler AR, Megson IL, Wright PG. Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochim Biophys Acta. 1998;1425:168–176. doi: 10.1016/s0304-4165(98)00065-8. [DOI] [PubMed] [Google Scholar]
- 51.Liao JC, Hein TW, Vaughn MW, Huang KT, Kuo L. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proc Natl Acad Sci U S A. 1999;96:8757–8761. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu C, Zhao W, Christ GJ, Gladwin MT, Kim-Shapiro DB. Nitric oxide scavenging by red cell microparticles. Free Radic Biol Med. 2013;65:1164–1173. doi: 10.1016/j.freeradbiomed.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem. 2005;51:434–444. doi: 10.1373/clinchem.2004.035154. [DOI] [PubMed] [Google Scholar]
- 55.Khandelwal S, Saxena RK. Assessment of survival of aging erythrocyte in circulation and attendant changes in size and CD147 expression by a novel two step biotinylation method. Exp Gerontol. 2006;41:855–861. doi: 10.1016/j.exger.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 56.Gokdemir MT, Sogut O, Kaya H, Sayhan MB, Cevik M, Dokuzoglu MA, Boleken ME. Role of oxidative stress in the clinical outcome of patients with multiple blunt trauma. J Int Med Res. 2012;40:167–173. doi: 10.1177/147323001204000117. [DOI] [PubMed] [Google Scholar]
- 57.Rana SV, Kashinath D, Singh G, Pal R, Singh R. Study on oxidative stress in patients with abdominal trauma. Mol Cell Biochem. 2006;291:161–166. doi: 10.1007/s11010-006-9210-y. [DOI] [PubMed] [Google Scholar]
- 58.Oldham KM, Wise SR, Chen L, Stacewicz-Sapuntzakis M, Burns J, Bowen PE. A longitudinal evaluation of oxidative stress in trauma patients. JPEN J Parenter Enteral Nutr. 2002;26:189–197. doi: 10.1177/0148607102026003189. [DOI] [PubMed] [Google Scholar]
- 59.Laplace C, Huet O, Vicaut E, Ract C, Martin L, Benhamou D, Duranteau J. Endothelial oxidative stress induced by serum from patients with severe trauma hemorrhage. Intensive Care Med. 2005;31:1174–1180. doi: 10.1007/s00134-005-2737-7. [DOI] [PubMed] [Google Scholar]
- 60.Vina J, Gomez-Cabrera MC, Lloret A, Marquez R, Minana JB, Pallardo FV, Sastre J. Free radicals in exhaustive physical exercise: mechanism of production, and protection by antioxidants. IUBMB Life. 2000;50:271–277. doi: 10.1080/713803729. [DOI] [PubMed] [Google Scholar]
- 61.Berzosa C, Gomez-Trullen EM, Piedrafita E, Cebrian I, Martinez-Ballarin E, Miana-Mena FJ, Fuentes-Broto L, Garcia JJ. Erythrocyte membrane fluidity and indices of plasmatic oxidative damage after acute physical exercise in humans. Eur J Appl Physiol. 2011;111:1127–1133. doi: 10.1007/s00421-010-1738-6. [DOI] [PubMed] [Google Scholar]
- 62.Xiong YL, Xiong YL, Li YJ, Tang FZ, Wang RF, Zhao YJ, Wang X. [Effects of exhaustive exercise-induced oxidative stress on red blood cell deformability] . Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2014;30:289–293. [PubMed] [Google Scholar]
- 63.Kameneva MV, Antaki JF, Borovetz HS, Griffith BP, Butler KC, Yeleswarapu KK, Watach MJ, Kormos RL. Mechanisms of red blood cell trauma in assisted circulation. Rheologic similarities of red blood cell transformations due to natural aging and mechanical stress. ASAIO J. 1995;41:M457–460. [PubMed] [Google Scholar]
- 64.Yen JH, Chen SF, Chern MK, Lu PC. The effect of turbulent viscous shear stress on red blood cell hemolysis. J Artif Organs. 2014;17:178–185. doi: 10.1007/s10047-014-0755-3. [DOI] [PubMed] [Google Scholar]
- 65.Koter M, Franiak I, Strychalska K, Broncel M, Chojnowska-Jezierska J. Damage to the structure of erythrocyte plasma membranes in patients with type-2 hypercholesterolemia. Int J Biochem Cell Biol. 2004;36:205–215. doi: 10.1016/s1357-2725(03)00195-x. [DOI] [PubMed] [Google Scholar]
- 66.Dimeski G, Mollee P, Carter A. Increased lipid concentration is associated with increased hemolysis. Clin Chem. 2005;51:2425. doi: 10.1373/clinchem.2005.058644. [DOI] [PubMed] [Google Scholar]
- 67.Broncel M, Balcerak M, Bala A, Koter-Michalak M, Duchnowicz P, Chojnowska-Jezierska J. [The erythrocyte membrane structure in patients with mixed hyperlipidemia] . Wiad Lek. 2007;60:4–9. [PubMed] [Google Scholar]
- 68.Jain SK. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem. 1989;264:21340–21345. [PubMed] [Google Scholar]
- 69.Resmi H, Akhunlar H, Temiz Artmann A, Guner G. In vitro effects of high glucose concentrations on membrane protein oxidation, G-actin and deformability of human erythrocytes. Cell Biochem Funct. 2005;23:163–168. doi: 10.1002/cbf.1129. [DOI] [PubMed] [Google Scholar]
- 70.Mohanty JG, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol. 2014;5:84. doi: 10.3389/fphys.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaya A, Rivera L, de la Espriella R, Sanchez F, Suescun M, Hernandez JL, Facila L. Red blood cell distribution width and erythrocyte deformability in patients with acute myocardial infarction. Clin Hemorheol Microcirc. 2015;59:107–14. doi: 10.3233/CH-131751. [DOI] [PubMed] [Google Scholar]
- 72.Keymel S, Heiss C, Kleinbongard P, Kelm M, Lauer T. Impaired red blood cell deformability in patients with coronary artery disease and diabetes mellitus. Horm Metab Res. 2011;43:760–765. doi: 10.1055/s-0031-1286325. [DOI] [PubMed] [Google Scholar]
- 73.Reid HL, Dormandy JA, Barnes AJ, Lock PJ, Dormandy TL. Impaired red cell deformability in peripheral vascular disease. Lancet. 1976;1:666–668. doi: 10.1016/s0140-6736(76)92778-1. [DOI] [PubMed] [Google Scholar]
- 74.Shin S, Ku YH, Ho JX, Kim YK, Suh JS, Singh M. Progressive impairment of erythrocyte deformability as indicator of microangiopathy in type 2 diabetes mellitus. Clin Hemorheol Microcirc. 2007;36:253–261. [PubMed] [Google Scholar]
- 75.Brown CD, Ghali HS, Zhao Z, Thomas LL, Friedman EA. Association of reduced red blood cell deformability and diabetic nephropathy. Kidney Int. 2005;67:295–300. doi: 10.1111/j.1523-1755.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 76.Blum A. The possible role of red blood cell microvesicles in atherosclerosis. Eur J Intern Med. 2009;20:101–105. doi: 10.1016/j.ejim.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 77.Giannopoulos G, Oudatzis G, Paterakis G, Synetos A, Tampaki E, Bouras G, Hahalis G, Alexopoulos D, Tousoulis D, Cleman MW, Stefanadis C, Deftereos S. Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty. Int J Cardiol. 2014;176:145–150. doi: 10.1016/j.ijcard.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 78.Gao C, Xie R, Yu C, Wang Q, Shi F, Yao C, Xie R, Zhou J, Gilbert GE, Shi J. Procoagulant activity of erythrocytes and platelets through phosphatidylserine exposure and microparticles release in patients with nephrotic syndrome. Thromb Haemost. 2012;107:681–689. doi: 10.1160/TH11-09-0673. [DOI] [PubMed] [Google Scholar]
- 79.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett. 1996;208:191–194. doi: 10.1016/0304-3940(96)12576-3. [DOI] [PubMed] [Google Scholar]
- 80.Naves LA, McCleskey EW. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res. 2005;38:1561–1569. doi: 10.1590/s0100-879x2005001100001. [DOI] [PubMed] [Google Scholar]


