Abstract
Pulsed electromagnetic fields (PEMF) have been shown to promote proliferation and regeneration in the damaged tissue. Here, we examined whether PEMF therapy improved postnatal neovascularization using murine model of hindlimb ischemia, and the underlying cellular/molecular mechanisms were further investigated. Hindlimb ischemia was induced by unilateral femoral artery resection using 6-8 week-old male C57BL6 mice. Then, mice were exposed to extracorporeal PEMF therapy (4 cycles, 8min/cycle, 30 ± 3 Hz, 5 mT) every day until day 14. Our data demonstrated that PEMF therapy significantly accelerated wound healing, decreased prevalence of gangrene and increased postnatal neovascularization. Moreover, the levels of vascular endothelial growth factor (VEGF), endothelial nitric oxide synthase (eNOS) and Akt phosphorylation in ischemic muscles were markedly enhanced following PEMF therapy. In vitro, PEMF inhibited the process of hypoxia-induced apoptosis and augmented tube formation, migration and proliferative capacities of human umbilical vein endothelial cells (HUVECs). Additionally, PEMF exposure increased VEGF secretion, as well as the eNOS and Akt phosphorylation, and these benefits could be blocked by either phosphoinositide 3-kinase (PI3K) or eNOS inhibitor. In conclusion, our data indicated that PEMF therapy enhanced ischemia-mediated angiogenesis, through up-regulating VEGF expression and activating the PI3K-Akt-eNOS pathway. Therefore, PEMF should be a valuable treatment for the patients with critical limb ischemia.
Keywords: Pulsed electromagnetic fields (PEMF), angiogenesis, hindlimb ischemia, VEGF, eNOS
Introduction
Peripheral artery disease (PAD) is an increasing clinical scenario defined as the impairment of blood flow to extremities, which is most common caused by atherosclerotic progression. The exacerbation of limb ischemia, which is associated with aging, black race/ethnicity, cigarette, diabetes, hypertension and hypercholesterolemia, would lead to critical limb ischemia (CLI) [1,2]. It is reported that the incidence of limb ischemia is 1.5 cases per 10000 persons per year in USA [3]. Despite achievements of therapeutic strategies against PAD, clinicians are still facing increasing challenges of refractory CLI. Recently, therapeutic angiogenesis have been applied to treat no-option patients with CLI.
Pulsed electromagnetic field (PEMF) is a non-invasive and non-pharmacological intervention and reported to repair damaged tissue [4-8]. Several clinical studies have demonstrated that PEMF effectively alleviate traumatic pain, tissue swell and promote wound healing [9]. PEMF also accelerates the fusion of fractured bones [10], as well as contributes to neuronal regeneration and differentiation [11,12]. Recent studies indicated that PEMF stimulated angiogenesis and improved microcirculation in patients with diabetes [13-15]. However the detailed mechanism remains modest understood.
We hypothesized that PEMF can promote PAD through accelerating angiogenesis. In the present study, we introduced PEMF treatment to a well-established mouse model of hindlimb ischemia and investigated the role PEMF in ischemia-mediated angiogenesis.
Materials and methods
Animals
Male, 8-week-old C57BL/6 mice (18-20 g) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China) and acclimated in an environmentally controlled breeding room for 5 days prior to the experiments. All the mice had free access to food/water supplies and were fasting overnight before experiments. The study was approved by the Animal Ethics Committee of the Shanghai Jiao Tong University and conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All surgeries were performed under anesthesia, and all efforts were made to minimize suffering.
Animal study protocol and sampling
Male C57BL/6 mice were randomly divided into 4 groups as follow: (1) skin cut without artery excision (sham group, n = 6), (2) sham operation but treated with PEMF (sham + PEMF group, n = 6), (3) left femoral artery resection without PEMF (ischemia group, n = 6), (4) left femoral artery resection treated with PEMF (ischemia + PEMF group, n = 6). Unilateral hindlimb ischemia was surgically induced and PEMF was performed among groups as previously described [8,16]. Wound healing and the gangrene occurrence was observed during the next 14 days after surgery. The blood perfusion was detected indirectly by the temperature of the limbs on postoperative day 0 and day 14 [17]. The rapid development of the infrared detection technology [18-20] promote the thermal infrared image to be a very useful noncontact temperature measurement method to get the temperature image of animals. This thermal infrared image method is used to determine the skin temperature of our studied mice. Animals were euthanized after finishing infrared spectrum imaging on postoperative day 14. The thigh adductor muscle was immersed in 4% formaldehyde and embedded in paraffin for immunohistochemistry analysis. For fluorescent immunohistochemistry, samples were snapped frozen in liquid nitrogen and stored at -80°C until analysis.
Hindlimb ischemia model
Unilateral hindlimb ischemia was surgically induced as previously described [16]. Mice were anesthetized with 4% chloral hydrate (40 mg/kg, i.p.) under sterile surgical conditions. We performed a longitudinal incision in the left hindlimb; the femoral artery was completely excised from its proximal origin to the point where it bifurcated into the popliteal and saphenous arteries including all branches of the femoral artery.
PEMF treatment
PEMF was generated by a commercially available healing device purchased from Biomobie Regenerative Medicine Technology (Shanghai, China). Fields were asymmetric and consisted of 4.5 ms pulses at 30 ± 3 Hz, with a magnetic flux density increasing from 0 to 5 mT in 400 μs as described before [8]. The mice among PEMF groups were housed in custom-designed cages and exposed to active PEMF for 32 minutes (4 cycles, 8 minutes per cycle) per day until day 14 after surgery.
Hindlimb ischemic score
The ischemia score was assessed as described previously [21]. Briefly, mice were investigated 14 days after surgery and assigned one of the following scores: 0, no necrosis or skin ulcers occurred; 1, skin ulcers; 2, below ankle amputation; and 3, above ankle amputation. Two researchers evaluated the ischemia score in a blinded manner and the average scores for each animal were used for quantitative analysis.
Thermal infrared imaging (TIRI) analysis
The skin temperatures of both hind limbs were measured by the thermal infrared imaging analyzer (Prism-DS 50137, FLIR Systems) to evaluate the blood perfusion [22,23]. Low temperature was displayed as dark-to-purple, whereas high temperature was displayed as red-to-white. We performed infrared radiation image detection over the same region of interest immediately after surgery (day 0) and day 14, temperature values was computed from histograms of the colored pixels. To minimize variations due to ambient light, the TIRI data was expressed as the ischemic/normal limb temperature ratio.
Immunofluorescence analysis
Immunofluorescence staining for capillary density was performed as described previously [24]. Briefly, 14 days after surgery, mice were sacrificed and the thigh adductor muscles were fixed in 4% paraformaldehyde for 24 h. Then, samples were washed, dehydrated in a graded ethanol series and embedded in paraffin. 5 μm-sections were cut and blocked with PBS containing 1% BSA and 0.1% Tween 20. The sections were incubated with goat anti-CD31 antibody (5 μg/ml, BD Biosciences, Franklin Lakes, NJ) at 4°C overnight. After washing with PBS containing 0.1% Tween 20, the sections were incubated with Alexa 488-conjugated anti-rat IgG goat polyclonal antibodies (250-fold dilution, Invitrogen, Carlsbad, CA) at room temperature for 30 minutes in a dark room. Capillary density was determined by counting of 10 randomly selected fields and is expressed as numbers of capillary/field (× 400 magnification) [25,26].
Cell culture
Human umbilical vein endothelial cells (HUVEC; ATCC, Cat. CRL1730) were purchased from cultured in DMEM (low-glucose plus 10% FBS) and supplemented with 100 U/ml penicillin and 100 U/ml streptomycin at 37°C under a humidified 95%: 5% (v/v) mixture of air and CO2. HUVECs were reseeded into plates overnight and stimulated at indicated time point flowing PEMF exposure 1-4 cycles/day for 3 days, 30 ± 3 Hz, 5 mT). Supernatant and cell lysates were collected for biological analysis.
Reagents
All drugs were obtained from Beyotime, Jiangsu, China. L-NAME, an eNOS inhibitor, was dissolved in PBS and used at a final concentration of 50 μM. LY294002, a phosphoinositide 3-kinase (PI3K) inhibitor, was dissolved in DMSO and used at a final concentration of 20 μM.
CCK-8 assay
Cell growth was analyzed using a WST-8 Cell Counting Kit-8 (CCK-8 kit, Beyotime). HUVECs (2 × 104/mL) were seeded in 96-well plates with 100 μL DMEM (with 10% FBS) and exposed to PEMF for 1-4 cycles The HUVECs were then incubated at 37°C for 4 h after the CCK-8 solution (10 μL) was added to each well. The absorbance of the reaction system at 0.5 h, 1 h, 2 h, and 4 h was measured at 450nm wavelength. Additionally, HUVECs (5 × 104/mL) suspended in DMEM (100 μL) were operated followed by the above-mentioned protocol for indicating the toxicity of PEMF.
Apoptosis analysis
HUVECs were seeded on sterile cover glasses placed in the 6-well plates and cultured in serum-free DMEM for 48 h. HUVECs were then treated with PEMF as indicated (1-4 cycles with/without L-NAME or LY294002 treatment) for consecutive 3 days. Next, cells were fixed with 4% paraformaldehyde, washed twice with PBS and stained with Hoechst 33258 staining solution according to the manufacturer instructions (Beyotime). Hoechst-stained pyknotic nuclei were counted as percentage of 100 cells in each well (× 400 magnification). Each group was studied at least in triplicate [27].
Migration assay
Migration assay was performed using 24-well Boyden Transwell chambers (Corning, Cambridge, MA) with 6.5-mm-diameter polycarbonate filters (8-μm pore size) [28]. Briefly, 600 μL DMEM containing 10% FBS was added into the lower compartment, HUVECs (3 × 105/mL) suspended in 100 μl DMEM (no serum) were seeded in upper compartment of the Transwell chambers. Cells were then treated with PEMF for different cycles as indicated with/without L-NAME or LY294002. After 8 h incubation, upper compartments were removed, whereas the cells that migrated through the membrane to the underside were fixed with cold 4% paraformaldehyde and stained with 0.1% crystal violet. Cell numbers were counted in 5 separate fields using light microscopy at × 200 magnification.
Wound healing assay
HUVECs were cultured and grown to 100% confluence in 6-well plates. A clear area was then scraped in the monolayer with a 200 μl pipette tip. After washed by PBS for three times, HUVECs were cultured with serum-free DMEM, treated with PEMF for different cycles as indicated. Migrated cells into wounded areas was evaluated with a phase contrast microscope and photographed 24 h and 48 h later (× 100 magnification). The healing rate was quantified with measurements of the narrowing down gap size by the formula: 100% - (width 24 h or 48 h/width 0 h) × 100%. Three different areas in each assay were randomly chosen.
Tube formation assay
The tube formation assay was performed as described previously [29]. Briefly, Matrigel-Matrix (BD Biosciences) was added in the well of a 48-well cell culture plate and HUVECs (5 × 105/mL) suspended in 50 μl DMEM (with 10% FBS) were seeded and treated with PEMF. After 6-8 h incubation, images were acquired under a fluorescent microscope (IX-71; Olympus, Tokyo, Japan) with 12.8 M pixel recording digital color cooled camera (DP72; Olympus). The tube formation was calculated as fold of control. Each experiment was repeated 4 times under similar conditions, images of tube morphology were taken and tube lengths were calculated under × 100 magnification.
Western blotting
Equal amounts of total protein from the extracts of thigh muscle and HUVECs were resolved in SDS 10% polyacrylamide gel and transferred to nitrocellulose membranes for Western blotting as described previously [16,30]. The primary antibodies used were as follows: anti-eNOS (Sigma, St. Louis, MO), anti-phosphor-eNOS (Sigma), anti-VEGF (Proteintech, Chicago, IL), anti-Akt, anti-phosphor-Akt (p-Akt) and anti-GAPDH (Cell Signaling Technology, Beverly, MA). Positive signals were visualized with a FluorChem E data system (Cell Biosciences, Santa Clara, CA) and quantified by densitometry using Quantity One 4.52 (Bio-Rad, Hercules, CA). We applied GAPDH as an internal control to standardize to the protein quantity.
Statistical analysis
One-way ANOVA analysis of variance with the post-hoc Tukey test was applied for multiple comparisons. SPSS software version 17.0 (SPSS Inc., Chicago, IL) was used. All experiments were performed at least in triplicate. A value of P < 0.05 was considered significant.
Results
PEMF reduced the prevalence of limb necrosis and ischemic ulcers
To investigate whether PEMF promoted wound healing, we observed the general recovery of ischemic limbs and recording the occurrence of skin ulcers and gangrene 14 days after hindlimb ischemia (Figure 1A). We found that the ischemic score among PEMF-treated mice was significantly lower than that in non-treated ones either between operation groups (P < 0.01) or sham groups (P < 0.05). These results indicated that PEMF could effectively accelerate skin wound healing and rescue ischemic limbs (Figure 1B).
Figure 1.
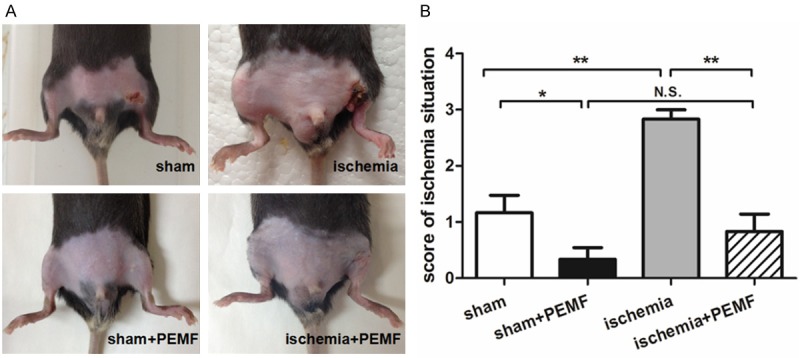
PEMF reduces the occurrence of necrosis or skin ulcers after hindlimb ischemia. At day 14 after induction of ischemia, the general condition of hindlimb was photographed (A), and, the ischemia was scored according to a recognized evaluation standards (B). Values are mean ± SEM; n = 6, N.S. means no significant difference, *means P < 0.05, **means P < 0.01.
PEMF improved angiogenesis in ischemic area
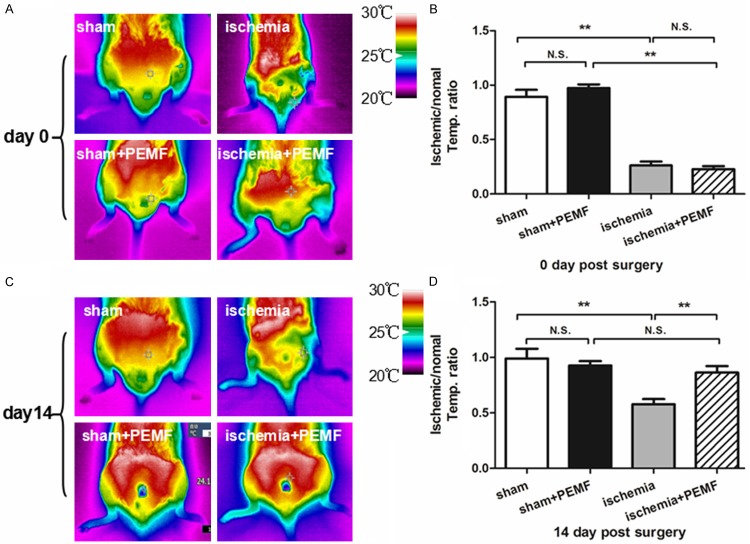
The representative photographs of infrared spectrum in day 0 and 14 were illustrated in Figure 2A and 2C. Fourteen days after PEMF therapy, the temperature ratio of PEMF-treated mice was dramatically increased in comparison with PEMF-free mice ischemic groups, suggested that PMEF accelerate the blood flow recovery of hindlimb ischemia (Figure 2B and 2C). To confirm our observation in microcirculation level, we preformed anti-CD31 immunofluorescence stainings to quantify the capillary density. We demonstrated that capillary density was increased in PEMF-treated mice compared with non-treated ones in ischemic group 14 days after operation (Figure 3).
Figure 2.
PEMF improves the blood flow of the ischemic hindlimb. Infrared spectrum imaging assay was performed on day 0 and day 14 after the hindlimb ischemia operation (A and C). The data was analyzed and illustrated as an ischemic/normal hindlimb temperature ratio (B and D). Values are mean ± SEM; n = 6 , N.S. means no significant difference,*means P < 0.05, **means P < 0.01.
Figure 3.
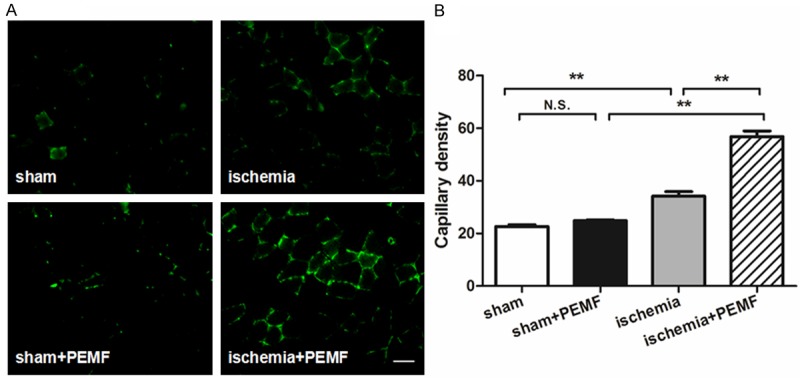
Effects of PEMF on capillary density in ischemic hindlimb. CD31-positive cells were stained in the left thigh adductor muscle at day 14 after induction of ischemia (A) Capillary density was identified with capillary per field (× 400 magnification, (B) Values are mean ± SEM; n = 6, N.S. means no significant difference, *means P < 0.05, **means P < 0.01. Scale bar indicated 20 μm.
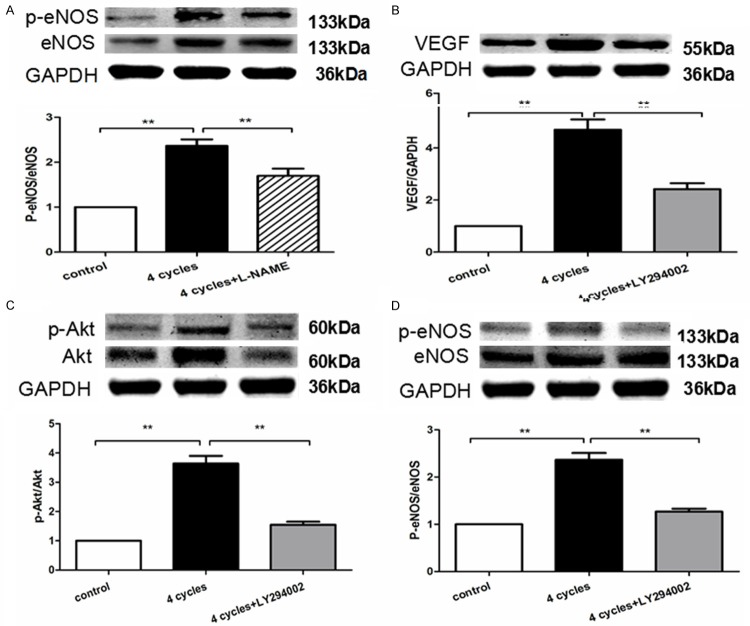
PEMF induced the expressions of angiogenic factors in vivo
To further examine the underlying mechanisms of PEMF-induced angiogenesis, we evaluated the expressions of several angiogenic factors. Although no difference of VEGF level could be found between 2 sham groups, PEMF treatment significantly increased VEGF expression in ischemic groups (Figure 4A). Moreover, the phosphorylation levels of Akt (p-Akt, Figure 4B) as well as eNOS (p-eNOS, Figure 4C) contained in the ischemic muscle were also up-regulated at postoperative day 14 in response to 4-cycle PEMF exposure.
Figure 4.
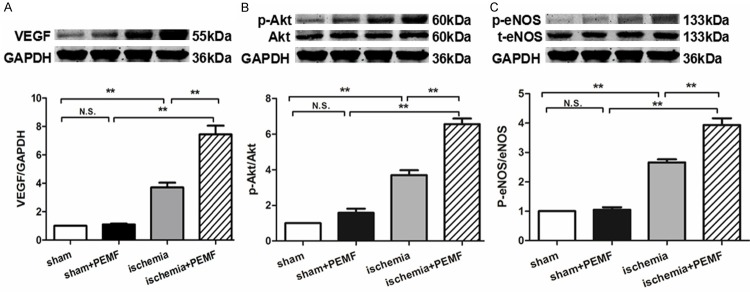
PEMF promotes the expression of VEGF, p-Akt and p-eNOS in vivo. Quantitative analysis of protein content of VEGF, Akt, phosphorylated Akt (p-Akt), eNOS and phosphorylated eNOS (p-eNOS) were analyzed 14 days after operation. Data of Western blotting was represented as fold of sham. Values are mean ± SEM; n = 6, N.S. means no significant difference, **means P < 0.01.
PEMF accelerated the proliferation of HUVECs
HUVECs were stimulated by PEMF in different duration (1-4 cycles, 8 min per cycle, 30 ± 3 Hz, 5 mT), cell growth was analyzed by CCK-8 assay in several time point as indicated. We found that PEMF promoted cell growth was promoted in treated groups from 1 h after the reaction to compare with the control group and became significantly higher 4 h later (Figure 5A). The HUVECs’ proliferation was enhanced by PEMF in a dose-dependent manner. During the process, there wasn’t any system toxicity exposed to the PEMF treatment (Figure 5B). This in vitro experiment further proved that PEMF could improve angiogenesis effectively.
Figure 5.
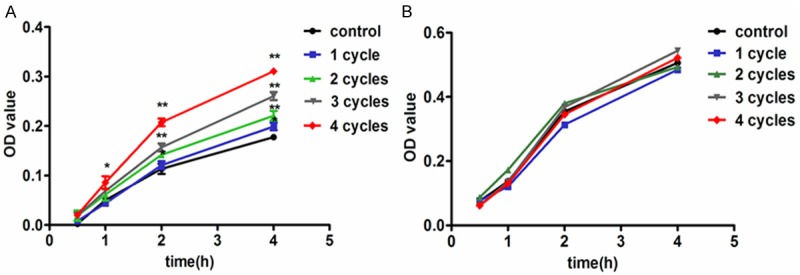
PEMF accelerates the proliferation of HUVECs without significant toxicity. HUVECs were stimulated by PEMF for 1-4 cycles and the proliferation was analyzed by Cell Count Kit-8 (CCK-8) in several time point as indicated (A). The cell toxicity was also checked (B). Values are mean ± SEM; n = 4, N.S. means no significant difference, *means P < 0.05, **means P < 0.01, vs. control group.
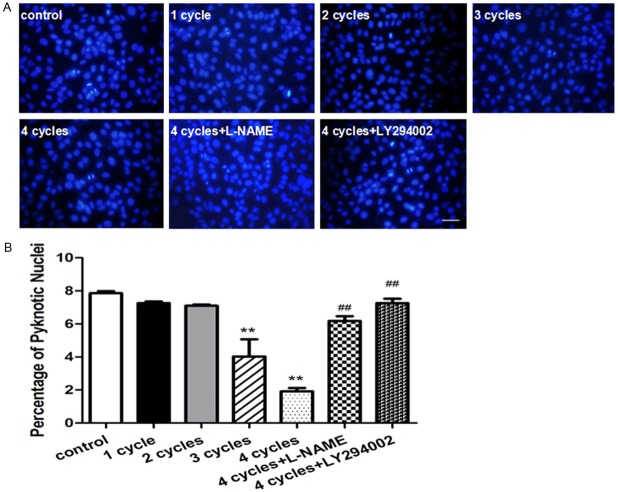
PEMF inhibited serum free-induced HUVEC apoptosis
As shown in Figure 6A, Hoechst staining was performed to determine the proportion of apoptotic cells by manually counting pyknotic nuclei. The proportions of apoptotic cells were dramatically decreased in the HUVECs that stimulated by PEMF for 3-4 cycles compared with others. Moreover, this benefit could be markedly attenuated using eNOS inhibitor L-NAME or PI3k inhibitor LY-294002 (Figure 6B).
Figure 6.
PEMF protects the apoptosis of HUVECs in vivo. Representative images of Hoechst33258 Staining in HUVECs were stimulated by PEMF for 1-4 cycles, and 4 cycles group treated with L-NAME or LY294002 and stained by Hoechst33258 (A) Quantitative analysis was represented as the apoptotic cells in the total cells per field (B) Values are mean ± SEM; n = 4, N.S. means no significant difference, ** means P < 0.01, vs. control; ## means P < 0.01, vs. 4 cycles group. Scale bar indicated 20 μm.
PEMF promotes migration and tube formation of HUVECs
Two kinds of migration assay were performed to evaluate endothelial migration ability in response to PEMF treatment. In transwell migration assay, both 3 cycle- and 4 cycle-treated groups showed significantly more migrated HUVECs than control group (Figure 7A and 7B). PEMF also accelerated scratch wound closure in comparison with control group following a dose dependent manner (Figure 7C and 7D) and these benefits could be blocked by L-NAME and LY294002. In tube formation assay, the HUVECs-formed micro-tubes were lengthened in response to PEMF exposure following a dose dependent manner via Akt-eNOS axis (Figure 8).
Figure 7.
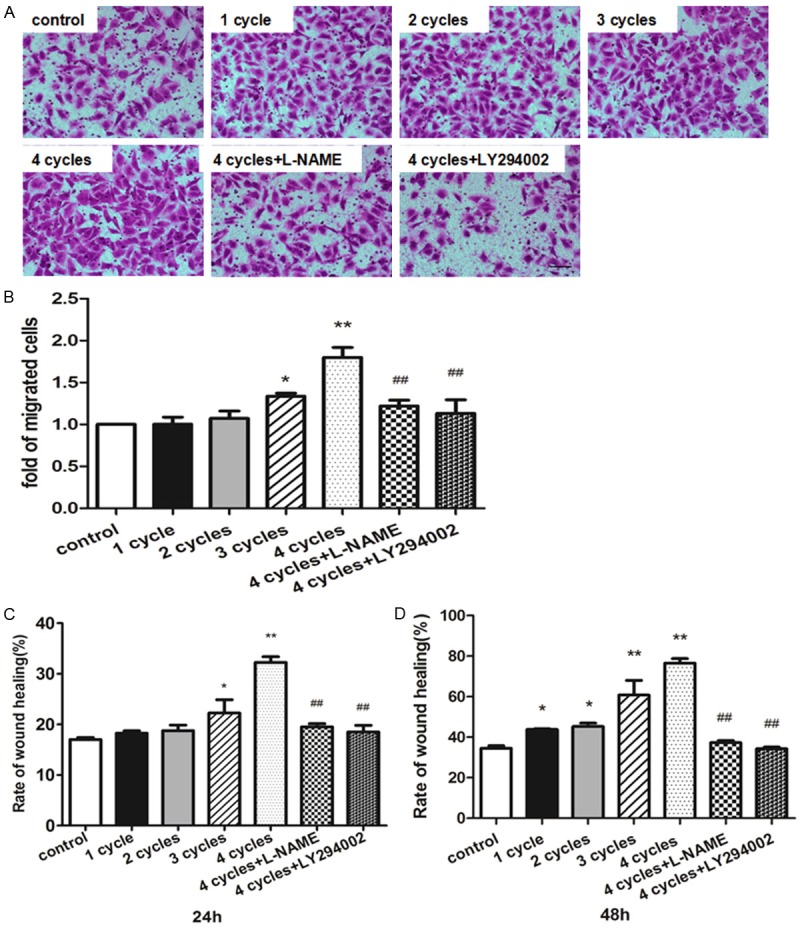
PEMF promotes the migration of HUVECs. HUVECs were plated in transwell and stimulated by PEMF for 1-4 cycles, and 4 cycles group treated with L-NAME or LY294002. Migrated cells were stained (A) and quantitative analysis of migrated cells was represented as fold of control (B). Wound healing analyze was performed 24 h and 48 h (C and D). Values are mean ± SEM; n = 4, N.S. means no significant difference, *means P < 0.05, **means P < 0.01, vs. control; ##means P < 0.01, vs. 4 cycles group. Scale bar indicated 50 μm.
Figure 8.
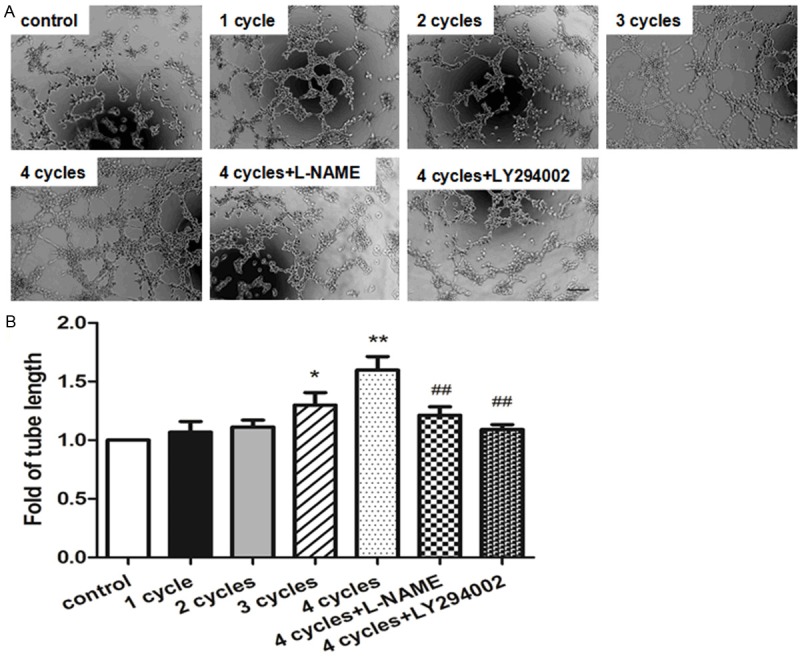
PEMF contributes to tube formation. Representative images of tube formation in HUVECs by stimulated PEMF for 1-4 cycles, and 4 cycles group treated with L-NAME or LY294002 (A) and quantitative analysis of tube length were represented as fold of control (× 100 magnification, B). Values are mean ± SEM; n = 4, *means P < 0.05, **means P < 0.01 vs. control; ##means P < 0.01, vs. 4 cycles group. Scale bar indicated 100 μm.
PEMF enhanced angiogenic factor expressions in vitro
PEMF promoted VEGF released from HUVECs in a dose dependent manner (Figure 9A). Furthermore, the phosphorylation of Akt and eNOS in HUVECs was also upregulated in response to PEMF following a dose dependent manner (Figure 9B and 9C). The PEMF’s benefits can be neutralized by administration of L-NAME and LY294002 (Figure 10). These results indicated that PEMF triggered angiogenesis via activation of Akt-eNOS-VEGF axis.
Figure 9.
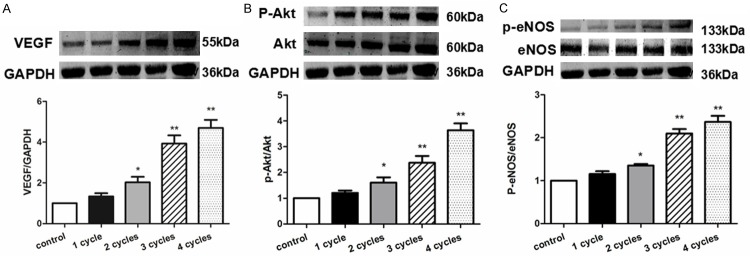
PEMF promotes the expression of VEGF, p-Akt and p-eNOS in vitro. Quantitative analysis of protein content of VEGF, Akt, p-Akt, eNOS and p-eNOS in HUVECs stimulated by PEMF for 1-4 cycles. Data of Western blotting were represented as fold of control. Values are mean ± SEM; n = 4, *means P < 0.05, **means P < 0.01, vs. control.
Figure 10.
Angiogenic factors of PEMF was eliminated by eNOS inhibitor or PI3K inhibitor. Quantitative analysis of protein content of VEGF, Akt, p-Akt, eNOS and p-eNOS in HUVECs stimulated by PEMF for 4 cycles treated with L-NAME (A) or LY294002 (B-D). Data of Western blotting were represented as fold of control. Values are mean ± SEM; n = 4, **means P < 0.01.
Discussion
In present study, we demonstrated that PEMF exposure improved ischemia-induced angiogenesis through enhancing endothelial proliferation, migration, survival and secretion via acting on the Akt-eNOS-VEGF pathway of endothelial cells.
Physiotherapy-mediated regenerative medicine takes several advantages compared with cell/pharmaco-mediated therapies. Extracorporeal PEMF was reported to enhance osteanagenesis [10], skin rapture healing [9] and neuronal regeneration [11,12], suggesting its regenerative potency. Previously, we reported that PEMF preserved heart systolic function and rescued apoptotic cardiomyocytes using mice model of myocardial infarction [8]. Present study demonstrated that PEMF therapy increased tissue repairmen and proliferation via promoting capillary endothelial growth and skin temperature of the ischemic hindlimb, even in sham operation group, suggesting that the PEMF-induced accelerated wound healing might due to increased blood perfusion to the ischemic limb.
To further explore the mechanism of PEMF-mediated neovascularization, we studied PEMF-induced endothelial biological modification in vitro. We clarified that PEMF improved multiple endothelial functions including migration, proliferation, tube formation and VEGF secretion, suggesting the PEMF-induced therapeutic potency targeted on pre-existing endothelial vasculature. On the other hand, we previously demonstrated PEMF exposure promoted sca-1+flk-1+ endothelial progenitor cells (EPCs) recruitment to the ischemic heart using mice model of myocardial infarction [8]. Goto and colleagues also reported that PEMF stimulation up-regulated the expressions of angiopoietin-2 and fibroblastic growth factor-2 in bone marrow, suggesting PEMF could promote the regenerative capacity of myeloid-derived cells (such as EPCs) in damaged tissue when recruited [4]. Taken together, PEMF triggered postnatal neovascularization through acting on both pre-existing endothelial cells and circulating EPCs.
To the best of our knowledge, we first revealed the molecular mechanism of PEMF-mediated angiogenesis in ischemic thigh. The PEMF-induced therapeutic benefits were abolished when either PI3K inhibitor (LY294002) or eNOS deactivator (L-NAME) was administrated to the culture dish. Our study provides convinced data that PEMF stimulated Akt-eNOS-VEGF axis using PI3K and eNOS pathway inhibitors in vitro. VGEF and nitric oxide (NO) appear to mediate distinct, but interdependent, pathways of angiogenesis in response to ischemic stress. VEGF increases the permeability and proliferation of endothelial cells so that triggers endothelial sprouting [31]; nitric oxide, on the other hand, induces mature endothelial markers expression (such as VE-cadherin) [32]. Our data suggested that the combination of VEGF and nitric oxide both induced by PEMF would certainly cause physical but pathological vascular extension [33]. Of course, other mechanisms involved in PEMF-mediated regeneration might also exist. For instance, Goto et al reported that PEMF induced cellular proliferation, as evidenced by cAMP activation and uptake of tritiated thymidine [4].
Several limitations of the present work should be pointed out. For instance, healthy mice model of hindlimb ischemia does not the best imitate for arteriosclerosis obliterans; and the therapeutic efficacy of PEMF on PAD patients with multiple background diseases remains yet clear.
In conclusion, our findings revealed that extracorporeal PEMF treatment stimulated multiple angiogenic pathways, effectively improved blood perfusion, reduced the occurrence of necrosis or skin ulcers and promoted angiogenesis. These findings highlighted the powerful therapeutic potential of PEMF for a safe and simple healing response with better blood perfusion in patients with critical limb ischemia.
Acknowledgements
This work was supported by the China National Natural Science Foundation (11374213) and Foundation of National Lab for Infrared Physics (200901).
Disclosure of conflict of interest
None.
References
- 1.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 2.Chang NT, Chan CL, Lu YT, Hsu JC, Hsu YN, Chu D, Yang NP. Invasively-treated incidence of lower extremity peripheral arterial disease and associated factors in Taiwan: 2000-2011 nationwide hospitalized data analysis. BMC Public Health. 2013;13:1107. doi: 10.1186/1471-2458-13-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366:2198–2206. doi: 10.1056/NEJMcp1006054. [DOI] [PubMed] [Google Scholar]
- 4.Goto T, Fujioka M, Ishida M, Kuribayashi M, Ueshima K, Ueshima K, Kubo T. Noninvasive up-regulation of angiopoietin-2 and fibroblast growth factor-2 in bone marrow by pulsed electromagnetic field therapy. J Orthop Sci. 2010;15:661–665. doi: 10.1007/s00776-010-1510-0. [DOI] [PubMed] [Google Scholar]
- 5.de Girolamo L, Stanco D, Galliera E, Vigano M, Colombini A, Setti S, Vianello E, Corsi Romanelli MM, Sansone V. Low frequency pulsed electromagnetic field affects proliferation, tissue-specific gene expression, and cytokines release of human tendon cells. Cell Biochem Biophys. 2013;66:697–708. doi: 10.1007/s12013-013-9514-y. [DOI] [PubMed] [Google Scholar]
- 6.Lei T, Jing D, Xie K, Jiang M, Li F, Cai J, Wu X, Tang C, Xu Q, Liu J, Guo W, Shen G, Luo E. Therapeutic effects of 15 Hz pulsed electromagnetic field on diabetic peripheral neuropathy in streptozotocin-treated rats. PLoS One. 2013;8:e61414. doi: 10.1371/journal.pone.0061414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross CL, Harrison BS. Effect of pulsed electromagnetic field on inflammatory pathway markers in RAW 264.7 murine macrophages. J Inflamm Res. 2013;6:45–51. doi: 10.2147/JIR.S40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao CN, Huang JJ, Shi YQ, Cheng XW, Li HY, Zhou L, Guo XG, Li RL, Lu W, Zhu YZ, Duan JL. Pulsed electromagnetic field improves cardiac function in response to myocardial infarction. Am J Transl Res. 2014;6:281–290. [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SS, Shin HJ, Eom DW, Huh JR, Woo Y, Kim H, Ryu SH, Suh PG, Kim MJ, Kim JY, Koo TW, Cho YH, Chung SM. Enhanced expression of neuronal nitric oxide synthase and phospholipase C-gamma1 in regenerating murine neuronal cells by pulsed electromagnetic field. Exp Mol Med. 2002;34:53–59. doi: 10.1038/emm.2002.8. [DOI] [PubMed] [Google Scholar]
- 10.Cheing GL, Li X, Huang L, Kwan RL, Cheung KK. Pulsed electromagnetic fields (PEMF) promote early wound healing and myofibroblast proliferation in diabetic rats. Bioelectromagnetics. 2014;35:161–169. doi: 10.1002/bem.21832. [DOI] [PubMed] [Google Scholar]
- 11.Weintraub MI, Herrmann DN, Smith AG, Backonja MM, Cole SP. Pulsed electromagnetic fields to reduce diabetic neuropathic pain and stimulate neuronal repair: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:1102–1109. doi: 10.1016/j.apmr.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Tepper OM, Callaghan MJ, Chang EI, Galiano RD, Bhatt KA, Baharestani S, Gan J, Simon B, Hopper RA, Levine JP, Gurtner GC. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 2004;18:1231–1233. doi: 10.1096/fj.03-0847fje. [DOI] [PubMed] [Google Scholar]
- 13.Yuan Y, Wei L, Li F, Guo W, Li W, Luan R, Lv A, Wang H. Pulsed magnetic field induces angiogenesis and improves cardiac function of surgically induced infarcted myocardium in Sprague-Dawley rats. Cardiology. 2010;117:57–63. doi: 10.1159/000321459. [DOI] [PubMed] [Google Scholar]
- 14.Pan Y, Dong Y, Hou W, Ji Z, Zhi K, Yin Z, Wen H, Chen Y. Effects of PEMF on microcirculation and angiogenesis in a model of acute hindlimb ischemia in diabetic rats. Bioelectromagnetics. 2013;34:180–188. doi: 10.1002/bem.21755. [DOI] [PubMed] [Google Scholar]
- 15.Delle Monache S, Alessandro R, Iorio R, Gualtieri G, Colonna R. Extremely low frequency electromagnetic fields (ELF-EMFs) induce in vitro angiogenesis process in human endothelial cells. Bioelectromagnetics. 2008;29:640–648. doi: 10.1002/bem.20430. [DOI] [PubMed] [Google Scholar]
- 16.Hao CN, Shintani S, Shimizu Y, Kondo K, Ishii M, Wu H, Murohara T. Therapeutic angiogenesis by autologous adipose-derived regenerative cells: comparison with bone marrow mononuclear cells. Am J Physiol Heart Circ Physiol. 2014;307:H869–879. doi: 10.1152/ajpheart.00310.2014. [DOI] [PubMed] [Google Scholar]
- 17.Hao CN, Huang ZH, Song SW, Shi YQ, Cheng XW, Murohara T, Lu W, Su DF, Duan JL. Arterial baroreflex dysfunction impairs ischemia-induced angiogenesis. J Am Heart Assoc. 2014;3:e000804. doi: 10.1161/JAHA.114.000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Li Z, Li N, Chen X, Chen P, Shen X, Lu W. High-polarization-discriminating infrared detection using a single quantum well sandwiched in plasmonic micro-cavity. Sci Rep. 2014;4:6332. doi: 10.1038/srep06332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Chen X, Yu A, Zhang Y, Ding J, Lu W. Highly sensitive and wide-band tunable terahertz response of plasma waves based on graphene field effect transistors. Sci Rep. 2014;4:5470. doi: 10.1038/srep05470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao KS, Li N, Zhou XH, Lu W. Extended mode in blocked impurity band detectors for terahertz radiation detection. Appl Phys Lett. 2014;105:143501. [Google Scholar]
- 21.Theurl M, Schgoer W, Albrecht K, Jeschke J, Egger M, Beer AG, Vasiljevic D, Rong S, Wolf AM, Bahlmann FH, Patsch JR, Wolf D, Schratzberger P, Mahata SK, Kirchmair R. The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circ Res. 2010;107:1326–1335. doi: 10.1161/CIRCRESAHA.110.219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Chen X, Yu A, Zhang Y, Ding J, Lu W. Highly sensitive and wide-band tunable terahertz response of plasma waves based on graphene field effect transistors. Sci Rep. 2014;4:5470. doi: 10.1038/srep05470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, Li Z, Li N, Chen X, Chen P, Shen X, Lu W. High-polarization-discriminating infrared detection using a single quantum well sandwiched in plasmonic micro-cavity. Sci Rep. 2014;4:6332. doi: 10.1038/srep06332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blunder S, Messner B, Aschacher T, Zeller I, Türkcan A, Wiedemann D, Andreas M, Blüschke G, Laufer G, Schachner T, Bernhard D. Characteristics of TAV- and BAV-associated thoracic aortic aneurysms--smooth muscle cell biology, expression profiling, and histological analyses. Atherosclerosis. 2012;220:355–361. doi: 10.1016/j.atherosclerosis.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 25.Duan J, Murohara T, Ikeda H, Sasaki K, Shintani S, Akita T, Shimada T, Imaizumi T. Hyperhomocysteinemia impairs angiogenesis in response to hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2000;20:2579–2585. doi: 10.1161/01.atv.20.12.2579. [DOI] [PubMed] [Google Scholar]
- 26.Duan J, Murohara T, Ikeda H, Katoh A, Shintani S, Sasaki K, Kawata H, Yamamoto N, Imaizumi T. Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia: nitric oxide-dependent mechanism. Circulation. 2000;102:III370–376. doi: 10.1161/01.cir.102.suppl_3.iii-370. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 28.Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-kappaB: implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24:1188–1202. doi: 10.1038/sj.onc.1208276. [DOI] [PubMed] [Google Scholar]
- 29.Chen JK, Deng YP, Jiang GJ, Liu YZ, Zhao T, Shen FM. Establishment of tube formation assay of bone marrow-derived endothelial progenitor cells. CNS Neurosci Ther. 2013;19:533–5. doi: 10.1111/cns.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XH, Lei H, Liu AJ, Zou YX, Shen FM, Su DF. Increased oxidative stress is responsible for severer cerebral infarction in stroke-prone spontaneously hypertensive rats. CNS Neurosci Ther. 2011;17:590–598. doi: 10.1111/j.1755-5949.2011.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, Isner JM. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- 32.Di Lorenzo A, Lin MI, Murata T, Landskroner-Eiger S, Schleicher M, Kothiya M, Iwakiri Y, Yu J, Huang PL, Sessa WC. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J Cell Sci. 2013;126:5541–5552. doi: 10.1242/jcs.115972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benest AV, Stone OA, Miller WH, Glover CP, Uney JB, Baker AH, Harper SJ, Bates DO. Arteriolar genesis and angiogenesis induced by endothelial nitric oxide synthase overexpression results in a mature vasculature. Arterioscler Thromb Vasc Biol. 2008;28:1462–8. doi: 10.1161/ATVBAHA.108.169375. [DOI] [PMC free article] [PubMed] [Google Scholar]