Abstract
Objective: To systemically explore effects of large dose of lubiprostone on gastrointestinal (GI) transit and contractions and its safety in dogs. Methods: 12 healthy dogs were studied. 6 dogs were operated to receive duodenal cannula and colon cannula and the other 6 dogs received gastric cannula. Lubiprostone was orally administrated at a dose of 24 µg or 48 µg 1 hr prior to the experiments. Gastric emptying (GE) of solids and small bowel transit were evaluated by collecting the effluents from the duodenal cannula and from the colon cannula. Gastric accommodation was measured by barostat. Gastric and intestinal contractions were by manometry. Colon transit was by X-ray pictures. Results: 1) Lubiprostone 48 µg not 24 µg accelerated GE. Atropine could block the effect; 2) Average motility index (MI) of gastric antrum in lubiprostone 48 µg session was significantly higher in both fasting state (P = 0.01) and fed state (P = 0.03). Gastric accommodation was not significantly different; 3) Lubiprostone 48 µg accelerated small bowel and colon transit. Atropine could block the effect on small bowel transit; 4) Lubiprostone 48 µg increased postprandial small bowel MI (P = 0.0008) and colon MI (P = 0.002). 5) No other adverse effects except for diarrhea were observed. Conclusion: Acute administration of lubiprostone at a dose of 48 µg accelerates GI motility and enhances GI contractions in the postprandial state. The findings suggest that lubiprostone may have an indirect prokinetic effects on the GI tract and vagal activity may be involved. Lubiprostone may be safely used.
Keywords: Constipation, gastric accommodation, gastrointestinal motility, lubiprostone
Introduction
Chronic constipation is a common clinical problem. 12-19% of the overall population experiences chronic constipation [1,2]. In 2006 and 2008, lubiprostone, a bicyclic fatty acid was approved by the US Food and Drug Administration to treat idiopathic chronic constipation and irritable bowel syndrome with predominant constipation (IBS-C). Lubiprostone became one of the few prescription drugs approved for IBS-C in the U.S.A. Lubiprostone is a selective type-2 chloride channel (ClC-2) activator. It can increase chloride ion transport and fluid secretion into the intestinal lumen to treat constipation [3,4]. However, few studies have reported its effects on gastrointestinal transit and contractions. No study on effect of lubiprostone on gastric accommodation, gastric antrum contractions and small bowel contractions was reported. An in-vitro study showed that lubiprostone could cause concentration-dependent contractions of gastric longitudinal muscle and circular muscle preparations via the EP1 receptor in rats and humans [5]. Cuppoletti et al showed that lubiprostone induced uterine smooth muscle membrane hyperpolarization mediated through lubiprostone’s highly selective activity on ClC-2 channels located on smooth muscle cells [6]. These studies suggest that lubiprostone maybe also have a prokinetic effect on gastrointestinal motility.
This study aimed to systematically investigate possible effects of lubiprostone on contractions and transit of the entire gastrointestinal tract using a well-established canine model [7,8]. We chose the doses of 24 µg or 48 µg of lubiprostone that is the standard dose in human and the larger dose in dogs to explore its prokinetic effects and its safety.
Methods
Animal preparation
Twelve healthy female hound dogs (2-3 years old, 19-26 kg) were studied. The dogs were operated under general anesthesia (with initial intravenous infusion of 5 mg/kg thiopental sodium and maintained on 1.5% isoflurane inhalation in 1:1 oxygen-nitrous oxide carrier gases) using a previously established method [9-11]. Laparotomy was performed. Two cannulas were placed in each of six dogs, one in the duodenum 10 cm distal to the gastric pylorus and the other in the ascending colon 5 cm distal to the cecum. A gastric cannula was placed in gastric anterior wall 10 cm above the pylorus in each of the other six dogs [12]. All dogs were given two weeks to recover from the surgery and all experiments were performed in the conscious state with the animal placed on an experimental table and slightly restrained. All dogs were free from any drugs within one week and were fasted overnight prior to the experiment. The protocol has been approved by the Animal Use and Care Committee of the University of Texas Medical Branch at Galveston.
Effects and mechanisms of lubiprostone on gastric solid emptying and small intestinal transit
The experiment was performed in five randomized sessions: control, lubiprostone (Amitiza, 24 µg/capsule, Sucampo Pharmaceuticals, Chicago, IL) 24 µg, lubiprostone 48 µg, atropine, atropine plus lubiprostone 48 µg. 6 dogs with duodenal and colon cannula were used. Lubiprostone or placebo was orally administrated 1 hr prior to the experiment. The dosage and timing of dosing were based on previous studies and the T1/2 (1.73-2.98 hr) and Tmax (2.38 hr) of lubiprostone [13,14]. Atropine of 0.2 mg/kg was intravenously administrated at the beginning of the experiment in atropine or atropine plus lubiprostone session and the dose was in the previous studies [15,16]. Gastric emptying (GE) was assessed by collecting gastric effluent from the duodenum cannula following ingestion of one can of dog food (Pedigree, Chopped Chicken). The collection was done at 15, 30, 45, 60, 90, 120, 150, 180 minutes after feeding for the lubiprostone sessions. For the control session, the collection lasted until no chyme containing any solid was seen. For every collection, the effluent was homogenized and the volume was recorded, 10 ml of sample was kept and the left effluent was immediately injected back into small bowel from the duodenal cannula. The samples were centrifuged, the liquid supernatant was discarded, and the remaining samples were removed from tube, spread on paper plates to air dry until the weight remained unchanged and then weighed in grams. The dried weight of every collection was calculated. The percentage of GE was defined as the ratio between the dried weight of the collection and the total dried weight of all samples in the control session. The percentage of GE at each time point is the accumulative percentage of GE up to that time point [11,16,17]. In control session, placebo capsule (vitamin E) was given orally.
Small bowel transit was measured at the same time when GE was monitored as follows: Immediately after feeding, 15 mg of phenol red mixed with 30 ml of saline was injected into the small intestine from the duodenum cannula. The colon cannula was opened and the effluent was collected every 5 minutes. The time when the phenol red began to appear from the colon cannula was confirmed by spectrophotometer method, and the time length between the injection of phenol red and the first appearance of phenol red collected from the distal cannula was defined as the small bowel transit time (SBTT).
Effects of lubiprostone on gastric tone and accommodation
Six dogs with gastric cannula were used in this experiment with control and lubiprostone session. Lubiprostone 48 µg/Placebo was given orally to each dog 1 hr prior to experiment. A barostat balloon (500 mL maximal volume, CT-BP800; H & A Mui Enterprise Inc., Mississauga, Ontario, Canada) was put into the proximal stomach from the gastric cannula. The insertion depth was examined during the surgery. The balloon was connected with a computerized electrical barostat device (Distender Series IIR; G & J Electronics Inc., Willowdale, Ontario, Canada) by a double-lumen catheter [18,19]. The balloon was deflated completely. The minimal distending pressure was determined by inflating the balloon in 1 mmHg increments until a pressure at which evident respiratory excursions were recorded. Gastric tone was recorded at an operating pressure of 2 mmHg higher than the minimal distending pressure [19]. Gastric tone was assessed from the balloon volume. After 30 min baseline recording of gastric tone, one can of food was provided to the dog and gastric tone was recorded for 30 minutes. Gastric accommodation was defined as the difference in average volume between the postprandial period and the fasting period.
Effects of lubiprostone on gastric antrum motility
Six dogs with gastric cannula were involved in the experiment with control and lubiprostone sessions. Lubiprostone 48 µg or placebo was given orally to each dog 1 hr before the experiment. Antral contractions was measured using one manometric catheter with four pressure sensors attached to a PC polygraph HR system and a microcapillary infusion system (model 8; Medtronic Synectics, Stockholm, Sweden). The catheter was put into the distal stomach to measure the antral motility. After 30 min baseline recording in the fasting state, one can of dog food was provided to each dog and postprandial motility was recorded for 60 minutes. The contractile activity was evaluated by using the mean area under curve (AUC) per second expressed as motility index (MI) computed by Polygram Function Testing Software (Medtronic, Shoreview, MN) [15,20].
Effects of lubiprostone on small bowel contractions
Six dogs with duodenal cannula were used in the experiment with control and lubiprostone 48 µg sessions. Lubiprostone or the placebo was orally administrated 1 hour prior to the experiment. After the dog was fed with the same food, the manometric recording was made for 60 min using an intra-lumen catheter inserted into the intestine through the duodenum cannula. The catheter contained five manometric sensors at an interval of 5 cm with the most proximal sensor 10 cm distal to the cannula. The manometric method was the same as Experiment 3. The small bowel contractile activity was evaluated by using the mean MI.
Effects of lubiprostone on colon transit
Six dogs with colon cannula were involved in the experiment with control and lubiprostone 48 µg sessions. The dog received the placebo/drug orally. Thirty minutes later, one capsule containing 24 radiopaque markers (1.5-5 mm, Sitzmarks-Konsyl Pharmaceuticals, Easton, MD, USA) were placed into the proximal colon via the colon cannula and one can of the same food was fed. The x-rays were taken at 2, 4, and 6 hours post feeding.
Colon transit was determined based on the localization and the number of markers in each x-ray picture using geometric center (GC) value. The GC is the weighted average of counts in the different colonic regions [21]: ascending (AC), hepatic flexure (HC), transverse (TC), splenic flexure (SC), descending (DC), rectosigmoid (RS), and stool. GC = (number in AC × 1 + number in HC × 2 + number in TC × 3 + number in SC × 4 + number in DC × 5 + number in RS × 6 + evacuated number × 7)/24. A high GC value implies faster colonic transit.
Effects of lubiprostone on colon manometry
Six dogs with colon cannula were used in the experiment with control and lubiprostone 48 µg sessions. The 3 hr postprandial manometric recording was made using an intraluminal catheter inserted into the colon through the colon cannula and the same manometric method was performed as in Experiment 3. The catheter contained three manometric side holes at an interval of 10 cm with the most proximal sensor 10 cm distal to the cannula. The contractile activity was assessed by using the mean MI.
Side effect observation
During the period of experiments, animal behaviors, vomiting, diarrhea were carefully observed and noted.
Statistical analysis
Data are reported as mean ± standard error. One-way ANOVA was applied to assess the difference among three sessions or phrases in the studies of GE, small bowel transit and colon transit. If the ANOVA shows p < 0.05, following Turkey’s test was applied to assess the difference between every two sessions. Student’s t-test was used to analyze the difference between two sessions in gastric volume, gastric manometry, the small bowel and colon manometry. A p-value of < 0.05 was used as a cutoff for statistical significance.
Results
Effects and mechanisms of lubiprostone on gastric solid emptying
Lubiprostone 48 µg significantly accelerated GE at all-time points with the largest increase noted at 120 min from 53% in the control session to 72% with the medication. Lubiprostone 24 µg didn’t significantly accelerated GE at all-time points (p > 0.05 vs. control). Lubiprostone 48 µg also had accelerated GE at all-time points compared with lubiprostone at 24 µg (p < 0.05). Atropine blocked the accelerative effect of lubiprostone 48 µg on GE. No difference was found in GE between the atropine session and lubiprostone 48 µg plus atropine session (p > 0.05) (see Table 1 and Figure 1). The finding suggested that vagal mechanism might be involved.
Table 1.
Gastric solid emptying (%) in different sessions (mean ± SD) (ANOVA P = 0.03)
| Control | Lub 24 µg | Lub 48 µg | Lub 48 µg plus atropine | Atropine | |
|---|---|---|---|---|---|
| 15 min | 1.3 ± 0.6 | 1.3 ± 0.4 | 2.2 ± 0.5* | 0.3 ± 0.1* | 0.2 ± 0.1* |
| 30 min | 6.9 ± 2.2 | 7.5 ± 3.8 | 13.0 ± 3.7* | 1.6 ± 0.8* | 0.8 ± 0.2* |
| 45 min | 15.5 ± 4.3 | 17.6 ± 5.6 | 23.8 ± 4.9* | 3.4 ± 1.5* | 2.2 ± 0.7* |
| 60 min | 25.6 ± 6.5 | 28 ± 7.5 | 37.6 ± 7.0* | 6.8 ± 2.8* | 3.7 ± 0.7* |
| 90 min | 42.6 ± 7.4 | 45 ± 10.6 | 58.6 ± 8.4* | 13.8 ± 5.1* | 9.8 ± 2.3* |
| 120 min | 53.1 ± 5.8 | 59.9 ± 9.4 | 72.2 ± 7.7* | 24.7 ± 7.7* | 24.1 ± 6.0* |
| 150 min | 64.0 ± 5.6 | 69.4 ± 7.5 | 79.6 ± 6.8* | 39.1 ± 8.7* | 42.9 ± 8.6* |
| 180 min | 68.9 ± 6.6 | 74.9 ± 6.6 | 83.3 ± 6.6* | 53.4 ± 9.5* | 54.9 ± 9.6* |
Footnotes: Lub = lubiprostone;
P < 0.05 vs. Control; P > 0.05: atropine session vs. atropine plus lubiprostone 48 µg at any time points; P < 0.05 Control, lubiprostone 24 µg, atropine, lubiprostone 48 µg plus atropine vs. lubiprostone 48 µg at any time points.
Figure 1.
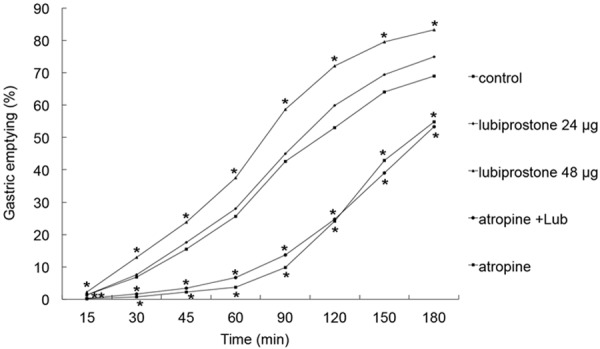
Effect of lubiprostone on gastric emptying of solids. Lubiprostone at 48 µg but not 24 µg significantly enhanced gastric solid emptying at any time points. Atropine blocked the effects. P < 0.05 Control, lubiprostone 24 µg, atropine, lubiprostone 48 µg plus atropine vs. lubiprostone 48 µg at any time points. *P < 0.05.
Effects of lubiprostone on gastric tone and accommodation
Lubiprostone 48 µg did not significantly alter gastric accommodation. In the fasting state, the average intragastric balloon volume was 116 ± 14 mL in the control session and 149 ± 18.4 mL with lubiprostone 48 µg (P > 0.05). In the fed state, the intragastric balloon volume was 437 ± 11.7 mL in the control session and 397.1 ± 47.9 mL in the lubiprostone session (P > 0.05). The gastric accommodation (the difference in gastric volume between postprandial and fasting states) was 321 ± 18 mL in the control session and 248 ± 30 mL with lubiprostone (P = 0.1).
Effects of lubiprostone on gastric antral contractions
Postprandial antral contractile activity was significantly increased with lubiprostone 48 µg. Compared with control session, the MI in the lubiprostone session was significantly higher in both fasting state (10.4 ± 0.7 vs. 6.9 ± 0.9, P = 0.01) and fed state (14.8 ± 1.3 vs. 12 ± 0.8, P = 0.03) (Figure 2).
Figure 2.
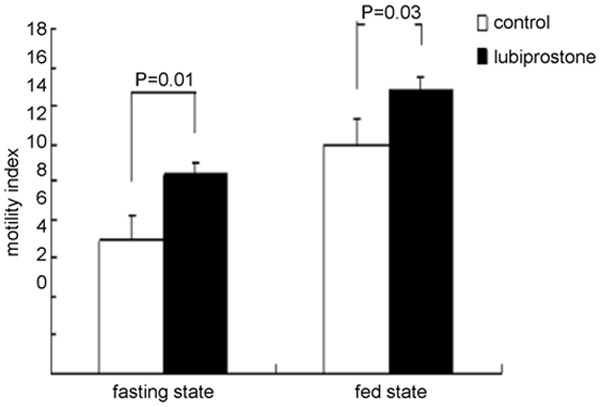
Effects of lubiprostone on gastric antral manometry. Lubiprostone 48 µg significantly increased gastric antral contractions in fasting state (P = 0.01) and in fed state (P = 0.03).
Effects and mechanisms of lubiprostone on small bowel transit
Lubiprostone at both 24 µg and 48 µg accelerated small bowel transit (P < 0.05). The small bowel transit time (SBTT) was 137.8 ± 19.3 min in the control session, 71.0 ± 28.9 min in the lubiprostone 24 µg session (P = 0.04 vs. control) and 82.5 ± 31.3 min in the lubiprostone 48 µg session (p = 0.04 vs. control). No difference was noted in the transit time between the two doses of lubiprostone (P > 0.05). Atropine could block the effects of lubiprostone 48 µg on small bowel transit. The SBTT was 208.8 ± 21.8 min in atropine session (P = 0.007 vs. control) and 205.6 ± 15.9 min in lubiprostone 48 µg plus atropine session (P = 0.006 vs. control; P = 0.002 vs. lubiprostone 48 µg). No difference was found between the two groups (P > 0.05) (Figure 3).
Figure 3.

Effects and mechanisms of lubiprostone on small bowel transit. *P < 0.05 vs. Control. Effects of lubiprostone on small bowel transit time (SBTT) (min). Both lubiprostone 24 µg and lubiprostone 48 µg significantly reduced small bowel transit time (P < 0.05). Atropine and atropine plus lubiprostone 48 µg significantly increased SBTT (P < 0.05).
Effects of lubiprostone on small bowel manometry
Lubiprostone 48 µg enhanced postprandial small bowel motility. The mean MI was 8.8 ± 0.6 in the control session and 13.2 ± 1.5 (P = 0.0008) in the lubiprostone session, an increase of about 50% in contractility (See Figure 4).
Figure 4.

Effect of lubiprostone 48 µg on tracing in small bowel manometry (30 minutes). Lubiprostone 48 µg significantly increased small bowel motility index (P < 0.001 vs. control). The average motility index increased 50% in the lubiprostone 48 µg session. This figure shows representative tracing in one dog.
Effects of lubiprostone on colon transit
Lubiprostone 48 µg accelerated colon transit. The GC values in the control session and the Lubiprostone session were 1.9 ± 0.6 and 3.0 ± 0.2 (P = 0.03) at 2 hrs, 3.9 ± 0.5 and 5.1 ± 0.3 (P = 0.03) at 4 hrs and 5.0 ± 0.1 and 5.7 ± 0.2 (P = 0.01) at 6 hrs after the insertion of the markers (ANOVA P < 0.001) (Figure 5).
Figure 5.

Effect of lubiprostone 48 µg on the geometric center values in colon transit. Lubiprostone 48 µg significantly promoted colon transit at 2, 4, 6 hours after the insertion of the markers and food feeding (*P < 0.05).
Effects of lubiprostone on colon manometry
Lubiprostone 48 µg improved postprandial colon contractions. The mean MI was 5.2 ± 0.3 in the control session and 6.5 ± 0.4 in the lubiprostone session (P = 0.002). The mean MI was increased by 26% with lubiprostone. Figure 6 presents typical colon contractile tracing in the postprandial state in the control and lubiprostone sessions.
Figure 6.
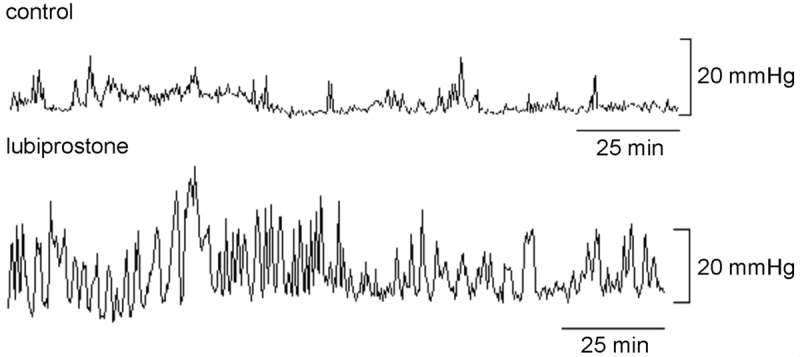
Effect of lubiprostone 48 µg on tracing in colon manometry (150 minutes). Lubiprostone 48 µg significantly increased colon contractions in the fed state. The average motility index increased 25.9% in the lubiprostone 48 µg session (P < 0.05 vs. control). This figure shows representative tracings in one dog.
Side effects
During the experiments, all the dogs were orally administrated with lubiprostone had diarrhea. No vomiting and other abnormal behaviors and adverse events were noted.
Discussion
This study demonstrates that lubiprostone 48 µg accelerated GE, small intestinal and colonic transit in healthy dogs. It enhanced gastric antral, small intestinal and colonic contractions in the postprandial state. It didn’t affect gastric accommodation. Lubiprostone 24 µg did not accelerate GE, but accelerated small intestinal transit. Atropine could block the accelerative effects of lubiprostone on GE and small bowel transit. No other side effects except for diarrhea were observed, suggesting lubiprostone might be used in safety.
A number of previous studies have confirmed the safety and efficacy of lubiprostone in treating the patients with idiopathic chronic constipation and irritable bowel syndrome with predominant constipation [9,22-25]. Lubiprostone is an oral bicyclic fatty acid ClC-2 channel activator that belongs to a new class of drugs called prostones derived from a metabolite of prostaglandin E1. ClC-2 channels are distributed throughout the body, including the GI tract (stomach, small intestine, colon), and have several essential functions, including maintaining the membrane potential of the cells, regulating pH and cell volume, and participating in Cl- transport and fluid secretion [26-31]. Lubiprostone selectively activates type-2 chloride channels located on intestinal epithelial cells leading to an active efflux of Cl- ions into the lumen of the GI tract, followed by Na+ ions and then water efflux [3,32]. Secretion of fluids promotes intestinal transit through stimulation of local receptors sensitive to stretch and distention [33,34]. Although the exact mechanism explaining lubiprostone’s efficacy is not completely understood, its primary action is possibly mediated via this mechanism [32]. However, few studies have been performed examining the effects of lubiprostone on gastrointestinal transit and motor functions.
In clinic, the common dose of lubiprostone is 24 µg twice daily for chronic idiopathic constipation. So we choose the doses of 24 µg and 48 µg to observe its effect on gastrointestinal motility. We found that lubiprostone 48 µg but not 24 µg accelerated GE. Camilleri et al found that lubiprostone 24 µg induced retarded GE in healthy human subjects but did decrease fullness 30 min after the fully satiating meal [35]. The differences of the effects on GE may be explained by the dose. The dose of 48 µg is a larger dose for one dog. We found lubiprostone at 24 µg did not affect GE in the dogs. The result was supported by our gastric antral manometric finding. We found lubiprostone 48 µg could increase gastric antral contractions. Interestingly, we found atropine could block the accelerative effect of lubiprostone on GE. It suggests lubiprostone possibly has an indirect prokinetic effect on stomach involving vagal activity. The specific mechanism isn’t clear. It’s proposed the effect may be via volume reflex. Recent study showed gastric distention-induced efferent chronotropic responses involve both increased parasympathetic and reduced sympathetic activity. The vagal excitatory reflex involves the nucleus ambiguus (NA) and atropine-methyl-bromide completely blocked the reflex [36]. Although there are contradictory studies in vitro regarding the direct activation of smooth muscle or activation of prostaglandin receptors by lubiprostone [5,6], our finding support an indirect prokinetic effect of lubiprostone. On the other hand, our results found lubiprostone 48 µg couldn’t improve gastric accommodation. The mechanism is not clear. To our knowledge, no previous studies were performed on the effects of lubiprostone on gastric accommodation. Our results in dogs need to be confirmed in clinic, especially at a higher dose.
We found that both lubiprostone 48 µg and 24 µg significantly accelerated small bowel transit. The results are similar to one previous study. Camilleri et al showed that lubiprostone accelerated small bowel transit in healthy human subjects [13]. In addition, we found atropine could block the accelerative effect of lubiprostone on small bowel transit, suggesting that lubiprostone may have indirect prokinetic effects by the peristaltic reflux mediated by muscle stretches and mucosal distention caused by fluid secretion. Vagal mechanisms may be involved in the reflux [21]. We further confirmed that lubiprostone enhanced small bowel contractile activity. No previous studies, to our knowledge, have addressed the effects of lubiprostone on small intestinal contractions.
We also found that lubiprostone significantly accelerated colon transit at 2, 4, and 6 hours post-feeding. Camilleri et al also found that lubiprostone accelerated colon transit in human subjects [13]. Our manometric study in the right colon further showed that lubiprostone increased local contractile activity. Sweetser et al found that lubiprostone could not increase contractile activity in the human left colon by placing a barostat-manometric tube under flexible sigmoidoscope [37]. The findings and the current findings suggest that the prokinetic effect of lubiprostone on the right colon possibly play an important role in its acceleration of colon transit. One recent 24-hour colonic manometry study in children patients with constipation also found that oral lubiprostone has a stimulant effects on colonic contraction [38]. Bassil et al showed that lubiprostone caused muscle contractions in rat colon longitudinal muscle and these excitatory effects tended to be inhibited by pretreatment with the EP1 receptor antagonist [5]. Recently, Jakab RL, et al found that lubiprostone induced contraction of villi and proximal colonic plicae and membrane trafficking of transporters, suppress fluid absorption, and enhance mucus-mobilization and mucosal contractility [39]. Consequently, lubiprostone has the prokinetic effects in colon. The mechanisms need further studies.
In conclusion, lubiprostone 48 µg accelerates GE of solids, small bowel transit and colon transit, and enhances gastric antral, small intestinal and colonic contractions in the postprandial state in dogs. Atropine could block the accelerative effect of lubiprostone on GE and small bowel transit, suggesting that lubiprostone may have an indirect prokinetic effects in stomach and intestine and vagal mechanism possibly was involved. More studies are needed to investigate the mechanisms involved in the prokinetic effects of lubiprostone.
Disclosure of conflict of interest
None.
References
- 1.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 2.Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ 3rd. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101:927–934. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]
- 3.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol. 2004;287:C1173–1183. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 4.Gale JD. The use of novel promotility and prosecretory agents for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation. Adv Ther. 2009;26:519–530. doi: 10.1007/s12325-009-0027-4. [DOI] [PubMed] [Google Scholar]
- 5.Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangiah R, Sung EZ, Lee K, Sanger GJ. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol. 2008;154:126–135. doi: 10.1038/bjp.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuppoletti J, Malinowska DH, Chakrabarti J, Ueno R. Effects of lubiprostone on human uterine smooth muscle cells. Prostaglandins Other Lipid Mediat. 2008;86:56–60. doi: 10.1016/j.prostaglandins.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Yin J, Chen J. Excitatory effects of synchronized intestinal electrical stimulation on small intestinal motility in dogs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1190–1195. doi: 10.1152/ajpgi.00092.2007. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Chen JD. Effects of cyclooxygenase-2 inhibitor on glucagon-induced delayed gastric emptying and gastric dysrhythmia in dogs. Neurogastroenterol Motil. 2007;19:144–151. doi: 10.1111/j.1365-2982.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Xing J, Chen JD. Effects of muscarinic receptor stimulation and nitric oxide synthase inhibition on gastric tone and gastric myoelectrical activity in canines. J Gastroenterol Hepatol. 2009;24:1130–1135. doi: 10.1111/j.1440-1746.2009.05843.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Koothan T, Chen JD. Synchronized gastric electrical stimulation improves vagotomy-induced impairment in gastric accommodation via the nitrergic pathway in dogs. Am J Physiol Gastrointest Liver Physiol. 2009;296:G310–318. doi: 10.1152/ajpgi.90525.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song J, Yin J, Chen JD. Acute and chronic effects of desvenlafaxine on gastrointestinal transit and motility in dogs. Neurogastroenterol Motil. 2013;25:824–e637. doi: 10.1111/nmo.12190. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Song GQ, Yin J, Koothan T, Chen JD. Electroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogs. Am J Physiol Gastrointest Liver Physiol. 2008;295:G614–620. doi: 10.1152/ajpgi.90322.2008. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Bharucha AE, Ueno R, Burton D, Thomforde GM, Baxter K, McKinzie S, Zinsmeister AR. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G942–947. doi: 10.1152/ajpgi.00264.2005. [DOI] [PubMed] [Google Scholar]
- 14.Sucompo pharmaceuticals, data on file. Bethesda MD: 2006. AMITIZEA(lubiprostone): Phase I ADME Study. [Google Scholar]
- 15.Qi H, Chen JD. Effects of intestinal electrical stimulation on postprandial small-bowel motility and transit in dogs. Am J Surg. 2006;192:e55–e60. doi: 10.1016/j.amjsurg.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Pasricha PJ, Sallam HS, Ma L, Chen JD. Clinical significance of quantitative assessment of rectoanal inhibitory reflex (RAIR) in patients with constipation. J Clin Gastroenterol. 2008;42:692–698. doi: 10.1097/MCG.0b013e31814927ba. [DOI] [PubMed] [Google Scholar]
- 17.Yin J, Chen JD. Electroacupuncture improves rectal distension-induced delay in solid gastric emptying in dogs. Am J Physiol Regul Integr Comp Physiol. 2011;301:R465–472. doi: 10.1152/ajpregu.00271.2010. [DOI] [PubMed] [Google Scholar]
- 18.Ouyang H, Xing J, Chen J. Electroacupuncture restores impaired gastric accommodation in vagotomized dogs. Dig Dis Sci. 2004;49:1418–1424. doi: 10.1023/b:ddas.0000042240.05247.01. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Chen JD. Peripheral mechanisms of sibutramine involving proximal gastric motility in dogs. Obesity (Silver Spring) 2006;14:1363–1370. doi: 10.1038/oby.2006.154. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Liu J, Chen JD. Neural mechanisms involved in the inhibition of intestinal motility induced by intestinal electrical stimulation in conscious dogs. Neurogastroenterol Motil. 2006;18:62–68. doi: 10.1111/j.1365-2982.2005.00739.x. [DOI] [PubMed] [Google Scholar]
- 21.Qi H, Brining D, Chen JD. Rectal distension inhibits postprandial small intestinal motor activity partially via the adrenergic pathway in dogs. Scand J Gastroenterol. 2007;42:807–813. doi: 10.1080/00365520601127257. [DOI] [PubMed] [Google Scholar]
- 22.Iwa M, Strickland C, Nakade Y, Pappas TN, Takahashi T. Electroacupuncture reduces rectal distension-induced blood pressure changes in conscious dogs. Dig Dis Sci. 2005;50:1264–1270. doi: 10.1007/s10620-005-2770-y. [DOI] [PubMed] [Google Scholar]
- 23.Lin X, Hayes J, Peters LJ, Chen JD. Entrainment of intestinal slow waves with electrical stimulation using intraluminal electrodes. Ann Biomed Eng. 2000;28:582–587. doi: 10.1114/1.294. [DOI] [PubMed] [Google Scholar]
- 24.Glia A, Lindberg G. Antroduodenal manometry findings in patients with slow-transit constipation. Scand J Gastroenterol. 1998;33:55–62. doi: 10.1080/00365529850166211. [DOI] [PubMed] [Google Scholar]
- 25.Preston DM, Lennard-Jones JE. Severe chronic constipation of young women: ‘idiopathic slow transit constipation’. Gut. 1986;27:41–48. doi: 10.1136/gut.27.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol. 2002;282:C805–816. doi: 10.1152/ajpcell.00291.2001. [DOI] [PubMed] [Google Scholar]
- 27.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 28.Sherry AM, Malinowska DH, Morris RE, Ciraolo GM, Cuppoletti J. Localization of ClC-2 Cl- channels in rabbit gastric mucosa. Am J Physiol Cell Physiol. 2001;280:C1599–1606. doi: 10.1152/ajpcell.2001.280.6.C1599. [DOI] [PubMed] [Google Scholar]
- 29.Roman RM, Smith RL, Feranchak AP, Clayton GH, Doctor RB, Fitz JG. ClC-2 chloride channels contribute to HTC cell volume homeostasis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G344–353. doi: 10.1152/ajpgi.2001.280.3.G344. [DOI] [PubMed] [Google Scholar]
- 30.Cuppoletti J, Tewari KP, Sherry AM, Kupert EY, Malinowska DH. ClC-2 Cl- channels in human lung epithelia: activation by arachidonic acid, amidation, and acid-activated omeprazole. Am J Physiol Cell Physiol. 2001;281:C46–54. doi: 10.1152/ajpcell.2001.281.1.C46. [DOI] [PubMed] [Google Scholar]
- 31.Ueno R. Comment and reply on: CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol. 2009;44:243–244. doi: 10.1080/10409230903195666. [DOI] [PubMed] [Google Scholar]
- 32.Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol. 2007;41:345–351. doi: 10.1097/01.mcg.0000225665.68920.df. [DOI] [PubMed] [Google Scholar]
- 33.Grider JR, Jin JG. Distinct populations of sensory neurons mediate the peristaltic reflex elicited by muscle stretch and mucosal stimulation. J Neurosci. 1994;14:2854–2860. doi: 10.1523/JNEUROSCI.14-05-02854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambizas EM, Ginzburg R. Lubiprostone: a chloride channel activator for treatment of chronic constipation. Ann Pharmacother. 2007;41:957–964. doi: 10.1345/aph.1K047. [DOI] [PubMed] [Google Scholar]
- 35.Abo M, Kono T, Wang Z, Chen JD. Impairment of gastric and jejunal myoelectrical activity during rectal distension in dogs. Dig Dis Sci. 2000;45:1731–1736. doi: 10.1023/a:1005590413490. [DOI] [PubMed] [Google Scholar]
- 36.Tjen-A-Looi SC, Hsiao AF, Longhurst JC. Central and peripheral mechanisms underlying gastric distention inhibitory reflex responses in hypercapnic-acidotic rats. Am J Physiol Heart Circ Physiol. 2011;300:H1003–12. doi: 10.1152/ajpheart.01131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweetser S, Busciglio IA, Camilleri M, Bharucha AE, Szarka LA, Papathanasopoulos A, Burton DD, Eckert DJ, Zinsmeister AR. Effect of a chloride channel activator, lubiprostone, on colonic sensory and motor functions in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G295–301. doi: 10.1152/ajpgi.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawal N, Desbiens J, Darbari A. Colonic manometry results of oral lubiprostone. Gastroenterology. 2010;136(Suppl 1):S229. [Google Scholar]
- 39.Bojo L, Cassuto J. Gastric reflex relaxation by colonic distension. J Auton Nerv Syst. 1992;38:57–64. doi: 10.1016/0165-1838(92)90216-4. [DOI] [PubMed] [Google Scholar]


