Abstract
Conflicting results have been reported regarding whether or not insulin-regulated glucose transporter 4 (GLUT4) is expressed in human and rodent endometria. There is an inverse relationship between androgen levels and insulin-dependent glucose metabolism in women. Hyperandrogenemia, hyperinsulinemia, and insulin resistance are believed to contribute to endometrial abnormalities in women with polycystic ovary syndrome (PCOS). However, it has been unclear in previous studies if endometrial GLUT4 expression is regulated by androgen-dependent androgen receptors (ARs) and/or the insulin receptor/Akt/mTOR signaling network. In this study, we demonstrate that GLUT4 is expressed in normal endometrial cells (mainly in the epithelial cells) and is down-regulated under conditions of hyperandrogenemia in tissues from PCOS patients and in a 5α-dihydrotestosterone-induced PCOS-like rat model. Western blot analysis revealed reduced endometrial GLUT4 expression and increased AR expression in PCOS patients. However, the reduced GLUT4 level was not always associated with an increase in AR in PCOS patients when comparing non-hyperplasia with hyperplasia. Using a human tissue culture system, we investigated the molecular basis by which GLUT4 regulation in endometrial hyperplasia tissues is affected by metformin in PCOS patients. We show that specific endogenous organic cation transporter isoforms are regulated by metformin, and this suggests a direct effect of metformin on endometrial hyperplasia. Moreover, we demonstrate that metformin induces GLUT4 expression and inhibits AR expression and blocks insulin receptor/PI3K/Akt/mTOR signaling in the same hyperplasia human tissues. These findings indicate that changes in endometrial GLUT4 expression in PCOS patients involve the androgen-dependent alteration of AR expression and changes in the insulin receptor/PI3K/Akt/mTOR signaling network.
Keywords: Glucose transporter 4, androgen receptor, insulin receptor, metformin, PCOS, endometrium
Introduction
Accumulating evidence suggests that uterine glucose metabolism plays an important physiological role during implantation, embryonic development, and pregnancy [1,2]. The regulation of glucose uptake in tissues and cells requires the facilitative glucose transporters (GLUT), and a number of GLUTs with different tissue expression, localization, and regulation profiles have been identified in humans and rodents [1]. Among them, tissue-specific insulin-regulated GLUT4 (SLC2A4) is a key contributor to glucose homeostasis under physiological and pathological conditions [3]. Although several methods such as quantitative real-time PCR, Northern blot, RNase protection assay, immunohistochemistry, and Western blot have been used to identify GLUT4 in the human and rodent uterus, these experiments have resulted in conflicting conclusions. While some studies have demonstrated the presence of GLUT4 mRNA and protein in human and rodent endometria and uterine stromal cells [4-7], other studies have indicated that the level of GLUT4 mRNA and protein is undetectable in human endometrial tissues and stromal cells [4,8-10]. In addition, although endometrial GLUT4 expression appears to be regulated in a menstrual cycle-dependent manner [6], there is no direct in vivo evidence that GLUT4 regulation is linked to human endometrial cellular function. Thus, the cellular expression and precise function of GLUT4 in the endometrium remain controversial or unknown.
Polycystic ovary syndrome (PCOS) is a common hormone-imbalance disease [11] that affects approximately 4%-18% of reproductive-aged women worldwide [12]. The etiology of PCOS is complex, and clinical data show that endocrine and metabolic abnormalities such as hyperandrogenemia, insulin resistance, and hyperinsulinemia commonly occur in this heterogeneous and chronic disease [11,13]. Although adipose and muscle tissues are the major sites of insulin resistance in women with PCOS, it has also been proposed that local insulin resistance exists in the endometrium of these patients. To support this, Fornes and colleagues have demonstrated aberrant endometrial insulin/insulin receptor signaling in PCOS patients with hyperinsulinemia [14]. However, whether or not alteration of the insulin/insulin receptor signaling network can directly regulate endometrial GLUT4 expression in the endometria of women with PCOS needs to be experimentally tested.
Metformin has been used clinically as a potential therapeutic agent to not only improve metabolic abnormalities-for example, by suppressing androgen levels [15]-but also to alleviate endometrial disorders such as endometrial hyperplasia [16,17] and early endometrial carcinoma [18-20] in PCOS patients with insulin resistance. Because an inverse relationship between androgen levels and insulin-dependent glucose metabolism exists in women [21], it is of great interest to analyze the possible mechanisms of metformin action on GLUT4 expression in the endometria of PCOS patients.
In this study, we examined whether GLUT4 is expressed in the endometrium and, if so, if its expression is altered in endometrial tissue from PCOS patients and in the 5α-dihydrotestosterone (DHT)-induced PCOS-like rat model. Because recent work from our lab has demonstrated that human and rat endometria express organic cation transporters (OCTs) [15], which are known to be involved in metformin uptake in cells [22], we further demonstrated the effect of metformin on OCT isoform expression in endometrial hyperplasia tissues in vitro. Finally, in addition to androgen receptors (ARs), we examined whether the insulin receptor/PI3K/Akt/mammalian target of rapamycin (mTOR) signaling network is involved in metformin-induced endometrial GLUT4 regulation.
Materials and methods
Animals and treatment
At 21 days of age, female Wistar rats had either a 90-day continuous-release pellet containing 7.5 mg DHT (daily dose = 83 μg, Sigma-Aldrich, St. Louis, MO) or a pellet containing only vehicle implanted subcutaneously in the back of the neck. All animals were maintained in standard cages under 12-hour cycles of light and dark with a 1-hour sunrise/sunset function. The cages were maintained at 21°C ± 2°C and a relative humidity between 45% and 55%, and the animals had ad libitum access to normal rodent chow and water. Control rats were killed at the diestrus stage to exclude variations from the estrous cycle. After 90 days of DHT treatment, uterine tissue was collected and embedded in paraffin for histochemical and immunofluorescence analysis. All experimental procedures and protocols used in the present study were approved by the local ethics committee of Shanghai Medical College, Fudan University, China.
Patient recruitment and endometrial tissue collection
All endometrial biopsies were obtained by curettage from the Obstetrics and Gynecology Hospital of Fudan University, Shanghai, with the approval of the institutional ethical review board. Informed consent was obtained from all patients. Each endometrial sample was diagnosed and staged by routine pathology analysis based on standard histological criteria [23], and the patient’s last reported menstrual period was recorded at the time of collection. Endometrial samples were obtained from fertile women at the proliferative stage of the menstrual cycle (n = 20, aged 26-50 years), women with PCOS at the proliferative stage of the menstrual cycle (n = 9, aged 25-36 years), and women with PCOS and hyperplasia (n = 2, aged 28 and 43 years). PCOS was diagnosed based on the Rotterdam criteria provided by the American Society for Reproductive Medicine and the European Society for Human Reproduction and Embryology [24]. A diagnosis of PCOS was made if at least two of the following criteria were met: 1) oligo/anovulation, 2) signs of hyperandrogenism (i.e., hirsutism and acne) and/or biochemical measurements, or 3) enhanced ovaries (at least 12 discrete follicles of 2-9 mm in diameter in one ovary or an ovarian volume > 10 cm3 observed by transvaginal ultrasonography). Women with other androgen-excess disorders or specific etiologies including congenital adrenal hyperplasia, Cushing’s syndrome, thyroid hormone abnormalities, hyperprolactinemia, or ovarian/adrenal tumors were excluded. All PCOS patients had no history of previous first-trimester miscarriage or pregnancy. No patients had received exogenous hormonal therapy for at least three months before the procedure.
Primary in vitro tissue culture
Briefly, endometrial tissues were obtained from the two PCOS patients with hyperplasia and placed in cold PBS in the operating room and immediately delivered to the laboratory after the surgery. Endometrial tissues were rinsed in PBS to remove blood and debris and then dissected into uniform 0.5-1 mm3 pieces with a fine scalpel under a stereomicroscope. Tissue samples were washed three times with RPMI-1640 medium (Sigma-Aldrich) and placed in 24-well tissue culture plates (Sarstedt, Newton, MA) containing RPMI-1640 medium with 100 IU/mL penicillin/streptomycin (GIBCO-BRL, San Francisco, CA) as described previously [25]. The endometrial tissues were treated with vehicle or metformin (20 mM, D-150959, Sigma-Aldrich) and were incubated in a humidified incubator (37°C, 95% O2, 5% CO2) for 24 hours. Metformin was dissolved in sterile water. Because a number of in vitro experiments have used doses of metformin from 1 mM to 40 mM, which is well above the feasible therapeutic plasma levels (2.8 µM-15 μM) in humans [26], we have selected to use 20 mM metformin in our culture study. At the end of the experiments, cultured tissues were snap-frozen in liquid nitrogen and stored at -70°C.
Protein isolation and Western blot analysis
Endometrial tissues were lysed using RIPA buffer (Sigma-Aldrich) supplemented with cOmplete mini protease inhibitor cocktail tablets (Roche Diagnostics, Mannheim, Germany) and PhosSTOP phosphatase inhibitor cocktail tablets (Roche Diagnostics). After incubation for 15 minutes on ice, tissue lysates were cleared by centrifugation at 13,000 rpm for 30 minutes at 4°C, and the total protein concentration of the supernatant was determined with a Direct Detect® spectrometer (EMD Millipore Corporation, Billerica, MA). Immunoblotting was performed as previously described [27]. Equal amounts of protein for each treatment group were resolved on NuPAGE 4-12% Bis-Tris gels (Invitrogen, Life Technologies Europe BV, Stockholm, Sweden), transferred onto PVDF membranes, and probed for GLUT4 (ab33780, Abcam, Cambridge, UK), AR (#5153, Cell Signaling Technology, Danvers, MA), OCT1 (AV41516, Sigma-Aldrich), OCT2 (HPA008567, Sigma-Aldrich), OCT3 (ab183071, Abcam), insulin receptor-β subunit (β-IR, #07-724, Millipore, Temecula, CA), Akt (#4691, Cell Signaling Technology), phospho-Akt (#12694, Cell Signaling Technology), S6 ribosomal protein (S6RB, #2217, Cell Signaling Technology), phospho-S6 ribosomal protein (p-S6RB, #4858, Cell Signaling Technology), eIF4E binding protein 1 (4EBP1, #9452, Cell Signaling Technology), phospho-4EBP1 (#9459, Cell Signaling Technology), or β-actin (P-0130, Sigma-Aldrich) at 1:1000-1:2000 dilutions in 0.01 M Tris-buffered saline supplemented with Triton X-100 (TBST) containing 5% nonfat dry milk. This was followed by anti-mouse IgG horseradish peroxidase (HRP)-conjugated goat antibody (A2304, 1:1000 dilution, Sigma-Aldrich) or anti-rabbit IgG HRP-conjugated goat antibody (A0545, 1:1000 dilution, Sigma-Aldrich). When necessary, PVDF membranes were stripped using Restore PLUS Western blot stripping buffer (Thermo Scientific, Rockford, IL) for 10 minutes at room temperature, washed twice in 0.01 M TBST, and reprobed.
Immunofluorescence analysis
Immunofluorescence was performed in the human and rat endometrial tissues as described previously [28]. Endometrial tissues were fixed in 4% formaldehyde neutral-buffered solution for 24 h at 4°C and were embedded in paraffin and cut into 5 μm sections. After deparaffinization and rehydration, antigen retrieval was completed with 10 mM sodium citrate buffer (pH 6.0) for 10 min in a 700 W microwave oven. After incubation with TBST containing 5% fat-free milk for 1 h at room temperature, slides were incubated with an antibody against GLUT4 (1:100 dilution in TBST containing 5% nonfat milk) overnight at 4°C. A secondary Alexa Fluor 594-conjugated goat polyclonal anti-mouse IgG (1:250 dilution, Invitrogen) was applied at room temperature for 1 h. After the sections were washed with TBST, they were re-suspended in mounting medium containing DAPI (4’,6’-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA) and examined under either a Nikon E-1000 microscope (Japan) and photomicrographed using Easy Image 1 (Bergström Instrument AB, Sweden) or under an Axiovert 200 confocal microscope (Zeiss, Jena, Germany) equipped with a laser-scanning confocal imaging LSM 700 inverted system (Zeiss) and photomicrographed. Background settings were adjusted based on the examination of negative control specimens. Images of positive staining were adjusted to make optimal use of the dynamic range of detection.
Statistical analysis
Results are presented as means ± SD. Statistical analyses were performed using SPSS version 21.0 statistical software for Windows (SPSS Inc., Chicago, IL). In all analyses, one-way ANOVA using Bonferroni’s multiple range test was used to compare treatment groups. A P-value less than 0.05 was considered statistically significant.
Results
Endometria from women with PCOS and from DHT-induced rats showed significantly reduced GLUT4 expression
The antibody against GLUT4 has been well characterized and its specificity has been confirmed in human and rat tissues [29-31]. We first sought to examine which endometrial cells expressed GLUT4 in healthy women during the proliferative phase of the menstrual cycle. Immunofluorescence analysis showed that GLUT4 was strongly expressed in the glandular epithelial cells but weakly expressed in the stromal cells (Figure 1A1, 1A2). Furthermore, the majority of the GLUT4 immunofluorescence was observed on the surface of epithelial and stromal cells while very little GLUT4-positive immunoreactivity could be detected in the cytoplasm of those cells, if at all (Figure 1C, left). The specificity of immunofluorescence staining was demonstrated by replacing the GLUT4 antibody with normal rabbit serum at the equivalent titer. Control sections did not show any evidence of positive labeling (Figure 1D).
Figure 1.
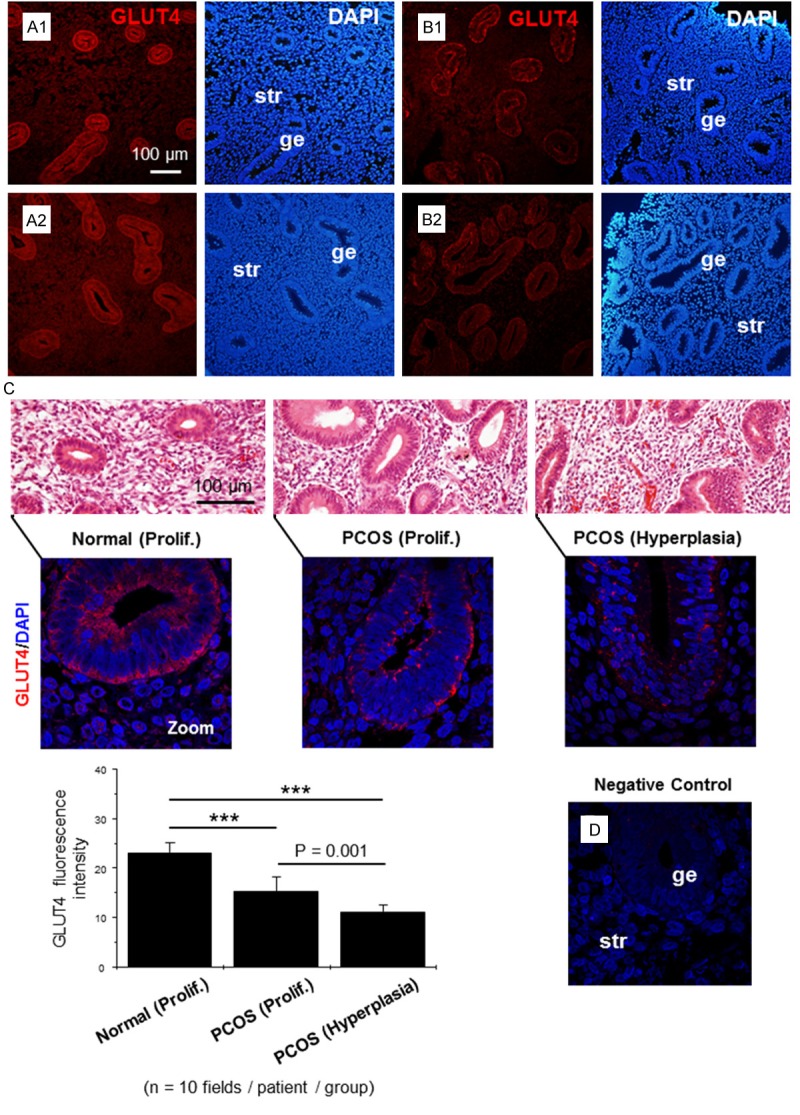
Immunofluorescence localization of GLUT4 in human endometrium. Representative paraffin-embedded endometrial sections in the proliferative stage of healthy women (A1, A2) and women with PCOS (B1) and in women with PCOS and hyperplasia (B2). GLUT4 was significantly decreased in glandular epithelial cells in women with PCOS (B1) and PCOS with hyperplasia (B2) compared to controls (A1, A2). The images are representative of those observed in numerous sections from multiple endometrial tissues. (C) Quantification of GLUT4 immunofluorescence intensity in human endometrium. The top row of images shows the histology of hematoxylin/eosin-stained human endometrial biopsy samples. The lower row consists of magnified images of the top row and shows immunostaining of GLUT4 (red) mainly in the membrane and cytoplasm. Ten fields were observed per patient in each group. Values are the mean ± SD, and significance was tested by one-way ANOVA with Bonferroni correction for multiple comparisons when appropriate. Experiments were performed using different endometrial donors with similar results. The image in the lower right shows the negative control. ***p < 0.001. Prolif., the proliferative phase; ge, glandular epithelial cells; str, stromal cells.
We next determined whether the GLUT4 expression was changed in the endometrial tissues from PCOS patients. As shown in Figure 1B1, 1B2 and by quantified computer image analysis in Figure 1C, the levels of epithelial GLUT4 were significantly reduced in PCOS patients with and without endometrial hyperplasia compared to menstrual-stage-matched normal women. There was little evidence of significant changes in stromal GLUT4 expression in PCOS patients.
The DHT-induced PCOS-like animal model has been developed to mimic the clinical situation of women with PCOS [32], and we used this model to investigate whether the reduction of endometrial GLUT4 expression was dependent on androgen stimulation. Immunofluorescence analysis revealed that GLUT4 was mainly localized in the luminal and glandular epithelial cells as well as in stromal cells that were located directly below the luminal epithelial cells in control animals at diestrus (Figure 2A1-A4) and at other estrous stages (data not shown). A significant reduction in epithelial and stromal GLUT4 expression was observed in DHT-treated animals compared to control animals (Figure 2B1-B4).
Figure 2.
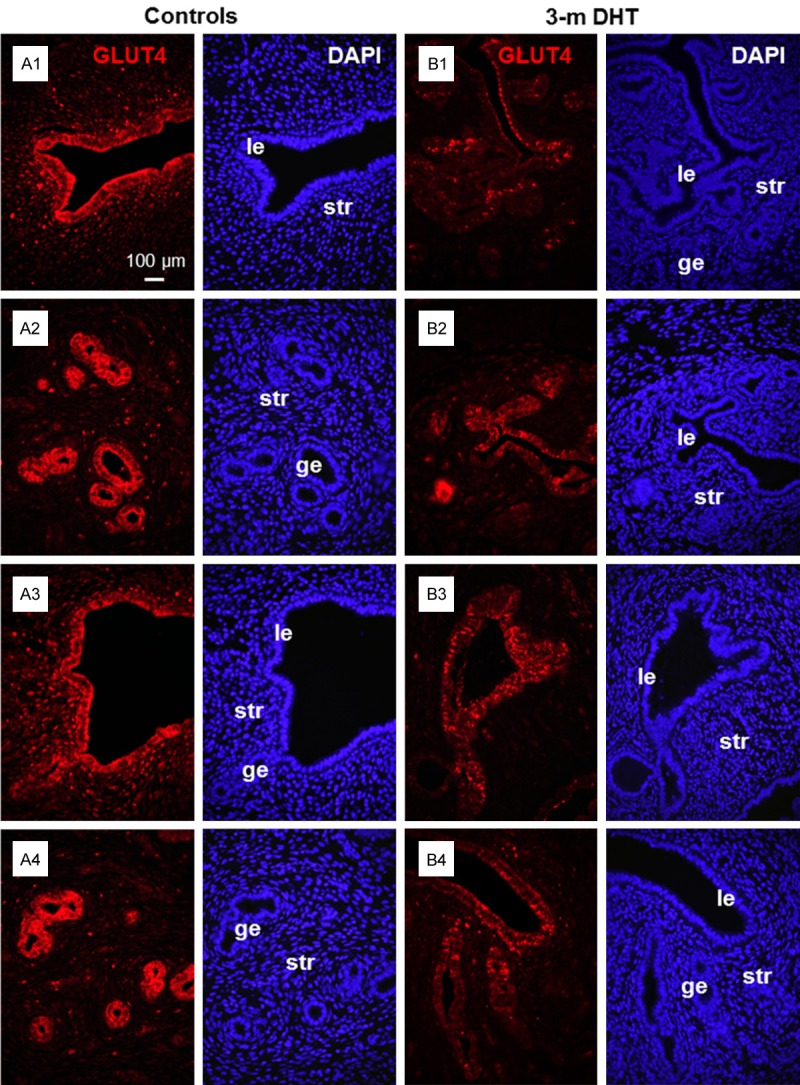
Immunofluorescence localization of uterine GLUT4 in DHT-induced PCOS-like rats. Representative paraffin-embedded uterine sections in rats treated without (A1-A4) and with 90 days of DHT (B1-B4) are shown. GLUT4 expression was significantly lower in luminal and glandular epithelial cells in DHT-induced PCOS-like rats than in controls. The images are representative of those observed in numerous sections from multiple uterine endometrial tissues. le, luminal epithelial cells; ge, glandular epithelial cells; str, stromal cells.
Both androgen/AR and insulin/insulin receptor signaling pathways were involved in the regulation of endometrial GLUT4 expression
With the observation of decreased endometrial GLUT4 expression in DHT-treated animals, we hypothesized that the changes in androgen-activated AR and its expression might be correlated with GLUT4 expression in PCOS patients. We performed Western blot analyses to assess the protein levels of endometrial GLUT4 and AR in healthy controls and in PCOS patients. As shown in Figure 3, PCOS patients with both non-hyperplasia and hyperplasia had a significant reduction of endometrial GLUT4 expression, and this was in accordance with the data obtained by immunofluorescence analysis (Figure 1B1, 1B2 and 1C). Furthermore, all PCOS patients regardless of hyperplasia showed an increase in endometrial AR expression compared to healthy women. These data suggest a direct link between PCOS-related hyperandrogenism and GLUT4 regulation in vivo. It is important to note that a different pattern of AR expression was found between non-hyperplasia and hyperplasia in women with PCOS. We showed that the increased AR level was not similar in PCOS patients under different stages of endometrial development (Figure 3). These results raised the question of which additional factor or factors might play a role in the regulation of GLUT4 expression in PCOS patients with endometrial hyperplasia.
Figure 3.
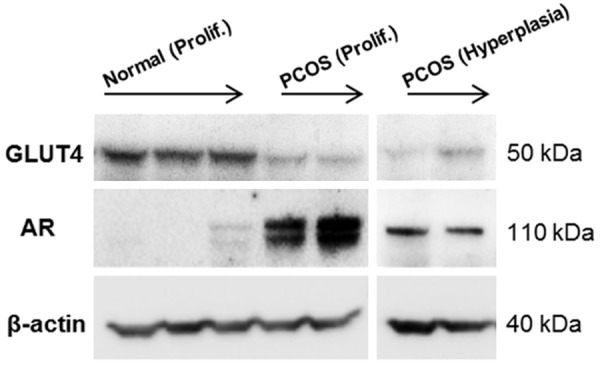
Distribution of GLUT4 proteins in human endometrial tissues in vivo. Western blot with endometrial homogenate samples from women with and without PCOS showed that the level of GLUT4 was decreased in women with PCOS regardless of whether hyperplasia was present. In contrast, the level of androgen receptor (AR) was increased in women with PCOS and was not associated with the level of GLUT4 when hyperplasia was present in the endometrium. β-actin was used as an internal control.
Because previous studies have shown that metformin, an oral biguanide insulin-sensitizing drug [33], decreased circulating insulin levels, increased tissue-specific insulin sensitivity [34,35], and decreased local androgen synthesis in human ovarian cells [36,37], it is possible that both androgen and insulin signaling are involved in the regulation of endometrial GLUT4 expression. To address this question, we treated and cultured endometrial hyperplasia tissues with 20 mM metformin. Our data indicated an enhancement of GLUT4 levels in parallel with a decreased AR level in endometrial hyperplasia tissues following 24-h metformin treatment (Figure 4A, left). These data suggested that metformin-induced regulation of endometrial GLUT4 and AR expression is time dependent.
Figure 4.
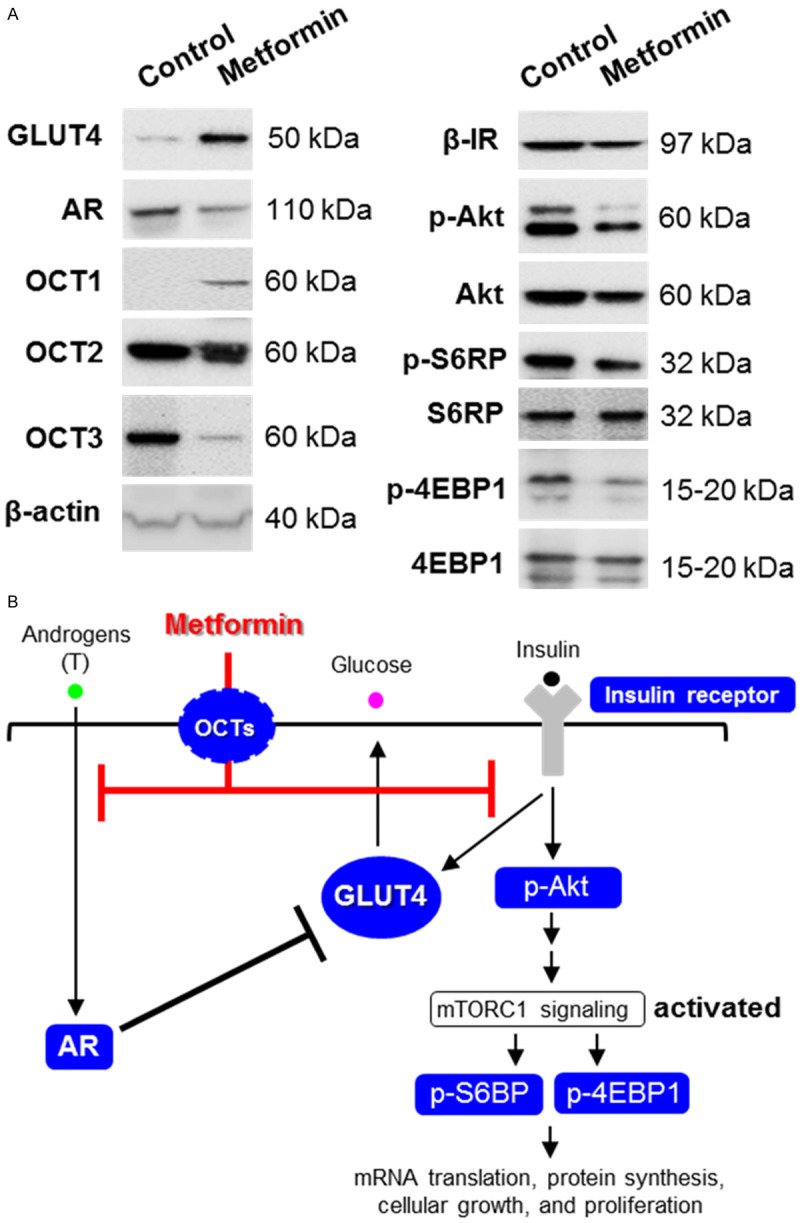
The direct effect of metformin in human endometrial tissues in vitro. A. Treatment of endometrial tissues from women with PCOS and hyperplasia with metformin (20 mM) for 24 h increased the levels of GLUT4 and decreased the levels of AR. The metformin treatment also resulted in an increased level of OCT1 and decreased levels of insulin receptor-β subunit (β-IR), phosphorylated Akt (p-Akt), phosphorylated S6 ribosomal protein (p-S6RP), and phosphorylated eIF4E binding protein 1 (p-4EBP1). No effect was seen on the level of OCT2. Levels of total Akt, S6RP, 4EBP1, and β-actin were used as internal controls. Experiments were performed using different endometrial donors with similar results. B. The proposed signaling network between AR and insulin receptor/Akt/mTORC1 in the endometrial tissues that is responsible for metformin’s effects in vitro. Blue-colored molecules represent those found to be regulated by metformin treatment in this study. T, testosterone; AR, androgen receptor; OCT, organic cation transporter; mTORC1, mammalian target of rapamycin complex 1.
The molecular mechanisms of metformin action in human endometrial hyperplasia
Because endometrial cells express OCT isoforms-which are the major uptake transporters for metformin in cells [15]-we further analyzed the expression of endometrial OCT1, OCT2, and OCT3 using a similar protocol as the in vitro metformin treatment. Here, we observed up-regulation of OCT1, down-regulation of OCT3, and no changes in OCT2 in response to 24-h metformin treatment (Figure 4A, left). To identify the signaling pathway leading to the changes of GLUT4 in endometrial hyperplasia tissues following 24-h metformin treatment, we next determined the levels of protein expression and/or phosphorylation of some key molecules in the insulin receptor/Akt/mTOR signaling network in the same tissue samples. Results from Western blot analysis showed that metformin treatment led to reduced levels of insulin receptor-β subunit (β-IR), phosphorylated Akt, phosphorylated S6 ribosomal protein (S6RP), and phosphorylated eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1, a translation repressor protein) in endometrial hyperplasia tissues (Figure 4A, right).
Discussion
The endometrium is a dynamic organ that is composed of several different cell types, including luminal and glandular epithelial cells and stroma cells [38,39]. In the present study, we showed that GLUT4 was expressed in normal human and rat endometria; however, there were appreciable differences in protein expression levels between epithelial and stromal cells. Although the expression of GLUT4 in endometrial stromal cells under physiological conditions is more controversial [4,9,14], we revealed the presence of stromal GLUT4 in human endometria using an immunofluorescence technique. Similarly, an in vitro study indicated that GLUT4 protein levels can be detected by Western blot analysis in human endometrial stromal cells [40]. Using the same antibody, we also found that GLUT4 was highly expressed in stromal cells that were located directly below the luminal epithelial cells in the rat uterus. Furthermore, in the normal rat and mouse uterine stromal cells GLUT4 is differentially expressed [4,5]. Thus, these in vivo and in vitro studies suggest that endometrial stromal GLUT4 expression under basal conditions might be species specific.
It has been assumed that PCOS-related implantation failure, recurrent miscarriage, and spontaneous abortion [2,41] are due at least in part to aberrant glucose metabolism in the endometrium [2]. Because hyperandrogenemia is negatively associated with glucose metabolism in women [21], numerous laboratories have taken great interest in the aberrant in vivo expression of GLUT4, a glucose transporter protein, in the endometria of women with PCOS [6,10,14,42-44]. We also found that the protein levels of GLUT4 were significantly reduced in PCOS patients compared to menstrual-stage-matched normal controls. However, these studies are unable to address the question if the aberrant expression of GLUT4 is a consequence of hyperandrogenemia and/or hyperinsulinemia and insulin resistance. In this study, we have used a DHT-induced PCOS-like rat model [32] to determine whether androgen initially reduces endometrial GLUT4 expression in vivo. We showed that endometrial GLUT4 levels were significantly decreased in DHT-treated rats, and the reduction of GLUT4 expression in the rat uterus after androgen stimulation is in agreement with in vivo association studies in PCOS patients. In a cell culture system, Zhang and Liao also found that treatment with testosterone resulted in a reduction of GLUT4 protein levels in human endometrial epithelial cells [45]. These observations suggest that hyperandrogenemia in PCOS patients might directly contribute to the down-regulation of endometrial GLUT4 expression. Androgen signaling is mediated through AR activation, and in agreement with this we observed contrasting protein expression patterns of endometrial GLUT4 and AR in PCOS patients. Future research with flutamide, an AR antagonist, should be able to establish whether AR acts directly on the endometrial GLUT4 expression. In addition, future research should focus on how AR activity as a transcription factor affects GLUT4-mediated impairments in cellular metabolism in the endometrium.
A highly significant correlation has also been noted between insulin resistance and PCOS [11,13], and approximately 50%-70% of all women with PCOS suffer from insulin resistance [46]. It has also been reported that women with insulin resistance exhibit decreased GLUT4 expression in adipose and muscle tissues [1]. Having observed a differential regulation pattern of AR expression in PCOS patients with and without hyperplasia, we hypothesized that some additional factors such as insulin-mediated insulin receptor signaling might also contribute to the differential regulation of endometrial GLUT4 expression in PCOS patients with and without hyperplasia. In fact, in vivo studies of PCOS patients have shown that endometrial tissues express insulin receptor and that hyperinsulinemia-associated decreases in endometrial GLUT4 expression can be reversed by metformin treatment [14,43]. In this study, we found that down-regulation of insulin receptor expression was associated with up-regulation of GLUT4 expression in endometrial hyperplasia tissues after metformin treatment in vitro. Thus, it appears that insulin-mediated insulin receptor signaling is involved in the regulation of endometrial GLUT4 expression in PCOS patients. It is known that the PI3K/Akt/mTOR signaling pathway is downstream of insulin-mediated insulin receptor signaling and that Akt participates in the process of insulin-stimulated glucose transport as well as contributes to cell growth, proliferation, and metabolism by directly phosphorylating mTOR in a rapamycin-sensitive complex [15,47]. While an increase in Akt phosphorylation was found in PCOS patients with endometrial hyperplasia in vivo [48,49], we demonstrated that treatment with metformin decreased Akt phosphorylation in endometrial hyperplasia tissues in vitro. In general, insulin-mediated activation of PI3K/Akt/mTOR signaling results in an increase in mRNA translation, protein synthesis, and cell growth and proliferation by inducing the p70 S6 kinase and the subsequent phosphorylation of S6BP and increased 4EBP1 phosphorylation [50]. However, the downstream effects of the mTOR complex in endometrial tissues in PCOS patients have, to our knowledge, not been reported previously. We showed that metformin had a similar effect on the phosphorylation of S6 and 4EBP1 in endometrial hyperplasia tissues. Our data together with other studies [14,40,43,44,49] suggest the feasibility of clinical trials of metformin in PCOS patients with endometrial disorders.
Recent studies undertaken in our laboratory have demonstrated that human and rat endometria express OCTs, which are known to be involved in metformin uptake in cells [15]. In the current study, we have provided direct in vitro evidence showing that treatment with metformin specifically increases OCT1 expression and decreases OCT3 expression in endometrial hyperplasia tissues. This suggests that the effects of metformin that lead to aberrant expression of endometrial GLUT4 are direct effects.
In summary, this study demonstrates that GLUT4 protein is expressed in human and rat endometria under physiological conditions. The levels of endometrial GLUT4 were found to be down-regulated by PCOS conditions, and this down-regulation in PCOS was associated with increased AR expression. Our in vitro experiments support our hypothesis that metformin is capable of regulating endometrial cell function directly and that the regulation of endometrial GLUT4 expression in women with PCOS is at least in part via the activation of androgen-dependent AR and insulin receptor/PI3K/Akt/mTOR signaling pathways (Figure 4B). Our data suggest the importance of investigating further the function of endometrial GLUT4 under physiological conditions and how the loss of GLUT4-mediated glucose metabolism affects PCOS patients.
Acknowledgements
We thank all our patients for their voluntary participation in this study. This work was supported by the Swedish Medical Research Council (5859 and 10380), the Swedish federal government under the LUA/ALF agreement (ALFGBG-147791), Jane and Dan Olsson’s Foundation, the Hjalmar Svensson Foundation, the Åke-Wiberg Foundation, and Clas Groschinsky’s Foundation to HB and RS, as well as the Shanghai Committee of Science and Technology, China, (124119a4002) and the Scientific Research Project of Shanghai Municipal Health Bureau, China, (20134264) to XL. The authors thank the Centre for Cellular Imaging of The Sahlgrenska Academy at Gothenburg University, Sweden.
Disclosure of conflict of interest
None.
References
- 1.Frolova AI, Moley KH. Glucose transporters in the uterus: an analysis of tissue distribution and proposed physiological roles. Reproduction. 2011;142:211–220. doi: 10.1530/REP-11-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulte MM, Tsai J, Moley KH. Obesity and PCOS: The Effect of Metabolic Derangements on Endometrial Receptivity at the Time of Implantation. Reprod Sci. 2015;22:6–14. doi: 10.1177/1933719114561552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- 4.Frolova AI, Moley KH. Quantitative analysis of glucose transporter mRNAs in endometrial stromal cells reveals critical role of GLUT1 in uterine receptivity. Endocrinology. 2011;152:2123–2128. doi: 10.1210/en.2010-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korgun ET, Demir R, Hammer A, Dohr G, Desoye G, Skofitsch G, Hahn T. Glucose transporter expression in rat embryo and uterus during decidualization, implantation, and early postimplantation. Biol Reprod. 2001;65:1364–1370. doi: 10.1095/biolreprod65.5.1364. [DOI] [PubMed] [Google Scholar]
- 6.Mioni R, Mozzanega B, Granzotto M, Pierobon A, Zuliani L, Maffei P, Blandamura S, Grassi S, Sicolo N, Vettor R. Insulin receptor and glucose transporters mRNA expression throughout the menstrual cycle in human endometrium: a physiological and cyclical condition of tissue insulin resistance. Gynecol Endocrinol. 2012;28:1014–1018. doi: 10.3109/09513590.2012.705367. [DOI] [PubMed] [Google Scholar]
- 7.Welch RD, Gorski J. Regulation of glucose transporters by estradiol in the immature rat uterus. Endocrinology. 1999;140:3602–3608. doi: 10.1210/endo.140.8.6923. [DOI] [PubMed] [Google Scholar]
- 8.von Wolff M, Ursel S, Hahn U, Steldinger R, Strowitzki T. Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. 2003;88:3885–3892. doi: 10.1210/jc.2002-021890. [DOI] [PubMed] [Google Scholar]
- 9.Mioni R, Chiarelli S, Xamin N, Zuliani L, Granzotto M, Mozzanega B, Maffei P, Martini C, Blandamura S, Sicolo N, Vettor R. Evidence for the presence of glucose transporter 4 in the endometrium and its regulation in polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2004;89:4089–4096. doi: 10.1210/jc.2003-032028. [DOI] [PubMed] [Google Scholar]
- 10.Mozzanega B, Mioni R, Granzotto M, Chiarelli S, Xamin N, Zuliani L, Sicolo N, Marchesoni D, Vettor R. Obesity reduces the expression of GLUT4 in the endometrium of normoinsulinemic women affected by the polycystic ovary syndrome. Ann N Y Acad Sci. 2004;1034:364–374. doi: 10.1196/annals.1335.038. [DOI] [PubMed] [Google Scholar]
- 11.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 12.Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011:CD007506. doi: 10.1002/14651858.CD007506.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 14.Fornes R, Ormazabal P, Rosas C, Gabler F, Vantman D, Romero C, Vega M. Changes in the expression of insulin signaling pathway molecules in endometria from polycystic ovary syndrome women with or without hyperinsulinemia. Mol Med. 2010;16:129–136. doi: 10.2119/molmed.2009.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao R, Li X, Feng Y, Lin JF, Billig H. Direct effects of metformin in the endometrium: a hypothetical mechanism for the treatment of women with PCOS and endometrial carcinoma. J Exp Clin Cancer Res. 2014;33:41. doi: 10.1186/1756-9966-33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen ZQ, Zhu HT, Lin JF. Reverse of progestin-resistant atypical endometrial hyperplasia by metformin and oral contraceptives. Obstet Gynecol. 2008;112:465–467. doi: 10.1097/AOG.0b013e3181719b92. [DOI] [PubMed] [Google Scholar]
- 17.Session DR, Kalli KR, Tummon IS, Damario MA, Dumesic DA. Treatment of atypical endometrial hyperplasia with an insulin-sensitizing agent. Gynecol Endocrinol. 2003;17:405–407. doi: 10.1080/09513590312331290298. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Guo JR, Lin JF, Feng Y, Billig H, Shao R. Combination of Diane-35 and metformin to treat early endometrial carcinoma in PCOS women with insulin resistance. J Cancer. 2014;5:173–181. doi: 10.7150/jca.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanosz S. An attempt at conservative treatment in selected cases of type I endometrial carcinoma (stage I a/G1) in young women. Eur J Gynaecol Oncol. 2009;30:365–369. [PubMed] [Google Scholar]
- 20.Stanosz S, von Mach-Szczypinski J, Sieja K, Kooeciuszkiewicz J. Micronized Estradiol and Progesterone Therapy in Primary, Preinvasive Endometrial Cancer (1A/G1) in Young Women With Polycystic Ovarian Syndrome. J Clin Endocrinol Metab. 2014;99:E2472–2476. doi: 10.1210/jc.2014-1693. [DOI] [PubMed] [Google Scholar]
- 21.Moghetti P, Tosi F, Castello R, Magnani CM, Negri C, Brun E, Furlani L, Caputo M, Muggeo M. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metab. 1996;81:952–960. doi: 10.1210/jcem.81.3.8772557. [DOI] [PubMed] [Google Scholar]
- 22.Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011:105–167. doi: 10.1007/978-3-642-14541-4_3. [DOI] [PubMed] [Google Scholar]
- 23.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 24.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 25.Shao R, Wang X, Weijdegard B, Norstrom A, Fernandez-Rodriguez J, Brannstrom M, Billig H. Coordinate regulation of heterogeneous nuclear ribonucleoprotein dynamics by steroid hormones in the human Fallopian tube and endometrium in vivo and in vitro. Am J Physiol Endocrinol Metab. 2012;302:E1269–1282. doi: 10.1152/ajpendo.00673.2011. [DOI] [PubMed] [Google Scholar]
- 26.Stambolic V, Woodgett JR, Fantus IG, Pritchard KI, Goodwin PJ. Utility of metformin in breast cancer treatment, is neoangiogenesis a risk factor? Breast Cancer Res Treat. 2009;114:387–389. doi: 10.1007/s10549-008-0015-4. [DOI] [PubMed] [Google Scholar]
- 27.Shao R, Ljungstrom K, Weijdegard B, Egecioglu E, Fernandez-Rodriguez J, Zhang FP, Thurin-Kjellberg A, Bergh C, Billig H. Estrogen-induced upregulation of AR expression and enhancement of AR nuclear translocation in mouse fallopian tubes in vivo. Am J Physiol Endocrinol Metab. 2007;292:E604–614. doi: 10.1152/ajpendo.00350.2006. [DOI] [PubMed] [Google Scholar]
- 28.Shao R, Norstrom A, Weijdegard B, Egecioglu E, Fernandez-Rodriguez J, Feng Y, Stener-Victorin E, Brannstrom M, Billig H. Distinct Expression Pattern of Dicer1 Correlates with Ovarian-Derived Steroid Hormone Receptor Expression in Human Fallopian Tubes during Ovulation and the Midsecretory Phase. J Clin Endocrinol Metab. 2011;96:E869–877. doi: 10.1210/jc.2010-2353. [DOI] [PubMed] [Google Scholar]
- 29.Janzen C, Lei MY, Cho J, Sullivan P, Shin BC, Devaskar SU. Placental glucose transporter 3 (GLUT3) is up-regulated in human pregnancies complicated by late-onset intrauterine growth restriction. Placenta. 2013;34:1072–1078. doi: 10.1016/j.placenta.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson J, Feng Y, Shao R, Lonn M, Billig H, Stener-Victorin E. Intense electroacupuncture normalizes insulin sensitivity, increases muscle GLUT4 content, and improves lipid profile in a rat model of polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2010;299:E551–559. doi: 10.1152/ajpendo.00323.2010. [DOI] [PubMed] [Google Scholar]
- 31.Johansson J, Manneras-Holm L, Shao R, Olsson A, Lonn M, Billig H, Stener-Victorin E. Electrical vs manual acupuncture stimulation in a rat model of polycystic ovary syndrome: different effects on muscle and fat tissue insulin signaling. PLoS One. 2013;8:e54357. doi: 10.1371/journal.pone.0054357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lonn M, Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 33.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358:47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 35.Palomba S, Falbo A, Zullo F, Orio F Jr. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev. 2009;30:1–50. doi: 10.1210/er.2008-0030. [DOI] [PubMed] [Google Scholar]
- 36.Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril. 2001;76:517–524. doi: 10.1016/s0015-0282(01)01975-6. [DOI] [PubMed] [Google Scholar]
- 37.Mansfield R, Galea R, Brincat M, Hole D, Mason H. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril. 2003;79:956–962. doi: 10.1016/s0015-0282(02)04925-7. [DOI] [PubMed] [Google Scholar]
- 38.Critchley HO, Saunders PT. Hormone receptor dynamics in a receptive human endometrium. Reprod Sci. 2009;16:191–199. doi: 10.1177/1933719108331121. [DOI] [PubMed] [Google Scholar]
- 39.Shao R, Cao S, Wang X, Feng Y, Billig H. The elusive and controversial roles of estrogen and progesterone receptors in human endometriosis. Am J Transl Res. 2014;6:104–113. [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira GD, Germeyer A, de Barros Machado A, do Nascimento TL, Strowitzki T, Brum IS, von Eye Corleta H, Capp E. Metformin modulates PI3K and GLUT4 expression and Akt/PKB phosphorylation in human endometrial stromal cells after stimulation with androgen and insulin. Eur J Obstet Gynecol Reprod Biol. 2014;175:157–162. doi: 10.1016/j.ejogrb.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361:1810–1812. doi: 10.1016/s0140-6736(03)13409-5. [DOI] [PubMed] [Google Scholar]
- 42.Zhai J, Liu CX, Tian ZR, Jiang QH, Sun YP. Effects of metformin on the expression of GLUT4 in endometrium of obese women with polycystic ovary syndrome. Biol Reprod. 2012;87:29. doi: 10.1095/biolreprod.112.099788. [DOI] [PubMed] [Google Scholar]
- 43.Carvajal R, Rosas C, Kohan K, Gabler F, Vantman D, Romero C, Vega M. Metformin augments the levels of molecules that regulate the expression of the insulin-dependent glucose transporter GLUT4 in the endometria of hyperinsulinemic PCOS patients. Hum Reprod. 2013;28:2235–2244. doi: 10.1093/humrep/det116. [DOI] [PubMed] [Google Scholar]
- 44.Kohan K, Carvajal R, Gabler F, Vantman D, Romero C, Vega M. Role of the transcriptional factors FOXO1 and PPARG on gene expression of SLC2A4 in endometrial tissue from women with polycystic ovary syndrome. Reproduction. 2010;140:123–131. doi: 10.1530/REP-10-0056. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Liao Q. Effects of testosterone and metformin on glucose metabolism in endometrium. Fertil Steril. 2010;93:2295–2298. doi: 10.1016/j.fertnstert.2009.01.096. [DOI] [PubMed] [Google Scholar]
- 46.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang HY, Zhang YF, Han YK, Xue FX, Zhao XH, Zhang XL. Activation and significance of the PI3K/Akt pathway in endometrium with polycystic ovary syndrome patients. Zhonghua Fu Chan Ke Za Zhi. 2012;47:19–23. [PubMed] [Google Scholar]
- 49.Villavicencio A, Goyeneche A, Telleria C, Bacallao K, Gabler F, Fuentes A, Vega M. Involvement of Akt, Ras and cell cycle regulators in the potential development of endometrial hyperplasia in women with polycystic ovarian syndrome. Gynecol Oncol. 2009;115:102–107. doi: 10.1016/j.ygyno.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hara K, Yonezawa K, Kozlowski MT, Sugimoto T, Andrabi K, Weng QP, Kasuga M, Nishimoto I, Avruch J. Regulation of eIF-4E BP1 phosphorylation by mTOR. J Biol Chem. 1997;272:26457–26463. doi: 10.1074/jbc.272.42.26457. [DOI] [PubMed] [Google Scholar]


