Abstract
Background: Ischemia related inflammation is the most critical factor for the survival of transplanted mesenchymal stem cells (MSCs), and strategies for controlling excessive inflammation after acute myocardial infarction (AMI) are essential and necessary for cell transplantation therapy. Our present study tested the effect of decreased Ly6Chigh monocytes on mouse MSCs transplantation after AMI. Methods: BALB/c AMI mice were treated systemically with a CCR2 antagonist (RS 504393, 2 mg/kg, subcutaneously) or normal saline (control group). Next, 105 EdU-labeled MSCs were administered by intramyocardial injection to the mice in each group. TUNEL kits were used to identify the apoptotic cardiomyocytes in the infarct. The slides of the infarct border zone were stained with wheat germ agglutinin to measure the vessel density, and anti-myosin heavy chain eFluor 660 was used to measure the cardiac myosin-positive area. A transwell chamber was used to examine the interactions between Ly6Chigh monocytes and MSCs. The inflammatory cytokines expressed by Ly6Chigh monocytes and the SDF-1 expressed by MSCs were detected using ELISA kits. MSC viability was further examined by MTT and mitochondrial membrane potential assays by flow cytometry using JC-1 kits. Results: We first observed the increased survival of transplanted MSCs (11.2 ± 3.4/mm2 vs. 3.5 ± 1.6/mm2, p < 0.001), and the decreased apoptosis of cardiomyocytes (11.20% ± 3.55% vs. 20.51% ± 8.17%, p < 0.001) in the infarcts at 3 days in the CCR2 antagonist group. An increased number of capillaries and small arterioles (139.6 ± 21.7/mm2 vs. 95.4 ± 17.6/mm2, p < 0.001) and an increased cardiac myosin-positive area (17.9% ± 6.6% vs. 11.8% ± 3.5%, p < 0.001) were also observed in the infarct zone at 21 days post MSC infusion in the CCR2 antagonist group. In addition, a significantly increased LvEF% (50.17 ± 10.06 vs. 45.44 ± 9.45, p < 0.001) was detected at the same time compared to the control mice. We further demonstrated that both the mitochondrial membrane potential of the MSCs (0.45 ± 0.11 vs. 3.4 ± 0.3, p < 0.001) and stromal cell-derived factor-1 (SDF-1) secreted by the MSCs significantly decreased (80.77 ± 39.02 pg/ml vs. 435.5 ± 77.41 pg/ml, p < 0.001) when co-cultured with Ly6Chigh monocytes. This is possibly mediated by the over-expressed cytokines secreted by the Ly6Chigh monocytes compared to the Ly6Clow monocytes, including IL-1 (139.45 ± 30.44 vs. 80.05 ± 19.33, p < 0.001), IL-6 (187.82 ± 40.43 vs. 135.5 ± 22.09, p < 0.001), TNF-α (121.77 ± 31.65 vs. 75.3 ± 22.14, p < 0.001) and IFN-γ (142.46 ± 27.55 vs. 88.25 ± 19.91, p < 0.001).
Keywords: Ly6Chigh monocytes, MSCs, AMI, CCR2
Introduction
Coronary artery disease (CAD) is likely the major cause of heart failure in both developed and developing countries [1]. Stem cells, such as bone marrow derived mesenchymal stem cells (MSCs), have been used to treat atherosclerotic heart disease and other diseases that involve acute injury of myocardium [2-4]. An attractive feature of MSCs is the lack of major histocompatibility complex (MHC) class II, rendering these cells relatively tolerant of immunological reactions. The paracrine effects of transplanted MSCs, including the secretion of a broad range of bioactive molecules, such as SDF-1, are now hypothesized to be the primary mechanisms by which MSCs achieve their therapeutic effect. However, previous attempts to use these cells have not been particularly successful [5]. Despite the advantages of MSC transplantation for heart disease, detailed in vivo observations have shown that the MSC survival time was short post-MSC transplantation due to harsh microenvironments that include ischemia, inflammation and anoikis in the infarcted myocardium. Strategies to improve the cell viability of transplanted MSCs are necessary to improve cardiac function after cell transplantation therapy. Spleen derived monocytes, particularly Ly6Chigh subsets are primarily involved in counteracting wound healing and contribute to left ventricular dilatation in acute myocardial infarction (AMI) [6,7]. Our present study tested the hypothesis that the inhibition of the mobilization of Ly6Chigh monocytes decreases the accumulation of cells in the infarcts, improves the efficacy of MSC transplantation and attenuates myocardial remodeling.
Materials and methods
AMI mice models and inhibition of CCR2
An anterior wall MI was induced by direct ligation of the left anterior descending (LAD) artery as previously described [8]. After anesthetization with pentobarbital sodium (50 mg/kg), the mice were endotracheally intubated and ventilated with room air at 110 breaths per minute using a rodent ventilator (Harvard Apparatus). A left thoracotomy was performed to expose the LV of the heart. The LAD coronary artery was visualized and ligated just below the left auricular level with an 8-0 nylon suture. Blanching and dysfunction of the anterior wall verified LAD ligation. The animal procedures were performed according to a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Southeast University.
For the pharmacological inhibition of CCR2 after AMI, the specific CCR2 antagonist, RS 504393, (Tocris, Bristol UK) was used (2 mg/kg, subcutaneous). The IC50 for CCR2 is 110 nmol/L. RS 504393 inhibits CCL2-induced chemotaxis with an IC50 of 360 nmol/L.
MSCs preparation, labeling and delivery
MSCs were isolated and cultured using standard protocols [9]. Femurs and tibias of 7- to 10-week-old mice were flushed using standard PBS (HyClone) to collect the bone marrow cells. FACSTM-Lysing solution (BD Biosciences) was used to deplete the erythrocytes. After centrifugation at 1000 rpm/min for 5 min, the cells were washed 3 times and then resuspended in minimal essential medium supplemented with 10% FBS (DMEM, Gibco) at a density of 5 × 106 cells/cm2. Non-adherent cells were removed at day 3, and the cells reached confluence after 2 weeks. Trypsin (0.25%) containing EDTA (HyClone) was used for the subsequent passaging. The monoclonal antibodies for Sca-1, CD105, CD90, CD31, CD34, and CD45 conjugated to FITC were used to identify the cells by flow cytometry (Becton Dickinson Inc. USA). The MSCs used for the experiments were from passages 5 to 6. Two days before infusion, the cells were freshly plated at a 1/3 ratio and incubated in complete medium with 10 μm/L [10,11] EdU (5-ethynyl-2-deoxyuridine, Invitrogen) for 48 hours to label the cells. Incorporation of EdU was detected by reaction with azide-conjugated Alexa Fluor 488. The AMI mice described above received 105 MSCs resuspended in 100 µl of PBS via intramyocardial injection.
Isolation of monocytes and co-culture
The monocytes were obtained as follows: First, 6-8-week-old BALB/c mice were sacrificed. The belly skin was then opened by pulling the skin apart on the left side of the abdomen, and the abdominal membrane was not broken until the spleen was excised. After organ harvest, single cell suspensions were obtained from the spleen by digestion with a cocktail of 450 U/ml collagenase II, 125 U/ml collagenase IV and 60 U/ml hyaluronidase (Sigma-Aldrich) for 30 min at 37°C with shaking. After filtration through a 200-mesh screen and centrifugation at 400 × g, the cells were positively selected using CD11b MACS beads (Miltenyi Biotec) and then stained with anti-Ly-6C Abs. The purified cells were cultured in RPMI medium 1640 supplemented with 10% FCS in 6-well plates. The monocytes used for the experiments were from passages 3 to 6. Transwell chambers (Corning) were used to examine the interactions between the monocytes and MSCs (co-culture for 24 h). Inflammatory cytokines, including IL1/6, TNF-α and IFN-γ, in the supernatant of the Ly6Chigh/low monocytes were further detected using ELISA kits (R&D).
Real-time PCR
Three days after AMI, the hearts were perfused with saline, and the infarcted left ventricles were removed below the ligation. The total RNA was extracted from the infarct with Trizol reagent according to the manufacturer’s instructions (Invitrogen). A QuantiTect Reverse Transcription Kit (Fermentas) was used for reverse transcription. Polymerase chain reaction was performed using Platinum SYBR Green qPCR SuperMix UDG (Applied Invitrogen) in a BIO-RAD MJ Mini Opticon Real-Time PCR System. The primer sequences were as follows: SDF-1 (NM_021704) 5’tgcccttcagattgttgcac3’ and 5’ccacggatgtcagccttcc3’; and angiopoietin-1 (NM_001286062.1) 5’cattcttcgctgccattctg3’ and 5’gcacattgcccatgttgaatc3’.
Immunostaining
The animals were sacrificed 3 days or 3 weeks after AMI. The tissues were fixed in formalin and embedded in paraffin blocks according to established protocols. The fixed hearts were serially cut 8 µm from the apex to below the coronary artery ligation site. After antigen retrieval, the specimens were incubated with 1% normal blocking serum in PBS for 60 min to suppress the nonspecific binding of IgGs. The slides were then incubated for 30-60 min with each mouse antibody or fluorescent reagents.
Three days after AMI, the infarction tissue was stained for EdU (Invitrogen) to calculate the live MSCs, and TUNEL kits (R&D Systems) were used to identify the nuclei of the apoptotic cardiac myocytes in the infarct border zone. Three weeks after AMI, the infarct border zone was stained with Texas Red-X conjugated wheat germ agglutinin (WGA, Invitrogen) to measure the vessel density. The tissue was further stained with anti-myosin heavy chain eFluor 660 (eBioscience) and 4,6-diamidino-2-phenylindole (DAPI, Roche) to measure the percent cardiac myosin-positive area in the infarct zone using Image Pro Plus software. Fluorescence microscopy (Olympus BX61) or a confocal laser scanning (CLS) microscopy system (Thorlabs, Inc.) was used as necessary for image acquisition of the immunostaining.
Flow cytometric analysis
One day after AMI, mouse blood was obtained and the monocytes were isolated through gradient density centrifugation following erythrocyte lysing. After two washes with cold PBS, the cells were resuspended at a concentration of 106 cells/ml. Single-cell suspensions were then incubated with FITC-rat anti-mouse CD11b and PE-rat anti-mouse Ly-6C (BD Biosciences). The CD11b+Ly6Chigh circulating monocytes and the CD11b+ monocytes in the infarcts were further identified using a FACS Calibur cytometer (Becton Dickinson). The mitochondrial membrane potential of the co-cultured MSCs was also determined using the FACSCalibur cytometer with JC-1 mitochondrial membrane potential assay kits (Abnova).
Echocardiography
At one and twenty-one days after AMI, 2-dimensional echocardiography was performed on the mice using a Vevo 770Rhigh-resolution Imaging System (Visual Sonic, Canada) and Vevo analysis software (Vevo 2.2.3) as previously described [12,13]. The LV dimensions and LV posterior wall thickness were quantified via digitally recorded 2-dimensional clips and Mmode images in a short axis view from the mid-LV just below the papillary muscles to allow for consistent measurements from the same anatomical location in each mouse. Each measurement in each mouse was repeated five to six times and performed using 3 randomly chosen m-Mode clips of the 5 recorded by the observer, who was blinded to the experiment group. The shortening fraction (FS) was calculated as (LvEDD-LvESD)/LvEDD × 100%.
Statistical analysis
Data management and statistical analysis were performed with Statistical Package for Social Sciences software (SPSS 18.0 for Windows, SPSS Inc.). Data are expressed as mean ± standard deviation. Categorical variables were compared using the χ2 test or Fisher’s exact test. For continuous data, group comparisons were performed using an unpaired t test or the Mann-Whitney U test. Results were considered statistically significant if the two sided P-value was ≤ 0.05.
Results
Inhibition of CCR2 ameliorates inflammation after AMI
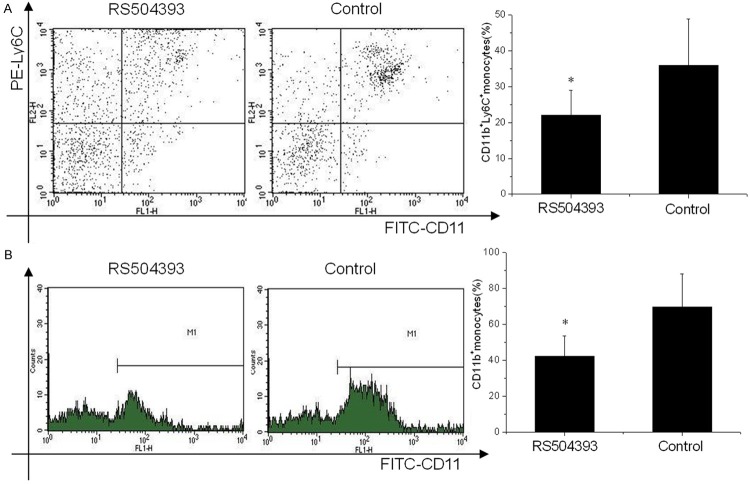
Ly6Chigh monocytes are potent inflammatory mediators and are a predominant source of inflammation after AMI. We tested the deployment of Ly6Chigh monocytes one day after AMI with the administration of RS504393 (2 mg/kg, subcutaneously). Flow cytometry analysis showed a significant reduction in circulating inflammatory Ly6Chigh monocytes (22.02% ± 7.05% vs. 35.87% ± 13.04%, p < 0.001) one day after AMI in the mice treated with RS504393 compared to the control group (Figure 1A). CD11b positive monocytes in the infarcts at this time were further examined, and the results revealed significantly reduced numbers of cells compared to the groups not treated with RS504393 (42.34% ± 11.04% vs. 69.54% ± 18.5%, p < 0.001) (Figure 1B).
Figure 1.
The effect of CCR2 inhibition on inflammatory infiltration post AMI (n = 20). A. Flow cytometry revealed different patterns of CD11b+Ly6Chigh cells in the circulation of AMI mice one day after treatment with RS504393. Significantly reduced cells counts were observed in the RS504393 group. B. Flow cytometry revealed reduced CD11b positive cells (monocytes and monocytes derived macrophages) in the infarct zone of AMI mice one day after treatment with RS504393. *p < 0.05 vs. the corresponding control group.
Increased survival of transplanted MSCs and decreased apoptosis of cardiomyocytes
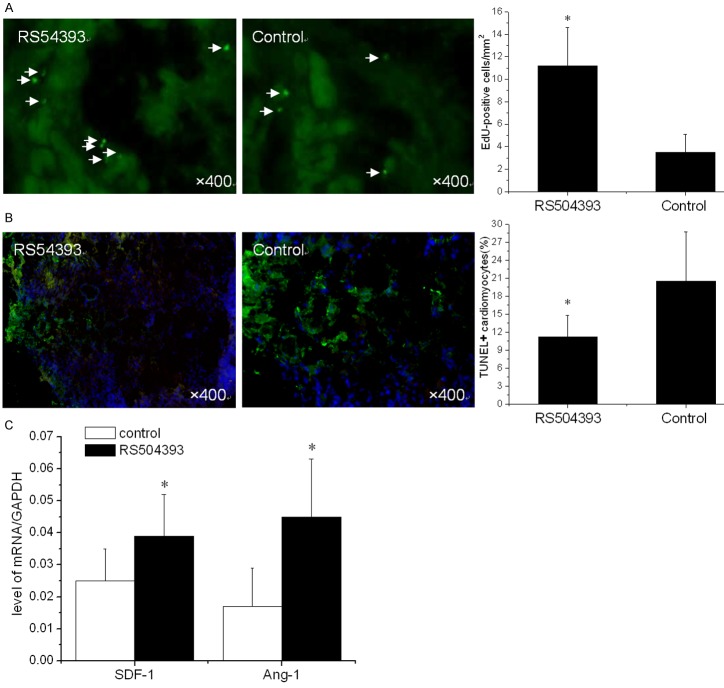
Current limitations of MSC therapy include the impact of the inflammatory microenvironment on cellular behavior. We next evaluated the therapeutic potential of MSCs when CCR2 was inhibited by RS504393. After infarction, the hearts were harvested and embedded in paraffin. The sections were incubated with azide-conjugated Alexa Fluor 488. We observed a significant difference in EdU-positive cells (Figure 2A) in the infarction (11.2 ± 3.4/mm2 vs. 3.5 ± 1.6/mm2, p < 0.001) between the two groups three days after MSC transplantation. Consistent with the results for the restored MSCs, we also observed a significant reduction in TUNEL+ cardiomyocytes (11.20% ± 3.55% vs. 20.51% ± 8.17%, p < 0.001) within the infarct zone compared to the control at three days (Figure 2B). In addition, increased levels of SDF-1 (0.039 ± 0.013 vs. 0.022 ± 0.01, p < 0.001) and Ang-1 (0.045 ± 0.018 vs. 0.017 ± 0.12, p < 0.001) mRNA in the infarct zone were also detected (Figure 2C).
Figure 2.
Inhibition of Ly6Chigh monocyte mobilization enhances the efficiency of MSC transplantation (n = 15). A. Surviving MSCs in the infarct zone following cell therapy are shown, with representative immunofluorescent staining for EdU (5-ethynyl-2’- deoxyuridine, green) within the infarct zone 3 days after cell transplantation. B. A representative image of immunofluorescent staining for TUNEL (Alexa Fluor 488, green) and DAPI in the infarct border zone 3 days after AMI. The number of TUNEL-positive cardiomyocytes has been calculated. C. The SDF-1 and Ang-1 mRNA levels in the infarct zone after MSC transplantation. The data represent the mean ± SD. *p < 0.05 vs. the corresponding control group.
Increased vascular density and cardiac myosin-positive area after MSC transplantation
Three weeks after MSC infusion, the vascular density in the border zone of the infarction was examined using WGA (Figure 3A). A significant increase in the number of capillaries and small arterioles was observed in the RS504393 group (139.6 ± 21.7/mm2 vs. 95.4 ± 17.6/mm2, p < 0.001). Consistent with the increased vascular density results, a significant increase of the cardiac myosin-positive area within the infarct zone in the RS504393 group (17.9% ± 6.6% vs. 11.8% ± 3.5%, p < 0.001) was also observed (Figure 3B). Finally, the LV (LvEF %) remodeling at three weeks after MSC infusion was measured and was significantly ameliorated compared to the control (50.17 ± 10.06 vs. 45.44 ± 9.45, p < 0.001) (Figure 3C). Taken together, these data demonstrate that Ly6Chigh monocytes play an adverse role in MSC transplantation post-AMI.
Figure 3.
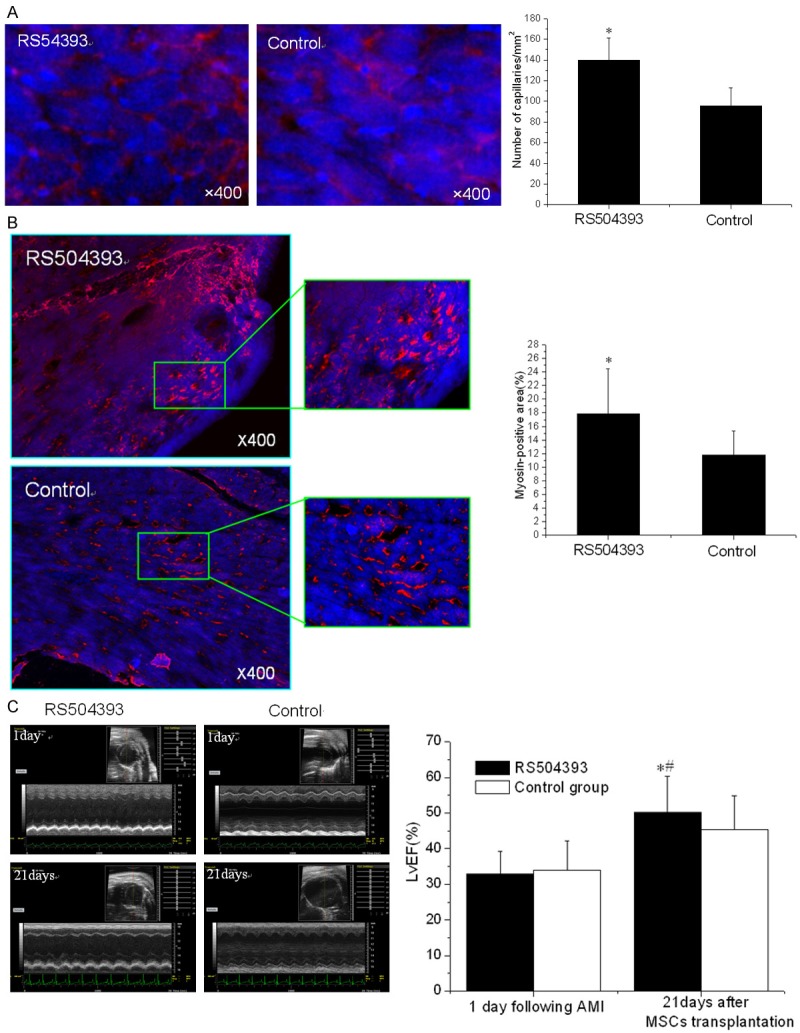
Reduced myocardial remodeling at three weeks (n = 15). A. The vascular density was determined using immunofluorescent staining for WGA (wheat germ agglutinin, red) and DAPI (blue) in the infarct zone 21 days post-MSC transplantation. B. A confocal image of representative immunofluorescent staining for cardiac myosin heavy chain (eFluor® 660, red) and DAPI (blue) within the infarct zone 21 days post-MSC transplantation. C. The ejection fraction at baseline and 21 days post-MSC transplantation. *p < 0.05 vs. the corresponding control group at 21 days; #p < 0.05 vs. the corresponding control group at 1 day post-AMI.
Decreased SDF-1 secretion by MSCs co-cultured with Ly6Chigh monocytes
Studies demonstrated [14,15] that the local trophic effects of MSC require cardiac progenitor cells and the expression of CM-CXCR4 and are also mediated by MSC stromal cell-derived factor-1 (SDF-1) secretion. After determining the levels of cytokines, including IL-1 (139.45 ± 30.44 vs. 80.05 ± 19.33, p < 0.001), IL-6 (187.82 ± 40.43 vs. 135.5 ± 22.09, p < 0.001), TNF-α (121.77 ± 31.65 vs. 75.3 ± 22.14, p < 0.001) and IFN-γ (142.46 ± 27.55 vs. 88.25 ± 19.91, p < 0.001) derived from the Ly6Chigh/low monocytes (Figure 4A), transwell chambers were used to examine the interactions between the cells. We observed that the SDF-1 secreted by the MSCs significantly decreased (280.77 ± 39.02 pg/ml vs. 435.5 ± 77.41 pg/ml, p < 0.001) when co-cultured with Ly6Chigh monocytes compared to the Ly6Clow monocytes (Figure 4B). After co-culture with the Ly6Chigh monocytes for 24 h, the cell viability of the co-cultured MSCs was determined using MTT (0.34 ± 0.06 vs. 0.71 ± 0.1, p < 0.001) and JC-1 mitochondrial membrane potential detection kits (0.45 ± 0.11 vs. 3.4 ± 0.3, p < 0.001). The results revealed the significantly decreased viability of the cells at this time compared to the control (Figure 4C).
Figure 4.
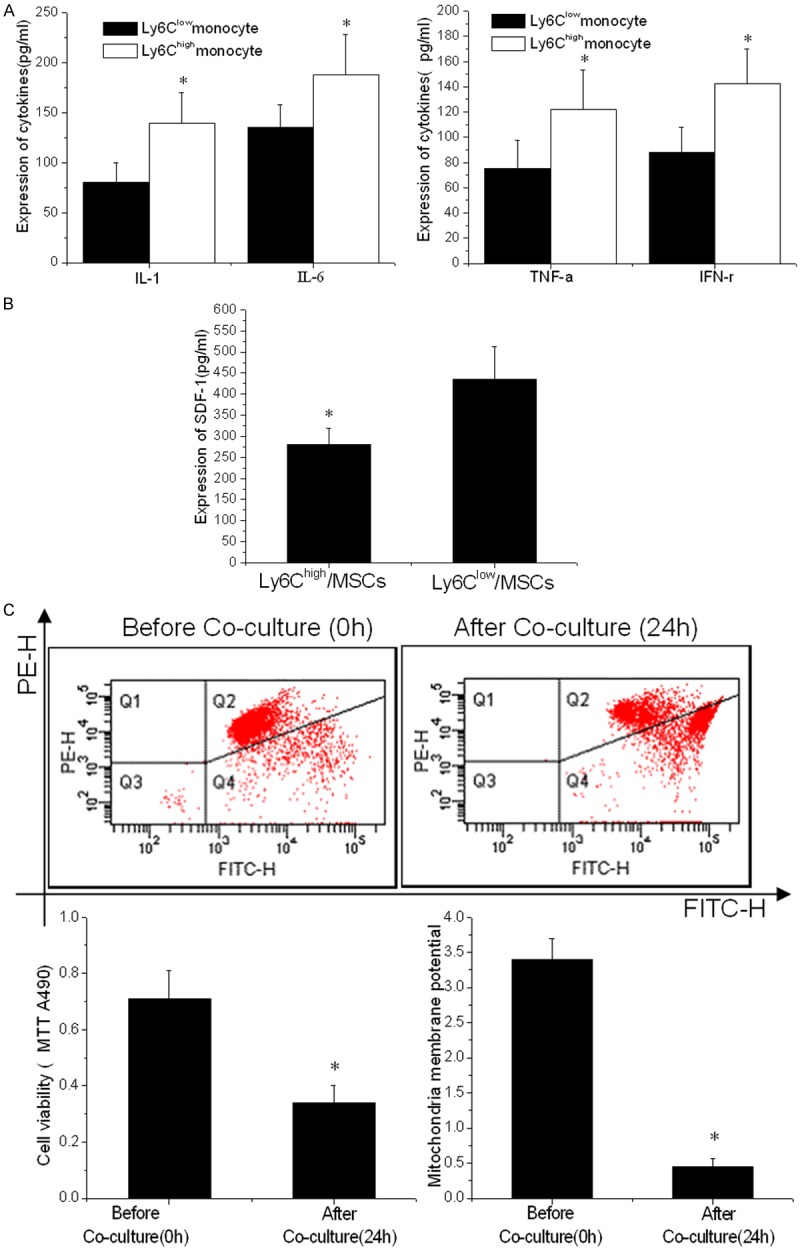
Decreased viability of MSCs when co-cultured with Ly6Chigh monocytes. A. The inflammatory cytokines, IL-1, IL-6, TNF-α and IFN-γ, in the supernatant of Ly6Chigh and Ly6Clow monocytes. B. The expression of SDF-1 in supernatant of MSCs determined by immunosorbent assay (ELISA) methods. C. MTT and mitochondria membrane assay measuring MSC viability before and after co-culture with Ly6Chigh monocytes. The conditioned medium from Ly6Chigh monocytes significantly reduced the MSC viability (p < 0.001). A representative measurement image of MSC mitochondria membrane potential determined by flow cytometry after co-culture with Ly6Chigh monocytes is shown. *p < 0.05 vs. the corresponding control group.
Discussion
The unique properties of MSCs [16,17], which include easy isolation and amplification from the bone marrow, immunological tolerance of allogeneic transplantation and multilineage potential, have led to their extensive investigation as a cell-based therapeutic strategy for cardiac repair. Although the causes of the decreased survival of transplanted MSCs have not yet been fully elucidated, inflammation, oxidative stress, and mitochondrial and energy metabolism dysfunction have been recognized as influential factors [18]. Current stem cell therapies are designed to address the genetic and protein modification of MSCs to enhance their anti-apoptotic ability [19-21] or decrease the influence of the local microenvironment via inflammation [20].
The present study used a CCR2 antagonist (RS 504393) to decrease the accumulation of Ly-6Chigh monocytes post-AMI and subsequent MSC transplantation and demonstrated the following: (1) the administration of the CCR2 antagonist intravenously decreased the mobilization of Ly-6Chigh monocytes from the spleen and decreased the counts in the circulation post-AMI; (2) AMI induced a local or systemic inflammatory response that was alleviated by the decreased level of Ly-6Chigh monocytes; (3) a marked upregulation of the MSC transplantation effect was observed after decreasing the level of Ly-6Chigh monocytes, as evidenced by the increased survival of MSCs and reduced apoptosis of cardiomyocytes at three days and the increased regeneration of capillaries and ameliorated LV remodeling at three weeks; and (4) over-expressed cytokines, including TNF-α, IL-1/6, and IFN-γ secreted by Ly6Chigh monocytes decreased the survival of MSCs and the subsequent secretion of SDF-1, which negatively affect the local trophic effects of transplanted MSCs.
The primary objective of this study was to establish an in vivo model of the influence of hypoxic and inflammatory environmental changes on transplanted MSCs. Previous studies have shown that AMI related inflammation is a pathological condition in which the oxygen demand of cells exceeds the supply, and the recruitment of inflammatory cells and the secretion of inflammatory factors is also observed [21]. As demonstrated, Ly6Chigh cells were deployed rapidly from the spleen in response to AMI, and the MSCs were influenced by the experimental conditions, which are vulnerable to severe continuous hypoxia and an inflammatory environment. The administration of CCR2 antagonist in this study led to the increased survival of transplanted MSCs in the infarcts, suggesting a requirement for the alleviation of ischemia related to the microenvironment in the infarct zone. In vivo, under the conditions of ischemia, MSCs underwent not only hypoxia but also a reduced supply of critical metabolic nutrients and inadequate metabolic waste removal [22]. Therefore, inhibition of the mobilization of Ly6Chigh monocytes was critical for the inflammatory cascade effect in the control of inflammation after AMI.
Here, we show that the CCR2 inhibitor significantly enhanced the efficiency of MSC transplantation and reduced myocardial remodeling, as demonstrated by the increased survival of transplanted MSCs and decreased apoptosis of cardiomyocytes at day 3, as well as the increased vascular density and cardiac myosin-positive area after MSC transplantation at 21 days. In addition, we showed significantly improved LVEF% in the mice treated with the CCR2 antagonist. These results provide a scientific foundation for the use of CCL2 and its cognate receptor CCR2 in the treatment of AMI. To further identify the mechanism of decreased monocyte numbers on the nutritional effects of MSCs, transwell chambers were used to examine the interactions between Ly6Chigh monocytes and MSCs. Notably, we not only observed a significant decrease in SDF-1 expression in the MSCs but also detected a reduced mitochondrial membrane potential in the cells. These results are consistent with recent reports that stroma cell-derived factor-1a (SDF-1a) is a cardioprotective chemokine, acting through its G-protein coupled receptor, CXCR4 [23]. Moreover, cardiac myocyte CXCR4 expression may act as a marker of injured or dysfunctional cardiac myocytes.
However, we must consider other factors, such as the inflammatory cytokines IL-1/6, TNF-α, and IFN-γ in Ly6Chigh and Ly6low monocytes to envision an ideal cellular therapy for AMI. Researchers [24,25] have shown that spleen-derived monocytes, particularly Ly6Chigh subsets, primarily oppose wound healing and contribute to left ventricular dilatation. In the setting of AMI, after these monocytes are recruited to the infarcted myocardium, they will release inflammatory mediators, such as TNF-α, IL-1/6, IFN-γ, inducible nitrous oxide synthase (iNOS) and matrix metalloproteinases (MMPs) [26]. Therefore, we conclude that the excessive inflammatory monocytes and the potent inflammatory microenvironment in the infarct are key factors that decrease the efficiency of stem cell transplantation.
Conclusion
We conclude that the mobilization of Ly6Chigh monocytes after AMI negatively affect the local trophic effects of transplanted MSCs, which may occur because the over-expressed TNF-α, IL-1/6, and INF-γ by the Ly6Chigh monocytes decreases the survival of MSCs and the subsequent secretion of SDF-1.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81300160).
Disclosure of conflict of interest
None.
References
- 1.Rahbarghazi R, Nassiri SM, Ahmadi SH, Mohammadi E, Rabbani S, Araghi A, Hosseinkhani H. Dynamic induction of pro-angiogenic milieu after transplantation of marrow-derived mesenchymal stem cells in experimental myocardial infarction. Int J Cardiol. 2014;173:453–66. doi: 10.1016/j.ijcard.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Pasternak J. Cardiac stem cells and their clinical use. Einstein (Sao Paulo) 2014;12:134–5. doi: 10.1590/S1679-45082014MD2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matar AA, Chong JJ. Stem cell therapy for cardiac dysfunction. Springerplus. 2014;3:440. doi: 10.1186/2193-1801-3-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goichberg P, Chang J, Liao R, Leri A. Cardiac stem cells: biology and clinical applications. Antioxid Redox Signal. 2014;21:2002–17. doi: 10.1089/ars.2014.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi E, Hosoda T. Myocyte renewal and therapeutic myocardial regeneration using various progenitor cells. Heart Fail Rev. 2014;19:789–97. doi: 10.1007/s10741-014-9430-2. [DOI] [PubMed] [Google Scholar]
- 6.Kamei M, Carman CV. New observations on the trafficking and diapedesis of monocytes. Curr Opin Hematol. 2010;17:43–52. doi: 10.1097/MOH.0b013e3283333949. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh M, Gerber C, Rahman MM, Vernier KM, Pereira FE, Subramani J, Caromile LA, Shapiro LH. Molecular mechanisms regulating CD13-mediated adhesion. Immunology. 2014;142:636–47. doi: 10.1111/imm.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cochain C, Auvynet C, Poupel L, Vilar J, Dumeau E, Richart A, Récalde A, Zouggari Y, Yin KY, Bruneval P, Renault G, Marchiol C, Bonnin P, Lévy B, Bonecchi R, Locati M, Combadière C, Silvestre JS. The Chemokine Decoy Receptor D6 Prevents Excessive Inflammation and Adverse Ventricular Remodeling After Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2012;32:2206–13. doi: 10.1161/ATVBAHA.112.254409. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen TO, Blois AL, Xue Y, Xing Z, Sun Y, Finne-Wistrand A, Lorens JB, Fristad I, Leknes KN, Mustafa K. Mesenchymal stem cells induce endothelial cellquiescence and promote capillary formation. Stem Cell Res Ther. 2014;5:23. doi: 10.1186/scrt412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ning H, Albersen M, Lin G, Lue TF, Lin CS. Effects of EdU Labeling on Mesenchymal Stem Cells. Cytotherapy. 2013;15:57–63. doi: 10.1016/j.jcyt.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin G, Huang YC, Shindel AW, Banie L, Wang G, Lue TF, Lin CS. Labeling and Tracking of Mesenchymal Stem Cells with EdU. Cytotherapy. 2009;11:864–73. doi: 10.3109/14653240903180084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng Z, Yao Y, Li Y, Yan F, Huang J, Ma G. Bradykinin Preconditioning Improves Therapeutic Potential of Human Endothelial Progenitor Cells in Infarcted Myocardium. PLoS One. 2013;8:e81505. doi: 10.1371/journal.pone.0081505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan F, Yao Y, Chen L, Li Y, Sheng Z, Ma G. Hypoxic Preconditioning Improves Survival of Cardiac Progenitor Cells: Role of Stromal Cell Derived Factor-1a-CXCR4 Axis. PLoS One. 2012;7:e37948. doi: 10.1371/journal.pone.0037948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rueda P, Richart A, Récalde A, Gasse P, Vilar J, Guérin C, Lortat-Jacob H, Vieira P, Baleux F, Chretien F, Arenzana-Seisdedos F, Silvestre JS. Homeostatic and Tissue Reparation Defaults in Mice Carrying Selective Genetic Invalidation of CXCL12/Proteoglycan Interactions. Circulation. 2012;126:1882–95. doi: 10.1161/CIRCULATIONAHA.112.113290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao S, Chang SL, Linderman JJ, Linderman JJ, Feng FY, Luker GD. A Comprehensive Analysis of CXCL12 Isoforms in Breast Cancer1,2. Transl Oncol. 2014 doi: 10.1016/j.tranon.2014.04.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams AR, Hare JM. Mesenchymal Stem Cells Biology, Pathophysiology, Translational Findings, and Therapeutic Implications for Cardiac Disease. Circ Res. 2011;109:923–40. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alfaro MP, Young PP. Lessons from genetically altered mesenchymal stem cells (MSCs): candidates for improved MSC-directed myocardial repair. Cell Transplant. 2012;21:1065–74. doi: 10.3727/096368911X612477. [DOI] [PubMed] [Google Scholar]
- 18.Cejkova J, Trosan P, Cejka C, Lencova A, Zajicova A, Javorkova E, Kubinova S, Sykova E, Holan V. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp Eye Res. 2013;116:312–23. doi: 10.1016/j.exer.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Reichert JC, Schmalzl J, Prager P, Gilbert F, Quent VM, Steinert AF, Rudert M, Nöth U. Synergistic effect of Indian hedgehog and bone morphogenetic protein-2 gene transfer to increase the osteogenic potential of human mesenchymal stem cells. Stem Cell Res Ther. 2013;4:105. doi: 10.1186/scrt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo T, Kubo M, Katsura S, Nishimoto A, Ueno K, Samura M, Fujii Y, Hosoyama T, Hamano K. Hypoxically preconditioned human peripheral blood mononuclear cells improve blood flow in hindlimb ischemia xenograft model. Am J Transl Res. 2014;6:570–579. [PMC free article] [PubMed] [Google Scholar]
- 21.Taghavi S, George JC. Homing of stem cells to ischemic myocardium. Am J Transl Res. 2013;5:404–411. [PMC free article] [PubMed] [Google Scholar]
- 22.Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142–9. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deschepper M, Oudina K, David B, Myrtil V, Collet C, Bensidhoum M, Logeart-Avramoglou D, Petite H. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J Cell Mol Med. 2011;15:1505–14. doi: 10.1111/j.1582-4934.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16:159–68. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 25.Hadad I, Veithen A, Springael JY, Sotiropoulou PA, Mendes Da Costa A, Miot F, Naeije R, De Deken X, Entee KM. Stroma cell-derived factor-1α signaling enhances calcium transients and beating frequency in rat neonatal cardiomyocytes. PLoS One. 2013;8:e56007. doi: 10.1371/journal.pone.0056007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–16. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu B, Kennedy JK, Wang Y, Sandoval-Garcia C, Cao L, Xiao S, Wu C, Elyaman W, Khoury SJ. Plasticity of Ly-6C(hi) myeloid cells in T cell regulation. J Immunol. 2011;187:2418–32. doi: 10.4049/jimmunol.1100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins CS, Chudnovskiy A, Rauch PJ, Figureueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C (high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–74. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]