Abstract
Neoadjuvant chemotherapy in osteosarcoma increased the long-term survival of patients with localized disease considerably but metastasizing osteosarcoma remained largely treatment resistant. Neuropilins, transmembrane glycoproteins, are important receptors for VEGF dependent hyper-vascularization in tumor angiogenesis and their aberrant expression promotes tumorigenesis and metastasis in many solid tumors. Our analysis of Neuropilin-1 (NRP1) and Neuropilin-2 (NRP2) immunostaining in a tissue microarray of 66 osteosarcoma patients identified NRP2 as an indicator of poor overall, metastasis-free and progression free survival while NRP1 had no predictive value. Patients with tumors that expressed NRP2 in the absence of NRP1 had a significantly worse prognosis than NRP1-/NRP2-, NRP1+ or NRP1+/NRP2+ tumors. Moreover, patients with overt metastases and with NRP2-positive primary tumors had a significantly shorter survival rate than patients with metastases but NRP2-negative tumors. Furthermore, the expression of both NRP1 and NRP2 in osteosarcoma cell lines correlated to a variable degree with the metastatic potential of the respective cell line. To address the functional relevance of Neuropilins for VEGF signaling we used shRNA mediated down-regulation and blocking antibodies of NRP1 and NRP2 in the metastatic 143B and HuO9-M132 cell lines. In 143B cells, VEGFA signaling monitored by AKT phosphorylation was more inhibited by blocking of NRP1, whereas in HuO9-M132 cells NRP2 blocking was more effective indicating that NRP1 and NRP2 can substitute each other in the functional interaction with VEGFR1. Altogether, these data point to NRP2 as a powerful prognostic marker in osteosarcoma and together with NRP1 as a novel target for tumor-suppressive therapy.
Keywords: Osteosarcoma, tissue microarray, Neuropilin, VEGF
Introduction
Osteosarcoma (OS) is the most frequent primary bone tumor with a peak incidence in the second decade of life. It is the major cause of cancer-related death in children in adolescence [1]. The introduction of multi-agent chemotherapy in the 1970s and refinements in the surgical techniques remarkably increased the long-term survival rate of patients with localized OS to approximately 60%. However, little has changed for patients with metastatic disease and their long-term survival rate remained at 25-30% [2]. Therefore, a more detailed understanding of the pathophysiological mechanisms in OS metastasis and of the biological roles of key regulators, considered as prognostic biomarkers, is needed for the development of new treatment strategies effectively targeting the complex metastatic processes in order to improve the outcome of OS patients with metastatic disease.
The Neuropilins (NRPs) NRP1 and NRP2 are type I transmembrane glycoproteins that have an important role in development, immunity and cancer [3-11]. NRP1 and NRP2 exhibit 44% amino acid sequence homology, share domains of similar structure and bind an overlapping set of ligands [3-6,10]. NRP1 and/or NRP2 are expressed in a variety of cells including neurons, endothelial cells, hepatocytes, melanocytes, osteoblasts, dendritic cells, thymocytes and regulatory T cells [6,12-18]. They form homo- and heterodimers [19] and, as coreceptors for various guidance molecules and growth factors, for example Semaphorin and proangiogenic VEGF isoforms (namely VEGF A), they enhance the biological effects of these effector molecules and are therefore important for axonal guidance and angiogenesis [20,21]. NRPs are capable to bind the different pro-angiogenic isoforms of VEGF and to recruit these ligands to the cell surface where they associate with VEGF receptors and form ternary VEGF/NRP/VEGFR complexes [20,22]. NRPs alone are unable to activate signalling pathways, but they promote the formation of and stabilize ternary VEGF/NRP/VEGFR signaling complexes [23-25].
Even though the role of Neuropilins in physiological processes is well described, less is known on their role in cancer biology. NRPs were reported to be important for VEGF dependent hyper-vascularisation in tumour angiogenesis [8]. The expression of NRPs varies considerably from one tumor to another, but their aberrant expression has been shown to promote tumorigenesis and metastasis in vivo in many solid tumors [5-7,26]. The expression of NRPs was shown to be critical for autocrine regulation of tumor cell activities including survival, growth and migration [27-30]. Since VEGF is considered a prime mediator of angiogenesis and is expressed in different isoforms, with some of them being ligands of NRPs, it is of great interest to investigate their expression in the light of Neuropilin mediated cancer progression. In OS, several studies have shown that the expression of VEGF in tumor tissue is a strong prognostic indicator for poor overall survival [31-33]. However, little is known about the expression and role of Neuropilins in OS progression. Therefore we investigated in the present study for the first time the expression of NRP1 and NRP2 in an OS tissue microarray and in a panel of osteosarcoma cell lines, and investigated in vitro the importance of Neuropilins in VEGFR signaling in the human metastatic 143B and HuO9-M132 OS cell lines.
Materials and methods
Charactersitics of OS patients
OS tissue samples were collected from 66 patients between June 1989 and June 2005 for diagnostic purposes The samples were fixed in 4% buffered formalin and embedded in paraffin. All patients were diagnosed with high-grade OS, according to valid WHO classifications. For detailed characteristics of the patients refer to Table 1. Most patients received standard neoadjuvant chemotherapy (52 were treated, 12 were not treated and 2 patients lack information about chemotherapy response) according to standard protocols (in course of COSS-91 and COSS-96) with methotrexate, cisplatin, ifosfamide and doxorubicin. Chemotherapy-induced tumor necrosis was evaluated according to the criteria of Salzer-Kuntschik [34]. Patients who showed tumor necrosis of 90% or higher were considered as responders, patients with tumor necrosis below 90% were classified as non-responders. The study was performed in accordance with the guidelines provided by the local ethic committee (approval reference number StV 41-2005).
Table 1.
Clinical characteristics of high grade osteosarcoma patients included in this study
| n | % | ||
|---|---|---|---|
| Totala | 66 | 100 | |
| NRP1 positivea | 7 | 11 | |
| NRP1 negativea | 58 | 89 | |
| NRP2 positive | 7 | 11 | |
| NRP2 negative | 59 | 89 | |
| Gender | female | 23 | 35 |
| male | 43 | 65 | |
| Age (years) | < 10 | 11 | 17 |
| 10-24 | 39 | 59 | |
| > 24 | 16 | 24 | |
| Tumor type | Osteoblastic | 45 | 68 |
| Chondroblastic | 11 | 17 | |
| Fibroblastic | 6 | 9 | |
| Telangiectatic | 4 | 6 | |
| Anatomical Site | Tibia/fibula/calcaneus | 21 | 32 |
| Femur | 24 | 36 | |
| Humerus/ulna | 6 | 9 | |
| Jaws/facies/vertebrae column/pelvis | 15 | 23 | |
| Chemotherapy response | Responders | 29 | 44 |
| Non-responders | 23 | 35 | |
| No neoadjuvant chemotherapy | 12 | 18 | |
| Missing information | 2 | 3 | |
| Metastasis | No metastasis | 43 | 65 |
| Total metastasis | 23 | 35 | |
| Metastasis at diagnosis | 5 | 8 | |
| Metastasis after diagnosis | 18 | 27 |
The total number of patients evaluated for NRP1 staining = 65.
Tissue microarray and immunohistochemistry
Tissue cores with a diameter of 0.6 mm were isolated from paraffin-embedded formalin fixed tissue and transferred into a recipient paraffin block as described earlier [35,36]. Tissue cores were selected by an experienced pathologist based on haematoxylin and eosin (H&E) stainings of tissue sections. A minimum of two cores per sample were selected, representing the tumor heterogeneity with more than 95% reliability [6,37].
Two µm or 4.5 µm sections of the tissue microarray (TMA) were de-paraffined in xylene and heated with the pretreatment buffer according to standard immunohistochemical protocols. The following primary antibodies were used for immunostaining: Rabbit polyclonal anti-NRP1 (1:50, ECM Biosciences LLC, Versailles, KY, USA) and rabbit polyclonal anti-NRP2 (H-300, 1:50, Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Primary antibodies were detected using standard indirect immunoperoxidase reactions and 3,3’-Diaminobenzidine (DAB) tetrahydrochloride as a substrate. Antibody binding was visualized with a iVIEW DAB Kit (Ventana Medical Systems Tucson, Arizona, USA) yielding a brown precipitate.
Analysis of the TMA was performed in a semi-quantitative manner. We developed our own MATLAB-analysis software tool (R2010b; Mathworks Inc., Natick, MA, USA) based on the principle of color deconvolution by Ruifrok and Johnston [38]. This tool was used to grade the immunostainings according to Gvozdenovic et al. [39]. Briefly, non-detectable immunostaining was considered as grade 1 whereas“weak staining” was defined as grade 2 and “strong staining” as grade 3. Based on the grading of 2-3 cores per tissue, the average staining intensity was calculated and an average grading above 1.5 was classified as NRP1/NRP2 positive and any value lower or equal than 1.5 was considered NRP1/NRP2 negative.
Statistical analysis
Overall survival, progression-free and metastases-free survival were calculated using Kaplan–Meier curves and statistical significance was assessed by log-rank tests. Overall survival was defined as percentage of patients who were alive at the latest follow-up. Progression-free survival (PFS) was defined as the percentage of patients who did not show a progression of disease between completion of the primary treatment and the last follow-up and metastases-free survival (MFS) denoted the percentage of patients who were free of metastases at the last follow-up.
Cell lines
MG-63 cells were kindly provided by Dr. G. Sarkar (Mayo Clinic, Rochester, MN), and MG63 M6 and M8 cells by Dr. W. T. Zhu (Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China). HuO9 cells and the sublines H3, M112, M132 were obtained from Dr. M. Tani (National Cancer Center Hospital, Tokyo, Japan). HOS, MNG/HOS and 143B cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD). The cell lines MG63 and HOS as well as their derivatives were grown in Dulbecco’s Modified Eagle Medium (DMEM) and F12 medium (Gibco, 1:1) supplemented with 10% FCS. HuO9 cells and the respective sublines HuO9-H3, -M112 and M132 were cultured in RPMI 1640 medium (Gibco, Basel, Switzerland), supplemented with 10% heat-inactivated fetal calf serum (FCS) and 1% glutamine (Invitrogen). Cells were cultured at 37°C in an atmosphere of 5% CO2 and 95% relative humidity.
Isolation of RNA and cDNA synthesis
Total RNA was isolated from cells grown to approximately 80% confluence with TRI reagent (Sigma-Aldrich, St. Louis, MO) as recommended by the supplier. The RNA was quantified by optical density measurements at 260 and 280 nm in a UV-spectrometer (Beckman Instruments Inc., Fullerton, CA). The integrity of RNA was assessed by standard agarose gel electrophoresis. cDNA was reverse transcribed from 1 μg of total RNA with the Stratagene first strand synthesizing system and random primers (Stratagene, La Jolla, CA) according to the protocol supplied by the manufacturer.
Cloning of shRNA constructs
Oligonucleotide sequences 5’-ATGCGAATGGCTGATTCAG-3’ and 5’-CTGGAGAACATATATGC-3’ designed to target NRP1 and NRP2 transcripts, respectively, were cloned into the pSIREN-RetroQ retroviral vector (Clontech Laboratories, USA). A control vector containing a scrambled oligonucleotide sequence was provided by the manufacturer. 143B cells were infected with these vectors and neomycin resistant cells were selected. The efficiency of NRP1 and NRP2 silencing was examined on Western blots of total cell extracts.
Polymerase chain reaction
PCR was performed in a total volume of 50 μl containing 2 μl of cDNA, equivalent to a concentration of 5 ng/μl of RNA in the reverse transcription (RT) reaction, 5 μl of 10x Taq Buffer advanced (Eppendorf, Hamburg, Germany), 1 μl of dNTPs (10 mM), 0,3 μl of each primer (0.2 μM), 0.5 μl (2.5 U) Taq DNAPolymerase (Eppendorf) with initial denaturation at 94°C for 3 min followed by 35 cycles of denaturation at 94°C for 40 s, annealing at the primer-dependent temperature for 40 s and elongation at 72°C for 20 s. PCR was completed by elongation at 72°C for 7 min. The annealing temperatures were 67°C for NRP1 and NRP2, 67°C for VEGFR-1 and VEGFR-2 64°C for VEGF and 66°C for GAPDH. The sequences of primers are shown in Supplemental Table 1. PCR products were analysed by 1.5% agarose gel electrophoresis at 120 V for approximately 90 min in the presence of ethidium bromide (0.1%).
RT/PCR products of transcripts encoding the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as controls for cDNA input in individual PCR reactions.
Western blot analysis
Proteins were extracted from indicated cell lines and immunoblotted as reported [40]. Rabbit antibodies to VEGF-A, VEGFR1, VEGFR2, NRP1, NRP2, phospho-Akt, Akt (Cell Signaling Technology, Danvers, MA, USA) and mouse antibodies to β-actin (Santa Cruz) were used at final dilutions of 1:1000. Subsequently, immunoblots were incubated at RT for 1 h with secondary horseradish peroxidase conjugated anti-rabbit or anti mouse IgG (Santa Cruz Biotechnology Inc., Heidelberg, Germany) at final dilutions of 1:2000. The proteins were then visualized with Immobilon chemiluminescence substrate (Millipore, Billerica) and quantified with a VersaDoc™ Imaging System (Bio-Rad Laboratories, Munich, Germany). In VEGF stimulation and NRP1 and -2 blocking experiments, the cells were pre-incubated at 37°C for 1 h with 20 µg/ml of NRP1 or NRP2 blocking antibodies (RD systems, USA) and subsequently stimulated at 37°C for 5 min with 10 ng/ml VEGF-A165 (Cell Signaling Technology, Danvers, MA, USA) in the presence of the blocking antibodies. The cells were then washed once with ice cold PBS and processed as described above.
Results
NRP2 expression in primary tumor tissue correlates with poor prognosis for osteosarcoma patients
Osteosarcomas are highly vascularized tumors and several studies showed correlations between VEGF and VEGFR expression and poor survival [31-33]. Based on the relevance of Neuropilins for VEGFR-1 signaling, they can be considered as potential prognostic markers in osteosarcoma. Consequently, we analyzed, by immunohistochemistry, the expression of NRP1 and NRP2 in primary tumor tissue on an OS tissue microarray (TMA) and performed Kaplan-Meier survival analyses.
Characteristics of the patients included in the TMA are summarized in Table 1. Sixty-five percent of the patients investigated were male and the largest subgroup of patients was that of adolescents of between 10 and 24 years of age (59%) followed by adults (24%, range: 27-66 years of age) and children (17%, range: 2-9 years of age). The majority of patients (68%) included in this study suffered from osteoblastic OS, and primary tumors were mainly located in long bones such as the femur (36%) or the tibia/fibula/calcaneus (32%). Seventy-nine percent of the patients received neoadjuvant chemotherapy. In the remaining group of patients, some were not treated because of their condition and for others the clinical information was missing. Furthermore, 65% of the patients remained metastases-free during the course of the disease, 8% presented with metastases at diagnosis and 27% developed metastases during follow up. Tissue specimens of 65 patients could be analyzed for immunoreactive NRP1 and/or NRP2. In the tumor of one additional patient only NRP2 immunostaining could be assessed. NRP1 immunostaining was found in tumors of 7 patients (11%) and an equal number of patients had tumors that stained for NRP2.
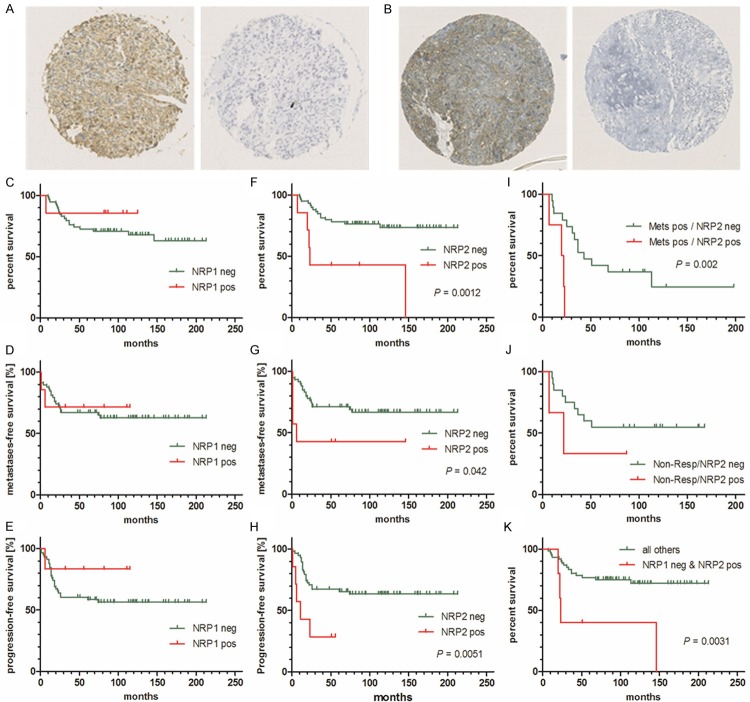
TMA tissue cores representative for positive or negative NRP1 and NRP2 immunostaining are shown in Figure 1A and 1B, respectively. Both, Figure 1A and 1B show in brown, extra-nuclear, positive staining, and staining considered as negative appears blue. A supplementary Figure (Supplemental Figure 1) shows representative high-resolution images of weak, moderate and strong NRP1 and NRP2 immunostaining of TMA cores. In Figure 1 is illustrated overall survival (Figure 1C and 1F), metastases-free (Figure 1D and 1G) and progression-free survival (Figure 1E and 1H) of patients who showed either positive or negative staining for NRP1 or for NRP2, respectively. Overall, metastasis-free and progression-free survival of patients with NRP1 positive tumors was not significantly different from that of patients with NRP1 negative tumors. However, patients with tumors that showed positive NRP2 immunostaining had a significantly shorter overall (P = 0.0012), metastases-free (P = 0.042) and progression-free survival (P = 0.0051) than those with NRP2 negative tumors, suggesting that the expression of NRP2 in OS primary tumors is a robust predictor for poor survival. Therefore, Kaplan-Meier survival analyses based on NRP2 immunostaining of primary tumors were extended to subgroups of patients. These included patients with metastatic disease and non-responders to chemotherapy. Interestingly, patients with metastases and positive immunostaining for NRP2 in primary tumors exhibited a significantly shorter survival than those with metastases and NRP2 negative tumors (Figure 1I). The data also showed a tendency to shorter survival of non-responders with NRP2 positive tumors compared to non-responders with NRP2 negative tumors, but the difference was not statistically significant (Figure 1J). The predictive power of NRP2 for OS patient survival was further emphasized by the results presented in Figure 1K. Interestingly, patients with tumors staining positive for NRP2 but negative for NRP1 had a significantly (P = 0.0031) shorter survival than all other patients with NRP1+/NRP2+, NRP1+/NRP2-, NRP1-/NRP2- staining patterns in their primary tumors. All findings taken together imply that the expression of NRP2, unlike that of NRP1, in OS primary tumors correlates with a poor prognosis for the patients, irrespective of metastatic or non-metastatic disease, the as of yet best established predictor for OS outcome.
Figure 1.
Prognostic power of NRP1 and NRP2 immunohistochemistry in a human osteosarcoma tissue microarray assessed by Kaplan-Meier survival analysis: Representative images of NRP1 (A) and NRP2 (B) positive and negative tissue cores, respectively. Overall survival (C), metastases free survival (D) and progression free survival (E) of patients with detectable or non-detectable NRP1 expression in primary tumor tissue. Overall survival (F), metastases free survival (G) and progression free survival (H) of patients with detectable or non-detectable NRP2 expression in primary tumor tissue. (I) OS patients with metastatic disease and detectable NRP2 have a shorter survival than those with non-detectable NRP2. (J) OS patients with a poor response to neoadjuvant chemotherapy (non-responders) and detectable NRP2 have a shorter survival than non-responders with non-detectable NRP2. (K) OS patients with detectable NRP2 and non-detectable NRP1 in their primary tumors have a shorter survival than all other patients (with and without metastases and responders and non-responders) with other patterns of NRP1 and NRP2 expression.
Neuropilin 1 and Neuropilin 2 are differentially expressed in osteosarcoma cell lines
Neuropilins have been reported to play an important role as co-receptors for VEGF in tumor growth and metastasis [22]. In the present study, the expression pattern of NRP1 and NRP2 was investigated in several OS cell line systems consisting of parental cell lines with low metastatic potential and corresponding derivative sub-lines with high metastatic potential. The different OS cell lines showed quite a heterogeneous pattern of NRP1 and NRP2 expression at the mRNA (Figure 2A) as well as at the protein level (Figure 2B). Interestingly, the highly metastatic sub-lines, except for the MG63 derivatives, showed higher expression of Neuropilins than their parental cell lines, particularly at the protein level. In addition, the highly metastatic 143B and the HuO9-H3, -M112 and -M132 cell lines showed higher levels of both NRP1 and NRP2 protein components than the respective parental HOS and HuO9 cell lines, which points to potentially important roles of NRP1 and NRP2 in the metastatic process in OS.
Figure 2.
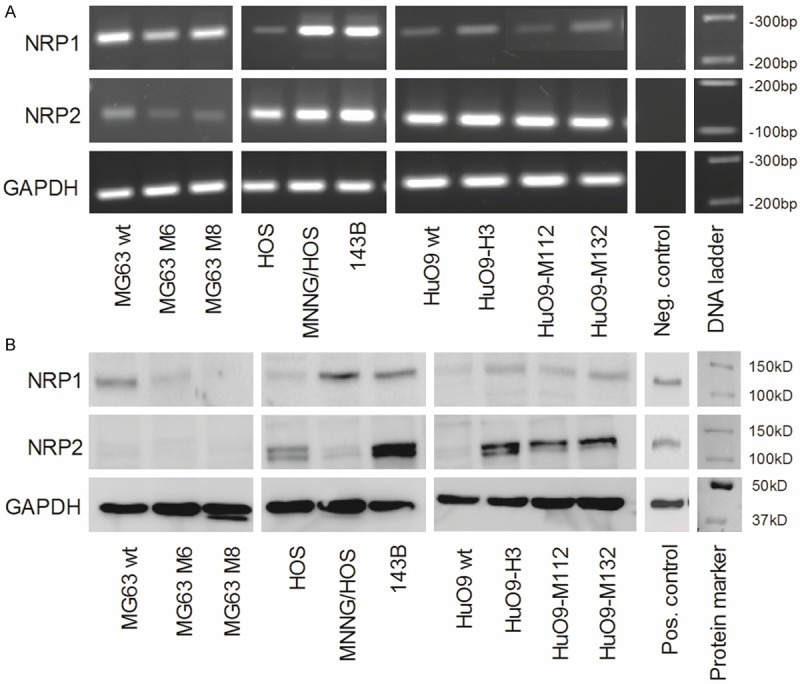
Differential expression of NRP1 and NRP2 in human osteosarcoma cell lines at the mRNA and protein level: A. Semi-quantitative RT-PCR analysis of NRP1, NRP2 and GAPDH (reference) encoding RNA in total RNA isolated from parental low-metastatic MG63, HOS and HuO9 and respective metastatic MG63M6 and MG63M8, MNNG/HOS and 143B, and HuO9-H3, HuO9-M112 and HuO9-M132 sublines. B. Western blot analysis of NRP1 and NRP2 levels in total protein extracts of indicated cell lines, β actin was used as protein loading control.
VEGF and VEGF receptors are expressed in osteosarcoma cell lines
In view of postulated malignancy enhancing properties of NRP1 and -2 as co-receptors of VEGF in interaction with VEGFR-1 and VEGFR-2 [22], we next analyzed the expression of VEGF A, which has been thoroughly described as an important ligand for Neuropilin 1 and 2 [22,41], and of its receptors VEGFR-1 and VEGFR-2 in the osteosarcoma cell lines investigated in the present study (Figure 3). VEGF A exists as different isoforms that are encoded by alternatively spliced primary VEGF A gene transcripts. Among the different splice variants of VEGF A, we analyzed the expression of the isoforms VEGF-A121, 145, 165 and 206 that contain domains required for the interaction with Neuropilins. Among these splice variants, transcripts for VEGF-A165, which is known to be the most potent VEGF A isoform [41], were uniformly expressed together with those encoding VEGF-A121 in all 10 osteosarcoma cell lines (Figure 3A). These findings were consistent with comparable levels of VEGF A immunoreactive components detected on Western blots of protein extracts of the 10 cell lines investigated (Figure 3B). Similarly, the VEGFR-1 was also found expressed at the transcript and the protein level in all tested cell lines and, interestingly, with a tendency for higher levels in the more aggressive cell lines (Figure 3A and 3B). VEGFR-2 encoding mRNA, on the other hand, was only detected in the three cell lines of the HOS system and, unexpectedly, it remained un-detectable at the protein level.
Figure 3.
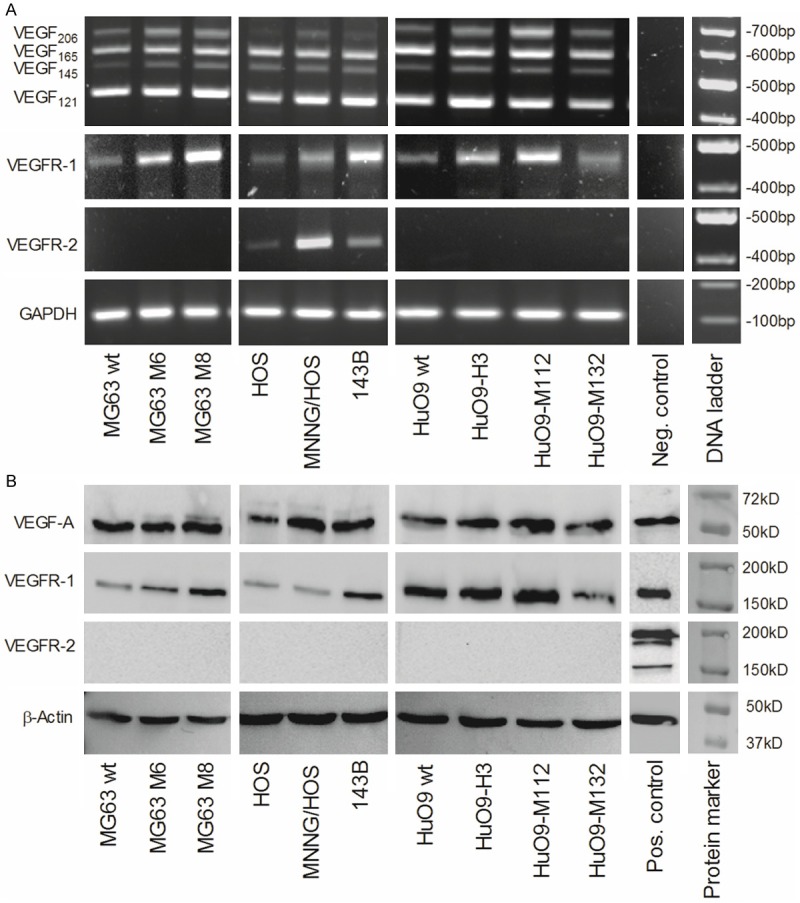
Differential expression of VEGFA and VEGF receptors in human osteosarcoma cell lines at the mRNA and protein level: A. Semi quantitative RT-PCR analysis of the expression of VEGFA isoform, VEGFR1 and VEGFR2 and GAPDH (reference) encoding RNA in total RNA isolated from parental low-metastatic MG63, HOS and HuO9 and respective metastatic MG63M6 and MG63M8, MNNG/HOS and 143B, and HuO9-H3, HuO9-M112 and HuO9-M132 sublines. B. Western blot analysis of VEGFA, VEGFR1 and VEGFR2 levels in total protein extracts of indicated cell lines, β-actin was used as a protein loading control.
Antibody blocking and/or downregulation by shRNA of NRP1/NRP2 in OS cell lines inhibits VEGFR1-mediated AKT phosphorylation
Even though Neuropilins are not linked to intracellular signaling cascades, it has been shown in various cell types that the Neuropilins, when associated as co-receptors with VEGF receptors, facilitate downstream signaling of VEGFR/VEGF/NRP ternary complexes [9,22,24]. NRP1 or NRP2 blocking antibodies that interfere with VEGF binding and thereby prevent the formation of VEGFR1/VEGF/NRP ternary complexes are expected to inhibit VEGFR1-mediated downstream signaling. To test this hypothesis, we pre-incubated serum-starved 143B cells in the absence and presence of NRP1 or NRP2 blocking antibodies and subsequently stimulated the cells with 10 ng/ml VEGF-A165 and analyzed AKT phosphorylation and NRP1 and NRP2 expression on Western blots (Figure 4A). VEGF stimulated the phosphorylation of AKT approximately 4-fold and pre-incubation of the cells with 20 µg/ml NRP1 antibody reduced the response significantly (P ≤ 0.05) by approximately 30%. Interestingly, pre-incubation with 20 µg/ml NRP2 blocking antibodies had no inhibitory effect on VEGF-evoked AKT phosphorylation in 143B cells. However, in HuO9-M132 cells, the result was just the opposite (Figure 4B). In these cells, VEGF stimulated AKT phosphorylation approximately 3-fold and pre-incubation with 20 µg/ml NRP2 antibodies inhibited the response significantly (P ≤ 0.05) by approximately 30%. NRP1 blocking antibodies, however, had no effect on VEGF-stimulated AKT phosphorylation. In both, the 143B and the HuO9-M132 cell lines, the treatment with NRP1 or NRP2 antibodies did not affect respective receptor expression levels (Figure 4A and 4B). The importance of NRP1 and NRP2 in VEGF-stimulated AKT phosphorylation in 143B cells was further investigated in cells in which the expression of NRP1 or NRP2 was selectively suppressed by stable expression of respective specific shRNAs. In these 143B-shNRP1 and 143B-shNRP2 cell lines, we repeated the experiments carried out with the non-manipulated 143B cells described above and used scrambled shRNA expressing 143B cells as a control (Figure 4C and 4D). Stable expression of NRP1-targeting shRNA in 143B cells reduced the level of NRP1 expression to approximately 20% of that detected in scrambled shRNA transduced 143B cells (control cells) and decreased, in parallel and significantly (P ≤ 0.05, n = 3), the VEGF-A165-stimulated phosphorylation of AKT to approximately 60% of that observed in control cells (Figure 4C) and to a level that was approximately 3-times higher than that in non-stimulated 143B-shNRP1 cells. Antibody blocking of NRP2 in these cells further reduced VEGF-A165 stimulated AKT phosphorylation significantly (P ≤ 0.05) by approximately 25% compared to non-blocked cells. In 143B cells that stably expressed NRP2-targeting shRNA the level of NRP2 expression was downregulated to approximately 10% of that detected in scrambled shRNA transduced 143B cells (control cells). Interestingly, the stimulation of AKT phosphorylation by 10 ng/ml VEGF-A165 in 143B-shNRP2 cells was only decreased to 80% of that observed in control cells, which was approximately 4-times higher than in non-stimulated 143B-shNRP2 cells. Antibody blocking of NRP1 in 143B-shNRP2 cells further reduced VEGF-A165 stimulated AKT phosphorylation significantly (P ≤ 0.05) by approximately 50% compared to non-blocked cells. Thus, shRNA-mediated downregulation of either NRP1 or NRP2 combined with antibody-blocking of the other NRP in 143B cells inhibited VEGF-A165 stimulated AKT phosphorylation similarly but not completely. Further targeting NRP1 alone by either shRNA or a blocking antibody was more effective than targeting NRP2 alone.
Figure 4.
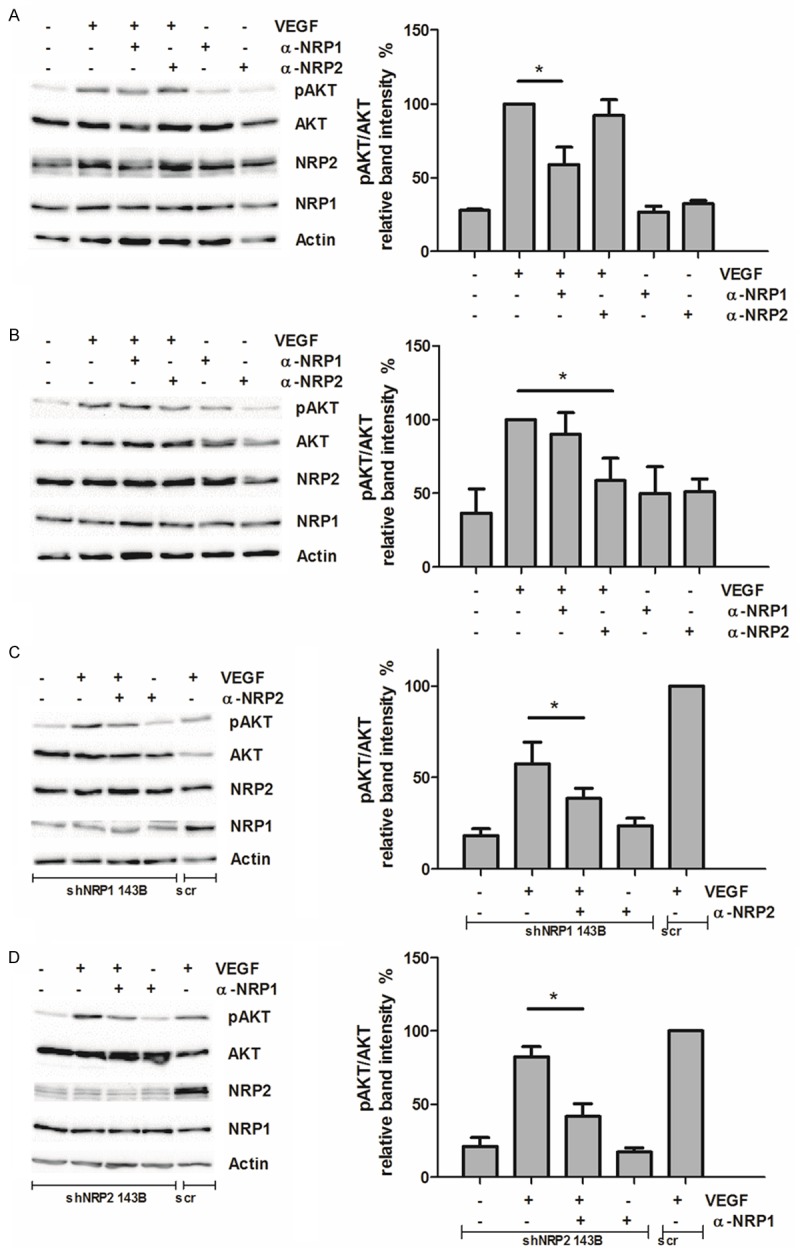
VEGF receptor activation stimulates NRP1/NRP2 dependent phosphorylation of AKT: Cells, serum-starved for 48 h and preincubated for 1 h with 20 µg/ml antibodies blocking NRP1 (α-NRP1) or NRP2 (α-NRP2), were stimulated with 10 ng/µl VEGF for 5 min. Western blot analysis (left) of AKT phosphorylation and expression of NRP1 and NRP2 after incubation in the absence and presence of VEGF and of α-NRP1 or α-NRP2 and densitometric quantification of phospho-AKT on Western blots of 3 independent experiments (*p ≤ 0.05) carried out with the indicated cell lines (right): (A) 143B cells, (B) HuO9-M132 cells, (C) 143B cells transduced with NRP1 silencing (shNRP1) or control (scr) RNA, (D) 143B cells transduced with NRP2 silencing (shNRP2) or scr RNA.
Discussion
Neoadjuvant chemotherapy in OS increased the long-term survival of patients with localized disease considerably from between 20 and 30% to approximately 60%, but metastasizing OS remained largely treatment resistant. Consequently, there is a need for reliable outcome predictive markers in primary tumor tissue and for more effective, tumor-selective and -tailored treatment modalities particularly in metastasizing OS.
Several recent studies showed that the expression of VEGF in OS tumor tissue is a strong prognostic indicator for poor overall survival of affected patients [31-33], but biological roles of VEGF signalling targets and components in OS have so far not been investigated. NRPs have been reported to be important for VEGF dependent hyper-vascularisation in tumour angiogenesis [8] and they were found to act as co-receptors for different pro-angiogenic isoforms of VEGF in ternary VEGF/NRP/VEGFR complexes [20,22]. In the present study, NRP1 and NRP2 were therefore evaluated as additional prognostic indicators and potentially novel tumour-selective treatment targets in OS.
The analysis of NRP1 and/or NRP2 immunostaining in a tissue micro array of 66 OS patients indeed identified NRP2 as an indicator of poor overall, metastases-free and progression-free survival, whereas NRP1 immunostaining had no predictive value. Interestingly, a recent study with RNA isolated from tumor tissue of 30 OS patients also revealed a correlation between increased NRP2 mRNA expression and poor patient survival [42]. More detailed Kaplan-Meier analyses of the TMA results in the present study even indicated that patients with tumors that expressed NRP2 in the absence of NRP1 had a significantly worse prognosis than the patients with NRP1 and -2 negative, NRP1 positive or NRP1 and -2 positive tumors. Moreover, patients with overt metastases and with NRP2 immunostaining in their primary tumors had a significantly shorter survival rate than patients with metastases but NRP2-negative tumors. This observation is particularly interesting because the presence of metastases in OS patients is well established as the most reliable predictor for poor survival. Thus, the results of this and the study by Handa et al. [42] suggest a crucial role of NRP2 in OS tumor progression and metastasis. This conclusion, for unexplained reasons, is however inconsistent with the results of a recent study that described NRP1 as an important prognostic marker in OS [43].
To further explore functions of NRP1 and NRP2 in VEGF signalling in OS and a putative biological relevance in OS progression and metastasis, we investigated the expression profiles of the neuropilins and of VEGF isoforms and receptors in several osteosarcoma cell line systems consisting of cell lines with low or high metastatic phenotypes. Interestingly, in two out of three cell line systems and in seven out of the ten cell lines investigated, the expression of both NRP1 and NRP2 at the mRNA and the protein level correlated to a variable degree with the metastatic potential of the respective cell line, suggesting a biological relevance of NRP1 and NRP2 in the metastatic process. A correlation between NRP expression and tumor aggressiveness and metastatic potential was also observed in a variety of solid tumors. Up-regulated expression of NRP1 correlated with metastatic phenotypes of prostate tumors [44] and in human colon cancer NRP1 plays a role in migration of cancer cells [45]. In non-small cell lung carcinoma co-expression of Neuropilin 1 and Neuropilin 2 correlated significantly with a poor prognosis for the patients [46]. A similar observation was made in other tumors such as renal cancer where NRP2 was found indicative for metastasis and overall survival [47].
NRP1 and NRP2 alone have been reported to be unable to mediate VEGF signaling. However, upon binding of particular proangiogenic VEGF A isoforms, NRPs bind to VEGFR both directly and via VEGF thereby forming a ternary complex. Even though VEGFA/VEGFR complexes alone are able to activate the AKT signaling cascade, additional binding of NRPs results in the formation of a stable ternary complex that enhances VEGFR signaling. It is important to note that in endothelial cells NRP1 is able to activate AKT signaling in the absence of the VEGFR2, which points to additional mechanisms of VEGF signaling in this particular cell type [48]. Here we have investigated in the panel of the ten OS cell lines the expression of the VEGFRs 1 and - 2 and of the VEGFA isoforms VEGF 121, 145, 165 and 206 known to bind to Neuropilins [22]. Among the four VEGFA splice variants, VEGF 121 and 165 encoding transcripts were rather uniformly expressed in all cell lines investigated, whereas VEGF 154 and 206 transcripts were expressed more heterogeneously and at lower levels. The findings were consistent with rather uniform levels of VEGFA immunoreactive components detected in extracts of all cell lines. The uniform and ubiquitous expression of VEGF 165 is of particular interest because it is known as the most potent VEGFA isoform and the only one which interacts with both NRP1 and -2 [22,41]. The observed robust expression of VEGFA isoforms in OS cell lines is also of relevance because it has been shown that up-regulated expression of VEGFA promotes metastasis in various tumor types [49]. At the level of receptors, VEGFR1 was found expressed at the transcript and protein levels in all cell lines examined with a tendency for higher expression levels in the metastatic sublines. RT/PCR products amplified from VEGFR2 transcripts were only recognized in the HOS cell line system, but, surprisingly, protein products remained undetectable. Altogether, the analysis in the three OS cell line systems showed robust expression of proangiogenic VEGFA isoforms and of the receptor components needed for efficient VEGF signaling particularly in the highly metastatic cell lines.
Consequently, such cell lines provided ideal experimental systems to investigate the functional relevance of NRP1 and -2 in VEGF signal transduction. This was examined in the present study with NRP1 and -2 ligand-blocking antibodies in the metastatic 143B and HuO9-M132 cell lines and by shRNA mediated downregulation of NRP1 and -2 combined with the ligand-blocking antibodies in the 143B cell line. Interestingly, in 143B cells VEGFA signaling, monitored by AKT phosphorylation, was more effectively inhibited by blocking or downregulation of NRP1 than of NRP2, whereas in HuO9-M132 cells NRP2 blocking antibodies were more effective than antibodies blocking NRP1. This finding in HuO9-M132 cells was consistent with considerably higher expression levels of NRP2 than of NRP1, whereas in 143B cells NRP1 and -2 were expressed at comparable levels. Consequently, inhibition of VEGFA signaling by combined antibody blocking and shRNA-mediated downregulation of NRP1 and NRP2 was carried out in 143B cells. These experiments demonstrated that NRP1 targeting in 143B cells inhibited AKT phosphorylation more effectively than blocking or downregulation of NRP2, confirming the result of the antibody blocking experiment. The data also indicated that NRP1 and -2 can substitute each other in the functional interaction with VEGFR1 with an apparently predominant role of NRP1 in 143B cells.
In conclusion, the expression of NRP2 in OS primary tumors was found to be a strong indicator for a poor prognosis for the patients. This even applied to patients with metastatic disease, to date the strongest predictor of survival in OS patients. Selective targeting of NRP1 or -2 with respective blocking antibodies in OS cell lines showed a cell line-dependent modulation of VEGF signaling by the individual NRPs. Putative distinct roles of NRP1 and NRP2 in OS pathophysiology remain to be investigated in more detail in future studies. The observed co-expression of NRP1, -2 and VEGFR1 in OS cell lines points to tumor promoting mechanisms of this signaling system other than VEGF-dependent hyper-vascularization in tumor angiogenesis. Such mechanisms may include a reported NRP2-mediated upregulation of metastatic genes through a mechanism involving β-catenin [50]. This and the results of the present and other recent studies point to NRP2 as a powerful prognostic marker in OS and, together with NRP1, as a novel target for tailored tumor-suppressive treatment.
Acknowledgements
Our work is supported by the University of Zurich, the Schweizerischer Verein Balgrist (Zurich, Switzerland), the Walter L. & Johanna Wolf Foundation (Zurich, Switzerland), the Highly Specialized Medicine for Musculoskeletal Oncology program of the Canton of Zurich, the Zurcher Krebsliga (Zurich, Switzerland), and the Swiss National Science Foundation SNF Nr.310030_149649.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Wang LL. Biology of osteogenic sarcoma. Cancer J. 2005;11:294–305. doi: 10.1097/00130404-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment - Where do we stand? A state of the art review. Cancer Treat Rev. 2014;40:523–32. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008;411:211–226. doi: 10.1042/BJ20071639. [DOI] [PubMed] [Google Scholar]
- 4.Uniewicz KA, Fernig DG. Neuropilins: a versatile partner of extracellular molecules that regulate development and disease. Front Biosci. 2008;13:4339–4360. doi: 10.2741/3008. [DOI] [PubMed] [Google Scholar]
- 5.Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res. 2006;312:584–593. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Ellis LM. The role of neuropilins in cancer. Mol Cancer Ther. 2006;5:1099–1107. doi: 10.1158/1535-7163.MCT-05-0538. [DOI] [PubMed] [Google Scholar]
- 7.Guttmann-Raviv N, Kessler O, Shraga-Heled N, Lange T, Herzog Y, Neufeld G. The neuropilins and their role in tumorigenesis and tumor progression. Cancer Lett. 2006;231:1–11. doi: 10.1016/j.canlet.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 8.Bagri A, Tessier-Lavigne M, Watts RJ. Neuropilins in tumor biology. Clin Cancer Res. 2009;15:1860–1864. doi: 10.1158/1078-0432.CCR-08-0563. [DOI] [PubMed] [Google Scholar]
- 9.Staton CA, Kumar I, Reed MW, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007;212:237–248. doi: 10.1002/path.2182. [DOI] [PubMed] [Google Scholar]
- 10.Koch S. Neuropilin signalling in angiogenesis. Biochem Soc Trans. 2012;40:20–25. doi: 10.1042/BST20110689. [DOI] [PubMed] [Google Scholar]
- 11.Zachary IC. How neuropilin-1 regulates receptor tyrosine kinase signalling: the knowns and known unknowns. Biochem Soc Trans. 2011;39:1583–1591. doi: 10.1042/BST20110697. [DOI] [PubMed] [Google Scholar]
- 12.Wild JR, Staton CA, Chapple K, Corfe BM. Neuropilins: expression and roles in the epithelium. Int J Exp Pathol. 2012;93:81–103. doi: 10.1111/j.1365-2613.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jubb AM, Strickland LA, Liu SD, Mak J, Schmidt M, Koeppen H. Neuropilin-1 expression in cancer and development. J Pathol. 2012;226:50–60. doi: 10.1002/path.2989. [DOI] [PubMed] [Google Scholar]
- 14.Jubb AM, Sa SM, Ratti N, Strickland LA, Schmidt M, Callahan CA, Koeppen H. Neuropilin-2 expression in cancer. Histopathology. 2012;61:340–349. doi: 10.1111/j.1365-2559.2012.04224.x. [DOI] [PubMed] [Google Scholar]
- 15.Corbel C, Lemarchandel V, Thomas-Vaslin V, Pelus AS, Agboton C, Romeo PH. Neuropilin 1 and CD25 co-regulation during early murine thymic differentiation. Dev Comp Immunol. 2007;31:1082–1094. doi: 10.1016/j.dci.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Lepelletier Y, Smaniotto S, Hadj-Slimane R, Villa-Verde DM, Nogueira AC, Dardenne M, Hermine O, Savino W. Control of human thymocyte migration by Neuropilin-1/Semap-horin-3A-mediated interactions. Proc Natl Acad Sci U S A. 2007;104:5545–5550. doi: 10.1073/pnas.0700705104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendes-da-Cruz DA, Lepelletier Y, Brignier AC, Smaniotto S, Renand A, Milpied P, Dardenne M, Hermine O, Savino W. Neuropilins, semaphorins, and their role in thymocyte development. Ann N Y Acad Sci. 2009;1153:20–28. doi: 10.1111/j.1749-6632.2008.03980.x. [DOI] [PubMed] [Google Scholar]
- 18.Dzionek A, Inagaki Y, Okawa K, Nagafune J, Rock J, Sohma Y, Winkels G, Zysk M, Yamaguchi Y, Schmitz J. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum Immunol. 2002;63:1133–1148. doi: 10.1016/s0198-8859(02)00752-8. [DOI] [PubMed] [Google Scholar]
- 19.Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell. 2011;22:2766–2776. doi: 10.1091/mbc.E09-12-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker MW, Guo HF, Li X, Linkugel AD, Vander Kooi CW. Function of members of the neuropilin family as essential pleiotropic cell surface receptors. Biochemistry. 2012;51:9437–9446. doi: 10.1021/bi3012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prud’homme GJ, Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget. 2012;3:921–939. doi: 10.18632/oncotarget.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shraga-Heled N, Kessler O, Prahst C, Kroll J, Augustin H, Neufeld G. Neuropilin-1 and neuropilin-2 enhance VEGF121 stimulated signal transduction by the VEGFR-2 receptor. FASEB J. 2007;21:915–926. doi: 10.1096/fj.06-6277com. [DOI] [PubMed] [Google Scholar]
- 24.Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85:357–368. doi: 10.1002/jcb.10140. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 26.Klagsbrun M, Takashima S, Mamluk R. The role of neuropilin in vascular and tumor biology. Adv Exp Med Biol. 2002;515:33–48. doi: 10.1007/978-1-4615-0119-0_3. [DOI] [PubMed] [Google Scholar]
- 27.Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]
- 28.Cao Y, E G, Wang E, Pal K, Dutta SK, Bar-Sagi D, Mukhopadhyay D. VEGF exerts an angiogenesis-independent function in cancer cells to promote their malignant progression. Cancer Res. 2012;72:3912–3918. doi: 10.1158/0008-5472.CAN-11-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–5233. [PubMed] [Google Scholar]
- 30.Bagci T, Wu JK, Pfannl R, Ilag LL, Jay DG. Autocrine semaphorin 3A signaling promotes glioblastoma dispersal. Oncogene. 2009;28:3537–3550. doi: 10.1038/onc.2009.204. [DOI] [PubMed] [Google Scholar]
- 31.Hassan SE, Bekarev M, Kim MY, Lin J, Piperdi S, Gorlick R, Geller DS. Cell surface receptor expression patterns in osteosarcoma. Cancer. 2012;118:740–749. doi: 10.1002/cncr.26339. [DOI] [PubMed] [Google Scholar]
- 32.Lammli J, Fan M, Rosenthal HG, Patni M, Rinehart E, Vergara G, Ablah E, Wooley PH, Lucas G, Yang SY. Expression of Vascular Endothelial Growth Factor correlates with the advance of clinical osteosarcoma. Int Orthop. 2012;36:2307–2313. doi: 10.1007/s00264-012-1629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu XW, Wu TY, Yi X, Ren WP, Zhou ZB, Sun YQ, Zhang CQ. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumour Biol. 2014;35:155–60. doi: 10.1007/s13277-013-1019-1. [DOI] [PubMed] [Google Scholar]
- 34.Salzer-Kuntschik M, Brand G, Delling G. [Determination of the degree of morphological regression following chemotherapy in malignant bone tumors] . Der Pathologe. 1983;4:135–141. [PubMed] [Google Scholar]
- 35.Bubendorf L, Nocito A, Moch H, Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001;195:72–79. doi: 10.1002/path.893. [DOI] [PubMed] [Google Scholar]
- 36.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 37.Camp RL, Charette LA, Rimm DL. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000;80:1943–1949. doi: 10.1038/labinvest.3780204. [DOI] [PubMed] [Google Scholar]
- 38.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- 39.Gvozdenovic A, Arlt MJ, Campanile C, Brennecke P, Husmann K, Li Y, Born W, Muff R, Fuchs B. CD44 enhances tumor formation and lung metastasis in experimental osteosarcoma and is an additional predictor for poor patient outcome. J Bone Miner Res. 2013;28:838–847. doi: 10.1002/jbmr.1817. [DOI] [PubMed] [Google Scholar]
- 40.Sabile AA, Arlt MJ, Muff R, Bode B, Langsam B, Bertz J, Jentzsch T, Puskas GJ, Born W, Fuchs B. Cyr61 expression in osteosarcoma indicates poor prognosis and promotes intratibial growth and lung metastasis in mice. J Bone Miner Res. 2012;27:58–67. doi: 10.1002/jbmr.535. [DOI] [PubMed] [Google Scholar]
- 41.Woolard J, Bevan HS, Harper SJ, Bates DO. Molecular diversity of VEGF-A as a regulator of its biological activity. Microcirculation. 2009;16:572–592. doi: 10.1080/10739680902997333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Handa A, Tokunaga T, Tsuchida T, Lee YH, Kijima H, Yamazaki H, Ueyama Y, Fukuda H, Nakamura M. Neuropilin-2 expression affects the increased vascularization and is a prognostic factor in osteosarcoma. Int J Oncol. 2000;17:291–295. doi: 10.3892/ijo.17.2.291. [DOI] [PubMed] [Google Scholar]
- 43.Zhu H, Cai H, Tang M, Tang J. Neuropilin-1 is overexpressed in osteosarcoma and contributes to tumor progression and poor prognosis. Clin Transl Oncol. 2014;16:732–8. doi: 10.1007/s12094-013-1141-y. [DOI] [PubMed] [Google Scholar]
- 44.Latil A, Bieche I, Pesche S, Valeri A, Fournier G, Cussenot O, Lidereau R. VEGF overexpression in clinically localized prostate tumors and neuropilin-1 overexpression in metastatic forms. Int J Cancer. 2000;89:167–171. doi: 10.1002/(sici)1097-0215(20000320)89:2<167::aid-ijc11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Ochiumi T, Kitadai Y, Tanaka S, Akagi M, Yoshihara M, Chayama K. Neuropilin-1 is involved in regulation of apoptosis and migration of human colon cancer. Int J Oncol. 2006;29:105–116. [PubMed] [Google Scholar]
- 46.Kawakami T, Tokunaga T, Hatanaka H, Kijima H, Yamazaki H, Abe Y, Osamura Y, Inoue H, Ueyama Y, Nakamura M. Neuropilin 1 and neuropilin 2 co-expression is significantly correlated with increased vascularity and poor prognosis in nonsmall cell lung carcinoma. Cancer. 2002;95:2196–2201. doi: 10.1002/cncr.10936. [DOI] [PubMed] [Google Scholar]
- 47.Cao Y, Hoeppner LH, Bach S, E G, Guo Y, Wang E, Wu J, Cowley MJ, Chang DK, Waddell N, Grimmond SM, Biankin AV, Daly RJ, Zhang X, Mukhopadhyay D. Neuropilin-2 promotes extravasation and metastasis by interacting with endothelial alpha5 integrin. Cancer Res. 2013;73:4579–4590. doi: 10.1158/0008-5472.CAN-13-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Dutta SK, Kojima T, Xu X, Khosravi-Far R, Ekker SC, Mukhopadhyay D. Neuropilin-1 modulates p53/caspases axis to promote endothelial cell survival. PLoS One. 2007;2:e1161. doi: 10.1371/journal.pone.0001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McColl BK, Stacker SA, Achen MG. Molecular regulation of the VEGF family -- inducers of angiogenesis and lymphangiogenesis. APMIS. 2004;112:463–480. doi: 10.1111/j.1600-0463.2004.apm11207-0807.x. [DOI] [PubMed] [Google Scholar]
- 50.Samuel S, Gaur P, Fan F, Xia L, Gray MJ, Dallas NA, Bose D, Rodriguez-Aguayo C, Lopez-Berestein G, Plowman G, Bagri A, Sood AK, Ellis LM. Neuropilin-2 mediated beta-catenin signaling and survival in human gastro-intestinal cancer cell lines. PLoS One. 2011;6:e23208. doi: 10.1371/journal.pone.0023208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.