Abstract
Background
The World Health Organization (WHO) recommends that people with uncomplicated Plasmodium falciparum malaria are treated using Artemisinin-based Combination Therapy (ACT). ACT combines three-days of a short-acting artemisinin derivative with a longer-acting antimalarial which has a different mode of action. Pyronaridine has been reported as an effective antimalarial over two decades of use in parts of Asia, and is currently being evaluated as a partner drug for artesunate.
Objectives
To evaluate the efficacy and safety of artesunate-pyronaridine compared to alternative ACTs for treating people with uncomplicated P. falciparum malaria.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; LILACS; ClinicalTrials.gov; the metaRegister of Controlled Trials (mRCT); and the WHO International Clinical Trials Search Portal up to 16 January 2014. We searched reference lists and conference abstracts, and contacted experts for information about ongoing and unpublished trials.
Selection criteria
Randomized controlled trials of artesunate-pyronaridine versus other ACTs in adults and children with uncomplicated P. falciparum malaria.
For the safety analysis, we also included adverse events data from trials comparing any treatment regimen containing pyronaridine with regimens not containing pyronaridine.
Data collection and analysis
Two authors independently assessed trial eligibility and risk of bias, and extracted data. We combined dichotomous data using risk ratios (RR) and continuous data using mean differences (MD), and presented all results with a 95% confidence interval (CI). We used the GRADE approach to assess the quality of evidence.
Main results
We included six randomized controlled trials enrolling 3718 children and adults.
Artesunate-pyronaridine versus artemether-lumefantrine
In two multicentre trials, enrolling mainly older children and adults from west and south-central Africa, both artesunate-pyronaridine and artemether-lumefantrine had fewer than 5% PCR adjusted treatment failures during 42 days of follow-up, with no differences between groups (two trials, 1472 participants, low quality evidence). There were fewer new infections during the first 28 days in those given artesunate-pyronaridine (PCR-unadjusted treatment failure: RR 0.60, 95% CI 0.40 to 0.90, two trials, 1720 participants, moderate quality evidence), but no difference was detected over the whole 42 day follow-up (two trials, 1691 participants, moderate quality evidence).
Artesunate-pyronaridine versus artesunate plus mefloquine
In one multicentre trial, enrolling mainly older children and adults from South East Asia, both artesunate-pyronaridine and artesunate plus mefloquine had fewer than 5% PCR adjusted treatment failures during 28 days follow-up (one trial, 1187 participants, moderate quality evidence). PCR-adjusted treatment failures were 6% by day 42 for these treated with artesunate-pyronaridine, and 4% for those with artesunate-mefloquine (RR 1.64, 95% CI 0.89 to 3.00, one trial, 1116 participants, low quality evidence). Again, there were fewer new infections during the first 28 days in those given artesunate-pyronaridine (PCR-unadjusted treatment failure: RR 0.35, 95% CI 0.17 to 0.73, one trial, 1720 participants, moderate quality evidence), but no differences were detected over the whole 42 days (one trial, 1146 participants, low quality evidence).
Adverse effects
Serious adverse events were uncommon in these trials, with no difference detected between artesunate-pyronaridine and comparator ACTs. The analysis of liver function tests showed biochemical elevation were four times more frequent with artesunate-pyronaridine than with the other antimalarials (RR 4.17, 95% CI 1.38 to 12.62, four trials, 3523 participants, moderate quality evidence).
Authors' conclusions
Artesunate-pyronaridine performed well in these trials compared to artemether-lumefantrine and artesunate plus mefloquine, with PCR-adjusted treatment failure at day 28 below the 5% standard set by the WHO. Further efficacy and safety studies in African and Asian children are required to clarify whether this combination is an option for first-line treatment.
Plain Language summary
Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria
What is uncomplicated malaria and how might artesunate-pyronaridine work
Uncomplicated malaria is the milder form of malaria which usually causes fever, with or without headache, tiredness, muscle pains, abdominal pains, nausea, and vomiting. If left untreated, uncomplicated malaria can rapidly develop into severe malaria with kidney failure, fitting, unconsciousness, and eventually death. Plasmodium falciparum is the most common parasite causing malaria in sub-Saharan Africa and causes most of the severe malaria worldwide.
The World Health Organization currently recommends countries use one of five different artemisinin-based combination therapies (ACTs) to treat malaria. These combinations contain an artemisinin component (artemether, dihydroartemisinin, or artesunate), which works quickly to clear the parasite from the person's blood, and a longer-acting drug which clears the remaining parasites from the blood and may prevent new Plasmodium infections for several weeks. Artesunate plus pyronaridine is a new combination and in this review we evaluate its effectiveness and safety compared to the other ACTs.
After examining the research published up to 16 January 2014, we included six randomized controlled trials, enrolling 3718 children and adults.
What the research says
Based on studies of mostly older children and adults living in Africa and Southeast Asia, artesunate-pyronaridine is probably as effective as artemether-lumefantrine at treating uncomplicated malaria and preventing further malaria infections after treatment (moderate quality evidence).
In a study primarily of older children and adults in Asia, artesunate-pyronaridine is probably as effective as artesunate plus mefloquine at treating P. falciparum malaria and preventing recurrent parasitaemias (moderate quality evidence).
Serious adverse events were rare in people treated with either artesunate-pyronaridine or other ACTs. However, short-lasting liver toxicity was more frequent in people treated with artesunate-pyronaridine than with the other antimalarials (moderate quality evidence).
Authors' conclusions
Artesunate-pyronaridine performed well compared to the other two ACT with which it has been compared, but further studies in African and Asian children are required to help clarify whether this combination is an option for first-line treatment.
Background
Description of the condition
Malaria continues to pose a serious global health challenge despite considerable progress over the past decade to control and eliminate malaria in some parts of the world. In 2010, there were an estimated 219 million malaria illness episodes, resulting in around 660,000 deaths (WHO 2012).
Five species of Plasmodium parasite cause malaria in humans; Plasmodium falciparum and P. vivax are the most common, and P. falciparum causes most of the severe disease cases (WHO 2012). Uncomplicated malaria is the mild form of the disease, typically characterized by fever with or without associated headache, tiredness, muscle pains, abdominal pains, rigors, nausea, and vomiting (WHO 2010a). If left untreated, uncomplicated malaria can rapidly develop into severe, life-threatening forms of the disease, particularly in people that have not acquired immunity. Effective immunity generally requires repeated infections over five to 10 years, and is reduced during pregnancy. Consequently in highly endemic settings, as seen in many areas of rural sub-Saharan Africa, young children and pregnant women are most at risk, while in settings with low or seasonal transmission, all age groups can be equally at risk (WHO 2010a).
In many parts of the world, P. falciparum has developed resistance to most antimalarial drugs used as monotherapy (White 2004; WHO 2010b). Consequently, the World Health Organization (WHO) now recommends that P. falciparum malaria is always treated with a combination of two drugs that act at different biochemical sites within the parasite (WHO 2010a). If a parasite mutation producing drug resistance arises spontaneously during treatment, the parasite should then be killed by the partner drug, thus reducing or delaying the development of resistance and increasing the useful lifetime of the individual drugs (White 1996; White 1999).
Five artemisinin-based combination therapies (ACTs) are now recommended for the first-line treatment of uncomplicated malaria; artemether-lumefantrine (AL), artesunate plus amodiaquine (AS+AQ), artesunate plus mefloquine (AS+MQ), artesunate plus sulfadoxine-pyrimethamine (AS+SP), and dihydroartemisinin-piperaquine (DHA-P) (WHO 2010a). The artemisinin components (artemether, artesunate, or dihydroartemisinin) are highly effective schizonticides, and over three days of treatment rapidly eliminate up to 90% of the blood stage asexual forms of P. falciparum. The partner drugs are longer-acting and are used to clear any residual infection (Nosten 2007; Kurtzhals 2008; WHO 2010a). The combinations with very long half-lives (AS+MQ and DHA-P) can provide a period of post-treatment prophylaxis which may last for up to six weeks (Sinclair 2009).
Resistance to the artemisinin-derivatives was first reported among P. falciparum strains in 2008 along the Thai-Cambodian border (Dondorp 2010; Lim 2010; WHO 2010b). This has led to global initiatives to contain the spread of artemisinin resistance, which includes the development of new drugs to partner and protect the artemisinin-derivatives in ACT (WHO 2011).
Description of the intervention
Pyronaridine is a benzonaphthyridine derivative first synthesized in China in 1970 (Fu 1991). It was used extensively as a monotherapy to treat P. falciparum and P. vivax infections in the Hunan and Yunan provinces of China for more than 20 years (Chen 1992), and to treat P. falciparum in some parts of Africa during the 1980s. Between 1985 and 1995, some in vitro pyronaridine-resistant strains of P. falciparum emerged along the China-Lao and China-Myanmar border areas (Yang 1997).
Elsewhere, in vitro studies using clinical isolates of P. falciparum from Africa, Cambodia, and Thailand in the 1990s demonstrated high activity of pyronaridine against chloroquine-sensitive and chloroquine-resistant P. falciparum strains (Childs 1988; Basco 1992; Chen 1992; Pradines 1998; Ringwald 1999), and more recent in vitro studies have also shown pyronaridine to be effective against multiple-drug resistant P. falciparum schizonts and gametocytes in Thailand and Indonesia (Chavalitshewinkoon-Petmitr 2000; Price 2010), and against chloroquine-resistant P. falciparum strains in Gabon (Kurth 2009). However, almost half of 28 P. falciparum isolates tested in vitro in Abidjan, Cote d'Ivoire, were resistant to pyronaridine and also showed some evidence of cross resistance to dihydroartemisinin (Brice 2010).
Pyronaridine interferes with the glutathione-dependent detoxification of haem and targeting of β-haematin formation (Auparakittanon 2006). Its activity in multi-drug resistant strains of P. falciparum is believed to be due to its ability to inhibit P-glycoprotein function and reverse multi-drug resistance in cell lines (Qi 2002; Pradines 2010).
Pyronaridine is structurally related to amodiaquine, leading to some concerns that pyronaridine may have similar toxicity related to the formation of a reactive metabolite (quinoneimine) in the liver and white blood cells. However, some studies suggest that pyronaridine and other bis-Mannich compounds are structurally advantaged and do not form the bioactive quinoneimine metabolite (Naisbitt 1998; Ruscoe 1998).
Assessment of antimalarial drug efficacy
The WHO recommends that new antimalarials should have a treatment failure rate of less than 5%, and that failure rates greater than 10% with existing first-line antimalarials should trigger a change in treatment policy (WHO 2010a).
Treatment failure can be classified as:
Early treatment failure:
the development of danger signs or severe malaria on days 1, 2, or 3 in the presence of parasitaemia;
parasitaemia on day 2 higher than on day 0;
parasitaemia and axillary temperature = 37.5 °C on day 3;
parasitaemia on day 3 = 20% of count on day 0.
Late treatment failure:
development of danger signs, or severe malaria, after day 3 with parasitaemia;
presence of P. falciparum parasitaemia and axillary temperature = 37.5 °C on or after day 4;
presence of P. falciparum parasitaemia after day 7.
The late reappearance of P. falciparum parasites in the blood of an infected person can be due to failure of the drug to completely clear the original parasite (a recrudescence) or due to a new infection, which is especially common in areas of high transmission. A molecular genotyping technique called polymerase chain reaction (PCR) can be used in clinical trials to distinguish between recrudescence and new infection, giving a clearer picture of the efficacy of the drug and its post-treatment prophylactic effect (White 2002; Cattamanchi 2003; WHO 2008).
The WHO recommends a minimum follow-up period of 28 days for antimalarial efficacy trials, but longer periods of follow-up may be required for antimalarials with long elimination half-lives (White 2002; Bloland 2003). Treatment failure due to true recrudescence of malaria parasites may be delayed until the drug concentration falls below the minimum concentration required to inhibit parasite multiplication, which may be beyond 28 days. The WHO recommends 42 days follow-up for trials involving lumefantrine and piperaquine and 63 days follow-up for trials of mefloquine (WHO 2010a).
Why it is important to do this review
Early studies of pyronaridine monotherapy conducted in Africa showing efficacy against chloroquine-resistant P. falciparum malaria (Ringwald 1999), and promising dose finding studies of the artesunate-pyronaridine combination from the Gabon (Ramharter 2008), have led to the promotion of artesunate-pyronaridine as a possible addition to the current list of recommended ACTs (Vivas 2008; Croft 2010).
This review aims to systematically evaluate the available trials on the effectiveness and safety of artemisinin plus pyronaridine for consideration by global and national policy makers.
Objectives
To evaluate the efficacy and safety of artesunate-pyronaridine compared to alternative ACTs for treating people with uncomplicated P. falciparum malaria.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Adults and children with uncomplicated P. falciparum malaria, as confirmed by either microscopy or rapid diagnostic tests.
Types of interventions
Intervention
Artesunate plus pyronaridine.
Control
WHO-recommended ACTs for treating malaria.
For an additional safety analysis we extended the inclusion criteria to all RCTs comparing pyronaridine alone or in combination with any other antimalarial.
Types of outcome measures
We used current WHO recommendations to guide the selection of outcomes for this review (Bloland 2003; WHO 2008).
Primary outcomes
Total treatment failure at day 28, 42, or 63 (PCR-unadjusted and PCR-adjusted).
Secondary outcomes
Early treatment failure
Parasite clearance
Fever clearance
Gametocyte carriage
Adverse events
Serious adverse events (leading to death, requiring hospitalization or prolongation of existing hospitalization, are life threatening, or result in persistent or significant disability or incapacity)
Adverse events leading to withdrawal from treatment (discontinuation of trial drug or withdrawal from trial)
Patient reported symptoms
Abnormal liver function tests (LFTs)
Abnormal WBC counts
Abnormal electrocardiogram (ECG) findings
Search methods for identification of studies
We attempted to find all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We updated previous literature searches done in February 2007 and August 2012 of the following databases using the search terms and strategy described in Appendix 1 up to 16 January 2014: Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; and LILACS. We also searched ClinicalTrials.gov, the metaRegister of Controlled Trials (mRCT) and the WHO's International Clinical Trials Registry Platform Search Portal for ongoing or recently completed trials using 'pyronaridine' and 'malaria' as search terms.
Searching other resources
Conference proceedings
We searched the following conference proceedings for relevant abstracts: The American Society of Tropical Medicine and Hygiene Annual Meetings (2007, 2008, 2009, and 2010); The Third ASEAN Congress of Tropical Medicine and Parasitology (ACTMP3); the MIM Pan-African Malaria Conference (2005 and 2009); the International Congress on Infectious Diseases (ICID) (2002, 2004, 2008, and 2010); the International Conference on Malaria: 125 years of Malaria Research 2005; the Keystone Symposia Global Health Series: and Malaria (Immunology, pathogenesis and perspectives) 2008.
Reference lists
We checked the reference lists of all trials identified by the above methods.
Contacting organizations and experts
We contacted the Medicines for Malaria Venture and the WHO for information about ongoing and unpublished trials.
Data collection and analysis
Selection of studies
Hasifa Bukirwa (HB) and Prathap Tharyan (PT) independently scanned the results of the search strategy and retrieved the full text articles of all potentially relevant trials, conscious of the possibility of multiple publications of the same trial. HB and PT independently assessed each potentially relevant trial for inclusion in the review using an eligibility form based on the inclusion criteria. There were no disagreements. We excluded studies that did not meet the eligibility criteria and listed the reasons for exclusion in the 'Characteristics of excluded studies' table.
Data extraction and management
HB and PT independently extracted the data from the trials using data extraction forms. We resolved disagreements through discussion. For dichotomous outcome measures, we recorded the number of participants experiencing the event and the number analysed in each group. For continuous outcome measures, we extracted arithmetic means and standard deviations for each group together with the numbers analysed in each group.
Primary outcome
Our primary analysis drew on the WHO's protocol for assessing and monitoring antimalarial drug efficacy (Bloland 2003). This protocol has been used to guide most efficacy trials since its publication in 2003, even though it was designed to assess the level of antimalarial resistance in the trial area rather than for comparative trials. As a consequence, a high number of randomized participants are excluded from the final efficacy outcome as losses to follow-up or voluntary or involuntary withdrawals (see Table 1).
PCR-unadjusted total failure
We calculated PCR-unadjusted total failure (P. falciparum) as the sum of early treatment failures and late treatment failures (without PCR adjustment). The denominator excludes participants for whom an outcome was not available (for example, those who were lost to follow-up, withdrew consent, took other antimalarials, or failed to complete treatment) and those participants who did not to fulfil the inclusion criteria after randomization.
PCR-adjusted total failure
We determined PCR-adjusted total failure (P. falciparum) as the sum of early treatment failures, and late treatment failures due to PCR-confirmed recrudescence. We treated participants with indeterminate PCR results, missing PCR results, or PCR-confirmed new infections as involuntary withdrawals and excluded them from the calculation. The denominator excludes participants for whom an outcome was not available (for example, those who were lost to follow-up, withdrew consent, took other antimalarials, or failed to complete treatment) and participants who did not fulfil the inclusion criteria after randomization.
These primary outcomes relate solely to failure due to P. falciparum. For both PCR-unadjusted and PCR-adjusted total failure, we retained in the calculation participants who developed P. vivax parasitaemia during follow-up if they were treated with chloroquine and continued to be monitored by the trialists. We classified them as treatment successes provided they did not go on to develop P. falciparum parasitaemia. We excluded from the calculation participants who developed P. vivax parasitaemia and were removed from the trial's follow-up at the time of P. vivax parasitaemia.
Assessment of risk of bias in included studies
For efficacy outcomes we assessed the risk of bias for each included trial using the Cochrane tool for assessing the risk of bias (Higgins 2011). For each of six domains; sequence generation; allocation concealment; blinding of participants, trial personnel and outcome assessors; incomplete outcome data; selective reporting; and other sources of bias, we assigned a judgment regarding the risk of bias. We classified these judgments as 'high risk', 'low risk ', or 'unclear risk' of bias. We recorded these assessments in the standard 'risk of bias' tables and summarized the risk of bias for each trial in a summary risk of bias graph.
For patient reported adverse events, we assessed the risk of bias by examining if monitoring was active or passive; whether participants and outcome assessors were blinded; whether the outcome data reporting was complete; whether all participants were included; and whether data analysis was independent of pharmaceutical companies (Table 2).
For laboratory reported adverse events, we assessed the risk of bias by examining which tests were performed, the timing of the tests, the completeness of reporting, and the independence of the data analysis (Table 2).
Measures of treatment effect
We extracted data from each included trial to calculate risk ratios, 95% confidence intervals (CIs) for dichotomous data, and mean differences with 95% CIs for continuous data.
Unit of analysis issues
We did not encounter any unit of analysis issues.
Dealing with missing data
If data from the trial reports were insufficient, unclear, or missing, we attempted to contact the trial authors for additional information. If we considered that the missing data rendered the result uninterpretable, we excluded the data from the meta-analysis and clearly stated the reason for exclusion. We explored the potential effects of missing data through a series of sensitivity analyses (Table 1).
Assessment of heterogeneity
We assessed heterogeneity amongst trials by inspecting the forest plots, applying the Chi² test with a 10% level of statistical significance, and also using the I² statistic with a value of 50% used to denote moderate levels of heterogeneity.
Assessment of reporting biases
There were too few trials to examine funnel plot asymmetry for evidence of small trial effects or publication bias.
Data synthesis
We analysed data using Review Manager 2011.
For the primary analysis we stratified by comparator ACT, and when outcomes were assessed and reported at different time-points, we also stratified the analyses by time point. We performed meta-analysis where appropriate after assessment and investigation of heterogeneity. In the first instance, we used a fixed-effect model and applied a random-effects model when the Chi² test P value was < 0.1 or the I² statistic was = 50%.
Arithmetic means and standard deviations used to summarize continuous data are assumed to be normally distributed; however, sometimes these summary statistics are incorrectly used when the data are not normally distributed. Therefore, when arithmetic means were reported, we checked the normality of the data by calculating the ratio of the mean over the standard deviation. If this ratio (mean/standard) was < 2, then it is likely that the data are skewed as the mean cannot then lie in the centre of a normal distribution. It is possible to combine data with less severe degrees of skew in meta-analyses and when ratio of the mean over the standard deviation was more than one (ratios less than one indicate that data were severely skewed), we combined data from these trials with normally distributed data.
Subgroup analysis and investigation of heterogeneity
There were too few trials to use subgroup analyses to explore the causes of heterogeneity. However, to explore the generalizability of the evidence we subgrouped the available data by age (< 5 years versus ≥ 5 years), country, and geographic region.
Sensitivity analysis
We assessed that all three trials were at low risk of bias so we did not perform a sensitivity analysis exploring effects of risk of bias.
To investigate the robustness of the methodology used in the primary analysis, we conducted a series of sensitivity analyses. The aim of this was to restore the integrity of the randomization process by adding excluded groups back into the analysis in a stepwise fashion (see Table 1 for details).
Quality of evidence
We assessed the quality of evidence across each outcome measure using the GRADE approach. The quality rating across studies has four levels: high, moderate, low, or very low. RCTs are initially categorized as high quality but can be downgraded after assessment of five criteria: risk of bias, consistency, directness, imprecision, and publication bias (Guyatt 2008).
Results
Description of studies
See Characteristics of included studies, and Characteristics of excluded studies sections.
Results of the search
Of the 52 reports we retrieved by the search, we identified 39 potentially relevant reports. Three trials comparing artesunate-pyronaridine with other ACTs met the inclusion criteria for the main review (Tshefu 2010; Kayentao 2012; Rueangweerayut 2012). We included three additional trials for a further assessment of the effect of pyronaridine on liver function (Ringwald 1996; Ringwald 1998; Poravuth 2011). We described the results of the search in a flow diagram (1)
figure 1.
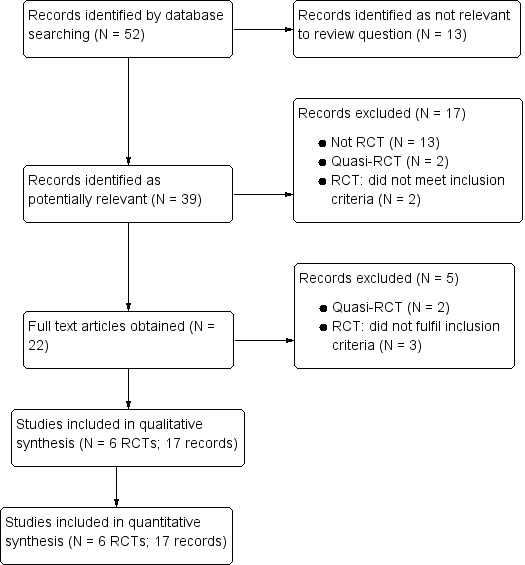
Flow diagram.
Included studies
Efficacy trials
The three efficacy trials were all Phase III non-inferiority trials conducted by the public-private partnership of Medicines for Malaria Venture (Switzerland) and Shin Poong Pharmaceuticals (Korea) for registration with the European Medicines Agency (Tshefu 2010; Kayentao 2012; Rueangweerayut 2012).
Artesunate-pyronaridine versus artemether-lumefantrine
Two multicentre trials that included 1807 participants evaluated this comparison (Tshefu 2010; Kayentao 2012).
Most participants (88.3%) were recruited from trial sites in Africa (Burkina Faso, Cote d'Ivoire, Democratic Republic of Congo, Gabon, The Gambia, Ghana, Kenya, Mali, Mozambique, and Senegal), with a small number (11.7%) from Southeast Asia (Indonesia and the Phillipines). All recruiting sites were endemic for P. falciparum malaria and most were reported as highly endemic.
Most participants were older children or adults, and only 232 children aged under five years, and 15 aged under one year were included.
Important exclusion criteria were severe malaria, cerebral malaria, severe anaemia, pregnant and lactating women, and people with hepatic, renal, or other disorders. Tshefu 2010 also excluded those with severe malnutrition and Kayentao 2012 excluded children with HIV infection.
In both trials, artesunate-pyronaridine was administered once daily for three days, and artemether-lumefantrine twice daily for three days in the standard dosing (see Table 3).
Artesunate-pyronaridine versus artemether plus mefloquine
A single multicentre trial, enrolling 1271 participants evaluated this comparison (Rueangweerayut 2012).
Most participants (81.3%) were from Southeast Asia (Cambodia, India, Thailand, and Vietnam), with a smaller number (18.7%) from Africa (Burkina Faso, Ivory Coast, and Tanzania). Malaria endemicity was high in most sites.
Although the trial planned to recruit participants aged between 3 to 60 years, the youngest participant was five years old.
Important exclusion criteria were severe malaria, cerebral malaria, severe anaemia, severe malnutrition, pregnant and lactating women, and people with hepatic or renal disorders.
Both artesunate-pyronaridine and artesunate plus mefloquine were administered once daily for three days (see Table 3).
Additional safety trials
The three additional safety trials compared artesunate-pyronaridine versus chloroquine (Poravuth 2011), and pyronaridine alone versus chloroquine (Ringwald 1996; Ringwald 1998).
Poravuth 2011 was conducted in Asia and primarily evaluated the effects of artesunate-pyronaridine on P. vivax malaria (Cambodia, India, Indonesia, and Thailand). Ringwald 1996 and Ringwald 1998 were conducted in Cameroon.
Poravuth 2011 randomized 456 participants aged seven years to 60 years; Ringwald 1996 randomized 96 adults aged 15 to 64 years, and Ringwald 1998 recruited 88 children only, aged five years to 15 years.
For further details of the included trials see the 'Characteristics of included studies' tables.
Excluded studies
We excluded 21 trials (22 records) for the reasons described in the 'Characteristics of excluded studies' table. In brief; 13 were not randomized, four were quasi-randomized (used alternation), and five did not have populations, comparisons, or outcomes of relevance to this review (1).
One trial comparing pyronaridine alone for three days versus dihydroartemisinin alone for seven days versus a combination of pyronaridine and dihydroartemisinin for three days did not meet the inclusion criteria for the primary efficacy analysis due to the lack of an appropriate comparison arm with an ACT, and was not included in the safety analysis as LFTs were not reported (Liu 2002).
Risk of bias in included studies
See 2.
figure 2.

Risk of bias summary table (Methodological quality summary): review authors' judgements about each methodological quality item for each included trial.
Allocation
All trials were at low risk of selection bias.
Blinding
The four non-inferiority trials were at low risk for performance and detection bias as they used double-dummy techniques, or independent outcome assessors and trial personnel who were not aware of allocation (Tshefu 2010; Poravuth 2011; Kayentao 2012; Rueangweerayut 2012).
The additional two safety trials (Ringwald 1996; Ringwald 1998) were open label or inadequately masked but were at low risk of bias, since blinding would not affect detection of the adverse outcomes sought in this review.
Incomplete outcome data
All of the included trials reported attrition with details of all randomized participants.
Selective reporting
Tshefu 2010; Poravuth 2011; Kayentao 2012 and Rueangweerayut 2012 were prospectively registered and appeared free of selective reporting, as ascertained from the data presented in the reports, the registration documents, and where available, the trial protocols.
Other potential sources of bias
We considered that Ringwald 1996; Ringwald 1998; and Tshefu 2010 had other potential biases (see Risk of bias tables) but the effects on these on outcomes are uncertain.
For adverse events, we conducted additional assessments of the adequacy of safety monitoring and the completeness of reporting. For patient reported adverse events, the method for monitoring adverse events was unclear in all six trials, the days monitoring occurred was unclear in five trials, and the day of outcome reporting unclear in all six trials (see Table 4). For biochemical adverse events, the frequency of testing was adequate in three trials (Tshefu 2010; Poravuth 2011; Kayentao 2012), and reporting was complete in two trials (Tshefu 2010; Poravuth 2011; see Table 5).
Effects of interventions
See: Summary of findings for the main comparison Artesunate-pyronaridine compared to artemether-lumefantrine for uncomplicated falciparum malaria; Summary of findings 2 Artesunate-pyronaridine compared to artesunate plus mefloquine for treating uncomplicated P. falciparum malaria; Summary of findings 3 Liver toxicity of pyronaridine compared to other antimalarials
Comparison 1. Artesunate-pyronaridine versus artemether-lumefantrine
Two trials, including 1595 participants from Africa and 212 from Southeast Asia, compared artesunate-pyronaridine with artemether-lumefantrine (Tshefu 2010; Kayentao 2012). Only Kayentao 2012 included children aged under five years (232 children), of which only 15 were aged under one year. Follow-up was until day 42.
Treatment failure
At day 28, the proportion of participants with recurrent parasitaemia was lower in those treated with artesunate-pyronaridine compared to artemether-lumefantrine (PCR-unadjusted treatment failure; RR 0.60, 95% CI 0.40 to 0.90; two trials, 1720 participants, Analysis 1.1, 3). However, after PCR-adjustment treatment failure, it was below 5% with both ACTs, with no differences between groups (PCR-adjusted treatment failure: two trials, 1650 participants, Analysis 1.1).
figure 3.
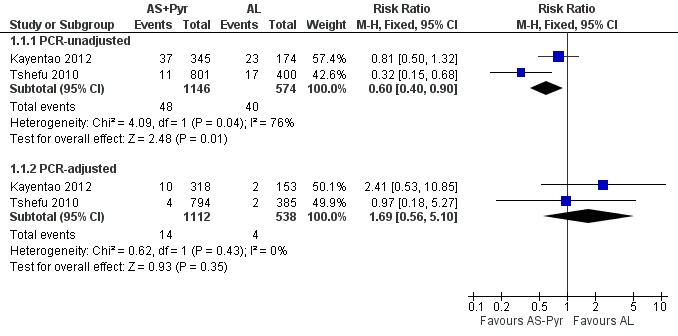
Forest plot of comparison: 1 Artesunate-pyronaridine versus artemether-lumefantrine, outcome: 1.1 Total failure (Day 28).
At day 42, there were no significant differences between artesunate-pyronaridine and artemether-lumefantrine for PCR-unadjusted (two trials, 1691 participants, Analysis 1.2) or PCR-adjusted treatment failure (two trials, 1472 participants, Analysis 1.2). PCR-adjusted treatment failure with artesunate-pyronaridine was marginally above 5% in one trial at this time-point (6.8%).
Only two people on artesunate-pyronaridine and one on artemether-lumefantrine experienced early treatment failure (two trials, 1676 participants, Analysis 1.3).
Parasite clearance
Both trials reported that artesunate-pyronaridine cleared parasites from the peripheral blood quicker than artemether-lumefantrine. Tshefu 2010 reported a slightly lower mean clearance time (MD 3.2 hours, 95% CI 4.38 to 2.02; one trial, 1170 participants; Analysis 1.4), and Kayentao 2012 reported a slightly lower median clearance time (24.1 hours, 95% CI 24.0 to 24.1 with artesunate-pyronaridine versus 24.2 hours, 95% CI 24.1 to 32.0 with artemether-lumefantrine; P = 0.02, authors' own figures, one trial, 535 participants, Table 6). These differences are probably not clinically important.
Fever clearance
Fever clearance times were similar between groups in both trials. Tshefu 2010 reported mean fever clearance time as marginally shorter following treatment with artesunate-pyronaridine than artemether-lumefantrine (MD 1.2 hours, 95% CI 2.38 to 0.02 hours, one trial, 1170 participants, Analysis 1.5), while Kayentao 2012 reported equal median clearance times (8.1 hours with artesunate-pyronaridine versus 8.1 hours with artemether-lumefantrine, P = 0.049, authors' own figures, one trial, 535 participants, Table 6).
Gametocyte clearance and carriage
In Tshefu 2010, 8% of participants given artesunate-pyronaridine and 5% of those given artemether-lumefantrine had peripheral gametocytaemia at baseline. The mean time to gametocyte clearance was 10.5 hours shorter with artesunate-pyronaridine (MD 10.5 hours, 95% CI 12.4 to 8.60; one trial, 1170 participants, Analysis 1.6).
In Kayentao 2012, 13% of participants had gametocytes at baseline. No subsequent statistically significant differences in gametocyte carriage, or gametocyte development were reported (one trial, 532 participants, Table 6).
Serious adverse events
Neither trial reported any deaths. There were six serious adverse events in total with no significant difference between groups (0.3% with artesunate-pyronaridine versus 0.3% with artemether-lumefantrine; two trials, 1787 participants, Analysis 1.7).
Adverse events leading to withdrawal from treatment
There was no significant difference between groups in the proportion of participants withdrawn from the trial due to adverse events (2.3% with artesunate-pyronaridine versus 1.7% with artemether-lumefantrine; two trials, 1787 participants, Analysis 1.8).
Patient-reported symptoms
There were no significant differences in patient-reported symptoms between the two ACTs (two trials, 1807 participants, Analysis 1.9, Analysis 1.10). The trial authors reported symptoms of vomiting, headache, abdominal pain, vertigo, haematuria, upper abdominal pain, and anorexia.
Biochemical monitoring and adverse events
Both trials measured biochemical LFTs in all participants at baseline and on days three and seven (Kayentao 2012 also measured LFTs on day 28), Although the two trials used slightly different grading scales, there were no significant differences between groups in grade 3 or 4 liver toxicity by any of the measures used (two trials, 1807 participants, Analysis 1.11, Analysis 1.12).
Haematological monitoring and adverse events
In both trials the mean haemoglobin fell compared to baseline during the first seven days after starting treatment, before recovering by day 28 (two trials, 1807 participants, Analysis 1.12). At day seven the reduction in haemoglobin was greater with artesunate-pyronaridine but this is unlikely to be of clinical significance (MD -0.16, 95% CI -0.28 to -0.05; two trials, 1741 participants, Analysis 1.12).
Kayentao 2012 also reported the occurrence of anaemia as an adverse event with no differences between groups (one trial, 535 participants, Analysis 1.13).
ECG monitoring and adverse events
Both trials conducted ECG monitoring at baseline, days 2, 7, 14 and 28. Tshefu 2010 reported two participants in each group having abnormal ECG readings and reported these as "mild". Kayentao 2012 reported that there were "no post-baseline clinically important abnormal ECG results" (see Table 7).
Subgroup analysis
We have presented a subgroup analysis of PCR-adjusted treatment failure at day 28 by age of participants in Analysis 2.1. This demonstrates the paucity of data for the under-five age group.
Further subgroup analyses by geographical region and country are in Analysis 2.2 and Analysis 2.3. Again, these demonstrate that the data remain severely underpowered to inform national decision-making. Primary outcome data was available for only 194 participants from East Africa, compared to 816 from West Africa, 490 from South-central Africa, and 175 from Asia.
Sensitivity analysis
We conducted a sensitivity analysis to explore the influence of different methods for analysing the primary outcome data. For PCR-unadjusted treatment failure, our primary analysis following the WHO guidelines for analysing trials of antimalarials was the least conservative (Analysis 3.1). The per-protocol and intention-to-treat analyses as presented by the trial authors, where missing data were considered treatment failure, were more conservative and the result did not reach statistical significance. For PCR-adjusted treatment failure, there were no substantial differences (Analysis 3.2).
We did not undertake a sensitivity analysis by risk of bias criteria as both of the included trials were at low risk of bias.
Comparison 2. Artesunate-pyronaridine versus artesunate plus mefloquine
Only one trial, enrolling 1033 participants from Asia and 238 from Africa, compared artesunate-pyronaridine versus artemether-lumefantrine (Rueangweerayut 2012). This trial excluded children under five years of age and follow-up was until day 42.
Treatment failure
At day 28, the proportion of participants with recurrent parasitaemia was lower in those treated with artesunate-pyronaridine compared to artesunate plus mefloquine (PCR-unadjusted treatment failure: RR 0.35, 95% CI 0.17 to 0.73; one trial, 1200 participants, Analysis 4.1). However, after PCR-adjustment treatment, failure was below 5% with both ACTs with no differences between groups (one trial, 1187 participants, Analysis 4.1).
At day 42, there were no statistically significant differences between artesunate-pyronaridine and artesunate plus mefloquine for PCR-unadjusted or PCR-adjusted treatment failure (one trial, 1146 participants, Analysis 4.2). At this time point, PCR-adjusted treatment failure was 5.8% with artesunate-pyronaridine versus 3.6% with artesunate plus mefloquine.
One person treated with artesunate plus mefloquine experienced early treatment failure and developed cerebral malaria (one trial, 1103 participants, Analysis 4.3).
Parasite clearance
The mean parasite clearance time was slightly lower with artesunate-pyronaridine compared to artesunate plus mefloquine (MD 2.60 hours, 95% CI 4.94 to 0.26, one trial, 1259 participants, Analysis 4.4).
Fever clearance
Fever clearance time was similar between treatment arms (one trial, 1051 participants, Analysis 4.5).
Gametocyte clearance and carriage
Rueangweerayut 2012 only reported the mean time to gametocyte clearance for the 27 participants (13 on artesunate-pyronaridine versus 14 on artesunate plus mefloquine) who cleared their gametocytes within the first 72 hours. There was no difference between groups (one trial, 27 participants, Analysis 4.6).
Serious adverse events
Rueangweerayut 2012 did not report any deaths. There were nine serious adverse events in total with no significant difference between groups (0.7% with artesunate-pyronaridine versus 0.7% with artesunate plus mefloquine; one trial, 1271 participants, Analysis 4.7).
Adverse events leading to withdrawal from treatment
There was no significant difference between groups in the proportion of participants withdrawn from the trial due to adverse events (0.6% with artesunate-pyronaridine versus 0.9% with artesunate plus mefloquine; one trial, 1271 participants, Analysis 4.8).
Patient-reported symptoms
Rueangweerayut 2012 only reported symptoms if they occurred in at least 2% of patients. Dizziness was twice as common in those treated with artesunate plus mefloquine than with artesunate-pyronaridine (RR 0.46, 95% CI 0.28 to 0.78; one trial, 1271 participants, Analysis 4.9). The other reported symptoms were headache, cough, diarrhoea, vomiting, and myalgia.
Biochemical monitoring and adverse events
Biochemical tests for liver function monitoring were performed on all participants on days 0, 3, 7, 28, and 42.
Artesunate-pyronaridine was associated with more participants recording elevated ALT and AST levels following treatment. For ALT, grade 2 toxicity (up to five times the upper limit of normal) was significantly higher with artesunate-pyronaridine (21/843 versus 0/417; RR 21.30, 95% CI 1.29 to 350.7; one trial, 1260 participants, Analysis 4.10), and grade 3 or 4 toxicity (= five times the upper limit of normal) approached statistical significance (15/843 versus 0/417; RR 7.41, 95% CI 0.98 to 55.98; one trial, 1260 participants, Analysis 4.11). There were no significant differences for other liver enzymes or bilirubin. No patients developed signs or symptoms of liver disease.
Haematological monitoring and adverse events
The mean haemoglobin level fell in both groups during the first seven days after starting treatment (Analysis 4.12) This drop was slightly larger with artesunate-pyronaridine compared to artesunate plus mefloquine (Day 3: MD -0.22 g/dL, 95% CI -0.36 to -0.08; one trial, participants, Analysis 4.12), but by day 28 mean haemoglobin levels were better than baseline in both groups. A similar pattern was observed with platelet counts (Analysis 4.13), and white cell counts (Analysis 4.14). However the differences were small and unlikely to be of clinical significance.
ECG monitoring and adverse events
Rueangweerayut 2012 conducted ECG monitoring on all participants in this trial but the timing and frequency of ECGs was unclear. The trial authors reported abnormal ECGs in under 1% of participants in both groups, and described all abnormalities as mild and transient (one trial, 1271 participants, Analysis 4.15).
Subgroup analysis
We did not conduct a subgroup analysis by age of participants as this trial did not include children aged under five years.
We have presented subgroup analyses by geographical region and country in Analysis 5.1 and Analysis 5.2. The majority of PCR-adjusted treatment failures occurred in Thailand and Cambodia, with almost none elsewhere. They also demonstrate the paucity of data from Africa.
Trial authors noted that participants enrolled in Pailin, Cambodia (an area of low-transmission forP. falciparum) had significantly longer parasite clearance times than people in the other trial sites; only 63% cleared parasites within 72 hours compared to 98% of participants in the other sites. Recrudescence at this site was reportedly higher with artesunate-pyronaridine than with artesunate plus mefloquine (10.2% versus 0%, P = 0.04; authors' own figures).
Sensitivity analysis
We conducted a sensitivity analysis to explore the influence of different methods for analysing the primary outcome data. For PCR-unadjusted treatment failure, our primary analysis following the WHO guidelines for analysing trials of antimalarials was similar to the per-protocol analysis of the trial authors (Analysis 6.1). In the most conservative estimates the effect size was dramatically reduced and the estimate was no longer statistically significant (Analysis 6.1). For PCR-adjusted treatment failure we did not observe any substantial differences (Analysis 6.2).
We did not perform any further sensitivity analyses as there was only one trial.
Part 3. Biochemical, haematological and ECG adverse events
In light of concerns about liver toxicity with pyronaridine, we included three additional RCTs of pyronaridine. Two trials compared pyronaridine alone to chloroquine (Ringwald 1996; Ringwald 1998) and one trial compared artesunate-pyronaridine to chloroquine (Poravuth 2011).
Biochemical monitoring and adverse events
The six trials reported abnormalities in liver functions in different ways. We assessed the adequacy of monitoring and completeness of results reporting in Table 5.
Artesunate-pyronaridine was associated with a four-fold increase in the incidence of ALT and AST grade 3 or 4 toxicity (elevations = five times the upper limit of normal) (ALT: RR 4.17, 95% CI 1.38 to 12.62, AST: RR 4.08, 95% CI 1.17 to 14.26; four trials, 3528 participants, Analysis 7.1). Grade 3 or 4 toxicity measured with ALP and bilirubin were not substantially different.
The three main efficacy trials also reported cases with both raised ALT (3 x ULN) and raised bilirubin (2 x ULN) as an indicator for drug induced liver injury (Tshefu 2010; Kayentao 2012; Rueangweerayut 2012). Only five of the 2052 participants in the artesunate-pyronaridine group and one of 1020 participants in the comparator groups had raised ALT and bilirubin. This difference was not statistically significant (three trials, 3072 participants, Analysis 7.2).
Ringwald 1996 reported that 5/40 participants given pyronaridine had elevated bilirubin levels compared to 0/41 with chloroquine but did not give any further details.
Renal function tests
Three trials reported serum creatinine levels as a measure of renal function. At day 7, creatinine values were marginally lower in the pyronaridine-treated group than in those treated with comparator regimens (artemether-lumefantrine, artesunate+mefloquine, chloroquine) (MD -2.76, 95% CI -4.58 to -0.94; three trials, 1808 participants, Analysis 7.3).
Haematological monitoring and adverse events
Four trials reported mean haemoglobin on days 0, 3, 7, and 28, and in all four trials the mean haemoglobin fell in both groups between day 0 and day 7 before recovering by day 28 (four trials, 3534 participants, Analysis 7.4). At day 7 the mean haemoglobin was ¼ gram lower in those treated with artesunate-pyronaridine (MD -0.24 g/dL, 95% CI -0.32 to -0.16; four trials, 3394 participants, Analysis 7.4).
ECG monitoring and adverse events
Four trials conducted ECG monitoring and ECG adverse effects were rare in all four trials (see Table 7). Prolonged QT interval was less common with artesunate-pyronaridine than comparators (RR 0.25, 95% CI 0.07 to 0.90; three trials, 2991 participants, Analysis 7.5).
Discussion
Summary of main results
Artesunate-pyronaridine versus artemether-lumefantrine
In two multicentre trials, enrolling mainly older children and adults from west and south-central Africa, both artesunate-pyronaridine and artemether-lumefantrine had fewer than 5% PCR adjusted treatment failures during 42 days of follow-up, with no differences between groups (low quality evidence). There were fewer new infections during the first 28 days in those given artesunate-pyronaridine (moderate quality evidence), but no difference was detected over the whole 42 day follow-up (moderate quality evidence).
Artesunate-pyronaridine versus artesunate plus mefloquine
In one multicentre trial, enrolling mainly older children and adults from South East Asia, both artesunate-pyronaridine and artesunate plus mefloquine had fewer than 5% PCR adjusted treatment failures during 28 days follow-up (moderate quality evidence). PCR-adjusted treatment failures had risen to 6% by day 42 in those treated with artesunate-pyronaridine, but this was not substantially different to artesunate plus mefloquine (low quality evidence). Again, there were fewer new infections during the first 28 days in those given artesunate-pyronaridine (moderate quality evidence), but no differences were detected over the whole 42 days (low quality evidence).
Adverse effects
Serious adverse events were rare in these trials with no statistically significant differences between artesunate-pyronaridine and the comparator ACTs. However, biochemical elevation of LFTs occurred four times more frequently with artesunate-pyronaridine than with the other antimalarials (moderate quality evidence).
Overall completeness and applicability of evidence
Artesunate-pyronaridine performed well in all three efficacy trials included in this review, with low levels of PCR-adjusted treatment failure at day 28 in all settings. All three trials were multicentre trials, with trial sites in 11 African countries and six countries in Asia, which broadens the applicability of the findings. However, the actual number of participants recruited from many trial sites was small and the trials were underpowered to evaluate either superiority or equivalence at country level. East Africa is particularly under represented, with only 232 participants from Kenya and Tanzania, and several of the West African countries recruited fewer than 100 participants.
The other major limitation on the applicability of these trials is the age of the participants. The trials predominantly recruited older children and adults. The combination appeared to be effective in these groups but little is known about the main target group; children aged under five years. These trials included only 232 children aged below five years compared to over 7000 in trials of dihydroartemisinin-piperaquine.
Notably, all three efficacy trials excluded people with known pre-existing liver disease, and one trial explicitly excluded those with raised LFTs at baseline. Screening of this kind may not be feasible in many malaria-endemic settings.
Quality of the evidence
We assessed the quality of the evidence in this review using the GRADE approach and presented it in two summary of findings tables for efficacy (Summary of findings for the main comparison; Summary of findings 2).
The evidence that artesunate-pyronaridine is equivalent to established ACTs at preventing PCR-adjusted treatment failures was of moderate quality due to two main concerns:
Indirectness: The trials to date have largely been conducted in older children and adults, with exclusion of young children who bear the greatest burden and risks of malaria infection and illness.
Imprecision: The trials were not powered to examine the efficacy of artesunate-pyronaridine in individual regions or countries. This is problematic for national decision-making, and limits the wider generalizability of these results. Larger trials would be required to have full confidence in these results.
We also assessed the quality of evidence on comparative adverse effects and presented these in Appendix 2 and Appendix 3. In general the evidence was of moderate to low quality, and downgraded for similar reasons.
Potential biases in the review process
The objectives of the review changed significantly between the published protocol and final review. The basis for the change was to focus on only interventions of relevance to current malaria treatment policies (see Differences between protocol and review). We used standard methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and complied with the Cochrane Collaboration's methodological standards for the conduct of new reviews of interventions (MECIR 2011).
We believe that we have identified all pyronaridine trials relevant to inform clinical decisions and policy regarding the use of pyronaridine combinations for the treatment of uncomplicated P. falciparum malaria. The three trials were all conducted under the auspices of the public-private partnership, Medicines for Malaria Venture, and Poong Pharmaceutical Company Ltd, Seoul, Republic of Korea.
Agreements and disagreements with other studies or reviews
We found one further systematic review of artesunate-pyronaridine published by authors from the Medicines for Malaria Venture (MMV), the co-developers of the artesunate-pyronaridine combination (Duparc 2013). The authors include four of the studies included here, plus one study we excluded as it was not randomized (Ramharter 2008), and one unpublished study. The authors conclude that 'Pyronaridine-artesunate was well tolerated with no safety concerns with the exception of mostly mild transient rises in transaminases. Efficacy was high and met the requirements for use as first-line therapy'. While we agree that artesunate-pyronaridine shows promise as a further addition to the ACT combinations, we think it requires further studies in the main target group, children aged less than five years, before countries consider this as a first-line treatment.
Authors' conclusions
Implications for practice
Artesunate-pyronaridine performed well in these trials compared to artemether-lumefantrine and artesunate-mefloquine, with PCR-adjusted treatment failure at day 28 below the 5% standard set by the WHO.
Artesunate-pyronaridine is well-tolerated, apart from transient gastrointestinal adverse effects, similar to other antimalarials. However, the potential for liver toxicity in people treated with artesunate-pyronaridine needs further investigation and will necessitate caution in using this treatment combination, particularly in people with pre-existing liver disorders.
Implications for research
Further efficacy and safety studies in African and Asian children are required before this combination could be established as a first or second-line treatment option.
Appendices
Appendix 1. Search methods: search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
|---|---|---|---|---|---|
| 1 | malaria | malaria | malaria | malaria | malaria |
| 2 | pyronaridine | pyronaridine | pyronaridine | pyronaridine | pyronaridine |
| 3 | 1 and 2 | 1 and 2 | NAPHTYRIDINES | PYRONARIDINE | 1 and 2 |
| 4 | — | — | 2 or 3 | 2 or 3 | — |
| 5 | — | — | 1 and 4 | 1 and 4 | — |
| 6 | — | — | Limit 5 to human | Limit 5 to human | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2005); upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 2. Artesunate-pyronaridine versus artemether-lumefantrine adverse events GRADE table
| Artesunate-pyronaridine compared to artemether-lumefantrine for treating uncomplicated P. falciparum malaria | |||||
| Patient or population: Patients with uncomplicated P. falciparum malaria Settings: Malaria endemic areas Intervention: Artesunate-pyronaridine (AS-Pyr) Comparison: Artemether-lumefantrine (AL6) | |||||
| Outcomes | Number of participants having adverse events (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | ||
| AL6 | AS-Pyr | ||||
| Serious adverse events (including deaths) | 3 per 1000 | 0 more per 1000 (From 2 fewer to 10 more) | 1787(2 trials) | low 1,2,3,4 | |
| Adverse events leading to withdrawal | 17 per 1000 | 6 more per 1000 (From 6 fewer to 31 more) | 1787(2 trials) | low 1,2,3,4 | |
| Gastroenterological | Vomiting | 4 per 100 | 2 more per 100 (From 1 fewer to 10 more) | 535(1 trial) | low 5,6,7 |
| Diarrhoea | — | — | — | —8 | |
| Abdominal pain | 5 per 100 | 0 more per 100 (From 2 fewer to 4 more) | 1272(1 trial) | low 5,9,10 | |
| Neuro-psychiatric | Headache | 3 per 100 | 0 more per 100 (From 1 fewer to 1 more) | 1272(1 trial) | low 5,9,10 |
| Dizziness | — | — | — | —8 | |
| Cardio-respiratory | Cough | 9 per 100 | 1 fewer per 100 (From 3 fewer to 2 more) | 1807(2 trials) | moderate 1,2,3,10 |
| ECG abnormality | 4 per 1000 | 2 fewer per 1000 (From 4 fewer to 10 more) | 1272(1 trial) | moderate 5,9,10,11 | |
| Prolonged QT interval | 0 per 1000 | 1 more per 1000 (From 0 fewer to 36 more) | 1272(1 trial) | moderate 5,9,10,11 | |
| Musculoskeletal/ dermatological | Myalgia | — | — | — | —8 |
| Biochemical | Alanine aminotransferase Grade 3 or 4 toxicity |
3 per 1000 | 3 more per 1000 (From 2 fewer to 25 more) | 1807(2 trials) | low 1,2,3,4 |
| Aspartate aminotransferase Grade 3 or 4 toxicity |
1 per 1000 | 3 more per 1000 (From 0 fewer to 20 more) | 1807(2 trials) | low 1,2,3,4 | |
| The assumed risk of adverse events in the artemether-lumefantrine group is the average risk across trials. The corresponding risk with artesunate-pyronaridine (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
| 1 No serious risk of bias: Both trials were at low risk of bias. 2 No serious inconsistency: Statistical heterogeneity was low. 3 Downgraded by one for serious indirectness: These two trials included only 232 children aged below five years. 4 Downgraded by one for imprecision: These trials do not exclude the possibility of rare but clinically important adverse effects. 5 No serious risk of bias: This single trial was at low risk of bias. 6 Downgraded by one for serious indirectness: This trial included only 232 children aged less than five years and only 15 less than one year. 7 Downgraded by one for serious imprecision: The 95% CI is wide and includes both no difference and clinically important differences. 8 This outcome was not reported. 9 Downgraded by two for very serious indirectness: This trial excluded children aged less than five years. 10 No serious imprecision: The finding is of no difference between treatments and the sample size is adequately powered to detect differences if they existed. 11 The second trial only reports that there were "no clinically important post baseline ECG results". | |||||
Appendix 3. Artesunate-pyronaridine versus artesunate plus mefloquine adverse event GRADE table
| Artesunate-pyronaridine compared to artesunate plus mefloquine for treating uncomplicated P. falciparum malaria | |||||
| Patient or population: Patients with uncomplicated P. falciparum malaria Settings: Malaria endemic areas Intervention: Artesunate-pyronaridine (AS-Pyr) Comparison: Artesunate plus mefloquine (AS+MQ) | |||||
| Outcomes | Number of participants having adverse events (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | ||
| AS+MQ | AS-Pyr | ||||
| Serious adverse events (including deaths) | 7 per 1000 | 0 more per 1000 (From 5 fewer to 21 more) | 1271(1 trial) | low 1,2,3,4 | |
| Adverse events leading to withdrawal | 9 per 1000 | 3 fewer per 1000 (From 7 fewer to 7 more) | 1271(1 trial) | low 1,2,3,4 | |
| Gastroenterological | Vomiting | 2 per 100 | 0 more per 100 (From 1 fewer to 2 more) | 1271(1 trial) | low 1,2,5,6 |
| Diarrhoea | 2 per 100 | 1 fewer per 100 (From 2 fewer to 0 more) |
1271(1 trial) | low 1,2,5,6 | |
| Abdominal pain | — | — | — | —7 | |
| Neuropsychiatric | Headache | 10 per 100 | 2 more per 100 (From 2 fewer to 6 more) | 1271(1 trial) | low 1,2,5,6 |
| Dizziness | 7 per 100 | 4 fewer per 100 (From 5 fewer to 2 fewer) | 1271(1 trial) | low 1,2,5,6 | |
| Cardiorespiratory | Cough | 2 per 100 | 2 more per 100 (From 1 fewer to 4 more) | 1271(1 trial) | low 1,2,5,6 |
| ECG abnormality | 7 per 1000 | 0 more per 1000 (From 7 fewer to 21 more) | 1271(1 trial) | low 1,2,3,8 | |
| Prolonged QT interval | 7 per 100 | 7 fewer per 100 (From 7 fewer to 4 more) | 1271(1 trial) | moderate 1,2,3,6 | |
| Musculoskeletal/ dermatological | Myalgia | 4 per 100 | 2 more per 100 (From 1 fewer to 5 more) | 1271(1 trial) | low 1,2,5,6 |
| Biochemical | Alanine aminotransferase Grade 3 or 4 toxicity |
2 per 1000 | 16 more per 1000 (From 0 fewer to 110 more) | 1271(1 trial) | low 1,2,3,8 |
| Aspartate aminotransferase Grade 3 or 4 toxicity |
0 per 1000 | 11 more per 1000 (From 0 more to 161 more) | 1271(1 trial) | low 1,2,3,8 | |
| The assumed risk of adverse events in the artesunate plus mefloquine group is the risk from the single trial. The corresponding risk with artesunate-pyronaridine (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
| 1 No serious risk of bias: This single trial is at low risk of bias. 2 No serious inconsistency: Not applicable as only one trial. 3 Downgraded by one for serious indirectness: This trial excluded children aged below five years. 4 Downgraded by one for imprecision: Trials of this size do not exclude the possibility of rare but clinically important adverse effects. 5 Downgraded by two for very serious indirectness: This trial excluded children aged less than five years. 6 No serious imprecision: The finding is of no difference between treatments and the sample size is adequately powered to detect differences if they existed. 7 This outcome was not reported. 8 Downgraded by one for serious imprecision: The 95% CI is wide and includes both no difference and clinically important differences. | |||||
Appendix 4. Descriptions of serious adverse events
| Trial ID | Number of participants | Comparator | All serious adverse events | Serious adverse events judged to be related to the medication | ||
| Artesunate-pyronaridine | Comparator | Artesunate-pyronaridine | Comparator | |||
| Kayentao 2012 | 535 | Artemether-lumefantrine | Complicated malaria (1) | None | None | None |
| Tshefu 2010 | 1272 | Artemether-lumefantrine | Parotitis (1) Typhoid fever (1) Urinary tract infection (1) |
Cerebral malaria (1)Immunosuppresion (1) | None | None |
| Rueangweerayut 2012 | 1271 | Artesunate-mefloquine | Autimmune haemolytic anaemia (1) Cholera (1) Pneumonia (1) Acute pyelonephritis (1) Wound infection (1) Abortion (1) Depression (1) |
Cerebral malaria (1) Seizure (1) Grand-mal seizure (1) |
None | Seizure (1) Grand-mal seizure (1) |
History
Protocol first published: Issue 1, 2007Review first published: Issue 3, 2014
| Date | Event | Description |
|---|---|---|
| 11 November 2008 | Amended | We converted to the new review format with minor editing. |
Contributions of authors
B Unnikrishnan (BU) and Suma Nair (SN) co-drafted the initial version of the protocol. HB revised the protocol, and together with PT independently selected trials, assessed quality, extracted and entered data that was checked by BU and SN. Christine Kramer extracted adverse events data. HB used GRADE profiler to create and import 'Summary of findings' tables. HB wrote the initial draft of the review and worked with all the authors to finalise the review. All authors approved the final review version.
Declarations of interest
None known.
Sources of support
Internal sources
-
Manipal University, India.
Employment and logistic support for Drs. Unnikrishanan and Nair
-
South Asian Cochrane Centre, Vellore, India.
Protocol Development and Review Completion workshops
-
Christian Medical College, Vellore, India.
Employment for Prof. Tharyan, and logistic support for the South Asian Cochrane Centre
External sources
-
Department for International Development (DFID), UK.
Fellowships to Drs. Unnikrishnan and Nair, via the Effective Health Care Research Partnership Consortium grant to Prof. Tharyan, to complete the review at the South Asian Cochrane Centre
-
Indian Council of Medical Research, India.
Funding for the Prof. BV Moses & ICMR Centre for Advanced Research and Training in Evidence-Informed Healthcare; CMC Vellore
Differences between protocol and review
We stated in the protocol that we intended to assess the methods used to generate the allocation sequence and conceal allocation concealment as adequate, inadequate, or unclear according to Jüni 2001, and note who was blinded to the interventions in each trial. However, since the introduction of Review Manager 2011, we made these assessments using the methods described in Higgins 2011.
In keeping with the Cochrane Collaboration policy to use 'Summary of findings' tables, which was introduced after publication of the protocol, we generated them using GRADE profiler (GRADE 2008) and interpreted the evidence for each outcome and comparison using the GRADE approach (Schünemann 2008).
We revised the list of outcomes to reflect current WHO standards for assessing outcomes in antimalarial trials.
Although gametocyte carriage was not included as an outcome in the protocol, we included it as a secondary outcome due to its importance in malaria transmission.
In the protocol we stated that we intended to assess the effectiveness of pyronaridine both as a monotherapy and in combination with an artemisinin. However, we revised this to focus only on pyronaridine-artemisinin combinations. In addition, due to concerns regarding pyronaridine's effect on the liver, assessment of the effects of the comparisons on liver function now include randomized comparisons in both falciparum and vivax malaria. Accordingly, we updated the background and methods sections considerably to reflect the changing scenario in malaria policies and epidemiology.
PT and HB joined the review team. Rajeev Aravindakshan withdrew from the team due to conflicting demands on his time.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kayentao 2012
| Methods |
Trial design: Randomized, multicentre, open-label, active-controlled, parallel group, non-inferiority trial Period of trial: November 2007 to November 2008 |
|
| Participants |
Number randomized: 535 Age: Three months to 12 years Gender: Both Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Randomized 2:1 to Intervention:
Control:
*Artesunate-pyronaridine was given once daily: 5 kg to < 9 kg, one sachet; 9 kg to <17 kg, two sachets; 17 kg to < 25 kg, three sachets (dose range 6.7/2.2 to 13.3/4.4 mg/kg/dose mixed in water, milk, or soup) **Artemether-lumefantrine was given twice daily crushed and shaken to a suspension in 50 mL water: 5 kg to < 15 kg, one tablet; 15 kg to < 25 kg, two tablets (dose range 1.3/8.0 to 4.0/24.0 mg/kg/dose); the second day 0 dose was 8 hrs after the first dose, the first Day 1 dose was 24 hrs after the first Day 0 dose, with all subsequent doses 12 hrs apart. |
|
| Outcomes | Primary outcomes: Efficacy
Safety
Secondary outcomes:
Exploratory efficacy outcomes:
Outcomes reported but not used in quantitative synthesis in this review
|
|
| Notes |
Countries of recruitment: Six countries in Africa (96.3%; Burkina Faso, Democratic Republic of Congo, Gabon, Côte d’Ivoire, Kenya, and Mali) and one in Asia (3.7%; The Philippines). Setting: Local hospitals and clinics at seven centres in six countries in Africa and one in the Philippines Funding: Medicines for Malaria Venture, Poong Pharmaceutical Company Ltd, Seoul, Republic of Korea Endemicity: High Duration of follow-up: 42 days Comment:
Trials registration: ClinicalTrials.gov: identifier NCT00541385 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "The sponsor provided a computer-generated randomisation schedule. Patients were randomised 2:1 to artesunate-pyronaridine or artemether-lumefantrine". |
| Allocation concealment (selection bias) | Low risk | Quotes from report: " Individually numbered treatment packs of similar appearance were masked on allocation." Quote from report: "The study sponsor remained blinded to treatment allocation". |
| Blinding (performance bias and detection bias) Objective outcomes: parasitological and biochemical | Low risk | Quote from report: "Drugs were given open-label". Quote from report: ""Clinical assessments and drug administration were performed by different clinical personnel." Comment: Unlikely to have introduced detection bias for objective outcomes. |
| Blinding (performance bias and detection bias) Subjective outcomes: adverse events | Low risk | Comment: Outcome assessors were blinded to allocation, and were not involved in drug administration. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: The participants randomized were accounted for in the trial report and missing data and participants were not differentially distributed in treatment arms, or substantial. |
| Selective reporting (reporting bias) | Low risk | Comment: This trial was prospectively registered and though some changes in the timing of assessments were noticed between the protocol and the trial report, these are not of much importance; all other pre-stated outcomes were adequately reported. |
| Other bias | Low risk | Quote: "The sponsors and study site principal investigators developed the protocol, interpreted the data and developed the report. The study sponsors were responsible for data collection and statistical analysis. All authors had access to the primary data, take responsibility for data reporting accuracy and completeness". Comment: Three of the authors are employed by the study sponsors. However, the report states the study sponsors were blind to treatment allocation, and the final report appears to have been approved by all authors. |
Poravuth 2011
| Methods |
Trial design: Randomized, multicentre, double-blind, double-dummy, parallel-group, non-inferiority trial Period of trial: March 2007 to March 2008 |
|
| Participants |
Number randomized: 456 Age range: Seven years to 60 years Gender: Both Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: Artesunate-pyronaridine tablets (180:60 mg) once daily for three days* (N = 228) Control: Chloroquine based on body weight once daily for three days** (N = 228) *For artesunate-pyronaridine, drug dose was based on body weight: 20 kg to 25 kg, 1 tablet; 26 kg to 44 kg, two tablets; 45 kg to 64 kg, three tablets; and 65 kg to 90 kg, four tablets, (giving a artesunate-pyronaridine target dose of between 7.2:2.4 mg/kg and13.8:4.6 mg/kg). **The chloroquine dose for adults was 620 mg on Day 0 and 1, and 310 mg on Day 2. The chloroquine target dose for children was 10 mg/kg on Days 0 and 1, and 5 mg/kg on Day 2. |
|
| Outcomes | Outcomes used in this review:
Outcomes reported but not used in this review:
|
|
| Notes |
Countries of recruitment: Four countries in Asia (Cambodia, India, Indonesia, and Thailand) Setting: Five local hospitals in four countries in Asia Funding: Medicines for Malaria Venture, Poong Pharmaceutical Company Ltd, Seoul, Republic of Korea Endemicity: High Duration of follow-up: Until day 42 Comment:
Trials registration: ClinicalTrials.gov identifier: NCT00440999 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "A computer-generated randomisation scheme was provided by the sponsor. Subjects were randomised 1:1 within each study site in blocks of six." |
| Allocation concealment (selection bias) | Low risk | Quotes from report: "Subjects were randomised...to receive either artesunate-pyronaridine plus matching chloroquine placebo or oral chloroquine plus matching artesunate-pyronaridine placebo". "The subject was allocated an individually numbered treatment pack, which contained sufficient tablets for 3 days' therapy plus an overage bottle containing tablets in case the subject vomited the first dose. All study investigators, laboratory technicians and patients were blind to treatment assignment". "Sealed opaque envelopes containing the study medication assignment for each subject were provided to the study site investigator for use in an emergency; no code breaks were required." |
| Blinding (performance bias and detection bias) Objective outcomes: parasitological and biochemical | Low risk | Quotes from report: "Study drugs were administered on a double-blind, double-dummy basis. The investigator calculated the appropriate dose and study drug was administered by a different member of staff, designated by the investigator". "Active drugs and placebos were packaged similarly." |
| Blinding (performance bias and detection bias) Subjective outcomes: adverse events | Low risk | Comment: The double-blind, double-dummy design used minimized the risk of performance and detection bias. Pruritis that is common with chloroquine could potentially compromise blinding but was not reported in = 2% of participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from report: " Most patients (83.3%) completed the study. A similar number of patients withdrew prematurely from the study in both groups." Comment: The results were assessed in per-protocol and intention-to-treat analyses. |
| Selective reporting (reporting bias) | Low risk | Comment: This trial was prospectively registered and reported all pre-stated outcomes adequately. |
| Other bias | Low risk | Quote from report: "The sponsors and study site principal investigators developed the protocol, interpreted the data and developed the report. The study sponsors were responsible for data collection and statistical analysis. All authors had access to the primary data, take responsibility for data reporting accuracy and completeness and had responsibility for the final decision to submit for publication." Comment: Some of the authors are employed by the trial sponsors but all authors had access to data and assumed responsibility for reporting accuracy. |
Ringwald 1996
| Methods |
Trial design: Randomized, parallel group, active controlled trial Duration of trial: Recruitment: April 1994 to May 1995 |
|
| Participants |
Number randomized: 96 Age: 15 to 64 years Gender: 42 males; 54 females
Exclusion criteria:
|
|
| Interventions | Intervention 1. Pyronaridine: 32 mg/kg in divided doses over 3 days* (N = 47) Control 2. Chloroquine: 25 mg/kg in divided doses over 3 days (N = 49) * Pyronaridine dose: 16 mg/kg on day 1 and 8 mg/kg on days 2 and 3 * Chloroquine dose: 10 mg/kg on days 1 and 2 and 5 mg/kg on day 3 |
|
| Outcomes | Outcome used in this review:
Outcomes reported but used in this review:
|
|
| Notes |
County of recruitment: Cameroon Setting: Nlongkak Catholic missionary dispensary in Yaounde; outpatients; all doses of drugs supervised Source of funding: French Ministere de la Cooperation (Grant 93A43); pyronaridine was supplied by the Institute of Parasitic Diseases, Chinese Academy of Preventive Medicine, Shangai, China Endemicity: High; 50% to 60% chloroquine resistant Duration of follow-up: Until day 14 (for all, and in four participants until day 238) Comment: Proportions with normal transaminase enzyme levels at baseline that were elevated in each arm at day 7 provided in the text of results were used for analysis; the extent of elevation was not reported. The values were normal on day 14 on whom the levels were repeated, but the numbers in whom these were repeated are not reported Trials registration: Nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "Patients were randomly assigned in blocks". Comment: Unpublished information provided through correspondence with authors reveal that randomisation was done "in blocks of 10". |
| Allocation concealment (selection bias) | Low risk | Comment: Not mentioned in report. Quote from correspondence with senior author: "central randomisation was used". |
| Blinding (performance bias and detection bias) Objective outcomes: parasitological and biochemical | Low risk | Comment: Not mentioned in report. Quote from correspondence: "It was blinded but the tablets were different and many patient treated with CQ suffered of pruritus". Comment: Blinding was probably compromised but risk of bias due to this is unlikely to have affected the biochemical outcomes assessing liver functions that were used in this review. |
| Blinding (performance bias and detection bias) Subjective outcomes: adverse events | Low risk | Comment: As above. Comment: Blinding was probably compromised and risk of bias due to this may have affected the reporting or detection of some subjective adverse events, but only biochemical liver functions were used in this review. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from report: "After enrolment, six patients treated with chloroquine and five patients treated with pyronaridine were lost to follow-up. Two additional patients from each group were withdrawn because of self-medication with quinine". Quote from correspondence: "Drop out were mainly lost to follow-up and most often after the patients were cured. We do not think that it was related to intervention." Comment: Equal numbers dropped out from each intervention arm and hence are unlikely to have differentially influenced liver toxicity outcomes used in this review. |
| Selective reporting (reporting bias) | Low risk | Comment: The trial was not prospectively registered and the trial protocol was not available, but all outcomes stated in methods were reported. |
| Other bias | Unclear risk | Comment: The extent of elevation in liver transaminases and the proportions re-tested at day 14 were not reported. |
Ringwald 1998
| Methods |
Trial design: Randomized, parallel group, active controlled trial Duration of trial: 1996; duration not stated |
|
| Participants |
Number randomized: 88 Age: Children in the age range five to 15 years Gender: Both Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: 1. Pyronaridine: 32 mg/kg (N = 48) (16 mg/kg on day 0, in two divided doses; 8 mg/kg on days 1 and 2) Control: 2. Chloroquine: 35 mg/kg (N = 48) (10 mg on days 0 and 1; 5 mg on day 2) |
|
| Outcomes | Outcomes used in this review: Proportions with normal serum aspartate aminotransferase (AST) enzyme levels at baseline and two-fold or greater elevations at day 7 Outcomes reported but not used in this review:
|
|
| Notes |
Country of recruitment: Cameroon Setting: Nlognkak Catholic missionary dispensary in Yaounde; outpatients. All interventions were supervised Source of funding: Pyronaridine provided by Institute of Parasitic Diseases, Chinese Academy of Preventive Medicine, Shanghai, China Endemicity: High; 50 to 60% chloroquine resistant Duration of follow-up: Until day 14 Comment: Proportions on whom transaminase enzyme levels were repeated on day 14 and in those in whom they were normal were not reported Trials registration: Nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "Patients were randomly assigned in blocks". Comment: Unpublished information provided by authors suggest that randomisation was done in "blocks of 10." |
| Allocation concealment (selection bias) | Low risk | Comment: Not mentioned in trial report. Quote from correspondence with authors: "central randomisation was used." |
| Blinding (performance bias and detection bias) Objective outcomes: parasitological and biochemical | Low risk | Comment: Not mentioned in report. Quote from correspondence: "It was blinded but the tablets were different and many patient treated with CQ suffered of pruritus". Comment: Blinding was probably compromised but risk of bias due to this may not have affected the reporting of liver enzymes that was the outcome used in this review. |
| Blinding (performance bias and detection bias) Subjective outcomes: adverse events | Low risk | Comment: As above. Comment: Blinding was probably compromised and risk of bias due to this may have affected the reporting or detection of some subjective adverse events, but not the objective outcome used in this review. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from report: "Of the 88 patients enrolled in the study, 81 completed the14-day follow-up (dropout rate, 8%). Three patients in the pyronaridine group and four in the chloroquine group were lost to follow-up." Quote from correspondence: "Drop out were mainly lost to follow-up and most often after the patients were cured. We do not think that it was related to intervention." Comment: Equal numbers dropped out from each intervention arm and hence are unlikely to have differentially influenced the outcomes used in this review. |
| Selective reporting (reporting bias) | Low risk | Comment: The trial was not prospectively registered and the trial protocol was not available, but all outcomes stated in methods were reported. |
| Other bias | Unclear risk | Comment: The proportions re-tested for liver transaminases at day 14 and the proportions in whom they were normal were not reported. |
Rueangweerayut 2012
| Methods |
Trial design: Randomized, multicentre, parallel-group, double-blind, double-dummy, non-inferiority trial Duration of trial: January 2007 to October 2008 |
|
| Participants |
Numbers randomized: 1271 Age: Four years to 59 years Gender: Both Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Randomized in a 2:1 ratio to: Intervention: 1. Artesunate-pyronaridine combination (7.2: 2.4 mg/kg respectively) once a day for three days (N = 848) Control: 2. Mefloquine plus artesunate combination (6.2 to 12.5 mg/kg and 2.2 to 5.0 mg/kg respectively) once a day for three days (N = 423) |
|
| Outcomes | Primary outcome
Secondary outcomes
Exploratory efficacy outcomes
Safety outcomes Incidence of adverse events Outcomes reported but not used in quantitative synthesis
|
|
| Notes |
Countries of recruitment: Four countries in Asia (Cambodia, India, Thailand, and Vietnam; 81.3%); and three countries in Africa (Bukina Faso, Ivory Coast, Tanzania; 18.7%) Setting: Local hospitals and health centres Endemicity: High in most sites Source of funding: Primary sponsor: Medicines for Malaria Venture; secondary sponsor: Shin Poong Pharmaceuticals Duration of follow-up: Until day 42 Comments:
Trials registration: ClinicalTrials.gov identifier: NCT00403260 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "Averion International (now part of Aptiv Solutions) provided the computer generated randomisation schedule." Quote from trial protocol, "Patients who meet all entry criteria and present no exclusion criteria will be randomised to receive either pyronaridine artesunate or mefloquine plus artesunate in a 2:1 ratio according to the randomisation scheme provided by the sponsor. Patients will be assigned, in ascending order, a randomisation number according to the order recruited". |
| Allocation concealment (selection bias) | Low risk | Quote from trial protocol: "The patient will be allocated an individual numbered treatment pack which contains sufficient tablets for 3 days therapy plus an overage bottle containing tablets in case the patient vomits the first dose." Quote from trial protocol, "Clinical study material will be administered using a third-party single blind design. That is: after determining the eligibility criteria, the investigator shall communicate the patient randomisation number to a qualified study team member (third party) who is not performing clinical assessments. The third party will open the study package and administer the correct amount of tablets as instructed by the investigator to ensure unbiased randomisation." |
| Blinding (performance bias and detection bias) Objective outcomes: parasitological and biochemical | Low risk | Quote from report: "Drugs were administered by an investigator who was aware of group assignments; clinical and parasitologic assessments were performed by investigators who were aware of group assignments". Comment: The use of blinded outcome assessors minimised the risk of detection bias for objective outcomes. |
| Blinding (performance bias and detection bias) Subjective outcomes: adverse events | Low risk | Quote from report: "Clinical and parasitological assessments were performed by investigators who were not aware of group assignments." Comment: Most of the outcomes used in this review were objective outcomes, so participant's knowledge of treatment allocation is not likely to introduce bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Data regarding all participants recruited provided in results for all outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: The trial was prospectively registered and the protocol was also available; all pre-stated outcomes were reported adequately. |
| Other bias | Low risk | Quote from report: "The study was designed by the authors and the study sponsors, the Medicines for Malaria Venture and Shin Poong Pharmaceutical Company. All the authors vouch for the completeness and accuracy of the data and the analysis and for the fidelity of the study to the protocol". Quote from report: "No potential conflict of interest relevant to this article was reported". Comment: Some of the authors are employed by the trial sponsors but all authors had access to data and assumed responsibility for reporting accuracy. |
Tshefu 2010
| Methods |
Trial design: Randomized, multi centre, parallel-group, double-blind, double-dummy, non-inferiority trial Period of trial: January 2007 to April 2008 |
|
| Participants |
Number randomized: 1272 Age range: Five to 60 years Gender: Both Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Randomized in a 2:1 ratio to: Intervention: 1. artesunate-pyronaridine combination (180 mg and 60 mg)* once a day for three days according to bodyweight (N = 849) Control: 2. Artemether-lumefantrine combination (20 mg and 120 mg)** twice a day for three days according to bodyweight (N = 423) *Average dose of pyronaridine: 9 mg/Kg body weight (range 13.8 to 7.2 mg/ Kg); artesunate doses ranged from 2.3 to 4.7 mg/kg body weight **Mean artemether dose: 1.7 mg/kg (range 0.9 to 2.4 mg/kg); lumefantrine doses ranged from 5 to 14.4 mg/kg |
|
| Outcomes | Primary outcome
(Sensitivity analysis done with crude APCR (non-PCR-corrected) at day 28) Secondary outcomes
Exploratory efficacy outcomes
Safety outcomes
Outcome reported but not used in quantitative synthesis in this review
|
|
| Notes |
Countries of recruitment: Seven countries in Africa (Democratic Republic of Congo, The Gambia, Ghana; Kenya; Mali; Mozambique; and Senegal) recruited over 1000 participants; remainder were from three sites in two countries in southeast Asia (two in Indonesia; one in the Phillipines) Setting: Local hospitals and clinics Source of funding: Primary sponsor: Medicines for Malaria Venture; secondary sponsor: Shin Poong Pharmaceuticals Endemicity: All are high endemic areas Comments:
Trials registration: ClinicalTrials.gov identifier: NCT00422084 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "A computer generated randomisation schedule was provided by Averion AG (Allschwil, Switzerland). Patients were assigned a randomisation code by the investigator in ascending order and allocated to treatment in blocks of nine by study centre." |
| Allocation concealment (selection bias) | Low risk | Quote from report: "Patients were allocated an individual numbered treatment pack containing sufficient tablets for 3 days therapy plus an overage bottle containing an extra dose in case the patient vomited the first dose. Study packages were allocated on the basis of patient randomisation number. A qualified study team member (third party) who was not undertaking clinical assessments opened the study package and administered the correct amount of tablets, based on patient weight at screening, as instructed by the investigator." |
| Blinding (performance bias and detection bias) Objective outcomes: parasitological and biochemical | Low risk | Quote from report: "All clinical and laboratory staff and patients were masked to treatment allocation." Quote from report: "Study drugs and placebos were presented in identical packaging. Artemether-lumefantrine placebo was dosed twice daily to maintain blinding. Placebos were of similar shape and colour to their respective active drug." Quote from report: "Sealed opaque envelopes of treatment allocation were provided for use in an emergency, although no code breaks were necessary." Quote from report: "Food was not required for artemether-lumefantrine dosing to retain blinding." |
| Blinding (performance bias and detection bias) Subjective outcomes: adverse events | Low risk | Comment: See quotes above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Data regarding all participants recruited provided in results for all outcomes. |
| Selective reporting (reporting bias) | Low risk | Quote from report: "There were no changes to study outcomes after trial commencement." Comment: The trial was prospectively registered. No changes were noted in the details provided in the registration document and the study report for outcomes. Comment: Day 42 efficacy outcomes and gametocyte counts were not listed in trial registration document and are listed in the report as exploratory. |
| Other bias | Unclear risk | Comment: Sponsors designed the trial, were responsible for data collection and analysis, and developed the report; all authors had access to trial data. Comment: Participants on artemether-lumefantrine were not expected to take medication after food; unclear if this reduced bioavailability of lumefantrine, particularly for day 42 outcomes and reinfection rate when lumefantrine levels may have been low. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cai 1999 | Not a RCT: Case series. |
| Chang 1997 | Not a RCT: Case series. |
| Che 1987 | Quasi-RCT. Odd and even numbers used for allocation. |
| Che 1990 | Not a RCT: Controlled clinical trial. |
| Chen 1989 | Not a RCT: Field trial. |
| Fleckenstein 2007 | Not a RCT: Controlled clinical trial evaluating drug pharmacokinetics. |
| Huang 1988 | Quasi-RCT. Randomized according to order of admission. |
| Huang 1989 | Quasi-RCT. Odd and even numbers used for allocation. Compared plain and enteric coated tablets of pyronaridine |
| Huang 1993 | RCT: Conducted in people with complicated falciparum malaria (malignant malaria). |
| Huang 1996 | RCT: Compared single dose versus two days of the same drug combination. |
| Liu 2002 | RCT: Compared pyronaridine + dihydroartemisinin versus dihydroartemisinin alone and with pyronaridine alone. No ACT comparator; no data on liver functions provided in report to include for "Adverse event affecting liver functions". |
| Looareesuwan 1996 | Not a RCT: Clinical trial of two doses of pyronaridine monotherapy with group given second dose recruited after results of first dose were analysed. |
| Looareesuwan 2007 | RCT: Phase II dose ranging trial. |
| NCT01156389 | RCT: ongoing trial evaluating drug interactions in healthy volunteers. |
| No authors listed 1985 | Not a RCT: field trial. |
| Pang 1989 | Not a RCT: controlled clinical trial comparing tablets versus intramuscular injections of pyronaridine/sulfadoxine/pyrimethamine. |
| Piola 2008 | Not a RCT: phase II dose ranging study. |
| Ramharter 2008 | Quasi-RCT. Sequential allocation; Phase II dose ranging trial. |
| Shao 1991 | Not a RCT: case series. |
| Tan 2008 | Not a RCT: Pharmacokinetic study in healthy volunteers. |
| Wattanavijitkul 2008 | Not a RCT: Pharmacokinetic study in healthy volunteers. |
Analysis 1.1.
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 1 Total failure (Day 28).
Analysis 1.2.
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 2 Total failure (Day 42).
Analysis 1.3.
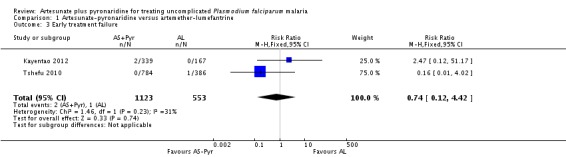
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 3 Early treatment failure.
Analysis 1.4.
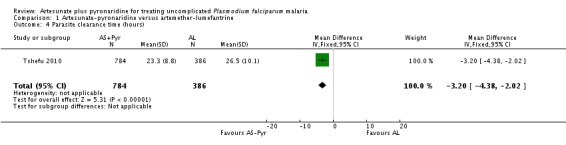
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 4 Parasite clearance time (hours).
Analysis 1.5.
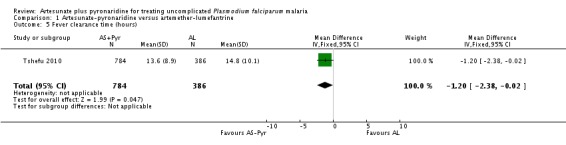
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 5 Fever clearance time (hours).
Analysis 1.6.
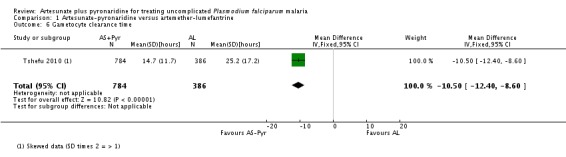
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 6 Gametocyte clearance time.
Analysis 1.7.
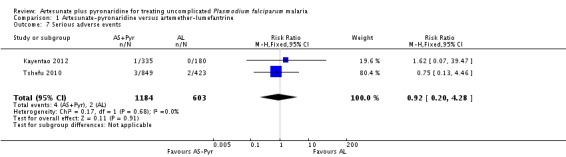
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 7 Serious adverse events.
Analysis 1.8.
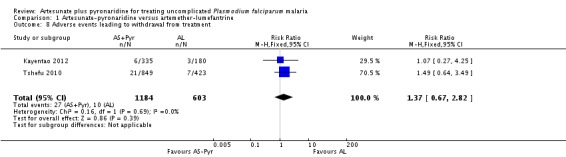
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 8 Adverse events leading to withdrawal from treatment.
Analysis 1.9.
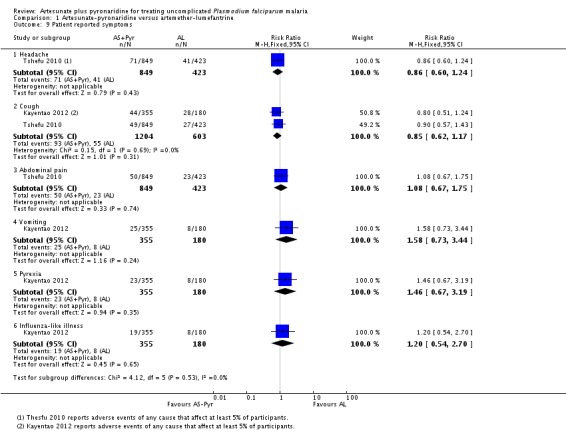
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 9 Patient reported symptoms.
Analysis 1.10.
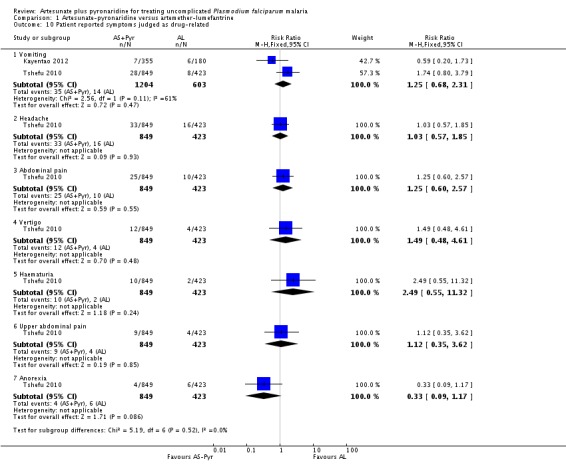
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 10 Patient reported symptoms judged as drug-related.
Analysis 1.11.
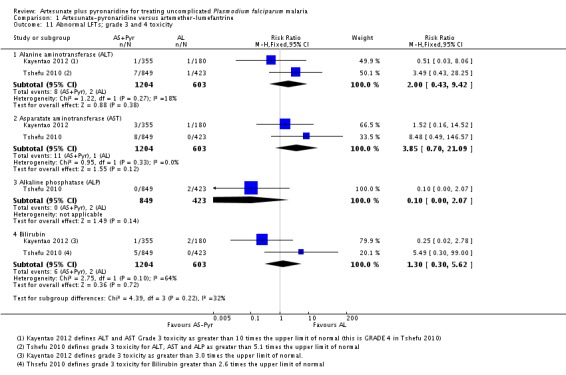
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 11 Abnormal LFTs; grade 3 and 4 toxicity.
Analysis 1.12.
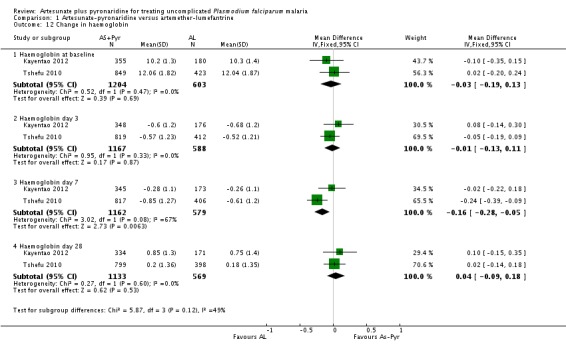
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 12 Change in haemoglobin.
Analysis 1.13.
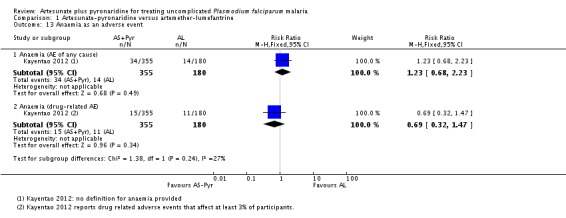
Comparison 1 Artesunate-pyronaridine versus artemether-lumefantrine, Outcome 13 Anaemia as an adverse event.
Analysis 2.1.
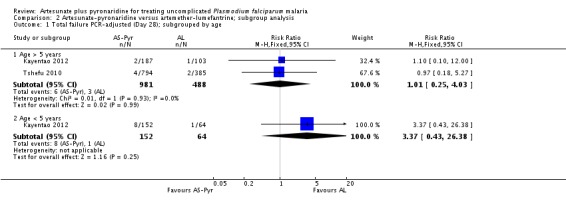
Comparison 2 Artesunate-pyronaridine versus artemether-lumefantrine; subgroup analysis, Outcome 1 Total failure PCR-adjusted (Day 28); subgrouped by age.
Analysis 2.2.
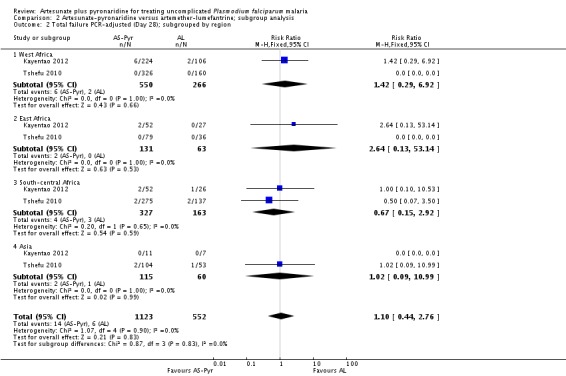
Comparison 2 Artesunate-pyronaridine versus artemether-lumefantrine; subgroup analysis, Outcome 2 Total failure PCR-adjusted (Day 28); subgrouped by region.
Analysis 2.3.
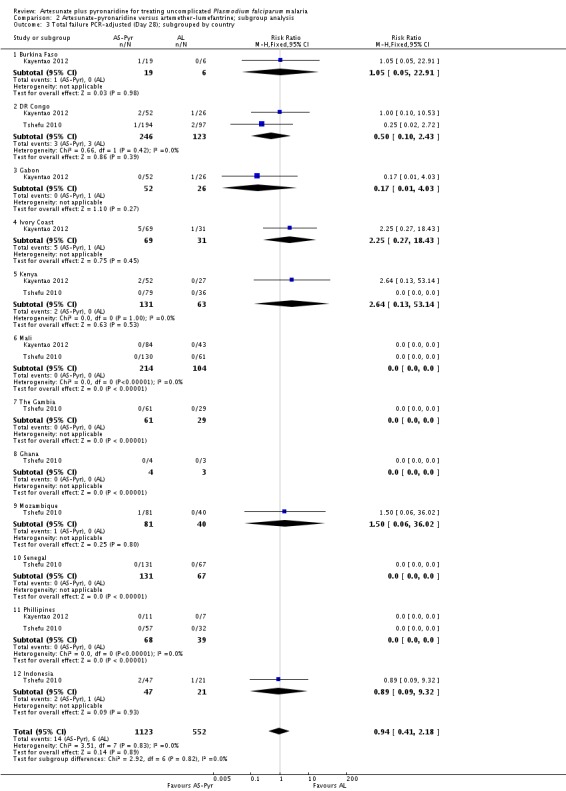
Comparison 2 Artesunate-pyronaridine versus artemether-lumefantrine; subgroup analysis, Outcome 3 Total failure PCR-adjusted (Day 28); subgrouped by country.
Analysis 3.1.
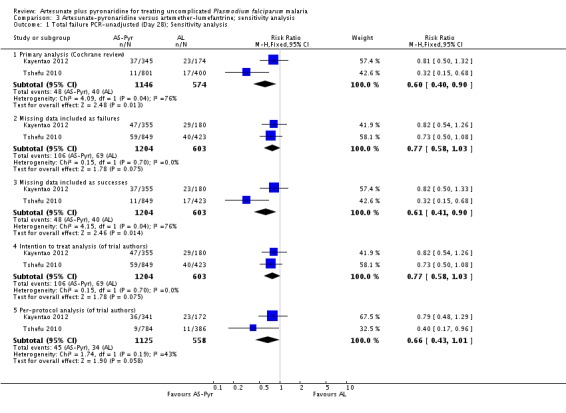
Comparison 3 Artesunate-pyronaridine versus artemether-lumefantrine; sensitivity analysis, Outcome 1 Total failure PCR-unadjusted (Day 28); Sensitivity analysis.
Analysis 3.2.
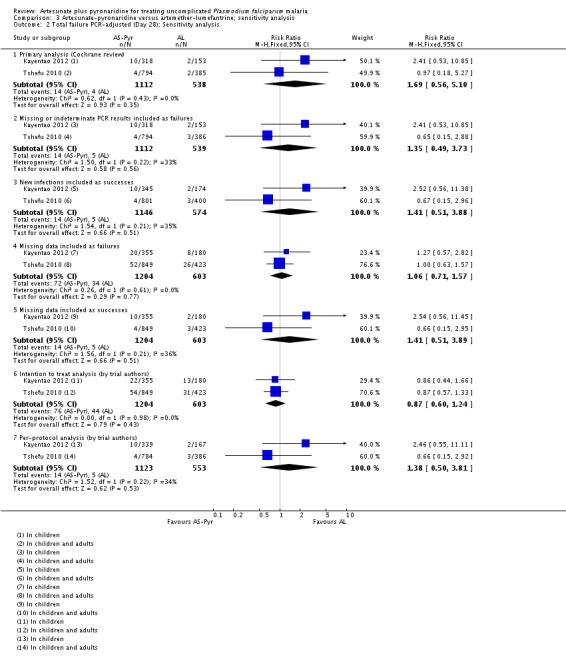
Comparison 3 Artesunate-pyronaridine versus artemether-lumefantrine; sensitivity analysis, Outcome 2 Total failure PCR-adjusted (Day 28); Sensitivity analysis.
Analysis 4.1.
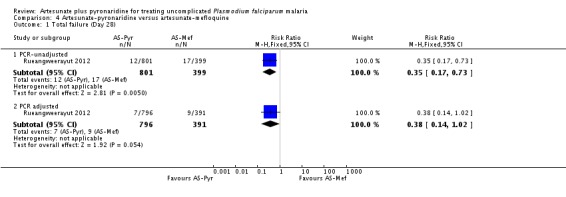
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 1 Total failure (Day 28).
Analysis 4.2.
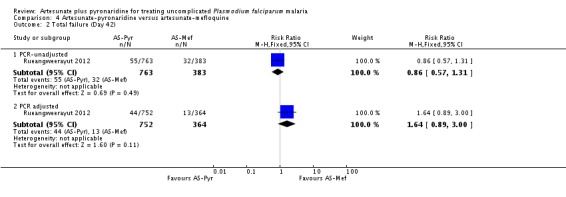
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 2 Total failure (Day 42).
Analysis 4.3.
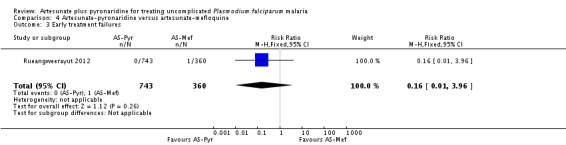
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 3 Early treatment failures.
Analysis 4.4.
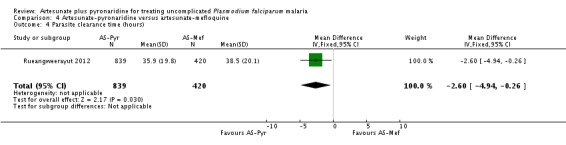
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 4 Parasite clearance time (hours).
Analysis 4.5.
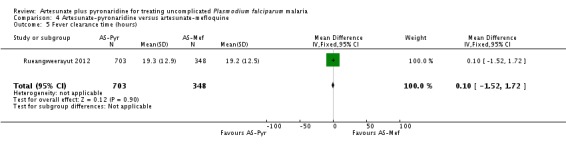
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 5 Fever clearance time (hours).
Analysis 4.6.
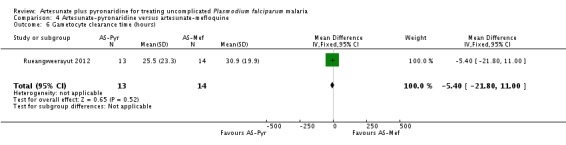
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 6 Gametocyte clearance time (hours).
Analysis 4.7.
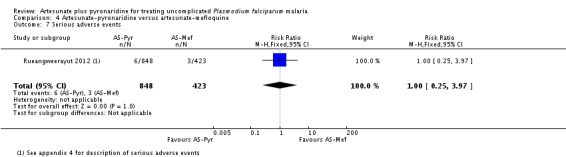
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 7 Serious adverse events.
Analysis 4.8.
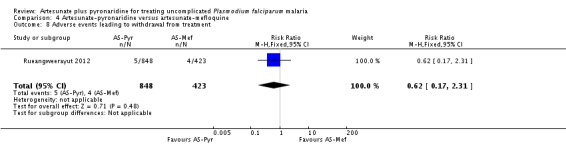
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 8 Adverse events leading to withdrawal from treatment.
Analysis 4.9.
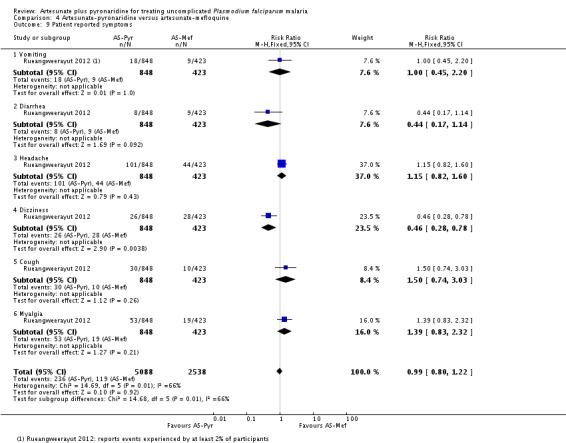
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 9 Patient reported symptoms.
Analysis 4.10.
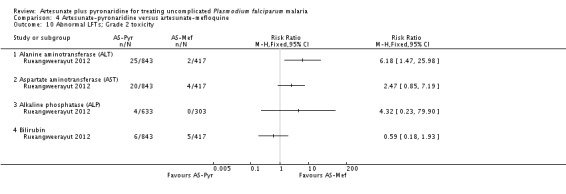
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 10 Abnormal LFTs; Grade 2 toxicity.
Analysis 4.11.
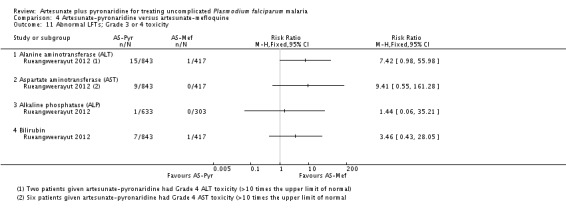
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 11 Abnormal LFTs; Grade 3 or 4 toxicity.
Analysis 4.12.
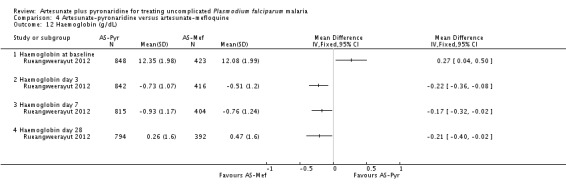
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 12 Haemoglobin (g/dL).
Analysis 4.13.
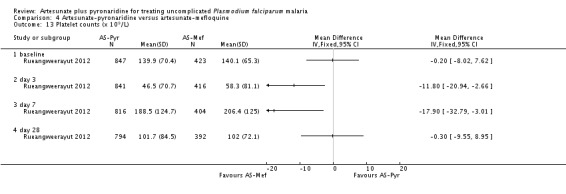
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 13 Platelet counts (x 109/L).
Analysis 4.14.
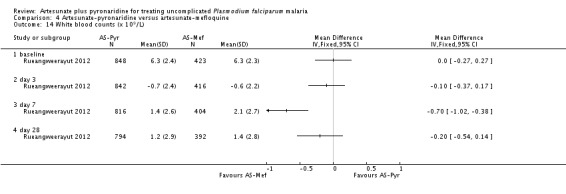
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 14 White blood counts (x 109/L).
Analysis 4.15.
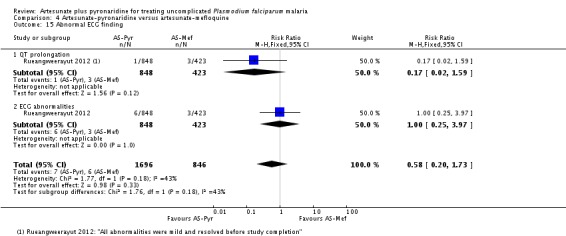
Comparison 4 Artesunate-pyronaridine versus artesunate-mefloquine, Outcome 15 Abnormal ECG finding.
Analysis 5.1.
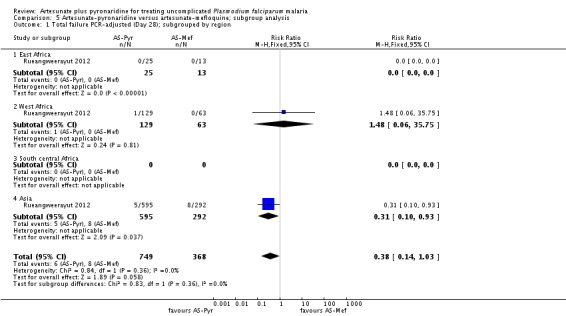
Comparison 5 Artesunate-pyronaridine versus artesunate-mefloquine; subgroup analysis, Outcome 1 Total failure PCR-adjusted (Day 28); subgrouped by region.
Analysis 5.2.
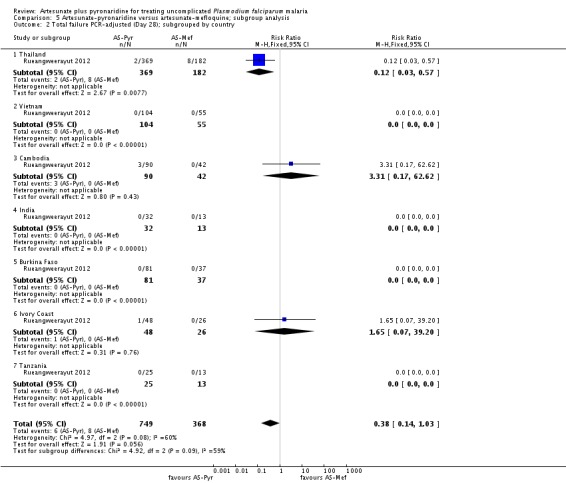
Comparison 5 Artesunate-pyronaridine versus artesunate-mefloquine; subgroup analysis, Outcome 2 Total failure PCR-adjusted (Day 28); subgrouped by country.
Analysis 6.1.
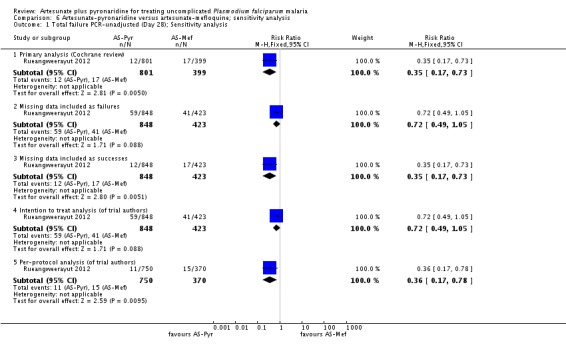
Comparison 6 Artesunate-pyronaridine versus artesunate-mefloquine; sensitivity analysis, Outcome 1 Total failure PCR-unadjusted (Day 28); Sensitivity analysis.
Analysis 6.2.
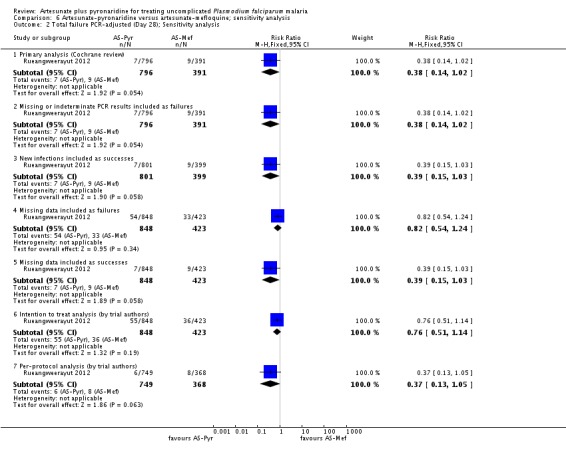
Comparison 6 Artesunate-pyronaridine versus artesunate-mefloquine; sensitivity analysis, Outcome 2 Total failure PCR-adjusted (Day 28); Sensitivity analysis.
Analysis 7.1.
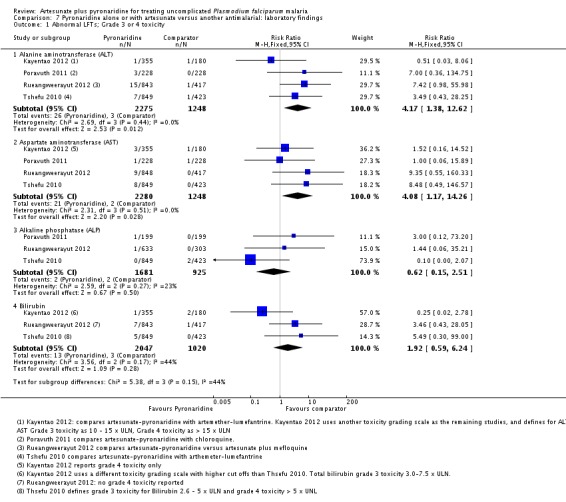
Comparison 7 Pyronaridine alone or with artesunate versus another antimalarial: laboratory findings, Outcome 1 Abnormal LFTs; Grade 3 or 4 toxicity.
Analysis 7.2.
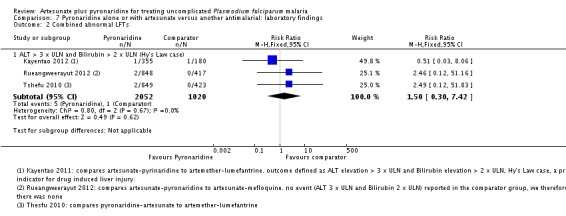
Comparison 7 Pyronaridine alone or with artesunate versus another antimalarial: laboratory findings, Outcome 2 Combined abnormal LFTs.
Analysis 7.3.
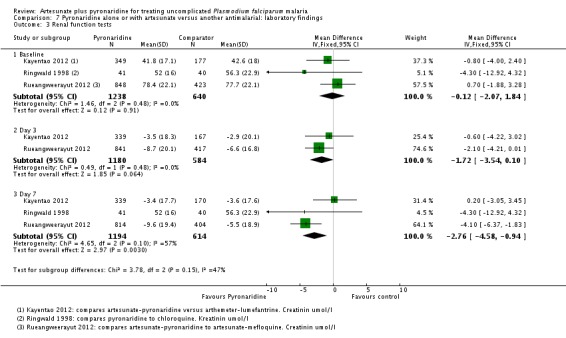
Comparison 7 Pyronaridine alone or with artesunate versus another antimalarial: laboratory findings, Outcome 3 Renal function tests.
Analysis 7.4.
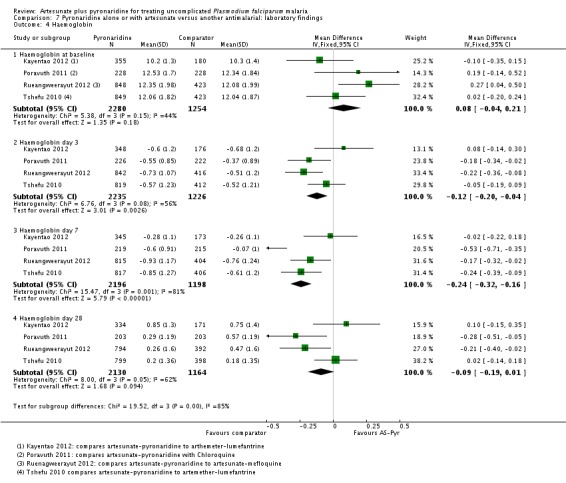
Comparison 7 Pyronaridine alone or with artesunate versus another antimalarial: laboratory findings, Outcome 4 Haemoglobin.
Analysis 7.5.
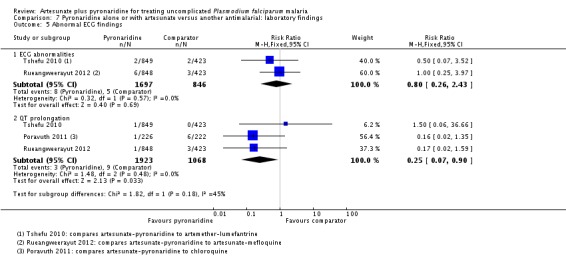
Comparison 7 Pyronaridine alone or with artesunate versus another antimalarial: laboratory findings, Outcome 5 Abnormal ECG findings.
Summary of Findings for the Main Comparison[Explanation]
| Artesunate-pyronaridine compared to artemether-lumefantrine for treating people with uncomplicated falciparum malaria | |||||
| Patient or population: Adults and children with uncomplicated falciparum malaria Settings: Malaria endemic areas in Africa and Asia Intervention: Artesunate-pyronaridine Comparison: Artemether-lumefantrine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect(95% CI) | No of participants(trials) | Quality of the evidence(GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Artemether-lumefantrine | Artesunate-pyronaridine | ||||
| Treatment failure (day 28) | PCR-unadjusted | RR 0.60 (0.40 to 0.90) | 1720(2 trials) | ⊕⊕⊕○ moderate 1,2,3,4 | |
| 7 per 100 | 4 per 100 (3 to 6) | ||||
| PCR-adjusted | RR 1.69 (0.56 to 5.10) | 1650(2 trials) | ⊕⊕⊕○ moderate 1,2,3,5 | ||
| 1 per 100 | 1 per 100 (0 to 4) | ||||
| Treatment failure (Day 42) | PCR-unadjusted | RR 0.85 (0.53 to 1.36) | 1691 (2 trials) |
⊕⊕⊕○ moderate 1,2,3,5 | |
| 17 per 100 | 15 per 100 (9 to 23) | ||||
| PCR-adjusted | RR 1.53 (0.73 to 3.19) | 1472(2 trials) | ⊕⊕○○low 1,6,3,5 | ||
| 2 per 100 | 3 per 100 (1 to 6) | ||||
| The assumed risk is the mean risk across the trials in those treated with artemether-lumefantrine. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: Both trials were well conducted and at low risk of bias.
2 No serious inconsistency: The trend was towards benefit with artesunate-pyronaridine in both trials but only reached statistical significance in one.
3 Downgraded by one for serious indirectness: The two trials were conducted in children aged between three months and 12 years and had trial sites in Africa and Asia. However across both trials only 152 children aged < five years received artesunate-pyronaridine, and only 115 children in total were randomized to artesunate-pyronaridine in Asia. Further adequately powered studies in children in Africa and adults and children in Asia would be needed to fully generalize this result.
4 No serious imprecision: The result is statistically significant and the meta-analysis is adequately powered. However, it should be noted that these multicentred trials are underpowered to show equivalence at the country level. We did not downgrade.
5 No serious imprecision: The finding is of no substantial difference between the two ACTs. However, it should it should be noted that these multicentred trials are underpowered to show equivalence at the country level. We did not downgrade.
6 Downgraded by one for serious inconsistency: Although statistical heterogeneity was low, PCR-adjusted treatment failure was above 5% in on the one trial recruiting children aged < five years.
7 For adverse events see the additional Summary of Findings table in Appendix 2.
Artesunate-pyronaridine compared to artesunate plus mefloquine for treating uncomplicated P. falciparum malaria
| Artesunate-pyronaridine compared to artesunate plus mefloquine for treating people with uncomplicated P. falciparum malaria | |||||
| Patient or population: People with uncomplicatedP. falciparum malaria Settings: Malaria endemic areas in Africa and Asia Intervention: Artesunate-pyronaridine Comparison: Artesunate plus mefloquine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect(95% CI) | No of participants(trials) | Quality of the evidence(GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Artesunate-mefloquine | Artesunate-pyronaridine | ||||
| Treatment failure (Day 28) | PCR-unadjusted | RR 0.35 (0.17 to 0.73) | 1200(1 trial) | ⊕⊕⊕○ moderate 1,2,3,4 | |
| 4 per 100 | 2 per 100 (1 to 2) | ||||
| PCR-adjusted | RR 0.38 (0.14 to 1.02) | 1187(1 trial) | ⊕⊕⊕○ moderate 1,2,3,5 | ||
| 2 per 100 | 1 per 100 (0 to 2) | ||||
| Treatment failure (Day 42) | PCR-unadjusted | RR 0.86 (0.57 to 1.31) | 1146(1 trial) | ⊕⊕⊕○ moderate 1,2,3,5 | |
| 8 per 100 | 7 per 100 (5 to 11) | ||||
| PCR-adjusted | RR 1.64 (0.89 to 3.00) | 1116(1 trial) | ⊕⊕○○low 1,2,3,5,6 | ||
| 4 per 1000 | 6 per 100 (3 to 11) | ||||
| The assumed risk is the risk in the group treated with artesunate plus mefloquine in the single trial. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: This trial was well conducted with low risk of bias.
2 No serious inconsistency: Not applicable as only one trial.
3 Downgraded by one for serious indirectness: Of the 1271 children and adults aged greater than five years enrolled in this trial, 81.3% (1033) were enrolled and treated in trial sites in Asia (Cambodia, India, Thailand, Vietnam), and only 18.7% (237) in Africa (Bukina Faso, Ivory Coast, and Tanzania). Further studies in African children are necessary to fully generalize this result.
4 No serious imprecision: The result is statistically significant and the meta-analysis is adequately powered. However, it should be noted that this multicentred trial is underpowered to show equivalence at the country level. Not downgraded.
5 No serious imprecision: The result is of no clinically important differences between ACTs. However, it should be noted that this multicentred trial is underpowered to show equivalence at the country level. Not downgraded.
6 PCR-adjusted treatment failure was just above 5% with artesunate-pyronaridine in this trial.
7 For adverse events see the additional Summary of Findings table in Appendix 3.
Liver toxicity of pyronaridine compared to other antimalarials
| Liver toxicity of pyronaridine compared to other antimalarials | |||||
| Patient or population: People with uncomplicated falciparum malaria Settings: High and low-transmission settings for P. falciparum and P. vivax malaria Intervention: Pyronaridine alone or with an artemisinin-derivative Comparison: Another antimalarial | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect(95% CI) | Number of participants(trials) | Quality of the evidence(GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Comparator antimalarial | Pyronaridine alone or with artesunate | ||||
| Elevated alanine aminotransaminase levels Grade 3,4 toxicity | 2 per 1000 | 10 per 1000 (3 to 30) | RR 4.17 (1.38 to 12.61) | 3523(4 trials) | ⊕⊕⊕○ moderate 1,2,3,4 |
|
Elevated aspartate aminotransferase levels Grade 3, 4 toxicity |
2 per 1000 | 8 per 1000 (2 to 29) | RR 4.08 (1.17 to 14.26) | 3528(4 trials) | ⊕⊕⊕○ moderate 1,2,3,4 |
| Elevated alkaline phosphatase levels Grade 3, 4 toxicity | 2 per 1000 | 1 per 1000 (0 to 5) | RR 0.62 (0.15 to 2.51) | 2606(3 trials) | ⊕⊕⊕○ moderate 1,2,3,5 |
| Elevated bilirubin Grade 3, 4 toxicity | 3 per 1000 | 6 per 1000 (2 to 19) | RR 1.92 (0.59 to 6.24) | 3067(3 trials) | ⊕⊕○○low 1,2,3,6 |
| *The basis for the assumed risk (for example, the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: Trials were well conducted, although the data analysis was not clearly independent of the drug manufacturer in three trials.
2 No serious inconsistency: Statistical heterogeneity was low.
3 Downgraded by one for serious indirectness: Only 232 children aged less than five years were included in these trials.
4 No serious imprecision: The 95% CI is wide, and there are few events. Larger trials would be necessary to have full confidence in this result but not downgraded.
5 No serious imprecision: The 95% CI is narrow and probably excludes clinically important differences.
6 Downgraded by one for serious imprecision: The 95% CI is wide and includes no difference and clinically important effects.
Data And Analysis
Comparison 1. Artesunate-pyronaridine versus artemether-lumefantrine
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total failure (Day 28) | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 PCR-unadjusted | 2 | 1720 | Risk Ratio (M-H, Fixed, 95% CI) | 0.60 [0.40, 0.90] |
| 1.2 PCR-adjusted | 2 | 1650 | Risk Ratio (M-H, Fixed, 95% CI) | 1.69 [0.56, 5.10] |
| 2 Total failure (Day 42) | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 PCR-unadjusted | 2 | 1691 | Risk Ratio (M-H, Random, 95% CI) | 0.85 [0.53, 1.36] |
| 2.2 PCR-adjusted | 2 | 1472 | Risk Ratio (M-H, Random, 95% CI) | 1.53 [0.73, 3.19] |
| 3 Early treatment failure | 2 | 1676 | Risk Ratio (M-H, Fixed, 95% CI) | 0.74 [0.12, 4.42] |
| 4 Parasite clearance time (hours) | 1 | 1170 | Mean Difference (IV, Fixed, 95% CI) | -3.20 [-4.38, -2.02] |
| 5 Fever clearance time (hours) | 1 | 1170 | Mean Difference (IV, Fixed, 95% CI) | -1.20 [-2.38, -0.02] |
| 6 Gametocyte clearance time | 1 | 1170 | Mean Difference (IV, Fixed, 95% CI) | -10.5 [-12.40, -8.60] |
| 7 Serious adverse events | 2 | 1787 | Risk Ratio (M-H, Fixed, 95% CI) | 0.92 [0.20, 4.28] |
| 8 Adverse events leading to withdrawal from treatment | 2 | 1787 | Risk Ratio (M-H, Fixed, 95% CI) | 1.37 [0.67, 2.82] |
| 9 Patient reported symptoms | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Headache | 1 | 1272 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.60, 1.24] |
| 9.2 Cough | 2 | 1807 | Risk Ratio (M-H, Fixed, 95% CI) | 0.85 [0.62, 1.17] |
| 9.3 Abdominal pain | 1 | 1272 | Risk Ratio (M-H, Fixed, 95% CI) | 1.08 [0.67, 1.75] |
| 9.4 Vomiting | 1 | 535 | Risk Ratio (M-H, Fixed, 95% CI) | 1.58 [0.73, 3.44] |
| 9.5 Pyrexia | 1 | 535 | Risk Ratio (M-H, Fixed, 95% CI) | 1.46 [0.67, 3.19] |
| 9.6 Influenza- like illness | 1 | 535 | Risk Ratio (M-H, Fixed, 95% CI) | 1.20 [0.54, 2.70] |
| 10 Patient reported symptoms judged as drug-related | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Vomiting | 2 | 1807 | Risk Ratio (M-H, Fixed, 95% CI) | 1.25 [0.68, 2.31] |
| 10.2 Headache | 1 | 1272 | Risk Ratio (M-H, Fixed, 95% CI) | 1.03 [0.57, 1.85] |
| 10.3 Abdominal pain | 1 | 1272 | Risk Ratio (M-H, Fixed, 95% CI) | 1.25 [0.60, 2.57] |
| 10.4 Vertigo | 1 | 1272 | Risk Ratio (M-H, Fixed, 95% CI) | 1.49 [0.48, 4.61] |
| 10.5 Haematuria | 1 | 1272 | Risk Ratio (M-H, Fixed, 95% CI) | 2.49 [0.55, 11.32] |
| 10.6 Upper abdominal pain | 1 | 1272 | Risk Ratio (M-H, Fixed, 95% CI) | 1.12 [0.35, 3.62] |
| 10.7 Anorexia | 1 | 1272 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.09, 1.17] |
| 11 Abnormal LFTs; grade 3 and 4 toxicity | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Alanine aminotransferase (ALT) | 2 | 1807 | Risk Ratio (M-H, Fixed, 95% CI) | 2.00 [0.43, 9.42] |
| 11.2 Asparatate aminotransferase (AST) | 2 | 1807 | Risk Ratio (M-H, Fixed, 95% CI) | 3.85 [0.70, 21.09] |
| 11.3 Alkaline phosphatase (ALP) | 1 | 1272 | Risk Ratio (M-H, Fixed, 95% CI) | 0.10 [0.00, 2.07] |
| 11.4 Bilirubin | 2 | 1807 | Risk Ratio (M-H, Fixed, 95% CI) | 1.30 [0.30, 5.62] |
| 12 Change in haemoglobin | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Haemoglobin at baseline | 2 | 1807 | Mean Difference (IV, Fixed, 95% CI) | -0.03 [-0.19, 0.13] |
| 12.2 Haemoglobin day 3 | 2 | 1755 | Mean Difference (IV, Fixed, 95% CI) | -0.01 [-0.13, 0.11] |
| 12.3 Haemoglobin day 7 | 2 | 1741 | Mean Difference (IV, Fixed, 95% CI) | -0.16 [-0.28, -0.05] |
| 12.4 Haemoglobin day 28 | 2 | 1702 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [-0.09, 0.18] |
| 13 Anaemia as an adverse event | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Anaemia (AE of any cause) | 1 | 535 | Risk Ratio (M-H, Fixed, 95% CI) | 1.23 [0.68, 2.23] |
| 13.2 Anaemia (drug- related AE) | 1 | 535 | Risk Ratio (M-H, Fixed, 95% CI) | 0.69 [0.32, 1.47] |
Comparison 2. Artesunate-pyronaridine versus artemether-lumefantrine; subgroup analysis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total failure PCR-adjusted (Day 28); subgrouped by age | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Age = 5 years | 2 | 1469 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.25, 4.03] |
| 1.2 Age < 5 years | 1 | 216 | Risk Ratio (M-H, Fixed, 95% CI) | 3.37 [0.43, 26.38] |
| 2 Total failure PCR-adjusted (Day 28); subgrouped by region | 2 | 1675 | Risk Ratio (M-H, Fixed, 95% CI) | 1.10 [0.44, 2.76] |
| 2.1 West Africa | 2 | 816 | Risk Ratio (M-H, Fixed, 95% CI) | 1.42 [0.29, 6.92] |
| 2.2 East Africa | 2 | 194 | Risk Ratio (M-H, Fixed, 95% CI) | 2.64 [0.13, 53.14] |
| 2.3 South-central Africa | 2 | 490 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.15, 2.92] |
| 2.4 Asia | 2 | 175 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.09, 10.99] |
| 3 Total failure PCR-adjusted (Day 28); subgrouped by country | 2 | 1675 | Risk Ratio (M-H, Fixed, 95% CI) | 0.94 [0.41, 2.18] |
| 3.1 Burkina Faso | 1 | 25 | Risk Ratio (M-H, Fixed, 95% CI) | 1.05 [0.05, 22.91] |
| 3.2 DR Congo | 2 | 369 | Risk Ratio (M-H, Fixed, 95% CI) | 0.5 [0.10, 2.43] |
| 3.3 Gabon | 1 | 78 | Risk Ratio (M-H, Fixed, 95% CI) | 0.17 [0.01, 4.03] |
| 3.4 Ivory Coast | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 2.25 [0.27, 18.43] |
| 3.5 Kenya | 2 | 194 | Risk Ratio (M-H, Fixed, 95% CI) | 2.64 [0.13, 53.14] |
| 3.6 Mali | 2 | 318 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 The Gambia | 1 | 90 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Ghana | 1 | 7 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 Mozambique | 1 | 121 | Risk Ratio (M-H, Fixed, 95% CI) | 1.5 [0.06, 36.02] |
| 3.10 Senegal | 1 | 198 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.11 Phillipines | 2 | 107 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.12 Indonesia | 1 | 68 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.09, 9.32] |
Comparison 3. Artesunate-pyronaridine versus artemether-lumefantrine; sensitivity analysis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total failure PCR-unadjusted (Day 28); Sensitivity analysis | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Primary analysis (Cochrane review) | 2 | 1720 | Risk Ratio (M-H, Fixed, 95% CI) | 0.60 [0.40, 0.90] |
| 1.2 Missing data included as failures | 2 | 1807 | Risk Ratio (M-H, Fixed, 95% CI) | 0.77 [0.58, 1.03] |
| 1.3 Missing data included as successes | 2 | 1807 | Risk Ratio (M-H, Fixed, 95% CI) | 0.61 [0.41, 0.90] |
| 1.4 Intention to treat analysis (of trial authors) | 2 | 1807 | Risk Ratio (M-H, Fixed, 95% CI) | 0.77 [0.58, 1.03] |
| 1.5 Per-protocol analysis (of trial authors) | 2 | 1683 | Risk Ratio (M-H, Fixed, 95% CI) | 0.66 [0.43, 1.01] |
| 2 Total failure PCR-adjusted (Day 28); Sensitivity analysis | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Primary analysis (Cochrane review) | 2 | 1650 | Risk Ratio (M-H, Fixed, 95% CI) | 1.69 [0.56, 5.10] |
| 2.2 Missing or indeterminate PCR results included as failures | 2 | 1651 | Risk Ratio (M-H, Fixed, 95% CI) | 1.35 [0.49, 3.73] |
| 2.3 New infections included as successes | 2 | 1720 | Risk Ratio (M-H, Fixed, 95% CI) | 1.41 [0.51, 3.88] |
| 2.4 Missing data included as failures | 2 | 1807 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.71, 1.57] |
| 2.5 Missing data included as successes | 2 | 1807 | Risk Ratio (M-H, Fixed, 95% CI) | 1.41 [0.51, 3.89] |
| 2.6 Intention to treat analysis (by trial authors) | 2 | 1807 | Risk Ratio (M-H, Fixed, 95% CI) | 0.87 [0.60, 1.24] |
| 2.7 Per-protocol analysis (by trial authors) | 2 | 1676 | Risk Ratio (M-H, Fixed, 95% CI) | 1.38 [0.50, 3.81] |
Comparison 4. Artesunate-pyronaridine versus artesunate-mefloquine
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total failure (Day 28) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 PCR-unadjusted | 1 | 1200 | Risk Ratio (M-H, Fixed, 95% CI) | 0.35 [0.17, 0.73] |
| 1.2 PCR adjusted | 1 | 1187 | Risk Ratio (M-H, Fixed, 95% CI) | 0.38 [0.14, 1.02] |
| 2 Total failure (Day 42) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 PCR-unadjusted | 1 | 1146 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.57, 1.31] |
| 2.2 PCR adjusted | 1 | 1116 | Risk Ratio (M-H, Fixed, 95% CI) | 1.64 [0.89, 3.00] |
| 3 Early treatment failures | 1 | 1103 | Risk Ratio (M-H, Fixed, 95% CI) | 0.16 [0.01, 3.96] |
| 4 Parasite clearance time (hours) | 1 | 1259 | Mean Difference (IV, Fixed, 95% CI) | -2.60 [-4.94, -0.26] |
| 5 Fever clearance time (hours) | 1 | 1051 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [-1.52, 1.72] |
| 6 Gametocyte clearance time (hours) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | -5.40 [-21.80, 11.00] |
| 7 Serious adverse events | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.25, 3.97] |
| 8 Adverse events leading to withdrawal from treatment | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 0.62 [0.17, 2.31] |
| 9 Patient reported symptoms | 1 | 7626 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 9.1 Vomiting | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.45, 2.20] |
| 9.2 Diarrhea | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 0.44 [0.17, 1.14] |
| 9.3 Headache | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 1.15 [0.82, 1.60] |
| 9.4 Dizziness | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 0.46 [0.28, 0.78] |
| 9.5 Cough | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 1.50 [0.74, 3.03] |
| 9.6 Myalgia | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 1.39 [0.83, 2.32] |
| 10 Abnormal LFTs; Grade 2 toxicity | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Alanine aminotransferase (ALT) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Aspartate aminotransferase (AST) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Alkaline phosphatase (ALP) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Bilirubin | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Abnormal LFTs; Grade 3 or 4 toxicity | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 11.1 Alanine aminotransferase (ALT) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Aspartate aminotransferase (AST) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Alkaline phosphatase (ALP) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Bilirubin | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Haemoglobin (g/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Haemoglobin at baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Haemoglobin day 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Haemoglobin day 7 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.4 Haemoglobin day 28 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Platelet counts (x 109/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 day 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 day 7 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.4 day 28 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 White blood counts (x 109/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 day 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 day 7 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.4 day 28 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Abnormal ECG finding | 1 | 2542 | Risk Ratio (M-H, Fixed, 95% CI) | 0.58 [0.20, 1.73] |
| 15.1 QT prolongation | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 0.17 [0.02, 1.59] |
| 15.2 ECG abnormalities | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.25, 3.97] |
Comparison 5. Artesunate-pyronaridine versus artesunate-mefloquine; subgroup analysis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total failure PCR-adjusted (Day 28); subgrouped by region | 1 | 1117 | Risk Ratio (M-H, Fixed, 95% CI) | 0.38 [0.14, 1.03] |
| 1.1 East Africa | 1 | 38 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 West Africa | 1 | 192 | Risk Ratio (M-H, Fixed, 95% CI) | 1.48 [0.06, 35.75] |
| 1.3 South central Africa | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Asia | 1 | 887 | Risk Ratio (M-H, Fixed, 95% CI) | 0.31 [0.10, 0.93] |
| 2 Total failure PCR-adjusted (Day 28); subgrouped by country | 1 | 1117 | Risk Ratio (M-H, Fixed, 95% CI) | 0.38 [0.14, 1.03] |
| 2.1 Thailand | 1 | 551 | Risk Ratio (M-H, Fixed, 95% CI) | 0.12 [0.03, 0.57] |
| 2.2 Vietnam | 1 | 159 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Cambodia | 1 | 132 | Risk Ratio (M-H, Fixed, 95% CI) | 3.31 [0.17, 62.62] |
| 2.4 India | 1 | 45 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Burkina Faso | 1 | 118 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Ivory Coast | 1 | 74 | Risk Ratio (M-H, Fixed, 95% CI) | 1.65 [0.07, 39.20] |
| 2.7 Tanzania | 1 | 38 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Artesunate-pyronaridine versus artesunate-mefloquine; sensitivity analysis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total failure PCR-unadjusted (Day 28); Sensitivity analysis | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Primary analysis (Cochrane review) | 1 | 1200 | Risk Ratio (M-H, Fixed, 95% CI) | 0.35 [0.17, 0.73] |
| 1.2 Missing data included as failures | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 0.72 [0.49, 1.05] |
| 1.3 Missing data included as successes | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 0.35 [0.17, 0.73] |
| 1.4 Intention to treat analysis (of trial authors) | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 0.72 [0.49, 1.05] |
| 1.5 Per-protocol analysis (of trial authors) | 1 | 1120 | Risk Ratio (M-H, Fixed, 95% CI) | 0.36 [0.17, 0.78] |
| 2 Total failure PCR-adjusted (Day 28); Sensitivity analysis | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Primary analysis (Cochrane review) | 1 | 1187 | Risk Ratio (M-H, Fixed, 95% CI) | 0.38 [0.14, 1.02] |
| 2.2 Missing or indeterminate PCR results included as failures | 1 | 1187 | Risk Ratio (M-H, Fixed, 95% CI) | 0.38 [0.14, 1.02] |
| 2.3 New infections included as successes | 1 | 1200 | Risk Ratio (M-H, Fixed, 95% CI) | 0.39 [0.15, 1.03] |
| 2.4 Missing data included as failures | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 0.82 [0.54, 1.24] |
| 2.5 Missing data included as successes | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 0.39 [0.15, 1.03] |
| 2.6 Intention to treat analysis (by trial authors) | 1 | 1271 | Risk Ratio (M-H, Fixed, 95% CI) | 0.76 [0.51, 1.14] |
| 2.7 Per-protocol analysis (by trial authors) | 1 | 1117 | Risk Ratio (M-H, Fixed, 95% CI) | 0.37 [0.13, 1.05] |
Comparison 7. Pyronaridine alone or with artesunate versus another antimalarial: laboratory findings
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Abnormal LFTs; Grade 3 or 4 toxicity | 4 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alanine aminotransferase (ALT) | 4 | 3523 | Risk Ratio (M-H, Fixed, 95% CI) | 4.17 [1.38, 12.62] |
| 1.2 Aspartate aminotransferase (AST) | 4 | 3528 | Risk Ratio (M-H, Fixed, 95% CI) | 4.08 [1.17, 14.26] |
| 1.3 Alkaline phosphatase (ALP) | 3 | 2606 | Risk Ratio (M-H, Fixed, 95% CI) | 0.62 [0.15, 2.51] |
| 1.4 Bilirubin | 3 | 3067 | Risk Ratio (M-H, Fixed, 95% CI) | 1.92 [0.59, 6.24] |
| 2 Combined abnormal LFTs | 3 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 ALT = 3 x ULN and Bilirubin = 2 x ULN (Hy's Law case) | 3 | 3072 | Risk Ratio (M-H, Fixed, 95% CI) | 1.50 [0.30, 7.42] |
| 3 Renal function tests | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Baseline | 3 | 1878 | Mean Difference (IV, Fixed, 95% CI) | -0.12 [-2.07, 1.84] |
| 3.2 Day 3 | 2 | 1764 | Mean Difference (IV, Fixed, 95% CI) | -1.72 [-3.54, 0.10] |
| 3.3 Day 7 | 3 | 1808 | Mean Difference (IV, Fixed, 95% CI) | -2.76 [-4.58, -0.94] |
| 4 Haemoglobin | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Haemoglobin at baseline | 4 | 3534 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [-0.04, 0.21] |
| 4.2 Haemoglobin day 3 | 4 | 3461 | Mean Difference (IV, Fixed, 95% CI) | -0.12 [-0.20, -0.04] |
| 4.3 Haemoglobin day 7 | 4 | 3394 | Mean Difference (IV, Fixed, 95% CI) | -0.24 [-0.32, -0.16] |
| 4.4 Haemoglobin day 28 | 4 | 3294 | Mean Difference (IV, Fixed, 95% CI) | -0.09 [-0.19, 0.01] |
| 5 Abnormal ECG findings | 3 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 ECG abnormalities | 2 | 2543 | Risk Ratio (M-H, Fixed, 95% CI) | 0.80 [0.26, 2.43] |
| 5.2 QT prolongation | 3 | 2991 | Risk Ratio (M-H, Fixed, 95% CI) | 0.25 [0.07, 0.90] |
Table 1.
Primary outcome measure (Total failure)
| Analysisa | Participants | PCRb-unadjusted | PCR-adjusted | ||
| Numerator | Denominator | Numerator | Denominator | ||
| Primary analysis | Exclusions after enrolment | Excludedc | Excluded | Excluded | Excluded |
| Missing or indeterminate PCR | Included as failures | Included | Excluded | Excluded | |
| New infections | Included as failures | Included | Excluded | Excluded | |
| Sensitivity analysis 1d | As 'Primary analysis' except: missing or indeterminate PCR | — | — | Included as failures | Included |
| Sensitivity analysis 2e | As 'Sensitivity analysis 1' except: new infections | — | — | Included as successes | Included |
| Sensitivity analysis 3f | As 'Sensitivity analysis 2' except: exclusions after enrolment | Included as failures | Included | Included as failures | Included |
| Sensitivity analysis 4g | As 'Sensitivity analysis 2' except: exclusions after enrolment | Included as successes | Included | Included as successes | Included |
aNote: we removed participants that did not satisfy the inclusion criteria after randomization from all calculations.
bPCR: polymerase chain reaction.
c'Excluded' means removed from the calculation.
dTo re-classify all indeterminate or missing PCR results as treatment failures in the PCR-adjusted analysis.
eTo re-classify all PCR-confirmed new infections as treatment successes in the PCR-adjusted analysis. (This analysis may overestimate efficacy as PCR is not wholly reliable and some recrudescences may be falsely classified as new infections. Also some participants may have gone on to develop a recrudescence after the new infection).
fTo re-classify all exclusions after enrolment (losses to follow-up, withdrawn consent, other antimalarial use, or failure to complete treatment) as treatment failures. For PCR-unadjusted total failure this represents a true worse-case scenario.
gTo re-classify all exclusions after enrolment (losses to follow-up, withdrawn consent, other antimalarial use, or failure to complete treatment) as treatment successes.
Table 2.
Adverse events risk of bias assessment methods
| Criterion | Assessment | Explanation |
| Patient-reported symptoms | ||
| Was monitoring active or passive? |
Active Passive Unclear |
We classified monitoring as 'active' when authors reviewed participants at set timepoints and enquired about symptoms. |
| Was blinding for participants and outcome assessors adequate? | Adequate Inadequate Unclear |
We classified blinding as 'adequate' when both participants and outcome assessors were blinded to the intervention group, and the methods of blinding (including use of a placebo) were described. |
| Was outcome data reporting complete or incomplete? | Complete Incomplete Unclear |
We classified outcome data reporting as 'complete' when data was presented for all the time-points where it was collected. |
| Were all participants included in reporting? | We report the percentage of randomized participants included in adverse event reporting. | |
| Was theanalysis independent of study sponsor? | Yes No Unclear |
We classified the analysis of trials sponsored by pharmaceutical companies as independent of the sponsor when it was clearly stated that the sponsor had no input to the trial analysis |
| Laboratory tests | ||
| Number of tests undertaken | — | We extracted the type and number of laboratory tests were taken. |
|
Timing of tests Was number and timing of tests adequate? |
Adequate Inadequate |
We classified the number and timing of tests as 'adequate', when tests were taken at baseline, plus two other timepoints within the first week after treatment, plus the last day of the study. We classed the number of test taken as "inadequate", if either the laboratory controls in the first week or controls at four weeks were not performed. |
|
Reporting of test results Was reporting of test results complete? |
Complete Incomplete |
We classified reporting as 'complete' when test results of all time points were reported. For the trials with inadequate number of tests taken, we considered completeness of reporting as inconsequential, and therefore did not record a judgement. |
|
Independence of data analysis Was data analysis independent? |
Yes No Unclear |
We classified the analysis of trials sponsored by pharmaceutical companies as independent of the sponsor when it was clearly stated that the sponsor had no input to the trial analysis |
Table 3.
Dosing regimens of artesunate-pyronaridine
| Trial ID | Actual or target dose | Intervention(mg/kg/dose) | Comparator(mg/kg/dose) | ||
| Artesunate | Pyronaridine | Artemisinin-derivative | Partner drug | ||
| Kayentao 2012 | Actual dose | 2.2 to 4.4 | 6.7 to 13.3 | 1.3 to 4.0 | 8.0 to 24.0 |
| Tshefu 2010 | Target dose | 2.4 to 4.6 | 7.2 to 13.8 | NS | NS |
| Rueangweerayut 2012 | Actual dose | 2.4 to 4.7 | 7.1 to 14.0 | 2.5 to 5.0 | 6.2 to 12.5 |
| Poravuth 2011 | Target dose | 2.4 to 4.6 | 7.2 to 13.8 | — | 10, 5, 5 |
| Ringwald 1996 | Target dose | — | 16, 8, 8 | — | 10, 5, 5 |
| Ringwald 1998 | Target dose | — | 8, 8, 8, 8 | — | 10, 10, 5 |
NS = Not specified.
Table 4.
Risk of bias for patient reported symptoms
| Trial ID | Monitoring active or passive? | Outcome data reporting | Blinding adequate? | % of participants included in AE reporting | Independent data analysis | ||||
| Days data collected | Days data reported | Patient | Clinician | Data analysis | AS-Pyr | Control | |||
| Tshefu 2010 | Unclear | 0 to 3, 7, 14, 21, 28, 35, 42 | Unclear | Yes | Yes | Unclear | 100% | 100% | No |
| Kayentao 2012 | Unclear | Unclear | Unclear | No | No | Yes | 100% | 100% | No |
| Rueangweerayut 2012 | Unclear | Unclear | Unclear | No | No | Unclear | 100% | 100% | No |
| Poravuth 2011 | Unclear | Unclear | Unclear | Yes | Yes | Yes | 100% | 100% | Yes |
| Ringwald 1996 | Unclear | Unclear | Unclear | No | No | Unclear | Unclear | Unclear | Yes |
| Ringwald 1998 | Unclear | Unclear | Unclear | No | No | Unclear | 100% | 100% | Yes |
Table 5.
Risk of bias table for biochemical liver function tests
| Trial ID | Number of tests | Days tested | Days reported | Days tested adequate? | For adequate testing, was reporting complete? | Data analysis independent of sponsor? |
| Tshefu 2010 | 41 | 0, 3, 7, 282 | 3, 7, 28 | Adequate | Complete | No |
| Kayentao 2012 | 41 | 0, 3, 7, 28, 42 | 3, 7 | Adequate | Incomplete 3 | No |
| Rueangweerayut 2012 | 41 | 0, 28, 42 | 0, “post baseline” | Inadequate | -4 | No |
| Poravuth 2011 | 41 | 0, 3, 7, 282 | 3, 7, 28 | Adequate | Complete | Yes |
| Ringwald 1996 | 41 | 0, 7 | 0, 7 | Inadequate | — | Yes |
| Ringwald 1998 | 51,5 | 0, 7 | 0, 7 | Inadequate | — | Yes |
1 Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (TBIL).
2 Plus day 42 if clinically indicated.
3 Does not report the outcome data for day 28 in additional file 3.
4 The trial did not report ALP values for all participants (ALT and AST values for 848 patients in the artesunate pyronaridine arm and for 423 participants in the artesunate mefloquine arm at baseline; AST values only for 635 participants and 308 participants respectively at baseline).
5 Plus conjugated bilirubin in addition.
Table 6.
Additional data from Kayentao 2012
| Trial ID | Outcome | Artesunate-pyronaridine | Artemether-lumefantrine | P value |
| Kayentao 2012 | Median parasite clearance time | 24.1 hours(95% CI 24.0 to 24.1) | 24.2 hours (95% CI 24.1 to 32.0) |
0.02 |
| Median fever clearance time | 8.1 hours(95% CI 8.0 to 8.1) | 8.1 hours(95% CI 8.0 to 15.8) | 0.049 | |
| "Post-baseline gametocytes" | 95/354 (26.8%) |
44/178(24.7%) | 0.6 | |
| Gametocyte development(in those negative at baseline) | 53/354(15%) | 20/178(11.2%) | 0.24 |
Table 7.
Summary of ECG monitoring and results
| Trial ID | Days tested | ECG results | |
| Pyronaridine arm | Comparator arm | ||
| Kayentao 2012 | 0, 2, 7, 14, and 28 |
"no post baseline clinically important ECG results" | "no post baseline clinically important ECG results" |
| Tshefu 2010 | 0, 2, 7, 14, and 28 | 1 patient with T-wave inversion at day 2 1 patient with ventricular premature complexes and extended QTc (manual reading QTcB 461 ms, QTcF 458 ms) at day 21 |
1 patient with sinus bradycardia and sinus arrhythmia on day 2 1 patient with sinus bradycardia on day 2 |
| Rueangweerayut 2012 | Unclear | 6/848 (0.7%) patients with abnormal ECGs- "All were mild and resolved before study completion"1/848 with QT prolongation- None had a QT interval that exceeded 480 msec | 3/423 (0.7%) patients with abnormal ECGs - "All were mild and resolved before study completion" 3/423 with long or prolonged QT interval - None had a QT interval that exceeded 480 msec |
| Poravuth 2011 | 0, 2, 7, 14, and 42 |
1/226 (0.4%) patients with QTc prolongations | 6/222 (2.7%) patients with QTc prolongations (1/222 not drug-related) |
Acknowledgments
We acknowledge the South Asian Cochrane Network & Centre; the Effective Health Care Research Programme Consortium (supported by the Department for International Development (DFID), UK); the Indian Council of Medical Research (ICMR) that funds the Prof. BV Moses & ICMR Centre for Advanced Research & Training in Evidence-Informed Healthcare at CMC Vellore. Thanks to Rajeev Aravindakshan for his initial contributions to the review; to Pascal Ringwald for unpublished data, and for additional references; Nitya Gogty, Mumbai, and editors of the Cochrane Infectious Diseases Group, for constructive comments as referees and editorial guidance; and Isabelle Borghini-Fuhrer, of Medicines for Malaria Venture, for a list of trials involving artesunate-pyronaridine. We are grateful to Yang Wu for translation of studies from Chinese. We are grateful to the considerable editorial support that ensured this review was completed and that the focus of this review reflected current issues in the treatment of malaria. The editorial base for the Cochrane Infectious Diseases Group is funded by UKaid from the UK Government for the benefit of developing countries.
References
- Kayentao K. Pyronaridine-artesunate versus artemether/lumefantrine: efficacy in malaria patients with uncomplicated acute falciparum malaria: results of a pivotal Phase III trial. 2008;79:114. American Journal of Tropical Medicine and Hygiene(6 Suppl) [Google Scholar]
- Kayentao K, Doumbo OK, Pénali LK, Offianan AT, Bhatt KM, Kimani J, et al. Pyronaridine-artesunate granules versus artemether-lumefantrine crushed tablets in children with Plasmodium falciparum malaria: a randomized controlled trial. Malaria Journal. 2012;11:364. doi: 10.1186/1475-2875-11-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nct00541385 Pyronaridine artesunate 3:1 granule formulation vs. Coartem© crushed tablets in P. falciparum malaria pediatric patients. www.clinicaltrials.gov/show/ NCT00541385 (accessed 30 November 2010).
- Duparc S, Borghini-Fuhrer I, Craft JC, Arbe-Barnes S, Miller RM, Shin CS, et al. Efficacy of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. 2009;81:51–100. American Journal of Tropical Medicine and Hygiene. [Google Scholar]
- Duparc S, Borghini-Fuhrer I, Craft JC, Arbe-Barnes S, Miller RM, Shin CS, et al. Safety of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. 2009;81:101–150. American Journal of Tropical Medicine and Hygiene. [Google Scholar]
- Poravuth Y, Socheat D, Rueangweerayut R, Uthaisin C, Pyae Phyo A, Valecha N, et al. Pyronaridine-artesunate versus chloroquine in patients with acute Plasmodium vivax malaria: a randomized, double-blind, non-inferiority trial. PLoS One. 2011;6(1):e14501. doi: 10.1371/journal.pone.0014501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwald P, Bickii J, Basco L. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet. 1996;347(8993):24–8. doi: 10.1016/s0140-6736(96)91558-5. [DOI] [PubMed] [Google Scholar]
- Ringwald P, Bickii, Basco LK. Efficacy of oral pyronaridine for the treatment of acute uncomplicated falciparum malaria in African children. Clinical Infectious Diseases. 1998;26(4):946–53. doi: 10.1086/513942. [DOI] [PubMed] [Google Scholar]
- Nct00403260 Pyronaridine artesunate (3:1) versus mefloquine artesunate in P. falciparum malaria patients. www.clinicaltrials.gov/show/ NCT00403260.
- Rueangweerayut R, Phyo AP, Uchaisin C, Poravuth Y, Quang Binh T, Tinto H, et al. A randomized clinical trial comparing the efficacy and safety of fixed-dose pyronaridine-artesunate versus mefloquine plus artesunate in uncomplicated Plasmodium falciparum malaria. Submitted under review; personal communication by email from Isabelle Borghini Fuhrer, Medicines for Malaria Venture.
- Rueangweerayut R, Phyo AP, Uchaisin C, Socheat D, Quang Binh T, Tinto H, et al. Efficacy and safety of pyronaridine/artesunate fixed-dose combination compared with mefloquine plus artesunate in patients with acute uncomplicated Plasmodium falciparum malaria: results of a pivotal phase III trial. 2009;14:30–97. Tropical Medicine and International Health Suppl 2: [Google Scholar]
- Rueangweerayut R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Tinto H, et al. Pyronaridine-artesunate versus mefloquine plus artesunate for malaria. New England Journal of Medicine. 2012;366(14):1298–309. doi: 10.1056/NEJMoa1007125. [DOI] [PubMed] [Google Scholar]
- Rueangweerayut R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Tinto H, et al. Supplement to: Pyronaridine–artesunate versus mefloquine plus artesunate for malaria. New England Journal of Medicine. 2012;366(14):1298–309. doi: 10.1056/NEJMoa1007125. [DOI] [PubMed] [Google Scholar]
- Duparc S, Borghini- Fuhrer I, Craft JC, Arbe- Barnes S, Miller RM, Shin CS, et al. Safety of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. 2009;81 Suppl:101–150. American Journal of Tropical Medicine and Hygiene. [Google Scholar]
- Duparc S, Borghini-Fuhrer I, Craft JC, Arbe-Barnes S, Miller RM, Shin CS, et al. Efficacy of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. 2009;81 Suppl:51–100. American Journal of Tropical Medicine and Hygiene. [Google Scholar]
- Tshefu AK, Gaye O, Kayentao K, Thompson R, Bhatt KM, Sesay SS, et al. Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a randomised non-inferiority trial. Lancet. 2010;375(9724):1457–67. doi: 10.1016/S0140-6736(10)60322-4. [DOI] [PubMed] [Google Scholar]
- Cai X, Chen C, Zheng X, Wang X. Preliminary study of dihydroartemisinin for treatment of falciparum malaria. Journal of Practical Parasitic Diseases. 1999;7(3):104–5. [Google Scholar]
- Chang C, et al. Analysis on the results of antimalarial pyronaridine combined with sulfadoxine and pyrimethamine to falciparum malaria. Journal of Practical Parasitic Diseases. 1997;5(3):104–7. [Google Scholar]
- Che LG, Huang KG, Yang HL, Yu L, Lin ZL, Huang R. Combined use of pyronaridine, sulfadoxine and primaquine in areas with chloroquine-resistant falciparum malaria. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chinese Journal of Parasitology and Parasitic Diseases] 1987;5(3):194–6. [ISSN: 1000-7423] [PubMed] [Google Scholar]
- Che L, Huang K, Ying D, Yang H, Yang P. Efficacy of two combined therapies for treatment of chloroquine-resistant falciparum malaria. Chinese Journal of Parasitic Disease Control. 1990;3(1):24–6. [Google Scholar]
- Chen L, Dai ZR, Qian YL, Ma ZM, Guo FC, Liao ZH, et al. Observation on the efficacy of combined use of some new antimalarials for the treatment of falciparum malaria in Hainan Province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1989;7(2):81–4. [PubMed] [Google Scholar]
- Fleckenstein L, Ramharter M, Kremsner PG. Pharmacokinetics of pyronaridine: Artesunate (Pyramax®) for treatment of children with acute uncomplicated Plasmodium falciparum malaria. 2007;12 Suppl 1:222. Tropical Medicine and International Health. [Google Scholar]
- Huang ZS, Shao BR, Meng F, Zeng LH, Ye XY, Huang J, et al. Effects of combined dose of pyronaridine/sulfadoxine/pyrimethamine on falciparum malaria. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chinese Journal of Parasitology and Parasitic Diseases] 1988;6(4):285–8. [PubMed] [Google Scholar]
- Huang ZS, Feng Z, Meng F, Zeng LH, Lin X, Zhen Y, Xing QF, Guo RN. Therapeutic effect of pyronaridine in plain tablets and enteric coated tablets in falciparum malaria patients. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chinese Journal of Parasitology and Parasitic Diseases] 1989;7(1):19–21. [PubMed] [Google Scholar]
- Huang Z, Shao B, Meng F, Shi X. Comparison of different regimen of pyronaridine and sulfadoxine combined with pyrimethamine in the treatment of malignant malaria. Chinese Journal of Infectious Diseases. 1993;11(Suppl 3):175–7. [: ISSN: CN-00256187] [Google Scholar]
- Haung Z, Meng F, Fu S. Comparative studies on the treatment of drug-resistant falciparum malaria with single-dose or two-day regimens of pyronaridine/sulfadoxine-pyrimethamine plus primaquine. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chinese Journal of Parasitology and Parasitic Diseases] 1996;14(4):314–7. [Google Scholar]
- Liu DQ, Lin SG, Feng XP, Chen WJ, Chen PL, Wu HM, et al. Study on Treatment of Multi-drug resistant Falciparum Malaria by Using a Combination of Dihydroartemisinin and Pyronaridine. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chinese Journal of Parasitology and Parasitic Diseases] 2002;20(4):193–6. [PubMed] [Google Scholar]
- Looareesuwan S, Kyle DE, Viravan C, Vanijanonta S, Wilairatana P, Wernsdorfer WH. Clinical study of pyronaridine for the treatment of acute uncomplicated falciparum malaria in Thailand. American Journal of Tropical Medicine and Hygiene. 1996;54(2):205–9. doi: 10.4269/ajtmh.1996.54.205. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S, Gaye O, Tjitra E, Bojang K, Socheat D, Piola P. Results of a randomized, multicentre, phase II, dose-ranging, clinical study to assess the safety and efficacy of fixed dose, orally administered pyronaridine and artesunate in adult patients with acute uncomplicated Plasmodium falciparum malaria. 2007. p. 287. Abstracts of the 56th Annual Meeting of the American Society of Tropical Medicine and Hygiene.
- Nct01156389 Pyronaridine/Artesunate-Ritonavir drug drug interaction study. www.clinicaltrials.gov/show/ NCT01156389 (accessed 09 August 2010).
- No authors listed. Efficacy of pyronaridine in 510 acute malaria cases. Zhonghua Nei Ke Za Zhi. 1985;24(11):646-7, 700. [PubMed] [Google Scholar]
- Pang X, Xing Q, Lin K, Li X, Ou F, Ou L, et al. Efficacy of combined use of various preparations of pyronaridine, sulfadoxine and pyrimethamine in the treatment of falciparum malaria. Chinese Journal of Parasitic Disease Control. 1989;2(3):167–8. [PubMed] [Google Scholar]
- Piola P, Fleckenstein L. Pharmacokinetics, clinical and safety outcomes of pyronaridine/artesunate treatment of acute Plasmodium falciparum malaria in Uganda. 2008. p. 252. Abstracts of the 57th Annual meeting of the American Society of Tropical Medicine and Hygiene. Available at: www.astmh.org: American Society of Tropical Medicine and Hygiene.
- Piola P, Fleckenstein L. Pharmacokinetics, clinical and safety outcomes of pyronaridine/artesunate treatment of acute Plasmodium falciparum malaria in Uganda. 2008;79:195. American Journal of Tropical Medicine and Hygiene. [Google Scholar]
- Ramharter M, Kurth F, Schreier AC, Nemeth J, Glasenapp Iv, Bélard S, et al. Fixed-dose pyronaridine-artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. Journal of Infectious Diseases. 2008;198(6):911–9. doi: 10.1086/591096. [DOI] [PubMed] [Google Scholar]
- Shao BR, Huang ZS, Shi XH, Meng F. A 5-year surveillance of sensitivity in vivo of Plasmodium falciparum to pyronaridine/sulfadoxine/pyrimethamine in Diaoluo area, Hainan Province. Southeast Asian Journal of Tropical Medicine and Public Health. 1991;22(1):65–7. [PubMed] [Google Scholar]
- Tan B, Fleckenstein lL, Yu KS, Jang IJ. Population pharmacokinetics of artesunate and dihydroartemisinin in healthy volunteers. 2008;79 Suppl:100–49. American Journal of Tropical Medicine and Hygiene. [Google Scholar]
- Wattanavijitkul T, Fleckenstein L, Yu KS, Jang IJ. Multiple-dose population pharmacokinetics of pyronaridine in healthy volunteers. 2008;79 Suppl:250–99. American Journal of Tropical Medicine and Hygiene. [Google Scholar]
- Auparakkitanon S, Chapoomram S, Kuaha K, Chirachariyavej T, Wilairat P. Targeting of hematin by the antimalarial pyronaridine. Antimicrobial Agents and Chemotherapy. 2006;50(6):2197–200. doi: 10.1128/AAC.00119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basco LK, Le Bras J. In vitro activity of pyronaridine against African strains of Plasmodium falciparum. Annals of Tropical Medicine and Parasitology. 1992;86(5):447–54. doi: 10.1080/00034983.1992.11812693. [DOI] [PubMed] [Google Scholar]
- Bloland PB. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria [WHO/HTM/RBM/2003.50] Geneva: World Health Organization; 2003. [Google Scholar]
- Brice BK, William Y, Lacina O, Félix Y, Hugues A, Léonardo B, et al. In vitro susceptibility of Plasmodium falciparum isolates from Abidjan, Cote d'Ivoire, to artemisinin, chloroquine, dihydroartemisinin and pyronaridine. Tanzanian Journal of Health Research. 2010;12(1):73–9. doi: 10.4314/thrb.v12i1.56364. [DOI] [PubMed] [Google Scholar]
- Cattamanchi A, Kyabayinze D, Hubbard A, Rosenthal PJ, Dorsey G. Distinguishing recrudescence from reinfection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of msp-1, msp-2, and glurp. American Journal of Tropical Medicine and Hygiene. 2003;68(2):133–9. [PubMed] [Google Scholar]
- Chavalitshewinkoon-Petmitr P, Pongvilairat G, Auparakkitanon S, Wilairat P. Gametocytocidal activity of pyronaridine and DNA topoisomerase II inhibitors against multidrug-resistant Plasmodium falciparum in vitro. Parasitology International. 2000;48(4):275–80. doi: 10.1016/s1383-5769(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Chang C, Lin-Hua T, Jantanavivat C. Studies on a new antimalarial compound: pyronaridine. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992;86(1):7–10. doi: 10.1016/0035-9203(92)90414-8. [DOI] [PubMed] [Google Scholar]
- Childs GE, Häusler B, Milhous W, Chen C, Wimonwattrawatee T, Pooyindee N, et al. In vitro activity of pyronaridine against field isolates and reference clones of Plasmodium falciparum. American Journal of Tropical Medicine and Hygiene. 1988;38(1):24–9. doi: 10.4269/ajtmh.1988.38.24. [DOI] [PubMed] [Google Scholar]
- Review of pyronaridine anti-malarial properties, product characteristics. Croft SL, Duparc S, Arbe-Barnes SJ, Craft JC, Shin CS, Fleckenstein L, et al. Malaria Journal. 2010;11:270. doi: 10.1186/1475-2875-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, et al. Artemisinin resistance: current status and scenarios for containment. Nature Reviews Microbiology. 2010;8(4):272–80. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- Duparc S, Borghini-Fuhrer I, Craft CJ, Arbe-Barnes S, Miller RM, Shin CS, et al. Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials. Malaria journal. 2013;12(70):Epub 2013/02/26. doi: 10.1186/1475-2875-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Xiao SH. Pyronaridine: a new antimalarial drug. Parasitology Today. 1991;7(11):310–3. doi: 10.1016/0169-4758(91)90267-r. [DOI] [PubMed] [Google Scholar]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann. 2008.
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is "quality of evidence" and why is it important to clinicians? BMJ. 2008;336(7651):995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Appendix 5b: Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. In: Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions Version 4.2.5 [updated May 2005]. The Cochrane Collaboration, 2005. Available from www.cochrane.org/resources/handbook/hbook.htm (accessed 17 June 2006).
- Higgins JPT, Altman DG, Sterne Jac (editors) Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
- Jüni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ. 2001;323(7303):42–6. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Pongratz P, Bélard S, Mordmüller B, Kremsner PG, Ramharter M. In vitro activity of pyronaridine against Plasmodium falciparum and comparative evaluation of anti-malarial drug susceptibility assays. Malaria Journal. 2009;8:79. doi: 10.1186/1475-2875-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzhals JAL. Importance of the long-acting partner drug in artemisinin-based combination therapy. Expert Reviews in Pharmacology. 2008;1(6):745–7. doi: 10.1586/17512433.1.6.745. [DOI] [PubMed] [Google Scholar]
- Lim P, Wongsrichanalai C, Chim P, Khim N, Kim S, Chy S, et al. Decreased in vitro susceptibility of Plasmodium falciparum isolates to artesunate, mefloquine, chloroquine, and quinine in Cambodia from 2001 to 2007. Antimicrobial Agents and Chemotherapy. 2010;54(5):2135–42. doi: 10.1128/AAC.01304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J, Churchill R, Higgins J, Lasserson T, Tovey D. Methodological Expectations of Cochrane Intervention Reviews (MECIR) Methodological standards for the conduct of new Cochrane Intervention Reviews. Version 2.1, 8 December 2011. Available at: http://www.editorial-unit.cochrane.org/sites/editorial-unit.cochrane.org/files/uploads/MECIR_conduct_standards%202.1.pdf (accessed on 4 November 2012).
- Naisbitt DJ, Williams DP, O'Neill PM, Maggs JL, Willock DJ, Pirmohamed M, et al. Metabolism-dependent neutrophil cytotoxicity of amodiaquine: A comparison with pyronaridine and related antimalarial drugs. Chemical Research in Toxicology. 1998;11(12):1586–95. doi: 10.1021/tx980148k. [DOI] [PubMed] [Google Scholar]
- Nosten N, White NJ. Artemisinin-based combination treatment of falciparum malaria. American Journal of Tropical Medicine and Hygiene. 2007;77(Suppl 6):181–92. [PubMed] [Google Scholar]
- Pradines B, Tall A, Parzy D, Spiegel A, Fusai T, Hienne R, et al. In-vitro activity of pyronaridine and amodiaquine against African isolates (Senegal) of Plasmodium falciparum in comparison with standard antimalarial agents. 1998;42(3):333–9. doi: 10.1093/jac/42.3.333. Journal of Antimicrobial Chemotherapy. [DOI] [PubMed] [Google Scholar]
- Pradines B, Briolant S, Henry M, Oeuvray C, Baret E, Amalvict R, et al. Absence of association between pyronaridine in vitro responses and polymorphisms in genes involved in quinoline resistance in Plasmodium falciparum. Malaria Journal. 2010;9:339. doi: 10.1186/1475-2875-9-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RN, Marfurt J, Chalfein F, Kenangalem E, Piera KA, Tjitra E, et al. In vitro activity of pyronaridine against multidrug-resistant Plasmodium falciparum and Plasmodium vivax. Antimicrobial Agents and Chemotherapy. 2010;54(12):5146–50. doi: 10.1128/AAC.00801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Yang CZ, Wang CY, Wang SB, Yang M, Wang JH. Function and mechanism of pyronaridine: a new inhibitor of P-glycoprotein-mediated multidrug resistance. Acta Pharmacologica Sinica. 2002;23(6):544–50. [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. 2011.
- Ringwald P, Eboumbou EC, Bickii J, Basco LK. In vitro activities of pyronaridine, alone and in combination with other antimalarial drugs, against Plasmodium falciparum. Antimicrobial Agents and Chemotherapy. 1999;43(6):1525–7. doi: 10.1128/aac.43.6.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscoe JE, Tingle MD, O'Neill PM, Ward SA, Park BK. Effect of disposition of mannich antimalarial agents on their pharmacology and toxicity. Antimicrobial Agents and Chemotherapy. 1998;42(9):2410–6. doi: 10.1128/aac.42.9.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. 2008. Available from www.cochrane-handbook.org. The Cochrane Collaboration.
- Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews. 2009;(3) doi: 10.1002/14651858.CD007483.pub2. [DOI: 10.1002/14651858.CD007483.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivas L, Rattray L, Stewart L, Bongard E, Robinson BL, Peters W, et al. Anti-malarial efficacy of pyronaridine and artesunate in combination in vitro and in vivo. Acta Tropica. 2008;105(3):222–8. doi: 10.1016/j.actatropica.2007.12.005. [DOI] [PubMed] [Google Scholar]
- White NJ, Olliaro PL. Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitology Today. 1996;12(10):399–401. doi: 10.1016/0169-4758(96)10055-7. [DOI] [PubMed] [Google Scholar]
- White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, Snow RW, et al. Averting a malaria disaster. Lancet. 1999;353(9168):1965–7. doi: 10.1016/s0140-6736(98)07367-x. [DOI] [PubMed] [Google Scholar]
- White NJ. The assessment of antimalarial drug efficacy. Trends in Parasitology. 2002;18(10):458–64. doi: 10.1016/s1471-4922(02)02373-5. [DOI] [PubMed] [Google Scholar]
- White NJ. Antimalarial drug resistance. Journal of Clinical Investigation. 2004;113(8):1084–92. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. http://whqlibdoc.who.int/publications/2008/9789241596305_eng.pdf (accessed 28 December 2010).
- World Health Organization. Malaria treatment guidelines - 2nd edition. www.who.int/malaria/publications/atoz/9789241547925/en/index.html (accessed 20 November 2010).
- World Health Organization. Global report on antimalarial drug efficacy and drug resistance 2000-2010. www.who.int/malaria/publications/atoz/9789241500470/en/index.html (accessed 28 December 2010).
- World Health Organization. Global plan for artemisinin resistance containment (GPARC) www.who.int/malaria/publications/atoz/9789241500838/en/index.html (accessed 4 February 2011).
- WHO Global Malaria Programme. World Malaria Report 2012. Geneva: World Health Organization; 2012. World Malaria Report 2012. [Google Scholar]
- Yang HL, Liu DQ, Yang YM, Huang KG, Dong Y, Yang PF, et al. In vitro sensitivity of Plasmodium falciparum to eight antimalarials in China-Myanmar and China-Lao PDR border areas. Southeast Asian Journal of Tropical Medicine and Public Health. 1997;28(3):460–4. [PubMed] [Google Scholar]


