Abstract
Background
Seborrhoeic dermatitis is a chronic inflammatory skin condition that is distributed worldwide. It commonly affects the scalp, face and flexures of the body. Treatment options include antifungal drugs, steroids, calcineurin inhibitors, keratolytic agents and phototherapy.
Objectives
To assess the effects of antifungal agents for seborrhoeic dermatitis of the face and scalp in adolescents and adults.
A secondary objective is to assess whether the same interventions are effective in the management of seborrhoeic dermatitis in patients with HIV/AIDS.
Search methods
We searched the following databases up to December 2014: the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 11), MEDLINE (from 1946), EMBASE (from 1974) and Latin American Caribbean Health Sciences Literature (LILACS) (from 1982). We also searched trials registries and checked the bibliographies of published studies for further trials.
Selection criteria
Randomised controlled trials of topical antifungals used for treatment of seborrhoeic dermatitis in adolescents and adults, with primary outcome measures of complete clearance of symptoms and improved quality of life.
Data collection and analysis
Review author pairs independently assessed eligibility for inclusion, extracted study data and assessed risk of bias of included studies. We performed fixed-effect meta-analysis for studies with low statistical heterogeneity and used a random-effects model when heterogeneity was high.
Main results
We included 51 studies with 9052 participants. Of these, 45 trials assessed treatment outcomes at five weeks or less after commencement of treatment, and six trials assessed outcomes over a longer time frame. We believe that 24 trials had some form of conflict of interest, such as funding by pharmaceutical companies.
Among the included studies were 12 ketoconazole trials (N = 3253), 11 ciclopirox trials (N = 3029), two lithium trials (N = 141), two bifonazole trials (N = 136) and one clotrimazole trial (N = 126) that compared the effectiveness of these treatments versus placebo or vehicle. Nine ketoconazole trials (N = 632) and one miconazole trial (N = 47) compared these treatments versus steroids. Fourteen studies (N = 1541) compared one antifungal versus another or compared different doses or schedules of administration of the same agent versus one another.
Ketoconazole
Topical ketoconazole 2% treatment showed a 31% lower risk of failed clearance of rashes compared with placebo (risk ratio (RR) 0.69, 95% confidence interval (CI) 0.59 to 0.81, eight studies, low-quality evidence) at four weeks of follow-up, but the effect on side effects was uncertain because evidence was of very low quality (RR 0.97, 95% CI 0.58 to 1.64, six studies); heterogeneity between studies was substantial (I² = 74%). The median proportion of those who did not have clearance in the placebo groups was 69%.
Ketoconazole treatment resulted in a remission rate similar to that of steroids (RR 1.17, 95% CI 0.95 to 1.44, six studies, low-quality evidence), but occurrence of side effects was 44% lower in the ketoconazole group than in the steroid group (RR 0.56, 95% CI 0.32 to 0.96, eight studies, moderate-quality evidence).
Ketoconozale yielded a similar remission failure rate as ciclopirox (RR 1.09, 95% CI 0.95 to 1.26, three studies, low-quality evidence). Most comparisons between ketoconazole and other antifungals were based on single studies that showed comparability of treatment effects.
Ciclopirox
Ciclopirox 1% led to a lower failed remission rate than placebo at four weeks of follow-up (RR 0.79, 95% CI 0.67 to 0.94, eight studies, moderate-quality evidence) with similar rates of side effects (RR 0.9, 95% CI 0.72 to 1.11, four studies, moderate-quality evidence).
Other antifungals
Clotrimazole and miconazole efficacies were comparable with those of steroids on short-term assessment in single studies.
Treatment effects on individual symptoms were less clear and were inconsistent, possibly because of difficulties encountered in measuring these symptoms.
Evidence was insufficient to conclude that dose or mode of delivery influenced treatment outcome. Only one study reported on treatment compliance. No study assessed quality of life. One study assessed the maximum rash-free period but provided insufficient data for analysis. One small study in patients with HIV compared the effect of lithium versus placebo on seborrhoeic dermatitis of the face, but treatment outcomes were similar.
Authors' conclusions
Ketoconazole and ciclopirox are more effective than placebo, but limited evidence suggests that either of these agents is more effective than any other agent within the same class. Very few studies have assessed symptom clearance for longer periods than four weeks. Ketoconazole produced findings similar to those of steroids, but side effects were fewer. Treatment effect on overall quality of life remains unknown. Better outcome measures, studies of better quality and better reporting are all needed to improve the evidence base for antifungals for seborrhoeic dermatitis.
Plain Language Summary
Antifungal treatments applied to the skin to treat seborrhoeic dermatitis
Background
Seborrhoeic dermatitis is a chronic inflammatory skin condition found throughout the world, with rashes with varying degrees of redness, scaling and itching. It affects people of both sexes but is more common among men. The disease usually starts after puberty and can lead to personal discomfort and cosmetic concerns when rashes occur at prominent skin sites. Drugs that act against moulds, also called antifungal agents, have been commonly used on their own or in combination.
Review question
Do antifungal treatments applied to the skin clear up the rashes and itching of seborrhoeic dermatitis?
Study characteristics
We included 51 studies with 9052 participants. Trials typically were four weeks long, and very few trials were longer. In all, 24 studies had some involvement of pharmaceutical companies such as funding or employment of the researchers.
Key results
Particpants taking ketoconazole were 31% less likely than those given placebo to have symptoms that persisted at four weeks of follow-up. This was seen in eight studies with 2520 participants, but wide variation was noted between studies. Ketoconazole was as effective as steroids but had 44% fewer side effects. Without causing more side effects, ciclopirox was 21% more effective than placebo in achieving clinical clearance of rashes. Treatment effect on redness, itching or scaling symptoms of the skin was less clear. Evidence was insufficient to conclude that that one antifungal was superior to other antifungals, but this observation was based on few studies. Ketoconazole and ciclopirox are the most heavily investigated antifungals and are more effective than placebo. Other antifungals might have similar effects, but data are insufficient to underpin this.
Common side effects were increased skin redness or itching, burning sensation and hair loss.
No studies measured quality of life. Only one study reported on percentage of compliance in different treatment groups. Other studies used surrogates such as acceptability to represent compliance. We therefore could not assess the effect of compliance on treatment outcomes. One study on patients with HIV reported no clear effects of treatments.
Quality of the evidence
Evidence for the effects of ketoconazole compared with placebo or a steroid was assessed to be of low quality. Evidence derived from comparison of ciclopirox versus placebo was assessed to be of moderate quality. Better quality studies with longer follow-up and better reporting are needed to enlarge the evidence base for antifungals.
Background
Definition
Seborrhoeic dermatitis is a common chronic inflammatory disease of the skin, which manifests as scaly reddish-brown itchy patches in sebaceous gland-rich regions of the scalp, face and trunk (Scaparro 2001).
Epidemiology
Seborrhoeic dermatitis has a worldwide distribution and affects all races. Global prevalence ranges from 2% to 5% (Aly 2003; Gupta 2004). Occurrence is common in the years following puberty (Burton 1983; Johnson 2000), but peak occurrence is seen around 40 years of age (Aly 2003). When the disease occurs in infancy, it is known as 'cradle cap’. Seborrhoeic dermatitis affects men more often than women (Gupta 2004; Johnson 2000).
Causes
The disease is caused by an interaction of endogenous (individual), environmental and general health factors (Johnson 2000). Presence of the yeast known as Malassezia species, which normally lives on the skin, is a finding that is often associated with seborrhoeic dermatitis, but this remains controversial (Bergrant 1996; Gaitanis 2013; Rigopoulos 2004). Changes in seasonal humidity are believed by some to worsen the symptoms (Scheinfeld 2005).
The fact that seborrhoeic dermatitis responds to antifungal medication strongly supports the role of yeast as a causal factor (Johnson 2000; Scaparro 2001). This theory is further supported by the observed reduction in the number of Malassezia yeast cells during treatment, which correlates with clinical improvement (Gupta 2004). Recurrence of the disease is observed following a rebound in the number of Malassezia yeast cells to pretreatment levels (Parry 1998). Evidence from research indicates that human sebocytes (fat-producing cells) respond to androgen stimulation, and their increased activity worsens the severity of seborrhoeic dermatitis (Johnson 2000).
Risk factors for this skin disorder include stress, fatigue, weather extremes, oily skin, obesity, infrequent skin cleaning and skin disorders such as acne. People with neurological conditions such as Parkinson’s disease, stroke, cranial nerve palsy and head injury appear to be more prone to this skin disease (Schwartz 2006). When co-existing conditions occur, seborrhoeic dermatitis tends to be more extensive and poorly responsive to treatment (Johnson 2000). Conflicting findings have been reported from various studies that have explored the role of the immune system in development of this disease. Although evidence for specific immunological influence remains inconclusive, the correlation with human immunodeficiency virus (HIV) infection gives credibility to this (Parry 1998). Occurrence and severity of seborrhoeic dermatitis increase with progression and severity of HIV infection (Parry 1998). Human immunodeficiency virus infection increases the prevalence of this condition to as much as 83% (Bergrant 1996; Scheinfeld 2005). The strong association between cancer of the head and neck and seborrhoeic dermatitis may be the result of an underlying immune distortion.
Several drugs have been known to provoke eruption of this rash; these include chlorpromazine, cimetidine, ethionamide, gold, griseofulvin, haloperidol, interferon-alpha, lithium, methoxsalen, methyldopa, phenothiazines, psoralens, stanozolol, thiothixene and trioxsalen (Scheinfeld 2005).
Description of the condition
Diagnosis
Seborrhoeic dermatitis is a mainly clinical diagnosis that is made on the basis of occurrence of characteristic rashes in areas rich in sebaceous glands. In adolescents and adults, it commonly presents as a scaling rash of the scalp (Schwartz 2006). The rash appears as areas of redness covered with greasy white or yellowish scales (Burton 1983). "The scaling is often concurrent with an oily complexion" (Schwartz 2006).
On the scalp, the rash spans the spectrum from mild dandruff to a more grievous oozy rash. On the face, it affects the eyebrows, the creases of the nose and the adjacent cheek, and occasionally the eyelids. Rashes are increasingly apparent when men grow moustaches or beards, and tend to disappear when facial hair is removed (Johnson 2000). Rashes may occur behind the ears, in the cup of the ears and within the ear canal, and can occur as red patches on the front of the chest or between the shoulder blades (Johnson 2000). In persons of colour, the rash sometimes appears as white, minimally scaly patches on the face, particularly around the eyebrows (Scheinfeld 2005). Flexure areas such as between the breasts and in the armpits, groin, abdominal folds and nappy area in infants can also be affected. The rashes are often non-itching in infants (Schwartz 2006) and tend to disappear spontaneously (Foley 2003; Naldi 2009).
Biopsies of affected skin may effectively distinguish seborrhoeic dermatitis from similar disorders. White blood cells (neutrophils) are characteristically found within the scale crusts (Schwartz 2006).
Impact
In its active phase in adolescents and adults, seborrhoeic dermatitis may manifest as unpleasant symptoms of burning, itching and scaling, causing much discomfort to those affected. Affected areas vary from mild patchy scaling to widespread thick adherent crusts and occasionally disfiguring plaques.
Serious cosmetic problems may arise for people living with this condition because of the prominent location of red greasy rashes on the scalp, back of the neck and ears, forehead, eyebrows, eyelashes or moustache and beard area (Burton 1983; Gupta 2004).
Those with seborrhoeic dermatitis can become increasingly frustrated by relapses following treatment and poor treatment outcomes, which can lead to psychosocial distress. Occasionally secondary bacterial infection may complicate the disease, leading to an oozing, crusting eczematous dermatitis.
Occasional co-existence of the disease with other disease conditions such as blepharitis (inflammation of the eyelids), meibomian gland (sebaceous glands within the eyelids), occlusion and abscess formation, external ear infection, acne vulgaris, psoriasis and pityriasis versicolour (fungus that commonly colonises the skin) can create further problems for those affected (Schwartz 2006).
Prognosis
Seborrhoeic dermatitis runs a chronic course. As the aetiology is not fully understood (Johnson 2000; Naldi 2009; Trznadel-Grodzka 2012), no medical cure has been developed. Available interventions are at best suppressive. Relapses are frequent. In severe cases, suppressive treatment may be followed by maintenance therapy that lasts for several years (Johnson 2000).
Description of the intervention
Treatment for seborrhoeic dermatitis aims to do the following.
Achieve remission of rashes.
Eliminate itching and burning sensations.
Reduce the severity of rashes.
Prevent recurrence of rashes.
A variety of drug and non-drug treatments have been tried for seborrhoeic dermatitis. Antifungal and anti-inflammatory drugs are probably the most widely applied (Naldi 2009). Various preparations are available for topical and oral application. Behavioural modifications such as frequent skin cleansing with soap, resolute commitment to personal hygiene and frequent outdoor recreation, especially in summer, have been found to lessen the symptoms (Johnson 2000). Other therapeutic modalities include salicylic acid, zinc pyrithione and coal tar, which are applied topically and function to soften and remove the thick hardened crusts that sometimes occur in seborrhoeic dermatitis (Schwartz 2006). Recalcitrant cases of this skin problem have been managed with phototherapy (i.e. ultraviolet B phototherapy) (Naldi 2009), as well as with isotretinoin therapy, which reduces sebaceous gland size and consequently sebum secretion (Johnson 2000).
In this review, we have focused on the more widespread topical application of topical antifungal agents such as ketoconazole, fluconazole and ciclopirox, which are available as ointments, creams, gels and shampoos (Gupta 2004a; Shuster 2005).
How the intervention might work
Based on the concept that Malassezia yeasts are involved in the pathogenesis of seborrhoeic dermatitis, antifungals have long been proposed as treatment that confers the same benefits as steroids but lacks associated adverse effects (Gupta 2004a). Antifungals can lead to inhibition of fungal growth, mainly by interaction with the fungal cell membrane through inhibition of sterol synthesis or inhibition of the synthesis of cell walls (Kathiravan 2012). In accordance with their chemical structure, antifungals are usually divided into azole-based antifungals such as ketoconazole, allylamines such as terbinafine, benzylamines such as butenafine and hydroxypyridones such as ciclopirox (Ghannoum 1999). Other drugs such as selenium sulphide or herbal agents and natural products such as honey have also been shown to influence fungal growth, but their mechanism of action is not clear (Gupta 2004a). In this review, we have selectively included herbal extracts that have well-documented antifungal properties.
Why it is important to do this review
The high global prevalence of seborrhoeic dermatitis, its explosive incidence rate in HIV/acquired immunodeficiency syndrome (AIDS) and its chronic course justify further research with the purpose of finding treatment options targeted to achieve effective control. Physicians are inclined to use different treatment regimens for management, and in some instances the long course of therapy may erode patient compliance. Furthermore, almost all treatments aim to obtain but not to maintain remission. Long-term control of the disease should be attainable.
A systematic review of current treatment options is the best means to explore evidence on efficacy and appropriateness of treatment. This review focuses on antifungal treatments and was originally published as the protocol 'Interventions for seborrhoeic dermatitis'. This topic was subsequently split into two reviews: 'Topical antifungals for seborrhoeic dermatitis' and 'Topical anti-inflammatory agents for seborrhoeic dermatitis'. The latter was published as a separate protocol in 2011 and later as a review (Kastarinen 2011). We are also aware of a related Cochrane review on infantile seborrhoeic dermatitis (including cradle cap) that is in preparation (Victoire 2014).
Objectives
To assess the effects of antifungal agents for seborrhoeic dermatitis of the face and scalp in adolescents and adults.
A secondary objective is to assess whether the same interventions are effective in the management of seborrhoeic dermatitis in patients with HIV/AIDS.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (including cross-over trials and cross-over trials of body parts) of antifungal agents for seborrhoeic dermatitis.
Types of participants
We included studies conducted with adults or adolescents who had been diagnosed by a healthcare practitioner, as explicitly stated or implied within context, as having seborrhoeic dermatitis (SD) of the scalp, face or both based on clinical case definition, with or without laboratory confirmation. The term 'healthcare practitioner' as used implies physicians or another cadre of care providers who used well-defined guidelines for making the diagnosis. We included studies that had described the diagnosis as seborrhoeic eczema or seborrhoeic dermatitis. No consensus has been reached on the difference between seborrhoeic dermatitis of the scalp and dandruff, which are seen by many as part of a continuous spectrum of dermatitis of the scalp. Therefore, we also included studies with patients who were diagnosed with dandruff.
Types of interventions
We included studies that had evaluated the effectiveness of topical antifungal drugs for seborrhoeic dermatitis, as well as studies that had compared interventions according to either of the following two schedules.
Any topical antifungal-based treatment versus no treatment or placebo.
Any topical antifungal-based treatment versus another treatment.
We defined antifungal drugs as drugs with an established antifungal mode of action. According to Gupta 2004, this included the following drug classes.
Imidazoles: bifonazole, climbazole, ketoconazole, miconazole.
Triazoles: fluconazole.
Allylamines: terbinafine.
Benzylamines: butenafine.
Hydroxypyrones: ciclopirox.
We found no consensus among study authors on how antifungal drugs were defined for use in trials. Therefore we included all studies in which study authors presented evidence that the drug had antifungal properties. We also included two herbal treatments with documented antifungal properties.
We excluded studies or treatment arms of studies that used a combination of antifungals and other drugs as the intervention, such as a combination of antifungals and steroids, because it would be unclear which of the active agents accounts for a given outcome and to what extent.
For topical applications, it is difficult to capture dose, as it is unclear how much a patient will need to apply to the skin. The only information available in studies was the strength of the drug given as a percentage and the frequency of application per day and per week. To calculate a dose that is comparable across studies, we multiplied the percentage by the frequency per day by the frequency per week. For example, 2% ketoconazole applied twice daily seven days a week would add up to 28 percentage points per week (%/wk).
Types of outcome measures
Primary outcomes
Percentage of persons who had clinical resolution (clearance) of all symptoms based on physician assessment.
Quality of life measured with any validated quality of life assessment index.
Secondary outcomes
Symptom severity scores for erythema, pruritus and scaling, measured with any type of systematic symptom severity assessment.
Side effects/intolerance to treatment.
Percentage of persons treated who comply with treatment regimens.
The longest rash-free period.
Timing of outcomes
Treatment effects were measured and combined at:
four weeks or less following commencement of treatment (short-term); and
more than four weeks following commencement of treatment (long-term).
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press or in progress).
Electronic searches
We searched the following databases up to 16 December 2014.
The Cochrane Skin Group Specialised Register using the following search terms: "seborrh* dermatitis" or "scalp dermatos*" or "scalp dermatitis" or "scalp eczema" or "cradle cap" or dandruff or malassezia or "seborrh* eczema".
The Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 11), using the search strategy presented in Appendix 1.
MEDLINE via Ovid (from 1946), using the strategy in Appendix 2.
EMBASE via Ovid (from 1974), using the strategy in Appendix 3.
Latin American and Caribbean Health Sciences Literature (LILACS) (from 1982), using the strategy in Appendix 4.
Trials registers
On 10 February 2015, we searched the following trials registers using the search terms 'seborrhoeic dermatitis, cradle cap, scalp dermatoses, and malassezia'.
The metaRegister of Controlled Trials (www.controlled-trials.com/).
The US National Institutes of Health ongoing trials register (www.clinicaltrials.gov/).
The Australian and New Zealand Clinical Trials Registry (www.anzctr.org.au/).
The World Health Organization International Clinical Trials Registry platform (apps.who.int/trialsearch/).
The Ongoing Skin Trials register (www.nottingham.ac.uk/ongoingskintrials/).
The EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
The International Federation of Pharmaceutical Manufacturers and Associations Clinical Trials Portal (clinicaltrials.ifpma.org/clinicaltrials/no_cache/en/myportal/index.htm).
The Clinical Trials Registry India (ctri.nic.in/Clinicaltrials/login.php).
Searching other resources
References from published studies
We checked the bibliographies of published studies for further references to potentially relevant trials.
Data collection and analysis
Selection of studies
Five review authors (EOO, JHV, JHR, OAO and VNB) working in independent pairs screened titles and abstracts of references to identify studies presented as RCTs or controlled trials. We further retrieved full-text articles of such references and ran in-depth checks on study methodology to support our decision on which to include. To ensure that the study selection process was systematic, we developed and used a study selection form that operationalised the inclusion and exclusion criteria. We discussed conflicts between pairs of review authors to resolve them, and when no consensus was reached, a third review author from another pair arbitrated. The same pair of review authors assessed studies for risk of bias with recourse to a third review author when conflicts arose. JHV, JHR and colleagues within The Cochrane Collaboration (see Acknowledgements) translated studies published in languages other than English.
Data extraction and management
We developed a detailed data extraction form and tested it on a subset of the included studies to ascertain its adequacy and useability. We made the necessary modifications before using the form to extract data from identified studies. EOO, JHV, JHR, OAO and VNB extracted data. We used the same review author pairing approach for data extraction that we had used for study selection. Whenever a pair of review authors produced discrepancies, one of the other review authors resolved them. EOO entered the data into RevMan, and JHV checked that they were correct.
Assessment of risk of bias in included studies
Assessment of risk of bias consisted of an evaluation of the following components for each included study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Method of generating randomisation sequence - We considered this adequate if a proper randomisation method such as a table of random numbers or a computer programme had been used. The randomisation sequence had to be generated away from the actual trial site.
Method of allocation concealment - We considered this adequate if the assignment could not be foreseen by trial participants or investigators, for example, through the use of identical bottles with codes that were indecipherable to both participants and investigators.
Blinding - We considered whether participants, care providers and outcome assessors were adequately blinded as to who received the intervention and who received placebo.
Avoidance of co-interventions - We assessed whether co-interventions were avoided or similar between comparison groups. This was not prespecified in the protocol.
Drop-out rate - We considered loss of 20% or less of trial participants, comparable among groups, as non-systematic and therefore not likely to bias results. This was not prespecified in the protocol.
Intention-to-treat analysis - We assessed whether participants were analysed in the groups to which they were originally randomly assigned.
Selective outcome reporting - If a protocol was available, we checked whether outcomes were reported as proposed in the protocol and adequately; if no protocol was available, we checked whether outcomes were adequately reported and were consistent with those proposed in the Methods section of the article. This was not prespecified in the protocol.
Baseline imbalance among participants - We assessed whether participants in the intervention and control groups suffered from seborrhoeic dermatitis to a similar degree, or if a considerable difference was obvious.
Compliance - We checked whether participants in intervention and control groups complied with their drug regimen over an equal duration. This was not prespecified in the protocol.
Measures of treatment effect
For the outcome 'clearance of symptoms', which was stated in the protocol, we used instead the number of persons not cleared of symptoms, id est 'Failure to achieve complete resolution', because this best represents the treatment effect or lack of such an effect; for clarity we labelled the analyses.
For dichotomous outcomes such as the proportion of participants with lack of clearance of symptoms, we expressed the estimate of effect as a risk ratio (RR) with 95% confidence interval (Cl) at both short-term follow-up (up to four weeks) and long-term follow-up (more than four weeks). Thus, RR < 1 indicates a beneficial effect of the treatment. We expressed summary estimates of dichotomous outcomes as number needed to treat for an additional beneficial outcome (NNTB) for statistically significant findings, when appropriate with 95% CI. We used the median control group risk in the comparison for NNTB calculations.
For continuous outcomes such as symptom scores for erythema, scaling and pruritus, we used the mean difference (MD) in summarising results. When similar outcomes were measured on different scales, we used the standardised mean difference (SMD) with its 95% CI.
Unit of analysis issues
We intended to analyse cross-over trials using techniques appropriate for paired designs, but the studies did not report sufficient data to facilitate this (see Description of studies).
We analysed studies with multiple treatment groups using pair-wise comparisons. When some studies compared an antifungal agent versus more than one control treatment, we considered each arm as a separate study comparing one active treatment versus one control treatment.
We avoided double counting of treatment and control groups of multiple treatment studies by equally dividing the number of control participants over the number of comparisons in the same meta-analysis.
Dealing with missing data
When we encountered missing data, we corresponded with study authors to request additional information. When data were reported only in figures, we extracted the data from the figures.
When standard errors were presented in figures, we recalculated these into standard deviations (SDs) using the RevMan calculator (RevMan 2011). For studies in which SDs were not given, we calculated these from P values.
Assessment of heterogeneity
We assessed statistical heterogeneity using the I² statistic, and judged heterogeneity between studies as considerable when the I² statistic was greater than 50%.
Data synthesis
We pooled risk ratios for studies with dichotomous outcomes and mean differences or, when appropriate, standardised mean differences for studies with continuous outcomes using their weighted average for treatment effect as implemented in the RevMan software (RevMan 2011). When heterogeneity was greater than 50%, we used a random-effects model. When heterogeneity was severe - I² statistic greater than 80% - we did not perform a meta-analysis but reported individual study results separately.
Grade
We used the programme GRADEPro to assess the quality of evidence across studies and to generate 'Summary of findings' tables for the most important comparisons that included a relevant number of studies. We started at a high level of quality because we included only randomised studies. We then used limitations in study design, consistency of results, directness, precision and publication bias to determine whether this should be downgraded by one or more levels. We reported our reasons for doing so as footnotes in the 'Summary of findings' table and in Table 1. We considered the study design to have limitations when most of the studies in a comparison had unclear or high risk of bias for randomisation, unclear allocation concealment or blinding of outcome assessment.
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis among HIV-positive participants with seborrhoeic dermatitis, but only one study included patients with HIV.
We conducted subgroup analyses based on conflicts of interest, dose and mode of delivery. These subgroup analyses were not planned in the protocol. Trial results were not presented in such a way as to allow subgroup analysis based on age, sex or presence of co-morbidity (significant co-morbidity was an exclusion criterion in many trials), as we had intended to do. Study factors (i.e. quality, design) that we had proposed as a basis for subgroup analysis were used instead for sensitivity analysis.
Sensitivity analysis
We attempted to carry out a sensitivity analysis by excluding studies that we judged to have high risk of bias based on inadequate randomisation, allocation concealment or absence of blinding. However, we found too few studies on subgroup categorisation to effectively perform this. We deemed exclusion on the basis of accuracy of diagnosis (as stated in the protocol) as not worthwhile because most trials did not explicitly state whether the diagnosis was made by a physician. We dropped other criteria as stated in the protocol because they were not feasible (see Differences between protocol and review).
Results
Description of studies
Results of the search
Our systematic searches, conducted between November 2009 and 17 December 2014, produced altogether 910 references. We identified 18 additional references by searching the reference lists of included studies, and 17 additional studies by searching trials registers. We screened these 945 references for inclusion on the basis of title and abstract. We were left with 220 articles that we then scrutinised in full text by using our study inclusion checklist. We present a more detailed picture of the screening process in figure 1.
figure 1.
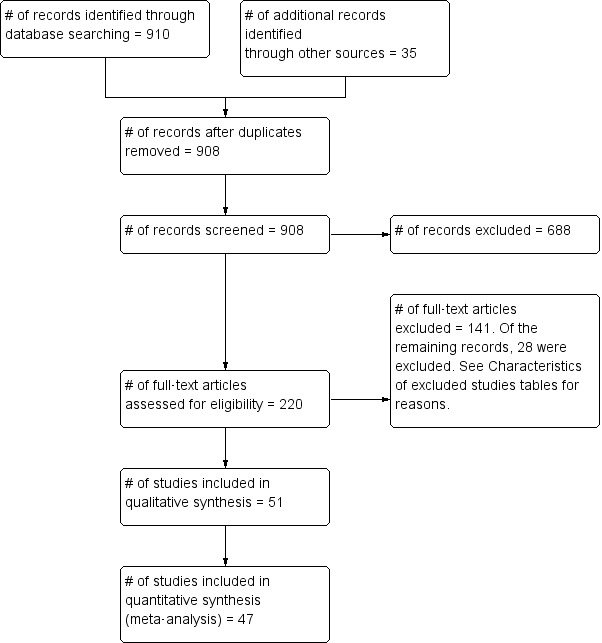
Flow diagram for study inclusion.
Altogether we included 51 studies in this review, of which 47 provided sufficient data to be included in the meta-analysis.
Included studies
Study design
Almost all studies were individual parallel-group RCTs, but one was a cross-over trial (Draelos 2005) and two were RCTs of body parts (Langtry 1997; Schofer 1988).
The cross-over trial by Draelos 2005 did not use a wash-out period between the first and second treatment periods. Therefore, we used only results from the first treatment period of one week.
In the RCTs of body parts, treatment was applied to one-half of the face, and the placebo to the other half of the face. As these were the same individuals, a matched-pairs analysis should have been used to assess outcomes. This was done in one trial (Langtry 1997), which used a paired t-test, but not in the other (Schofer 1988), which reported a dichotomous outcome. For both of these studies, we did not find sufficient data to correct for the unpaired analysis, and we accepted that this would lead to underestimation of the real effects of treatment.
Participants
In total, studies included 9052 participants. Of these, 4164 were included in the main intervention group (participants receiving treatment that is primarily being tested by the investigators) and 3701 in the largest control group. Multi-arm studies included 1985 participants in a second control group and an additional 202 participants in a third control group, altogether including 4888 control participants.
We grouped the diagnoses of trial participants as follows.
Seborrhoeic dermatitis or dandruff of the scalp.
Seborrhoeic dermatitis of the scalp and face.
Seborrhoeic dermatitis of the face.
It was not always clear within studies to what extent the disease also affected the trunk of a participant's body. Few studies stated this clearly (Green 1987; Ortonne 1992; Pari 1998; Pierard 1991; Stratigos 1988; Swinyer 2007; Van't Veen 1998), and we assumed that when the face was involved, the trunk might also be affected. Therefore we did not make further distinctions between seborrhoeic dermatitis of the face or scalp exclusively and seborrhoeic dermatitis of these parts with truncal involvement.
One study specifically recruited patients with HIV with seborrhoeic dermatitis as participants. These investigators recruited most participants from outpatient departments of hospitals.
Trial settings and diagnoses
Eight studies were conducted in the USA; six each in Germany and France; five in the UK; four in Turkey; three each in Greece and Belgium; two each in India, Iran, Israel, Mexico, the Netherlands and Sweden; and one study each in Argentina, Australia, Canada, Finland, Italy, Japan and Korea.
Included studies were conducted between 1985 and 2013, with 27 studies conducted from the year 2000 onward.
Interventions
Of the 51 included studies, eight studies included three intervention arms (Attarzadeh 2013; Danby 1993; Diehl 2013; Faergermann 1986; Ratnavel 2007; Shuster 2005; Shuttleworth 1998; Unholzer 2002(I)) and four included four intervention arms (Abeck 2004; Altmeyer 2004; Elewski 2007; Ortonne 2011). We excluded arms from Faergermann 1986 and Ortonne 2011 that tested mixed compounds. Of the multi-arm trials, two (Abeck 2004; Altmeyer 2004) compared various doses of the same intervention drug, and another (Elewski 2007) compared various forms (e.g. foam, gel) of the same drug but not different drugs.
Included trials assessed the effectiveness of the following imidazole drugs: ketoconazole, miconazole, bifonazole, climbazole and cotrimoxazole. For the hydroxypyridone group, studies focused on ciclopirox. Additional studies examined zinc pyrithione and lithium. Even though lithium is not typically used as an antifungal, many believe that it has antifungal properties (Dreno 2002).
No included studies evaluated drugs in the triazole group such as fluconazole, no studies examined drugs in the allylamine group such as terbinafine and no studies focused on drugs in the benzylamine group such as butenafine.
Ketoconazole
Ketoconazole was used in 33 studies and in 37 study arms; 12 studies compared it directly versus placebo, nine studies versus a steroid, one study versus pimecrolimus, three studies versus zinc pyrithione, six versus ciclopirox, one versus climbazole, one versus metronidazole, one versus lithium, two versus herbal medicines, one versus a different dose of ketoconazole and one versus a different formulation.
Ketoconazole was administered in widely varying doses. For ketoconazole, the most frequent dose was 2% twice daily every day for the face, adding up to 28%/wk and 2% twice a week for the scalp, amounting to 4%/wk. However, for the face studies, trialists also used a dose of 4%, 6% or 14%/wk. For the scalp, doses varied from 2% to 7%/wk but with less variation. Doses were similar for studies that used ketoconazole as a control intervention. Across studies, the average was 14.4%/wk.
Ciclopirox
Ciclopirox was used in 13 studies in 22 study arms and was compared with placebo in 11 study arms, with ketoconazole in six studies, with a different dose in four study arms and with Quassia amara in one.
Ciclopirox was also administered in varying doses. For the scalp, this varied from 1% twice a week to twice daily; for the face, it was once a day or twice a day, amounting to 14%/wk. Across studies, the average was 8.2%/wk.
Bifonazole
Bifonazole was used in two studies - one that used it for the face (one a day) and another that used it for the scalp (twice a day, three times a week). It used only as a 1% solution.
Climbazole
Climibazole was used in one study that compared it with ketoconazole. The dose used was 1% once daily for the scalp.
Clotrimazole
Clotriamazole was used in two study arms that compared it versus steroids and versus emu oil, which has been shown to have anti-inflammatory properties (Attarzadeh 2013). The dose used was 1% once daily for the face.
Lithium
Lithium salts were used in three studies that compared them versus placebo and versus ketoconazole. The dose used was 8% twice daily for the face.
Miconazole
Micoconazole was used in two studies that compared it versus steroids and versus a combination of shampoo and rinse, both for the scalp. The dose in one study was 2% twice daily, but the dose was unclear in the other study.
Zinc pyrithione
Zinc pyrithione was used in one study that compared it with ketoconazole. The dose used was 1% once daily for the scalp.
Quassia amara
One study evaluated the effect of Quassia amara, an extract reported to have antifungal properties, compared with ketoconazole 2%. We included this because it was listed by the US Food and Drug Administration (FDA) (USFDA 1987).
Solanum chrysotrichum
This herbal extract was investigated in one study in which its mycological action was compared with that of ketoconazole. It is widely used in Mexico, and its antifungal action has been reported in some studies (Herrera-Arellano 2013; Zamilpa 2002).
Outcomes of included studies
A total of 31 studies assessed complete clearance of symptoms, which was our prespecified first primary outcome: Abeck 2004; Altmeyer 2004; Aly 2003; Berger 1990; Chosidow 2003; Dreno 2003; Dupuy 2001; Elewski 2007; Faergermann 1986; Go 1992; Green 1987; Herrera-Arellano 2004; Hersle 1996; Katsambas 1989; Lebwohl 2004; Lopez-Padilla 1996; Ortonne 1992; Pari 1998; Piepponen 1992; Pierard 1991; Pierard-Franchimont 2001; Piérard-Franchimont 2002; Schofer 1988; Shuttleworth 1998; Skinner 1985; Stratigos 1988; Unholzer 2002(I); Unholzer 2002(II); Van't Veen 1998; Vardy 2000; Zienicke 1993.
In all, 14 studies assessed symptom severity score for redness (erythema), which was part of our prespecified first secondary outcome: Aly 2003; Elewski 2006; Hersle 1996; Koc 2009; Kousidou 1992; Langtry 1997; Ortonne 1992; Piepponen 1992; Pierard 1991; Satriano 1987; Segal 1992; Shuttleworth 1998; Stratigos 1988; Vardy 2000.
A total of 18 studies assessed symptom severity score for scaling (desquamation), which was part of our prespecified first secondary outcome: Aly 2003; Danby 1993; Draelos 2005; Elewski 2006; Faergermann 1986; Hersle 1996; Kousidou 1992; Langtry 1997; Ortonne 1992; Piepponen 1992; Pierard 1991; Piérard-Franchimont 2002; Ratnavel 2007; Satriano 1987; Shuttleworth 1998; Stratigos 1988; Van't Veen 1998; Vardy 2000.
In all, 11 studies assessed symptom severity score for itching (pruritus), which was part of our prespecified first secondary outcome: Elewski 2006; Kousidou 1992; Ortonne 1992; Piepponen 1992; Pierard 1991; Ratnavel 2007; Satriano 1987; Seckin 2007; Segal 1992; Stratigos 1988; Van't Veen 1998.
A total of 7 studies assessed clearance of individual symptoms, which was not prespecified as an outcome: Abeck 2004; Dreno 2002; Dreno 2003; Elewski 2007; Lopez-Padilla 1996; Ortonne 2011; Zienicke 1993.
No study assessed quality of life, which was our prespecified second primary outcome.
A total of 32 studies reported occurrence of side effects, which was a prespecified second secondary outcome, but only 27 studies specified their incidence in comparison groups. Most studies simply reported the total number of participants who had side effects without separating them into specific side effects and incidence within groups. We believe this missing information was crucial, as side effects had a bearing on tolerability of the interventions. The overall low numbers of cases reported may raise questions about the accuracy of these reports. We therefore analysed side effects simply using reported proportion within study groups. Only one study (Dreno 2003) assessed participants' treatment compliance as a formal variable. No study assessed the longest rash-free period.
Length of follow-up
Six studies followed participants for less than four weeks, and 37 followed them for exactly four weeks. We regarded these as short-term studies. Seven studies measured the outcome between four and eight weeks, and one study followed participants for a little over 17 weeks. We regarded these as long-term studies.
Excluded studies
We excluded 51 studies. See Characteristics of excluded studies for details.
Most of the excluded studies were non-randomised studies. Some studies involved skin conditions other than seborrhoeic dermatitis. When no indicator existed to show that seborrhoeic dermatitis was seen in at least 75% of total trial participants, we excluded these studies. We also excluded studies in which more than 25% of participants were younger than 10 years of age and those in which the composition of control groups was unclear.
We excluded nine studies that compared a combination of drugs, because of the uncertainty of the contribution of each component drug to the observed effect. This exclusion was not prespecified in the protocol.
We excluded studies that had used an outcome measure combining severity scores for erythema, pruritus and scaling. We did not see this index as objective, as there is no way of knowing what weight different symptoms are given in such sum scores. The following studies were excluded because they used such a composite symptom score: Amos 1994; Boyle 1986; Brown 1990; Cauwenbergh 1986; Comert 2007; Ermosilla 2005; Koca 2003; Kozlowska 2007; Peter 1995; Pierard-Franchimont 2002b; Vena 2005. This exclusion was not prespecified in the protocol.
Studies awaiting classification
Ten studies are awaiting classification. We are unable to make a decision whether to include them until we receive answers to our requests for more information, or until we have them translated. Please see Characteristics of studies awaiting classification for details.
Ongoing studies
We identified five studies through trials registries. Even though we tried to contact all of the investigators at once, we did not succeed in getting any information on whether results of these trials were available. We doubt if these results will ever be available. Please see Characteristics of ongoing studies for details.
Risk of bias in included studies
Please see figure 2 for the 'Risk of bias' summary, which includes our judgements about each risk of bias item for each included study, and figure 3 for the 'Risk of bias' graph, which includes our judgements about each risk of bias item presented as percentages across all included studies.
figure 2.
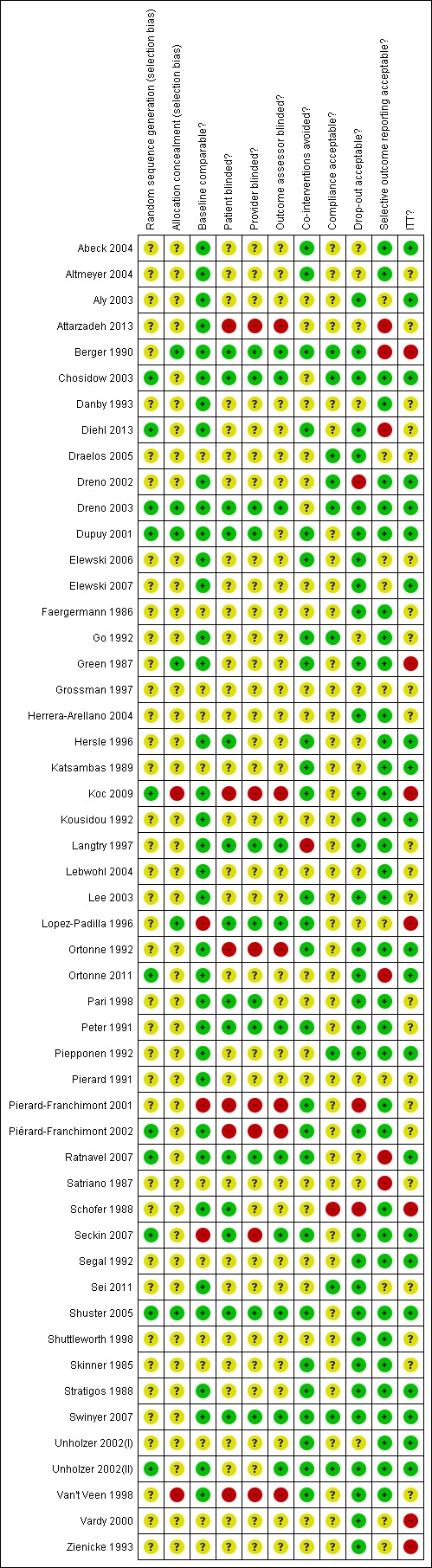
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
figure 3.
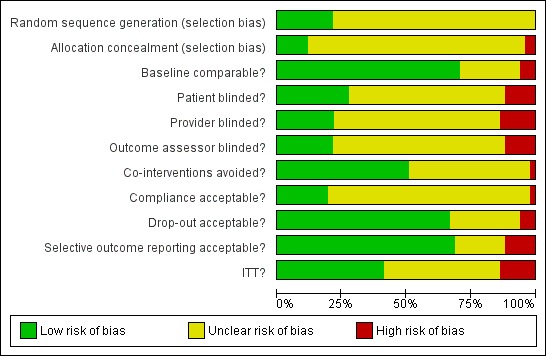
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
A total of 11 studies gave an account of the generation of randomisation sequence, so we rated these as having low risk of bias for this item: Chosidow 2003; Diehl 2013; Dreno 2003; Dupuy 2001; Koc 2009; Ortonne 2011; Piérard-Franchimont 2002; Ratnavel 2007; Seckin 2007; Shuster 2005; Unholzer 2002(II).
Allocation concealment procedure
Six studies documented an actual procedure for allocation concealment, so we rated these as having low risk of bias for this item: Berger 1990; Dreno 2003; Dupuy 2001; Green 1987; Lopez-Padilla 1996; Shuster 2005.
Blinding
Most studies did not report actual procedures for blinding. We assessed nine studies as having low risk of bias across the three domains that we labelled 'participant blinded?', 'provider blinded?' and 'outcome assessor blinded?': Berger 1990; Chosidow 2003; Dreno 2003; Langtry 1997; Lopez-Padilla 1996; Peter 1991; Ratnavel 2007; Shuster 2005; Swinyer 2007.
The above nine studies and two others reported using similar looking containers: Dupuy 2001; Pari 1998.
Other studies did not elaborate beyond stating that the study was "double-blind".
Incomplete outcome data
A total of 33 studies had acceptable drop-out rates within treatment groups, so we rated them as having low risk of bias for this domain.
Selective reporting
Six studies did not report all proposed outcome measures, so we rated them at high risk of reporting bias: Attarzadeh 2013; Berger 1990; Diehl 2013; Ortonne 2011; Ratnavel 2007; Satriano 1987.
It was difficult to judge from the articles whether other outcomes had been measured but were simply not reported, so we judged 10 as having unclear risk of bias and the rest as having low risk of bias.
Other potential sources of bias
Reporting of treatment compliance was generally unsatisfactory. In our domain labelled 'compliance acceptable?', we rated 10 studies at low risk of bias.
Reporting of side effects of treatment was generally unsatisfactory, and one study used a very small sample size (Green 1987), but we did not assess these issues in our 'Risk of bias' table.
Effects of interventions
See: Summary of findings for the main comparison Ketoconazole compared with placebo for seborrhoeic dermatitis; Summary of findings 2 Ketoconazole compared with steroids for seborrhoeic dermatitis; Summary of findings 3 Ketoconazole compared with ciclopirox for seborrhoeic dermatitis; Summary of findings 4 Ciclopirox compared with placebo for seborrhoeic dermatitis
We have addressed the outcomes of this review in relation to the following comparisons.
Ketoconazole versus placebo.
Ketoconazole versus steroids.
Ketoconazole versus zinc pyrithione.
Ketoconazole versus ciclopirox.
Ketoconazole versus metronidazole.
Ketoconazole versus climbazole.
Ketoconazole versus Solanum chrysotricum.
Ketoconazole versus pimecrolimus.
Ketoconazole versus lithium.
Ketoconazole versus selenium sulphide.
Ketoconazole versus Quassia amara.
Ketoconazole foam versus ketoconazole cream.
Ketoconazole (2%) versus ketoconazole (1%).
Bifonazole versus placebo.
Clotrimazole versus steroid.
Clotrimazole versus Emu oil.
Miconazole versus steroids.
Miconazole shampoo plus rinse versus shampoo alone.
Ciclopirox versus placebo.
Ciclopirox versus Quassia amara.
Ciclopirox versus ciclopirox (in different doses).
Lithium salts versus placebo.
Ketoconazole versus placebo
Primary outcomes
Participants without complete resolution
Nine studies compared a topical ketoconazole preparation with a topical placebo (Berger 1990; Elewski 2007 (gel and foam); Go 1992; Green 1987; Pierard 1991; Schofer 1988; Skinner 1985; Swinyer 2007; Unholzer 2002(I)). Two studies evaluated the effect on the scalp only (RR 0.71, 95% CI 0.31 to 1.61) with similar outcomes. For face and scalp application, three studies (four comparisons) found a beneficial effect of ketoconazole (RR 0.72, 95% CI 0.51 to 0.84). For application to the face only, two studies yielded an effect of similar size (RR 0.73, 95% CI 0.51 to 1.05). All studies combined in a random-effects meta-analysis showed that fewer patients taking ketoconazole had failed clearance of symptoms compared with those given placebo (RR 0.69, 95% CI 0.59 to 0.81 (Analysis 1.1); NNTB 5, 95% CI 4 to 8). However, heterogeneity was considerable (I² = 75%). We could not explain the heterogeneity with the total dose applied; Go 1992 had the lowest dose (eight percentage points) and Skinner 1985 had the highest dose (112 percentage points).
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
Two studies (Satriano 1987; Shuttleworth 1998) compared ketoconazole versus placebo and used a continuous outcome measure. Results showed high statistical heterogeneity (I² = 93%), which we could not explain by any study characteristic, so we did not combine the studies in a meta-analysis. These studies (Satriano 1987; Shuttleworth 1998) showed that ketoconazole was statistically significantly more effective in reducing erythema when compared with placebo in the short term (up to four weeks) (Analysis 1.2). Two additional studies reported effects on erythema score, but because of missing SDs, their results could not be used in the meta-analysis. Elewski 2006 reported a mean erythema score for people taking ketoconazole of -1.23, and the mean score for people receiving placebo was -1.13. Pierard 1991 reported a decrease in mean erythema score of -1.39 for the ketoconazole group and -0.43 for the placebo group.
One study (Shuttleworth 1998) assessed the erythema score in the long term (more than four weeks); ketoconazole yielded a statistically significantly higher score reduction than was seen with placebo (SMD -0.69, 95% CI -1.20 to -0.18) (Analysis 1.3).
Two trials (Peter 1991; Ratnavel 2007) reported erythema reduction as a discrete variable. These data could not be pooled because of high heterogeneity (I² = 80%). Peter 1991 found a lower (erythema) failed clearance rate in the ketoconazole group (5/30; 17%) than in the placebo group (15/29; 52%), and the difference was statistically significant (RR 0.32, 95% CI 0.13 to 0.77; NNTB 3, 95% CI 1 to 8) (Analysis 1.4). Ratnavel also reported a lower failed clearance rate with ketoconazole (RR 0.78, 95% CI 0.66 to 0.92) (Analysis 1.4).
Pruritus score
On short-term (up to four weeks) assessment, three studies reported treatment effects on pruritus as absolute scores. Satriano 1987 reported mean endpoint pruritus scores, whereas Elewski 2006 and Ratnavel 2007 reported changes in mean pruritus score. Only Satriano 1987 found a statistically significant effect for ketoconazole (SMD -2.06, 95% CI -2.84 to -1.28) (Analysis 1.5). Elewski 2006 did not provide SDs but reported a mean pruritus score of -1.9 for 229 participants using ketoconazole, and a mean pruritus score of -1.04 for 230 participants given placebo. Ratnavel 2007 obtained comparable results for both treatments (MD -0.30, 95% CI -0.62 to 0.02) (Analysis 1.5). Pierard 1991 reported a decrease in mean pruritus score of -1.25 for 23 persons in the ketoconazole group and of -0.57 for 16 persons in the placebo group, but no SDs.
One trial (Ratnavel 2007) compared long-term (more than four weeks) effects of ketoconazole and placebo on pruritus score, and reported values on a continuous scale. Ketoconazole induced a greater reduction in symptom score, but the difference was not statistically significant (MD -6.40, 95% CI -21.23 to 8.43) (Analysis 1.6).
Two studies analysed pruritus score as a discrete outcome (Green 1987; Peter 1991). A meta-analysis showed that fewer participants taking ketoconazole had failed resolution of itch compared with participants in the placebo group, and the difference was statistically significant (RR 0.38, 95% CI 0.21 to 0.69; NNTB 2, 95% CI 2 to 5; I² = 0) (Analysis 1.7).
Scaling score
Six trials (Danby 1993; Elewski 2006; Pierard 1991; Ratnavel 2007; Satriano 1987; Shuttleworth 1998) assessed short-term (up to four weeks) effects of scalp treatment with ketoconazole on a mean scaling score. Results could not be combined in a meta-analysis because of insufficient reporting, differences in reporting and high heterogeneity.
Elewski and Ratnavel reported mean changes in scaling score, and the other studies reported endpoint mean scaling scores. Elewski 2006, Danby 1993 and Pierard 1991 reported only mean scores without SDs. Danby reported a mean ketoconazole score of 6.57 for a total of 97 trial participants and a mean placebo score of 14.78 for a total of 49 participants. Elewski 2006 reported a mean decrease of -1.55 for 229 participants taking ketoconazole, and a mean decrease of -1.31 for 230 participants given placebo. Pierard 1991 reported a decrease in mean scaling score of -1.68 for 23 persons in the ketoconazole group and of -0.98 for 16 persons in the placebo group.
Ratnavel 2007 could not be pooled with other studies because the outcome was very different from those of Satriano 1987 and Shuttleworth 1998, with an MD of -17.90 (95% CI -33.82 to -1.98). Satriano 1987 reported that ketoconazole reduced scaling better than placebo, with a difference that was statistically significant (MD -1.25, 95% CI -1.61 to -0.89) (Analysis 1.8). Shuttleworth 1998 had similar findings (MD -0.75, 95% CI -1.29 to -0.21) (Analysis 1.8). These two studies showed high statistical heterogeneity (I² = 89%) and so were not combined.
Two trials (Ratnavel 2007; Shuttleworth 1998) compared long-term (more than four weeks) effects of ketoconazole on scaling score versus those of placebo. These data could not be combined because Ratnavel 2007 measured the decrease in mean differences of scaling scores, and Shuttleworth 1998 recorded absolute scores before and after treatment with widely varying results. Ketoconazole was better than placebo in both trials, showing statistically significant differences (Ratnavel 2007: MD -18.90, 95% CI -35.05, to -2.75; Shuttleworth 1998: MD -0.98, 95% CI -1.48 to -0.48) (Analysis 1.9).
Three studies (Green 1987; Peter 1991; Ratnavel 2007) presented dichotomous outcome measures as complete clearance of scaling. Peter 1991 data could not be pooled with those of the other studies because of high heterogeneity (I² = 83%); data showed better clearance of scaling with ketoconazole, and the difference was statistically significant (RR 0.22, 95% CI 0.09 to 0.52; NNTB 2, 95% CI 2 to 4). Pooling of Ratnavel 2007 and Green 1987 data (I² = 0) revealed better remission with ketoconazole (RR 0.77, 95% CI 0.67 to 0.87; NNTB 6, 95% CI 4 to 11) (Analysis 1.10).
Side effects/intolerance to treatment
Side effects
Six studies (Elewski 2006; Go 1992; Peter 1991; Ratnavel 2007; Schofer 1988; Shuttleworth 1998) documented side effects of treatment with ketoconazole versus placebo: Side effects were comparable in both treatment groups (RR 0.97, 95% CI 0.58 to 1.64; I² = 45%) (Analysis 1.11).
Ketoconazole versus steroids
Primary outcomes
Participants without complete resolution
Six trials (Hersle 1996; Katsambas 1989; Kousidou 1992; Pari 1998; Stratigos 1988; Van't Veen 1998) compared short-term (up to four weeks) assessment of the effect of ketoconazole versus a steroid on resolution of seborrhoeic dermatitis rashes. A meta-analysis of these studies showed that rashes resolved better with steroids, but the difference was not statistically significant (RR 1.17, 95% CI 0.95 to 1.44; I² = 11%) (Analysis 2.1).
Hersle 1996 and Pari 1998 compared long-term (more than four weeks) effects of ketoconazole versus those of a steroid. Data from these two studies could not be pooled because of high heterogeneity (I² = 86%). Hersle 1996 found an RR of 3.44 in favour of steroids (95% CI 1.47 to 8.06; NNTB 3, 95% CI 2 to 5) (Analysis 2.2). By contrast, Pari 1998 found ketoconazole to be more effective than steroid, but the difference was not statistically significant (RR 0.67, 95% CI 0.28 to 1.59) (Analysis 2.2).
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
Three trials (Hersle 1996; Kousidou 1992; Piepponen 1992) compared short-term (up to four weeks) effects on erythema of a ketoconazole-based preparation versus a steroid preparation. Hersle 1996 and Kousidou 1992 recorded actual mean scores after treatment. These data were pooled together, and results showed that the two drugs had comparable efficacy (SMD 0.12, 95% CI -0.30 to 0.53; I² = 0) (Analysis 2.3). Piepponen 1992 presented his results as a change in mean score following treatment; this also showed comparability of effect on erythema of the scalp between both types of treatment (SMD -0.12, 95% CI -0.51 to 0.27) (Analysis 2.3).
Hersle 1996 assessed the long-term (more than four weeks) effects of ketoconazole on erythema in comparison with a steroid and found a non-statistically significant difference between treatments (MD 0.20, 95% CI -0.43 to 0.83) (Analysis 2.4).
Two studies (Ortonne 1992; Ortonne 2011) reporting erythema as a discrete outcome for ketoconazole versus steroid were combined in a random-effects meta-analysis, which revealed a non-significant difference (RR 0.51, 95% CI 0.19 to 1.38; I² = 50) (Analysis 2.5).
Pruritus score
Four trials (Hersle 1996; Kousidou 1992; Piepponen 1992; Van't Veen 1998) compared the effect of ketoconazole versus a steroid on reduction of pruritus. We pooled results from these studies excluding Piepponen 1992 (who reported a decrease in mean pruritus score) and found weak evidence that steroid-based treatment reduced pruritus better than ketoconazole (SMD 0.23, 95% CI -0.08 to 0.54; I² = 0) (Analysis 2.6). Piepponen 1992 found the two treatments to be of comparable efficacy (SMD 0.03, 95% CI -0.36 to 0.42) (Analysis 2.6).
Hersle 1996 compared the effects of ketoconazole versus steroid treatments on long-term (more than four weeks) application. Results showed statistically significantly lower pruritus scores for participants in the steroid group (MD 0.30, 95% CI 0.20 to 0.40) (Analysis 2.7).
Ortonne 1992 and Ortonne 2011 reported the effects of ketoconazole versus a steroid on itch as a discrete outcome. Failure of resolution of itch was less in the ketoconazole group (RR 0.53, 95% CI 0.34 to 0.84; I² = 0; NNTB 3, 95% CI 2 to 9) (Analysis 2.8).
Scaling score
We pooled results data from four trials (Hersle 1996; Kousidou 1992; Stratigos 1988; Van't Veen 1998) in a random-effects meta-analysis. We found that ketoconazole was similar to steroid-based treatment in reducing scaling (SMD 0.27, 95% CI -0.11 to 0.65; I² = 50) (Analysis 2.9). Piepponen 1992 was not combined with the rest because it compared mean reduction in scaling scores between ketoconazole and steroids, rather than absolute scores. Piepponen found the two treatments to be of comparable efficacy (SMD -0.06, 95% CI -0.45 to 0.33) (Analysis 2.9).
Hersle 1996 and Stratigos 1988 compared long-term effects (more than four weeks) of ketoconazole versus steroids on scaling. The two trials could not be combined because effects varied widely between them (I² = 95%). Hersle 1996 found a lower scaling mean score with steroid application, which was statistically significant (SMD 1.96, 95% CI 1.27 to 2.65) (Analysis 2.10). Stratigos 1988 found comparable effects with the two treatments (SMD 0.08, 95% CI -0.42 to 0.57) (Analysis 2.10).
Two studies (Ortonne 1992; Ortonne 2011) reported scaling as a discrete outcome: The ketoconazole group had less scaling, but the difference was not statistically significant (RR 0.78, 95% CI 0.54 to 1.12; I² = 0) (Analysis 2.11).
Side effects/intolerance to treatment
Side effects
Pooled data from eight studies (Hersle 1996; Katsambas 1989; Kousidou 1992; Ortonne 1992; Ortonne 2011; Piepponen 1992; Stratigos 1988; Van't Veen 1998) showed greater frequency of side effects for participants receiving steroids (29/304; 10%) compared with ketoconazole (15/292; 5%). The difference was statistically significant (RR 0.56, 95% CI 0.32 to 0.96; I² = 0; NNTB 3, 95% CI 2 to 36) (Analysis 2.12).
Ketoconazole versus zinc pyrithione
Primary outcomes
Participants without complete resolution
Three studies (Draelos 2005; Grossman 1997; Piérard-Franchimont 2002) in all made this comparison, but Grossman 1997 reported insufficient data to be included in the meta-analysis.
In one study (Piérard-Franchimont 2002), ketoconazole showed a lower remission failure rate compared with zinc pyrithione, with a statistically significant difference (RR 0.85, 95% CI 0.72 to 0.99 (Analysis 3.1); NNTB 10, 95% CI 5 to 139). With long-term (more than four weeks) use of both treatments, ketoconazole still showed a lower remission failure rate with a statistically significant difference (RR 0.87, 95% CI 0.78 to 0.97 (Analysis 3.2); NNTB 10, 95% CI 7 to 46).
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
One trial (Draelos 2005) compared ketoconazole shampoo versus zinc pyrithione shampoo on short-term (up to four weeks) application of these treatments. On assessment, a mean erythema score of 0.111 and a standard deviation of 0.333 were recorded for 20 participants in the ketoconazole group, and in the zinc pyrithione group, both mean erythema score and standard deviation were zero for the 20 participants. Because of SDs of 0, the results could not be used in a meta-analysis.
Pruritus score
None of the studies measured a pruritus score.
Scaling score
Two trials (Draelos 2005; Piérard-Franchimont 2002) compared the effects of ketoconazole and zinc pyrithione on scaling. These trials could not be pooled because although Draelos reported the mean score following treatment, which showed comparability of treatment effects (MD 0.08, 95% CI -0.09 to 0.24 (Analysis 3.3)), the bigger study (Piérard-Franchimont 2002), which reported the mean change in scaling score, showed a lower score with ketoconazole with a statistically significant difference (MD -2.74, 95% CI -4.51 to -0.97) (Analysis 3.3).
One study (Piérard-Franchimont 2002) also assessed scaling over the long term (more than four weeks); ketoconazole still performed better than zinc pyrithione (MD -2.55, 95% CI -4.66 to -0.44) (Analysis 3.4), and this result was statistically significant.
Side effects/intolerance to treatment
Side effects
No significant difference in side effects was reported in the study by Piérard-Franchimont 2002 when ketoconazole was compared with zinc pyrithione (RR 1.43, 95% CI 0.24 to 8.66) (Analysis 3.5).
Ketoconazole versus ciclopirox
Primary outcomes
Participants without complete resolution
Three studies (Chosidow 2003; Diehl 2013; Unholzer 2002(I)) compared effectiveness of ketoconazole versus that of ciclopirox. Among participants taking ciclopirox, 58% (133/228) did not have resolution of their seborrhoeic dermatitis compared with 63% (139/219) taking ketoconazole, but the difference was not statistically significant (RR 1.09, 95% CI 0.95 to 1.26; I² = 32%) (Analysis 4.1).
Chosidow 2003 and Diehl 2013 assessed comparative effectiveness of these treatments on long-term (more than four weeks) application and found that ciclopirox was better, with fewer participants exhibiting persistence of their seborrhoeic dermatitis again compared with ketoconazole, but the difference was not statistically significant (RR 1.10, 95% CI 0.88 to 1.36; I² = 51%) (Analysis 4.2).
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
One study (Shuttleworth 1998) comparing ketoconazole and ciclopirox showed a decrease in erythema score on ciclopirox, but the effect was not statistically significant (MD -0.21, 95% CI -1.09 to 0.67) (Analysis 4.3).
Shuttleworth 1998 also assessed long-term (more than four weeks) effectiveness of these treatments on erythema; results showed less erythema in the ketoconazole group, but the difference was not statistically significant (MD -0.28, 95% CI -1.16 to 0.60) (Analysis 4.4).
One trial (Ratnavel 2007) reported treatment effect on SD erythema as a discrete outcome. Treatment effects were comparable between the ketoconazole group (98/150; 65%) and the ciclopirox group (105/150; 70%), and the difference was not statistically significant (RR 0.93, 95% CI 0.08 to 1.09) (Analysis 4.5).
Pruritus score
Two studies (Lee 2003; Ratnavel 2007) compared ketoconazole and ciclopirox. Lee reported pruritus scores as endpoint absolute values, and Ratnavel reported them as change in mean value. Ratnavel found weak evidence for reduced pruritus with ciclopirox use (MD 5.00, 95% CI -6.03 to 16.03) (Analysis 4.6). Lee 2003 data were omitted from the data table because no SDs were available for mean scores. Pruritus scores were 2.2 (group total = 30) for the ketoconazole group and 1.6 (group total = 17) for the ciclopirox group (Analysis 4.6).
Long-term (more than four weeks) assessment from two trials (Ratnavel 2007; Shuttleworth 1998) showed less pruritus in the ketoconazole group (Ratnavel 2007: MD -8.00, 95% CI -19.24 to 3.24; Shuttleworth 1998: MD -0.14, 95% CI -0.53 to 0.25) (Analysis 4.7); the difference was not statistically significant. Effects in these studies were assessed differently and could not be combined. Lee 2003 data were omitted from the data tables because of absence of standard deviation, but mean pruritus scores of 2 for 30 participants taking ketoconazole and 2.7 for 27 participants taking ciclopirox were reported.
Scaling score
Two studies (Ratnavel 2007; Shuttleworth 1998) compared the effects of ketoconazole versus ciclopirox on scaling score. Ratnavel reported mean reduction in scaling score (MD 4.30, 95% CI -6.08 to 14.68) (Analysis 4.8), and Shuttleworth reported the endpoint mean score following treatment (MD -0.14, 95% CI -0.53 to 0.25) (Analysis 4.8). Neither of these studies found a statistically significant difference between the effects of the two drugs. On long-term (more than four weeks) assessments in both studies, ketoconazole reducing scaling similarly to ciclopirox (Ratnavel 2007: MD -4.90, 95% CI -16.18 to 6.38; Shuttleworth 1998: MD -0.14, 95% -0.53 to 0.25) (Analysis 4.9).
Ratnavel 2007 reported treatment effect on scaling as a discrete outcome. The failure rate of scaling resolution was comparable in the ketoconazole and ciclopirox treatment groups (RR 0.93, 95% CI 0.81 to 1.07) (Analysis 4.10).
Side effects/intolerance to treatment
Side effects
A meta-analysis of two studies (Chosidow 2003; Ratnavel 2007) comparing side effects of ciclopirox when applied to the scalp versus ketoconazole showed no statistically significant differences between the two treatments (RR 1.35, 95% CI 0.54 to 3.38; I² = 62%) (Analysis 4.11).
Ketoconazole versus metronidazole
Primary outcomes
Participants without complete resolution
Seckin 2007 compared effects of ketoconazole on rash clearance versus metronidazole, but no statistically significant difference was observed between treatments (RR 0.84, 95% CI 0.41 to 1.72) (Analysis 5.1).
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
None of the included studies reported an erythema score.
Pruritus score
One trial (Seckin 2007) showed no statistically significant differences between ketoconazole and metronidazole in ameliorating pruritus (MD -0.10, 95% CI -1.10 to 0.90) (Analysis 5.2).
Scaling score
None of the included studies reported a scaling score.
Side effects/intolerance to treatment
Side effects
Seckin 2007 compared the side effects of treatment with ketoconazole versus metronidazole and found comparable rates (RR 1.82, 95% CI 0.60 to 5.48) (Analysis 5.3).
Ketoconazole versus climbazole
Primary outcomes
Participants without complete resolution
Lopez-Padilla 1996 compared the effects of ketoconazole and climbazole over the long term (more than four weeks). Only 20% (6/30) of participants taking ketoconazole only failed to achieve complete resolution of rashes compared with 86% (26/30) of those taking climbazole, which reflected a statistically significant difference (RR 0.23, 95% CI 0.11 to 0.48 (Analysis 6.1); NNTB 2, 95% CI 2 to 3).
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
On short-term (up to four weeks) application, one trial (Lopez-Padilla 1996) found lower failed erythema remission rates with ketoconazole compared with climbazole (RR 0.47, 95% CI 0.24 to 0.92) (Analysis 6.2). Rates were comparable on long-term (more than four weeks) application (RR 0.25, 95% CI 0.06 to 1.08) (Analysis 6.3).
Scaling score
Lopez-Padilla 1996 compared the effects of ketoconazole and climbazole on scaling. On short-term (up to four weeks) use, the failed scaling remission rate was lower with ketoconazole than with climbazole, with a statistically significant difference (RR 0.52, 95% CI 0.32 to 0.84) (Analysis 6.4). The difference remained on long-term assessment (RR 0.26, 95% CI 0.12 to 0.55) (Analysis 6.5).
Lopez-Padilla 1996 did not report on the secondary outcomes of pruritus and side effects.
Side effects/intolerance to treatment
Side effects
No side effects were reported for this comparison.
Ketoconazole versus Solanum chrysotricum
Primary outcomes
Participants without complete resolution
One trial (Herrera-Arellano 2004) compared ketoconazole shampoo versus Solanum chrysotricum shampoo. Although 8% (4/51) of those taking ketoconazole failed to achieve complete resolution compared with 13% (7/52) taking Solanum chrysotricum, the difference was not statistically significant (RR 0.58, 95% CI 0.18 to 1.87) (Analysis 7.1).
Herrera-Arellano 2004 did not report any of our secondary outcomes.
Ketoconazole versus pimecrolimus
Koc 2009 compared ketoconazole versus pimecrolimus but did not report either of our primary outcomes.
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
One trial (Koc 2009) assessed the long-term (more than four weeks) effect of ketoconazole application, in comparison with pimecrolimus, on erythema score. Ketoconazole led to a greater decrease in erythema, which was statistically significant (MD -0.30, 95% CI -0.58 to -0.02) (Analysis 8.1).
None of the included studies reported a pruritus score.
Scaling score
Koc 2009 compared the ability of ketoconazole to reduce scaling with long-term (more than four weeks) use versus that of pimecrolimus; no significant difference was observed between the two groups (MD -0.04, 95% CI -0.27 to 0.19) (Analysis 8.2).
Side effects/intolerance to treatment
Side effects
Koc 2009 found ketoconazole to be more tolerable than pimecrolimus; statistically significantly fewer side effects were observed in the ketoconazole group (RR 0.31, 95% CI 0.12 to 0.82; NNTB 3, 95% CI 2 to 9) (Analysis 8.3).
Ketoconazole versus lithium
Primary outcome
Participants without complete resolution
Dreno 2003 compared effects of ketoconazole and lithium gluconate on facial seborrhoeic dermatitis. Of participants taking lithium, 73% did not achieve complete resolution compared with 85% of those taking ketoconazole who did not achieve complete resolution (RR 1.16, 95% CI 1.03 to 1.30; NNTB 9, 95% CI 42 to 5) (Analysis 9.1). Long-term (more than four weeks) outcome was also better with lithium gluconate (RR 1.47, 95% CI 1.21 to 1.78) (Analysis 9.2).
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
Dreno 2003 observed no statistically significant differences in erythema clearance from the face when ketoconazole was compared with lithium gluconate in the short term (RR 1.13, 95% CI 0.96 to 1.33)(Analysis 9.3) but in the long term (more than four weeks), erythema was less persistent with lithium gluconate (RR 1.50, 95% CI 1.14 to 1.98; NNTB 6, 95% CI 17 to 4) (Analysis 9.4).
Pruritus score
Dreno 2003 found no differences between treatment groups in remission of itch in the short term (up to four weeks) (RR 1.43, 95% CI 0.81 to 2.53) (Analysis 9.5) or over the long term (more than four weeks) (RR 1.20, 95% CI 0.59 to 2.47) (Analysis 9.6).
Scaling score
Less scaling (Dreno 2003) was reported in the ketoconazole group (RR 0.40, 95% CI 0.32 to 0.50; NNTB 2, 95% CI 2 to 3) (Analysis 9.7), and this statistically significant effect was maintained over the long term (RR 0.46, 95% CI 0.36 to 0.58; NNTB 2, 95% CI 2 to 3) (Analysis 9.8).
Side effects/intolerance to treatment
Side effects
The difference between trial participants experiencing side effects while taking ketoconazole (34/136; 25%) compared with lithium gluconate (40/152; 26%) was not statistically significant (RR 0.95, 95% CI 0.64 to 1.41) (Analysis 9.9).
Ketoconazole versus selenium sulphide
Secondary outcomes
Scaling score
One study (Danby 1993) compared effects of ketoconazole and selenium sulphide on scalp scaling. Endpoint scaling scores were 6.57 for a total of 97 persons in the ketoconazole group and 7.91 for 100 persons in the selenium sulphide group. No standard deviations were given for these scores (Analysis 10.1).
Ketoconazole versus Quassia amara
Primary outcomes
Participants without complete resolution
Diehl 2013 compared seborrhoeic dermatitis rash clearance effects of ketoconazole versus Quassia amara. Weak evidence showed better action with Quassia amara, but this finding was not statistically significant (RR 1.30, 95% CI 0.96 to 1.78) (Analysis 11.1). Long-term (more than four weeks) use showed a better effect of Quassia amara (RR 2.27, 95% 1.24 to 4.15) (Analysis 11.2).
No secondary outcomes were recorded for this comparison.
Ketoconazole foam versus ketoconazole cream
Elewski 2007 compared two modes (foam and cream) of delivery of ketoconazole.
Primary outcomes
Participants without complete resolution
These modes of delivery had comparable efficacy for complete resolution of SD rashes of the face and scalp (RR 1.00, 95% CI 0.83 to 1.21) (Analysis 12.1).
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
Elewski 2007 found that erythema remission was similar with the two different preparations (RR 0.97, 95% 0.79 to 1.20) (Analysis 12.2).
Pruritus score
Elewski 2007 reported comparable efficacy of ketoconazole cream and foam for pruritus resolution (RR 1.02, 95% CI 0.85 to 1.22) (Analysis 12.3).
Scaling score
No statistically significant differences between two ketoconazole preparations in resolving scaling were observed (RR 0.97, 95% CI 0.79 to 1.20) (Analysis 12.4).
No side effects were recorded for this comparison.
Ketoconazole (2%) versus ketoconazole (1%)
Primary outcomes
Participants without complete resolution
Pierard-Franchimont 2001 found that with ketoconazole (2%) 48% (16/33) of participants failed to achieve complete resolution of seborrhoeic dermatitis compared with 87% (29/33) taking ketoconazole (1%) - a difference that was statistically significant (RR 0.55, 95% CI 0.38 to 0.80; NNTB 3, 95% CI 2 to 5) (Analysis 13.1). This study also showed that the higher dose of ketoconazole was statistically significantly better in clearing SD rashes on long-term (more than four weeks) application (RR 0.61, 95% CI 0.45 to 0.83; NNTB 3, 95% CI 2 to 6) (Analysis 13.2).
No secondary outcomes were reported for this comparison.
Bifonazole versus placebo
Primary outcomes
Participants without complete resolution
One trial (Zienicke 1993) compared the short-term (up to four weeks) effects of bifonazole and placebo. Among participants taking bifonazole, 64% (29/45) failed to achieve complete resolution of rashes versus 79% (37/47) of those given placebo; the difference was not statistically significant (RR 0.82, 95% CI 0.63 to 1.06) (Analysis 14.1). Segal 1992 made a similar comparison in which he assessed effects on long-term (more than four weeks) application; in this study, bifonazole was more effective than placebo, and the difference was statistically significant (RR 0.40, 95% CI 0.19 to 0.84; NNTB 2, 95% CI 2 to 8) (Analysis 14.2).
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
One study (Zienicke 1993) assessed decreases in erythema in bifonazole and placebo groups on short-term (up to four weeks) application. No statistically significant difference was observed between groups (MD -0.13, 95% CI -0.42 to 0.16) (Analysis 14.3). Segal 1992 compared groups on long-term (more than four weeks) treatment and found no statistically significant difference in erythema score (MD -0.50, 95% CI -1.04 to 0.04) (Analysis 14.4).
Pruritus score
Zienicke 1993 found that participants who received bifonazole experienced less itch than those receiving placebo, but the difference was not statistically significant (MD -0.21, 95% CI -0.51 to 0.09) (Analysis 14.5). Segal 1992 found less itch with long-term (more than four weeks) use of bifonazole compared with placebo, and this finding was statistically significant (MD -0.85, 95% CI -1.39 to -0.31) (Analysis 14.6).
Scaling score
Participants who received bifonazole treatment for a short term (up to four weeks) (Zienicke 1993) experienced less scaling than those given placebo (MD -0.32, 95% CI -0.59 to -0.05) (Analysis 14.7). A similar finding was reported in Segal 1992, where, on long-term assessment, less scaling was seen in the bifonazole group as compared with the placebo group (MD -0.92, 95% CI -1.46 to -0.38) (Analysis 14.8). Differences between treatments in these studies were statistically significant.
Side effects/intolerance to treatment
Side effects
Two studies (Segal 1992; Zienicke 1993) recorded more side effects with bifonazole than with placebo (RR 2.19, 95% CI 0.75 to 6.37) (Analysis 14.9), but this finding was not statistically significant.
Clotrimazole versus steroid
No primary outcomes were assessed in this comparison.
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
One trial (Attarzadeh 2013) compared short-term (up to four weeks) and long-term treatments with clotrimazole versus steroid treatments. The treatments were comparable in efficacy (MD 0.04, 95% -0.16 to 0.24) (Analysis 15.1).
Pruritus score
Steroid treatment yielded a lower pruritus mean score than was attained with clotrimazole (MD 1.09, 95% 0.71 to 1.47) (Analysis 15.2) (Attarzadeh 2013).
Scaling score
Attarzadeh found no evidence for better remission of scaling with topical clotrimazole use (MD -0.11, 95% CI -0.29 to 0.07) (Analysis 15.3).
Clotrimazole versus Emu oil
No primary outcomes were reported for this comparison.
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
One trial (Attarzadeh 2013) provided weak evidence of better reduction in erythema score with Emu oil when applied for a short term (up to four weeks) in comparison with clotrimazole (MD 0.17, 95% CI -0.00 to 0.34) (Analysis 16.1).
Pruritus score
Topical Emu oil achieved greater reduction in pruritus score than clotrimazole, but the difference was not statistically significant (MD 0.17, 95% -0.24 to 0.58) (Analysis 16.2).
Scaling score
Clotrimazole yielded better reduction in pruritus score than Emu oil with statistically significant differences (MD -0.35, 95% CI -0.54 to -0.16) (Analysis 16.3).
No side effects were reported for this comparison.
Miconazole versus steroids
Primary outcomes
Participants without complete resolution
One trial (Faergermann 1986) compared 2% miconazole solution versus 1% hydrocortisone solution and reported similar outcomes for both drugs (RR 1.09, 95% CI 0.46 to 2.61) (Analysis 17.1).
On long-term follow-up, miconazole induced complete resolution better than the steroid did (RR 0.68, 95% CI 0.46 to 0.99 (Analysis 17.2); NNTB 4, 95% CI 2 to 15).
No secondary outcomes were reported for this comparison.
Miconazole shampoo plus rinse versus shampoo alone
No primary outcomes were reported for this comparison.
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Pruritus score
Sei 2011 found that miconazole and placebo had similar efficacy in clearance of itch (RR 0.72, 95% CI 0.30 to 1.71) (Analysis 18.1).
Scaling score
Sei 2011 also reported similar efficacy for clearance of scaling with miconazole and placebo (RR 0.84, 95% CI 0.34 to 2.10) (Analysis 18.2).
Ciclopirox versus placebo
Primary outcomes
Participants without complete resolution
Eight studies (Abeck 2004; Altmeyer 2004; Aly 2003; Dupuy 2001; Shuster 2005; Unholzer 2002(I); Unholzer 2002(II); Vardy 2000) compared the effects of ciclopirox versus placebo with regard to resolution of seborrhoeic dermatitis rash. Abeck 2004, contributed three study arms to this comparison because different intensities of application of ciclopirox were compared with placebo. In a random-effects meta-analysis, ciclopirox produced failure of clearance of 21% (RR 0.79, 95% CI 0.67 to 0.94; I² = 81%) (Analysis 19.1). However, Altmeyer 2004, which was a clear outlier, reported 62% lower risk of failure to clear rashes than was seen with placebo (RR 0.38, 95% CI 0.25 to 0.57) (Analysis 19.1). Thus Altmeyer 2004 was omitted from the meta-analysis, which still showed that fewer participants on ciclopirox failed to achieve complete resolution compared with those given placebo - a difference that was still statistically significant (RR 0.84, 95% CI 0.72 to 0.98 (Analysis 19.1); I² = 74%; NNTB 9, 95% CI 5 to 73).
Vardy 2000 found no statistically significant differences between the two treatments on long-term follow-up (RR 0.89, 95% CI 0.78 to 1.01) (Analysis 19.2).
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
Shuttleworth 1998 and Vardy 2000 compared the effects of ciclopirox and placebo on mean erythema score. These studies were combined in a fixed-effect meta-analysis and showed that ciclopirox achieved better reduction in erythema score and the difference was statistically significant (SMD -0.68, 95% CI -1.00 to -0.37; I² = 0) (Analysis 19.3). On long-term follow-up, pooled data from both studies showed that ciclopirox reduced erythema better than placebo did - a result that was statistically significant (SMD -0.44, 95% CI -0.75 to -0.13) (Analysis 19.4).
Lebwohl 2004 and Ratnavel 2007 performed a similar comparison, reporting erythema as a dichotomous outcome. These studies showed high heterogeneity (I² = 98%) and so could not be combined. Ratnavel 2007 reported better lower failure of erythema clearance with placebo, which was statistically significant (RR 6.88, 95% CI 3.39 to 13.93; NNTB 6, 95% CI 4 to 10) (Analysis 19.5). Lebwohl 2004 obtained contradictory results: This study found that participants taking ciclopirox had lower failed clearance rates than those given placebo (RR 0.77, 95% CI 0.68 to 0.87; NNTB 6, 95% CI 4 to 10) (Analysis 19.5).
Pruritus score
One trial (Vardy 2000), which compared the effects of ciclopirox and placebo on pruritus, found that ciclopirox improved itching symptoms better than placebo did (MD -0.34, 95% CI -0.66 to -0.02) (Analysis 19.6). On long-term follow-up (more than 4-weeks), the difference between the two treatment groups was comparable (MD -0.12, 95% CI -27.56 to 27.32) (Analysis 19.7).
Lebwohl 2004 made a similar comparison, reporting pruritus as a dichotomous outcome. Less itching was reported in the ciclopirox than in the placebo group - a result that was statistically significant (RR 0.74, 95% CI 0.64 to 0.86 (Analysis 19.8); NNTB 4, 95% CI 5 to 3).
Scaling score
Three trials (Ratnavel 2007; Shuttleworth 1998; Vardy 2000) explored the differences in reduction of scaling with ciclopirox and placebo use. Data from Ratnavel 2007 could not be pooled with data from the other studies because Ratnavel reported mean change in scaling score, and the other studies reported the endpoint scaling score. Ratnavel found greater scaling reduction in the placebo group, but this finding was not statistically significant (SMD 0.09, 95% CI -0.13 to 0.32) (Analysis 19.9). Pooling of the other studies in a fixed-effect model showed that ciclopirox produced lower pruritus scores than were seen with placebo with statistically significant differences (SMD -0.84, 95% CI-1.16 to -0.52; I² = 0) (Analysis 19.9).
At more than four weeks follow-up, Unholzer 2002(I) and Vardy 2000 found that ciclopirox had better reduction of scaling than placebo with statistically significant differences (SMD -0.67, 95% CI -0.98 to -0.35) (Analysis 19.10).
Lebwohl 2004 and Ratnavel 2007 reported the effects of ciclopirox on scaling remission. These were combined in a fixed-effect meta-analysis, which showed less scaling in the ciclopirox group than in the placebo group - a result that was statistically significant (RR 0.86, 95% CI 0.79 to 0.94 (Analysis 19.11); NNTB 10, 95% CI 7 to 18).
Side effects/intolerance to treatment
Side effects
A fixed-effect meta-analysis of four studies (Aly 2003; Dupuy 2001; Lebwohl 2004; Vardy 2000) found more side effects with ciclopirox use, but this finding was not statistically significant (RR 0.90, 95% CI 0.72 to 1.11) (Analysis 19.12).
Ciclopirox versus Quassia amara
Primary outcomes
Participants without complete resolution
One trial (Diehl 2013) compared the effects of ciclopirox versus Quassia amara in inducing complete clearance of seborrhoeic dermatitis rash. Short-term (up to four weeks) assessment showed that although failure to achieve complete resolution was less for Quassia amara, the difference was not statistically significant (RR 1.31, 95% CI 0.97 to 1.78) (Analysis 21.1). Long-term (more than four weeks) assessment yielded less failed clearance of rashes in participants placed on Quassia amara, and the difference was statistically significant (RR 2.30, 95% CI 1.26 to 4.19) (Analysis 21.2).
Ciclopirox (higher dose) versus ciclopirox (lower dose)
Primary outcomes
Participants without complete resolution
Two studies (Altmeyer 2004; Shuster 2005) compared the effects of higher doses of treatment using ciclopirox versus lower doses of the same drug. The two studies were analysed separately because they determined the compared dosages using different methods. Altmeyer 2004 compared ciclopirox 1% against ciclopirox 0.3% and ciclopirox 0.1%. We found that the larger dose resulted in better treatment effect more often than the lower doses, but the difference was not statistically significant (RR 0.49, 95% CI 0.32 to 0.76) (Analysis 20.1).
One study (Shuster 2005) compared a twice-weekly application regimen of 1% ciclopirox versus a once-weekly application regimen. No statistically significant difference in induction of complete resolution was noted between the two regimens (RR 0.93, 95% CI 0.86 to 1.0) (Analysis 20.1).
However, combining these studies (Altmeyer 2004; Shuster 2005) in a random-effects meta-analysis did not yield statistically significant differences in effects between high and low doses (RR 0.65, 95% CI 0.37 to 1.13; I² = 79%) (Analysis 20.1).
No secondary outcomes were reported for this comparison.
Lithium salts versus placebo
Primary outcomes
Participants without complete resolution
Dreno 2002 compared lithium gluconate versus placebo. At short-term (up to four weeks) follow-up, lithium resulted in a higher remission rate (i.e. fewer participants taking lithium failed to achieve complete resolution compared with participants given placebo), but the difference was not statistically significant (RR 0.94, 95% CI 0.85 to 1.04) (Analysis 22.1). However, at long-term (more than four weeks) follow-up, lithium was found to be statistically significantly better than placebo (RR 0.74, 95% CI 0.63 to 0.86) (Analysis 22.2).
Secondary outcomes
Symptom severity scores for erythema, pruritus, scaling measured with any type of systematic symptom severity assessment
Erythema score
One study (Langtry 1997) compared the effects on erythema of lithium preparations versus placebo. Results provided weak evidence of better erythema reduction with lithium (MD -3.90, 95% CI -16.91 to 9.11) (Analysis 22.3). A similar effect was observed at long-term follow-up (MD -6.20, 95% CI -20.49 to 8.09) (Analysis 22.4).
Dreno 2002 analysed erythema as a discrete outcome and found that lithium gluconate produced better clearance of erythema at eight weeks of follow-up when compared with placebo (RR 0.69, 95% CI 0.57 to 0.84) (Analysis 22.5).
Scaling score
Results from Langtry 1997 show that trial participants taking lithium had lower scaling scores than those given placebo, but the difference was not statistically significant (MD -5.00, 95% CI -18.78 to 8.78) (Analysis 22.6). Langtry 1997 conducted a long-term (more than four weeks) assessment that yielded a similar result (MD -10.60, 95% CI -27.84 to 6.64) (Analysis 22.7).
Dreno 2002 reported scaling as a discrete variable and found that participants taking lithium had less scaling than those given placebo with statistically significant differences (RR 0.58, 95% CI 0.41 to 0.81) (Analysis 22.8).
Side effects
Dreno 2002 documented fewer side effects with lithium use, but the difference was not statistically significant (RR 0.69, 95% CI 0.30 to 1.61) (Analysis 22.9).
Subgroup analyses
We intended to perform a subgroup analysis to compare effects in patients with HIV versus those with no other co-morbidities. Only one study (Langtry 1997) fell into this category with 12 participants, among whom investigators did not find a considerable effect of lithium on symptoms. We investigated significant heterogeneity using the following parameters: analysis by conflict of interest; by dosage; and by mode of delivery for our main comparisons.
Analysis by conflict of interest
Ketoconazole versus placebo
We appraised five studies reporting complete remission as having no conflict of interest (COI) (Go 1992; Pierard 1991; Schofer 1988; Skinner 1985; Unholzer 2002(I)). We pooled results in a fixed-effect meta-analysis and found that fewer participants taking ketoconazole failed to achieve complete resolution compared with those given placebo. The difference was statistically significant (RR 0.54, 95% CI 0.46 to 0.64; I² = 33%) (Analysis 23.1).
We assessed four studies as potentially having COI (Berger 1990; Elewski 2007; Green 1987; Swinyer 2007). Combining these studies in a random-effects meta-analysis revealed that fewer participants taking ketoconazole failed to achieve complete resolution compared with those given placebo; the difference was statistically significant (RR 0.78, 95% CI 0.73 to 0.83; I² = 58%) (Analysis 23.1). The difference in effects between the two subgroups was statistically significant.
We found no study that assessed erythema in this comparison to be without a potential COI (Analysis 23.2). The same finding applied to studies assessing pruritus (Analysis 23.3) and scaling.
Two studies (Go 1992; Schofer 1988) reported no potential conflicts of interest. No heterogeneity was observed between these studies, and they described greater numbers of side effects among participants using ketoconazole (RR 1.82, 95% CI 1.07 to 3.09) (Analysis 23.4). Four studies (Elewski 2006; Peter 1991; Ratnavel 2007; Shuttleworth 1998) were assessed as having conflicts of interest. Pooling these together in a fixed-effect meta-analysis showed comparable occurrence of side effects in participants receiving these treatments (RR. 0.75, 95% CI 0.52 to 1.09) (Analysis 23.4). No heterogeneity was noted between studies in this subgroup. Tests for subgroup differences yielded very high heterogeneity (I² = 86.1%).
Ketoconazole versus steroids
We assessed two studies (Kousidou 1992; Pari 1998) as potentially having no conflicts of interest out of six that had compared the effectiveness of ketoconazole and steroids for complete remission of seborrhoeic dermatitis. When we pooled study results, we found no heterogeneity (I² = 0). Meta-analysis showed that fewer participants taking ketoconazole failed to achieve complete resolution of their seborrhoeic dermatitis (23%) compared with those taking steroids (33%), but the difference was not statistically significant (RR 0.68, 95% CI 0.32 to 1.47) (Analysis 24.1).
The remaining studies (Hersle 1996; Katsambas 1989; Stratigos 1988; Van't Veen 1998) that were assessed as having potential COI showed only minimal heterogeneity (I² = 8%) (Analysis 24.1). Meta-analysis of study results showed a statistically significant difference favouring steroids (RR 1.28, 95% CI 1.04 to 1.58) (Analysis 24.1). The subgroups were not statistically significantly different.
On long-term follow-up assessment of complete seborrhoeic dermatitis remission, we judged one study (Pari 1998) as potentially having no COI, and another study (Hersle 1996) as potentially having COI. Therefore we could not carry out a subgroup analysis for this outcome (Analysis 24.2).
Studies that reported erythema and pruritus scores for this comparison showed no heterogeneity in meta-analysis.
Five studies compared the effects of ketoconazole and steroids on mean scaling score. Only one study (Kousidou 1992) was assessed as having no potential COI (Analysis 24.3). Studies that reported side effects did not show heterogeneity in meta-analysis (Analysis 2.12).
Analysis by dosage
We considered the following treatment regimens for topical ketoconazole.
In total, 28% (of 2% ketoconazole) per week for four weeks (Elewski 2007; Pari 1998; Peter 1991; Satriano 1987; Skinner 1985).
In total, 28% (of 2% ketoconazole) per week for two weeks (Katsambas 1989).
In total, 14% (of 2% ketoconazole) per week for four weeks (Elewski 2006; Kousidou 1992; Pierard 1991; Schofer 1988; Stratigos 1988; Unholzer 2002(I)).
In total, 7% (of 2% ketoconazole) per week for two weeks (Swinyer 2007).
In total, 4% to 6% (of 2% ketoconazole) per week for four weeks (Berger 1990; Danby 1993; Green 1987; Hersle 1996; Ortonne 1992; Ortonne 2011; Piepponen 1992; Ratnavel 2007; Shuttleworth 1998; Van't Veen 1998).
In total, 2% (of 1% ketoconazole) per week for four weeks (Go 1992).
Most of the trial participants receiving ketoconazole were treated for four weeks. Data presented from all of these studies pertained to participant evaluation at four weeks or more from commencement of treatment, except for Katsambas 1989, in which participants were evaluated on the 14th day of treatment.
Ketoconazole versus placebo
We categorised the treatment regimen into three broad dosage groups on the basis of total dose applied per week: 28% per week, 14% per week and 2% to 7% per week.
Dosage categories did not explain heterogeneity between studies assessing complete resolution at four weeks: 60%, 65% and 80%, respectively, within subgroups. It is notable that the 'test for subgroup differences' yielded an I² value of zero (Analysis 25.1).
The effectiveness of 2% ketoconazole in reducing erythema was compared with that of placebo in three studies (Elewski 2006; Satriano 1987; Shuttleworth 1998). Elewski 2006 was omitted from the data table because of incomplete data, Satriano 1987 fell into the '28% per week' category and Shuttleworth 1998 fell into the '2-7% per week' category (Analysis 25.2). Although improvement in erythema was significantly better in the study that used the higher dose, no meaningful subgroup analysis could be done, as each group included single studies.
Two studies assessed erythema outcome as a discrete variable. Peter 1991 fell into the 28% per week category, and Ratnavel 2007 fell into the 2% to 7% category. The small number of studies did not allow for meaningful subgroup analysis by dose (Analysis 25.3).
Each of the three studies (Elewski 2006; Ratnavel 2007; Satriano 1987) that assessed pruritus each fell into a different category. The study using the highest dose had significantly better outcomes than the others, but no difference was noted between the studies using lower doses (Analysis 25.4). Two trials (Green 1987; Peter 1991) assessed pruritus clearance (Analysis 25.5).
Only one study assessed long-term improvement in pruritus score (Analysis 25.6).
The two studies (Ratnavel 2007; Shuttleworth 1998) that carried out long-term assessment of scaling score fell into the same dosage category (2% to 7%/wk) (Analysis 25.7).
Three studies (Green 1987; Peter 1991; Ratnavel 2007) that assessed treatment effect on scaling clearance fell into the highest and lowest dosage subgroups. The study in the highest dosage group had a greater effect than others (Analysis 25.8).
No statistically significant differences were observed between subgroups of studies that reported side effects (Analysis 25.9).
Ketoconazole versus steroids
All studies within this comparison used 2% ketoconazole applied in different regimens. Analysing complete remission of seborrhoeic dermatitis rash resulted in low heterogeneity between all studies (I² = 11%), and no difference in effect size was noted between subgroups (Analysis 26.1). Single studies in the highest and lowest dose subgroups could not facilitate subgroup analysis for studies that assessed rash clearance in the long-term assessment (Analysis 26.2).
The two studies (Ortonne 1992; Ortonne 2011) that carried out long-term comparative assessment of ketoconazole and steroid effect on erythema (as a discrete outcome) fell into the same dosage category (2% to 7%/wk)(Analysis 26.3).
No heterogeneity was observed among studies reporting erythema and pruritus scores. Although heterogeneity was substantial among studies that assessed short-term scaling score, subgroups showed no significant differences (Analysis 26.4). Meaningful subgroup analysis could not be done for long-term assessment of scaling.
The main analyses (not subgroup) showed no heterogeneity between studies assessing side effects (Analysis 2.12).
Analysis by mode of delivery
Topical preparations were delivered in the following forms: shampoos, gels, demulcents (cream, ointment, lotion or liniment), foam and alcohol solution. Only one study (Elewski 2007) explored differences between modes of delivery, namely, foam and gel. Very limited data suggest that differences in drug kinetics evident between gels, creams, ointments and liniments could cause heterogeneity in study outcomes. The main consideration is that the formulation with the active ingredient delivered to affected sites may have implications for safety and user compliance (Elewski 2007), thereby affecting outcomes. Analysis of these subgroups in many instances left just one study within a subgroup.
Ketoconazole versus placebo
In assessing complete remission, investigators used such preparations as shampoos, demulcents, foams and gels. All showed better induction of remission by ketoconazole over placebo (i.e. fewer participants in the ketoconazole group failed to achieve complete resolution of seborrhoeic dermatitis compared with those in the placebo groups: shampoo (Berger 1990; Go 1992; Green 1987) (RR 0.62, 95% CI 0.39 to 0.99; I² = 64%); demulcent (Elewski 2007; Pierard 1991; Schofer 1988; Skinner 1985; Unholzer 2002(I)) (RR 0.61, 95% CI 0.50 to 0.74; I² = 20%). Foam and gel subgroups each included only one study showing better results with ketoconazole use. Between-subgroup heterogeneity was significant (I² = 67.6%) and showed slightly better treatment effects for shampoo and demulcent than for foam and gel (Analysis 27.1).
Most subgroups of studies assessing other outcomes included single studies within subgroups; this could not facilitate meaningful analysis (Analysis 27.2; Analysis 27.3; Analysis 27.4; Analysis 27.5; Analysis 27.6).
Subgroup analysis of side effects showed no heterogeneity between subgroups. The incidence of side effects with ketoconazole or placebo use was comparable, irrespective of the formulation applied (Analysis 27.7).
Ketoconazole versus steroids
Of the six studies (Hersle 1996; Katsambas 1989; Kousidou 1992; Pari 1998; Stratigos 1988; Van't Veen 1998) assessing incidence of complete seborrhoeic dermatitis rash resolution between ketoconazole and steroid, only Hersle 1996 used a shampoo preparation. The other studies used demulcents. The single study (Kousidou 1992) that showed a direction of effect that was different from the others used a demulcent. Therefore, subgroup analysis by mode of delivery did not explain the heterogeneity (Analysis 28.1).
For symptom-based outcomes, no heterogeneity was observed among studies assessing erythema and pruritus scores. Studies assessing scaling score had considerable heterogeneity, which was not explained by mode of delivery (Analysis 28.2). Studies reporting side effects showed no heterogeneity (Analysis 2.12).
Sensitivity analyses
We considered two comparisons to contain sufficient studies for sensitivity analysis, namely, 'ketoconazole versus placebo' and 'ketoconazole versus steroids'. We intended to analyse differences in outcomes by conducting adequate randomisation, allocation concealment and blinding. However for these two comparisons, we found no study or at most two studies that had low risk of bias for these domains. We therefore refrained from drawing conclusions regarding the influence of risk of bias on review results.
Publication bias
Funnel plots for the main comparisons that included sufficient studies for assessment did not reveal a strong indication of publication bias (figure 4).
figure 4.
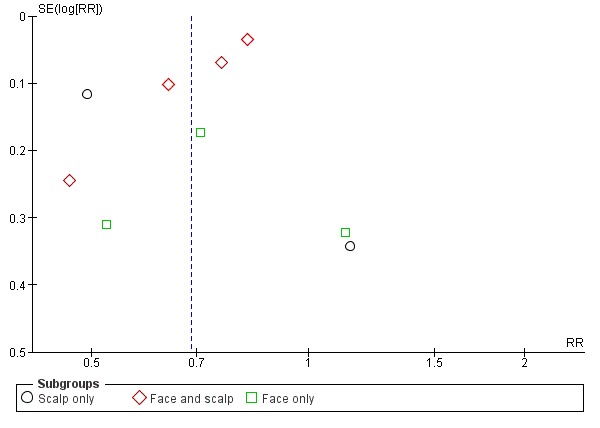
Funnel plot of comparison: 1 Ketoconazole vs placebo, outcome: 1.1 Failure to achieve complete resolution.
Grading of the evidence
Only one study had no limitations regarding randomisation, allocation concealment and blinding of the outcome assessor (Dreno 2003). Therefore we downgraded the evidence for all comparisons on the basis of limitations in study design. For further downgrading decisions, see Table 1.
Discussion
Summary of main results
We found studies on the effects of ketoconazole, bifonazole, metronidazole, clotrimazole and ciclopirox in alleviating symptoms of seborrhoeic dermatitis. Ketoconazole led to a lower incidence of failure to achieve complete resolution than was seen with placebo, but the results were statistically heterogeneous and could not be explained by subgroup analyses of dose, mode of delivery nor conflict of interest. Evidence was considered to be of moderate or low quality.
Treatment with ketoconazole yielded less failure of total rash clearance than was observed with placebo. Evidence for this was of low quality. Ketoconazole reduced erythema and scaling better than placebo did, but the two treatments had a comparable effect on pruritus. Participants taking ketoconazole had comparable risk of side effects across all reporting studies when compared with those given placebo.
Ketoconazole was less effective than steroids in yielding complete remission of rashes, but this finding was not statistically significant. Ketoconazole and steroids showed similar effects on improvement of erythema, pruritus and scaling symptoms. Statistical heterogeneity was high, and the evidence was judged to be of low quality. Participants taking ketoconazole had a 44% lower risk of side effects than those taking steroids.
Ketoconazole was comparable with ciclopirox in eliminating symptoms of seborrhoeic dermatitis. Evidence for this was graded as low. The two drugs were comparable when assessed in terms of symptom-specific outcomes. The incidence of side effects was comparable for the two drugs. Evidence for this was graded as low.
Compared with other antifungals, ketoconazole showed similar or slightly better effects.
Ciclopirox was more effective than placebo in yielding total clearance and in improving symptoms of erythema, pruritus and scaling. Occurrence of side effects was similar with the two treatments. Evidence was considered to be of moderate quality.
Bifonazole was better for all outcomes when compared with placebo, but statistically significant effects were seen most often in longer-term assessments of outcomes. Bifonazole was not as well tolerated as placebo.
Risk of bias in included studies was difficult to ascertain from reports on the articles, but in general was assumed to be high or at best unclear. Only 11 (Berger 1990; Chosidow 2003; Dreno 2003; Dupuy 2001; Langtry 1997; Peter 1991; Ratnavel 2007; Seckin 2007; Shuster 2005; Swinyer 2007; Unholzer 2002(II) of the 51 included articles fulfilled more than five of the 11 'risk of bias' criteria.
Overall completeness and applicability of evidence
Given the extensive search and absence of language restrictions, we are confident that we located most of the studies on topical antifungal treatments for seborrhoeic dermatitis. However, we found sufficient evidence to draw conclusions only for ketoconazole-, ciclopirox- and bifonazole-based treatments. For several classes of antifungals, no studies at all were conducted. Studies with long-term follow-up were particularly sparse. Various studies were carried out over a wide time span, with the oldest study dating as far back as 1985. Many studies did not report our primary outcomes; when this occurred, we included studies that reported only our secondary outcomes. When studies were so poorly reported that we could not use the data in meta-analyses, we reported study findings in the text of this review. Studies used a wide range of doses and application modes of topical antifungal agents. We included studies on seborrhoeic dermatitis of the face and scalp, and on dandruff, which is considered a mild form of seborrhoeic dermatitis of the scalp. Therefore, we are confident that we have included all available evidence.
In most studies, participants were of widely ranging age groups and of both sexes. Results from most studies were given for all participants without stratification on the basis of sex, age and so forth. Thus we could not explore the role of these personal characteristics in treatment outcomes. Most studies used pregnancy as an exclusion criterion; therefore it is unclear whether antifungals are efficacious in pregnant women within a similar range as in non-pregnant women, given known changes in hormone profiles.
Studies included in this review were conducted in different countries, but these were nations with predominantly light-skinned populations. No study analysed outcomes on the basis of ethnicity of participants; thus it was unclear which segments of study participants were of darker skin. It should be borne in mind that seborrhoeic dermatitis in people with darker skin is less easy to diagnose than in those with lighter skin.
Although studies have documented side effects and tolerability concerns, we found only one study (Dreno 2003) that reported actual compliance rates. We believe that this could be a parameter that would explain some of the large heterogeneity that we encountered. Wide-ranging modes of delivery of the active agent across studies may also have accounted for contrasting findings. Alhough a subgroup analysis of these left only single studies in most groups, it remains unclear how this factor impacted trial outcomes.
We did not undertake a subgroup analysis for people having seborrhoeic dermatitis within a background of HIV/AIDS, as only one trial (Langtry 1997) in this category met the inclusion criteria.
Quality of the evidence
Overall, the quality of the evidence was low. In general, studies were badly reported, and missing standard deviations were the most common problem. Often results were presented only as figures. None of the studies used a clear case definition of seborrhoeic dermatitis. The level of evidence was downgraded most often because of risk of bias in the studies, lack of precision of the results and large heterogeneity of effects.
The description of procedures for selection of participants in many studies lacked detail, with particular emphasis on diagnosis. In many articles, it was simply stated that patients with seborrhoeic dermatitis were included without any reference as to how and by whom the diagnosis was made. The description of randomisation and allocation procedures was absent in most articles, with studies simply labelled as "double-blind". Few studies provided details on how sample sizes were determined. Sample sizes varied to a great extent, with both very small and very big studies present.
The rationale for certain treatment regimens was seldom given; most studies simply stated that participants were treated for four weeks with a 2% solution to be applied twice daily. However, wide variation in dose and mode of delivery was observed without a clear explanation for this. It is interesting no relationship between dose and the outcome 'clearance of symptoms' was obvious at the study level as an indirect comparison. It was only when individual symptom outcomes were assessed that higher doses seemed to produce a better treatment effect. However, this observation was based only on single studies. In direct comparisons of dose effect, no reason was found to conclude that different doses had different treatment effects.
The major problem with the quality of the evidence was how outcomes were measured. We failed to identify any validated outcome measure for seborrhoeic dermatitis or outcome measures conventionally endorsed by expert committees or ranking specialist fora. This situation also applies to other dermatological disorders, as our consultation with experts in the field revealed. Our principal outcome measure, namely, total clearance, has face validity, but we do not know how reliably it can be measured. Global severity scores have the drawback that they can be based on assessment of any symptom/affected area combination, which could weaken the reliability of the measure. Therefore, we excluded studies that measured outcomes in this way. They are listed under excluded studies. Although we undertook rigorous assessment of treatment outcomes, considerable heterogeneity was observed in most comparisons, which we could explain only by attributing differences to the absence of validated outcome measures.
Reliable quality of life measurements, which constitute one of our prespecified outcome measures, could prevent in part measurement at symptom level and indicate how treatment influences a more general outcome. However no studies used this outcome. In addition, what constitutes a clinically relevant change in scores for symptoms is unclear. A validated scale should take the clinically relevant change into account.
Potential biases in the review process
We minimised the effect of reporting bias by including studies published in any language. However it was difficult to find available translators for Chinese language articles, and several of these are still on the list awaiting assessment. We avoided reporting bias by including in the review studies with insufficient data and imputing missing data to enable inclusion within meta-analyses.
A small number of studies used a split-face cross-over design. Even though we intended to analyse them with paired t-tests, lack of detail in the study reports prevented us from doing this. Because these studies accounted for only a small proportion of all studies, we believe that this has not essentially influenced our results.
Outcome measures varied greatly across studies, with most seen as a four-step scale ranging from no symptoms present to mild, moderate or severe symptoms present. We treated this, as the study authors did, as a continuous scale ranging from 0 to 3 or from 1 to 4. Therefore, we used total clearance as our primary outcome, because we believe that this can be more reliably assessed. We have no data to underpin this. Given the much higher heterogeneity in meta-analyses involving symptom scores compared with those involving total clearance of symptoms, in hindsight this seems to be a wise decision.
Lack of a good case definition of seborrhoeic dermatitis was a difficult problem. We dealt with this by including studies in which trial authors included participants with a diagnosis of dandruff, as this can be seen as a mild form of seborrhoeic dermatitis, and we had many studies in which investigators had included participants with seborrhoeic dermatitis of the scalp, which in our view is an identical disease entity. We then categorised studies according to the affected area of the body and analysed them separately in subgroups as scalp, scalp and face or face only. With involvement of the face, study authors sometimes mentioned other areas of the body that were affected in addition to the face. We judged that available information on flexures and other areas of the body was insufficient to create another subgroup.
Agreements and disagreements with other studies or reviews
We located only one systematic review with a similar topic (Apasrawirote 2011). Review authors searched only MEDLINE through PubMed and included only nine studies. They concluded that ketoconazole, metronidazole, bifonazole and ciclopirox had better effects on seborrhoeic dermatitis than were seen with placebo. Their conclusion is consistent with our findings that ketoconazole had greater consistency of effect upon comparison with other antifungals. Another systematic review (Kastarinen 2011), which compared steroids versus azoles (mainly ketoconazole) for different treatment outcomes with seborrhoeic dermatitis, found steroids and ketoconazole to be of comparable efficacy.
Authors' conclusions
Implications for practice
Ketoconazole was more effective than placebo at four weeks of follow-up and possibly at three months of follow-up, but few longer-term studies have been conducted. Evidence for this was of low quality. Evidence was insufficient to suggest a dose effect. The most often applied dose was 2%, but the frequency of application of treatments varied between studies from once or twice daily to once or three times weekly for varying lengths of time, and it is unclear which regimen works best.
Ketoconazole did not cause more side effects than were observed with placebo. Topical ketoconazole showed similar efficacy when compared with steroids, but steroids showed a two-fold greater risk of side effects than was seen with ketoconazole. Compared with other antifungals, we cannot say that ketoconazole consistently resulted in a more or less effective outcome because most of these comparisons involved single studies.
Ciclopirox was more effective than placebo but with a comparable incidence of side effects. Evidence was insufficient to reveal an effect of increased dose. Evidence was of moderate quality. Ciclopirox showed effects similar to those of ketoconazole. No comparisons of ciclopirox versus steroids were reported.
Bifonazaole was also found to be more effective than placebo.
Outome variables in this review were stratified according to site (scalp, face or scalp and face). Treatment outcomes were fairly consistent for ketoconazole and other antifungals across different application sites. Studies provided insufficient evidence that the mode of delivery accounted for consistent differences in treatment effect.
Implications for research
The following issues should be attended to in future trials.
Methodological quality - Trial investigators should describe random sequence allocation, allocation concealment and blinding when reporting trials, as would this would make for greater certainty of conclusions.
Completeness of reporting - Side effects and conflicts of interest should be better reported.
Validated outcome measures - This review has emphasised the applicability of validated outcome measures. Expert committees of dermatologists should consider what outcome measures would most objectively assess treatment efficacy in seborrhoeic dermatitis. These should be streamlined and validated. In the interim, all trials should report the proportions of participants with complete clearance of symptoms.
Participant-oriented outcome variables - Measures such as quality of life index would enhance the objectivity of the assessment of efficacy and would provide participants' perspectives on level of efficacy. Future research should consider using these measures, albeit in a standardised way, for outcome assessment.
Compliance with treatment regimen - This clearly impacts outcomes for any mode of treatment. A summary documentation of actual compliance among participants completing trials could be used to stratify analyses of efficacy.
Longer-term assessments with follow-up of at least one year are needed because seborrhoic dermatitis is a chronic condition with a high relapse rate. This plan will also enable better long-term assessment of side effects. A treatment regimen is needed for the intermittent delivery of active agent to a site at a rate that would compromise neither efficacy nor participant compliance. This consideration would address and define parameters for sustained remission.
Economic evaluations - As most of the included studies were conducted in high-income countries, the suitability of evidence so obtained for providers in resource-constrained settings, where prescribers often have to decide between effectiveness and affordability of care, remains questionable. Good economic evaluations would give an indication regarding which option would best suit the collective objectives of patients, providers and the financing system.
We found various kinds of placebo favoured by different trial investigators. Given the high rate of resolution of symptoms under placebo treatment (about 25%), it is important to find out which aspects of treatment could account for this. Some of these placebos were vehicles and bases commonly used as carriers for the active agent. Specific formulations of many placebos were unstated. We considered that the formulation of the placebo may have implications for efficacy. Although this review did not include an analysis based on choice of comparative placebo, it would seem a reasonable undertaking. Subsequent reviews on this topic should explore this question.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: (Malassezia] this term only#2 ("scalp dermatoses" or "scalp dermatosis" or "scalp dermatitis" or "scalp eczema"):ti,ab,kw#3 ("seborrheic dermatitis" or "seborrhoeic dermatitis" or malassezia or "cradle cap" or dandruff or "seborrheic eczema" or "seborrhoeic eczema"):ti,ab,kw#4 MeSH descriptor: (Dermatitis, Seborrheic] this term only#5 MeSH descriptor: (Scalp Dermatoses] this term only#6 #1 or #2 or #3 or #4 or #5
Appendix 2. MEDLINE (Ovid) search strategy
1. exp Dermatitis, Seborrheic/ 2. seborrh$ dermatitis.mp. 3. scalp dermatos$.mp. 4. exp Scalp Dermatoses/ 5. scalp dermatitis.mp. 6. scalp eczema.mp. 7. dandruff.mp. 8. Malassezia.mp. or exp Malassezia/ 9. cradle cap.mp. 10. seborrh$ eczema.mp. 11. or/1-10 12. randomized controlled trial.pt. 13. controlled clinical trial.pt. 14. randomized.ab. 15. placebo.ab. 16. clinical trials as topic.sh. 17. randomly.ab. 18. trial.ti. 19. 12 or 13 or 14 or 15 or 16 or 17 or 18 20. exp animals/ not humans.sh. 21. 19 not 20 22. 11 and 21
Appendix 3. EMBASE (Ovid) search strategy
1. random$.mp.2. factorial$.mp.3. (crossover$ or cross-over$).mp.4. placebo$.mp. or PLACEBO/5. (doubl$ adj blind$).mp. 6. (singl$ adj blind$).mp. 7. (assign$ or allocat$).mp.8. volunteer$.mp. or VOLUNTEER/9. Crossover Procedure/10. Double Blind Procedure/11. Randomized Controlled Trial/12. Single Blind Procedure/13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 1214. Seborrh$ dermatitis.ti,ab.15. scalp dermatitis.ti,ab.16. scalp eczema.ti,ab.17. cradle cap.ti,ab.18. exp *dandruff/19. exp *Malassezia/20. dandruff.ti,ab.21. malassezia.ti,ab.22. exp *seborrheic dermatitis/23. scalp dermatos$.ti,ab.24. seborrh$ eczema.ti,ab.25. or/14-2426. 13 and 25
Appendix 4. LILACS search strategy
((Pt RANDOMIZED CONTROLLED TRIAL OR Pt CONTROLLED CLINICAL TRIAL OR Mh RANDOMIZED CONTROLLED TRIALS OR Mh RANDOM ALLOCATION OR Mh DOUBLE-BLIND METHOD OR Mh SINGLE-BLIND METHOD OR Pt MULTICENTER STUDY) OR ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((CT ANIMALS OR MH ANIMALS OR CT RABBITS OR CT MICE OR MH RATS OR MH PRIMATES OR MH DOGS OR MH RABBITS OR MH SWINE) AND NOT (CT HUMAN AND CT ANIMALS)) [Words] and “seborrh$ dermatitis” or seborreico or dandruff or caspa or “cradle cap” or “costra lactea” or malassezia or “scalp dermatos$” or “eczema seborreico” or “dermatitis seborreica” [Words]
Appendix 5. Glossary
Erythema: reddish discolouration of the skin or mucous membrane.
Calcineurin inhibitors: drugs that inhibit the immunostimulatory effect of the protein calcineurin, which plays a role in generating the symptoms seen in seborrhoeic dermatitis.
Dandruff: an inflammatory skin condition that causes increased shedding and flaking of dead skin from the scalp.
Desquamation: scaling of outermost devitalised layers of the skin.
Keratolytic agent: drug with the ability to dissolve keratin (a structural protein found in the outermost skin layer), so that healthier skin underneath can thrive.
Phototherapy: use of light of specific wavelengths for topical treatment of skin disorders.
Pruritus: sensation of itch.
Sebocyte: cells found in the epithelium of the skin that produce the oily substance, sebum, which serves to moisturise the skin.
Steroids: chemical substances with a cyclic structure, which regulate metabolism, immunity, inflammation, salt and water balance and secondary sex characteristics.
Contributions of authors
EOO was the contact person with the editorial base.EOO coordinated contributions from the co-authors and wrote the final draft of the review.EOO, JHV, JHR, OO and VNB screened papers against eligibility criteria.EOO and JHV obtained data on ongoing and unpublished studies.EOO, JHR and JHV appraised the quality of papers.EOO and JHV extracted data for the review and sought additional information about papers.EOO and JHV entered data into RevMan.EOO and JHV analysed and interpreted data.EOO and JHV worked on the Methods sections.EOO and JHV drafted the clinical sections of the background and responded to the clinical comments of the referees.EOO and JHV responded to the methodology and statistics comments of the referees.This review had no consumer co-author.EOO is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Enembe O Okokon: nothing to declare.Jos H Verbeek: nothing to declare.Jani H Ruotsalainen: nothing to declare.Olumuyiwa A Ojo: nothing to declare.Victor Nyange Bakhoya: nothing to declare.Clinical referee, Rod Hay: "I have been a paid consultant for both P and G and L’Oreal to provide advice on the pathogenesis of seborrhoeic dermatitis but not its treatment. I have been consulted, as an expert adviser (unpaid), by a borderline products investigation by the European Commission on the effect of antifungal products – the index product was climbazole - in cosmetics including shampoos on wider antifungal drug resistance."
Sources of support
Internal sources
-
The Nigerian Branch of the South African Cochrane Centre, Nigeria.
Capacity building in research synthesis by way of a training workshop on protocol development.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Differences between protocol and review
Title
We changed the review title from 'Interventions for seborrhoeic dermatitis' to 'Topical antifungals for seborrhoeic dermatitis'. In the protocol, we presented this review as an all-encompassing interventions review for seborrhoeic dermatitis. We had to modify that goal and limit ourselves to topical antifungal agents used for treatment of seborrhoeic dermatitis. This decision was made because of the multiplicity of comparisons and the equally diverse outcome variables that we encountered. We reasoned that for meaningful comparisons, leading to coherent conclusions, it was best to split the review into segments focusing on major classes of treatment as we identified them from the trials that we scoured, which will be published in series, at the end of which an overview can be written.
Searches
We had proposed to conduct a search for side effects of various interventions used to treat seborrhoeic dermatitis. We did not carry out this search because we lacked the resources. In the included studies, adverse effects that were reported were non-specific; therefore with hindsight, we believe that a search for specific adverse effects would have been difficult to perform. We also decided that searching grey literature and conference proceedings, as proposed in the protocol, would not yield extra information. The quality of reporting of published trials was already low, which made analysis difficult. Conference proceedings that were covered by the electronic search provided very little in terms of data, and we believe that it was not useful to further pursue this search approach.
Excluded studies
We decided to exclude studies in which antifungals were combined with other active medicines in the same treatment; this was not specified in the protocol. This decision was made when it became clear that with these combination treatments, treatment effect could not be attributed to the antifungal when in combination with an active agent of another class, or to a specific antifungal when in combination with another antifungal.
Interventions
We set out to include all interventions for seborrhoeic dermatitis but later reconsidered this proposal and rather split the review into two parts. This part is related only to topical antifungals.
Outcomes
We made some changes to the secondary outcome measures. Because we reasoned that global severity scores cannot be assessed in a valid way, we chose to drop the outcomes measures listed below.
Mean change in global severity score from baseline as assessed by the physician.
Mean change in global severity score from baseline as assessed by the participant.
We replaced these measures with severity scores for erythema, pruritus and scaling, which are cardinal symptoms of seborrhoeic dermatitis and unarguably the most investigated. We deemed these measures adequate to objectively capture treatment effect and enable comparisons across trials when they were derived on different scales. This decision was made after due consultation with experts in this field, including the Co-ordinating Editor of the Skin Group. The consultation was conducted to clarify which measure of treatment effect was objective enough to facilitate comparisons across studies. It was informed by the observation that global severity scores were measured on the basis of different combinations of affected areas of the skin and various possible symptoms. Thus we excluded studies that used only composite scores for treatment outcomes, as they did not all represent the same thing. Such studies are listed under the heading Excluded studies. Studies were included only if investigators had measured complete clearance of symptoms or a change in at least one of the cardinal symptoms of seborrhoeic dermatitis.
Subgroup analysis
In the review we added conflicts of interest to the parameters on which we based our subgroup analysis.
Searches
In the protocol in error, we omitted that we planned to search LILACS, which is an important source of records from South America; therefore we searched this database for this review.
GRADE
Within the time period that we needed to complete the review, assessing quality of evidence using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach became established practice. Therefore, we used GRADE to assess the quality of evidence, and we prepared 'Summary of findings' tables. These were not specified in the protocol.
Sensitivity analysis
We proposed to conduct a sensitivity analysis based on the presence of co-morbidities such as HIV, participants' use of drugs other than prescriptions for seborrhoeic dermatitis and the professional cadre of the diagnostician. Only one study recruited participants who also had HIV infection. Most studies included use of other drugs as an exclusion criterion. Very few studies have identified the cadre of the care provider who made the diagnosis. We therefore dropped these original criteria for these reasons.
Notes
The original protocol was split into 2 separate protocols - 1 on antifungal agents and the other on anti-inflammatory agents. This was done because of the large number of studies retrieved and the multiplicity of outcome measures used. See Differences between protocol and review.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abeck 2004
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis: patients with seborrhoeic dermatitis of the scalp (physician's diagnosis implied) with scores of 2 to 4 for scaling and inflammation at baseline Exclusion: persons with psoriasis, atopic dermatitis, long hair; those treated with systemic antibiotics or antifungals 2 weeks or less before commencement of study; pregnancy/breast feeding; child-bearing potential without adequate contraception or irregular menstrual cycles; history of drug or alcohol use; and many others (see Table 1, page 14) Sex: male (109), female (74) |
|
| Interventions | Intervention: ciclopirox 1% shampoo applied 3 times weekly for 28 days (n = 45) Controls:
|
|
| Outcomes | Complete clearance | |
| Notes | Country: Germany; conflict of interest: none; side effects: 27 participants overall had side effects, which included skin and appendage disorders, pruritus, mild hair loss, severe parietal erythema and moistness of scalp | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomized into four parallel groups ..." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | "The differences in baseline characteristics among groups were minor and unlikely to affect results" |
| Patient blinded? | Unclear risk | "... randomized, double-blind, vehicle controlled four arm trial ..." |
| Provider blinded? | Unclear risk | "... randomized, double-blind, vehicle controlled four arm trial ..." |
| Outcome assessor blinded? | Unclear risk | "... randomized, double-blind, vehicle controlled four arm trial ..." |
| Co-interventions avoided? | Low risk | "No other cosmetic nor non-cosmetic treatment of the scalp or hair was permitted" |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Unclear risk | Not reported |
| Selective outcome reporting acceptable? | Low risk | All outcomes were reported |
| ITT? | Low risk | "All analyses were performed for the ITT population ..." Results confirm this |
Altmeyer 2004
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis (Dx): seborrhoeic dermatitis of the scalp (physician diagnosis implied from text) Exclude if patient has psoriasis, atopic dermatitis, long hair, pregnancy and others (see Table 1, page 10) |
|
| Interventions | Intervention (Int): ciclopirox 1% shampoo applied twice weekly to scalp for 28 days (n = 51) Control:
We combined second and third control groups into a single meta-analysis |
|
| Outcomes | Total clearance | |
| Notes | Country: Germany; conflict of interest (COI): none This study randomly assigned 203 participants to 4 groups, but the number of participants in each group is not given. We therefore assumed that they were equally shared among the groups. We assigned a total of 50 to the intervention (ciclopirox 1%) group and 51 to each of the control groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... were randomised into four parallel groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | Duration and sex similar, but age not reported |
| Patient blinded? | Unclear risk | "... double-blind ..." |
| Provider blinded? | Unclear risk | "... double-blind ..." |
| Outcome assessor blinded? | Unclear risk | "... double-blind ..." |
| Co-interventions avoided? | Low risk | "... concomitant medications were not allowed" |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Unclear risk | Not reported |
| Selective outcome reporting acceptable? | Low risk | All proposed outcomes were reported |
| ITT? | Unclear risk | Unclear from analysis |
Aly 2003
| Methods | Multi-centre trial | |
| Participants | Dx: seborrhoeic dermatitis (SD) of the scalp (physician diagnosis implied from context); baseline score of at least 4 Exclusion criteria: individuals receiving concomitant products that may interfere with outcomes Severity score: 6 |
|
| Interventions | Intervention (Int): ciclopirox gel applied to scalp twice daily for 28 days (n = 89) Control: vehicle gel applied similarly (n = 89) |
|
| Outcomes | Complete clearance | |
| Notes | Country: USA; no conflict of interest Side effects: Int group (13%), Control group (9%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized, double-blind" |
| Allocation concealment (selection bias) | Unclear risk | "randomized, double-blind" |
| Baseline comparable? | Low risk | No differences between groups for demographic data |
| Patient blinded? | Unclear risk | "double-blind" |
| Provider blinded? | Unclear risk | "double-blind" |
| Outcome assessor blinded? | Unclear risk | "double-blind" |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | "Only 7 ciclopirox and 11 vehicle subjects did not complete the entire 4 weeks" |
| Selective outcome reporting acceptable? | Unclear risk | Not reported |
| ITT? | Low risk | "All 89 subjects were included in the safety analysis and analysis of signs and symptoms" |
Attarzadeh 2013
| Methods | Randomised controlled trial of body parts | |
| Participants | Diagnosis: patients diagnosed by dermatologist as having seborrhoeic dermatitis involving the face with skin types II to IV on Fitzpatrick's scale Exclusion criteria: medical therapy within 4 weeks preceding recruitment into study Sex: M:F = 57 (45%):69 (55%); age: 14 to 60 years |
|
| Interventions | Intervention: clotrimazole 1% applied to the left half of the face for 30 days twice daily (n = 62) Control: topical hydrocortisone 1% applied to the left side of the face twice daily for 30 days (n = 64) Second control group treated with Emu oil excluded |
|
| Outcomes | Symptom severity scores for erythema, pruritus and scaling | |
| Notes | Country: Iran; COI: none stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | For age, sex and severity |
| Patient blinded? | High risk | All participants were aware of the treatment given |
| Provider blinded? | High risk | All participants were aware of the treatment given |
| Outcome assessor blinded? | High risk | All participants were aware of the treatment given |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Unclear risk | Not reported |
| Selective outcome reporting acceptable? | High risk | No information on side effects |
| ITT? | Unclear risk | No report on missing data or loss to follow-up |
Berger 1990
| Methods | Individual randomised controlled trials | |
| Participants | Dx: dandruff, minimum score for dandruff severity, with or without SD. Physician diagnosis implied in text Exclusions: pregnancy, infection, immunodeficiencies, psoriasis Sex: keto - male (13/28), placebo - male (12/24) Mean age: 41 years; duration: ketoconazole (13.6 years), placebo (14.8 years) |
|
| Interventions | Intervention: 2% ketoconazole shampoo applied twice weekly to scalp for 28 days (n = 29) Control: placebo shampoo applied similarly (n = 24) |
|
| Outcomes | Total cure | |
| Notes | Country: USA Shampoo provided by Janssen |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... randomised..." |
| Allocation concealment (selection bias) | Low risk | "... identically appearing placebo" |
| Baseline comparable? | Low risk | Age, sex, duration |
| Patient blinded? | Low risk | Identical bottles |
| Provider blinded? | Low risk | Identical bottles |
| Outcome assessor blinded? | Low risk | Not reported |
| Co-interventions avoided? | Low risk | "... no other medication allowed..." |
| Compliance acceptable? | Low risk | "... technician applied shampoo ..." |
| Drop-out acceptable? | Low risk | 1 drop-out |
| Selective outcome reporting acceptable? | High risk | Arbitrary cutoff points used |
| ITT? | High risk | 1 participant who did not complete the trial was excluded |
Chosidow 2003
| Methods | Individual randomised controlled trial | |
| Participants | Dx: mild to moderate seborrhoeic dermatitis of the nasolabial folds, alae nasi and/or eyebrows (test lesions) in patients older than 18 years. Physician diagnosis implied from context Exclude patients with psoriasis, contact dermatitis; "patients who had taken systemic antibiotics or had used topical corticosteroids, topical antifungals, tar, zinc pyrithione, selenium, salicylates or antiseptics on their test lesions within 7 days prior to study entry" and those who had taken oral retinoids Sex: ciclo (male - 93:154), keto (male - 88:149); age: ciclo (41 ± 1.17), keto (43.2 ± 1.17); lesional score: ciclo (6.03 ± 0.119), keto (6.15 ± 0.126); duration: ciclo (98.2 ± 7.82 months), keto (86.3 ± 7.3 months) |
|
| Interventions | Int: ciclopirox 1% cream applied to face twice daily for 28 days, then once daily for the next 28 days (n = 154) Control: ketoconazole 2% gel applied twice weekly to face for 28 days, then once weekly for the next 28 days (n = 149) |
|
| Outcomes | Complete remission of rashes | |
| Notes | Country: France; COI: sponsorship by Pierre-Fabre Dermatology Laboratory Investigators C. Maurette and P. Dupuy were employees of the Pierre-Fabre Research Institute at the time the study was conducted Side effects: ciclo (31:154), keto (57:149) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "According to a computer-generated randomization schedule (blocks of 4 assignments/centre), patients applied either the CIC 1% cream twice a day or the KC 2% foaming gel twice a week...” |
| Allocation concealment (selection bias) | Unclear risk | "Drugs were prepared at the clinical pharmacy of the Pierre-Fabre Research Institute according to the randomization code and had a secondary identical packaging ..." |
| Baseline comparable? | Low risk | See Table 1 |
| Patient blinded? | Low risk | "Drugs were prepared at the clinical pharmacy of the Pierre-Fabre Research Institute according to the randomization code and had a secondary identical packaging ..." |
| Provider blinded? | Low risk | "Drugs were prepared at the clinical pharmacy of the Pierre-Fabre Research Institute according to the randomization code and had a secondary identical packaging ..." |
| Outcome assessor blinded? | Low risk | "Drugs were prepared at the clinical pharmacy of the Pierre-Fabre Research Institute according to the randomization code and had a secondary identical packaging ..." |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Low risk | See Figure 3 |
| Drop-out acceptable? | Low risk | "CIC(7), Keto(14) excluded from analysis; premature withdrawal due to local side effects occurred in 21 patients in the CIC group and 19 patients in the KC group" |
| Selective outcome reporting acceptable? | Low risk | All proposed outcomes were reported |
| ITT? | Low risk | "The two analysis populations, i.e. the intent-to-treat (ITT) population and the per protocol population (PP), were equally studied" |
Danby 1993
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis: male and female patients with moderate to severe dandruff (i.e. dandruff score higher than 14 on a scale of 0 to 60) Exclusion criteria: psoriasis, atopic dermatitis, tinea capitis, Parkinson's disease, immunodeficiency, pregnancy and lactation, sensitivity or allergy to shampoos or soaps, persons on antimycotics or antibiotics |
|
| Interventions | Intervention: ketoconazole 2% shampoo applied twice weekly to scalp at the study facility for 28 days (n = 97) Control 1: placebo shampoo applied similarly (n = 100) Control 2: selenium sulphide 2.5% shampoo applied similarly (n = 47) |
|
| Outcomes | Change in symptom (scaling) severity score | |
| Notes | Country: Canada; COI: support provided by Janssen Pharmaceutica Inc Side effects: pruritus or burning, eruption near hairline, psoriasis, lightening/bleaching of hair colour, orange staining of scalp, chemical taste on being shampooed. All of these occurred in the selenium sulphide group Endpoint mean scaling scores were reported without standard deviations; therefore the results were not analysed quantitatively but were reported qualitatively in relevant sections. The study also reported pruritus outcomes for subsamples of the comparison group. This result could not be used because the number of persons affected in each group was not explicitly stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated simply as randomised study |
| Allocation concealment (selection bias) | Unclear risk | "...double-blind ..." |
| Baseline comparable? | Low risk | "The three treatment groups did not differ statistically with respect to sex distribution, age, racial background, concomitant medications, disease duration and adherent dandruff severity score" |
| Patient blinded? | Unclear risk | "...double-blind ..." |
| Provider blinded? | Unclear risk | "...double-blind ..." |
| Outcome assessor blinded? | Unclear risk | "...double-blind ..." |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Unclear risk | Not reported |
| Selective outcome reporting acceptable? | Low risk | All proposed outcomes were reported |
| ITT? | Unclear risk | Not reported |
Diehl 2013
| Methods | Individual randomised controlled trials | |
| Participants | Inclusion criteria: patients older than 18 years with facial seborrhoeic dermatitis, diagnosis confirmed by investigator that they were not on any treatment that could interfere with test products Exclusion criteria: pregnant women, immunocompromised persons, patients with previous history of cancer Age: QX (15.7 ± 6.99), 2% keto (14.64 ± 8.33) Sex, M:F: QX (9:11), 2% keto (10:10) |
|
| Interventions | Intervention: aqueous gel containing 4% extract of Quassia amara applied twice a day to the face for 28 days (n = 20) Control 1: ketoconazole 2% gel applied similarly (n = 20) Control 2: ciclopirox olamine 1% gel applied similarly (n = 20) |
|
| Outcomes | Complete remission as determined by the investigator | |
| Notes | Country: Argentina; COI: no disclosure of COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used a tool, the Researcher Randomizer, version 3.0; http:.//www.randomizer.org |
| Allocation concealment (selection bias) | Unclear risk | "randomized double blind study" |
| Baseline comparable? | Low risk | Ketoconazole patients on average 5 years older; gender, duration, previous treatment similar |
| Patient blinded? | Unclear risk | Not reported |
| Provider blinded? | Unclear risk | Not reported |
| Outcome assessor blinded? | Unclear risk | Not reported |
| Co-interventions avoided? | Low risk | No other treatment allowed |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | 3/20 in ketoconazole group, 2/20 in Quassia group, 1/20 in ciclopirox group |
| Selective outcome reporting acceptable? | High risk | Table 4 is missing; not all remission categories reported |
| ITT? | Unclear risk | Not reported and unclear imputation for missing data |
Draelos 2005
| Methods | Randomised cross-over trial | |
| Participants | Diagnosis: mild to moderate SD. Hair of sufficient length Sex: male (40/80) |
|
| Interventions | Intervention: 2% ketoconazole shampoo applied daily to scalp for 1 week (n = 20) Control: 1% ZnPTO applied similarly (n = 20) |
|
| Outcomes | Reduction in symptom severity score for erythema and scaling | |
| Notes | Country: USA; poor documentation of methodology; wash-out period not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Unclear risk | Not reported |
| Patient blinded? | Unclear risk | Not reported |
| Provider blinded? | Unclear risk | Not reported |
| Outcome assessor blinded? | Unclear risk | Not reported |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Low risk | "All subjects completed the study with no adverse effects" |
| Drop-out acceptable? | Low risk | Same as above |
| Selective outcome reporting acceptable? | Unclear risk | Not reported |
| ITT? | Unclear risk | Not reported |
Dreno 2002
| Methods | Individual randomised controlled trial | |
| Participants | DX: seborrhoeic dermatitis of the face, physician diagnosis implied from text Exclude if participant has psoriasis or atopic dermatitis or is taking any of the following drugs: lithium or rapid-release corticosteroid therapy 2 weeks preceding the study, or slow-release corticosteroid less than 2 months preceding the study |
|
| Interventions | Intervention: lithium gluconate (8%) ointment applied twice daily to the face for 8 weeks (n = 66) Control: vehicle topically applied similarly (n = 63) |
|
| Outcomes | Global evaluation and scaling severity score | |
| Notes | Country: France; COI: sponsorship received from Labcatel; side effects: lithium (8/66), vehicle (11/63) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | "No significant difference in demography, baseline characteristics, medical history and clinical examination among the 2 populations" |
| Patient blinded? | Unclear risk | "... double-blinded" |
| Provider blinded? | Unclear risk | "... double-blinded" |
| Outcome assessor blinded? | Unclear risk | "... double-blinded" |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Low risk | Compliance with treatment was good and similar in both groups |
| Drop-out acceptable? | High risk | "... 22 patients did not complete the study 10 in LiG group and 12 in placebo group" |
| Selective outcome reporting acceptable? | Low risk | All proposed outcomes were reported |
| ITT? | Low risk | "... efficacy was assessed in the intention-to-treat population." Results support this |
Dreno 2003
| Methods | Individual randomised controlled trials | |
| Participants | Dx: facial seborrhoeic dermatitis of at least 2 months' duration in male and female patients between 18 and 65 years of age; moderate to severe redness and scaling. Physician diagnosis implied from context Exclude patients with allergy to test products; patients with scalp SD requiring therapy; patients with Parkinson's disease, HIV, ear, nose and throat cancer, and with severe recurrent illness Sex: lithium (male - 63.8%), keto (male - 63.2%); age: lithium (39.2 ± 11.7), keto (41.3 ± 11.2); duration: lithium (3.5 ± 1.0 years), keto (3.6 ± 0.9 years); previous treatment: lithium (75%), keto (72.8%) |
|
| Interventions | Int: Lithium gluconate 8% applied twice daily for 8 weeks (n = 152) Control: ketoconazole 2% emulsion applied twice weekly to face for 28 days and then once weekly for the next 28 days (n = 136) |
|
| Outcomes |
|
|
| Notes | Country: France; COI: sponsorship by Laboratoire Labcatal, producer of the brand of lithium gluconate used Sample size derivation was elucidated; compliance with regimen was also a study objective; side effects: lithium (26.3%), keto (25%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization method used computer generated blocks .." |
| Allocation concealment (selection bias) | Low risk | "The randomization code was concealed in sealed envelopes ..." |
| Baseline comparable? | Low risk | See Table 1 |
| Patient blinded? | Low risk | "Investigators provided patients with sealed boxes ... these boxes were similar in appearance ..." |
| Provider blinded? | Low risk | "Investigators provided patients with sealed boxes ... these boxes were similar in appearance ..." |
| Outcome assessor blinded? | Low risk | "The randomization code was concealed in sealed envelopes ..." |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Low risk | 80% compliance with protocol: lithium (93.3%), keto (93%) |
| Drop-out acceptable? | Low risk | Lithium (17/152), keto (17/136) |
| Selective outcome reporting acceptable? | Low risk | All outcomes were reported |
| ITT? | Low risk | Number of participants evaluated corresponds with the number randomly assigned. Refer to Figures 4, 5 and 6 |
Dupuy 2001
| Methods | Individual randomised controlled trial | |
| Participants | Patients over 18 years of age with mild to moderate seborrhoeic dermatitis of the nasolabial folds and or the eyebrows (test lesions); 39.5 average age; 65% male; average duration of symptoms: 80 months | |
| Interventions | Intervention: ciclopiroxolamine 1% cream applied to affected areas for 28 days twice daily (n = 57) Control: matched vehicle cream applied similarly (n = 72) |
|
| Outcomes | Complete clearance | |
| Notes | Country: France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Code kept by research institute. Allocation for each participant was concealed using opaque envelopes |
| Baseline comparable? | Low risk | Comparable for age, sex, lesion score; duration 20 months less in control group |
| Patient blinded? | Low risk | Matched vehicle |
| Provider blinded? | Low risk | Treatment allocation was concealed for each participant during the study |
| Outcome assessor blinded? | Unclear risk | Not reported |
| Co-interventions avoided? | Low risk | Patients with other treatments excluded |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | No drop-outs |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Low risk | ITT was done |
Elewski 2006
| Methods | Individual randomised controlled trials | |
| Participants | Dx: seborrhoeic dermatitis (physician diagnosis implied from text) Exclude if patient has other skin conditions, is allergic to agents used, is pregnant, has used systemic or topical antiseborrhoeic treatment within 30 to 14 days of trial, respectively Sex: keto - male (59.4%), vehicle - male (59.1%); age: keto (52 ± 17.8), vehicle (50 ± 17.2); duration: keto (12.2 ± 13.4), vehicle (11.1 ± 12.2); previous treatment: keto (64.6%), vehicle (68.7%) |
|
| Interventions | Intervention: ketoconazole 2% gel applied once daily to face for 14 days (n = 229) Control: vehicle gel applied similarly (n = 230) |
|
| Outcomes | Symptom severity scores for erythema, pruritus and scaling | |
| Notes | Country: United Kingdom; COI: sponsorship by Barrier Therapeutics; side effects: application site burning and erythema - keto (35), vehicle (44) This study reported symptom scores but did not provide standard deviations. For this reason, we omitted the study from the data tables and reported data in text in appropriate sections |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | Refer to Table 1 |
| Patient blinded? | Unclear risk | "... double-blind ..." |
| Provider blinded? | Unclear risk | "... double-blind ..." |
| Outcome assessor blinded? | Unclear risk | "... double-blind ..." |
| Co-interventions avoided? | Low risk | "No other topical medications or moisturizers were to be applied to affected area(s) during the study, and medicated shampoos were prohibited" |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | "... 442 (96.3%) completed the study... 222 (96.9%) in the ketoconazole group and 220 (95.7%) in the vehicle group" |
| Selective outcome reporting acceptable? | Unclear risk | Not reported |
| ITT? | Unclear risk | Not reported |
Elewski 2007
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis: mild to moderate SD of the scalp, body and face (physician diagnosis implied in text). Participants must be immunocompetent, older than 11 years of age Exclusion criteria: other skin conditions, allergies, use of investigational therapy within 8 weeks before the study |
|
| Interventions | Intervention: keto 2% foam applied to the scalp and face twice daily for 4 weeks (n = 427) Control 1: vehicle foam applied similarly (n = 105) Control 2: 2% keto cream applied similarly (n = 420) |
|
| Outcomes | Overall clearance, symptom clearance | |
| Notes | Country: USA Conflict of interest: study conducted by employees and consultants to Stieffel Laboratories Multi-centre study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... randomized ... study" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | Refer to Table 1 |
| Patient blinded? | Unclear risk | "... double-blinded study" |
| Provider blinded? | Unclear risk | "... double-blinded study" |
| Outcome assessor blinded? | Unclear risk | "... double-blinded study" |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | "... low drop-out rate with 96% ... completing ..." |
| Selective outcome reporting acceptable? | Unclear risk | Not reported |
| ITT? | Low risk | "The ITT population included all 1162 ..." |
Faergermann 1986
| Methods | Individual randomised controlled trials | |
| Participants | Dx: SD and dandruff of the scalp (dx made by physician as implied in text) Male (50%); age (mean): 38 years |
|
| Interventions | Control 2: 2% miconazole base applied to the scalp once daily for 21 days (n = 23) Intervention: 2% miconazole + 1% hydrocortisone in alcohol solution applied to scalp once daily for 21 days (n = 23) (excluded) Control 1: 1% hydrocortisone in alcohol solution applied to scalp once daily for 21 days (n = 23) |
|
| Outcomes | Complete remission | |
| Notes | Country: Sweden Medication provided by Janssen |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | "... double-blind ..." |
| Baseline comparable? | Unclear risk | Not reported |
| Patient blinded? | Unclear risk | "... double-blind ..." |
| Provider blinded? | Unclear risk | "... double-blind ..." |
| Outcome assessor blinded? | Unclear risk | "... double-blind ..." |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | Miconazole + hydrocortisone (2); hydrocortisone (1) |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Unclear risk | Not reported |
Go 1992
| Methods | Individual randomised controlled trials | |
| Participants | Dx: dandruff Exclusion criteria: pregnancy, younger than 18 years of age, not using topical steroids in the past 2 weeks |
|
| Interventions | Intervention: 1% keto - shampoo applied 2× weekly for 4 weeks (n = 88) Control: vehicle shampoo applied similarly (n = 88) |
|
| Outcomes | Complete clearance | |
| Notes | Country: The Netherlands COI: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated to either .." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Baseline comparable? | Low risk | "At the start the 2 treatment showed no significant difference in history of dandruff and severity?" |
| Patient blinded? | Unclear risk | ''double blind study..'' |
| Provider blinded? | Unclear risk | "double blind study..'' |
| Outcome assessor blinded? | Unclear risk | "double blind study..'' |
| Co-interventions avoided? | Low risk | "The patient who used a corticosteroid concomitantly was removed from the efficacy analysis" |
| Compliance acceptable? | Low risk | "... one subject stopped treatment after one week because of an adverse effect" |
| Drop-out acceptable? | Unclear risk | "Only one patient in the KTZ group stopped treatment due to greasy ..." |
| Selective outcome reporting acceptable? | Low risk | All outcomes were reported |
| ITT? | Unclear risk | Not stated |
Green 1987
| Methods | Individual randomised controlled trials | |
| Participants | Dx: SD of the face, scalp and trunk (physician diagnosis implied in text) Sex: male (10/20); age: 16 to 76 years, mean 34 years |
|
| Interventions | Intervention: 2% keto cream and shampoo applied to face and scalp 2 to 3 times weekly for 4 weeks (n = 10) Control: placebo cream or shampoo applied similarly (n = 10) |
|
| Outcomes | Complete clearance | |
| Notes | Country: UK Support from Janssen Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ''Patients were allocated at random to receive either..'' |
| Allocation concealment (selection bias) | Low risk | ''Placebo of identical appearance to the KTZ?'' |
| Baseline comparable? | Low risk | ''There was no significant difference between the 2 groups?'' |
| Patient blinded? | Unclear risk | ''Randomized, double-blinded?'' |
| Provider blinded? | Unclear risk | ''Randomized, double-blinded?'' |
| Outcome assessor blinded? | Unclear risk | ''Randomized, double-blinded?'' |
| Co-interventions avoided? | Low risk | ''All topical Rx were stopped at least 2 weeks before?'' |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | "Only one drop out as described above ..." |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | High risk | Not reported |
Grossman 1997
| Methods | Individual randomised controlled trial | |
| Participants | Dx: patients with moderate to severe dandruff | |
| Interventions | Intervention: ketoconazole 1% shampoo applied daily for 28 days (frequency of daily application not stated) Control: zinc pyrithione 1% shampoo applied daily for 28 days (frequency of daily application not stated) Total number of participants = 230 |
|
| Outcomes |
|
|
| Notes | Country: USA. This article was a stub with no useable data that could be added to the data table. For this reason, we report the study qualitatively. Participants were assessed on days 7, 14, 28 and 42 after discontinuation of treatment. During 6 weeks of follow-up, the ketoconazole 1% group took longer time to relapse with statistically significant differences. After 4 weeks of follow-up, less relapse of itching was reported in the ketoconazole 1% group compared with the zinc pyrithione group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... double-blind, multicentre, randomised study ..." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Unclear risk | Not reported |
| Patient blinded? | Unclear risk | "... double-blind, multicentre, randomised study ..." |
| Provider blinded? | Unclear risk | "... double-blind, multicentre, randomised study ..." |
| Outcome assessor blinded? | Unclear risk | "... double-blind, multicentre, randomised study ..." |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Unclear risk | Not reported |
| Selective outcome reporting acceptable? | Unclear risk | Not reported |
| ITT? | Unclear risk | Not reported |
Herrera-Arellano 2004
| Methods | Individual randomised controlled trials | |
| Participants | Dx: pityriasis capitis, or dandruff, as diagnosed by physician Exclusion criteria: other skin conditions, allergy to shampoos, pregnancy and lactation, co-infection with bacterial or other mycological infection of the scalp Sex: S. chrysotricum (male - 16/51), keto (male - 16/52) Age: S. chrysotricum (33.78 ± 12.74 years); keto (35.94 ± 11.57 years)Duration: S. chrysotricum (16.62 ± 18.73 months); keto (19.21 ± 18.82 months) |
|
| Interventions | Intervention: Solanum chrysotricum shampoo applied every 3 days to scalp for 4 weeks (n = 51) Control: 2% ketoconazole shampoo applied similarly (n = 52) |
|
| Outcomes | Complete clearance | |
| Notes | Country: Mexico | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... subjects were randomized into..." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Baseline comparable? | Unclear risk | ''No significant differences between the 2 Rx groups were found'' |
| Patient blinded? | Unclear risk | Not stated |
| Provider blinded? | Unclear risk | Not stated |
| Outcome assessor blinded? | Unclear risk | Not stated |
| Co-interventions avoided? | Unclear risk | Not stated |
| Compliance acceptable? | Unclear risk | Compliance was checked but was not described formally |
| Drop-out acceptable? | Low risk | ''2 patients withdrew due to?'' |
| Selective outcome reporting acceptable? | Low risk | All outcome variables were reported |
| ITT? | Unclear risk | Not stated |
Hersle 1996
| Methods | Individual randomised controlled trials | |
| Participants | Inclusion criteria: moderate to severe SD (physician diagnosis implied from text), stable and deteriorating disease Sex: male 40/54; age: mean 58 years (22 to 85) Exclusion criteria: patients on therapy that may interfere with trial medication |
|
| Interventions | Intervention: 0.1% mometasone shampoo applied once daily to scalp for 28 days (n = 22) Control: 2% ketoconazole shampoo applied twice weekly for 28 days (n = 27) |
|
| Outcomes | Global evaluation of healing; symptom severity score for scaling, erythema and pruritus | |
| Notes | Country: Sweden Support by Schering-Plough AB, Sweden Adverse effects: depression Rate: mometazone (3.7%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | "The 2 treatment groups were well matched with respect to demographic and baseline status" |
| Patient blinded? | Low risk | Coding of shampoo containers |
| Provider blinded? | Unclear risk | "...double-masked study ..." |
| Outcome assessor blinded? | Unclear risk | "...double-masked study ..." |
| Co-interventions avoided? | Low risk | "During the study, participants were not allowed to use concomitant medication ...." |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Unclear risk | Not reported |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Low risk | Implied from Table 1 (page 516) |
Katsambas 1989
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis: seborrhoeic dermatitis of the scalp and face | |
| Interventions | Intervention: ketoconazole 2% cream applied to scalp and face twice daily for 14 days (n = 24) Control: hydrocortisone 1% cream applied similarly (n = 26) |
|
| Outcomes | Global evaluation of improvement | |
| Notes | Country: Greece | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised fashion" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Unclear risk | Not reported |
| Patient blinded? | Unclear risk | "...assigned tubes of either 2% ketoconazole cream or 1% hydrocortisone cream in a randomised fashion" |
| Provider blinded? | Unclear risk | "...assigned tubes of either 2% ketoconazole cream or 1% hydrocortisone cream in a randomised fashion" |
| Outcome assessor blinded? | Unclear risk | "...assigned tubes of either 2% ketoconazole cream or 1% hydrocortisone cream in a randomised fashion" |
| Co-interventions avoided? | Low risk | No other medication was allowed |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Unclear risk | Not reported |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Low risk | All participants included |
Koc 2009
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis: SebDerm Exclusion criteria: patients with severe SebDerm, other skin conditions including psoriasis and acne; patients allergic to chemicals used in shampoos; patients who have used systemic treatments for SD within the past month Implied from context that this was SD of the face Sex: M (34/38); age: 21 to 42 years |
|
| Interventions | Intervention: pimecrolimus 1% cream applied twice daily for 42 days (n = 23) Control: ketoconazole 2% cream applied twice daily for 42 days (n = 25) |
|
| Outcomes | Symptom severity score for erythema and scaling at 12 weeks | |
| Notes | Country: Turkey Adverse effects: keto (1/24), hydrocortisone (2/26) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized into two treatment groups according to a random digits table" (page 5) |
| Allocation concealment (selection bias) | High risk | "In this 6 week open label, randomized comparative study ..." |
| Baseline comparable? | Low risk | "The treatment groups were not statistically significantly different at baseline with respect to age, sex, mean disease duration and with regards to the criteria (erythema, scaling and infiltration) (p = 0.05)" (page 5) |
| Patient blinded? | High risk | "In this 6 week open label, randomized comparative study ..." |
| Provider blinded? | High risk | "In this 6 week open label, randomized comparative study ..." |
| Outcome assessor blinded? | High risk | "In this 6 week open label, randomized comparative study ..." |
| Co-interventions avoided? | Low risk | "No other medications for SD were allowed during the trial, except for anti-dandruff shampoos started more than 1 month in advance and not used on the face" (page 5) |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | Pimecrolimus 5/21, ketoconazole 4/22 (page XX) |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | High risk | Not reported |
Kousidou 1992
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis: SebDerm Excluded: patients currently on corticosteroids or antibiotics Sex: M (21/40); age (33.7 ± 10.2 years); severity (?) |
|
| Interventions | Intervention: ketoconazole cream 2% applied facially once daily for 28 days (n = 20) Control: hydrocortisone cream 1% applied facially once daily for 28 days (n = 20) |
|
| Outcomes |
|
|
| Notes | Country: Greece | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were randomized to two groups?" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | "without any statistically significant difference in mean scores" |
| Patient blinded? | Unclear risk | Not reported |
| Provider blinded? | Unclear risk | Not reported |
| Outcome assessor blinded? | Unclear risk | Not reported |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | "One patient in the ketoconazole group stopped treatment after two weeks because of dermatitis" |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Low risk | Not reported |
Langtry 1997
| Methods | RCT of body parts (face halves) | |
| Participants | Dx: homosexual men with advanced AIDS having facial SD; physician diagnosis implied within context | |
| Interventions | Intervention: lithium succinate 8% ointment applied to one-half of the face twice daily for 8 weeks (n = 12) Control: ointment base applied similarly to the opposite half of the face (n = 12) |
|
| Outcomes | Erythema and scaling clearance rates. We used the following MD and P values from the paired t-test to calculate the MD (100-mm VAS score) and the SE to be put into RevMan using the general inverse variance method.
|
|
| Notes | Study was conducted in the UK; COI: grant received from Scotia Pharmaceuticals; side effect was reported for a single participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | Each participant was his own control |
| Patient blinded? | Low risk | Use of identical containers |
| Provider blinded? | Low risk | Use of identical containers |
| Outcome assessor blinded? | Low risk | Use of identical containers |
| Co-interventions avoided? | High risk | "... and remained on all other medication which included azidothymidine (AZT) for seven patients" |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | "During the first week of treatment one patient died of an opportunistic infection and two others dropped out ... Ten patients were thus available for assessment" |
| Selective outcome reporting acceptable? | Low risk | All outcomes were reported |
| ITT? | Unclear risk | "The scores for each assessment were expressed as a percentage of baseline and the mean differences between the percentage changes for active and placebo treatment were assessed ..." |
Lebwohl 2004
| Methods | Individual randomised controlled trial | |
| Participants | Dx: SD of the scalp (physician diagnosis implied) Exclusion criteria: psoriasis, atopic dermatitis, previous use of systemic antimycotic medication and lots more (see Table 1, page 18) Sex: ciclo (male 46%), vehicle (male 49%) |
|
| Interventions | Intervention: ciclopirox (1%) shampoo applied twice weekly to scalp for 28 days (n = 250) Control: vehicle shampoo applied similarly (n = 249) |
|
| Outcomes | Complete clearance | |
| Notes | Country: USA Conflict of interest: The second study author is employed by Medicis Pharmaceutical Corporation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The present study was a randomized double blind, vehicle-controlled multicenter trial" |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Baseline comparable? | Low risk | Baseline characteristics reported and similar |
| Patient blinded? | Unclear risk | "This randomized, double blind, vehicle-controlled study was conducted ..." |
| Provider blinded? | Unclear risk | "This randomized, double blind, vehicle-controlled study was conducted ..." |
| Outcome assessor blinded? | Unclear risk | "This randomized, double blind, vehicle-controlled study was conducted ..." |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Mean treatment duration was 27 days for both groups |
| Drop-out acceptable? | Unclear risk | Not reported |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Unclear risk | Not reported |
Lee 2003
| Methods | Individual randomised controlled trial | |
| Participants | Dx: dandruff (diagnosis by a physician implied) Exclusion: concomitant medication |
|
| Interventions | Intervention: 1.5% ciclopirox shampoo applied to scalp 3× weekly for 28 days (n = 33) Control: 2% ketoconazole shampoo applied similarly (n = 31) |
|
| Outcomes | Symptom severity score for pruritus (short-term assessment); symptom clearance (long-term assessment) | |
| Notes | Country: South Korea No conflict of interest Symptom scores were reported as endpoint mean pruritus score. No standard deviations were provided; therefore results were omitted from the data tables and reported qualitatively in the relevant section |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ''During treatment period, they were randomized equally into?'' |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Baseline comparable? | Low risk | ''These 2 treatment groups did not differ in?'' |
| Patient blinded? | Unclear risk | ''A double blind study?'' |
| Provider blinded? | Unclear risk | Not stated |
| Outcome assessor blinded? | Unclear risk | Not stated |
| Co-interventions avoided? | Low risk | ''Other shampoos were not allowed during this?'' |
| Compliance acceptable? | Unclear risk | Not stated |
| Drop-out acceptable? | Low risk | 7 participants did not go through follow-up (number within each group was not given) |
| Selective outcome reporting acceptable? | Low risk | Objectives correspond to what was reported in results |
| ITT? | Unclear risk | Not reported |
Lopez-Padilla 1996
| Methods | Individual randomised controlled trials | |
| Participants | Dx: SD and dandruff as diagnosed by physician Exclusion criteria: psoriasis, contact dermatitis, HIV infection Male: 62%; age: 29 years on average |
|
| Interventions | Intervention: 1% climbazole shampoo applied daily for 3 weeks (n = 30) Control: 1% ketoconazole applied similarly (n = 30) |
|
| Outcomes | Full remission of all symptoms, independent remission of pruritus and desquamation | |
| Notes | Country: Mexico | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assigned randomly |
| Allocation concealment (selection bias) | Low risk | Not reported |
| Baseline comparable? | High risk | Sex differed |
| Patient blinded? | Low risk | Use of identical bottles |
| Provider blinded? | Low risk | Use of identical bottles |
| Outcome assessor blinded? | Low risk | Use of identical bottles |
| Co-interventions avoided? | Low risk | Not allowed |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Unclear risk | Not reported |
| Selective outcome reporting acceptable? | Unclear risk | Not reported |
| ITT? | High risk | Persons who did not complete the study were excluded |
Ortonne 1992
| Methods | Individual randomised controlled trials | |
| Participants | Dx: dandruff and SD of the scalp, face and trunk Exclusion criteria: HIV, pityriasis, pregnancy, lactation Sex: male (66%), female (34%); age (35 to 41 years); duration: 9 years and 10 years on average for keto and betamethasone, respectively |
|
| Interventions | Intervention: 2% ketoconazole foaming gel applied to face and scalp cyclically twice weekly for 4 weeks. Maintenance phase - once weekly for 3 months (n = 31) Control: 0.05% betamethasone lotion once daily for first week, once every other day second week, then twice weekly till end of first month. Maintenance phase - once weekly for 3 months (n = 31) |
|
| Outcomes | Total clearance; symptom remission for erythema, scaling and pruritus | |
| Notes | Country: France; no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... were randomized ..." |
| Allocation concealment (selection bias) | Unclear risk | "...were randomized..." |
| Baseline comparable? | Low risk | Age, sex, severity |
| Patient blinded? | High risk | "... single-blind ..." |
| Provider blinded? | High risk | "... single-blind ..." |
| Outcome assessor blinded? | High risk | "... single-blind ..." |
| Co-interventions avoided? | Low risk | Not allowed |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | Keto (83%), beta (67%) at the end of 5 months |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Low risk | Not reported |
Ortonne 2011
| Methods | Individual randomised controlled trials | |
| Participants | Dx: SD of the scalp in persons 18 years (physician diagnosis implied from text) and older; investigator global assessment of 3 or 4 Exclusion criteria: pregnancy, nursing mothers, HIV infection Age: C2 (44.9), K2 (44.7) Sex: C2 (M = 54%), K2 (M = 55%) Severity: C2 (6.9 ± 1.0), K2 (7 ± 0.9) |
|
| Interventions | Intervention: clobetasol (0.05%) shampoo applied to scalp 2× weekly for 4 weeks (n = 82) Control: ketoconazole (2%) shampoo applied to scalp similarly (n = 80) |
|
| Outcomes | Symptom clearance | |
| Notes | Country: Belgium, France, Germany, Mexico, South Korea No conflict of interest stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomized in a 1:1:1:1 ratio by a designated statistician (using a central computed randomization list that generated treatment numbers in a block of 4) ...." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | All groups were comparable at baseline (Table 1, page 173) |
| Patient blinded? | Unclear risk | Not reported |
| Provider blinded? | Unclear risk | Not reported |
| Outcome assessor blinded? | Unclear risk | Not reported |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | C2 (6), K2 (6), C2 + K2 (8), C4 + K2 (6) (see Figure 1, page 173) |
| Selective outcome reporting acceptable? | High risk | All outcomes reported |
| ITT? | Low risk | "... in the intent to treat population (ITT) consisting of all subjects who were randomized ..." |
Pari 1998
| Methods | Individual randomised controlled trials | |
| Participants | Dx: SD of the face and trunk (physician diagnosed as implied in text) | |
| Interventions | Intervention: 2% keto cream applied to face and trunk twice daily for 28 days (n = 17) Control: 0.05% clobetasol cream applied similarly (n = 19) |
|
| Outcomes | Complete remission | |
| Notes | Country: India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... stratified block random method" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | Only severity reported |
| Patient blinded? | Low risk | Identical tubes |
| Provider blinded? | Low risk | Identical tubes |
| Outcome assessor blinded? | Unclear risk | Identical tubes |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | Ketoconazole (2), clobetasol (3) |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Unclear risk | Not reported |
Peter 1991
| Methods | Individual randomised controlled trials | |
| Participants | Dx: SD as diagnosed by physician Exclusion: intercurrent illness or therapy, severe SD Male: 25%; age: 31 years on average |
|
| Interventions | Intervention: 2% keto cream applied to face twice daily for 4 weeks (n = 30) Control: cream base applied similarly (n = 30) |
|
| Outcomes | Total remission of symptoms (erythema, pruritus, scaling) | |
| Notes | Country: Germany Conflict of interest: free drugs provided by Janssen Pharmacy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random plan |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | Age, duration and sex |
| Patient blinded? | Low risk | Blinding and randomisation of containers |
| Provider blinded? | Low risk | Blinding and randomisation of containers |
| Outcome assessor blinded? | Low risk | Blinding and randomisation of containers |
| Co-interventions avoided? | Low risk | Not allowed |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | 1/30 participants in control group |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Unclear risk | Not reported |
Piepponen 1992
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis: SebDerm with desquamation Excluded: persons with dandruff, pregnancy; persons on concomitant antidandruff agent; uncooperative persons Sex: Intervention - male (21/51), Control - male (17/50); age: Intervention (52.6 ± 21 years), Control (53.6 ± 22.9 years); mean duration: 5 years |
|
| Interventions | Intervention: ketoconazole 2% shampoo applied to scalp 2× weekly for 28 days (n = 51) Control: hydrocortisone 1% liniment applied similarly for 28 days (n = 50) |
|
| Outcomes |
|
|
| Notes | Country: Finland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "this randomized, double-blind (double-dummy) parallel-group..." (page 119) Comment: insufficient information in the report to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | No significant differences between groups with respect to age, sex and dandruff history (Table 1, page 120) |
| Patient blinded? | Unclear risk | Double-blind, double-dummy |
| Provider blinded? | Unclear risk | Double-blind, double-dummy |
| Outcome assessor blinded? | Unclear risk | Double-blind, double-dummy |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Low risk | "2 ketoconazole patients discontinued treatment due to irritations, and 2 hydrocortisone patients discontinued treatment...." |
| Drop-out acceptable? | Low risk | "2 ketoconazole patients discontinued treatment due to irritations, and 2 hydrocortisone patients discontinued treatment...." |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Low risk | "An intention to treat approach with all patients included" |
Pierard 1991
| Methods | Individual randomised controlled trial | |
| Participants | Dx: Seborrhoeic dermatitis lesions present at the hairline, eyebrow, forehead, nasolabial folds and retroauricular, presternal and intracapsular areas (physician diagnosis implied) Excluded: patients treated with antifungals or corticosteroids 2 weeks before start of the study, patients with only seborrhoeic dermatitis of the scalp, patients with serious concomitant disease, patients known to be unreliable |
|
| Interventions | Intervention: ketoconazole 2% emulsion applied to site twice daily for 28 days (n = 23) Control: placebo emulsion applied similarly (n = 16) |
|
| Outcomes |
|
|
| Notes | Country: Belgium COI: No conflicts of interest were declared Symptom scores were reported as endpoint mean decreases in symptom score. No standard deviations were provided; therefore results were omitted from the data tables and were reported qualitatively in the relevant section |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | "Both groups were comparable for sex, age, weight, height and duration of infection" |
| Patient blinded? | Unclear risk | Not reported |
| Provider blinded? | Unclear risk | Not reported |
| Outcome assessor blinded? | Unclear risk | Not reported |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Unclear risk | 2 drop-outs in the ketoconazole group vs 9 in the placebo group |
| Selective outcome reporting acceptable? | Unclear risk | All outcomes were reported |
| ITT? | Unclear risk | Not reported |
Pierard-Franchimont 2001
| Methods | Individual randomised controlled trials | |
| Participants | Dx: SD of the scalp or dandruff as diagnosed by physician. Dandruff severity score = 25; = 200 Malasseizia yeasts/mm2 of scalp Excluded: taking antidandruff or antifungal medication 2 weeks before run-in Sex: keto 2% (male 17/33), keto 1% (male 14/33); age: keto 2% (20 to 69), keto 1% (20 to 67); duration: keto 2% (3.3 months), keto 1% (7.8 months) |
|
| Interventions | Intervention: 2% ketoconazole shampoo applied to scalp twice weekly for 4 weeks (n = 33) Control: 1% ketoconazole applied similarly (n = 33) |
|
| Outcomes | Complete cure | |
| Notes | Country: Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... randomized parallel group trial ..." |
| Allocation concealment (selection bias) | Unclear risk | "... randomized parallel group trial ..." |
| Baseline comparable? | High risk | Difference in duration of illness between groups |
| Patient blinded? | High risk | "... open randomized parallel group trial ..." |
| Provider blinded? | High risk | "... open randomized parallel group trial ..." |
| Outcome assessor blinded? | High risk | "... open randomized parallel group trial ..." |
| Co-interventions avoided? | Low risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | High risk | 12% in keto 2% group, 33% in keto 1% group |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Unclear risk | Not reported |
Piérard-Franchimont 2002
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis: SebDerm or non-inflammatory dandruff as diagnosed by physician Age: 17 to 69 years; mean duration: Intervention (78.2), Control (77.8); previous treatment: Schwarzkopf shampoo; sex: keto (male 96/171), ZnPTO (male 105/160); mean duration: keto (78.2 months), ZnPTO (77.8 months) |
|
| Interventions | Intervention: ketoconazole 2% shampoo applied to scalp 2× weekly for 28 days (n = 171) Control: zinc pyrithione 1% shampoo applied to scalp 2× weekly for 28 days (n = 176) |
|
| Outcomes |
|
|
| Notes | Country: Belgium Relapse rate: keto (60/155), ZnPTO (73/142); adverse effects: itching and erythema - keto (2%), ZnPTO (1%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects were then allocated ... according to a computer generated randomized code ..." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | "The subjects were then allocated ... according to a computer generated randomized code ..." "The demographic and baseline observations were similar in the 2 treatment groups" |
| Patient blinded? | High risk | The design part of the clinical trial was open because the 2 test formulations ... had different colours and smells |
| Provider blinded? | High risk | Open-label trial |
| Outcome assessor blinded? | High risk | Open-label trial |
| Co-interventions avoided? | Low risk | "... neutral Scarzkopf shampoo was allowed to be used as an additional shampoo ..." |
| Compliance acceptable? | Unclear risk | Table 3, page 437 |
| Drop-out acceptable? | Low risk | Table 3, page 437 |
| Selective outcome reporting acceptable? | Low risk | All outcomes were reported |
| ITT? | Unclear risk | "The efficacy analysis were carried out on both the intent-to-treat and on-protocol populations of randomized subjects" Results in Table 3 suggest a per protocol analysis, but it is unclear whether subsequent table and figure and results text refer to an ITT analysis |
Ratnavel 2007
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis: SD as diagnosed by physician Sex: keto (male - 31%), placebo (male - 30%) Mean age: 69.5 ± 50.4, 76.9 ± 52.1 |
|
| Interventions | Intervention: 1.5% ciclopirox olamine shampoo applied thrice weekly to scalp for 4 weeks (n = 150) Control 1: 2% ketoconazole shampoo applied thrice weekly for 4 weeks (n = 150) Control 2: placebo shampoo applied similarly (n = 50) |
|
| Outcomes | Mean change in symptom severity score for scaling and pruritus; technician's assessment of clearance for scaling and erythema | |
| Notes | Country: United Kingdom Support by Stiefel International R & D Adverse effects: CPO (5%), keto (5%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were sequentially randomized into treatment according to a computer generated randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | Not reported |
| Patient blinded? | Low risk | ".... double blinding maintained by identical packing of shampoo" |
| Provider blinded? | Low risk | ".... double blinding maintained by identical packing of shampoo" |
| Outcome assessor blinded? | Low risk | ".... double blinding maintained by identical packing of shampoo" |
| Co-interventions avoided? | Low risk | Not reported |
| Compliance acceptable? | Unclear risk | "The PP population excluded patients who ... did not use study shampoo according to protocol" |
| Drop-out acceptable? | Unclear risk | Not reported |
| Selective outcome reporting acceptable? | High risk | Not reported |
| ITT? | Low risk | "... tested using intention to treat and per protocol populations ..." |
Satriano 1987
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis: SebDerm of duration over 5 years Sex: M (28/40); age: 29.3 years on average; duration: 100% longer than 5 years |
|
| Interventions | Int: ketoconazole cream 2% applied facially 2× daily for 28 days; ketoconazole 1% shampoo applied to scalp 2× weekly for 28 days (n = 20) Placebo: not specified but applied similarly (n = 20) |
|
| Outcomes | Symptom severity score for erythema, desquamation and pruritus at 4 weeks | |
| Notes | Country: Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... in two randomised groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Unclear risk | Not reported |
| Patient blinded? | Unclear risk | Double-blind |
| Provider blinded? | Unclear risk | Double-blind |
| Outcome assessor blinded? | Unclear risk | Double-blind |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Unclear risk | Not reported |
| Selective outcome reporting acceptable? | High risk | Global evaluation reported only for open part of trial |
| ITT? | Unclear risk | Not reported |
Schofer 1988
| Methods | RCT of body parts | |
| Participants | Diagnosis: SebDerm Excluded: patients taking concurrent systemic antibacterial or antimycotic treatment Sex: M (23/29); age: mean 40 years; duration: 2.7 years on average |
|
| Interventions | Int: ketoconazole 2% cream applied to one-half of face 1× daily for 28 days (n = 15) Control: vehicle applied similarly to opposite half of face (n = 15) |
|
| Outcomes | Global evaluation of improvement at 4 weeks | |
| Notes | Country: Germany COI: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "According to randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | Left vs right side of face |
| Patient blinded? | Low risk | "Two externally identical tubes" |
| Provider blinded? | Unclear risk | "double-blind" |
| Outcome assessor blinded? | Unclear risk | "double-blind" |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | High risk | "average treatment three weeks" |
| Drop-out acceptable? | High risk | 9/29 lost to follow-up; details on group loss not given |
| Selective outcome reporting acceptable? | Low risk | Everything reported |
| ITT? | High risk | Analysed per protocol |
Seckin 2007
| Methods | Individual randomised controlled trial; double-dummy technique | |
| Participants | Diagnosis: SebDermExcluded: those with severe seborrhoeic dermatitis requiring systemic therapy or very mild disease with a baseline score less than 5; other skin conditions such as rosacea; use of topical treatments in the previous 2 weeks and systemic treatments in the previous 4 weeks; HIV positivity; allergy to imidazoles; acne vulgaris Sex: M (124/200); age: 16 to 81; duration: 6 years average; severity (?) |
|
| Interventions | Int: both metronidazole 0.75% gel 1× per day and cream vehicle applied facially 1× daily for 28 days (n = 30) Control: both ketoconazole 2% cream 1× per day and gel vehicle 1× per day for 28 days (n = 30) |
|
| Outcomes |
|
|
| Notes | Country: Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized to two groups according to a random digits table" (page 346) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | High risk | Yes for mean disease duration, baseline clinical severity score and pruritus score; no for age and sex |
| Patient blinded? | Low risk | "Vehicles of both agents were identical in appearance to their original forms?" (page 346) Double-dummy technique: Participants used active drug, and vehicle of the other drug was gel or cream |
| Provider blinded? | High risk | Investigator gave instructions during treatment. We believe this was based on knowledge of the treatment |
| Outcome assessor blinded? | Low risk | "All of the efficacy assessments were carried out by an investigator (DS) who was unaware of which group patients were allocated to" (page 346) |
| Co-interventions avoided? | Low risk | "Patients who had used any topical and systemic treatments in the previous 2 and 4 weeks respectively were not enrolled in the study" |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | 10% overall |
| Selective outcome reporting acceptable? | Low risk | Everything reported |
| ITT? | Low risk | Number of participants evaluated corresponds with the number randomly assigned |
Segal 1992
| Methods | Individual randomised controlled trial | |
| Participants | Diagnosis: SebDerm affecting scalp with seborrhoea of scalp | |
| Interventions | Int: bifonazole 1% shampoo; 2 applications to scalp 3× weekly for 42 days (n = 22) Control: shampoo base applied similarly (for 5 minutes on each occasion) for 42 days (n = 22) |
|
| Outcomes | Mean symptom severity score for erythema, pruritus and desquamation as observed at week 6 | |
| Notes | Country: Israel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They [patients] were randomly allocated to one of two groups?" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Unclear risk | Not reported |
| Patient blinded? | Unclear risk | Not reported |
| Provider blinded? | Unclear risk | Not reported |
| Outcome assessor blinded? | Unclear risk | Not reported |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | Not reported |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Low risk | Not reported |
Sei 2011
| Methods | Individual randomised controlled trial | |
| Participants | Diagnosis: dandruff (n = 40), implied physician diagnosis Male: Int 12/17, Control 12/18; age: Int 43 ± 25, Control 46 ± 22; severity: moderate: Int 15/19, Control 12/16 Excluded: patients taking antifungal argent orally or topically on their head and/or patients who did not use rinse in washing their hair |
|
| Interventions | Int: miconazole nitrate: Rinse together with miconazole nitrate shampoo (n = 17); mean use: 5.8 ± 1.8 days per week during 4 weeks (n = 19) Control: placebo rinse with miconazole nitrate shampoo (n = 14); mean use 5.9 ± 2.4 days per week during 4 weeks (n = 18) |
|
| Outcomes | Dandruff eliminated after 4 weeks; itching eliminated after 4 weeks | |
| Notes | Country: Japan COI: not reported Translation by native speaker |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "used block randomisation with a block size of four" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | See participant characteristics |
| Patient blinded? | Unclear risk | Not reported |
| Provider blinded? | Unclear risk | Not reported |
| Outcome assessor blinded? | Unclear risk | Not reported |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Low risk | Yes, used shampoo 5 out of 7 days on average |
| Drop-out acceptable? | Low risk | 20% |
| Selective outcome reporting acceptable? | Unclear risk | Not clear if all outcomes reported |
| ITT? | Unclear risk | No imputation for loss to follow-up |
Shuster 2005
| Methods | Multi-centre trial | |
| Participants | Diagnosis: SD of the scalp (physician diagnosis implied from context) Exclusion: psoriasis, asthma, diabetes Severity score approximately 9 for all groups |
|
| Interventions | Intervention: 1% ciclopirox shampoo applied twice weekly to scalp for 28 days (n = 376) Control 1: vehicle shampoo applied 2× weekly to scalp for 28 days (n = 190) Control 2: 1% ciclopirox shampoo applied once weekly to scalp for 28 days (n = 376) |
|
| Outcomes | Outcome: complete clearance | |
| Notes | Country: England, Austria, Germany, France No conflict of interest Side effects: seborrhoea, rhinitis, shock, skin ulcer, anxiety Numbers of cases in each group were not given, but overall 120 participants had side effects |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients in segment A and segment B were randomized separately using different sets of randomization numbers." The research organization and sponsors held an identical set of envelopes. The randomization envelopes were not opened until the day of study |
| Allocation concealment (selection bias) | Low risk | See above |
| Baseline comparable? | Low risk | "... no difference in severity, no difference in duration of previous treatment" |
| Patient blinded? | Low risk | "The patients were required to use 2 applications per week strictly alternating the use of bottles A1 and A2" |
| Provider blinded? | Low risk | "The patients were required to use 2 applications per week strictly alternating the use of bottles A1 and A2" |
| Outcome assessor blinded? | Low risk | "The patients were required to use 2 applications per week strictly alternating the use of bottles A1 and A2" |
| Co-interventions avoided? | Low risk | "Patients were not allowed to receive concomitant topical treatment of the scalp or any non-systemic treatment..." |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | "4% overall ..." |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Low risk | Number of patients evaluated corresponds with the number randomly assigned |
Shuttleworth 1998
| Methods | Individual randomised controlled trials | |
| Participants | Diagnosis: dandruff diagnosed by a physician. Participant must have dandruff on both sides of the scalp, females must be on contraception Exclusion criteria: allergies to shampoos; pregnancy, lactation; recent use of medication that can affect test shampoo; eye disease that could be exacerbated by study; history of photosensitivity and history of corticosteroid use in the 2 weeks preceding the study |
|
| Interventions | Intervention: 1.5% ciclopirox olamine shampoo applied twice weekly to scalp (n = 22) Control 1: unmedicated (placebo) shampoo base applied twice weekly to scalp for 4 weeks (n = 22) Control 2: ketoconazole 2% applied twice weekly to scalp for 4 weeks (n = 22) |
|
| Outcomes | Symptom severity score for erythema and scaling | |
| Notes | Country: UK Sponsorship by Stiefel Labs; study was conducted by Pharmaco UK Ltd Adverse effect: scalp irritation in keto group (2/32) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... according to a predetermined randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Unclear risk | "The groups were balanced with respect to age, sex, presence of seborrhoeic dermatitis ...." No tabulated data from which to ascertain this |
| Patient blinded? | Unclear risk | ".. double blinded ..." |
| Provider blinded? | Unclear risk | ".. double blinded ..." |
| Outcome assessor blinded? | Unclear risk | ".. double blinded ..." |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | ".. two subjects failed to complete the study for personal reasons unrelated to the shampoo" |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Unclear risk | Not reported |
Skinner 1985
| Methods | Individual randomised controlled trial | |
| Participants | Dx: seborrhoeic dermatitis (physician diagnosis implied in text) | |
| Interventions | Intervention: 2% ketoconazole cream applied twice daily to scalp, face and ears for 4 weeks (n = 20) Control: vehicle cream applied on similar regimen (n = 17) |
|
| Outcomes | Total clearance | |
| Notes | Country: USA No conflict of interest. No baseline information on participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... assigned ... in a randomized fashion" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Unclear risk | Not reported |
| Patient blinded? | Unclear risk | "... double-blind ..." |
| Provider blinded? | Unclear risk | "... double-blind ..." |
| Outcome assessor blinded? | Unclear risk | "... double-blind ..." |
| Co-interventions avoided? | Low risk | "... other medication not allowed" |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | No drop-out |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Unclear risk | Not reported |
Stratigos 1988
| Methods | Individual randomised controlled trials | |
| Participants | Dx: SD of the scalp, face and trunk Male (36/72); age: 33 years on average; duration: 16 months on average |
|
| Interventions | Intervention: 2% keto cream applied once daily to affected area for 1 month (n = 29) Control: 1% hydrocortisone cream applied similarly (n = 34) |
|
| Outcomes | Total clearance and symptom scores for erythema, scaling and pruritus | |
| Notes | Country: Greece, Austria and Belgium Sponsored by Janssen; adverse effects: dryness and skin tension - ketoconazole (1), hydrocortisone (2) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "assigned ... in a randomized fashion" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | Age, sex and severity |
| Patient blinded? | Unclear risk | "... double-blind ..." |
| Provider blinded? | Unclear risk | "... double-blind ..." |
| Outcome assessor blinded? | Unclear risk | "... double-blind ..." |
| Co-interventions avoided? | Low risk | Not allowed |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | Not reported |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Low risk | Not reported |
Swinyer 2007
| Methods | Individuat RCT | |
| Participants | Inclusion criteria: moderate to severe SD affecting scalp hairline, postauricular area, eyebrows and/or bridge of the nose, nasolabial folds and sternum Exclusion criteria: other skin conditions Intervention group: sex - M 61%, F 39%; age: 47.7 ± 17.6 years; duration: 9.7 ± 11.4 months; previously treated: 375 (69%) Control group: sex - M 61%, F 39%; age: 48.0 ± 17.5 years; duration: 10.1 ± 11.4 months; previously treated: 271 (70%) |
|
| Interventions | Intervention group: anhydrous ketoconazole gel 2% applied to the scalp and face once daily for 14 days (N = 545) Control group: vehicle gel applied once daily for 14 days (N = 388) |
|
| Outcomes | Erythema and scaling clearance | |
| Notes | Coutry: This was a multi-centre trial conducted in the USA; other countries not mentioned COI: "... studies were supported by an unrestricted educational grant from Barrier Therapeutics …" 1 trialist was a consultant for Barrier Therapeutics, and another was an employee of Barrier Therapeutics Adverse effects included application site reaction, burning, dermatitis, discharge, dryness, erythema, irritation, pain and pruritus. 40 participants in the intervention group had adverse effects, and 25 participants in the control group had adverse effects |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | In the pivotal study, participants were assigned in a 1:1 fashion to ketoconazole gel 2% or vehicle gel; in the 2 supporting studies, participants were assigned in a 2:2:1:1 fashion for treatment with ketoconazole gel 2%, ketoconazole 2% plus desonide gel 0.05% (combination gel), desonide gel 0.05% or vehicle gel |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | See Table 2, page 477 |
| Patient blinded? | Low risk | "… blinding was maintained by a 2-part tear-off labelling system where the study drug identity was hidden under a scratch-off layer" |
| Provider blinded? | Low risk | "… blinding was maintained by a 2-part tear-off labeling system where the study drug identity was hidden under a scratch-off layer" |
| Outcome assessor blinded? | Low risk | "… blinding was maintained by a 2-part tear-off labeling system where the study drug identity was hidden under a scratch-off layer" |
| Co-interventions avoided? | Low risk | "The use of systemic antifungal agents and corticosteroids was not permitted within 30 days of the baseline visit. Furthermore, the use of other local treatments for seborrheic dermatitis was not permitted within 14 days of baseline. During these studies, application of other topical medications or moisturizers to the affected areas was not permitted, and if the administration of other medication became necessary, it was reported" |
| Compliance acceptable? | Low risk | Table 2, page 477 |
| Drop-out acceptable? | Low risk | Ketoconazole = 24, vehicle = 14 |
| Selective outcome reporting acceptable? | Low risk | All proposed outcomes were reported |
| ITT? | Low risk | Yes, Tables 3 and 4 (pages 478 and 480, respectively) |
Unholzer 2002(I)
| Methods | Individual randomised controlled trial | |
| Participants | Diagnosios: mild, moderate or severe seborrhoeic dermatitis involving facial skin in persons 18 years of age and older Exclusion criteria: patient has other skin conditions, HIV, pregnancy, breastfeeding; patient has participated in another clinical trial within the past 30 days; patient has received treatment with topical antimycotic agent or systemic antihistamine in the past 7 days to 2 months |
|
| Interventions | Intervention: ciclopirox 1% cream applied once daily to face for 28 days (n = 55) Control 1: vehicle cream applied similarly (n = 57) Control 2: ketoconazole 2% cream applied similarly (n = 53) |
|
| Outcomes | Complete remission | |
| Notes | Country: Germany; COI: sponsorship by Aventis Pharma; side effects: ciclopirox (1), vehicle (5), ketoconazole (2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... Randomly assigned test population ..." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Unclear risk | Age |
| Patient blinded? | Unclear risk | "... double-blind ..." |
| Provider blinded? | Unclear risk | "... double-blind ..." |
| Outcome assessor blinded? | Unclear risk | "... double-blind ..." |
| Co-interventions avoided? | Low risk | "... concomitant topical or systemic application of corticosteroids or antimycotics was not allowed" |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Unclear risk | 21/165: It is unclear how attrition was distributed between comparison groups |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Low risk | All randomly assigned participants were included in the results |
Unholzer 2002(II)
| Methods | Individual randomised controlled trial | |
| Participants | Dx: adult males and females with seborrhoeic dermatitis of the face Excluded: patients with other skin conditions, HIV, allergies to active agents; pregnant or lactating; patient has participated in other trials within the past 30 days |
|
| Interventions | Intervention: ciclopirox 1% cream applied once daily to the face for 28 days (n = 97) Control: vehicle cream applied similarly (n = 92) |
|
| Outcomes | Global evaluation of cure | |
| Notes | Country: Australia and New Zealand; COI: sponsorship by Aventis Pharma; side effects: ciclopirox (10), vehicle (9) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization performed using an internally validated software" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Low risk | Age, severity |
| Patient blinded? | Unclear risk | "... block sizes were generally different in order to blind the investigator" |
| Provider blinded? | Unclear risk | "... block sizes were generally different in order to blind the investigator" |
| Outcome assessor blinded? | Low risk | "... block sizes were generally different in order to blind the investigator" |
| Co-interventions avoided? | Low risk | "... concomitant topical or systemic application of corticosteroids, antimycotics or acne medication ... was not allowed" |
| Compliance acceptable? | Low risk | "No patient was definitely non-compliant" |
| Drop-out acceptable? | Low risk | None reported |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Low risk | All randomly assigned participants were included in the results |
Van't Veen 1998
| Methods | Individual randomised controlled trials | |
| Participants | Dx: SD and dandruff (physician dx implied from text) Excluded: pregnant or breastfeeding Male: beta (50%), keto (46%); age: beta (47 ± 16 years), keto (40 ± 17 years); duration: beta (11 months), keto (17 months) |
|
| Interventions | Intervention: 0.1% betamethasone lotion applied twice daily (week 1), once daily (week 2), twice weekly (weeks 3 and 4) (n = 34) Control: 2% ketoconazole hydrogel applied twice weekly for 4 weeks (n = 35) |
|
| Outcomes | Global evaluation of cure, symptom score for scaling and pruritus | |
| Notes | Country: The Netherlands; multi-centre study Financial support by Glaxo; adverse effect: folliculitis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... randomly allocated..." |
| Allocation concealment (selection bias) | High risk | "... randomly allocated..." |
| Baseline comparable? | Low risk | Age, sex and severity |
| Patient blinded? | High risk | Not reported |
| Provider blinded? | High risk | Not reported |
| Outcome assessor blinded? | High risk | Not reported |
| Co-interventions avoided? | Low risk | Not allowed |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | Beta (5/34), keto (3/35) |
| Selective outcome reporting acceptable? | Low risk | All outcomes reported |
| ITT? | Unclear risk | Not reported |
Vardy 2000
| Methods | Individual randomised controlled trials | |
| Participants | Dx: mild to moderate SD of the scalp in persons 15 years of age and older (physician diagnosis stated in article) Excluded: women who are pregnant and lactating, etc |
|
| Interventions | Intervention: ciclopirox olamine 1% shampoo applied twice daily to scalp for 28 days (n = 53) Control: placebo shampoo applied similarly (n = 44) |
|
| Outcomes |
|
|
| Notes | Country: Israel; COI: Shampoo was provided by Trima, Israel Pharmaceutical Products; side effects: ciclo (2:53), placebo (1:49) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Unclear risk | "No significant differences in age, sex, severity of each symptom, overall severity were found between the 2 groups" |
| Patient blinded? | Unclear risk | Not reported |
| Provider blinded? | Unclear risk | Not reported |
| Outcome assessor blinded? | Unclear risk | Not reported |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | Ciclo (6:53), placebo (5:44) |
| Selective outcome reporting acceptable? | Unclear risk | All outcomes were reported |
| ITT? | High risk | Totals in result tables show per-protocol analysis |
Zienicke 1993
| Methods | Individual randomised controlled trials | |
| Participants | Dx: SD; participants must be 16 years of age and older Excluded: pregnant persons, HIV-positive persons, those with allergy to imidazoles, those given topical treatment 2 weeks before start of study, etc |
|
| Interventions | Intervention: 1% bifonazole ointment applied once daily to the face for 28 days (n = 45) Control: vehicle applied similarly (n = 47) |
|
| Outcomes | Total remission of symptoms, symptom severity score | |
| Notes | Country: Germany; no conflict of interest; side effects: Unwanted effects were recorded 7 times in the bifonazole group and 4 times in the control group. No mention is made of the actual number of participants in each group who were affected. Side effects included itch, erythema, tightness of the skin, burning, papules and scaling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "One hundred patients were enrolled and treated according to a random plan ... This was a controlled, double-blind multi centre trial ..." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline comparable? | Unclear risk | "There were no differences between the verum-treated group and the control group in terms of age ..." |
| Patient blinded? | Unclear risk | "... double blind ..." |
| Provider blinded? | Unclear risk | "... double blind ..." |
| Outcome assessor blinded? | Unclear risk | "... double blind ..." |
| Co-interventions avoided? | Unclear risk | Not reported |
| Compliance acceptable? | Unclear risk | Not reported |
| Drop-out acceptable? | Low risk | Bifonazole (17%), vehicle (8.51%) |
| Selective outcome reporting acceptable? | Unclear risk | Not reported |
| ITT? | High risk | 92 participants were evaluated in all; totals provided in tables show that ITT analysis was not done |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alizadeh 2014 | Used oral medication |
| Amos 1994 | Used a composite symptom score only |
| Attila 1993 | Used a mixture of drugs |
| Boyle 1986 | Used a composite outcome score only |
| Brown 1990 | Used a composite outcome score only |
| Cauwenbergh 1986 | Used a composite outcome score only |
| Chappell 2014 | Used a composite outcome score only |
| Cheng 2001 | Not a randomised trial (as assessed by native speaker) |
| Cicek 2009 | Does not involve antifungal drugs |
| Comert 2007 | Used a composite outcome score only |
| CTRI/2009/091/001079 | Used a combination of antifungals for treatment |
| Davies 1999 | Combined antifungal with another drug having a different mode of action |
| Efalith Trial Group 1992 | Used a mixture of lithium and zinc |
| Emad 2000 | Abstract with insufficient data; no full text retrieved |
| Emtestam 2012 | Drugs used in this trial were not antifungal |
| Ermosilla 2005 | Outcome variable used is a composite of symptom scores; this falls within our exclusion criteria |
| Ernst 1990 | Non-randomised study |
| Ford 1984 | Evaluated orally administered ketoconazole |
| Gayko 2006 | Ketoconazole was compared with a combination of ketoconazole and another drug |
| Goldust 2013(a) | No outcomes reported |
| Gupta 2006 | This was a qualitative review |
| Humke 2002 | Conference abstract; not able to retrieve full text |
| Iraji 2005 | Abstract; did not provide enough data; not able to retrieve full text |
| Iudica 2001 | This was a commentary on another article (Parsad 2001a) |
| Jensen 2009 | Abstract with insufficient data; not able to retrieve full text |
| Kaszuba 2005 | Study evaluated orally administered Itraconazole |
| Koca 2003 | Used a composite outcome score only |
| Kozlowska 2007 | Used a composite outcome score only |
| Li 1996 | Not a randomised trial as assessed by native speaker |
| Loden 2000 | Combined antifungal with a drug having a different mode of action |
| Lorette 2006 | Combined 2 antifungals versus a third antifungal (or placebo) (ciclopiroxolamine/zinc pyrithione) |
| Meyer-Rohn 1979 | This study deals with patients with non-seborrhoeic dermatitis |
| NCT00703846 | Open-label phase 4 trial without control group |
| NCT01139749 | Used oral medication |
| Ozcan 2007 | Metronidazole is not an antifungal |
| Parsad 2001 | Metronidazole is not an antifungal |
| Pedrinazzi 2009 | Used peat; we excluded herbal treatments |
| Peter 1995 | Used a composite outcome score only |
| Pierard-Franchimont 2002b | Used a composite outcome score only |
| Pierard-Franchimont 2002c | Outcome variables in this study were sebum excretion rate and percentage of anagen hair, which did not fulfil our inclusion criteria |
| Prensner 2003 | This is a commentary on a study that was previously conducted |
| Quadri 2005 | Authors (Milani) were contacted; data not available anymore |
| Rigoni 1989 | Non-randomised study |
| Salmanpoor 2012 | This was a Letter to the Editor |
| Scaparro 2001 | Used oral medication |
| Schmidt-Rose 2011 | Used a composite outcome score only |
| Schwartz 2013 | Combined antifungal with a drug having a different mode of action (zinc pyrithione and climbazole) |
| Seite 2009 | Combined antifungal with a drug having a different mode of action |
| Siadat 2006 | Metronidazole is not an antifungal |
| Sparavigna 2013 | Combined 2 antifungals vs placebo |
| Squire 2002 | Combined antifungal with a drug having a different mode of action |
| Syed 2008 | Herbal extract |
| Trznadel-Grodzka 2012 | This study was not a clinical trial |
| Vena 2005 | Used a composite outcome score only |
| Xu 1996 | Not a randomised trial (as assessed by native speaker) |
Characteristics of studies awaiting assessment [ordered by study ID]
Feng 2012
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation from Chinese |
Goldust 2013(b)
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting assessment |
Goldust 2013(c)
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting assessment |
Gould 1988
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting retrieval |
IRCT138807202581N1
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting retrieval |
IRCT2013072314117N1
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting retrieval |
Kim 2008
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation from Korean |
Li 1999
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation from Chinese |
Liu 1997
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation from Chinese |
Mao 1999
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation from Chinese |
Nong 1996
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation from Chinese |
Sun 1994
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation from Chinese |
Turlier 2014
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting assessment |
Xia 1998
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Chinese; awaiting translation |
Xu 2000
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation from Chinese |
Characteristics of ongoing studies [ordered by study ID]
EUCTR2005-001371-35
| Trial name or title | A Double-Blind, Placebo-Controlled, Half-Head Design, CPO Solution Dose Ranging-Finding Study in Patients With Seborrhoeic Dermatitis of the Scalp |
| Methods | Randomised double-blind parallel-group trial |
| Participants | Diagnosis: male or female participant aged 16 years and older with seborrhoeic dermatitis of the scalp with minimum visual scaling score of 5, differing between bilateral study sites by a score of no more than 17, will be willing to have their hair washed only 3 or 4 times during the treatment phase of the study - and always at the study site - and who will be willing to have a small section of hair clipped to enable removal of scales for yeast samplingExclusion criteria: patients with acute weeping or infected scalp dermatoses, patients with a history of known intolerance to any of the investigational products, patients who have received any unlicensed drug within the previous 30 days or who are scheduled to receive an investigative drug other than the study medication during the period of the study, patients with systemic diseases that may adversely influence their participation in the trial, female patients who are pregnant or lactating and many others |
| Interventions | Intervention: ciclopirox olamine 1.5% solution applied to scalp for 4 weeks Control:
|
| Outcomes | "The primary end point will be the change in area of seborrhoeic dermatitis from day 01 to day 29" |
| Starting date | 18-05-2005 |
| Contact information | euctr@ema.europa.eu euctr@ema.europa.eu |
| Notes | Country: United Kingdom. We were not able to trace whether the trial has been published |
| Trial name or title | Seborrheic Dermatitis: Ketoconazole 2% Foam Versus Ketoconazole 2% Shampoo |
| Methods | Individual randomised controlled trial |
| Participants | Diagnosis: seborrhoeic dermatitis of the scalp in African American females aged 18 to 89 years, with symptom score of 50 to 200, who are willing to not grease or oil scalp Exclusion criteria: age younger than 18 years or older than 89 years; history of psoriasis, diabetes mellitus, immunosuppression, neurological disorders or chronic disease not stabilised by medication; persons taking oral steroids and/or antifungals within 30 days before enrolment; sensitivity to any formulation of ketoconazole foam or shampoo or sulphur; use of any topical medications indicated for the treatment of seborrhoeic dermatitis within 14 days of enrolment; pregnant women or women who plan on becoming pregnant; breastfeeding women |
| Interventions | Intervention: ketoconazole 2% shampoo applied twice weekly to scalp for 4 weeks Control: ketoconazole 2% foam applied to the scalp twice daily for 4 weeks |
| Outcomes | Sympton severity score, compliance |
| Starting date | September 2010 |
| Contact information | Jeaneen A Chappell, MD; jchappe1@slu.edu |
| Notes | Study is being conducted by Louis University Department of Dermatology, USA. Results have been requested but have not yet been received |
Analysis 1.1.
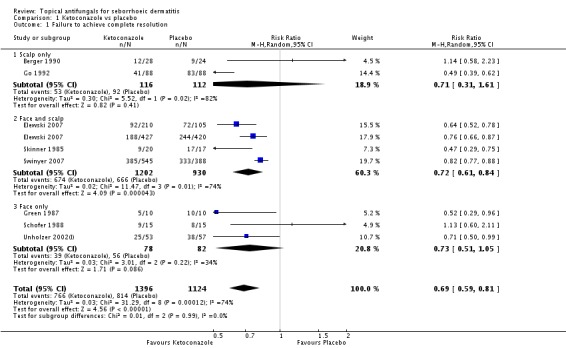
Comparison 1 Ketoconazole vs placebo, Outcome 1 Failure to achieve complete resolution.
Analysis 1.2.
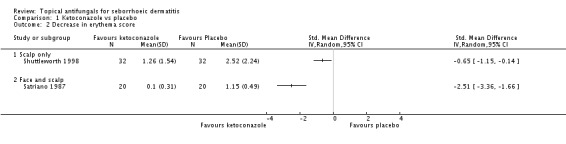
Comparison 1 Ketoconazole vs placebo, Outcome 2 Decrease in erythema score.
Analysis 1.3.
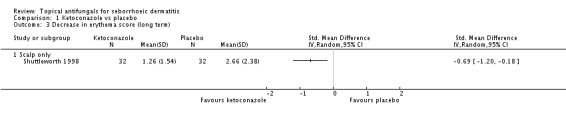
Comparison 1 Ketoconazole vs placebo, Outcome 3 Decrease in erythema score (long term).
Analysis 1.4.
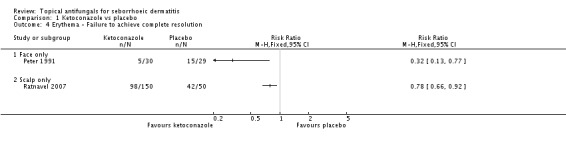
Comparison 1 Ketoconazole vs placebo, Outcome 4 Erythema - Failure to achieve complete resolution.
Analysis 1.5.
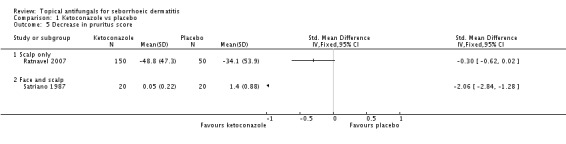
Comparison 1 Ketoconazole vs placebo, Outcome 5 Decrease in pruritus score.
Analysis 1.6.
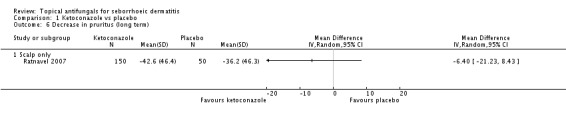
Comparison 1 Ketoconazole vs placebo, Outcome 6 Decrease in pruritus (long term).
Analysis 1.7.
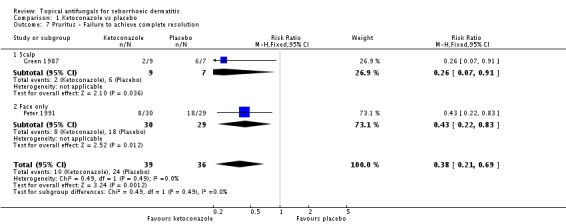
Comparison 1 Ketoconazole vs placebo, Outcome 7 Pruritus - Failure to achieve complete resolution.
Analysis 1.8.
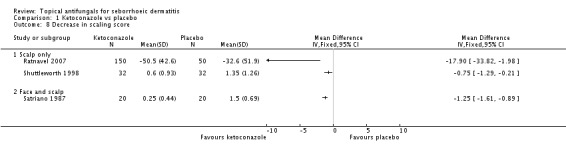
Comparison 1 Ketoconazole vs placebo, Outcome 8 Decrease in scaling score.
Analysis 1.9.
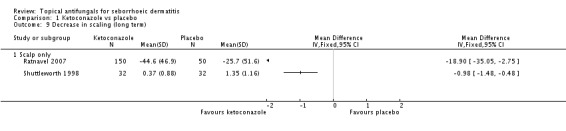
Comparison 1 Ketoconazole vs placebo, Outcome 9 Decrease in scaling (long term).
Analysis 1.10.
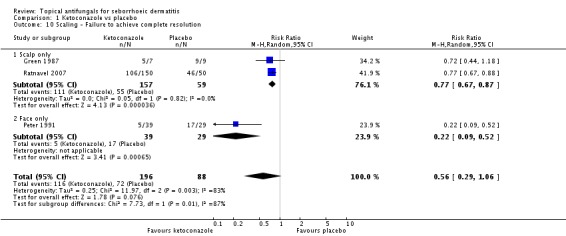
Comparison 1 Ketoconazole vs placebo, Outcome 10 Scaling - Failure to achieve complete resolution.
Analysis 1.11.
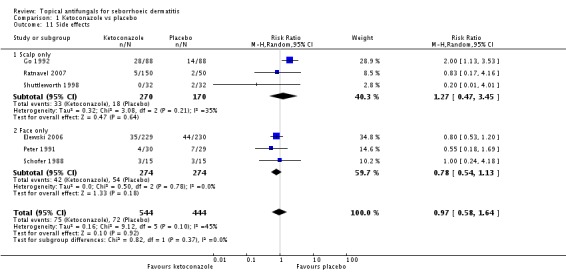
Comparison 1 Ketoconazole vs placebo, Outcome 11 Side effects.
Analysis 2.1.
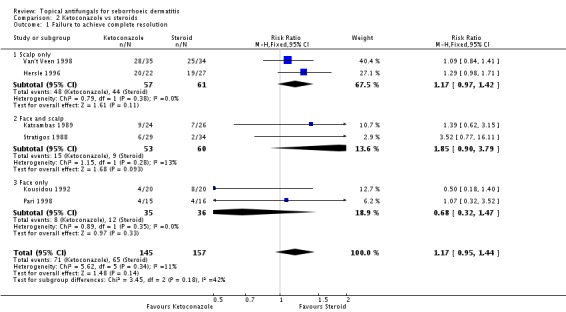
Comparison 2 Ketoconazole vs steroids, Outcome 1 Failure to achieve complete resolution.
Analysis 2.2.
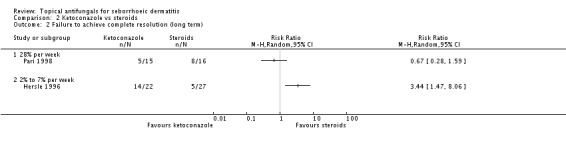
Comparison 2 Ketoconazole vs steroids, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 2.3.
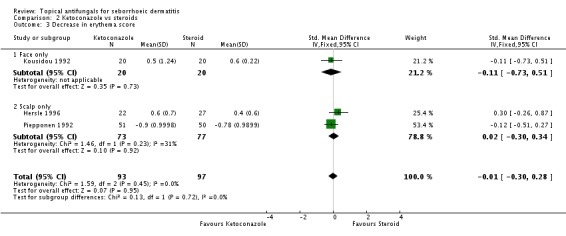
Comparison 2 Ketoconazole vs steroids, Outcome 3 Decrease in erythema score.
Analysis 2.4.
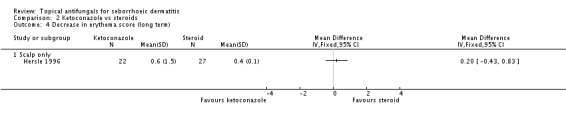
Comparison 2 Ketoconazole vs steroids, Outcome 4 Decrease in erythema score (long term).
Analysis 2.5.
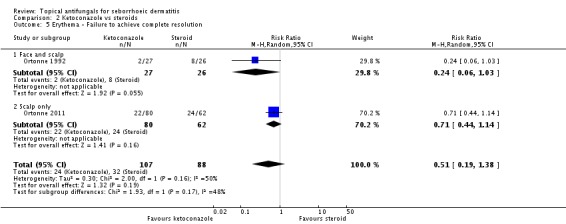
Comparison 2 Ketoconazole vs steroids, Outcome 5 Erythema - Failure to achieve complete resolution.
Analysis 2.6.
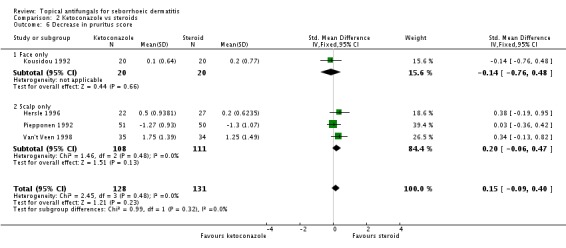
Comparison 2 Ketoconazole vs steroids, Outcome 6 Decrease in pruritus score.
Analysis 2.7.
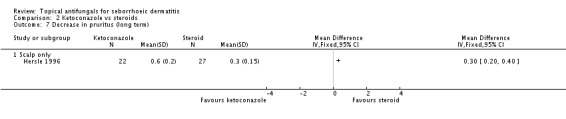
Comparison 2 Ketoconazole vs steroids, Outcome 7 Decrease in pruritus (long term).
Analysis 2.8.
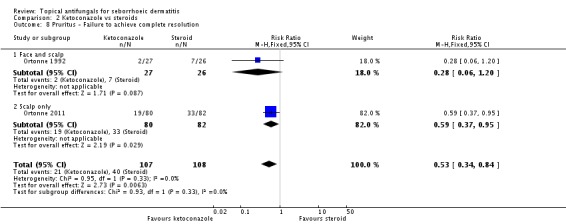
Comparison 2 Ketoconazole vs steroids, Outcome 8 Pruritus - Failure to achieve complete resolution.
Analysis 2.9.
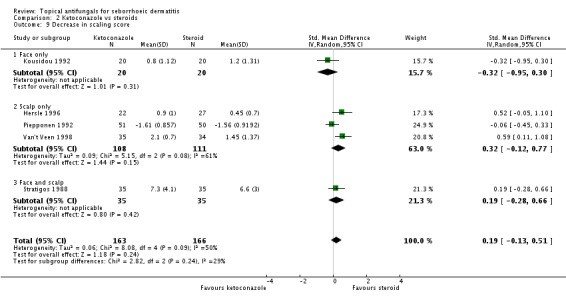
Comparison 2 Ketoconazole vs steroids, Outcome 9 Decrease in scaling score.
Analysis 2.10.
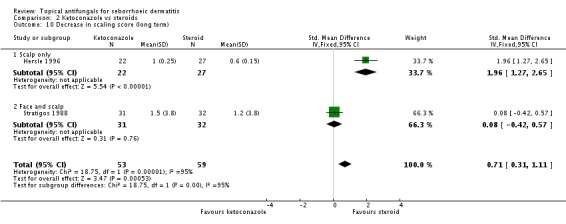
Comparison 2 Ketoconazole vs steroids, Outcome 10 Decrease in scaling score (long term).
Analysis 2.11.
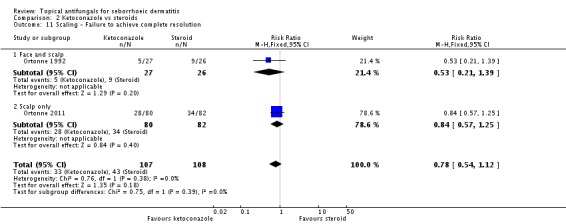
Comparison 2 Ketoconazole vs steroids, Outcome 11 Scaling - Failure to achieve complete resolution.
Analysis 2.11.
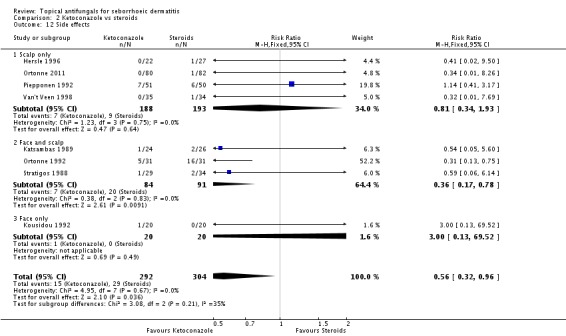
Comparison 2 Ketoconazole vs steroids, Outcome 12 Side effects.
Analysis 3.1.
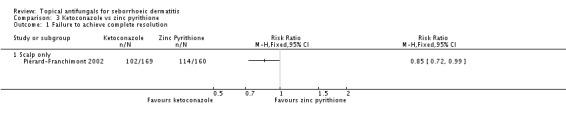
Comparison 3 Ketoconazole vs zinc pyrithione, Outcome 1 Failure to achieve complete resolution.
Analysis 3.2.
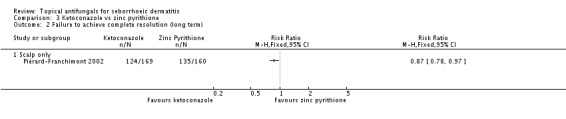
Comparison 3 Ketoconazole vs zinc pyrithione, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 3.3.
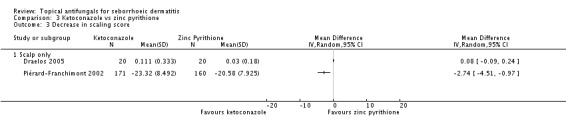
Comparison 3 Ketoconazole vs zinc pyrithione, Outcome 3 Decrease in scaling score.
Analysis 3.4.
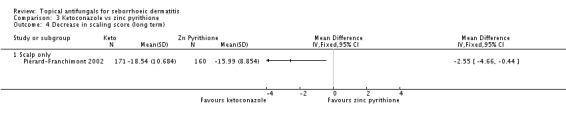
Comparison 3 Ketoconazole vs zinc pyrithione, Outcome 4 Decrease in scaling score (long term).
Analysis 3.5.
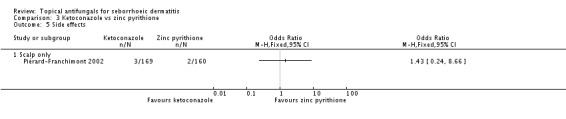
Comparison 3 Ketoconazole vs zinc pyrithione, Outcome 5 Side effects.
Analysis 4.1.
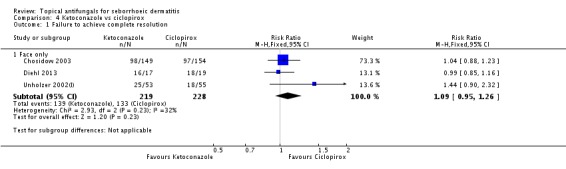
Comparison 4 Ketoconazole vs ciclopirox, Outcome 1 Failure to achieve complete resolution.
Analysis 4.2.
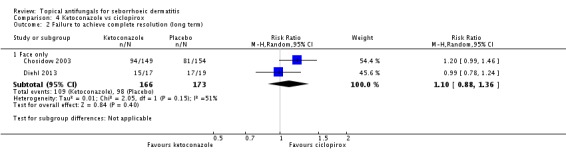
Comparison 4 Ketoconazole vs ciclopirox, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 4.3.
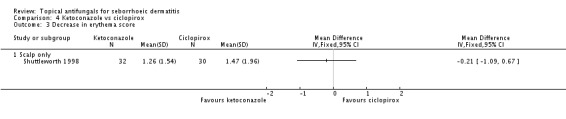
Comparison 4 Ketoconazole vs ciclopirox, Outcome 3 Decrease in erythema score.
Analysis 4.4.
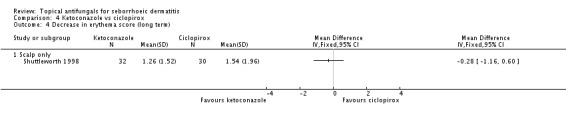
Comparison 4 Ketoconazole vs ciclopirox, Outcome 4 Decrease in erythema score (long term).
Analysis 4.5.
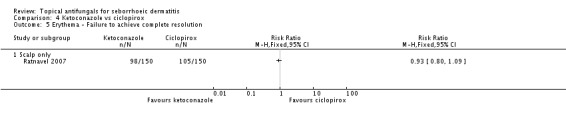
Comparison 4 Ketoconazole vs ciclopirox, Outcome 5 Erythema - Failure to achieve complete resolution.
Analysis 4.6.
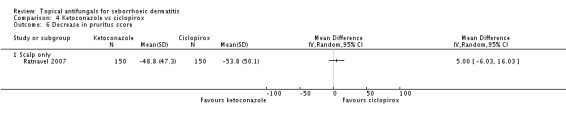
Comparison 4 Ketoconazole vs ciclopirox, Outcome 6 Decrease in pruritus score.
Analysis 4.7.
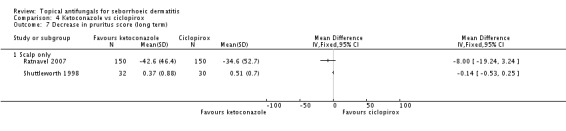
Comparison 4 Ketoconazole vs ciclopirox, Outcome 7 Decrease in pruritus score (long term).
Analysis 4.8.
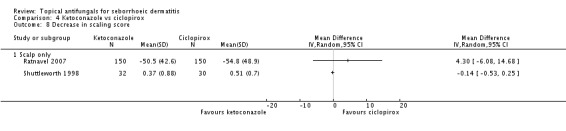
Comparison 4 Ketoconazole vs ciclopirox, Outcome 8 Decrease in scaling score.
Analysis 4.9.
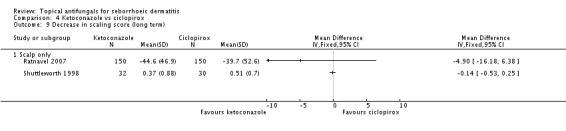
Comparison 4 Ketoconazole vs ciclopirox, Outcome 9 Decrease in scaling score (long term).
Analysis 4.10.
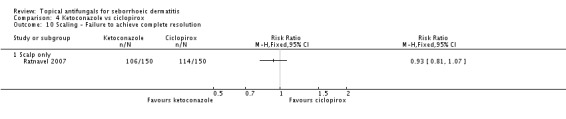
Comparison 4 Ketoconazole vs ciclopirox, Outcome 10 Scaling - Failure to achieve complete resolution.
Analysis 4.11.
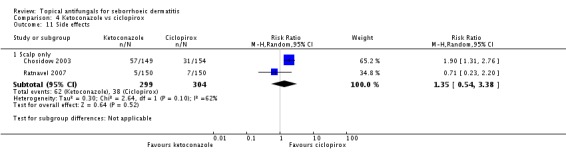
Comparison 4 Ketoconazole vs ciclopirox, Outcome 11 Side effects.
Analysis 5.1.
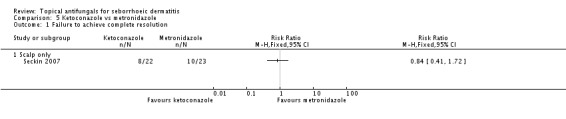
Comparison 5 Ketoconazole vs metronidazole, Outcome 1 Failure to achieve complete resolution.
Analysis 5.2.
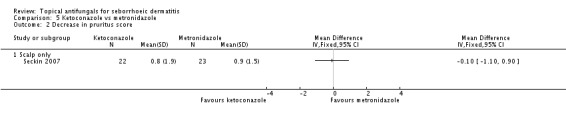
Comparison 5 Ketoconazole vs metronidazole, Outcome 2 Decrease in pruritus score.
Analysis 5.3.
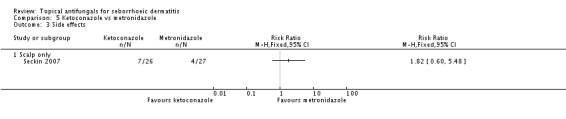
Comparison 5 Ketoconazole vs metronidazole, Outcome 3 Side effects.
Analysis 6.1.
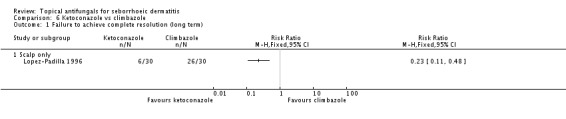
Comparison 6 Ketoconazole vs climbazole, Outcome 1 Failure to achieve complete resolution (long term).
Analysis 6.2.
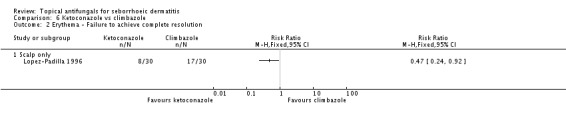
Comparison 6 Ketoconazole vs climbazole, Outcome 2 Erythema - Failure to achieve complete resolution.
Analysis 6.3.
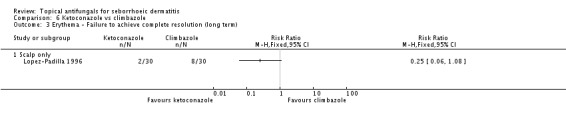
Comparison 6 Ketoconazole vs climbazole, Outcome 3 Erythema - Failure to achieve complete resolution (long term).
Analysis 6.4.
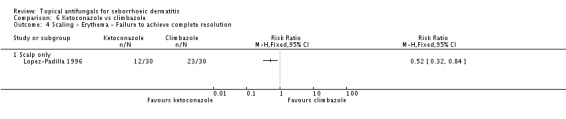
Comparison 6 Ketoconazole vs climbazole, Outcome 4 Scaling - Erythema - Failure to achieve complete resolution.
Analysis 6.5.
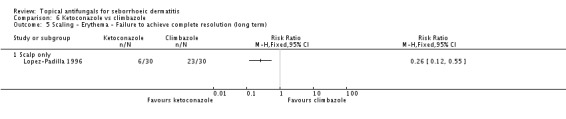
Comparison 6 Ketoconazole vs climbazole, Outcome 5 Scaling - Erythema - Failure to achieve complete resolution (long term).
Analysis 7.1.
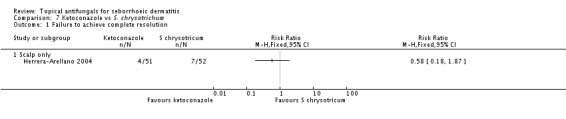
Comparison 7 Ketoconazole vs S. chrysotrichum, Outcome 1 Failure to achieve complete resolution.
Analysis 8.1.
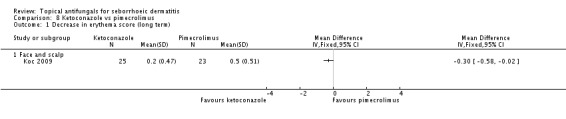
Comparison 8 Ketoconazole vs pimecrolimus, Outcome 1 Decrease in erythema score (long term).
Analysis 8.2.
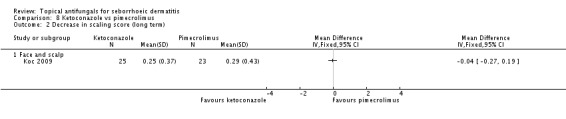
Comparison 8 Ketoconazole vs pimecrolimus, Outcome 2 Decrease in scaling score (long term).
Analysis 8.3.
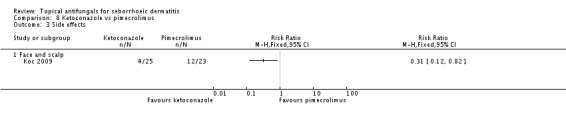
Comparison 8 Ketoconazole vs pimecrolimus, Outcome 3 Side effects.
Analysis 9.1.
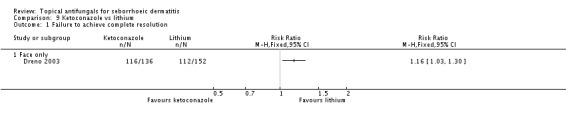
Comparison 9 Ketoconazole vs lithium, Outcome 1 Failure to achieve complete resolution.
Analysis 9.2.
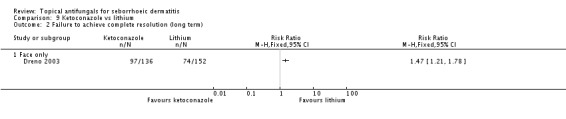
Comparison 9 Ketoconazole vs lithium, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 9.3.
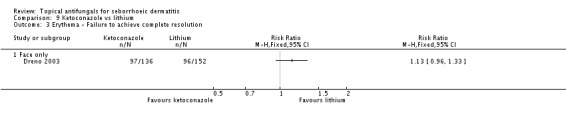
Comparison 9 Ketoconazole vs lithium, Outcome 3 Erythema - Failure to achieve complete resolution.
Analysis 9.4.
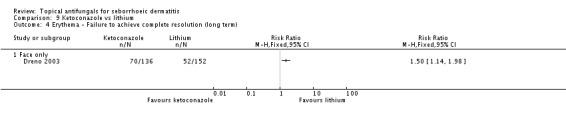
Comparison 9 Ketoconazole vs lithium, Outcome 4 Erythema - Failure to achieve complete resolution (long term).
Analysis 9.5.
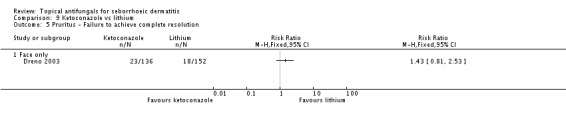
Comparison 9 Ketoconazole vs lithium, Outcome 5 Pruritus - Failure to achieve complete resolution.
Analysis 9.6.
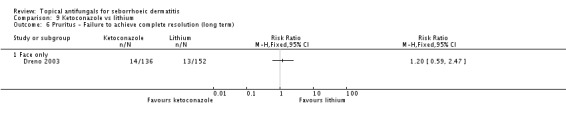
Comparison 9 Ketoconazole vs lithium, Outcome 6 Pruritus - Failure to achieve complete resolution (long term).
Analysis 9.7.
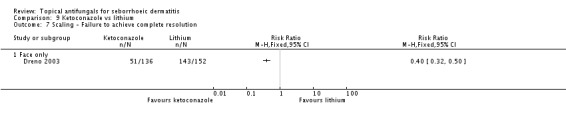
Comparison 9 Ketoconazole vs lithium, Outcome 7 Scaling - Failure to achieve complete resolution.
Analysis 9.8.
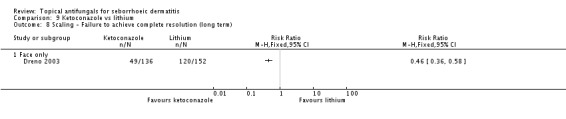
Comparison 9 Ketoconazole vs lithium, Outcome 8 Scaling - Failure to achieve complete resolution (long term).
Analysis 9.9.
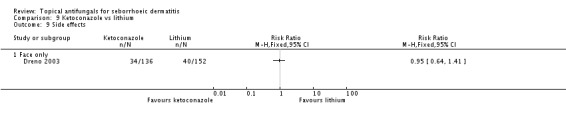
Comparison 9 Ketoconazole vs lithium, Outcome 9 Side effects.
Analysis 10.1.
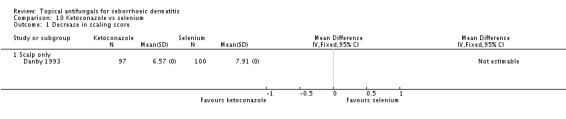
Comparison 10 Ketoconazole vs selenium, Outcome 1 Decrease in scaling score.
Analysis 11.1.
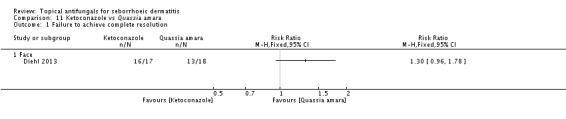
Comparison 11 Ketoconazole vs Quassia amara, Outcome 1 Failure to achieve complete resolution.
Analysis 11.2.
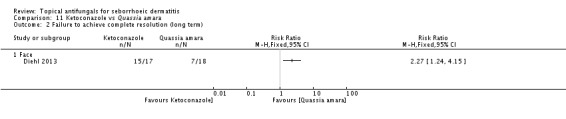
Comparison 11 Ketoconazole vs Quassia amara, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 12.1.
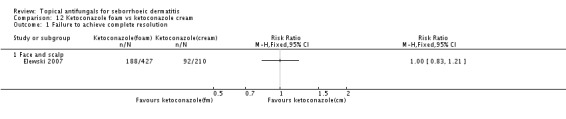
Analysis 12.1. Comparison 12 Ketoconazole foam vs ketoconazole cream, Outcome 1 Failure to achieve complete resolution.
Analysis 12.2.
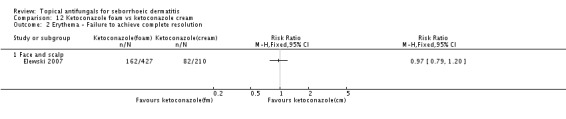
Comparison 12 Ketoconazole foam vs ketoconazole cream, Outcome 2 Erythema - Failure to achieve complete resolution.
Analysis 12.3.
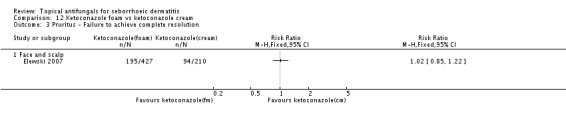
Comparison 12 Ketoconazole foam vs ketoconazole cream, Outcome 3 Pruritus - Failure to achieve complete resolution.
Analysis 12.4.
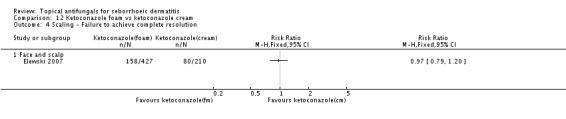
Comparison 12 Ketoconazole foam vs ketoconazole cream, Outcome 4 Scaling - Failure to achieve complete resolution.
Analysis 13.1.
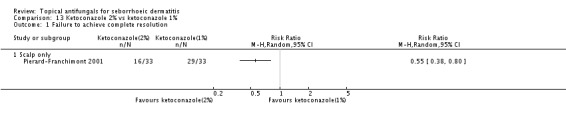
Comparison 13 Ketoconazole 2% vs ketoconazole 1%, Outcome 1 Failure to achieve complete resolution.
Analysis 13.2.
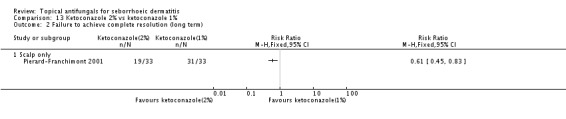
Comparison 13 Ketoconazole 2% vs ketoconazole 1%, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 14.1.
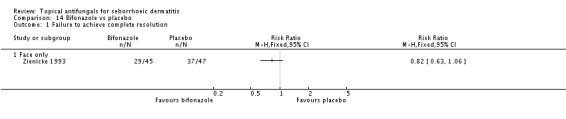
Comparison 14 Bifonazole vs placebo, Outcome 1 Failure to achieve complete resolution.
Analysis 14.2.
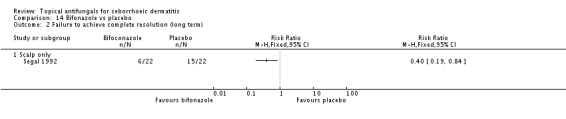
Comparison 14 Bifonazole vs placebo, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 14.3.
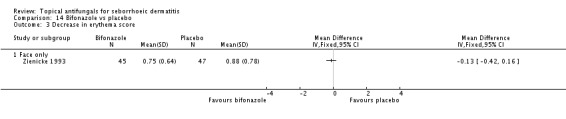
Comparison 14 Bifonazole vs placebo, Outcome 3 Decrease in erythema score.
Analysis 14.4.
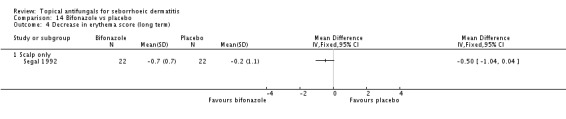
Comparison 14 Bifonazole vs placebo, Outcome 4 Decrease in erythema score (long term).
Analysis 14.5.
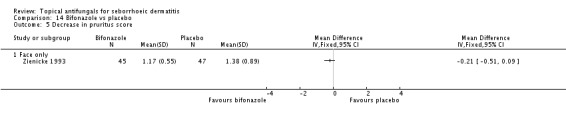
Comparison 14 Bifonazole vs placebo, Outcome 5 Decrease in pruritus score.
Analysis 14.6.
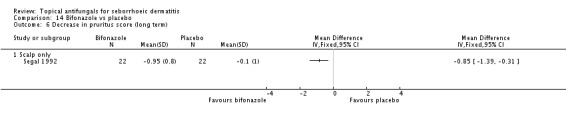
Comparison 14 Bifonazole vs placebo, Outcome 6 Decrease in pruritus score (long term).
Analysis 14.7.
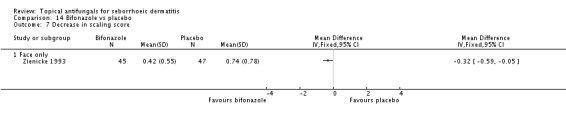
Comparison 14 Bifonazole vs placebo, Outcome 7 Decrease in scaling score.
Analysis 14.8.
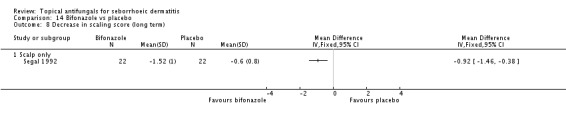
Comparison 14 Bifonazole vs placebo, Outcome 8 Decrease in scaling score (long term).
Analysis 14.9.
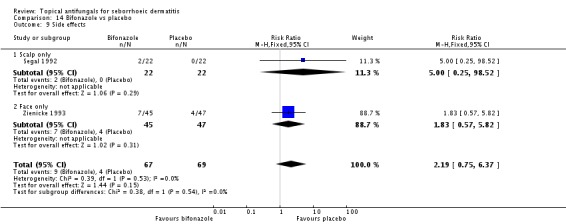
Comparison 14 Bifonazole vs placebo, Outcome 9 Side effects.
Analysis 15.1.
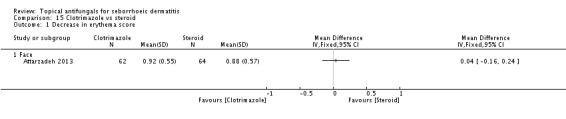
Comparison 15 Clotrimazole vs steroid, Outcome 1 Decrease in erythema score.
Analysis 15.2.
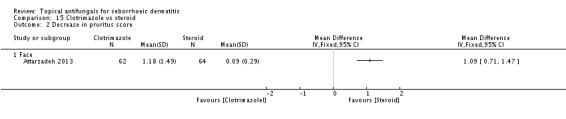
Comparison 15 Clotrimazole vs steroid, Outcome 2 Decrease in pruritus score.
Analysis 15.3.
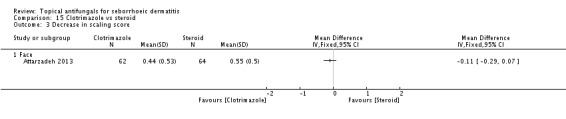
Comparison 15 Clotrimazole vs steroid, Outcome 3 Decrease in scaling score.
Analysis 16.1.
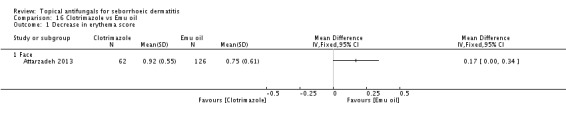
Comparison 16 Clotrimazole vs Emu oil, Outcome 1 Decrease in erythema score.
Analysis 16.2.
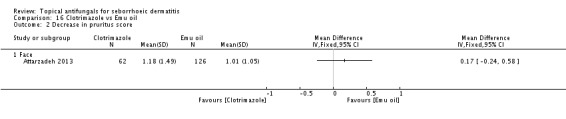
Comparison 16 Clotrimazole vs Emu oil, Outcome 2 Decrease in pruritus score.
Analysis 16.3.
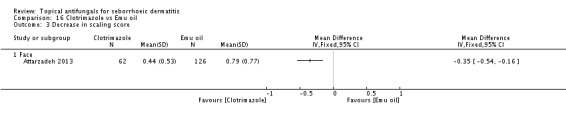
Comparison 16 Clotrimazole vs Emu oil, Outcome 3 Decrease in scaling score.
Analysis 17.1.
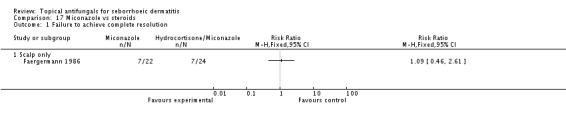
Comparison 17 Miconazole vs steroids, Outcome 1 Failure to achieve complete resolution.
Analysis 17.2.
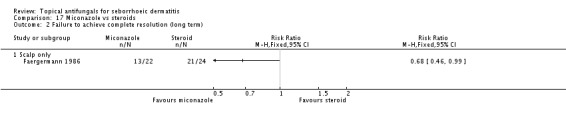
Comparison 17 Miconazole vs steroids, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 18.1.
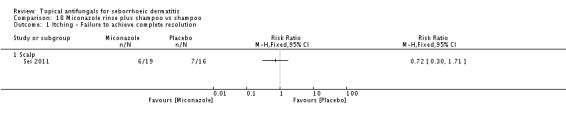
Comparison 18 Miconazole rinse plus shampoo vs shampoo, Outcome 1 Itching - Failure to achieve complete resolution.
Analysis 18.2.
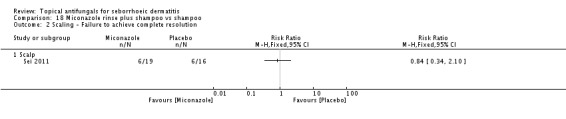
Comparison 18 Miconazole rinse plus shampoo vs shampoo, Outcome 2 Scaling - Failure to achieve complete resolution.
Analysis 19.1.
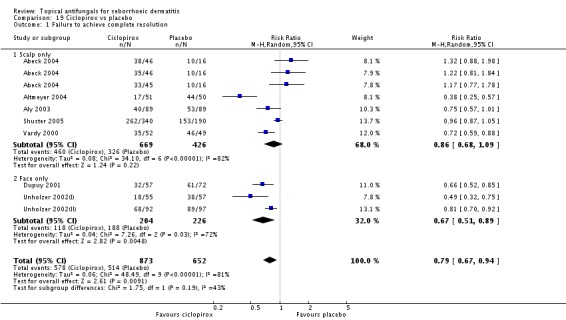
Comparison 19 Ciclopirox vs placebo, Outcome 1 Failure to achieve complete resolution.
Analysis 19.2.
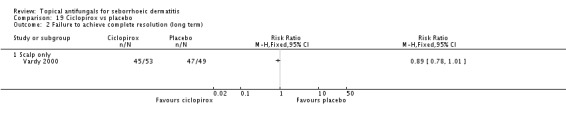
Comparison 19 Ciclopirox vs placebo, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 19.3.
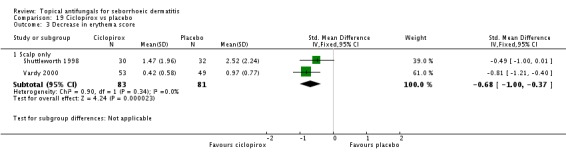
Comparison 19 Ciclopirox vs placebo, Outcome 3 Decrease in erythema score.
Analysis 19.4.
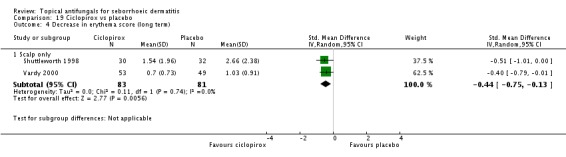
Comparison 19 Ciclopirox vs placebo, Outcome 4 Decrease in erythema score (long term).
Analysis 19.5.
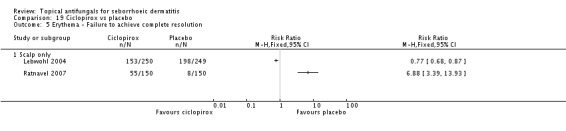
Comparison 19 Ciclopirox vs placebo, Outcome 5 Erythema - Failure to achieve complete resolution.
Analysis 19.6.
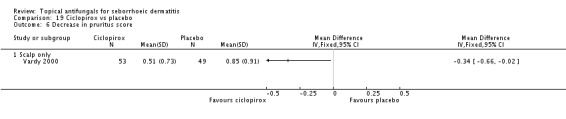
Comparison 19 Ciclopirox vs placebo, Outcome 6 Decrease in pruritus score.
Analysis 19.7.
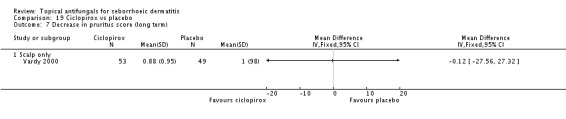
Comparison 19 Ciclopirox vs placebo, Outcome 7 Decrease in pruritus score (long term).
Analysis 19.8.
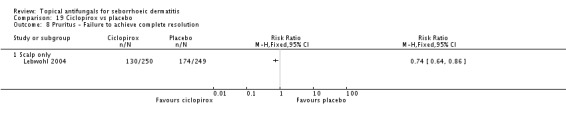
Comparison 19 Ciclopirox vs placebo, Outcome 8 Pruritus - Failure to achieve complete resolution.
Analysis 19.9.
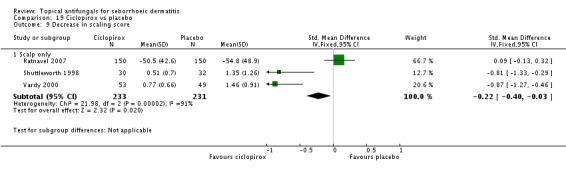
Comparison 19 Ciclopirox vs placebo, Outcome 9 Decrease in scaling score.
Analysis 19.10.
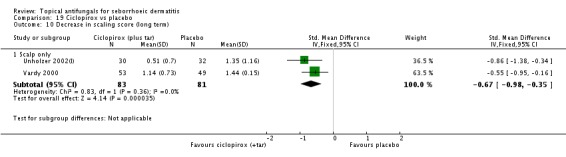
Comparison 19 Ciclopirox vs placebo, Outcome 10 Decrease in scaling score (long term).
Analysis 19.11.
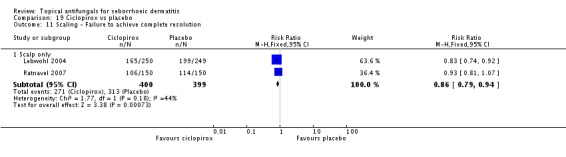
Comparison 19 Ciclopirox vs placebo, Outcome 11 Scaling - Failure to achieve complete resolution.
Analysis 19.12.
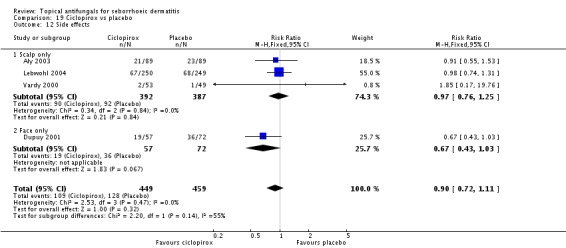
Comparison 19 Ciclopirox vs placebo, Outcome 12 Side effects.
Analysis 20.1.
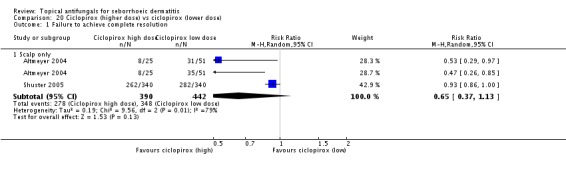
Comparison 20 Ciclopirox (higher dose) vs ciclopirox (lower dose), Outcome 1 Failure to achieve complete resolution.
Analysis 21.1.
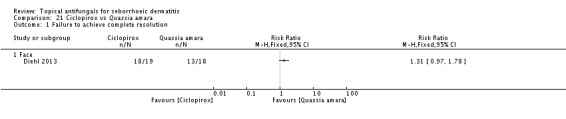
Comparison 21 Ciclopirox vs Quassia amara, Outcome 1 Failure to achieve complete resolution.
Analysis 21.2.
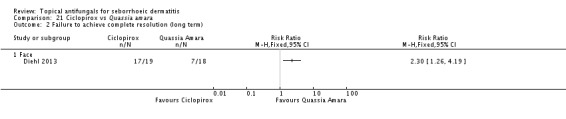
Comparison 21 Ciclopirox vs Quassia amara, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 22.1.
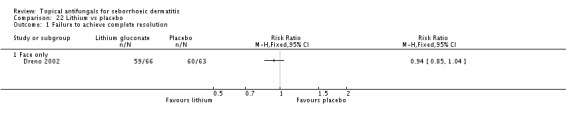
Comparison 22 Lithium vs placebo, Outcome 1 Failure to achieve complete resolution.
Analysis 22.2.
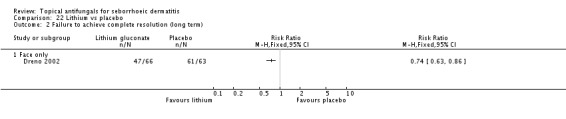
Comparison 22 Lithium vs placebo, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 22.3.
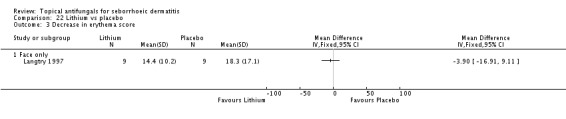
Comparison 22 Lithium vs placebo, Outcome 3 Decrease in erythema score.
Analysis 22.4.
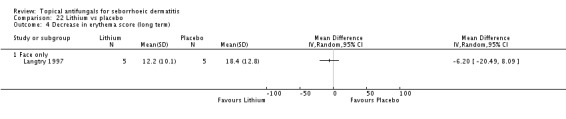
Comparison 22 Lithium vs placebo, Outcome 4 Decrease in erythema score (long term).
Analysis 22.5.
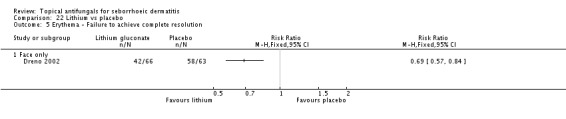
Comparison 22 Lithium vs placebo, Outcome 5 Erythema - Failure to achieve complete resolution.
Analysis 22.6.
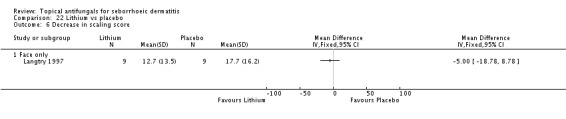
Comparison 22 Lithium vs placebo, Outcome 6 Decrease in scaling score.
Analysis 22.7.
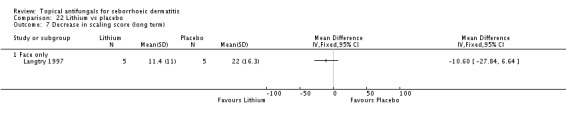
Comparison 22 Lithium vs placebo, Outcome 7 Decrease in scaling score (long term).
Analysis 22.8.
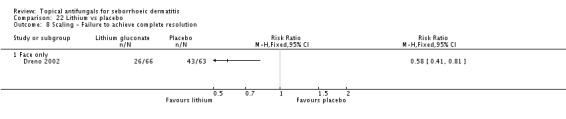
Comparison 22 Lithium vs placebo, Outcome 8 Scaling - Failure to achieve complete resolution.
Analysis 22.9.
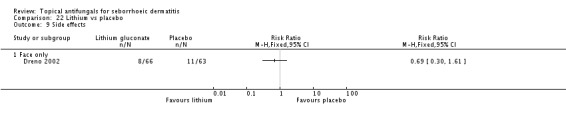
Comparison 22 Lithium vs placebo, Outcome 9 Side effects.
Analysis 23.1.
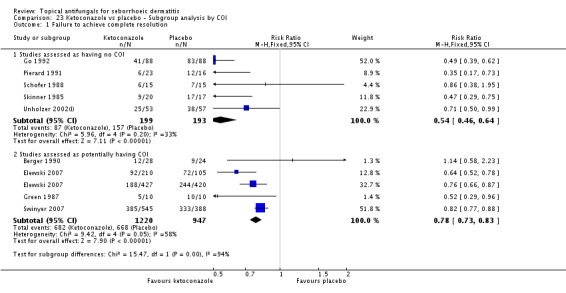
Comparison 23 Ketoconazole vs placebo - Subgroup analysis by COI, Outcome 1 Failure to achieve complete resolution.
Analysis 23.2.
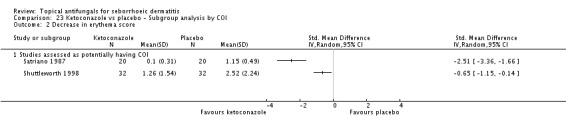
Comparison 23 Ketoconazole vs placebo - Subgroup analysis by COI, Outcome 2 Decrease in erythema score.
Analysis 23.3.
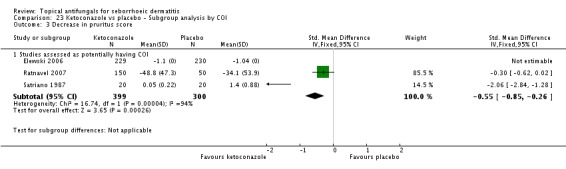
Comparison 23 Ketoconazole vs placebo - Subgroup analysis by COI, Outcome 3 Decrease in pruritus score.
Analysis 23.4.
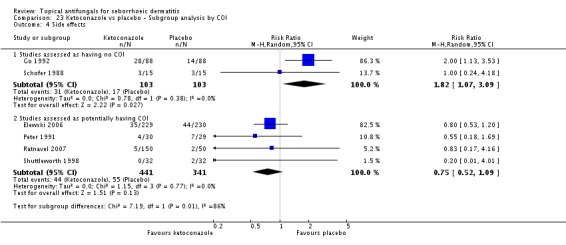
Comparison 23 Ketoconazole vs placebo - Subgroup analysis by COI, Outcome 4 Side effects.
Analysis 24.1.
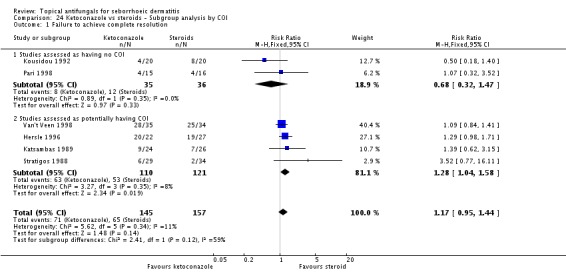
Comparison 24 Ketoconazole vs steroids - Subgroup analysis by COI, Outcome 1 Failure to achieve complete resolution.
Analysis 24.2.
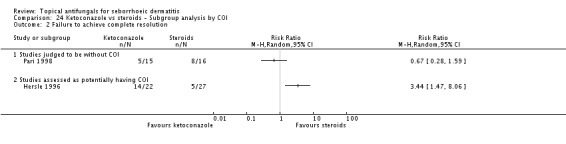
Comparison 24 Ketoconazole vs steroids - Subgroup analysis by COI, Outcome 2 Failure to achieve complete resolution.
Analysis 24.3.
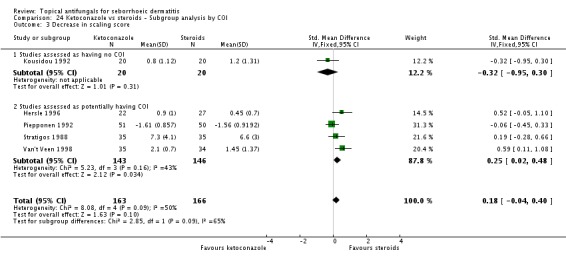
Comparison 24 Ketoconazole vs steroids - Subgroup analysis by COI, Outcome 3 Decrease in scaling score.
Analysis 25.1.
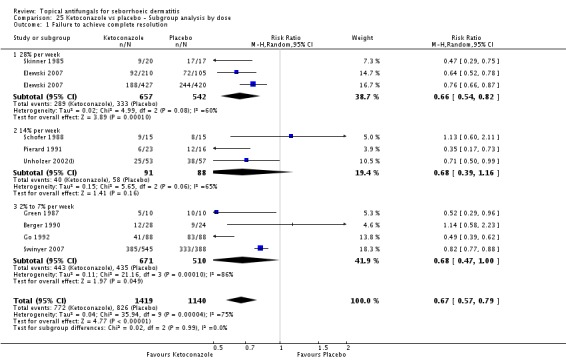
Comparison 25 Ketoconazole vs placebo - Subgroup analysis by dose, Outcome 1 Failure to achieve complete resolution.
Analysis 25.2.
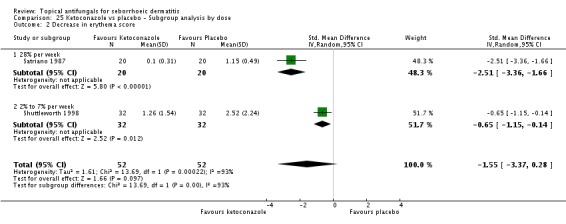
Comparison 25 Ketoconazole vs placebo - Subgroup analysis by dose, Outcome 2 Decrease in erythema score.
Analysis 25.3.
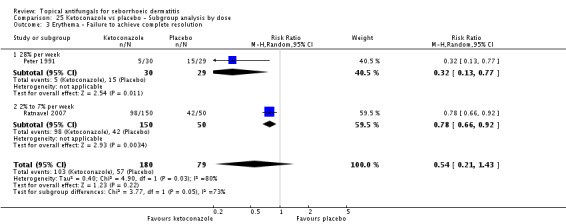
Comparison 25 Ketoconazole vs placebo - Subgroup analysis by dose, Outcome 3 Erythema - Failure to achieve complete resolution.
Analysis 25.4.
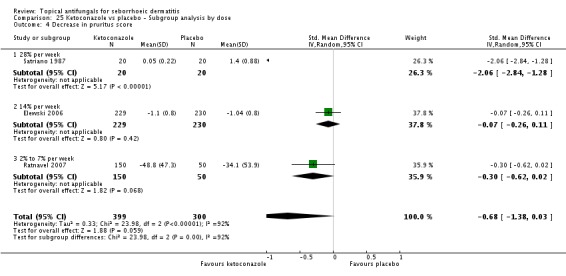
Comparison 25 Ketoconazole vs placebo - Subgroup analysis by dose, Outcome 4 Decrease in pruritus score.
Analysis 25.5.
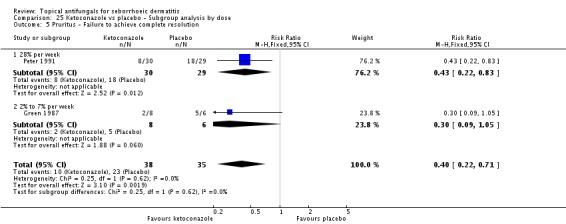
Comparison 25 Ketoconazole vs placebo - Subgroup analysis by dose, Outcome 5 Pruritus - Failure to achieve complete resolution.
Analysis 25.6.
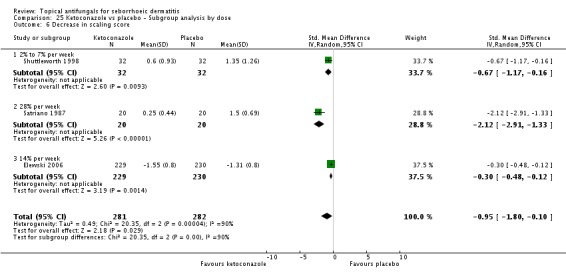
Comparison 25 Ketoconazole vs placebo - Subgroup analysis by dose, Outcome 6 Decrease in scaling score.
Analysis 25.7.
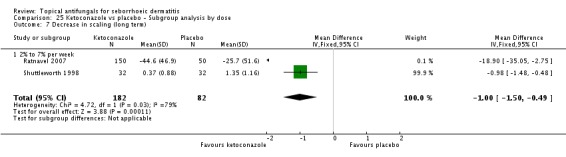
Comparison 25 Ketoconazole vs placebo - Subgroup analysis by dose, Outcome 7 Decrease in scaling (long term).
Analysis 25.8.
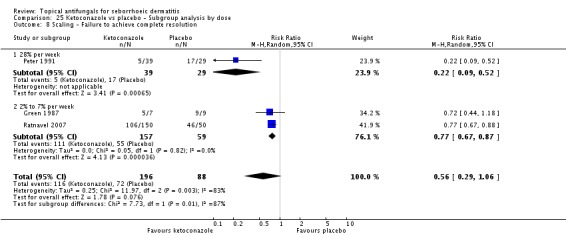
Comparison 25 Ketoconazole vs placebo - Subgroup analysis by dose, Outcome 8 Scaling - Failure to achieve complete resolution.
Analysis 25.9.
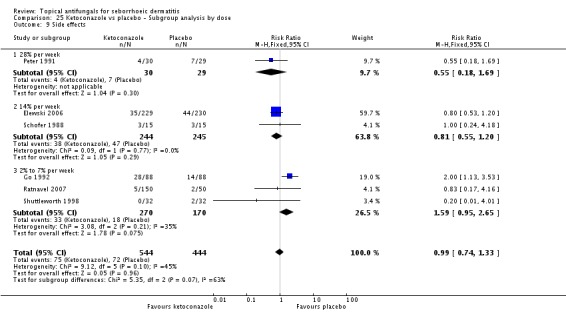
Comparison 25 Ketoconazole vs placebo - Subgroup analysis by dose, Outcome 9 Side effects.
Analysis 26.1.
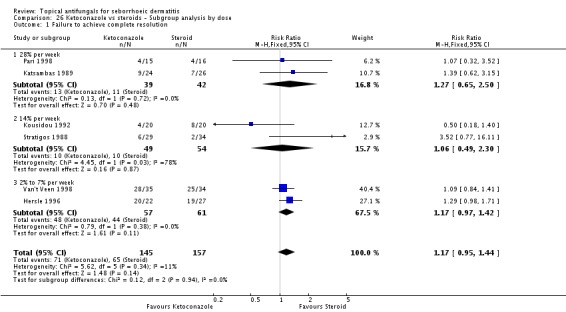
Comparison 26 Ketoconazole vs steroids - Subgroup analysis by dose, Outcome 1 Failure to achieve complete resolution.
Analysis 26.2.
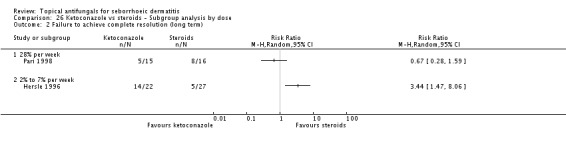
Comparison 26 Ketoconazole vs steroids - Subgroup analysis by dose, Outcome 2 Failure to achieve complete resolution (long term).
Analysis 26.3.
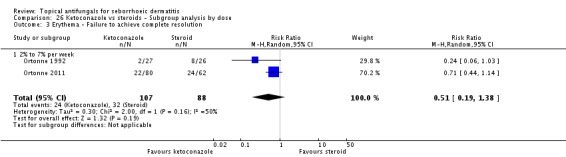
Comparison 26 Ketoconazole vs steroids - Subgroup analysis by dose, Outcome 3 Erythema - Failure to achieve complete resolution.
Analysis 26.4.
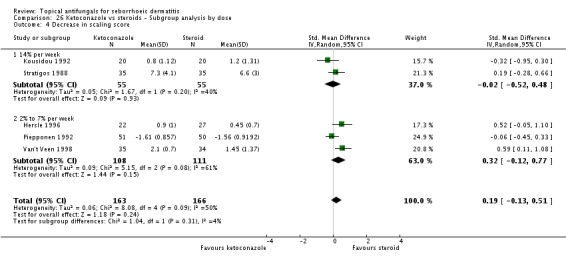
Comparison 26 Ketoconazole vs steroids - Subgroup analysis by dose, Outcome 4 Decrease in scaling score.
Analysis 27.1.
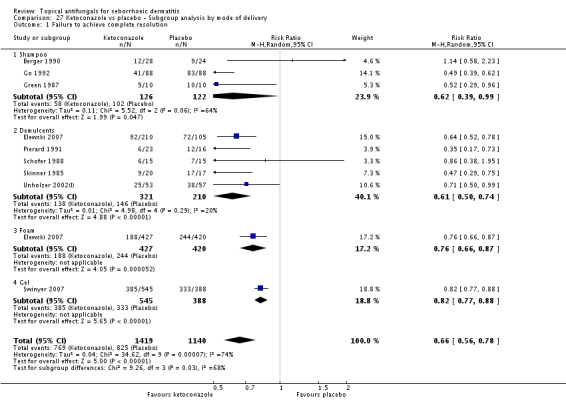
Comparison 27 Ketoconazole vs placebo - Subgroup analysis by mode of delivery, Outcome 1 Failure to achieve complete resolution.
Analysis 27.2.
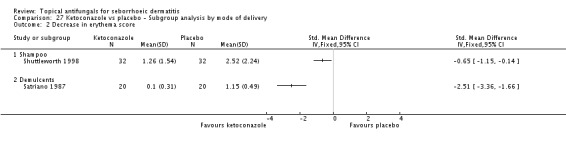
Comparison 27 Ketoconazole vs placebo - Subgroup analysis by mode of delivery, Outcome 2 Decrease in erythema score.
Analysis 27.3.
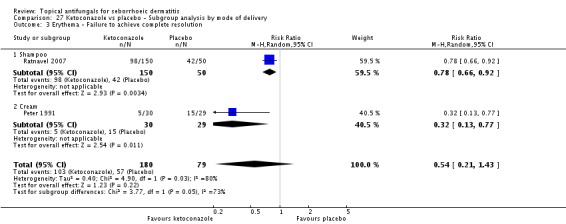
Comparison 27 Ketoconazole vs placebo - Subgroup analysis by mode of delivery, Outcome 3 Erythema - Failure to achieve complete resolution.
Analysis 27.4.
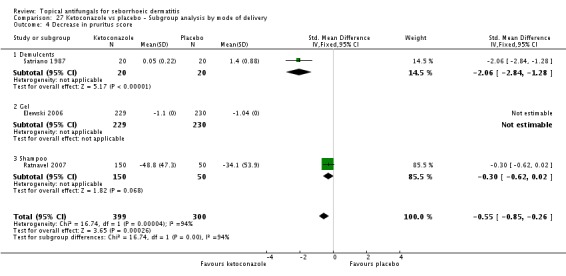
Comparison 27 Ketoconazole vs placebo - Subgroup analysis by mode of delivery, Outcome 4 Decrease in pruritus score.
Analysis 27.5.
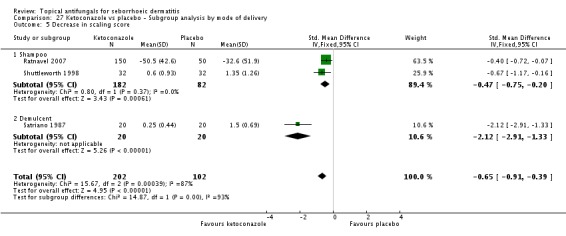
Comparison 27 Ketoconazole vs placebo - Subgroup analysis by mode of delivery, Outcome 5 Decrease in scaling score.
Analysis 27.6.
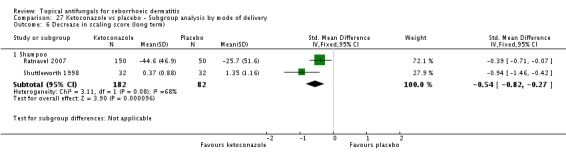
Comparison 27 Ketoconazole vs placebo - Subgroup analysis by mode of delivery, Outcome 6 Decrease in scaling score (long term).
Analysis 27.7.
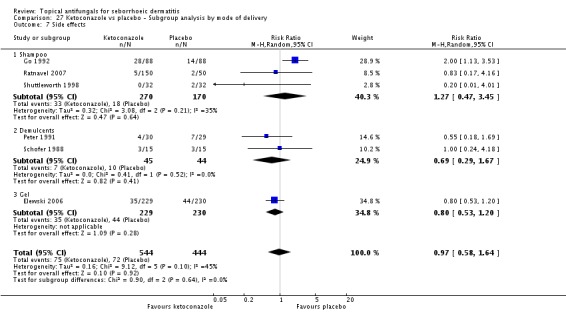
Comparison 27 Ketoconazole vs placebo - Subgroup analysis by mode of delivery, Outcome 7 Side effects.
Analysis 28.1.
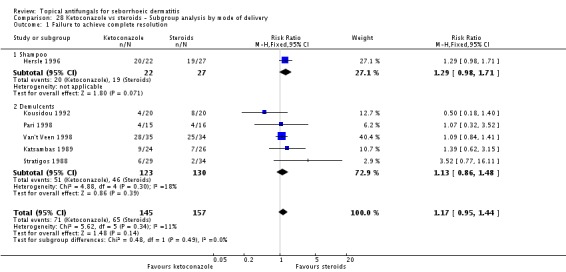
Comparison 28 Ketoconazole vs steroids - Subgroup analysis by mode of delivery, Outcome 1 Failure to achieve complete resolution.
Analysis 28.2.
Comparison 28 Ketoconazole vs steroids - Subgroup analysis by mode of delivery, Outcome 2 Decrease in scaling score.
Summary of Findings for the main Comparison[Explanation]
| Ketoconazole compared with placebo for seborrhoeic dermatitis | ||||||
| Patient or population: patients with seborrhoeic dermatitis Settings: Intervention: ketoconazole Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect(95% CI) | Number of participants(studies) | Quality of the evidence(GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ketoconazole | |||||
| Failure to achieve complete resolution Clinical assessmentFollow-up: mean 4 weeks | Study population | RR 0.69 (0.59 to 0.81) | 2520(8 studies) | ⊕○○○ Low a,b | ||
| 724 per 1000 | 500 per 1000 (427 to 587) | |||||
| Moderate | ||||||
| 686 per 1000 | 473 per 1000 (405 to 556) | |||||
| Side effects Self reportFollow-up: mean 4 weeks | Study population | RR 0.97 (0.58 to 1.64) | 988(6 studies) | ⊕○○○ Very low a,c,d | ||
| 162 per 1000 | 157 per 1000 (94 to 266) | |||||
| Moderate | ||||||
| 175 per 1000 | 170 per 1000 (101 to 287) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Downgraded 1 level because most studies were at high or unclear risk of bias.
Downgraded 1 level because of high heterogeneity (I² = 74%).
Downgraded 1 level because of high heterogeneity: One study reported twice as many side effects for ketoconazole as the other studies, which report no or decreased risk.
Downgraded 1 level because of wide confidence intervals including greater side effect risk for both control and intervention groups.
| Ketoconazole compared with steroids for seborrhoeic dermatitis | ||||||
| Patient or population: patients with seborrhoeic dermatitis Intervention: ketoconazole Comparison: steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect(95% CI) | Number of participants(studies) | Quality of the evidence(GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Steroids | Ketoconazole | |||||
| Failure to achieve complete resolution (combined for face and scalp) Clinical assessmentFollow-up: mean 4 weeks | Study population | RR 1.17 (0.95 to 1.44) | 302(6 studies) | ⊕⊕○○ Low a,b | ||
| 414 per 1000 | 484 per 1000 (393 to 596) | |||||
| Moderate | ||||||
| 335 per 1000 | 392 per 1000 (318 to 482) | |||||
| Failure to achieve complete resolution (long term, combined for face and scalp) Clinical assessmentFollow-up: mean 8 weeks | See comment | See comment | Not estimable | 80(2 studies) | ⊕○○○ Very low a,c,d | Studies could not be combined because of high heterogeneity |
| Side effects (combined for face and scalp) Self reportFollow-up: mean 4 weeks | Study population | RR 0.56 (0.32 to 0.96) | 596(8 studies) | ⊕⊕⊕○ Moderate a | ||
| 95 per 1000 | 53 per 1000 (31 to 92) | |||||
| Moderate | ||||||
| 48 per 1000 | 27 per 1000 (15 to 46) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Downgraded 1 level because most studies were at high or unclear risk of bias.
Downgraded 1 level because of lack of precision: N = 302; confidence Interval overlaps with both 1 and 1.25.
Downgraded 1 level because of heterogeneity: Included studies had opposite results.
Downgraded 1 level because of lack of precision: only 79 participants.
| Ketoconazole compared with ciclopirox for seborrhoeic dermatitis | ||||||
| Patient or population: patients with seborrhoeic dermatitis Intervention: ketoconazole Comparison: ciclopirox | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect(95% CI) | Number of participants(studies) | Quality of the evidence(GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ciclopirox | Ketoconazole | |||||
| Failure to achieve complete resolution - Face only Clinical assessmentFollow-up: mean 4 weeks | Study population | RR 1.09 (0.95 to 1.26) | 447(3 studies) | ⊕⊕○○ Low a,b | ||
| 583 per 1000 | 636 per 1000 (554 to 735) | |||||
| Moderate | ||||||
| 630 per 1000 | 687 per 1000 (598 to 794) | |||||
| Failure to achieve complete resolution (long term) - Face only Clinical assessmentFollow-up: mean 4 weeks | Study population | RR 1.16 (0.98 to 1.38) | 339(2 studies) | ⊕⊕○○ Low a,c | ||
| 566 per 1000 | 657 per 1000 (555 to 782) | |||||
| Moderate | ||||||
| 710 per 1000 | 824 per 1000 (696 to 980) | |||||
| Side effects - Scalp only Self reportedFollow-up: mean 4 weeks | Study population | RR 1.35 (0.54 to 3.38) | 603(2 studies) | ⊕○○○ Very low a,d,e | ||
| 125 per 1000 | 169 per 1000 (68 to 423) | |||||
| Moderate | ||||||
| 124 per 1000 | 167 per 1000 (67 to 419) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Downgraded 1 level because most studies had high or unclear risk of bias.
Downgraded 1 level because of lack of precision: 272 participants. CI overlaps with RR = 1 and RR = 1.25.
Downgraded 1 level because of lack of precision: RR overlaps with 1 and with 1.25. N = 339 participants.
Downgraded 1 level because of heterogeneity: I² = 62%; 1 study shows no difference and 1 study favours ciclopirox.
Downgraded 1 level because of lack of precision: wide confidence interval overlapping with 1 and 1.25 and 0.75.
| Ciclopirox compared with placebo for seborrhoeic dermatitis | ||||||
| Patient or population: patients with seborrhoeic dermatitis Intervention: ciclopirox Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect(95% CI) | Number of participants(studies) | Quality of the evidence(GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ciclopirox | |||||
| Failure to achieve complete resolution (combined for face and scalp) Clinical assessmentFollow-up: mean 4 weeks | Study population | RR 0.79 (0.67 to 0.94) | 1525(8 studies) | ⊕⊕⊕○ Moderate a | ||
| 788 per 1000 | 623 per 1000 (528 to 741) | |||||
| Moderate | ||||||
| 736 per 1000 | 581 per 1000 (493 to 692) | |||||
| Side effects (combined for face and scalp) Self reportedFollow-up: mean 4 weeks | Study population | RR 0.9 (0.72 to 1.11) | 908(4 studies) | ⊕⊕⊕○ Moderate b | ||
| 279 per 1000 | 251 per 1000 (201 to 310) | |||||
| Moderate | ||||||
| 266 per 1000 | 239 per 1000 (192 to 295) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Downgraded 1 level because of high heterogeneity (I² = 75%).
Downgraded 1 level because most studies were at risk of bias.
Data Analyses
Comparison 1.Ketoconazole vs placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 8 | 2520 | Risk Ratio (M-H, Random, 95% CI) | 0.69 [0.59, 0.81] |
| 1.1 Scalp only | 2 | 228 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.31, 1.61] |
| 1.2 Face and scalp | 3 | 2132 | Risk Ratio (M-H, Random, 95% CI) | 0.72 [0.61, 0.84] |
| 1.3 Face only | 3 | 160 | Risk Ratio (M-H, Random, 95% CI) | 0.73 [0.51, 1.05] |
| 2 Decrease in erythema score | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Scalp only | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Face and scalp | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Decrease in erythema score (long term) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Scalp only | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Erythema - Failure to achieve complete resolution | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Decrease in pruritus score | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Scalp only | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Face and scalp | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Decrease in pruritus (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Scalp only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Pruritus - Failure to achieve complete resolution | 2 | 75 | Risk Ratio (M-H, Fixed, 95% CI) | 0.38 [0.21, 0.69] |
| 7.1 Scalp | 1 | 16 | Risk Ratio (M-H, Fixed, 95% CI) | 0.26 [0.07, 0.91] |
| 7.2 Face only | 1 | 59 | Risk Ratio (M-H, Fixed, 95% CI) | 0.43 [0.22, 0.83] |
| 8 Decrease in scaling score | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Scalp only | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Face and scalp | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Decrease in scaling (long term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Scalp only | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Scaling - Failure to achieve complete resolution | 3 | 284 | Risk Ratio (M-H, Random, 95% CI) | 0.56 [0.29, 1.06] |
| 10.1 Scalp only | 2 | 216 | Risk Ratio (M-H, Random, 95% CI) | 0.77 [0.67, 0.87] |
| 10.2 Face only | 1 | 68 | Risk Ratio (M-H, Random, 95% CI) | 0.22 [0.09, 0.52] |
| 11 Side effects | 6 | 988 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.58, 1.64] |
| 11.1 Scalp only | 3 | 440 | Risk Ratio (M-H, Random, 95% CI) | 1.27 [0.47, 3.45] |
| 11.2 Face only | 3 | 548 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.54, 1.13] |
Comparison 2. Ketoconazole vs steroids
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 6 | 302 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.95, 1.44] |
| 1.1 Scalp only | 2 | 118 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.97, 1.42] |
| 1.2 Face and scalp | 2 | 113 | Risk Ratio (M-H, Fixed, 95% CI) | 1.85 [0.90, 3.79] |
| 1.3 Face only | 2 | 71 | Risk Ratio (M-H, Fixed, 95% CI) | 0.68 [0.32, 1.47] |
| 2 Failure to achieve complete resolution (long term) | 2 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 2.1 28% per week | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 2% to 7% per week | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Decrease in erythema score | 3 | 190 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.01 [-0.30, 0.28] |
| 3.1 Face only | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.11 [-0.73, 0.51] |
| 3.2 Scalp only | 2 | 150 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [-0.30, 0.34] |
| 4 Decrease in erythema score (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Erythema - Failure to achieve complete resolution | 2 | 195 | Risk Ratio (M-H, Random, 95% CI) | 0.51 [0.19, 1.38] |
| 5.1 Face and scalp | 1 | 53 | Risk Ratio (M-H, Random, 95% CI) | 0.24 [0.06, 1.03] |
| 5.2 Scalp only | 1 | 142 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.44, 1.14] |
| 6 Decrease in pruritus score | 4 | 259 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [-0.09, 0.40] |
| 6.1 Face only | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.14 [-0.76, 0.48] |
| 6.2 Scalp only | 3 | 219 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [-0.06, 0.47] |
| 7 Decrease in pruritus (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Pruritus - Failure to achieve complete resolution | 2 | 215 | Risk Ratio (M-H, Fixed, 95% CI) | 0.53 [0.34, 0.84] |
| 8.1 Face and scalp | 1 | 53 | Risk Ratio (M-H, Fixed, 95% CI) | 0.28 [0.06, 1.20] |
| 8.2 Scalp only | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 0.59 [0.37, 0.95] |
| 9 Decrease in scaling score | 5 | 329 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [-0.13, 0.51] |
| 9.1 Face only | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | -0.32 [-0.95, 0.30] |
| 9.2 Scalp only | 3 | 219 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [-0.12, 0.77] |
| 9.3 Face and scalp | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [-0.28, 0.66] |
| 10 Decrease in scaling score (long term) | 2 | 112 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.71 [0.31, 1.11] |
| 10.1 Scalp only | 1 | 49 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.96 [1.27, 2.65] |
| 10.2 Face and scalp | 1 | 63 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [-0.42, 0.57] |
| 11 Scaling - Failure to achieve complete resolution | 2 | 215 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.54, 1.12] |
| 11.1 Face and scalp | 1 | 53 | Risk Ratio (M-H, Fixed, 95% CI) | 0.53 [0.21, 1.39] |
| 11.2 Scalp only | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 0.84 [0.57, 1.25] |
| 12 Side effects | 8 | 596 | Risk Ratio (M-H, Fixed, 95% CI) | 0.56 [0.32, 0.96] |
| 12.1 Scalp only | 4 | 381 | Risk Ratio (M-H, Fixed, 95% CI) | 0.81 [0.34, 1.93] |
| 12.2 Face and scalp | 3 | 175 | Risk Ratio (M-H, Fixed, 95% CI) | 0.36 [0.17, 0.78] |
| 12.3 Face only | 1 | 40 | Risk Ratio (M-H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
Comparison 3. Ketoconazole vs zinc pyrithione
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Decrease in scaling score | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Scalp only | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Decrease in scaling score (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Side effects | 1 | Odds Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Scalp only | 1 | Odds Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Ketoconazole vs ciclopirox
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 3 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Face only | 3 | 447 | Risk Ratio (M-H, Fixed, 95% CI) | 1.09 [0.95, 1.26] |
| 2 Failure to achieve complete resolution (long term) | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Face only | 2 | 339 | Risk Ratio (M-H, Random, 95% CI) | 1.10 [0.88, 1.36] |
| 3 Decrease in erythema score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Decrease in erythema score (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Erythema - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Decrease in pruritus score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Scalp only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Decrease in pruritus score (long term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Scalp only | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Decrease in scaling score | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Scalp only | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Decrease in scaling score (long term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Scalp only | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Scaling - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Side effects | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 11.1 Scalp only | 2 | 603 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [0.54, 3.38] |
Comparison 5. Ketoconazole vs metronidazole
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Decrease in pruritus score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Side effects | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Ketoconazole vs climbazole
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Erythema - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Erythema - Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Scaling - Erythema - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Scaling - Erythema - Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Ketoconazole vs S. chrysotrichum
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Ketoconazole vs pimecrolimus
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Decrease in erythema score (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Face and scalp | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Decrease in scaling score (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Face and scalp | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Side effects | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Face and scalp | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Ketoconazole vs lithium
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Erythema - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Erythema - Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pruritus - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pruritus - Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Scaling - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Scaling - Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Side effects | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Ketoconazole vs selenium
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Decrease in scaling score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Ketoconazole vs Quassia amara
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Face | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Face | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Ketoconazole foam vs ketoconazole cream
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Face and scalp | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Erythema - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Face and scalp | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pruritus - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Face and scalp | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Scaling - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Face and scalp | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. Ketoconazole 2% vs ketoconazole 1%
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 1.1 Scalp only | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. Bifonazole vs placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Decrease in erythema score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Face only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Decrease in erythema score (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Decrease in pruritus score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Face only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Decrease in pruritus score (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Decrease in scaling score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Face only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Decrease in scaling score (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Side effects | 2 | 136 | Risk Ratio (M-H, Fixed, 95% CI) | 2.19 [0.75, 6.37] |
| 9.1 Scalp only | 1 | 44 | Risk Ratio (M-H, Fixed, 95% CI) | 5.0 [0.25, 98.52] |
| 9.2 Face only | 1 | 92 | Risk Ratio (M-H, Fixed, 95% CI) | 1.83 [0.57, 5.82] |
Comparison 15. Clotrimazole vs steroid
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Decrease in erythema score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Face | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Decrease in pruritus score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Face | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Decrease in scaling score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Face | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 16. Clotrimazole vs Emu oil
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Decrease in erythema score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Face | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Decrease in pruritus score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Face | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Decrease in scaling score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Face | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 17. Miconazole vs steroids
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 18. Miconazole rinse plus shampoo vs shampoo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Itching - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Scalp | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Scaling - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Scalp | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 19. Ciclopirox vs placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 8 | 1525 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.67, 0.94] |
| 1.1 Scalp only | 5 | 1095 | Risk Ratio (M-H, Random, 95% CI) | 0.86 [0.68, 1.09] |
| 1.2 Face only | 3 | 430 | Risk Ratio (M-H, Random, 95% CI) | 0.67 [0.51, 0.89] |
| 2 Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Decrease in erythema score | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Scalp only | 2 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.68 [1.00, -0.37] |
| 4 Decrease in erythema score (long term) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Scalp only | 2 | 164 | Std. Mean Difference (IV, Random, 95% CI) | -0.44 [-0.75, -0.13] |
| 5 Erythema - Failure to achieve complete resolution | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Scalp only | 2 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Decrease in pruritus score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Decrease in pruritus score (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Scalp only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Pruritus - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Scalp only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Decrease in scaling score | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Scalp only | 3 | 464 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.22 [-0.40, -0.03] |
| 10 Decrease in scaling score (long term) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Scalp only | 2 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.67 [-0.98, -0.35] |
| 11 Scaling - Failure to achieve complete resolution | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Scalp only | 2 | 799 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 12 Side effects | 4 | 908 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.72, 1.11] |
| 12.1 Scalp only | 3 | 779 | Risk Ratio (M-H, Fixed, 95% CI) | 0.97 [0.76, 1.25] |
| 12.2 Face only | 1 | 129 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.43, 1.03] |
Comparison 20. Ciclopirox (higher dose) vs ciclopirox (lower dose)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Scalp only | 2 | 832 | Risk Ratio (M-H, Random, 95% CI) | 0.65 [0.37, 1.13] |
Comparison 21. Ciclopirox vs Quassia amara
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Face | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Face | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 22. Lithium vs placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure to achieve complete resolution (long term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Decrease in erythema score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Face only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Decrease in erythema score (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Face only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Erythema - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Decrease in scaling score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Face only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Decrease in scaling score (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Face only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Scaling - Failure to achieve complete resolution | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Side effects | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Face only | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 23. Ketoconazole vs placebo - Subgroup analysis by COI
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 9 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Studies assessed as having no COI | 5 | 392 | Risk Ratio (M-H, Fixed, 95% CI) | 0.54 [0.46, 0.64] |
| 1.2 Studies assessed as potentially having COI | 4 | 2167 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.73, 0.83] |
| 2 Decrease in erythema score | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Studies assessed as potentially having COI | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Decrease in pruritus score | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Studies assessed as potentially having COI | 3 | 699 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.55 [-0.85, -0.26] |
| 4 Side effects | 6 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Studies assessed as having no COI | 2 | 206 | Risk Ratio (M-H, Random, 95% CI) | 1.82 [1.07, 3.09] |
| 4.2 Studies assessed as potentially having COI | 4 | 782 | Risk Ratio (M-H, Random, 95% CI) | 0.75 [0.52, 1.09] |
Comparison 24. Ketoconazole vs steroids - Subgroup analysis by COI
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 6 | 302 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.95, 1.44] |
| 1.1 Studies assessed as having no COI | 2 | 71 | Risk Ratio (M-H, Fixed, 95% CI) | 0.68 [0.32, 1.47] |
| 1.2 Studies assessed as potentially having COI | 4 | 231 | Risk Ratio (M-H, Fixed, 95% CI) | 1.28 [1.04, 1.58] |
| 2 Failure to achieve complete resolution | 2 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 2.1 Studies judged to be without COI | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Studies assessed as potentially having COI | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Decrease in scaling score | 5 | 329 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [-0.04, 0.40] |
| 3.1 Studies assessed as having no COI | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.32 [-0.95, 0.30] |
| 3.2 Studies assessed as potentially having COI | 4 | 289 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.02, 0.48] |
Comparison 25. Ketoconazole vs placebo - Subgroup analysis by dose
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 9 | 2559 | Risk Ratio (M-H, Random, 95% CI) | 0.67 [0.57, 0.79] |
| 1.1 28% per week | 2 | 1199 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.54, 0.82] |
| 1.2 14% per week | 3 | 179 | Risk Ratio (M-H, Random, 95% CI) | 0.68 [0.39, 1.16] |
| 1.3 2% to 7% per week | 4 | 1181 | Risk Ratio (M-H, Random, 95% CI) | 0.68 [0.47, 1.00] |
| 2 Decrease in erythema score | 2 | 104 | Std. Mean Difference (IV, Random, 95% CI) | -1.55 [-3.37, 0.28] |
| 2.1 28% per week | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | -2.51 [-3.36, -1.66] |
| 2.2 2% to 7% per week | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | -0.65 [-1.15, -0.14] |
| 3 Erythema - Failure to achieve complete resolution | 2 | 259 | Risk Ratio (M-H, Random, 95% CI) | 0.54 [0.21, 1.43] |
| 3.1 28% per week | 1 | 59 | Risk Ratio (M-H, Random, 95% CI) | 0.32 [0.13, 0.77] |
| 3.2 2% to 7% per week | 1 | 200 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.66, 0.92] |
| 4 Decrease in pruritus score | 3 | 699 | Std. Mean Difference (IV, Random, 95% CI) | -0.68 [-1.38, 0.03] |
| 4.1 28% per week | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | -2.06 [-2.84, -1.28] |
| 4.2 14% per week | 1 | 459 | Std. Mean Difference (IV, Random, 95% CI) | -0.07 [-0.26, 0.11] |
| 4.3 2% to 7% per week | 1 | 200 | Std. Mean Difference (IV, Random, 95% CI) | -0.30 [-0.62, 0.02] |
| 5 Pruritus - Failure to achieve complete resolution | 2 | 73 | Risk Ratio (M-H, Fixed, 95% CI) | 0.40 [0.22, 0.71] |
| 5.1 28% per week | 1 | 59 | Risk Ratio (M-H, Fixed, 95% CI) | 0.43 [0.22, 0.83] |
| 5.2 2% to 7% per week | 1 | 14 | Risk Ratio (M-H, Fixed, 95% CI) | 0.3 [0.09, 1.05] |
| 6 Decrease in scaling score | 3 | 563 | Std. Mean Difference (IV, Random, 95% CI) | -0.95 [-1.80, -0.10] |
| 6.1 2% to 7% per week | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | -0.67 [-1.17, -0.16] |
| 6.2 28% per week | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | -2.12 [-2.91, -1.33] |
| 6.3 14% per week | 1 | 459 | Std. Mean Difference (IV, Random, 95% CI) | -0.30 [-0.48, -0.12] |
| 7 Decrease in scaling (long term) | 2 | 264 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [-1.50, -0.49] |
| 7.1 2% to 7% per week | 2 | 264 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [-1.50, -0.49] |
| 8 Scaling - Failure to achieve complete resolution | 3 | 284 | Risk Ratio (M-H, Random, 95% CI) | 0.56 [0.29, 1.06] |
| 8.1 28% per week | 1 | 68 | Risk Ratio (M-H, Random, 95% CI) | 0.22 [0.09, 0.52] |
| 8.2 2% to 7% per week | 2 | 216 | Risk Ratio (M-H, Random, 95% CI) | 0.77 [0.67, 0.87] |
| 9 Side effects | 6 | 988 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.74, 1.33] |
| 9.1 28% per week | 1 | 59 | Risk Ratio (M-H, Fixed, 95% CI) | 0.55 [0.18, 1.69] |
| 9.2 14% per week | 2 | 489 | Risk Ratio (M-H, Fixed, 95% CI) | 0.81 [0.55, 1.20] |
| 9.3 2% to 7% per week | 3 | 440 | Risk Ratio (M-H, Fixed, 95% CI) | 1.59 [0.95, 2.65] |
Comparison 26. Ketoconazole vs steroids - Subgroup analysis by dose
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 6 | 302 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.95, 1.44] |
| 1.1 28% per week | 2 | 81 | Risk Ratio (M-H, Fixed, 95% CI) | 1.27 [0.65, 2.50] |
| 1.2 14% per week | 2 | 103 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.49, 2.30] |
| 1.3 2% to 7% per week | 2 | 118 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.97, 1.42] |
| 2 Failure to achieve complete resolution (long term) | 2 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 2.1 28% per week | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 2% to 7% per week | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Erythema - Failure to achieve complete resolution | 2 | 195 | Risk Ratio (M-H, Random, 95% CI) | 0.51 [0.19, 1.38] |
| 3.1 2% to 7% per week | 2 | 195 | Risk Ratio (M-H, Random, 95% CI) | 0.51 [0.19, 1.38] |
| 4 Decrease in scaling score | 5 | 329 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [-0.13, 0.51] |
| 4.1 14% per week | 2 | 110 | Std. Mean Difference (IV, Random, 95% CI) | -0.02 [-0.52, 0.48] |
| 4.2 2% to 7% per week | 3 | 219 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [-0.12, 0.77] |
Comparison 27. Ketoconazole vs placebo - Subgroup analysis by mode of delivery
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 9 | 2559 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.56, 0.78] |
| 1.1 Shampoo | 3 | 248 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.39, 0.99] |
| 1.2 Demulcents | 5 | 531 | Risk Ratio (M-H, Random, 95% CI) | 0.61 [0.50, 0.74] |
| 1.3 Foam | 1 | 847 | Risk Ratio (M-H, Random, 95% CI) | 0.76 [0.66, 0.87] |
| 1.4 Gel | 1 | 933 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.77, 0.88] |
| 2 Decrease in erythema score | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Shampoo | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Demulcents | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Erythema - Failure to achieve complete resolution | 2 | 259 | Risk Ratio (M-H, Random, 95% CI) | 0.54 [0.21, 1.43] |
| 3.1 Shampoo | 1 | 200 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.66, 0.92] |
| 3.2 Cream | 1 | 59 | Risk Ratio (M-H, Random, 95% CI) | 0.32 [0.13, 0.77] |
| 4 Decrease in pruritus score | 3 | 699 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.55 [-0.85, -0.26] |
| 4.1 Demulcents | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | -2.06 [-2.84, -1.28] |
| 4.2 Gel | 1 | 459 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Shampoo | 1 | 200 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.30 [-0.62, 0.02] |
| 5 Decrease in scaling score | 3 | 304 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.65 [-0.91, -0.39] |
| 5.1 Shampoo | 2 | 264 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.47 [-0.75, -0.20] |
| 5.2 Demulcent | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | -2.12 [-2.91, -1.33] |
| 6 Decrease in scaling score (long term) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Shampoo | 2 | 264 | Std. Mean Difference (IV, Fixed, 95% CI) | -0.54 [-0.82, -0.27] |
| 7 Side effects | 6 | 988 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.58, 1.64] |
| 7.1 Shampoo | 3 | 440 | Risk Ratio (M-H, Random, 95% CI) | 1.27 [0.47, 3.45] |
| 7.2 Demulcents | 2 | 89 | Risk Ratio (M-H, Random, 95% CI) | 0.69 [0.29, 1.67] |
| 7.3 Gel | 1 | 459 | Risk Ratio (M-H, Random, 95% CI) | 0.80 [0.53, 1.20] |
Comparison 28. Ketoconazole vs steroids - Subgroup analysis by mode of delivery
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to achieve complete resolution | 6 | 302 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.95, 1.44] |
| 1.1 Shampoo | 1 | 49 | Risk Ratio (M-H, Fixed, 95% CI) | 1.29 [0.98, 1.71] |
| 1.2 Demulcents | 5 | 253 | Risk Ratio (M-H, Fixed, 95% CI) | 1.13 [0.86, 1.48] |
| 2 Decrease in scaling score | 5 | 329 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [-0.13, 0.51] |
| 2.1 Shampoo | 2 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [-0.37, 0.76] |
| 2.2 Demulcent | 3 | 179 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [-0.30, 0.67] |
Additional Tables
Table 1.
Grading of quality of evidence
| Comparison | Risk of bias | Consistency | Directness | Precision | Publication bias | Grade quality |
| Ketoconazole vs placebo | Most studies had unclear risk of bias: Downgrade 1 level | High heterogeneity (I² = 50%): Downgrade 1 level | No indirect comparison: no downgrading | 2520 participants, no overlap with 1: no downgrading | Funnel plot does not indicate publication bias: no downgrading | Low: downgraded for risk of bias, consistency |
| Ketoconazole vs steroids | Most studies had unclear risk of bias: Downgrade 1 level | Consistent: no downgrading | No indirect comparison: no downgrading | 302 participants. CI overlaps with RR = 1 and RR = 1.25: Downgrade 1 level | Funnel plot does not indicate publication bias: no downgrading | Low: downgraded for risk of bias, precision |
| Ketoconazole vs ciclopirox | Most studies had unclear risk of bias: Downgrade 1 level | Consistent: no downgrading | No indirect comparison: no downgrading | 272 participants. CI overlaps with RR = 1 and RR = 1.25: Downgrade 1 level | Funnel plot does not indicate publication bias: no downgrading | Low: downgraded for risk of bias, precision |
| Ciclopirox vs placebo | Most studies had low or unclear risk of bias: Downgrade 1 level | High heterogeneity (I² = 75%): Downgrade 1 level | No indirect comparison: no downgrading | 1525 participants, no overlap with 1: no downgrading | Funnel plot does not indicate publication bias: no downgrading | Low: downgraded for risk of bias, consistency |
Acknowledgments
The authors would like to thank Martin Meremikwu and Angela Oyoita for their encouragement, which led to conception of this review. We also thank Erin Dixon and Oliver Chosidow for their input to the protocol. Kenneth Ochieng gave support during development and testing of the data extraction tools. We would like to thank immensely the Cochrane Occupational Safety and Health Review Group editorial base in Kuopio, Finland, for their hospitality and support. Their initiative, encouragement and expertise was crucial in building this review beyond the protocol stage. We also appreciate Piotr Sakowski and Gosia Bala, who helped with translation of some articles.
The Cochrane Skin Group editorial base wishes to thank Luigi Naldi, who was the Cochrane Dermatology Editor for this review; Jo Leonardi-Bee, who was the Statistical Editor; Philippa Middleton, who was Methods Editor; the clinical referees, Roderick Hay and Noah Scheinfeld; and the consumer referee, Shirley Manknell.
References
- Abeck D Loprox Shampoo Dosing Study Group. Rationale of frequency of use of ciclopirox 1% shampoo in the treatment of seborrhoeic dermatitis: results of a double-blind, placebo-controlled study comparing the efficacy of once, twice and three times weekly usage. International Journal of Dermatology. 2004;43(Suppl 1):13–6. doi: 10.1111/j.1461-1244.2004.02382.x. [MEDLINE: 15271195] [DOI] [PubMed] [Google Scholar]
- Altmeyer P, Hoffmann K Loprox Shampoo Dosing Concentration Study Group. Efficacy of different concentrations of ciclopirox shampoo for the treatment of seborrheic dermatitis of the scalp: results of a randomized, double-blind, vehicle-controlled trial. International Journal of Dermatology. 2004;43(Suppl 1):9–12. doi: 10.1111/j.1461-1244.2004.02381.x. [MEDLINE: 15271194] [DOI] [PubMed] [Google Scholar]
- Aly R, Katz HI, Kempers SE, Lookingbill DP, Lowe N, Menter A, et al. Ciclopirox gel for seborrhoeic derrmatitis of the scalp. International Journal of Dermatology. 2003;42(Suppl 1):19–22. doi: 10.1046/j.1365-4362.42.s1.5.x. [MEDLINE: 12895183] [DOI] [PubMed] [Google Scholar]
- Attarzadeh Y, Asilian A, Shahmoradi Z, Adibi N. Comparing the efficacy of Emu oil with clotrimazole and hydrocortisone in the treatment of seborrheic dermatitis: a clinical trial. Journal of Research in Medical Sciences. 2013;18(6):477–81. [MEDLINE: 24250695] [PMC free article] [PubMed] [Google Scholar]
- Berger R, Mills OH, Jones EL, Mrusek S. Double-blind, placebo-controlled trial of ketoconazole 2% shampoo in the treatment of moderate to severe dandruff. Advances in Therapy. 1990;7(5):247–56. [EMBASE: 1990377776] [Google Scholar]
- Chosidow O, Maurette C, Dupuy P. Randomized, open-labeled, non-inferiority study between ciclopiroxolamine 1% cream and ketoconazole 2% foaming gel in mild to moderate facial seborrheic dermatitis. Dermatology. 2003;206(3):233–40. doi: 10.1159/000068904. [MEDLINE: 12673081] [DOI] [PubMed] [Google Scholar]
- Danby FW, Maddin WS, Margesson LJ, Rosenthal D. A randomized, double-blind, placebo-controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2.5% shampoo in the treatment of moderate to severe dandruff. Journal of the American Academy of Dermatology. 1993;29(6):1008–12. doi: 10.1016/0190-9622(93)70282-x. [MEDLINE: 8245236] [DOI] [PubMed] [Google Scholar]
- Diehl C, Ferrari A. Efficacy of topical 4% Quassia amara gel in facial seborrheic dermatitis: a randomized, double-blind, comparative study. Journal of Drugs in Dermatology. 2013;12(3):312–5. [MEDLINE: 23545914] [PubMed] [Google Scholar]
- Draelos ZD, Kenneally DC, Hodges LT, Billhimer W, Copas M, Margraf C. A comparison of hair quality and cosmetic acceptance following the use of two anti-dandruff shampoos. Journal of Investigative Dermatology. Symposium Proceedings. 2005;10(3):201–4. doi: 10.1111/j.1087-0024.2005.10127.x. [MEDLINE: 16382664] [DOI] [PubMed] [Google Scholar]
- Dreno B. Lithium gluconate 8% ointment in the treatment of moderate to severe seborrhoeic dermatitis. Journal of the European Academy of Dermatology and Venereology. 2002;16(Suppl s1):153. [Google Scholar]
- Dreno B. Lithium gluconate in seborrheic dermatitis. Journal of Investigative Dermatology. 1997;108(3):389. [Google Scholar]
- Dreno B. Lithium vs ketoconazole in seborrheic dermatitis: a multicenter, randomized, controlled study. Annales de Dermatologie et de Venereologie. 2002;129:P1664. [Google Scholar]
- Dreno B, Moyse D. Lithium gluconate in the treatment of seborrhoeic dermatitis: a multicenter, randomised, double-blind study versus placebo. European Journal of Dermatology. 2002;12(6):549–52. [MEDLINE: 12459525] [PubMed] [Google Scholar]
- Dreno B, Chosidow O, Revuz J, Moyse D The Study Investigator Group. Lithium gluconate 8% vs. ketoconazole 2% in the treatment of seborrhoeic dermatitis: a multicentre, randomized study. British Journal of Dermatology. 2003;148(6):1230–6. doi: 10.1046/j.1365-2133.2003.05328.x. [PUBMED: 12828753] [DOI] [PubMed] [Google Scholar]
- Dupuy P, Maurette C, Amoric JC, Chosidow O Study Investigator Group. Randomized, placebo-controlled, double-blind study on clinical efficacy of ciclopiroxolamine 1% cream in facial seborrhoeic dermatitis. British Journal of Dermatology. 2001;144(5):1033–7. doi: 10.1046/j.1365-2133.2001.04194.x. [MEDLINE: 11359393] [DOI] [PubMed] [Google Scholar]
- Elewski B, Ling MR, Phillips TJ. Efficacy and safety of a new once-daily topical ketoconazole 2% gel in the treatment of seborrhoeic dermatitis: a phase III trial. Journal of Drugs in Dermatology. 2006;5(7):646–50. [MEDLINE: 16865870] [PubMed] [Google Scholar]
- Elewski BE, Abramovits W, Kempers S, Schlessinger J, Rosen T, Gupta AK, et al. A novel foam formulation of ketoconazole 2% for the treatment of seborrheic dermatitis on multiple body regions. Journal of Drugs in Dermatology. 2007;6(10):1001–8. [MEDLINE: 17966177] [PubMed] [Google Scholar]
- Faergemann J. Seborrhoeic dermatitis and Pityrosporum orbiculare: treatment of seborrhoeic dermatitis of the scalp with miconazole-hydrocortisone (Daktacort), miconazole and hydrocortisone. British Journal of Dermatology. 1986;114(6):695–700. doi: 10.1111/j.1365-2133.1986.tb04878.x. [MEDLINE: 2941051] [DOI] [PubMed] [Google Scholar]
- Go IH, Wientjens DP, Koster M. A double-blind trial of 1% ketoconazole shampoo versus placebo in the treatment of dandruff. Mycoses. 1992;35(3-4):103–5. doi: 10.1111/j.1439-0507.1992.tb00828.x. [MEDLINE: 1435847] [DOI] [PubMed] [Google Scholar]
- Green CA, Farr PM, Shuster S. Treatment of seborrhoeic dermatitis with ketoconazole: II. Response of seborrhoeic dermatitis of the face, scalp and trunk to topical ketoconazole. British Journal of Dermatology. 1987;116(2):217–21. doi: 10.1111/j.1365-2133.1987.tb05815.x. [MEDLINE: 2950915] [DOI] [PubMed] [Google Scholar]
- Grossman R, Gisoldi E, Phillips S, Cauwenbergh G. A comparative efficacy study of two dandruff shampoos [Abstract 1206]. The 19th World Congress of Dermatology 15-20 June, 1997 Sydney, Australia. Australasian Journal of Dermatology. 1997;38(Suppl 2):94. [Google Scholar]
- Herrera-Arellano A, Jiménez-Ferrer E, Vega-Pimentel AM, Martínez-Rivera Mde L, Hernández-Hernández M, Zamilpa A, et al. Clinical and mycological evaluation of therapeutic effectiveness of Solanum chrysotrichum standardized extract on patients with pityriasis capitis (dandruff). A double blind and randomized clinical trial controlled with ketoconazole. Planta Medica. 2004;70(6):483–8. doi: 10.1055/s-2004-827145. [MEDLINE: 15241887] [DOI] [PubMed] [Google Scholar]
- Hersle K, Mobacken H, Nordin P. Mometasone furoate solution 0.1% compared with ketoconazole shampoo 2% for seborrhoeic dermatitis of the scalp. Current Therapeutic Research - Clinical & Experimental. 1996;57(7):516–22. [MEDLINE: 1996233065] [Google Scholar]
- Katsambas A, Antoniou C, Frangouli E, Avgerinou G, Michailidis D, Stratigos J. A double-blind trial of treatment of seborrhoeic dermatitis with 2% ketoconazole cream compared with 1% hydrocortisone cream. British Journal of Dermatology. 1989;121(3):353–7. doi: 10.1111/j.1365-2133.1989.tb01429.x. [MEDLINE: 2529893] [DOI] [PubMed] [Google Scholar]
- Koc E, Arca E, Kose O, Akar A. An open, randomized, prospective, comparative study of topical pimecrolimus 1% cream and topical ketoconazole 2% cream in the treatment of seborrheic dermatitis. Journal of Dermatological Treatment. 2009;20(1):4–9. doi: 10.1080/09546630802286993. [MEDLINE: 18677657] [DOI] [PubMed] [Google Scholar]
- Kousidou T, Panagiotidou D, Boutli F, Mourellou O, Ioannidis D, Fotidou D, et al. A double-blind comparison of 2% ketoconazole cream and 1% hydrocortisone cream in the treatment of seborrheic dermatitis. Current Therapeutic Research, Clinical & Experimental. 1992;51(5):723–9. [EMBASE: 1992159176] [Google Scholar]
- Langtry JA, Rowland Payne CM, Staughton RC, Stewart JC, Horrobin DF. Topical lithium succinate ointment (Efalith) in the treatment of AIDS-related seborrhoeic dermatitis. Clinical & Experimental Dermatology. 1997;22(5):216–9. [MEDLINE: 9536541] [PubMed] [Google Scholar]
- Lebwohl M, Plot T. Safety and efficacy of ciclopirox 1% shampoo for the treatment of seborrheic dermatitis of the scalp in the US population: results of a double-blind, vehicle-controlled trial. International Journal of Dermatology. 2004;43(Suppl 1):17–20. doi: 10.1111/j.1461-1244.2004.02409.x. [MEDLINE: 15271196] [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee HS, Eun HC, Cho KH. Successful treatment of dandruff with 1.5% ciclopirox olamine shampoo in Korea. Journal of Dermatological Treatment. 2003;14(4):212–5. [MEDLINE: 14660265] [PubMed] [Google Scholar]
- Lopez-Padilla SO, Carvajal A. Ketoconazole 1% shampoo vs. climbazole shampoo on the treatment of seborrhoeic dermatitis in scalp skin [Ketoconazole al 1% champú vs. climbazole champú en el tratamiento de la dermatitis seborreica en piel cabelluda] Dermatologia Revista Mexicana. 1996;40(3):190–5. [EMBASE: 1996238292] [Google Scholar]
- Ortonne JP, Lacour JP, Vitetta A, Le Fichoux Y. Comparative study of ketoconazole 2% foaming gel and betamethasone dipropionate 0.05% lotion in the treatment of seborrhoeic dermatitis in adults. Dermatology. 1992;184(4):275–80. doi: 10.1159/000247566. [MEDLINE: 1386766] [DOI] [PubMed] [Google Scholar]
- Ortonne JP, Nikkels AF, Reich K, Ponce Olivera RM, Lee JH, Kerrouche N, et al. Efficacious and safe management of moderate to severe scalp seborrhoeic dermatitis using clobetasol propionate shampoo 0.05% combined with ketoconazole shampoo 2%: a randomized controlled study. British Journal of Dermatology. 2011;165(1):171–6. doi: 10.1111/j.1365-2133.2011.10269.x. [MEDLINE: 21707573] [DOI] [PubMed] [Google Scholar]
- Pari T, Pulimood S, Jacob M, George S, Jeyaseelan I, Thomas K. Randomized double blind controlled trial of 2% ketoconazole cream versus 0.05% clobetasol 17-butyrate cream in seborrhoeic dermatitis. Journal of the European Academy of Dermatology & Venereology. 1998;10(1):89–90. doi: 10.1111/j.1468-3083.1998.tb00938.x. [MEDLINE: 9552768] [DOI] [PubMed] [Google Scholar]
- Peter RU, Korting HC. Treatment of seborrhoeic eczema with ketoconazole in comparison with an active agent-free cream [Behandlung des seborrhoischen Ekzems mit Ketoconazol im Vergleich zu einer Wirkstoff-freien Pflegecreme] Arzneimittel-Forschung. 1991;41(8):852–4. [MEDLINE: 1838256] [PubMed] [Google Scholar]
- Piepponen T, Suhonen R, Rantanen T, Blomqvist K, Pajarre R, Lehtonen L. Treatment of dandruff with a ketoconazole 2% shampoo. Journal of Dermatological Treatment. 1992;3(3):119–23. [EMBASE: 1992296410] [Google Scholar]
- Pierard GE, Pierard-Franchimont C, Van Cutsem J, Rurangirwa A, Hoppenbrouwers ML, Schrooten P. Ketoconazole 2% emulsion in the treatment of seborrhoeic dermatitis. International Journal of Dermatology. 1991;30(11):806–9. doi: 10.1111/j.1365-4362.1991.tb04793.x. [MEDLINE: 1836780] [DOI] [PubMed] [Google Scholar]
- Pierard G. A trial comparing 2% Nizoral shampoo with Head & Shoulders in severe dandruff/seborrhoeic dermatitis. Journal of the European Academy of Dermatology and Venereology. 1999;12(Suppl 2):S230. [Google Scholar]
- Pierard-Franchimont C, Pierard GE, Arrese JE, De Doncker P. Effect of ketoconazole 1% and 2% shampoos on severe dandruff and seborrhoeic dermatitis: clinical, squamometric and mycological assessments. Dermatology. 2001;202(2):171–6. doi: 10.1159/000051628. [MEDLINE: 11306850] [DOI] [PubMed] [Google Scholar]
- Piérard-Franchimont C, Goffin V, Decroix J, Piérard GE. A multicenter randomized trial of ketoconazole 2% and zinc pyrithione 1% shampoos in severe dandruff and seborrhoeic dermatitis. Skin Pharmacology & Applied Skin Physiology. 2002;15(6):434–41. doi: 10.1159/000066452. [MEDLINE: 12476017] [DOI] [PubMed] [Google Scholar]
- Ratnavel RC, Squire RA, Boorman GC. Clinical efficacies of shampoos containing ciclopirox olamine (1.5%) and ketoconazole (2.0%) in the treatment of seborrhoeic dermatitis. Journal of Dermatological Treatment. 2007;18(2):88–96. doi: 10.1080/16537150601092944. [MEDLINE: 17520465] [DOI] [PubMed] [Google Scholar]
- Satriano RA, Florio M, Grimaldi Filioli F, Gregori S. Seborrheic dermatitis: use of ketoconazole cream and shampoo. Double-blind study versus placebo [Dermatite seborroica: uso di una crema e di uno shampoo al ketoconazolo. Studio in doppio cieco versus placebo.] Giornale Italiano di Dermatologia e Venereologia. 1987;122(11):LVII–LX. [MEDLINE: 2966107] [PubMed] [Google Scholar]
- Schofer H. Treatment of seborrhoeic dermatitis with ketoconazole cream. A randomised double blind study. (Half side comparison) [KETOCONAZOL-CREME BEIM SEBORRHOISCHEN EKZEM. ERGEBNISSE EINER SEITENVERGLEICHSSTUDIE] Aktuelle Dermatologie. 1988;14(Suppl 1):412–5. [EMBASE: 1989013802] [Google Scholar]
- Seckin D, Gurbuz O, Akin O. Metronidazole 0.75% gel vs. ketoconazole 2% cream in the treatment of facial seborrheic dermatitis: a randomized, double-blind study. Journal of the European Academy of Dermatology & Venereology. 2007;21(3):345–50. doi: 10.1111/j.1468-3083.2006.01927.x. [MEDLINE: 17309456] [DOI] [PubMed] [Google Scholar]
- Segal R, David M, Ingber A, Lurie R, Sandbank M. Treatment with bifonazole shampoo for seborrhea and seborrheic dermatitis: a randomized, double-blind study. Acta Dermato-Venereologica. 1992;72(6):454–5. [MEDLINE: 1362843] [PubMed] [Google Scholar]
- Sei Y, Kobayashi M, Soude E. Study on the usefulness of rinse containing miconazole nitrate for treatment of dandruff - a double-blind, comparative study. Medical Mycology Journal. 2011;52(3):229–37. doi: 10.3314/mmj.52.229. [MEDLINE: 21891985] [DOI] [PubMed] [Google Scholar]
- Shuster S, Meynadier J, Kerl H, Nolting S. Treatment and prophylaxis of seborrheic dermatitis of the scalp with antipityrosporal 1% ciclopirox shampoo. Archives of Dermatology. 2005;141(1):47–52. doi: 10.1001/archderm.141.1.47. [MEDLINE: 15655141] [DOI] [PubMed] [Google Scholar]
- Shuttleworth D, Squire RA, Boorman GC, Goode K. Comparative clinical efficacy of shampoos containing ciclopirox olamine (1.5%) or ketoconazole (2%; Nizoral(TM)) for the control of dandruff/seborrhoeic dermatitis. Journal of Dermatological Treatment. 1998;9(3):157–62. [EMBASE: 1998346162] [Google Scholar]
- Skinner RB, Noah PW, Taylor RM, Zanolli MD, West S, Guin JD, et al. Double-blind treatment of seborrhoeic dermatitis with 2% ketoconazole cream. Journal of American Academy of Dermatology. 1895;12(5 Pt 1):852–6. doi: 10.1016/s0190-9622(85)70107-7. [MEDLINE: 3159759] [DOI] [PubMed] [Google Scholar]
- Stratigos JD, Antoniou C, Katsambas A, Bohler K, Fritsch P, Schmolz A, et al. Ketoconazole 2% cream versus hydrocortisone 1% cream in the treatment of seborrhoeic dermatitis: a double blind comparative study. Journal of the American Academy of Dermatology. 1988;19(5 Pt 1):850–3. doi: 10.1016/s0190-9622(88)70244-3. [MEDLINE: 2973476] [DOI] [PubMed] [Google Scholar]
- Blockhuys S, Legendre R, Beger B, Barranco C. Three double blind studies comparing 2% ketoconazole anhydrous gel with its gel vehicle in patients with seborrheic dermatitis. Journal of the European Academy of Dermatology and Venereology. 2005;19(Suppl 2):120. [Google Scholar]
- Swinyer LJ, Decroix J, Langner A, Quiring JN, Blockhuys S. Ketoconazole gel 2% in the treatment of moderate to severe seborrheic dermatitis. Cutis. 2007;79(6):475–82. [EMBASE: 17713152] [PubMed] [Google Scholar]
- Unholzer A, Schinzel S, Nietsch KH, Jung GE, Korting HC. Ciclopiroxolamine cream 1% in the treatment of seborrhoeic dermatitis: a double-blind, parallel-group comparison with ketoconazole and vehicle in a confirmatory trial. Clinical Drug Investigation. 2002;22(3):167–72. [EMBASE: 2002127872] [Google Scholar]
- Unholzer A, Varigos G, Nicholls D, Schnizel S, Nietsch KH, Ulbricht H, et al. Ciclopiroxamine cream for treating seborrhoeic dermatitis: a double-blind parallel group comparison. Infection. 2002;30(6):373–6. doi: 10.1007/s15010-002-2196-9. [PUBMED: 12478328] [DOI] [PubMed] [Google Scholar]
- Van't Veen AJ, Prevoo RLMA, Velders AJ, Jagtman BA, Van Niel JCG, Stolz E. Betamethasone-17-valerate compared with ketoconazole for topical treatment of seborrhoeic dermatitis of the scalp in adults. Results of a Dutch multicentre study. Journal of Dermatological Treatment. 1998;9(4):239–45. [EMBASE: 1999025626] [Google Scholar]
- Vardy DA, Zvulunov A, Tchetov T, Biton A, Rosenman D. A double-blind, placebo-controlled trial of a ciclopirox olamine 1% shampoo for the treatment of scalp seborrheic dermatitis. Journal of Dermatological Treatment. 2000;11(2):73–7. [EMBASE: 2000266084] [Google Scholar]
- Zienicke H, Korting HC, Braun-Falco O, Effendy I, Hagedorn M, Küchmeister B, et al. Comparative efficacy and safety of bifonazole 1% cream and the corresponding base preparation in the treatment of seborrhoeic dermatitis. Mycoses. 1993;36(9-10):325–31. doi: 10.1111/j.1439-0507.1993.tb00776.x. [MEDLINE: 8015566] [DOI] [PubMed] [Google Scholar]
- Alizadeh N, Monadi Nori H, Golchi J, Eshkevari SS, Kazemnejad E, Darjani A. Comparison of the efficacy of fluconazole and terbinafine in patients with moderate to severe seborrheic dermatitis. Dermatology Research & Practice 2014 Feb 18 [Epub ahead of print]. [MEDLINE: 24696675] [DOI] [PMC free article] [PubMed]
- Amos HE, MacLennan AI, Boorman GC. Clinical efficacy of Polytar AF (Fongitar) and Nizoral scalp treatments in patients with dandruff/seborrhoeic dermatitis. Journal of Dermatological Treatment. 1994;5(3):127–30. [EMBASE: 1994316061] [Google Scholar]
- Attila P, Forstrom L, Hannuksela M, Karvonen J, Lehtonen L, Salo OP. Clotrimazole-hydrocortisone, hydrocortisone and propylene glycol liniment in the treatment of seborrhoeic dermatitis of the scalp. Indian Journal of Dermatology, Venerology & Leprology. 1993;59:157. [CENTRAL: CN-00692692] [Google Scholar]
- Boyle J, Burton JL, Faergemann J. Use of topical lithium succinate for seborrhoeic dermatitis. British Medical Journal - Clinical Research Ed. 1986;292(6512):28. doi: 10.1136/bmj.292.6512.28. [MEDLINE: 2935222] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Evans TW, Poyner T, Tooley PJH. The role of ketoconazole 2% shampoo in the treatment and prophylactic management of dandruff. Journal of Dermatological Treatment. 1990;1(4):177–9. [EMBASE: 1990343403] [Google Scholar]
- Cauwenbergh G, De Doncker P, Schrooten P, Degreef H. Treatment of dandruff with a 2% ketoconazole scalp gel. A double-blind placebo-controlled study. International Journal of Dermatology. 1986;25(8):541. doi: 10.1111/j.1365-4362.1986.tb00877.x. [MEDLINE: 3533805] [DOI] [PubMed] [Google Scholar]
- Chappell J, Mattox A, Simonetta C, Armbrecht E, Guo M. Seborrheic dermatitis of the scalp in populations practicing less frequent hair washing: ketoconazole 2% foam versus ketoconazole 2% shampoo. Three-year data. 72nd Annual Meeting of the American Academy of Dermatology Denver, CO United States. Conference Start: 20140321 Conference End: 20140325. Journal of the American Academy of Dermatology. 2014;70(5 Suppl 1):AB54. [EMBASE: 71390306] [Google Scholar]
- Cheng Y, Hu P, Hu Y. Clinical observation on effect of bifonazole cream in treating seborrhoeic dermatitis on face (Chinese) China Journal of Leprosy & Skin Diseases. 2001;17(1):26. [Google Scholar]
- Cicek D, Kandi B, Bakar S, Turgut D. Pimecrolimus 1% cream, methylprednisolone acetonate 0.1% cream and metronidazole 0.75% gel in the treatment of seborrhoeic dermatitis: a randomized clinical study. Journal Dermatological Treatment. 2009;20(6):344–9. doi: 10.3109/09546630802687349. [PUBMED: 19954391] [DOI] [PubMed] [Google Scholar]
- Comert A, Bekiroglu N, Gurbuz O, Ergun T. Efficacy of oral fluconazole in the treatment of seborrhoeic dermatitis: a placebo controlled study. American Journal of Clinical Dermatology. 2007;8(4):235–8. doi: 10.2165/00128071-200708040-00005. [PUBMED: 17645378] [DOI] [PubMed] [Google Scholar]
- Ctri/2009/091/001079 Efficacy, safety and tolerability of sertaconazole 2% plus zinc pyrithione 1% shampoo vs ketoconazole 2% shampoo in adult patients with dandruff. apps.who.int/trialsearch/Trial.aspx?TrialID=CTRI/2009/091/001079 (accessed 10 February 2015).
- Davies DB, Boorman GC, Shuttleworth D. Comparative efficacy of shampoos containing coal tar (4.0% w/w; Tarmed(TM)), coal tar (4.0% w/w) plus ciclopirox olamine (1.0% w/w; Tarmed(TM) AF) and ketoconazole (2.0% w/w; Nizoral(TM)) for the treatment of dandruff/seborrhoeic dermatitis. Journal of Dermatological Treatment. 1999;10(3):177–83. [EMBASE: 1999324981] [Google Scholar]
- Efalith Multicentre Trial Group. A double-blind, placebo-controlled, multicenter trial of lithium succinate ointment in the treatment of seborrheic dermatitis. Journal of the American Academy of Dermatology. 1992;26(3 Pt 2):452–7. doi: 10.1016/0190-9622(92)70071-m. [PUBMED: 1532964] [DOI] [PubMed] [Google Scholar]
- Emad M. Comparison of topical and oral ketoconazole in the treatment of intractable seborrheic dermatitis (Persian) Iranian Journal of Dermatology. 2000;3(3):4. [Google Scholar]
- Emtestam L, Svensson A, Rensfeldt K. Treatment of seborrhoeic dermatitis of the scalp with a topical solution of urea, lactic acid, and propylene glycol (K301): results of two double-blind, randomised, placebo-controlled studies. Mycoses. 2012;55(5):393–403. doi: 10.1111/j.1439-0507.2011.02126.x. [DOI] [PubMed] [Google Scholar]
- Ermosilla VE, Lorette GL, Doss ND, Morinet PM, Sibaud VS. Seborrheic dermatitis of the scalp: results of a clinical study comparing a shampoo with ciclopiroxolamine 1.5% and pirythione zinc 1% to ketoconazole 2% shampoo and to a placebo. [Abstract P16.22]. The 14th Congress of the European Academy of Dermatology and Venereology, London,UK. 12-15th October 2005. Journal of the European Academy of Dermatology & Venereology. 2005;19(Suppl 2):388. [Google Scholar]
- Ernst Th-M. Local treatment of seborrheoic eczema with a new external application containing clotrimazol [Die Lokalbehandlung des seborrhoischen ekzems mit einem neuen clotrimazolhaltigen externum] Aktuelle Dermatologie. 1990;16(7):209–11. [EMBASE: 1990243639] [Google Scholar]
- Ford GP, Farr PM, Ive FA, Shuster S. The response of seborrhoeic dermatitis to ketoconazole. British Journal of Dermatology. 1984;111(5):603–7. doi: 10.1111/j.1365-2133.1984.tb06631.x. [MEDLINE: 6093845] [DOI] [PubMed] [Google Scholar]
- Gayko G, Warnecke J, Zelenkova H. The use of a pale type of Ichthyol in cosmetic dermatology. Cosmetic, single-centre, controlled and randomized double blind study for proof of efficacy and tolerance of a combination of ichthyol pale 0.5% and ketoconazole 0.5% vs. ketoconazole 1.0% shampoo in treatment of moderate to severe dandruff. Dermatologia Kliniczna. 2006;8(4):243–7. [EMBASE: 2007006167] [Google Scholar]
- Goldust M, Rezaee E, Rouhani S. Double blind study of sertaconazole 2% cream vs clotrimazole 1% cream in treatment of seborrheic dermatitis. Annals of Parasitology. 2013;59(1):25–9. [EMBASE: 23829055] [PubMed] [Google Scholar]
- Gupta AK, Nicol KA. Ciclopirox 1% shampoo for the treatment of seborrheic dermatitis. International Journal of Dermatology. 2006;45(1):66–9. doi: 10.1111/j.1365-4632.2004.02331.x. [EMBASE: 2006190414] [DOI] [PubMed] [Google Scholar]
- Humke S, Buddie MA, Richard A, Rougier A, Krutman J. 2002. Efficacy and tolerability of a new shampoo in the treatment of dandruff [Abstract] p. P0425. 20th World Congress of Dermatology Paris 1st to 5th July 2002 Not applicable:[CENTRAL: CN-00454339] [PubMed] [Google Scholar]
- Iraji F, Shamoradi Z, Taheri A. Metronidazole gel in seborrhoic dermatitis: a double blind study. Journal of the European Academy of Dermatology & Venereology. 2005;19(Suppl 2):218–9. [Google Scholar]
- Iudica AC. Does treatment with topical metronidazole improve seborrheic dermatitis? Journal of Family Practice. 2001;50(6):492. [MEDLINE: 11401733] [PubMed] [Google Scholar]
- Jensen J, Clausen C, Folster-Holst R, Proksch E. Seborrhoeic eczema responds more quickly to treatment with pimecrolimus cream than treatment with ketoconazole cream [Abstract P165]. 36th Annual Meeting of the Arbeitsgemeinschaft Dermatologische Forschung (ADF), Heidelberg, Germany – March 05–07, 2009. Experimental Dermatology. 2009;18(3):302. [DOI: 10.1111/j.1600-0625.2008.00834.x"] [Google Scholar]
- Kaszuba A, Kusiba-Charaziak A, Bialek E, Kozlowska M, Kaszuba A. The efficacy of itraconazole in the treatment of seborrheic dermatitis: own clinical trials [Skutecznosc leczenia tojotokowego zapalenia skory itrakonazolem - Doswiadczenia wlasne] Mikologia Lekarska. 2005;12(1):43–7. [EMBASE: 2005181689] [Google Scholar]
- Koca R, Altinyazar HC, Estürk E. Is topical metronidazole effective in seborrheic dermatitis? A double-blind study. International Journal of Dermatology. 2003;42(8):632–5. doi: 10.1046/j.1365-4362.2003.01981.x. [PUBMED: 12890109] [DOI] [PubMed] [Google Scholar]
- Kozlowska M, Kaszuba A, Michalak I, Trznadel-Budzko E, Bartkowiak R. The efficacy of orally fluconazole and topically 1% clotrimazole cream and shampoo containing 2% ketoconazole in the treatment of moderate to severe types of seborrheic dermatitis [Skutecznosc doustnego flukonazolu oraz miejscowo stosowanego 1% kremu klotrimazol i szamponu z 2% ketokonazolem w leczenlu skojarzonym umiarkowanych do ciez[dot]kich postaci lojotokowego zapalenia skory] Mikologia Lekarska. 2007;14(2):111–6. [EMBASE: 2007228017] [Google Scholar]
- Li TS. Ketoconazole 2% lotion in the treatment of 30 cases of seborrheic dermatitis (Chinese) Chinese Journal of Dermatology. 1996;29(5):318. [Google Scholar]
- Loden M, Wessman C. The antidandruff efficacy of a shampoo containing piroctone olamine and salicylic acid in comparison to that of a zinc pyrithione shampoo. International Journal of Cosmetic Science. 2000;22(4):285–9. doi: 10.1046/j.1467-2494.2000.00024.x. [MEDLINE: 18503415] [DOI] [PubMed] [Google Scholar]
- Lorette G, Ermosilla V. Clinical efficacy of a new ciclopiroxolamine/zinc pyrithione shampoo in scalp seborrheic dermatitis treatment. European Journal of Dermatology. 2006;16(5):558–64. [MEDLINE: 17101479] [PubMed] [Google Scholar]
- Meyer-Rohn J. Piadar (Ladar): new antimycotic drug containing the antifungal agent siccanin [Piadar (Ladar): Ein neues Antimykoticum mit dem Wirkstoff Siccanin] Mykosen. 1979;22(7):255–8. [MEDLINE: 381921] [PubMed] [Google Scholar]
- NCT00703846 (previously study NCT01337284) Study to assess the long-term safety of Extina (Ketoconazole) foam, 2% clinicaltrials.gov/ct2/show/ NCT00703846 (accessed 24 November 2011).
- Nct01139749 Efficacy and safety of low-dose oral isotretinoin for seborrhea. clinicaltrials.gov/ct2/show/ NCT01139749 (accessed 24 November 2011).
- Ozcan H, Seyhan M, Yologlu S. Is metronidazole 0.75% gel effective in the treatment of seborrhoeic dermatitis? A double-blind placebo controlled study. European Journal of Dermatology. 2007;17(4):313–6. doi: 10.1684/ejd.2007.0206. [MEDLINE: 17540638] [DOI] [PubMed] [Google Scholar]
- Parsad D, Pandhi R, Negi KS, Kumar B. Topical metronidazole in seborrheic dermatitis – a double-blind study. Dermatology. 2001;202(1):35–7. doi: 10.1159/000051582. [MEDLINE: 11244226] [DOI] [PubMed] [Google Scholar]
- Pedrinazzi C, Andreoli S, Battistini E, D'Errigo ML, Gregotti C, Richelmi P. Efficacy of a peat and salsobromoiodic water mask in the treatment of seborrhoeic dermatitis of the face [Efficacia de una maschera di torba e acqua termale Salsobromoiodica nel trattamento della dermatitie seborroica del viso] Journal of Plastic Dermatology. 2009;5(3):293–8. [EMBASE: 2010142548] [Google Scholar]
- Peter RU, Richarz-Barthauer U. Successful treatment and prophylaxis of scalp seborrhoeic dermatitis and dandruff with 2% ketoconazole shampoo: results of a multicentre, double-blind, placebo-controlled trial. 1995. pp. 441–5. [MEDLINE: 7718463] British Journal of Dermatology; Vol. 132, issue 3: [DOI] [PubMed]
- Pierard-Franchimont C, Pierard GE. A double-blind placebo controlled study of ketoconazole + desonide gel combination in the treatment of facial seborrhoeic dermatitis. Dermatology. 2002;204(4):344–7. doi: 10.1159/000063382. [MEDLINE: 12077544] [DOI] [PubMed] [Google Scholar]
- Pierard-Franchimont C, Goffin V, Henry F, Uhoda I, Braham C, Pierard GE. Nudging hair shedding by antidandruff shampoos. A comparison of 1% ketoconazole, 1% piroctone olamine and zinc pyrithione formulations. International Journal of Cosmetic Science. 2002;24(5):249–56. doi: 10.1046/j.1467-2494.2002.00145.x. [MEDLINE: 18498517] [DOI] [PubMed] [Google Scholar]
- Prensner R. Does 5% tea tree oil shampoo reduce dandruff? Journal of Family Practice. 2003;52(4):285–6. [MEDLINE: 12681088] [PubMed] [Google Scholar]
- Quadri G, Cavallero W, Milani M. Efficacy of a new antidandruff thermophobic foam: a randomized, controlled investigator-blind trial vs ketoconazole scalp fluid. Journal of Cosmetic Dermatology. 2005;4(1):23–6. doi: 10.1111/j.1473-2165.2005.00154.x. [MEDLINE: 17134417] [DOI] [PubMed] [Google Scholar]
- Rigoni C, Toffolo P, Cantu A, Beretta D, Terenzio C. 1% Econazole hair-shampoo in the treatment of pityriasis capitis: a comparative study between econazole 1% shampoo and zinc-pyrithione shampoo [1% Hair-Shampoo Nel Trattamento Della Pityriasis Capitis; Studio Di Confronto vs Zinco-Piritione Shampoo] Giornale Italiano di Dermatologia e Venereologia. 1989;124(11-2):LXVII–LXX. [MEDLINE: 2638641] [PubMed] [Google Scholar]
- Salmanpoor R, Saki N, Mahmoodi G. Liquorice 7% versus selenium sulfide 1% shampoos in the treatment of dandruff: a clinical trial. Iranian Journal of Dermatology. 2012;15(62):144–5. [EMBASE: 2013309268] [Google Scholar]
- Scaparro E, Quadri G, Virno G, Orifici C, Milani M. Evaluation of the efficacy and tolerability of oral terbinafine (Daskil) in patients with seborrhoeic dermatitis. A multicentre, randomized, investigator-blinded, placebo-controlled trial. British Journal of Dermatology. 2001;144(4):854–7. doi: 10.1046/j.1365-2133.2001.04144.x. [MEDLINE: 11298548] [DOI] [PubMed] [Google Scholar]
- Schmidt-Rose T, Braren S, Folster H, Hillemann T, Oltrogge B, Philipp P, et al. Efficacy of a piroctone olamine/climbazol shampoo in comparison with a zinc pyrithione shampoo in subjects with moderate to severe dandruff. International Journal of Cosmetic Science. 2011;33(3):276–82. doi: 10.1111/j.1468-2494.2010.00623.x. [MEDLINE: 21272039] [DOI] [PubMed] [Google Scholar]
- Schwartz JR, Bacon RA, Shah R, Mizoguchi H, Tosti A. Therapeutic efficacy of anti-dandruff shampoos: a randomized clinical trial comparing products based on potentiated zinc pyrithione and zinc pyrithione/climbazole. International Journal of Cosmetic Science. 2013;35(4):381–7. doi: 10.1111/ics.12055. [EMBASE: 2013439432] [DOI] [PubMed] [Google Scholar]
- Seite S, Rougier A, Talarico S. Randomized study comparing the efficacy and tolerance of a lipohydroxy acid shampoo to a ciclopiroxolamine shampoo in the treatment of scalp seborrhoeic dermatitis. Journal of Cosmetic Surgery. 2009;8(4):249–53. doi: 10.1111/j.1473-2165.2009.00460.x. [PUBMED: 19958427] [DOI] [PubMed] [Google Scholar]
- Siadat AH, Iraji F, Shahmoradi Z, Enshaieh S, Taheri A. The efficacy of 1% metronidazole gel in facial seborrhoeic dermatitis: a double blind study. Indian Journal of Dermatology Venereology & Leprology. 2006;72(4):266–9. doi: 10.4103/0378-6323.26745. [MEDLINE: 16880571] [DOI] [PubMed] [Google Scholar]
- Sparavigna A, Setaro M, Caserini M, Bulgheroni A. Assessment of the antidandruff activity of a new shampoo: a randomized, double-blind, controlled study by clinical and instrumental evaluations. Skinmed. 2013;11(2):85–91. [EMBASE: 23745226] [PubMed] [Google Scholar]
- Squire RA, Goode K. A randomised, single-blind, single-centre clinical trial to evaluate comparative clinical efficacy of shampoos containing ciclopirox olamine (1.5%) and salicylic acid (3%), or ketoconazole (2%, Nizoral) for the treatment of dandruff/seborrhoeic dermatitis. Journal of Dermatological Treatment. 2002;13(2):51–60. doi: 10.1080/095466302317584395. [MEDLINE: 12060502] [DOI] [PubMed] [Google Scholar]
- Syed TA, Aly R, Govil V, Ahmad SA, Andersson TS. Clinical evaluation of 2% analogue of green tea extract in a hydrophilic cream for treating seborrheic dermatitis: a placebo-controlled, double-blind study. Journal of Investigative Dermatology. 2008;128(Suppl 1):S51. [Google Scholar]
- Trznadel-Grodzka E, Błaszkowski M, Rotsztejn H. Investigations of seborrheic dermatitis. Part II. Influence of itraconazole on the clinical condition and the level of selected cytokines in seborrheic dermatitis [Badania nad ³ojotokowym zapaleniem skóry. Czêœæ II.Wp³yw itrakonazolu na stan kliniczny chorych oraz poziomwybranych cytokin w ³ojotokowym zapaleniu skóry] Postepy Hig Med Dosw (Online) 2012;66:848–54. doi: 10.5604/17322693.1019648. [PUBMED: 23175341] [DOI] [PubMed] [Google Scholar]
- Vena GA, Micali G, Santoianni P, Cassano N, Peruzzi E. Oral terbinafine in the treatment of multi-site seborrhoeic dermatitis: a multicentre, double-blind placebo-controlled study. International Journal of Immunopathology & Pharmacology. 2005;18(4):745–53. doi: 10.1177/039463200501800418. [MEDLINE: 16388724] [DOI] [PubMed] [Google Scholar]
- Xu G. A randomised controlled trial of 2% ketoconazole lotion versus 2,5% selenium sulphide lotion in the treatment of seborrheic dermatitis (Chinese) Journal of Clinical Dermatology. 1996;25(2):97. [Google Scholar]
- Feng H, Hu YH, Wang JL, Wang TL, Wang Q. Clinical efficacy of combination therapy with 0,1% tacrolimus ointment and selenium sulfide lotion in seborrheic dermatitis of the scalp. Journal of Clinical Dermatology. 2012;41(2):117–8. [Google Scholar]
- Goldust M, Rezaee E, Masoudnia S, Raghifar R. Clinical study of sertaconazole 2% cream vs. hydrocortisone 1% cream in the treatment of seborrheic dermatitis. Annals of Parasitology. 2013;59(3):119–23. [MEDLINE: 24881281] [PubMed] [Google Scholar]
- Goldust M, Rezaee E, Raghifar R. A double blind study of the effectiveness of sertaconazole 2% cream vs. metronidazole 1% gel in the treatment of seborrheic dermatitis. Annals of Parasitology. 2013;59(4):173–7. [MEDLINE: 24791343] [PubMed] [Google Scholar]
- Gould DJ. Topical lithium succinate - a safe and effective treatment for seborrhoeic dermatitis in adults. British Journal of Dermatology. 1988;119(Suppl 33):27–8. [Google Scholar]
- Irct138807202581N1 Efficacy of topical terbinafine 1% cream in the treatment of facial seborrhoeic dermatitis. apps.who.int/trialsearch/Trial.aspx?TrialID=IRCT138807202581N1 (accessed 10 February 2015).
- Irct2013072314117N1 To investigate the effectiveness of tea tree oil gel in the treatment of mild to moderate facial seborrheic dermatitis in comparison with placebo. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013072314117N1 (accessed 10 February 2015).
- Kim CH, Hwang DS, Kim JT, Jung HA, Roh SS, Lim NK. [A randomized study, double-blind, placebo-controlled study of herbal shampoo & essence about dandruff] The Journal of Korean Oriental Medical Ophthalmology & Otolaryngology & Dermatology [taehan hanbang an’ibiinhupibu gwa hakhoe chi] 2008;20(3):222–35. [Google Scholar]
- Li YN. A randomised controlled trial of 2% Triatop (ketoconazole) versus sulfur ointment in the treatment of scalp seborrheic dermatitis. Journal of Clinical Dermatology. 1999;28(5):308. [Google Scholar]
- Liu YF, Li CX, Che NZ. Ketoconazole 2% lotion in the treatment of seborrheic dermatitis [Chinese] Chinese Journal of Dermatology. 1997;30(2):145. [Google Scholar]
- Mao HJ. A clinical controlled trial of 2% ketoconazole versus in the treatment of scalp scale dermatitis. Journal of Clinical Dermatology. 1999;28(6):369. [Google Scholar]
- Nong DC. A clinical controlled trial of nasturtium herbs extraction versus resorcinol in the treatment of seborrheic dermatitis [Chinese] Journal of Clinical Dermatology. 1996;25(2):122. [Google Scholar]
- Sun RD, Chu WY, Guo SM, Yang GK, Gu J, Wang WZ. Pyrithionc zinc cream in the treatment of 65 cases of scalp seborrheic dermatitis [Chinese] Chinese Journal of Dermatology. 1994;27(6):372. [Google Scholar]
- Turlier V, Viode C, Durbise E, Bacquey A, LeJeune O, Oliveira Soares R, et al. Clinical and biochemical assessment of maintenance treatment in chronic recurrent seborrheic dermatitis: randomized control study. Dermatology and Therapy. 2014;4(1):43–59. doi: 10.1007/s13555-014-0047-0. [MEDLINE: 24643869] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JP, Sun WL, Chen B, Liu GH. A randomised controlled trial of 2% ketoconzole lotion versus 2.5% sulphide selenium lotion in the treatment of seborrheic dermatitis and scalp pityriasis. Journal of Clinical Dermatology. 1998;27(2):100–1. [Google Scholar]
- Xu G, Huang YL, Wang J, Xiao CZ. Observation on effects of terbinafine cream in treating seborrheic dermatitis on face (Chinese) Chinese Journal of Leprosy & Skin Disease. 2000;16(1):27–8. [Google Scholar]
- 2005-001371-35 A double-blind, placebo controlled, half-head design, CPO solution dose ranging-finding study in patients with seborrhoeic dermatitis of the scalp. clinicaltrialsregister.eu/ctr-search/trial/2005-001371-35/GB#C (accessed 10 February 2015).
- Nct01203189 Seborrheic dermatitis: ketoconazole 2% foam versus ketoconazole 2% shampoo. clinicaltrials.gov/ct2/show/ NCT01203189 (accessed 10 February 2015).
- Apasrawirote W, Udompataikul M, Rattanamongkolgul S. Topical antifungal agents for seborrheic dermatitis: systematic review and meta-analysis. Journal of the Medical Association of Thailand. 2011;94(6):756–60. [PubMed] [Google Scholar]
- Bergrant IM, Faegemann J, Voog E, Lowhagen GB. Pytirosporum ovale and seborrhoeic dermatitis in HIV-seropositive and HIV-seronegative men. Quantitative cultures and serological studies. Journal of the European Academy of Dermatology & Venereology. 1996;6(2):147–51. [DOI: 10.1111/j.1468-3083.1996.tb00158.x] [Google Scholar]
- Burton JL, Pye RJ. Seborrhoea is not a feature of seborrhoeic dermatitis. British Medical Journal. 1983;286(6372):1169–70. doi: 10.1136/bmj.286.6372.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley P, Zuo Y, Plunkett A, Merlin K, Marks R. The frequency of common skin conditions in pre-school-aged children in Australia:seborrheic dermatitis and pityriasis capitis (cradle cap) Archives of Dermatology. 2003;139(3):318–22. doi: 10.1001/archderm.139.3.318. [DOI] [PubMed] [Google Scholar]
- Gaitanis G, Velegraki A, Mayser P, Bassukas ID. Skin diseases associated with Malassezia yeasts: facts and controversies. Clinics in Dermatology. 2013;31(4):455–63. doi: 10.1016/j.clindermatol.2013.01.012. [MEDLINE: 23806162] [DOI] [PubMed] [Google Scholar]
- Ghannoum M, Rice LB. Anitfungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clinical Microbiology Reviews. 1999;12(4):501–17. doi: 10.1128/cmr.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Batra R, Bluhm R, Boekhout T, Dawson TL. Skin diseases associated with Malssezia species. Journal of the American Academy of Dermatology. 2004;51(5):785–98. doi: 10.1016/j.jaad.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Nicol K, Batra R. Role of antifungal agents in the treatment of seborrheic dermatitis. American Journal of Clinical Dermatology. 2004;5(6):417–22. doi: 10.2165/00128071-200405060-00006. [DOI] [PubMed] [Google Scholar]
- Herrera-Arellano A, Lopez-Villegas EO, Rodriguez-Tovar A, Zamilpa A, Jiménez-Ferrer E, Tortoriello J, et al. Use of antifungal saponin SC-2 of Solanum chrysotrichum for the treatment of vulvovaginal dandidiasis: in vitro studies and clinical experiences. African Journal Traditional Complementary and Alternative Medicines. 2013;10(3):410–7. [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. www.cochrane-handbook.org. The Cochrane Collaboration.
- Johnson BA, Nunley JR. Treatment of seborrheic dermatitis. American Family Physician. 2000;61(9):2703-10, 2713-4. [PUBMED: 10821151] [PubMed] [Google Scholar]
- Kastarinen H, Oksanen T, Okokon EO, Kiviniemi VV, Airola K, Jyrkkä J, et al. Topical anti-inflammatory agents for seborrhoeic dermatitis of the face or scalp. Cochrane Database of Systematic Reviews. 2014;(5) doi: 10.1002/14651858.CD009446.pub2. [CENTRAL: CD009446; DOI: 10.1002/14651858.CD009446.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, et al. The biology and chemistry of antifungal agents: a review. Bioorganic & Medical Chemistry. 2012;20:5678–98. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Naldi L, Rebora A. Clinical practice. Seborrheic dermatitis. New England Journal of Medicine. 2009;360(4):387–96. doi: 10.1056/NEJMcp0806464. [MEDLINE: 19164189] [DOI] [PubMed] [Google Scholar]
- Parry ME, Sharpe GR. Seborrhoeic dermatitis is not caused by an altered immune response to Malassezia yeast. British Journal of Dermatology. 1998;139(2):254–63. doi: 10.1046/j.1365-2133.1998.02362.x. [PUBMED: 9767239] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). 5.1. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration. 2011.
- Rigopoulos D, Ioannides D, Kolageromitros D, Gregoriou S, Katsambas A. Pimecrolimus cream 1% vs. betamethasone 17-valerate 0.1% cream in the treatment of seborrhoeic dermatitis. A randomized open-label clinical trial. British Journal of Dermatology. 2004;151(5):1071–5. doi: 10.1111/j.1365-2133.2004.06208.x. [MEDLINE: 15541087] [DOI] [PubMed] [Google Scholar]
- Scheinfeld NS. Seborrhoeic dermatitis. SKINMed. 2005;4(1):49–50. doi: 10.1111/j.1540-9740.2005.03961.x. [PUBMED: 15654167] [DOI] [PubMed] [Google Scholar]
- Schwartz RA, Janusz CK, Janniger CK. Seborrheic dermatitis: an overview. American Family Physician. 2006;74(1):125–30. [PUBMED: 16848386] [PubMed] [Google Scholar]
- United States Food, Drug Administration. Part 172 food additives permitted for direct addition to food for human consumption. In: Michele Bugenhagen., editor. Code of Federal Regulations: Title 21-Food and Drugs. Vol. 21. Washington: US Government Printing Office; 1987. p. 58. [: 21CFR172.510] [Google Scholar]
- Victoire A, Magin P, Coughlan J, van Driel ML. Interventions for infantile seborrhoeic dermatitis (including cradle cap) Cochrane Database of Systematic Reviews. 2014;(11) doi: 10.1002/14651858.CD011380.pub2. [DOI: 10.1002/14651858.CD011380] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamilpa A, Tortoriello J, Navarro V, Delgado G, Alvarez L. Five new steroidal saponins from Solanum chrysotrichum leaves and their antimycotic activity. Journal of Natural Products. 2002;65(12):1815–9. doi: 10.1021/np020261h. [DOI] [PubMed] [Google Scholar]


