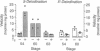Abstract
The type III iodothyronine 5-deiodinase metabolizes thyroxine and 3,5,3'-triiodothyronine to inactive metabolites by catalyzing the removal of iodine from the inner ring. The enzyme is expressed in a tissue-specific pattern during particular stages of development in amphibia, birds, and mammals. Recently, a PCR-based subtractive hybridization technique has been used to isolate cDNAs prepared from Xenopus laevis tadpole tail mRNA that represent genes upregulated by thyroid hormone during metamorphosis. Sequence analysis of one of these cDNAs (XL-15) revealed regions of homology to the mRNA encoding the rat type I (outer ring) 5'-deiodinase, including a conserved UGA codon that encodes selenocysteine in the mammalian enzyme. We report here that the protein encoded by the XL-15 cDNA efficiently catalyzes the (inner ring) 5-deiodination of 3,5,3'-triiodothyronine with a Km value of 2 nM and is resistant to inhibition by propylthiouracil and aurothioglucose. Our analysis confirms that the UGA codon encodes a selenocysteine that is critical for the catalytic activity of the enzyme. In addition, the direct induction of XL-15 mRNA levels by thyroid hormone in X. laevis tadpole tail tissue and cultured cell lines correlates closely with increases in 5- (but not 5'-) deiodinase activity. These findings indicate that the XL-15 cDNA encodes a type III 5-deiodinase and underscores the importance of the trace element selenium in thyroid hormone metabolism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
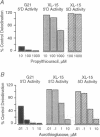
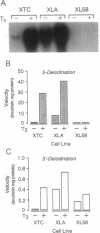
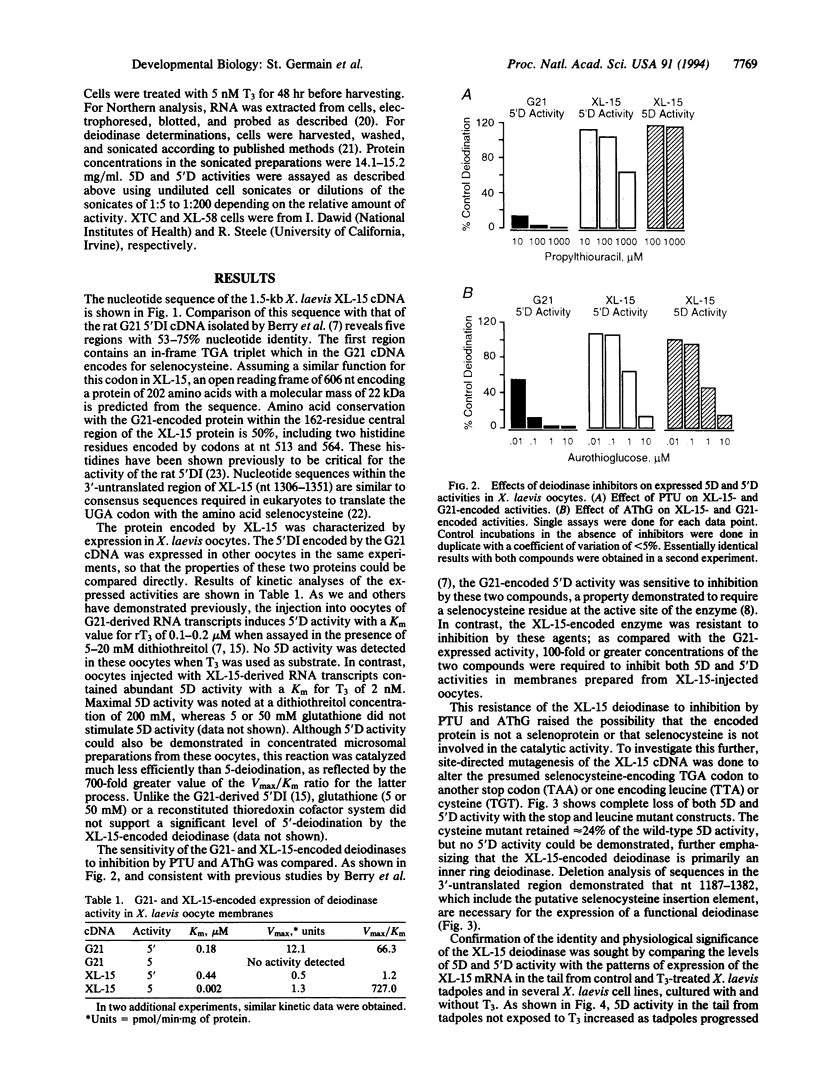
- Berry M. J., Banu L., Harney J. W., Larsen P. R. Functional characterization of the eukaryotic SECIS elements which direct selenocysteine insertion at UGA codons. EMBO J. 1993 Aug;12(8):3315–3322. doi: 10.1002/j.1460-2075.1993.tb06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Larsen P. R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991 Jan 31;349(6308):438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- Berry M. J. Identification of essential histidine residues in rat type I iodothyronine deiodinase. J Biol Chem. 1992 Sep 5;267(25):18055–18059. [PubMed] [Google Scholar]
- Berry M. J., Kieffer J. D., Harney J. W., Larsen P. R. Selenocysteine confers the biochemical properties characteristic of the type I iodothyronine deiodinase. J Biol Chem. 1991 Aug 5;266(22):14155–14158. [PubMed] [Google Scholar]
- Berry M. J., Kieffer J. D., Larsen P. R. Evidence that cysteine, not selenocysteine, is in the catalytic site of type II iodothyronine deiodinase. Endocrinology. 1991 Jul;129(1):550–552. doi: 10.1210/endo-129-1-550. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buckbinder L., Brown D. D. Thyroid hormone-induced gene expression changes in the developing frog limb. J Biol Chem. 1992 Dec 25;267(36):25786–25791. [PubMed] [Google Scholar]
- Galton V. A., Hiebert A. The ontogeny of the enzyme systems for the 5'- and 5-deiodination of thyroid hormones in chick embryo liver. Endocrinology. 1987 Jun;120(6):2604–2610. doi: 10.1210/endo-120-6-2604. [DOI] [PubMed] [Google Scholar]
- Galton V. A., McCarthy P. T., St Germain D. L. The ontogeny of iodothyronine deiodinase systems in liver and intestine of the rat. Endocrinology. 1991 Apr;128(4):1717–1722. doi: 10.1210/endo-128-4-1717. [DOI] [PubMed] [Google Scholar]
- Galton V. A., St Germain D. Putative nuclear triiodothyronine receptors in tadpole liver during metamorphic climax. Endocrinology. 1985 Sep;117(3):912–916. doi: 10.1210/endo-117-3-912. [DOI] [PubMed] [Google Scholar]
- Kanamori A., Brown D. D. Cultured cells as a model for amphibian metamorphosis. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6013–6017. doi: 10.1073/pnas.90.13.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol K., Kaptein E., Darras V. M., de Greef W. J., Kühn E. R., Visser T. J. Different thyroid hormone-deiodinating enzymes in tilapia (Oreochromis niloticus) liver and kidney. FEBS Lett. 1993 Apr 26;321(2-3):140–144. doi: 10.1016/0014-5793(93)80095-c. [DOI] [PubMed] [Google Scholar]
- Safran M., Farwell A. P., Leonard J. L. Evidence that type II 5'-deiodinase is not a selenoprotein. J Biol Chem. 1991 Jul 25;266(21):13477–13480. [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Santini F., Chopra I. J., Hurd R. E., Solomon D. H., Teco G. N. A study of the characteristics of the rat placental iodothyronine 5-monodeiodinase: evidence that it is distinct from the rat hepatic iodothyronine 5'-monodeiodinase. Endocrinology. 1992 Apr;130(4):2325–2332. doi: 10.1210/endo.130.4.1547744. [DOI] [PubMed] [Google Scholar]
- Sharifi J., St Germain D. L. The cDNA for the type I iodothyronine 5'-deiodinase encodes an enzyme manifesting both high Km and low Km activity. Evidence that rat liver and kidney contain a single enzyme which converts thyroxine to 3,5,3'-triiodothyronine. J Biol Chem. 1992 Jun 25;267(18):12539–12544. [PubMed] [Google Scholar]
- St Germain D. L., Croteau W. Expression of phenolic and tyrosyl ring iodothyronine deiodinases in Xenopus laevis oocytes is dependent on the tissue source of injected poly(A)+ RNA. Mol Endocrinol. 1989 Dec;3(12):2049–2053. doi: 10.1210/mend-3-12-2049. [DOI] [PubMed] [Google Scholar]
- St Germain D. L. The effects and interactions of substrates, inhibitors, and the cellular thiol-disulfide balance on the regulation of type II iodothyronine 5'-deiodinase. Endocrinology. 1988 May;122(5):1860–1868. doi: 10.1210/endo-122-5-1860. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Schoenmakers C. H. Characteristics of type III iodothyronine deiodinase. Acta Med Austriaca. 1992;19 (Suppl 1):18–21. [PubMed] [Google Scholar]
- Wang Z., Brown D. D. A gene expression screen. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11505–11509. doi: 10.1073/pnas.88.24.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Brown D. D. Thyroid hormone-induced gene expression program for amphibian tail resorption. J Biol Chem. 1993 Aug 5;268(22):16270–16278. [PubMed] [Google Scholar]