Abstract
Background
Adult cardiac surgery is significantly associated with the development of acute kidney injury (AKI). Still, the incidence and outcomes of AKI vary according to its definition. Our retrospective monocentric study comparatively investigates the yield of RIFLE definition, which is based on the elevation of serum creatinine levels (SCr) or the reduction of urine output (UO), taking into account only one or both criteria. Pre- and per-operative risk factors for post-operative AKI were evaluated.
Methods
All adult patients undergoing cardiac surgery, with or without cardiopulmonary bypass, from April 2008 to March 2009 were included. Clinical, biological and surgical features were recorded. Baseline serum creatinine was determined as its value on day 7 before surgery. Post-operative AKI was diagnosed and scored based upon the highest serum creatinine and/or the lowest urine output.
Results
443 patients (Male/Female ratio, 2.3; median age, 69y) were included, with 221 (49.9 %) developing postoperative AKI. Elevated serum creatinine (AKISCr) and oliguria (AKIUO) was observed in 9.7 % and 40.2 %, respectively. AKI patients had a significantly higher BMI and baseline SCr. In comparison to AKIUO, AKISCr mostly occurred in patients with co-morbidities, and was associated with an increased mortality at 1-year post surgery.
Conclusions
The use of standard RIFLE definition of AKI in a cohort of 443 patients undergoing cardiac surgery resulted in an incidence reaching 50 %. Still, significant discrepancies were found between AKISCr and AKIUO regarding the incidence and outcomes. In line with previous reports, our data questions the utility of urine output as a criterion for AKI diagnosis and management after cardiac surgery.
Keywords: Cardiac Surgery, Cardiopulmonary Bypass, Acute Kidney Injury, RIFLE, Serum Creatinine, Urine Output, Outcome, Mortality
Background
Adult cardiac surgery, with or without cardiopulmonary bypass (CPB), remains significantly associated with acute kidney injury (AKI). The incidence of post-surgery AKI varies from 5 to 42 % according to recent studies [1, 2]. In order to properly assess and compare the incidence and outcomes of AKI worldwide, the Acute Dialysis Quality Initiative Group proposed in 2004 a standard classification termed “RIFLE”, which stands for the acronym “Risk, Injury, Failure, Loss of kidney function and End stage kidney disease”, and is based on 2 criteria: serum creatinine levels (SCr) and urine output (UO) [3]. The main objectives of the present retrospective monocentric study were to (i) evaluate the incidence of AKI following cardiac surgery using the conventional RIFLE classification, and (ii) compare the yield of either RIFLE criteria to identify the patients developing AKI during the postoperative period. Next, we assessed the impact of AKI defined using either the full RIFLE classification or only one of either criterion on the length of in-ICU (intensive care unit) and in-hospital stays and on mortality at 1 year. Finally, we looked for the pre- and per-operative risk factors for post-operative AKI in our cohort.
Method
Patients
The present retrospective study was approved by the University Hospital of Liège Ethical Board (Ref. B70720108229). Data were collected from the database of University of Liège Hospital, and encompassed all adult patients who underwent heart surgery, including on- and off-pump coronary bypass grafting (CABG), isolated aortic or mitral valve reparation/replacement, or combined surgery (valve + CABG), from April 2008 to March 2009. Eight patients under chronic haemodialysis prior to surgery were excluded.
Types of anaesthesia and surgery
Anaesthesia was classically performed through target-controlled infusions of propofol and remifentanil. Tranexamic acid was used as antifibrinolytic agent (2.5 g injected prior to the start of surgery and 2.5 g injected at the end of CPB). The volume of priming was 1600 mL consisting of 150 mL of Mannitol® 15 % and 1450 mL of Volulyte® 6 %, 1 g of tranexamic acid, as well as 5000 IU of unfractioned heparine. Minimal haemodilution level was 20 % haematocrit. Standard cannulation was carried out (i.e. into the ascending aorta for reinjection and single cannulation of the right auricle for On-pump CABG and aortic valve surgery, double venous cannulation for mitral valve surgery). CPB (roller pump) was initiated to maintain a flow between 2.4 L/m2/min and 3.2 L/m2/min. Most interventions were conducted using normothermia, with intermittent cold crystalloid cardioplegia. Blood discharged from the left ventricle was collected in the venous reservoir of the CPB. The pericardial blood was suctioned in a separate reservoir, treated through an autologous transfusion system and re-transfused into the CPB circuit or directly to the patient after neutralisation of heparin for On-pump CABG. For isolated valve or combined surgery, the pericardial blood was re-infused to the CPB’s venous reservoir.
Clinical and biological variables
The following variables were analysed: gender (male vs female), age (years), body mass index (BMI, kg/m2), presence of high blood pressure (arterial pressure ≥140/90 mmHg) and/or treatment for high blood pressure (yes vs no), history of chronic obstructive pulmonary disease (COPD, yes vs no), angina pectoris (absence vs presence), myocardial infarction prior surgical intervention (yes vs no), diabetes mellitus (DM, yes vs no), emergency surgery (yes vs no), preoperative glomerular filtration rate (GFR, mL/min) and hemoglobin (g/dL), left ventricular ejection fraction < 60 % (LVEF < 60 %, yes vs no), surgical delay (days between the diagnostic coronarography and surgical intervention), type of operation (off-pump CABG, on-pump CABG, isolated valve, combined surgery), duration of aortic clamping (minutes), duration of CPB (minutes), nadir hematocrit during CPB and nadir mean arterial pressure (MAP, mmHg) during surgery. For each patient, the mortality scores (Parsonnet [4], EuroSCORE1 [5]) were calculated. In-ICU and in-hospital lengths of stay, as well as in-hospital mortality rates, were considered. Patients’ 1-year survival rate was established on the basis of post-operative follow-up and/or phone contact.
Assessment of renal function at baseline and after surgery
Baseline serum creatinine levels were systematically obtained at the time of the preoperative consultation in Anaesthesiology (i.e. 7 days before surgery) and measured by the isotope dilution mass spectrometry traceable Jaffe method from Roche®. GFR was estimated using the Modification of the Diet in Renal Disease (MDRD) formula. Following surgery, AKI was diagnosed within the first 7 days post-surgery. Severity of AKI according to the RIFLE classification was established by using the highest serum creatinine level and/or the lowest urine output. Stages L and E of the RIFLE classification, i.e. complete loss of renal function > 4 weeks or > 3 months, respectively, were not included in the analysis. The management of patients, including the use of diuretics and renal replacement therapy (RRT), was at the discretion of the physicians in charge.
Statistics
Results were expressed as median and inter-quartile ranges (IQR) and as counts and proportions (%) for qualitative variables. Non parametric Kruskal-Wallis and Wilcoxon tests were used for comparing samples from different groups. Proportions were compared using the chi-squared test for contingency tables. The relationship between patients’ postoperative renal status and a set of covariates was tested using binary logistic regression analysis. Results were expressed in terms of the odds ratio (OR) together with its 95 % CI. Survival data were illustrated using the Kaplan-Meier method, with the Cox proportional hazard (PH) model used to assess the effect of covariates on survival. All results were considered to be significant at the 5 % critical level (p < 0.05). Data analysis was carried out using, Satistica (version 10), SAS (version 9.3 for Windows) and S-PLUS (version 8.1) statistical softwares.
Results
Characteristics of the cohort
On the basis of the selection criteria, 443 patients were included during the study period. Baseline demographic, clinical, biological and operative characteristics of this cohort are summarized in Table 1. Proportion of male patients was predominant (70.0 %). The median age was 69 years [60–76]. Preoperative diagnostic of hypertension was present in 81.0 % of the patients. Most patients (76.7 %) had a normal renal function before surgery. Patients presenting with preoperative chronic kidney disease (CKD) were significantly older than individuals with normal kidney function (74-y vs 66-y, p = 0.0001), and included a higher proportion of females (41.7 % vs 26.5 %, p < 0.0001). The median mortality risks assessed by Parsonnet and EuroSCORE1 scores were 6.30 (3.10-13.6) and 4.00 (2.10-7.50), respectively, for the entire cohort. Two hundred and forty patients (54.2 %) were operated by CABG alone and 58 (13.1 %) of them were off-pump procedures.
Table 1.
Baseline, clinical and operative characteristics (N = 443)
| Variable | |
|---|---|
| Age (years) | 69.0 (60.0 - 76.0) |
| Gender (Female) (%) | 133 (30.0) |
| BMI (kg/m2) | 26.2 (23.7 - 29.4) |
| Hypertension (%) | 351 (81.0) |
| COPD (%) | 84 (19.0) |
| Angina pectoris (%) | 197 (44.5) |
| Myocardial infarction previous surgery (%) | 116 (26.2) |
| Diabete mellitus (%) | 100 (22.6) |
| Emergency (%) | 12 (2.70) |
| Preoperative GFR (mL/min) | 78.0 (64.0 - 92.0) |
| Preoperative hemoglobin (g/dL) | 13.9 (12.6 - 14.8) |
| LVEF < 60 % (%) | 114 (25.7) |
| Logistic Parsonnet (%) | 6.30 (3.10 - 13.6) |
| Logistic EuroSCORE1 (%) | 4.00 (2.10 - 7.50) |
| Surgical delay (days) | 21.0 (7.00 - 42.0) |
| Type of surgery: | |
| OPCABG (%) | 58 (13.1) |
| CABG (%) | 182 (41.1) |
| Valve surgery (%) | 148 (33.4) |
| Valve and CABG (%) | 55 (12.4) |
| CPB time (min.)* | 87.0 (73.0 - 104.0) |
| Aortic cross clamp time (min.)* | 55.0 (41.0 - 69.0) |
| Nadir hematocrit (%) | 21.0 (19.0 - 24.0) |
| Nadir MAP during surgery (mmHg) | 45.0 (40.0 - 55.0) |
| Days in ICU | 2.0 (2 - 4) |
| Days in hospital | 12.0 (10 - 18) |
Data are expressed as median (25-75th percentiles) or proportion of patients in %
*Data are calculated as median (25-75th percentiles) only for cases with cardiopulmonary bypass (n = 385)
BMI: body mass index; COPD: chronic obstructive pulmonary disease; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction; surgical delay: delay between diagnostic coronarography and surgery; OPCABG: off-pump coronary bypass graft; CABG: coronary artery bypass graft with cardiopulmonary bypass; Valve surgery: aortic valve replacement or mitral valve repair/replacement surgery; Valve and CABG: aortic valve replacement or mitral valve repair/replacement surgery combined with coronary artery bypass graft; CPB: cardiopulmonary bypass; MAP: mean arterial pressure.*: n = 385; ICU: intensive care unit
Incidence of AKI post cardiac surgery using combined or isolated criteria of the RIFLE classfication
Among the cohort of 443 patients, 221 (49.9 %) developed AKI within the week following surgery according to the conventional RIFLE classification. Eighty patients (36.2 %) reached stage RISK, 114 (51.6 %) stage INJURY and 27 (12.2 %) stage FAILURE. Stages L and E of the RIFLE classification were not included in our analysis. Of note, no patient actually reached L and E stages. Table 2 summarizes the clinical and operative features of the patients with or without postoperative AKI. Patients developing AKI were significantly older and heavier, with a lower preoperative GFR than non-AKI patients. Their logistic Parsonnet and EuroSCORE1 scores, as well as their program of surgery, were significantly worse. Of note, 8 patients (1.08 % of the cohort) required renal replacement therapy (RRT). Only 2 of them recovered normal renal function, while the other 6 died during their in-hospital stay.
Table 2.
Distribution of preoperative and operative data according to occurence or not of AKI allocated to RIFLE (Risk, Injury and Failure grade) and by the serum creatinine (SCr) or urine output (UO) criteria AKI
| Variable | No AKI | AKI | p-value | AKI SCr | AKI UO | p-value |
|---|---|---|---|---|---|---|
| (N = 222) | (N = 221) | (N = 43) | (N = 178) | |||
| Age (years) | 65.0 (59.0 - 74.0) | 71.0 (63.0 - 73.0) | < 0.001 | 71.0 (60.0 - 77.0) | 71.0 (63.0 - 77.0) | 0.98 |
| Gender (Female) (%) | 61 (27.5) | 72 (32.5) | 0.21 | 12 (27.9) | 60 (33.7) | 0.47 |
| BMI (kg/m²) | 25.6 (22.9 - 28.3) | 26.9 (24.3 - 30.5) | < 0.001 | 28.3 (24.8 - 30.9) | 26.6 (24.4 - 30.4) | 0.25 |
| Hypertension (%) | 178 (80.2) | 173 (78.3) | 0.62 | 40 (93.0) | 133 (74.7) | < 0.001 |
| COPD (%) | 39 (17.6) | 45 (20.4) | 0.45 | 14 (32.6) | 31 (17.4) | 0.03 |
| Angina pectoris (%) | 106 (47.7) | 91 (41.2) | 0.16 | 16 (37.2) | 75 (42.1) | 0.55 |
| MI previous surgery (%) | 67 (30.2) | 49 (22.2) | 0.06 | 10 (23.3) | 39 (21.9) | 0.85 |
| Diabetes mellitus (%) | 44 (19.8) | 56 (25.3) | 0.16 | 14 (32.6) | 42 (23.6) | 0.22 |
| Emergency (%) | 8 (3.60) | 4 (1.81) | 0.24 | 0 (0.00) | 4 (2.25) | 0.32 |
| Preoperative GFR | ||||||
| (mL/min) | 83.0 (73 - 97) | 72.0 (56 - 89) | < 0.001 | 67.0 (49 - 78) | 73.5 (56 - 89) | 0.06 |
| Preoperative | ||||||
| Hemoglobin (g/dL) | 14.0 (12.7 - 14.9) | 13.5 (12.4 - 14.7) | 0.10 | 12.9 (11.5 - 14.6) | 13.8 (12.5 - 14.8) | 0.06 |
| LVEF < 60% (%) | 58 (26.1) | 56 (25.3) | 0.85 | 13 (30.2) | 43 (24.2) | 0.41 |
| Logistic | ||||||
| Parsonnet (%) | 5.12 (2.65 - 10.8) | 7.60 (4.05 - 17.3) | < 0.001 | 8.41 (5.16 - 15.9) | 7.47 (4.00 - 18.7) | 0.71 |
| Logistic | ||||||
| EuroSCORE1 (%) | 3.51 (2.01 - 6.48) | 4.79 (2.28 - 8.58) | 0.004 | 5.29 (2.73 - 11.0) | 4.65 (2.21 - 8.44) | 0.40 |
| Surgical delay (days) | 20.0 (6 - 39) | 22.0 (8 - 45) | 0.18 | 17.0 (5 - 35) | 27.0 (8 - 48) | 0.02 |
| Type of surgery | ||||||
| OPCABG (%) | 37 (16.7) | 21 (9.50) | < 0.001 | 6 (14.0) | 15 (8.43) | 0.13 |
| CABG (%) | 102 (45.9) | 80 (36.1) | 13 (30.2) | 67 (37.6) | ||
| Valve surgery (%) | 74 (33.3) | 74 (33.5) | 19 (44.2) | 55 (30.9) | ||
| Valve and CABG (%) | 9 (4.05) | 46 (20.8) | 5 (11.6) | 41 (23.0) | ||
| CPB time (min.)* | 85.0 (70 - 100) | 90.0 (75.5 - 108.0) | 0.02 | 89.0 (81.0 - 100.0) | 90.0 (74.0 - 109.0) | 0.80 |
| Aortic cross | ||||||
| Clamp time (min.)* | 52.0 (39.0 - 64.0) | 58.0 (44.0 - 75.0) | 0.003 | 58.0 (45.0 - 71.0) | 58.5 (43.5 - 76.0) | 0.83 |
| Nadir hematocrit (%) | 22.0 (19 - 24) | 20.0 (19 - 24) | 0.06 | 20.0 (19.0 - 24.0) | 20.0 (19.0 - 24.0) | 0.83 |
| Nadir MAP | ||||||
| During surgery (mmHg) | 45 (40 -55) | 45 (40 -55) | 0.57 | 45 (40 -55) | 45 (40 -50) | 0.50 |
| Days in ICU | 2.0 (2 - 3) | 2.0 (2 -4) | < 0.001 | 3.0 (2 - 6) | 2.0 (2 - 4) | 0.07 |
| Days in hospital | 12.0 (10 - 16) | 13.0 (10 - 18) | 0.55 | 14.0 (11 - 22) | 12.0 (10 - 18) | 0.07 |
Data are expressed as median (25-75th percentiles) or proportion of patients in %
*Data are calculated as median (25-75th percentiles) only for cases with cardiopulmonary bypass (n = 385)
BMI: body mass index; COPD: chronic obstructive pulmonary disease; MI: myocardial infarction; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction; Surgical delay: delay between diagnostic coronarography and surgery; OPCABG: off-pump coronary artery bypass graft; CABG: Coronary artery bypass graft with cardiopulmonary bypass; Valve surgery: aortic valve replacement or mitral valve repair/replacement surgery; Valve and CABG: aortic valve replacement or mitral valve repair/replacement surgery combined with coronary artery bypass graft; CPB: cardiopulmonary bypass; MAP: mean arterial pressure; ICU: intensive care unit
The RIFLE criterion SCr only identified 9.7 % of patients developing postoperative AKI, whereas the UO criterion diagnosed AKI in 40.2 % of cases (Table 2). Patients developing AKIUO presented a significantly lower rate of preoperative hypertension (p < 0.001) and COPD (p < 0.05) than patients developing AKISCr. Preoperative GFR and hemoglobin levels were higher in the AKIUO than AKISCr patients. The delay between cardiologic exploration and heart surgery was also significantly longer in the AKIUO group (p < 0.05).
Influence of AKI definition on the length of in-hospital and ICU stays and mortality
On the basis of the full RIFLE classification, the duration of the ICU stay was shorter for patients who did not develop AKI after surgery than for AKI patients (2 [2 - 3] vs 3 [2 - 4] days; p < 0.001). However, no significant difference was observed regarding the length of in-hospital stay. The ICU period significantly increased with the degree of AKI severity based on the RIFLE stages: 2.0 (2–3) days for AKI RISK (n = 80), 3.0 (2 - 5) days for AKI INJURY (n = 114), and 5.0 (3 - 12) days for AKI FAILURE (n = 27); p < 0.001. When comparing either criterion of the RIFLE classification as the sole indicator of AKI, the duration of ICU stay tended to be slightly longer in AKISCr as compared to AKIUO (3.0 [2 - 6] vs 3.0 [2 - 4]; p = 0.07). Concerning mortality, 14 patients (3.16 %) died during their in-hospital stay. Causes of in-hospital mortality were: cardiogenic shock (n = 5), sepsis (n = 4), hemorrhagic shock (n = 3), stroke (n = 1), and aortic dissection (n = 1).
At one year post surgery, the follow-up was available for 364 patients of the cohort (82.2 %), and a sum of 20 deceased patients was recorded. Preoperative characteristics which were significantly associated with an increased risk for 1-year mortality included: age, COPD, CKD, anaemia, LEVF < 60 % and surgical delay (Table 3). The complexity of surgery, including the nadir of per-operative haematocrit, was also identified as a significant risk factor for death at 1-year post cardiac surgery. Postoperative AKI was also associated with an increased risk for 1-year mortality (p < 0.05, Table 3), which was further accentuated upon AKI severity score (p < 0.01, Fig. 1). One-year mortality rate was significantly higher in AKISCr patients in comparison to AKIUO group (p < 0.05, Fig. 2).
Table 3.
Distribution of preoperative and operative data according to status (death or alive) at one year after surgery
| Variable | Death | Alive | p-value |
|---|---|---|---|
| (N = 20) | (N = 344) | ||
| Age (years) | 77.0 (69.5 - 79.5) | 68.5 (60.0 - 75.0) | 0.004 |
| Gender (Female) (%) | 9 (45.0) | 94 (27.3) | 0.89 |
| BMI (kg/m2) | 24.5 (22.6 - 27.9) | 26.2 (23.6 - 29.3) | 0.28 |
| Hypertension (%) | 7 (35.0) | 56 (16.3) | 0.74 |
| COPD (%) | 7 (35.0) | 56 (16.3) | 0.03 |
| Angina pectoris (%) | 9 (45.0) | 149 (43.3) | 0.88 |
| MI previous surgery (%) | 3 (15.0) | 87 (25.3) | 0.30 |
| Diabetes (%) | 4 (20.0) | 80 (23.3) | 0.74 |
| Emergency (%) | 1 (5.00) | 8 (2.33) | 0.45 |
| Preoperative GFR (mL/min) | 60.0 (43.0 - 75.0) | 79.5 (65.0 - 93.0) | 0.002 |
| Preoperative hemoglobin (g/dL) | 11.4 (9.9 - 13.0) | 13.9 (12.6 - 14.9) | < 0.001 |
| LVEF < 60 % | 10 (50.0) | 82 (23.9) | 0.008 |
| Surgical delay (days) | 14.0 (4.0 - 19.0) | 22.0 (8.0 - 45.0) | 0.008 |
| Type of surgery | |||
| OPCABG (%) | 1 (5.00) | 42 (12.2) | 0.41 |
| CABG (%) | 6 (30.0) | 142 (41.3) | |
| Valve surgery (%) | 9 (45.0) | 116 (33.7) | |
| Valve and CABG (%) | 4 (20.0) | 44 (12.8) | |
| CPB time (min.)* | 89.5 (79.0 - 110.0) | 85.0 (66.5 - 102.5) | 0.19 |
| Aortic cross clamp time (min.)* | 59.5 (44.5 - 78.0) | 51.0 (35.0 - 67.0) | 0.13 |
| Nadir hematocrit (%) | 19.0 (18.0 -19.0) | 21.0 (19.0 - 24.0) | < 0.001 |
| Nadir MAP during surgery (mmHg) | 45.0 (40–53) | 45.0 (40–55) | 0.68 |
| Postoperative AKI (%) | 15 (75.0) | 175 (50.9) | 0.04 |
Data are expressed as median (25-75th percentiles) or proportion of patients in %
*Data are calculated as median (25-75th percentiles) only for cases with cardiopulmonary bypass (n=385)
BMI: body mass index; COPD: chronic obstructive pulmonary disease; MI: myocardial infarction; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction; Surgical delay: delay between diagnostic coronarography and surgery; OBCABG: off-pump coronary artery bypass graft; CABG: Coronary artery bypass graft with cardiopulmonary bypass; Valve surgery: aortic valve replacement or mitral valve repair/replacement surgery; Valve and CABG: aortic valve replacement or mitral valve repair/replacement surgery combined with coronary artery bypass graft; CPB: cardiopulmonary bypass; MAP: mean arterial pressure
Fig. 1.
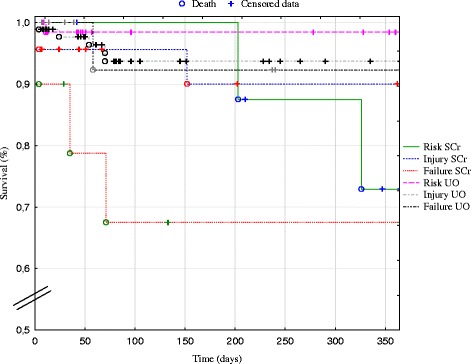
Survival post cardiac surgery according to acute kidney injury severity. Kaplan-Meier survival curve post cardiac surgery in patients with increasing RIFLE stages of acute kidney injury on the basis of elevated serum creatinine (SCr) or decreased urine output (UO)
Fig. 2.
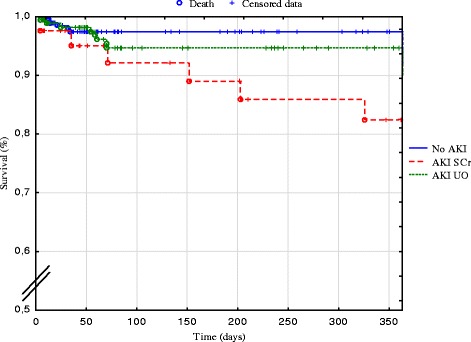
Survival post cardiac surgery according to AKI status. Kaplan-Meier survival curve post cardiac surgery in patients with no acute kidney injury (AKI, blue line), AKI with elevated serum creatinine (SCr, dotted red line) and AKI with decreased urine output (UO, dotted green line)
Predictive pre- and per-operative risk factors for AKI
In univariate analysis adjusted for preoperative CKD, six variables were positively associated with the occurrence of AKI defined upon the conventional RIFLE classification: age (OR = 1.02 [IC95 % 1.01-1.04], p = 0.016), BMI (OR = 1.08 [IC95 % 1.03-1.13], p = 0.001), logistical Parsonnet score (OR = 1.05 [IC95 % 1.03-1.09], p < 0.0001), combined surgery (OR = 6.26 [IC95 % 2.88-13.6], p < 0.0001), duration of aortic clamping (OR = 1.01 [IC95 % 1.01-1.02], p = 0.007) and duration of CPB (OR = 1.02 [IC95 % 1.01-1.03], p = 0.001). In multivariate setting, the occurrence of AKI increased with BMI (OR = 1.12 [IC95 1.06-1.18], p < 0.001), the type of surgery (combined surgery (OR = 10.03 [IC95: 3.11-33.9], p < 0.001)), and the lower preoperative GFR (OR = 0.98 [IC95: 0.97-0.99], p = 0.004) (Table 4).
Table 4.
Multivariate binary logistic regression of AKI development according to perioperative factors
| OR | 95 % CI | p-value | |
|---|---|---|---|
| Age (years) | 1.02 | 1.00 - 1.04 | 0.11 |
| Gender (Female vs Male) | 0.91 | 0.52 - 1.59 | 0.73 |
| BMI (kg/m2) | 1.12 | 1.06 - 1.18 | < 0.001 |
| Hypertension (Yes vs No) | 0.75 | 0.44 - 1.28 | 0.28 |
| COPD (Yes vs No) | 1.18 | 0.67 - 2.09 | 0.56 |
| Angina pectoris (Yes vs No) | 0.70 | 0.64 - 1.77 | 0.80 |
| MI previous surgery (Yes vs No) | 0.74 | 0.44 - 1.27 | 0.28 |
| Diabetes mellitus (Yes vs No) | 1.11 | 0.64 - 1.91 | 0.72 |
| Emergency (Yes vs No) | 0.39 | 0.09 - 1.72 | 0.21 |
| Preoperative GFR (mL/min.) | 0.98 | 0.97 - 0.99 | 0.004 |
| Preoperative hemoglobin (g/dL) | 0.02 | 0.87 - 1.19 | 0.84 |
| LVEF <60 % (Yes vs No) | 1.01 | 0.60 - 1.71 | 0.97 |
| Surgical delay (days) | 1.00 | 1.00 - 1.01 | 0.47 |
| Type of surgery: | |||
| Valve (vs CABG) | 1.56 | 0.70 - 3.51 | 0.28 |
| Valve and CABG (vs CABG) | 10.3 | 3.11 - 33.9 | < 0.001 |
| CPB time (min.)* | 1.01 | 1.00 - 1.02 | 0.17 |
| Aortic cross clamp time (min.)* | 0.99 | 0.96 - 1.01 | 0.23 |
| Nadir hematocrit (%) | 1.01 | 0.94 - 1.08 | 0.79 |
| Nadir MAP during surgery (mmHg) | 0.99 | 0.96 - 1.01 | 0.36 |
OR: odds ratio ± 95 % confidence interval; BMI: body mass index; COPD: chronic obstructive pulmonary disease; MI: myocardial infarction; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction; Surgical delay: delay between diagnostic coronarography and surgery; CABG: coronary artery bypass graft with cardiopulmonary bypass; Valve surgery: aortic valve replacement or mitral valve repair/replacement surgery; Valve and CABG: aortic valve replacement or mitral valve repair/replacement surgery combined with coronary artery bypass graft; CPB: cardiopulmonary bypass; MAP: mean arterial pressure; *, n=385
Discussion
The present retrospective monocentric study includes 443 patients undergoing elective or emergent cardiac surgery, and investigates the incidence and outcomes of postoperative AKI, as well as the pre- and per-operative risk factors for AKI. The incidence and outcomes of AKI vary according to its definition. In the present study, we first used the conventional 2004 RIFLE classification which is based on the elevation of SCr and the decrease of UO [6]. This classification has been approved for cardiac surgery [1, 7–9]. In an original approach, we compared AKI incidence and outcomes according to SCr or UO criteria. We observed a 7-day incidence of AKI reaching 50 % by taking into account both RIFLE criteria. This percentage of postoperative AKI is rather high, as compared to previous reports. This may be partly explained by the use of all daily values of SCr and UO for each patient over the 7-day period following surgery. In a similar retrospective study, D’Onofrio et al. reported an incidence of postoperative AKI of 23.5 % [7]. These authors only tracked the single variation in SCr during the length of hospitalisation. Conversely, by using the complete RIFLE classification, Hobson et al. reported an incidence of AKI reaching 43.0 % after cardiac surgery, which corresponds with our observations [1]. Interestingly, each component of RIFLE, i.e. SCr or UO, differentially contributes to AKI incidence and outcomes, as previously illustrated by McIlroy et al. [10] and Wlodzimirow, et al. [11]. In our series, the elevation of SCr was observed in 9.7 %, whereas a decreased UO was noted in 40.2 %. Such a 4-fold increase in AKI incidence when the UO criterion is taken into account provides cause for concern regarding its validity and the true significance of diagnosed AKIUO. To apply the SCr criteria of RIFLE, information on prior renal function is needed. When a pre-ICU admission SCr is not available, RIFLE group suggests that the baseline SCr be estimated from the MDRD formula [3]. Still, mathematical estimations of baseline SCr have been associated with both over- or under-diagnosis of AKI [12, 13]. Here, baseline SCr systematically corresponded with its value 7 days before surgery. Patients developing AKISCr showed a significantly higher preoperative morbidity, including hypertension, COPD, CKD and anaemia, than AKIUO patients (Table 2). Moreover, 1-year mortality rate was significantly higher in the case of AKISCr compared to AKIUO (Fig. 1). Systematic reviews investigating the validity of RIFLE classification in general ICU patients concluded that the mortality risk was higher in the absence of UO criterion in the AKI definition [14, 15]. Still, discarding the UO criterion has been suggested to delay the diagnosis of AKI, thereby increasing the mortality in general ICU patients [11]. In the particular settings of patients undergoing cardiac surgery, oliguria has also been associated with an increased incidence of AKI, but with a reduced discriminant utility for mortality in comparison to elevated SCr [10, 16]. As a whole, the inclusion of UO consensus criterion causes a questionable impact on the overestimated incidence of AKI after cardiac surgery.
The pathophysiology of postoperative AKI is multifactorial [17]. In our cohort, patients developing post surgery AKI were older and more fragile according to the Parsonnet and EuroSCORE1 scores, and presented with a higher BMI. Furthermore, increased BMI is significantly associated with DM (29.0 [25.8 - 31.6] vs 25.7 [23.1 - 28.3], p < 0.001) and hypertension (26.4 [23.9 - 29.9] vs 25.7 [23.0 - 27.8], p < 0.02). These risk factors were further highlighted by our multivariate model (Table 4). Pre-operative CKD reflected by a lower GFR before surgery was also associated with an increased risk for AKI. We estimated the pre-operative GFR using the MDRD formula based on serum creatinine concentration 7 days before surgery [18]. Most patients (76.7 %) showed a GFR within the normal range, i.e. > 60 ml/min. Patients presenting with impaired kidney function before surgery were significantly older (74 [68 - 78] years) than patients with GFR > 60 mL/min (66 [59 - 74] years, p < 0.001). This represents a selection bias. Indeed, given the impact of age in the multivariate logistical regression Parsonnet model, the predicted mortality rate at 30 days post surgery was higher in the CKD group compared to the rest of the cohort. The same pattern was observed with the logistical model EuroSCORE1 [5]. Similarly, we observed a lower 1-year survival probability in patients with a pre-operative GFR <60 mL/min [19].
The type and complexity of cardiac surgery was identified as an independent predictor of post-operative AKI upon our multivariate model (Table 3). At our institution, the choice between Off-pump and On-pump procedures essentially depends on the technical feasibility of myocardial revascularisation on the basis of coronarography images. In the present cohort, the types of surgeries, with or without CPB, are not distributed homogeneously, with more patients with normal renal function (15.3 %) being operated on using the Off-pump technique compared to CKD patients (5.83 %). Still, we did not observe a significantly higher incidence of AKI in On-pump versus Off-pump groups, in contrast to previous reports [20–22]. Patients with normal pre-operative renal function were mostly operated for isolated CABG, whereas only a minority (10.0 %) underwent combined surgery. Conversely, the distribution of surgery types appears more balanced in the CKD group, with 39.8 % of patients operated for CABG or isolated valve and 20.4 % for combined surgery. Such an imbalance between non-CKD and CKD patients may represent an additional selection bias in our analysis since the type of surgery influences the duration of aortic clamping and CPB, which in turn may favor AKI development.
In the present cohort, post-operative AKI was associated with a significantly longer stay in the ICU, with no impact on the entire in-hospital period. Moreover, the ICU period significantly increased with the degree of AKI severity based on the RIFLE stages. Elevation of SCr appeared to be associated with a prolonged ICU stay in comparison to UO, although this observation did not reach statistical significance (p = 0.07). Requirement for RRT was associated with a dramatic increase in the in-hospital mortality (75.5 %), as was previously reported [23–25].
The present study has several limitations, including its retrospective and monocentric design with a limited number of patients. Secondly, the majority of surgical interventions were planned (97.3 %), which limits the extension of the observations to emergency surgeries. Indeed, the need for nephrotoxic contrast medium at the time of preoperative coronarography may precipitate AKI development in cases of early cardiac surgery [26, 27]. Thirdly, the priming solution of CPB circuit most often consists of Volulyte 6 % (4th generation balanced hydroxyethyl starch). Recently, the use of such a starch has been associated with an increased incidence of AKI, as well as with postoperative bleeding [28]. Volulyte 6 % solution was chosen for CPB priming in order to reduce the risk for anaphylactoid shocks observed with some colloids or gelatins. New generation starches appear to be less nephrotoxic, provided that the perfusion is not extended beyond 24 h [29–31]. The optimal priming solution for CPB circuits remains a matter of debate.
Conclusions
The use of the conventional RIFLE classification in a cohort of 443 patients undergoing cardiac surgery found an incidence of AKI reaching 50 %. When each RIFLE criterion was considered individually, a significant discrepancy was found between AKISCr and AKIUO regarding AKI incidence and outcomes. AKISCr most often occurs in patients with a high-risk pre-operative profile, and is associated with an increased 1-year mortality compared to AKIUO. In line with previous reports, our present study urges the need for appropriately powered multi-centric follow-up trials to clarify the actual utility of UO as a criterion for AKI diagnosis and management in patients undergoing cardiac surgery.
Acknowledgements
The authors are grateful to Théophile Amand, Marie Erpicum, Marie-Pierre Fissette and Dominique Hella for their help in data recording.
F.J. is an MD Postdoctoral Fellow of the Fonds National de la Recherche Scientifique (FNRS) in the unit GIGA Cardiovascular Sciences of the University of Liège, and receives a financial support from the FNRS (Research Credit 3309), the University of Liège (Fonds Spéciaux à la Recherche) and from the Fonds Léon Fredericq.
Abbreviations
- AKI
Acute kidney injury
- CABG
Coronary artery bypass graft
- CKD
Chronic kidney disease
- COPD
Chronic obstructive pulmonary disease
- CPB
Cardioplumonary bypass
- GFR
Glomerular filtration rate
- ICU
Intensive care unit
- MAP
Mean arterial pressure
- MDRD
Modified of the died in renal disease
- OPCAB
Off-pump coronary artery bypass graft
- RIFLE
Risk, injury, failure, loss of function, end stage kidney disease
- RRT
Renal replacement therapy
- SCr
Serum creatinine
- UO
Urine output
- LVEF
Left ventricular ejection fraction
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MGL, FJ, JNK and FB designed and performed the study, analysed the data and wrote the paper; AF.D. and A.A. performed the bio-statistical analyses; LR, JMK and JOD designed the study, analysed the data and wrote the paper. All authors discussed the results and implications, and commented on the manuscript at all stages. All authors read and approved the final manuscript.
Contributor Information
Marc-Gilbert Lagny, Email: mglagny@chu.ulg.ac.be.
François Jouret, Email: francois.jouret@chu.ulg.ac.be.
Jean-Noël Koch, Email: jnkoch@alumni.ulg.ac.be.
Francine Blaffart, Email: francine.blaffart@chu.ulg.ac.be.
Anne-Françoise Donneau, Email: afdonneau@ulg.ac.be.
Adelin Albert, Email: aalbert@ulg.ac.be.
Laurence Roediger, Email: loroediger@yahoo.fr.
Jean-Marie Krzesinski, Email: jm.krzesinski@chu.ulg.ac.be.
Jean-Olivier Defraigne, Email: jo.Defraigne@chu.ulg.ac.be.
References
- 1.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–53. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 2.Arora P, Kolli H, Nainani N, Nader N, Lohr J. Preventable risk factors for acute kidney injury in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2012;26:687–97. doi: 10.1053/j.jvca.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R, Ronco C, Kellum J, Mehta R, Palevsky P, workgroup ADQI Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsonnet V, Dean D, Bernstein AD. A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation. 1989;79(6 Pt 2):I3–12. [PubMed] [Google Scholar]
- 5.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/S1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 6.Kellum JA. Acute kidney injury. Crit Care Med. 2008;36(4 Suppl):S141–5. doi: 10.1097/CCM.0b013e318168c4a4. [DOI] [PubMed] [Google Scholar]
- 7.D’Onofrio A, Cruz D, Bolgan I, Auriemma S, Cresce GD, Fabbri A, et al. RIFLE criteria for cardiac surgery-associated acute kidney injury: risk factors and outcomes. Congest Heart Fail. 2010;16(Suppl 1):S32–6. doi: 10.1111/j.1751-7133.2010.00170.x. [DOI] [PubMed] [Google Scholar]
- 8.Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, et al. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care. 2011;15:R16. doi: 10.1186/cc9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Delgado JC, Esteve F, Torrado H, Rodriguez-Castro D, Carrio ML, Farrero E, Javierre C, Ventura JL, Manez R: Influence of acute kidney injury on short- and long-term outcomes in patients undergoing cardiac surgery: risk factors and prognostic value of a modified RIFLE classification. Crit Care Lond Engl 2013, 17:R293. [DOI] [PMC free article] [PubMed]
- 10.McIlroy DR, Argenziano M, Farkas D, Umann T, Sladen RN. Incorporating oliguria into the diagnostic criteria for acute kidney injury after on-pump cardiac surgery: impact on incidence and outcomes. J Cardiothorac Vasc Anesth. 2013;27:1145–52. doi: 10.1053/j.jvca.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Wlodzimirow KA, Abu-Hanna A, Slabbekoorn M, Chamuleau RA, Schultz MJ, Bouman CS. A comparison of RIFLE with and without urine output criteria for acute kidney injury in critically ill patients. Crit Care. 2012;16:R200. doi: 10.1186/cc11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zavada J, Hoste E, Cartin-Ceba R, Calzavacca P, Gajic O, Clermont G, et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2010;25:3911–8. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- 13.Bagshaw SM, Uchino S, Cruz D, Bellomo R, Morimatsu H, Morgera S, et al. A comparison of observed versus estimated baseline creatinine for determination of RIFLE class in patients with acute kidney injury. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2009;24:2739–44. doi: 10.1093/ndt/gfp159. [DOI] [PubMed] [Google Scholar]
- 14.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538–46. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 15.Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692–702. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 16.Haase M, Bellomo R, Matalanis G, Calzavacca P, Dragun D, Haase-Fielitz A. A comparison of the RIFLE and Acute Kidney Injury Network classifications for cardiac surgery-associated acute kidney injury: a prospective cohort study. J Thorac Cardiovasc Surg. 2009;138:1370–6. doi: 10.1016/j.jtcvs.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Erpicum P, Detry O, Weekers L, Bonvoisin C, Lechanteur C, Briquet A, et al. Mesenchymal stromal cell therapy in conditions of renal ischaemia/reperfusion. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2014;29:1487–93. doi: 10.1093/ndt/gft538. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Brown JR, Cochran RP, MacKenzie TA, Furnary AP, Kunzelman KS, Ross CS, et al. Long-term survival after cardiac surgery is predicted by estimated glomerular filtration rate. Ann Thorac Surg. 2008;86:4–11. doi: 10.1016/j.athoracsur.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Massoudy P, Wagner S, Thielmann M, Herold U, Kottenberg-Assenmacher E, Marggraf G, et al. Coronary artery bypass surgery and acute kidney injury–impact of the off-pump technique. Nephrol Dial Transpl. 2008;23:2853–60. doi: 10.1093/ndt/gfn153. [DOI] [PubMed] [Google Scholar]
- 21.Kolli H, Rajagopalam S, Patel N, Ranjan R, Venuto R, Lohr J, et al. Mild acute kidney injury is associated with increased mortality after cardiac surgery in patients with eGFR < 60 mL/min/1.73 m(2) Ren Fail. 2010;32:1066–72. doi: 10.3109/0886022X.2010.510616. [DOI] [PubMed] [Google Scholar]
- 22.Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489–97. doi: 10.1056/NEJMoa1200388. [DOI] [PubMed] [Google Scholar]
- 23.Ho J, Reslerova M, Gali B, Nickerson PW, Rush DN, Sood MM, et al. Serum creatinine measurement immediately after cardiac surgery and prediction of acute kidney injury. Am J Kidney Dis. 2012;59:196–201. doi: 10.1053/j.ajkd.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Rosner M, Portilla D, Okusa M. Cardiac surgery as a cause of acute kidney injury: pathogenesis and potential therapies. J Intensive Care Med. 2008;23:3–18. doi: 10.1177/0885066607309998. [DOI] [PubMed] [Google Scholar]
- 25.Mariscalco G, Lorusso R, Dominici C, Renzulli A, Sala A. Acute kidney injury: a relevant complication after cardiac surgery. Ann Thorac Surg. 2011;92:1539–47. doi: 10.1016/j.athoracsur.2011.04.123. [DOI] [PubMed] [Google Scholar]
- 26.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–9. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 27.Medalion B, Cohen H, Assali A, Vaknin Assa H, Farkash A, Snir E, et al. The effect of cardiac angiography timing, contrast media dose, and preoperative renal function on acute renal failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2010;139:1539–44. doi: 10.1016/j.jtcvs.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Hecht-Dolnik M, Barkan H, Taharka A, Loftus J. Hetastarch increases the risk of bleeding complications in patients after off-pump coronary bypass surgery: a randomized clinical trial. J Thorac Cardiovasc Surg. 2009;138:703–11. doi: 10.1016/j.jtcvs.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 29.Blasco V, Leone M, Antonini F, Geissler A, Albanese J, Martin C. Comparison of the novel hydroxyethylstarch 130/0.4 and hydroxyethylstarch 200/0.6 in brain-dead donor resuscitation on renal function after transplantation. Br J Anaesth. 2008;100:504–8. doi: 10.1093/bja/aen001. [DOI] [PubMed] [Google Scholar]
- 30.Van Der Linden P, James M, Mythen M, Weiskopf RB. Safety of modern starches used during surgery. Anesth Analg. 2013;116:35–48. doi: 10.1213/ANE.0b013e31827175da. [DOI] [PubMed] [Google Scholar]
- 31.Godet G, Girard C, Guidet B, Ichai C, Lehot JJ, Leone M, et al. Hydroxyethylstarches and renal failure: to keep the reason is a necessity. Ann Fr Anesth Reanim. 2013;32:535–8. doi: 10.1016/j.annfar.2013.07.792. [DOI] [PubMed] [Google Scholar]


