Abstract
Background:
An alarming increase in infections due to penicillin non-susceptible pneumococci (PNSP) has been documented in nearly all countries. Increasingly, PNSP are also resistant to other antibiotics, and a growing number of clinical failures following the use of these agents have been reported.
Aims:
To determine the resistance pattern of pneumococcal isolates from patients with invasive pneumococcal infection in North West Nigeria.
Materials and Methods:
In a cross-sectional study clinical specimens were obtained from patients with community acquired pneumonia (CAP), meningitis and bacteraemia over a 2 year period. Pneumococcus strains were identified. Isolates were tested against a panel of antibiotics using E-test strips, and interpreted according to the CLSI criteria. 0.06 μg/ml was used as break point for penicillin. Analysis was carried out using descriptive statistics; relationships determined using chi-squared or Fisher's exact tests, with P < 0.05 regarded as significant.
Results:
Total number of isolates was 132. Twenty-two (16.7%) of the isolates were fully sensitive to penicillin while 73 (55.3%) and 37 (28.0%) were intermediately and fully resistant, respectively. One hundred and twenty-seven (96.2%) of the isolates were fully resistant to trimethoprim–sulphamethoxazole. Eleven (8.5%) were fully resistant to amoxicillin and 104 (78.8%) and 17 (12.9%) were intermediately resistant and fully susceptible. One hundred and six (80.3%) of the isolates were fully susceptible to chloramphenicol. Resistance to penicillin was shown to infer resistance to other antibiotics.
Conclusions:
Pneumococcal resistance is common in North West Nigeria. Ceftriaxone retains excellent activity against most of the invasive isolate, while trimethoprim-sulphamethoxazole is almost uniformly resistant.
Keywords: Antimicrobial, invasive, pneumococcus, resistance
INTRODUCTION
Streptococcus pneumoniae otherwise call ‘pneumococcus’ has remained an extremely important human bacterial pathogen since its initial recognition in the late 1800s and remained an important public health concern throughout the world. Globally it is the most common cause of community-acquired pneumonia (CAP), sporadic bacterial meningitis and bacteraemia.[1,2] While pneumococcal disease is a significant cause of morbidity and mortality worldwide, some groups bear a disproportionate share of the burden, such as the very young, the elderly, the economically disadvantaged, Africans,[3,4] and those with HIV/AIDS.[5,6]
From the beginning of the antibiotic era to the mid-1970s, pneumococcus remained uniformly susceptible to all classes of antibiotics that had been active against the organism, with the possible exception of tetracycline. However, in 1977 and 1978 an outbreaks of penicillin-resistance pneumococci (PRP) were reported in Durban and Johannesburg, South Africa.[7,8] Although they were originally called PRP, these organisms appeared to have acquired genetic material that encoded resistance to both penicillin and other commonly used antibiotics. The major mechanism of resistance involves the introduction of mutations in genes encoding penicillin-binding proteins and selective pressure is thought to play an important role. High-level penicillin resistance, macrolide resistance and multidrug-resistance often complicate the management of pneumococcal infection and make choosing empiric antimicrobial therapy for suspected cases of meningitis and pneumonia increasingly difficult. An alarming increase in infections due to penicillin non-susceptible pneumococci (PNSP) has been documented in nearly all countries in the world.[9,10] Benbachir et al. reported 30.4% of pneumococci as non-susceptible to penicillin in four African cities (Abidjan, Casablanca, Dakar, Tunisia).[11] In Kano, North-West Nigeria, 93%, 92%, 84%, 53% and 21% of pneumococci were non-susceptible to penicillin, co-trimoxazole, tetracycline and ampicillin, respectively.[12] Penicillin non-susceptible rates of 18%, 29.72%, 11.76% and 67% were also reported by different workers from different regions in Nigeria.[13,14,15,16] The rapid emergence of antibiotic resistance is adding to the burden of pneumococcal disease by significantly contributing to the numbers of treatment failures and deaths from this disease.
The previous studies in North-West Nigeria used disc diffusion method which is inadequate in distinguishing intermediate resistance from absolute resistance among pneumococcal isolates.[17,18] However, an E-test has been shown to be a good alternative for testing pneumococcal MIC when compared with the standard agar dilution method.[19] Hence, the main objective of the present study was to determine the resistance pattern of pneumococcal isolates from patients with invasive pneumococcal disease (IPD) in North West Nigeria using E-test strip.
MATERIALS AND METHODS
The study was a cross-sectional study among patients with community acquired pneumococcal pneumonia, meningitis or bacteraemia. The study was conducted in a tertiary referral centre in North West Nigeria over the period June 2009 to January 2011. The hospital is among the major referral centers for North-West states of Nigeria and neighboring countries like Niger republic. It has 550 beds and offers specialist inpatient and outpatient care, across various specialties.
All adult patients who were admitted with features compatible with case definition of community acquired pneumonia, meningitis and bacteraemia were recruited consecutively into the study. A sputum specimen was collected in a clean, sterile container from patients with clinical evidence of pneumonia. Blood samples were collected from all the patients, and inoculated directly into each of brain–heart infusion and thioglycolate culture media, with the use of standard aseptic procedures for aerobic and anaerobic cultures, respectively. A lumber puncture was conducted on all the patients presenting with clinical features suggestive of meningitis if there were no contraindication, the CSF samples were collected in a clean, sterile container and was sent to the laboratories for microbiological analysis and for glucose and protein measurement. A blood sample was taken for random plasma glucose measurement just before the lumbar puncture was performed, for comparison with the CSF glucose level. All samples were taken before administering antibiotic whenever feasible and transported to the laboratory immediately.
The laboratory operates 24 hour per day and able to process CSF specimen within 30 min of receipt. All the sputum specimen and CSF sample were inoculated onto 5% sheep blood agar and incubated at 37°C. Inoculated plates were incubated in a candle jar so as to create a reduced oxygen tension (5-10% additional CO2 tension). Inoculated blood culture bottles were incubated in the laboratory at 37°C and observed for bacterial growth within 24 to 72 hours and at day 7 if there was no bacterial growth. Subcultures of inoculated media were done twice, on days 2 and 3 after incubation, and were inoculated onto blood agar plates and incubated as far CSF and sputum. Plates were examined after incubation for bacterial pathogens, by the use of standard procedures. Samples of all typical pneumococcal colonies obtained from the plates were subjected to pneumococcal identification methods of α-hemolysis, colony morphology and ethylhydrocupreine hydrochloride (optochin).
Microbial susceptibility tests was carried out on all confirmed pneumococcal isolate to Penicillin G, Amoxycillin, Amoxycillin–Clvulanic acid, Cefuroxime, Ceftriaxone, Tetracycline, Trimethoprim/sulfamethoxazole (TPM/SMX), Azithromycin, ofloxacin and Chloramphenicol using Etest strips (Manufactured by AB BIODISK, Sweden). Minimum inhibitory concentrations (MICs) were measured and strains were divided into resistant, intermediate or sensitive according to the CLSI guidelines.[20]
Pneumococcal pneumonia was defined based on clinical findings plus a chest radiograph consistent with pneumonia, in addition to Gram positive diplococci on microscopy and positive culture of pneumococcus from an ideal sputum specimen defined as the presence of more than 25 white cells and less than 10 squamous epithelial cells per low power field. Pneumococcal meningitis was defined as isolation of pneumococcus with or without appearance of gram positive diplococci on microscopy from CSF or a CSF pleocytosis (>5 white blood cells/mm3) plus a blood culture positive for pneumococcus in a patient with clinical evidence of meningitis. Pneumococcal bacteremia was defined as isolation of pneumococcus from blood only.
Ethical clearance was obtained from the ethics committee of AKTH.
Analysis was carried out using descriptive statistics with differences and relationships determined using Student t-test, chi-squared and Fisher's exact tests as appropriate, with P < 0.05 regarded as significant.
RESULTS
The ages of the patients ranged from 18 to 82 years, with a mean age of 42.7 years ± 18.74. The peak age groups affected were 55-64 years and ≥65 years. There were 72 (54.5%) males and 60 (45.5%) females with a male to female ratio of 1:2.
A total of 132 pneumococcal isolate were recovered from different clinical specimens. One hundred and seventeen (88.6%) were recovered from sputum, 7 (5.3%) from CSF and 8 (6.1%) from blood.
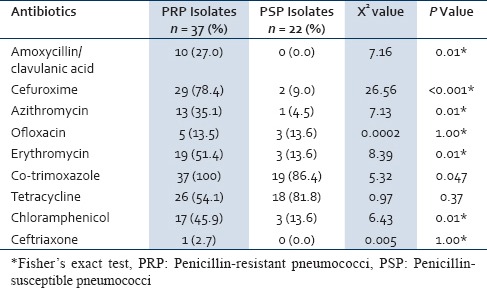
Twenty-two (16.7%) of the pneumococcal isolates were fully sensitive to penicillin while 73 (55.3%) were intermediately resistant and 37 (28%) were fully resistant. Only 5 (3.8%) of the isolates were sensitive to cotrimoxazole. The susceptibility profile of amoxicillin and amoxicillin/clavulanic acid was the same with 104 (78.8%) of the isolate been sensitive while 17 (12.9%) and 11 (8.5%) were intermediately resistant and fully resistant, respectively [Table 1]. Of the 132 isolates, 19 (14.4%) were resistant to only one class of antibiotic, 42 (31.8%) were resistant to two classes antibiotics while 71 (53.8%) were resistant to three or more antibiotics; none of the isolate was susceptible to the entire antibiotic tested. Penicillin-resistant pneumococcus is also resistant to more other antibiotics compared to penicillin susceptible pneumococcus [Table 2].
Table 1.
Susceptibility pattern of the pneumococcal isolates
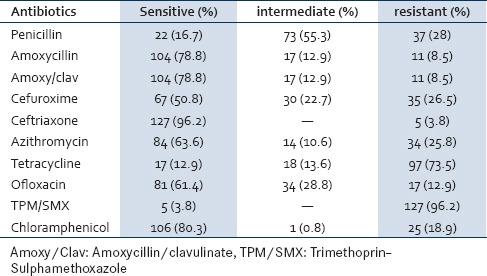
Table 2.
Resistance pattern of PRP and PSP against other antibiotics
DISCUSSION
The pattern of prescription of drugs by non-qualified personnel, under dosing, over prescription and outright fake drug racketeering has become profound in Nigeria.[21,22,23] These factors lead to development of resistance by bacteria to drugs that otherwise would have been helpful in treating infection.[24] We demonstrated pneumococcal resistance to commonly used antibiotics in a tertiary referral center in north-west Nigeria [Table 1].
In this study 28% of the isolate were fully resistance to penicillin and 55.3% were of intermediate resistance. Similarly, a previous study using disc diffusion method reported 95% of pneumococcal isolate to be non-susceptible to penicillin in Kano, Nigeria.[12] Akpede et al.[16] in Maiduguri north-east Nigeria reported penicillin non-susceptible rate of 67% among pediatric pneumococcal isolates, while in north central Nigeria Akanbi et al., in Ilorin reported resistance rate of 83%,[25] which is similar to our report. Furthermore, a recent multicenter study in two major cities in south west Nigeria reported 100% of pneumococcal isolates to be resistant to penicillin,[26] however, a decade earlier a resistance rate of 36%[27] was reported from this region thus highlighting the rapid spread of PRP in Nigeria. A lower resistance rate of 29.7% and 11.76% were reported among non-invasive nasopharyngeal isolate in North Central and South West Nigeria, respectively.[14,15] The high prevalence of PRP in Nigeria is not surprising as penicillin are the most widely used antibiotics. The greater the quantity and the longer the duration an antibiotic have been in use, the more likely resistant strains emerge to that particular antibiotic.[28]
The high prevalence of HIV infection and an attending prophylactic usage of TMP-SMX in AIDS patients has been linked to the wide spread pneumococcal resistance to TMP-SMX.[29] This antibiotic had been recommended by WHO for the treatment of pneumococcal disease in HIV/AIDS patients.[30] We found high prevalence of TMP-SMX (96.2%) resistance which is comparable to report from different part of Nigeria,[12,31,32] even though some studies in Nigeria reported a lower figure.[26,27] Amoxicillin and Amoxicillin/clavulanic acid showed relatively good activity in this study with 78.8% of the isolates being sensitive while an excellent activity was seen with ceftriaxone, with only 5% being fully resistance. Other Nigerian studies also reported a good susceptibility rate to these antibiotics.[12,22,27,32] However, contrary to our findings Bamidele et al.[26] reported a resistance rate of 55.6% among invasive pneumococcal isolates to amoxicillin–clavulinate, while Akanbi et al.[25] in a hospital-based study reported a resistance rate of 28.0% to ceftriaxone. This excellent activity seen with ceftriaxone might be related to the cost of the drug which is prohibiting in Nigeria, therefore not usually abuse. The macrolides have long been important in treating CAP, because of their excellent activity against pneumococci. This activity has been eroded in the past few years by the proliferation of macrolide-resistant strains. The prevalence of these resistance strains continues to increase in many parts of the world. In this study the resistance rate to azithromycin was 25.8%. A higher resistance rate of 56.6% and 66.7% to erythromycin were reported by other workers in Nigeria.[25,26] Contrary to our findings, Onipede et al.[33] reported 100% of invasive pneumococcal isolates from children to be sensitive to erythromycin which is also similar to findings by Adeleye et al.,[32] among sputum isolates from HIV-infected pediatrics and adult patients. Chloramphenicol is an old antibiotic that has been so much abused in the past, as a first-line treatment for typhoid fever in most developing countries, however, with the appearance of cheaper generic forms of quinolones, and appearance of chloramphenicol-resistant Salmonella typhi, prescribers have moved away from chloramphenicol and this relief of pressure on the drug, may be the reason for the good performance of chloramphenicol in this study with 80.3% being fully susceptible, with a reciprocal increase in ofloxacin non-susceptibility (41.7%). This corroborate with findings by other workers in Nigeria.[25,26,32]
It has been reported that pneumococci resistant to penicillin are more likely to become resistant to other antibiotic than penicillin-susceptible strains.[34] This is shown in this study where the resistance percentages among the cefuroxime, erythromycin, chloramphenicol, amoxicillin–clavulanic acid and azithromycin were consistently higher among penicillin-resistant strains than among penicillin-susceptible isolates [Table 2]. Kandakai et al. also reported similar findings.[14]
The heavy burden of community-acquired infectious diseases in most developing countries dictates a heavy requirement for antibiotics, often empirically. Chloramphenicol and TPM/SMX are commonly use as first-line drugs in the integrated management of childhood illness (IMCI) scheme.[22] In addition to TPM/SMX, penicillin and erythromycin are also widely use in sickle cell anemia and HIV/AIDS patient to prevent opportunistic infections.[35] These coupled with wide spread antibiotic self-medication,[23,36] may be promoting rapid emergence of antibiotic resistance in Nigeria.
In the first-line treatment of severe invasive pneumococcal infection in our environment we recommend the use of ceftriaxone; however, in a resource-constrained settings chloramphenicol, amoxicillin or amoxicillin–clavulanic acid may be an alternative. Cefotaxime was not studied in this study however it is expected to have similar spectrum to ceftriaxone, hence may be considered were indicated. With high prevalence of penicillin resistance in this study the use of penicillin for empiric treatment of suspected pneumococcal infection is no longer recommended. The findings of our study even though limited by small sample size also underscored the need for a comprehensive action plan in Nigeria to convert rapid spread of antimicrobial resistance among common bacteria pathogens such as pneumococcus and its associated morbidity and mortality. These should include promotion of rational use of antimicrobials, strengthening of antimicrobial resistance surveillance, and antimicrobial legislation among others.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Marston BJ, Plouffe JF, File TM, Jr, Hackman BA, Salstrom SJ, Lipman HB, et al. Incidence of community-acquired pneumonia requiring hospitalization: Results of population based active surveillance study in Ohio. Arch Intern Med. 1997;157:1709–18. [PubMed] [Google Scholar]
- 2.Schuchat A, Robinson K, Wenger JD, Harrison LH, Farley MM, Reingold AL, et al. Bacterial meningitis in United states in 1995. N Engl J Med. 1997;337:970–6. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 3.Center for Disease Control and Prevention. Prevention of pneumococcal disease: Recommendations of advisory committee on immunization practice (ACIP) MMWR Morb Mortal Wkly Rep. 1997;46:1–24. [Google Scholar]
- 4.Nourti P, Butler J, Farley M, Harrison LH, McGeer A, Kolazak MS, et al. Cigarette smoking and Invasive pneumococcal disease. N Engl J Med. 2000;342:681–9. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 5.Janoff EN, O’Brien J, Thompson P, Ehret J, Meiklejohn G, Duvall G, et al. Streptococcal pneumonia colonization, bacteraemia, and immune response among persons with human immunodeficiency virus infection. J Infect Dis. 1993;167:49–6. doi: 10.1093/infdis/167.1.49. [DOI] [PubMed] [Google Scholar]
- 6.Frankel RE, Virata M, Hardalo C, Altice FL, Freidland G. Invasive pneumococcal disease: Clinical features, serotype, and antimicrobial resistance patterns in cases involving patients with and without human immunodeficiency virus infection. Clin Infect Dis. 1996;23:577–84. doi: 10.1093/clinids/23.3.577. [DOI] [PubMed] [Google Scholar]
- 7.Appelbaum PC, Bhamjee A, Scragg JN, Hallett AF, Bowen AJ, Cooper RC. Streptococcus pneumonia resistant to penicillin and chloramphenicol. Lancet. 1977;2:995. doi: 10.1016/s0140-6736(77)92892-6. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs MR, Koornhof HJ, Robins-Browne RM, Stevenson CM, Vermaak ZA, Frieman I, et al. Emergence of multiply resistant pneumococci. N Engl J Med. 1978;299:735–40. doi: 10.1056/NEJM197810052991402. [DOI] [PubMed] [Google Scholar]
- 9.Whitney CG, Farley MM, Hadler J, Harrison LH, Lexau C, Reingold A, et al. Increasing prevalence of multidrug- resistance Streptococcus pneumonia in the United State. New Engl J Med. 2000;343:1917–24. doi: 10.1056/NEJM200012283432603. [DOI] [PubMed] [Google Scholar]
- 10.Doern GV, Heilmann KP, Huynh HK, Rhomberg PR, Coffman SL, Brueggemann AB, et al. Antimicrobial resistance among clinical isolates of Streptococcus pneumonia in the United State during 1999-2000, including a comparison of resistance rates since 1994-1995. Antimicrob Agents Chemother. 2001;45:1712–9. doi: 10.1128/AAC.45.6.1721-1729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benbachir M, Benredjeb S, Boye CS, Dosso M, Bellabbes H, Kamoun A. Two-year Surveillence of Antibiotic Resistance in Streptococcus pneumonia in Four African Cities. Antimicrob Agents Chemother. 2001;45:627–9. doi: 10.1128/AAC.45.2.627-629.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habib AG, Nwokedi EE, Ihesiulor IU, Mohammed A, Habib ZG. Widespread antibiotic resistance in savannah Nigeria. Afr J Med Med Sci. 2003;32:303–5. [PubMed] [Google Scholar]
- 13.Emele FE. Etiologic spectrum and pattern of antimicrobial susceptibility in bacterial meningitis in Sokoto, Nigeria. Acta Paediatr. 2000;89:942–6. doi: 10.1080/080352500750043396. [DOI] [PubMed] [Google Scholar]
- 14.Kandakai-Olukemi YT, Diso MS. Antimicrobial resistant profile of Streptococcus pneumoniae isolated from the nasopharynx of secondary school students in Jos. Ann Afr Med. 2009;8:10–3. doi: 10.4103/1596-3519.55757. [DOI] [PubMed] [Google Scholar]
- 15.Oduyebo OO, Nwaka DU, Nwaowolo C, Ogunsola FT. Resistance patterns of Streptococcus pneumonia isolated from the upper respiratory track of persons attending various clinics of a University Teaching Hospital in Lagos, Nigeria. Afr J Cln Exper Microbiol. 2006;7:89–97. [Google Scholar]
- 16.Akpede GO, Adeyemi O, Abba AA, Sykes RM. Pattern and antibiotic susceptibility of bacteria in pyogenic meningitis in a children's emergency room population in Maiduguri, Nigeria, 1988-1992. Acta Paediatrica. 1994;83:719–23. doi: 10.1111/j.1651-2227.1994.tb13126.x. [DOI] [PubMed] [Google Scholar]
- 17.Kanungo R, d’Lima D, Rajalakshmi B, Kumar A, Badrinath S. Emerging antibiotic resistant pneumococci in invasive infections in South India: Need for monitoring. Ind J Pharmacol. 2002;34:38–43. [Google Scholar]
- 18.Livin G, Ashkenzi S, Lev B, Harari S, Samra Z. The increasing penicillin resistant of Streptococcus pneumoniae in central Israel from 1988-2002. Int Pediatr. 2004;19:20–3. [Google Scholar]
- 19.Lalitha MK, Manayani DJ, Priya L, Jesudason MV, Thomas K, Steinhoff MC. E test as an alternative to conventional MIC determination for surveillance of drug resistant Streptococcus pneumoniae. Indian J Med Res. 1997;106:500–3. [PubMed] [Google Scholar]
- 20.Wayne, PA: CLSI; 2006. Clinical and Laboratory Standard Institute(CLSI). Performance Standards for Antimicrobial Susceptibility Testing: Sixteenth Informational Supplement. CLSI document M100-S16. (ISBN 1-56238-588-7) [Google Scholar]
- 21.Akpede O, Abiodun PO, Sykes M, Salami CE. Childhood bacterial meningitis beyond the neonatal period in southern Nigeria: Changes in organisms/antibiotic susceptibility. East Afr Med J. 1994;71:14–20. [PubMed] [Google Scholar]
- 22.Obaro S, Lawson L, Essen U, Ibrahim K, Brooks K, Otuneye A, et al. Community acquired bacteremia in young children from central Nigeria: A pilot study. BMC. Infect Dis. 2011;11:137. doi: 10.1186/1471-2334-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arikpo EA, Eja ME, Enyi-Idoh KH, Akubuenyi F, Ngang U, Akam C, et al. Patterns of antibiotics drug use in southern Nigerian communities. World J Appl Sci Technol. 2011;3:86–92. [Google Scholar]
- 24.Okeke N, Sosa A. Antibiotic resistance in Africa- discerning the enemy and plotting a defense. Afr Health. 2003;25:10–5. [Google Scholar]
- 25.Akanbi II, Taiwo AA, Babatunde SS, Onilke SK, Abdulraheem IS. Antibiotic susceptibility pattern of Streptococcus pneumoniae in Ilorin, Nigeria. Afr J Clin Exp Microbiol. 2004;5:173–6. [Google Scholar]
- 26.Iwalokun BA, Fowora M, Akinloye O, Oluwadun A, Antonio M, Adegbola RA. A retrospective study of clinical Streptococcus pneumoniaeisolates from four health facilities in South-West Nigeria. Int J Med Med Sci. 2012;4:160–70. [Google Scholar]
- 27.Fashae KF, Ogunsola FT, Salawu OM, Dada AO, Popoola O. A possible outbreak of Streptococcus pneumoniae invasive infection in children in Ibadan, Nigeria. Afr J Med Med. 2002;31:141–3. [PubMed] [Google Scholar]
- 28.Klugman KP. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–96. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soeters HM, Gottberg A, Cohen C, Quan V, Klugman KP. Epidemiology and Surveillance Trimethoprim-Sulfamethoxazole Prophylaxis and Antibiotic Nonsusceptibility in Invasive Pneumococcal Disease. Antimicrob Agents Chemother. 2012;56:1602–5. doi: 10.1128/AAC.05813-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geneva: 2005. [Last accessed on 11 June 2014]. WHO expert consultation on Cotrimoxazole pophylaxix in HIV infection. WHO technical report series. Available from: http://www.who.int/hiv/pub/meetingreports/ctx/en . [Google Scholar]
- 31.Falade AG, Lagunju IA, Bakare RA, Odekanmi AA, Adegbola RA. Invasive pneumococcal disease in children aged, 5 years admitted to 3 urban hospitals in Ibadan, Nigeria. Clin Infect Dis. 2009;48:190–6. doi: 10.1086/596500. [DOI] [PubMed] [Google Scholar]
- 32.Adeleye A, Uju L, Idika N, Sobande O. Cotrimoxazole Resistance in Streptococcus pneumoniaeIsolated from Sputum of HIV-positive Patients. West Indian Med J. 2008;57:497–9. [PubMed] [Google Scholar]
- 33.Onipede AO, Onayade AA, Elusiyan JB, Obiajunwa PO, Ogundare EO, Olaniran OO, et al. Invasive bacteria isolates from children with severe infections in a Nigerian hospital. J Infect Dev Ctries. 2009;3:429–36. doi: 10.3855/jidc.413. [DOI] [PubMed] [Google Scholar]
- 34.Doern GV, Brueggemann A, Holley HP, Rauch AM. Antimicrobial resistance of streptococcus pneumoniae recovered from out patients in the united states during the winter month of 1994-1995: Results of a 30 center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–13. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grange AO, Iroha EO, Akinsulie AO, Temiye EO, Ezeaka VC, Adetifa IM. Guidelines for the management of HIV/AIDs in infants and older children in Lagos University Teaching Hospital. Nig Qt J Hosp Med. 2003;13:8–13. [Google Scholar]
- 36.Iwalokun BA, Oluwadun A, Iwalokun O, Olukosi YA, Agomo PU. Reduction in Febrile Episodes and Dynamics of Pyrogenic Threshold in Nigerian Children with Plasmodium falciparum Malaria. J Paediatr Infect Dis. 2011;6:185–94. [Google Scholar]


