Abstract
Studies were carried out to assess the influence of nutrients, dissolved oxygen (DO) concentration, and nickel (Ni) on river biofilm development, structure, function, and community composition. Biofilms were cultivated in rotating annular reactors with river water at a DO concentration of 0.5 or 7.5 mg liter−1, with or without a combination of carbon, nitrogen, and phosphorus (CNP) and with or without Ni at 0.5 mg liter−1. The effects of Ni were apparent in the elimination of cyanobacterial populations and reduced photosynthetic biomass in the biofilm. Application of lectin-binding analyses indicated changes in exopolymer abundance and a shift in the glycoconjugate makeup of the biofilms, as well as in the response to all treatments. Application of the fluorescent live-dead staining (BacLight Live-Dead staining kit; Molecular Probes, Eugene, Oreg.) indicated an increase in the ratio of live to dead cells under low-oxygen conditions. Nickel treatments had 50 to 75% fewer ‘live’ cells than their corresponding controls. Nickel at 0.5 mg liter−1 corresponding to the industrial release rate concentration for nickel resulted in reductions in carbon utilization spectra relative to control and CNP treatments without nickel. In these cases, the presence of nickel eliminated the positive influence of nutrients on the biofilm. Other culture-dependent analyses (plate counts and most probable number) revealed no significant treatment effect on the biofilm communities. In the presence of CNP and at both DO levels, Ni negatively affected denitrification but had no effect on hexadecane mineralization or sulfate reduction. Analysis of total community DNA indicated abundant eubacterial 16S ribosomal DNA (rDNA), whereas Archaea were not detected. Amplification of the alkB gene indicated a positive effect of CNP and a negative effect of Ni. The nirS gene was not detected in samples treated with Ni at 0.5 mg liter−1, indicating a negative effect on specific populations of bacteria, such as denitrifiers, resulting in a reduction in diversity. Denaturing gradient gel electrophoresis revealed that CNP had a beneficial impact on biofilm bacterial diversity at high DO concentrations, but none at low DO concentrations, and that the negative effect of Ni on diversity was similar at both DO concentrations. Notably, Ni resulted in the appearance of unique bands in 16S rDNA from Ni, DO, and CNP treatments. Sequencing results confirmed that the bands belonged to bacteria originating from freshwater and marine environments or from agricultural soils and industrial effluents. The observations indicate that significant interactions occur between Ni, oxygen, and nutrients and that Ni at 0.5 mg liter−1 may have significant impacts on river microbial community diversity and function.
The impact of environmental stresses on microbially dominated systems such as rivers is poorly understood. It is also necessary to assess the effects of combinations of stresses, including dissolved oxygen (DO) levels, metals, and nutrients, which may be introduced due to municipal, industrial, and agricultural activities. Further questions arise regarding whether these stresses operate additively or whether they may act to mask or reduce the effects of each other. To effectively study these questions, there is a need to look at parts of aquatic ecosystems that are abundant, ubiquitous, stationary, exposed to stress, and integral to ecosystem function. Microorganisms, particularly those growing as biofilms, meet all of these criteria and are also an important source of food within rivers. Within riverine systems, microbial aggregates and biofilms are important in terms of fundamental processes, such as biogeochemical cycling and biodegradation activity (45). Hence, these communities represent excellent potential indicators of changes in ecosystem health. However, experiments have typically involved short-term exposures, applied specific bacteria, or other taxa as indicators and usually addressed a single stress (5, 21, 44). Further, previous studies of biofilm metal interactions have largely focused on sorption of the metals (see, for example, reference 15).
Recently, we have developed in situ techniques to investigate the structure of complex interfacial microbial biofilms grown in rotating annular bioreactors suitable for replicated biofilm studies (42). It is possible to apply multichannel imaging and confocal laser microscopy to assess biofilm parameters such as bacterial biomass, exopolymeric substance (EPS) biomass, cyanobacterial biomass, and algal biomass (41), allowing microscale analyses of complex microbial communities. In addition, the detection and quantification of cellular and polymeric compounds in biofilms (52, 53) and in situ visualization and localization of contaminants in biofilm systems (43) are possible. Techniques have also been developed to facilitate the quantitative characterization of EPS in situ by using fluor-conjugated lectins (54). Molecular tools and techniques also exist that capitalize on known catabolic and other genes. For example, catabolic genes from the microbial degradation pathways for simple aliphatic hydrocarbons (i.e., alkB) or biogeochemical cycles (i.e., nirS) may be used to detect specific populations (25, 73, 74). Fluorescent in situ hybridization may also be used to assess community composition (50). Additional analyses can be performed with total community DNA. After amplification by PCR, fragments can be subjected to analysis by using denaturing gradient gel electrophoresis (DGGE) (17, 32, 65). Sequencing information may also provide additional resolution on the nature of changes in these microbial communities (14).
Using these approaches, we investigated the impact of the single and combined effects of nutrients, nickel, and oxygen level on the form, arrangement, composition, and nutrient cycling of river biofilms grown in replicated rotating annular biofilm reactors.
MATERIALS AND METHODS
Microbial cultures.
Lotic biofilms were grown in rotating annular biofilm reactors as described in detail by Lawrence et al. (42). Natural river water (South Saskatchewan River, Saskatoon, Saskatchewan, Canada) was used as inoculum and as a source of carbon and nutrients. The bioreactors held 12 polycarbonate slides (1 by 11 cm) that could be removed for microscopic examination of the biofilms. The experimental setup included temperature-controlled reservoirs one (10 liters) for every two reactors (17 ± 2°C) for the raw and nutrient amended river water, a peristaltic pump for circulating the water, and the rotating annular reactor with motor and control for adjusting the speed of the rotating inner cylinder. All reactor components were surface sterilized with 1.2% sodium hypochlorite for 1 h. The reactors were operated at 100 rpm (a surface velocity of 1.9 km h−1). The water in the reservoirs was replaced with fresh river water and nutrients throughout the experimental period every 7 days. Nutrient levels were assessed as described by Chenier et al. (8). The water was pumped through the reactors at a rate of 500 ml per day (one reactor volume) by using a multichannel peristaltic pump (Watson Marlow, Wilmington, Mass.). A NiCl2 · 6H2O solution was added via syringe pump directly into the reactors at a rate that provided a constant 0.5-mg liter−1 nickel concentration in the reactor. Nickel levels were analyzed for dissolved metals by inductively coupled plasma mass spectrometry.
Treatments included a control (river water alone) with or without nickel (Ni) added at 0.5 mg liter−1, river water amended with carbon added as glucose at 66.7 μmol of C liter−1, nitrogen added as ammonium chloride at 80 μmol of N liter−1, phosphorus added as potassium phosphate at 5 μmol of P liter−1 in combination for the CNP application, whereas oxygen concentration was controlled at either 7.5 or 0.5 mg liter−1 in the reactor waters. Oxygen was removed by subjecting input water to vacuum stripping (48) with a reverse oxygen-free nitrogen gas flow, and low-oxygen reactors were placed in a sealed aquarium under an oxygen-free nitrogen atmosphere created by pumping oxygen-free nitrogen through the low-oxygen treatment aquarium with a peristaltic pump. Oxygen levels in the reactor were routinely monitored by using a StrathKelvin (Glasgow, United Kingdom) model 781b oxygen meter.
CLSM and image analysis.
Examination of all stained and control materials was carried out with an MRC 1024 confocal laser scanning microscope (Bio-Rad, Hemel Hempstead, United Kingdom) attached to a Microphot SA microscope (Nikon, Tokyo, Japan). Slides from each of the replicate reactors were cut into ∼1-cm2 pieces and mounted in small petri dishes by using Dow Corning #3140 acid-free silicone coating (WPI, Inc., Sarasota, Fla.) and then stained and analyzed according to the following procedures. For observation, the following water-immersible lenses were used: ×63 0.9 NA (Zeiss, Jena, Germany) and ×40 0.55 NA (Nikon). The biofilms were observed by using a double labeling procedure and three-channel recording: bacteria were stained with the fluorescent nucleic acid stain (SYTO 9) (excitation wavelength, 488 nm [ex 488]; emission wavelength, 522 to 532 nm [em 522/32]); a lectin probe (Triticum vulgaris-TRITC [tetramethyl rhodamine isothiocyanate]; ex 568, em 605/32) was used to visualize exopolymer, and autofluorescence (ex 647, em 680/32) was used to detect algal and cyanobacterial cells. Digital image analysis of the confocal laser scanning microscopy (CLSM) optical thin sections in each of the three channels was used to determine such parameters as biofilm depth, bacterial cell area (biomass), exopolymer biomass, cyanobacterial biomass, and total photosynthetic biomass at various depths. Image analyses were performed by using NIH Image version 1.61 (http://rsb.info.nih.gov/nih-image/) with macros written for semiautomated quantification as described in Manz et al. (50). In addition, three color red-green-blue projections of the biofilms were computed.
Exopolymer analyses.
Lectins with fluorescein isothiocyanate or TRITC labeling were purchased (Sigma, St. Louis, Mo.). Cy5 labeling was done by using a commercial labeling kit according to the instructions (Research Organics, Cleveland, Ohio). The lectins Arachis hypogaea, Canavalia ensiformis, Glycine max, and Ulex europaeus were used alone or in combination for in situ analyses of polymer composition as described by Neu et al. (54). Image analyses and calculations of lectin binding volumes were carried out by using the equations of Neu et al. (54).
Live-dead analyses.
A portion of the replicate biofilm strips (1 cm2) was stained with the BacLight Live-Dead staining kit (Molecular Probes, Eugene, Oreg.) as described by the manufacturer and enumerated by CLSM imaging and digital image analyses. The method follows that described by Neu and Lawrence (52). A semiautomated macro developed in NIH Image 1.6.1 was used to determine the numbers of live and dead cells in confocal image stacks as described above.
Carbon utilization spectra.
Carbon utilization spectra were determined for biofilm samples by using commercial Eco-Plates (BIOLOG, Hayward, Calif.) (20). Biofilm coupons were scraped with a sterile silicon rubber spatula to remove the biofilm and sonicated in a Bransonic 5120 water bath sonifier (Branson Ultrasonics, Danbury, Conn.) for 5 min to disperse the cells, and appropriate dilutions (10−4) were inoculated (150 μl) into all 96 wells of the BIOLOG microtiter plates and then incubated at 23 ± 3°C under atmospheric oxygen. The plates were read by using a standard microtiter plate reader each day until a stable result was obtained (7 days).
Plate counts.
Biofilm subsamples (1 cm2) were prepared by sterile scraping and sonication (as described above) prior to dilution and subsequent inoculation of a variety of media for both most probable number (MPN) and plate count estimation of specific populations. Tenfold dilutions were prepared in 9 ml of sterile pH 7.0 river water blanks. Dilutions were spread plated in triplicate onto 10% tryptic soy agar, actinomycete isolation agar, Rose Bengal agar, and peptone, tryptone, yeast extract, and glucose (PTYG) agar. All dried medium components were obtained from Difco (Detroit, Mich.). Counts of a variety of aerobic physiological groups were carried out by using a MPN method and appropriate media. These included thiosulfate oxidizers (40), nitrifying bacteria (64), and methylotrophic bacteria (58). Media were also inoculated to enumerate Fe(III)-reducing populations (47), sulfate-reducing bacteria (75), and denitrifying bacteria (71).
Biogeochemical cycling assays: microcosm preparation.
After the 8-week growth period in the bioreactors, fresh biofilm samples on polycarbonate strips were used in microcosms to assess the impact of nickel on bacterial activity. The polycarbonate strips were aseptically cut (2 cm2), and each piece with its associated biofilm was transferred into a 27-ml, crimpable, glass vial with 5 ml of the same river water supplemented with the same amendments as those used in the bioreactors. Microcosms were prepared, incubated, and analyzed as described by Chenier et al. (8). Hexadecane mineralization results are expressed as the cumulative percentage of 14CO2 produced from the [14C]-1-hexadecane (specific activity, 2.2 mCi mmol−1; Sigma-Aldrich, Mississauga, Ontario, Canada) initially added (100 mg of hexadecane liter−1 and 50,000 dpm), as measured by liquid scintillation spectrometry (Tri-Carb 2100TR; Packard Instruments, Downers Grove, Ill.). Negative controls were autoclaved. For nitrification, NH4Cl (BDH, Toronto, Ontario, Canada) was added at a concentration of 5 mmol liter−1, and microcosms were analyzed by high-pressure liquid chromatography (HPLC) for the production of NO3−. For denitrification, NaNO3 (BDH) was added at a concentration of 5 mmol liter−1, and microcosms were analyzed by gas chromatography for N2O production. For nitrification and denitrification, acetylene (C2H2; Prodair, Montreal, Quebec, Canada) was added as inhibitor in negative controls.
Molecular analyses. (i) Total community DNA extraction.
For each bioreactor, a frozen polycarbonate strip was aseptically cut (2 cm2) and transferred into a 50-ml polypropylene tube (Falcon; Becton Dickinson, Franklin Lakes, N.J.). Bacterial cells from the frozen biofilm samples were lysed by enzymatic treatment (lysozyme and proteinase K), DNA extraction was performed by phenol-chloroform treatment and alcohol precipitation, and the resulting pellet was dissolved in 30 μl of Tris-EDTA as described by Chenier et al. (8).
(ii) PCR amplification.
To assess the metabolic potential of the biofilm, extracts of the following target genes were analyzed by PCR amplification. The alkB gene encodes for alkane hydroxylase, a monooxygenase that is the first enzyme of the hexadecane mineralization pathway (37); the nirS gene encodes for cytochrome cd1-containing nitrite reductase, which reduces nitrite to nitric oxide during denitrification (7). The gene encoding for eubacterium 16S rRNA was also amplified to perform DGGE and sequencing. The PCR amplification procedure was described in detail by Chenier et al. (8). Essentially, 1 μl of DNA was added to 49 μl of reaction mix (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) containing 0.5 μmol of each primer liter−1 for eubacterial 16S ribosomal DNA (rDNA) or 1 μmol of each primer liter−1 for functional genes (alkB and nirS), as described in Table 1 and 2.5 U of Taq DNA polymerase (Amersham Pharmacia Biotech). The PCR products were electrophoresed in 1.4% agarose (Wisent, St-Bruno, Quebec), stained with ethidium bromide, and photographed (8).
TABLE 1.
Oligonucleotide primers used for PCR amplification of bacterial genes
| Target gene and orientation | Primer consensus sequence, position (length in nucleotides)b | Reference organism | PCR fragment size (bp) | Target bacteria (target enzyme) |
|---|---|---|---|---|
| 16S rDNA | ||||
| Forwarda | 5′-CCT ACG GGA GGC AGC AG-3′, 341-357 (17) | Pseudomonas putida ATCC 17484 | 460 | Bacteria (16S rDNA) |
| Reverse | 5′-CTA CCA GGG TAT CTA ATC C-3′, 740-758 (19) | Pseudomonas putida ATCC 17484 | 460 | Bacteria (16S rDNA) |
| alkB | ||||
| Forward | 5′-CIG IIC ACG AII TIG GIC ACA AGA AGG-3′, 495-521 (27) | Rhodococcus sp. strain Q15 | 550 | Hexadecane degraders (alkane hydroxylase) |
| Reverse | 5′-IGC ITG ITG ATC III GTG ICG CTG IAG-3′, 1018-1044 (27) | Rhodococcus sp. strain Q15 | 550 | Hexadecane degraders (alkane hydroxylase) |
| nirS | ||||
| Forward | 5′-CGG CTA CGC GGT GCA TAT CTC GCG TCT GTC-3′, 572-601 (30) | Pseudomonas stutzeri ATCC 14405 | 320 | Nitrite oxidizers (cd1 nitrite reductase) |
| Reverse | 5′-GAT GGA CGC CAC GCG CGG CTC GGG GTG GTA-3′, 864-893 (30) | Pseudomonas stutzeri ATCC 14405 | 320 | Nitrite oxidizers (cd1 nitrite reductase) |
Preceded by a GC clamp for DGGE (not for sequencing): CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG G (40 nucleotides).
I, inosine.
(iii) DGGE.
PCR products were prepared according to a modification of Teske et al. (70). The 16S PCR products were precipitated with 1/10 volume of 3 mol of sodium acetate (pH 5.2) liter−1 and 2.5 volumes of ethanol at −20°C for 1 h. The samples were centrifuged at 15,800 × g for 15 min, and the pellets were washed with 70% ethanol and dried under vacuum. The pellets were resuspended in 15 μl of pH 8.0 T10E0.1 (10 mmol of Tris-Cl [pH 8.0] and 0.1 mmol of EDTA liter−1 [pH 8.0]) and quantitated by Picogreen as described by the manufacturer. A total of 600 ng of the PCR products was loaded onto the gel. DGGE was performed by using a Bio-Rad DCode Universal Mutation Detection System (Bio-Rad) as described by the manufacturer. To avoid disturbing the gradient during comb insertion, PCR products were first loaded on a 6% (wt/vol) stacking polyacrylamide gel (no denaturant) (33), and then separated on an 8% (wt/vol) polyacrylamide gel with 40 to 65% denaturant (where 100% denaturant contained 7 mol of urea liter−1 and 40% formamide) for 16 h at 80 V and 60°C, as modified from Juck et al. (32). The gels were then stained with Vistra Green (Amersham Pharmacia Biotech, Inc., Baie d'Urfé, Quebec, Canada) and visualized with a FluorImager 595 (Molecular Dynamics, Sunnyvale, Calif.) with a 488-nm excitation filter and a 530-nm emission filter. Dendrogram analysis of DGGE banding patterns was performed by using the Dendron 3.0 software package (Solltech, Inc., Oakdale, Iowa) (32).
(iv) Sequencing.
Twenty-three individual bands (two to five from each experimental condition) were excised from the DGGE gel and macerated with 50 μl of sterile deionized water at 37°C overnight to elute the DNA. The acrylamide was removed by centrifugation. The DNA obtained from the excised bands was reamplified with the same primer pair in order to obtain >250 ng of DNA. Quantification was done by agarose gel electrophoresis as described above with 8.2, 16.4, and 24.6 μg of GeneRuler 100-bp DNA ladder. The PCR products corresponding to each specific band were pooled, precipitated by adding 1 μl of 20 mg of glycogen (Roche Diagnostics Corp., Indianapolis, Ind.) liter−1, a 0.5 volume of ammonium acetate (>97%; Merck-EM Science, Darmstadt, Germany) at 7.5 mol liter−1, and 1 volume of cold 2-propanol (HPLC grade; Fisher Scientific, Fairlawn, N.J.), followed by incubation at −20°C overnight. After centrifugation (15 min at 16,060 × g at 4°C), the DNA pellet was washed three times with 500 μl of 70% ethanol, air dried, and resuspended in sterile deionized water. The DNA was then purified by using the QIAquick PCR purification kit (Qiagen) and quantified as described above.
The sequencing reaction was performed with the BigDye terminator cycle sequencing kit (version 3.1; Applied Biosystems, Foster City, Calif.) as described by the manufacturer with 25 ng of purified DNA and 15 μmol of either U341 or U758 universal eubacterial 16S rDNA primer liter−1. The reaction program consisted of 25 cycles of 10 s at 96°C, 5 s at 50°C, and 4 min at 60°C. The sequencing products were purified with Centri-Sep columns as described by the manufacturer (Princeton Separations, Inc., Adelphia, N.J.) in order to remove excess terminators. Samples were dried and sequenced, and the sequences were assigned GenBank accession numbers AY487119 to AY487139.
(v) Phylogenetic analysis.
To confirm the identity of the bands excised from the DGGE gel, bidirectional DNA sequence alignment and correction was first performed by using the AssemblyLign software. Corrected sequences were then analyzed for chimeras by using the Ribosomal Database Project 2 (RDP 2) Chimera Check program (publicly available at http://rdp/cme.msu.edu/html/) (11). Finally, the sequences were identified and confirmed by using the standard nucleotide BLAST program (http://www.ncbi.nlm.nih.gov/) (1).
Statistical analyses.
The experimental design consisted of four low-oxygen and four normal oxygen treatments, each performed in an identical aquarium (block). Each nutrient and nickel treatment had three identical replicate reactors randomly assigned to it in each aquarium (replications). Each analysis was done on subsamples from randomly selected biofilm coupons from among the 12 identical coupons in each replicate reactor. The imaging was done at five random locations at five positions in a transect across the 1-cm2 piece of the biofilm coupon. Analysis of variance was used to detect significant differences among sample means at P < 0.05. Analyses were carried out by using a commercial package (MiniTab, State College, Pa.).
RESULTS
Biofilm architecture and community composition.
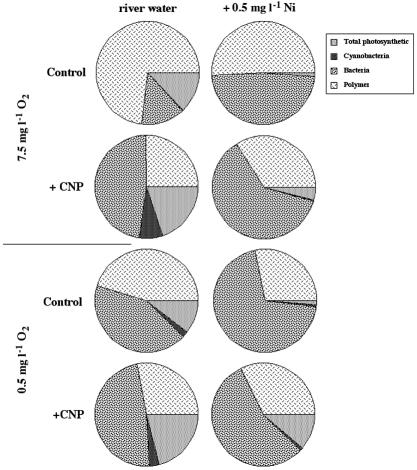
South Saskatchewan river water was used as an inoculum and the sole source of nutrient supply to allow the development of complex microbial biofilm communities with a diversity of bacterial and photosynthetic (cyanobacteria and algae) members. A visual comparison of the effects of treatments on biofilm development in the presence of 7.5 and 0.5 mg of oxygen liter−1 and nutrient supplementation with or without nickel is presented as three-color projections in Fig. 1. Quantitative comparisons (Fig. 2) indicated that at 7.5 mg of O2 liter−1, the control biofilms were 75% exopolymer, with the remainder equally divided between phototrophic algal and bacterial biomass. The addition of nickel resulted in a reduction of exopolymer to 50% of total biomass and an increase in bacterial contribution to 48%, with only a small remnant algal component. The addition of CNP resulted in a shift in the biofilm community to one dominated by bacteria (50%), with a concomitant decline in exopolymer and increases in the phototrophic biomass (25%) with the appearance of a substantial cyanobacterial biomass. The combination of CNP plus 0.5 mg of nickel liter−1 resulted in a further increase in bacterial biomass (66%) with a notable reduction in cyanobacterial and algal biomass. The manipulation of oxygen levels to 0.5 mg liter−1 resulted in a reduction in the level of exopolymer from 75 to 40% and a tripling of the percentage of bacterial biomass in the biofilm over control biofilms cultivated at 7.5 mg of oxygen liter−1. The addition of nickel resulted in a community very similar to that observed in the 7.5-mg liter−1 oxygen treatments, with 70 to 75% bacterial biomass and reductions in cyanobacteria, algae, and exopolymer components. Similarly, the addition of nutrients in low-oxygen conditions resulted in a community like that observed with CNP at ambient oxygen levels with or without nickel (Fig. 2).
FIG. 1.
Confocal image stacks illustrating the effects of nickel (0.5 mg liter−1), with or without nutrients and at high and low oxygen concentrations, on biofilm composition and architecture for high-oxygen control (7.5 mg of O2 liter−1) (A), 7.5 mg of O2 liter−1 plus Ni (B), 7.5 mg liter−1 O2 plus CNP (C), 7.5 mg of O2 liter−1 plus CNP plus Ni (D), low-oxygen control (0.5 mg of O2 liter−1) (E), 0.5 mg of O2 liter−1 plus Ni (F), 0.5 mg of O2 liter−1 plus CNP (G), and 0.5 mg of O2 liter−1 plus CNP plus Ni (H) after 8 weeks of development.
FIG. 2.
Results of image analyses of confocal laser micrographs illustrating the effects of the treatments on the relative abundance of algae, cyanobacteria, bacteria, and exopolymers in the river biofilms by treatment.
Statistically significant effects relative to control biofilms were observed with the additions of CNP to the river biofilms. At ambient oxygen, CNP significantly increased the algal, cyanobacterial, bacterial, and exopolymer components of the biofilm (P < 0.05), and treatment with 0.5 mg of nickel liter−1 only resulted in a significant change in the amount of exopolymer (Fig. 3). When CNP and nickel were added in combination, the resultant biofilm community was not significantly different from the control biofilm; however, these biofilms were significantly different from the CNP-alone treatment in all aspects measured. The application of low oxygen resulted in no detectable change in biofilm community composition. Similarly, nickel at 0.5 mg liter−1 did not result in biofilms significantly different from the control biofilms or biofilms grown at 0.5 mg of oxygen liter−1. In keeping with the results for 7.5-mg liter−1 oxygen treatments, the addition of nutrients resulted in significant increases in biomass of algae, bacteria, and exopolymer. The combined addition of CNP and nickel again resulted in biofilms that were not significantly different (P < 0.05) from the controls. However, again the combined CNP plus Ni treatment was significantly different from the CNP-alone treatment, with significant reductions in photosynthetic biomass, bacterial biomass, and total exopolymer. The biofilm thickness also varied between treatments, although it was not consistently related to overall biomass distribution. Specifically, the biofilm thicknesses, grouped by treatment, were as follows: control, 62 ± 29 μm; Ni, 26 ± 14 μm; CNP, 44 ± 22 μm; and CNP plus Ni, 24 ± 8 μm. For the low-oxygen treatment, the thicknesses were as follows: 0.5 O2, 51 ± 20 μm; O2 plus Ni, 38 ± 16 μm; CNP, 41 ± 16 μm; and CNP plus Ni, 27 ± 13 μm. In summary, the two oxygen levels are significantly different, the high-O2 and low-O2 CNP treatments are different from the controls but not from each other, and the addition of nickel results in a significant reduction (P < 0.05) in average thickness of the biofilms.
FIG. 3.

Influence of nutrient additions, oxygen level, and the presence of nickel on the biofilm community structure as assessed at the microscale. Treatments with the same letter from a to d for each parameter (in columns vertically) are not statistically significantly different at P < 0.05.
Cultivation-based analyses.
Traditional plate count and MPN results revealed few significant effects of any of the treatments relative to the control or between treatments (data not shown).
Exopolymer composition.
The application of lectin-binding analyses to define exopolymer composition was used to provide a further index of the changes in community composition. The exopolymer composition was observed to change in response to all treatments. At 7.5 mg of oxygen liter−1 in the control, the dominant lectin binding was with Canavalia ensiformis and Glycine max. The addition of CNP resulted in a shift to an Arachis hypogaea-dominated biofilm, whereas the addition of nickel resulted in biofilms overwhelmingly dominated by Canavalia ensiformis lectin-binding sites (50 to 70% of total detectable sites) (Fig. 4). In contrast, the oxygen treatment at 0.5 mg liter−1 and treatment with oxygen at 0.5 mg liter−1 plus nickel at 0.5 mg liter−1 resulted in biofilms dominated by binding of the lectin Ulex europeaus, followed by Arachis hypogaea binding. In this instance, the addition of CNP did not change the composition of the community, as detected by binding of the four-lectin panel. The combination of CNP plus nickel resulted in a shift to near-equal binding of Canavalia ensiformis, Arachis hypogeae, and Ulex europeaus. A statistical treatment of the quantitative data presented above indicated significant changes (P < 0.05) in the amount of Canavalia ensiformis and Glycine max binding in all treatments relative to the control biofilms. In the low-oxygen treatments significant increases were observed in the binding of Ulex europeaus in nickel- or CNP-only treatments. In contrast, the combination of CNP plus nickel resulted in a decrease in these binding sites relative to the other treatments in this group (Table 2).
FIG. 4.

Graphs showing the effect of the treatments, nutrient additions, two oxygen levels, and the presence of nickel on the composition of river biofilm exopolymer as determined by in situ lectin-binding analyses. A panel of four lectins was used to assess the nature of the glycoconjugates present in the biofilms.
TABLE 2.
Statistical comparison of the lectin-binding analyses for the biofilm treatments
| Treatment | Lectin binding (μm3 μm−2 of lectin binding)a
|
|||
|---|---|---|---|---|
| Arachis hypogaea | Canavalia ensiformis | Glycine max | Ulex europaeus | |
| Control | 0.10a | 0.71d | 0.55d | 0.32a |
| Ni | 0.07a | 0.43bc | 0.09ab | 0.04a |
| CNP | 0.53a | 0.41bc | 0.21abc | 0.35a |
| CNP, Ni | 0.01a | 0.15a | 0.04a | 0.09a |
| Low O2 | 0.10a | 0.38bc | 0.87e | 1.11b |
| Low O2, Ni | 0.07a | 0.43bc | 0.91e | 1.25b |
| Low O2, CNP | 0.14a | 0.62cd | 0.51d | 1.50b |
| Low O2, CNP, Ni | 0.02a | 0.33b | 0.28c | 0.30a |
Values followed by the same lowercase letter are not significantly different from one another (P < 0.05).
Community carbon utilization spectra.
BIOLOG plates were used to measure carbon utilization spectra of each biofilm community. The results (Fig. 5) show a variety of negative and positive effects of the treatments which may also be compound specific. The treatments of oxygen at 0.5 mg liter−1 and of low oxygen plus nickel both generally resulted in a significant (P < 0.05) depression of utilization of polymers, carboxylic acids, amino acids, amines, and esters. When CNP was added, there was an increase in carbohydrate utilization that was suppressed by nickel addition, amino acid utilization was increased or not affected but significantly depressed by the nickel treatment, and polymer utilization was not significantly impacted by either treatment. When the influence of oxygen level was removed, there was a clearer segregation of treatments with nickel alone depressing carbohydrate, carboxylic acids, and amine utilization (Fig. 5). In general, the CNP treatment increased utilization of the tested substrates, whereas the concomitant addition of nickel resulted in levels similar to the control or intermediate to those observed for the control treatment and the treatment with nickel alone at 0.5 mg liter−1 (Fig. 5).
FIG. 5.
Differential display of the carbon utilization spectra of control biofilms and those growing with oxygen, nutrient, and nickel treatments at 7.5 mg liter of oxygen−1 (A), 0.5 mg liter of oxygen−1 plus nickel (B), and 0.5 mg liter of oxygen−1 plus nutrients and nickel (C). Circled datum points are significantly different from their respective control value (P ≤ 0.05).
Live-dead analyses.
The two-component fluorescent stain BacLight was applied to assess the effects of treatments on the number of viable cells, their distribution in the biofilm, and the ratio of live to dead bacterial cells in the biomass. In the control treatment and the treatment with oxygen at 7.5 mg liter−1, both live and dead cells were distributed throughout the vertical profile of the biofilm community (Fig. 6A). The highest ratio of live to dead cells was generally observed in the upper 10 to 20 μm of the biofilms. The control and CNP-plus-nickel treatments had similar ratios of approximately 2:1, whereas the nickel-alone and CNP-alone treatments had ratios of 3 to 4:1. The oxygen treatment at 0.5 mg liter−1 was similar to the oxygen control treatment (7.5 mg liter−1), with an even vertical distribution of live and dead cells and a typical ratio of 2:1 (Fig. 6B). In contrast, the other low-oxygen treatments exhibited an uneven distribution with depth, with typical ratios of 2:1 at the base but increasing through the biofilm to values in excess of 5:1 and as high as 10:1 in nickel treatments. Statistical comparisons of the quantitative values indicated that at both oxygen levels, CNP treatment resulted in a significant increase in the numbers of live cells detected (Table 3). The addition of nickel resulted in a 75% decline in live cells at 7.5-mg liter−1 oxygen concentrations and a 50% decline in live cells at 0.5-mg liter−1 oxygen concentrations relative to the control. Relative to their respective CNP treatments, Ni-CNP treatments had 50 and 65% declines at 7.5- and 0.5-mg liter−1 oxygen concentrations. However, they were not significantly different (P < 0.05) from the levels observed in the controls (Table 3).
FIG. 6.
Graphs presenting the results of live-dead analyses on the river biofilm communities at 7.5 mg liter of oxygen−1 (A) and 0.5 mg liter of oxygen−1 (B). The results are expressed as the ratio of live to dead cells, as detected by the commercial Live-Dead system. Vertical distribution represents the maximum biofilm thickness and mean determination at each depth.
TABLE 3.
Statistical comparisons of live-dead analyses for biofilm communities by treatment group
| Treatment | Live-dead comparisons (μm3 μm−2 of bacterial cells)a
|
||
|---|---|---|---|
| Live | Dead | Live/dead ratio | |
| Control | 1.0a | 0.55ab | 1.8 |
| Ni | 0.24a | 0.09a | 2.7 |
| CNP | 2.5b | 0.73b | 3.5 |
| CNP, Ni | 1.3ab | 0.62ab | 2.1 |
| Low O2 | 0.31a | 0.11ab | 2.8 |
| Low O2, Ni | 0.16a | 0.05a | 3.2 |
| Low O2, CNP | 1.4ab | 0.39ab | 3.6 |
| Low O2, CNP, Ni | 0.51a | 0.16ab | 3.2 |
Values in a column followed by the same lowercase letter are not significantly different from one another (P < 0.05).
Metabolic activity.
Nitrification, denitrification, sulfate reduction, and hexadecane mineralization for all biofilm communities were measured in microcosms. There was a substantial effect of treatments on denitrification and nitrification activity in the biofilm communities as measured in microcosms. In the presence or absence of nickel and at both oxygen levels, CNP was required by the biofilms for nitrate production, since virtually no activity was observed with biofilms grown in the absence of CNP (Fig. 7A and B). Nickel appeared to have no impact on the low-oxygen biofilms, whereas it slightly reduced nitrate production in high-oxygen biofilms. In the presence of CNP and whether nickel was present or not, a lag phase of 16 days occurred before NO3− could be detected. The NO3− production rates were greater at low DO between 16 and 24 days, whereas the rates were greater at high DO between 24 and 35 days. In these experimental groups, the NO3− concentration reached similar levels at the end of the incubation period, and no plateau was reached. No nitrate was produced in the presence of C2H2, regardless of the nickel, nutrient, and oxygen status.
FIG. 7.
Effect of nickel, nutrients, and DO on NO3− production during nitrification by biofilms. The insert indicates the conditions under which the biofilms were grown in the bioreactors. (A and B) Nitrate production; (C and D) nitrous oxide production. Each datum point is the average of six replicate microcosms (three bioreactors, each in duplicate), and the bars represent one standard deviation.
Some N2O also accumulated in the microcosms, which is indicative of heterotrophic nitrification (Fig. 7C and D). A comparison of Fig. 7A and C shows that NO3− and N2O production, which are both products of the oxidation of NH4+, were related. This suggests that N2O production by the biofilms is nitrification dependent. However, N2O concentrations were very low compared to the NO3− concentrations, showing that heterotrophic nitrification does not account for much of the total nitrification activity. N2O production was higher when biofilms were incubated in the presence of CNP, whatever the DO level, and whether Ni was present or not. In most experimental groups, there was a lag phase of 5 days before N2O could be detected, and a plateau was reached after 23 days of incubation. N2O production was lower in the presence than in the absence of C2H2.
CNP stimulated denitrification at both oxygen levels whether nickel was present or not (Fig. 8A and B). Nickel negatively affected denitrification, whether nutrients were present or not and at both DO levels, but much more at the low DO in the presence of CNP (Fig. 8A and B). In the presence of CNP and at both DO levels, a lag phase of at least 2 days occurred before N2O was produced, and the activity was constant until a plateau was reached after 3 weeks of incubation. In the other experimental groups showing significant activity, a lag phase of about 13 days was observed, after which denitrification activity was constant until the end of incubation, with the exception of the CNP-Ni biofilms with a high DO concentration. In the absence of C2H2, there was no accumulation of N2O, regardless of the nickel, nutrient, and DO status. There was no sulfate reduction detected under any conditions (data not shown).
FIG. 8.
Effect of nickel, nutrients, and DO on biofilm activities. The legend indicates the conditions under which the biofilms were grown in the bioreactors. Each datum point is the average of six replicate microcosms (three bioreactors, each in duplicate), and the bars represent one standard deviation. (A) Nitrous oxide concentration during denitrification without acetylene inhibition; (B) nitrous oxide concentration during denitrification with acetylene inhibition; (C) mineralization of hexadecane by bioreactor biofilms. The initial hexadecane concentration in the liquid fraction was 100 mg liter−1 with 32,180 dpm of 14C-labeled hexadecane.
In the presence or absence of nickel and at both DO levels, CNP was required by the biofilms for detectable hexadecane mineralization, since only very weak activity was observed in the absence of nutrients (Fig. 8C). Nickel had no significant impact on mineralization at both oxygen levels. The high oxygen slightly stimulated mineralization in the presence or absence of nickel. Mineralization began after 1 to 2 days of lag phase, and even though no plateau was reached after 6 weeks of incubation, mineralization rates decreased constantly throughout the incubation period. In autoclaved microcosms, the cumulative background of 14C radioactivity found in the KOH traps at the end of the incubation period was always <0.1% of that initially added (data not shown).
In summary, Ni negatively affected denitrification but had no detectable effect on hexadecane mineralization or sulfate reduction.
Analyses of community DNA.
Analysis of total community DNA is summarized in Table 4. Eubacterial 16S rDNA was detected in biofilms grown under all of the conditions, whereas no Archaea were detected (data not shown). Also, there were essentially no differences in detection between the low- and high-oxygen-grown biofilms. The 16S rDNA amplification worked well for essentially all of the samples regardless of growth conditions, indicating that total community DNA had been successfully extracted and purified. The alkB gene was amplified poorly in the control and Ni samples and much better in the CNP-grown biofilms regardless of the DO level (Fig. 9). However, when Ni was combined with CNP, there was less amplification of the alkB fragment. Amplification of the alkB gene indicated a positive effect of nutrients and a negative effect of Ni on hexadecane-degrading bacterial populations. Globally, this correlates well with Fig. 8C, in which mineralization is shown to be dramatically greater when nutrients were present but only slightly affected by Ni.
TABLE 4.
Impact of nutrients, nickel, and DO level on the presence of 16S rDNA and functional genes in the bioreactor biofilms
| Bioreactor condition | Gene presencea
|
||||
|---|---|---|---|---|---|
| Eubacteria | Archaea | alkB | nirS | nirK | |
| Control | +/+s | −/− | w/w | +/+ | +s/ws |
| Ni | +s/+ | −/− | w/− | w/ws | ws/−s |
| CNP | +s/+s | −/− | +/+ | +/+s | ws/+s |
| CNP-Ni | +/+ | −/− | +/+ | −/− | ws/ws |
Gene presence is expressed as 0.5 mg of O2 liter−1/7.5 mg of O2 liter−1, using the following scores: +, amplification of the target gene by PCR; w, weak amplification of the target gene by PCR; s, presence of secondary bands; and −, no amplification of the target gene.
FIG. 9.

Agarose gel electrophoresis of the alkB gene, involved in alkane degradation, amplified from biofilms exposed to nickel, nutrients, and DO treatments. Total community DNA extracts were amplified by PCR with the alkB consensus primers. The asterisk indicates the 500-bp molecular weight marker, and the arrow indicates the amplified 550-bp alkB fragment (550 bp). Samples are indicated by the conditions under which the biofilms were grown (control, no nutrients; Ni, nickel alone; CNP, all nutrients; CNP+Ni, all nutrients plus Ni) in biofilm grown under low (0.5 mg liter−1)- or high (7.5 mg liter−1)-DO conditions. M, molecular weight marker; −, negative PCR control.
The effect of Ni was most pronounced on detection of the nirS fragment (Fig. 10). In the presence of Ni, there was essentially no amplification of the nirS gene regardless of the presence of CNP and of the oxygen level. The results suggest that Ni may have a negative effect on specific populations of bacteria (such as nirS-positive bacteria), which was detected as a negative impact on function as well.
FIG. 10.

Agarose gel electrophoresis of the nirS gene, involved in denitrification, amplified from biofilms exposed to nickel, nutrients, and DO treatments. Total community DNA extracts were amplified by PCR with the nirS consensus primers. The asterisk indicates the 500-bp molecular weight marker, and the arrow indicates the amplified 320-bp nirS fragment. Samples are indicated by the conditions under which the biofilms were grown (control, no nutrients; Ni, nickel alone; CNP, nutrients; CNP+Ni, nutrients plus Ni) in biofilm grown under the low (0.5 mg liter−1)- or high (7.5 mg liter−1)-DO conditions. M, molecular weight marker; +, positive control (50 ng of Pseudomonas stutzeri ATCC 14405 DNA template).
A comparative DGGE analysis of DNA extracted from the biofilm was performed to examine the effects of Ni, nutrients and DO on biofilm community composition (Fig. 11). PCR products specified by the eubacterial 16S rDNA U341-U758 primers were amplified from all eight treatments. Separation of these fragments by DGGE produced distinct patterns comprising between 15 and 30 bands for each treatment. Some bands appeared to be common among treatments (for example, bands 3, 8, 12, and 17), whereas other bands were unique for specific treatments (for example, band 16 in the high-DO control). The diversity, based on the number of bands, was lower at both DO levels when Ni was present. This was overcome by the inclusion of CNP at the higher DO level. At both DO levels, the number of bands between the control and CNP treatments was similar, although some bands were more intense in the presence of CNP (band 21), and some bands were unique to the control (band 2) or CNP (bands 6 and 20) treatments.
FIG. 11.

DGGE of PCR-amplified 16S rDNA fragments from river biofilm communities in each treatment category. Samples are indicated by the treatment conditions under which the biofilms were grown at DO concentrations of 0.5 and 7.5 mg liter−1: Co, control with no nutrients; CNP, nutrients alone; Ni, nickel alone; CNP+Ni, nutrients plus Ni. Bands which were isolated and sequenced are identified by number.
Banding patterns generated by DGGE for each site were used for cluster analysis. The dendrogram representing the similarities in the banding patterns indicated that the high-DO CNP treatment was distinctly different from the other treatments (data not shown). The other treatments formed two separate clusters. The small cluster, comprising the low-DO CNP and low-DO CNP-Ni treatments, showed the highest similarity coefficient. In the large cluster, pairs grouped according to their DO level (high-DO Ni combined with high-DO CNP-Ni and low-DO control combined with low-DO Ni), with the exception of the high DO control treatment. Figure 11 and the cluster analysis demonstrate that the studied variables did have noticeable effects on the biofilm community structure which could be detected by DGGE analysis.
Twenty-three selected bands (two to five bands from each treatment) were excised from the DGGE gel and sequenced (Table 5). Sequencing results revealed that bands 15 and 18, which migrated to the same position on the gel, were identical (100%) to each other and to uncultured bacterial strains from a drinking water distribution system. In addition, bands 8 and 12, 9 and 14, and 4 and 19, which also migrated to the same positions in the gel, but for different treatments, were identified as being identical (Table 5). Of interest, bands 4 and 19, which were identified as drinking water distribution system bacteria, appeared to be unique to the Ni treatment at both DO levels. Bands 10 and 21, which are unique bands that clearly migrated to different positions on the gel, shared 89 and 88% homology, respectively, with a lake water Sphingomonas sp. strain, KIN169, a dominant isolate under nutrient-limiting conditions.
TABLE 5.
Nucleotide sequence analysis of excised DGGE bands
| DGGE band | Database similarity (accession no.) | Similarity (%) | Characteristics |
|---|---|---|---|
| 1 | α-Proteobacteria zo12 and zj94 (AF531000 and AF530964) | 99 | Natural bacterioplankton from lake water enrichments |
| 2 | Sphingomonas sp. strain KIN169 (AY136096) | 94 | Lake water isolate dominant under nutrient-limiting conditions |
| 3 | Marine bacterium HP33 AY239011) | 97 | Isolated from marine particles, German Wadden sea |
| 4 | Uncultured bacteria strains DSSD56 and DSSD11 AY328754 and AY328710) | 93 | Drinking water distribution system |
| 5 | Uncultured bacterium DSSF16 AY328639) | 97 | Found in drinking water distribution system simulator |
| 6 | Unidentified eubacterium L10944) | 89 | Aggregate associated with marine bacterial assemblages |
| 8 | Uncultured β-proteobacterium AJ583175) | 97 | Groundwater of a deep-well injection site |
| 9 | Sphingomonas aquatilis JSS-7 AF131295) | 100 | Isolated from natural mineral water |
| 10 | Sphingomonas sp. strain KIN169 AY136096) | 89 | Lake water isolate dominant under nutrient-limiting conditions |
| 11 | Uncultured bacterium PHOS-HE21 AF314419) | 88 | Involved in enhanced biological phosphorus removal from a wastewater sequencing batch reactor |
| 12 | Uncultured bacterium clone D141 AY274146) | 87 | Heavy metal contaminated mine tailings |
| Uncultured β-proteobacterium AJ583175) | 87 | Groundwater of a deep-well injection site | |
| 13 | α-proteobacteria AF540047) | 99 | Found in a plug-flow bioreactor fed with lake water |
| 14 | Sphingomonas aquatilis JSS-7 AF131295) | 91 | Isolated from natural mineral water |
| 15 | Uncultured bacteria strains DSSD 64, 60, 15, and 3 AY328762) | 100 | Drinking water distribution system bacteria |
| 16 | Hyphomicrobium vulgareX53182) | 90 | Budding and prosthecate, nonphototrophic eubacterium |
| 17 | Sphingomonas sp. strain AV6C AF434172) | 98 | Hydroxylated and methoxylated monocyclic aromatic degrader from olive mill wastewater |
| 18 | Uncultured bacteria strains DSSD 64, 60, 15, and 3 AY328762) | 100 | Drinking water distribution system bacteria |
| 19 | Uncultured bacteria strains DSSD56 and DSSD11 (AY328754 and AY328710) | 95 | Drinking water distribution system |
| 21 | Sphingomonas sp. strain KIN169 AY136096) | 88 | Lake water isolate dominant under nutrient-limiting conditions |
| 22 | Uncultured α-proteobacterial clones Fuku S56 AJ290014), NE41 AJ575707), and SW22 AJ575705) | 90 | Lake Fuchskuhle, lake water enrichment, and lake water enrichment, respectively |
| 23 | Uncultured bacterium clone BCM3S-13B AY102903) | 92 | Associated with periphyton in a freshwater marsh |
| Rhodobacter blasticusD16429) | 92 | Phototrophic bacterium |
DISCUSSION
Laboratory experiments conducted under controlled conditions are required to confirm the effects of stresses and assess potential interactions. We sought to analyze the complex response of a river biofilm microbial community to combined stresses, including nickel, nutrients, and oxygen, by focusing on a representative river community with algal, cyanobacterial, and bacterial components. The reactor system provided samples with a reduced variability that were amenable to microscale and molecular analyses (42). The biofilm communities could then be allowed to develop under conditions simulating stresses and combinations of stresses representative of natural systems. A combination of techniques, including recently developed microscopic and molecular approaches, were then applied to allow us to detect community and population responses to the single and combined stresses. Furthermore, relevant interactions between the stresses could be detected.
Several studies have reported that metals such as Zn and Cd cause changes in the species composition of the periphyton in streams (21, 22, 55). The studies concentrated on the effects on algal taxa and the development of different algal communities in response to increasing metal concentrations. The validity of, for example, diatoms as an indicator of metal pollution may be questionable due to the range of interacting factors that influence biofilm development. However, Gold et al. (22) supported the contention that changes in periphytic diatom communities could be used as indicators of metal pollution. Examining only the abundance of phototrophic organisms (algae/cyanobacteria), we found that nickel had a negative effect on the phototrophic component of the river biofilm communities and that this was most evident when nutrient enrichment was occurring. This was true at both oxygen concentrations, suggesting that the nutrient enrichment may result in a community that was more sensitive to the nickel addition. It is notable that without an additional nutrient control, the effect of Ni on the biofilm phototrophic component would have been undetectable. Most studies of algal response to nickel contamination point to losses of photosynthetic biomass and very clear changes in the species composition (76), which is in general agreement with our observations for the phototrophic component of the biofilm community.
A wide range of environmental factors may influence the abundance of bacteria in aquatic systems such as rivers. It is likely that biofilm bacteria are affected by temperature, nutrients, and dissolved carbon levels (45, 59). An additional highly relevant factor is the linkage between bacteria and algae in streams. Studies have indicated a positive relationship between phototrophic and heterotrophic organisms (27, 28). There are at least three linkages between these groups: bacterial use of algal produced dissolved organic matter, (26, 34), the cycling of inorganic nutrients and micronutrients within the biofilm (46), and provision of substrata for colonization of the surface by algae for bacteria. The importance of these linkages may be controlled by the abundance of algae in the system. Bacteria may be tightly linked when the algal biomass is high and not when it is low (66), or there may be threshold levels as suggested by Findlay et al. (16) before this coupling occurs. This interaction may be critical in terms of assessing the detectable effects of single and combined stresses on river biofilms. We found that the biofilm community in the South Saskatchewan River was highly responsive to both single-nutrient (carbon, nitrogen, or phosphorus) and combined-nutrient (CNP) additions. Here we see that treatments that resulted in an increase of algal biomass also resulted in an increase in bacterial biomass and activity. An increase in algal biomass, as measured by chlorophyll a, in the epilithon of Rocky Mountain streams (Colorado) had a consistently positive effect on nitrification potential (57). However, on a percentage basis the treatments with CNP, nickel, or low oxygen resulted in bacterially dominated communities. It was observed by Williams and Mount (76) that introduction of zinc resulted in a shift of the biofilm community from predominantly autotrophic to heterotrophic. This negative impact on the phototrophic community contributes to a cascade effect on the heterotrophic community and will contribute significantly to the changes observed in bacterial populations.
We observed that the nickel-containing treatments resulted in biofilms with a substantially reduced proportion of exopolymeric substances. The lectin panel also revealed that significant changes occurred in the composition of the exopolymeric matrix of the community. Nutrients and other stresses have previously been reported to influence the EPS composition of both pure culture and river biofilm communities (54; T. R. Neu, G. D. W. Swerhone, and J. R. Lawrence, unpublished data). Carbon sources influence the composition and nature of the exopolymers of microbial communities (6) and may alter the community structure of river biofilms, as well as the quantities of exopolymer produced (51). In contrast, marine biofilms changed their structure and increased production of exopolymers by >100% in the presence of Cd, Cu, Pb, Zn, Al, and Cr (13). Jang et al. (31) demonstrated changes in EPS composition in response to metals. However, most work on exopolymers and metals has concentrated on their role in the sorption and removal of metals from water and waste waters, whereas studies dealing with the effect of metals on EPS production and composition are limited. In an experimental assessment of the effect of heavy metals (Cu, Pb, and Ni) on biofilm polymers, it was found that the carbohydrate/protein ratios of Cu- and Pb-treated biofilms were lower than those of control or nickel-treated biofilms. Increased production of exopolymers or a shift in composition may be associated with increased resistance to metal toxicity, whereby metals are sequestered in complexes in the exopolymer of the bacteria. This could occur by a change in community structure or by the induction of specific resistance mechanisms in community members. In pure culture studies, it has been found that biofilm grown cells are 2 to 600 times more resistant to metal stress than planktonic cells. The suggested mechanism was that the extracellular polymers protected the cells by binding the heavy metals and retarding their diffusion within the biofilm (69). These mechanisms may also be active within natural communities and would favor selection of bacteria with compositionally or quantitatively appropriate exopolymer production. A critical role of exopolymers in river biofilms is that of a sink or reservoir of available carbon for the community that allows long-term stability (18). The observed reduction in the exopolymer component would have implications for bacterial survival, community composition, and the quality of the biofilm as a food resource for aquatic grazers. This latter factor provides a mechanism for contaminant effects at higher trophic levels through food quality and quantity, as well as potential transfer of the sorbed contaminants. Effects of contaminants on food quality have been shown to influence the development and growth of aquatic invertebrates (49).
Heterotrophic processes are generally negatively impacted by the presence of heavy metals. Chua and Hua (9) reported that inhibition may occur at concentrations of >10 mg liter−1 for cadmium, chromium, and nickel, whereas Bitton (4) indicated that anaerobic treatment processes were sensitive to even trace levels of metals. Degradation of dissolved organic matter and specific processes such as nitrification can be significantly affected by the presence of a variety of metals. Chua et al. (10) reported that metals significantly affected these processes in wastewater treatment systems. In the case of biofilms and flocs, it has been observed that metal ions acted as a strong competitor against organic compounds for active sites on bioflocs instead of acting as a toxic microbial inhibitor, hampering organic adsorption, and degradation (10). This mechanism may be important in naturally occurring biofilms but has not been assessed. In general, heavy metal toxicity occurs through the induction of oxidative stress and interference with protein folding and function (56).
Based on the expected toxicity of Ni, we anticipated that analyses such as heterotrophic plate counts and assessments of bacterial viability would indicate the impact of nickel and the other stresses on the microbial community. Conventional culture-dependent methods did not indicate any statistically significant changes in the culturable microbial community or specific populations that were assessed. In contrast, other studies have detected changes in microbial community structure and diversity based on plate counts, phospholipid fatty acid signatures, biomass, and activity measurements in response to the presence of toxic metals (3, 19, 29). We found that in situ determination of bacterial viability was more sensitive to the effects of the various treatments, showing a clear trend for both reduced biomass and numbers of viable cells detected in the biofilms treated with nickel relative to controls and biofilms receiving nutrients alone. Thus, there was a detectable toxicity of nickel for the bacterial component of the biofilm community based on this assay. The assay has been used to assess viability in river biofilms previously (52), and the results observed for river biofilms were similar to those reported here for the control biofilm communities. The Live-Dead assay has also proven to be sensitive to the impacts of other metals on bacteria. For example, Sani et al. (63) reported that Cu(II), Zn(II), and Pb(II) inhibited growth of Desulfovibrio desulfuricans. However, these metals did not cause a loss of D. desulfuricans membrane integrity. Based on the pattern of effects, they suggested that the impact of Cu was through a different mechanism than Zn and Pb. Teitzel and Parsek, (69) found that Live-Dead staining revealed that exposure of P. aeruginosa biofilms to Cu and Zn resulted in an outer layer of cells that were primarily dead with the number of viable cells increasing toward the substratum. Other authors have reported on the phenomenon of hindered penetration of metals into biofilms and flocs. Rose and Cushing (60) used radiotracers to show hindered penetration of metals into periphyton. This has been suggested as a major mechanism of resistance to metals for bacteria and in particular bacteria in biofilms. However, in our experimental series the biofilms developed in the presence of the stress had new biomass at the periphery of the biofilm with the highest ratio of live to dead cells. This was, however, consistent for all treatments and the control and was in agreement with previous observations on the distribution of viable cells for river biofilms reported by Neu and Lawrence (52). Of interest was the observation that in the Live-Dead profiles an effect of oxygen level could be detected, with higher ratios of live to dead cells associated with low oxygen. However, the overall effect of nickel was a 50 to 75% decline in the number of viable cells detected relative to respective control biofilms. This may be related to toxicity of nickel at reduced oxygen levels or effects on carbon substrates available for use at these oxygen levels.
Potential changes in catabolic activities induced by treatments were analyzed by using the carbon sources provided by the BIOLOG system. The limits of the technique have been reviewed (38), establishing the strengths and weaknesses and the interpretation. Specifically, the method is biased toward gram-negative bacteria over gram-positive bacteria and is based on the physiologically active component of the community (30). It may, however, be a sensitive indicator of the functional spectrum and the impact of specific stresses. Variations in the complex metabolic fingerprints demonstrated inhibition of many catabolic pathways after the application of nickel. Baath et al. (2) reported that the BIOLOG system indicated general inhibition of pathways by metal contaminants; however, these authors could not determine significant effects due to high variation between replicates. In contrast, we were able to detect many significant effects of nickel and nutrients on catabolic pathways in the biofilm communities. In general, nutrients had a positive effect, nickel had a negative impact on carbon utilization, and nickel in the presence of nutrients abolished the positive effect of the nutrient addition. This was particularly clear in an examination of carbohydrate utilization but was consistent for all categories of carbon substrates. Predominantly, these results indicate a repression of catabolic potential by nickel, although at low oxygen levels the utilization of substrates such as I-erythritol, α-ketobutryic acid, d-malic acid, d-galactosonic acid, l-threonine, l-phenylalanine, and d,l-α-glycerol phosphate were not detectable. In contrast, at ambient oxygen levels the impact of nickel was such that only 2-hydroxy benzoic acid utilization was below detection. Ecologically, the reduction or loss of catabolic functions would be considered an undesirable effect. In terms of the community this may reflect a loss of a functional group or a loss of diversity of functional groups. Consistent with the observations for viability, these results also indicate a more significant effect of low oxygen on the community and suggest a greater effect of metals on anaerobic or low-oxygen-tension systems (9).
As observed above, biomass yield, exopolymer production, and other catabolic processes may be negatively influenced by the presence of nickel at even trace levels. Specific processes such as nitrification may be very sensitive to nutrients and the presence of inhibitors such as metals. Our metabolic studies indicated that the presence of nickel had a deleterious effect mainly on denitrification but also on NO3− production and, to a lesser extent, on hexadecane mineralization. In contrast, nutrient additions enhanced or made these processes detectable in the biofilms. This latter result was consistent with the reports of Chenier et al. (8) and of others who observed enhancement of biogeochemical activities in the presence of increased nutrients. In the Upper Bann River (Ireland), it was shown that glucose was efficient in stimulating denitrification, that glycine had a limited stimulatory effect, and that formate and acetate partially inhibited denitrification (35). Denitrification and nitrification have been reported to be sensitive to the presence of selected organic contaminants such as TNT and hexadecane (61, 65). In addition, significant reductions of ammonia oxidizers have been reported for metal-contaminated soils (67). Both processes are, as observed in the present study, favored by reduced oxygen and the presence of nutrients (36, 39). Sakadevan et al. (62) showed that the addition of Cd, Cu, or Zn inhibited denitrification and increased the concentration of ammonium in the sediment-water environment. Similarly, nitrification potential by epilithon from Rocky Mountain streams in Colorado was undetectable at Zn concentrations greater than 2 mg liter−1 (57). In contrast, hexadecane mineralization was enhanced by nutrients with only a minor reduction of the rate in the presence of nickel. Thus, these metabolic processes showed a range of responses to the treatments and to nickel specifically.
Community-level analyses such as DGGE, which produces a genetic fingerprint of the microbial diversity in a sample, yield findings that are indicative of the number of operational taxonomic units present in detectable quantities and thus allow visualization of the genetic diversity of microbial populations. DGGE profiles of amplified DNA extracts from each biofilm treatment were compared in Fig. 11. Analysis of these profiles revealed clear differences in the banding patterns and the intensities for each of the treatments, with evidence of the appearance and disappearance of specific bands indicative of changes in microbial community structure and diversity in response to each treatment. We observed that the presence of nickel reduced the diversity (number of bands) within biofilms, with the exception of the CNP-Ni biofilms at a high DO concentration. In addition, bands that had relatively high intensities were identified that appeared to be unique to the Ni treatment. Accordingly, during the study of the microbial ecology of the Tinto River (Spain), an extremely acidic environment in a mining region, it was found that bacteria related to the iron cycle accounted for most of the detected prokaryotic microorganisms (23). In the present study, the identities of a significant number of the sequenced bands were aquatic microorganisms, some of which were associated with biofilm and potable water distribution systems, which would tend to be the bacterial types one would expect to find in biofilm developed from surface water.
Community-level responses are an integration of both the response of individual populations and unique community-level mechanisms (55). Populations may acclimate physiologically through adoption of metal-resistant phenotypes. This has been reported in pure culture biofilm studies in which Pseudomonas aeruginosa adopted a metal-resistant phenotype when exposed to metal contamination (69). Colwell et al. (12) found that the numbers of Zn-tolerant bacteria increased with time in periphyton exposed to 1 mg of Zn liter−1. Similarly, Vaccaro et al. (72) found an increase in Cu tolerance of heterotrophs during a marine mesocosm experiment. In the more general pollution instance, specific organisms with potential to act as indicators of pollution have been suggested by Lemke et al. (44). These authors found that Acinetobacter calcoaceticus was most abundant at polluted sites, indicating tolerance or an ability to utilize specific nutrients at these sites. In contrast, Pseudomonas putida and Burkholderia cepacia were unresponsive to the pollution stress. In the case of nickel, resistant bacterial species appear relatively frequently in naturally enriched habitats and contaminated sites (68). At the community level, there may be a replacement of “sensitive” taxa by more resistant species or stress may result in a simplification of community structure characterized by a loss of specific species or a loss of functional redundancy in the community (55). The results of the amplification of the nirS gene and DGGE suggest that the community response to nickel may be via adaptation by substitution of responsive or resistant populations. In the case of nirS, the gene is not detectable in the presence of nickel and yet denitrification occurs, suggesting a loss of functional redundancy. In the case of the DGGE analyses we see both a loss of bands and the appearance of new unique bands. This is in agreement with both a potential loss of redundancy and recruitment of resistant taxa. It is also important to note here that the interaction between different biological components of the community (bacteria, microalgae, and protozoa) and sorption of contaminants by the biofilm will complicate the community responses and their interpretation (12, 24, 55).
Thus, we have demonstrated here that Ni treatments eliminated cyanobacterial populations and reduced photosynthetic biomass in the biofilm. There were changes in exopolymer abundance and a shift in the glycoconjugate make up of the biofilms in response to all treatments. Nickel resulted in reductions in carbon utilization spectra at 0.5 mg liter−1, a level corresponding to an industrial release rate concentration for nickel. CNP alleviated the negative effects of nickel. Ni negatively affected denitrification but had no effect on hexadecane mineralization or sulfate reduction. Amplification of the alkB gene indicated a positive effect of nutrients and a negative effect of Ni. The nirS gene was not detected in samples treated with 0.5 mg of Ni liter−1, indicating a negative effect on specific populations of bacteria, such as denitrifiers. DGGE analyses indicated effects of both nutrients and Ni, with 0.5 mg of Ni liter−1, resulting in the appearance of unique bands in DNA from Ni, DO, and CNP treatments. Overall, the results indicate the importance of considering interactions between metals, DO, and nutrients. The masking effects of nutrients on the detectable impacts of nickel are shown, as is the significance of the oxygen level in determining outcomes. Finally, Ni concentrations, such as the industrial release rate of 0.5 mg of Ni liter−1, may have significant impacts on river microbial community diversity and function.
Acknowledgments
We gratefully acknowledge Suzanne Labelle, Anca Mihoc, Claude Masson, Danielle Ouellette, Sylvie Sanschagrin, Dong Ning He, and Gesine Wisse for excellent technical assistance; Alain Corriveau for performing HPLC analyses; and George Swerhone for biofilm cultivation.
This research was supported by Health Canada through the Toxic Substances Research Initiative, by the National Water Research Institute of Environment Canada, by the National Research Council of Canada, and by a FCAR-MRST grant to M.R.C.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Baath, E., M. Di'Az-Ravin, A. A. Frostegard, and C. D. Campell. 1998. Effect of metal-rich sludge amendments on the soil microbial community. Appl. Environ. Microbiol. 64:238-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babich, H., and G. Stotzky. 1985. Heavy metal toxicity to microbial-mediated ecological processes: a review and potential application to regulatory policies. Environ. Res. 36:111-137. [DOI] [PubMed] [Google Scholar]
- 4.Bitton, G. 1994. Waste water microbiology, p. 333-335. John Wiley & Sons, Inc., New York, N.Y.
- 5.Blanck, H. and S.-Å Wängberg. 1988. The validity of an ecotoxicological test system: short-term and long-term effects of arsenate on marine periphyton communities in laboratory systems. Can. J. Fish. Aquat. Sci. 45:1807-1815. [Google Scholar]
- 6.Bonet, R., M. P. Simon-Pujol, and F. Congregado. 1993. Effects of nutrients on exopolysaccharide production and surface properties of Aeromonas salmonicida. Appl. Environ. Microbiol. 59:2437-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bothe, H., G. Jost, M. Schloter, B. B. Ward, and K. P. Witzel. 2000. Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiol. Rev. 24:673-690. [DOI] [PubMed] [Google Scholar]
- 8.Chenier, M. R., D. Beaumier, R. Roy, B. T. Driscoll, J. R. Lawrence, and C. W. Greer. 2003. Impact of seasonal variations and nutrient inputs on the cycling of nitrogen and the degradation of hexadecane by replicated river biofilms. Appl. Environ. Microbiol. 69:5170-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chua, H., and F. L. Hua. 1996. Effects of a heavy metal (zinc) on organic adsorption capacity and organic removal in activated sludge. Appl. Biochem. Biotechnol. 58:845-849. [Google Scholar]
- 10.Chua, H., P. H. F. Yu., S. N. Sin, and M. W. L Cheung. 1999. Sublethal effects of heavy metals on activated sludge microorganisms. Chemosphere 39:2681-2692. [DOI] [PubMed] [Google Scholar]
- 11.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal DatabaseProject 2 (RDP-2): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colwell, F. S., S. G. Hornor, and D. S. Cherry. 1989. Evidence of structural and functional adaptation in epilithon exposed to zinc. Hydrobiologia 171:79-90. [Google Scholar]
- 13.Fang, H. H. P., L.-C. Xu, and K.-Y. Chan. 2002. Effects of toxic metals and chemicals on biofilm and biocorrosion. Water Res. 36:4709-4716. [DOI] [PubMed] [Google Scholar]
- 14.Feris, K., P. Ramsey, C. Frazar, J. N. Moore, J. E. Gannon, and W. E. Holben. 2003. Differences in hyporheic-zone microbial community structure along a heavy-metal contamination gradient. Appl. Environ. Microbiol. 69:5563-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferris, F. G., S. Schultze, T. C. Witten, W. S. Fyfe, and T. J. Beveridge. 1989. Metal interactions with microbial biofilms in acidic and neutral pH environments. Appl. Environ. Microb. 55:1249-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Findlay, S., K. Howe, and D. Fontvieille. 1993. Bacterial-algal relationships in streams of the Hubbard Brook experimental forest. Ecology 74:2326-2336. [Google Scholar]
- 17.Fischer, S. G., and L. S. Lerman. 1983. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc. Natl. Acad. Sci. USA 80:1579-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman, C., and M. A. Lock. 1995. The biofilm polysaccharide matrix: a buffer against changing organic substrate supply. Limnol. Oceanogr. 40:273-278. [Google Scholar]
- 19.Frostegard, A., A. Tunlid, and E. Baath. 1993. Phospholipid fatty acid composition, biomass and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 59:3605-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garland, J. L., and A. L. Mills. 1991. Classification and characterization of heterotrophic microbial communities based on patterns of community-level sole carbon-source utilization. Appl. Environ. Microbiol. 57:2351-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genter, R. B., D. S. Cherry, E. P. Smith, and J. Cairns, Jr. 1987. Algal-periphyton population and community changes from zinc stress in stream mesocosms. Hydrobiologia 153:261-275. [Google Scholar]
- 22.Gold, C., A. Feurtet-Mazel, M. Coste, and A. Boudou. 2003. Effects of cadmium stress on periphytic diatom communities in indoor artificial streams. Freshwater Biol. 48:316-328. [Google Scholar]
- 23.Gonzalez-Toril, E., E. Lloblet-Brossa, E. O. Casamayor, R. Amann, and R. Amils. 2003. Microbial ecology of an extreme acidic environment, the Tinto River. Appl. Environ. Microbiol. 69:4853-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray, B. R., and W. R. Hill. 1995. Nickel sorption by periphyton exposed to different light intensities. J. N. Am. Benthol. Soc. 14:299-305. [Google Scholar]
- 25.Greer, C. W., L. Masson, Y. Comeau, R. Brousseau, and R. Samson. 1993. Application of molecular biology techniques for isolating and monitoring pollutant-degrading bacteria. Water Pollut. Res. J. Can. 28:275-287. [Google Scholar]
- 26.Haak, S. K., and G. A. McFeters. 1982. Microbial dynamics of an epilithic mat community in a high alpine stream. Appl. Environ. Microbiol. 43:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haak, S. K., and G. A. McFeters. 1982. Nutritional relationships among microorganisms in an epilithic biofilm community. Microb. Ecol. 8:115-126. [DOI] [PubMed] [Google Scholar]
- 28.Hepinstall, J. A., and R. L. Fuller. 1994. Periphyton reactions to different light and nutrient levels and the response of bacteria to these manipulations. Arch. Hydrobiol. 131:161-173. [Google Scholar]
- 29.Héry, M., S. Nazaret T. Jaffré, P. Normand, and E. Navarro. 2003. Adaptation to nickel spiking of bacterial communities in neocaledonian soils. Environ. Microbiol. 5:3-12. [DOI] [PubMed] [Google Scholar]
- 30.Heuer, H., and K. Smalla. 1997. Evaluation of community-level catabolic profiling using BIOLOG GN microplates to study community changes in potato phyllosphere. J. Microbiol. Methods 30:49-61. [Google Scholar]
- 31.Jang, A., S. M., Kim, S. Y. Kim, S. G. Lee, and I. S. Kim. 2001. Effect of heavy metals (Cu, Pb, Ni) on the compositions of EPS in biofilms. Water Sci. Technol. 43:41-48. [PubMed] [Google Scholar]
- 32.Juck, D., T. C. Charles., L. G. Whyte, and C. W. Greer. 2000. Polyphasic microbial community analysis of petroleum hydrocarbon-contaminated soils from two northern Canadian communities. FEMS Microbiol. Ecol. 33:241-249. [DOI] [PubMed] [Google Scholar]
- 33.Juck, D., B. T. Driscoll, T. C. Charles, and C. W. Greer. 2003. Effect of experimental contamination with the explosive hexahydro-1,3,5-trinitro-1,3,5-triazine on soil bacterial communities. FEMS Microbiol. Ecol. 43:255-262. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan, L. A., and T. L. Bott. 1989. Diel fluctuations in bacterial activity on streambed substrata during vernal algal blooms: effects of temperature, water chemistry, and habitat. Limnol. Oceanogr. 34:718-733. [Google Scholar]
- 35.Kelso, B. H. L., R. V. Smith, and R. J. Laughlin. 1999. Effects of carbon substrates on nitrite accumulation in freshwater sediments. Appl. Environ. Microbiol. 65:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knowles, R. 1982. Denitrification. Microb. Rev. 46:43-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kok, M., R. Oldenhuis, M. P. G. van der Linden, P. Raatjees, J. Kingma, P. H. van Lelyveld, and B. Witholt. 1989. The Pseudomonas oleovorans alkane hydroxylase gene. J. Biol. Chem. 264:5435-5441. [PubMed] [Google Scholar]
- 38.Konopka, A., A. L. Oliver, and R. F. Turco. 1998. The use of carbon substrate utilization patterns in environmental and ecological microbiology. Microb. Ecol. 35:103-115. [DOI] [PubMed] [Google Scholar]
- 39.Kuenen, J. G., and L. A. Robertson. 1988. Ecology of nitrification and denitrification, p. 161-218. In J. A. Cole and S. J. Ferguson (ed.), The nitrogen and sulfur cycles. Society for General Microbiology/Cambridge University Press, Cambridge, Mass.
- 40.Lawrence, J. R., and J. J. Germida. 1991. Enumeration of sulfur oxidizing populations in Saskatchewan agricultural soils. Can. J. Soil Sci. 71:127-136. [Google Scholar]
- 41.Lawrence, J. R., T. R. Neu, and G. D. W. Swerhone. 1998. Application of multiple parameter imaging for the quantification of algal, bacterial, and exopolymer components of microbial biofilms. J. Microb. Methods 32:253-261. [Google Scholar]
- 42.Lawrence, J. R., G. D. W. Swerhone, and T. R. Neu. 2000. Design and evaluation of a simple rotating annular reactor for replicated biofilm studies. J. Microb. Methods 42:215-224. [DOI] [PubMed] [Google Scholar]
- 43.Lawrence, J. R., G. Kopf, J. V. Headley, and T. R. Neu. 2001. Sorption and metabolism of selected herbicides in river biofilm communities. Can. J. Microbiol. 47:634-641. [DOI] [PubMed] [Google Scholar]
- 44.Lemke, M. J., B. J. Brown, and L. G. Leff. 1997. The response of three bacterial populations to pollution in a stream. Microb. Ecol. 34:224-231. [DOI] [PubMed] [Google Scholar]
- 45.Lock, M. A., R. R. Wallace, J. W. Costerton, R. M. Ventullo, and S. E. Charlton. 1984. River epilithon: toward a structural-functional model. Oikos 42:10-22. [Google Scholar]
- 46.Lock, M. A. 1993. Attached microbial communities in rivers, p. 113-138. In T. E. Ford (ed.), Aquatic microbiology: an ecological approach. Blackwell Scientific Publications, Boston, Mass.
- 47.Lovley, D. R., F. H. Chapelle, and E. J. P. Phillips. 1990. Fe (III)-reducing bacteria in deeply buried sediments of the Atlantic Coastal Plain. Geomicrobiol. J. 18:954-957. [Google Scholar]
- 48.Lowell, R. B., and J. M. Culp. 1998. Cumulative effects of multiple effluent and low dissolved oxygen stressors on mayflies at cold temperatures. Can. J. Fish. Aquat. Sci. 56:1624-1630. [Google Scholar]
- 49.Lowell, R. B., J. C. Culp, and F. J. Wrona. 1995. Stimulation of increased short-term growth and development of mayflies by pulp mill effluent. Environ. Toxicol. Chem. 14:1529-1541. [Google Scholar]
- 50.Manz, W., K. Wendt-Potthoff, T. R. Neu, U. Szewzyk, and J. R. Lawrence. 1999. Phylogenetic composition, spatial structure, and dynamics of lotic bacterial biofilms investigated by fluorescent in situ hybridization and confocal laser scanning microscopy. Microb. Ecol. 37:225-237. [DOI] [PubMed] [Google Scholar]
- 51.Mohamed M. N., J. R. Lawrence, and R. D. Robarts. 1998. Phosphorus limitation of heterotrophic biofilms from the Fraser river, British Columbia, and the effect of pulp mill effluent. Microb. Ecol. 36:121-130. [DOI] [PubMed] [Google Scholar]
- 52.Neu, T. R., and J. R. Lawrence. 1997. Development and structure of microbial stream biofilms as studied by confocal laser scanning microscopy. FEMS Microb. Ecol. 24:11-25. [Google Scholar]
- 53.Neu, T. R., and J. R. Lawrence. 1999. Lectin binding: analysis in biofilm systems. Methods Enzymol. 310:145-151. [DOI] [PubMed] [Google Scholar]
- 54.Neu, T. R., G. D. W. Swerhone, and J. R. Lawrence. 2001. Assessment of lectin-binding-analysis for in situ detection of glycoconjugates in biofilm systems. Microbiology 147:299-313. [DOI] [PubMed] [Google Scholar]
- 55.Niederlehner B. R., and J. Cairns, Jr. 1992. Community response to cumulative toxic impact: effects of acclimation on zinc tolerance of Aufwuchs. Can. J. Fish. Aquat. Sci. 49:2155-2163. [Google Scholar]
- 56.Nies, D. H. 1999. Microbial heavy metal resistance. Appl. Microbiol. Biotechnol. 51:730-750. [DOI] [PubMed] [Google Scholar]
- 57.Niyogi, D. K., W. M. Lewis, Jr., and D. M. McKnight. 2003. Direct and indirect effects of mine drainage on bacterial processes in mountain streams. J. N. Am. Benthol. Soc. 22:276-291. [Google Scholar]
- 58.Raj, H. D. 1989. Oligotrophic methylotrophs: Ancylobacter (basonym “Microcyclus” Ørskov) Raj gen. Nov. Crit. Rev. Microbiol. 17:89-106. [DOI] [PubMed] [Google Scholar]
- 59.Rier, S. T., and R. J. Stevenson. 2001. Relation of environmental factors to density of epilithic lotic bacteria in 2 ecoregions. J. N. Am. Benthol. Soc. 20:520-532.
- 60.Rose, F. L., and C. E. Cushing. 1970. Periphyton: autoradiography of zinc-65 adsorption. Science 168:576-577. [DOI] [PubMed] [Google Scholar]
- 61.Roy, R., and C. W. Greer. 2000. Hexadecane mineralization and denitrification in two diesel fuel-contaminated soils. FEMS Microbiol. Ecol. 32:17-23. [DOI] [PubMed] [Google Scholar]
- 62.Sakadevan, K., Z. Huang, and H. J. Bavor. 1999. Impact of heavy metals on denitrification in surface wetland sediments receiving wastewater. Water Sci. Technol. 40:347-349. [Google Scholar]
- 63.Sani, R. K., B. M. Peyton, and L. T. Brown. 2001. Copper-induced inhibition of growth of Desulfovibrio desulfuricans G20: assessment of its toxicity and correlation with those of zinc and lead. Appl. Environ. Microbiol. 67:4765-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidt, E. L., and L. W. Belser. 1982. Nitrifying bacteria, p. 1027-1042. In A. C. Page, R. H. Miller, and D. R. Keeney (ed.), Methods of soil analysis: chemical and microbiological properties. American Society of Agronomy, Madison, Wis.
- 65.Siciliano, S. D., R. Roy, and C. W. Greer. 2000. Reduction in denitrification activity in field soils exposed to long-term contamination by 2,4,6,-trinitrotoluene (TNT). FEMS Microbiol. Ecol. 32:61-68. [DOI] [PubMed] [Google Scholar]
- 66.Sobczak, W. V., and T. M. Burton. 1996. Epilithic bacterial and algal colonization in a streamrun, riffle, and pool: a test of biomass covariation. Hydrobiologia 332:159-166. [Google Scholar]
- 67.Stephen, J. R., Y.-J. Chang, S. J., Macnaughton., G. A. Kowalchuk, K. T. Leung, C. A. Flemming, and D. C. White. 1999. Effect of toxic metals on indigenous soil b-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Appl. Environ. Microbiol. 65:95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stoppel, R.-D., and H. G. Schlegel. 1995. Nickel-resistant bacteria from anthropogenically nickel-polluted and naturally nickel-percolated ecosystems. Appl. Environ. Microbiol. 61:2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teitzel, G. M., and M. R. Parsek. 2003. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 69:2313-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teske, A., C. Wawer, G. Muyzer, and N. B. Ramsing. 1996. Distribution of sulfate-reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most probable number counts and denaturing gradient gel electrophoresis of PCR-amplified ribosomal DNA fragments. Appl. Environ. Microbiol. 62:1405-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tiedje, J. M. 1982. Denitrification, p. 1011-1026. In A. C. Page, R. H. Miller, and D. R. Keeney (ed.), Methods of soil analysis: chemical and microbiological properties. American Society of Agronomy, Madison, Wis.
- 72.Vaccaro, R. F., F. Azam, and R. Hodson. 1977. Response of natural marine bacterial populations to copper: controlled ecosystem pollution experiment. Bull. Mar. Sci. 27:17-22. [Google Scholar]
- 73.Whyte, L. G., C. W. Greer, and W. E. Inniss. 1996. Assessment of the biodegradation potential of psychrotrophic microorganisms. Can. J. Microbiol. 42:99-106. [DOI] [PubMed] [Google Scholar]
- 74.Whyte, L. G., L. Bourbonniere, and C. W. Greer. 1997. Biodegradation of petroleum hydrocarbons by psychrotrophic Pseudomonas strains possessing both alkane (alk) and naphthalene (nah) catabolic pathways. Appl. Environ. Microbiol. 63:3719-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3378. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-L. Schleifer (ed.), The prokaryotes. Springer-Verlag, New York, N.Y.
- 76.Williams, L. G., and D. I. Mount. 1965. Influence of zinc on periphytic communities. Am. J. Bot. 52:26-34. [Google Scholar]