Abstract
Parvibaculum lavamentivorans DS-1T utilized the commercial surfactant linear alkylbenzenesulfonate (LAS) (20 congeners with C10 to C13 side chains) as a carbon and energy source by shortening the side chain, and sulfophenylcarboxylates (SPCs) and similar compounds (e.g., α,β-unsaturated SPCs [SPC-2Hs]) were excreted with quantitative recovery of the sulfophenyl moiety. 2-(4-Sulfophenyl)decane (2-C10-LAS) was converted largely to 3-(4-sulfophenyl)butyrate (3-C4-SPC), as were 2-C12-LAS and 2-C14-LAS; the other products were 5-C6-SPC (SPC+2C) and 3-C4-SPC-2H. 2-C11-LAS was converted largely to 4-C5-SPC with the corresponding SPC+2C and SPC-2H; similarly, 3-C12-LAS yielded 4-C6-SPC with the corresponding SPC+2C and SPC-2H. This pattern of products confirmed that LAS is degraded by ω-oxygenation and chain shortening through β-oxidation. At least nine major SPCs were formed from commercial LAS. The novel isolates Comamonas testosteroni SPB-2 and KF-1 utilized 3-C4-SPC; Delftia acidovorans SPH-1 utilized 4-C6-SPC enantioselectively. The substrate-dependent oxygen uptake of whole cells of strain SPB-2 indicated that there was inducible oxygenation of 3-C4-SPC and of 4-sulfophenol in whole cells of the strains of C. testosteroni during growth with 3-C4-SPC or 4-sulfophenol. The degradative pathways apparently involved 4-sulfocatechol and 4-sulfocatechol 1,2-dioxygenase. Strain SPB-2 and strain DS-1T grew together in LAS-salts medium, and only seven of the nine major SPCs were recovered. Strain SPB-2 utilized 3-C4-SPC, 3-C5-SPC, and 3-C4-SPC-2H. Strain SPH-1 grew together with strain DS-1T in LAS-salts medium, and a different set of seven major SPCs was recovered. Strain SPH-1 utilized 4-C6-SPC, 4-C5-SPC, 4-C6-SPC-2H, and 4-C5-SPC-2H. A three-member community consisting of strains DS-1T, SPB-2, and SPH-1 utilized four major SPCs. We inferred that this community mineralized the major SPCs derived from 8 of the 20 LAS congeners.
Linear alkylbenzenesulfonate (LAS) is the major synthetic surfactant in use worldwide (18). In western Europe, for example, the current LAS disposal rate is 3.1 g/person/day (www.lasinfo.org), and this represents some 4% of the organic input into the sewerage system, where it is presumably the highest-bulk compound to be degraded. Biodegradation of LAS was proven by Sawyer and Ryckman in 1957 (26). Nonetheless, a complete degradative pathway for LAS, backed by scientific evidence, is only now becoming available. Thus, it took many years after the work of Sawyer and Ryckman (26) before the requirement for communities for degradation of the commercial product was recognized (15, 16, 33). At that time, however, routine analytical-chemical methods for LAS-derived compounds were largely unavailable in microbiology laboratories (6, 22, 33), and also the lack of pure cultures (14, 28, 30, 32) prevented advances. The first suggestions concerning community structure were, however, made (4, 5, 14, 39).
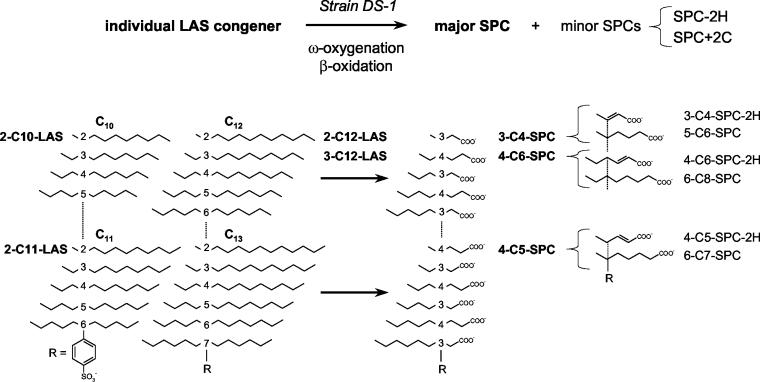
Commercial LAS is ideally a mixture of 20 congeners (Fig. 1). These congeners are degraded to a mixture of sulfophenylcarboxylates (SPCs), sulfophenyldicarboxylates (SPdCs), and α,β-unsaturated sulfophenylcarboxylates (SPC-2Hs) by Parvibaculum lavamentivorans DS-1T in a pattern that confirms that the degradative pathway involves ω-oxygenation and β-oxidation (5, 30), as first illustrated in mixed cultures by Eichhorn and Knepper (6). This mixture of products is termed SP(d)C. Strain DS-1T generates mainly 4-(4-sulfophenyl)hexanoate (4-C6-SPC) from 3-(4-sulfophenyl)dodecane (3-C12-LAS) (5, 28). Cook and Hrsák (4) deduced a three-tier model for the degradation of LAS initiated by methanotrophic bacteria and completed by heterotrophic bacteria and for purely heterotrophic degradation in which SPdCs played an important role. This model is still valid for the defined methanotrophic community, but the organisms used were a very limited range of organisms (e.g., Delftia acidovorans SPB1) able to mineralize only one SPC, 2-(4-sulfophenyl)butyrate (2-C4-SPC), via 4-sulfocatechol and ortho ring cleavage with subsequent desulfonation (32).
FIG. 1.
Diagrammatic representation of the 20 congeners of commercial LAS and of the corresponding major SPC known or presumed to be formed from each congener by P. lavamentivorans DS-1T. Corresponding congeners of LAS (e.g., 2-C10-LAS, 2-C12-LAS, and 2-C14-LAS) (see Fig. 4) yield the same major SPCs (in this example 3-C4-SPC) and the same minor SPCs (in this example 3-C4-SPC-2H, an α,β-unsaturated SPC [generalized as SPC-2H], and 4-C6-SPC [generalized as SPC+2C]). The LAS congeners used in this work are labeled, as are the corresponding major and minor products.
Here we report that two-tier, heterotrophic bacterial communities can completely degrade three LAS congeners, which we believe (in terms of degradative pathways) represent 8 of the 20 congeners of LAS. The first organism in each community is P. lavamentivorans DS-1T (28, 30), which yields one major and several minor products from each LAS congener. The second organism degrades one major SPC, the corresponding SPC-2H, and a second major SPC via oxygenation of the SPC and ortho cleavage of 4-sulfocatechol. Due to the relatively high specificity of SPC degradation, we concluded that a large community is needed to completely degrade all 20 congeners of commercial LAS, which may proceed via at least 11 major SPCs.
MATERIALS AND METHODS
Materials.
Commercial LAS (C10 to C13) with a mean side chain length of 11.4 carbon atoms (21) (Marlon A350) was provided by Hüls (Marl, Germany). The single LAS congeners 2-C10-LAS, 2-C12-LAS, and 2-C14-LAS (each >98% pure) were gifts from Petresa (Madrid, Spain), and 3-C12-LAS (about 95% pure) was made available by CONDEA-Vista (Austin, Tex.) (5). Commercial LAS and the single LAS congeners were each prepared as a 10 mM stock solution in distilled water and diluted in minimal salts medium to obtain a final concentration of 1 mM; 10 mM 2-C14-LAS precipitated at room temperature and was redissolved by heating it to over 60°C (with a microwave oven) prior to dilution. Racemic 2-(4-sulfophenyl)butyrate (2-C4-SPC) was available in the laboratory (32), and racemic 3-C4-SPC was synthesized by reacting sulfuric acid with the corresponding phenylcarboxylic acid, followed by recrystallization as described by Schulz et al. for synthesis of 2-C4-SPC (32). The compound obtained was about 95% pure [the major by-product was 2-(2-sulfophenyl)butyrate], and its identity was confirmed by electrospray mass spectrometry (ES-MS) and nuclear magnetic resonance (32).
4-Sulfocatechol that was about 95% pure was kindly supplied by B. Feigel (8); the major impurity was 3-sulfocatechol. Agarose (ultrapure electrophoresis grade; FMC BioProducts) was used to solidify SPC-salts medium for selective plating. The starting materials for chemical synthesis were purchased from Fluka (Buchs, Switzerland), and the sources of routine chemicals have been given elsewhere (20, 32).
Analytical methods.
LAS and SPCs were routinely analyzed by gradient reversed-phase high-performance liquid chromatography (HPLC) coupled to a UV (diode array) detector with gradient system I (28). HPLC for mass spectrometry (gradient system II) and determination of LAS and SPC via ES-MS in the negative-ion mode were performed as described by Eichhorn and Knepper (6). LAS and SPCs were tentatively identified by the m/z values of the ions formed from the deprotonated molecules, and the identities were confirmed when fragments with m/z values of 183 (4-styrenesulfonate) and 119 (4-styrenephenolate) were observed (6). SPC-2Hs were detected by m/z values of the deprotonated molecular ions that were 2 U lower than those of the corresponding SPCs, and the identities were confirmed when the characteristic fragmentation pattern was observed, as follows: (i) loss of m/z 44 (elimination of CO2), (ii) formation of ions at m/z 211, 209, and 195 (olefinic 4-benzenesulfonates), (iii) formation of an ion at m/z 145 (olefinic 4-benzenephenolate), and (iv) absence of a dominant ion from SPC species at m/z 183 (see above) (6).
The separation of SPCs with HPLC gradient system II (UV detector) was less efficient than that with HPLC gradient system I. This was not a problem for HPLC-ES-MS identifications (gradient system II), in which individual ions were traced. However, comparisons between the routine separations when the biological experiments were done with gradient system I (as shown here) and the detailed analyses and identification in the liquid chromatography (LC)-MS laboratory required experience (for a comparison see reference 5). The most important comparisons of data between methods were made in experiments in which the SP(d)Cs were derived from individual congeners of LAS; here the chromatograms were simple enough and the relative amounts of different compounds were sufficiently different to allow robust identification of peaks from the UV detector to be made.
HPLC for enantioselective separation of R-4-C6-SPC and S-4-C6-SPC (gradient system III) was done by using a method adapted from the method of Schulz et al. (32). Samples (100 μl) were loaded onto a β-pm-Nucleodex column (200 mm; diameter, 4 mm; particle size, 5 μm; Macherey Nagel, Düren, Germany) that was equilibrated with 100 mM potassium phosphate buffer (pH 6.0), and after 5 min a linear gradient to 50% methanol over 15 min was applied and maintained for 5 min. The R and S enantiomers eluted after 19.1 and 19.9 min, respectively, assuming that the same enantiomer (the R enantiomer in this case) of a homologous compound always eluted first (32).
The total LAS and SPC concentrations in solutions were estimated photometrically by using A220 and pure compounds (2-C12-LAS and 3-C4-SPC) as standards. Growth was expressed as optical density at 580 nm (OD580) and was quantified as protein by using a Lowry-type reaction (17). Sulfate was determined turbidimetrically by generating a suspension of BaSO4 (34). Substrate-dependent oxygen uptake by washed whole cells (or cell extracts) and the concomitant substrate degradation were determined as described elsewhere (27). Anoxic cell suspensions were prepared as described elsewhere (27).
Purification of 2-C11-LAS.
2-C11-LAS was purified from commercial LAS by semipreparative HPLC by using a method adapted from the HPLC gradient system I method (see above). A semipreparative C18 HPLC column (Ultraprep C18; 150 by 21.2 mm; particle size, 10 μm; Beckman, Fullerton, Calif.) and a preparative HPLC system (Beckman System Gold, Preparative μ-Flow) were used in combination with a fraction collector set in the automated peak-cutting mode (Pharmacia LKB FRAC-100); LAS was detected at 260 nm. The flow rate was 5 ml/min, and the column was equilibrated with 65% 0.11 M sodium perchlorate (buffer A) and 35% acetonitrile. Portions (5 ml) of 1 mM commercial LAS (acidified to pH 2 with 1 M HCl) were injected in each run, and after 5 min the acetonitrile gradient was ramped to 70% in 5 min and to 75% in 10 min and then maintained at 75% for 10 min. LAS eluted as sets of peaks between 18 and 36 min; 2-C11-LAS eluted as a single peak at 26.5 min (Fig. 2A), which was collected. The initial conditions were restored within 5 min, and the column was reequilibrated for 10 min prior to the next injection. Samples of 2-C11-LAS from 20 separations were pooled, the volume was reduced to one-third in a rotary evaporator at 60°C (by removal of acetonitrile), and the preparation was diluted in the same volume of distilled water prior to adjustment of the pH to 2 with 1 M HCl. The partially purified 2-C11-LAS was subjected to a second purification (as described above) to reduce impurities consisting largely of 3- and 4-C11-LAS. The fractions were pooled, and acetonitrile was removed as described above. The material was desalted by solid-phase extraction as described elsewhere (29). The concentration of 2-C11-LAS was determined photometrically (220 nm) with 2-C10-LAS as the standard.
FIG. 2.
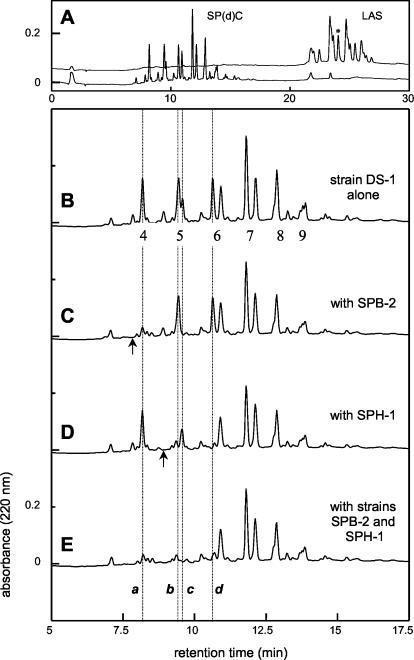
HPLC chromatograms of commercial LAS in salts medium (A, upper chromatogram) and of the medium after growth of P. lavamentivorans DS-1T (A, lower chromatogram, and B), after growth of strains DS-1T and SPB-2 (C), after growth of strains DS-1T and SPH-1 (D), and after growth of strains DS-1T, SPB-2, and SPH-1 (E). The asterisk indicates 2-C11-LAS, which was isolated in this study. The numbers 4 to 9 in panel B indicate the chain lengths of the major SPCs formed. The letters a to d in panel E indicate peaks that represent identified major SPCs which were subject to degradation (a, 3-C4-SPC; b, 4-C5-SPC; c, 3-C5-SPC; d, 4-C6-SPC), whereas the arrows indicate the minor products (SPC-2Hs) which were degraded (see text). Analyses were done with HPLC gradient system I.
Growth conditions for strain DS-1T.
A mineral salts medium containing 50 mM potassium phosphate (pH 7.2) was used (37), and strain DS-1T was routinely grown with 1 mM commercial LAS (210 mg of dissolved organic carbon/liter) or an LAS congener(s) in buffered mineral salts medium that was supplemented with suspended glass particles (1 mg/ml) to allow growth of strain DS-1T in the presence of the surfactant (28, 30). The cultures were incubated at 30°C in the dark and were aerated on a roller when they were grown at the 3-ml scale (in 30-ml screw-cap tubes) or on an orbital shaker when they were grown at the 250-ml scale (in 1-liter Erlenmeyer flasks). When SPC-salts medium was to be generated, strain DS-1T was grown with 2-C11-LAS, 2-C12-LAS, or 3-C12-LAS (or with commercial LAS), and effectively complete conversion of LAS to SPC after growth was confirmed by HPLC (gradient system I). Bacteria and glass particles were collected by centrifugation (10,000 × g, 60 min, 4°C), and the supernatant fluid was filtered (pore size, 0.45 μm) and autoclaved. This SPC-salts medium was used for carbon-limited enrichment of SPC-degrading bacteria (see below).
Enrichment and isolation of SPC-degrading bacteria.
Enrichment cultures (3 ml) for bacteria able to utilize 3-C4-SPC or 4-C6-SPC as a sole source of carbon and energy for growth were grown in 1 mM SPC-salts medium incubated at 30°C on a rotary shaker in the dark; an untreated sample (30 μl) from the aeration tank of a sewage works was used as the inoculum. The positive enrichments were subcultured several times in fresh selective medium. Bacteria were then plated on SPC-salts medium solidified with agarose (1%) and on agarose-salts medium with no additional carbon source (negative control), on which pinpoint colonies formed. A representative macrocolony from the SPC-salts medium was picked into the corresponding SPC-salts medium, and after growth was observed, substrate utilization was confirmed by HPLC (gradient system I). After several rounds of selective plating and picking, bacteria were plated on Luria-Bertani medium (9) to confirm the purity of the culture. The isolates were designated strain SPB-2 (from medium containing 3-C4-SPC) and strain SPH-1 (from medium containing 4-C6-SPC). Strain KF-1 was isolated essentially as described above, but the inoculum was biomass derived from a trickling filter able to mineralize commercial LAS (5) and the salts medium contained 6 mM 3-C4-SPC from chemical synthesis. The identity of each isolate was determined by the German Collection of Microorganisms and Cell Cultures (DSMZ) (Braunschweig, Germany) after sequencing of a partial 16S rRNA gene sequence, which was aligned and compared as described elsewhere (24, 25).
Generation of growth curves.
Samples were taken at intervals from 50-ml cultures, and the samples were used for HPLC analyses, for determination of sulfate, and for protein assays. Strain SPB-2, KF-1, or SPH-1 was grown in SPC-salts medium generated from LAS by using strain DS-1T (see above). The culture medium used for growth of communities involving strain DS-1T and an SPC degrader(s) in the presence of 1 mM LAS was supplemented with suspended glass particles (1 mg/ml) (28, 30).
RESULTS
Formation of SPCs from commercial LAS and from individual LAS congeners during growth of strain DS-1T. Strain DS-1T grew with commercial LAS (Fig. 2A, upper chromatogram; see Fig. 1 for structures), which essentially disappeared completely (>95%) (Fig. 2A, lower chromatogram), yielding about 9 major peaks and at least 15 minor peaks (Fig. 2B), which were shown in previous work to be SPCs, SPC-2Hs, and SPdCs (5, 28). When a single congener of LAS (e.g., 3-C12-LAS) was used as the growth substrate, the congener disappeared, and a major product (4-C6-SPC) was formed, along with two minor products (Table 1) (6-C8-SPC and 4-C6-SPC-2H [see Fig. 1 for structures]) that were attributable to degradation of 3-C12-LAS. 4-C6-SPC-2H showed a UV spectrum different from that of 4-C6-SPC (see below). Significant amounts of C5-SPC and C7-SPC were also detected, but they were attributed to the 5% impurities in the LAS (5, 6). The mass spectral identification data (Table 1) confirmed previous data (5). The molar growth yield with 3-C12-LAS was 39 g of protein/mol of LAS, which confirmed previous data (28) and represented about 6 mol of C utilized/mol of LAS, because the normal growth yield is 6 g of protein/mol of C (2). Thus, there was mass balance in the growth experiment; 12 carbon atoms were recovered in the 4-C6-SPC, and the remaining 6 carbon atoms accounted for the cell material and its formation.
TABLE 1.
Retention times (with gradient system I) of SPCs formed during growth of P. lavamentivorans DS-1T with single LAS congeners (Fig. 4), contributions of the relevant peaks to the total SPC peak area (A220), the corresponding m/z signals ([M-H]) observed by LC-ES-MS (with gradient system II), the general characteristics of the SPC, and the presumed identities of the compounds
| Growth substrate | Product
|
Interpretationa | |||
|---|---|---|---|---|---|
| Retention time (min)b | Peak area (%)c | m/z | SPCd | ||
| 3-C12-LASg | 9.3 | 2 | 243 | C5 | 3-C5-SPCe |
| 9.6f | 2 | 269 | C6-2H | 4-C6-SPC-2H | |
| 10.4 | 85 | 271 | C6 | 4-C6-SPC | |
| 11.7 | 3 | 285 | C7 | 5-C7-SPCe | |
| 12.6 | 7 | 299 | C8 | 6-C8-SPC | |
| 2-C12-LAS | 7.4f | 13 | 241 | C4-2H | 3-C4-SPC-2H |
| 7.9 | 70 | 243 | C4 | 3-C4-SPC | |
| 9.2 | 2 | 257 | C5 | 4-C5-SPCh | |
| 10.3 | 12 | 271 | C6 | 5-C6-SPC | |
| 2-C10-LAS, 2-C12-LAS, | 7.4f | 9 | 241 | C4-2H | 3-C4-SPC-2H |
| and 2-C14-LAS | 7.9 | 62 | 243 | C4 | 3-C4-SPC |
| 9.2 | 5 | 257 | C5 | 4-C5-SPCh | |
| 10.3 | 21 | 271 | C6 | 5-C6-SPC | |
| 2-C11-LAS | 8.7f | 31 | 255 | C5-2H | 4-C5-SPC-2H |
| 9.2 | 53 | 257 | C5 | 4-C5-SPC | |
Interpretation derived from the LC-ES-MS data, the UV spectrum, the retention time, the substrate ranges of SPC-degrading isolates, and the concept that the substituent does not migrate on the chain.
Only retention times clearly attributable to products resulting from degradation of the relevant LAS congener(s) are shown.
Percentage of the total SPC peak area.
Derived from the LC-ES-MS signal.
The material probably arose largely from contaminants in the LAS.
The material showed a UV spectrum which differed from that of LAS and known SPCs (Fig. 5).
The data represent an evaluation of a new version of an experiment described by Dong et al. (5).
The product was presumably formed after an α-oxidation step (see Discussion).
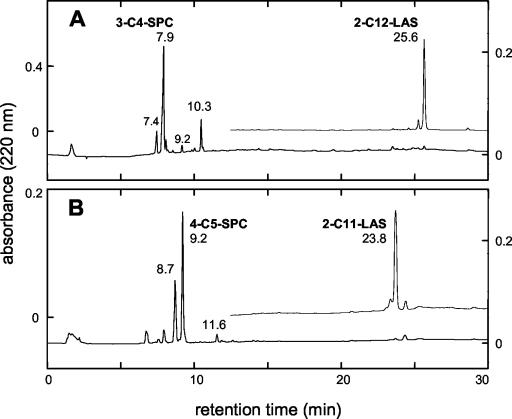
When supplied with 1 mM 2-C10-LAS, 1 mM 2-C12-LAS, or 1 mM 2-C14-LAS in salts medium, strain DS-1T generated 39, 47, or 61 μg of protein/ml (each value is the mean of three experiments), and it produced the same major SPC in each case, as illustrated by an experiment with 2-C12-LAS (Fig. 3A) or all three congeners in growth medium (the results were indistinguishable from those in Fig. 3A). This major product cochromatographed with authentic 3-C4-SPC and had the same UV spectrum as the authentic material, and the identification was confirmed by LC-ES-MS (Table 1). The growth yields were consistent with this identification, because they indicated that there was utilization of 6, >7, and > 9 carbon atoms from the C10-LAS, C12-LAS and C14-LAS, respectively.
FIG. 3.
HPLC chromatograms of individual congeners of LAS in salts medium (upper data set) and of the culture medium after growth of P. lavamentivorans DS-1T (lower data set). (A) 1 mM 2-C12-LAS; (B) 0.5 mM 2-C11-LAS. Analyses were done with HPLC gradient system I.
The major product from these three congeners was 3-C4-SPC, but as observed with 3-C12-LAS, there were several other products (Fig. 3A). The peak at 10.3 min was a C6-SPC as identified by LC-ES-MS, and it could be separated from 4-C6-SPC (Table 1), so it was presumably 5-C6-SPC (the SPC from the previous round of β-oxidation [SPC+2C]) (see Fig. 4 for the structure), and it represented about 20% of the relevant products when a mixture of congeners 2-C10-LAS, 2-C12-LAS, and 2-C14-LAS (0.33 mM each) was utilized (Table 1). The peak at 7.4 min (Fig. 3A) was shown to correspond to an α,β-unsaturated C4-SPC by LC-ES-MS and was presumably 3-C4-SPC-2H (see Fig. 4 for the structure). The peak at 9.2 min (Fig. 3A) was a C5-SPC, as shown by LC-ES-MS, and the compound coeluted with 4-C5-SPC (see below). The impurities in the LAS were negligible, especially when the mixture of 2-C10-LAS, 2-C12-LAS, and 2-C14-LAS was used, and we presumed that the 4-C5-SPC was a minor product generated from 2-C10-LAS, 2-C12-LAS, and 2-C14-LAS. This is the first evidence of a trace of α-oxidation (see Discussion) in P. lavamentivorans DS-1T.
FIG. 4.
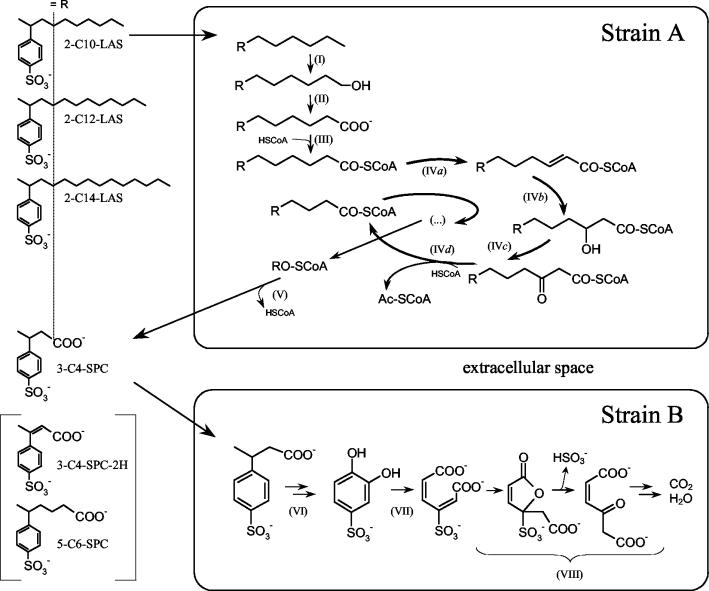
Sketch showing the conversion of LAS (2-C10-LAS, 2-C12-LAS, and 2-C14-LAS) to SPCs by P. lavamentivorans DS-1T and complete degradation of the major SPC (3-C4-SPC) by C. testosteroni strains SPB-2 and KF-1 (i.e., a two-tier bacterial community). Degradation is initiated by ω-oxygenation (reaction I) and oxidation (reaction II) of LAS to the corresponding SPC (5), which is considered to be thioesterified (reaction III) and to undergo β-oxidation (reactions IVa to IVd) until the reaction is hindered by the 4-sulfophenyl-substituent; the unsubstituted SPC is then excreted (reaction V). The SPC is apparently oxygenated (reaction VI), possibly via 4-sulfophenol, to 4-sulfocatechol, which is subject to ortho cleavage (reaction VII) and degradation (reaction VIII). Ac, acetyl; CoA, coenzyme A.
2-C11-LAS from commercial LAS was available at a purity of about 80% (the major impurities were 3-C11-LAS, 4-C11-LAS, and 6-C12-LAS). Strain DS-1T converted this material during growth to a major product (Fig. 3B, peak at 9.2 min) identified as a C5-SPC by LC-ES-MS (Table 1), which we presumed to be 4-C5-SPC (see Fig. 1 for the structure). The product with a retention time of 8.7 min (Fig. 3B) was an α,β-unsaturated C5-SPC (Table 1), presumably 4-C5-SPC-2H (Fig. 1). The minor products observed in this experiment (Fig. 3B) were a C7-SPC (putatively 6-C7-SPC [Fig. 1]) (11.6 min), which was presumably formed from 2-C11-LAS, and a C4-SPC (7.9 min) of unknown origin.
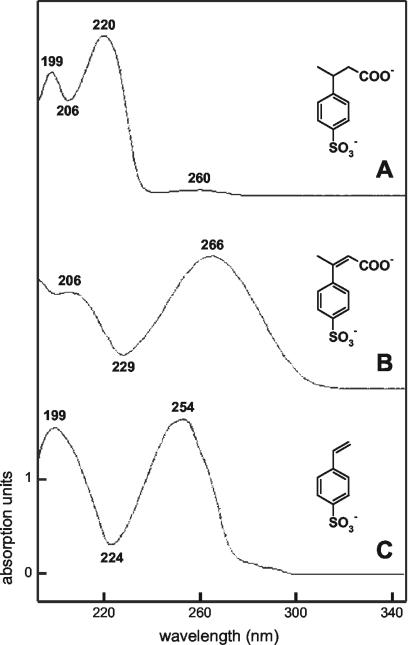
UV spectra of the SPC-2Hs.
Previously, it was reported that the UV spectra of products from LAS resembled that of, e.g., 3-C4-SPC (Fig. 5A) (28), and the SPCs identified in this work confirmed this (data not shown). However, with access to separated, low-quantity products, we observed that 3-C4-SPC-2H had a different UV spectrum (Fig. 5B). The maxima and minima at higher wavelengths were consistent with a larger system of delocalized electrons, analogous to the delocalization in 4-sulfostyrene and its UV spectrum (Fig. 5C). The UV spectrum of 4-C5-SPC-2H (maxima at 204 and 264 nm, minimum at 237 nm) was similar to that of 3-C4-SPC-2H (Fig. 5B).
FIG. 5.
UV spectra of 3-C4-SPC (A), 3-C4-SPC-2H (B), and 4-styrenesulfonate (C) obtained after separation with gradient system I. 3-C4-SPC and 3-C4-SPC-2H were generated from 1 mM 2-C12-LAS, and the spectra were obtained from separations of 10-fold-diluted (A) and undiluted (B) samples, respectively (Fig. 3A). The spectrum of 4-styrenesulfonate (C) was obtained after separation of a sample containing 60 μM 4-styrenesulfonate.
Enrichment cultures for SPC-degrading bacteria.
We enriched from municipal sewage sludge bacteria that were able to grow with 4-C6-SPC (major SPC from 3-C12-LAS) or with 3-C4-SPC (major SPC from 2-C12-LAS) as a sole source of carbon and energy. The growth of bacteria (mostly rods) in each enrichment culture was observed after 3 days, and no bacteria grew in the negative control (no carbon source). After several transfers into fresh medium, the cultures were plated on medium containing 4-C6-SPC (or 3-C4-SPC) solidified with agarose; agarose-salts medium without an added carbon source was used as the negative control. Growth of large colonies of bacteria (diameter, 3 mm) was observed on 4-C6-SPC (or 3-C4-SPC) plates, whereas pinpoint colonies were found on the negative control plates. Only the large colonies (rods) grew in selective liquid medium, in which we observed growth and substrate disappearance (as determined by HPLC). After several rounds of selective plating and picking, the culture was streaked on complex medium, on which homogeneous colony morphology was observed. Bacteria grew when they were transferred to selective medium, and they utilized the substrate.
The isolate which utilized 4-C6-SPC was designated strain SPH-1. It was a gram-negative, motile rod. The partial 16S rRNA gene sequence showed 100% sequence identity with that of Delftia acidovorans DSM 39T. D. acidovorans SPH-1 was deposited in the DSMZ as DSM 14801.
The isolate which utilized 3-C4-SPC was designated strain SPB-2. It was a gram-negative, motile rod. The partial 16S rRNA gene sequence showed 100% sequence identity with that of Comamonas testosteroni ATCC 11996T. C. testosteroni SPB-2 was deposited in the DSMZ as DSM 14802.
We obtained a second bacterium, strain KF-1, that was able to utilize 3-C4-SPC. This strain was isolated from a trickling filter which degraded LAS quantitatively (5). The organism was a gram-negative, motile rod whose partial 16S rRNA gene sequence showed 100% sequence identity with that of C. testosteroni ATCC 11996T. C. testosteroni KF-1 was deposited in the DSMZ as DSM 14576. C. testosteroni strains KF-1 and SPB-2 showed different colony morphologies when they were grown on Luria-Bertani medium plates (vaulted dense and spreading diffuse colonies, respectively). Both organisms utilized benzoate as a carbon source and seemed to lack meta cleavage of protocatechuate (see below), so they were atypical for C. testosteroni in these characters (12).
Growth of SPC-degrading bacteria.
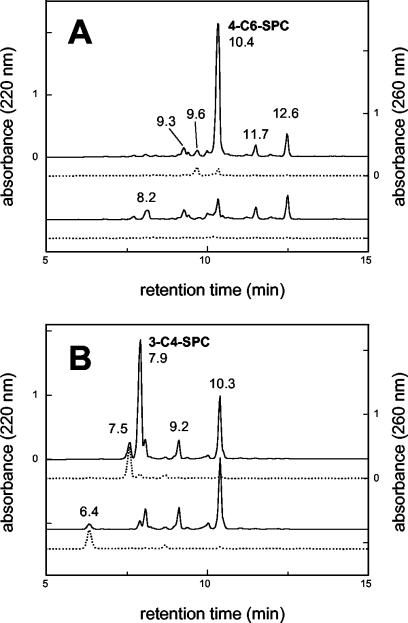
D. acidovorans SPH-1 was grown in minimal salts medium, which contained 4-C6-SPC (and the minor SPCs) generated from 3-C12-LAS (Table 1). Comparison of the medium before and after growth (Fig. 6A) showed that only 4-C6-SPC and one minor product from LAS, 4-C6-SPC-2H, were degraded. 6-C8-SPC (Table 1) and other minor products were not utilized. Corresponding to this observation, the residual level of dissolved organic carbon in the culture medium was about 20% of the initial level, which was consistent with the residual peak area. When strain SPH-1 was incubated in the minimal salts medium containing 3-C4-SPC (and the minor SPCs) generated from 2-C12-LAS (Table 1), only 4-C5-SPC (the product resulting from α-oxidation [see above]) disappeared, but 3-C4-SPC, 3-C4-SPC-2H, and 5-C6-SPC were not utilized; 2-C4-SPC also was not utilized when it was tested. The utilization of 4-C5-SPC and 4-C5-SPC-2H by strain SPH-1 was confirmed by analysis of the single growth experiment possible with the small amount of 4-C5-SPC medium, which was generated by using purified 2-C11-LAS (data not shown); no other assays were possible. The organism utilized benzoate, phenylacetate, 2- and 3-hydroxyphenylacetate, 4-hydroxybenzoate, 3,4-dihydroxybenzoate (protocatechuate), 4-phenylbutyrate, and 4-sulfocatechol as sole carbon sources for growth, but it did not utilize 4-sulfophenol, 4-sulfobenzoate, 3-phenylbutyrate, or 4-sulfostyrene.
FIG. 6.
HPLC chromatograms (gradient system I) of culture medium before (upper chromatograms) and after (lower chromatograms) growth of D. acidovorans SPH-1 with 4-C6-SPC (A) and growth of C. testosteroni SPB-2 with 3-C4-SPC (B). The detector was operated at 220 nm (solid lines) and 260 nm (dotted lines) to distinguish peaks with different absorption characteristics (SPC and SPC-2H).
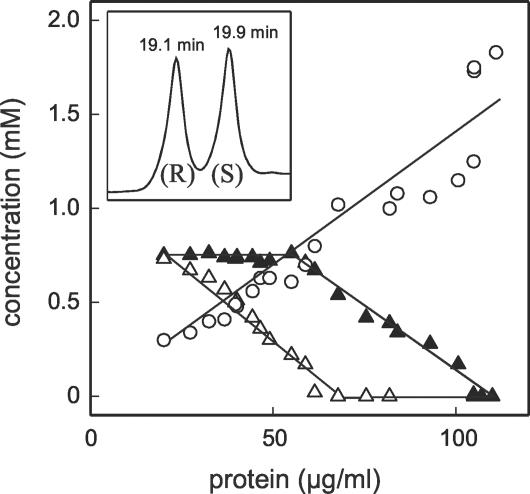
The R and S enantiomers of 4-C6-SPC were subjected to separation (Fig. 7, inset) with HPLC gradient system III. Strain SPH-1 initially utilized only the putative S enantiomer (Fig. 7); then it utilized both enantiomers until the S enantiomer was exhausted, and finally it utilized the residual R enantiomer alone. The molar growth yield (5.3 g of protein/mol of C) indicated that there was quantitative substrate utilization (2). The sulfonate sulfur was released concomitant with growth and was recovered, essentially quantitatively, as sulfate (Fig. 7). There was thus essentially a complete mass balance for the utilization of 4-C6-SPC, so a minor product (retention time, 8.2 [Fig. 6A]; UV spectrum of an SPC), which accumulated concomitant with growth, made an insignificant contribution to the total carbon.
FIG. 7.
Plot of concentration versus protein concentration during growth of D. acidovorans SPH-1 in 4-C6-SPC-salts medium, with enantiomeric separation of R- and S-4-C6-SPC (inset). Symbols: ▵, putative S-4-C6-SPC; ▴, putative R-4-C4-SPC; ○, sulfate.
C. testosteroni SPB-2 (or C. testosteroni KF-1 [data not shown]) was grown in the minimal salts medium containing 3-C4-SPC (and the minor SPCs) generated from 2-C12-LAS (Table 1). Comparison of the medium before and after growth (Fig. 6B) showed that only 3-C4-SPC and 3-C4-SPC-2H were degraded. 5-C6-SPC and other minor products were not utilized. The level of residual dissolved organic carbon, about 25% of the initial value, corresponded to this observation. When strain SPB-2 or KF-1 was incubated in the minimal salts medium which contained 4-C6-SPC (and the minor SPCs) generated from 3-C12-LAS (Table 1), 3-C5-SPC disappeared, but 4-C6-SPC, 4-C6-SPC-2H, and 6-C8-SPC did not disappear (data not shown); 2-C4-SPC was also not utilized. Each organism utilized 4-sulfophenol, benzoate, 4-hydroxybenzoate, 3,4-dihydroxybenzoate, and 2- and 3-hydroxyphenylacetate as sole sources of carbon and energy for growth. The organisms did not utilize 4-sulfobenzoate, phenylacetate, 3- or 4-phenylbutyrate, or 4-sulfostyrene; strain SPB-2 utilized 3-phenylpropionate, whereas strain KF-1 did not.
We could not separate the enantiomers of 3-C4-SPC. During exponential growth of strain SPB-2 (specific growth rate, 0.22 h−1) (data not shown) in medium containing 3-C4-SPC derived from 2-C10-LAS, the utilization of 3-C4-SPC (and of 3-C4-SPC-2H) was concomitant with growth and with the excretion of sulfate. Recovery of sulfate (83% of the total sulfonate) and the molar growth yield (6.1 g of protein/mol of C) indicated that there was quantitative substrate utilization and mass balance. The metabolic product, which eluted at 6.4 min (Fig. 6B), thus represented a negligible amount of carbon.
Tolerance of strains SPH-1, SPB-2, and KF-1 to LAS.
The new isolates grew without clumping in a suspension culture with, e.g., succinate as the sole source of carbon and energy. The organisms could grow in the presence of LAS at concentrations up to 0.5 mM, but the growth was accompanied by formation of a biofilm. D. acidovorans SPH-1 tended to form wall growth under these conditions, whereas C. testosteroni strains SPB-2 and KF-1 tended to form clumps. Addition of glass particles (1 mg/ml) to the culture medium containing LAS allowed the organisms to grow with a shorter lag phase; clumping of the glass particles during growth was due to the development of biofilm on the solid support (data not shown). C. testosteroni KF-1 grew in a uniform suspension in 3-C4-SPC-salts medium, whereas strain SPB-2 tended to form clumps, so we largely used strain KF-1 for experiments with whole-cell suspensions (see below).
Mineralization of 3-C12-LAS or 2-C12-LAS in defined two-member communities.
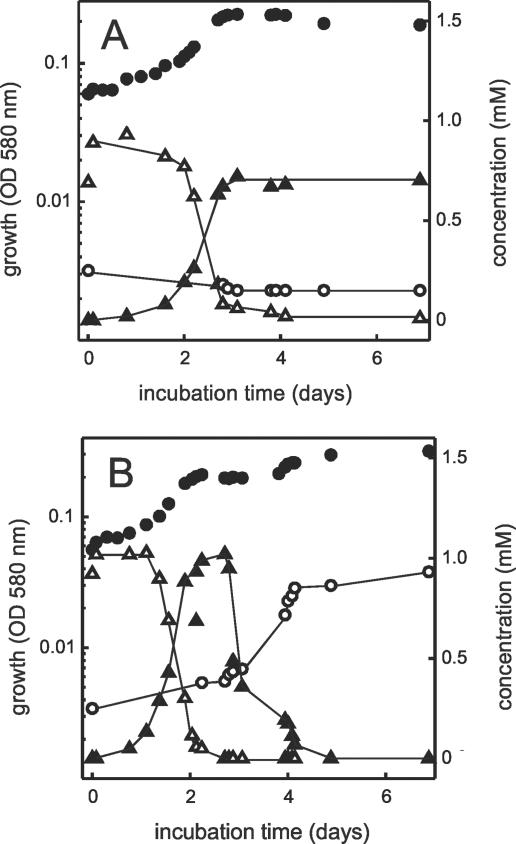
P. lavamentivorans DS-1T utilized 3-C12-LAS as the sole added growth substrate in a single growth phase and excreted largely 4-C6-SPC (and the minor SPCs) with utilization of some sulfate (Fig. 8A); the organism grew in a biofilm on glass particles, from which it could be shaken loose for turbidity measurements, as described previously (28, 30). When D. acidovorans SPH-1 was also present, a two-phase growth curve was observed (Fig. 8B). In the first phase, LAS was utilized and 4-C6-SPC was excreted, but negligible turnover of the latter compound occurred, as shown by the negligible release of sulfate in the presence of LAS and by the quantitative release of the SPC (at about 3 days). The SPC was utilized quantitatively in the second phase of growth, which appeared to be uncoupled from substrate disappearance (during day 3) but to be simultaneous with release of sulfate (day 4). This was probably due to extreme clumping in the culture, which was not detected as turbidity until the clumps disintegrated and presumably released sulfate. We could not determine growth directly by measuring protein, because there was insufficient 3-C12-LAS to allow an experiment of that magnitude. For the same reason, we could not monitor the behavior of the enantiomers of the SPC (Fig. 7).
FIG. 8.
Growth of P. lavamentivorans DS-1T (A) and of the community containing strain DS-1T and D. acidovorans SPH-1 (B) in 3-C12-LAS-salts medium. Symbols: •, turbidity; ▵, 3-C12-LAS; ▴, 4-C6-SPC; ○, sulfate. OD 580 nm, optical density at 580 nm.
Similar experiments were done with 2-C12-LAS, P. lavamentivorans DS-1T, and C. testosteroni SPB-2. The data for this community also showed that there was two-phase growth with quantitative utilization of both LAS and the corresponding 3-C4-SPC, as well as quantitative release of sulfate (data not shown).
Mineralization of several congeners of LAS in two- and three-member communities.
Strain DS-1T was grown in salts medium containing 1 mM commercial LAS alone, which yielded a mixture of SP(d)Cs (Fig. 2B), or in the presence of C. testosteroni SPB-2 (Fig. 2C) or D. acidovorans SPH-1 (Fig. 2D) or both strains (Fig. 2E). The initial experiments (see above) indicated that strains SPB-2 and SPH-1 have narrow substrate ranges for SPCs, although the ranges were not as narrow as the single SPC substrate known for the older isolate, D. acidovorans SPB1 (32). Strain SPB-2 utilized only 3-C4-SPC (peak a in Fig. 2), 3-C4-SPC-2H (arrow in Fig. 2C), and 3-C5-SPC (peak c in Fig. 2). Strain SPH-1 probably utilized four compounds, 4-C6-SPC (peak d in Fig. 2), 4-C5-SPC (peak b in Fig. 2), and 4-C5-SPC-2H (arrow in Fig. 2D); 4-C6-SPC-2H, which was also subject to degradation (see Fig. 6A), was not detected in the chromatograms shown in Fig. 2. The three-member community degraded precisely the same six SPCs (peaks a to d and arrows) (Fig. 2E), so neither interference nor synergy among community members was detected.
Oxygenase activities detected in SPC-degrading organisms.
Whole cells of D. acidovorans SPH-1 showed 4-sulfocatechol-dependent oxygen uptake (0.2 mkat/kg of protein) when they were grown with 4-C6-SPC. No development of a yellow color was detected, which might indicate meta-ring cleavage, and the results were considered to be compatible with ortho-ring cleavage of 4-sulfocatechol. The very limited amounts of biomass from strain SPH-1 available under these conditions precluded further direct experiments. However, strain SPH-1 grown with protocatechuate, phenylacetate, or succinate did not show 4-sulfocatechol-dependent oxygen uptake, so we presumed that the degradative pathway for 4-sulfocatechol is inducible.
3-C4-SPC-grown cells of C. testosteroni KF-1 (or of strain SPB-2 [data not shown]) showed substrate-dependent oxygen uptake after addition of 3-C4-SPC, 4-sulfophenol, 4-sulfocatechol, or protocatechuate (Table 2) but not after addition of 2-C4-SPC, 4-C6-SPC (Table 2), 4-sulfobenzoate, or benzoate. Substrate disappearance during oxygen uptake was confirmed by HPLC (gradient system I), and all reaction mixtures remained colorless. No substrate disappeared in the absence of oxygen (see below), but addition of oxygen led to a reaction. We inferred that oxygenases were involved in these reactions. The oxygenases were present at usually negligible levels in succinate-grown cells, so we presumed that the enzymes are inducible.
TABLE 2.
Specific activities of enzymes in whole cells of C. testosteroni KF-1 grown with different substratesa
| Substrate in reaction mixture | Specific oxygen uptake rates in reactions with cells grown with the following substrates (mkat/kg of protein):
|
|||
|---|---|---|---|---|
| 3-C4-SPC | 4-Sulfophenol | Protocatechuate | Succinate | |
| 3-C4-SPC | 1.1b | 1.2b,c | 0.1 | 0.4 |
| 2-C4-SPC | <0.01 | <0.01 | NDd | ND |
| 4-C6-SPC | <0.01 | <0.01 | ND | ND |
| 4-Sulfophenol | 0.1b | 0.1b,c | <0.01 | <0.01 |
| 4-Sulfocatechol | 0.3b | 0.3b,c | <0.01 | <0.01 |
| Protocatechuate | ND | 0.4 | 2.4 | <0.01 |
No reaction mixture developed a yellow color.
Substrate disappearance was confirmed by HPLC.
Anoxic cell suspensions (N2) did not convert the substrate, but addition of air enabled a reaction.
ND, not determined.
3-C4-SPC oxygenase, 4-sulfophenol oxygenase, 4-sulfocatechol oxygenase, and protocatechuate oxygenase were detected when 4-sulfophenol-grown cells of C. testosteroni KF-1 (or strain SPB-2) were examined (Table 2), whereas protocatechuate-grown cells exhibited a high level of protocatechuate oxygenase only, again with a colorless reaction mixture. Growth with the commercially available 4-sulfophenol allowed preliminary examination of these oxygenases. Cell suspensions incubated under anoxic conditions (N2 gas phase) did not degrade 3-C4-SPC, 4-sulfophenol, or 4-sulfocatechol until air was added to the reaction mixtures (data not shown), so each reaction presumably involved an oxygenase.
The ortho-ring cleavage of 4-sulfocatechol was confirmed with crude extracts prepared from 4-sulfophenol-grown cells of strain KF-1; extracts from succinate-grown and protocatechuate-grown cells did not catalyze the reaction. Disappearance of 4-sulfocatechol was detected photometrically as a decrease in absorption at 238 and 283 nm, while concomitant formation of 3-sulfo-cis,cis-muconate was detected as an increase in absorption at 257 nm; the same phenomenon was observed by Schulz et al. (32) and by Feigel (7). Substrate disappearance and product formation were detected by HPLC, as observed by Schulz et al. (32). The activity of 4-sulfocatechol 1,2-dioxygenase was determined to be about 0.2 mkat/kg of protein at the oxygen electrode. 3-C4-SPC oxygenase and 4-sulfophenol oxygenase activities were not detected in crude extracts.
DISCUSSION
The data show that commercial LAS is converted to about nine major SPCs (and many minor products) by P. lavamentivorans DS-1T (Fig. 2A and B) and that each individual LAS congener which we could test was converted to one major SPC (and many minor products) (Fig. 3 and Table 1). When LAS congeners with even chain lengths and the same substitution (position 2 on the chain) were available, the same major SPC was formed (Table 1), so we presumed that this is true for each relevant substituent position (positions 2 to 6 in Fig. 1) and also for the odd chain lengths for all 20 LAS congeners. The data (Table 1) (5, 6) show that β-oxidation is the major pathway of chain shortening, as observed repeatedly (31, 35) by authors who formulated the distance principle, which states that the methyl group that is more distant from the sulfophenyl substituent is attacked. Furthermore, where the identity of the SPC has been thoroughly determined, as it has been with 3-C4-SPC from 2-C10-LAS, from 2-C12-LAS, and from 2-C14-LAS (Fig. 3A and Table 1) or with 4-C6-SPC from 3-C12-LAS (28), β-oxidation stops two and three carbon atoms from the substituent when the attacked portion of the LAS side chain has an even number of carbon atoms and an odd number of carbon atoms, respectively, and there is no evidence of movement of the substituent on the chain. This allows the major SPC generated from each LAS congener by strain DS-1T to be predicted (Fig. 1). Thus, there are 11 major SPC products, one C4-SPC and two each for the C5-, C6-, C7-, C8-, and C9-SPCs. Our initial interpretation of Fig. 2B (about nine major products from C4-SPC to C9-SPC) was a conservative estimate. Given the uneven distribution of the positions of the sulfophenyl substituent in commercial LAS (Fig. 2A) and the generation of SPdCs from the long-chain SPCs (5), largely from C8- and C9-SPCs (Schleheck, unpublished data), the residual amounts of long-chain SPCs are smaller than those of the smaller SPCs.
The metabolism shown in Fig. 1 and 4 implies the presence of a large number of intermediates in P. lavamentivorans DS-1T, almost none of which have been observed. The (mono)oxygenation of LAS is presumed, because no reaction occurs in the absence of oxygen (35; Schleheck, unpublished). The presumed product of oxygenation (the alcohol) has not been observed, and the aldehyde is presumed from standard biochemical reactions leading to the acid; this SPC has been observed, usually in complex matrices (5, 10, 14). None of the many thioesters needed for β-oxidation has been observed, but the patterns of excreted intermediates (Table 1), especially the SPC-2Hs, make β-oxidation the only option (discussed in reference 5). Much work remains to be done to confirm the biochemical reactions catalyzed by strain DS-1T. In addition, there is no information on the transport mechanisms involved in bringing LAS to the oxygenase or the excretion of SPCs from the cytoplasm, where the β-oxidation enzymes may be expected.
The SPC-2Hs, which we observed (Table 1), are presumed to carry the Δ-2 double bond, which arises in β-oxidation (Fig. 4) and which gives a wider delocalization of π electrons and the shift of the UV spectrum to longer wavelengths in the example shown in Fig. 5B (3-C4-SPC-2H). The fact that 4-C6-SPC-2H and 4-C5-SPC-2H also give this UV spectrum, where the Δ-2 double bond should not lead to delocalization of electrons into the ring (see Fig. 1 for structures), shows that these compounds need to be isolated and examined in more detail to establish their identities and their generation.
Whereas most of the LAS was metabolized by strain DS-1T via the β-oxygenation pathway, a small portion (<5%) may have been subject to α-oxidation (Table 1). This reaction, however catalyzed, is readily seen in some environmental compartments (23). α-Oxidation can presumably be studied best in an isolated organism which uses the reaction as the major pathway to degrade LAS.
Transport systems for organosulfonates are axomatic (11), and the first transport system for an arenesulfonate is being elucidated in C. testosteroni T-2 (24a, 38). There is no indication yet how LAS is transported into the P. lavamentivorans or how SPCs are exported. Similarly, there is no indication how SPCs are transported into, e.g., C. testosteroni KF-1, or how sulfite and sulfate are exported.
The degradability of SPCs, SPdCs, and the recently discovered SPC-2Hs has been recognized implicitly for many years, because LAS (as shown in Fig. 1) is fully biodegradable (21, 22), and degradative organisms were isolated in laboratories which did not have extensive analytical capabilities to explore their physiology in detail (13, 16, 33). Schulz et al. (32) found that their isolate, D. acidovorans SPB1, has a very narrow substrate spectrum for SPCs, namely, RS-2-C4-SPC, which are degraded sequentially, the S enantiomer first. The new isolates, D. acidovorans SPH-1 and C. testosteroni strains SPB-2 and KF-1, also have narrow substrate ranges for SPCs, but they seem to degrade three or four compounds each (4-C6-SPC, 4-C6-SPC-2H, 4-C5-SPC, and 4-C5-SPC-2H for strain SPH-1; and 3-C4-SPC, 3-C4-SPC-2H, and 3-C5-SPC for strains SPB-2 and KF-1). D. acidovorans SPH-1 utilizes RS-4-C6-SPC enantiomer specifically, in that the putative S enantiomer is utilized first (Fig. 7), and preliminary data from H.-P. E. Kohler (EAWAG, Dübendorf, Switzerland) (personal communication) indicate that C. testosteroni KF-1 utilizes 3-C4-SPC enantiomer specifically. It seems likely that many SPCs are utilized enantiomer specifically, but the mechanisms involved (two enantiomer-specific transport systems and two enantiomer-specific oxygenases, as described by Kohler [19]) remain to be explored.
The degradative pathway(s) for SPCs was the subject of much speculation (3) until Schulz et al. (32) showed the involvement of inducible 4-sulfocatechol 1,2-dioxygenase in the degradation of 2-C4-SPC and Dong et al. (5) found high activity of the enzyme in a mixed culture which degraded SP(d)Cs. The new isolates, strains SPH-1, SPB-2, and KF-1, all express 4-sulfocatechol 1,2-dioxygenase inducibly during degradation of the appropriate SPC (Table 2; see Results), so we consider it highly likely that all SPCs are degraded via 4-sulfocatechol and the ortho pathway elucidated by Feigel and Knackmuss (1, 8), whose central reactions, including desulfonation, are shown in Fig. 4. Further work on this ortho pathway showed that the ring cleavage enzyme of Hydrogenophaga intermedia S1 is almost identical to the 3,4-dihydroxybenzoate 3,4-dioxygenase in the same organism; whereas the 3,4-dihydroxybenzoate dioxygenase cleaves only 3,4-dihydroxybenzoate, the 4-sulfocatechol 1,2-dioxygenase can cleave both 3,4-dihydroxybenzoate and 4-sulfocatechol (1). The same phenomenon is observed in C. testosteroni KF-1 (Table 2) and in strain SPB-2.
The shortages of SPCs and 4-sulfocatechol led us to explore 4-sulfophenol as a potential, commercially available growth substrate with which to obtain 4-sulfocatechol-1,2-dioxygenase. Whereas strain SPH-1 failed to grow, strains SPB-2 and KF-1 grew with 4-sulfophenol and expressed 4-sulfocatechol 1,2-dioxygenase (Table 2). Presumably, 4-sulfophenol is oxygenated by a 4-sulfophenol 2-monooxygenase (Table 2) to 4-sulfocatechol. This is the first demonstration of a degradative pathway for 4-sulfophenol, whose degradation was first observed in the 1960s (36).
Growth of C. testosteroni KF-1 (or strain SPB-2) with 4-sulfophenol gratuitously induces the 3-C4-SPC oxygenase(s) (Table 2). The simplest degradative pathway for 3-C4-SPC that we can postulate is oxygenolytic cleavage of the side chain to yield 4-sulfophenol and a C4 carboxylic acid. The latter compound is utilized for growth, as indicated by the molar growth yield for 3-C4-SPC (about 6 g of protein/mol of C). 4-Sulfophenol is oxidized via 4-sulfophenol 2-monooxygenase. We know of no precedent for the reaction postulated for 3-C4-SPC, except the cleavage of 2-C4-SPC in strain SPB1 (32) and the corresponding reactions with 3-C4-SPC in strain SPB-2 and with 4-C6-SPC in strain SPH-1 (this paper). This inducible reaction is not yet available cell free.
C. testosteroni KF-1 grew uniformly in suspended cultures with 3-C4-SPC, 4-sulfophenol, and compounds like benzoate, but when small amounts of LAS, which the organism does not attack, were added to the culture, the organism started to form a biofilm. Presumably this is a stress response, which is also detected in strains SPB-2 and SPH-1. P. lavamentivorans DS-1T degrades LAS only when it can form a biofilm, so the biofilm in LAS-degrading cocultures of, e.g., strains KF-1 and DS-1T is presumably contributed to by both organisms.
The representative degraders of SPCs, strains SPH-1 and KF-1 (or strain SPB-2), degrade 4 (Fig. 2E) of the 11 major SPCs (Fig. 1) produced from commercial LAS by strain DS-1T. These four degraded SPCs thus represent the main products from 8 of the 20 LAS congeners. If this specificity of SPC degraders is generally true, at least four more organisms are needed to degrade the other major SPCs that are detected. We have no evidence for the degradation of SPdCs under these conditions (Fig. 2), so many more organisms are presumably needed to degrade the minor components of the mixture of compounds generated from LAS by strain DS-1T. The ready biodegradability of commercial LAS masks a complex community of microorganisms and many poorly understood biochemical reactions.
Acknowledgments
We are grateful to J. Müller for performing the LC-MS analyses, to H.-H. Stabel, Bodensee Wasserversorgung (Sipplingen, Germany), for arranging the analyses of dissolved organic carbon, to L. Elsgaard (DIAS, Tjele, Denmark) for improving the text, and to K. Denger and H.-P. E. Kohler for helpful discussions.
D.S. was supported by funds from the European Union program SUITE (ENV4-CT98-0723), BASF/BMBF, ECOSOL, and the University of Konstanz. Work with HPLC-ES-MS was supported by the European Union program P-THREE (grant to T.P.K.). K.F. was supported by funds from the LBS Stiftung Umwelt und Wohnen.
REFERENCES
- 1.Contzen, M., S. Bürger, and A. Stolz. 2001. Cloning of the genes for a 4-sulphocatechol-oxidizing protocatechuate 3,4-dioxygenase from Hydrogenophaga intermedia S1 and identification of the amino acid residues responsible for the ability to convert 4-sulphocatechol. Mol. Microbiol. 41:199-205. [DOI] [PubMed] [Google Scholar]
- 2.Cook, A. M. 1987. Biodegradation of s-triazine xenobiotics. FEMS Microbiol. Rev. 46:93-116. [Google Scholar]
- 3.Cook, A. M. 1998. Sulfonated surfactants and related compounds: facets of their desulfonation by aerobic and anaerobic bacteria. Tenside Surfactants Deterg. 35:52-56. [Google Scholar]
- 4.Cook, A. M., and D. Hrsák. 2000. The complete degradation of LAS is becoming better understood with pure cultures of bacteria. CLER Rev. 6:46-53. [Google Scholar]
- 5.Dong, W., P. Eichhorn, S. Radajewski, D. Schleheck, K. Denger, T. P. Knepper, J. C. Murrell, and A. M. Cook. 2004. Parvibaculum lavamentivorans converts linear alkylbenzenesulfonate surfactant to sulfophenylcarboxylates, α,β-unsaturated sulfophenylcarboxylates and sulfophenyldicarboxylates, which are degraded in communities. J. Appl. Microbiol. 96:630-640. [DOI] [PubMed] [Google Scholar]
- 6.Eichhorn, P., and T. P. Knepper. 2002. α,β-Unsaturated sulfophenylcarboxylate intermediates detected during aerobic degradation of linear alkylbenzenesulfonate (LAS) surfactant: direct evidence for ω-oxygenation followed by β-oxidations by liquid chromatography-electrospray mass spectrometry. Environ. Toxicol. Chem. 21:1-8. [PubMed] [Google Scholar]
- 7.Feigel, B. 1990. Synergistischer Abbau von 4-Aminobenzolsulfonat durch Hydrogenophaga palleronii und Agrobacterium radiobacter. Ph.D. thesis. University of Stuttgart, Stuttgart, Germany.
- 8.Feigel, B. J., and H.-J. Knackmuss. 1993. Syntrophic interactions during degradation of 4-aminobenzenesulfonic acid by a two species bacterial culture. Arch. Microbiol. 159:124-130. [DOI] [PubMed] [Google Scholar]
- 9.Gerhardt, P., R. G. E. Murray, W. A. Wood, and N. R. Krieg. 1994. Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 10.González-Mazo, E., M. Honing, D. Barceló, and A. Gómez-Parra. 1997. Monitoring long-chain intermediate products from the degradation of linear alkylbenzene sulfonates in the marine environment by solid-phase extraction followed by liquid chromatography/ionspray mass spectrometry. Environ. Sci. Technol. 31:504-510. [Google Scholar]
- 11.Graham, D. E., H. Xu, and R. H. White. 2002. Identification of coenzyme M biosynthetic phosphosulfolactate synthase: a new family of sulfonate biosynthesizing enzymes. J. Biol. Chem. 277:13421-13429. [DOI] [PubMed] [Google Scholar]
- 12.Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, and S. T. Williams. 1994. Bergey's manual of determinative bacteriology, 9th ed. Williams & Wilkins, Baltimore, Md.
- 13.Hrsák, D., and A. Begonja. 1998. Growth characteristics and metabolic activities of the methanotrophic-heterotrophic groundwater community. J. Appl. Microbiol. 85:448-456. [DOI] [PubMed] [Google Scholar]
- 14.Hrsák, D., and A. Begonja. 2000. Possible interactions within a methanotrophic-heterotrophic groundwater community able to transform linear alkylbenzenesulfonates. Appl. Environ. Microbiol. 66:4433-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hrsák, D., and D. Grbic-Galic. 1995. Biodegradation of linear alkylbenzenesulfonates (LAS) by mixed methanotrophic-heterotrophic cultures. J. Appl. Bacteriol. 78:487-494. [DOI] [PubMed] [Google Scholar]
- 16.Jiménez, L., A. Breen, N. Thomas, T. W. Federle, and G. S. Sayler. 1991. Mineralization of linear alkylbenzene sulfonate by a four-member aerobic bacterial consortium. Appl. Environ. Microbiol. 57:1566-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy, S. I. T., and C. A. Fewson. 1968. Enzymes of the mandelate pathway in bacterium N.C.I.B. 8250. Biochem. J. 107:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knepper, T. P., and J. L. Berna. 2003. Surfactants: properties, production, and environmental aspects, p. 1-49. In T. P. Knepper, D. Barceló, and P. de Voogt (ed.), Analysis and fate of surfactants in the aquatic environment. Elsevier, Amsterdam, The Netherlands.
- 19.Kohler, H.-P. E. 1999. Sphingomonas herbicidovorans MH: a versatile phenoxyalkanoic acid herbicide degrader. J. Ind. Microbiol. Biotechnol. 23:336-340. [DOI] [PubMed] [Google Scholar]
- 20.Kölbener, P., U. Baumann, A. M. Cook, and T. Leisinger. 1994. 3-Nitrobenzenesulfonic acid and 3-aminobenzenesulfonic acid in a laboratory trickling filter: biodegradability with different activated sludges. Water Res. 28:1855-1860. [Google Scholar]
- 21.Kölbener, P., U. Baumann, T. Leisinger, and A. M. Cook. 1995. Linear alkylbenzenesulfonate (LAS) surfactants in a simple test to detect refractory organic carbon (ROC): attribution of recalcitrants to impurities in LAS. Environ. Toxicol. Chem. 14:571-577. [Google Scholar]
- 22.Kölbener, P., U. Baumann, T. Leisinger, and A. M. Cook. 1995. Non-degraded metabolites arising from the biodegradation of commercial linear alkylbenzenesulfonate (LAS) surfactants in a laboratory trickling filter. Environ. Toxicol. Chem. 14:561-569. [Google Scholar]
- 23.León, V. M., and E. González-Mazo. 2003. Degradation of LAS in the marine environment, p. 591-606. In T. P. Knepper, P. DeVoogt, and D. Barceló (ed.), Analysis and fate of surfactants in the aquatic environment. Elsevier, Amsterdam, The Netherlands.
- 24.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Mampel, J., E. Maier, T. Tralau, J. Ruff, R. Benz, and A. M. Cook. A novel outer-membrane anion channel (porin) as part of the putatively two-component transport system for p-toluenesulfonate in Comamonas testosteroni T-2. Biochem. J., in press. [DOI] [PMC free article] [PubMed]
- 25.Rainey, F. A., N. Ward-Rainey, R. M. Kroppenstedt, and E. Stackebrandt. 1996. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 46:1088-1092. [DOI] [PubMed] [Google Scholar]
- 26.Sawyer, C. N., and D. W. Ryckman. 1957. Anionic synthetic detergents and water supply problems. J. Am. Water Works Assoc. 49:480-490. [Google Scholar]
- 27.Schleheck, D., and A. M. Cook. 2003. Saccharin as a sole source of carbon and energy for Sphingomonas xenophaga SKN. Arch. Microbiol. 179:191-196. [DOI] [PubMed] [Google Scholar]
- 28.Schleheck, D., W. Dong, K. Denger, E. Heinzle, and A. M. Cook. 2000. An α-proteobacterium converts linear alkylbenzenesulfonate (LAS) surfactants into sulfophenylcarboxylates and linear alkyldiphenyletherdisulfonate surfactants into sulfodiphenylethercarboxylates. Appl. Environ. Microbiol. 66:1911-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schleheck, D., M. Lechner, R. Schönenberger, M. J.-F. Suter, and A. M. Cook. 2003. Desulfonation and degradation of sulfodiphenylethercarboxylates from linear alkyldiphenyletherdisulfonate surfactants. Appl. Environ. Microbiol. 69:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schleheck, D., B. J. Tindall, R. Rosselló-Mora, and A. M. Cook. Parvibaculum lavamentivorans gen. nov., sp. nov., a novel heterotroph that initiates catabolism of linear alkylbenzenesulfonate. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 31.Schöberl, P. 1989. Basic principles of LAS biodegradation. Tenside Surfactants Deterg. 26:86-94. [Google Scholar]
- 32.Schulz, S., W. Dong, U. Groth, and A. M. Cook. 2000. Enantiomeric degradation of 2-(4-sulfophenyl)butyrate via 4-sulfocatechol in Delftia acidovorans SPB1. Appl. Environ. Microbiol. 66:1905-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigoillot, J.-C., and M.-H. Nguyen. 1992. Complete oxidation of linear alkylbenzene sulfonate by bacterial communities selected from coastal seawater. Appl. Environ. Microbiol. 58:1308-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sörbo, B. 1987. Sulfate: turbidimetric and nephelometric methods. Methods Enzymol. 143:3-6. [DOI] [PubMed] [Google Scholar]
- 35.Swisher, R. D. 1987. Surfactant biodegradation, 2nd ed. Marcel Dekker, New York, N.Y.
- 36.Symons, J. M., and L. A. del Valle-Rivera. 1961. Metabolism of organic sulfonates by activated sludge. Eng. Ext. Ser. Purdue Univ. 109:555-571. [Google Scholar]
- 37.Thurnheer, T., T. Köhler, A. M. Cook, and T. Leisinger. 1986. Orthanilic acid and analogues as carbon sources for bacteria: growth physiology and enzymic desulfonation. J. Gen. Microbiol. 132:1215-1220. [Google Scholar]
- 38.Tralau, T., A. M. Cook, and J. Ruff. 2003. An additional regulator, TsaQ, is involved with TsaR in regulation of transport during the degradation of p-toluenesulfonate in Comamonas testosteroni T-2. Arch. Microbiol. 180:319-326. [DOI] [PubMed] [Google Scholar]
- 39.van Ginkel, C. G. 1996. Complete degradation of xenobiotic surfactants by consortia of aerobic organisms. Biodegradation 7:151-164. [DOI] [PubMed] [Google Scholar]