Abstract
Biofilms from drains in food processing facilities with a recent history of no detectable Listeria monocytogenes in floor drains were cultured for microorganisms producing antilisterial metabolites. A total of 413 microbial isolates were obtained from 12 drain biofilm samples and were assayed at 15 and 37°C for activities that were bactericidal or inhibitory to L. monocytogenes, by two agar plate assays. Twenty-one of 257 bacterial isolates and 3 of 156 yeast isolates had antilisterial activity. All 24 isolates which produced metabolites inhibitory to L. monocytogenes were assayed for antilisterial activity in coinoculated broth cultures containing tryptic soy broth with yeast extract (TSB-YE). A five-strain mixture of 103 CFU of L. monocytogenes/ml and 105 CFU of the candidate competitive-exclusion microorganism/ml was combined in TSB-YE and incubated at 37°C for 24 h, 15°C for 14 days, 8°C for 21 days, and 4°C for 28 days. Substantial inhibition of L. monocytogenes growth (4 to 5 log CFU/ml) was observed for nine bacterial isolates at 37°C, two at 15 and 8°C, and three at 4°C. The inhibitory isolates were identified as Enterococcus durans (six isolates), Lactococcus lactis subsp. lactis (two isolates), and Lactobacillus plantarum (one isolate). The anti-L. monocytogenes activity of these isolates was evaluated in biofilms of L. monocytogenes on stainless steel coupons at 37, 15, 8, and 4°C. Results revealed that two isolates (E. durans strain 152 and L. lactis subsp. lactis strain C-1-92) were highly inhibitory to L. monocytogenes (growth inhibition of >5 log10 CFU of L. monocytogenes/cm2). These two bacterial isolates appear to be excellent competitive-exclusion candidates to control L. monocytogenes in biofilms at environmental temperatures of 4 to 37°C.
Listeria monocytogenes is distributed widely in the environment, being able to survive and grow in soil and aqueous environments (15, 21). L. monocytogenes has been isolated from 8 to 44% of samples obtained from grain fields, pastures, mud, animal feces, wildlife feeding grounds, and related sources and can survive in moist soils for more than 295 days (22).
Recent outbreak investigations revealed that recontamination after a listerial treatment is applied is the primary source of L. monocytogenes in many commercially prepared ready-to-eat processed foods (4, 8, 11, 14). Studies have revealed that certain strains of L. monocytogenes can become well established in a food processing facility in locations such as floor drains and remain members of the resident microbial flora for months or years (13, 21). Although significant improvements in plant layout and equipment design and procedures for cleaning and sanitizing have been made, it is believed that L. monocytogenes will continue to be introduced into the environment in which ready-to-eat foods are exposed for further processing and packaging (21).
The objective of this study was to isolate, from biofilms obtained from floor drains of processing plants that were negative for Listeria species, microorganisms that produce metabolites bactericidal or inhibitory to L. monocytogenes and determine their ability to kill or suppress growth of L. monocytogenes when cocultured in a liquid growth medium and in a biofilm.
MATERIALS AND METHODS
Preparation of L. monocytogenes cultures.
A five-strain mixture of L. monocytogenes, including LM101 (serotype 4b, salami isolate), LM112 (serotype 4b, salami isolate), LM113 (serotype 4b, pepperoni isolate), H9666 (serotype 1/2c, human isolate), and ATCC 5779 (serotype 1/2c, cheese isolate), from the University of Georgia Center for Food Safety culture collection was used. Each strain was individually grown in tryptic soy broth with 0.6% yeast extract (TSB-YE; Becton Dickinson, Sparks, Md.) at 37°C for 16 h. The cultures were sedimented by centrifugation at 8,000 × g for 20 min and resuspended in 0.1% peptone. The optical density of each strain was adjusted in a spectrophotometer with 0.1% peptone to an optical density reading of 0.5 (ca. 108 CFU/ml) at 630 nm. Approximately the same cell numbers of each strain were mixed and combined to provide a five-strain mixture.
Isolation and screening of microorganisms for metabolites inhibitory to L. monocytogenes.
Biofilm samples collected from floor drains at different food processing plants having a recent history of no detectable L. monocytogenes were used to obtain isolates of bacteria and yeasts. Two methods, including direct plating and enrichment culture, were used to isolate these microorganisms. TSB (10 ml) was added to each biofilm sample (ca. 1 g), and biofilm preparations were serially diluted (1:10) in 0.1% peptone to 10−3 CFU/ml. A volume of 0.1 ml of each dilution was plated on dichloran rose bengal chloramphenicol agar (DRBC) and tryptic soy agar (TSA) plates in duplicate. DRBC plates were incubated at 30°C for 72 h, and TSA plates were incubated at 37°C for 24 h. A biofilm preparation (1 ml) also was added to 9 ml of TSB and incubated at 37°C for 24 h for enrichment. Enrichment cultures were serially diluted in 0.1% peptone, and 0.1-ml portions from dilutions of 10−5 to 10−8 were plated onto TSA and DRBC plates and incubated according to the conditions described above. Thirty to forty colonies per biofilm specimen were randomly selected from plates and streaked for isolation. Two methods, including a spot-on-lawn assay and a double-layer assay, and two temperatures (37 and 15°C) were used to screen isolates for antilisterial activity.
For the spot-on-lawn assay, 0.1 ml of approximately 107 cells of the five-strain mixture of L. monocytogenes was plated in duplicate onto the surface of TSA plates. Candidate competitive-exclusion isolates were grown individually in TSB at 37°C for 24 h, cells were sedimented by centrifugation (4,000 × g for 20 min), and the supernatant fluid of each culture was filter sterilized (0.22-μm-pore-size cellulose acetate membrane; Nalgene Co., Rochester, N.Y.). A 12-mm-diameter disk (Dispens-O-Disk; Difco Laboratories, Detroit, Mich.) was placed onto the surface of each TSA plate, and 0.1 ml of filter-sterilized supernatant from a single culture was applied to the surface of the disk. In addition, a disk with nisin (3.125 μg; Sigma, St. Louis, Mo.) was used as the positive control and a disk with 0.1 M phosphate-buffered saline (PBS), pH 7.2, was used as the negative control. The plates were incubated at 37°C for 24 h and then observed for zones of inhibition (≥0.5 mm).
The double-layer assay, also a two-step procedure, involved first growing a spot-inoculated candidate competitive-exclusion isolate on TSA and then applying a second layer of growth medium containing the five-strain mixture of L. monocytogenes. Specifically, an individual colony of candidate competitive-exclusion bacterium was inoculated in the center of each of two TSA plates and incubated for 24 h at 37°C. The five-strain mixture of L. monocytogenes was added at 106 CFU/ml to brain heart infusion with 0.4% agar (Difco) at 50°C and stirred for 1 min at 200 rpm with a magnetic stir bar. Approximately 8 ml of this preparation was poured onto the surface of each TSA plate as a second layer and allowed to cool to room temperature. The cultures were then incubated for 24 h at 37°C and observed for zones of inhibition (≥0.5 mm). A nisin A and B gene-carrying Lactococcus lactis subsp. lactis strain (ATCC 11454) was used as the positive control, and a yeast isolate, which was isolated in this work and confirmed to produce no extracellular antimicrobials to L. monocytogenes, was used as the negative control.
Competitive growth inhibition of L. monocytogenes in broth cultures at different temperatures:.
All isolates with anti-L. monocytogenes activity were further tested in TSB-YE for competitive growth with L. monocytogenes at 37, 15, 8, and 4°C. Approximately 106 CFU (0.1 ml) of a potential competitive-exclusion microorganism having anti-L. monocytogenes activity and 0.1 ml of 103 CFU of the five-strain mixture of L. monocytogenes were added to 10 ml of TSB-YE and incubated at 37, 15, 8, or 4°C. Cultures (1 ml) were sampled at intervals of 8 and 24 h for 37°C; at 1, 2, 3, 7, 10, and 14 days for 15°C; at 1, 7, 14, and 21 days for 8°C; and at 2, 7, 14, 21, and 28 days for 4°C incubation and enumerated for L. monocytogenes on modified Oxford agar (Difco) held at 37°C for 48 h and for total bacterial counts (L. monocytogenes and competitive-exclusion microorganisms) and competitive-exclusion microorganism counts (competitive-exclusion microorganisms only) on TSA held at 37°C for 48 h.
Identification of competitive-exclusion microorganisms.
Isolates having anti-L. monocytogenes activity at all four temperatures tested were identified by Gram staining, biochemical assays (API CHB and API CHL; bioMérieux Industry, Marcy l'Etoile, France), and 16S rRNA gene alignment profile analysis (Midi Labs, Newark, Del.).
Preparation of stainless steel coupons.
Stainless steel (T-304; Tull Metals Company, Atlanta, Ga.) coupons (4 by 2.5 cm) were washed by a 10-min immersion with agitation (150 rpm) in 1,000 ml of an aqueous 2% RBS 35 detergent concentrate solution (20 ml of RBS 35 concentrate per liter of tap water at 50°C; Pierce, Rockford, Ill.), and rinsed by immersion in 1,000 ml of tap water (initially at 50°C) with agitation (150 rpm) for 25 min. Five additional 1-min immersions with agitation (150 rpm) in 1,000 ml of distilled water at ambient temperature were performed. The coupons were dried, and a hydrophobic marker was used to encircle an area of 1.13 cm in diameter. The coupons were then individually wrapped and autoclaved at 121°C for 30 min.
Competitive exclusion of L. monocytogenes in a biofilm.
Biofilms were grown using modifications of the protocols described by Leriche and Carpentier (10) and Chae and Schraft (5). An inoculum of 0.1 ml of ca. 106 to 108 CFU of candidate competitive-exclusion microorganisms and ca. 103 to 104 CFU of the five-strain mixture of L. monocytogenes was deposited within the marked area of the stainless steel coupon and then placed in a humidity-controlled incubator (approximately 95% relative humidity) at 37, 15, 8, or 4°C for 6 h. Nonadherent bacteria were removed by vacuum aspiration after 6 h of incubation and replaced with 0.1 ml of fresh TSB medium. The stainless steel coupons were reincubated at the same temperature, and the medium was replaced every 1, 3, 3, and 7 days for incubation at 37, 15, 8, and 4°C, respectively. At each sampling time, selected coupons in duplicate were transferred to a laminar flow hood in which weakly adherent bacteria were removed by washing the marked area of each coupon three times with PBS, and then remaining liquid from the marked area was removed by vacuum aspiration. The coupons were each placed in a 50-ml centrifuge tube containing 9.9 ml of PBS and ca. 30 glass beads (5-mm diameter; Fisher Scientific, Norcross, Ga.). The tubes were agitated by a Vortex mixer (Fisher Scientific) for 2 min to disrupt bacteria in the adherent biofilm. The suspended bacteria were serially diluted (1:10) in 0.1% peptone and plated in duplicate on TSA for competitive-exclusion microorganism counts and total bacterial counts and on modified Oxford agar for L. monocytogenes counts. The plates were incubated for 48 h at 37°C, and competitive-exclusion microorganism counts, total bacterial counts, and L. monocytogenes counts were determined. Coupons inoculated with only ca. 103 to 104 CFU of L. monocytogenes served as positive controls, whereas coupons inoculated with only ca. 106 to 108 competitive-exclusion microorganisms served as negative controls. All experiments were duplicated, and results are presented as the means plus standard deviations.
Identification of nisin-encoding genes by competitive-exclusion microorganisms.
A PCR assay was used to identify nisin-encoding genes in competitive-exclusion microorganisms. Bacterial DNA was extracted with a microbial genomic DNA isolation kit according to the procedures described by the manufacturer (Mo Bio Laboratories, Inc., Solana Beach, Calif.). The oligonucleotide sequences of the primers used were as given in parentheses: NisA (5-CGGCTCTGATTAAATTCTGAAG and 5-CGGTTGAGCTTTAAATGAAC) and NisB (5-AGAGAAGTTATTTACGATCAAC and 5-ATCTGACAACAAATCTTTTTGT) (16). PCR was performed by using an Icycler 96-well reaction module (Bio-Rad Laboratories, Hercules, Calif.) according to the protocols described by Olasupo et al. (16).
Statistical analysis:.
Least-square means of L. monocytogenes counts in biofilm samples with and without competitive-exclusion microorganisms were analyzed using the general linear model of the Statistical Analysis System procedure (SAS Institute, Cary, N.C.). The criterion for significance of difference was P < 0.05 for all tests.
RESULTS
A total of 12 biofilm samples from floor drains of four different food processing facilities were screened for microorganisms inhibitory to L. monocytogenes. A total of 156 yeasts and 257 bacterial isolates were obtained from the biofilms and assayed for inhibitory activity against L. monocytogenes. Twenty-four isolates, including three of yeast and 21 of bacteria, were inhibitory to L. monocytogenes (0.5- to 3.5-mm zone of inhibition), with none of the bacteria and three of the yeasts being identified by the spot-on-lawn assay and 21 of the bacteria and none of the yeasts being identified by the double-layer assay.
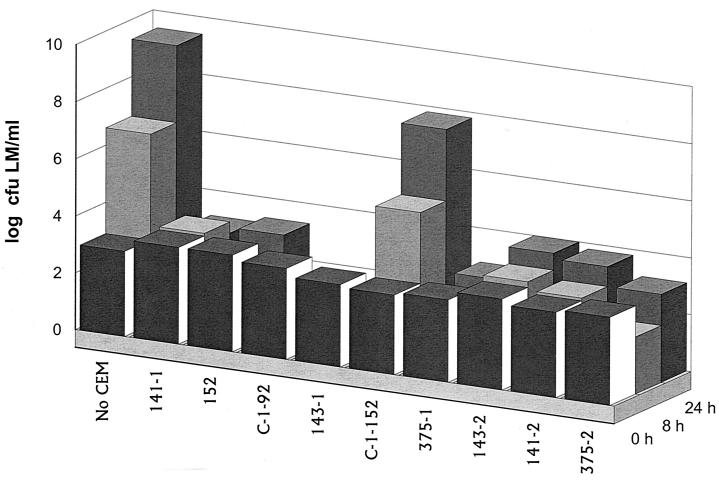
All isolates inhibitory to L. monocytogenes were evaluated individually for their ability to inhibit growth of or kill a five-strain mixture of L. monocytogenes in TSB-YE at 37°C. Under these conditions, two yeast isolates were weakly inhibitory to L. monocytogenes, repressing growth of listeriae by 0.7 log10 CFU/ml compared to the positive control of L. monocytogenes only. This possibly may be attributed to the slower growth of these yeast strains than of bacteria in TSB-YE. In contrast, nine of the bacterial isolates were strongly inhibitory to L. monocytogenes, with each providing a >5-log10-CFU/ml differential within 24 h compared with the L. monocytogenes-only positive control (Fig. 1).
FIG. 1.
Inhibition at 37°C of L. monocytogenes (LM) by competitive-exclusion microorganisms (not including yeast isolates) in TSB-YE.
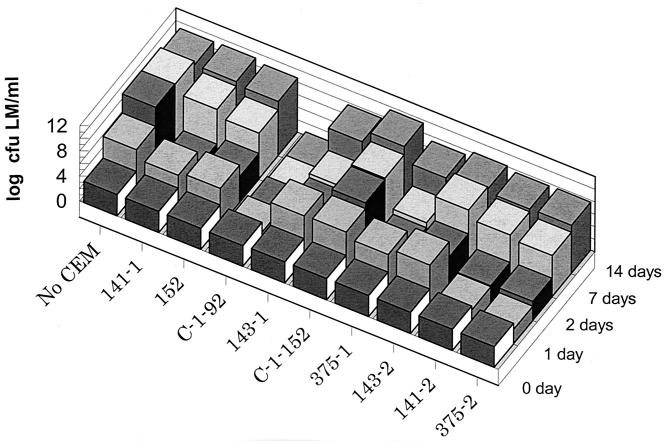
Twelve isolates were assayed under the same conditions but at 15°C. Three of the isolates were highly inhibitory and/or bactericidal to L. monocytogenes, with a >4-log10-CFU L. monocytogenes differential in growth at day 7 compared to the L. monocytogenes-only positive control, and one strain, C-1-92, was exceptionally bactericidal, with no detectable L. monocytogenes present (>8-log10-L. monocytogenes CFU/ml differential in growth compared with the positive control) at 7 and 14 days (Fig. 2).
FIG. 2.
Inhibition at 15°C of L. monocytogenes (LM) by competitive-exclusion microorganisms (not including yeast isolates) in TSB-YE.
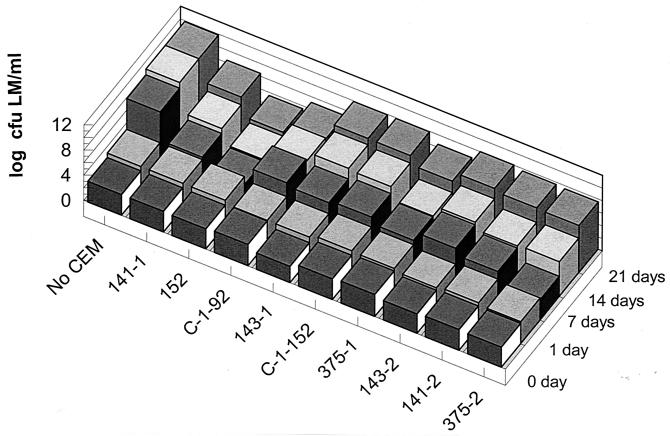
These same 12 isolates were assayed for activity inhibitory to L. monocytogenes in TSB-YE at 8°C. Six of the isolates were highly inhibitory, with a >4-log10-L. monocytogenes CFU/ml differential in growth at 14 days compared to the L. monocytogenes-only positive control, and one strain, 152, was exceptionally antimicrobial, with a 6.3-log10-L. monocytogenes CFU differential in growth at 21 days (Fig. 3).
FIG. 3.
Inhibition at 8°C of L. monocytogenes (LM) by competitive-exclusion microorganisms (not including yeast isolates) in TSB-YE.
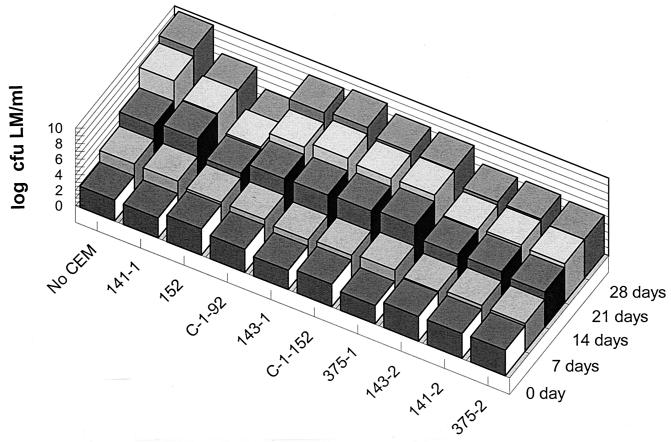
The nine isolates with activity inhibitory to L. monocytogenes at all three temperatures were assayed for their activity against L. monocytogenes at 4°C. Three isolates were highly inhibitory, with a 4-log10-L. monocytogenes CFU/ml differential in growth at 28 days compared to the positive control, and one isolate, 152, was exceptionally antimicrobial, with a 6-log10-CFU differential in growth at 28 days (Fig. 4).
FIG. 4.
Inhibition at 4°C of L. monocytogenes (LM) by competitive-exclusion microorganisms (not including yeast isolates) in TSB-YE.
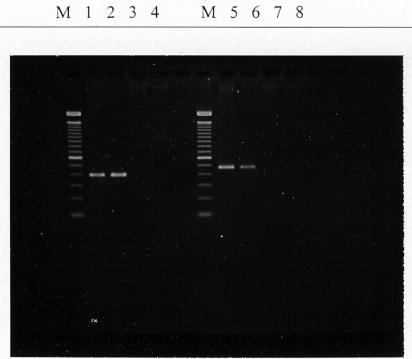
Identification of the nine most antagonistic cultures revealed that six (isolates 141-1, 141-2, 143-2, 152, 375-1, and 375-2) were Enterococcus durans, and 16S rRNA analysis indicated that all were indistinguishable (results not shown). Two (isolates C-1-92 and C-1-152) were identified as Lactococcus lactis subsp. lactis, and one (isolate 143-1) was Lactobacillus plantarum. Isolate C-1-92 possesses nisin A and B genes (Fig. 5).
FIG. 5.
PCR amplification of nisin A (lanes 1 to 4) and nisin B (lanes 5 to 8) genes of competitive-exclusion microorganisms and genes of nisin A and B (ATCC 11454, control). Lanes: M, 100-bp DNA ladder; 1 and 5, ATCC 11454; 2 and 6, C-1-92; 3 and 7, C-1-152; 4 and 8, 143.
The nine competitive-exclusion bacterial isolates and two yeast isolates were evaluated at two different population combinations (high, having 8.4 to 6.9 log10 competitive-exclusion microorganism CFU/cm2 and 4.6 log10 L. monocytogenes CFU/cm2; and low, having 6.4 to 6.5 log10 competitive-exclusion microorganism CFU/cm2 and 2.9 log10 L. monocytogenes CFU/cm2) for their ability to control L. monocytogenes in a biofilm on stainless steel at 37°C. Results of studies with the high combination of microbial populations revealed a >7-log10-L. monocytogenes CFU/cm2 (to an undetectable level by a direct plating method) differential compared to the positive control for eight isolates at 37°C for 24 h and a 5-log10-L. monocytogenes CFU/cm2 differential for one isolate (data not shown). There was only a 0.5- and 0.2-log10-L. monocytogenes CFU/cm2 differential for the two yeast isolates (data not shown). Studies with the low combination of microbial populations revealed that all nine competitive-exclusion bacterial isolates provided a >6-log10-L. monocytogenes CFU/cm2 (to an undetectable level by a direct plating method) differential compared to the L. monocytogenes-only positive control (Table 1).
TABLE 1.
Inhibition at 37°C of L. monocytogenes (LM) by competitive-exclusion microorganisms (CEM) in biofilms formed on stainless steel coupons
| CEM strain no. |
L. monocytogenes or CEM count (log10 CFU/cm2)a
|
|||||
|---|---|---|---|---|---|---|
| 0 h
|
24 h
|
|||||
| LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | |
| 141-1 | 2.9 ± 0.1 | 2.9 ± 0.1 | 6.5 ± 0.4 | 7.7 ± 0.2 | <1.7b | 7.9 ± 0.5 |
| 152 | 2.9 ± 0.1 | 2.9 ± 0.1 | 6.6 ± 0.6 | 7.7 ± 0.5 | <1.7 | 7.8 ± 0.7 |
| 143-1 | 2.9 ± 0.1 | 2.9 ± 0.1 | 6.4 ± 0.4 | 7.7 ± 0.5 | <1.7 | 7.3 ± 0.2 |
| C-1-152 | 2.9 ± 0.1 | 2.9 ± 0.1 | 6.4 ± 0.3 | 7.7 ± 0.3 | <1.7 | 7.8 ± 0.4 |
| 375-1 | 2.9 ± 0.1 | 2.9 ± 0.1 | 6.4 ± 0.5 | 7.7 ± 0.7 | <1.7 | 8.0 ± 0.5 |
| 143-2 | 2.9 ± 0.1 | 2.9 ± 0.1 | 6.5 ± 0.6 | 7.7 ± 0.4 | <1.7 | 8.0 ± 0.3 |
| 141-2 | 2.9 ± 0.1 | 2.9 ± 0.1 | 6.5 ± 0.2 | 7.7 ± 0.3 | <1.7 | 7.8 ± 0.6 |
| 375-2 | 2.9 ± 0.1 | 2.9 ± 0.1 | 6.6 ± 0.5 | 7.7 ± 0.5 | <1.7 | 7.9 ± 0.4 |
| C-1-92 | 2.9 ± 0.1 | 2.9 ± 0.1 | 6.4 ± 0.3 | 7.1 ± 0.4 | <1.7 | 7.2 ± 0.5 |
LM only, L. monocytogenes count in biofilm in which only L. monocytogenes bacteria were applied to coupons; LM + CEM, L. monocytogenes count in biofilm in which both L. monocytogenes and CEM were applied to coupons; CEM only, competitive-exclusion microorganism count in biofilm in which only CEM were applied to coupons.
Minimum detection limit by direct plating method (<1.7 log10 CFU/cm2).
Six competitive-exclusion bacterial isolates were evaluated under similar conditions (initial cell numbers of 3.7 log10 CFU of L. monocytogenes and 6.3 to 6.5 log10 CFU of competitive-exclusion microorganisms) at 15°C, of which two isolates (C-1-92 and C-1-152) reduced L. monocytogenes populations to an undetectable level (>7.8-log10-L. monocytogenes CFU/cm2 differential) through 28 days, the end of the study (Table 2). Six competitive-exclusion bacterial isolates were tested at 8°C with the same protocol, and four isolates provided substantial reduction (>7.1 log10 CFU of L. monocytogenes/cm2) on day 28 compared with the L. monocytogenes-only control (Table 3). Six competitive-exclusion bacterial isolates (at initial populations of 6.3 to 6.6 log10 CFU/cm2) were evaluated in combination with an initial population of 2.6 log10 L. monocytogenes CFU/cm2 on stainless steel coupons held at 4°C. One competitive-exclusion isolate (C-1-92) was exceptionally effective in killing L. monocytogenes, with no detectable L. monocytogenes (differential of >5.1 log10 L. monocytogenes CFU/cm2 compared to the L. monocytogenes-only positive control) at 35 days (Table 4). Interestingly, strain C-1-92 did not grow but rather declined in cell numbers (3.6- to 3.8-log10-CFU/cm2 reduction) during 35 days at 4°C, whereas cell populations of all five other competitive-exclusion microorganisms increased by 1 to 2 log10 CFU/cm2 under the same conditions. The other five competitive microorganisms also were inhibitory to L. monocytogenes through 35 days at 4°C, with differentials of L. monocytogenes cell populations in biofilms compared to L. monocytogenes-only positive controls ranging from 3.4 to 4.4 log10 CFU/cm2. Statistical analysis results of competitive-exclusion microorganisms tested in biofilms at four temperatures compared with control groups (no competitive-exclusion microorganisms) revealed that all treatments with the six competitive-exclusion microorganisms were significantly different (P < 0.05). A comparison of the inhibitory activities of two superior competitive-exclusion microorganisms (strains C-1-92 and 152) against L. monocytogenes in broth culture and in biofilms is shown in Table 5.
TABLE 2.
Inhibition at 15°C of L. monocytogenes (LM) by competitive-exclusion microorganisms (CEM) in biofilms formed on stainless steel coupons
| CEM strain no. |
L. monocytogenes or CEM (log10 CFU/cm2) at daya:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
7
|
14
|
28
|
|||||||||
| LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | |
| 141-1 | 3.7 ± 0.1 | 3.7 ± 0.1 | 6.5 ± 0.2 | 8.5 ± 0.4 | <1.7b | 8.1 ± 0.4 | 9.2 ± 0.4 | 5.3 ± 0.1 | 8.2 ± 0.6 | 9.5 ± 0.3 | 6.6 ± 0.3 | 8.8 ± 0.2 |
| 152 | 3.7 ± 0.1 | 3.7 ± 0.1 | 6.3 ± 0.3 | 8.5 ± 0.4 | 1.7 | 7.9 ± 0.3 | 9.2 ± 0.4 | 4.3 ± 0.2 | 8.7 ± 0.3 | 9.5 ± 0.3 | 6.5 ± 0.4 | 8.4 ± 0.4 |
| 375-1 | 3.7 ± 0.1 | 3.7 ± 0.1 | 6.4 ± 0.3 | 8.5 ± 0.4 | 2.7 ± 0.2 | 7.7 ± 0.6 | 9.2 ± 0.4 | 5.3 ± 0.3 | 8.7 ± 0.1 | 9.5 ± 0.3 | 6.6 ± 0.3 | 9.0 ± 0.2 |
| C-1-92 | 3.7 ± 0.1 | 3.7 ± 0.1 | 6.6 ± 0.4 | 8.5 ± 0.4 | <1.7 | 6.7 ± 0.2 | 9.2 ± 0.4 | <1.7 | 8.6 ± 0.3 | 9.5 ± 0.3 | <1.7 | 8.5 ± 0.7 |
| 143-1 | 3.7 ± 0.1 | 3.7 ± 0.1 | 6.5 ± 0.2 | 8.5 ± 0.4 | 4.7 ± 0.4 | 7.7 ± 0.4 | 9.2 ± 0.4 | 2.6 ± 0.1 | 7.8 ± 0.2 | 9.5 ± 0.3 | 6.4 ± 0.2 | 9.0 ± 0.3 |
| C-1-152 | 3.7 ± 0.1 | 3.7 ± 0.1 | 6.3 ± 0.3 | 8.5 ± 0.4 | <1.7 | 7.5 ± 0.2 | 9.2 ± 0.4 | <1.7 | 6.7 ± 0.7 | 9.5 ± 0.3 | <1.7 | 8.3 ± 0.6 |
LM only, L. monocytogenes count in biofilm in which only L. monocytogenes bacteria were applied to coupons; LM + CEM, L. monocytogenes count in biofilm in which both L. monocytogenes and CEM were applied to coupons; CEM only, competitive-exclusion microorganism count in biofilm in which only CEM were applied to coupons.
Minimum detection limit by direct plating method (<1.7 log10 CFU/cm2).
TABLE 3.
Inhibition at 8°C of L. monocytogenes (LM) by competitive-exclusion microorganisms (CEM) in biofilms formed on stainless steel coupons
| CEM strain no. |
L. monocytogenes or CEM count (log10 CFU/cm2) at daya:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
7
|
14
|
21
|
28
|
|||||||||||
| LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | |
| 141-1 | 3.7 ± 0.2 | 3.7 ± 0.2 | 6.0 ± 0.4 | 6.8 ± 0.3 | 3.7 ± 0.1 | 7.4 ± 0.4 | 8.5 ± 0.2 | 3.3 ± 0.1 | 8.4 ± 0.3 | 8.5 ± 0.2 | <1.7b | 8.0 ± 0.4 | 8.8 ± 0.3 | <1.7 | 7.7 ± 0.2 |
| 152 | 3.7 ± 0.2 | 3.7 ± 0.2 | 6.4 ± 0.6 | 6.8 ± 0.3 | 3.9 ± 0.2 | 7.5 ± 0.2 | 8.5 ± 0.2 | 3.5 ± 0.3 | 8.2 ± 0.1 | 8.5 ± 0.2 | <1.7 | 7.6 ± 0.2 | 8.8 ± 0.3 | <1.7 | 7.8 ± 0.3 |
| 375-1 | 3.7 ± 0.2 | 3.7 ± 0.2 | 6.5 ± 0.3 | 6.8 ± 0.3 | 3.7 ± 0.2 | 7.6 ± 0.3 | 8.5 ± 0.2 | 3.0 ± 0.2 | 8.5 ± 0.2 | 8.5 ± 0.2 | <1.7 | 7.8 ± 0.2 | 8.8 ± 0.3 | <1.7 | 7.8 ± 0.2 |
| C-1-92 | 3.7 ± 0.2 | 3.7 ± 0.2 | 6.7 ± 0.6 | 6.8 ± 0.3 | <1.7 | 4.7 ± 0.5 | 8.5 ± 0.2 | <1.7 | 6.5 ± 0.1 | 8.5 ± 0.2 | <1.7 | 5.4 ± 0.3 | 8.8 ± 0.3 | <1.7 | 4.7 ± 0.1 |
| 143-1 | 3.7 ± 0.2 | 3.7 ± 0.2 | 6.5 ± 0.2 | 6.8 ± 0.3 | 3.9 ± 0.1 | 7.2 ± 0.3 | 8.5 ± 0.2 | 4.4 ± 0.4 | 8.1 ± 0.3 | 8.5 ± 0.2 | 5.9 ± 0.3 | 7.5 ± 0.3 | 8.8 ± 0.3 | 3.3 ± 0.3 | 7.7 ± 0.6 |
| C-1-152 | 3.7 ± 0.2 | 3.7 ± 0.2 | 6.4 ± 0.5 | 6.8 ± 0.3 | 3.1 ± 0.3 | 7.4 ± 0.3 | 8.5 ± 0.2 | 4.5 ± 0.2 | 8.1 ± 0.2 | 8.5 ± 0.2 | 4.9 ± 0.3 | 7.8 ± 0.2 | 8.8 ± 0.3 | 6.0 ± 0.5 | 7.7 ± 0.3 |
LM only, L. monocytogenes count in biofilm in which only L. monocytogenes bacteria were applied to coupons; LM + CEM, L. monocytogenes count in biofilm in which both L. monocytogenes and CEM were applied to coupons; CEM only, competitive-exclusion microorganism count in biofilm in which only CEM were applied to coupons.
Minimum detection limit by direct plating method (<1.7 log10 CFU/cm2).
TABLE 4.
Inhibition at 4°C of L. monocytogenes (LM) by competitive-exclusion microorganisms (CEM) in biofilms formed on stainless steel coupons
| CEM strain no. |
L. monocytogenes or CEM count (log10 CFU/cm2) at daya:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
14
|
21
|
28
|
35
|
|||||||||||
| LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | LM only | LM + CEM | CEM only | |
| 141-1 | 2.6 ± 0.1 | 2.6 ± 0.1 | 6.4 ± 0.3 | 3.7 ± 0.1 | 2.2 ± 0.0 | 6.4 ± 0.6 | 4.5 ± 0.1 | 2.3 ± 0.1 | 7.0 ± 0.6 | 6.0 ± 0.5 | 2.3 ± 0.1 | 7.6 ± 0.4 | 6.8 ± 0.5 | 3.2 ± 0.1 | 7.9 ± 0.5 |
| 152 | 2.6 ± 0.1 | 2.6 ± 0.1 | 6.6 ± 0.4 | 3.7 ± 0.1 | <1.7b | 6.6 ± 0.2 | 4.5 ± 0.1 | <1.7 | 7.3 ± 0.4 | 6.0 ± 0.5 | 2.7 ± 0.0 | 7.8 ± 0.1 | 6.8 ± 0.2 | 2.5 ± 0.0 | 7.8 ± 0.2 |
| 375-1 | 2.6 ± 0.1 | 2.6 ± 0.1 | 6.5 ± 0.2 | 3.7 ± 0.1 | 2.2 ± 0.1 | 6.2 ± 0.2 | 4.5 ± 0.1 | 2.2 ± 0.0 | 7.0 ± 0.3 | 6.0 ± 0.5 | <1.7 | 7.3 ± 0.5 | 6.8 ± 0.4 | 2.4 ± 0.0 | 7.8 ± 0.7 |
| C-1-92 | 2.6 ± 0.1 | 2.6 ± 0.1 | 6.3 ± 0.2 | 3.7 ± 0.1 | <1.7 | 3.8 ± 0.3 | 4.5 ± 0.1 | <1.7 | 3.9 ± 0.2 | 6.0 ± 0.5 | <1.7 | 3.5 ± 0.2 | 6.8 ± 0.4 | <1.7 | 2.5 ± 0.2 |
| 143-1 | 2.6 ± 0.1 | 2.6 ± 0.1 | 6.5 ± 0.2 | 3.7 ± 0.1 | <1.7 | 5.9 ± 0.1 | 4.5 ± 0.1 | 2.2 ± 0.0 | 6.5 ± 0.1 | 6.0 ± 0.5 | <1.7 | 6.9 ± 0.7 | 6.8 ± 0.2 | 3.0 ± 0.2 | 7.5 ± 0.3 |
| C-1-152 | 2.6 ± 0.1 | 2.6 ± 0.1 | 6.5 ± 0.3 | 3.7 ± 0.1 | 2.0 ± 0.0 | 6.1 ± 0.2 | 4.5 ± 0.1 | <1.7 | 6.4 ± 0.4 | 6.0 ± 0.5 | 2.5 ± 0.2 | 7.2 ± 0.4 | 6.8 ± 0.5 | 3.4 ± 0.2 | 7.4 ± 0.6 |
LM only, L. monocytogenes count in biofilm in which only L. monocytogenes bacteria were applied to coupons; LM + CEM, L. monocytogenes count in biofilm in which both L. monocytogenes and CEM were applied to coupons; CEM only, competitive-exclusion microorganism count in biofilm in which only CEM were applied to coupons.
Minimum detection limit by direct plating method (<1.7 log10 CFU/cm2).
TABLE 5.
Comparison of inhibitory activities of two superior competitive-exclusion microorganisms (CEM) against L. monocytogenes in TSB and biofilms
| CEM strain |
L. monocytogenes count (log10 CFU/ml) in TSB at temp:
|
L. monocytogenes count (log10 CFU/cm2) in biofilm at temp:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 37°C
|
15°C
|
8°C
|
4°C
|
37°C
|
15°C
|
8°C
|
4°C
|
|||||||||
| Zero time | 24 h | Zero time | 14 days | Zero time | 21 days | Zero time | 28 days | Zero time | 24 h | Zero time | 28 days | Zero time | 28 days | Zero time | 35 days | |
| C-1-92 | 3.2 ± 0.3 | 0.7 ± 0.1 | 3.4 ± 0.2 | <0.7a | 3.4 ± 0.1 | 5.1 ± 0.2 | 3.0 ± 0.2 | 8.0 ± 0.4 | 2.9 ± 0.1 | <1.7b | 3.7 ± 0.1 | <1.7 | 3.7 ± 0.2 | <1.7 | 2.6 ± 0.1 | <1.7 |
| 152 | 3.4 ± 0.2 | 3.3 ± 0.2 | 3.3 ± 0.2 | 8.6 ± 0.3 | 3.2 ± 0.2 | 3.5 ± 0.3 | 3.4 ± 0.1 | 3.9 ± 0.1 | 2.9 ± 0.1 | <1.7 | 3.7 ± 0.1 | 6.5 ± 0.4 | 3.7 ± 0.2 | <1.7 | 2.6 ± 0.1 | 2.5 ± 0.0 |
| No CEM (control) | 3.3 ± 0.3 | 9.4 ± 0.5 | 3.4 ± 0.2 | 9.0 ± 0.5 | 3.3 ± 0.1 | 9.8 ± 0.7 | 3.4 ± 0.1 | 9.9 ± 0.3 | 2.9 ± 0.1 | 7.7 ± 0.2 | 3.7 ± 0.1 | 9.5 ± 0.3 | 3.7 ± 0.2 | 8.8 ± 0.3 | 2.6 ± 0.1 | 6.8 ± 0.5 |
Minimum detection limit by direct plating method (<0.7 log10 CFU/ml).
Minimum detection limit by direct plating method (<1.7 log10 CFU/cm2).
DISCUSSION
Controlling the widely distributed psychrotrophic L. monocytogenes in food processing facilities has been a formidable challenge for the entire food industry, from the smallest to the largest food processor (21). Besides the pathogen's widespread occurrence in nature, it is nonfastidious, grows at refrigeration temperature, forms protective biofilms, and thrives in moist environments (15, 18). Floor drains in food processing facilities are a particularly important niche for existence and can be a site critical to the control of contamination of the processing plant environment and food products (13, 21).
Decontaminating floor drains of listeriae is especially challenging because, when entrapped in a biofilm (5), L. monocytogenes is afforded unusual protection against disinfectants and treatments available to control pathogens on environmental surfaces (6, 7, 20, 21). Our goal was to isolate and characterize microorganisms that would thrive in combination with L. monocytogenes within its biofilm at a wide range of temperatures that occur in food processing facilities (especially refrigeration conditions) and would compete to control listerial growth and possibly eliminate the pathogen.
With the use of biofilms from floor drains of food processing facilities having a history of no L. monocytogenes contamination as the source of candidate competitive-exclusion microorganisms, 413 microbial isolates were obtained for evaluation. Initial screening identified 24 promising candidates with antilisterial activity. Further competitive testing between the candidate microorganisms and L. monocytogenes in broth and in biofilms at different temperatures identified nine bacterial isolates that effectively reduced, controlled, or eliminated detectable L. monocytogenes depending on environmental conditions. One strain in particular, L. lactis subsp. lactis C-1-92, was exceptionally effective in controlling L. monocytogenes when in biofilms for long periods of time, including at 4°C. This strain uniquely encodes nisin A and B, which are inhibitory to L. monocytogenes (16). Interestingly, this isolate did not grow at 4°C but apparently produced antilisterial metabolites at this temperature to maintain L. monocytogenes populations in biofilms below the detectable limit. Similarly, Amézquita and Brashears (1) observed that some lactic acid bacteria could competitively inhibit L. monocytogenes in ready-to-eat meats at refrigeration temperature even though the competitive bacteria did not grow.
Another isolate, E. durans 152, also was very effective in controlling L. monocytogenes in biofilms. Enterococcus spp. are sometimes used as starter cultures for meat fermentations when acid production is of primary importance (12). Metabolites, including enterocins, of Enterococcus spp. have been documented to be bactericidal to L. monocytogenes (2, 3, 9, 19).
Lactic acid bacteria and their products have been well documented for their antimicrobial activity against the growth of L. monocytogenes (1, 16, 17). However, their application in the food industry to control L. monocytogenes has been limited because of their cost, variable effect, fastidious growth requirements, and potential spoilage of foods. The lactic acid bacteria that we isolated from biofilms formed in floor drains have likely adapted to the environmental conditions present in floor drains, thereby enabling them to grow or compete at a wide range of temperatures, including refrigeration temperature, and also to form a biofilm to enable attachment to equipment and drain surfaces. Two lactic acid bacterial isolates, in particular, were identified as highly effective in controlling, and perhaps eliminating, L. monocytogenes in biofilms at different temperatures. Additional studies are needed to determine the efficacy of these strains in controlling listeriae when different cell numbers (including exceptionally high populations, e.g., 108 CFU/cm2) of L. monocytogenes are present in biofilms, when different nutrients and environmental conditions are present, and when different combinations of competitive-exclusion bacteria are used, especially in the food processing plant environment. However, our initial studies are very encouraging and indicate that at least two of the lactic acid bacteria that we have isolated and characterized are promising candidates for controlling L. monocytogenes in biofilms in food processing facilities.
Acknowledgments
We thank R. Bruce Tompkin for insightful comments and enlightening thoughts regarding control of L. monocytogenes in food processing facilities.
This study was supported by grants from the American Meat Institute Foundation and the State of Georgia's Traditional Industries Program for Food Processing.
REFERENCES
- 1.Amézquita, A., and M. M. Brashears. 2002. Competitive inhibition of Listeria monocytogenesListeria monocytogenes in ready-to-eat meat products by lactic acid bacteria. J. Food Prot. 65:316-325. [DOI] [PubMed]
- 2.Aymerich, T., H. Holo, L. S. Håvarstein, M. Hugas, M. Garriga, and I. F. Nes. 1996. Biochemical and genetic characterization of enterocin A from Enterococcus faeciumEnterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 62:1676-1682. [DOI] [PMC free article] [PubMed]
- 3.Balla, E., L. M. T. Dicks, M. Du Toit, M. J. Van Der Merwe, and W. H. Holzapfel. 2000. Characterization and cloning of the genes encoding enterocin 1071A and enterocin 1071B, two antimicrobial peptides produced by Enterococcus faecalisEnterococcus faecalis BFE 1071. Appl. Environ. Microbiol. 66:1298-1304. [DOI] [PMC free article] [PubMed]
- 4.Bula, C. J., J. Bille, and M. P. Glauser. 1995. An epidemic of food borne listeriosis in Western Switzerland: description of 57 cases involving adults. Clin. Infect. Dis. 20:66-72. [DOI] [PubMed]
- 5.Chae, M. S., and H. Schraft. 2001. Cell viability of Listeria monocytogenesListeria monocytogenes biofilms. Food Microbiol. 18:103-112. [DOI] [PubMed]
- 6.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed]
- 7.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed]
- 8.Goulet, V., A. Lapoutre, P. Rocourt, A. Courtieu, P. Dehaumont, and P. Veit. 1993. Epidemie de Listeriose en France-bilin final et resultat de l'enquète epidemiologique. Bull. Epidemiol. Hebdom. 4:13-14.
- 9.Joosten, H. M. L. J., M. Nuñez, B. Devreese, J. V. Beeumen, and J. D. Narugg. 1996. Purification and characterization of enterocin 4, a bacteriocin produced by Enterococcus faecalisEnterococcus faecalis INIA 4. Appl. Environ. Microbiol. 62:4220-4223. [DOI] [PMC free article] [PubMed]
- 10.Leriche, V., and B. Carpentier. 1995. Viable but non-culturable Salmonella typhimuriumSalmonella typhimurium in single- and binary-species biofilms in response to chlorine treatment. J. Food Prot. 58:1186-1191. [DOI] [PubMed]
- 11.Linnan, M. J., L. Mascola, X. D. Lou, V. Goulet, S. May, G. Salminen, D. W. Hird, M. L. Yonekura, P. Hayes, R. Weaver, A. Audurier, B. D. Plikaytis, S. L. Fannin, A. Kleks, and C. V. Broome. 1988. Epidemic listeriosis associated with Mexican-style cheese. N. Engl. J. Med. 319:823-828. [DOI] [PubMed]
- 12.London, J. 1990. Uncommon pathways of metabolism among lactic acid bacteria. FEMS Microbiol. Lett. 87:103-112. [DOI] [PubMed]
- 13.McBain, A. J., R. G. Bartolo, C. E. Catrenich, D. Charbonneau, R. G. Ledder, A. H. Richard, S. A. Wymmons, and P. Gilbert. 2003. Microbial characterization of biofilms in domestic drains and the establishment of stable biofilm microcosms. Appl. Environ. Microbiol. 69:177-185. [DOI] [PMC free article] [PubMed]
- 14.McLauchlin, J., S. M. Hall, S. K. Velani, and R. J. Gilbert. 1991. Human listerosis and pate: a possible association. Br. Med. J. 303:773-775. [DOI] [PMC free article] [PubMed]
- 15.Miettinen, M. K., L. Palmu, K. J. Bjőrkroth, and H. Korkeala. 2001. Prevalence of Listeria monocytogenesListeria monocytogenes in broilers at the abattoir, processing plant, and retail level. J. Food Prot. 64:994-999. [DOI] [PubMed]
- 16.Olasupo, N. A., U. Schillinger, A. Narbad, H. Dodd, and W. H. Holzapfel. 1999. Occurrence of nisin Z production in Lactococcus lactisLactococcus lactis BFE 1500 isolated from warawara, a traditional Nigerian cheese product. Int. J. Food Microbiol. 53:141-152. [DOI] [PubMed]
- 17.Pleasants, A. B., T. K. Soboleva, G. A. Dykes, R. J. Jones, and A. E. Filippov. 2001. Modelling of the growth of populations of Listeria monocytogenesListeria monocytogenes and a bacteriocin-producing strain of LactobacillusLactobacillus in pure and mixed cultures. Food Microbiol. 18:605-615.
- 18.Schlech, W. F., III, P. M. Lavigne, R. A. Bortolussi, A. C. Allen, E. V. Haldane, A. J. Wort, A. W. Hightower, S. E. Johnson, S. H. King, E. S. Nicholls, and C. V. Broome. 1983. Epidemic listeriosis—evidence for transmission by food. N. Engl. J. Med. 308:203-206. [DOI] [PubMed]
- 19.Siragusa, G. 1992. Production of bacteriocin inhibitory to ListeriaListeria species by Enterococcus hiraeEnterococcus hirae. Appl. Environ. Microbiol. 58:3508-3513. [DOI] [PMC free article] [PubMed]
- 20.Spoering, A. L., and K. Lewis. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosaPseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183:6746-6751. [DOI] [PMC free article] [PubMed]
- 21.Tompkin, R. B. 2002. Control of Listeria monocytogenesListeria monocytogenes in the food-processing environment. J. Food Prot. 65:709-725. [DOI] [PubMed]
- 22.Welshimer, H. J. 1960. Survival of Listeria monocytogenesListeria monocytogenes in soil. J. Bacteriol. 80:316-320. [DOI] [PMC free article] [PubMed]