Abstract
Background
We conducted a meta-analysis to evaluate the association between traffic-related air pollution and lung cancer in order to provide evidence for control of traffic-related air pollution.
Methods
Several databases were searched for relevant studies up to December 2013. The quality of articles obtained was evaluated by the Strengthening the Reporting of Observational Studies in Epidemiology checklist. Statistical analysis, including pooling effective sizes and confidential intervals, was performed.
Results
A total of 1106 records were obtained through the database and 36 studies were included in our analysis. Among the studies included, 14 evaluated the association between ambient exposure to traffic-related air pollution and lung cancer and 22 studies involved occupational exposure to air pollution among professional drivers. Twenty-two studies were marked A level regarding quality, 13 were B level, and one was C level. Exposure to nitrogen dioxide (meta-odds ratio [OR]: 1.06, 95% confidence interval [CI]: 0.99–1.13), nitrogen oxide (meta-OR: 1.04, 95% CI: 1.01–1.07), sulfur dioxide (meta-OR: 1.03, 95% CI: 1.02–1.05), and fine particulate matter (meta-OR: 1.11, 95% CI: 1.00–1.22) were positively associated with a risk of lung cancer. Occupational exposure to air pollution among professional drivers significantly increased the incidence (meta-OR: 1.27, 95% CI: 1.19–1.36) and mortality of lung cancer (meta-OR: 1.14, 95% CI: 1.04–1.26).
Conclusion
Exposure to traffic-related air pollution significantly increased the risk of lung cancer.
Keywords: Lung cancer, meta-analysis, traffic-related air pollution
Introduction
It is estimated that there were 1.825 million lung cancer cases globally in 2012, accounting for 13.0% of all cancer cases, and 1.59 million deaths from lung cancer, responsible for 19.4% of deaths from all cancers.1 Air pollution is currently the principal issue in the field of environmental health, among which outdoor air pollution causes 1.3 million deaths in urban areas worldwide and indoor air pollution is responsible for two million premature deaths in developing countries.2 Vehicle emissions are a major source of outdoor air pollution, producing gaseous and particulate pollutants including carbon monoxide, ozone, particulate matter, nitrogen dioxide aldehydes, benzene, 1,3 – butadiene, polycyclic aromatic hydrocarbons, and metals.3 Pollution from vehicles causes a broad range of acute and chronic diseases, including lung cancer. It was estimated that 11 395 deaths and 232 646 disability adjusted life years (DALYs) were attributed to motorized road transport globally in 2010.4 In Western countries, the histological distribution of lung cancer has changed during the past decades, showing an increase in adenocarcinomas and a decrease in squamous-cell carcinomas; this transition is associated with tobacco blends5 and ambient air pollution.6,7 People inhale 10 000 liters of air per day and even though the concentration of harmful substances in the air seems trivial, the amount breathed in per day cannot be ignored. Too few data are available to draw meaningful inferences of non-occupational exposure to traffic-related air pollution and lung cancer. Most studies respecting traffic-related air pollution in occupational settings also have failed to adequately account for confounding in their analyses, despite the availability in many cases of a large amount of data on potential confounders and effect modifiers.8 Additionally, varied methods and measurements are employed in studies. Therefore, the objective of this meta-analysis is to clarify the potential association between pollutants of traffic-related air pollution with lung cancer, and also the risk of lung cancer among professional drivers occupationally exposed to vehicle emissions.
Materials and methods
Data sources and searches
We searched PubMed, Embase, and the Cochrane library for studies published in English, as well as the China National Knowledge Infrastructure, Wanfang, and SINOMED databases for studies published in Chinese, up to December 2013, evaluating the association between traffic-related air pollution and lung cancer incidence and mortality. Literature research was performed using keywords including: “traffic related;” “motor vehicles;” “lung cancer;” “air pollution;” “carbon monoxide;” “oxides;” “particulate matter;” “ozone;” “sulfur dioxide;” “relative risks;” “incidence;” “mortality;” and corresponding keywords in Chinese. Specific search strategies are presented in detail in Appendix S1. We also screened the reference lists and included additional relevant studies.
Study selection
Inclusion criteria
Observational epidemiological studies (case-control, cohort, nested case-control studies) were included in our analysis. Effect sizes with corresponding 95% confidence intervals (CIs) indicating association between traffic-related air pollution and lung cancer (odds ratio [OR], hazard ratio [HR], relative risk [RR], standardized mortality ratio [SMR], standardized incidence ratio [SIR]) are reported, as well as methods used to adjust confounders. Except for studies on occupational exposure to air pollution, the method and period of measurement of each pollutant was required. Traffic-related air pollutants included carbon monoxide (CO), nitrogen monoxide (NO), nitrogen dioxide (NO2), nitrogen oxides (NOX), sulfur dioxide (SO2), ozone (O3), particulate matter with an aerodynamic diameter of less than 10 μm (PM10), and particulate matter with an aerodynamic diameter of less than 2.5 μm (PM2.5). In terms of studies on occupational exposure to air pollution, the specific occupation and location of exposure was required. The criteria for selection of lung cancer cases was also required, and the number of lung cancer cases had to be larger than 30.
Exclusion criteria
Studies with poor quality (ranked C) and/or insufficient data, and duplicate publications were excluded from our analysis.
We included only one article for each study considering the time published, calculation methods, and participants. With respect to studies of ambient exposure reporting effective amounts of air pollution with both lung cancer incidence and mortality, we only included effective numbers of lung cancer incidence once pooled. If a study reported effective numbers of different categories of professional drivers with lung cancer, we included all of these.
Data extraction and analysis
Two of the authors extracted data independently from each article based on study design, age, sampling of participants, measurement of pollutants, source of lung cancer cases, effect sizes, and corresponding confidential intervals, with covariates adjusted. Discrepancies were resolved through discussion and consultation with a third author where necessary. We performed meta-analysis to obtain the weighted average of effect measures using RevMan V.5.2 (The Cochrane Collaboration, Oxford, UK). A Cochran Q statistic test was employed to evaluate heterogeneity between study results. Statistic significance was defined as <0.10. The percentage of variation as a result of heterogeneity was tested with I2 statistics. Effect sizes weighted by inverse variances were pooled with a fixed effect model when there was less than 50% variation because of heterogeneity and P > 0.10, otherwise a random effect model was employed.
Quality assessment of studies
The quality of reporting was evaluated using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklist for cohort, case-control, and cross-sectional studies, version 4.9 Two authors evaluated each article independently and counted the number of STROBE criteria fulfilled. Considering that STROBE criteria are normally used to evaluate the quality of observational epidemiological studies, with respect to studies of pooled analysis and re-analysis when extracting data from other studies, STROBE criteria were adjusted. Specifically, item No.10, item No.14, and item No. 12c-No. 13c were not used while evaluating the quality of studies related to arrival of study size, dealing with missing data, and characteristics of participants, which were reported in previous articles. The studies were classified as having: A, more than 80% of STROBE criteria fulfilled; B, 60–80% of STROBE criteria fulfilled; or C, less than 60% of STROBE criteria fulfilled.10
Results
A total of 1106 articles were identified, including 370 from Pubmed, 694 from Embase, and 45 from Chinese databases, with no Cochrane library articles (Fig 1). After reading full texts, 39 studies were left; however, the effect sizes of two articles were not measured by every 10 unit increments,11,12 and one article ranked “C” in terms of the quality of the study.13 Therefore, 36 studies were finally included in our pooled analysis, among which 14 evaluated the association of ambient exposure to traffic-related air pollution,14–27 and 22 reported professional drivers’ risk of lung cancer.28–49 Two articles included data from the European Prospective Investigation into Cancer and Nutrition;15,21 to avoid duplication we included data of SO2 exposure from one21 and NO2 exposure from the other. 15
Figure 1.
Flow diagram of study selection.
Study characteristics
With respect to studies on ambient exposure to traffic-related air pollution, seven were conducted in Europe: four cohort studies,18,20,24,25 two case-control studies,21,27 and a pooled analysis.15 Five studies were conducted in North America: four cohort studies,16,22,23,26 and one case-control study.17 Two cohort studies were conducted in Asia.14,19 Table 1 provides details of these studies.
Table 1.
Characteristics and evaluation of quality of 14 studies on ambient exposure to traffic-related air pollution
| Study ID (Reporting quality) | Location and study design | Age (years) | Total participants | Lung cancer cases | Exposure† (μg/m3) | Exposure assessment | Outcome | Outcome assessment | Covariates adjusted for |
|---|---|---|---|---|---|---|---|---|---|
| Yorifuji et al. 201314 (A) | Shizuoka, Japan, Cohort | 65–84 | 14001 | 116 | NO2: 35.11 | LUR modeling | Lung cancer and hemorrhagic stroke | Obtained from the database of the Ministry of Health,Labor and Welfare of Japan | Age, sex, smoking, BMI, hypertension, diabetes, financial capability and area mean taxable income |
| Raaschou-Nielsen et al. 201315 (A)†† | European, 17 cohorts | 42.8–73.1 (mean age) | 2380–108018 | 18–678 | PM10: 13.5–48.1, PMcoarse:4.0– 20.8PM2.5: 6.6–31.0, PM2·5absorbance: 0.5-3.2 NO2: 5.2–59.8 NOX: 8.7–107.3 |
LUR model | Lung cancer | Histology | Age, sex, calendar time, smoking related variables, occupation, fruit intake, marital status, educational level, employment status and area-level socioeconomic status |
| Jerrett et al. 201316 (B) | California, U.S. Cohort | ≥30 | 73711 | 1481 | PM2.5: 14.09 NO2: 12.27‡ O3: 50.35‡ |
Monthly average monitoring data and LUR models | All cause of death including lung cancer | Ascertained by volunteers and using the National Death Index | Lifestyle, dietary, demographic, occupational and educational factors |
| Hystad et al. 201317 (A) | 8 provinces, Canada, Case-control | 63.5 and 59.0(mean age for cases and controls) | 5897 | 2390 | PM2.5: 11.9 NO2: 15.4‡ O3: 20.3 |
Fixed site monitoring data and proximity measures | Lung cancer | Histology | Age, sex, educational attainment, smoking related variables, alcohol and meat consumption, occupational exposure and geographic covariates. |
| Cesaroni et al. 201318 (B) | Roma, Italy, Cohort | ≥30 | 1265058 | 12208 | NO2: 44 PM2.5: 23 |
LUR modeling and PM2.5 dispersion model | All cause of death including lung cancer | Obtained from Lazio regional health information system | Sex, marital status, place of birth, education, occupation, and area-based socioeconomic position |
| Cao et al. 201019 (B) | 17 provinces, China, Cohort | 55.8 (mean age) | 70947 | 624 | TSP: 289 SO2: 73 NOX: 50 |
Fixed-site monitoring data | All cause of death including lung cancer | Hospital records and death certificates | Age, sex, BMI, physical activity, education, smoking status, age at starting to smoke, cigarettes per day, alcohol intake, and hypertension |
| Beelen et al. 200820 (A) | Netherlands, Case-Cohort | 55–69 | 120852 | 2183 | BS: 11.6, NO2: 36.9 SO2: 13.7 PM2.5: 28.2 |
Regulatory monitoring data and LUR models | Lung cancer | Histopathology and cytopathology | Full cohort: age, sex, smoking status and area level indicators of socioeconomics |
| Vineis et al. 200621 (A) | 9 countries, Europe, Nested case-control | 60.4 and 60.0 (mean ages for cases and controls) | 1008 | 271 | NO2: 12.0–64.7§ PM10: 19.9–73.4§ SO2: 1.1–30.6§ |
Home addresses and data from monitoring stations | Lung cancer | Histological conformation | Age, sex, country, smoking status, time since recruitment, education, BMI, physical activity, cotinine, occupational index and intake of fruit, vegetables, meat, and alcohol ) |
| Laden et al. 200622 (B) | 6 cities, U.S. Cohort | 25–74 | 8096 | 226 | PM2.5: nearly 10–40§ | Fixed air-monitoring station | All cause of death including lung cancer | Data obtained from National Death Index | Current or former smoking, number of pack-years of smoking for former and current smokers separately, education, and body mass index |
| Jerrett et al. 200523 (A)†† | Los Angeles, U.S. Cohort | NA | 22905 | 434 | PM2.5: 9.0–27.1§ | Data from state and local district monitoring stations | All cause of death including lung cancer | NA | Age, sex, age, O3(average of 4 highest 8 h maxima) and 44 other covariates including lifestyle, dietary, demographic, occupational and educational factors |
| Filleul et al. 200524 (A) | 7 towns, France, Cohort | 25–29 | 14284 | 178 | SO2: 17–85 TSP: NA BS: 18–152 NO2: 12–61 NO: NA |
Data from centrally located pollution monitoring station | All cause of death including lung cancer | Data from specialized department of the National Institute of Health and Medical Research (INSERM) | Age, smoking habits, body mass index, educational level, occupational exposure, and stratified by sex |
| Nafstad et al. 200325 (A) | Oslo, Norway, Cohort | 40–49 | 16209 | 422 | NOX: 10.7¶ SO2: 9.4¶ |
Model calculations using data for observed concentrations and emission from point sources | Lung cancer | Obtained from Norwegian cancer register | Age, smoking habits, physical activity, occupation, height and weight |
| Pope et al. 200226 (A) | 50 states. U.S. Cohort | ≥30 | Approximately 500000 | NA | PM2.5: 17.7 | Inhalable particle monitoring network and National Aerometric Database | All cause of death including lung cancer | Death certificates | Age, sex, race, smoking, education, marital status, body mass, alcohol consumption, occupational exposure and the diet |
| Nyberg et al. 200027 (A) | Stockholm, Sweden, Case-control | 40–75 | 3406 | 1042 | NO2: 19.85¶ SO2: 52.75¶ |
Source-specific emission data and dispersion modeling | Lung cancer | Histology and cytology | Age, selection year, smoking, radon, socioeconomic grouping, occupational exposure to diesel exhaust, other combustion products and asbestos, and employment in risk occupation. |
†Mean concentration of exposure. ‡Exposure concentration is measured by ppb. §Range of exposure concentration. ¶Median concentration of exposure. ††Studies evaluated with modified STROBE items. BS, black smoke; LUR, land-use regression; TSP, total suspended particles.
Respecting studies on professional drivers, 11 were conducted in Europe: five cohort studies,28,29,34,41,42 five case-control studies,30,33,35,39,43 and a pooled analysis.37 Ten studies were conducted in America: four cohort studies,30,32,47,49 five case-control studies,38,40,44–46 and one pooled analysis.48 One case-control study was conducted in Asia.36 Table 2 provides details of these studies.
Table 2.
Characteristics and evaluation of quality of 22 studies on occupational exposure to traffic-related air pollution
| Study | Location and study design | Age (years) | Total participants | Lung cancer cases of drivers | Type of drivers | Duration of employment | Covariance | outcome assessment |
|---|---|---|---|---|---|---|---|---|
| Petersen et al. 201028 (A) | 3 cities, Denmark cohort | 22–67 | 2037 | 100 | Bus drivers | 0–44 years | Age, calendar time, city of employment, bus route and smoking habits | Data obtained from the Danish Cancer Registry |
| Merlo et al. 201029 (A) | Genoa, Italy cohort | NA | 9267 | 235 | Bus drivers | >6 months | length of employment, time since first employment and job title | death certificates |
| Consonni et al. 201030 (A) | Lombardy, Italy case-control | 35–79 | 4220 | 149 | Bus and truck drivers | >6 months | Residence, age, smoking, number of jobs held, and education | Pathology, cytology and clinical records |
| Birdsey et al. 201031 (B) | U.S. cohort | 25–74 | 156241 | 557 | truck drivers | 6 years | age, racial group, sex and calendar period | Obtained from Social security Administration and the National Death Index |
| Garshick et al. 200832 (B) | U.S. cohort | >40 | 31135 | 323 | Long-haul drivers | nearly 15 years | age, calendar, decade of hire, race, region, company and smoking | Obtained from National Death Index |
| Richiardi et al. 200633 (A) | Turin, Italy case-control | <76 | 1440 | 70 | Professional drivers and transport conductors | >20 years | Age, cigarette consumption, exposure to occupations, education | Radiology, histology and cytology |
| Jarvholm and Silverman 200334 (B) | Sweden cohort | 33–40 (mean) | 140712 | 61 incident cases and 57 deaths | Truck drivers | not clear | Age, time period and smoking | Obtained from National Cancer registry and National death Registry |
| Soll-Johanning et al. 200335 (A) | Copenhagen, Denmark nested case-control | 20–68 | 843 | 153 | Bus drivers or tramway employees | 13 years | Smoking | Obtained from Danish Cancer Registry |
| Elci et al. 200336 (B) | Turkey case-control | NA | 2873 | 88 | Unspecified | NA | age and smoking | Histology |
| Bruske-Hohlfeld et al. 199937 (A)† | Germany pooled case-control | 60.5 for cases and 60.4 for controls | 7039 | 3498 | Professional drivers | nearly 16.0 for cases and 14.2 for controls | Smoking and asbestos exposure | Histology and cytology |
| Pezzotto and Poletto 199938 (A) | Rosario, Argentina case-control | 60.1 and 60.1 for cases and controls | 943 | 367 | Unspecified | >33 years | age, smoking habit and lifelong cigarette consumption | histology and pathology |
| Hansen et al. 199839 (A) |
Denmark case-control | 18–66 | 28744 | 2251 | Lorry, bus, taxi and unspecified drivers | NA | Obtained from Danish Cancer Registry | |
| Muscat et al. 199840 (B) | U.S. case-control | 58.9 for male cases and 58.6 for female cases | 936 | 550 | Unspecified | NA | Age, education, cumulative smoking | Histology |
| Jakobsson et al. 199741 (B) | 4 counties, Sweden cohort | 20–64 | 96438 | 604 | Taxi drivers, long distance lorry drivers and short distance lorry drivers | >13 years | smoking | Obtained from National Swedish Cancer registry |
| Borgia et al. 199442 (B) | Rome, Italy cohort | 40 (median) | 2311 | 76 | Taxi drivers | >13 years | NA | Obtained from Registry Office |
| Alfredsson et al. 199343 (B) | 4 counties, Sweden cohort | 20–64 | 9446 | 334 | Bus drivers | >15 years | age, county | Obtained from National Cause of Death Registry |
| Burns and Swanson 199144 (B) | Detroit, U.S. case-referent | >40 | 9891 | 238 | Unspecified | NA | diagnosis, race and smoking | Obtained from MDCSS system |
| Steenland et al. 199045 (A) | U.S. case-control | NA | 2081 | 730 | Long haul drivers and short haul drivers | 23.4 for long haul drivers and 24.2 for others | age, smoking and asbestos | Death certificates |
| Boffetta et al. 199046 (A) | 6 cities, U.S. case-control | nearly 60 | 7683 | 114 | Truck drivers | NA | smoking, education, race, age, year of interview | Histology |
| Paradis et al. 198947 (A) | Montreal, Canada cohort | NA | 2134 | 78 | Bus drivers | >5 years | age, sex, cause of death | obtained from death registries |
| Hayes et al. 198948 (B)f | 3 states, U.S. pooled case-control | NA | 4861 | 320 | Truck, bus, and taxi drivers, and chauffeur | >10 years | birth cohort, daily cigarette use and state | not clear |
| Boffetta et al. 198849 (A) | U.S. cohort | 40–79 | 461981 | 48 | Truck drivers | >6 years | age and smoking | Obtained from State Health Departments |
Studies evaluated with modified STROBE items.
Exposure to nitrogen dioxide and lung cancer
The association between ambient exposure to nitrogen dioxide and lung cancer was estimated in five studies.14,15,18,24,27 Considering significant heterogeneity (P = 0.05, I2 = 59%), pooled effect size with a random effect model showed that ambient exposure to nitrogen dioxide increased the risk of lung cancer (meta-OR: 1.06, 95% CI: 0.99–1.13). (Fig 2)
Figure 2.
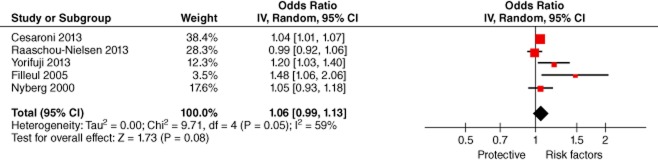
Lung Cancer and NO2 (odds ratio per 10 μg/m3). CI, confidence interval.
Exposure to nitrogen oxides and lung cancer
The relationship between ambient exposure to nitrogen oxides (mainly NO and NO2) was examined in two studies;19,25 a fixed effect model was employed and the result showed an increased risk of lung cancer exposure to nitrogen oxides (meta-OR: 1.04, 95% CI: 1.01–1.07). (Fig 3)
Figure 3.

Lung Cancer and NOX (odds ratio per 10 μg/m3). CI, confidence interval.
Exposure to sulfur dioxide and lung cancer
The association of ambient exposure to sulfur dioxide and lung cancer was estimated in five studies.19,21,24,25,27 Considering no heterogeneity (P = 0.48, I2 = 0%), the effect size was pooled with a fixed effect model, which showed an increased risk of lung cancer exposure to sulfur dioxide (meta-OR: 1.03, 95% CI: 1.02–1.05). (Fig 4)
Figure 4.
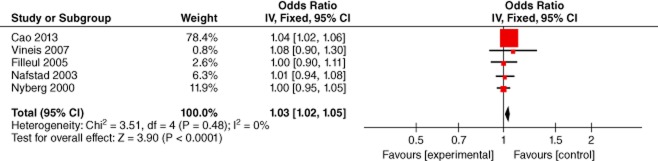
Lung Cancer and SO2 (odds ratio per 10 μg/m3). CI, confidence interval.
Exposure to fine particulate matter and lung cancer
The relationship between ambient exposure to fine particulate matter and lung cancer was examined in six studies.17,18,20,22,23,26 As a result of heterogeneity (P = 0.02, I2 = 64%), the pooled effect with a random effect model revealed an increased risk of lung cancer exposure to fine particulate matter (P = 0.02, I2 = 64%). (Fig 5)
Figure 5.
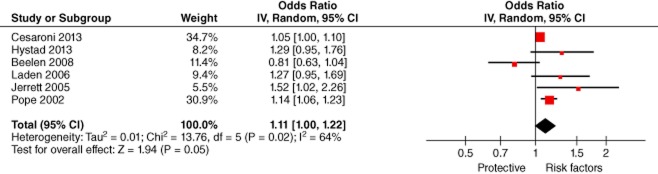
Lung Cancer and PM2.5 (odds ratio per 10 μg/m3). CI, confidence interval.
Exposure to other pollutants and lung cancer
Some studies reported the association between exposure to coarse particulate matter and ozone with lung cancer,11,12,15–17 but effect sizes calculated with varied measurements could not be pooled in our meta-analysis; therefore, we collected all pollutant-specific effect sizes calculated with different measurements. These are listed in Table 3.
Table 3.
Association between air pollution and lung cancer with varied measurements of effect sizes
| Increase | Number of studies | OR 95%CI |
|---|---|---|
| NO2 | ||
| 4.1167 ppb | 1 | 1.11 (1.02, 1.21) |
| 8 ppb | 1 | 1.06 (0.97, 1.15) |
| 10 ppb | 1 | 1.11 (1.00, 1.24) |
| 10 μg/m3 | 6 | 1.04 (1.01, 1.07) |
| 16 μg/m3 | 1 | 1.46 (0.92, 2.32) |
| 30 μg/m3 | 2 | 0.88 (0.75, 1.04) |
| NOX | ||
| 10 μg/m3 | 2 | 1.04 (1.01, 1.07) |
| 20 μg/m3 | 1 | 1.01 (0.95, 1.07) |
| SO2 | ||
| 4 ppb | 1 | 1.09 (0.98, 1.21) |
| 10 μg/m3 | 5 | 1.03 (1.02, 1.05) |
| 20 μg/m3 | 2 | 0.88 (0.75, 1.04) |
| PM 2.5 | ||
| 5.3037 ppb | 1 | 1.06 (0.95, 1.18) |
| 10 μg/m3 | 6 | 1.08 (1.04, 1.12) |
| O3 | ||
| 10 ppb | 1 | 1.09 (0.85, 1.39) |
| 24.1782ppb | 1 | 0.86 (0.75, 0.99) |
| PM10 | ||
| 6 μg/m3 | 1 | 1.00 (0.92,1.08) |
| 7 μg/m3 | 1 | 1.84 (1.23,2.74) |
| 10 μg/m3 | 1 | 1.22 (1.03, 1.45) |
Risk of lung cancer among professional drivers
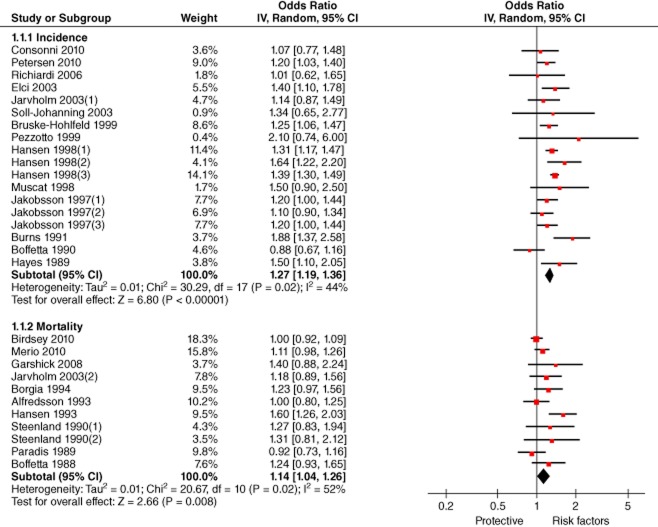
The risk of lung cancer incidence among professional drivers was examined by 14 studies.28,30,33–41,44,46,48 Considering heterogeneity (P = 0.02, I2 = 44%), the pooled effect size with a random effect model showed an increased risk (meta-OR: 1.27, 95% CI: 1.19–1.36). (Fig 6)
Figure 6.
Forest plot of the association of occupational exposure of drivers and lung cancer incidence and mortality. CI, confidence interval.Note:1. Jakobsson et al.41 evaluated the risk of lung cancer among three kinds of professional drivers, taxi drivers, long distance lorry drivers and short distance lorry drivers, and we included three effect sizes, which were noted as Jakobsson et al.41 (1), Jakobsson et al.41 (2) and Jakobsson et al.41 (3). Similar treatment was applied to Hansen et al.39 and Steenland et al.452: As all effect sizes were represented with two decimal places, because of the difference in calculation precision, several odds ratios were slightly different from those reported originally, such as Pezzotto and Poletto.38
The risk of lung cancer mortality was evaluated by 10 studies.13,29,31,32,34,42,43,45,47,49 The pooled effect size with a random model revealed an increased risk (meta-OR: 1.14, 95% CI: 1.04–1.26). (Fig 6)
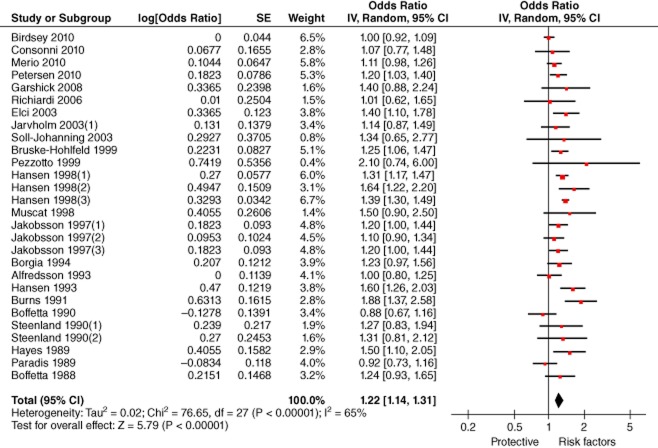
Our results illustrated that no significant difference existed between risks of professional drivers developing and dying of lung cancer (confidence intervals overlap). We pooled the effect sizes respecting incidence and mortality, which showed a significantly higher risk (meta-OR: 1.22, 95% CI: 1.14–1.31). (Fig 7) Studies on occupations other than professional drivers were also identified in our literature search, such as truck industry workers,50 railway workers,51 and filling station attendants.52 However this data was not included in our meta-analysis, because there were limited articles after duplicate exclusion or the effect size could not be extracted, particularly for professional drivers.
Figure 7.
Forest plot of the risk of lung cancer among professional drivers (pooled effect sizes of incidence and mortality).Note: Jarvholm and Silverman34 reported effect sizes of both lung cancer incidence and mortality, but we only included incidence data in this figure, noted as Jarvholm and Silverman34 (1). CI, confidence interval.
Discussion
Outdoor air pollution is derived from resources other than vehicle emissions, including industry, energy, and household heating. However, vehicle emissions account for 25–40% of air pollution.3 The International Agency for Research on Cancer recently reviewed toxicological and epidemiologic evidence and classified diesel engine exhaust as carcinogenic to humans (Group 1).53
The results of our meta-analysis indicate that ambient exposure to nitrogen oxides, sulfur dioxide, and fine particulate matters significantly increase the risk of lung cancer. Most ambient nitrogen dioxide is derived from oxidation of nitrogen monoxide, which is mainly produced by vehicle emissions. Nitrogen dioxide involves a series of photochemical reactions induced by sunlight. During the process, nitrate, sulphate, and organic aerosol are produced which further promote the formation of particulate matter and harmful secondary pollutants.54 Animal studies indicate that the inhalation of sulfur dioxide causes multi-organ DNA lesions, including in the lung, which can develop into mutation, cancers, and relevant diseases.55 The surfaces of fine particles can absorb various chemicals. Compared with coarse particles, fine particles are more likely to pervade indoors and be inhaled deeply in the lung; therefore ambient exposure to fine particles is more prevalent.56 According to the latest cancer registry data, in China the incidence and mortality rates of lung cancer both ranked first among cancers.57 In 2010, air pollution was the fourth leading risk factor for the disease in China.58 Thus, the association between air pollution and lung cancer should be viewed as a major public health threat. Despite this data, of the studies we obtained through our literature search, only one cohort study was conducted in China.15 However, Zhang et al. examined the correlation of ambient SO42− level and lung cancer in Beijing, and according to Zhou et al., a higher exposure to particulate air pollution increased the risk of cardiopulmonary mortality among Chinese men.59,60 Considering various components, distributions of air pollution geologically, and different effects of air pollution on people in varied age groups,61 the results of studies conducted in Western populations cannot be directly extrapolated to China. Surveillance data indicates that the exposure level of air pollution in China is much higher than in Western countries. For instance, during the first half of 2013, the average concentration of PM2.5 and PM10 in 74 Chinese cities were 76 μg/m3 and 123 μg/m3 respectively,62 but PM2.5 and PM10 in nine European regions reported by Raaschou-Nielsen15 ranged from 6.6–31.0 μg/m3 and 13.5–48.1 μg/m3, respectively. In light of our results that the risk of lung cancer increases with a higher exposure level, the association between air pollution and lung cancer may be much stronger in heavily polluted areas.63 In order to provide basic data for scientific research and policymaking aimed to prevent air pollution, more environmental monitoring stations need to be established in China, especially in rural areas.19 More studies need to be conducted to illustrate the distribution of varied pollutants and their relationships with diseases. China will soon implement the fifth set of light vehicle emission limits and measurement methods; however, these do not provide limits for sulfur dioxide emissions.64 Considering the significant association between exposure to sulfur dioxide and lung cancer, the government and relevant associations should limit vehicle emissions of sulfur dioxide and strengthen the management of vehicle emissions.
Through our literature review, the evaluations of the risk of lung cancer among professional drivers are relatively consistent, which might be attributed to a higher exposure to relevant pollutants and longer duration compared with controls. In some studies, the association between professional exposure to air pollution and lung cancer was found to be insignificant. However, as hazardous pollutants including carbon monoxide, nitrogen oxides, particulate matter, and polycyclic aromatic hydrocarbons are produced in the process of gasoline and diesel combustions,3 the government should cooperate with the automobile industry, energy department, and transportation companies to promote the consumption of cleaner fuels, such as natural gas and electricity. As professional drivers must pass regular examinations to get their driver's licenses, they must maintain a certain level of health to perform their jobs, known as the healthy worker effect.65 However, the general population includes individuals unemployed as a result of poor health and related conditions. The duration of employment might not be an accurate predictor of cumulative exposure to traffic-related air pollution, which potentially leads to an underestimation of the risk of lung cancer because of exposure misclassification.28
Because of the limited studies obtained, we were not able to employ subgroup analysis by regions, gender, and smoking status. We could not use controls for these variables with multi-regression models, which potentially leads to bias to some extent. As some studies did not provide effect sizes measured by every 10 μg/m3 increment of exposure, the exclusion of such studies might also cause a selection bias. Considering the existence of interactions between pollutants, individual analysis of one particular pollutant might overestimate its effect on lung cancer.66
Conclusion
Exposure to nitrogen dioxide, nitrogen oxide, sulfur dioxide, and fine particulate matter were positively associated with a risk of lung cancer. Occupational exposure to air pollution among professional drivers significantly increased the incidence and mortality of lung cancer.
Acknowledgments
This study is a part of the “Strengthen Capacity of Study and Application on Burden of Disease in Health Care System of China (12-107),” supported by CMB. We are grateful to Aaron J Cohen of the Health Effects Institute and Ghassan Hamra of the International Agency for Research on Cancer who provided commends on our draft.
Disclosure
No authors report any conflict of interest.
Supporting Information
Appendix S1 Strategy of literature search.
References
- International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [Cited 1 May 2014.] Available from URL: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- World Health Organization. 2008. Ambient (outdoor) air quality and health. Fact sheet number 313. Updated March 2014. [Cited 1 May 2014.] Available from URL: http://www.who.int/mediacentre/factsheets/fs313/en/
- Straif K, Cohen A, Samet J. 2013. Air pollution and cancer. IARC Scientific Publication No. 161. [Cited 1 May 2014.] Available from URL: http://www.iarc.fr/en/publications/books/sp161/index.php.
- Bhalla K, Shotten M, Cohen A, et al. Transport for Health: The Global Burden of Disease from Motorized Road Transport. Washington, DC: The World Bank; 2014. [Google Scholar]
- Gabrielson E. Worldwide trends in lung cancer pathology. Respirology. 2006;11:533–538. doi: 10.1111/j.1440-1843.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Chen F, Cole P, Bina WF. Time trend and geographic patterns of lung adenocarcinoma in the United States, 1973–2002. Cancer Epidemiol Biomarkers Prev. 2007;16:2724–2729. doi: 10.1158/1055-9965.EPI-07-0455. [DOI] [PubMed] [Google Scholar]
- Chen F, Jackson H, Bina WF. Lung adenocarcinoma incidence rates and their relation to motor vehicle density. Cancer Epidemiol Biomarkers Prev. 2009;18:760–764. doi: 10.1158/1055-9965.EPI-08-0741. [DOI] [PubMed] [Google Scholar]
- Health Effects Institute. 2010. Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. Special report 17, January. [Cited 1 May 2014.] Available from URL: http://pubs.healtheffects.org/view.php?id=334.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Gasana J, Dillikar D, Mendy A, Forno E, Ramos Viera E. Motor vehicle air pollution and asthma in children: a meta-analysis. Environ Res. 2012;117:36–45. doi: 10.1016/j.envres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Heinrich J, Thiering E, Rzehak P, et al. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup Environ Med. 2013;70:179–186. doi: 10.1136/oemed-2012-100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med. 2011;183:73–78. doi: 10.1164/rccm.200912-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen ES. A follow-up study on the mortality of truck drivers. Am J Ind Med. 1993;23:811–821. doi: 10.1002/ajim.4700230514. [DOI] [PubMed] [Google Scholar]
- Yorifuji T, Kashima S, Tsuda T, et al. Long-term exposure to traffic-related air pollution and the risk of death from hemorrhagic stroke and lung cancer in Shizuoka, Japan. Sci Total Environ. 2013;443:397–402. doi: 10.1016/j.scitotenv.2012.10.088. [DOI] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Beckerman BS, et al. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med. 2013;188:593–599. doi: 10.1164/rccm.201303-0609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hystad P, Demers PA, Johnson KC, Carpiano RM, Brauer M. Long-term residential exposure to air pollution and lung cancer risk. Epidemiology. 2013;24:762–772. doi: 10.1097/EDE.0b013e3182949ae7. [DOI] [PubMed] [Google Scholar]
- Cesaroni G, Badaloni C, Gariazzo C, et al. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 2013;121:324–331. doi: 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Yang C, Li J, et al. Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study. J Hazard Mater. 2011;186:1594–1600. doi: 10.1016/j.jhazmat.2010.12.036. [DOI] [PubMed] [Google Scholar]
- Beelen R, Hoek G, van den Brandt PA, et al. Long-term exposure to traffic-related air pollution and lung cancer risk. Epidemiology. 2008;19:702–710. doi: 10.1097/EDE.0b013e318181b3ca. [DOI] [PubMed] [Google Scholar]
- Vineis P, Hoek G, Krzyzanowski M, et al. Air pollution and risk of lung cancer in a prospective study in Europe. Int J Cancer. 2006;119:169–174. doi: 10.1002/ijc.21801. [DOI] [PubMed] [Google Scholar]
- Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Ma R, et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005;16:727–736. doi: 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]
- Filleul L, Rondeau V, Vandentorren S, et al. Twenty five year mortality and air pollution: results from the French PAARC survey. Occup Environ Med. 2005;62:453–460. doi: 10.1136/oem.2004.014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafstad P, Haheim LL, Oftedal B, et al. Lung cancer and air pollution: a 27 year follow up of 16 209 Norwegian men. Thorax. 2003;58:1071–1076. doi: 10.1136/thorax.58.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CR3rd, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg F, Gustavsson P, Järup L, et al. Urban air pollution and lung cancer in Stockholm. Epidemiology. 2000;11:487–495. doi: 10.1097/00001648-200009000-00002. [DOI] [PubMed] [Google Scholar]
- Petersen A, Hansen J, Olsen JH, Netterstrøm B. Cancer morbidity among Danish male urban bus drivers: a historical cohort study. Am J Ind Med. 2010;53:757–761. doi: 10.1002/ajim.20837. [DOI] [PubMed] [Google Scholar]
- Merlo DF, Stagi E, Fontana V, et al. A historical mortality study among bus drivers and bus maintenance workers exposed to urban air pollutants in the city of Genoa, Italy. Occup Environ Med. 2010;67:611–619. doi: 10.1136/oem.2009.050377. [DOI] [PubMed] [Google Scholar]
- Consonni D, De Matteis S, Lubin JH, et al. Lung cancer and occupation in a population-based case-control study. Am J Epidemiol. 2010;171:323–333. doi: 10.1093/aje/kwp391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsey J, Alterman T, Li J, Petersen MR, Sestito J. Mortality among members of a truck driver trade association. AAOHN J. 2010;58:473–480. doi: 10.3928/08910162-20101018-01. [DOI] [PubMed] [Google Scholar]
- Garshick E, Laden F, Hart JE, et al. Lung cancer and vehicle exhaust in trucking industry workers. Environ Health Perspect. 2008;116:1327–1332. doi: 10.1289/ehp.11293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richiardi L, Mirabelli D, Calisti R, et al. Occupational exposure to diesel exhausts and risk for lung cancer in a population-based case-control study in Italy. Ann Oncol. 2006;17:1842–1847. doi: 10.1093/annonc/mdl307. [DOI] [PubMed] [Google Scholar]
- Järvholm B, Silverman D. Lung cancer in heavy equipment operators and truck drivers with diesel exhaust exposure in the construction industry. Occup Environ Med. 2003;60:516–520. doi: 10.1136/oem.60.7.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll-Johanning H, Bach E, Jensen SS. Lung and bladder cancer among Danish urban bus drivers and tramway employees: a nested case-control study. Occup Med (Lond) 2003;53:25–33. doi: 10.1093/occmed/kqg004. [DOI] [PubMed] [Google Scholar]
- Elci OC, Akpinar-Elci M, Alavanja M, Dosemeci M. Occupation and the risk of lung cancer by histologic types and morphologic distribution: a case control study in Turkey. Monaldi Arch Chest Dis. 2003;59:183–188. [PubMed] [Google Scholar]
- Brüske-Hohlfeld I, Möhner M, Ahrens W, et al. Lung cancer risk in male workers occupationally exposed to diesel motor emissions in Germany. Am J Ind Med. 1999;36:405–414. doi: 10.1002/(sici)1097-0274(199910)36:4<405::aid-ajim1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Pezzotto SM, Poletto L. Occupation and histopathology of lung cancer: a case-control study in Rosario, Argentina. Am J Ind Med. 1999;36:437–443. doi: 10.1002/(sici)1097-0274(199910)36:4<437::aid-ajim4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Hansen J, Raaschou-Nielsen O, Olsen JH. Increased risk of lung cancer among different types of professional drivers in Denmark. Occup Environ Med. 1998;55:115–118. doi: 10.1136/oem.55.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat JE, Stellman SD, Richie JP, Jr, Wynder EL. Lung cancer risk and workplace exposures in black men and women. Environ Res. 1998;76:78–84. doi: 10.1006/enrs.1997.3787. [DOI] [PubMed] [Google Scholar]
- Jakobsson R, Gustavsson P, Lundberg I. Increased risk of lung cancer among male professional drivers in urban but not rural areas of Sweden. Occup Environ Med. 1997;54:189–193. doi: 10.1136/oem.54.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgia P, Forastiere F, Rapiti E, et al. Mortality among taxi drivers in Rome: a cohort study. Am J Ind Med. 1994;25:507–517. doi: 10.1002/ajim.4700250405. [DOI] [PubMed] [Google Scholar]
- Alfredsson L, Hammar N, Hogstedt C. Incidence of myocardial infarction and mortality from specific causes among bus drivers in Sweden. Int J Epidemiol. 1993;22:57–61. doi: 10.1093/ije/22.1.57. [DOI] [PubMed] [Google Scholar]
- Burns PB, Swanson GM. The Occupational Cancer Incidence Surveillance Study (OCISS): risk of lung cancer by usual occupation and industry in the Detroit metropolitan area. Am J Ind Med. 1991;19:655–671. doi: 10.1002/ajim.4700190510. [DOI] [PubMed] [Google Scholar]
- Steenland NK, Silverman DT, Hornung RW. Case-control study of lung cancer and truck driving in the Teamsters Union. Am J Public Health. 1990;80:670–674. doi: 10.2105/ajph.80.6.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P, Harris RE, Wynder EL. Case-control study on occupational exposure to diesel exhaust and lung cancer risk. Am J Ind Med. 1990;17:577–591. doi: 10.1002/ajim.4700170504. [DOI] [PubMed] [Google Scholar]
- Paradis G, Theriault G, Tremblay C. Mortality in a historical cohort of bus drivers. Int J Epidemiol. 1989;18:397–402. doi: 10.1093/ije/18.2.397. [DOI] [PubMed] [Google Scholar]
- Hayes RB, Thomas T, Silverman DT, et al. Lung cancer in motor exhaust-related occupations. Am J Ind Med. 1989;16:685–695. doi: 10.1002/ajim.4700160608. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Stellman SD, Garfinkel L. Diesel exhaust exposure and mortality among males in the American Cancer Society prospective study. Am J Ind Med. 1988;14:403–415. doi: 10.1002/ajim.4700140405. [DOI] [PubMed] [Google Scholar]
- Garshick E, Laden F, Hart JE, Davis ME, Eisen EA, Smith TJ. Lung cancer and elemental carbon exposure in trucking industry workers. Environ Health Perspect. 2012;120:1301–1306. doi: 10.1289/ehp.1204989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laden F, Hart JE, Eschenroeder A, Smith TJ, Garshick E. Historical estimation of diesel exhaust exposure in a cohort study of U.S. railroad workers and lung cancer. Cancer Causes Control. 2006;17:911–919. doi: 10.1007/s10552-006-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Andersen O. Lung cancer in filling station attendants. Am J Ind Med. 1991;20:763–768. doi: 10.1002/ajim.4700200607. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans. Vol 105: Diesel and Gasoline Engine Exhausts and Some Nitroarenes. Lyon: IARC; 2013. [Google Scholar]
- WHO Working Group. Health Aspects of Air Pollution with Particulate Matter, Ozone and Nitrogen Dioxide. Report on A WHO Working Group. EUR/03/5042688. Copenhagen: WHO Regional Office for Europe; 2003. ; [Cited 1 May 2014.] Available from URL: http://www.euro.who.int/document/e79097.pdf. [Google Scholar]
- Meng Z, Qin G, Zhang B. DNA damage in mice treated with sulfur dioxide by inhalation. Environ Mol Mutagen. 2005;46:150–155. doi: 10.1002/em.20142. [DOI] [PubMed] [Google Scholar]
- Pope CR3rd. Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who's at risk? Environ Health Perspect. 2000;108(Suppl. 4):713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center of Cancer; Disease Prevention and Control Bureau, Ministry of Health. Chinese Cancer Registry Annual Report in 2012. Beijing: Publishing House of Military Science; 2012. [Google Scholar]
- Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Song H, Tong S, Li L, Liu B, Wan L. Ambient sulfate concentration and chronic disease mortality in Beijing. Sci Total Environ. 2000;262:63–71. doi: 10.1016/s0048-9697(00)00573-8. [DOI] [PubMed] [Google Scholar]
- Zhou M, Liu Y, Wang L, Kuang X, Xu X, Kan H. Particulate air pollution and mortality in a cohort of Chinese men. Environ Pollut. 2014;186:1–6. doi: 10.1016/j.envpol.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Katsouyanni K, Touloumi G, Samoli E, et al. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology. 2001;12:521–531. doi: 10.1097/00001648-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Ministry of Environmental Protection of the People's Republic of China. 2013. . [ National environmental quality in the first half of 2013 (CHINA 49).]. [Cited 1 May 2014.] Available from URL: http://www.mep.gov.cn/gkml/hbb/bgg/201308/t20130820_257686.htm. (In Chinese.)
- Beeson WL, Abbey DE, Knutsen SF. Long-term concentrations of ambient air pollutants and incident lung cancer in California adults: results from the AHSMOG study.Adventist Health Study on Smog. Environ Health Perspect. 1998;106:813–822. doi: 10.1289/ehp.106-1533247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Environmental Protection of the People's Republic of China. 2013. . [ Limits and measurement methods for emissions from light-duty vehicles (CHINA 5).]. [Cited 1 May 2014.] Available from URL: http://kjs.mep.gov.cn/hjbhbz/bzwb/dqhjbh/dqydywrwpfbz/201309/t20130917_260352.htm. (In Chinese.)
- Pearce N, Checkoway H, Kriebel D. Bias in occupational epidemiology studies. Occup Environ Med. 2007;64:562–568. doi: 10.1136/oem.2006.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoralas-Alises S, Diaz-Lobato S. Air pollution and lung cancer. Curr Respir Med Rev. 2012;8:418–429. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Strategy of literature search.