Abstract
Background
Non-small cell lung cancer (NSCLC) accounts for 80% of lung cancers, and lung squamous cell carcinoma (SQCC) is one of the main types. Advances in the treatment of lung SQCC are lacking when compared to lung adenocarcinoma. The main treatment for early-stage SQCC is surgery. However, factors affecting the efficacy of surgical treatments for early-stage lung SQCC remain unclear. In this study, we examined the significance of commonly used lung SQCC diagnostic markers p63, p40, and cytokeratin (CK)5/6 in prognosis.
Methods
Seventy-six cases of early-stage lung SQCC (N0) were obtained from our lung cancer database (January 2000 to December 2009). Tissue microarray and immunohistochemical (IHC) staining were used to detect the expression of p63, p40, and CK5/6. The effect of the expression level of each marker on patients' survival was examined.
Results
Sensitivity and specificity of each marker for detecting lung SQCC was 87.0% and 81.0% for p63, 75.9% and 97.9% for p40, and 78.9% and 97.7% for CK5/6. Survival rates of patients with high expression levels of p63 or CK5/6 or both were higher than in patients with low expression levels (P < 0.05). Expression levels of p40 had no effect on survival (P > 0.05). Multivariate analysis showed that high levels of p63 expression p63+CK5/6 co-expression were independent prognostic factors for good survival.
Conclusion
IHC staining detection of p63 and CK5/6 in specimens should be routinely performed in postoperative early-stage lung SQCC patients. Its significance lies not only in differential diagnosis, but also in determining prognosis.
Keywords: Immunohistochemistry, lung squamous cell carcinoma, non-small cell lung cancer, prognosis
Introduction
Pneumonectomy was successfully used to treat lung cancer in 1933, and thereafter, surgery has been used extensively as a lung cancer treatment. In 1968, different treatment principles emerged for non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). For many years, treatment of NSCLC has depended on anatomy-based tumor node metastasis (TNM) staging to determine the methods used. Along with an increased understanding of the genetic background of lung cancer, more theories have been added to the basis for choosing methods for treating lung cancer. Present treatment methods include: antifolate chemotherapy drug Pemetrexed, which is only effective for treating lung adenocarcinoma (ADC);1 Bevacizumab, which should be avoided in lung squamous cell carcinoma (SQCC);1 epidermal growth factor receptor–tyrosine-kinase inhibitor (EGFR–TKI) therapy, which is only effective in treating lung ADC with EGFR gene mutations;2 and Crizotinib, which is only effective in treating patients with anaplastic lymphoma kinase (ALK) gene rearrangement.3 Therefore, an accurate clinical distinction between SQCC (non-SQCC) and ADC (non-ADC) is necessary. In most cases, hematoxylin and eosin (H&E) staining is sufficient to distinguish these two tissue types. However, immunohistochemical (IHC) staining-assisted evaluation may be required in the following situations: small biopsy specimens or cytological specimens, in which observation of the tissue is limited; or poorly differentiated carcinomas, where the direction of differentiation is difficult to determine.4 The commonly used markers for distinguishing between lung SQCC and ADC are p63, p40, and cytokeratin (CK)5/6 (SQCC-specific), and thyroid transcription factor 1 (TTF-1), Napsin A, and CK7 (ADC-specific). Numerous studies and reliable results have been reported in this field.5 The progress in lung ADC research means that characteristics, including clinical and imaging features, pathological growth types, gene expression, and molecular biomarkers, are used to divide ADC into several subtypes related to prognosis, and guide clinical treatment decisions. However, treatment decisions for lung SQCC still mostly depend on anatomy-based TNM staging for two reasons: first, the study of the molecular background of SQCC is not as advanced as that of lung ADC; and second, lung SQCC is called “dirty cancer” and the genes involved in this cancer are more complicated than those involved in lung ADC. Nevertheless, studies on the clinical markers of lung SQCC with potential prognostic value continue to remain a focus for research. p63, p40, and CK5/6 have been widely used as specific markers for lung SQCC diagnosis. However, few studies on the relationship between the expression of SQCC diagnostic markers and post-surgery survival have been reported. Therefore, this was the focus of the current study.
Materials and methods
Clinical data
The Institutional Review Board of the Peking Cancer Hospital, Peking University approved this retrospective study. A single surgery group at the Cancer Hospital of Peking University chose patient data from the prospectively maintained database for lung cancer.
Recruitment criteria
Between January 2000 and December 2009, 395 patients received surgical treatment for lung cancer. Patient inclusion criteria were: (i) clear diagnosis; (ii) primary lung cancer of the SQCC pathological type and pathological tumor-node-metastasis (pTNM) stage N0; (iii) surgery performed by a single group; (iv) anatomic lung resection (pneumonectomy or lobectomy); (v) R0 resection; and (vi) complete specimen inventory. Patient exclusion criteria were: (i) perioperative death; (ii) missing paraffin blocks or pathology sections in the inventory; and (iii) an insufficient number of typical cancer cells in the paraffin blocks for tissue microarray (TMA) analysis. A total of 76 cases met the study criteria.
Study subjects
The group consisted of 76 cases, including 70 men (92.1%) and six women (7.9%). The male to female ratio was 11.7:1. The age range was between 38 and 78 years; the mean age was 60 years; and the median age was 61 years. According to the 7th Edition of the Union for International Cancer Control TNM Classification of Lung Cancer (2009), 52 cases (68.4%) were stage I, 16 cases (21.1%) were stage II, and eight cases (10.5%) were stage III (T4N0). All patients in this group received anatomic pulmonary resection.
Pathological differentiation types
According to the World Health Organization standard (2004), among the 76 cases of SQCC, five (6.6%) were well differentiated, 32 (42.1%) were moderately differentiated, and 39 cases (51.3%) were poorly differentiated. If the degree of differentiation was between the grades, it was classified as the lesser differentiated grade.
Follow-up method
Postoperative follow-ups were conducted mainly through clinic visits (accounting for 86.2%). Follow-up for those who did not come to the clinic according to the schedule, were conducted via telephone calls or letters. The total follow-up rate was 97.8%. Follow-up intervals were every three months for the first two years after surgery, every six months from two to five years, and once a year after five years. The deadline for follow-up in this study was 3 September 2013.
Tissue microarray preparation
One hundred and twenty SQCC cases and 194 ADC cases were selected from the database, and their paraffin specimens were prepared for TMA analysis. All of the tumor sections of each specimen were re-evaluated by two experienced pathologists. Sections with intact and typical cancer tissue were selected, and the areas covering the tumor were marked. The corresponding areas in the paraffin blocks for TMA sampling were then labeled. A MiniCore automatic TMA instrument (Alphelys, Plaisir, France) was used for TMA sample preparation, with a needle diameter of 1 mm. Samples of the whole group were assembled into four TMA paraffin blocks, and two cancer tissue cores were extracted for each specimen. The quality was controlled by H&E staining of the sections. The criteria for effective samples were the number of cancer cells of ≥20 in at least one cancer tissue core in each specimen. A total of 76 effective N0 lung SQCC specimens were confirmed.
Immunohistochemical (IHC) analysis
IHC staining of the TMA sections was performed using the Ventana automated IHC staining system (Ventana Medical Systems, Inc., Tucson, AZ, USA). The antibodies used are listed in Table 1. All antibody labeling was detected using the Ventana 3,3′– diaminobenzidine (DAB) detection kit. The positive controls for p63, p40, and CK5/6 were lung SQCC positive sections, and phosphate-buffered saline (PBS) was used instead of primary antibodies for the negative controls.
Table 1.
Antibodies used in this Study (TMA)
| Antibodies | Mono/Polyclonal | Source | Clone | Dilution |
|---|---|---|---|---|
| p63 | m | Dako, Glostrup Denmark | DAK-p63 | Working solution |
| p40 | p | MAIXIN-BIO, Fuzhou China | — | 1:200 |
| CK5/6 | m | Dako, Glostrup Denmark | D5/16 B4 | Working solution |
CK, cytokeratin; TMA, tissue microarray.
Two highly qualified pathologists who had not been informed about the ordering rules of the TMA samples or the clinical data evaluated the results of the IHC analysis of cancer cells in each spot. The criteria for IHC staining evaluation for each selected marker was: (i) p63- and p40-positive cells: staining was located in cancer cell nuclei and cells with brown particles in the nuclei were positive (Fig 1a,b), whereas cells without brown particles were negative; and (ii) CK5/6-positive cells: staining was mainly located on the plasma membrane of cancer cells, and cells with brown particles on the plasma membrane were considered positive (Fig 1c), whereas cells without brown particles were negative.
Figure 1.
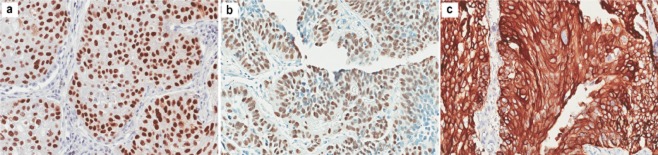
Representative sections (×200) of positive immunostaining of (a) p63 diffuse nuclear staining; (b) p40 diffuse nuclear staining; and (c) cytokeratin (CK)5/6 diffuse membrane staining, in lung squamous cell carcinoma (SQCC).
The two pathologists evaluated sections independently after agreeing on standards. When different results were obtained, consensus was reached through a discussion. The staining intensity and area of the sections were evaluated under low magnification (×20). Histologic (H) scores were conducted following the recognized method.6 A 4-level scoring scale for intensity was used (0, negative; 1+, weak; 2+, moderate; 3+, strong). The expression intensity (range: 0–300) was determined by multiplying the scores by the percentage of positive areas (0–100%). Sections with H scores of >150 were considered to show a high expression, and those with H scores of ≤150 were considered to show a low expression.7
Statistical analysis
Statistical analysis was performed using SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA). The relationship between the expression levels of each IHC marker and the clinical factors in each group was examined using the chi-square and Fisher's exact tests. The correlation of the expression levels among markers was analyzed using Spearman's rank correlation. The Kaplan–Meier method and log-rank test were used to determine the significance of the expression levels of IHC markers in each group in the prognosis of N0 lung SQCC patients. Cox proportional hazard modeling was used to analyze the independent prognostic factors in N0 lung SQCC patients. Statistical results with a P-value of <0.05 were considered statistically significant.
Results
Follow-up results
The follow-up rate of the whole patient group was 97.4%. The median follow-up period was 62.2 months (8.3–152.7 months). The five-year survival rate of the whole group was 66.9 ± 5.8%. Among those, 52 were stage I patients with a five-year survival rate of 71.4 ± 8.2%; and 24 were stage II–III patients with a five-year survival rate of 63.3 ± 8.0%. The total number of deaths was 28, including 19 deaths in stage I patients and nine in stage II–III patients. The median survival time could not be determined because the number of end-point events did not reach half the total number.
IHC staining results of the specimens from 76 N0 lung squamous cell carcinoma (SQCC) patients
Clear IHC staining results were obtained for each antibody (Fig 1a–c). The sensitivity and specificity of the three IHC markers in SQCC diagnosis in this study are listed in Table 2.
Table 2.
IHC-marker diagnostic sensitivity/specificity for SQCC
| Marker | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| p63 | 87.0 | 81.0 | 73.5 | 91.1 |
| p40 | 75.9 | 97.9 | 95.7 | 86.9 |
| CK5/6 | 78.9 | 97.7 | 95.6 | 88.1 |
CK, cytokeratin; IHC, immunohistochemistry; NPV, negative predictive value; PPV, positive predictive value; SQCC, squamous cell carcinoma.
In SQCC diagnosis, the sensitivity of p63 was the highest, at 87.0%, while the diagnostic specificity of p40 and CK5/6 were higher, 97.9% and 97.7%, respectively.
Expression of the three IHC markers
The ratios of high to low expression for each IHC marker are as follows: p63 (total 73 cases), 58 (79.5%)/15 (20.5%); p40 (total 74 cases), 30 (40.5%)/44 (59.5%); and CK5/6 (total 68 cases), 41 (60.3%)/27 (39.7%). No significant difference was observed for clinical factors including age, smoking history, primary tumor (pT) stage, and histological grade in each group (P > 0.05) (Table 3). The independent expression of p63 and CK5/6 perfectly correlated with the co-expression of p63+CK5/6, with a Spearman correlation coefficient of rs = 1 (P < 0.001).
Table 3.
Patient characteristics according to IHC marker expression
| Variable | p63 (n = 73) | P* | p40 (n = 74) | P* | CK5/6 (n = 68) | P* | |||
|---|---|---|---|---|---|---|---|---|---|
| H >150 n (%) (n = 58) | H ≤150 n (%) (n = 15) | H >150 n (%) (n = 30) | H ≤150 n (%) (n = 44) | H >150 n (%) (n = 41) | H ≤150 n (%) (n = 27) | ||||
| Age† | |||||||||
| ≤61 y | 25 (43.1) | 9 (60.0) | 0.242 | 15 (50.0) | 18 (40.9) | 0.440 | 15 (36.6) | 14 (51.9) | 0.213 |
| >61 y | 33 (56.9) | 6 (40.0) | 15 (50.0) | 26 (59.1) | 26 (63.4) | 13 (48.1) | |||
| Gender | |||||||||
| Male | 57 (98.3) | 12 (80.0) | 0.033 | 29 (96.7) | 40 (90.9) | 0.619 | 41 (100.0) | 22 (81.5) | 0.008 |
| Female | 1 (1.7) | 3 (20.0) | 1 (3.3) | 4 (9.1) | 0 (0.0) | 5 (18.5) | |||
| Smoking history | |||||||||
| Ever | 46 (79.3) | 9 (60.0) | 0.226 | 23 (76.7) | 32 (72.7) | 0.703 | 32 (78.0) | 17 (63.0) | 0.175 |
| Never | 12 (20.7) | 6 (40.0) | 7 (23.3) | 12 (27.3) | 9 (22.0) | 10 (37.0) | |||
| pT stage | |||||||||
| pT1 | 24 (41.4) | 6 (40.0) | 0.923 | 13 (43.3) | 18 (40.9) | 0.836 | 15 (36.6) | 15 (55.6) | 0.123 |
| pT2–4 | 34 (58.6) | 9 (60.0) | 17 (56.7) | 26 (59.1) | 26 (63.4) | 12 (44.4) | |||
| Histological grade | |||||||||
| Well, moderately | 31 (53.4) | 5 (33.3) | 0.165 | 18 (40.9) | 18 (60.0) | 0.107 | 20 (48.8) | 12 (44.4) | 0.726 |
| Poorly | 27 (46.6) | 10 (66.7) | 12 (59.1) | 26 (40.0) | 21 (51.2) | 15 (55.6) | |||
*Statistical significance was calculated by the χ2 test or Fisher's exact test. †The median age of the entire population is indicated. H, histologic scores; IHC, immunohistochemistry.
Relationship between the expression of IHC markers and the prognosis in N0 lung SQCC patients
Results of univariate analysis
Univariate analysis was performed to examine the relationship between the high expression levels of p63, p40, CK5/6, and p63+CK5/6 and N0 lung SQCC prognosis.
The expression of p63 was analyzed in 73 cases (73/76) of N0 lung SQCC. Among these, 58 cases had high levels (H > 150), and 15 cases had low levels of p63 expression (H ≤ 150). Patients with high levels of p63 expression had better survival rates than those with low expression levels, and the difference was statistically significant (P = 0.002; hazard ratio [HR] 0.288; 95% confidence interval [CI], 0.127–0.655) (Fig 2a).
The expression of p40 was analyzed in 74 cases (74/76) of N0 lung SQCC. Among these, 30 cases had high levels (H > 150), and 44 cases had low levels of p40 expression (H ≤ 150), and the difference of survival between the two groups was not statistically significant (P = 0.632; HR 0.827; 95% CI, 0.380–1.800) (Fig 2b).
The expression levels of CK5/6 were analyzed in 68 cases (68/76) of N0 lung SQCC. Among these, 41 cases had high levels (H >150), and 27 cases had low levels of CK5/6 expression (H ≤150). Patients with high CK5/6 expression levels had better rates of survival than those with low expression levels, and the difference between the two was statistically significant (P = 0.008; HR 0.352; 95% CI, 0.158–0.786) (Fig 2c).
The co-expression of p63+CK5/6 in N0 lung SQCC was analyzed in 67 cases (67/76). Among these, 39 cases had high levels of p63+CK5/6 co-expression (H >150), 17 cases had high levels of p63 or CK5/6 expression, and 11 cases had low levels of p63+CK5/6 co-expression (H ≤150). Patients with high co-expression levels had better survival rates than those with low co-expression levels, and the difference was statistically significant (P = 0.002) (Fig 2d).
Figure 2.
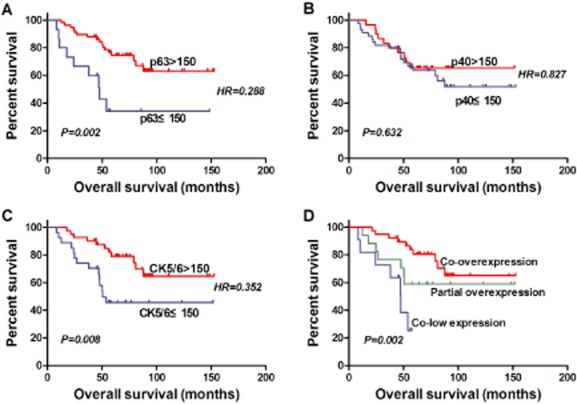
Kaplan-Meier survival curves for N0 lung squamous cell carcinoma (SQCC) patients according to expression level of (a) p63, (b) p40, (c) CK5/6, and (d) p63+CK5/6; survival difference tested by log-rank test (co-overexpression, H scoresp63 > 150 and H scoresCK5/6 > 150; co-low expression, H scoresp63 ≤ 150 and H scoresCK5/6 ≤ 150; partial overexpression, H scoresp63 > 150 or H scoresCK5/6 > 150.).
Results of Cox multivariate regression analysis
Univariate analysis showed that both high levels of p63 and CK5/6 expression and p63+CK5/6 co-expression correlated with the prognosis of N0 lung SQCC patients. In addition, the co-expression of p63+CK5/6 perfectly correlated with the expression of p63 and CK5/6 (rs = 1). Therefore, the expression of p63 and CK5/6 and the co-expression of p63+CK5/6 were considered prognostic factors and separately included in the Cox regression analysis. Other included factors were age, gender, smoking history, (pT) stage, and degree of differentiation.
High expression levels of p63 (HR 0.182, 95% CI, 0.055–0.602, P = 0.005), advanced age (>61 years) (HR 2.984, 95% CI,1.146–7.773, P = 0.025), and smoking (HR 11.240, 95% CI, 1.382–91.402, P = 0.024) were independent prognostic factors for the survival of N0 lung SQCC patients. High expression levels of p63 correlated with better prognosis, whereas advanced age (>61 years) and smoking correlated with poorer prognosis (Table 4).
High levels of p63+CK5/6 co-expression (P = 0.002) were an independent prognostic factor for the survival of N0 lung SQCC patients. High levels of p63+CK5/6 co-expression correlated with good prognosis (Table 5).
Table 4.
Multivariate Cox model of overall survival to expression of p63 and CK5/6 (n = 67)
| Variable | HR* | 95% CI | P* |
|---|---|---|---|
| Age: >61 y versus ≤61 y† | 2.984 | 1.146–7.773 | 0.025 |
| Gender: Male versus female | 0.109 | 0.010–1.193 | 0.069 |
| Smoking history: Ever versus never | 11.240 | 1.382–91.402 | 0.024 |
| pT stage: pT2–4 versus pT1 | 2.487 | 0.941–6.576 | 0.066 |
| Histological grade: Well/moderately versus poorly | 0.431 | 0.160–1.162 | 0.096 |
| p63: H >150 versus H ≤150 | 0.182 | 0.055–0.602 | 0.005 |
| CK5/6: H >150 versus H ≤150 | 0.373 | 0.137–1.015 | 0.054 |
*Statistical significance was calculated by the Cox proportional hazards model. †The median age of the entire population is indicated. CI, confidence interval; CK, cytokeratin; H, histologic scores; HR, hazard ratio.
Table 5.
Multivariate Cox model of overall survival to co-expression of p63 and CK5/6 (n = 67)
| Variable | HR* | 95% CI | P* |
|---|---|---|---|
| Age: >61 y versus ≤61 y† | 2.694 | 1.035–7.011 | 0.042 |
| Gender: Male versus female | 0.117 | 0.011–1.305 | 0.081 |
| Smoking history: Ever versus never | 11.519 | 1.418–93.594 | 0.022 |
| pT stage: pT2–4 versus pT1 | 2.601 | 0.983–6.879 | 0.054 |
| Histological grade: Well/moderately versus poorly | 0.454 | 0.171–1.205 | 0.113 |
| p63+CK5/6 co-expression | 0.002 | ||
| Co-low expression versus co-overexpression | 15.394 | 3.392–69.859 | 0.000 |
| Partial overexpression versus co-overexpression | 2.556 | 0.873–7.487 | 0.087 |
Co-overexpression, H scoresp63 > 150 and H scoresCK5/6 > 150; co-low expression, H scoresp63 ≤ 150 and H scoresCK5/6 ≤ 150; partial overexpression, H scoresp63 > 150 or H scoresCK5/6 > 150. *Statistical significance was calculated by the Cox proportional hazards model. †The median age of the entire population is indicated. CI, confidence interval; CK, cytokeratin; HR, hazard ratio.
Discussion
p63, p40, and cytokeratin (CK)5/6 are specific diagnostic markers for lung SQCC
The p63 gene is located on chromosome 3q27-29, and it shares structural homology with p53. The p63 gene has two promoters, and two corresponding isoforms are produced through alternative splicing: the full-length protein TAp63 (containing the transactivation domain) and the truncated protein ΔNp63 (lacking the transactivation domain). TAp63 has a transactivation domain (TA) similar to that of p53, which regulates the expression of growth-suppressing genes; the ΔNp63 isoform has an inactive ΔN domain resulting from alternative splicing, which antagonizes the activity of TAp63 and p53.8 Therefore, the p63 protein includes two subtypes with opposite functions: TAp63, a p53-like tumor suppressor gene transcription factor and ΔNp63, an oncogene transcription factor. The antibody 4A4 recognizes not only TAp63 but also ΔNp63, whereas the p40 antibody only recognizes ΔNp63.9 Studies indicate that the diagnostic sensitivity of p63 protein for SQCC is 95–100%.10,11 However, the specificity is low, with a value of 60–86%.11–13 Both the sensitivity and specificity of p40 for SQCC diagnosis are better than those of p63, reaching 100% and 98–100%,9,14,15 respectively. In this study, the sensitivity of p63 in identifying lung SQCC from lung ADC was 87.0%, and the specificity was 81.0%, whereas the sensitivity of p40 was 75.9%, and the specificity was as high as 97.9%.
The large family of CKs is composed of 20 polypeptides of different molecular weights and isoelectric points. CK proteins are grouped into two types: type 1 CKs (CK9–20) are small acidic polypeptides, and type 2 CKs (CK1–8) are large neutral polypeptides.16 CK5/6 is an acidic cytokeratin of intermediate size. In normal tissues, CK5/6 is mainly expressed in keratinized (epithelium) and non-keratinized (mucosa) squamous epithelium, as well as in the basal myoepithelial cell layer of the prostate, mammary, and salivary glands. In addition, CK5/6 is also expressed in benign and malignant tumors originating in the epithelium, squamous mucosa, and myoepithelium,17,18 for example, basal-like carcinoma and mesothelioma in breast cancer.19,20 CK5/6 is a sensitive marker for squamous differentiation. Its reported sensitivity for lung SQCC diagnosis was 75–100%,19,21,22 and its specificity in the study by Whithaus et al.11 reached 96%. In the current study, the sensitivity of CK5/6 for identifying lung SQCC from lung ADC was 78.9%, and the specificity was as high as 97.7%.
p63 and CK5/6 are also prognostic factors for lung SQCC
Studies have suggested that p63 proteins consist not only of an isoform with p53-like tumor-suppressing function, but also an isoform with oncogene-promoting function. The CK family of proteins is expressed in epithelial cells, and play a specific role in maintaining malignant transformation of normal cells. Because of the important functions of these three proteins in the regulation of tumor differentiation, growth, and malignant transformation, it is not surprising that they are mainly used as lung SQCC diagnostic markers, and their expression levels may be related to prognosis. Therefore, in this study, homogeneous samples were selected with identical pathological stage (pTN0), surgery type (single surgery group), long-term follow-up, and high follow-up rate, to analyze the significance of these three markers in the prognosis of early stage SQCC. The results indicate that patients with high levels of p63 expression have better survival rates than those with low expression. In studies by Renouf et al. and Au et al., univariate analysis showed that high levels of p63 expression are an indicator for good prognosis.23,24 However, multivariate analysis did not show a significant difference. In this study, univariate analysis showed that no significant difference was found in patients' survival between groups of high and low p40 expression; that is, the expression level of p40 did not affect the long-term survival of patients. The results are similar to those from the study by Iwata et al.25
In addition, univariate analysis showed that patients with high levels of CK5/6 expression had better survival rates than those with low expression. However, to our knowledge, no study on the role of CK5/6 expression levels in prognosis has been reported.
Combined and weighted stratified analysis of p63 and CK5/6 showed that survival was different between groups of high and low-level co-expression of p63+CK5/6. Multivariate analysis further found that in early-stage lung SQCC patients, high expression levels of p63 and high co-expression levels of p63+CK5/6 are independent prognostic factors for good survival.
A limitation of this study was its small sample size, which can result in bias. Although the data were from a prospective database, the study was essentially retrospective. Therefore, this study requires prospective data, a large sample size, and randomized, controlled clinical trials for verification.
Conclusion
p63, p40, and CK5/6 are all specific indicators in lung SQCC diagnosis. In addition, high levels of p63 and CK5/6 expression are indicators for good prognosis.
Acknowledgments
This work was supported by Beijing Academic Leaders Program (Grant 2009-2-17), Beijing Natural Science Foundation (Grant 7102029), Capital Medical Developed Research Found (Grant 2007-1023), New Scholar Star Program of Ministry of Education, and National Basic Research Program of China (Grant 2011CB504300), Specialized Research Fund for the Doctoral Program of Higher Education (Grant 20130001110108), National Natural Science Foundation for Distinguished Young Scholars (Grant81301748), Science Fund for Creative Research Groups of the National Natural Science Foundation of China (GrantIRT13003).
Disclosure
No authors report any conflict of interest.
References
- Linardou H, Dahabreh IJ, Kanaloupiti D, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008;9:962–972. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98:1817–1824. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- Pelosi G, Sonzogni A, Viale G. The classification of lung carcinoma: time to change the morphology-based approach? Int J Surg Pathol. 2010;18:161–172. doi: 10.1177/1066896910361736. [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis LM, Behrens C, Dong W, et al. Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res. 2010;16:3743–3753. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad RS, Liu YL, Han H, Landreneau RJ, Silverman JF. Prognostic significance of thyroid transcription factor-1 expression in both early-stage conventional adenocarcinoma and bronchioloalveolar carcinoma of the lung. Hum Pathol. 2004;35:3–7. doi: 10.1016/j.humpath.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Crum CP, McKeon FD. p63 in epithelial survival, germ cell surveillance, and neoplasia. Annu Rev Pathol. 2010;5:349–371. doi: 10.1146/annurev-pathol-121808-102117. [DOI] [PubMed] [Google Scholar]
- Nobre AR, Albergaria A, Schmitt F. p40: a p63 isoform useful for lung cancer diagnosis - a review of the physiological and pathological role of p63. Acta Cytol. 2013;57:1–8. doi: 10.1159/000345245. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Katzenstein AL. Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: utility of an immunohistochemical panel containing TTF-1, napsin A, p63, and CK5/6. Am J Surg Pathol. 2011;35:15–25. doi: 10.1097/PAS.0b013e3182036d05. [DOI] [PubMed] [Google Scholar]
- Whithaus K, Fukuoka J, Prihoda TJ, Jagirdar J. Evaluation of napsin A, cytokeratin 5/6, p63, and thyroid transcription factor 1 in adenocarcinoma versus squamous cell carcinoma of the lung. Arch Pathol Lab Med. 2012;136:155–162. doi: 10.5858/arpa.2011-0232-OA. [DOI] [PubMed] [Google Scholar]
- Kwak EL, Bang YJ, Camidge DR. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Tsuta K, Watanabe S. Frequent ALK rearrangement and TTF-1/p63 co-expression in lung adenocarcinoma with signet-ring cell component. Lung Cancer. 2011;72:309–315. doi: 10.1016/j.lungcan.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol. 2012;25:405–415. doi: 10.1038/modpathol.2011.173. [DOI] [PubMed] [Google Scholar]
- Nonaka D. A study of DeltaNp63 expression in lung non-small cell carcinomas. Am J Surg Pathol. 2012;36:895–899. doi: 10.1097/PAS.0b013e3182498f2b. [DOI] [PubMed] [Google Scholar]
- Sundström BE, Stigbrand TI. Cytokeratins and tissue polypeptide antigen. Int J Biol Markers. 1994;9:102–108. doi: 10.1177/172460089400900207. [DOI] [PubMed] [Google Scholar]
- Cooper D, Schermer A, Sun TT. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985;52:243–256. [PubMed] [Google Scholar]
- Miettinen M. Keratin immunohistochemistry: update of applications and pitfalls. Pathol Annu. 1993;28(Pt 2):113–143. [PubMed] [Google Scholar]
- Kaufmann O, Fietze E, Mengs J, Dietel M. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol. 2001;116:823–830. doi: 10.1309/21TW-2NDG-JRK4-PFJX. [DOI] [PubMed] [Google Scholar]
- Carella R, Deleonardi G, D'Errico A, et al. Immunohistochemical panels for differentiating epithelial malignant mesothelioma from lung adenocarcinoma: a study with logistic regression analysis. Am J Surg Pathol. 2001;25:43–50. doi: 10.1097/00000478-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Downey P, Cummins R, Moran M, Gulmann C. If it's not CK5/6 positive, TTF-1 negative it's not a squamous cell carcinoma of lung. APMIS. 2008;116:526–529. doi: 10.1111/j.1600-0463.2008.00932.x. [DOI] [PubMed] [Google Scholar]
- Kargi A, Gurel D, Tuna B. The diagnostic value of TTF-1, CK 5/6, and p63 immunostaining in classification of lung carcinomas. Appl Immunohistochem Mol Morphol. 2007;15:415–420. doi: 10.1097/PAI.0b013e31802fab75. [DOI] [PubMed] [Google Scholar]
- Renouf DJ, Wood-Baker R, Ionescu DN, et al. BCL-2 expression is prognostic for improved survival in non-small cell lung cancer. J Thorac Oncol. 2009;4:486–491. doi: 10.1097/JTO.0b013e318199e03a. [DOI] [PubMed] [Google Scholar]
- Au NH, Cheang M, Huntsman DG, et al. Evaluation of immunohistochemical markers in non-small cell lung cancer by unsupervised hierarchical clustering analysis: a tissue microarray study of 284 cases and 18 markers. J Pathol. 2004;204:101–109. doi: 10.1002/path.1612. [DOI] [PubMed] [Google Scholar]
- Iwata T, Uramoto H, Sugio K, et al. A lack of prognostic significance regarding DeltaNp63 immunoreactivity in lung cancer. Lung Cancer. 2005;50:67–73. doi: 10.1016/j.lungcan.2005.03.039. [DOI] [PubMed] [Google Scholar]


