Abstract
Background
To address the question of how much chest-wall (CW) resections and prosthetic reconstructions influence functional outcome.
Methods
We retrospectively reviewed 175 patients who underwent surgery for CW tumors. The clinical, histological, surgical, oncological, and functional factors were analyzed.
Results
We performed: 75 rib resections; 20 sternal resections; 15 combined resections; and 27 lung resections. In 39 cases (22.2%) CW was stabilized with non-rigid prosthesis (Vicryl-mesh: 8 patients; Goretex-mesh: 31 patients). Postoperative complications occurred in 22 cases (12.6%): a correlation with lung resection was evidenced by multivariate analysis (P = 0.025). Five-year survival for primary and secondary tumors was 50% and 36%, respectively: multivariate analysis (P = 0.048) showed a worse survival in men only. In the prosthesis subset, pulmonary function tested as percentage of forced expiratory volume in one second (%FEV1) (pre: 87.1 ± 18.9%; post: 82.3 ± 23.0%, P = ns), percentage of forced vital capacity (pre: 94.1 ± 19.3%; post: 82.0 ± 21.6%, P = ns), diffusing capacity of the lungs for carbon monoxide (pre: 15.7 ± 7.4; post: 12.1 ± 4.1, P = ns) and paO2 (pre: 82.6 ± 10.9 mmHg; post: 83.9 ± 7.3 mmHg, P = ns) was slightly modified from pre to postoperative. Interestingly, the decline of FEV1% was lower in the prosthesis-subset (4.1 ± 15.9%) compared with the subgroup who did not undergo prosthetic stabilization (17.5 ± 16.2%), but this difference was not statistically significant (P = ns).
Conclusion
Because of the low decrease of lung parameters, CW prosthetic reconstruction could be helpful for avoiding postoperative worsening of functional outcome, mostly in patients with pre-existing pulmonary diseases.
Keywords: Chest wall tumor, FEV1, prosthesis, PTFE
Introduction
Chest wall tumors (CWTs) encompass neoplasms of various origins, including bone and cartilage, soft tissue (muscle, vessel, nerve), and even some hematologic diseases.1 CWTs have long represented challenging clinical entities for surgeons because of the high rate of incorrect diagnosis, incomplete resections or the inability to perform successful reconstructions of chest wall (CW) defects after extensive demolitions. CW resection may be necessary for a variety of reasons including malignancy, radio-necrosis, infection or trauma.2 On the other hand, CW reconstruction is recommended for restoring its primary functions: (i) protection of the intra-thoracic and upper abdominal contents; (ii) respiration; (iii) support of shoulders and upper extremities; and (iv) for aesthetic purposes. In literature, a variety of philosophies about the amount of chest wall stabilization exist. Many authors have focused on the in-hospital period only,3–5 while in other studies, the data mainly refer to survival, recurrence, and metastasis rates.6,7 Only sporadic reports focusing on quality of life and postoperative pulmonary function exist.8,9
There is a body of evidence to support that the degree of lung resection is associated with varying degrees of pulmonary and functional status impairment.10,11 Additional CW resection could potentially worsen the pulmonary reserve in such patients.
A retrospective study of CWTs was conducted to review their clinical, radiological, and pathological features, as well as the early and long-term results of surgical management. Moreover, this study addresses the relevant question of how much resections and reconstructions with prostheses influence pulmonary function parameters.
Materials and methods
After approval of this study by our institutional review board, the medical records of all patients referred for CWTs at the Department of Thoracic Surgery at Agostino Gemelli General Hospital from 1 January 1998 to 31 December 2010 were retrospectively reviewed. Patients undergoing CW resection for post-thoracotomy empyema, radionecrosis or infections were excluded from this study. The data collected in the pre-op setting included patient demographics, medical comorbidities, and the use of pre-operative chemotherapy or radiotherapy. Follow-up data regarding the clinical course and outcome were collected from the patients' charts and interviews with the patients, their relatives, and their general practitioners. Surgical data were obtained from operative reports and included: (i) the location of the CW lesion; (ii) the number of ribs resected; (iii) whether lung resection was also performed; and (iv) the methodology chosen for the CW reconstruction. The size of the CW defect and the histological diagnosis were obtained from the final pathology report. Skeletal CW reconstruction was performed with non-rigid prostheses (Vicryl mesh or expanded polytetrafluoroethylene [PTFE]/Gore-Tex mesh). Postoperative analgesia was provided as a continuous narcotic infusion through a peridural catheter (when peridural analgesia was precluded by technical problems, intravenous patient-controlled analgesia was used). Complications were considered as perioperative if they occurred within 30 days of surgery. Pulmonary function was measured using a volume displacement body plethysmograph (Platinum Elite, Medical Graphics Corporation, St. Paul, MN, USA). Lung volumes and single-breath diffusion capacity for carbon monoxide (DLCO) were measured according to American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines.12 Functional tests were expressed as absolute values and as a percent of the predicted; these were performed before surgery and three months after discharge. Oncological follow-up was completed in 155 patients (88.6%). When considering only those patients who underwent extended CW resection (81 patients: 39 undergoing a prosthesis reconstruction and 42 a sternal or rib resection without prosthetic repair), the pneumological data (lung volumes, diffusing capacity of the lungs for carbon monoxide [DLCO], and blood gas parameters) were complete in 74.1% of cases (60 patients).
Statistical analysis
The sample characteristics were summarized by absolute and relative frequencies for categorical variables and by means ± standard deviations for continuous variables. Continuous variables were compared with the Student's t test; the Pearson χ2 test was applied for categorical variables.
The occurrence of postoperative complications and the analysis of predictors of relapse were investigated by means of a logistic regression analysis. Potentially associated factors considered for these analyses were: age; gender; co-morbidity; rib, sternal and lung resection; use of prosthesis; and histology.
Overall survival and recurrence rates were investigated by a survival analysis. Socio-demographic and clinically meaningful factors, potentially associated to these two outcomes, were: age; gender; rib, sternal and lung resection; postoperative complications; and histology. Kaplan-Meier analysis was performed and survival curves were compared with the log-rank test. A simple Cox model was also fitted to all factors listed above and the model with the factor predictive of the highest statistically significant hazards of mortality was selected; multivariable Cox regression analysis was also performed in a forward-stepwise fashion: all remaining factors were fitted, one at a time, to the selected model and the new models evaluated for their statistical significance and reliability in the prediction of mortality hazards.
The groups of subjects who underwent extended CW resection (patients with and without prosthesis reconstruction) were compared with regards to the percentage of forced expiratory volume in one second (FEV1) on the decline from the baseline; this was calculated as the decline of FEV 1% = (pre-operative – postoperative value).
The limit for statistical significance was set at P < 0.05. For statistical analysis, SPSS version 18.0 for Windows (SPSS, Chicago, USA) was used.
Results
Clinical data
During the study period, 175 patients underwent CW resection at the Agostino Gemelli General Hospital. There were 78 male (44.6%) and 97 female patients (55.4%), with a median age of 57 years (range, 7 to 88). The indications for resection were primary CWT in 111 patients (63.4%) and secondary tumor in 64 (36.6%). Among primary CWT, there were 86 benign tumors (77.5%) and 25 malignancies (22.5%). There were 30 (46.9%) secondary tumors arising from a primary lung cancer, and 14 (21.8%) CWTs for recurrent breast cancer. The most common CWTs were elastofibroma dorsi, non-small-cell lung carcinoma, breast carcinoma, and bone or soft tissue sarcoma (Table 1). Prior pre-operative therapy was given to a total of 53 patients (30.3%), of whom 24 (13.7%) received chemotherapy only, seven (4%) received radiation only in the area of resection, and 22 (12.6%) received both. Co-morbidities included chronic obstructive pulmonary disease (COPD) in nine patients, coronary artery disease (CAD) in seven, diabetes mellitus (DM) in 13, and hypertension in 57.
Table 1.
Histology of resected chest wall tumors
| Benign tumors (49.1%) | Malignant tumors (50.9%) | ||||
|---|---|---|---|---|---|
| Primary tumors (28.1%) | Secondary tumors (71.9%) | ||||
| Elastofibroma | 55 | Synovial sarcoma | 3 | Lung cancer | 30 |
| Osteomyelitis | 10 | Myeloma | 3 | - Adenocarcinoma | 18 |
| Lipoma | 4 | Chondrosarcoma | 3 | - Squamous cell carcinoma | 8 |
| Bone cyst | 4 | Malignant solitary fibrous tumor | 2 | - Sarcomatoid carcinoma | 3 |
| Osteochondroma | 2 | Ewing's sarcoma | 2 | - Undifferentiated carcinoma | 1 |
| Neurofibroma | 2 | Leiomyosarcoma | 2 | Breast cancer | 14 |
| Fibroma | 2 | Fibrous histiocytoma | 2 | Melanoma | 5 |
| Lymphangioma | 1 | Askin's tumor | 2 | Cervix/uterus cancer | 4 |
| Osteoma | 1 | Dermatofibrosarcoma | 2 | Thymoma | 3 |
| Desmoid tumor | 1 | Melanoma | 2 | Mesothelioma | 2 |
| Fibrous dysplasia | 1 | Fibrosarcoma | 1 | Prostate cancer | 2 |
| Fibromatosis multiple | 1 | Liposarcoma | 1 | Solitary fibrous tumor | 2 |
| Chondroma | 1 | Kidney cancer | 1 | ||
| Schwannoma | 1 | Epithelioid sarcoma | 1 | ||
Swelling (29.3%) and pain (24.6%) were the most common symptoms detected, while 41.7% of patients were asymptomatic at the moment of diagnosis. More than half of the tumors affected the right CW (54.3%), left side (32%), and bilateral (6.8%) while the sternum was affected in only 6.3% of cases.
Pre-operative diagnosis was suggested by radiological examinations (ultrasonography, chest X-ray, computed tomography [CT] scan and magnetic resonance imaging [MRI]) in 92.6% of patients. Positron emission tomography (PET) and bone scintigraphy were used in 43.4% of cases. Pre-op pathological diagnosis was carried out by fine-needle aspiration or surgical biopsy, in 5.7% and 0.6% of patients, respectively.
Surgical data
In our series, 75 patients underwent rib resection while sternal resection was executed in 20 cases; a combined resection (rib plus sternum) was performed in 15 patients. On average, three rib segments were resected (range: 0–7) with a multiple rib resection (equal or more than 3 ribs) in 41.1% of cases (Fig 1). In 86.7% of patients the size of the CW defects was larger than 4 cm. Concomitant lung resection was performed in 27 patients (15.4%): six wedge resections, 18 lobectomies, one bilobectomy, and two pneumonectomies. In 39 patients (22.2% of the total sample) the CW was stabilized using a non-rigid prosthetic mesh (Vicryl mesh: 8 patients; Gore-Tex Mesh: 31 patients). A myocutaneous flap was used 15 times to cover the CW defect. In the remaining cases no flap was necessary and the defects could be closed primarily by tissue advancement.
Figure 1.
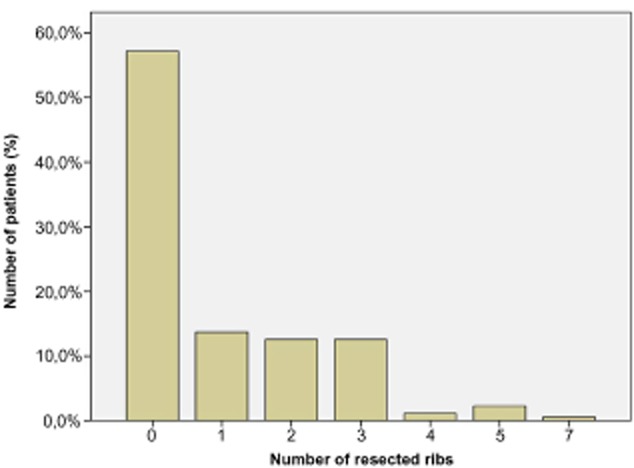
Number of resected ribs and relative frequency in the patient population.
At final pathology, mean tumor size was 6.2 ± 3.3 cm (benign tumors: 6.9 cm – malignant tumors: 5.6 cm; P = 0.013). Surgery was radical in 150 cases (85.7%): R0 resection was achieved in 79 benign lesions, as well as in 71 malignant ones (P = 0.018).
Complications and hospital stay
Our routine perioperative management consisted of early extubation, usually in the operating room at the end of surgery. For 51 patients, the length of stay in the intensive care unit (ICU) (15 patients with myocutaneous flap, 22 patients with prosthesis reconstruction, and 14 without flap or prothesis) was 2.4 days on average and ranged from one to 14 days.
Complications occurred in 22 patients (12,6%) within the postoperative period (Table 2). The most common complications were anemia occurring in seven patients (4%) and seroma in five cases (2.8%). Respiratory failure and atelectasis occurred in four patients (2.3%). Factors associated with postoperative complications were analyzed (Table 2). Age, gender, comorbidities, the need for sternal or rib resection, use of prosthesis, and histology were not significant predictors of postoperative complications. Univariate analysis identified only lung resection to be a significant predictor of postoperative complications (P = 0.001). Multivariate analysis confirmed lung resection as the only factor significantly impacting the complication rate in such patients (P = 0.025).
Table 2.
Post-op complications after chest wall resection
| Complications | Patients |
|---|---|
| Anemia | 7 (31.8%) |
| Seroma | 5 (22.7%) |
| Hematoma | 2 (9.1%) |
| Arrhythmia | 2 (9.1%) |
| Respiratory failure | 2 (9.1%) |
| Atelectasis | 1 (4.5%) |
| Pulmonary embolism | 1 (4.5%) |
| Sepsis | 1 (4.5%) |
| Fever | 1 (4.5%) |
| Analysis on predictors of post-op complications (n = 22) | |||
|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||
| Variables | P – value* | HR [95% CI] | P – value* |
| Age (≥ 60 years) | 0.207 | 1.30 [0.45–3.73] | 0.630 |
| Male sex | 0.738 | 1.18 [0.42–3.28] | 0.756 |
| Comorbidities | 0.315 | 0.57 [0.19–1.71] | 0.312 |
| Rib resection (≥ 3) | 0.796 | 1.61 [0.14–18.43] | 0.699 |
| Sternal resection | 0.255 | 2.52 [0.28–23.14] | 0.413 |
| Lung resection | 0.001 | 5.42 [1.24–23.75] | 0.025 |
| Prosthesis | 0.641 | 0.63 [0.17–2.24] | 0.475 |
| Histology (malignant secondary tumors) | 0.232 | 1.25 [0.32–4.94] | 0.753 |
CI, confidence interval; HR, hazard ratio.
The median length of hospital stay for all patients was 5.3 days (range: 1–21). Univariate analysis showed male sex (P = 0.04); the need for rib resection (P < 0.001); use of prosthesis (P < 0.001); associated lung resection (P < 0.001); histology consistent with secondary malignant tumors (P < 0.001); and complications (P < 0.001) to be significant predictors of higher stay. Multivariate analysis confirmed that male sex (P = 0.039); lung resection (P < 0.001); rib resection (P < 0.001); use of prosthesis (P < 0.001); and histology consistent with secondary malignant tumors (P < 0.001) were significant predictors of higher stay.
Thirty-day mortality for all patients was 0.6% (1 patient who died because of a systemic inflammatory response syndrome).
Pulmonary function
Among the subgroup of patients undergoing CW reconstruction with prostheses, pulmonary function tests revealed that pre-operative and postoperative FEV1 ranged from 87.1 ± 18.9% to 82,3 ± 23.0% (P = ns), forced vital capacity (FVC) from 94.1 ± 19.3% to 82.0 ± 21.6% (P = ns), DLCO from 15.7 ± 7.4 to 12.1 ± 4.1 (P = ns), and pO2-value from 82.6 ± 10.9 mmHg to 83.9 ± 7.3 mmHg (P = ns) (Fig 2). Interestingly, the mean reduction of FEV1 in this subgroup of patients, who had prosthesis reconstructions, was 4.1 ± 15.9% and this reduction was not significantly different from the reduction in the subgroup that did not undergo prosthesis stabilization (17.5 ± 16.2%; P = ns) (Fig 3). Moreover, rib resection (more than 4 ribs) did not affect the mean reduction of pulmonary function tests (P = ns), while lung resection (P < 0.001) and location of CW defect (P = 0.026) seemed to affect it; sternal resection shows an interesting trend (P = 0.079) as well. This evidence was not confirmed at multivariate analysis (Table 3).
Figure 2.
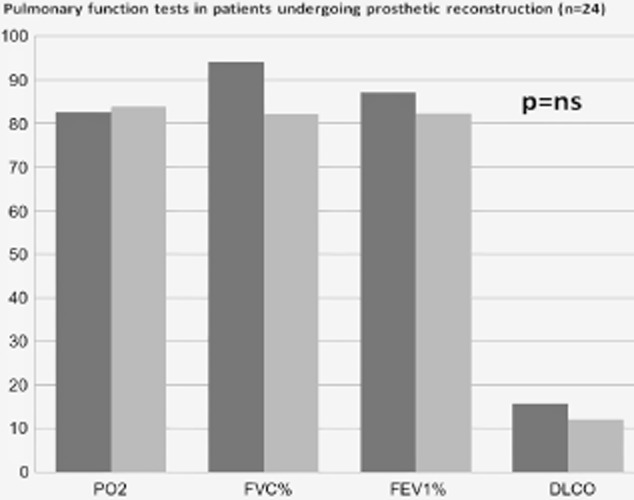
Change in respiratory function among the subgroup of patients undergoing chest wall reconstruction with prostheses.  , pre-operative tests;
, pre-operative tests;  , post-operative tests.
, post-operative tests.
Figure 3.
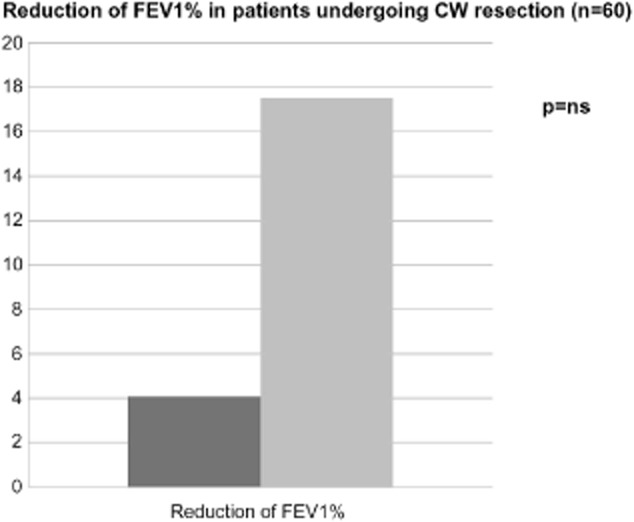
Comparison between the decrease of FEV1% in patients undergoing extended chest wall resection with and without prosthetic reconstruction.  , prosthesis group (n=24);
, prosthesis group (n=24);  , no-prosthesis group (n=36).
, no-prosthesis group (n=36).
Table 3.
Analysis on factors affecting mean decline of FEV1% (n = 60)
| Variables |
Univariate analysis P – value* |
|---|---|
| Rib resection (≥ 3) | 0.638 |
| Sternal resection | 0.079 |
| Location of chest wall defect (antero-lateral) | 0.026 |
| Lung resection | <0.001 |
Statistical tests: Analysis of variance; statistical significance: P < 0.05. FEV1, forced expiratory volume in one second.
Recurrence and survival
The progression-free interval for all patients who had undergone CW resection was 22.5 ± 21.0 months on average (range: 60 days to 88 months), while in malignant tumors it was 15.0 ± 14.7 (range: 60 days to 46 months). We observed a recurrence in 24 patients (13.7%): six were affected by benign lesions, four by primary malignant tumors, and 14 by secondary tumors. Factors associated with recurrence development were analyzed (Table 4). Tumor size, positive margins, sternal or rib resection (equal or more than 3), and histology consistent with primary tumors were not significant predictors of recurrence. Univariate analysis identified histology consistent with malignant tumors (P = 0.007) and secondary tumors (P = 0.013) to be the only significant predictors of relapse (Table4). Multivariate analysis showed no factors affecting relapse.
Table 4.
Analysis on predictors of recurrence (n = 24)
| Univariate analysis | Multivariate analysis | ||
|---|---|---|---|
| Variables | P – value* | HR [95% CI] | P – value* |
| Rib resection (≥ 3) | 0.137 | 2.50 [0.33–18.89] | 0.374 |
| Capsulated tumors | 0.172 | 0.71 [0.19–2.61] | 0.605 |
| Radical excision | 0.166 | 1.59 [0.46–5.44] | 0.463 |
| Histology (malignant tumors) | 0.007 | 0.35 [0.07–1.81] | 0.211 |
| Histology (malignant secondary tumors) | 0.013 | 0.73 [0.19–2.82] | 0.645 |
Statistical tests: Logistic Regression Analysis; statistical significance: P < 0.05. CI, confidence interval; HR, hazard ratio.
| Analysis on survival in patients affected by malignant chest wall tumors (n = 89 pts) | |||||
|---|---|---|---|---|---|
| Variables | Survival proportion | Univariate analysis | Multivariate analysis | ||
| 1 year % | 5 years % | P – value* | HR [95% CI] | P-value ** | |
| Gender | |||||
| Male | 45 | 29 | 0.027 | 0.41 [0.17–0.99] | 0.048 |
| Female | 78 | 56 | 1 | ||
| Age | |||||
| ≤60 years | 43 | 43 | 0.204 | 1.99 [0.76–5.28] | 0.163 |
| >60 years | 77 | 42 | 1 | ||
| Lung resection | |||||
| Yes | 46 | 46 | 0.649 | 1.90 [0.66–5.44] | 0.230 |
| No | 68 | 43 | 1 | ||
| Rib resection | |||||
| ≤3 | 59 | 42 | 0.597 | 1.21 [0.28–5.17] | 0.795 |
| >3 | 56 | 28 | 1 | ||
| Sternal resection | |||||
| Yes | 88 | 88 | 0.111 | 4.86 [056–41.97] | 0.150 |
| No | 55 | 34 | 1 | ||
| Use of prosthesis | |||||
| Yes | 60 | 43 | 0.659 | 1.05 [0.41–2.70] | 0.911 |
| No | 58 | 40 | 1 | ||
| Post-op complications | |||||
| Yes | 64 | 0 | 0.526 | 0.80 [0.27–2.35] | 0.698 |
| No | 58 | 43 | 1 | ||
Statistical tests: Kaplan-Meier Analysis and Log-Rank Test; statistical significance: P < 0.05. **Statistical tests: Cox Regression Analysis; statistical significance: P < 0.05. CI, confidence interval; HR, hazard ratio.
Overall, the five-year survival rate was 40%. In patients who were treated surgically for primary (malignant) tumors, one-year and five-year survival was 75% and 50%, respectively. Patients treated for secondary neoplasms, with a one-year survival of 54% and a five-year survival of 36%, experienced poorer survival rates even if they were not statistically significant (Fig 4, P = ns).
Figure 4.
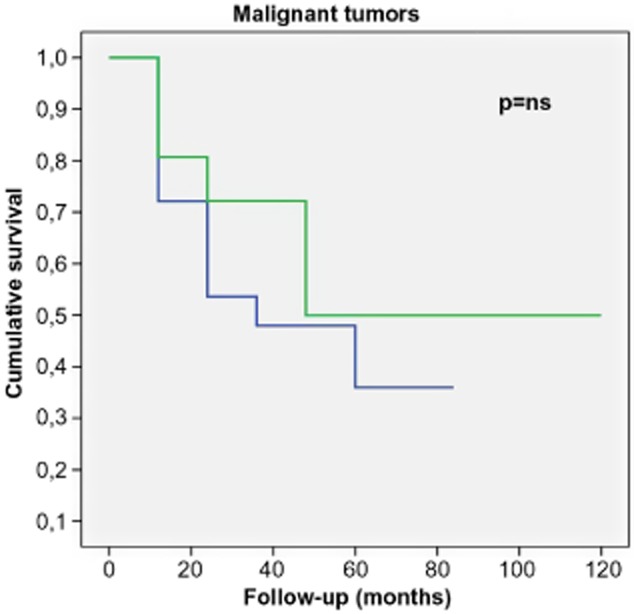
Survival of malignant chest wall tumors.  , primary tumors;
, primary tumors;  , secondary tumors.
, secondary tumors.
Age, rib resection higher than three ribs, sternal resection, use of prosthesis, postoperative complications, and a concurrent lung resection were not significantly associated to worse survival. Univariate analysis showed a worse survival rate in men only (P = 0.027). Multivariate analysis confirmed this evidence (P = 0.048) (Table 4).
Discussion
During the last century, improvements in surgical techniques for the reconstruction, use of prosthetic meshes, use of antibiotic prophylaxis, development of anesthesia, and intensive care units have made CW extensive resections substantially safe and feasible. Despite the fact that surgery is widely considered the main treatment for most malignant tumors of the CW, scarce systematic data actually exist concerning the impact of skeletal chest wall resection on respiratory function.
Larson and McMurtrey first described the respiratory outcome in a series of 50 patients who had undergone CW reconstruction with musculo-cutaneous flaps.13 Reconstruction with musculo-cutaneous flaps alone resulted in postoperative improvement, implying that these large flaps supplied sufficient CW rigidity to maintain adequate pulmonary mechanics. Although FEV1 and vital capacity were measured, the degree of postoperative ventilator dependency was not accurately defined in that study, nor were study patients matched for outcome with patients who had similar chest wounds, but had undergone skeletal reconstruction. Meadows et al. investigated the modifications of pulmonary function occurring after the removal of the sternum and manubrium and repair by pectoralis major muscle transposition in a cohort of six patients.14 The authors showed that minor pulmonary function changes related to CW resection are generally well tolerated. However, in “frail” patients (because of their poor general clinical condition), minor changes in pulmonary function may be a relevant element, such as in patients with COPD. In this setting, Ringelman et al. analyzed a cohort of 133 patients who had undergone debridement and flap reconstruction of their infected median sternotomy wounds.15 In this series, 36% of patients reported an inability to perform the same pre-operative activities: in fact, when midline sternal continuity is not restored, patients suffer from acute discomfort caused by shifting of the two halves of the CW. Similarly, Kohman et al. performed a comparative analysis of cardio-respiratory function in 13 patients before and after sternal resection with muscle flap closure, as well as with a control group of 15 patients undergoing similar procedures without complication.16 They concluded that exercise tolerance and pulmonary function may not differ from a control group of cardiac surgical patients, despite the altered composition of the CW. Lardinois et al. analyzed the outcome of 26 patients who underwent CW reconstruction by use of methyl methacrylate (MMA) mesh.8 In this series, the authors did not find any significant difference between pre-operative and postoperative FEV1 in patients with associated pulmonary resections. Klesius et al. reported a cohort of 12 patients who had undergone complete sternal resection with plastic reconstruction by bilateral pectoralis major flaps for mediastinal wound infection and sternum necrosis.17 They described a satisfactory CW stability without impairment of pulmonary function or significant limitations in quality of life. Recently, Daigeler et al. analyzed 92 consecutive patients with full thickness CW resections.9 Pulmonary function data were available for only 27 patients. The authors showed that pulmonary function parameters were only moderately reduced and not significantly affected by the size or localization of the resection.
These studies revealed only a moderate change in respiratory function among patients with CW resection and the real effects of CW stabilization with prosthetic material were not investigated. In our subset of 39 patients who had undergone Gore-tex and Vicryl mesh repair, we observed a decrease of all functional parameters from pre-operative to postoperative, especially regarding FEV1 and FVC, as previously reported.18 This may be interpreted as a consequence of impaired thoracic stability, loss of intercostal muscles, and increase of restrictive ventilatory patterns as a result of CW fibrosis and, thereby, disturbed respiratory biomechanics. Regardless of these functional effects, our data showed no statistical difference between pre-operative and postoperative pulmonary functional tests. Moreover, the mean reduction of FEV1 was substantially comparable between the prosthesis subset and the subgroup that did not undergo prosthesis stabilization. In the debate about how much CW demolition influences the outcome,18 our findings suggested that prosthesis stabilization did not significantly affect functional parameters. Although a comparative analysis among different prosthetic materials was not investigated, these data are similar to those reported by Macedo-Neto et al.: in fact, after surgical repair with mesh, despite elastic changes and an increase of pressure dissipated against viscoelastic/inhomogeneous segments of the CW, the PTFE patch (non-rigid prosthesis) seems to induce less important mechanical modifications than the Marlex mesh associated with MMA (rigid prosthesis), thus, better conveying the restoration of physiologic function.19
These results should also be taken into account when the effects of CW instability on postoperative ventilation are considered. In literature, in fact, there are few reports on the need for ventilator support after CW stabilization with mesh. In this setting, Hanna et al. reported that the incidence of admission to the ICU for ventilator support was higher in the no-mesh-cohort than in the mesh-group (60% vs. 33%, respectively).20 This evidence is similar to that found in our study: only 22 patients (56%) with mesh repair were admitted to the ICU compared to 29 (69%) without prostheses. However, further studies are needed to investigate this issue.
Although we advocate a CW stabilization with mesh for reducing the need for postoperative ventilation, our study is limited by a lack of information concerning the length of intubation. Moreover, the location of the CW defect, the number of ribs removed, and the body habitus of the patient are factors that cannot be ignored when planning reconstruction. In this debate, preoperative spirometry could be helpful in assisting with patient selection, preparation for resection, determination of the extent of resection, and the technique of reconstruction. Although our study confirmed only moderate changes in pulmonary function from pre-operative to postoperative, the decrease of FEV1 and FVC may be clinically significant, particularly in patients with pre-existing pulmonary disease. In the light of new evidence reported,21 further studies are needed to evaluate the effect of pre-operative and postoperative pulmonary rehabilitation in patients undergoing CW reconstruction.
In literature, complications after CW resection are common and range from 46% to 69% in two of the largest recent series of CW resection.2,22 Respiratory complications, including pneumonia, acute respiratory distress syndrome (ARDS), and atelectasis are by far the most common.2 In our study, complications occurred in 22 patients (12,6%) and respiratory complications were found in four patients (2.3%). Few prior series have analyzed the possible risk factors contributing to postoperative complications after CW resection.23 Risk factors that may predispose postoperative complications, either by altering the respiratory mechanics or the wound environment, include: comorbidities; the need for sternal or rib resection; use of prosthesis; histology; and concomitant lung resection. Similarly to that reported by others,22,23 in our series, pulmonary resection only was a significant predictor of postoperative complications. That is not surprising as these patients have compromised respiratory function not only because of a reduction in lung volume, but also as a result of changes to the rigidity of the CW.
The results of the present study are subjected to several limitations. First, because this study is retrospective, there is a possibility of selection bias. Second, possible oncological treatment of the study patients was not standardized. Third, the number of patients may not be large enough for evaluation by multivariate logistic regression analysis. Moreover, the patients are not homogeneous because of the extreme heterogeneity and the different behaviors of CWTs. Further studies with a larger number of cases are needed to investigate the impact of CW resection and reconstruction with prosthetic material on respiratory function; unfortunately, randomized controlled trials are not always possible because of ethical considerations, clinical judgment, and unwillingness on the part of the investigators or patients.24 On the other hand, we believe that the present study has the great merit to perform a deep and systematic analysis on the pulmonary function modification after surgical procedures on the CW, identifying predictors of worse pattern (i.e. concomitant lung resection).
Conclusion
In conclusion, CW stabilization with non-rigid prosthesis is associated with a low rate of complication and causes only moderate changes in pulmonary function according to pre-operative and postoperative baseline values. Because of the low decrease of lung parameters, prosthetic reconstruction could be helpful in avoiding postoperative worsening of functional outcome and reducing the need of postoperative ventilation, particularly in patients with pre-existing pulmonary disease. Further studies are needed to estimate the need for pre-operative or postoperative pulmonary rehabilitation in patients undergoing CW reconstruction.
Disclosure
No authors report any conflict of interest.
References
- Pairolero PC, Arnold PG. Chest wall tumors. Experience with 100 consecutive patients. J Thorac Cardiovasc Surg. 1985;90:367–372. [PubMed] [Google Scholar]
- Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg. 2002;73:1720–1726. doi: 10.1016/s0003-4975(02)03527-0. [DOI] [PubMed] [Google Scholar]
- Losken A, Thourani VH, Carlson GW, et al. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br J Plast Surg. 2004;57:295–302. doi: 10.1016/j.bjps.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg. 1996;98:804–810. doi: 10.1097/00006534-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Chang RR, Mehrara BJ, Hu QY, Disa JJ, Cordeiro PG. Reconstruction of complex oncologic chest wall defects: a 10-year experience. Ann Plast Surg. 2004;52:471–479. doi: 10.1097/01.sap.0000122653.09641.f8. [DOI] [PubMed] [Google Scholar]
- Chapelier A, Macchiarini P, Rietjens M, et al. Chest wall reconstruction following resection of large primary malignant tumors. Eur J Cardiothorac Surg. 1994;8:351–356. doi: 10.1016/1010-7940(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Athanassiadi K, Kalavrouziotis G, Rondogianni D, Loutsidis A, Hatzimichalis A, Bellenis I. Primary chest wall tumors: early and long-term results of surgical treatment. Eur J Cardiothorac Surg. 2001;19:589–593. doi: 10.1016/s1010-7940(01)00655-8. [DOI] [PubMed] [Google Scholar]
- Lardinois D, Müller M, Furrer M, et al. Functional assessment of chest wall integrity after methylmethacrylate reconstruction. Ann Thorac Surg. 2000;69:919–923. doi: 10.1016/s0003-4975(99)01422-8. [DOI] [PubMed] [Google Scholar]
- Daigeler A, Druecke D, Hakimi M, et al. Reconstruction of the thoracic wall-long-term follow-up including pulmonary function tests. Langenbecks Arch Surg. 2009;394:705–715. doi: 10.1007/s00423-008-0400-9. [DOI] [PubMed] [Google Scholar]
- Sarna L, Evangelista L, Tashkin D, et al. Impact of respiratory symptoms and pulmonary function on quality of life of long-term survivors of non-small cell lung cancer. Chest. 2004;125:439–445. doi: 10.1378/chest.125.2.439. [DOI] [PubMed] [Google Scholar]
- Sarna L, Padilla G, Holmes C, Tashkin D, Brecht ML, Evangelista L. Quality of life of long-term survivors of non-small-cell lung cancer. J Clin Oncol. 2002;20:2920–2929. doi: 10.1200/JCO.2002.09.045. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Larson DL, McMurtrey MJ. Musculocutaneous flap reconstruction of chest-wall defects: an experience with 50 patients. Plast Reconstr Surg. 1984;73:734–740. doi: 10.1097/00006534-198405000-00003. [DOI] [PubMed] [Google Scholar]
- Meadows JA, III, Staats BA, Pairolero PC, Rodarte JR, Arnold PG. Effect of resection of the sternum and manubrium in conjunction with muscle transposition on pulmonary function. Mayo Clin Proc. 1985;60:604–609. doi: 10.1016/s0025-6196(12)60984-7. [DOI] [PubMed] [Google Scholar]
- Ringelman PR, Vander Kolk CA, Cameron D, Baumgartner WA, Manson PN. Long-term results of flap reconstruction in median sternotomy wound infections. Plast Reconstr Surg. 1994;93:1208–1214. [PubMed] [Google Scholar]
- Kohman LJ, Auchincloss JH, Gilbert R, Beshara M. Functional results of muscle flap closure for sternal infection. Ann Thorac Surg. 1991;52:102–106. doi: 10.1016/0003-4975(91)91428-x. [DOI] [PubMed] [Google Scholar]
- Klesius AA, Dzemali O, Simon A, et al. Successful treatment of deep sternal infections following open heart surgery by bilateral pectoralis major flaps. Eur J Cardiothorac Surg. 2004;25:218–223. doi: 10.1016/j.ejcts.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Leuzzi G, Cesario A, Novellis P, et al. Chest wall reconstruction: impact of Gore-Tex(®) dual-mesh prosthesis on respiratory function. Ann Thorac Cardiovasc Surg. 2012;18:582–583. doi: 10.5761/atcs.le.12.01902. [DOI] [PubMed] [Google Scholar]
- Macedo-Neto AV, Santos LV, Menezes SL, Paiva DS, Rocco PR, Zin WA. Respiratory mechanics after prosthetic reconstruction of the chest wall in normal rats. Chest. 1998;113:1667–1672. doi: 10.1378/chest.113.6.1667. [DOI] [PubMed] [Google Scholar]
- Hanna WC, Ferri LE, McKendy KM, Turcotte R, Sirois C, Mulder DS. Reconstruction after major chest wall resection: can rigid fixation be avoided? Surgery. 2011;150:590–597. doi: 10.1016/j.surg.2011.07.055. [DOI] [PubMed] [Google Scholar]
- Cesario A, Ferri L, Galetta D, et al. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer. 2007;57:118–119. doi: 10.1016/j.lungcan.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Deschamps C, Tirnaksiz BM, Darbandi R, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg. 1999;117:588–591. doi: 10.1016/s0022-5223(99)70339-9. [DOI] [PubMed] [Google Scholar]
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg. 2006;81:279–285. doi: 10.1016/j.athoracsur.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]


