Abstract
Background
The treatment status of small cell lung cancer (SCLC) in Mainland China has never been reported; therefore, this study is the first multicenter survey investigating the status of epidemiology and treatment options of SCLC in Mainland China.
Methods
Two questionnaires were designed to obtain information in 12 medical centers in five major Chinese cities. The hospital information questionnaire was designed to outline SCLC patients' characteristics and treatment preferences in each medical institution, and the patient information questionnaire collected detailed treatment information of 298 SCLC cases in these hospitals.
Results
SCLC represented 13.7% and 18.3% of all lung cancer patients in 2005 and 2010, respectively. Clinical management of SCLC follows mainstream clinical guidelines in general. The most widely applied first-line treatment mode for limited-stage patients was combined chemoradiotherapy (66.2%), while 77.0% of the extensive-stage patients received chemotherapy alone as initial treatment. Etoposide with cisplatin or carboplatin were the most accepted first-line chemotherapy regimens. The objective response rate was 58.3% after first-line chemotherapy and 23% of the patients who responded well to first-line treatment received prophylactic cranial irradiation. As for second-line chemotherapy, single regimen topotecan or a combined regimen containing topotecan were preferred (53.0%).
Conclusions
The treatment options indicated in our study are in accordance with the international clinical guidelines, which is valuable for the improvement of future guidelines, health care standard, and even the better distribution of health care resources in China.
Keywords: Chemotherapy, Chinese population, epidemiology, radiotherapy, small cell lung cancer
Introduction
Lung cancer has become the most common cancer and the leading cause of cancer-related death in the world.1 In 2010, both the incidence and mortality rates of lung cancer in China ranked first among all malignancies.2 As a distinct histological subtype of lung cancer, small cell lung cancer (SCLC) accounts for 14% of all newly diagnosed cases of lung cancer worldwide. The relatively poor prognosis, in part because of its aggressive clinical course and widespread metastases at diagnosis, makes the disease a serious public health issue.3
Evidence-based guidelines are essential in today's clinical practice. Currently, treatment guidelines on SCLC are available in the United States and Europe, as well as in China.4–6 However, clinical practice patterns may deviate from guidelines because of a variety of factors in the diverse health care settings in China. Because the real treatment situations of SCLC in China have never been reported, adherence to clinical guidelines is beyond our knowledge.
Therefore, we implemented a survey to probe the current status of SCLC treatment patterns in China. The data revealed in this study may help provide references for the refinement of future clinical guidelines and health care policies.
Methods
The survey was conducted between 2011 and 2012 in five major cities in Mainland China, including Beijing, Shanghai, Hangzhou, Jinan, and Changsha. Twelve hospitals including comprehensive cancer centers, chest hospitals, and general hospitals were involved in the study. Two questionnaires were designed to obtain information. The hospital information questionnaire was designed to outline SCLC patients' characteristics and treatment preferences in each hospital, and the patient information questionnaire collected both diagnoses and regimens of each patient (Table 1).
Table 1.
Essential questionnaire items
| Hospital information questionnaire |
|
| Patient information questionnaire |
|
LS, limited stage; SCLC, small cell lung cancer.
Results
Twelve medical centers submitted the hospital information questionnaire and the treatment options of 298 SCLC cases were collected.
Results of the hospital information questionnaire
Epidemiology and patients' characteristics
In 2005 and 2010, the number of lung cancer cases was 8748 and 11 671, respectively, while the number of SCLC cases was 1197 and 2139, respectively. SCLC represented 13.7% and 18.3% of all lung cancer patients in 2005 and 2010, respectively (Fig 1).
Figure 1.
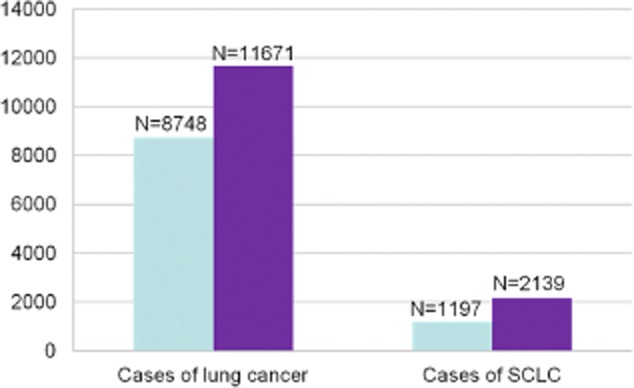
Numbers of lung cancer and small cell lung cancer (SCLC) patients in 2005 and 2010.  , 2005;
, 2005;  , 2010.
, 2010.
SCLC patients' characteristics including age, gender, stage, and brain metastasis status are shown in Table 2.
Table 2.
SCLC patients' characteristics
| Year | 2005 | 2010 |
|---|---|---|
| Age | ||
| <65 y/o cases (%) | 755 (63.1) | 1431 (66.9) |
| ≥65 y/o cases (%) | 442 (36.9) | 708 (33.1) |
| Gender | ||
| Male cases (%) | 984 (82.2) | 1753 (82.0) |
| Female cases (%) | 213 (17.8) | 386 (18.0) |
| M/F | 4.6 | 4.5 |
| Limited stage cases† (%) | 465 (41.7) | 857 (40.1) |
| Cases with brain metastasis at diagnosis‡ (%) | 222 (20.1) | 445 (20.8) |
†Data was provided from 10 hospitals in 2005. ‡Data was provided from nine hospitals in 2005. SCLC, small cell lung cancer.
Overview of therapeutic options
Among 12 hospitals, seven preferred concurrent chemoradiotherapy and five preferred sequential chemoradiotherapy as first-line treatments for limited stage (LS)-SCLC patients. All centers preferred etoposide with cisplatin or carboplatin (EP/CE) as a first-line chemotherapy regimen. The preferred second-line chemotherapy regimen was single-agent topotecan in eight centers, and topotecan-based combined therapy in four centers. Other options for second-line chemotherapy provided in the questionnaire include single-agent irinotecan, irinotecan-based combined chemotherapy, and chemotherapy regimens for treating non-small cell lung cancer (NSCLC).
A number of essential factors that should be considered in chemotherapy regimen selection were listed in the questionnaire. The ranking of these factors is summarized is as follows (from the most significant to the least): effectiveness; hematological toxicity; gastrointestinal adverse reaction; hepatic and renal toxicity; cost of the drug; and neurotoxicity and passage through the blood-brain barrier (BBB). In addition, the ranking of common indicators in the evaluation of the efficacy of treatment is (from the most significant to the least): time to progression (TTP)/progression-free survival (PFS); response rate (RR); overall survival (OS); and quality of life (QOL). We also inquired into the reason behind the uniform choice of topotecan as a second-line regimen. Efficacy was of first concern, followed by lower hematological toxicity, better passage through BBB, and less non-hematologic toxicity.
Results of the patient information questionnaire
Patients' characteristics
Of the 298 SCLC patients that participated in this survey, 235 were male (78.9%), and 215 (72.1%) were younger than 65 at the time of diagnosis. As for their clinical characteristics, 208 patients (69.8%) had a smoking history, and 41 (13.8%) reported a family history of cancer. Pathology revealed that 277 cases (93.0%) were pure SCLC, 18 (6%) had an NSCLC component, and the information of three patients was not provided. One hundred and thirty cases (43.6%) were clinically staged as LS-SCLC and 166 (55.7%) as extensive stage (ES). Staging details were not available for two patients. Four of the 130 LS-SCLC patients (3.0%) and 26 of the 166 (15.7%) ES-SCLC patients had brain metastasis at the time of diagnosis.
First-line treatment modalities
Eighty-six of the 130 LS-SCLC patients (66.2%) began their treatment with combined chemoradiotherapy, including 34 cases (26.2%) with sequential chemoradiotherapy, 33 (25.4%) with concurrent chemoradiotherapy, and 19 (14.6%) with chemo-radio-chemo “sandwich” therapy. Thirty patients (23.1%) were treated with chemotherapy alone. Other treatment methods used were as follows: four cases (3.1%) received surgery alone, six (4.6%) received surgery with chemotherapy, three (2.3%) received surgery with chemoradiotherapy, and one case (0.7%) radiotherapy alone.
One hundred and twenty-seven of the 165 evaluable ES-SCLC patients (77.0%) received chemotherapy alone as first-line treatment (6 patients were enrolled in clinical trials). Thirty-eight patients (23.0%) received combined therapy, including 25 cases (15.2%) with sequential chemoradiotherapy, seven (4.2%) with concurrent chemoradiotherapy, five (3.0%) with chemo-radio-chemo “sandwich” therapy, and one (0.6%) with surgery. First-line treatment information was not available for one patient (Table 3).
Table 3.
First-line treatment modalities†
| Treatment modalities | ||
|---|---|---|
| LS | ES | |
| Cases (%) | Cases (%) | |
| Chemotherapy alone | 30 (23.1) | 127 (77.0) |
| Clinical trials | 0 (0) | 6 (3.6) |
| Combined chemoradiotherapy | 86 (66.2) | 37 (22.4) |
| Sequential chemo-radiation therapy | 34 (26.2) | 25 (15.2) |
| Concurrent chemoradiotherapy | 33 (25.4) | 7 (4.2) |
| Chemo-radio-chemo “sandwich” therapy | 19 (14.6) | 5 (3.0) |
| Surgery alone | 4 (3.1) | 1 (0.6) |
| Surgery with chemotherapy | 6 (4.6) | 0 |
| Surgery with chemoradiotherapy | 3 (2.3) | 0 |
| Radiotherapy alone | 1 (0.7) | 0 |
| Total | 130 | 165 |
Staging data was not available for two patients and first-line treatment information was not available for one patient. ES, extensive stage; LS, limited stage.
First-line chemotherapy regimens
The details of chemotherapy regimens were collected in 149 of the 151 patients who received chemotherapy alone as their first-line treatment (patients who participated in clinical trials were excluded). In 138 out of 149 (92.6%) cases, patients received an etoposide and platinum regimen, including an EP regimen in 113 (75.8%) cases and CE in 25 (16.8%). Other regimens used were as follows: four cases (2.7%) with amrubicin/cisplatin (AP), two (1.3%) with etoposide/cisplatin/cyclophosphamide (EPI), one (0.7%) with topotecan/cisplatin regimen, and four (2.7%) with other regimens.
There were 123 patients treated initially with combined chemoradiotherapy and the first-line chemotherapy regimen was recorded in 121 cases. An EP regimen was adopted in 85 out of 121 cases (70.2%), and CE in 34 of 121 cases (28.1%). The other two patients received EPI and venorelbine/ifosfamide/cisplatin (NIP) regimens.
We also analyzed the chemotherapy regimen of 40 patients during the course of concurrent chemoradiotherapy. Twenty-nine were treated with EP and nine with a CE regimen. The treatment regimens of two cases were not available.
The number of chemotherapy cycles in first-line treatment
The total cycles of first-line chemotherapy were reflected in the questionnaire for 274 patients and the median number of first-line chemotherapy cycles was four. Fifty-three patients (19.3%) received fewer than four cycles of chemotherapy. Reasons for discontinuation included 21 cases (39.6%) with disease progression, 14 with poor tolerance (26.4%), 16 patients (30.2%) refused further therapy, and two (3.8%) had incomplete concurrent chemoradiotherapy (Table 4).
Table 4.
The number of cycles in first-line chemotherapy (n = 274)
| Cycles | Cases (%) |
|---|---|
| 1 | 5 (1.8) |
| 2 | 22 (8.0) |
| 3 | 26 (9.5) |
| 4 | 85 (31.0) |
| 5 | 122 (44.5) |
| 6 | 14 (5.2) |
The efficacy evaluation of first-line treatment
The efficacy of first-line treatment was evaluated for 286 patients, including 25 (8.7%) cases with complete response (CR), 142 (49.6%) with partial response (PR), 62 (21.7%) with stable disease (SD), and 57 (20.0%) with progressive disease (PD). The median time to progression (TTP) was 6.8 (0.7–21) months.
Second-line treatment
Second-line treatment for 157 patients was recorded in the questionnaire. Of 134 evaluable cases, 90 (67.2%) received chemotherapy alone, 32 (23.9%) received chemoradiotherapy, and 12 (8.9%) received radiotherapy alone.
Of the 122 patients whose second-line treatment contained chemotherapy, the regimen of 102 cases was provided (Table 5). The total cycles of second-line chemotherapy were recorded in 115 patients: including 30 cases (26.1%) who completed one cycle, 47 cases (40.9%) with two cycles, and 38 (33%) who received more than two cycles of chemotherapy. Efficacy to second-line treatment was evaluated in 106 patients, including 14 PR (13.2%), 30 SD (28.3%), and 62 PD (58.5%). The median TTP of second-line treatment in 78 patients was 2.1 (1–9) months.
Table 5.
Second-line chemotherapy regimens (n = 102)
| Regimen | Cases (%) |
|---|---|
| Single agent topotecan | 38 (37.3) |
| Combined regimen containing topotecan | 16 (15.7) |
| EP | 17 (16.7) |
| CE | 6 (5.9) |
| IP | 9 (8.8) |
| CAV | 2 (1.9) |
| Single agent etoposide or teniposide | 2 (1.9) |
| Other regimens | 12 (11.8) |
EP, etoposide with cisplatin; CAV, cyclophosphamide/adriamycin/vincristine; CE, etoposide with carboplatin; IP, irinotecan/cisplatin.
Prophylaxis and treatment of brain metastasis
After first line treatment in patients who had a good response to initial therapy (CR and PR), 148 cases were free of brain metastasis at diagnosis, and were potential candidates for prophylactic cranial irradiation (PCI). According to questionnaire results, 34 of 148 cases (23.0%) were eventually administered PCI, 109 (73.6%) were not, and five patients' (3.4%) data was not available.
Thirty of 298 patients (10.1%) developed brain metastasis at the time of diagnosis (13 of them had clinical symptoms). Twenty-two of 30 cases (73.3%) received radiotherapy, including 19 cases (63.3%) with whole brain radiation therapy (WBRT) and three (10%) with stereotactic radiotherapy (SRT). Chemotherapy drugs that may pass the BBB, such as temozolomide, lomustine, topotecan and irinotecan, were not used.
Discussion
This is the first multicenter survey investigating the status of epidemiology and treatment options of SCLC in Mainland China. Our survey reveals a drastic increase in the number of both lung cancer and SCLC patients in 2010 compared to 2005, a vivid reflection of the soaring incidence of lung cancer in recent years. The percentage of SCLC patients of all lung cancer patients is noteworthy as it is relatively constant over the years, meaning that the incidence of SCLC has increased simultaneously with NSCLC in China in the past five years. The majority SCLC patients were male in both 2005 and 2010. As has been reported in the US and Europe, the incidence of SCLC is decreasing; however, incidence in women is increasing.7,8 Because a large number of SCLC cases are attributable to cigarette smoking,9 the decreasing incidence of SCLC in developed countries reflects the reduction in both smoking prevalence and in the tar-concentration in cigarettes.7,8 However, a smoking prevalence remains high in the Chinese population and the consumption of tobacco continues to rise,10,11 which may imply a potential increase in the incidence of SCLC in China.
Lüchtenborg et al. reported that approximately 1% of SCLC patients were treated surgically;12 however, the results of our survey (4.7%, 14/298) were significantly higher, probably a result of the growing popularity of low-dose computed tomography screening for lung cancer; therefore solitary pulmonary nodules can be detected earlier. Surgeons are more likely to take active treatment when they find solitary pulmonary nodules. For LS-SCLC patients, the combined treatment of chemotherapy and concurrent chemoradiotherapy has gained worldwide acceptance and is recommended in several guidelines.4–6 In our study, the results of the hospital information questionnaire also revealed that chemoradiotherapy was the most accepted treatment mode for LS-SCLC patients, further verified in the analysis of the patient information questionnaire in our study. Concurrent chemoradiotherapy is the most recommended in guidelines.4,5 In our survey, however, sequential chemoradiotherapy, chemo-radio-chemo “sandwich” therapy, and chemotherapy alone were all represented. Potential factors that might influence the choice of treatment mode include performance status and the tolerability of the patient; most SCLC patients are elderly with basic diseases and poor heart and lung function, which influence the implementation of concurrent chemoradiotherapy. For patients with ES-SCLC, chemotherapy alone is the standard treatment.4–6 In our survey, radiotherapy was used in a proportion of patients with extensive stage (22.4%). These patients received palliative radiotherapy in addition to chemotherapy in order to ease local symptoms, which was still in accordance with the principles addressed in guidelines.
EP/CE are the most recommended first-line chemotherapy regimens for SCLC.4–6 In our survey, 92.6% of the patients were treated with an EP/CE regimen as first-line therapy, reflecting strict adherence to guidelines. PCI is recommended in patients who respond well to first-line treatment.4–6 The relatively low percentage (23%) of patients who eventually received PCI in our survey was mainly ascribed to the poor performance status of these patients. Single-agent topotecan and CAV are recommended in second-line chemotherapy.4,5 The results shown in our survey are primarily in line with these suggestions.
The medical centers that participated in our survey are located in major cities with large populations and health care resources, and generally reflect the real world situation of epidemiology and the treatment of SCLC in Mainland China. The basic health care situation provided in small cities and rural areas was not included in our survey, the limitation of which should not be overlooked. Considering that a majority of cancer patients generally pursue medical service in local or national medical centers, our study is still of great significance in reporting SCLC treatment status. This survey was not a standard cross-sectional study and had a relatively small sample size. Considering that all centers involved in our survey are normalized cancer-treating hospitals with large numbers of cancer patients from all over China, the results shown in our survey are representative of the status of SCLC treatment, in accordance with international clinical guidelines, and can serve as a reference for guiding further clinical practice in China.
Conclusions
Guidelines direct clinical practice, but the limited knowledge of the present situation of clinical practice for SCLC patients in Mainland China serves as invaluable feedback for further clinical study. Our survey is the first study providing a revealing insight into the epidemiology and treatment status of SCLC in Mainland China, which is valuable for the improvement of future guidelines, health care standards, and the better distribution of health care resources.
Acknowledgments
We thank all of the doctors and patients who participated in the study. This study was supported by Beijing Municipal Science and Technology Project (D141100000214005) and GlaxoSmithKline (GSK) China.
Disclosure
No authors report any conflict of interest.
References
- International Agency for Research on Cancer. Globocan 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [Cited 25 Oct 2014.] Available from URL: http://globocan.iarc.fr.
- Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26:48–58. doi: 10.3978/j.issn.1000-9604.2014.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. 2013. NCCN Clinical Practice Guidelines in Oncology: small cell lung cancer. Version 1. [Cited 16 Dec 2014.] Available from URL: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl.
- Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;24(Suppl 6):vi99–vi105. doi: 10.1093/annonc/mdt178. [DOI] [PubMed] [Google Scholar]
- Zhi XY, Wu YL, Bu H, et al. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2011) J Thorac Dis. 2012;4:677–688. doi: 10.3978/j.issn.2072-1439.2010.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- Riaz SP, Lüchtenborg M, Coupland VH, Spicer J, Peake MD, Moller H. Trends in incidence of small cell lung cancer and all lung cancer. Lung Cancer. 2012;75:280–284. doi: 10.1016/j.lungcan.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- Yang GH, Ma JM, Liu N, Zhou LN. Smoking and passive smoking in Chinese, 2002. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26:77–83. (In Chinese.) [PubMed] [Google Scholar]
- Dan X, Yuankai S, Chen W. Tobacco in China. Lancet. 2014;383:2045–2046. doi: 10.1016/S0140-6736(14)60995-8. [DOI] [PubMed] [Google Scholar]
- Lüchtenborg M, Riaz SP, Lim E, et al. Survival of patients with small cell lung cancer undergoing lung resection in England, 1998–2009. Thorax. 2014;69:269–273. doi: 10.1136/thoraxjnl-2013-203884. [DOI] [PMC free article] [PubMed] [Google Scholar]


